Abstract
Schisandra chinensis, a widely used Chinese herbal medicine, was considered as central nervous system (CNS) drug for years. Both ethanol extracts (EES) and water extracts (WES) of it were applied clinically. Unfortunately, the difference of their efficacy and even effective material foundation of S. chinensis remains obscure. In this study, to explore the active constituents of S. chinensis, we compared pharmacodynamics and chemical profiles in vitro/in vivo of EES/WES for the first time using multiple chemical analysis, pharmacological and data processing approaches. It was proved that there was no significant difference in the anti-depressive effects between WES and EES. However, the contents of most components in vitro and in plasma were higher in EES than those in WES, which was unconvincing for their similar efficacy. Therefore, we further explored components of S. chinensis targeted onto brain and the results showed that 5 lignans were identified with definite absorptivity respectively both in EES and WES caused by the limitation of blood−brain barrier. Moreover, bioinformatic analysis predicted their anti-depressive action. Above all, the systematic strategy screened 5 brain-targeted effective substances of S. chinensis and it was suggested that exploring the components into nidi would promote the studies on herbs effective material basis.
KEY WORDS: Schisandra chinensis, Ethanol extracts, Water extracts, Pharmacodynamics, Chemical profiles, Components into nidi, Effective material basis, Traditional Chinese medicine
Graphical abstract
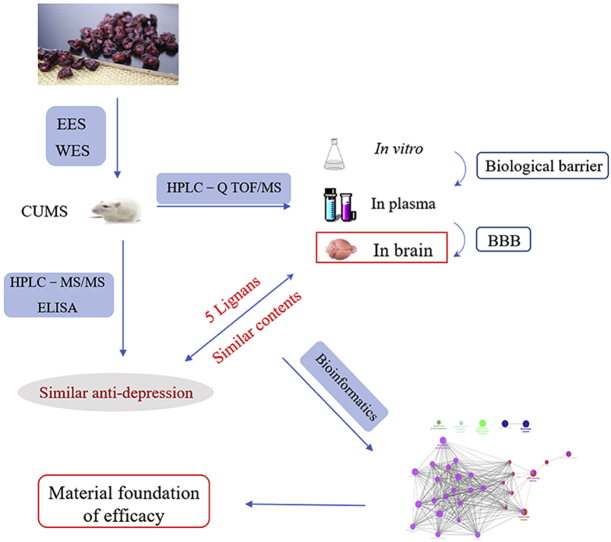
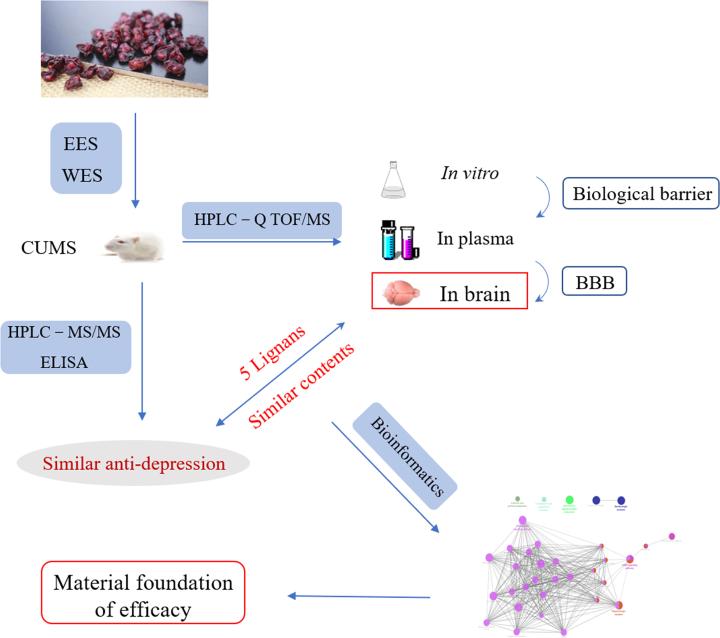
The research strategy for finding the material basis for efficacy of Chinese traditional medicine Schisandra chinensis was studied. The chemical profiles and pharmacodynamics of ethanol extracts (EES) and water extracts (WES) were compared and analyzed to achieve this goal.
1. Introduction
Schisandra chinensis (Turcz.) Baill, a Magnoliaceae family traditional Chinese medicine (TCM), was abundant in orient with related records traced back before Christ1,2. Based on extensive studies done by researchers, complex and various chemical ingredients have been identified3, 4, 5, abounding in triterpenoids, lignans, polysaccharides, organic acids, and essential oils. Recently, S. chinensis has attracted increasing attention for its high nutritional and officinal values6,7. In previous reports, S. chinensis has been proved to be effective in alleviating diverse central nervous system (CNS) diseases and our past research demonstrated that S. chinensis has satisfying anti-depression action8, 9, 10, 11. Over the years, ethanol extracts (EES) and water extracts (WES) have been widely applied clinically, whereas WES was considered as a traditional administration for humans for thousands of years. However, the difference of the pharmacodynamics of these refinements has always been neglected and even the active constituents of S. chinensis has been indistinct for now.
Interestingly, our study suggested that there was no significant difference in the anti-depressive effects between WES and EES though contents of most components in EES were higher than those in WES in vitro and in plasma samples. According to published literatures, blood−brain barrier (BBB) possesses high selectivity for foreign materials, limiting the delivery of CNS drugs to reach the site of action in brain12,13. Thus, we speculated that as a natural anatomical gateway, BBB may adjust the type and content of S. chinensis components into the nidi to further display pharmacodynamic.
In present research, we explored the selection of BBB on the constituents of S. chinensis by comparing the therapeutic mechanism and chemical contents in vitro/in vivo of EES/WES, to ascertain its brain-targeted effective substances. The exploration strategy was shown as Fig. 1. In brief, we proved the similar anti-depression action of WES and EES on chronic unpredictable mild stress (CUMS) depressive rats by measuring the levels of neurotransmitters and cytokines. Then, after contrast research on the chemical profiles of EES and WES in vitro/in vivo, only 5 lignans, schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N, could be identified in the brain lesions with similar contents in both EES and WES, which might be selected and leveled by BBB independent on their contents in vitro or in plasma. It was indicated that the 5 brain-oriented components might contribute to the similar effect of WES and EES greatly. Furthermore, bioinformatics was employed to predict the pharmacology of these 5 potential active ingredients, suggesting their potential anti-depression effects on modulating neurotransmitters and inflammatory pathways respectively. This research suggested that ingredients pointed at lesions were vital for the pharmacodynamics of TCM and the established integrated strategy on nidi-directed ingredients was significant for the development of studies on herbs effective material basis.
Figure 1.
The research strategy for finding the material basis for efficacy of Schisandra chinensis by comparing the chemical profiles and pharmacodynamics of EES and WES.
2. Materials and methods
2.1. Materials and reagents
The fruits of S. chinensis were purchased from the TCM dispensary—Tongrentang (Shenyang, China) grown in Ji'an, Jilin, China. Fluoxetine, bifendatatum, dopamine (DA), norepinephrine (NE), serotonin (5-HT) were all purchased from Sigma (St. Louis, MO, USA). The reference substances of schisandrol A, gomisin D, gomisin J, schisandrol B, gomisin O, epigomisin O, schisandrin A, schisandrin B and gomisin N were obtained from Yuanye of Biological Engineering (Shanghai, China). The commercial enzyme-linked immunosorbent assay (ELISA) kits of corticosterone (CORT), interleukin-1b (IL-1β), cyclooxygenase-2 (COX-2), tumor necrosis factor α (TNF-α) and tyrosine protein kinase (TPK) were purchased from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China).
