Abstract
Background
Alzheimer's disease is the commonest cause of dementia affecting older people. One of the therapeutic strategies aimed at ameliorating the clinical manifestations of Alzheimer's disease is to enhance cholinergic neurotransmission in the brain by the use of cholinesterase inhibitors to delay the breakdown of acetylcholine released into synaptic clefts. Tacrine, the first of the cholinesterase inhibitors to undergo extensive trials for this purpose, was associated with significant adverse effects including hepatotoxicity. Other cholinesterase inhibitors, including rivastigmine, with superior properties in terms of specificity of action and lower risk of adverse effects have since been introduced. Rivastigmine has received approval for use in 60 countries including all member states of the European Union and the USA.
Objectives
To determine the clinical efficacy and safety of rivastigmine for patients with dementia of Alzheimer's type.
Search methods
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 2 March 2015 using the terms: Rivastigmine OR exelon OR ENA OR "SDZ ENA 713". ALOIS contains records of clinical trials identified from monthly searches of a number of major healthcare databases (Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL, LILACS), numerous trial registries and grey literature sources.
Selection criteria
We included all unconfounded, double‐blind, randomised, controlled trials in which treatment with rivastigmine was administered to patients with dementia of the Alzheimer's type for 12 weeks or more and its effects compared with those of placebo in a parallel group of patients, or where two formulations of rivastigmine were compared.
Data collection and analysis
One review author (JSB) applied the study selection criteria, assessed the quality of studies and extracted data.
Main results
A total of 13 trials met the inclusion criteria of the review. The trials had a duration of between 12 and 52 weeks. The older trials tested a capsule form with a dose of up to 12 mg/day. Trials reported since 2007 have tested continuous dose transdermal patch formulations delivering 4.6, 9.5 and 17.7 mg/day.
Our main analysis compared the safety and efficacy of rivastigmine 6 to 12 mg/day orally or 9.5 mg/day transdermally with placebo.
Seven trials contributed data from 3450 patients to this analysis. Data from another two studies were not included because of a lack of information and methodological concerns. All the included trials were multicentre trials and recruited patients with mild to moderate Alzheimer's disease with a mean age of about 75 years. All had low risk of bias for randomisation and allocation but the risk of bias due to attrition was unclear in four studies, low in one study and high in two studies.
After 26 weeks of treatment rivastigmine compared to placebo was associated with better outcomes for cognitive function measured with the Alzheimer's Disease Assessment Scale‐Cognitive (ADAS‐Cog) score (mean difference (MD) ‐1.79; 95% confidence interval (CI) ‐2.21 to ‐1.37, n = 3232, 6 studies) and the Mini‐Mental State Examination (MMSE) score (MD 0.74; 95% CI 0.52 to 0.97, n = 3205, 6 studies), activities of daily living (SMD 0.20; 95% CI 0.13 to 0.27, n = 3230, 6 studies) and clinician rated global impression of changes, with a smaller proportion of patients treated with rivastigmine experiencing no change or a deterioration (OR 0.68; 95% CI 0.58 to 0.80, n = 3338, 7 studies).
Three studies reported behavioural change, and there were no differences compared to placebo (standardised mean difference (SMD) ‐0.04; 95% CI ‐0.14 to 0.06, n = 1529, 3 studies). Only one study measured the impact on caregivers using the Neuropsychiatric Inventory‐Caregiver Distress (NPI‐D) scale and this found no difference between the groups (MD 0.10; 95% CI ‐0.91 to 1.11, n = 529, 1 study). Overall, participants who received rivastigmine were about twice as likely to withdraw from the trials (odds ratio (OR) 2.01, 95% CI 1.71 to 2.37, n = 3569, 7 studies) or to experience an adverse event during the trials (OR 2.16, 95% CI 1.82 to 2.57, n = 3587, 7 studies).
Authors' conclusions
Rivastigmine (6 to 12 mg daily orally or 9.5 mg daily transdermally) appears to be beneficial for people with mild to moderate Alzheimer's disease. In comparisons with placebo, better outcomes were observed for rate of decline of cognitive function and activities of daily living, although the effects were small and of uncertain clinical importance. There was also a benefit from rivastigmine on the outcome of clinician's global assessment. There were no differences between the rivastigmine group and placebo group in behavioural change or impact on carers. At these doses the transdermal patch may have fewer side effects than the capsules but has comparable efficacy. The quality of evidence is only moderate for all of the outcomes reviewed because of a risk of bias due to dropouts. All the studies with usable data were industry funded or sponsored. This review has not examined economic data.
Plain language summary
Rivastigmine for people with Alzheimer's disease
Review question
We reviewed evidence comparing the effectiveness and safety of rivastigmine with placebo in people with Alzheimer's disease. Background
Alzheimer's disease is the commonest cause of dementia affecting older people. As the disease progresses, people lose the ability to remember, communicate, think clearly and perform the usual daily activities. Their behaviour or personality may also change. In severe Alzheimer's disease, the patients lose the ability to care for themselves and require full time care.
Currently, there is no cure available for Alzheimer's disease, but a few pharmacological interventions are available to alleviate symptoms.
The symptoms are caused by the loss of a type of nerve cell in the brain called cholinergic neurons. Rivastigmine, an acetylcholinesterase inhibitor, works by increasing the levels of a brain chemical called acetylcholine which allows the nerve cells to communicate. This may improve the symptoms of dementia. Rivastigmine can be taken orally, either as capsules or a liquid, or by applying a patch on the skin. Its effectiveness in improving the symptoms of Alzheimer's disease and safety were evaluated in this review.
Study characteristics
This review included double‐blinded randomised controlled trials, and the evidence was searched for up to March 2015 using the standard Cochrane methods. The review included studies conducted for at least 12 weeks that compared the safety and effectiveness of rivastigmine compared with placebo. Thirteen studies that met these criteria were found. Most of these studies involved people with mild to moderate Alzheimer's disease with an average age of around 75 years.
Key results
Results from seven trials showed that patients on rivastigmine (6 to 12 mg/day by mouth, or 9.5 mg/day by skin patch) were better for three outcomes than those on placebo, after six months of treatment. The differences were quite small for cognitive function (2 points, using the ADAS‐Cog which has a range of 70 points) and activities of daily living (standardised mean difference (SMD) of 0.20, which is considered a small effect). Patients on rivastigmine were more likely to show overall improvement compared with those on placebo (odds ratio of 1.47, 95% confidence interval (CI) of 1.25 to 1.72) . However, there was no difference for behavioural changes (reported by three trials) or impact on carers (reported by one trial). Patients on rivastigmine were also about twice as likely to experience adverse events, although this risk might have been slightly less for patients using patches compared with capsules. It was possible that certain types of adverse events were less in people using patches than taking capsules (nausea, vomiting, weight loss, dizziness).
In summary, rivastigmine may be of benefit to people with Alzheimer's disease. It is possible that the using a patch is associated with reduced side effects compared to using oral capsules.
Quality of evidence
The quality of the evidence for most of the outcomes reviewed was moderate. The main factors affecting our confidence in the results included relatively high number of patients dropping out in some of the trials (the rates of dropout in the rivastigmine arms were higher). There were also concerns about the applicability of the evidence for the long term treatment of Alzheimer's disease since data from double‐blinded randomised controlled trials were only available for up to 12 months. All the data included in the main analysis of this review came from studies either sponsored or funded by the drug manufacturer (Novartis Pharma).
Summary of findings
Summary of findings for the main comparison. Rivastigmine compared to placebo for Alzheimer's disease.
| Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.8 mg/day) patch) compared to placebo for Alzheimer's disease | |||||||
| Patient or population: patients with Alzheimer's disease, mild to moderate Settings: multicentre, mostly in Europe or United States Intervention: rivastigmine (capsules 6 to 12 mg/day in 2 divided doses or 10 cm2 patch) for 24 to 26 weeks Comparison: placebo for 24 to 26 weeks | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Placebo | Rivastigmine (capsules 6 to 12 mg/day b.i.d. or 10 cm2 patch) | ||||||
|
Cognitive function (change from baseline at 26 weeks using ADAS‐Cog) |
The mean score in the rivastigmine group was 1.79 lower (2.21 to 1.37 lower) | 3232 (6 studies) | ⊕⊕⊕⊝ moderate1,2, | ADAS‐Cog score has a maximum of 70 points, the lower score of the rivastigmine group indicates greater improvement | |||
|
Cognitive function (change from baseline at 26 weeks using MMSE) |
The mean score in the rivastigmine group was 0.74 higher (0.52 to 0.97 higher) | 3205 (6 studies) | ⊕⊕⊕⊝ moderate1,2 |
MMSE has a maximum score of 30 points, a lower score indicates greater impairment. treatment effect was in favour of rivastigmine | |||
|
Activities of daily living (change from baseline at 26 weeks measured using various scales) |
The mean score in the rivastigmine group was 0.2 standard deviations higher (0.13 to 0.27 higher) | 3230 (6 studies) | ⊕⊕⊕⊝ moderate1 | SMD 0.2 (0.13 to 0.27) A SMD of 0.2 is considered a small effect size. Treatment effect in favour of rivastigmine |
|||
| Physician rated global impression tests (no change or worse compared with baseline, measured using Global Impression of Change at 26 weeks) | 810 per 1000 | 744 per 1000 (712 to 773) | OR 0.68 (0.58 to 0.8) | 3338 (7 studies) | ⊕⊕⊕⊝ moderate1 | Treatment effect was in favour of rivastigmine | |
| Behavioural symptoms (change from baseline at 26 weeks measured using various scales) |
The mean score in the rivastigmine group was 0.04 standard deviations lower (0.14 lower to 0.06 higher) | 1529 (3 studies) | ⊕⊕⊕⊝ moderate1,3 | SMD ‐0.04 (‐0.14 to 0.06) A SMD of 0.2 is considered a small effect size. The size of this SMD and its small confidence interval suggests that there is no difference between the two groups |
|||
| Acceptability of treatment (as measured by withdrawals from trials before end of treatment at 26 weeks) | 149 per 1000 | 260 per 1000 (230 to 293) | OR 2.01 (1.71, 2.37) | 3569 (7 studies) | ⊕⊕⊕⊝ moderate1 | Withdrawals significantly more frequent in rivastigmine group compared with placebo group | |
| Incidence of adverse events (at least one adverse event by 26 weeks) | 761 per 1000 | 870 per 1000 (850 to 888) |
OR 2.14 (1.80 to 2.53) |
3587 (7 studies) | ⊕⊕⊕⊝ moderate1 | Adverse events significantly more frequent in rivastigmine group compared with placebo group | |
| Quality of life of patients or carers (measured using NPI‐D carer distress scale (change from baseline at 24 weeks) | The mean score in the rivastigmine group was 0.1 higher (0.91 lower to 1.11 higher) | 529 (1 study) | ⊕⊕⊕⊝ moderate1 | The size of this MD and its small confidence interval suggests that there is no difference between the two groups | |||
| *The assumed risk used the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | |||||||
1 Confidence in estimate of effect lowered due to relatively high dropout rates across studies, which are higher in the treatment group. The ITT analysis in these studies used LOCF (last observed carried forward) imputations. In addition, results are available up to only 26 weeks, longer term data would be more applicable.
2 There was high heterogeneity the ADAS‐Cog outcome due to B352, which had high dropout rates and showed a difference of 3.8 points, compared to 1.2 to 1.6 points for the other studies. However, evidence not further downgraded; removal of this study from the analysis will only result in a small change of estimate by about 0.35 points. 3 Three studies (IDEAL; Lopez‐Pousa 2005; Nakamura 2011) reported a scale measuring behavioural disturbance. 4 The protocol for most studies had some measures related to quality of life or impact on carers, but only one study reported this (IDEAL).
Background
Description of the condition
Alzheimer's disease (AD), alone or in combination with other brain conditions, is the commonest cause of dementia affecting older people. It is associated with the loss of cholinergic neurons in parts of the brain subserving aspects of memory. As the disease progresses, people lose the ability to remember, communicate, think clearly and perform their usual daily activities. Their behaviour or personality may also change. In severe AD, people lose the ability to care for themselves and require full time care.
Currently there is no cure available for AD, but a few pharmacological interventions are available to alleviate symptoms.
Description of the intervention
Acetylcholinesterase inhibitors, such as rivastigmine, delay the breakdown of acetylcholine released into synaptic clefts and may enhance cholinergic neurotransmission.
Tacrine, the first of the acetylcholinesterase inhibitors to undergo extensive trials for this purpose, was associated with significant disadvantages, including low oral bioavailability and metabolism involving hepatic microsomal enzymes with a consequent risk of interactions with other drugs. Tacrine was also associated with adverse effects including hepatotoxicity. Several other acetylcholinesterase inhibitors, including rivastigmine, galantamine, and donepezil, have now been introduced. They are believed to have superior properties in terms of specificity of action and low incidence of adverse effects.
Rivastigmine is a 'pseudo‐irreversible' inhibitor of acetyl and butyrylcholinesterases with a phenylcarbamate structure, the metabolism of which is almost totally independent of the hepatic cytochrome P450 system. After binding to cholinesterase, the carbamate portion of rivastigmine is slowly hydrolysed, cleaved, conjugated to a sulphate and excreted. Rivastigmine has an oral bioavailability of 0.355 and low (40%) binding to plasma proteins. Its elimination half‐life is around two hours. Its disposition is essentially unaltered in patients with renal or hepatic impairment (Jann 2000) and the risk of interactions with other drugs is low (Grossberg 2000). This is of particular relevance for elderly patients with AD, some of whom may also need medications for other conditions. The drug is selective both to the central nervous system (CNS) and within it. In studies in human volunteers the inhibition of central acetylcholinesterase was substantially greater than the inhibition of peripheral acetylcholinesterase or butyrylcholinesterase (Kennedy 1999). Evidence from animal studies suggests that rivastigmine is a more potent inhibitor of acetylcholinesterase in the cortex and hippocampus, the brain regions most affected by AD (Polinsky 1998). Rivastigmine also preferentially inhibits the G1 enzymatic form of acetylcholinesterase, which predominates in the brains of patients with AD (Polinsky 1998). Rivastigmine is long‐acting and readily penetrates the CNS after parenteral or oral administration. The duration of cholinesterase inhibition by rivastigmine is approximately 10 hours.
Rivastigmine can be administered orally as capsules or liquid or from a transdermal patch, which has been developed more recently. Based on pharmacokinetic principles, the transdermal patch form was postulated to have advantages over the oral form. Adherence was expected to be improved by once daily dosing. Tolerance was also expected to be improved as the patch delivers a more steady concentration of rivastigmine to the body and has a lower equivalent dose to the oral form (9.5 mg as a transdermal patch is equivalent to 12 mg daily in the oral form).
Why it is important to do this review
Large multicentre trials have been completed in the USA, Canada, Europe, Australia and South Africa. Rivastigmine has received approval for use in 60 countries including all the member states of the European Union and in the USA, where it received approval from the Food and Drugs Administration (FDA) in April 2000. It is important to assess the safety and efficacy of this intervention in a systematic review.
Objectives
To determine the clinical efficacy and safety of rivastigmine for patients with dementia of Alzheimer's type
To compare the efficacy and safety of the oral and transdermal formulations of rivastigmine
Methods
Criteria for considering studies for this review
Types of studies
We included double‐blind, randomised controlled trials in which rivastigmine was administered for 12 weeks or longer and compared with placebo; or rivastigmine patches were compared with rivastigmine capsules. Trials in which the allocation to treatment was not randomised, or in which treatment allocation was not concealed, were excluded. This was because prior knowledge of treatment allocation may lead to biased allocation of patients (Schulz 1995).
Types of participants
The patients in trials to be included were diagnosed with probable AD according to internationally accepted criteria such as the Diagnostic and Statistical Manual of Mental Disorders DSM‐IV (DSM IV) and National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRDA) criteria (McKhann 1984).
Types of interventions
Objective 1
Intervention: rivastigmine given at any dose, using any method of administration
Comparison: placebo
Objective 2
Intervention: rivastigmine patches at the manufacturer's recommended dose
Comparison: rivastigmine capsules at the manufacturer's recommended dose
Types of outcome measures
In the original protocol and during the review, we looked for all the following outcomes:
cognitive function (as measured by psychometric tests);
functional performance;
global impression;
behavioural disturbance;
acceptability of treatment as measured by withdrawal from trials;
incidence of adverse effects;
effect on carers;
death;
institutionalisation rates;
quality of life;
dependency.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register, on 2 March 2015. The search terms used were: Rivastigmine OR exelon OR ENA OR "SDZ ENA 713".
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies are identified from:
monthly searches of a number of the major healthcare databases, MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS;
monthly searches of a number of the trial registers, ISRCTN, UMIN (Japan's Trial Register), the World Health Organization (WHO) Clinical Trials Registry Platform portal (which covers ClinicalTrials.gov, ISRCTN, the Chinese Clinical Trials Register, the German Clinical Trials Register, the Iranian Registry of Clinical Trials, and the Netherlands National Trials Register, plus others);
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library;
six‐monthly searches of a number of grey literature sources, ISI Web of Knowledge Conference Proceedings, Index to Theses, Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
The latest search for this review (March 2015) retrieved a total of 17 results for consideration.
Searching other resources
In addition, the search engines Copernic and Google were used to find evidence of unreported or unpublished trials using the word rivastigmine and its synonyms. Novartis websites, the Food and Drug Administration (FDA), European Medicines Agency (EMEA) and National Institute for Health and Care Excellence (NICE) websites were searched for data and evidence of trials.
1. Reference searching The references of all identified studies were inspected for more studies.
2. Pharmaceutical companies Novartis, the developer of rivastigmine, was contacted for information about any unpublished and published trials.
Data collection and analysis
Selection of studies
Irrelevant citations were discarded by a review of the title of the publication and its abstract. In the presence of any suggestion that the article could possibly be relevant, it was retrieved in full for further assessment. In the later versions of the review, one review author (JSB) selected the trials for inclusion in the review from the culled citation list.
There were multiple publications for most of the industry sponsored trials, often reporting different aspects (outcomes) of the studies or different lengths of follow up.
Data extraction and management
Data were extracted from the published reports in journals and unpublished company reports using data collection forms. One review author (JSB) extracted information from the reports of each study.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, the following summary statistics, required for each trial and each outcome, were extracted.
For continuous data, mean change from baseline, the standard deviation, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and number of patients for each treatment group at each time point were extracted, if available.
For binary data, the numbers in each treatment group and the numbers experiencing the outcome of interest were sought.
For ordinal variables which can be approximated to continuous variables, the main outcomes of interest were the assessment score at the time point being considered and the change from baseline (i.e. pre‐randomisation or at randomisation) at this time point. For some binary and ordinal outcomes the endpoint category relative to the baseline category was the outcome of interest. For other categorical outcomes, such as the Clinical Global Impression of Change (CIBIC‐Plus), the endpoint itself was of clinical relevance as all patients had begun, by definition, at the same baseline score.
The baseline assessment score was the latest available score, no longer than two months prior to the randomisation. Studies may have included a titration period prior to the randomisation phase of the study. Data from any open follow‐on phase, after the randomised phase, were not used to assess safety or efficacy.
For each outcome measure, data were sought on every patient assessed. To allow an intention‐to‐treat analysis (ITT), the data were sought irrespective of compliance and whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow up. If ITT data were not available, an analysis of patients who completed treatment was conducted.
Assessment of risk of bias in included studies
The risk of bias assessment was conducted using the standard recommended approach for assessing the risk of bias in studies included in Cochrane reviews. The Cochrane Collaboration risk of bias tool is available in RevMan 5.2 and assesses the following domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcomes assessment;
incomplete outcome data;
selective outcome reporting;
'other bias'.
We made a judgement about the risk of bias in each domain, assigning it to one of three categories: 'high', 'low' or 'unclear' risk of bias. These assessments were based on the criteria for making judgements that are listed in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions. The criteria focus on whether the risk is of importance (that is whether the presence of the risk could have an important impact on the results or the outcomes of the trial) rather than whether a risk of bias is present or not (Higgins 2011). The levels of risk may be different for different outcomes and this was considered during the assessment.
If insufficient detail was reported to make a judgement, this was usually considered as an ‘unclear' risk of bias. An ‘unclear’ judgement was also used in situations where it was clear what happened in the study but its likely impact on the study results was not known.
Measures of treatment effect
For dichotomous outcomes (where the outcome of interest was either present or absent), the estimate of treatment effect of the intervention was expressed as the Peto odds ratio (OR) together with the 95% confidence interval (CI).
For continuous data the measure of treatment effect was the mean difference (MD) or the standardised mean difference (SMD).
Unit of analysis issues
The review only included parallel‐group, double‐blinded randomised controlled trials (RCTs), with individual patients randomised. No unit of analysis issues were expected or encountered.
Dealing with missing data
Where data were missing from the published report of a trial, the authors or the study sponsors were contacted to obtain the data and to clarify any uncertainty.
We made no attempts at data imputation, except for the estimation of standard deviations for continuous data using the methods detailed in section 7.7.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Where possible we reported ITT analyses. We conducted sensitivity analyses to compare methods of dealing with missing data.
Assessment of heterogeneity
Potential differences between the included studies in the types of participants, interventions or control used were assessed before pooling data. No subgroup analyses were planned.
We assessed heterogeneity between studies using the Chi2 test (with a significance level set at P < 0.10) and the I2 statistic, which calculates the percentage of variability due to heterogeneity rather than to chance, with I2 values over 50% suggesting substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
Outcomes reported in a trial were compared with the protocol, whenever possible, to examine whether all of the study's pre‐specified outcomes that were of interest to the review had been reported.
Data synthesis
For ordinal variables, such as psychometric test scores, functional and quality of life scales, where there are a large number of possible scores, the measure was treated as continuous and the mean difference or the SMD was calculated.
For ordinal variables with only a small number of possible values, such as the Clinical Global Impression of Change, the data were reduced to a binary variable. The two classes were improvement compared with no change or worse. For all binary variables the Peto method of the typical OR was used.
The duration of the trials varied between 12 weeks and 1 year. Separate meta‐analyses were conducted for endpoints of 12 weeks, 24 to 26 weeks and 52 weeks. Some trials contributed data to more than one meta‐analysis if multiple assessments had been done.
A weighted estimate of the typical treatment effect across trials was calculated. Overall estimates of the treatment difference are presented. In all cases the overall estimate from a fixed‐effect model was presented.
Subgroup analysis and investigation of heterogeneity
Heterogeneity were examined both visually and using the I2 statistic. Where there was evidence of heterogeneity of the treatment effect between trials then sensitivity analyses were conducted, where only homogeneous results were pooled.
There were no pre‐identified subgroups for subgroup analysis.
Sensitivity analysis
This review sought to analyse data using ITT data whenever possible. Some studies reported both an ITT analysis that included all patients randomised and a per protocol analysis. The ITT analysis results reported in studies often involved data imputation techniques such as the last observation carried forward (LOCF) for patients who did not complete the study. The impact of different ways of dealing with missing data were investigated using a sensitivity analysis of as observed, ITT and per protocol analyses. These results were tabulated and any important discrepancies discussed.
Summary of findings table
We summarised the data on the efficacy and safety of the currently recommended dose of rivastigmine (6 to 12 mg/day orally or 9.5 mg/day transdermally) in the summary of findings table using GRADE methods (Guyatt 2008) to assess the overall quality of the evidence.
Results
Description of studies
Results of the search
The updated searches performed in 2011, 2013, 2014 and 2015 retrieved a total of 112 references. The full texts of 42 references were read and, of these, 10 were of studies that could be included or additional reports of studies already included, and 32 were of studies that were excluded.
Included studies
The characteristics of the 13 included trials are summarised in Characteristics of included studies.
Design, participants, samples sizes and setting
Important details of study design (number of participants, duration of follow up, mean Mini‐Mental State Examination (MMSE) of participants at baseline and description of interventions) are summarised in Table 2 and the objectives of the trials in Table 3.
1. Description of the included studies at baseline.
| Study | Duration (weeks) | Participants | Mean age (SD) | % males | Mean MMSE (SD) | country | Number of centres | Treatment groups |
| Oral (different doses versus placebo ) | ||||||||
|
B103 (Phase II) |
13 | 402 | 69.4 | 44 | ‐ | Europe | 54 |
|
|
B104 (Phase II) |
18 | 114 | 71.2 (7.5) | 39 | 19.5 (3.7) | Belgium, France, UK, Norway, Canada | 11 |
|
|
B303/B305* (Phase III) |
26 | 725 | 72.0 (8.1) | 41 | 20.0 (4.5) | France, Germany, Austria, Switzerland, Canada, USA | 44 |
|
|
B304* (Phase III) |
26 | 677 | 71.4 (8.2) | 41 | 18.5 (4.5) | UK, Ireland, Australia, Canada, RSA, Italy | 37 |
|
|
B351* (Phase III) |
26 | 702 | 74.1 (8.3) | 44 | 20.0 (4.4) | USA | 14 |
|
|
B352* (Phase III) |
26 | 699 | 74.5 (7.4) | 39 | 19.7 (4.5) | USA | 22 |
|
| Ballard 2005 | 26 | 93 | 83.8 (7.7) | 20 | ‐ | UK | ‐ |
|
| Karaman 2005* | 52 | 44 | 73.8 | 45 | 12.2 | Turkey | 1 |
|
| Lopez‐Pousa 2005* | 26 | 218 | 77.6 | 23 | 8.8 | Spain | 21 |
|
| Mowla 2007 | 12 | 122 | 69.2 | 46 | 16.1 (4.0) | Iran | ‐ |
|
| Tai 2000 | 26 | 80 | ‐ | ‐ | ‐ | Taiwan | ‐ |
|
| Oral and patches | ||||||||
|
IDEAL* (Phase III) |
24 | 1195 | 73.3 (7.8) | 33 | 16.5 (3.0) | North, Central and South America, Asia, Europe | 100 |
|
| Patches | ||||||||
| Nakamura 2011 | 24 | 859 | 74.6 (7.2) | 31.7 | 16.6 (3.0) | Japan | multicentre |
|
* These studies met the inclusion criteria of the main analysis comparing rivastigmine at the therapeutic doses versus placebo.
b.i.d = bis in die in Latin, this means that a medication is taken two times a day, dividing the total daily dose into two doses.
t.i.d = ter in die in Latin, this means that a medication is taken three times a day, dividing the total daily dose into three doses.
MMSE = Mini‐Mental Health State Examination. The score range from 0 ( severe impairment) to 30 (normal).
2. Objectives of included studies.
| Study | Objective |
| B103 | To assess the short term (3 months) symptomatic efficacy and tolerability of rivastigmine 4 and 6 mg/day compared with placebo in patients with AD |
| B104 | Primary: to determine the maximum tolerated dose (MTD) of rivastigmine in patients with mild to moderate dementia of the Alzheimer type (DAT) |
| Secondary: to determine ‐ a) whether tolerability is different when the drug is administered twice daily (b.i.d.) or three times daily (t.i.d.) ‐ b) if nausea and vomiting, associated with cholinesterase inhibition, can be controlled with antiemetics thereby increasing the MTD, and ‐ c) to assess the efficacy of rivastigmine at its MTD in comparison with that of placebo in the treatment of DAT | |
| B303/B305 | Primary 1: to evaluate the efficacy of two non‐overlapping dose ranges of rivastigmine (1 to 4mg daily and 6 to 12 mg daily) versus placebo over a 26 week treatment period as assessed by two primary measures of outcome; change from baseline in ADAS‐Cog score and the CIBIC‐Plus score at week 26 |
| Primary 2: to evaluate the safety of the study medication as assessed by incidence of adverse events, clinical laboratory evaluations , vital signs, ECG recordings, and the results of physical examination made at baseline and throughout the study | |
| Secondary: to assess dose‐efficacy and dose‐safety relationships for rivastigmine | |
| B304 | Primary: to evaluate the efficacy and safety of individual highest well‐tolerated doses (range 6 to 12 mg daily) of rivastigmine given b.i.d. or t.i.d. for 26 weeks compared with placebo in the therapy of patients with probable Alzheimer's disease |
| Secondary: to compare the twice daily and three times daily dosing regimens with respect to efficacy and safety to evaluate changes in activities of daily living (ADL) | |
| B351 | Primary: to evaluate the efficacy and safety of three fixed doses of rivastigmine (3, 6 and 9 mg/day) and placebo for 26 weeks of treatment |
| Secondary: to assess the dose‐efficacy and dose‐safety relationships for rivastigmine | |
| Tertiary: to explore the pharmacokinetics of rivastigmine at doses of 3, 6 and 9 mg daily | |
| B352 | Primary: to evaluate the efficacy and safety of two non‐overlapping dose ranges of rivastigmine (1 to 4 mg daily and 6 to 12 mg daily) and placebo for 26 weeks of treatment |
| Secondary: to assess the dose‐efficacy and dose‐safety relationships of rivastigmine. To investigate the relationship between plasma concentrations of rivastigmine and efficacy and safety | |
| Tertiary: to explore the pharmacokinetics of rivastigmine at doses of 1 to 4 and 6 to 12 mg daily | |
| IDEAL | To compare the efficacy,safety and tolerability of a novel rivastigmine transdermal patch with conventional rivastigmine capsules and placebo in patients with AD |
| Karaman 2005 | To evaluate the efficacy of rivastigmine for a period of 12 months in patients with advanced moderate AD |
| Lopez‐Pousa 2005 | To evaluate the safety and efficacy of rivastigmine in patients with more advanced AD |
| Mowla 2007 | To assess the effect of serotonin augmentation on cognition and ADL of patients with AD |
| Ballard 2005 | To determine whether rivastigmine was better than placebo for agitation and cognition |
| Tai 2000 | To evaluate the safety and efficacy of Exelon compared with placebo in patients with probable Alzheimer's disease who had dementia ranging from mild to moderate degree |
| Nakamura 2011 | To evaluate the efficacy, safety, and tolerability of the 5 cm2 (9 mg loading dose, 4.6 mg/24 h delivery rate) and 10 cm2 (18 mg loading dose, 9.5 mg/day delivery rate) rivastigmine patch in Japanese patients with AD |
Only randomised, double‐blinded placebo controlled trials or studies comparing different formulations were included in this review. Thirteen studies met the inclusion criteria of the review.
