Abstract
Malaria remains a major cause of mortality in African children, with no adjunctive treatments currently available to ameliorate the severe clinical forms of the disease. Rosetting, the adhesion of infected erythrocytes (IEs) to uninfected erythrocytes, is a parasite phenotype strongly associated with severe malaria, and hence is a potential therapeutic target. However, the molecular mechanisms of rosetting are complex and involve multiple distinct receptor–ligand interactions, with some similarities to the diverse pathways involved in P. falciparum erythrocyte invasion. This review summarizes the current understanding of the molecular interactions that lead to rosette formation, with a particular focus on host uninfected erythrocyte receptors including the A and B blood group trisaccharides, complement receptor one, heparan sulphate, glycophorin A and glycophorin C. There is strong evidence supporting blood group A trisaccharides as rosetting receptors, but evidence for other molecules is incomplete and requires further study. It is likely that additional host erythrocyte rosetting receptors remain to be discovered. A rosette-disrupting low anti-coagulant heparin derivative is being investigated as an adjunctive therapy for severe malaria, and further research into the receptor–ligand interactions underlying rosetting may reveal additional therapeutic approaches to reduce the unacceptably high mortality rate of severe malaria.
Key words: ABO blood group, adjunctive therapy, cell adhesion, Plasmodium, receptors, severe malaria pathogenesis
Introduction
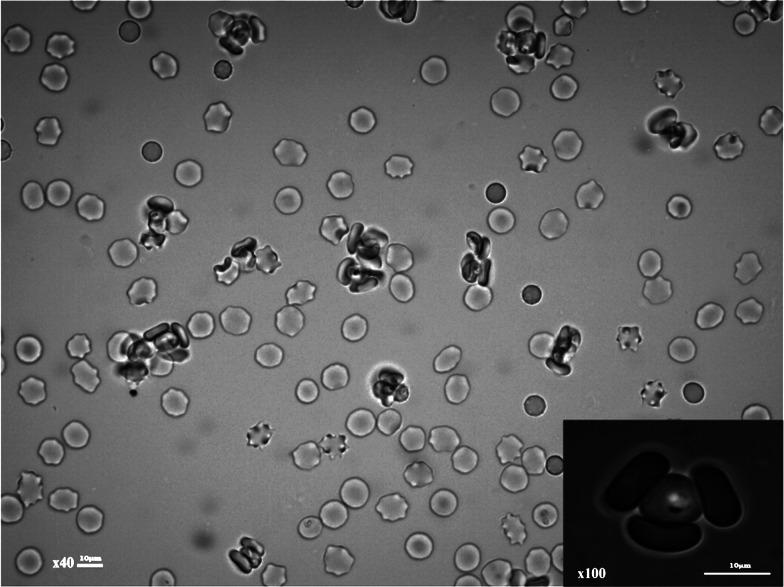
Rosetting is a Plasmodium falciparum infected erythrocyte (IE) adhesion phenotype that is associated with severe malaria in sub-Saharan Africa (summarized in Doumbo et al., 2009). It is a form of cell adhesion in which erythrocytes infected with mature, asexual parasites bind to uninfected erythrocytes to form clusters of cells (Fig. 1). Rosetting is a phenotypically variable property, which is common in parasite isolates collected from severe malaria patients, but infrequent in parasites from uncomplicated malaria cases. For culture-adapted P. falciparum isolates, only a subset of parasite lines can be selected in vitro for the rosetting phenotype, and many of the commonly used laboratory strains such as 3D7, rosette poorly or not at all. The relative rarity of rosetting in culture-adapted parasite lines may explain why rosetting is studied infrequently, despite being a virulence-associated phenotype in clinical isolates.
Fig. 1.
Plasmodium falciparum rosetting in an in vitro culture. Rosettes consisting of clusters of infected and uninfected erythrocytes are shown. Inset image shows a single infected erythrocyte (centre) and three adherent uninfected erythrocytes. Images were taken using a Yenway microscope camera on a Leica DM LB2 fluorescent microscope using the ×40 and ×100 (inset) objectives.
Rosetting can contribute to IE sequestration and microvascular congestion, leading to obstruction to blood flow (Kaul et al., 1991), one of the major pathological events in severe falciparum malaria contributing to inflammation, tissue damage and organ failure (Miller et al., 2002; White et al., 2013). Rosetting also causes membrane changes in uninfected erythrocytes that may contribute to phagocytic removal and anaemia (Uyoga et al., 2012). In Africa, high levels of rosetting occur in parasites sampled from severe malaria patients with all clinical types of disease including cerebral malaria (Carlson et al., 1990; Treutiger et al., 1992; Ringwald et al., 1993; Rowe et al., 1995; Kun et al., 1998; Doumbo et al., 2009), severe malarial anaemia (Newbold et al., 1997; Doumbo et al., 2009) and respiratory distress (Warimwe et al., 2012). Rosette-like clusters of cells have been seen in the microvasculature in histological studies of fatal malaria cases (Dondorp et al., 2004; Barrera et al., 2018). The major Plasmodium species that infect humans are all able to form rosettes (Udomsanpetch et al., 1995; Angus et al., 1996; Chotivanich et al., 1998; Lowe et al., 1998). However, the link between severity of disease and rosetting is confined to P. falciparum, possibly due to the unique ability to bind both endothelial cells and uninfected erythrocytes simultaneously (Udomsangpetch et al., 1992; Adams et al., 2014), such that P. falciparum rosetting IEs are sequestered and are not seen in peripheral blood. Recently it has been suggested that rosetting may contribute to anaemia in Plasmodium vivax infections (Marín-Menéndez et al., 2013).
The biological function of rosetting in vivo remains unknown. Rosettes may shield IEs from host immune attack, or close contact with uninfected erythrocytes in rosettes might enhance merozoite invasion (Wahlgren et al., 1989; Deans and Rowe, 2006). However, firm evidence to support either of these hypotheses is lacking. Most rosetting parasite isolates form larger, stronger rosettes with blood group A erythrocytes compared to other blood groups (Carlson and Wahlgren, 1992), and these group A rosettes may shield IEs to reduce antibody binding to parasite variant surface antigens (VSAs) (Moll et al., 2015). Whether this translates into the reduced clearance of IEs and subsequent higher parasite burdens in vivo is unclear, although some studies have noted a positive correlation between rosetting and parasitaemia (Rowe et al., 2002). Another study showed that rosetting does not prevent IgG-mediated phagocytosis of IEs (Stevenson et al., 2015a), although experiments were only performed in group O cells. Parasite invasion of erythrocytes is not increased in vitro in rosetting compared to isogenic non-rosetting parasites (Clough et al., 1998; Deans and Rowe, 2006; Ribacke et al., 2013), nor in the presence of larger rosettes (Moll et al., 2015). However, in vivo studies using splenectomized Saimiri sciureus monkeys demonstrated a 1.5 times higher parasite multiplication rate with rosetting compared to isogenic non-rosetting parasites (Le Scanf et al., 2008). This suggests either increased invasion or decreased clearance of rosetting parasites in vivo, which requires further investigation.
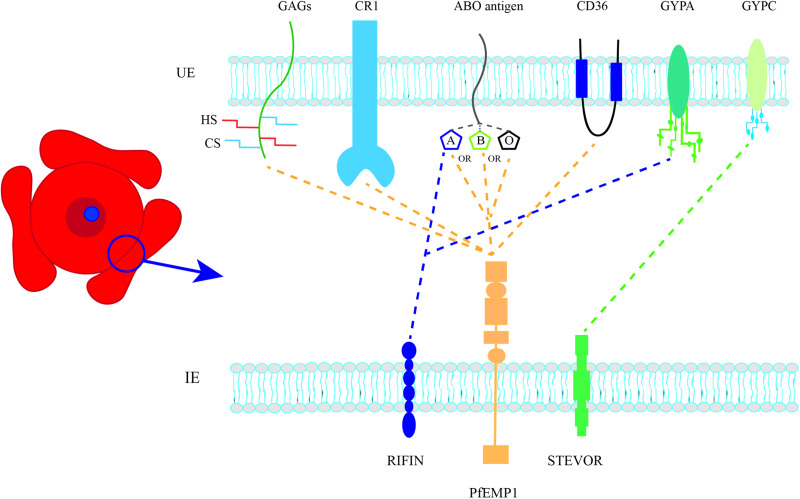
This review will discuss the molecular mechanisms of rosetting and describe recent advances exploring the potential of rosetting as a therapeutic target in severe P. falciparum malaria. Rosetting is a complex cell adhesion phenotype involving parasite adhesion molecules on the IE surface and host receptors on uninfected erythrocytes (Fig. 2). Current evidence suggests that there are multiple distinct pathways of rosette formation, similar to the diverse pathways involved in merozoite invasion of erythrocytes (Cowman et al., 2017). Interestingly, although the parasite molecules that mediate rosetting are different from those involved in merozoite invasion, both sets of proteins have ‘Duffy-Binding-Like’ adhesion domains and many of the same host erythrocyte receptors are used (e.g. glycophorin A, glycophorin C and complement receptor one). The diversity in P. falciparum merozoite invasion pathways is thought to have evolved to allow parasites to successfully establish infections despite host genetic variation and/or development of host antibodies blocking single pathways. The same arguments can be applied to rosetting, and the existence of multiple rosetting pathways suggests that there has been significant selection pressure in favour of the phenotype, and that rosetting somehow improves parasite fitness.
Fig. 2.
Parasite-derived adhesion ligands and host receptors that interact to form rosettes. UE, uninfected erythrocyte; IE, infected erythrocyte; GAGs, glycosaminoglycans; HS, heparan sulphate; CS, chondroitin sulphate; CR1, complement receptor 1; GYPA, glycophorin A; GYPC, glycophorin C. Dotted lines represent proposed host receptors for each parasite ligand.