2.2. Animals
Male Sprague–Dawley rats weighing between 200–250 g were used in present study. Standards for Animal Experimentation of Shenyang Pharmaceutical University were used in all experiments, which was in line with the rules of the Animal Ethics Committee of the institution (Shenyang, China). Before the experiments, rats were housed in groups at indoor temperature with 40%–60% humidity for seven days. Food and water were available ad libitum. Rats were divided into control, CUMS, WES, EES and positive groups.
2.2.1. CUMS procedure
CUMS protocol used in this study was derived from a model established by Willner14,15. Rats were suffered from various stressors for 28 days. The process of CUMS in the first 14 days was shown as Supporting Information Table S1 and was repeated in the next 14 days. The experiment process was shown as Supporting Information Fig. S1. The rats in CUMS, WES, EES and positive groups were placed in a different room from the rats in control group, and they were bear two or three stressors every single day. In additional, all stressors were inflicted randomly.
2.2.2. Behavioral tests
Forced-swim test (FST), locomotor activity test and novelty suppressed feeding (NSF) test were conducted during the animals’ light cycle. Rats were gavage ahead of time and given 1 h to habituate to the room before behavioral tests.
2.2.2.1. FST test
It was carried out as designed before. Rats were coerced to swim separately in a metallic box (18 cm in diameter × 40 cm in height) full with water (approximately 30 cm deep at 25 °C). The observation time on the rats was 6 min and the total immobile time in last 4 min was recorded, which was defined as short of activities in spite of needful movements avoiding their heads into water.
2.2.2.2. Locomotor activity test
Spontaneous locomotor activity of the experimental rats was proceeded in an open filed analysis system (Jiliang Bio-Apparatus Co. Ltd., Shanghai, China) to exclude the possibility that the change of the immobility time in the FST was resulted by the locomotor activity. The analysis system was placed in a dark and quiet room. The total path of spontaneous locomotive was observed for 5 min. The equipment was cleaned using 75% ethanol after each test.
2.2.2.3. NSF test
Rats were abstained from food for 24 h and placed in a corner of a top-opening square box individually. The box covered with 2 cm padding and 12 food pellets placed in the center. The feeding latency period to eat (up to 5 min) was recorded. Eating was defined as chewing or biting, not merely sniffing or toying with the food.
2.3. Quantitative analysis of neurotransmitters using HPLC–MS/MS
2.3.1. Working standard solutions and sample solutions
We prepared the standard stock solutions (1.0 g/mL) of NE, DA, 5-HT and internal standard isoprenaline stock solution in methanol respectively (stored at 4 °C). In addition, we prepared the mixed brain tissue standard solution with different concentrations as follow: DA of 1.00, 2.00, 10.00, 20.00, 100.00 and 200.00 μg/mL; NE, 5-HT of 5.00, 10.00, 50.00, 100.00, 500.00 and 1000.00 μg/mL. Besides, the quality control (QC) samples were prepared ditto. The working solution of the internal standard (IS) at 10 μg/mL was prepared.
Twenty-four hours after all the behavior tests, all the experimental rats were killed and brain samples were stored at –80 °C. The brain samples were homogenized in a 10-fold volume of methanol and centrifuged at 4 °C, 12,000 rpm (TGL-16, Cence Xiangyi, Hunan, China) for 10 min. 600 μL supernatant was transferred to a 1.5 mL centrifugal tube, then added 60 μL IS solution and 60 μL methanol into it and the mixture was vortexed for 45 s. Next, the solution was blown with air at 35 °C to dry. After that, the residue was dissolved in 100 μL methanol. Then, the solution was vortexed for 5 min and centrifuged at 4 °C, 12,000 rpm (TGL-16) for 10 min. Finally, 3 μL supernatants was used for the HPLC–MS/MS analysis.
2.3.2. Conditions of HPLC–MS/MS
In this study, an XR LC-20AD Prominence™ HPLC system (Shimadzu, Kyoto, Japan) combined with a QTRAP™ 4000 MS/MS system (AB Sciex, Redwood City, CA, USA) was used. The chromatographic separation was proceeded on a Phenomenex OOD-4496-EO (2.6 μm, 100 mm × 4.6 mm) column and the column temperature was set at 30 °C. The mobile phase was water–0.1% formic (A) and acetonitrile (B) at 0.4 mL/min. The gradient elution program was performed as follows (t in min): 10% B maintained for 0.5 min, increased to 20% at 1.0 min, increased to 90% at 3.0 min, and then decreased to 10% and held for 2.0 min for equilibration. The MS was operated in positive ion multiple reaction monitoring mode. Supporting Information Table S2 showed all optimized parameters. The other parameters were listed as follows: ion spray voltage of 4500 V, curtain gas of 20 psi; source temperature of 500 °C; ion source gas 1 and gas 2 of 50 psi.
We abide by the U.S. Food and Drug Administration Guidance for Industry Bioanalytical Method Validation and European Medicines Agency Guidelines Principle in this study. The inspection items included calibration curve, selectivity, accuracy and precision, lower limit of quantification (LLOQ), matrix effect and stability. Samples were prepared according to “sample preparation”. On every validation day, a series stock solution was added to brain samples to prepare the QC and calibration standards samples. The concentrations of neurotransmitters were calculated by calibration curves.
2.4. Qualitative and semi-quantitative analysis of contents of S. chinensis using HPLC–Q-TOF/MS
2.4.1. Preparation of samples
Samples were smashed into powder and sieved with 60 mesh. The medicine powder was extracted by reflux in 75% ethanol or water for 1 h for 3 times. The extracts were collected and being filtered. Then, under vacuum, the filtrate was concentrated. The residues were then dissolved in water at concentration of 0.9 g/mL as the intragastric solutions (WES or EES). The dosage used for rats was according with human dosage listed on the Chinese Pharmacopoeia. To ensure the parallelity of the detected samples in vivo and in vitro, the method for preparation of test solution of WES and EES in vitro was same as the intragastric solutions.
One hour after the last administration, we collected plasma samples from suborbital vein and transferred into heparinized tubes. The plasma samples were immediately centrifuged at 4000 rpm (TGL-16) for 10 min and then stored at −80 °C. Protein precipitation was adopted to extract the plasma samples in this study. 1 mL supernatant of plasma was evaporated to dryness under a gentle stream of nitrogen at 35 °C. Then, the residue was reconstituted with 100 μL solution of bifendatatum (1 mg/mL) and vortex-mixed for 3 min, and sonicated for 5 min followed by centrifuged at 12,000 rpm (TGL-16) for 5 min. Finally, 5 μL supernatants was injected into the HPLC–Q-TOF/MS system for analysis.
The rats were weighed and anesthetic using chloral hydrate. Then, we opened the rats’ abdominal cavity and freed the heart tissue from their chest. Opened the constant-current pump to pump 200 mL of warm normal saline from the heart to clear the blood. Rapid whitening of liver, eyes and claws is an effective observation index for blood expulsion. At last, rat brain tissues were collected and stored at −80 °C. Five-fold methanol was added to the brain samples, and the mixtures were homogenized on the ice and then centrifugated at 4 °C, 12,000 rpm (TGL-16) for 10 min. The following procedures were the same as that for the plasma samples.