Six trials, phase II and III, were all supported by Novartis Pharmaceuticals Corporation and were completed by 1996. They are identified by their Novartis or ADENA code (ADENA was the name given by Novartis to the Exelon Phase III clinical trials programme). The two phase II trials were designed to assess the tolerability, efficacy and safety of rivastigmine over three to four months. The four phase III trials were designed to assess the efficacy and safety of rivastigmine in patients with mild to moderately severe AD over six months. The trials had many features in common. They were all multicentre, randomised, double‐blind, parallel‐group trials. All trials compared rivastigmine with placebo, with at least two treatment groups of different rivastigmine regimens.
Of the seven later trials, three were also sponsored by Novartis (IDEAL; Lopez‐Pousa 2005; Nakamura 2011). The key information about these seven trials is summarised as follows.
There is limited information available about Tai 2000, which has been published only as an abstract. This trial appeared to be an independent trial carried out in Taiwan. Eighty participants with mild to moderate AD were treated with rivastigmine or placebo for 26 weeks. No data were available to include in the meta‐analyses.
Ballard 2005 was a small 26 week trial (n = 93) with three treatment arms, rivastigmine, quetiapine and placebo, of equal size. The objective was to compare the efficacy of rivastigmine and quetiapine for agitation in people with possible or probable AD who were living in institutions. We did not include any data from this trial in the meta‐analyses because of concerns about a high risk of attrition bias and exclusion of the most severely impaired patients from the analyses.
Karaman 2005 and Lopez‐Pousa 2005 aimed to investigate the efficacy of rivastigmine for patients with more advanced disease than those previously tested.
Karaman 2005 was a small 12 month trial (n = 44, mean baseline MMSE = 12.2). We did not include data from this trial in our meta‐analyses due to concern about a high risk of bias.
Lopez‐Pousa 2005 was a 6 month trial (n = 218, mean baseline MMSE = 8.8). In addition to the outcomes of cognitive function, activities of daily living and global clinical change, Lopez‐Pousa 2005 was the earliest included trial to assess behavioural symptoms.
Mowla 2007 was a 12 week trial in mild to moderate AD with three treatment groups, rivastigmine, rivastigmine plus fluoxetine and placebo. The rivastigmine plus fluoxetine group was not included in this review. There were 82 participants in total in the rivastigmine and placebo groups. We were not able to include any data from this trial in the meta‐analyses due to incomplete reporting of results.
IDEAL and Nakamura 2011 were the only trials to include transdermal rivastigmine.
IDEAL was a 6 month study (n = 1195) in mild to moderate AD, with 4 treatments arms, rivastigmine capsules, 2 doses of transdermal rivastigmine and placebo.
Nakamura 2011 was a 24 week dose finding trial in mild to moderate AD (n = 859) with 3 treatment arms, 2 doses of transdermal rivastigmine and placebo..
All studies used current diagnostic criteria for dementia (DSM‐IV) and probable AD (NINCDS‐ADRDA) (McKhann 1984) except Tai 2000, which did not give its diagnostic criteria. The severity of disease was mostly assessed by the MMSE rating scale, and patients that were included had MMSE scores of 10 to 26 inclusive apart from 2 studies (Karaman 2005; Lopez‐Pousa 2005), which randomised patients with MMSE scores of 3 to 12. The list of exclusions was not extensive. Patients with severe and unstable illnesses (cardiovascular or pulmonary disease, unstable diabetes mellitus, peptic ulceration within the preceding five years, evidence of alcohol or substance abuse) were excluded, as were individuals taking medications such as anticholinergic drugs, acetylcholine precursor health food supplements, memory enhancers, insulin and psychotropic drugs. The procedures followed were in accordance with the ethical standards of the relevant institutional committees on human experimentation and with the Declaration of Helsinki (Helsinki declaration).
Interventions
Information about treatment groups and actual doses achieved are tabulated in Table 2 and Table 4 respectively.
3. Mean daily dose (mg/day) of rivastigmine achieved in the studies at different time points.
| Time (weeks) | treatment group | B103 | B104 | B303/B305 | B304 | B351 | B352 | IDEAL | Karaman 2005 | Lopez‐Pousa 2005 | Nakamura 2011 |
| 10 to 12 | low b.i.d. | 4 | ‐ | 3.8 | ‐ | 2.9 | 3.6 | ||||
| medium b.i.d. | 6 | ‐ | ‐ | ‐ | 5.7 | ‐ | |||||
| high b.i.d. | ‐ | 9.6 | 10.4 | 9.5 | 8.8 | 10.1 | 6.1 | ||||
| high t.i.d. | ‐ | 10.2 | ‐ | 9.7 | ‐ | ‐ | |||||
| 26 | low b.i.d. | ‐ | ‐ | 3.7 | ‐ | 2.8 | 3.5 | ||||
| medium b.i.d. | ‐ | ‐ | ‐ | ‐ | 5.7 | ‐ | |||||
| high b.i.d. | ‐ | ‐ | 10.4 | 9.3 | 8.5 | 9.7 | 9.7 | 8.3 | 9.8 | ||
| high t.i.d. | ‐ | ‐ | ‐ | 9.6 | ‐ | ‐ | |||||
| low patch | 4.6 | ||||||||||
| medium patch | 9.5 | 9.5 | |||||||||
| high patch | 16.5 | ||||||||||
| 48 | medium patch | ||||||||||
| high patch | |||||||||||
| 52 | high b.i.d. | 10.7 |
Exact doses not available for B103, Ballard 2005, Tai 2000, Mowla 2007.
Twelve studies investigated the oral form of rivastigmine, and one of these studies also included an arm randomised to a rivastigmine patch (IDEAL).
Earlier industry sponsored trials investigated a range of doses, from 2 mg/day to 12 mg/day in two or three divided doses. In later trials (Ballard 2005; Karaman 2005; Lopez‐Pousa 2005; Mowla 2007; IDEAL) only the dose range of 6 to 12 mg/day was used to compare against placebo. Tai 2000 investigated doses of 3 to 6 mg/day in two divided doses. All studies with high oral doses achieved a mean daily dose of between 9.3 to 10.7 mg/day, except for Karaman 2005 (8.3 mg/day) and B351 (8.5 mg/day). The mean daily doses achieved for medium doses were between 5.7 and 6 mg/day. Further information on the doses achieved was not available for four trials (B103; Ballard 2005; Mowla 2007; Tai 2000).
Two studies evaluated the safety and efficacy of patches. IDEAL investigated 6 to 12 mg/day capsules in 2 doses and the other 2 arms tested rivastigmine patches, a 10 cm2 patch which delivered 9.5 mg/day and a 20 cm2 patch which delivered 17.4 mg/day. Patients were titrated to their target dose in four week steps. Patients in the patch groups started with a 5 cm2 patch until the target dose was achieved; in the capsule group they began with 3 mg/day, increased by steps of 3 mg/day. All patients had a rivastigmine or placebo patch once a day and a rivastigmine or placebo capsule twice a day. Nakamura 2011 investigateda 10 cm2 patch which delivered 9.5 mg/day, a 5 cm2 patch which delivered 4.6 mg/day and a placebo arm. Patients were titrated to their target patch dose over four week intervals, followed by an eight week maintenance period.
Outcomes
The trials examined cognitive, functional and global effects, behavioural symptoms, as well as the safety and tolerability of rivastigmine.
Apart from the outcome measures related to safety or adverse effects, all the outcomes for the effectiveness of rivastigmine were measured by questionnaires or psychometric tests. Different types of instruments were utilised to measure each outcome. The details of the outcomes measured and reported in each trial are summarised in Table 5.
4. Measured outcomes.
| Outcomes assessed | Cognitive function | Activities of daily lIving | Behavioural symptoms | Physician rated global impression of change | Other domains | ||||
| Study | ADAS‐Cog | MMSE | Others | PDS | Others | CIBIC‐Plus | Others | ||
| B103 | X | OE, TMT, NOSGER, DSST, VRT | CGIC | ||||||
| B104 | X | Wechsler psychometric tests, NOSGER | X | ||||||
| B303/B305 | X | X | ADAS‐CogA | X | CAS | X | GDS | ||
| B304 | X | X | ADAS‐CogA | X | CAS | X | GDS | ||
| B351 | X | X | ADAS‐CogA | X | CAS | X | GDS | ||
| B352 | X | X | ADAS‐CogA | X | CAS | X | GDS | ||
| Ballard 2005 | SIB | CMAI | |||||||
| Karaman 2005 | X | X | X | ACDS‐ADL, DAD | X | GDS | |||
| IDEAL | X | X | CLOCK DRAWING, TMT | ACDS‐ADL | NPI‐12 | ADCS‐CGIC | |||
| Lopez‐Pousa 2005 | X | SIB, BLESSED DEMENTIA SCALE | ACDS‐ADL | NPI‐10, NPI‐4 | GDS ADCS‐CGIC |
||||
| Mowla 2007 | WMS‐III, | ADL | CGI | Hamilton score | |||||
| Tai 2000 | X | NPT | X | GDS | |||||
| Nakamura 2011 | X | X | MENFIS | DAD | BEHAVE‐AD | X | |||
x indicated that the study measured this outcome.
The full names of these scales and their properties are described in Types of outcome measures.
1. Cognitive function
Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984). ADAS‐Cog comprises 11 individual tests: spoken language ability (0 to 5), comprehension of spoken language (0 to 5), recall of test instructions (0 to 5), word finding difficulty (0 to 5), following commands (0 to 5), naming object (0 to 5), construction drawing (0 to 5), ideational praxis (0 to 5), orientation (0 to 8), word recall (0 to 10) and word recognition (0 to 12). The total score ranges from 0 to 70, the higher the score indicating greater impairment.
The ADAS‐CogA total score is the ADAS‐Cog plus the attention item from the ADAS‐Noncog.
The Mini‐Mental State Examination (MMSE) (Folstein 1975) evaluates cognition in five areas: orientation, immediate recall, attention and calculation, delayed recall and language. The test takes only 15 minutes to administer and the scores range from 0 (severe impairment) to 30 (normal).
The Severe Impairment Battery (SIB) (Panisset 1994; Saxton 1990) is a 40‐item questionnaire designed to assess the severity of cognitive dysfunction in advanced AD and is divided into 9 domains: memory, language, orientation, attention, praxis, vasospastically, construction, orientation to name and social interaction. The score ranges from 0 (greatest impairment) to 100 (no impairment).
The Revised Wechsler Memory Scale (WMS‐R) (Wechsler 1987) comprises a series of brief subtests, some taken from the WMS and each measuring a different facet of memory, which are summarised into five composite scores and finally two major scores using weights prescribed by Wechsler. Some of the tests were used in B103.
The Fuld Object‐Memory Test (OME) (Fuld 1981) evaluates short term memory and learning by measuring the recall of 10 previously viewed objects.
The Benton Visual Retention Test (VRT) (Benton 1974) evaluates visual memory by assessing the accuracy of reproduction of each of 10 designs shown briefly to the individual.
The Trail Making Test (TMT) (Reitan 1958) assesses the time taken to connect a series of 25 numbered dots.
The Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC) (Schneider 1997) provides a single global rating of change from baseline, rated by an independent observer who has no access to the other efficacy or safety data.
The Ten‐Point Clock Drawing Test (Watson 1993) assesses visuospatial and executive functions.
The Mental Function Impairment (MENFIS) (Homma 1991) evaluates core symptoms of dementia including cognitive, motivational and emotional aspects based on an interview with the patient and carer. The score ranges from 0 to 78 (greater functional deficit).
Digital substitution test (DSST).
2. Activities of daily living
The Progressive Deterioration Scale (PDS) (DeJong 1989) is an instrument with 29 items assessing the activities of daily living as rated by a carer. Each item is scored on a visual analogue scale of 0 to 100, and the total score is the mean item score. The score of 0 to 100 decreases with severity of dementia.
The Alzheimer's Disease Cooperative Study activities of daily living inventory for severe Alzheimer's disease (ADCS‐ADL) (Galasko 1997). This is a 19‐item scale for basic and complex abilities validated in patients with moderate to severe dementia. The total score ranges from 0 to 54 (no impairment). Items include basic activities of daily living (eating, bathing) and complex activities (operating taps, switching lights).
The Caregiver Activity Survey (CAS) is completed by the caregiver and includes six items for which the caregiver estimates the amount of time spent in the previous 24 hours helping the patient with activities of daily living.
The Nurses' Observation Scale for Geriatric Patients (NOSGER) (Brunner 1990) is designed to assess various cognitive functions and behaviour as related to activities of daily living and as assessed by a caregiver who sees the patient frequently. The NOSGER contains 6 x 5 = 30 items which were selected to assess the following dimensions: (a) memory, (b) self‐care, (c) instrumental activities of daily life, (d) mood, (e) disturbing behaviour, (f) social behaviour.The Disability Assessment for Dementia (DAD) is a 46‐item structured interview for the carer, scored 0 to 100 (least impairment), to evaluate activities of daily living (Gelinas 1999).
3. Behavioural symptoms
The Neuropsychiatric Instrument (NPI) (Cummings 1994) is a 12‐item, carer rated instrument to evaluate behavioural and neuropsychiatric symptoms, including delusions, hallucinations, agitation and aggression, depression or dysphoria, anxiety, elation or euphoria, apathy, disinhibition, irritability, aberrant motor behaviour, night‐time behaviour and appetite or eating disorder. The frequency is rated from 1 (occasional, less than once a week) to 4 (very frequent) and severity from 1 (mild) to 3 (severe). The product of frequency and severity ranges from 1 to 12, with a total score ranging from 12 to 120 for the 10 domains summed. A lower score indicates improvement.
The Cohen‐Mansfield Agitation Inventory (CMAI) (Cohen‐Mansfield 1995) scale, range from 29 to 203, is widely used in nursing homes to assess agitation. The scale examines 29 types of agitated behaviour, including pacing, verbal or physical aggression, performing repetitious mannerisms, screaming, and general restlessness. The frequency of these behaviours is measured on a 7‐point scale, ranging from 1 (never occurs) to 7 (occurs several times an hour, and includes cluster scores for physical and verbal aggression, and total aggression.
The Behavioural Pathology in AD (BEHAVE‐AD) assesses potentially remediable behavioural problems (agitation, aggression, affect, psychosis) in patients with AD. It consists of 22 symptoms grouped into 7 categories, each scored by a carer on a 4‐point scale (Reisberg 1989).
4. Physician rated global impression tests
A Clinician's Interview‐Based Impression of Change scale (CIBIC‐Plus) (Reisberg 1994) includes information supplied by the caregiver and patient. It provides a global rating of patient function in four areas: general, cognitive, behaviour and activities of daily living. All patients are scored as 4 at baseline; subsequent assessment on a scale of 1 to 7 is relative to baseline, with 1 showing marked improvement and 7 marked worsening.
The Global Deterioration Scale (GDS) (Reisberg 1982) is reported as a score from 1 to 7, 1 indicating normality to 7 indicating very severe dementia, and is a global assessment carried out by a clinician who has access to all information about a patient.
The Clinical Global Impression of Change (CGIC) (Guy 1976) is a global rating of all domains of a patient's current condition in comparison with baseline. It is a 7‐point scale ranging from 1 (very much improved) to 7 (very much worse), with 4 indicating no change. The assessment is conducted by the same clinician at both time points with input from relatives or carers.
5. Acceptability of treatment, as measured by withdrawal from trial
In anticipation of the typical gastrointestinal adverse events associated with cholinesterase inhibitors, which can be dose‐dependent, the various arms of the older trials compared both different doses and twice or thrice daily dosage schedules. Three fixed doses were tested in B351, but the other trials aimed for a maximum tolerated dose within a prescribed range. The period of titration was longer for larger doses and varied between 3 and 12 weeks. The later trials tested a transdermal patch formulation which provided continuous delivery of the drug with the objective of improving tolerability. The mean daily doses of rivastigmine at different time points are presented in Table 4. Safety and tolerability were evaluated by recording adverse events and serious adverse events. In addition, routine physical examinations with blood and urine analyses were performed and vital signs and electrocardiograms were checked at all clinic visits. Seven trials reported the withdrawal rate at 26 weeks (B303/B305; B304; B351; B352; IDEAL; Lopez‐Pousa 2005; Nakamura 2011).
6. Incidence of adverse events
The studies reported the types of adverse events reported by patients, and the number of patients experiencing these events, usually focusing on the most commonly experienced adverse events. A wide range of adverse events which were consistent with the anticholinergic properties of rivastigmine were reported, including gastrointestinal adverse events such as nausea, vomiting, abdominal pain or discomfort, and diarrhoea. Other adverse events reported included falls, insomnia, agitation, weight loss, headache, dizziness, and cutaneous adverse events where patches were used.
The same seven studies which reported on withdrawal from the trial before completion of the study also reported the number of patients who experienced at least one adverse event. Most of these studies had defined a safety population which is the basis for the adverse events analyses.
7. Quality of life of patients and carers
Only one study reported changes in the NPI‐D carer distress scale. This study reported the change from baseline at 24 weeks (IDEAL).
Excluded studies
Please see Characteristics of excluded studies.
Risk of bias in included studies
1.
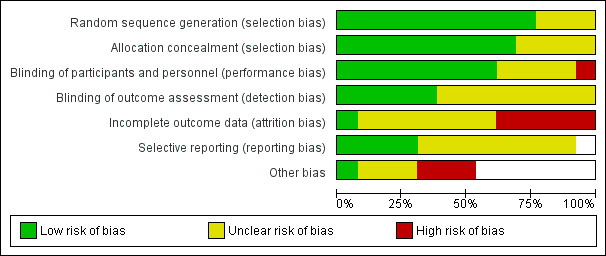
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
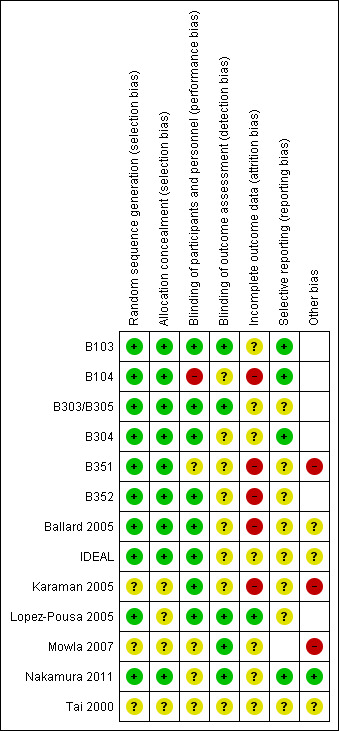
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All the trials sponsored by Novartis were considered to be at low risk of bias for randomisation and allocation concealment, other than Lopez‐Pousa 2005 where it was difficult to be sure whether allocation was concealed effectively.
Of the independent trials, Ballard 2005 had a low risk of allocation bias, with clearly described procedures. However, the risk of bias in this domain was unclear for Tai 2000 (an abstract), Karaman 2005 and Mowla 2007 because there were no descriptions of methods. Karaman 2005 was of particular concern as only "participants who tolerated the drug well and perceived benefit were invited to continue rivastigmine treatment" after eight weeks.
Blinding
All trials were double‐blinded and placebo controlled, with precautions taken to maintain the blinding such as ensuring the placebo was identical in appearance to the active treatment. However, in B104 the placebo group received the treatment twice daily whereas one of the treatment arms received the intervention three times daily. There were no descriptions of additional steps taken to mask this. The difference in the number of times the capsules were taken could have unmasked the three times per day group. The effectiveness of double‐blinding in Mowla 2007 was also unclear because all patients in this study had received the placebo during the six week pre‐randomisation run‐in period.
Of the two studies testing patches, IDEAL was considered to be at low risk of bias for blinding as a double dummy was used. Nakamura 2011 stated that "patients, investigator staff, persons performing the assessments and data analysts are all blinded", but it was unclear how this was achieved since the study had used different patch sizes (2.5, 5, 7.5 and 10 cm2) to achieve the target dose.
Incomplete outcome data
Attrition bias was a major concern. There were substantial losses from Ballard 2005 where 19% (6/31) of those randomised to rivastigmine did not start treatment compared with 6% of those randomised to placebo. Only 18/31 in the rivastigmine group completed the trial compared with 27/31 in the placebo group. Those with a low baseline score on the Severe Impairment Battery (SIB) were not included in the analyses. These concerns led us to exclude data from Ballard 2005 from the meta‐analyses.
Karaman 2005, although the longest duration included trial (52 weeks), lost very few patients: only 3 of 24 in the rivastigmine group and none from the placebo group. This was a much lower rate of loss than for any other trial.
For the other 11 studies missing assessments caused major problems in the analysis and interpretation of the results. Approximately 17% of patients from the 1 to 4 mg daily and placebo groups and 35% of patients from the 6 to 12 mg daily groups left the trial before completing treatment. If patients dropped out at random from each group, that is the dropout was not associated with the treatment, the comparisons between groups are not biased but estimates of differences are reduced in precision. However, the dropout rates were not random and were related to treatment. Various methods were used in the trials for dealing with missing data.
The older trials (B303/B305; B304; B351; B352) reported in detail the methods using for dealing with missing data. Approximately a third of the patients who dropped out contributed endpoint data (retrieved drop out (RDO)). The ITT analyses included the completers (observed cases (OC)) data and the RDO data, and for the remainder of the patients the last available assessment (last observation carried forward (LOCF)). This remainder comprised approximately 6% of the patients in the placebo and 1 to 4 mg daily groups, and 24% for the 6 to 12 mg daily group at 26 weeks. An overestimate of the outcome effect would be expected.
In order to compare the different methods of dealing with missing assessments, for two outcomes (ADAS‐Cog and CIBIC‐Plus) we conducted meta‐analyses on three different groups of patients: OC only, RDO + OC, and ITT (OC + RDO + LOCF). The results are presented in Table 6. These analyses showed that compared with OC or RDO + OC, the ITT analyses did not produce results favouring rivastigmine, indeed the opposite was true but the differences between results were small. Therefore, the ITT analyses were considered satisfactory and were reported for all other outcomes. Further analysis of the data from the ITT, the OC and RDO + OC analyses to investigate the size and direction of the bias due to differential dropouts from the arms of the rivastigmine trials (Birks 2008) led to the conclusion that the absolute size of the bias was small and the direction could not be ascertained.
5. Comparison of different methods of dealing with missing values.
| Time point | population | rivastigmine n | placebo n | result | probability level | 95% confidence limits |
| 1 to 4 mg daily versus placebo, ADAS‐Cog measured as change from baseline | ||||||
| 12 weeks | ITT | 650 | 643 | favours rivastigmine WMD ‐0.31 | 0.30 | ‐0.87, 0.25 |
| OC | 589 | 598 | favours rivastigmine WMD ‐0.46 | 0.14 | ‐1.08, 0.15 | |
| RDO + OC | 616 | 615 | favours rivastigmine WMD ‐0.37 | 0.20 | ‐0.96, 0.23 | |
| 18 weeks | ITT | 650 | 643 | favours rivastigmine WMD ‐1.07 | 0.0004 | ‐1.66, ‐0.48 |
| OC | 558 | 552 | favours rivastigmine WMD ‐1.19 | 0.0005 | ‐1.86, ‐0.52 | |
| RDO + OC | 573 | 572 | favours rivastigmine WMD ‐1.33 | 0.00008 | ‐1.99, ‐0.67 | |
| 26 weeks | ITT | 650 | 644 | favours rivastigmine WMD ‐0.84 | 0.01 | ‐1.48, ‐0.19 |
| OC | 519 | 526 | favours rivastigmine WMD ‐0.96 | 0.01 | ‐1.72, ‐0.21 | |
| RDO + OC | 559 | 564 | favours rivastigmine WMD ‐1.07 | 0.004 | ‐1.80, ‐0.34 | |
| 6 to 12 mg daily versus placebo, ADAS‐Cog measured as change from baseline | ||||||
| 12 weeks | ITT | 1054 | 863 | favours rivastigmine WMD ‐1.49 | <0.00001 | ‐1.96, ‐1.01 |
| OC | 843 | 803 | favours rivastigmine WMD ‐1.80 | <0.00001 | ‐2.33, ‐1.27 | |
| RDO + OC | 967 | 828 | favours rivastigmine WMD ‐1.38 | <0.00001 | ‐1.89, ‐0.88 | |
| 6 to 12 mg daily versus placebo, ADAS‐Cog measured as change from baseline | ||||||
| 18 weeks | ITT | 1054 | 863 | favours rivastigmine WMD ‐1.79 | <0.00001 | ‐2.30,‐ 1.29 |
| OC | 732 | 742 | favours rivastigmine WMD ‐2.36 | <0.00001 | ‐2.96, ‐1.76 | |
| RDO + OC | 837 | 772 | favours rivastigmine WMD ‐2.12 | <0.00001 | ‐2.69, ‐1.55 | |
| 26 weeks | ITT | 1054 | 863 | favours rivastigmine WMD ‐2.09 | <0.00001 | ‐2.65, ‐1.54 |
| OC | 670 | 709 | favours rivastigmine WMD ‐2.62 | <0.00001 | ‐3.29, ‐1.94 | |
| RDO + OC | 788 | 759 | favours rivastigmine WMD ‐2.39 | <0.00001 | ‐3.03, ‐1.74 | |
| 1 to 4 mg daily versus placebo, CIBIC‐Plus measured as no change or worse | ||||||
| 12 weeks | ITT | 608 | 612 | favours rivastigmine Peto OR 0.93 |
0.60 | 0.72, 1.21 |
| OC | 583 | 596 | favours rivastigmine Peto OR 0.95 |
0.70 | 0.72, 1.23 | |
| RDO + OC | 609 | 612 | favours rivastigmine Peto OR 0.94 |
0.60 | 0.72, 1.22 | |
| 18 weeks | ITT | 614 | 620 | favours rivastigmine Peto OR 0.98 |
0.90 | 0.75, 1.26 |
| OC | 556 | 554 | favours placebo Peto OR 1.04 |
0.80 | 0.80, 1.37 | |
| RDO + OC | 570 | 576 | favours placebo Peto OR 1.02 |
0.90 | 0.78, 1.34 | |
| 26 weeks | ITT | 614 | 623 | favours rivastigmine Peto OR 0.71 |
0.01 | 0.55, 0.93 |
| OC | 513 | 523 | favours rivastigmine Peto OR 0.67 |
0.006 | 0.50, 0.89 | |
| RDO + OC | 544 | 549 | favours rivastigmine Peto OR 0.68 |
0.008 | 0.52, 0.91 | |
| 1 to 4 mg daily versus placebo, CIBIC‐Plus measured as no change or worse | ||||||
| 12 weeks | ITT | 950 | 825 | favours rivastigmine Peto OR 0.74 |
0.008 | 0.60, 0.92 |
| OC | 831 | 799 | favours rivastigmine Peto OR 0.72 |
0.005 | 0.58, 0.91 | |
| RDO + OC | 952 | 825 | favours rivastigmine Peto OR 0.75 |
0.01 | 0.60, 0.93 | |
| 18 weeks | ITT | 970 | 835 | favours rivastigmine Peto OR 0.81 |
0.06 | 0.65, 1.01 |
| OC | 720 | 741 | favours rivastigmine Peto OR 0.72 |
0.005 | 0.57, 0.91 | |
| RDO + OC | 820 | 772 | favours rivastigmine Peto OR 0.77 |
0.02 | 0.62, 0.97 | |
| 26 weeks | ITT | 973 | 839 | favours rivastigmine Peto OR 0.68 |
0.0007 | 0.55, 0.85 |
| OC | 660 | 693 | favours rivastigmine Peto OR 0.63 |
0.0004 | 0.49, 0.81 | |
| RDO + OC | 784 | 758 | favours rivastigmine Peto OR 0.65 |
0.0003 | 0.51, 0.82 | |
The results for two outcomes, ADAS‐Cog and CBIC at 12, 18 and 26 weeks, have been pooled for 3 studies, B303/B305, B351. B352. These studies reported results for 3 populations, intention‐to‐treat (ITT), completers (OC), and completers + retrieved dropout (RDO + OC). The table reports the results of the meta‐analyses for 2 comparisons (1 to 4 mg daily versus placebo and 6 to 12 mg/day versus placebo) for the 3 populations at the 3 time points.
Selective reporting
For most of the studies the risk of reporting bias across all outcomes was difficult to judge. A few of the studies had listed the Caregiver Activities Survey (CAS) as an outcome in their protocols but these were not reported in the study results. In addition B304 and B351, two large randomised trials, were not published. Our data were obtained from information provided by Novartis Ltd.
For three of the studies (B104; B304; Nakamura 2011) sufficient information was available from the study protocols and we considered these as low risk of bias. However, there was insufficient information to assess the risk of reporting bias in the other studies.
Other potential sources of bias
Out of these 13 studies included in the review, only four (Ballard 2005; Karaman 2005; Mowla 2007; Tai 2000) were conducted without direct sponsorship or funding from the manufacturer, Novartis Pharma, but none provided data that could be included in the review.
Karaman 2005 reported standard deviations for the outcome measures that were an order of magnitude smaller than those seen in any other trial. We have asked the authors for clarification of these unusual findings but have not received a reply.