Several recent reviews have discussed the parasite adhesion molecules involved in rosetting (Hviid and Jensen, 2015; Wang and Hviid, 2015; Yam et al., 2017), so these will not be described in detail here. Briefly, multiple studies have identified members of the VSA family P. falciparum erythrocyte membrane protein one (PfEMP1) as rosette-mediating adhesion molecules (Rowe et al., 1997; Vigan-Womas et al., 2008, 2011; Albrecht et al., 2011; Ghumra et al., 2012), and recent reports suggest that other VSAs such as RIFIN (Goel et al., 2015) and STEVOR (Niang et al., 2014) may also contribute to rosette formation. Further work is needed to determine the relative contributions of the different VSAs to rosetting, especially in clinical isolates.
Host serum proteins such as IgM, α2macroglobulin, albumin and fibrinogen also contribute to rosetting, either by binding directly to parasite adhesion molecules or by non-specific erythrocyte aggregating effects (Scholander et al., 1996; Treutiger et al., 1999; Luginbuhl et al., 2007; Ghumra et al., 2008, 2012; Semblat et al., 2015; Stevenson et al., 2015a, 2015b). The extent to which host serum proteins influence rosetting, sequestration and microvascular obstruction in vivo is unknown, and would be a valuable area of future study.
Rosetting receptors on host erythrocytes
A number of different molecules on uninfected erythrocytes have been proposed as receptors for P. falciparum rosetting (Fig. 2 and Table 1), and multiple receptor–ligand interactions may contribute to rosetting in any given parasite isolate. Some of the proposed rosetting receptor molecules, including blood group A and B sugars, heparan sulphate (HS)-like molecules and complement receptor one (CR1) are widely accepted as having a role in rosetting, whereas other recent candidates such glycophorin A (GYPA) and glycophorin C (GYPC) are less well-authenticated. However, a close examination of the underlying data shows that in most cases, the evidence is incomplete, as discussed in detail below.
Table 1.
Summary of host erythrocyte receptors for Plasmodium falciparum rosetting
| Name | Characteristics | Studiesa | Comments |
|---|---|---|---|
| ABO blood group antigens | Differ based on terminal sugar: A = N-acetyl-D-galactosamine, B = D-galactose, O (H-antigen) = L-Fucose O is a predominant blood group in sub-Saharan Africa Blood group O protects against severe malaria (Rowe et al., 2007; Fry et al., 2008; Tekeste and Petros, 2010; Rout et al., 2012; Malaria Genomic Epidemiology Network, 2014; Ndila et al., 2018; Degarege et al., 2019) |
Larger rosettes in parasites cultured in A, B, AB compared to O (Carlson and Wahlgren, 1992; Udomsangpetch et al., 1993; Barragan et al., 2000b) Parasites from group O patients have lower mean rosette frequencies than those from non-O patients (Rowe et al., 1995; Rowe et al., 2007; Rout et al., 2012) Rosettes from group O patients are more easily disrupted by immune sera and removal of A/B antigen decreases rosette size (Barragan et al., 2000b) Blood group antigen binding site mapped to NTS-DBLα the domain of PfEMP1-VarO (Vigan-Womas et al., 2012) |
Blood group A antigen is the most well-validated host rosetting receptor Both PfEMP1 (Vigan-Womas et al., 2012) and RIFINs (Goel et al., 2015) may interact with A antigen Challenging to manipulate therapeutically |
| Complement receptor 1 (CR1) | Membrane glycoprotein responsible for regulating the complement system (Thielen et al., 2018) Polymorphisms affect CR1 copy number, molecular weight and sequence (Schmidt et al., 2015) RBC CR1 deficiency protects in medium-high (Cockburn et al., 2004; Sinha et al., 2009; Rout et al., 2011; Panda et al., 2012) but not low malaria transmission areas (Nagayasu et al., 2001; Teeranaipong et al., 2008). CR1 Knops blood group polymorphisms associated with severe malaria (Opi et al., 2018) |
Rosetting reduced in CR1 deficient erythrocytes (Rowe et al., 1997) Soluble CR1 and CR1 antibodies disrupt rosettes in some parasite isolates (Rowe et al., 1997; Rowe et al., 2000; Vigan-Womas et al., 2012) Essential region mapped to the C3b binding site on CR1 (Rowe et al., 2000) |
Further work needed to assess the relative importance of CR1 in rosetting isolates and potential as a therapeutic target Soluble recombinant CR1 has been considered for therapeutic use in humans, e.g. cardiac and renal disease (Li et al., 2006; Reddy et al., 2017) |
| Heparan sulphate (HS)b | Glycosaminoglycan Heparin is a highly sulfated form of HS that is only found in mast cells HS is a receptor for P. falciparum sporozoite invasion of hepatocytes (Frevert et al., 1993) HS is a receptor for infected erythrocyte cytoadherence to endothelial cells (Vogt et al., 2003; Adams et al., 2014) |
Heparin partially disrupts rosettes in some isolates (Udomsangpetch et al., 1989; Carlson et al., 1992; Rogerson et al., 1994; Rowe et al., 1994; Barragan et al., 1999) Heparinase treatment reported to reduce rosetting in two culture-adapted parasite lines (Barragan et al., 1999) Heparin binds to rosetting IE (Barragan et al., 2000a; Heddini et al., 2001) and to rosette-mediating PfEMP1 (Barragan et al., 2000a; Vogt et al., 2003; Juillerat et al., 2010; Juillerat et al., 2011; Adams et al., 2014) |
Limited evidence that HS is present on mature RBCs (Vogt et al., 2004) Further work needed to determine whether HS is present on normal erythrocytes and acts as a rosetting receptor Therapeutic potential due to PfEMP1 binding and rosette disruption. Clinical trials of low anticoagulant heparin ongoing (Leitgeb et al., 2017) |
| Chondroitin sulphate (CS) | Glycosaminoglycan Receptor for infected erythrocyte placental sequestration in pregnancy malaria (Fried and Duffy, 1996) |
Soluble CS did not disrupt rosettes (Rogerson et al., 1994; Rowe et al., 1994) Chondroitinase treatment reduced rosetting in one parasite line only (Barragan et al., 1999) |
No evidence that CS is present on mature RBC Minimal evidence for a role in rosetting |
| CD36 | Widely distributed membrane protein and scavenger receptor (Silverstein and Febbraio, 2009) Deficiency is common in Africa but not associated with severe malaria (Fry et al., 2009) |
Antibodies disrupt rosettes in single culture-adapted line only (Handunnetti et al., 1992) PfEMP1 variants that mediate rosetting are group A types that do not bind CD36 (Robinson et al., 2003) |
Minimal evidence for a widespread role in rosetting |
| Glycophorin C (GYPC) | Red cell membrane protein responsible for Gerbich blood group (Jaskiewicz et al., 2018) Receptor for merozoite invasion of erythrocytes (Maier et al., 2003) ‘Gerbich-negative’ blood group common in Melanesians (Patel et al., 2001), but no evidence yet for association with protection against severe malaria |
Reduced rosetting with GYPC antibodies and GYPC knockdown RBCs (Niang et al., 2014) (single culture-adapted parasite line tested) Gerbich-negative erythrocytes formed rosettes normally with five P. falciparum lines (Rowe et al., 1997) Possible role in P. vivax rosetting (Lee et al., 2014) |
Further work needed to assess the relative importance of GYPC in P. falciparum rosetting isolates and potential as a therapeutic target |
| Glycophorin A (GYPA) | Sialoglycoprotein which, along with glycophorin B, constitutes the MNS blood group Receptor for merozoite invasion of erythrocytes (Sim et al., 1994) GYPA polymorphisms are associated with protection against severe malaria (Band et al., 2015; Leffler et al., 2017). |
GYPA-deficient erythrocytes showed reduced rosetting with RIFIN transfected parasites (Goel et al., 2015) GYPA antibodies had no inhibitory effect on rosetting (Lee et al., 2014) (Niang et al., 2014) GYPA null erythrocytes formed rosettes with five culture-adapted P. falciparum lines (Rowe et al., 1997) |
Further work needed to assess the relative importance of GYPA in P. falciparum rosetting isolates and potential as a therapeutic target |
| Unknown receptor/s | Possibly carbohydrate or protease-resistant protein | Protease and heparinase treated erythrocytes capable of forming rosettes (Udomsangpetch et al., 1989; Rowe et al., 1994) | Further work needed to identify novel rosetting receptors |
Parasite strains used are not consistent between studies with a wide range of culture-adapted and clinical isolates in use. Results are therefore not necessarily generalizable from single studies.
Many studies included here use heparin instead of/in addition to heparan sulphate.
Evidence needed to establish a role for a specific host receptor in rosetting
In order to prove that a particular molecule acts as a host receptor for P. falciparum rosetting, a variety of different types of evidence have been provided. Essential data include proof that the molecule in question is found on normal human erythrocytes and that erythrocytes lacking the molecule show reduced/absent rosetting. Direct binding between IEs and/or recombinant parasite adhesion proteins and the receptor molecule should be demonstrated. Ideally, a crystal structure of the parasite adhesion molecule–host receptor complex should show the precise binding interaction site. Supportive evidence includes the ability of antibodies against the receptor or soluble receptor proteins to inhibit rosetting, and biochemical approaches to remove or alter the receptor on erythrocytes. Human genetic evidence can also provide indirect supportive evidence that particular molecules are important in life-threatening malaria. Several putative rosetting receptors have high-frequency polymorphisms in populations from malaria endemic regions that reduce rosetting and are associated with protection against severe malaria and death [reviewed in Rowe et al. (2009a, 2009b)]. These various lines of evidence are summarized below for each potential host rosetting receptor.