2.4.2. Conditions of HPLC–Q-TOF/MS
The constituents of S. chinensis in vivo/in vitro were analyzed on the Agilent 1260 HPLC system (Santa Clara, CA, USA) interfaced with a Triple TOF 5600+ (AB Sciex, Redwood City, CA, USA) outfitted with a DuoSpray™ ion source (Sciex). The Phenomenex OOD-4496-EO (2.6 μm, 100 mm × 4.6 mm) column was used for the separation at a flow rate of 0.8 mL/min in LC−MS grade water (A) and acetonitrile (B). The gradient elution program was as follows: 10% held for 5.0 min; increased to 40% at 10 min; increased to 57% at 75 min; increased to 90% at 85 min and held for 5 min followed by 10% for 5.0 min for equilibration. The scan acquisition for the analysis was over the range of m/z 100–2000 in positive ion mode. The other parameters were listed as follows: ion spray voltage of 5500 V; declustering potential (DP) of 80 V; the turbo spray temperature of 550 °C; curtain gas of 30 psi; ion source gas 1 and gas 2 of 50 psi. Nitrogen was kept as nebulizer and auxiliary gas. SciexPeakView® (version 2.2) and Master View™ (version 1.1) software were used for the further data process.
2.5. Statistical analyses
All values were showed as means ± SD in the study. GraphPad Prism (GraphPad software, San Diego, CA, USA) and SPSS (SPSS Inc., Armonk, NY, USA) were used for the data process.
3. Results
3.1. Exploration of the anti-depressant effects of EES and WES
3.1.1. Improvement of the behavioral deficits induced by CUMS
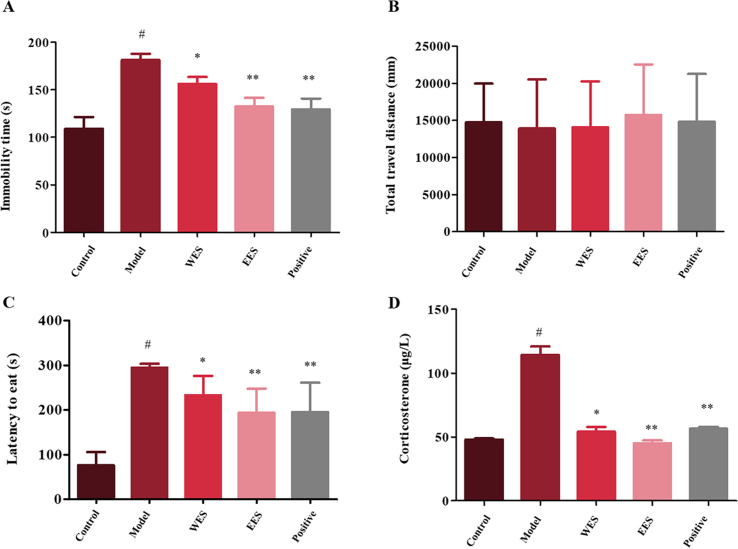
After 4-week CUMS process and EES/WES/fluoxetine (10 mg/kg) treatment, rats were assessed on their behaviors and the level of corticosterone in plasma to evaluate the success of depression model. All treatments showed no significant alterations to the total path in spontaneous locomotive activity test, which suggesting that the locomotor activity has few impacts on the alteration of immobility time in FST. Moreover, the process of CUMS successfully induced depression on rats according to depressive symptoms showed in behaviors (immobility time and latency time remarkably prolonged, Fig. 2A and C, n = 6, P < 0.01), and increased level of corticosterone in rats’ plasma samples compared with the control group (Fig. 2D, n = 6, P < 0.01). However, they were consistently restored after pharmacological treatments (Fig. 2A, C and D, n = 6, *P < 0.05, **P < 0.01), and have no significant difference between WES and EES groups (Fig. 2).
Figure 2.
(A) The immobility time in FST of groups. (B) The travel paths in the locomotor activity test. (C) The latency time to feed in NSF test. (D) The level of corticosterone in plasma samples. Values shown are means ± SD, n=6; #P < 0.01 compared with control group; *P < 0.05, **P < 0.01 compared with model group.
3.1.2. Effects of WES and EES on neurotransmitters and inflammation markers
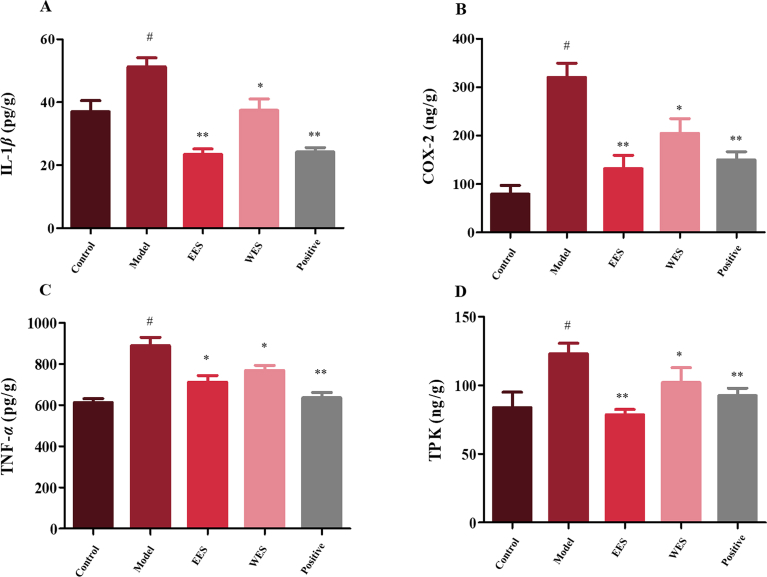
In this study, an HPLC–MS/MS method was employed to detect 5-HT, NE and DA in rat brain samples. The retention time of analytes or IS was without interference based on the chromatography (Supporting Information Fig. S2). The developed method was validated with respect to the lower limit of quantification, calibration curve, accuracy and precision, matrix effect and stability (Supporting Information Tables S3–7). It was suggested that the HPLC–MS/MS method was well validated to quantify the target analytes in this study. Besides, inflammatory enzyme COX-2, TNF-α, TPK and IL-1β were measured in brain samples using ELISA assays. Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), was selected as the positive drug.
In CUMS group, obvious reduction of the expression of neurotransmitters and significant elevation of the levels of inflammatory markers were observed as shown in Table 1 and Fig. 3. However, the samples from both EES and WES groups showed significantly reverse tendency in both two tests based on the results of t test, and there was no obvious deference between them.
Table 1.
The concentrations of 5-HT, DA, NE in samples WES and EES.
| Analyte | Control group (ng/mg) | CUMS group (ng/mg) | WES (ng/mg) | EES (ng/mg) | Fluoxetine group (ng/mg) |
|---|---|---|---|---|---|
| 5-HT | 19.9±4.94 | 4.34±1.89## | 8.04±2.14* | 15.20±3.77** | 13.9±2.26** |
| DA | 5.49±2.29 | 2.40±1.22# | 3.54±1.28 | 6.60±2.04* | 5.51±3.13 |
| NE | 6.93±1.08 | 2.41±0.97# | 5.30±1.60* | 7.82±1.16** | 5.50±1.38* |
For statistical significance **P < 0.01, *P < 0.05 compared with CUMS group, ##P < 0.01, #P < 0.05, compared with control group.
Figure 3.
Effects of EES and WES on inflammation markers. Values shown are means ± SD; #P < 0.01 compared with control group; *P < 0.05, **P < 0.01 compared with CUMS.