Effects of interventions
See: Table 1
There are 13 included trials but 4 (Ballard 2005; Karaman 2005; Mowla 2007; Tai 2000) did not contribute to the analyses. Data from Ballard 2005 was excluded because of the high attrition rate from the rivastigmine group and concern over the elimination from the analyses of patients with a low baseline score. Data from Mowla 2007 could not be included due to incomplete reporting. No data could be used from Tai 2000 as the trial report provided insufficient information. The data from Karaman 2005 were of concern because of the potential for biased results and were omitted from the analyses. Although the longest duration trial, 52 weeks, only 3 of 24 in the rivastigmine group and none from the placebo group were lost. This was a much lower rate of loss than for any other trial. The numbers randomised were not reported but it was stated that patients were excluded at eight weeks if they did not appear to benefit.
In order to meet the objectives of the review we conducted analyses comparing various doses and formulations of rivastigmine with placebo or comparing different formulations of rivastigmine.
The rating scales and cognitive tests used differ in the direction representing improvement. A decrease in score indicates clinical improvement with the ADAS‐Cog, the CIBIC‐Plus and the GDS, while an increase shows improvement for the PDS and MMSE.
Comparison of rivastigmine (6 to 12 mg/day twice daily capsules or 10 cm2 (9.5 mg/day) patch) with placebo
Cognitive function
The meta‐analysis, using weighted mean differences (WMDs), revealed a benefit on cognitive function as measured by the ADAS‐Cog test scores for rivastigmine compared with placebo at 26 weeks (ITT analysis, WMD ‐1.79; 95% CI ‐2.21 to ‐1.37, P < 0.00001, 6 studies).
The MMSE showed similar results in favour of rivastigmine at 26 weeks compared with placebo (ITT analysis, WMD 0.74; 95% CI 0.52 to 0.97, P < 0.00001, 6 studies).
Activities of daily living
The meta‐analysis, using standardised mean differences (SMDs), showed an improvement associated with rivastigmine compared with placebo at 26 weeks (ITT analysis, WMD 0.20; 95% CI 0.13 to 0.27, P < 0.00001, 6 studies).
Global assessment
The seven‐point CIBIC‐Plus scale, or the ADCS‐CGIC scale, measuring global clinical state was dichotomized by counting those showing no change or decline against those showing improvement. There were benefits associated with rivastigmine compared with placebo at 26 weeks (ITT analysis, 1339/1848 rivastigmine, 1197/1490 placebo) (OR 0.68; 95%CI 0.58 to 0.80, P < 0.00001, 7 studies).
Behavioural symptoms
Three studies (IDEAL; Lopez‐Pousa 2005; Nakamura 2011) assessed behavioural symptoms using the Neuropsychiatric Instrument (NPI‐10 and NPI‐12). There was no difference between rivastigmine and placebo at 26 weeks.
Withdrawals before the end of treatment
The meta‐analysis of withdrawals before the end of treatment showed a significant difference in favour of placebo compared with rivastigmine 26 weeks (571/2038 rivastigmine, 240/1531 placebo) (OR 2.06; 95%CI 1.74 to 2.45, P < 0.00001, 7 studies).
Adverse events
The meta‐analysis of numbers of patients with at least one adverse event showed that at 26 weeks there was a significant difference between the rivastigmine and placebo groups in favour of placebo (1637/2025 rivastigmine, 1123/1562 placebo) (OR 2.16; 95%CI 1.82 to 2.57, P < 0.00001, 7 studies).
Quality of life of carers
One study reported the changes in NPI‐D carer distress scale from baseline and this was reported at 24 weeks (IDEAL). No significant difference was detected (MD 0.10; 95% CI ‐0.91 to 1.11, 1 study).
Comparison of rivastigmine (1 to 4 mg/day and 6 to 12 mg/day twice daily capsules) with placebo
Cognitive function
The meta‐analysis, using WMDs, revealed a benefit on cognitive function as measured by ADAS‐Cog test scores for the lower dose rivastigmine compared with placebo at 26 weeks, but not at 12 weeks; and for the higher dose at 12 and 26 weeks:
rivastigmine 1 to 4 mg/day at 12 weeks (ITT analysis, WMD ‐0.31; 95% CI ‐0.87 to 0.25, P = 0.01, 3 studies);
rivastigmine 6 to 12 mg/day at 12 weeks (ITT analysis, WMD ‐1.49; 95% CI ‐1.96 to ‐1.01, P < 0.00001, 4 studies);
rivastigmine 1 to 4 mg/day at 26 weeks (ITT analysis, WMD ‐0.84; 95% CI ‐1.48 to ‐0.19, P = 0.01, 3 studies);
rivastigmine 6 to 12 mg/day at 26 weeks (ITT analysis, WMD ‐1.99; 95% CI ‐2.49 to ‐1.50, P < 0.00001, 5 studies).
The MMSE showed similar results in favour of lower dose rivastigmine at 26 weeks and higher dose rivastigmine at 26 weeks, compared with placebo:
rivastigmine 1 to 4 mg/day at 26 weeks (ITT analysis, WMD 0.43; 95% CI 0.08 to 0.78, P = 0.02, 3 studies);
rivastigmine 6 to 12 mg/day at 26 weeks (ITT analysis, WMD 0.82; 95% CI 0.56 to 1.08, P < 0.00001, 5 studies).
One study (Lopez‐Pousa 2005) used the Severe Impairment Battery (SIB), which showed benefit associated with higher dose rivastigmine compared with placebo at 26 weeks (MD 4.53; 95% CI 0.47 to 8.59, P = 0.03).
Activities of daily living
The PDS (carer assessment of the activities of daily living) showed an improvement associated with higher dose, but not lower dose, rivastigmine compared with placebo at 12 and 26 weeks:
rivastigmine 1 to 4 mg/day at 12 weeks (WMD ‐0.77; 95% CI ‐1.84 to 0.30, 3 studies);
rivastigmine 1 to 4 mg/day at 26 weeks (WMD ‐0.38; 95% CI ‐1.61 to 0.84) (3 studies);
rivastigmine 6 to 12 mg/day at 12 weeks (WMD 1.08; 95% CI 0.19 to 1.98, P = 0.02, 4 studies);
rivastigmine 6 to 12 mg/day at 26 weeks (WMD 2.15; 95% CI 1.13 to 3.16, P < 0.0001, 4 studies).
One study (IDEAL) assessing activities of daily living (ADL) using the ADCS‐ADL scale showed benefit for 6 to 12 mg/day at 24 weeks (MD 1.80; 95% CI 0.20 to 3.40, P = 0.03).
Global assessment
The seven‐point CIBIC‐Plus scale, or the ADCS‐CGIC scale, measuring global clinical state was dichotomized by counting those showing no change or decline against those showing improvement (as set out in the study protocols by Novartis) and analysed using the Peto OR. There were benefits associated with lower dose rivastigmine compared with placebo at 26 weeks, but not at 12 weeks; and benefits with the higher dose at both 12 and 26 weeks compared with placebo:
rivastigmine 14 mg/day at 12 weeks (ITT analysis, 456/608 rivastigmine, 466/612 placebo) (OR 0.93; 95% CI 0.72 to 1.21, 3 studies);
rivastigmine 6 to 12 mg/day at 12 weeks (ITT analysis, 688/950 rivastigmine, 645/825 placebo) (OR 0.74; 95% CI 0.60 to 0.92, P = 0.008, 4 studies);
rivastigmine 1 to 4 mg/day at 26 weeks (ITT analysis, 457/614 rivastigmine, 500/623 placebo) (OR 0.71; 95% CI 0.55 to 0.93, P = 0.01, 3 studies);
rivastigmine 6 to 12 mg/day at 26 weeks (ITT analysis, 957/1330 rivastigmine, 971/1223 placebo) (OR 0.66; 95% CI 0.55 to 0.79, P < 0.00001, 6 studies).
The GDS (global assessment) carried out at 26 weeks by a clinician who had access to all information about a patient was dichotomized by counting those showing moderately severe, severe or very severe dementia against those showing moderate or mild dementia. Using the Peto OR to compare with placebo, there were benefits associated with 6 to 12 mg daily rivastigmine (ITT analysis, 579/1056 on rivastigmine showed the worse condition compared to 511/868 on placebo) (OR 0.78; 95% CI 0.64 to 0.94, P = 0.01, 4 studies) but not with 1 to 4 mg daily rivastigmine.
Behavioural symptoms
Two studies (IDEAL; Lopez‐Pousa 2005) assessed behavioural symptoms using the NPI (NPI‐10 and NPI‐12). There was no difference between rivastigmine and placebo:
rivastigmine 6 to 12 mg/day at 26 weeks (ITT analysis, WMD ‐0.06; 95% CI ‐0.20 to 0.09, 2 studies).
Withdrawals before the end of treatment
The meta‐analyses of withdrawals before the end of treatment showed no significant differences between withdrawals from the 1 to 4 mg daily rivastigmine group and from the placebo group at 12 and 26 weeks. There were significant differences for the higher dose group in favour of placebo at 12 and 26 weeks:
rivastigmine 1 to 4 mg/day at 12 weeks (17/136 rivastigmine, 8/133 placebo) (OR 2.15; 95% Cl 0.95 to 4.89, 1 study);
rivastigmine 1 to 4 mg/day at 26 weeks (113/644 rivastigmine, 113/646 placebo) (OR 1.01; 95% CI 0.75 to 1.34, 3 studies);
rivastigmine 6 to 12 mg/day at 12 weeks (20/133 rivastigmine, 8/133 placebo) (OR 2.60; 95% CI 1.19 to 5.68, P = 0.02, 1 study);
rivastigmine 6 to 12 mg/day at 26 weeks (448/1458 rivastigmine, 1194/1243 placebo) (OR 2.19; 95% CI 1.83 to 2.63, P < 0.00001, 6 studies).
Adverse events
Most adverse events occurred within the titration period. The meta‐analyses of numbers of patients with at least one adverse event showed that by the end of the titration period and at 26 weeks there were no significant differences between the lower dose rivastigmine and placebo groups. There were, however, significant differences between the higher dose rivastigmine and placebo groups in favour of placebo by the end of the titration period and at 26 weeks:
rivastigmine 1 to 4 mg/day at the end of the titration period (440/644 rivastigmine, 437/646 placebo) (OR 1.04; 95% Cl 0.82 to 1.31, 3 studies);
rivastigmine 1 to 4 mg/day at 26 weeks (509/644 rivastigmine, 518/646 placebo) (OR 0.93; 95% CI 0.71 to 1.23, 3 studies);
rivastigmine 6 to 12 mg/day at the end of the titration period (920/1072 rivastigmine, 584/878 placebo) (OR 2.96; 95% CI 2.39 to 3.68, P < 0.00001, 4 studies);
rivastigmine 6 to 12 mg/day at 26 weeks (1242/1450 rivastigmine, 901/1276 placebo) (OR 2.49; 95% CI 2.05 to 3.02, P < 0.00001, 6 studies).
A similar pattern was seen for the number of patients with at least one severe adverse event. The rivastigmine 1 to 4 mg daily group did not differ significantly from the placebo group, but there were significant differences between the rivastigmine 6 to 12 mg daily and placebo groups in favour of the latter for the titration period:
rivastigmine 1 to 4 mg/day at the end of the titration period (48/644 rivastigmine, 51/646 placebo) (OR 0.94; 95% CI 0.62 to 1.42, 3 studies);
rivastigmine 6 to 12 mg/day at the end of the titration period (130/1052 rivastigmine versus 61/868 placebo) (OR 1.88; 95% CI 1.39 to 2.55, P < 0.0001, 4 studies).
There were many types of adverse events reported and only the significant results are reported here. There were significant differences in favour of placebo for the rivastigmine 6 to 12 mg daily group by the end of the titration period and by 26 weeks for the number of patients suffering nausea, vomiting, diarrhoea, anorexia, headache, syncope, abdominal pain and dizziness. There were significant differences in favour of placebo for the rivastigmine 1 to 4 mg daily group compared to placebo by the end of the titration period and by 26 weeks for the number of patients suffering nausea, vomiting, diarrhoea and anorexia.
Withdrawals before the end of treatment due to adverse events
The meta‐analyses of withdrawals at 26 weeks due to adverse events showed no significant differences in withdrawals from the lower dose rivastigmine and placebo groups. There were, however, significant differences between the rivastigmine 6 to 12 mg daily and placebo groups in favour of placebo (291/1453 versus 94/1276) (OR 2.73, 95% CI 2.19 to 3.41, P < 0.00001, 6 studies).
Comparison of rivastigmine (20 cm2 (17.4 mg/day) patch) with placebo
Cognitive function
The meta‐analysis, using MDs, showed that rivastigmine had a benefit compared with placebo for cognitive function as measured by the ADAS‐Cog at 24 weeks:
rivastigmine (ITT analysis, MD ‐2.60; 95% CI ‐3.72 to ‐1.48, P < 0.00001, 1 study).
The MMSE showed similar results in favour of rivastigmine at 26 weeks, compared with placebo:
rivastigmine (ITT analysis, MD 0.90; 95% CI 0.32 to 1.48, P = 0.002, 1 study).
The TMT‐A showed similar results in favour of rivastigmine at 26 weeks, compared with placebo:
rivastigmine (ITT analysis, MD ‐14.20; 95% CI ‐24.11 to ‐4.29, P = 0.005, 1 study).
There was no significant difference between rivastigmine and placebo for the clock drawing test.
Activities of daily living
The ADCS‐ADL showed benefit in favour of rivastigmine compared with placebo at 24 weeks:
rivastigmine (ITT analysis, MD 2.30; 95% CI 0.52 to 4.08, P = 0.01, 1 study).
Behavioural symptoms
One study assessed behavioural symptoms using the NPI (NPI‐12). There was no difference between rivastigmine and placebo (ITT analysis, MD ‐0.60; 95% CI ‐2.88 to 1.68, 1 study).
Withdrawals before the end of treatment
There was a significant difference between rivastigmine and placebo in favour of placebo for total withdrawals before the end of treatment (62/303 rivastigmine compared with 36/302 placebo) (OR 1.90; 95% CI 1.22 to 2.97, P = 0.005).
Adverse events
There was a significant difference between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event by 24 weeks (200/303 rivastigmine compared with 139/302 placebo) (OR 2.28; 95% CI 1.64 to 3.16, P < 0.00001).
There was a significant difference between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event of dizziness (21/303 compared with 7/302) (ITT analysis, OR 3.14; 95% CI 1.31 to 7.50, P = 0.01), nausea (64/303 compared with 15/302) (OR 5.12; 95% CI 2.85 to 9.22, P < 0.00001), vomiting (57/303 compared with 10/302) (ITT analysis, OR 6.77; 95% CI 3.38 to 13.53, P < 0.00001), weight decrease (23/303 compared with 4/302) (ITT analysis, OR 6.12; 95% CI 2.09 to 17.92, P = 0.0009), and decreased appetite (15/303 compared with 3/302) (ITT analysis, OR 5.19; 95% CI 1.49 to 18.12, P = 0.01, 1 study).
Withdrawals before the end of treatment due to adverse events
The meta‐analyses of withdrawals at 26 weeks due to adverse events showed no significant differences in withdrawals from the rivastigmine and placebo groups (26/303 rivastigmine compared with 15/302 placebo) (OR 1.80; 95% CI 0.93 to 3.46, 1 study).
Quality of life of carers
One study assessed the NPI‐D carer distress scale at 24 weeks (IDEAL). No significant difference between rivastigmine and placebo was detected (ITT analysis, MD 0.00; 95% CI ‐1.07 to 1.07).
Comparison of rivastigmine (10 cm2 (9.5 mg/day) patch) with placebo
Cognitive function
The meta‐analysis, using WMDs and MDs, showed a benefit of the 10 cm2 rivastigmine patch on cognitive function as measured by the ADAS‐Cog, MMSE, TMT‐A and MENFIS at 24 weeks:
ADAS‐cog (ITT analysis, WMD ‐1.34; 95% CI ‐2.02 to ‐0.66, P = 0.0001, 2 studies);
MMSE (ITT analysis, WMD 0.64; 95% CI 0.26 to 1.02, P = 0.0009, 2 studies);
TMT‐A (ITT analysis, MD ‐20.0; 95% CI ‐29.8 to ‐10.2, P < 0.0001, 1 study);
MENFIS (ITT analysis, MD ‐1.30; 95% CI ‐2.32 to ‐0.28, P = 0.01, 1 study).
Activities of daily living
The ADCS‐ADL showed benefit in favour of rivastigmine at 24 weeks (ITT analysis, MD 2.20; 95% CI 0.62 to 3.78, P = 0.006, 1 study).
The DAD showed benefit in favour of rivastigmine at 24 weeks (ITT analysis, MD 2.3; 95% CI 0.34 to 4.26, P = 0.02, 1 study).
Global assessment
The seven‐point CIBIC‐Plus scale measuring global clinical state was dichotomized by counting those showing no change or decline against those showing improvement and analysed using the Peto OR. There was no difference between rivastigmine and placebo at 24 weeks (382/518 rivastigmine, 426/545 placebo) (ITT analysis, OR 0.77; 95% CI 0.58 to 1.02, P = 0.07, 2 studies).
Withdrawals before the end of treatment
There was a significant difference between rivastigmine and placebo in favour of placebo for total withdrawals before the end of treatment (123/580 rivastigmine compared with 82/590 placebo) (OR 1.67; 95% CI 1.23 to 2.26, P = 0.001, 2 studies).
Adverse events
There were significant differences between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event by 24 weeks (395/578 rivastigmine compared with 361/588 placebo) (OR 1.39; 95% CI 1.08 to 1.80, P = 0.01, 2 studies) and withdrawals due to adverse events (62/580 rivastigmine compared with 36/590 placebo) (OR 1.84; 95% CI 1.20 to 2.82, P = 0.005, 2 studies).
There were significant differences between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event at the application site: erythema (113/287 compared with 55/286) (OR 2.73; 95% CI 1.87 to 3.98, P < 0.00001, 1 study), application site pruritis (100/287 compared with 61/286) (OR 1.97; 95% CI 1.36 to 2.86, P = 0.0004, 1 study), application site oedema (31/287 compared with 7/286) (OR 4.83; 95% CI 2.09 to 11.15, P = 0.0002, 1 study), application site exfoliation (11/282 compared with 4/286) (OR 3.68; 95% CI 1.20 to 1.33, P = 0.02), contact dermatitis (68/287 compared with 40/286) (OR 1.91; 95% CI 1.24 to 2.94, P = 0.003, 1 study), nausea (41/578 compared with 24/588) (OR 1.80; 95% CI 1.07 to 3.02, P = 0.03, 2 studies) and vomiting (41/578 compared with 21/588) (OR 2.06; 95% CI 1.20 to 3.53, P = 0.009, 2 studies).
Withdrawals before the end of treatment due to adverse events
There was a significant difference between rivastigmine and placebo in favour of placebo for withdrawals due to adverse events (62/580 rivastigmine compared with 36/590 placebo) (OR 1.84; 95% CI 1.20 to 2.82, P = 0.005, 2 studies).
Comparison of rivastigmine (5 cm2 (4.6 mg/day) patch) with placebo
This comparison was made in one study (Nakamura 2011).
Cognitive function
There was no difference between rivastigmine and placebo at 24 weeks for cognitive function measured using the ADAS‐Cog scale (ITT analysis, MD 0.80; 95% CI ‐1.62 to 0.02), MMSE ITT analysis, MD 0.00; 95% CI ‐0.52 to 0.52) and MENFIS (ITT analysis, MD ‐0.70; 95% CI ‐0.70, 95% CI ‐1.72 to 0.32).
Activities of daily living
There was no difference between rivastigmine and placebo at 24 weeks for activities of daily living measured using the DAD scale (ITT analysis, MD 1.20; 95% CI ‐0.73 to 3.13).
Global assessment
There was no difference between rivastigmine and placebo at 24 weeks for global assessment measured using the CIBIC‐plus J scale (212/269 rivastigmine, 226/267 placebo) (ITT analysis, OR 0.67; 95% CI 0.43 to 1.05).
Behavioural symptoms
There was no difference between rivastigmine and placebo at 24 weeks for behavioural symptoms measured using the BEHAVE‐AD scale (ITT analysis, MD 0.00; 95% CI ‐0.67 to 0.67).
Withdrawals before the end of treatment
There was a significant difference between rivastigmine and placebo in favour of placebo for total withdrawals before the end of treatment (64/284 rivastigmine compared with 46/288 placebo) (OR 1.53; 95% CI 1.01 to 2.33, P = 0.05).
Adverse events
There was a significant difference between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event at 24 weeks (243/282 rivastigmine compared with 222/286 placebo) (OR 1.80; 95% CI 1.16 to 2.78, P = 0.009), but no difference for deaths.
There were significant differences between rivastigmine and placebo in favour of placebo for the total number of patients that had at least one adverse event at the application site: erythema (106/282 compared with 55/286) (OR 2.53; 95% CI 1.73 to 3.70, P < 0.00001, 1 study), application site pruritis (92/282 compared with 61/286) (OR 1.79; 95% CI 1.23 to 2.60, P = 0.003, 1 study), application site oedema (35/282 compared with 7/286) (OR 5.65; 95% CI 2.46 to 12.94, P < 0.0001, 1 study), application site exfoliation (14/282 compared with 4/286) (OR 3.68; 95% CI 1.20 to 11.35, P = 0.02), contact dermatitis (69/282 compared with 40/286) (OR 1.99; 95% CI 1.30 to 3.06, P = 0.002, 1 study); but no difference between rivastigmine and placebo for adverse events of nasopharyngitis, nausea, vomiting and diarrhoea.
Withdrawals before the end of treatment due to adverse events
There was a significant difference between rivastigmine and placebo in favour of placebo for withdrawals due to adverse events (38/284 rivastigmine compared with 21/288 placebo) (OR 1.96; 95% CI 1.12 to 3.44, P = 0.02).
Comparison of rivastigmine (10 cm2 (9.5 mg/day) patch) with rivastigmine (6 to 12 mg/day twice daily) capsules
Cognitive function
One study (IDEAL) showed no difference between the rivastigmine patch and rivastigmine capsules on cognitive function as measured by the ADAS‐Cog, MMSE, TMT‐A and MENFIS at 24 weeks:
ADAS‐cog (ITT analysis, MD 0.0; 95% CI ‐1.10 to 1.10, P = 1.0, 1 study);
MMSE (ITT analysis, MD 0.30; 95% CI ‐0.27 to 0.87, P = 0.30, 1 study);
TMT‐A (ITT analysis, MD ‐2.6; 95% CI ‐13.5 to 8.3, P = 0.64, 1 study);
clock drawing (ITT analysis, MD 0.1; 95% CI ‐0.5 to 0.7, P = 0.73, 1 study).
Activities of daily living
The ADCS‐ADL showed no difference between the rivastigmine patch and rivastigmine capsules at 24 weeks (ITT analysis, MD 0.40; 95% CI ‐1.23 to 2.03, P = 0.63, 1 study).
Global assessment
The seven‐point CIBIC‐Plus scale measuring global clinical state was dichotomized by counting those showing no change or decline against those showing improvement and analysed using the Peto OR. There was no difference between the rivastigmine patch and rivastigmine capsules at 24 weeks (171/248 rivastigmine patch, 161/267253 rivastigmine capsules) (ITT analysis, OR 1.27; 95% CI 0.88 to 1.84, P = 0.21, 1 study).
Behavioural symptoms
One study assessed behavioural symptoms using the NPI (NPI‐12). There was no difference between the rivastigmine patch and rivastigmine capsules (ITT analysis, MD 0.50; 95% CI ‐1.55 to 2.55, P = 0.63, 1 study).
Withdrawals before the end of treatment
There was no significant difference between rivastigmine and placebo for withdrawals before the end of treatment (64/293 compared with 63/297) (OR 1.09; 95% CI 0.70 to 154, P = 0.85, 1 study).
Adverse events
There was a significant difference between the rivastigmine patch and rivastigmine capsules in favour of the patch for the total number of patients that had at least one adverse event by 24 weeks (147/291 rivastigmine compared with 186/294 placebo) (OR 0.59; 95% CI 0.43 to 0.82, P = 0.002, 1 study).
There were significant differences between the rivastigmine patch and rivastigmine capsules in favour of the patch for the total number of patients that had at least one adverse event of decreased appetite (2/291 compared with 12/294) (OR 0.16; 95% CI 0.04 to 0.73, P = 0.02, 1 study), dizziness (7/291 compared with 22/294) (OR 0.30; 95% CI 0.13 to 0.72, P = 0.007, 1 study), asthenia (5/291 compared with 17/294) (OR 0.28; 95% CI 0.10 to 0.78, P = 0.01, 1 study), nausea (21/291 compared with 68/294) (OR 0.26; 95% CI 0.15 to 0.43, P < 0.001, 1 study) and vomiting (18/291 compared with 50/294) (OR 0.32; 95% CI 0.18 to 0.57, P < 0.001, 1 study).
Withdrawals before the end of treatment due to adverse events
There was no significant difference between rivastigmine and placebo for withdrawals due to adverse events (28/293 rivastigmine compared with 24/297 placebo) (OR 1.20; 95% CI 0.68 to 2.13, P = 0.53, 1 study).
Discussion
Summary of main results
The results of the review showed the following main findings.
The currently recommended doses of rivastigmine (6 to 12 mg/day in two divided doses for capsules and 9.5 mg/day for transdermal patches) have some benefits compared to placebo at 26 weeks for cognitive function, activities of daily living and the physician rated global impression scales. No difference was found for behavioural symptoms or the impact on carers. Patients on rivastigmine are about twice as likely (OR of about 2) to experience adverse events or to withdraw from the trial before the end of the study.
Limited evidence from one trial suggests that the transdermal formulation (9.5 mg/day) is as effective as the oral formulation (6 to 12 mg/day) and is associated with a lower incidence of adverse events but does not affect the rate of withdrawals due to adverse events.
Outcomes
The two cognitive tests used, the MMSE and ADAS‐Cog, assess similar domains and a high correlation between the results would be expected. The results from 5 studies show that 6 to 12 mg daily of oral rivastigmine improved the cognitive function of patients with mild to moderate probable Alzheimer's disease treated over a period of 26 weeks, by 0.8 points on the MMSE (range 0 to 30) and by 2.0 points on the ADAS‐Cog (range 0 to 70), when compared with placebo. The results from 2 studies show that the 9.5 mg/day of rivastigmine in a transdermal patch improved cognitive function by 0.6 points on the MMSE and 1.4 points on the ADAS‐cog when compared with placebo. Pooling the data showed a treatment effect of 0.7 points on the MMSE and 1.8 points on the ADAS‐Cog. There was a smaller effect on cognitive function in the 1 to 4 mg daily oral treatment group.
Four studies assessed the effect of 6 to 12 mg daily oral rivastigmine on activities of daily living as reported by a carer using the PDS rating scale (range 0 to 100). Rivastigmine showed a benefit of 2.2 points compared with placebo, but the difference between placebo and 1 to 4 mg daily rivastigmine was not significant. The 10 cm2 (9.5 mg/day) patch showed a benefit of 2.2 points on the ADCS‐ADL scale (range 0 to 54) when compared with placebo.
The US Food and Drug Administration (FDA) requires an independent clinician to assess global clinical state after interviewing the patient and the carer at baseline and the endpoint. When the results of global impression measures were dichotomized to compare the number of patients who improved with the numbers who showed no change or whose condition had deteriorated, the 6 to 12 mg daily group was significantly better than the placebo group at 12 and 26 weeks, and there was a similar significant difference favouring the 1 to 4 mg daily group over placebo at 26 weeks. The 10 cm2 (9.5 mg/day) patch was also significantly better than placebo at 24 weeks.The clinician and carer, whilst following the guidelines for the application of the CIBIC‐Plus, are essentially making an assessment of whether the patient has improved or not based on criteria relevant to them. This is perhaps closest to what is commonly meant by the term 'meaningful improvement'.
Minimal clinically important differences (MCID), patient derived scores that represent changes in a score that have meaning for patients, have been suggested for the ADAS‐cog (3 points in severe AD (Howard 2011)) and MMSE (1.4 points in mild AD (Schrag 2012)). Comparing our findings with these we might conclude that the treatment effects for cognitive function are unlikely to be clinically relevant.
Adverse effects
When taking capsules, a fairly lengthy titration period of up to 12 weeks is needed to develop tolerance and to minimize adverse effects such as nausea, vomiting, diarrhoea, abdominal pain, dizziness, headache and anorexia. The target was to treat patients with a maximum tolerated dose administered in two divided doses, the upper limit being 12 mg per day. There were significantly more total dropouts and dropouts due to adverse events from the 6 to 12 mg daily dose groups than from placebo groups and therefore adverse effects remain a clinical issue. There was no hepatotoxicity associated with rivastigmine and no statistically or clinically significant changes in vital signs.
The continuous dose patch was introduced to improve tolerability. One study (IDEAL) tested two sizes of rivastigmine patch, one delivering a higher dose than previously tested in a 20 cm2 patch (17.4 mg/day) and one 10 cm2 patch (9.5 mg/day), a dose similar to the usual oral dose. Another study (Nakamura 2011) tested 5 cm2 (4.6 mg/day) and 10 cm2 (9.5 mg/day) patches. The smallest patch showed no treatment effect when compared with placebo for cognition, global function and activities of daily living. The efficacy of the 9.5 mg/day patch was comparable to that of the capsules with a similar daily dose, but was associated with significantly fewer adverse events of nausea, vomiting, dizziness and asthenia. There was no difference in the number of withdrawals due to adverse events. Therefore, the 9.5 mg/day patch appears to have advantages compared with both the higher dose patches and the 6 to 12 mg/day capsules in terms of the overall incidence of adverse events, but it may not reduce the incidence of the more serious events that lead to cessation of treatment.