Blood group A and B trisaccharides
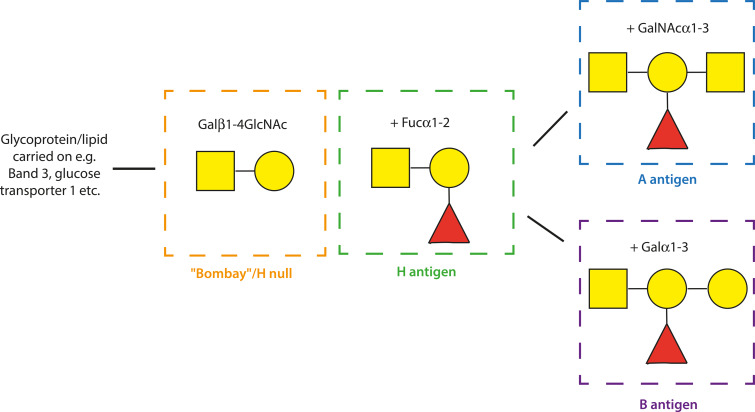
The most well-validated rosetting receptors are the blood group A and B trisaccharides (Fig. 3). In vitro experiments have shown that rosetting parasites have a ‘preference’ for blood groups A, B or AB rather than O (Carlson and Wahlgren, 1992; Udomsangpetch et al., 1993; Barragan et al., 2000b; Pipitaporn et al., 2000; Vigan-Womas et al., 2012; Moll et al., 2015). This varies by parasite genotype, with A-preference being the commonest. Clinical isolates from non-O (i.e. A, B or AB) patients show higher levels of rosetting than isolates from group O patients in studies from sub-Saharan Africa (Rowe et al., 1995, 2007) and India (Rout et al., 2012), although the same result was not seen in one Thai study (Lee et al., 2014). When parasites are cultured in their ‘preferred’ blood group, they form larger, stronger rosettes that are more resistant to disruption by antibodies or chemical agents than in group O cells (Carlson and Wahlgren, 1992; Barragan et al., 2000b; Ch'ng et al., 2016). Enzymatic removal of the terminal sugars (N-acetyl-D-galactosamine for A and D-galactose for B) results in smaller, weaker rosettes, equivalent to those seen in group O erythrocytes (Barragan et al., 2000b). Rosettes do, however, still occur with blood group O erythrocytes (that express the H antigen), and also in Bombay phenotype red cells that lack the ABO blood group core fucose residue (Fig. 3) (Carlson and Wahlgren, 1992; Rowe et al., 1997). This indicates that other red cell surface molecules in addition to the A and B antigens can act as host receptors for rosette formation.
Fig. 3.
Diagram of the ABO blood group sugars. Schematic representation of the terminal structure of the A (blue square), B (purple) H (green; H is the antigen carried on blood group O erythrocytes) and Bombay (orange) antigens. Yellow circle: D-Galactose (Gal), yellow square: N-acetyl-D-galactosamine (GalNac), red triangle: L-Fucose (Fuc). The symbols α and β indicate the position of the hydroxyl group and the numbers indicate the specific carbon atoms that are linked between the sugars. The H, A and B antigens are synthesized by a series of glycosyltransferase enzymes that add monosaccharides to create oligosaccharide chains attached to lipids and proteins in the erythrocyte membrane.
For the blood group A-preferring parasite line, Palo Alto 89F5, direct binding between the VarO PfEMP1 adhesion molecule and the blood group A trisaccharide was shown by Surface Plasmon Resonance (Vigan-Womas et al., 2012). The VarO PfEMP1 variant also binds to the B trisaccharide, but with lower affinity (Vigan-Womas et al., 2012). A crystal structure of the PfEMP1 N-terminal region was obtained and the A-trisaccharide binding site mapped (Vigan-Womas et al., 2012). A recent study suggests that P. falciparum RIFIN molecules may also be able to interact with blood group A sugars to contribute to rosette formation (Goel et al., 2015), although direct RIFIN-A trisaccharide interaction was not shown.
The importance of the A and B antigens in rosetting is emphasized by the fact that the non-O blood groups are associated with increased risk of severe malaria and death compared to O (Rowe et al., 2007; Fry et al., 2008; Tekeste and Petros, 2010; Rout et al., 2012; Malaria Genomic Epidemiology Network, 2014; Ndila et al., 2018; Degarege et al., 2019). Reduced rosetting in blood group O, and therefore reduced microvascular obstruction and reduced downstream pathological effects, is the proposed mechanism for the protective association with group O (Udomsangpetch et al., 1993; Rowe et al., 2007). ABO blood group does not influence parasite burden (Rowe et al., 2007; Degarege et al., 2019), and evidence for an effect of ABO on P. falciparum invasion or other host–parasite interactions is conflicting and requires further study (Chung et al., 2005; Wolofsky et al., 2012; Pathak et al., 2016; Theron et al., 2018). The ABH antigens are known to be present on endothelial cells (Ito et al., 1990) and it is likely, but has not been shown experimentally, that cytoadhesion and overall levels of sequestration of rosetting parasites are enhanced in group A/B/AB patients compared to O.
Despite the progress in identifying the A and B trisaccharides as rosetting receptors and key genetic determinants of host susceptibility to severe malaria, there have been no attempts to develop specific therapies to block P. falciparum interaction with A/B antigens. The PfEMP1-blood group A trisaccharide binding pair described above (Vigan-Womas et al., 2012) remains the most clearly defined molecular interaction between parasite ligand and host receptor in rosetting, and could be used as a starting point to develop rosette-blocking therapeutics. Vigan-Womas et al. did report that the interaction between PfEMP1 and the A and B trisaccharides is indirectly inhibited by heparin (Vigan-Womas et al., 2012), and the development of a heparin-derivative as a potential adjunctive therapy for severe malaria is described below (Leitgeb et al., 2017).
Complement receptor one (CR1, CD35)
CR1 is a red cell membrane glycoprotein that regulates complement activation on cell surfaces (Thielen et al., 2018) and carries the Knops Blood Group antigens (Moulds, 2010). In malaria, CR1 plays a role in both rosetting and parasite invasion of erythrocytes (Schmidt et al., 2015). CR1 was first identified as a rosetting receptor from a screen of 23 naturally occurring erythrocyte null mutants, each missing a particular blood group molecule or membrane glycoprotein (Rowe et al., 1997). The only variant to show substantially reduced rosetting with five P. falciparum parasite lines was Knops null cells, which are deficient in CR1. Normally, erythrocytes have between 100 and 1000 molecules of CR1 per cell (Wilson et al., 1986), whereas Knops null cells have fewer than 100 molecules per cell (Moulds et al., 1992). Erythrocytes with fewer than 50 CR1 molecules per cell form rosettes poorly (Rowe et al., 1997), with normal rosetting occurring above a threshold of around 100 molecules per cell (JA Rowe, unpublished data).
Soluble CR1 and CR1 antibodies were shown to inhibit rosetting in some but, not all P. falciparum rosetting laboratory strains and clinical isolates, with only monoclonal antibodies (mAbs) that map to the C3b binding site on CR1 being effective inhibitors (Rowe et al., 1997, 2000; Vigan-Womas et al., 2012). A recent paper suggested that the commercially available CR1 mAb E11 that recognizes epitopes outside the C3b binding site (Nickells et al., 1998) may inhibit P. falciparum rosetting (Lee et al., 2014), but this was not seen in our hands (Rowe et al., 2000). Further evidence of a role for CR1 in rosetting came from the expression of recombinant PfEMP1 domains in COS-7 cells, which bound to normal erythrocytes but not to CR1-deficient cells (Rowe et al., 1997).
Despite these supportive data, direct binding of IEs to CR1 protein has not been demonstrated, and recombinant rosette-mediating PfEMP1 proteins produced in E. coli (Ghumra et al., 2012) do not bind to CR1 in Surface Plasmon Resonance experiments (Tetteh-Quarcoo et al., 2012). This could reflect a genuine lack of interaction between the two molecules, or could be due to technical reasons (e.g. the recombinant CR1 used in experiments was produced in mouse rather than human cells, whereas CR1 glycosylation, which may affect function, is cell-type specific) (Lublin et al., 1986). It is also possible that a serum protein mediates the interaction between PfEMP1 on IEs and CR1 on uninfected erythrocytes, as the original experiments were all performed in the presence of serum (Rowe et al., 1997).
Human genetic studies provide additional support for the importance of CR1 in malaria host–parasite interactions. Erythrocyte CR1 deficiency is common in some malaria-endemic countries such as Papua New Guinea (Cockburn et al., 2004) and India (Sinha et al., 2009), and is associated with protection against severe malaria in medium to high transmission areas (Cockburn et al., 2004; Sinha et al., 2009; Rout et al., 2011; Panda et al., 2012). However, erythrocyte CR1 deficiency may be detrimental in areas such as Thailand, where malaria transmission is low (Nagayasu et al., 2001; Teeranaipong et al., 2008). There is also evidence that the CR1 Swain Langley 2 (Sl2) Knops blood group polymorphism that is common in African populations (Moulds, 2010) is associated with protection against severe malaria (Thathy et al., 2005; Opi et al., 2018). Red cells carrying the Sl2 antigen on CR1 show reduced rosetting (Rowe et al., 1997; Opi et al., 2018), and Sl2 may have additional effects on complement activation and regulation (Opi et al., 2018).
Overall, the ability of CR1 mAbs and soluble protein to reverse rosettes suggests that CR1 plays a role in rosetting for some P. falciparum isolates. However, further work is needed to fully investigate the molecular interactions between parasite adhesion molecules and CR1, and to explore the potential for CR1 reagents (Li et al., 2006; Reddy et al., 2017) as therapeutic disruptors of rosetting.
Heparan sulphate and chondroitin sulphate
The glycosaminoglycans HS and chondroitin sulphate (CS) are found on cell surfaces and in the extracellular matrix of many tissues, and have a role in multiple aspects of the P. falciparum life cycle including hepatocyte invasion (Frevert et al., 1993), endothelial cell cytoadherence (Vogt et al., 2003; Adams et al., 2014) and, for CS, placental sequestration (Fried and Duffy, 1996). A number of papers have showed that heparin (which is a highly-sulphated form of HS found only in mast cells) can partially disrupt rosettes in about one-third to one-half of P. falciparum clinical isolates in vitro (Udomsangpetch et al., 1989; Carlson et al., 1992; Rogerson et al., 1994; Rowe et al., 1994; Barragan et al., 1999). It was shown that treating erythrocytes with heparinase III, which selectively cleaves HS chains, reduces rosetting in two P. falciparum lines (Barragan et al., 1999), and therefore suggested that ‘HS-like’ molecules on red cells are receptors for rosetting (Chen et al., 2000). However, there has been only one paper reporting the existence of HS on normal human erythrocytes (Vogt et al., 2004) and we have been unable to confirm this, and unable to detect any rosette-reducing effect of heparinase III in a range of parasite lines (McQuaid and Rowe, unpublished data).