3.2. Analysis of chemical profiles in vivo and in vitro using HPLC–Q-TOF/MS
3.2.1. Identification of constituents in WES and EES
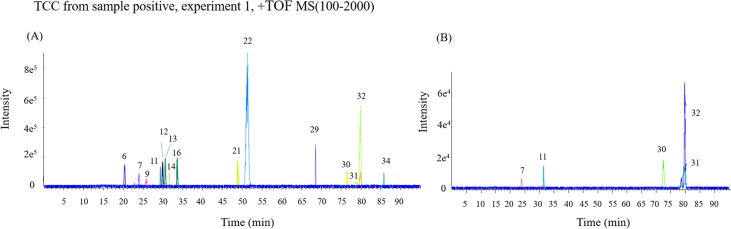
An efficient and selective HPLC–Q-TOF/MS method was employed to identify and semi-qualify the ingredients of WES and EES in vitro/in vivo. The absolute value of errors was defined lower than 3. Total 45 constituents including 33 lignans, 10 triterpenes and 2 organic acids were identified in EES, while 36 components (the first 36 ingredients in Table 2, including 30 lignans, 4 triterpenes and 2 organics) of them were also found in WES, all their structures were shown in Supporting Information Fig. S3. Moreover, 15 lignans were found in the plasma samples and 5 of them (schisandrol A, gomisin J, schisandrin A, gomisin N and schisandrin B) were found in the brain samples both in EES and WES. All components of EES and WES in vitro/in vivo were listed in Table 2 and total compound chromatogram (TCC) for ingredients in vivo shown as Fig. 4. Nine lignans were confirmed with standard substance among them (Supporting Information Fig. S4).
Table 2.
The components in WES and EES in vitro/in vivo.
| Peak No. | Identification | Error ▵ (ppm) | Retention time (min) | [M+H]+/[M+Na]+ (m/z) | Fragment ions (m/z) |
|---|---|---|---|---|---|
| 1 | Citric/isocitric acid | 2.2 | 2.02 | 193.0278 [M+H]+ | 216.1779, 148.1526, 129.0188, 111.0081, 87.0087 |
| 2 | 1,5-Dimethyl citrate | 0.7 | 5.05 | 221.0583 [M+H]+ | 185.0400, 157.0493, 129.0185, 111.0082, 87.0088 |
| 3 | Schindilactone D | 2 | 15.05 | 561.2341 [M+H]+ | 501.2136, 423.1792, 401.1073, 320.6077, 242.2852 |
| 4 | Schisanwilsonin B | 0.0 | 15.06 | 501.2046 [M+H]+ | 483.1986, 455.2089, 437.1956, 365.1784, 317.1752 |
| 5 | PRXFPPAVWQDTHT-UHFFFAOYSA-N | 0.7 | 16.82 | 419.1992 [M+H]+ | 426.1799, 401.1980, 395.1494, 369.1716, 337.1453 |
| 6 | Schisanwilsonin Da | 0.6 | 18.39 | 503.2203 [M+H]+ | 525.2078, 425.1567, 354.1457, 339.1205, 266.0913 |
| 7 | Schisandrol Ab | 0.4 | 24.49 | 433.2148 [M+H]+ | 400.1885, 384.1930, 346.1413, 331.1184, 300.0996 |
| 8 | Schisantherin E | 0.8 | 27.35 | 539.2203 [M+H]+ | 561.2099, 439.1719, 417.1922, 357.1347, 342.1487 |
| 9 | Gomisin Da | 0.9 | 27.79 | 531.2152 [M+H]+ | 485.2177, 467.2040, 401.1594, 326.1139, 353.1371 |
| 10 | Rubrisandrin A | 1.5 | 29.14 | 389.1886 [M+H]+ | 389.1977, 357.1684, 325.1451, 288.0977, 227.0713 |
| 11 | Gomisin Jb | 1.5 | 29.44 | 389.1886 [M+H]+ | 357.1708, 326.1457, 319.1162, 287.0908, 255.0646 |
| 12 | Gomisin Oa | 0.8 | 30.02 | 417.1835 [M+H]+ | 399.1813, 369.1630, 337.1419, 316.0960, 277.1228 |
| 13 | Epigomisin Oa | 0.8 | 30.33 | 417.1835 [M+H]+ | 399.1813, 369.1630, 337.1419, 316.0960, 277.1228 |
| 14 | Wilsonilignan Ca | 0.8 | 31.12 | 417.1835 [M+H]+ | 399.1813, 368.1681, 355.1648, 314.1040, 299.0895 |
| 15 | Schisphenlignan A | 1.7 | 31.59 | 523.189 [M+H]+ | 423.1425, 383.1530, 352.0834, 341.1024, 326.1151 |
| 16 | Schisandrol Ba | 0.8 | 31.90 | 417.1835 [M+H]+ | 399.1804, 369.1700, 330.1100, 229.0921, 263.1079 |
| 17 | Angeloygomisin H/Tigloylgomisin H | 1.0 | 40.69 | 501.2412 [M+H]+ | 483.2374, 451.2159, 401.1963, 370.1779, 337.1443 |
| 18 | Benzoylgomisin H | −0.4 | 43.94 | 523.2254 [M+H]+ | 545.2141, 515.1654, 503.1874, 455.1392, 355.1554 |
| 19 | Benzoylgomisin Q | −0.6 | 44.56 | 553.2359 [M+H]+ | 453.1896, 387.1882, 356.1620, 343.1177, 301.1071 |
| 20 | Angeloylgomisin Q/Tigloylgomisin Q | −0.5 | 46.07 | 531.2516 [M+H]+ | 553.2389, 453.1875, 431.2050, 371.1477, 341.1371 |
| 21 | Gomisin Ca | 0.2 | 50.60 | 537.2046 [M+H]+ | 559.1936, 437.7814, 415.1754, 341,1380, 299.0911 |
| 22 | Benzoylgomisin Pa | 0.2 | 50.62 | 537.2046 [M+H]+ | 437.1569, 415.1754, 371.1482, 340.1303, 310.1189 |
| 23 | Schisanhenol | 0.0 | 62.02 | 403.2042 [M+H]+ | 371.1845, 340.1667, 301.1063, 271.0948, 270,0885 |
| 24 | Schisantherin D | −2.9 | 64.26 | 521.1733 [M+H]+ | 421.1237, 399.1439, 355.1172, 325.1084, 295.0084 |
| 25 | gomisinE | −0.5 | 69.17 | 515.2203 [M+H]+ | 469.2176, 438.1765, 423.1801, 385.1647, 355.1543 |
| 26 | Schisantherin B | −0.5 | 69.20 | 515.2203 [M+H]+ | 469.2207, 421.1801, 385,1635, 355,1531, 302.0772 |
| 27 | Gomisin F | −0.5 | 69.25 | 515.2203 [M+H]+ | 469.2176, 438.2017, 385.1638, 355.1537, 315.0859 |
| 28 | Schisantherin C | −0.5 | 70.17 | 515.2203 [M+H]+ | 469.2222, 438.1801, 385.1635, 355.1531, 316.0940 |
| 29 | Gomisin Ga | 0.0 | 71.18 | 537.2046 [M+H]+ | 491.2034, 475.2089, 407.1452, 316.0940, 302.0772 |
| 30 | Schisandrin Ab | −0.9 | 77.81 | 417.2199 [M+H]+ | 417.2251, 402.2034, 386.2088, 316.1291, 301.1059 |
| 31 | Gomisin Nb | −0.5 | 80.27 | 401.1886 [M+H]+ | 386.1718, 370.1772, 300.4007, 285.0755, 242.0929 |
| 32 | Schisandrin Bb | −0.4 | 80.36 | 401.1886 [M+H]+ | 386.1718, 370.1772, 300.4007, 285.0755, 242.0929 |
| 33 | BenzoylisogomisinO | −0.9 | 84.84 | 521.2097 [M+H]+ | 543.1984, 421.1605, 399.1779, 369.1692, 315.0846 |
| 34 | Schisandrin Ca | −0.1 | 85.09 | 385.1573 [M+H]+ | 385.1636, 355.1532, 338.1132, 315.0857, 299.0876 |
| 35 | Schisanlactone E | −0.5 | 85.60 | 469.3242 [M+H]+ | 451.3176, 433.3098, 423.3237, 337.2525, 311.2370 |
| 36 | Mexicanolide | −0.8 | 86.61 | 469.2148 [M+H]+ | 491.2019, 476.0341, 435.3217, 387.1814, 339.1210 |
| 37 | Lancifodilactone C | 0.9 | 14.64 | 545.2386 [M+H]+ | 527.2292, 485.2170, 467,2073, 439.2131, 403.1909 |
| 38 | Schisanlactone B | 1.5 | 15.52 | 501.2119 [M+H]+ | 487.2031, 425.1936, 345.1683, 379.1521, 288.0826 |
| 39 | Schisanwilsonin A | 2.8 | 16.94 | 523.1914 [M+Na]+ | 469.1657, 425.1433, 353.0876, 337.1054, 311.0901 |
| 40 | Schindilactone A | 0.7 | 20.30 | 543.2152 [M+H]+ | 563.2048, 503.1967, 463.1752, 437.2334, 407.1871 |
| 41 | Gomisin S | 2.9 | 30.27 | 419.2077 [M+H]+ | 402.2005, 369.1720, 337.1448, 323.1274, 301.1042 |
| 42 | Pregomisin | −0.3 | 38.36 | 413.1934 [M+Na]+ | 391.1702, 359.1829, 351.1555, 327.1581, 299.1555 |
| 43 | Kadsuphilactone B | 0.7 | 51.14 | 505.2924 [M+H]+ | 483.3090, 464.2474, 438.6657, 425.1936, 345.1663 |
| 44 | Propindilactone G | −0.2 | 69.49 | 514.2566 [M+H]+ | 479.4076, 400.1871, 384.1931, 369.1698, 338.1511 |
| 45 | Schisanlactone D | −2.5 | 91.21 | 453.3325 [M+H]+ | 435.3240, 313.2222, 270.1898, 245.1885, 111.1018 |
Identified in plasma samples.