Overall completeness and applicability of evidence
We were able to include evidence from both published and unpublished trials in this systematic review. There were 3319 participants. Most participants were in industry sponsored trials. Data from two independent trials (n = 162) were not available and we excluded data from two other independent trials (n = 75) from our analyses because of concerns about risk of bias. The participants in the included trials had mainly mild to moderate dementia due to Alzheimer's disease. They were not highly selected with respect to their general health so that all but the seriously ill were included. Only two trials included patients with severe dementia, and we excluded the data from one of these leaving data on only 218 patients with severe dementia included in the analyses.
The main limitations in the completeness and applicability of the evidence were the lack of long term data beyond 26 weeks and the limited range of outcomes measured. Beyond 26 weeks, some trials continued as an open label, extension phase. There were very few data on outcomes important to patients and carers, such as quality of life.
Quality of the evidence
The quality of the evidence at 26 weeks is moderate for most outcomes. Our main concern for the evidence is that only seven studies have contributed data to the meta‐analysis, and all of these studies were either industry sponsored or industry funded. In addition, withdrawals from these studies were of concern.
The results from B352 nearly always showed greater benefits for rivastigmine on each outcome than demonstrated in B303/B305, B304 and B351. B352 was responsible for the heterogeneity between trials that was reported for some of the measures of cognitive function. There are no obvious differences between B352 and the other phase III trials. It was conducted only in the US, but so was B351. The doses reached by patients in B352 were higher than those of B351, by 1.2 mg per day on average. Results from B352 have been more extensively reported than the other three phase III trials but there is no reason to suppose that this trial is of any more importance in the overall assessment of rivastigmine. We have not downgraded the evidence based on this heterogeneity concern since the impact on the overall pooled results is small and does not change the interpretation of any of the results.
Outcomes such as behavioural symptoms and quality of life are important to patients but these were only reported by three studies and one study, respectively. Hence, the quality of evidence for these was lower.
Potential biases in the review process
The initial protocol of the review, which was published in 1998, had aimed to include all double‐blinded randomised controlled trials (RCTs) of rivastigmine with a minimum study period of two weeks, regardless of the doses or formulations used. However, this resulted in a large number of possible comparisons. In addition, studies often used multiple instruments to report the same outcome, for example cognitive function was measured using the MMSE, ADAS‐Cog and other tests. For some of these outcomes we decided to use only the most commonly used tests in the main analysis.
In this update we decided to concentrate on the currently recommended doses (6 and 12 mg/day for oral doses, and 9.5 mg/day for transdermal patches), and a minimum treatment duration of six months for the main analysis. We considered the decision to focus on longer term data was clinically sensible since a titration period was required to reach the target doses.
Mowla 2007, Karaman 2005, Ballard 2005 and Tai 2000, all non‐industry funded studies, did not provide data that could be included in the review.
Agreements and disagreements with other studies or reviews
Most patients from the four phase III trials continued in an open label phase for a further 26 weeks during which the maximum tolerated dose was administered. Results from these extension phases have been described as showing a possible beneficial effect of rivastigmine on disease progression (Product monograph, Novartis 1998). Reported results showed that patients who had received placebo or rivastigmine 1 to 4 mg daily in the randomised phase showed initial improvement on the ADAS‐Cog before declining at the same rate as the 6 to 12 mg daily group, although remaining more impaired by approximately 1.5 points. These results must be interpreted with caution. The randomised, double‐blind conditions no longer prevailed. There had been differential dropout from the groups and there was no placebo group for the comparison. An imputed rate of decline for placebo patients was obtained by extrapolating from the randomised phase and not from actual observations.
There is much interest in the identification of patient characteristics that might predict a response to a cholinesterase inhibitor. Burns 2004 reported that cholinesterase inhibitors may be effective in patients with more severe disease. Data were pooled from three studies (B303/B305; B351; B352) for those with a baseline MMSE of 10, 11 or 12 (n = 117), in the group treated with rivastigmine (6 to 12 mg/day) or placebo, and the analysis showed that rivastigmine benefited those with more severe disease. This result has not added anything substantial to what was known already. The analysis of the total dataset from these trials demonstrated that rivastigmine was of benefit to the population randomised.
Erkinjuntti 2002, funded by Novartis Pharmaceuticals, investigated the response to rivastigmine of those without hypertension compared to those with using the data from B303/B305. They reported that particular benefits may be observed in those with vascular risk factors. These results are based on retrospective analysis of the study data and there has been no study confirming this finding using prospective data.
Farlow 2003a retrieved dropouts from the studies B303/B305, B351 and B352. These patients stopped treatment before the end of the trials but were invited back for assessment at the endpoint. Farlow concluded that those who had been in the rivastigmine groups had deteriorated less than those from the placebo groups, and therefore rivastigmine had provided a beneficial delay in disease progression. The two groups cannot be compared. The participants belong to a highly selected group, those who stopped treatment and agreed to return. The placebo group was much smaller than the rivastigmine group (38 compared with 88). Those who left the trial from the placebo group may have done so because their illness was more severe. This may have applied to some of those in the rivastigmine group who left but, in addition, there were those who left because they suffered from adverse effects. It is not possible to compare these two groups in a meaningful way.
Grossberg 2000 was a Novartis funded extension study examining the data from the four phase III studies and the related open label extension studies. Those who had been taking rivastigmine continuously for two years were compared with historical controls and the study concluded that rivastigmine has a beneficial effect on cognitive performance for up to two years in patients with Alzheimer's disease. These results must be treated with caution as the two groups are not comparable.
Several reviews of rivastigmine have been published. Schneider 1998 and Spencer 1998 both limited analysis and interpretation to the three trials B352, B303/B305 and B351. Spencer reported that "individual and pooled results indicate that rivastigmine usually produces cognitive, global and functional changes that indicate significantly less deterioration than was observed with placebo". Schneider reported that "the pooled analyses confirm the efficacy of rivastigmine in the treatment of both the cognitive and functional deficits of mild to moderately severe AD". Clegg 2002 was the report from NICE (National Institute of Clinical Excellence, UK) of the systematic review on which the decision was made that the cholinesterase inhibitors would be available on the National Health Service to treat those with Alzheimer's disease. Williams 2003 was a review of all aspects of rivastigmine, and summarised the clinical trials but without meta‐analyses.
Hauber 2000 calculated the potential savings costs using rivastigmine compared with no treatment for Alzheimer's disease. Hauber used a disease stage model. Results from two phase III trials of rivastigmine, together with extrapolation beyond the six month duration of the trial, identified the stage of disease using the MMSE assessments. Costs of healthcare resource use was estimated as a function of MMSE, using data from Canadian sources. Rivastigmine was judged to be cost effective due to the delay in disease progression. The analysis was repeated in a UK and US setting. These results are not based on randomised evidence and rest on many assumptions. It would be unwise to base decisions on whether rivastigmine should be prescribed to patients on the basis of cost‐effectiveness studies such as these. Fillit 2004 presents an excellent summary of the cost‐effectiveness studies and of the assumptions on which they are based. Fillit concludes that the results from these studies are not reliable and that outcomes related to costs and healthcare resource use must be assessed in randomised clinical trials.
Authors' conclusions
Implications for practice.
Use of rivastigmine in doses of 6 to 12 mg daily is associated with statistically significant benefits in terms of cognitive function. Benefits are also seen in the activities of daily living and clinician rated global impression scale ratings, which suggests that they may be of clinical as well as statistical significance. At lower doses (4 mg or less total daily dose) differences were in the same direction and were significant for cognitive function. Significant differences in the CIBIC‐Plus were seen at 26 weeks but not earlier. The 10 cm2 (9.5 mg/day) patch has been tested in two placebo controlled trials and shows similar benefits to the 6 to 12 mg oral dose. One double‐blind placebo controlled study of longer than 26 weeks is included in this review, but the data were not included in the meta‐analyses due to concerns about the study. This present review has not examined economic data.
Side effects observed were predictably related to the cholinergic actions of the drug. They may be related to the pharmacokinetics of the drug and merit further study. Three sizes of transdermal patch have been tested in two trials, and there is evidence that the 9.5 mg/day patch is associated with fewer side effects than the capsules or the higher dose larger patches and has comparable efficacy to all three.
Implications for research.
Longer term studies with a focus on clinically significant endpoints need to be linked to economic analyses to generate information on cost‐utility.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2020 | Amended | One word changed/corrected in PLS |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 10 September 2015 | Amended | Third author added (previously omitted in error); Implications for research section edited to remove incorrect text. |
| 10 September 2015 | New citation required but conclusions have not changed | Third author added (previously omitted in error); Implications for research section edited to remove incorrect text. |
| 2 March 2015 | New search has been performed | A pre‐publication search was run for this review on 2 March 2015. All results were assessed and no new studies were identified |
| 2 March 2015 | New citation required but conclusions have not changed | Conclusions unchanged |
| 24 January 2014 | New search has been performed | An update search was performed for this review on 24 January 2014. |
| 15 February 2013 | New search has been performed | A pre‐publication search was performed for this review on 15 February 2013 |
| 10 May 2011 | New search has been performed | An update search was performed for this review on 16 February 2011 |
| 24 March 2009 | Amended | Table 1 and Discussion have been amended |
| 18 December 2008 | New search has been performed | Update searches were run in March 2008 |
| 4 September 2008 | New citation required and conclusions have changed | An update search was performed on 27 March 2008. Two new studies have been included, IDEAL and Mowla 2007. |
| 15 June 2006 | New search has been performed | Update 2006. Two new trials in more severe dementia, Karaman 2005 and Lopez‐Pousa 2005, were included. We have contacted the authors of Karaman 2005 for clarification of their unusual drop out rates and unusually small standard deviations of outcome measures before drawing firm conclusions from the data, but have not received a reply. |
| 30 August 2000 | New citation required and conclusions have changed | Substantive amendment |
Notes
Update 2014
Additional studies were included: Mowla 2007, Nakamura 2011
Update 2005
One new trial, Ballard 2005, met the inclusion criteria for the review but its results could not be included in the analyses. There were substantial losses from the trial, and of concern was the elimination of those participants with low baseline scores from the analyses.
November 2003: following an update search, one additional trial, Tai 2000, was added. There is only limited information available about this trial. It appears to be an independent trial carried out in Taiwan. No results could be used from Tai 2000.
The review authors dealt with the consumer editor and peer reviewer comments.
Acknowledgements
Novartis (Pharma UK) has provided access to all the company results from the four phase III studies. Novartis (Pharma UK) and Novartis (Hellas) have provided abstracts of conference presentations, and lists and descriptions of trials.
The authors also wish to acknowledge the assistance of Lon Schneider in the development of the protocol for this review.
The authors acknowledge the significant contribution made by Jenny McCleery to the 2015 update.
Appendices
Appendix 1. Searches: February 2013, January 2014, March 2015
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [Searched on 02 March 2015; up‐to‐date: 01 March 2015] |
rivastigmine OR "SDZ ENA 713" OR exelon | Feb 2013: Jan 2014: 5 March 2015: 17 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE Feb 2013: 1950‐present (OvidSP) Jan 2014: 1950‐present [24 January 2014] (OvidSP) |
1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. "benign senescent forgetfulness".mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. or/1‐8 10. Rivastigmin*.ti,ab. 11. exelon*.ti,ab. 12. (ENA or "SDZ ENA 713").ti,ab. 13. *Cholinesterase Inhibitors/ 14. or/10‐13 15. 9 and 14 16. controlled trial.pt. 17. controlled clinical trial.pt. 18. .ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 28. (2011* or 2012* or 2013*).ed. 29. 27 and 28 |
Feb 2013: 299 Jan 2014: 144 |
| 3. EMBASE Feb 2013: 1974‐2013 Feb 14 (OvidSP) Jan 2014: 1974‐2014 January 23 (OvidSP) |
1. cognitive defect/ 2. dement*.mp. 3. alzheimer*.mp. 4. ("organic brain disease" or "organic brain syndrome").mp. 5. (cerebr* adj2 deteriorat*).mp. 6. (cerebral* adj2 insufficient*).mp. 7. Alzheimer disease/ 8. AD.ab. 9. or/1‐8 10. RIVASTIGMINE/ 11. rivastigmin*.ti,ab. 12. exelon*.ti,ab. 13. (ENA or "SDZ ENA 713").ti,ab. 14. or/10‐13 15. 9 and 14 16. controlled trial/ 17. controlled clinical trial/ 18. placebo.ab. 19. randomly.ab. 20. trial.ab. 21. ("double‐blind*" or "double‐mask*").ti,ab. 22. or/16‐21 23. 15 and 22 24. (2011* or 2012* or 2013*).em. 25. 23 and 24 |
Feb 2013: 135 Jan 2014: 79 |
| 4. PsycINFO Feb 2013: 1806‐February week 2 2013 (OvidSP) Jan 2014: 1806‐January week 3 2014 (OvidSP) |
1. alzheimer*.mp. 2. ("organic brain disease" or "organic brain syndrome").mp. 3. (cerebr* adj2 deteriorat*).mp. 4. (cerebral* adj2 insufficient*).mp. 5. Alzheimer's Disease/ 6. AD.ab. 7. or/1‐6 8. rivastigmin*.ti,ab. 9. exelon*.ti,ab. 10. (ENA or "SDZ ENA 713").ti,ab. 11. or/8‐10 12. 7 and 11 13. (2011* or 2012* or 2013*).up. 14. 12 and 13 |
Feb 2013: 56 Jan 2014: 28 |
| 5. CINAHL (EBSCOhost) Feb 2013: all dates to February week 1 2013 |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 |
Feb 2013: 50 |
| 6. Web of Science and conference proceedings Feb 2013: 1950 to Feb 14 2013 Jan 2014: 1950 to Jan 24 2014 |
Topic=(dement* OR alzheimer* OR "lewy bod*" OR DLB OR "vascular cognitive impairment*" OR FTD OF FTLD OR "cerebrovascular insufficienc*") AND Topic=(rivastigmin* OR exelon OR "SDZ ENA 713") AND Topic=(random* OR placebo OR "double‐blind*" OR trial OR RCT OR CCT) AND Year Published=(2011‐2013) Timespan=All Years. Databases=SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH. |
Feb 2013: 102 Jan 2014: 54 |
| 7. LILACS (BIREME) Feb 2013: all dates to 14 February 2013 Jan 2014: all dates to 24 January 2014 |
rivastigmine OR rivastigmine OR "SDZ ENA 713" OR exelon [Words] | Feb 2013: 9 Jan 2014: 3 |
| 8. CENTRAL (The Cochrane Library) Feb 2013: Issue 4 of 12, 2013 Jan 2014: Issue 1 of 12, 2014 |
#1 MeSH descriptor: [Dementia] explode all trees #2 MeSH descriptor: [Delirium] this term only #3 MeSH descriptor: [Wernicke Encephalopathy] this term only #4 MeSH descriptor: [Delirium, Dementia, Amnestic, Cognitive Disorders] this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 rivastigmin* or exelon* or "SDZ ENA 713" #22 #20 and #21 from 2011 to 2013, in Trials |
Feb 2013: 7 Jan 2014: 12 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) ‐ all dates |
rivastigmine OR exelon OR "SDZ ENA 713" | Interventional Studies | dementia OR alzheimer OR alzheimers OR lewy OR vascular cognitive impairment | Adult, Senior | received from 01/01/2011 to 02/15/2013 | Feb 2013: 16 Jan 2014: 0 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] ‐ all dates |
Advanced search: (rivastigmine OR exelon OR "SDZ ENA 713" | Interventional Studies) AND (received from 01/01/2011 to 02/15/2013) | Feb 2013: 136 Jan 2014: 2 |
| TOTAL before de‐duplication | Feb 2013: 922 Jan 2014: 327 |
|
| TOTAL after de‐dupe and first assess | Feb 2013: 36 Jan 2014: 24 March 2015: 17 |
|
Appendix 2. Update search: February 2011
| Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | Advanced search: Study design: RCT AND Health status: Alzheimer AND Intervention: rivastigmine | 45 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) | 1. exp Dementia/ 2. Delirium, Dementia, Amnestic, Cognitive Disorders/ 3. dement*.mp. 4. alzheimer*.mp. 5. ("organic brain disease" or "organic brain syndrome").mp. 6. "benign senescent forgetfulness".mp. 7. (cerebr* adj2 deteriorat*).mp. 8. (cerebral* adj2 insufficient*).mp. 9. or/1‐8 10. Rivastigmin*.ti,ab. 11. exelon*.ti,ab. 12. (ENA or "SDZ ENA 713").ti,ab. 13. *Cholinesterase Inhibitors/ 14. or/10‐13 15. 9 and 14 16. controlled trial.pt. 17. controlled clinical trial.pt. 18. .ab. 19. placebo.ab. 20. drug therapy.fs. 21. randomly.ab. 22. trial.ab. 23. groups.ab. 24. or/16‐23 25. (animals not (humans and animals)).sh. 26. 24 not 25 27. 15 and 26 28. (2008* or 2009* or 2010* or 2011*).ed. 29. 27 and 28 |
445 |
| 3. EMBASE 1980‐2011 week 6 (OvidSP) |
1. cognitive defect/ 2. dement*.mp. 3. alzheimer*.mp. 4. ("organic brain disease" or "organic brain syndrome").mp. 5. (cerebr* adj2 deteriorat*).mp. 6. (cerebral* adj2 insufficient*).mp. 7. Alzheimer disease/ 8. AD.ab. 9. or/1‐8 10. RIVASTIGMINE/ 11. rivastigmin*.ti,ab. 12. exelon*.ti,ab. 13. (ENA or "SDZ ENA 713").ti,ab. 14. or/10‐13 15. 9 and 14 16. randomised controlled trial/ 17. controlled clinical trial/ 18. placebo.ab. 19. randomly.ab. 20. trial.ab. 21. ("double‐blind*" or "double‐mask*").ti,ab. 22. or/16‐21 23. 15 and 22 24. (2008* or 2009* or 2010* or 2011*).em. 25. 23 and 24 |
226 |
| 4. PsycINFO 1806‐February week 2 2011 (OvidSP) |
1. alzheimer*.mp. 2. ("organic brain disease" or "organic brain syndrome").mp. 3. (cerebr* adj2 deteriorat*).mp. 4. (cerebral* adj2 insufficient*).mp. 5. Alzheimer's Disease/ 6. AD.ab. 7. or/1‐6 8. rivastigmin*.ti,ab. 9. exelon*.ti,ab. 10. (ENA or "SDZ ENA 713").ti,ab. 11. or/8‐10 12. 7 and 11 13. (2008* or 2009* or 2010* or 2011*).up. 14. 12 and 13 |
98 |
| 5. CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 |
120 |
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] | Topic=(rivastigmine OR exelon OR ena OR "SDZ ENA 713") AND Topic=(alzheimer* OR AD OR "ADD") AND Topic=(random* OR placebo OR trial OR "double‐blind*") AND Year Published=(2008‐2011) |
191 |
| 7. LILACS (BIREME) | rivastigmine OR exelon | 7 |
| 8. CENTRAL (The Cochrane Library) (Issue 4 of 4, Oct 2010) | #1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 rivastigmin* OR Exelon* OR “SDZ ENA 713” #22 #21 AND #20 |
40 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | Advanced search: Intervention: rivastigmine OR Exelon OR “SDZ ENA 713” AND Condition: Alzheimer OR Alzheimer’s OR ad OR dementia OR alzheimers | 18 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | (Alzheimer OR Alzheimer’s OR ad OR dementia OR alzheimers) AND (rivastigmine OR Exelon) AND (2008‐2011) | 5 |
| TOTAL before de‐duplication | 1195 | |
| TOTAL after de‐dupe and first‐assess | 45 | |
Data and analyses
Comparison 1. Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 24‐26 weeks) ITT | 6 | 3232 | Mean Difference (IV, Fixed, 95% CI) | ‐1.79 [‐2.21, ‐1.37] |
| 2 MMSE (change from baseline at 24‐26 weeks) ITT | 6 | 3205 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.52, 0.97] |
| 3 Activities of daily living (change from baseline at 24‐26 weeks ) ITT | 6 | 3230 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [0.13, 0.27] |
| 4 Clinical Global Impression (no change or worse at 24‐26 weeks ) ITT | 7 | 3338 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.80] |
| 5 Behavioural symptoms (change from baseline at 24‐26 weeks) ITT | 3 | 1529 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 6 Withdrawals before end of treatment at 24‐26 weeks | 7 | 3569 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.74, 2.45] |
| 7 at least one adverse event by 24‐26 weeks | 7 | 3587 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.16 [1.82, 2.57] |
| 8 NPI‐D carer distress scale (change from baseline at 24‐26 weeks) ITT | 1 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.91, 1.11] |
1.1. Analysis.
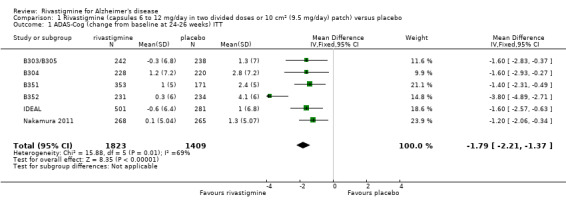
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 1 ADAS‐Cog (change from baseline at 24‐26 weeks) ITT.
1.2. Analysis.
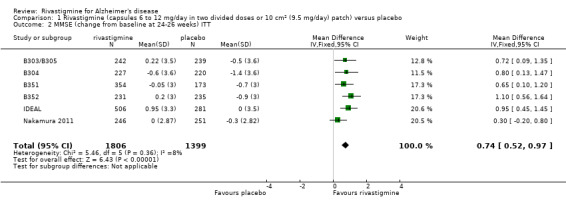
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 2 MMSE (change from baseline at 24‐26 weeks) ITT.
1.3. Analysis.
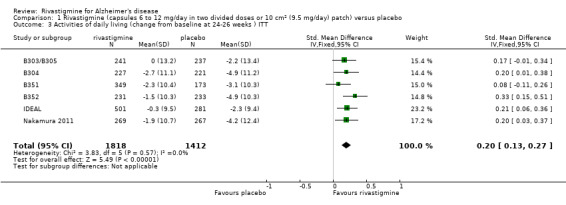
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 3 Activities of daily living (change from baseline at 24‐26 weeks ) ITT.
1.4. Analysis.
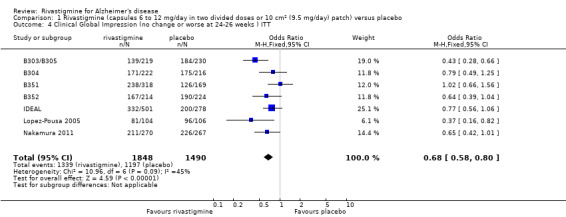
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 4 Clinical Global Impression (no change or worse at 24‐26 weeks ) ITT.
1.5. Analysis.
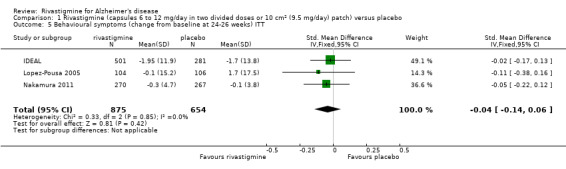
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 5 Behavioural symptoms (change from baseline at 24‐26 weeks) ITT.
1.6. Analysis.
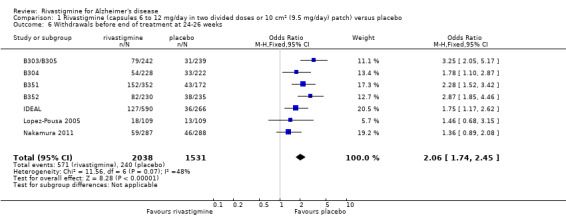
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 6 Withdrawals before end of treatment at 24‐26 weeks.
1.7. Analysis.
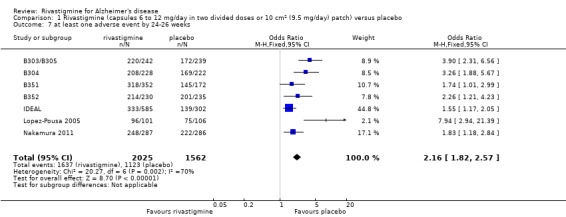
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 7 at least one adverse event by 24‐26 weeks.
1.8. Analysis.
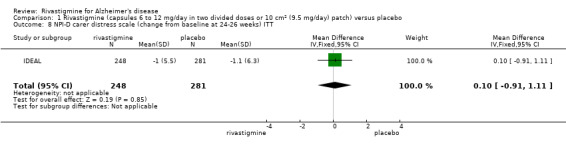
Comparison 1 Rivastigmine (capsules 6 to 12 mg/day in two divided doses or 10 cm2 (9.5 mg/day) patch) versus placebo, Outcome 8 NPI‐D carer distress scale (change from baseline at 24‐26 weeks) ITT.