Fluorescently-labelled heparin does bind to the surface of erythrocytes infected with rosetting parasites more than non-rosetting lines (Barragan et al., 2000a; Heddini et al., 2001), and some rosette-mediating PfEMP1 variants bind directly to heparin (Barragan et al., 2000a; Vogt et al., 2003; Juillerat et al., 2010, 2011; Adams et al., 2014). The heparin binding site in the N-terminal region of the varO PfEMP1 variant was mapped onto a crystal structure (Juillerat et al., 2011), and shown to be on the opposite side of the molecule from the erythrocyte binding site (Vigan-Womas et al., 2012). Hence, the rosette-disrupting effect of heparin is not due to direct blocking of receptor binding, but may result from aggregating PfEMP1 monomers and preventing their interaction with erythrocyte receptors (Vigan-Womas et al., 2012). Similarly, for another rosette-mediating PfEMP1 variant IT4var60, site-directed mutagenesis studies of recombinant proteins showed that mutations that disrupt heparin binding are distinct from mutations that disrupt erythrocyte binding, indicating that heparin-like molecules are not the main host rosetting receptor in this case (Angeletti et al., 2015).
Overall, whether HS is present on normal erythrocytes and is a host receptor for rosetting requires further confirmation. HS in present on the luminal surface of microvascular endothelial cells (albeit at a much lower density than on basolateral surfaces) (de Agostini et al., 1990; Stoler-Barak et al., 2014), therefore interactions between IE and endothelial HS (Vogt et al., 2003; Adams et al., 2014) are physiologically relevant and are likely to contribute to cytoadherence and sequestration in vivo.
Despite the uncertainty on the precise role of HS as an erythrocyte rosetting receptor, heparin and other sulphated glycoconjugate compounds have clear potential as adjunctive therapies for severe malaria due to their rosette-disrupting effects (Udomsangpetch et al., 1989; Carlson et al., 1992; Rogerson et al., 1994; Rowe et al., 1994; Kyriacou et al., 2007). There are reports of successful heparin treatment in severe malaria (Rampengan, 1991) but its use is not recommended due to a high incidence of bleeding complications (World Health Organisation, 1986). As an alternative, Wahlgren and coworkers have developed a low anti-coagulant heparin derivative, Sevuparin, that reverses rosetting and cytoadherence in some P. falciparum isolates (Leitgeb et al., 2011; Saiwaew et al., 2017) and also blocks merozoite invasion (Leitgeb et al., 2017). Sevuparin has been shown to be safe in adults with uncomplicated malaria (Leitgeb et al., 2017), but has not yet been tested in severe malaria patients.
The evidence for CS as a rosetting receptor is minimal. One report shows that rosetting in the P. falciparum line TM284 was partially inhibited by soluble CS and by chondroitinase enzyme treatment of erythrocytes, and that several clinical isolates showed reduced rosetting in the presence of CS (Barragan et al., 1999). However, other studies have found no effect of CS on rosetting in a variety of culture-adapted lines and clinical isolates (Rogerson et al., 1994; Rowe et al., 1994). There is also no convincing evidence that CS is found on the surface of normal human erythrocytes. Overall, current data do not support a role for CS in rosetting.
CD36
The membrane glycoprotein CD36 is a scavenger receptor for oxidized lipoproteins and a fatty acid translocase (Silverstein and Febbraio, 2009). It is expressed on a variety of cell types including monocytes, macrophages, platelets, microvascular endothelial cells and adipocytes (Silverstein and Febbraio, 2009), and at low levels on erythrocytes (van Schravendijk et al., 1992). The binding of PfEMP1 (group B and C variants) to CD36 on microvascular endothelial cells plays a major role in P. falciparum sequestration (Baruch et al., 1996; Robinson et al., 2003). Almost all P. falciparum isolates bind to CD36, and increased CD36 binding (Newbold et al., 1997; Ochola et al., 2011) and predominant expression of group B and C PfEMP1 (Kraemer and Smith, 2006; Kyriacou et al., 2006) are associated with uncomplicated malaria.
The role of CD36 in rosetting is less clear. Anti-CD36 mAbs are capable of disrupting rosettes in a single culture-adapted parasite line, Malayan Camp (Handunnetti et al., 1992), but not in a wide range of other laboratory lines or clinical isolates (Udomsangpetch et al., 1989; Wahlgren et al., 1992; Rowe et al., 2000; Niang et al., 2014). The PfEMP1 variants identified as parasite rosetting ligands (Rowe et al., 1997; Vigan-Womas et al., 2011; Ghumra et al., 2012) are mostly of the group A type, which do not bind to CD36 (Robinson et al., 2003).
Intriguingly, while CD36 deficiency is fairly common in African populations, large-scale genetic studies have shown that CD36 polymorphisms do not influence severe malaria risk (Fry et al., 2009). There is some evidence that interaction between IEs and CD36 may benefit the host, as CD36 may contribute to innate immune clearance of IEs and platelet-mediated parasite death (McGilvray et al., 2000; McMorran et al., 2012; Cabrera et al., 2014). Overall, it is unlikely that CD36 is a clinically significant rosetting receptor or a useful therapeutic target in severe malaria (Cabrera et al., 2014).
Glycophorin C (GYPC; GPC; CD236)
GYPC is a red cell membrane glycoprotein that carries the Gerbich blood group antigens (Jaskiewicz et al., 2018). It is a P. falciparum invasion receptor bound by the merozoite protein EBA-140/BAEBL (Maier et al., 2003; Mayer et al., 2006). Recently, two studies have suggested that GYPC is a rosetting receptor for both P. falciparum (Niang et al., 2014) and P. vivax (Lee et al., 2014; Niang et al., 2014). Niang et al. showed that the rosetting of a 3D7-derived P. falciparum laboratory strain (5A-R+) was partially inhibited by a GYPC mAb (clone Ret40f) and by soluble recombinant GYPC (Niang et al., 2014). Furthermore, cultured GYPC knockdown erythrocytes failed to rosette, providing strong evidence that GYPC is an essential rosetting receptor for 5A-R+ parasites (Niang et al., 2014). Other parasite lines or clinical isolates were not tested, therefore the wider role of GYPC in P. falciparum rosetting was not determined.
Lee et al. (2014) focussed mainly on P. vivax, but also assessed the ability of GYPC mAb fragments to inhibit rosette formation in ten P. falciparum clinical isolates from Thailand. A significant decrease in rosetting was reported with GYPC mAb BRIC 4, although the reduction in the median rosette frequency was small (from 11.5 to 5.5%), and was based on a single count for each isolate with no replication (Lee et al., 2014). The biological significance of these results is difficult to assess, given the low starting rosette frequencies and inherent variation in the rosetting assay. Lee et al. also used a different definition of rosetting to all previous studies, defining a rosette as an IE binding one or more uninfected erythrocytes. The usual definition requires the binding of two or more uninfected erythrocytes, which helps to identify genuine cell–cell interactions and avoid spurious identification of rosettes due to close packing of cells under the coverslip during microscopy.
For P. vivax, Lee et al. showed that the GYPC mAb reduced the median rosette frequency from 30 to 22% when tested on 11 Thai isolates, and that GYPC knockdown cultured erythrocytes formed rosettes poorly compared to GYPC-positive control cells (median rosette frequency 6.2 vs. 35.4% in controls, tested on three isolates). Plasmodium falciparum isolates were not tested with the GYPC knockdown erythrocytes.
If GYPC is a rosetting receptor, it is possible that the ‘Gerbich-negative’ blood group type, which is common in Melanesian populations (Patel et al., 2001), might influence rosetting. As part of a screen of null blood group erythrocytes with five high-rosetting P. falciparum culture-adapted parasite lines, Rowe et al. (1997) tested two donors with the Gerbich-negative blood group (formed by deletion of exon 3 of the GYPC gene on chromosome 2, giving a truncated protein with altered glycosylation). Gerbich-negative erythrocytes formed rosettes normally with the five parasite lines tested. Goel et al. also report normal rosetting of Gerbich-negative erythrocytes from two donors (Goel et al., 2015). The true null phenotype for GYPC, called the Leach phenotype (which arises due to the deletion of exon 3 and exon 4, encoding the transmembrane and cytoplasmic domains, respectively) is rare and has not been tested in rosetting assays to our knowledge.
Taking into account all existing evidence, further investigation of a wider range of parasite lines is needed to determine whether GYPC is an important host receptor for both P. falciparum and P. vivax rosetting.
Glycophorin A (GYPA, GPA, CD235a)
GYPA is a highly-expressed erythrocyte surface glycoprotein that carries the MNS blood group antigens. It is known to be a receptor for P. falciparum erythrocyte invasion (Sim et al., 1994), and polymorphisms in GYPA are associated with resistance to severe malaria (Band et al., 2015; Leffler et al., 2017).
There is some limited evidence to suggest that GYPA may have a role in rosetting. Parasites of the strain FCR3S1.2 transfected with a specific RIFIN gene formed rosettes that were largely dependent on blood group A (Goel et al., 2015). However, rosetting of the RIFIN-transfected parasites was significantly reduced with GYPA null cells from blood group O and B donors, whereas blood group A GYPA null erythrocytes formed rosettes normally. These data suggest that GYPA may have an accessory role for RIFIN-mediated rosetting in the absence of the A antigen (Goel et al., 2015), although whether this applies to rosetting in non-genetically manipulated parasites in unknown.