Identified in both plasma and brain samples.
Figure 4.
TCC of (A) plasma and (B) brain samples in positive ion mode.
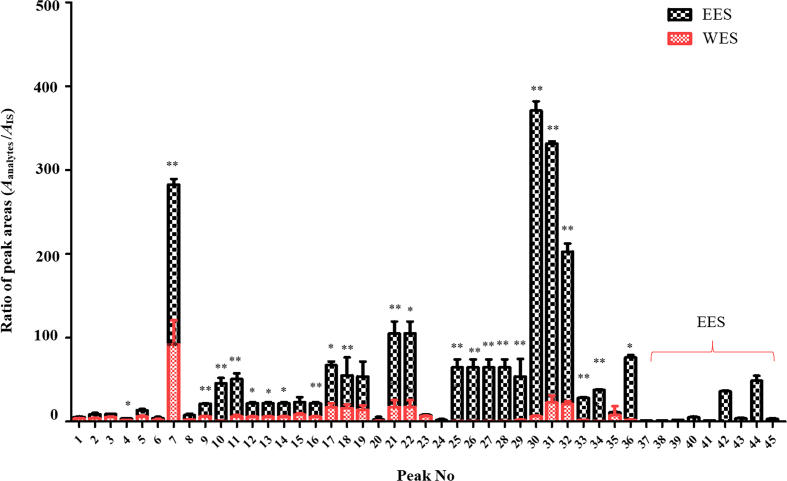
3.2.2. Semi-quantitation of constituents in EES and WES
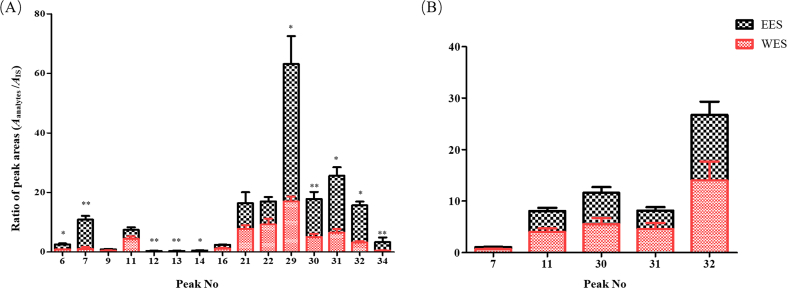
In present study, bifendate was taken as the IS for semi-quantitative analysis. The contents of all lignans except for 9 included benzoylgomisin Q, angeloylgomisin Q, schisantherin D, schisantherin E, schisanhenol, schisanwilsonin D, schindilactone D, schisphenlignan A and schisanlactone E were decreased significantly in WES but those of organic acids and triterpenes were similar in EES and WES in vitro (Fig. 5). In addition, in the plasma samples, contents of 10 lignans, schisanwilsonin D, schisandrol A, gomisin O, epigomisin O, wilsonilignan C, gomisin G, schisandrin A, gomisin N, schisandrin B and schisandrin C, in EES were obviously higher than those in WES, whereas others were basically equal in both groups. However, schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N were figured out not only in the brain samples of EES but in WES, and there was no significant difference between their contents (Fig. 6), which might be caused by the selectivity of BBB and they might be the direct acting components for CNS disease of S. chinensis. Bioinformatics was adopted following to predict the pharmacodynamics of these 5 lignans.
Figure 5.
Peak areas of lignans corrected by Bifendate in vitro. For statistical significance, *P < 0.05, **P < 0.01.
Figure 6.
Peak areas of lignans corrected by Bifendate in vivo. (A) Contents in plasma samples. (B) Contents in brain samples. For statistical significance, *P < 0.05, **P < 0.01.
3.2.3. Targets prediction of contents in brain by bioinformatic analysis
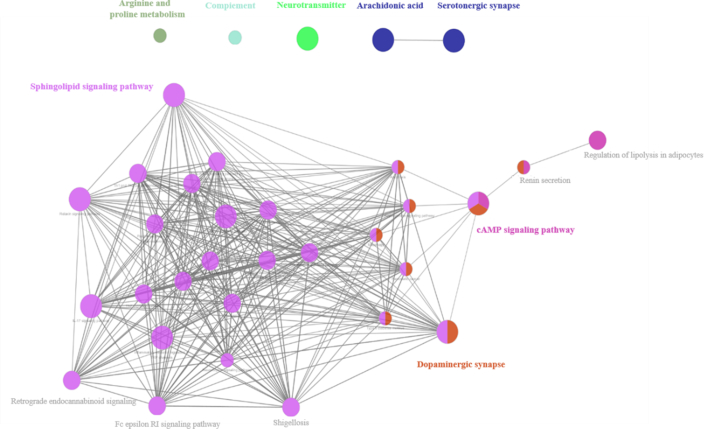
The prognostic gene targets of these constituents were analyzed by Swiss Target Prediction (http://www.swisstargetprediction.ch/). Furthermore, pathway enrichment analysis was proceeded on Cytoscape using Cluego. The target pathway network of the 5 potential active chemical compositions was shown as Fig. 7.
Figure 7.
Pathways of all active chemical compositions based on bioinformation analysis.