Comparison 2. Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 12 weeks) ITT | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.87, 0.25] |
| 1.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1917 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐1.96, ‐1.01] |
| 2 ADAS‐Cog (change from baseline at 26 weeks) ITT | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1293 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.48, ‐0.19] |
| 2.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2451 | Mean Difference (IV, Fixed, 95% CI) | ‐1.99 [‐2.49, ‐1.50] |
| 3 MMSE (change from baseline at 26 weeks) ITT | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1297 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.08, 0.78] |
| 3.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2458 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [0.56, 1.08] |
| 4 SIB (change from baseline at 26 weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Rivastigmine 6‐12 mg/day | 1 | 210 | Mean Difference (IV, Fixed, 95% CI) | 4.53 [0.47, 8.59] |
| 5 ADCS‐ADL (change from baseline at 26 weeks) ITT | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 rivastigmine (6‐12 mg/d) vs placebo | 1 | 535 | Mean Difference (IV, Fixed, 95% CI) | 1.80 [0.20, 3.40] |
| 6 PDS (change from baseline at 12 weeks ) ITT | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1288 | Mean Difference (IV, Fixed, 95% CI) | ‐0.77 [‐1.84, 0.30] |
| 6.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1912 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.19, 1.98] |
| 7 PDS (change from baseline at 26 weeks ) ITT | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1288 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.61, 0.84] |
| 7.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1912 | Mean Difference (IV, Fixed, 95% CI) | 2.15 [1.13, 3.16] |
| 8 Clinical Global Impression (no change or worse at 12 weeks) ITT | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1220 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 8.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1775 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.60, 0.92] |
| 9 Clinical Global Impression (no change or worse at 26 weeks ) ITT | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.55, 0.93] |
| 9.2 rivastigmine (6‐12 mg/d) vs placebo | 6 | 2553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.55, 0.79] |
| 10 GDS( moderately severe, severe, or very severe dementia at 26 weeks) ITT | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 10.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1296 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 10.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1923 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.64, 0.94] |
| 11 CGIC (little or no improvement, or worse at 12 weeks) ITT | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 rivastigmine (1‐4 mg/d) vs placebo | 1 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.60, 1.77] |
| 11.2 rivastigmine (6‐12 mg/d) vs placebo | 1 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.43, 1.22] |
| 12 Behavioural disturbance NPI‐10 or NPI‐12 (change from baseline at 26 weeks) ITT | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Rivastigmine (6‐12 mg/day) vs placebo | 2 | 744 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.20, 0.09] |
| 13 withdrawals before end of treatment at 12 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 13.1 rivastigmine (1‐4 mg/d) vs placebo | 1 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.15 [0.95, 4.89] |
| 13.2 rivastigmine (6‐12 mg/d) vs placebo | 1 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.60 [1.19, 5.67] |
| 14 withdrawals before end of treatment at 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 14.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.75, 1.34] |
| 14.2 rivastigmine (6‐12 mg/d) vs placebo | 6 | 2701 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.19 [1.83, 2.63] |
| 15 at least one adverse event by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 15.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.82, 1.31] |
| 15.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.96 [2.39, 3.68] |
| 16 at least one adverse event by 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 16.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.71, 1.23] |
| 16.2 rivastigmine (6‐12mg/d) vs placebo | 6 | 2726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [2.05, 3.02] |
| 17 dropouts due to adverse events by 12 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 17.1 rivastigmine (4mg/d) vs placebo | 1 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [1.06, 6.84] |
| 17.2 rivastigmine (6mg/d) vs placebo | 1 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.11 [1.28, 7.56] |
| 18 dropouts due to adverse events by 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 18.1 rivastigmine (1‐4 mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.69, 1.52] |
| 18.2 rivastigmine (6‐12 mg/d) vs placebo | 6 | 2729 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [2.19, 3.41] |
| 19 at least one adverse event of decreased appetite by 26 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 19.1 rivastigmine (6‐12 mg/d) vs placebo | 1 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.51 [1.26, 9.79] |
| 20 at least one adverse event of weight decrease by 26 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 20.1 rivastigmine (6‐12mg/d) vs placebo | 1 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.55 [1.46, 8.66] |
| 21 at least one adverse event of nausea by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 21.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [1.36, 2.52] |
| 21.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.57 [4.59, 6.75] |
| 22 at least one adverse event of nausea by 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 22.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [1.28, 2.36] |
| 22.2 rivastigmine (6‐12mg/d bid) vs placebo | 6 | 2726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.36 [4.50, 6.40] |
| 23 at least one adverse event of vomiting by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 23.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.22, 3.16] |
| 23.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2187 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.72 [4.48, 7.29] |
| 24 at least one adverse event of vomiting by 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 24.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.65 [1.08, 2.52] |
| 24.2 rivastigmine (6‐12mg/d) vs placebo | 6 | 2726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.15 [4.20, 6.32] |
| 25 at least one adverse event of diarrhoea by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 25.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.68, 1.42] |
| 25.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.51, 2.57] |
| 26 at least one adverse event of diarrhoea by 26 weeks | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 26.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.93 [0.67, 1.31] |
| 26.2 rivastigmine (6‐12mg/d) vs placebo | 5 | 2516 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [1.39, 2.24] |
| 27 at least one adverse event of anorexia by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 27.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [1.24, 3.95] |
| 27.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.94 [3.56, 6.85] |
| 28 at least one adverse event of anorexia by 26 weeks | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 28.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.13 [1.29, 3.52] |
| 28.2 rivastigmine (6‐12mg/d) vs placebo | 5 | 2130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.46 [3.34, 5.95] |
| 29 at least one adverse event of headache by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 29.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.69, 1.37] |
| 29.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.64 [1.26, 2.14] |
| 30 at least one adverse event of headache by 26 weeks | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 30.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.84, 1.64] |
| 30.2 rivastigmine (6‐12mg/d) vs placebo | 5 | 2516 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.34, 2.21] |
| 31 at least one adverse event of insomnia by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 31.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.64, 1.67] |
| 31.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.94, 2.09] |
| 32 at least one adverse event of insomnia by 26 weeks | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 32.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.70, 1.58] |
| 32.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.33 [0.95, 1.87] |
| 33 at least one adverse event of syncope by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 33.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.43, 5.20] |
| 33.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.16 [0.99, 4.68] |
| 34 at least one adverse event of syncope by 26 weeks | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 34.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.37, 2.69] |
| 34.2 rivastigmine (6‐12mg/d bid) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.96, 3.11] |
| 35 at least one adverse event of abdominal pain by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 35.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.72, 1.88] |
| 35.2 rivastigmine (6‐12mg/d) vs placebo | 5 | 2186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.50 [1.80, 3.48] |
| 36 at least one adverse event of abdominal pain by 26 weeks | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 36.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.77, 1.87] |
| 36.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [1.65, 3.05] |
| 37 at least one adverse event of dizziness by the end of titration period | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 37.1 rivastigmine (1‐4mg/d) vs placebo | 4 | 1559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.70, 1.39] |
| 37.2 rivastigmine (6‐12 mg/d) vs placebo | 5 | 2186 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.38 [1.86, 3.04] |
| 38 at least one adverse event of dizziness by 26 weeks | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 38.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.25 [0.91, 1.72] |
| 38.2 rivastigmine (6‐12mg/d) vs placebo | 5 | 2516 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.24 [1.78, 2.82] |
| 39 at least one adverse event of bone fracture by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 39.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.25, 2.72] |
| 39.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.37, 2.46] |
| 40 at least one adverse event of bone fracture by 26 weeks | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 40.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.27, 1.34] |
| 40.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.34, 1.42] |
| 41 at least one adverse event of asthenia by 26 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 41.1 rivastigmine (6‐12mg/d) vs placebo | 1 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.37 [1.79, 10.65] |
| 42 at least one severe adverse event by the end of titration period | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 42.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.62, 1.42] |
| 42.2 rivastigmine (6‐12 mg/d) vs placebo | 4 | 1920 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.88 [1.39, 2.55] |
| 43 at least one serious adverse event by 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 43.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.70, 1.36] |
| 43.2 rivastigmine (6‐12mg/d) vs placebo | 6 | 2726 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.93, 1.47] |
| 44 deaths before end of treatment at 12 weeks | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 44.1 rivastigmine (1‐4mg/d) vs placebo | 1 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.34 [0.76, 71.14] |
| 44.2 rivastigmine (6‐12 mg/d) vs placebo | 1 | 266 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.46, 119.66] |
| 45 deaths before end of treatment at 26 weeks | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 45.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.98 [0.20, 19.15] |
| 45.2 rivastigmine (6‐12mg/d) vs placebo | 6 | 2737 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.40, 3.37] |
| 46 CIBIC‐Plus (no change or worse at 12 weeks) OC | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 46.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.72, 1.23] |
| 46.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1630 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.58, 0.91] |
| 47 CIBIC‐Plus (no change or worse at 26 weeks) OC | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 47.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1036 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.50, 0.89] |
| 47.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1353 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.49, 0.81] |
| 48 CIBIC‐Plus (no change or worse at 12 weeks) OC+RDO | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 48.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.72, 1.22] |
| 48.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1777 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.60, 0.93] |
| 49 CIBIC‐Plus (no change or worse at 26 weeks)OC+RDO | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 49.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1093 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.52, 0.91] |
| 49.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1542 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.51, 0.82] |
| 50 CIBIC‐Plus (no change or worse at 12 weeks) ALL+OC | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 50.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1293 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.77, 1.30] |
| 50.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1917 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 51 CIBIC‐Plus (no change or worse at 26 weeks) ALL+OC | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 51.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1297 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| 51.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1921 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 52 ADAS‐Cog (change from baseline at 12 weeks) OC | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 52.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1187 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.08, 0.15] |
| 52.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1646 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.33, ‐1.27] |
| 53 ADAS‐Cog (change from baseline at 26 weeks) OC | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 53.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1045 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.72, ‐0.21] |
| 53.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1379 | Mean Difference (IV, Fixed, 95% CI) | ‐2.62 [‐3.29, ‐1.94] |
| 54 ADAS‐Cog (change from baseline at 12 weeks) OC+RDO | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 54.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1231 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐0.96, 0.23] |
| 54.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1795 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐1.89, ‐0.88] |
| 55 ADAS‐Cog (change from baseline at 26 weeks) OC+RDO | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 55.1 rivastigmine (1‐4mg/d) vs placebo | 3 | 1123 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐1.80, ‐0.34] |
| 55.2 rivastigmine (6‐12mg/d) vs placebo | 4 | 1547 | Mean Difference (IV, Fixed, 95% CI) | ‐2.39 [‐3.03, ‐1.74] |
2.1. Analysis.
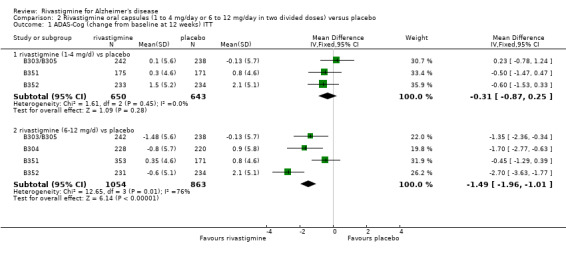
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 1 ADAS‐Cog (change from baseline at 12 weeks) ITT.
2.2. Analysis.
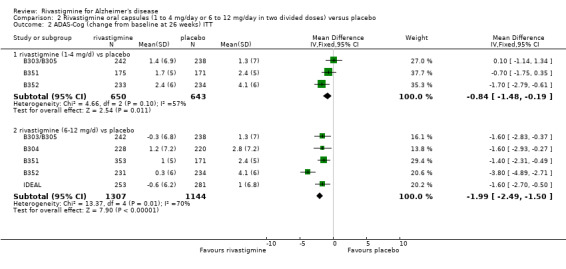
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 2 ADAS‐Cog (change from baseline at 26 weeks) ITT.
2.3. Analysis.
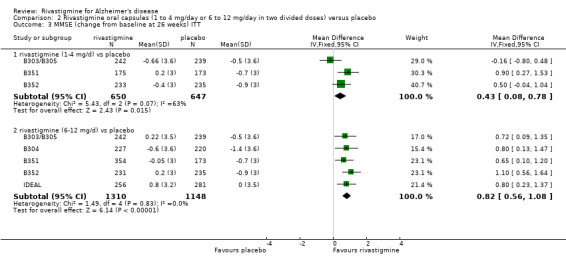
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 3 MMSE (change from baseline at 26 weeks) ITT.
2.4. Analysis.
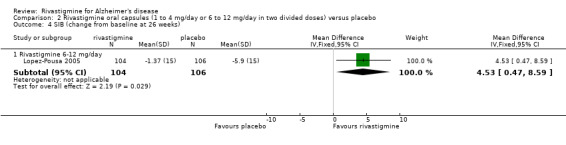
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 4 SIB (change from baseline at 26 weeks).
2.5. Analysis.
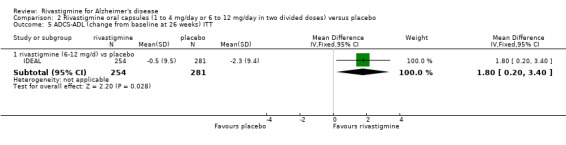
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 5 ADCS‐ADL (change from baseline at 26 weeks) ITT.
2.6. Analysis.
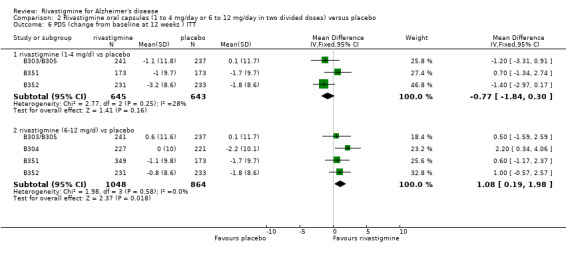
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 6 PDS (change from baseline at 12 weeks ) ITT.
2.7. Analysis.
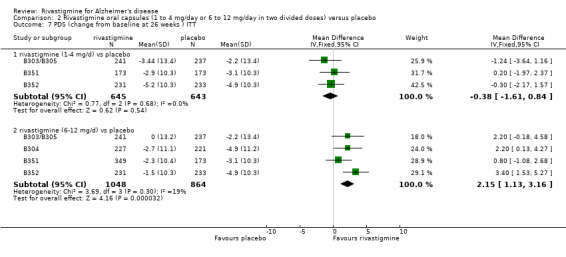
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 7 PDS (change from baseline at 26 weeks ) ITT.
2.8. Analysis.
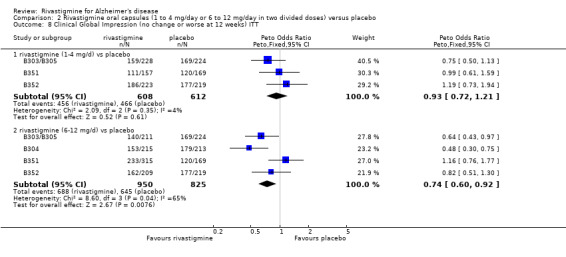
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 8 Clinical Global Impression (no change or worse at 12 weeks) ITT.
2.9. Analysis.
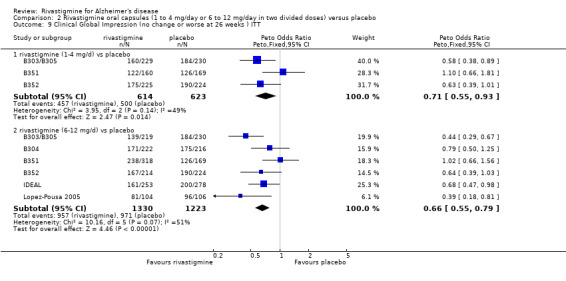
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 9 Clinical Global Impression (no change or worse at 26 weeks ) ITT.
2.10. Analysis.
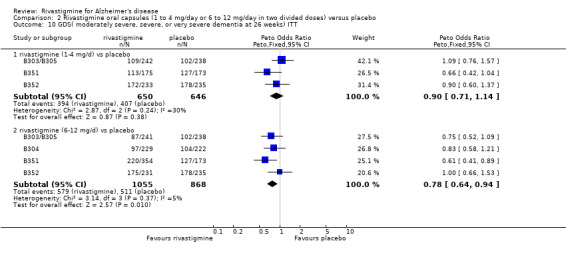
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 10 GDS( moderately severe, severe, or very severe dementia at 26 weeks) ITT.
2.11. Analysis.

Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 11 CGIC (little or no improvement, or worse at 12 weeks) ITT.
2.12. Analysis.
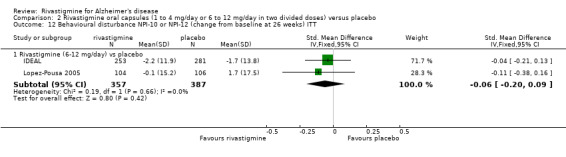
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 12 Behavioural disturbance NPI‐10 or NPI‐12 (change from baseline at 26 weeks) ITT.
2.13. Analysis.
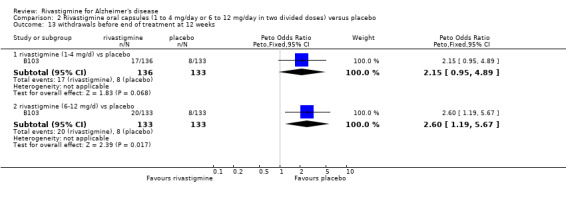
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 13 withdrawals before end of treatment at 12 weeks.
2.14. Analysis.
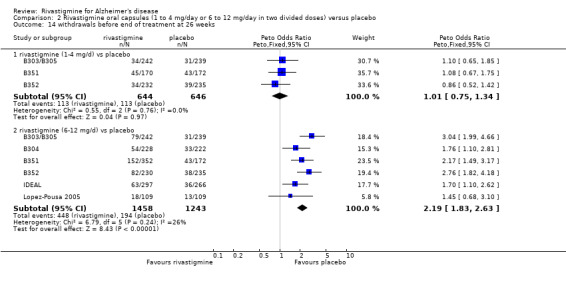
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 14 withdrawals before end of treatment at 26 weeks.
2.15. Analysis.
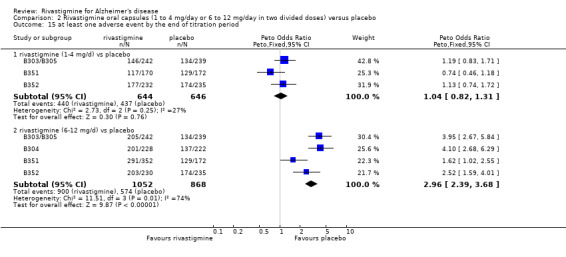
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 15 at least one adverse event by the end of titration period.
2.16. Analysis.
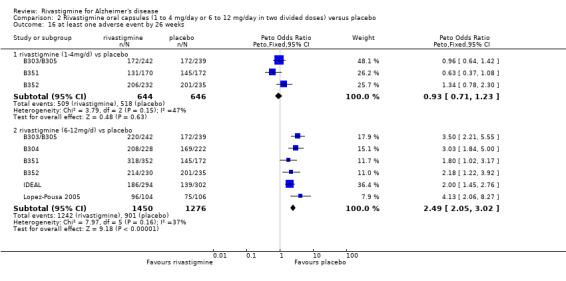
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 16 at least one adverse event by 26 weeks.
2.17. Analysis.
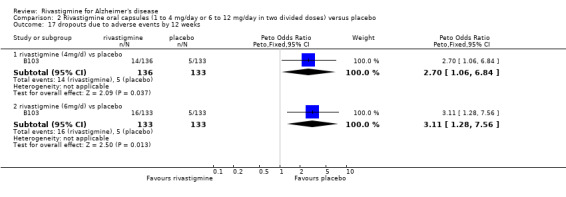
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 17 dropouts due to adverse events by 12 weeks.
2.18. Analysis.
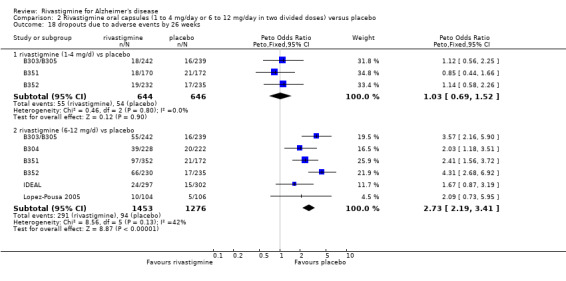
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 18 dropouts due to adverse events by 26 weeks.
2.19. Analysis.
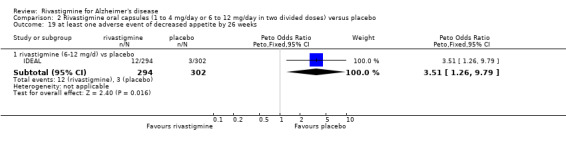
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 19 at least one adverse event of decreased appetite by 26 weeks.
2.20. Analysis.
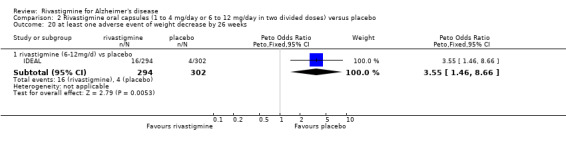
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 20 at least one adverse event of weight decrease by 26 weeks.
2.21. Analysis.
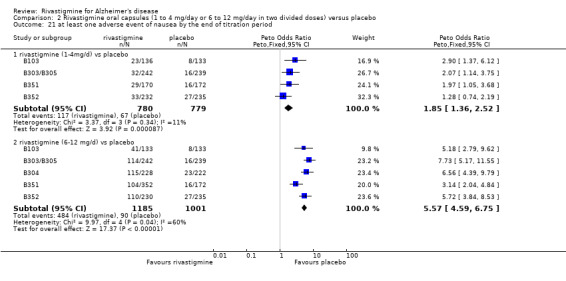
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 21 at least one adverse event of nausea by the end of titration period.
2.22. Analysis.
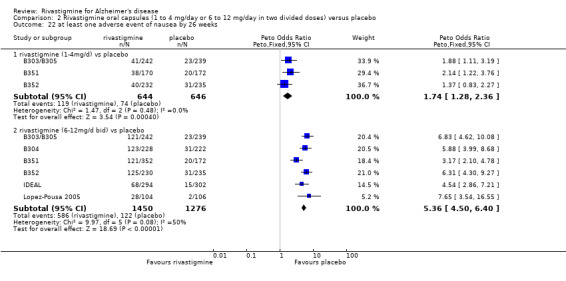
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 22 at least one adverse event of nausea by 26 weeks.
2.23. Analysis.
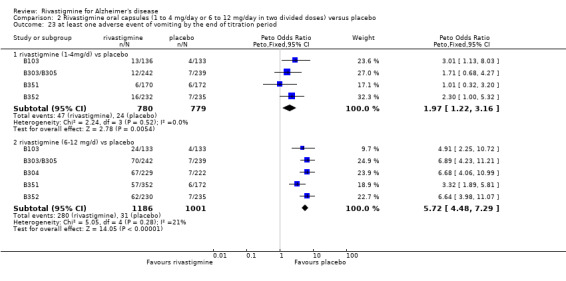
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 23 at least one adverse event of vomiting by the end of titration period.
2.24. Analysis.
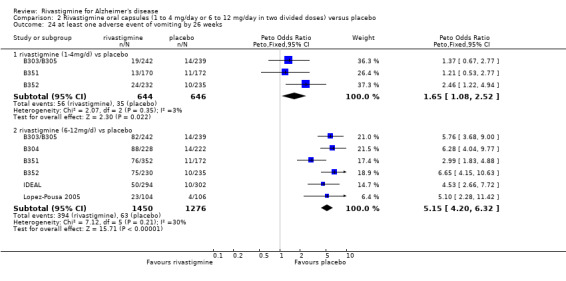
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 24 at least one adverse event of vomiting by 26 weeks.
2.25. Analysis.
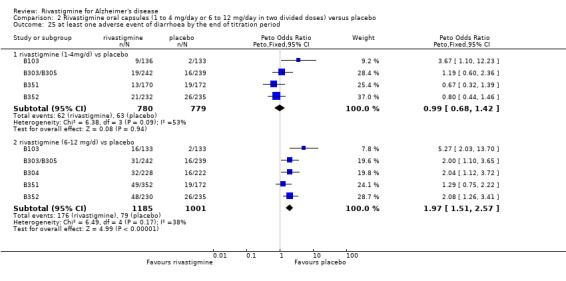
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 25 at least one adverse event of diarrhoea by the end of titration period.
2.26. Analysis.
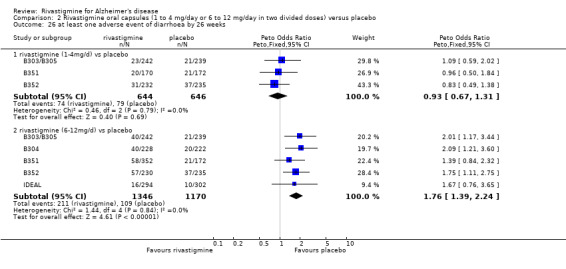
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 26 at least one adverse event of diarrhoea by 26 weeks.
2.27. Analysis.
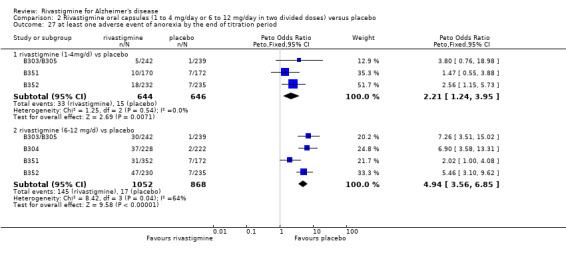
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 27 at least one adverse event of anorexia by the end of titration period.
2.28. Analysis.
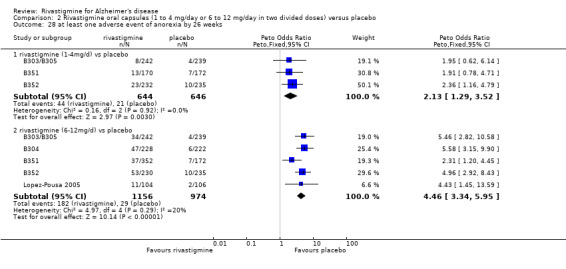
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 28 at least one adverse event of anorexia by 26 weeks.
2.29. Analysis.
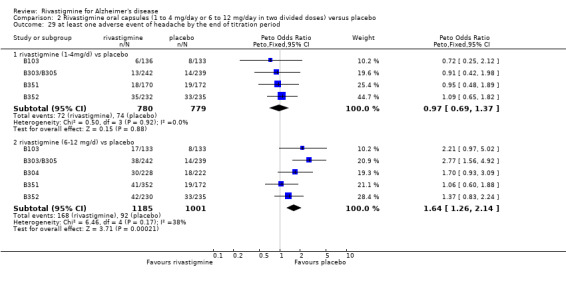
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 29 at least one adverse event of headache by the end of titration period.
2.30. Analysis.
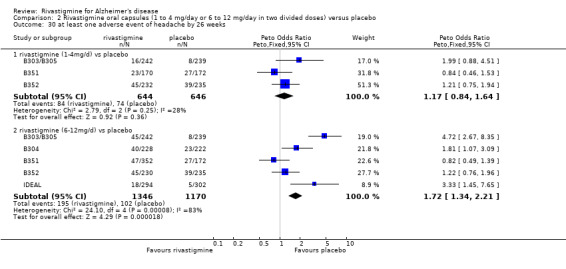
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 30 at least one adverse event of headache by 26 weeks.
2.31. Analysis.
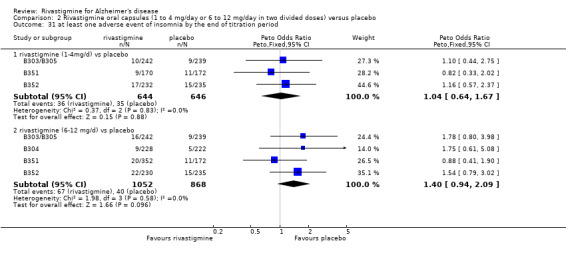
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 31 at least one adverse event of insomnia by the end of titration period.
2.32. Analysis.
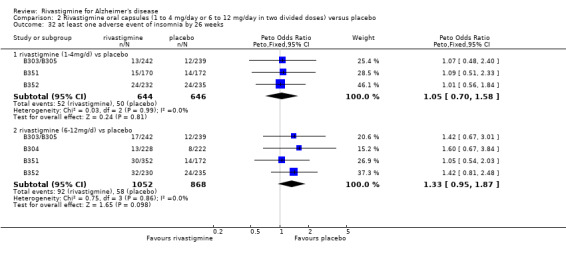
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 32 at least one adverse event of insomnia by 26 weeks.
2.33. Analysis.
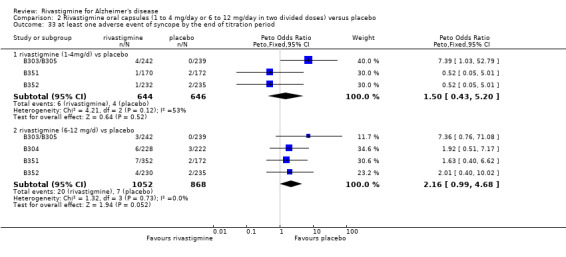
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 33 at least one adverse event of syncope by the end of titration period.
2.34. Analysis.
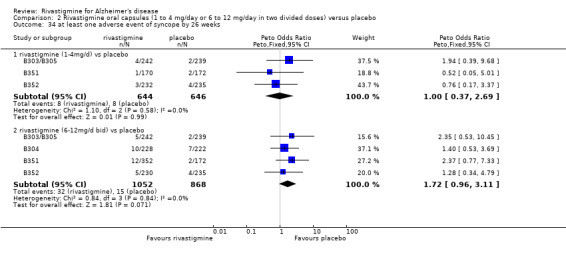
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 34 at least one adverse event of syncope by 26 weeks.
2.35. Analysis.
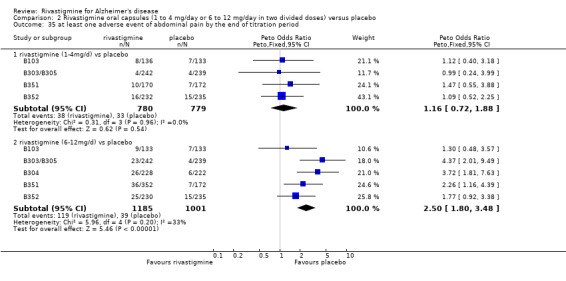
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 35 at least one adverse event of abdominal pain by the end of titration period.
2.36. Analysis.
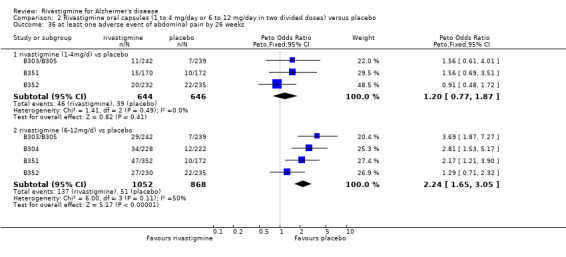
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 36 at least one adverse event of abdominal pain by 26 weeks.
2.37. Analysis.
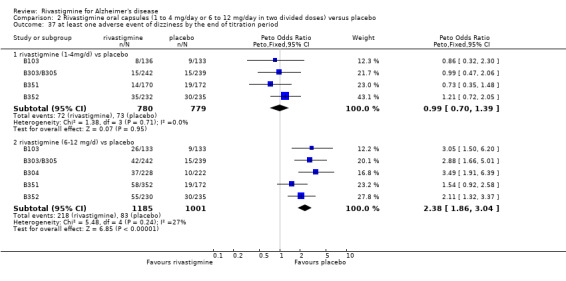
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 37 at least one adverse event of dizziness by the end of titration period.
2.38. Analysis.
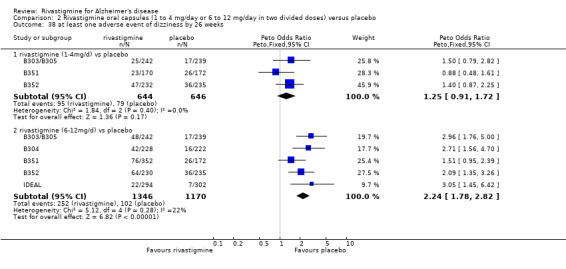
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 38 at least one adverse event of dizziness by 26 weeks.
2.39. Analysis.
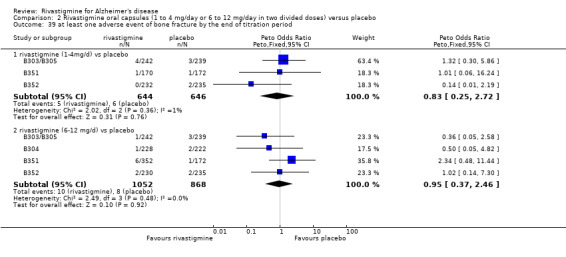
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 39 at least one adverse event of bone fracture by the end of titration period.
2.40. Analysis.
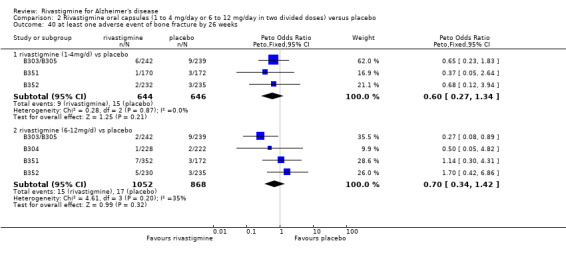
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 40 at least one adverse event of bone fracture by 26 weeks.
2.41. Analysis.
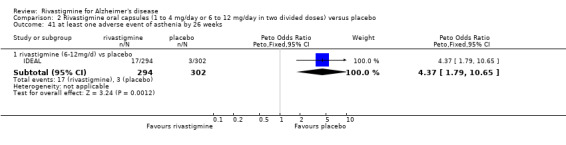
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 41 at least one adverse event of asthenia by 26 weeks.
2.42. Analysis.
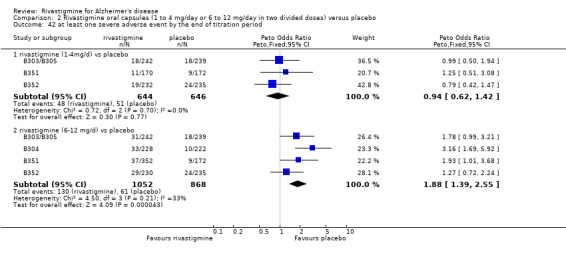
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 42 at least one severe adverse event by the end of titration period.