Despite the above positive evidence, there are no other data supporting a role for GYPA in rosetting. GYPA mAb fragments had no inhibitory effect on rosetting in ten P. falciparum and 11 P. vivax clinical isolates (Lee et al., 2014), and a GYPA mAb did not inhibit 3D7 5A-R+ rosettes (Niang et al., 2014). Furthermore, GYPA null erythrocytes (MkMk cells, lacking both GYPA and glycophorin B) formed rosettes with five culture-adapted P. falciparum lines (Rowe et al., 1997). Overall, existing evidence does not support a major role for GYPA in rosetting, but as with GYPC, further investigation is needed.
New receptors and new approaches
None of the receptors described above fully account for the adhesion interactions between infected and uninfected erythrocytes, and it is likely that other host rosetting receptors remain to be identified. There is evidence to suggest that these unknown host receptors are carbohydrates or protease-resistant proteins, because uninfected group O erythrocytes treated with trypsin and other proteases are still able to form rosettes (Udomsangpetch et al., 1989; Rowe et al., 1994).
In order to progress rosetting research, alternative methods are needed. Rosetting experiments with GYPC and CR1 knockdown cultured human red cells derived from CD34+ haematopoetic stem cells have been performed (Lee et al., 2014; Niang et al., 2014), using lentiviral transduction of short hairpin RNA (Bei et al., 2010). However, these cultured erythrocytes have a short life-span, limiting their usefulness. The development of immortalized erythroid lines (Kurita et al., 2013; Kanjee et al., 2017; Trakarnsanga et al., 2017; Scully et al., 2019) may overcome this limitation. Nevertheless, attention must be paid to the subtle but real differences between mature erythrocytes and these, still relatively immature, immortalized CD34+ derived cells (Wilson et al., 2016; Dankwa et al., 2017; Trakarnsanga et al., 2017). CRISPR-Cas9 technology (Doudna and Charpentier, 2014) has led to an explosion in the ability to genetically manipulate multiple cell types, including erythrocyte precursors and immortalized haematopoietic lines (Song et al., 2015; Kanjee et al., 2017; Hawksworth et al., 2018; Chung et al., 2019; Scully et al., 2019), potentially giving the opportunity to generate multiple knockout lines for rosetting research. A consistent supply of knockout erythrocytes would allow large-scale screens for new rosetting receptors using cells as close to their normal physiological form as possible, raising exciting prospects for future work.
Conclusions
Of the rosetting receptors described over the past 30 years, only the blood group A trisaccharide has been authenticated by a variety of methodological approaches from a range of different investigators. For all other potential rosetting receptors, the evidence remains fragmentary (Table 1) and further research is needed (Table 2). Recent technical advances in genetic manipulation of red cell precursors and immortalised lines should enable reverse genetic studies to bring further clarity to this biologically important topic.
Table 2.
Key areas for future research on rosetting receptors
| Determine the relative importance of known host erythrocyte receptors |
| Develop screening technologies to identify novel host rosetting receptors |
| Use immortalized erythroid lines for reverse genetic studies in rosetting |
| Develop novel rosette-disrupting adjunctive therapies |
| Develop in vivo models to test rosette-disrupting adjunctive therapies |
Financial support
This work was supported by the Wellcome Trust (PhD studentship grant number 108685/Z/15/Z).
Conflict of interest
None.
Ethical standards
Not applicable.
References
- Adams Y, Kuhnrae P, Higgins MK, Ghumra A and Rowe JA (2014) Rosetting Plasmodium falciparum-infected erythrocytes bind to human brain microvascular endothelial cells in vitro, demonstrating a dual adhesion phenotype mediated by distinct P. falciparum erythrocyte membrane protein 1 domains. Infection and Immunity 82, 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht L, Moll K, Blomqvist K, Normark J, Chen Q and Wahlgren M (2011) Var gene transcription and PfEMP1 expression in the rosetting and cytoadhesive Plasmodium falciparum clone FCR3S1.2. Malaria Journal 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti D, Sandalova T, Wahlgren M and Achour A (2015) Binding of subdomains 1/2 of PfEMP1-DBL1alpha to heparan sulfate or heparin mediates Plasmodium falciparum rosetting. PLoS ONE 10, e0118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus BJ, Thanikkul K, Silamut K, White NJ and Udomsangpetch R (1996) Short report: rosette formation in Plasmodium ovale infection. American Journal of Tropical Medicine and Hygiene 55, 560–561. [DOI] [PubMed] [Google Scholar]
- Band G, Rockett KA, Spencer CC, Kwiatkowski DP and Network MGE (2015) A novel locus of resistance to severe malaria in a region of ancient balancing selection. Nature 526, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragan A, Spillmann D, Kremsner PG, Wahlgren M and Carlson J (1999) Plasmodium falciparum: molecular background to strain-specific rosette disruption by glycosaminoglycans and sulfated glycoconjugates. Experimental Parasitology 91, 133–143. [DOI] [PubMed] [Google Scholar]
- Barragan A, Fernandez V, Chen Q, von Euler A, Wahlgren M and Spillmann D (2000a) The duffy-binding-like domain 1 of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) is a heparan sulfate ligand that requires 12 mers for binding. Blood 95, 3594–3599. [PubMed] [Google Scholar]
- Barragan A, Kremsner PG, Wahlgren M and Carlson J (2000b) Blood group A antigen is a coreceptor in Plasmodium falciparum rosetting. Infection and Immunity 68, 2971–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera V, MacCormick IJC, Czanner G, Hiscott PS, White VA, Craig AG, Beare NAV, Culshaw LH, Zheng Y, Biddolph SC, Milner DA, Kamiza S, Molyneux ME, Taylor TE and Harding SP (2018) Neurovascular sequestration in paediatric P. falciparum malaria is visible clinically in the retina. Elife 7, e32208. doi: 10.7554/eLife.32208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DI, Gormely JA, Ma C, Howard RJ and Pasloske BL (1996) Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proceedings of the National Academy of Sciences USA 93, 3497–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei AK, Brugnara C and Duraisingh MT (2010) In vitro genetic analysis of an erythrocyte determinant of malaria infection. Journal of Infectious Diseases 202, 1722–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A, Neculai D and Kain KC (2014) CD36 and malaria: friends or foes? A decade of data provides some answers. Trends in Parasitology 30, 436–444. [DOI] [PubMed] [Google Scholar]
- Carlson J and Wahlgren M (1992) Plasmodium falciparum erythrocyte rosetting is mediated by promiscuous lectin-like interactions. Journal of Experimental Medicine 176, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM and Wahlgren M (1990) Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336, 1457–1460. [DOI] [PubMed] [Google Scholar]
- Carlson J, Ekre HP, Helmby H, Gysin J, Greenwood BM and Wahlgren M (1992) Disruption of Plasmodium falciparum erythrocyte rosettes by standard heparin and heparin devoid of anticoagulant activity. American Journal of Tropical Medicine and Hygiene 46, 595–602. [DOI] [PubMed] [Google Scholar]
- Chen Q, Heddini A, Barragan A, Fernandez V, Pearce SF and Wahlgren M (2000) The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. Journal of Experimental Medicine 192, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch'ng JH, Moll K, Quintana MEP, Chan SC, Masters E, Moles E, Liu J, Eriksson AB and Wahlgren M (2016) Rosette-disrupting effect of an anti-plasmodial compound for the potential treatment of Plasmodium falciparum malaria complications. Scientific Reports 6, 29317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotivanich KT, Pukrittayakamee S, Simpson JA, White NJ and Udomsangpetch R (1998) Characteristics of Plasmodium vivax-infected erythrocyte rosettes. American Journal of Tropical Medicine and Hygiene 59, 73–76. [DOI] [PubMed] [Google Scholar]
- Chung WY, Gardiner DL, Hyland C, Gatton M, Kemp DJ and Trenholme KR (2005) Enhanced invasion of blood group A1 erythrocytes by Plasmodium falciparum. Molecular and Biochemical Parasitology 144, 128–130. [DOI] [PubMed] [Google Scholar]
- Chung JE, Magis W, Vu J, Heo SJ, Wartiovaara K, Walters MC, Kurita R, Nakamura Y, Boffelli D, Martin DIK, Corn JE and DeWitt MA (2019) CRISPR-Cas9 interrogation of a putative fetal globin repressor in human erythroid cells. PLoS ONE 14, e0208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough B, Atilola FA and Pasvol G (1998) The role of rosetting in the multiplication of Plasmodium falciparum: rosette formation neither enhances nor targets parasite invasion into uninfected red cells. British Journal of Haematology 100, 99–104. [DOI] [PubMed] [Google Scholar]
- Cockburn IA, Mackinnon MJ, O'Donnell A, Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC and Rowe JA (2004) A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria. Proceedings of the National Academy of Sciences USA 101, 272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman AF, Tonkin CJ, Tham WH and Duraisingh MT (2017) The molecular basis of erythrocyte invasion by malaria parasites. Cell Host and Microbe 22, 232–245. [DOI] [PubMed] [Google Scholar]