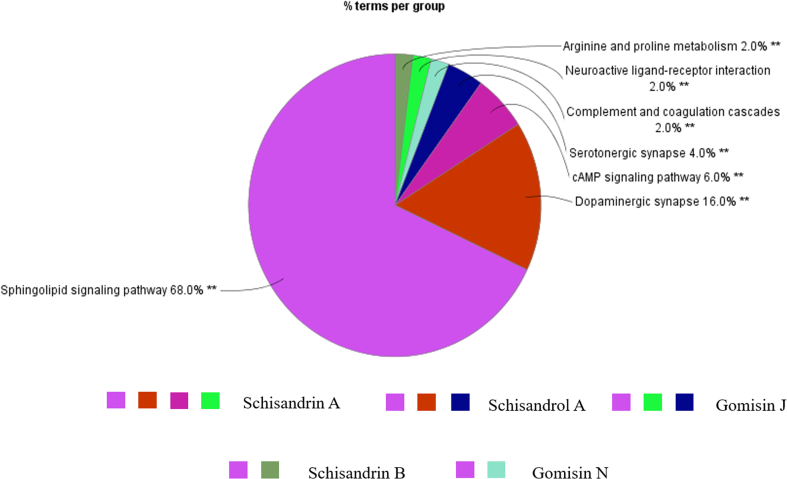
The main functional groups in vivo modulated by the 5 lignans, schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N, were shown as Fig. 8. The predicted pathways were mainly concentrated on dopaminergic synapse, serotoninergic synapse, cAMP signaling pathway and sphingolipid signaling pathway, which were largely involved in neurogenic diseases plus the inflammation, and they all have relevant to the etiology of depression. This result showed their potential antidepressant effects and indicated that they were the strongest candidates for the effective constituents of S. chinensis.
Figure 8.
The enrichment degree of target pathways on function groups. Corresponding functional groups related to schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N respected with relevant color, respectively.
4. Discussion
4.1. Comparing the anti-depressive effects of EES and WES
In this study, we compared the antidepressant effects of WES and EES first. The results of behavioral tests and the level of CORT were indicated the success of CUMS model and the similar activity of anti-depression of EES/WES preliminarily. Previous researches have been demonstrated that neurotransmitters and inflammation were all involved in the etiology of depression16, 17, 18. Therefore, we detected neurotransmitters 5-HT, DA, NE and cytokines IL-1β, Cox-2, TPK, TNF-α in brain to further compare the pharmacodynamics of these two groups at the molecular level. It was proved that EES showed a little bit stronger effect with no significant difference (P > 0.05) on depression than WES in reversing the levels of analyzed endogenous substances. Thereof, it was speculated that resemblance effective material basic might be responsible for the similar effects of EES and WES. For exploring the active substances of S. chinensis, we following proceeded comparative studies on the chemical ingredients in vitro/in vivo of EES and WES.
4.2. Chemical profiles of EES and WES in vitro/in vivo
It has been figured out 45 constituents in EES, whereas 36 of them were identified both in EES and WES. The 9 components peculiar to EES covered 6 triterpenes and 3 lignans with low-polarity and low-contents, which might be resulted in their inexistence in WES. Furthermore, as shown in Fig. 5, the results of semi-quantification illuminated that the contents of 33 ingredients (27 lignans and 6 triterpenes) were higher in EES. From the above, not only the compound types but also the contents of most components in EES were more than WES. Considering their similar anti-depressive activity, it was suggested that the highly concentrated ingredients in EES in vitro might not be absorbed in vivo in quantity and some of them even couldn't reach the nidus on account of the selectivity of diverse biological barriers. The exploration of chemical profiles of EES and WES in vivo was conducted next.
The components in plasma are the potential direct acting ingredients of TCM19, 20, 21. In this study, 15 lignans have been identified in the plasma samples of both WES and EES group. Among them, contents of 10 lignans were significantly higher in EES than WES with varied absorptivity independent upon their contents in vitro, while others were nearly equal, as shown in Table 3. It was indicated the selectivity of organisms on ingredients of S. chinensis and there might be saturation points for the absorption of part of components. Besides, the complicated physiological process, including metabolism, tissue distribution and such, for each constituents according to their characteristic physicochemical property based on the theory of TCM serum pharmaco-chemistry might also be an important factor resulted in their diverse absorptivity22. For the stability of dibenzocyclooctene lignans’ parent nucleus, the metabolic pathways of the lignans were mainly focused on their side chains, included hydroxylation, demethylation, phosphorylation, dehydration and even the mutual transformation between prototypes. The proposed metabolic pathways of transform process between schisandrols A/B and schisandrins A/B were shown as the example of lignans metabolism in Supporting Information Fig. S5. However, the specific metabolic mechanism (involved with the metabolic rate, distribution in bodies and so on) of these bioactive components needs further investigation. Although these results might lead to preferable anti-depressant effect of EES, they were farfetched explaining the similar efficacy of these two groups. It was worthy to comparatively investigate the constituents, permeated BBB absorbed in the position of depression—brain, of WES and EES since they might contribute to the effects of S. chinensis greatly. To avoid the influence of the constituents in numerous blood capillary in the brain tissue, the in vivo perfusion method was used to wash away the blood in rats in this study.
Table 3.
The ratio of Aanalytes/AIS of EES and WES in vitro/in vivo.
| Lignan |
In vitro (Aanalytes/AIS) |
Ratio (E/W) | Plasma (Aanalytes/AIS) |
Ratio (E/W) | Brain (Aanalytes/AIS) |
Ratio (E/W) | |||
|---|---|---|---|---|---|---|---|---|---|
| WES | EES | WES | EES | WES | EES | ||||
| 6 | 1.84±0.58 | 2.19±0.27 | 1.19 | 0.84±0.01 | 1.76±0.54 | 2.08* | – | – | – |
| 7 | 92.07±18.61 | 190.60±6.71 | 2.07** | 1.02±0.38 | 9.88±2.13 | 9.67** | 0.64±0.30 | 0.41±0.36 | 0.64 |
| 9 | 6.13±0.59 | 15.32±0.37 | 2.50** | 0.41±0.35 | 0.54±0.20 | 1.32 | – | – | – |
| 11 | 6.17±0.26 | 44.75±6.62 | 7.25** | 4.42±1.37 | 3.02±1.54 | 0.68 | 3.98±2.30 | 4.10±1.30 | 1.03 |
| 12 | 5.79±0.59 | 16.72±1.68 | 2.89* | 0.02±0.01 | 0.41±0.12 | 20.50** | – | – | – |
| 13 | 5.69±0.67 | 16.07±1.48 | 2.83* | 0.02±0.01 | 0.38±0.18 | 19.06** | – | – | – |
| 14 | 6.03±0.81 | 17.17±2.06 | 2.85* | 0.12±0.05 | 0.52±0.20 | 4.33* | – | – | – |
| 16 | 5.53±0.61 | 17.07±1.62 | 3.09** | 1.13±0.53 | 1.29±0.28 | 1.14 | – | – | – |
| 21 | 17.53±7.12 | 87.62±14.15 | 5.00** | 7.79±2.68 | 8.62±1.33 | 1.11 | – | – | – |
| 22 | 20.03±7.48 | 85.12±11.97 | 4.25* | 9.19±4.56 | 7.82±3.31 | 0.85 | – | – | – |
| 29 | 1.71±0.79 | 52.19±20.88 | 30.58** | 16.92±3.48 | 46.23±18.76 | 2.73* | – | – | – |
| 30 | 6.45±0.75 | 364.49±10.98 | 56.52** | 5.04±0.84 | 13.06±2.97 | 2.60** | 5.53±3.74 | 6.07±3.54 | 1.10 |
| 31 | 22.73±8.63 | 308.96±2.78 | 13.59** | 6.47±2.28 | 19.13±4.94 | 2.96* | 4.54±3.77 | 3.62±2.11 | 0.80 |
| 32 | 20.79±3.48 | 181.85±9.68 | 8.75** | 3.19±1.09 | 12.47±2.87 | 3.91** | 13.99±5.78 | 12.74±3.13 | 0.91 |
| 34 | 0.56±0.12 | 37.11±0.96 | 66.22** | 0.48±0.23 | 2.83±2.73 | 11.63** | – | – | – |
For statistical significance, *P < 0.05, **P < 0.01. –Not applicable.