2.43. Analysis.
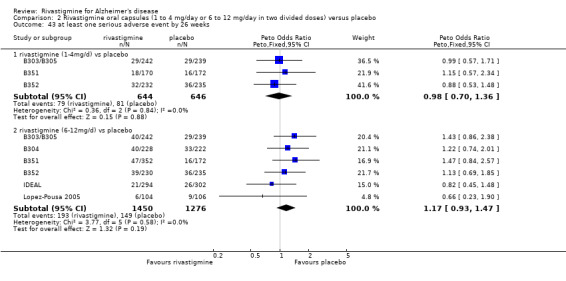
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 43 at least one serious adverse event by 26 weeks.
2.44. Analysis.
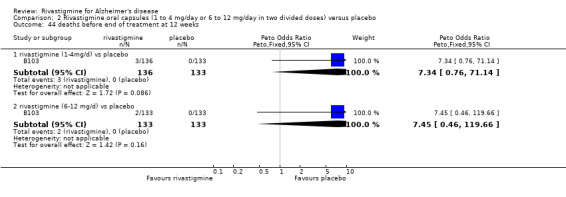
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 44 deaths before end of treatment at 12 weeks.
2.45. Analysis.
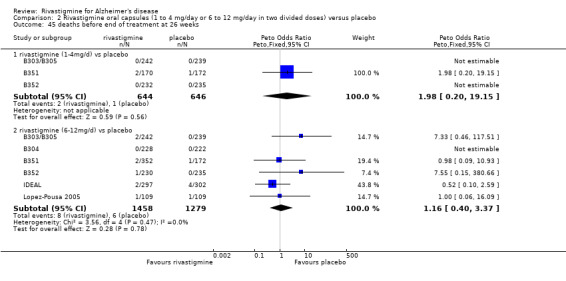
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 45 deaths before end of treatment at 26 weeks.
2.46. Analysis.
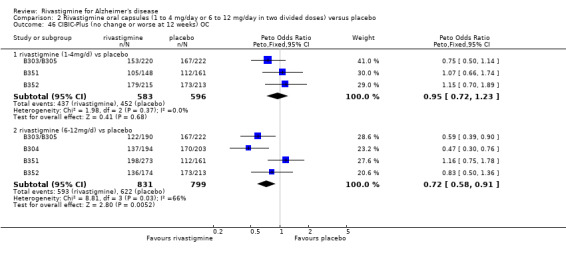
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 46 CIBIC‐Plus (no change or worse at 12 weeks) OC.
2.47. Analysis.
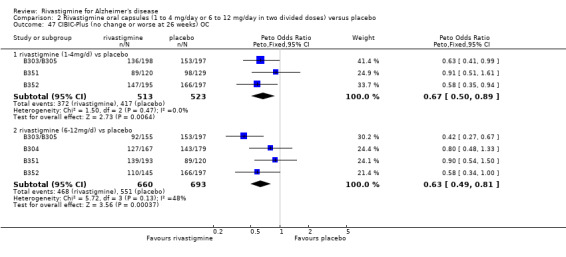
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 47 CIBIC‐Plus (no change or worse at 26 weeks) OC.
2.48. Analysis.
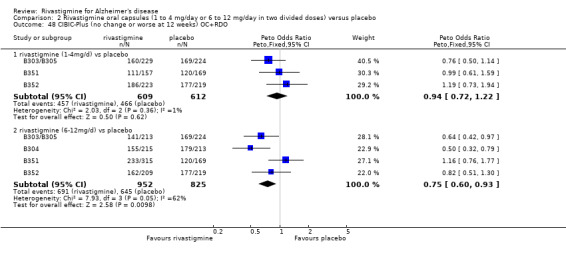
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 48 CIBIC‐Plus (no change or worse at 12 weeks) OC+RDO.
2.49. Analysis.
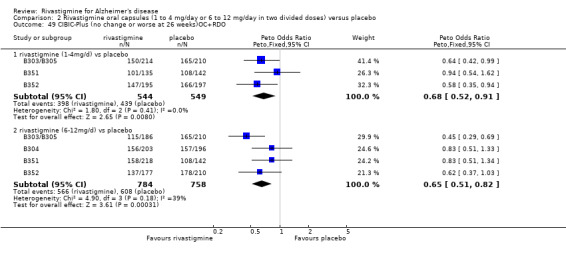
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 49 CIBIC‐Plus (no change or worse at 26 weeks)OC+RDO.
2.50. Analysis.
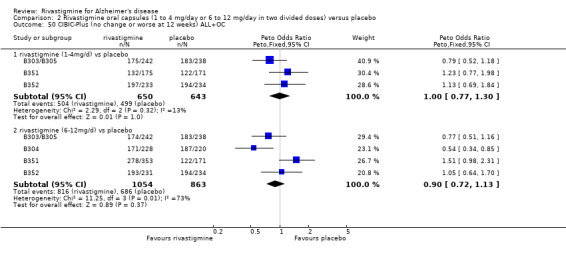
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 50 CIBIC‐Plus (no change or worse at 12 weeks) ALL+OC.
2.51. Analysis.
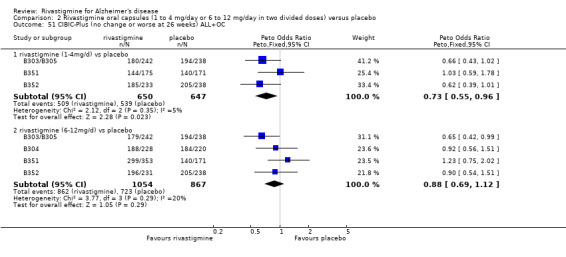
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 51 CIBIC‐Plus (no change or worse at 26 weeks) ALL+OC.
2.52. Analysis.
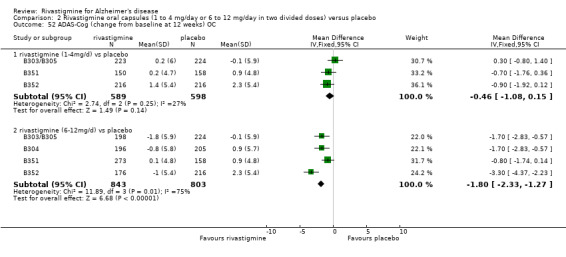
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 52 ADAS‐Cog (change from baseline at 12 weeks) OC.
2.53. Analysis.
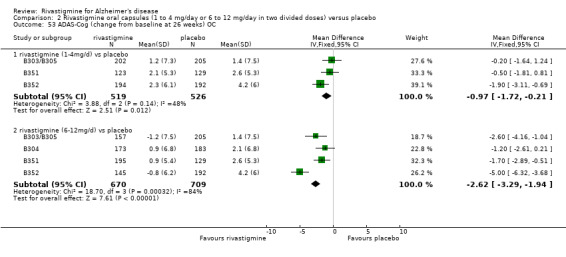
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 53 ADAS‐Cog (change from baseline at 26 weeks) OC.
2.54. Analysis.
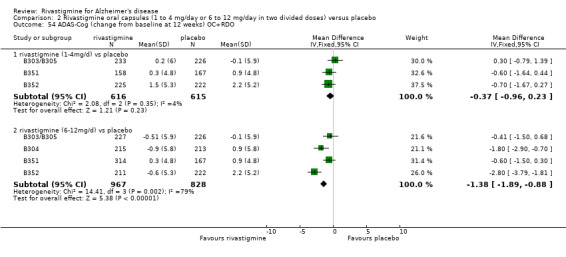
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 54 ADAS‐Cog (change from baseline at 12 weeks) OC+RDO.
2.55. Analysis.
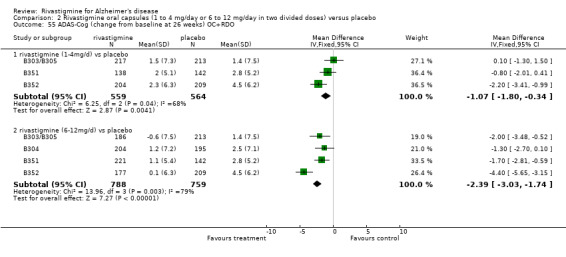
Comparison 2 Rivastigmine oral capsules (1 to 4 mg/day or 6 to 12 mg/day in two divided doses) versus placebo, Outcome 55 ADAS‐Cog (change from baseline at 26 weeks) OC+RDO.
Comparison 3. Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 24 weeks) ITT | 1 | 543 | Mean Difference (IV, Fixed, 95% CI) | ‐2.6 [‐3.72, ‐1.48] |
| 2 TMT‐A (change from baseline at 24 weeks) ITT | 1 | 496 | Mean Difference (IV, Fixed, 95% CI) | ‐14.2 [‐24.11, ‐4.29] |
| 3 clock drawing (change from baseline at 24 weeks) ITT | 1 | 520 | Mean Difference (IV, Fixed, 95% CI) | 0.2 [‐0.34, 0.74] |
| 4 MMSE (change from baseline at 24 weeks) ITT | 1 | 543 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.32, 1.48] |
| 5 ADCS‐ADL (change from baseline at 24 weeks) ITT | 1 | 544 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [0.52, 4.08] |
| 6 NPI‐12 (change from baseline at 24 weeks) ITT | 1 | 544 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.88, 1.68] |
| 7 withdrawals before end of treatment at 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.22, 2.97] |
| 8 at least one adverse event by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.64, 3.16] |
| 9 withdrawals due to an adverse event before end of treatment at 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.93, 3.46] |
| 10 at least one adverse event of dizziness by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.31, 7.50] |
| 11 at least one adverse event of nausea by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.12 [2.85, 9.22] |
| 12 at least one adverse event of vomiting by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.77 [3.38, 13.53] |
| 13 at least one adverse event of weight decrease by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.12 [2.09, 17.92] |
| 14 at least one adverse event of decreased appetite by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.19 [1.49, 18.12] |
| 15 at least one adverse event of headache by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.94, 7.56] |
| 16 at least one adverse event of asthenia by 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.82, 11.38] |
| 17 deaths before end of treatment at 24 weeks | 1 | 605 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.40, 7.06] |
| 18 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT | 1 | 544 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.07, 1.07] |
3.1. Analysis.
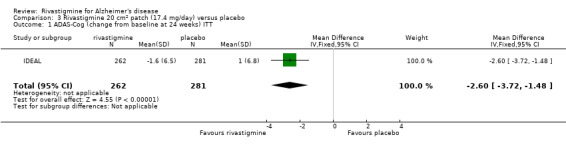
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 1 ADAS‐Cog (change from baseline at 24 weeks) ITT.
3.2. Analysis.
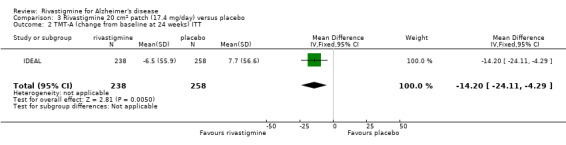
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 2 TMT‐A (change from baseline at 24 weeks) ITT.
3.3. Analysis.
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 3 clock drawing (change from baseline at 24 weeks) ITT.
3.4. Analysis.
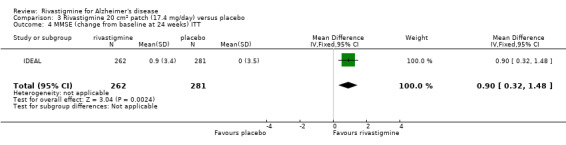
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 4 MMSE (change from baseline at 24 weeks) ITT.
3.5. Analysis.
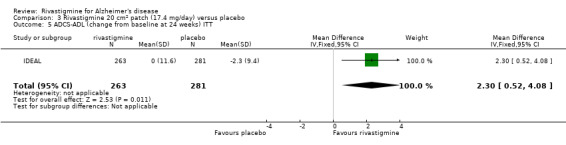
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 5 ADCS‐ADL (change from baseline at 24 weeks) ITT.
3.6. Analysis.
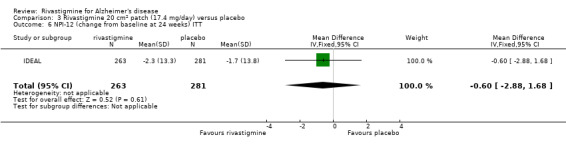
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 6 NPI‐12 (change from baseline at 24 weeks) ITT.
3.7. Analysis.
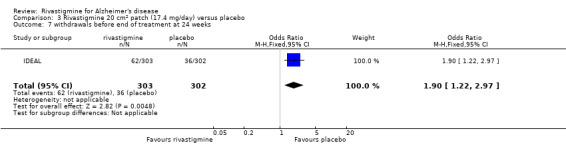
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 7 withdrawals before end of treatment at 24 weeks.
3.8. Analysis.
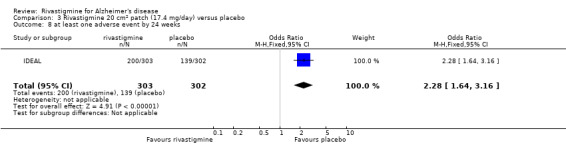
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 8 at least one adverse event by 24 weeks.
3.9. Analysis.
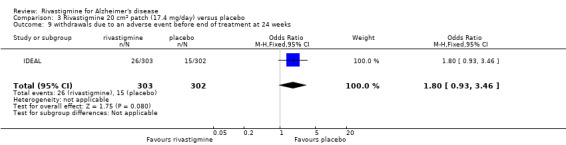
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 9 withdrawals due to an adverse event before end of treatment at 24 weeks.
3.10. Analysis.
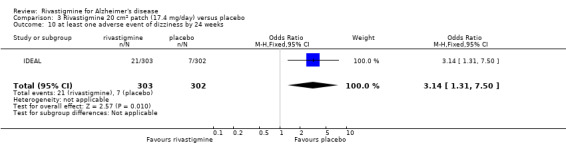
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 10 at least one adverse event of dizziness by 24 weeks.
3.11. Analysis.
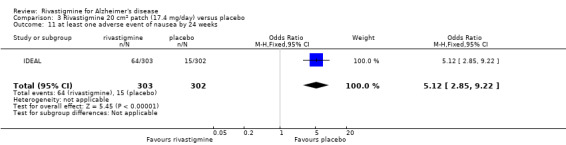
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 11 at least one adverse event of nausea by 24 weeks.
3.12. Analysis.
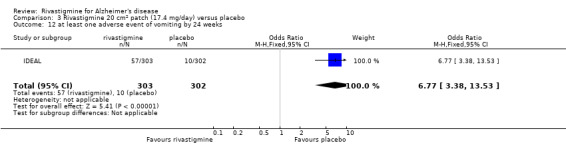
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 12 at least one adverse event of vomiting by 24 weeks.
3.13. Analysis.
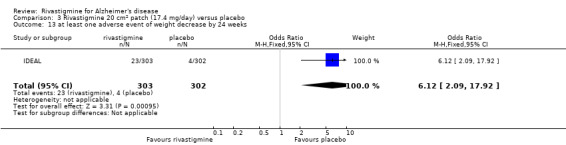
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 13 at least one adverse event of weight decrease by 24 weeks.
3.14. Analysis.
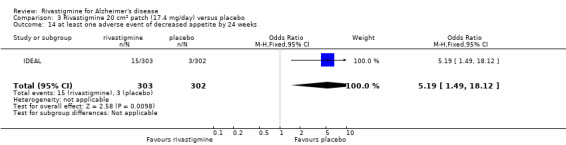
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 14 at least one adverse event of decreased appetite by 24 weeks.
3.15. Analysis.
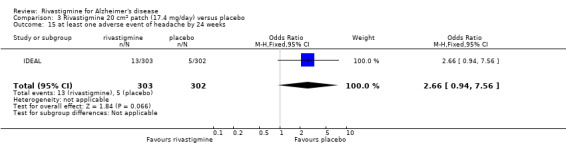
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 15 at least one adverse event of headache by 24 weeks.
3.16. Analysis.
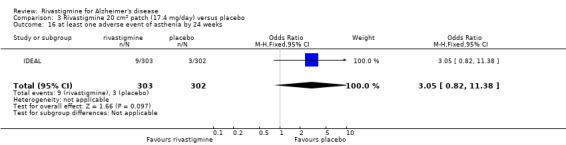
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 16 at least one adverse event of asthenia by 24 weeks.
3.17. Analysis.
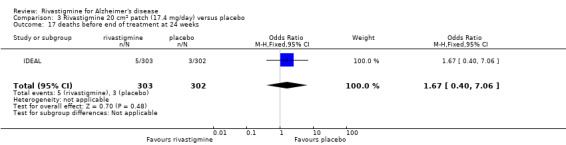
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 17 deaths before end of treatment at 24 weeks.
3.18. Analysis.
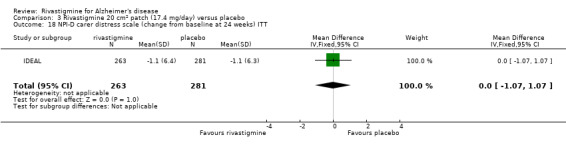
Comparison 3 Rivastigmine 20 cm2 patch (17.4 mg/day) versus placebo, Outcome 18 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT.
Comparison 4. Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 24 weeks) ITT | 2 | 1062 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐2.03, ‐0.66] |
| 2 MMSE (change from baseline at 24 weeks) ITT | 2 | 1028 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [0.26, 1.02] |
| 3 clock drawing (change from baseline at 24 weeks) ITT | 1 | 514 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐0.17, 0.97] |
| 4 TMT‐A (change from baseline at 24 weeks) ITT | 1 | 499 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐29.80, ‐10.20] |
| 5 Mental Function Impairment MENFIS (change from baseline at 24 weeks) ITT | 1 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.32, ‐0.28] |
| 6 ADCS‐ADL (change from baseline at 24 weeks) ITT | 1 | 528 | Mean Difference (IV, Fixed, 95% CI) | 2.20 [0.62, 3.78] |
| 7 Disability Assessment for Dementia (DAD) (change from baseline at 24 weeks) ITT | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [0.34, 4.26] |
| 8 BEHAVE‐AD (change from baseline at 24 weeks) ITT | 1 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.92, 0.52] |
| 9 NPI‐12 (change from baseline at 24 weeks) ITT | 1 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.16, 2.16] |
| 10 Clinical Global Impression (no change or worse at 24 weeks) | 2 | 1063 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.02] |
| 11 withdrawals before end of treatment at 24 weeks | 2 | 1170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.23, 2.26] |
| 12 at least one adverse event by 24 weeks | 2 | 1460 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.29, 2.06] |
| 13 withdrawals due to an adverse event before end of treatment at 24 weeks | 2 | 1170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.20, 2.82] |
| 14 at least one adverse event of application site erythema by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.87, 3.98] |
| 15 at least one adverse event of application site pruritis by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.36, 2.86] |
| 16 at least one adverse event of application site edema by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.83 [2.09, 11.15] |
| 17 at least one adverse event application site exfoliation by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.88, 8.93] |
| 18 at least one adverse event of dermatitis contact by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.24, 2.94] |
| 19 at least one adverse event of nasopharyngitis by 24 weeks | 1 | 573 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.73] |
| 20 at least one adverse event of nausea by 24 weeks | 2 | 1166 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.07, 3.02] |
| 21 at least one adverse event of vomiting by 24 weeks | 2 | 1166 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.20, 3.53] |
| 22 at least one adverse event of diarrhoea by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.87, 4.24] |
| 23 at least one adverse event of weight decrease by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.63, 7.07] |
| 24 at least one adverse event of dizziness by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.36, 3.00] |
| 25 at least one adverse event of decreased appetite by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.11, 4.16] |
| 26 at least one adverse event of headache by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.71, 6.26] |
| 27 at least one adverse event of asthenia by 24 weeks | 1 | 593 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.41, 7.36] |
| 28 deaths before end of treatment at 24 weeks | 2 | 1170 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.28, 3.81] |
| 29 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT | 1 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.91, 1.11] |
4.1. Analysis.
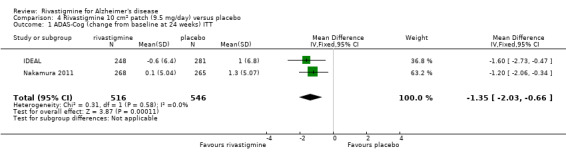
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 1 ADAS‐Cog (change from baseline at 24 weeks) ITT.
4.2. Analysis.
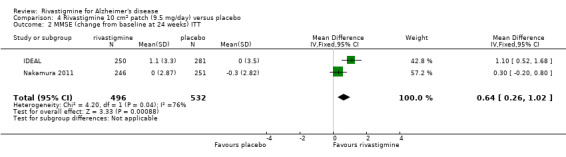
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 2 MMSE (change from baseline at 24 weeks) ITT.
4.3. Analysis.
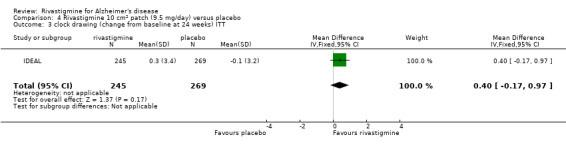
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 3 clock drawing (change from baseline at 24 weeks) ITT.
4.4. Analysis.
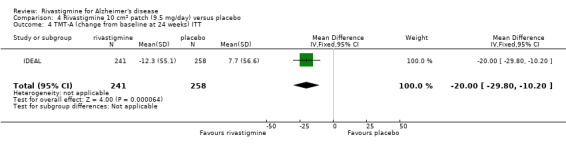
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 4 TMT‐A (change from baseline at 24 weeks) ITT.
4.5. Analysis.
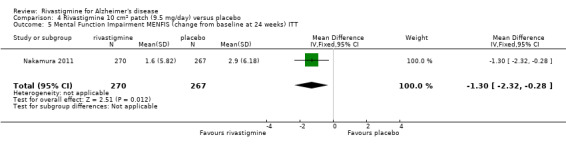
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 5 Mental Function Impairment MENFIS (change from baseline at 24 weeks) ITT.
4.6. Analysis.
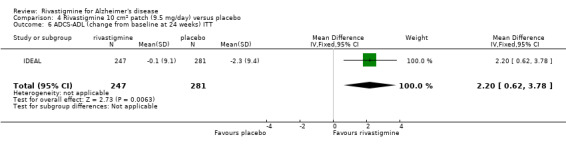
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 6 ADCS‐ADL (change from baseline at 24 weeks) ITT.
4.7. Analysis.
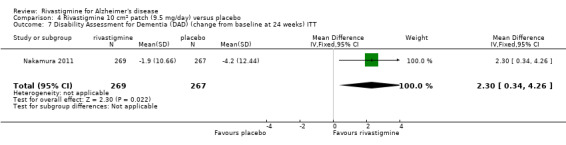
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 7 Disability Assessment for Dementia (DAD) (change from baseline at 24 weeks) ITT.
4.8. Analysis.
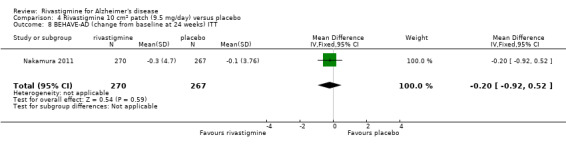
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 8 BEHAVE‐AD (change from baseline at 24 weeks) ITT.
4.9. Analysis.
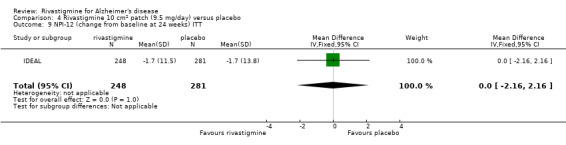
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 9 NPI‐12 (change from baseline at 24 weeks) ITT.
4.10. Analysis.
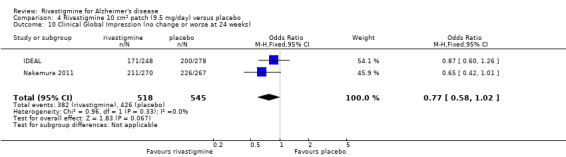
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 10 Clinical Global Impression (no change or worse at 24 weeks).
4.11. Analysis.
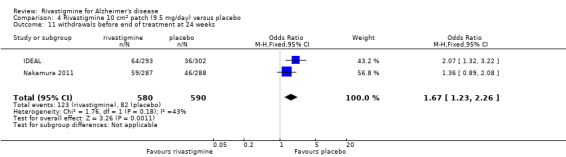
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 11 withdrawals before end of treatment at 24 weeks.
4.12. Analysis.
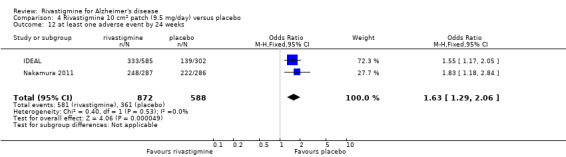
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 12 at least one adverse event by 24 weeks.
4.13. Analysis.
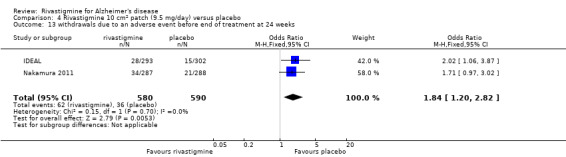
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 13 withdrawals due to an adverse event before end of treatment at 24 weeks.
4.14. Analysis.
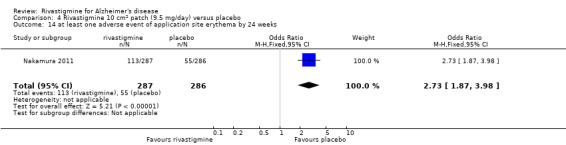
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 14 at least one adverse event of application site erythema by 24 weeks.
4.15. Analysis.
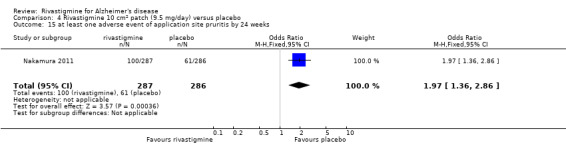
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 15 at least one adverse event of application site pruritis by 24 weeks.
4.16. Analysis.
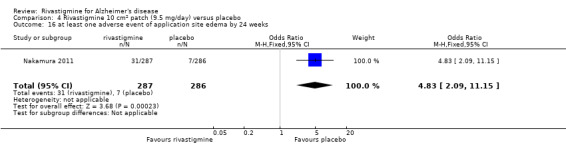
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 16 at least one adverse event of application site edema by 24 weeks.
4.17. Analysis.
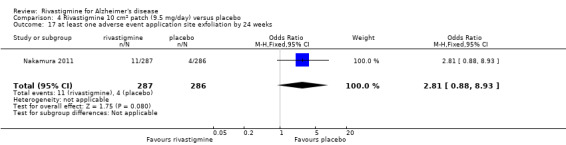
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 17 at least one adverse event application site exfoliation by 24 weeks.
4.18. Analysis.
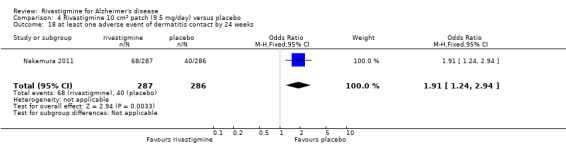
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 18 at least one adverse event of dermatitis contact by 24 weeks.
4.19. Analysis.
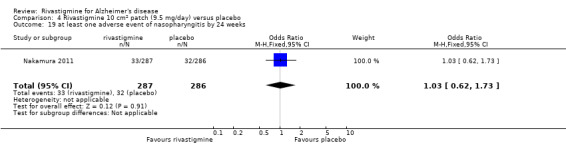
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 19 at least one adverse event of nasopharyngitis by 24 weeks.
4.20. Analysis.
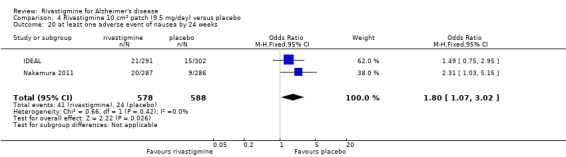
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 20 at least one adverse event of nausea by 24 weeks.
4.21. Analysis.
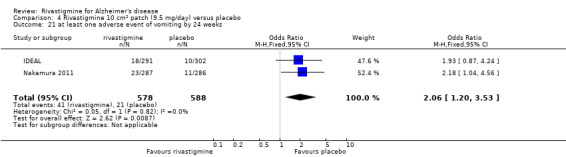
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 21 at least one adverse event of vomiting by 24 weeks.
4.22. Analysis.
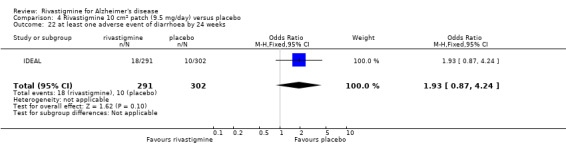
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 22 at least one adverse event of diarrhoea by 24 weeks.
4.23. Analysis.
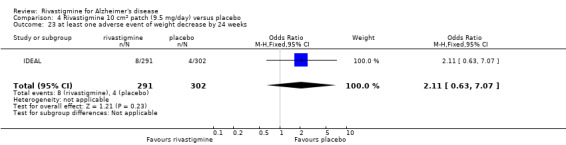
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 23 at least one adverse event of weight decrease by 24 weeks.
4.24. Analysis.
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 24 at least one adverse event of dizziness by 24 weeks.
4.25. Analysis.
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 25 at least one adverse event of decreased appetite by 24 weeks.
4.26. Analysis.
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 26 at least one adverse event of headache by 24 weeks.
4.27. Analysis.
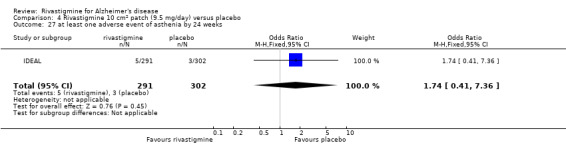
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 27 at least one adverse event of asthenia by 24 weeks.