- Dankwa S, Chaand M, Kanjee U, Jiang RHY, Nobre LV, Goldberg JM, Bei AK, Moechtar MA, Grüring C, Ahouidi AD, Ndiaye D, Dieye TN, Mboup S, Weekes MP and Duraisingh MT (2017) Genetic evidence for erythrocyte receptor glycophorin B expression levels defining a dominant Plasmodium falciparum invasion pathway into human erythrocytes. Infection and Immunity 85, e00074-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Agostini AI, Watkins SC, Slayter HS, Youssoufian H and Rosenberg RD (1990) Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. Journal of Cell Biology 111, 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans AM and Rowe JA (2006) Plasmodium falciparum: rosettes do not protect merozoites from invasion-inhibitory antibodies. Experimental Parasitology 112, 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degarege A, Gebrezgi MT, Ibanez G, Wahlgren M and Madhivanan P (2019) Effect of the ABO blood group on susceptibility to severe malaria: a systematic review and meta-analysis. Blood Reviews 33, 53–62. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Pongponratn E and White NJ (2004) Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Tropica 89, 309–317. [DOI] [PubMed] [Google Scholar]
- Doudna JA and Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. [DOI] [PubMed] [Google Scholar]
- Doumbo OK, Thera MA, Koné AK, Raza A, Tempest LJ, Lyke KE, Plowe CV and Rowe JA (2009) High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. American Journal of Tropical Medicine and Hygiene 81, 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Sinnis P, Cerami C, Shreffler W, Takacs B and Nussenzweig V (1993) Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. Journal of Experimental Medicine 177, 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M and Duffy PE (1996) Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science 272, 1502–1504. [DOI] [PubMed] [Google Scholar]
- Fry AE, Griffiths MJ, Auburn S, Diakite M, Forton JT, Green A, Richardson A, Wilson J, Jallow M, Sisay-Joof F, Pinder M, Peshu N, Williams TN, Marsh K, Molyneux ME, Taylor TE, Rockett KA and Kwiatkowski DP (2008) Common variation in the ABO glycosyltransferase is associated with susceptibility to severe Plasmodium falciparum malaria. Human Molecular Genetics 17, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AE, Ghansa A, Small KS, Palma A, Auburn S, Diakite M, Green A, Campino S, Teo YY, Clark TG, Jeffreys AE, Wilson J, Jallow M, Sisay-Joof F, Pinder M, Griffiths MJ, Peshu N, Williams TN, Newton CR, Marsh K, Molyneux ME, Taylor TE, Koram KA, Oduro AR, Rogers WO, Rockett KA, Sabeti PC and Kwiatkowski DP (2009) Positive selection of a CD36 nonsense variant in sub-Saharan Africa, but no association with severe malaria phenotypes. Human Molecular Genetics 18, 2683–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumra A, Semblat JP, McIntosh RS, Raza A, Rasmussen IB, Braathen R, Johansen FE, Sandlie I, Mongini PK, Rowe JA and Pleass RJ (2008) Identification of residues in the Cmu4 domain of polymeric IgM essential for interaction with Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). Journal of Immunology 181, 1988–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, Claessens A, Anong DN, Bull PC, Fennell C, Arman M, Amambua-Ngwa A, Walther M, Conway DJ, Kassambara L, Doumbo OK, Raza A and Rowe JA (2012) Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathogens 8, e1002665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, Moradi N, Öjemalm K, Westman M, Angeletti D, Kjellin H, Lehtiö J, Blixt O, Ideström L, Gahmberg CG, Storry JR, Hult AK, Olsson ML, von Heijne G, Nilsson I and Wahlgren M (2015) RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nature Medicine 21, 314–317. [DOI] [PubMed] [Google Scholar]
- Handunnetti SM, van Schravendijk MR, Hasler T, Barnwell JW, Greenwalt DE and Howard RJ (1992) Involvement of CD36 on erythrocytes as a rosetting receptor for Plasmodium falciparum-infected erythrocytes. Blood 80, 2097–2104. [PubMed] [Google Scholar]
- Hawksworth J, Satchwell TJ, Meinders M, Daniels DE, Regan F, Thornton NM, Wilson MC, Dobbe JG, Streekstra GJ, Trakarnsanga K, Heesom KJ, Anstee DJ, Frayne J and Toye AM (2018) Enhancement of red blood cell transfusion compatibility using CRISPR-mediated erythroblast gene editing. EMBO Molecular Medicine 10, e8454. doi: 10.15252/emmm.201708454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddini A, Pettersson F, Kai O, Shafi J, Obiero J, Chen Q, Barragan A, Wahlgren M and Marsh K (2001) Fresh isolates from children with severe Plasmodium falciparum malaria bind to multiple receptors. Infection and Immunity 69, 5849–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L and Jensen AT (2015) Pfemp1 – a parasite protein family of key importance in Plasmodium falciparum malaria immunity and pathogenesis. Advances in Parasitology 88, 51–84. [DOI] [PubMed] [Google Scholar]
- Ito N, Nishi K, Kawahara S, Okamura Y, Hirota T, Rand S, Fechner G and Brinkmann B (1990) Difference in the ability of blood group-specific lectins and monoclonal antibodies to recognize the ABH antigens in human tissues. Histochemical Journal 22, 604–614. [DOI] [PubMed] [Google Scholar]
- Jaskiewicz E, Peyrard T, Kaczmarek R, Zerka A, Jodlowska M and Czerwinski M (2018) The Gerbich blood group system: old knowledge, new importance. Transfusion Medicine Reviews 32, 111–116. [DOI] [PubMed] [Google Scholar]
- Juillerat A, Igonet S, Vigan-Womas I, Guillotte M, Gangnard S, Faure G, Baron B, Raynal B, Mercereau-Puijalon O and Bentley GA (2010) Biochemical and biophysical characterisation of DBL1alpha1-varO, the rosetting domain of PfEMP1 from the VarO line of Plasmodium falciparum. Molecular and Biochemical Parasitology 170, 84–92. [DOI] [PubMed] [Google Scholar]
- Juillerat A, Lewit-Bentley A, Guillotte M, Gangnard S, Hessel A, Baron B, Vigan-Womas I, England P, Mercereau-Puijalon O and Bentley GA (2011) Structure of a Plasmodium falciparum PfEMP1 rosetting domain reveals a role for the N-terminal segment in heparin-mediated rosette inhibition. Proceedings of the National Academy of Sciences USA 108, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjee U, Grüring C, Chaand M, Lin KM, Egan E, Manzo J, Jones PL, Yu T, Barker R, Weekes MP and Duraisingh MT (2017) CRISPR/cas9 knockouts reveal genetic interaction between strain-transcendent erythrocyte determinants of. Proceedings of the National Academy of Sciences USA 114, E9356–E9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Roth EF, Nagel RL, Howard RJ and Handunnetti SM (1991) Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78, 812–819. [PubMed] [Google Scholar]
- Kraemer SM and Smith JD (2006) A family affair: var genes, PfEMP1 binding, and malaria disease. Current Opinion in Microbiology 9, 374–380. [DOI] [PubMed] [Google Scholar]
- Kun JF, Schmidt-Ott RJ, Lehman LG, Lell B, Luckner D, Greve B, Matousek P and Kremsner PG (1998) Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambaréné, Gabon. Transactions of the Royal Society of Tropical Medicine and Hygiene 92, 110–114. [DOI] [PubMed] [Google Scholar]
- Kurita R, Suda N, Sudo K, Miharada K, Hiroyama T, Miyoshi H, Tani K and Nakamura Y (2013) Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE 8, e59890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou HM, Stone GN, Challis RJ, Raza A, Lyke KE, Thera MA, Koné AK, Doumbo OK, Plowe CV and Rowe JA (2006) Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Molecular and Biochemical Parasitology 150, 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriacou HM, Steen KE, Raza A, Arman M, Warimwe G, Bull PC, Havlik I and Rowe JA (2007) In vitro inhibition of Plasmodium falciparum rosette formation by Curdlan sulfate. Antimicrobial Agents and Chemotherapy 51, 1321–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Scanf C, Vigan-Womas I, Contamin H, Guillotte M, Bischoff E and Mercereau-Puijalon O (2008) Rosetting is associated with increased Plasmodium falciparum in vivo multiplication rate in the Saimiri sciureus monkey. Microbes and Infection 10, 447–451. [DOI] [PubMed] [Google Scholar]
- Lee WC, Malleret B, Lau YL, Mauduit M, Fong MY, Cho JS, Suwanarusk R, Zhang R, Albrecht L, Costa FT, Preiser P, McGready R, Renia L, Nosten F and Russell B (2014) Glycophorin C (CD236R) mediates vivax malaria parasite rosetting to normocytes. Blood 123, e100–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler EM, Band G, Busby GBJ, Kivinen K, Le QS, Clarke GM, Bojang KA, Conway DJ, Jallow M, Sisay-Joof F, Bougouma EC, Mangano VD, Modiano D, Sirima SB, Achidi E, Apinjoh TO, Marsh K, Ndila CM, Peshu N, Williams TN, Drakeley C, Manjurano A, Reyburn H, Riley E, Kachala D, Molyneux M, Nyirongo V, Taylor T, Thornton N, Tilley L, Grimsley S, Drury E, Stalker J, Cornelius V, Hubbart C, Jeffreys AE, Rowlands K, Rockett KA, Spencer CCA, Kwiatkowski DP and Malaria Genomic Epidemiology Network (2017) Resistance to malaria through structural variation of red blood cell invasion receptors. Science 356, eaam6393. doi: 10.1126/science.aam6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitgeb AM, Blomqvist K, Cho-Ngwa F, Samje M, Nde P, Titanji V and Wahlgren M (2011) Low anticoagulant heparin disrupts Plasmodium falciparum rosettes in fresh clinical isolates. American Journal of Tropical Medicine and Hygiene 84, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitgeb AM, Charunwatthana P, Rueangveerayut R, Uthaisin C, Silamut K, Chotivanich K, Sila P, Moll K, Lee SJ, Lindgren M, Holmer E, Färnert A, Kiwuwa MS, Kristensen J, Herder C, Tarning J, Wahlgren M and Dondorp AM (2017) Inhibition of merozoite invasion and transient de-sequestration by sevuparin in humans with Plasmodium falciparum malaria. PLoS ONE 12, e0188754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JS, Jaggers J and Anderson PA (2006) The use of TP10, soluble complement receptor 1, in cardiopulmonary bypass. Expert Reviews in Cardiovascular Therapy 4, 649–654. [DOI] [PubMed] [Google Scholar]
- Lowe BS, Mosobo M and Bull PC (1998) All four species of human malaria parasites form rosettes. Transactions of the Royal Society of Tropical Medicine and Hygiene 92, 526. [DOI] [PubMed] [Google Scholar]
- Lublin DM, Griffith RC and Atkinson JP (1986) Influence of glycosylation on allelic and cell-specific Mr variation, receptor processing, and ligand binding of the human complement C3b/C4b receptor. Journal of Biological Chemistry 261, 5736–5744. [PubMed] [Google Scholar]