BBB, a high selectivity biological barrier structure, was composed of microvascular endothelial cells connected by tight junctions of the cerebral capillary endothelium and plenty of transporters, prevented substances in circulations from CNS. In present research, only 5 lignans (schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N), infiltrated BBB were figured out in rat brains with almost equivalent contents both in EES and WES, while those were all higher in plasma samples of EES (Table 3). It was demonstrated that the species absorbed in brain were definite and even their absorptive amount were leveled severally by BBB. Moreover, it was also indicated the limitation of BBB on medicines mainly according to their characteristic instead of their contents in blood or in vitro, which was of great significant for exploring the effective ingredients of neuroprotective TCM. Above all, these 5 lignans might be the chemical material foundation for similar effects of EES and WES. In order to ascertain the active substances of S. chinensis, the pharmacodynamics of potential active ingredients need to be explicit in further.
4.3. Ascertaining the active substances of S. chinensis
The bioinformatics analysis predicted the anti-depression effects of 5 lignans absorbed in brain on modulating the neurotransmitter and inflammation pathways. Besides, according to previous researches, CNS disease lead to the elevation of mitochondrial membrane potential-9 (MMP-9) and the degradation of tight junction proteins, which were responsible for the BBB destruction. Under the pathological state, antidepressants like fluoxetine and reboxetine could perfusion in the pathological brain to attenuate the damage of BBB23. Schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N are the liposoluble constituents and their molecular weight are close to fluoxetine, which was supported that their mechanisms of permeating BBB were similar with fluoxetine. In addition, neuroinflammation was caused by impaired BBB through releasing an excess of inflammatory factors24, 25, 26. Moreover, cytokines could in turn modulate MMP-9 activation and subsequent BBB disruption27. It has been demonstrated that schisandrol A, schisandrin A, schisandrin B, gomisin J and gomisin N all have anti-inflammatory and neuroprotective effects on LPS-induced inflammation in microglia in vitro mainly by inhibiting the expression of nuclear translocation of nuclear factor-кB (NF-кB) and diverse inflammatory markers as well as the mitogen-activated protein kinases (MAPK) signaling pathway, and then relieving the destruction of BBB28, 29, 30, 31, 32. Furthermore, accompanied by impaired BBB, the redox balance as well as mitochondrial function are disrupted, which is resulted in the inhibition of antioxidant enzymes expression simultaneously33,34. As reported, schisandrin A and schisandrin B manifested anti-oxidative effects on regulating the levels of diverse enzymes35,36. Besides, TLR4, belonging to Toll-like receptors (TLRs), is widely expressed in the central nervous system and plays an important role in inflammatory responses37. After interaction of TLR4 with ligands, the NF-κB was stimulated by MyD88-dependent pathways, which also caused damage of BBB38 (Supporting Information Fig. S6). Schisandrin B could downregulate the expression of proteins TLR4 and MyD88, also the levels of NF-κB based on previous investigations39. Moreover, schisandrol A, schisandrin A, schisandrin B, gomisin J and gomisin N could modulate the levels of neurotransmitters in CNS disorder rats38,40. Given the pharmacodynamic studies as above and the similar antidepressant effects of WES and EES concluded in this study, it was indicated that schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N were the pharmacodynamic substances for CNS diseasess of S. chinensis.
In conclusion, the study demonstrated that the absorptivity of TCM constituents in vivo did not depend on their contents in vitro entirely because of the high selectivity of organisms. Furthermore, the biological barriers in bodies could limit not only the species but also the quantity of components absorbed in vivo, and the constituents targeted on lesions were crucial for the effects of TCM.
5. Conclusions
The current study has demonstrated that BBB could level the quantity of schisandrol A, gomisin J, schisandrin A, schisandrin B and gomisin N respectively in brain samples in both EES and WES, which might be resulted in their similar antidepressant effects. Based on bioinformatics analysis and lots of previous researches, it has been suggested that the 5 lignans were the strong candidates for active substances of S. chinensis. Furthermore, the research proved the selectivity of bodied on TCM ingredients and provided a novel perspective for exploring the material foundation of TCM efficacy by considering the limitation of diverse biological barriers in organisms. It was suggested that the chemical constituents directed into locations of lesion were vital for TCM effects, which was expected to further advance the development of the study on effective substance basis of TCM.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (U1508220); Liaoning Distinguished Professor Project for Qing Li (2017, China) and Shenyang Pharmaceutical University Innovative Research Team.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.10.008.
Author contributions
Yiwen Zhang and Kaishun Bi conceived and designed the experiments; Xinyan Lv, Jiameng Qu, Xin Zhang, Mingyang Zhang, Qian Zhang and Hao Gao performed the experiments; Ran Liu, Huarong Xu, Yiwen Zhang and Qing Li analyzed the data; Yiwen Zhang wrote the paper.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supporting information
The following is the Supplementary data to this article:
References
- 1.Li M., Du Y., Wang L., Jiang L., Ma X., Zhou P. Efficient discovery of quality control markers for gastrodia elata tuber by fingerprint-efficacy relationship modelling. Phytochem Anal. 2017;28:351–359. doi: 10.1002/pca.2682. [DOI] [PubMed] [Google Scholar]
- 2.Wei B., Liu M., Chen Z., Wei M. Schisandrin ameliorates cognitive impairment and attenuates Aβ deposition in APP/PS1 transgenic mice: involvement of adjusting neurotransmitters and their metabolite changes in the brain. Acta Pharmacol Sin. 2018;39:616–625. doi: 10.1038/aps.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L., Li B., Liu X., Huang G., Meng X. Purification of six lignans from the stems of Schisandra chinensis by using high-speed counter-current chromatography combined with preparative high-performance liquid chromatography. Food Chem. 2015;186:146–152. doi: 10.1016/j.foodchem.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Wang Y., Wu W., Song J., Ruan H. Triterpenoids and lignans from the fruit of Schisandra sphenanthera. Fitoterapia. 2017;116:10–16. doi: 10.1016/j.fitote.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Jiang P., Lu Y., Chen D. Authentication of Schisandra chinensis and Schisandra sphenanthera in Chinese patent medicines. J Pharm Biomed Anal. 2016;131:263–271. doi: 10.1016/j.jpba.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Piao J., Liu L., Wang S., Shang H., He M., Quan N. Magnetic separation coupled with high-performance liquid chromatography-mass spectrometry for rapid separation and determination of lignans in Schisandra chinensis. J Sep Sci. 2018;41:2056–2063. doi: 10.1002/jssc.201701098. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y.H. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed Pharmacother. 2018;106:902–909. doi: 10.1016/j.biopha.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y., Lv X., Liu R., Zhang M., Liu H., Gao H. An integrated strategy for ascertaining quality marker of Schisandra chinensis (Turcz.) Baill based on correlation analysis between depression-related monoaminergic metabolites and chemical components profiling. J Chromatogr A. 2019;1598:122–131. doi: 10.1016/j.chroma.