4.28. Analysis.
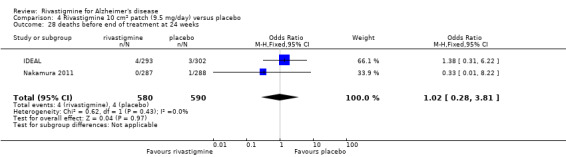
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 28 deaths before end of treatment at 24 weeks.
4.29. Analysis.
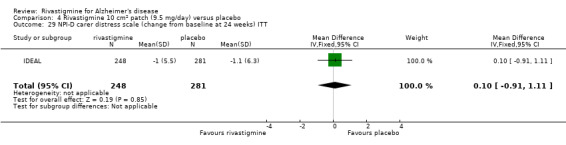
Comparison 4 Rivastigmine 10 cm2 patch (9.5 mg/day) versus placebo, Outcome 29 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT.
Comparison 5. Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐J Cog (change from baseline at 24 weeks) ITT | 1 | 531 | Mean Difference (IV, Fixed, 95% CI) | ‐0.8 [‐1.62, 0.02] |
| 2 MMSE (change from baseline at 24 weeks) ITT | 1 | 487 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.52, 0.52] |
| 3 Mental Function Impairment MENFIS (change from baseline at 24 weeks) ITT | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.72, 0.32] |
| 4 Disability Assessment for Dementia (DAD) (change from baseline at 24 weeks) ITT | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐0.73, 3.13] |
| 5 CIBIC‐Plus J (no change or worse at 24 weeks) ITT | 1 | 536 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.05] |
| 6 BEHAVE‐AD (change from baseline at 24 weeks) ITT | 1 | 536 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.67, 0.67] |
| 7 withdrawals before end of treatment at 24 weeks | 1 | 572 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.01, 2.33] |
| 8 at least one adverse event by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.16, 2.78] |
| 9 withdrawals due to an adverse event before end of treatment at 24 weeks | 1 | 572 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.12, 3.44] |
| 10 at least one adverse event of application site erythema by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.73, 3.70] |
| 11 at least one adverse event of application site pruritis by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.23, 2.60] |
| 12 at least one adverse event of application site edema by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.65 [2.46, 12.94] |
| 13 at least one adverse event application site exfoliation by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.68 [1.20, 11.33] |
| 14 at least one adverse event of dermatitis contact by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.30, 3.06] |
| 15 at least one adverse event of nasopharyngitis by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.19] |
| 16 at least one adverse event of nausea by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.09, 1.24] |
| 17 at least one adverse event of vomiting by 24 weeks | 1 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.43, 2.38] |
| 18 deaths before end of treatment at 24 weeks | 1 | 572 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.29] |
5.1. Analysis.
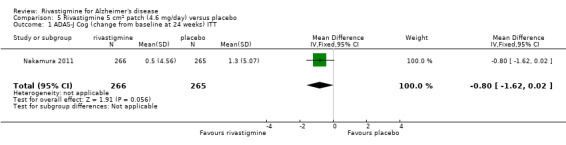
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 1 ADAS‐J Cog (change from baseline at 24 weeks) ITT.
5.2. Analysis.
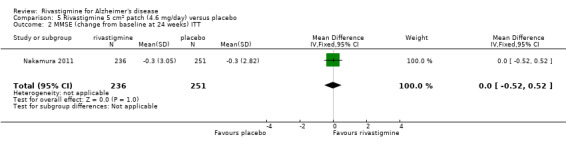
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 2 MMSE (change from baseline at 24 weeks) ITT.
5.3. Analysis.
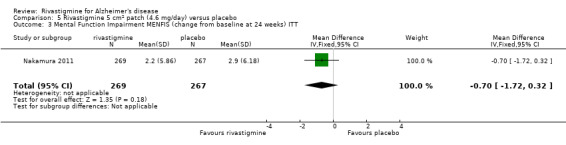
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 3 Mental Function Impairment MENFIS (change from baseline at 24 weeks) ITT.
5.4. Analysis.
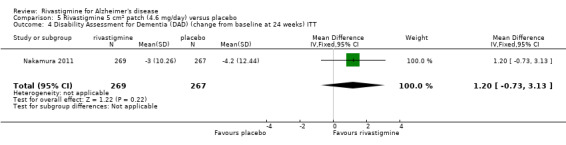
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 4 Disability Assessment for Dementia (DAD) (change from baseline at 24 weeks) ITT.
5.5. Analysis.
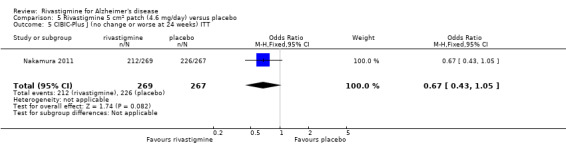
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 5 CIBIC‐Plus J (no change or worse at 24 weeks) ITT.
5.6. Analysis.
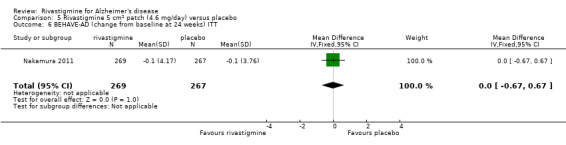
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 6 BEHAVE‐AD (change from baseline at 24 weeks) ITT.
5.7. Analysis.
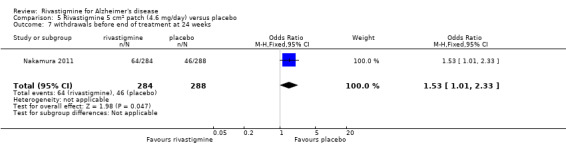
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 7 withdrawals before end of treatment at 24 weeks.
5.8. Analysis.
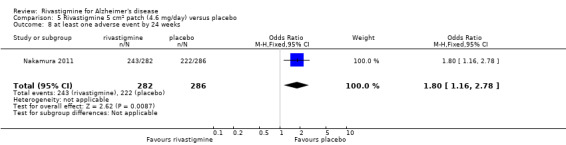
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 8 at least one adverse event by 24 weeks.
5.9. Analysis.
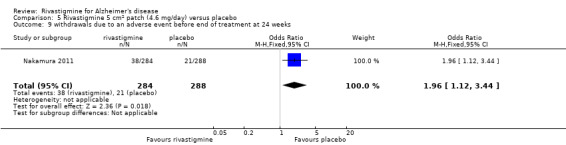
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 9 withdrawals due to an adverse event before end of treatment at 24 weeks.
5.10. Analysis.
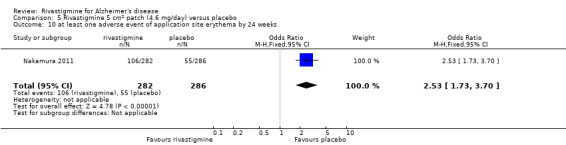
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 10 at least one adverse event of application site erythema by 24 weeks.
5.11. Analysis.
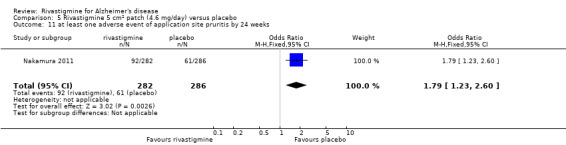
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 11 at least one adverse event of application site pruritis by 24 weeks.
5.12. Analysis.
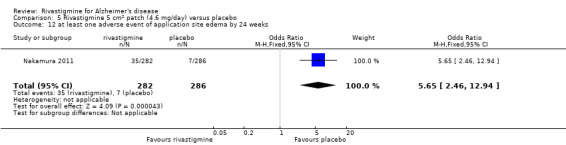
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 12 at least one adverse event of application site edema by 24 weeks.
5.13. Analysis.
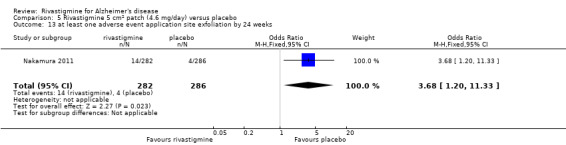
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 13 at least one adverse event application site exfoliation by 24 weeks.
5.14. Analysis.
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 14 at least one adverse event of dermatitis contact by 24 weeks.
5.15. Analysis.
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 15 at least one adverse event of nasopharyngitis by 24 weeks.
5.16. Analysis.
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 16 at least one adverse event of nausea by 24 weeks.
5.17. Analysis.
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 17 at least one adverse event of vomiting by 24 weeks.
5.18. Analysis.
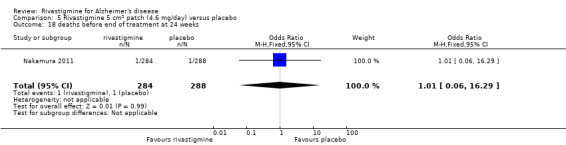
Comparison 5 Rivastigmine 5 cm2 patch (4.6 mg/day) versus placebo, Outcome 18 deaths before end of treatment at 24 weeks.
Comparison 6. Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADAS‐Cog (change from baseline at 24 weeks) ITT | 1 | 501 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.10, 1.10] |
| 2 MMSE (change from baseline at 24 weeks) ITT | 1 | 506 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.27, 0.87] |
| 3 clock drawing (change from baseline at 24 weeks) ITT | 1 | 491 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.46, 0.66] |
| 4 TMT‐A (change from baseline at 24 weeks) ITT | 1 | 481 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐13.48, 8.28] |
| 5 ADCS‐ADL (change from baseline at 24 weeks) ITT | 1 | 501 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐1.23, 2.03] |
| 6 Clinical Global Impression (no change or worse at 24 weeks) | 1 | 501 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.88, 1.84] |
| 7 NPI‐12 (change from baseline at 24 weeks) ITT | 1 | 501 | Mean Difference (IV, Fixed, 95% CI) | 0.50 [‐1.55, 2.55] |
| 8 withdrawals before end of treatment at 24 weeks | 1 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.54] |
| 9 at least one adverse event by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.82] |
| 10 withdrawals due to an adverse event before end of treatment at 24 weeks | 1 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.68, 2.13] |
| 11 at least one adverse event of nausea by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.15, 0.43] |
| 12 at least one adverse event of vomiting by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.57] |
| 13 at least one adverse event of diarrhoea by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.57, 2.29] |
| 14 at least one adverse event of weight decrease by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.21, 1.17] |
| 15 at least one adverse event of dizziness by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.13, 0.72] |
| 16 at least one adverse event of decreased appetite by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.73] |
| 17 at least one adverse event of headache by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.20] |
| 18 at least one adverse event of asthenia by 24 weeks | 1 | 585 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.10, 0.78] |
| 19 deaths before end of treatment at 24 weeks + 30 days | 1 | 590 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.49, 13.31] |
| 20 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT | 1 | 501 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.96, 1.16] |
6.1. Analysis.
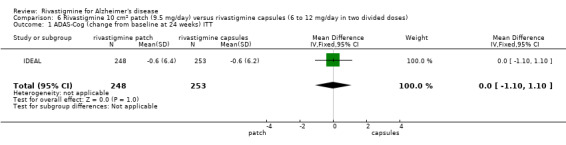
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 1 ADAS‐Cog (change from baseline at 24 weeks) ITT.
6.2. Analysis.
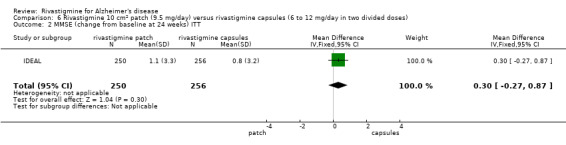
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 2 MMSE (change from baseline at 24 weeks) ITT.
6.3. Analysis.
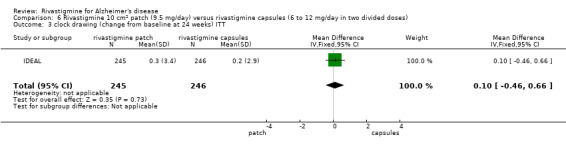
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 3 clock drawing (change from baseline at 24 weeks) ITT.
6.4. Analysis.
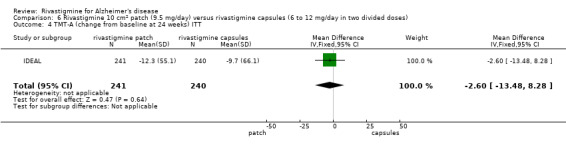
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 4 TMT‐A (change from baseline at 24 weeks) ITT.
6.5. Analysis.
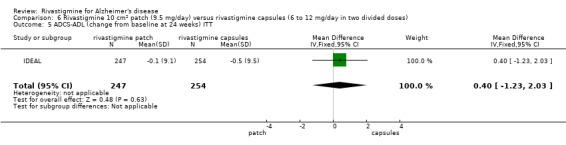
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 5 ADCS‐ADL (change from baseline at 24 weeks) ITT.
6.6. Analysis.
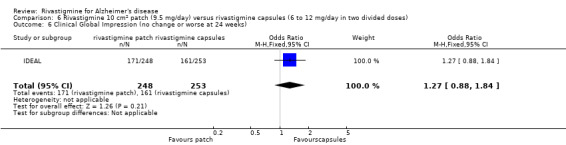
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 6 Clinical Global Impression (no change or worse at 24 weeks).
6.7. Analysis.
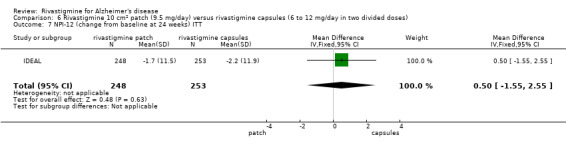
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 7 NPI‐12 (change from baseline at 24 weeks) ITT.
6.8. Analysis.
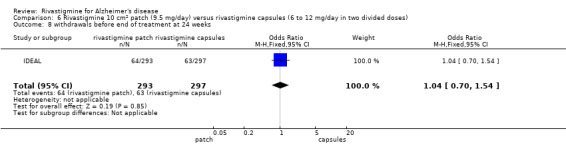
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 8 withdrawals before end of treatment at 24 weeks.
6.9. Analysis.
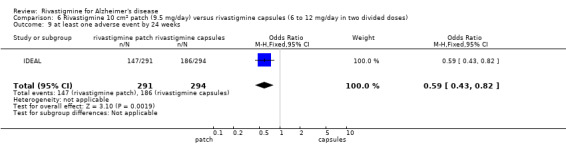
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 9 at least one adverse event by 24 weeks.
6.10. Analysis.
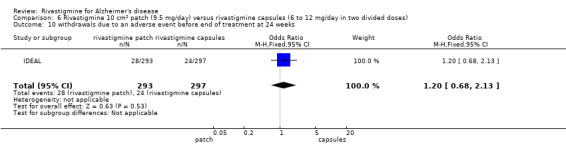
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 10 withdrawals due to an adverse event before end of treatment at 24 weeks.
6.11. Analysis.
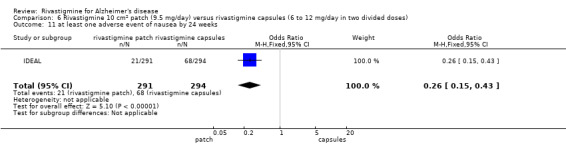
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 11 at least one adverse event of nausea by 24 weeks.
6.12. Analysis.
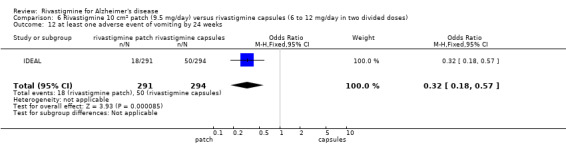
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 12 at least one adverse event of vomiting by 24 weeks.
6.13. Analysis.
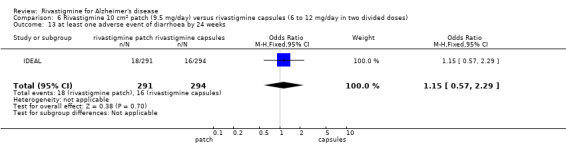
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 13 at least one adverse event of diarrhoea by 24 weeks.
6.14. Analysis.
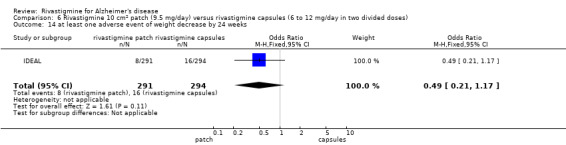
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 14 at least one adverse event of weight decrease by 24 weeks.
6.15. Analysis.
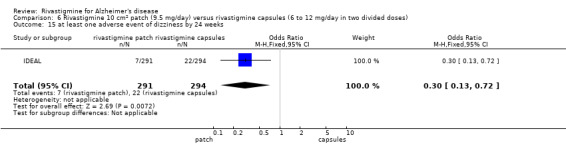
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 15 at least one adverse event of dizziness by 24 weeks.
6.16. Analysis.
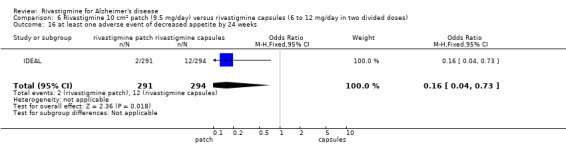
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 16 at least one adverse event of decreased appetite by 24 weeks.
6.17. Analysis.
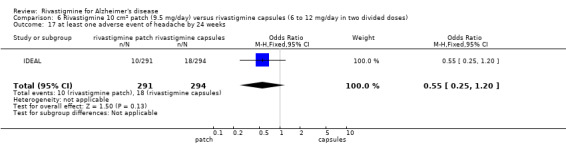
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 17 at least one adverse event of headache by 24 weeks.
6.18. Analysis.
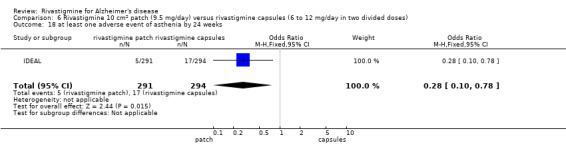
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 18 at least one adverse event of asthenia by 24 weeks.
6.19. Analysis.
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 19 deaths before end of treatment at 24 weeks + 30 days.
6.20. Analysis.
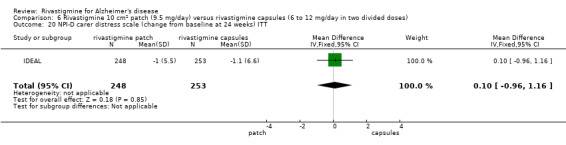
Comparison 6 Rivastigmine 10 cm2 patch (9.5 mg/day) versus rivastigmine capsules (6 to 12 mg/day in two divided doses), Outcome 20 NPI‐D carer distress scale (change from baseline at 24 weeks) ITT.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
B103.
| Methods | Double‐blinded, 3 arm, parallel‐group randomised controlled trial 13 week treatment followed by 2 weeks of washout with placebo with an optional double‐masked extension | |
| Participants |
Setting: Europe and UK; 54 centres, between March 1991 and March 1992
Sample size: 402 participants (226 female, 176 male), 133 in the 6 mg/day group, 136 in the 4 mg/day group and 133 in the placebo group
Age: range 50 to 90 years, mean age 69.4 years
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine: 4 mg/day divided into 2 doses (titrated to target dose in 1 week) 2. Rivastigmine: 6 mg/day divided into 2 doses (titrated to target dose in 3 weeks) 3. Placebo (identical) taken twice daily Doses maintained for 10 weeks after titration period to week 13. All patients then had a 2 week washout period with placebo (single‐blinded) | |
| Outcomes | Outcomes measured at baseline and at 13 weeks 1. Cognitive function
2. Activities of daily living
3. Behavioural symptoms
4. Physician rated global impression tests
5. Incidence of adverse events
6. Discontinuation
|
|
| Source of funding | Novartis Pharma Ltd | |
| Declaration of interest | Study sponsored by Novartis Pharma | |
| Notes | Primary hypothesis:to assess short term (3 month) symptomatic efficacy of rivastigmine 4 and 6 mg/d compared with placebo in patients with AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned a randomisation number by the investigator in chronological order according to a list generated by study sponsor (Novartis) |
| Allocation concealment (selection bias) | Low risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Active medication and placebo capsules had the same physical appearance, and the number of capsules for each dose intake was the same in all three groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 346/402 (86%) patients completed study. Analyses done with ITT population with imputations for missing values and an observed case population |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in protocol were reported |
B104.
| Methods | Double‐blinded, 3 arm parallel‐group randomised controlled trial 18 week treatment | |
| Participants |
Setting: Europe and Canada; 11 centres, between January 1993 and September 1993 Sample size: 114 participants Age: range years, mean age years Inclusion criteria:
Exclusion criteria: concomitant conditions or medications that may confound assessment of dementia; current diagnosis or history of significant medical, neurological or psychiatric disorder |
|
| Interventions | 1. Rivastigmine: 6 to 12 mg/day divided into 2 doses 2. Rivastigmine: 6 to 12 mg/day divided into 3 doses 3. Placebo (identical looking) twice daily Titration to a maximum dose of 12 mg/day or maximum tolerated dose during weeks 1 to 10, followed by 8 weeks maintenance of dose | |
| Outcomes | Measured at 18 weeks 1. Cognitive function
2. Activities of daily living
3.Global evaluation (physician rated)
4. Behavioural symptoms
5. Incidence of adverse events 6. Discontinuation
|
|
| Source of funding | Novartis Pharma Ltd | |
| Declaration of interest | Sponsored by Novartis Pharma Ltd | |
| Notes | Main hypothesis: to investigate tolerability of rivastigmine 10 week titration phase to a maximum of 12 mg daily or maximum tolerated dose, then 8 weeks maintenance phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described "patients were randomly assigned to one of three treatment groups" |
| Allocation concealment (selection bias) | Low risk | Not described Comment: likely to be low risk since this is a large multicentre trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Identifical placebo used, taken twice daily Comment: this effectively unblinded the group assigned to three times daily regimen |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Clinician who rated the CIBIC did not have access to baseline results and psychometric tests and also did not ask questions; however, it was unclear how effective this was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 85/114 (75%) completed the study. Numbers of participants who completed the study were different between groups: 92% for placebo, 89% for three times daily group and 78% for twice daily group. The efficacy analysis was conducted only in "valid" patients, defined as all those patients who had completed the study according to protocol |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in protocol reported |
B303/B305.
| Methods | Double‐blinded, placebo controlled 3 arm parallel‐group randomised controlled trial 26 weeks of treatment |
|
| Participants |
Setting: 45 centres, Europe and North America
Sample size: 725 participants (428 female, 297 male)
Age: 45 to 95 years, mean age 72 years
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine 1 to 4 mg/day divided into 2 doses 2. Rivastigmine 6 to 12 mg/day divided into 2 doses 3. Placebo Doses increased weekly in steps of 1.5 mg/day during weeks 1 to 12, but had to be within target range by week 7; thereafter, multiple single level dose increases or decreases were permitted provided patients remained within their assigned dose range (otherwise patients discontinued study, but were asked to return for scheduled efficacy evaluations) | |
| Outcomes | Assessments made and reported at baseline, 12, 18, 26 weeks 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
4. Adverse events |
|
| Source of funding | Novartis Pharma | |
| Declaration of interest | Sponsored by Novartis Pharma | |
| Notes | Main hypothesis: to assess the effects of rivastigmine on the core domains of AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated ... according to a computer generated randomisation code at Novartis Pharma" |
| Allocation concealment (selection bias) | Low risk | Not described Comment: likely to be low risk since this is a large multicentre trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo, and "the number taken were the same at each dose for all groups" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An 80% completion (581/725). Proportion of participant completions was unbalanced across groups: 203/239 (87%) in placebo, 209/243 (86%) in low dose rivastigmine (1 to 4 mg/day) and 164/243 (67.5%) in higher dose (6 to 2 mg/day) group |
| Selective reporting (reporting bias) | Unclear risk | One of the outcomes listed in protocol, CAS, was not reported |
B304.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 26 week treatment and follow up |
|
| Participants |
Setting: 37 centres, Australia, Canada, Italy, South Africa, UK and Ireland
Sample size: 678 participants out of 788 screened Age: mean age 71.4 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine 2 to 12 mg/day divided into 2 doses 2. Rivastigmine 2 to 12 mg/day divided into 3 doses 3. Placebo Started at 2 mg/day, and increased at weekly intervals in 1 mg/day steps until reaching the maximum tolerated dose. Titration lasted 10 days to 12 weeks. Patients who could not tolerate 2 mg/day by 10 days were withdrawn from study. Tolerability could be optimised by maintaining a dose level for periods of up to 2 weeks. During maintenance, dose variation allowed | |
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
4. Adverse events |
|
| Source of funding | Funded by Novartis | |
| Declaration of interest | Sponsored by Novartis Pharma | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of individual highest well tolerated doses (range 2 to 12 mg/d) of rivastigmine twice or three times daily for 26 weeks compared to placebo, in the therapy of patients with probable AD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not described, likely to be low risk; large multicentre trial |
| Allocation concealment (selection bias) | Low risk | Comment: not described, likely to be low risk, large multicentre trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Administration of each dose level was achieved by using a combination of active medication and placebo so that the appropriate daily dose was presented as 2 capsules t.i.d" Comment: used matching placebo, number of capsules taken were the same at each dose |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 82% completion, 83% in three times daily group, 76% in two times daily group, 85% in placebo group. Comment: data were imputed using 'retrieved dropout' assessment, or last observed carried forward (LOCF) if retrieved dropout was not available |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in protocol reported except for CAS (Caregiver Activity Survey) |
B351.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 26 week treatment and follow up |
|
| Participants |
Setting: USA, 14 centres between December 1994 to 22 March 1996
Sample size: 702 participants (393 female, 309 male)
Age: range 45 to 89 years, mean 74.5 years
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine: 3 mg/day divided into 2 doses
2. Rivastigmine: 6 mg/day divided into 2 doses
3. Rivastigmine: 9 mg/day divided into 2 doses
4. Placebo
Titration during weeks 1 to 12 to the fixed dose, fixed dose between week 13 and 26, no dose reductions allowed Patients who discontinued prematurely were asked to return at weeks 12, 18, and 26 for efficacy evaluation |
|
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
4. Adverse events |
|
| Source of funding | Novartis Pharma | |
| Declaration of interest | Novartis Pharma | |
| Notes | Main hypothesis: to evaluate the efficacy and safety of 3 fixed doses of rivastigmine (3, 6, 9 mg/day) and placebo for 26 weeks of treatment, and dose‐efficacy and dose‐safety relationships in patients with probable mild to moderate AD Assessments: baseline, 12,18, 26 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described, likely low risk |
| Allocation concealment (selection bias) | Low risk | Not described, likely low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A 66% completion rate overall. Higher percentage in the 9 mg group (49%) and 6 mg (37%) group discontinued compared to the placebo group (25%) |
| Selective reporting (reporting bias) | Unclear risk | Study data were not published, obtained from the company. Only data pooled with other studies were published by the pharmaceutical company (pooled with B303 and B352) |
| Other bias | High risk | |
B352.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 26 week treatment and follow up |
|
| Participants |
Setting: USA, 22 centres
Sample size: 699 participants (426 female, 273 male)
Age: range 45 to 89 years, mean 74.5 years
Inclusion criteria:
Exclusion:
|
|
| Interventions | 1. Rivastigmine 1 to 4 mg/day divided into 2 doses 2. Rivastigmine 6 to 12 mg/day divided into 2 doses 3. Placebo Titration phase (fixed dose) weeks 0 to 7, flexible phase weeks 8 to 26, dose twice daily with food | |
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
Assessments: baseline, 12, 18, 26 weeks |
|
| Source of funding | Novartis Pharma | |
| Declaration of interest | Investigator and author received payment or employed by Novartis | |
| Notes | The study had an open label extension after 16 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation procedures managed by Statistical Programming Group at Corning‐Besselaar, independent of sponsor and investigating centres |
| Allocation concealment (selection bias) | Low risk | "At each study site, the research coordinator accessed an interactive voice response system that assigned the next available patient randomisation number, thus maintaining the blind in assigning medication to patients throughout the study and serving as a tracking system for all randomised patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Throughout the study all patients received two capsules twice daily Comment: identifical placebo appearance, dosing schedule and good allocation concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Rate of completion differed between arms, 197/235 (84%) in placebo, 199/233 (85%) in low dose arm, and 149/231 (65%) in the high dose arm |
| Selective reporting (reporting bias) | Unclear risk | CAS scale listed in protocol but not reported in study |
Ballard 2005.