- Luginbuhl A, Nikolic M, Beck HP, Wahlgren M and Lutz HU (2007) Complement factor D, albumin, and immunoglobulin G anti-band 3 protein antibodies mimic serum in promoting rosetting of malaria-infected red blood cells. Infection and Immunity 75, 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier AG, Duraisingh MT, Reeder JC, Patel SS, Kazura JW, Zimmerman PA and Cowman AF (2003) Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nature Medicine 9, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaria Genomic Epidemiology Network (2014) Reappraisal of known malaria resistance loci in a large multicenter study. Nature Genetics 46, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Menéndez A, Bardají A, Martínez-Espinosa FE, Bôtto-Menezes C, Lacerda MV, Ortiz J, Cisteró P, Piqueras M, Felger I, Müeller I, Ordi J, del Portillo H, Menéndez C, Wahlgren M and Mayor A (2013) Rosetting in Plasmodium vivax: a cytoadhesion phenotype associated with anaemia. PLoS Neglected Tropical Diseases 7, e2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DC, Jiang L, Achur RN, Kakizaki I, Gowda DC and Miller LH (2006) The glycophorin C N-linked glycan is a critical component of the ligand for the Plasmodium falciparum erythrocyte receptor BAEBL. Proceedings of the National Academy of Sciences USA 103, 2358–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray ID, Serghides L, Kapus A, Rotstein OD and Kain KC (2000) Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96, 3231–3240. [PubMed] [Google Scholar]
- McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G and Foote SJ (2012) Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 338, 1348–1351. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K and Doumbo OK (2002) The pathogenic basis of malaria. Nature 415, 673–679. [DOI] [PubMed] [Google Scholar]
- Moll K, Palmkvist M, Ch'ng J, Kiwuwa MS and Wahlgren M (2015) Evasion of immunity to Plasmodium falciparum: rosettes of blood group A impair recognition of PfEMP1. PLoS ONE 10, e0145120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulds JM (2010) The Knops blood-group system: a review. Immunohematology 26, 2–7. [PubMed] [Google Scholar]
- Moulds JM, Moulds JJ, Brown M and Atkinson JP (1992) Antiglobulin testing for CR1-related (Knops/McCoy/Swain-Langley/York) blood group antigens: negative and weak reactions are caused by variable expression of CR1. Vox Sanguinis 62, 230–235. [DOI] [PubMed] [Google Scholar]
- Nagayasu E, Ito M, Akaki M, Nakano Y, Kimura M, Looareesuwan S and Aikawa M (2001) CR1 density polymorphism on erythrocytes of falciparum malaria patients in Thailand. American Journal of Tropical Medicine and Hygiene 64, 1–5. [DOI] [PubMed] [Google Scholar]
- Ndila CM, Uyoga S, Macharia AW, Nyutu G, Peshu N, Ojal J, Shebe M, Awuondo KO, Mturi N, Tsofa B, Sepulveda N, Clark TG, Band G, Clarke G, Rowlands K, Hubbart C, Jeffreys A, Kariuki S, Marsh K, Mackinnon M, Maitland K, Kwiatkowski DP, Rockett KA, Williams TN and Malaria GENC (2018) Human candidate gene polymorphisms and risk of severe malaria in children in Kilifi, Kenya: a case-control association study. Lancet Haematology 5, e333–e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold C, Warn P, Black G, Berendt A, Craig A, Snow B, Msobo M, Peshu N and Marsh K (1997) Receptor-specific adhesion and clinical disease in Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene 57, 389–398. [DOI] [PubMed] [Google Scholar]
- Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, Gunalan K, Amaladoss A, Yeo KP, Bob NS, Malleret B, Duraisingh MT and Preiser PR (2014) STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host & Microbe 16, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells M, Hauhart R, Krych M, Subramanian VB, Geoghegan-Barek K, Marsh HC Jr. and Atkinson JP (1998) Mapping epitopes for 20 monoclonal antibodies to CR1. Clinical and Experimental Immunology 112, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochola LB, Siddondo BR, Ocholla H, Nkya S, Kimani EN, Williams TN, Makale JO, Liljander A, Urban BC, Bull PC, Szestak T, Marsh K and Craig AG (2011) Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS ONE 6, e14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opi DH, Swann O, Macharia A, Uyoga S, Band G, Ndila CM, Harrison E, Thera MA, Kone AK, Diallo DA, Doumbo OK, Lyke KE, Plowe C, Moulds JM, Shebbe M, Mturi N, Peshu N, Maitland K, Raza A, Kwiatkowski DP, Rockett KA, Williams T and Rowe JA (2018) Two complement receptor one alleles have opposing associations with cerebral malaria and interact with α + thalassaemia. Elife 7, e31579. doi: 10.7554/eLife.31579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda AK, Panda M, Tripathy R, Pattanaik SS, Ravindran B and Das BK (2012) Complement receptor 1 variants confer protection from severe malaria in Odisha, India. PLoS ONE 7, e49420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SS, Mehlotra RK, Kastens W, Mgone CS, Kazura JW and Zimmerman PA (2001) The association of the glycophorin C exon 3 deletion with ovalocytosis and malaria susceptibility in the Wosera, Papua New Guinea. Blood 98, 3489–3491. [DOI] [PubMed] [Google Scholar]
- Pathak V, Colah R and Ghosh K (2016) Correlation between ‘H’ blood group antigen and Plasmodium falciparum invasion. Annals of Hematology 95, 1067–1075. [DOI] [PubMed] [Google Scholar]
- Pipitaporn B, Sueblinvong T, Dharmkrong-at A and Udomsangpetch R (2000) Rosetting of Plasmodium falciparum required multiple components of the uninfected erythrocytes. Asian Pacific Journal of Allergy and Immunology 18, 29–35. [PubMed] [Google Scholar]
- Rampengan TH (1991) Cerebral malaria in children. Comparative study between heparin, dexamethasone and placebo. Paediatrica Indonesiana 31, 59–66. [PubMed] [Google Scholar]
- Reddy YN, Siedlecki AM and Francis JM (2017) Breaking down the complement system: a review and update on novel therapies. Current Opinion in Nephrology and Hypertension 26, 123–128. [DOI] [PubMed] [Google Scholar]
- Ribacke U, Moll K, Albrecht L, Ahmed Ismail H, Normark J, Flaberg E, Szekely L, Hultenby K, Persson KE, Egwang TG and Wahlgren M (2013) Improved in vitro culture of Plasmodium falciparum permits establishment of clinical isolates with preserved multiplication, invasion and rosetting phenotypes. PLoS ONE 8, e69781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald P, Peyron F, Lepers JP, Rabarison P, Rakotomalala C, Razanamparany M, Rabodonirina M, Roux J and Le Bras J (1993) Parasite virulence factors during falciparum malaria: rosetting, cytoadherence, and modulation of cytoadherence by cytokines. Infection and Immunity 61, 5198–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BA, Welch TL and Smith JD (2003) Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Molecular Microbiology 47, 1265–1278. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Reeder JC, al-Yaman F and Brown GV (1994) Sulfated glycoconjugates as disrupters of Plasmodium falciparum erythrocyte rosettes. American Journal of Tropical Medicine and Hygiene 51, 198–203. [DOI] [PubMed] [Google Scholar]
- Rout R, Dhangadamajhi G, Mohapatra BN, Kar SK and Ranjit M (2011) High CR1 level and related polymorphic variants are associated with cerebral malaria in eastern-India. Infection Genetics and Evolution 11, 139–144. [DOI] [PubMed] [Google Scholar]
- Rout R, Dhangadamajhi G, Ghadei M, Mohapatra BN, Kar SK and Ranjit M (2012) Blood group phenotypes A and B are risk factors for cerebral malaria in Odisha, India. Transactions of the Royal Society of Tropical Medicine and Hygiene 106, 538–543. [DOI] [PubMed] [Google Scholar]
- Rowe A, Berendt AR, Marsh K and Newbold CI (1994) Plasmodium falciparum: a family of sulphated glycoconjugates disrupts erythrocyte rosettes. Experimental Parasitology 79, 506–516. [DOI] [PubMed] [Google Scholar]
- Rowe A, Obeiro J, Newbold CI and Marsh K (1995) Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infection and Immunity 63, 2323–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JA, Moulds JM, Newbold CI and Miller LH (1997) P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388, 292–295. [DOI] [PubMed] [Google Scholar]
- Rowe JA, Rogerson SJ, Raza A, Moulds JM, Kazatchkine MD, Marsh K, Newbold CI, Atkinson JP and Miller LH (2000) Mapping of the region of complement receptor (CR) 1 required for Plasmodium falciparum rosetting and demonstration of the importance of CR1 in rosetting in field isolates. Journal of Immunology 165, 6341–6346. [DOI] [PubMed] [Google Scholar]
- Rowe JA, Obiero J, Marsh K and Raza A (2002) Short report: positive correlation between rosetting and parasitemia in Plasmodium falciparum clinical isolates. American Journal of Tropical Medicine and Hygiene 66, 458–460. [DOI] [PubMed] [Google Scholar]
- Rowe JA, Handel IG, Thera MA, Deans AM, Lyke KE, Koné A, Diallo DA, Raza A, Kai O, Marsh K, Plowe CV, Doumbo OK and Moulds JM (2007) Blood group O protects against severe Plasmodium falciparum malaria through the mechanism of reduced rosetting. Proceedings of the National Academy of Sciences USA 104, 17471–17476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JA, Claessens A, Corrigan RA and Arman M (2009a) Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Reviews in Molecular Medicine 11, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JA, Opi DH and Williams TN (2009b) Blood groups and malaria: fresh insights into pathogenesis and identification of targets for intervention. Current Opinion in Hematology 16, 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiwaew S, Sritabal J, Piaraksa N, Keayarsa S, Ruengweerayut R, Utaisin C, Sila P, Niramis R, Udomsangpetch R, Charunwatthana P, Pongponratn E, Pukrittayakamee S, Leitgeb AM, Wahlgren M, Lee SJ, Day NP, White NJ, Dondorp AM and Chotivanich K (2017) Effects of sevuparin on rosette formation and cytoadherence of Plasmodium falciparum infected erythrocytes. PLoS ONE 12, e0172718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Kennedy AT and Tham WH (2015) More than just immune evasion: Hijacking complement by Plasmodium falciparum. Molecular Immunology 67, 71–84. [DOI] [PubMed] [Google Scholar]