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 9.Wei M., Liu Y., Pi Z., Li S., Hu M., He Y. Systematically characterize the anti-Alzheimer’s disease mechanism of lignans from S. chinensis based on in-vivo ingredient analysis and target-network pharmacology strategy by UHPLC–Q-TOF-MS. Molecules. 2019;24:1203. doi: 10.3390/molecules24071203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi Y., Cheng X., Jing H., Yan T., Xiao F., Wu B. Effect of Alpinia oxyphylla—Schisandra chinensis herb pair on inflammation and apoptosis in Alzheimer's disease mice model. J Ethnopharmacol. 2019;237:28–38. doi: 10.1016/j.jep.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M., Xu L., Yang H. Schisandra chinensis fructus and its active ingredients as promising resources for the treatment of neurological diseases. Int J Mol Sci. 2018;19:E1970. doi: 10.3390/ijms19071970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeniya S., Kuwahara H., Daizo K., Watari A., Kondoh M., Yoshida-Tanaka K. Angubindin-1 opens the blood−brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J Control Release. 2018;283:126–134. doi: 10.1016/j.jconrel.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson S., Gustavsson T., Roshanbin S., Hultqvist G., Hammarlund-Udenaes M., Sehlin D. Blood−brain barrier integrity in a mouse model of Alzheimer's disease with or without acute 3D6 immunotherapy. Neuropharmacology. 2018;143:1–9. doi: 10.1016/j.neuropharm.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Huang B., Zha Q., Chen T., Xiao S., Xie Y., Luo P. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC−QTOF/MS coupled with OPLS-DA. Phytomedicine. 2018;45:8–17. doi: 10.1016/j.phymed.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z., Zhang Y., Liu Y., Zhao N., Zhang Y., Yuan L. RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects. Br J Pharmacol. 2013;168:1001–1014. doi: 10.1111/j.1476-5381.2012.02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ries A.S., Hermanns T., Poeck B., Strauss R. Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat Commun. 2017;8:15738. doi: 10.1038/ncomms15738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klavir O., Prigge M., Sarel A., Paz R., Yizhar O. Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nat Neurosci. 2017;20:836–844. doi: 10.1038/nn.4523. [DOI] [PubMed] [Google Scholar]
- 18.Lorsch Z.S., Loh Y.E., Purushothaman I., Walker D.M., Parise E.M., Salery M. Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat Commun. 2018;9:1116. doi: 10.1038/s41467-018-03567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L., Di Y., Shergis J.L., Li Y., Zhang A., Lu C. A systematic review of acupuncture and Chinese herbal medicine for postpartum depression. Complement Ther Clin Pract. 2018;33:85–92. doi: 10.1016/j.ctcp.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Liu C., Guo D., Liu L. Quality transitivity and traceability system of herbal medicine products based on quality markers. Phytomedicine. 2018;44:247–257. doi: 10.1016/j.phymed.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Boyle S.P., Doolan P.J., Andrews C.E., Reid R.G. Evaluation of quality control strategies in Scutellaria herbal medicines. J Pharm Biomed Anal. 2011;54:951–957. doi: 10.1016/j.jpba.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Ju Z., Li J., Han H., Yang L., Wang Z. Analysis of bioactive components and multi-component pharmacokinetics of saponins from the leaves of Panax notoginseng in rat plasma after oral administration by LC−MS/MS. J Sep Sci. 2018;41:1512–1523. doi: 10.1002/jssc.201701042. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.Y., Lee H.E., Kang S.R., Choi H.Y., Ryu J.H., Yune T.Y. Fluoxetine inhibits transient global ischemia-induced hippocampal neuronal death and memory impairment by preventing blood−brain barrier disruption. Neuropharmacology. 2014;79:161–171. doi: 10.1016/j.neuropharm.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Feiler S., Plesnila N., Thal S.C., Zausinger S., Scholler K. Contribution of matrix metalloproteinase-9 to cerebral edema and functional outcome following experimental subarachnoid hemorrhage. Cerebrovasc Dis. 2011;32:289–295. doi: 10.1159/000328248. [DOI] [PubMed] [Google Scholar]
- 25.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood−brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sozen T., Tsuchiyama R., Hasegawa Y., Suzuki H., Jadhav V., Nishizawa S. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki R., Hiraki Y., Nishida S., Inatomi Y., Yabe T. Gomisin N ameliorates lipopolysaccharide-induced depressive-like behaviors by attenuating inflammation in the hypothalamic paraventricular nucleus and central nucleus of the amygdala in mice. J Pharmacol Sci. 2016;132:138–144. doi: 10.1016/j.jphs.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Hu D., Li C., Han N., Miao L., Wang D., Liu Z. Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Aβ1−42-induced memory impairment in mice. Planta Med. 2012;78:1332–1336. doi: 10.1055/s-0032-1315019. [DOI] [PubMed] [Google Scholar]
- 30.Szopa A., Dziurka M., Warzecha A., Kubica P., Klimek-Szczykutowicz M., Ekiert H. Targeted lignan profiling and anti-inflammatory properties of Schisandra rubriflora and Schisandra chinensis extracts. Molecules. 2018;23:E3103. doi: 10.3390/molecules23123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee T.H., Jung C.H., Lee D.H. Neuroprotective effects of schisandrin B against transient focal cerebral ischemia in Sprague−Dawley rats. Food Chem Toxicol. 2012;50:4239–4245. doi: 10.1016/j.fct.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 32.Park S.Y., Park S.J., Park T.G., Rajasekar S., Lee S.J., Choi Y.W. Schizandrin C exerts anti-neuroinflammatory effects by upregulating phase II detoxifying/antioxidant enzymes in microglia. Int Immunopharmacol. 2013;17:415–426. doi: 10.1016/j.intimp.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 33.Sun Z., Zhang H., Wang X., Wang Q., Zhang C., Wang J. TMCO1 is essential for ovarian follicle development by regulating ER Ca2+ store of granulosa cells. Cell Death Differ. 2018;25:1686–1701. doi: 10.1038/s41418-018-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J. Autophagy and ethanol neurotoxicity. Autophagy. 2014;10:2099–2108. doi: 10.4161/15548627.2014.981916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giridharan V.V., Thandavarayan R.A., Arumugam S., Mizuno M., Nawa H., Suzuki K. Schisandrin B ameliorates ICV-infused amyloid β induced oxidative stress and neuronal dysfunction through inhibiting RAGE/NF-κB/MAPK and up-regulating HSP/Beclin expression. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song F., Zeng K., Liao L., Yu Q., Tu P., Wang X. Schizandrin A inhibits microglia-mediated neuroninflammation through inhibiting TRAF6-NF-κB and Jak2-Stat3 signaling pathways. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Nardo D. Toll-like receptors: activation, signalling and transcriptional modulation. Cytokine. 2015;74:181–189. doi: 10.1016/j.cyto.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Wang P., Xiong X., Chen J., Wang Y., Duan W., Yang Q. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: from preconditioning to treatment. J Neuroinflammation. 2015;12:80. doi: 10.1186/s12974-015-0301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu N., Zheng J., Zhuang Y., Zhou Z., Zhao J., Yang L. Anti-inflammatory effects of schisandrin B on LPS-stimulated BV2 microglia via activating PPAR-γ. Inflammation. 2017;40:1006–1011. doi: 10.1007/s10753-017-0544-2. [DOI] [PubMed] [Google Scholar]
- 40.Li N., Liu J., Wang M., Yu Z., Zhu K., Gao J. Sedative and hypnotic effects of schisandrin B through increasing GABA/Glu ratio and upregulating the expression of GABAA in mice and rats. Biomed Pharmacother. 2018;103:509–516. doi: 10.1016/j.biopha.2018.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.