- Scholander C, Treutiger CJ, Hultenby K and Wahlgren M (1996) Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nature Medicine 2, 204–208. [DOI] [PubMed] [Google Scholar]
- Scully EJ, Shabani E, Rangel GW, Gruring C, Kanjee U, Clark MA, Chaand M, Kurita R, Nakamura Y, Ferreira MU and Duraisingh MT (2019) Generation of an immortalized erythroid progenitor cell line from peripheral blood: a model system for the functional analysis of Plasmodium spp. invasion. American Journal of Hematology 94, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semblat JP, Ghumra A, Czajkowsky DM, Wallis R, Mitchell DA, Raza A and Rowe JA (2015) Identification of the minimal binding region of a Plasmodium falciparum IgM binding PfEMP1 domain. Molecular and Biochemical Parasitology 201, 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL and Febbraio M (2009) CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science Signaling 2, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim BK, Chitnis CE, Wasniowska K, Hadley TJ and Miller LH (1994) Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264, 1941–1944. [DOI] [PubMed] [Google Scholar]
- Sinha S, Jha GN, Anand P, Qidwai T, Pati SS, Mohanty S, Mishra SK, Tyagi PK, Sharma SK, Venkatesh V and Habib S (2009) CR1 levels and gene polymorphisms exhibit differential association with falciparum malaria in regions of varying disease endemicity. Human Immunology 70, 244–250. [DOI] [PubMed] [Google Scholar]
- Song B, Fan Y, He W, Zhu D, Niu X, Wang D, Ou Z, Luo M and Sun X (2015) Improved hematopoietic differentiation efficiency of gene-corrected beta-thalassemia induced pluripotent stem cells by CRISPR/Cas9 system. Stem Cells and Development 24, 1053–1065. [DOI] [PubMed] [Google Scholar]
- Stevenson L, Huda P, Jeppesen A, Laursen E, Rowe JA, Craig A, Streicher W, Barfod L and Hviid L (2015a) Investigating the function of Fc-specific binding of IgM to Plasmodium falciparum erythrocyte membrane protein 1 mediating erythrocyte rosetting. Cellular Microbiology 17, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson L, Laursen E, Cowan GJ, Bandoh B, Barfod L, Cavanagh DR, Andersen GR and Hviid L (2015b) α2-Macroglobulin can crosslink multiple Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) molecules and may facilitate adhesion of parasitized erythrocytes. PLoS Pathogens 11, e1005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler-Barak L, Moussion C, Shezen E, Hatzav M, Sixt M and Alon R (2014) Blood vessels pattern heparan sulfate gradients between their apical and basolateral aspects. PLoS ONE 9, e85699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeranaipong P, Ohashi J, Patarapotikul J, Kimura R, Nuchnoi P, Hananantachai H, Naka I, Putaporntip C, Jongwutiwes S and Tokunaga K (2008) A functional single-nucleotide polymorphism in the CR1 promoter region contributes to protection against cerebral malaria. Journal of Infectious Diseases 198, 1880–1891. [DOI] [PubMed] [Google Scholar]
- Tekeste Z and Petros B (2010) The ABO blood group and Plasmodium falciparum malaria in Awash, Metehara and Ziway areas, Ethiopia. Malaria Journal 9, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh-Quarcoo PB, Schmidt CQ, Tham WH, Hauhart R, Mertens HD, Rowe A, Atkinson JP, Cowman AF, Rowe JA and Barlow PN (2012) Lack of evidence from studies of soluble protein fragments that Knops blood group polymorphisms in complement receptor-type 1 are driven by malaria. PLoS ONE 7, e34820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathy V, Moulds JM, Guyah B, Otieno W and Stoute JA (2005) Complement receptor 1 polymorphisms associated with resistance to severe malaria in Kenya. Malaria Journal 4, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theron M, Cross N, Cawkill P, Bustamante LY and Rayner JC (2018) An in vitro erythrocyte preference assay reveals that Plasmodium falciparum parasites prefer Type O over Type A erythrocytes. Scientific Reports 8, 8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielen AJF, Zeerleder S and Wouters D (2018) Consequences of dysregulated complement regulators on red blood cells. Blood Reviews 32, 280–288. [DOI] [PubMed] [Google Scholar]
- Trakarnsanga K, Griffiths RE, Wilson MC, Blair A, Satchwell TJ, Meinders M, Cogan N, Kupzig S, Kurita R, Nakamura Y, Toye AM, Anstee DJ and Frayne J (2017) An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nature Communications 8, 14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood BM and Wahlgren M (1992) Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. American Journal of Tropical Medicine and Hygiene 46, 503–510. [DOI] [PubMed] [Google Scholar]
- Treutiger CJ, Scholander C, Carlson J, McAdam KP, Raynes JG, Falksveden L and Wahlgren M (1999) Rouleaux-forming serum proteins are involved in the rosetting of Plasmodium falciparum-infected erythrocytes. Experimental Parasitology 93, 215–224. [DOI] [PubMed] [Google Scholar]
- Udomsangpetch R, Wåhlin B, Carlson J, Berzins K, Torii M, Aikawa M, Perlmann P and Wahlgren M (1989) Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. Journal of Experimental Medicine 169, 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R, Webster HK, Pattanapanyasat K, Pitchayangkul S and Thaithong S (1992) Cytoadherence characteristics of rosette-forming Plasmodium falciparum. Infection and Immunity 60, 4483–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R, Todd J, Carlson J and Greenwood BM (1993) The effects of hemoglobin genotype and ABO blood group on the formation of rosettes by Plasmodium falciparum-infected red blood cells. American Journal of Tropical Medicine and Hygiene 48, 149–153. [DOI] [PubMed] [Google Scholar]
- Udomsanpetch R, Thanikkul K, Pukrittayakamee S and White NJ (1995) Rosette formation by Plasmodium vivax. Transactions of the Royal Society of Tropical Medicine and Hygiene 89, 635–637. [DOI] [PubMed] [Google Scholar]
- Uyoga S, Skorokhod OA, Opiyo M, Orori EN, Williams TN, Arese P and Schwarzer E (2012) Transfer of 4-hydroxynonenal from parasitized to non-parasitized erythrocytes in rosettes. Proposed role in severe malaria anemia. British Journal of Haematology 157, 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schravendijk M, Handunnetti S, Barnwell J and Howard R (1992) Normal human erythrocytes express CD36, an adhesion molecule of monocytes, platelets and endothelial cells. Blood 80, 2105–2114. [PubMed] [Google Scholar]
- Vigan-Womas I, Guillotte M, Le Scanf C, Igonet S, Petres S, Juillerat A, Badaut C, Nato F, Schneider A, Lavergne A, Contamin H, Tall A, Baril L, Bentley GA and Mercereau-Puijalon O (2008) An in vivo and in vitro model of Plasmodium falciparum rosetting and autoagglutination mediated by varO, a group A var gene encoding a frequent serotype. Infection and Immunity 76, 5565–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigan-Womas I, Guillotte M, Juillerat A, Vallieres C, Lewit-Bentley A, Tall A, Baril L, Bentley GA and Mercereau-Puijalon O (2011) Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes. PLoS ONE 6, e16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigan-Womas I, Guillotte M, Juillerat A, Hessel A, Raynal B, England P, Cohen JH, Bertrand O, Peyrard T, Bentley GA, Lewit-Bentley A and Mercereau-Puijalon O (2012) Structural basis for the ABO blood-group dependence of Plasmodium falciparum rosetting. PLoS Pathogens 8, e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt AM, Barragan A, Chen Q, Kironde F, Spillmann D and Wahlgren M (2003) Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1alpha domain of PfEMP1. Blood 101, 2405–2411. [DOI] [PubMed] [Google Scholar]
- Vogt AM, Winter G, Wahlgren M and Spillmann D (2004) Heparan sulphate identified on human erythrocytes: a Plasmodium falciparum receptor. Biochemical Journal 381, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M, Carlson J, Udomsangpetch R and Perlmann P (1989) Why do Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes? Parasitology Today 5, 183–185. [DOI] [PubMed] [Google Scholar]
- Wang CW and Hviid L (2015) Rifins, rosetting, and red blood cells. Trends in Parasitology 31, 285–286. [DOI] [PubMed] [Google Scholar]
- Warimwe GM, Fegan G, Musyoki JN, Newton CR, Opiyo M, Githinji G, Andisi C, Menza F, Kitsao B, Marsh K and Bull PC (2012) Prognostic indicators of life-threatening malaria are associated with distinct parasite variant antigen profiles. Science Translational Medicine 4, 129ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ, Turner GD, Day NP and Dondorp AM (2013) Lethal malaria: Marchiafava and Bignami were right. Journal of Infectious Diseases 208, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Murphy EE, Wong WW, Klickstein LB, Weis JH and Fearon DT (1986) Identification of a restriction fragment length polymorphism by a CR1 cDNA that correlates with the number of CR1 on erythrocytes. Journal of Experimental Medicine 164, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, Trakarnsanga K, Heesom KJ, Cogan N, Green C, Toye AM, Parsons SF, Anstee DJ and Frayne J (2016) Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Molecular and Cellular Proteomics 15, 1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolofsky KT, Ayi K, Branch DR, Hult AK, Olsson ML, Liles WC, Cserti-Gazdewich CM and Kain KC (2012) ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathogens 8, e1002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (1986) Severe and complicated malaria. World Health Organization Malaria Action Programme. Transactions of the Royal Society of Tropical Medicine and Hygiene 80(suppl.), 3–50. [PubMed] [Google Scholar]
- Yam XY, Niang M, Madnani KG and Preiser PR (2017) Three is a crowd – new insights into rosetting in Plasmodium falciparum. Trends in Parasitology 33, 309–320. [DOI] [PubMed] [Google Scholar]