| Methods | Double‐blinded, placebo controlled 3 arm, randomised controlled trial 26 weeks |
|
| Participants |
Setting: UK, participants lived in care facilities
Sample size: 93 (74 female, 19 male), 31 in each group Age: mean 83.8 (SD 7.7) years Inclusion criteria:
Exclusion:
|
|
| Interventions | 1. Quetiapine (50 to 100 mg/day in two doses)
2. Rivastigmine (6 to 12 mg/day in two doses)
3. Placebo Investigators aimed to reach the target dose at week 12 |
|
| Outcomes | 1. Cogntive function
2. Behavioral symptoms
Patients were evaluated at 6, 12 and 26 weeks |
|
| Source of funding | Alzheimer's Research Trust, general donation to the main investigator's (Clive Ballard) research programme and profits from previously completed commercial trials | |
| Declaration of interest | Main investigator has received honoraria and research donations to support this research programme from Novartis and Astra Zeneca (manufacturers) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated with block randomisation (block sizes of three and six)" done by study statistician using Stata |
| Allocation concealment (selection bias) | Low risk | "The randomisation clinician faxed a form to the statistician, who communicated allocation to the pharmacy, ensuring concealment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy design used, placebo for both types of drugs used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Method not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of participants not completing treatment. Outcomes were only available for about half of all patient randomised for the SIB (cognitive function) and CMAI (behavioural disturbance) at 26 weeks |
| Selective reporting (reporting bias) | Unclear risk | Unclear why report emphasised 6 week results (still within titration period), rather than 26 week data Comment ‐ insufficient information to determine |
| Other bias | Unclear risk | Unclear. Some patients were excluded from trial |
IDEAL.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 24 weeks |
|
| Participants |
Setting: North, South and Central America, Asia and Europe, 21 countries, 100 centres
Sample size: 1195 participants (796 female, 399 male) out of 1464 screened
Age: range 50 to 85 years, mean 73.3 (SD 7.8) years MMSE mean 16.5 (SD 3.0) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine patch 10 cm2 (9.5 mg/24h)
2. Rivastigmine patch 20 cm2 (17.4 mg/24h) 3. Rivastigmine capsules 6 to 12mg/day divided into 2 doses 4. Placebo Titration phase weeks 0 to 7, flexible phase weeks 8 to 26, dose twice daily with food |
|
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
4. Behavioural disturbances
|
|
| Source of funding | "Study supported by Novartis Pharma AG, Basel, Switzerland. Data were collected by investigators and co‐investigators, entered into a central database using electronic data capture software, and analysed by Novartis Pharma AG, which vouches for the data and the analysis" | |
| Declaration of interest | Some of the investigators were employees of Novartis Pharma | |
| Notes | There was a 4‐week screening period prior to randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " Patients were sequentially assigned the lowest available identification number at each centre, Automated random assignment of treatment using interactive voice‐response system." |
| Allocation concealment (selection bias) | Low risk | automated random assignment of treatment using interactive voice‐response system.Independent rater at 16 and 24 weeks who had no access to other data. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | double dummy used, patients received placebo capsule and/or patch |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described ‐ insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | About 79% completion in active treatment arms, and 88% in placebo arm at 26 weeks. Main ITT analysis used LOCF imputation. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine risk |
| Other bias | Unclear risk | None identified. |
Karaman 2005.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 52 weeks | |
| Participants |
Setting: Turkey
Sample size: 44 participants (24 female, 20 male), mean MMSE 12.2
Age: mean age 73.8 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine 12 mg/day divided into 2 doses 2. Placebo Titration phase weeks 0 to 2, 1.5 mg twice daily Week 3 to 4, 3.0 twice daily; week 5 to 6, 4.5 mg twice daily; week 7 to 8, 6 mg twice daily | |
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
|
|
| Source of funding | Not stated | |
| Declaration of interest | Not stated | |
| Notes | Baseline study characteristics reported were those of randomised patients who had received trial medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to receive treatment with rivastigmine or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "identical tablets were given" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Rivastigmine and placebo were administered as identical tablets taken twice daily. In the rivastigmine group, patients received rivastigmine twice daily with food" There was no indication in paper how the investigators or outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from 21/24 patients in the rivastigmine group (and all the placebo patients) were available at 52 weeks. There was no information about loss to follow up, but the following was stated in the methods section: "At the conclusion of the 8‐week study visit, participants who tolerated the drug well and perceived benefit were invited to continue rivastigmine treatment." It is unclear how many patients were excluded because they did not 'benefit' or 'tolerate' the drug well |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to determine |
| Other bias | High risk | As this study only extended the continuation to those who had perceived to benefit at the 8 week visit, this potentially introduced bias and broke the randomisation. It was not reported how many patients who were randomised initially continued to the 52 week study |
Lopez‐Pousa 2005.
| Methods | Double‐blinded, placebo controlled, parallel‐group randomised controlled trial 26 weeks |
|
| Participants |
Setting: Spain, 21 centres
Sample size: 218 participants, 77% female, mean MMSE 8.8 Age: mean age 77.6 years Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine 12 mg/day divided into 2 doses 2. Placebo Titration phase weeks 0 to 4, 1.5 mg twice daily Weeks 5 to 8, 3.0 mg twice daily; weeks 9 to 12, 4.5 mg twice daily; weeks 13 to 16, 6 mg twice daily | |
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Behavioural symptoms
4. Physician rated global impression tests
Other scales: Blessed Dementia Scale (Blessed 1968), a multidimensional performance scale |
|
| Source of funding | Not stated in study publication | |
| Declaration of interest | Likely to be linked to pharmaceutical company, 2 of the 4 authors were employees of Novartis, randomisation scheme generated by a contract research organisation but was "reviewed" by Novartis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation schedule", in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | "Eligible patients, identified at an initial screening visit were allocated randomisation number at second visit" Unclear whether this allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Rivastigmine and placebo hard capsules were identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Medical monitors, centre personnel, patients and caregivers were blinded to treatment". Randomisation data were not available until after the study had been completed and the database locked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An 83.5% completion in treatment arm, 88.1% completion in placebo ITT population was 104/109 in the treatment arm, 106/109 in the placebo arm Comment: early withdrawals were followed up. Documented, small percentage of missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
Mowla 2007.
| Methods | Double‐blinded, 3 arm, placebo controlled, parallel‐group randomised controlled trial 12 week study; 6 week single‐blind placebo run‐in period was included to exclude responders |
|
| Participants |
Setting: Iran Sample size: 122 patients, 41 in rivastigmine group, 41 in placebo group Age: mean age 69.2 years, 53.5% female Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | 1. Rivastigmine 6 to 12 mg/day, twice daily 2. Rivastigmine 6 to 12 mg/day twice daily + fluoxetine 20 mg/day 2. Placebo No information was given as to whether there was any titration |
|
| Outcomes | 1. Cognitive function
2. Activities of daily living (ADL)
3. Physician rated global impression tests
|
|
| Source of funding | Shiraz University of Medical Science Grant 83‐421 | |
| Declaration of interest | None stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Same number of drugs were given to the patients of the 3 groups", “ there were no significant difference between the groups with respect to taking other medications” Comment: mentioned use of placebo, but unclear if these were identical to active treatments ‐ all participants had received placebo during a 6 week run‐in period |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Same number of drugs were given to the patients of the 3 groups" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate was 7/41 (17%) in the rivastigmine group, and 8/40(20%) in the placebo group. Stated that “major cause of withdrawal was adverse events compared with loss of efficacy as the most common cause for group C”. Actual causes of loss to follow up not reported, relatively high numbers of loss to follow up for a short study of 12 weeks |
| Other bias | High risk | "a single blind placebo 6 week run in period was included to exclude placebo responders" |
Nakamura 2011.
| Methods | Multicentre, randomised, double‐blind, placebo controlled, 3 arm, parallel‐group trial of 24 weeks A dose finding study (NCT00423085) |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Participants were titrated to their target dose at 4 week intervals over 16 weeks, followed by an 8 week maintenance dose at weeks 17 to 24 |
|
| Outcomes | 1. Cognitive function
2. Activities of daily living
3. Physician rated global impression tests
4. Behavioural symptoms
Results were reported as intention‐to‐treat last observation carried forward at 24 weeks. Assessments were done during weeks 8, 16 and 24 |
|
| Source of funding | A total of 4/9 authors had no interest to declare; the rest were employees of either Novartis or ONO Alpha‐Plus Medical Communications Ltd (UK) provided medical writing and editorial support in the production of this manuscript; this service was sponsored by Novartis and ONO |
|
| Declaration of interest | Sponsored by Novartis and ONO Pharmaceutical Ltd (they jointly developed and marketed the transdermal patch in Japan) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patient registration centre provided a randomisation number to the eligible participants and randomisation lists were generated by a Drug ALlocation Controller". A dynamic allocation was utilised, using body weight (< 45, 45 to < 55 and ≥ 55 kg) and MMSE score (≤ 15, > 15 points) as factors |
| Allocation concealment (selection bias) | Low risk | Likely to be low risk. Investigators had to offer enrolment to all eligible patients. Allocation number provided after eligibility criteria confirmed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Randomisation data kept strictly confidential by the Study Drug Allocation Controller until the time of unblinding" (only permitted during emergencies and at conclusion of study) However, 3 different patch sizes were used, 2.5 cm2, 5 cm2, 7.5 cm2 and 10 cm2 Since the higher dose group (10 cm2) used bigger patches than the maximum for the 5 cm2 group, unclear how blinding was maintained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "patients, investigator staff, persons performing the assessments and data analysts were all blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | An 80.3% completion rate of study; 88% in placebo, about 80% in active treatment available as ITT‐observed cases at week 24 |
| Selective reporting (reporting bias) | Low risk | Outcomes listed in protocol were reported |
| Other bias | Low risk | None identified |
Tai 2000.
| Methods | Double‐blinded randomised controlled trial 26 weeks follow up |
|
| Participants |
Setting: Taiwan
Sample size: 80 Inclusion criteria:
|
|
| Interventions | 1. Rivastigmine: 3 mg/day divided into 2 doses, escalating by 3 mg/day not faster than every two weeks until a dose that was not tolerated was reached 2. Placebo | |
| Outcomes | 1. Cognitive Function
2.Activities of daily living ‐ none stated 3. Physician rated global impression tests
5. Frequency of adverse events 6. Withdrawal due to adverse events
|
|
| Source of funding | Not stated | |
| Declaration of interest | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. Only abstract available |
| Allocation concealment (selection bias) | Unclear risk | No information. Only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. Only abstract available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Only abstract available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to judge. Only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to judge. Only abstract available |
| Other bias | Unclear risk | Insufficient information to judge. Only abstract available |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTION | This trial compared two sizes of rivastigmine patches, but there was no placebo group |
| Almkvist 2004 | This trial studied particular aspects of memory, but used historical controls as the untreated group |
| Auriacombe 2002 | Open label, 6 month study of rivastigmine for patients who had failed to benefit from donepezil. Later extended to 12 months |
| B105 | THis was a randomised, placebo controlled trial, but the duration was only 9 weeks |
| Bilikiewicz 2002 | Open label study of rivastigmine in community setting |
| Blesa Gonzalez 2011 | Trial investigating adverse events on changing to patches from tablets. There were 2 groups using patches, and one group continuing on tablets, but no placebo group. The trial was open label, and data were not reported for all groups, limiting usable comparisons |
| Brassen 2003 | An unblinded study. Open controlled design of rivastigmine compared with donepezil, 35 AD patients |
| Caffarra 2007 | There was no placebo group. Comparison of rivastigmine with donepezil, retrospective study |
| Cummings 2000 | Open label study of nursing home patients |
| Cutler 1998 | Open label non‐randomised study |
| Cutler 2000 | Open label study investigating the pharmacokinetics of oral and intravenous rivastigmine |
| Dantoine 2006 | Comparison of rivastigmine with rivastigmine plus memantine in AD patients previously failing to respond on donepezil or galantamine, open label study |
| Doraiswamy 2000a | Open label extension to either B303 or B352 |
| Edwards 2002 | Open label study of nursing home patients, all on rivastigmine. Outcome was assessment of use of psychotropics |
| EXCEED | Donepezil compared with rivastigmine. A 24 month randomised controlled trial with no placebo group |
| Frankfort 2007 | Study of effect of rivastigmine on specific cognitive domains. This is not an randomised controlled trial, treatment group compared with historical controls |
| Fuschillo 2001 | Randomised study of donepezil compared with rivastigmine for AD. No placebo group |
| Holmes 2007 | Randomised study of rivastigmine compared with risperidone, no placebo group |
| InDDEx | Randomised placebo controlled study of rivastigmine for patients with mild cognitive impairment but not dementia |
| Kim 2002 | A 24 week open label study, all on rivastigmine |
| Malsch 2001 | Open label 8 week study, patients randomised to two different titration schemes |
| McMillan 1999 | Open label study of early non‐responders |
| Novartis 2005 | Open label extension study |
| OPTIMA | This trial compared two sizes of rivastigmine patches, but there was no placebo group |
| Potkin 1999a | Investigation of brain metabolism using positron emission tomography (PET) scans from 27 patients chosen non‐randomly from study B351 |
| Riepe 2005 | This is not a randomised controlled trial; 12 week open label study of rivastigmine + memantine |
| Rozzini 2002 | Randomised trial comparing rivastigmine with donepezil. No placebo group |
| Schmidt 2002 | Open label study on the use of rivastigmine in routine clinical practice |
| Shanks 2001 | Open label study, all on rivastigmine, assessing cerebral flow only |
| Shua‐Haim 2002a | A 5 month study, comparing donepezil with rivastigmine with galantamine. No placebo group |
| Shua‐Haim 2002b | Open label treatment of agitation in patients with AD |
| Shua‐Haim 2002c | Donepezil, compared with rivastigmine, compared with galantamine |
| Small 2005 | A pooled study of two open label extension studies of rivastigmine |
| Sobow 2002 | Retrospective review of patients who had been prescribed rivastigmine or donepezil |
| Stefanova 2002 | Rivastigmine compared with tacrine in matched groups |
| Thomas 2001 | Open label trial |
| Tsolaki 2002 | Retrospective study, comparing donepezil with rivastigmine |
| Venneri 2002 | Non‐randomised study of 4 patients. Rates of progression of disease in those treated with rivastigmine compared with untreated patients |
| Wang 2001 | Open label, randomised study, comparing rivastigmine with donepezil |
| Wang 2003 | Open label study of rivastigmine |
| Weiser 2002 | Open label pilot study. Patients randomised to rivastigmine and risperidone, alone or in combination |
| Werber 2002 | Non‐randomised study of donepezil compared with tacrine, with rivastigmine. Outcome is cognition related brain evoked potential |
| Wilkinson 2002 | Randomised, 12 week, open label study comparing donepezil with rivastigmine. No placebo group |
Differences between protocol and review
In the 2014 update of the review, the results were reorganised to focus on currently recommended doses. The main analysis was done for the 26 week period and seven outcomes were prioritised for meta‐analysis. The other analyses done in earlier versions are retained in the appendix.
The risk of bias assessment of individual studies was also expanded for this update, with additional assessments on blinding, selective reporting and other biases carried out.
Contributions of authors
This updated review was prepared by J Birks and J Grimley Evans. V Iakovidou and M Tsolaki made contributions to the original review.
Contact editor: Frans Verhey.
Consumer editor: Mervyn Richardson.
The review has been peer reviewed anonymously.
The 2014 update was undertaken by J Birks.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR, UK.
This review update was supported by the National Institute for Health Research, via a Cochrane Programme Grant to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
None known
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
B103 {published data only}
- Agid Y, Dubois B on behalf of the International Rivastigmine Investigators, Anand R, Gharabawi G. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Current Therapeutic Research 1998;59(12):837‐45. [Google Scholar]
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's Disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16. [Google Scholar]
B104 {published data only}
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's Disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16. [Google Scholar]
- Forette F, Anand R, Gharabawi G. A phase II study in patients with Alzheimer's disease to assess the preliminary efficacy and maximum tolerated dose of rivastigmine (Exelon®). European Journal of Neurology 1999;6:423‐9. [DOI] [PubMed] [Google Scholar]
B303/B305 {published and unpublished data}
- Anand R, Hartman R, Graham S. Effects of Alzheimer's disease severity on activities of daily living with long‐term rivastigmine treatment. Journal of the American Geriatrics Society 2001;49(4):S151. [Google Scholar]
- Anand R, Messina J, Veach J, Hartman R. Effects of rivastigmine in patients with moderately severe Alzheimer's disease. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:199.
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Potential long‐term effects of rivastigmine on disease progression may be linked to drug effects on vascular changes in Alzheimer brains. International Journal of Clinical Practice 2003;57(9):756‐60. [PubMed] [Google Scholar]
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer's disease and concurrent hypertension. International Journal of Clinical Practice 2002;56(10):791‐6. [PubMed] [Google Scholar]
- Farlow M, Anand R, Messina J, Hartman R. Increased cognitive efficacy of rivastigmine in patients with moderate to severe Alzheimer's disease with co‐existing vascular risk. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:206.
- Farlow M, Messina J, Anand R, Hartman R, Veach J. Dose dependent effect of rivastigmine on progression of cognitive deterioration in Alzheimer's disease. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000. 2000:172.
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003;60:843‐8. [DOI] [PubMed] [Google Scholar]
- Hebert M. [Alzheimer disease: efficacy and tolerance of rivastigmine] [Maladie d'Alzheimer: efficacite et tolerance de la rivastigmine]. Presse Medicale 1999;28(32):1757‐8. [PubMed] [Google Scholar]
- Kumar V, Messina J, Hartman R, Anand R. Long‐term cognitive benefits of rivastigmine in Alzheimer's disease patients with vascular risk. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden. 2000:215.
- Lindesay J. An open label one‐year extension of SDZ‐ENA‐713 studies B303, B304 and B305 to prospectively evaluate long‐term safety, tolerability and efficacy of SDZ‐ENA‐713 in out‐patients with probable Alzheimer's disease. National Research Register 2000.
- Roesler M, Retz W, Retz Junginger P, Dennler HJ. Effect of two‐year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer's disease. Behavioural‐Neurology 1998;11(4):211‐6. [DOI] [PubMed] [Google Scholar]
- Rösler M. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: International randomised controlled trial [Erratum]. BMJ 2001; Vol. 322, issue 7300:1456. [DOI] [PMC free article] [PubMed]
- Rösler M, Anand R, Cicin‐Sain A, Gauthier S, Agid Y, Dal‐Bianco P, et al on behalf of the B303 Exelon Study Group. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 1999;318(7184):633‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler M, Dennler H, Retz W, Gastpar W. A double‐blind placebo controlled study of ENA 713 in Alzheimer's disease (DAT). Pharmacopsychiatry 1997;30:212. [MEDLINE: ] [Google Scholar]
- Rösler M, Retz W, Retz Junginger P, Dennler HJ. Effects of two‐year treatment with the cholinesterase inhibitor rivastigmine on behavioural symptoms in Alzheimer's disease. Behavioural‐Neurology 1998;11(4):211‐6. [DOI] [PubMed] [Google Scholar]
- Vincent S, Andrews C, Lane R. Rivastigmine shows particular efficacy in Alzheimer patients with concomitant hypertension. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer therapy, 2002 Apr 3‐6, Geneva. 2002:253.
- Wilkinson DG. Rivastigmine was effective and safe in Alzheimer disease. ACP Journal Club 1999;131(2):34. [MEDLINE: ] [Google Scholar]
B304 {published and unpublished data}
- Feldman HH, Lane R. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry 2007;78(10):1056‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindesay J. An open label one‐year extension of SDZ‐ENA‐713 studies B303, B304 and B305 to prospectively evaluate long‐term safety, tolerability and efficacy of SDZ‐ENA‐713 in out‐patients with probable Alzheimer's disease. National Research Register 2000.
- Novartis. No title. Unpublished. Data provided by Novartis. No year.
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
B351 {unpublished data only}
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. [DOI] [PubMed] [Google Scholar]
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003;60:843‐8. [DOI] [PubMed] [Google Scholar]
- Novartis. No title. Unpublished. Data provided by Novartis. No year.
- Novartis Pharmaceuticals. ADENA Programme. Unpublished Data 1998.
B352 {published data only}
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. [DOI] [PubMed] [Google Scholar]
- Corey‐Bloom J, Anand R, Veach J for ENA 713 B352 Study Group. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. International Journal of Geriatric Psychopharmacology 1998;1:55‐65. [Google Scholar]
- Doraiswamy M. The effects of rivastigmine on the Alzheimer's disease assessment scale‐cognitive subscale items scores of patients with Alzheimer's disease. Health in aging the challenge and promise of new decade. Proceedings of the annual scientific meeting of the American Geriatrics Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:173.
- Doraiswamy PM, Anand R, Hartman R. Cognitive effects of rivastigmine in patients with mild to moderate alzheimer's disease compared to those with moderately‐severe to severe AD. Clinical Neuropschological Assessment 2000;1(6):12. [Google Scholar]
- Doraiswamy PM, Anand R, Hartman R. Long term cognitive effects in Alzheimer's disease patients stratified by vascular risk score and treated for 1 year with rivastigmine. Clinical Neuropschological Assessment 2000;1(6):14. [Google Scholar]
- Farlow M, Hake A, Messina J, Veach J, Anand R. The response of patients with Alzheimer's disease to rivastigmine treatment is predicted by the rate of disease progression. Neurology 2000; Vol. 54 Suppl 3:A469. [DOI] [PubMed]
- Farlow M, Messina J, Anand R. Long term cognitive benefits associated with the use of rivastigmine in the treatment of Alzheimer's disease results following two years of treatment. Proceedings of the Annual Scientific Meeting of the American Geriatric Society and the American Federation for Aging Research; 2000 May 17‐21, Nashville. 2000:172.
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003;60:843‐8. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Hake A, Messina J, Hartman R, Veach J, Anand R. Response of patients with Alzheimer disease to rivastigmine treatment is predicted by the rate of disease progression. Archives of Neurology 2001;58(3):417‐22. [DOI] [PubMed] [Google Scholar]
- Ferris S. Improving day to day functioning in patients with AD. Proceedings of the Ninth Congress of the International Psychogeriatric Association; 1999 Aug 15‐20, Vancouver. 1999:77.
- Krishnan KR, Dorasiswamy PM, Messina J, Veach J, ENA 713 B352 Study Group. Rivastigmine slows stage specific global deterioration in Alzheimer's disease. Journal of the American Geriatrics Society 1999;47:S3. [Google Scholar]
- Kumar V, Anand R, Messina J, Hartman R, Veach J. An efficacy and safety analysis of Exelon in Alzheimer's disease patients with concurrent vascular risk factors. European Journal of Neurology 2000;7(2):159‐69. [DOI] [PubMed] [Google Scholar]
- Veach KR, Doraiswamy PM. Rivastigmine slows stage‐specific global deterioration in Alzheimer's disease. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20, Washington DC. 1999. [MEDLINE: ]
Ballard 2005 {published data only}
- Ballard C, Margallo‐Lana M, Juszczak E, Douglas S, Swann A, Thomas A, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. BMJ 2005;330:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
IDEAL {published data only}
- Alva G, Grossberg G, Schmitt F, Olin J. Influence of rivastigmine on activities of daily living: item responder analyses targeting improvement and atability. Annals of Neurology 2009; Vol. 66:S49.
- Alva G, Grossberg GT, Schmitt FA, Meng X, Olin JT. Efficacy of rivastigmine transdermal patch on activities of daily living: item responder analyses. International Journal of Geriatric Psychiatry 2011; Vol. 26, issue 4:356‐63. [DOI] [PubMed]
- Blesa R, Ballard C, Orgogozo JM, Lane R, Thomas SK. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer's disease. Neurology 2007;69(4 Suppl 1):S23‐8. [DOI] [PubMed] [Google Scholar]
- Cummings J, Winblad B. A rivastigmine patch for the treatment of Alzheimer's disease and Parkinson's disease dementia. Expert Review on Neurotherapeutics 2007;7(11):1457‐63. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Farlow MR, Meng X, Tekin S, Olin JT. Rivastigmine transdermal patch skin tolerability: results of a 1‐year clinical trial in patients with mild‐to‐moderate Alzheimer's disease. Clinical Drug Investigation 2010; Vol. 30, issue 1:41‐9. [DOI] [PubMed]
- Cummings JL, Ferris SH, Farlow MR, Olin JT, Meng XY. Effects of rivastigmine transdermal patch and capsule on aspects of Clinical Global Impression of Change in Alzheimer's disease: a retrospective analysis. Dementia and Geriatric Cognitive Disorders 2010; Vol. 29, issue 5:406‐12. [DOI] [PubMed]
- Farlow M, Cummings J, Olin J. Conference publication. American Journal of Geriatric Psychiatry. Conference: AAGP Annual Meeting 2009 Honolulu, HI United States. Conference Start: 20090305 Conference End: 20090308. Conference Publication 2009; Vol. 17:A58‐9.
- Farlow MR, Grossberg GT, Meng X, Olin J, Somogyi M. Rivastigmine transdermal patch and capsule in Alzheimer's disease: influence of disease stage on response to therapy. International Journal of Geriatric Psychiatry 2011; Vol. 26, issue 12:1236‐43. [DOI] [PubMed]
- Grossberg GT, Olin JT, Somogyi M, Meng X. Dose effects associated with rivastigmine transdermal patch in patients with mild‐to‐moderate Alzheimer's disease. International Journal of Clinical Practice 2011; Vol. 65, issue 4:465‐71. [DOI] [PubMed]
- Grossberg GT, Schmitt FA, Meng X, Tekin S, Olin J. Reviews: effects of transdermal rivastigmine on ADAS‐Cog items in mild‐to‐moderate Alzheimers disease. American Journal of Alzheimer's Disease and Other Dementias 2010; Vol. 25, issue 8:627‐33. [DOI] [PMC free article] [PubMed]
- Lee JH, Sevigny J. Effects of body weight on tolerability of rivastigmine transdermal patch: a post‐hoc analysis of a double‐blind trial in patients with Alzheimer disease. Alzheimer Disease and Associated Disorders 2011; Vol. 25, issue 1:58‐62. [DOI] [PubMed]
- Orgogozo J‐M. Clinical studies investigating dose‐related outcomes of cholinesterase inhibition with rivastigmine patch. European Journal of Neurology 2012; Vol. Conference: 16th Congress of the European Federation of Neurological Societies, EFNS Stockholm Sweden. Conference Start: 20120908 Conference End: 20120911. Conference Publication:, issue var.pagings.
- Winblad B, Cummings J, Andreasen N, Grossberg G, Onofrj M, Sadowsky C, et al. A six‐month double‐blind, randomized, placebo‐controlled study of a transdermal patch in Alzheimer's disease ‐ rivastigmine patch versus capsule. International Journal of Geriatric Psychiatry 2007;22(5):456‐67. [DOI] [PubMed] [Google Scholar]
- Winblad B, Grossberg G, Frolich L, Farlow M, Zechner S, Nagel J, Lane R. IDEAL: a 6‐month, double‐blind, placebo‐controlled study of the first skin patch for Alzheimer's disease. Neurology 2007;69(4 Suppl 1):S14‐S22. [DOI] [PubMed] [Google Scholar]
- Winblad B, Kawata AK, Beusterien KM, THomas SK, Wimo A, Lane R, et al. Caregiver preference for rivastigmine patch relative to capsules for treatment of probable Alzheimer's disease. International Journal of Geriatric Psychiatry 2007;22(5):485‐91. [DOI] [PubMed] [Google Scholar]
Karaman 2005 {published data only}
- Karaman Y, Erdogan F, Koseoglu E, Turan T, Ersoy AO. A 12‐month study of the efficacy of rivastigmine in patients with advanced moderate Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2005;19(1):51‐6. [DOI] [PubMed] [Google Scholar]
Lopez‐Pousa 2005 {published data only}
- Lopez‐Pousa S. Pilot, multicenter, randomized, double‐blind, controlled, parallel efficacy and safety study of rivastigmine vs placebo in the treatment of cognitive and non‐cognitive symptoms in patients with moderate‐to‐severe Alzheimer's disease. IFPMA Register 2005.
Mowla 2007 {published data only}
- Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer's dementia? A double‐blind, placebo‐controlled clinical trial. Journal of Clinical Psychopharmacology 2007;27(5):484‐7. [DOI] [PubMed] [Google Scholar]
Nakamura 2011 {published data only}
- Nakamura Y, Imai Y, Shigeta M, Graf A, Shirahase T, Kim H, et al. A 24‐week, randomized, double‐blind, placebo‐controlled study to evaluate the efficacy, safety and tolerability of the rivastigmine patch in Japanese patients with Alzheimer's disease. Dementia and Geriatric Cognitive Disorders Extra 2011; Vol. 1, issue 1:163‐79. [DOI] [PMC free article] [PubMed]
Tai 2000 {published data only}
- Tai CT, Liu CK, Sung SM, Pai MC, Hsu CY. The safety and efficacy of Exelon in Alzheimer's patients: A multicentre, randomized, 26‐week study in Taiwan. International Journal of Neuropsychopharmacology 2000;3 Suppl 1:S356. [MEDLINE: ] [Google Scholar]
References to studies excluded from this review
ACTION {published data only}
- Alva G, Cummings J, Galvin J, Meng X, Somogyi M. Infrequent skin reactions at the application site of the rivastigmine patch (4.6, 9.5 or 13.3 mg/24 h): Analysis of two clinical studies revealed most were tolerable and manageable across all doses. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P666. [Google Scholar]
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somgyi M. Long‐term safety and efficacy of 13.3 mg/24 h rivastigmine patch in severe Alzheimer's disease: ACTivities of daily living and cognitION (ACTION) study. Journal of the Neurological Sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication:(var.pagings):e339. [Google Scholar]
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somogyi M. A 24‐week, open‐label extension to the activities of daily living and cognition (ACTION) study: Long‐term safety, tolerability and efficacy of a 13.3 mg/24h rivastigmine patch in people with severe Alzheimer's disease. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P655. [Google Scholar]
- Farlow M, Grossberg G, Sadowsky C, Meng X, Somogyi M. Long‐term safety, tolerability and efficacy of 13.3 mg/24 h rivastigmine patch in patients with severe Alzheimer's disease. Clinical Pharmacology in Drug Development 2013;Conference: 2013 American College of Clinical Pharmacology Annual Meeting Bethesda, MD United States. Conference Start: 20130922 Conference End: 20130924. Conference Publication:(var.pagings):43. [Google Scholar]
- Farlow M, Meng X, Somogyi M. Efficacy, safety and tolerability of rivastigmine patch 13.3 mg/24 h (15 cm2) versus 4.6 mg/24 h (5 cm2) in patients with severe Alzheimer's disease: Results of the activities of daily living and cognition (ACTION) study. American journal of geriatric psychiatry 2013;Conference: 2013 AAGP Annual Meeting Los Angeles, CA United States. Conference Start: 20130314 Conference End: 20130317(var.pagings):S139‐40. [Google Scholar]
- Farlow M, Sadowsky C, Velting D, Meng X, Islam Z. Predictors of response to 13.3 mg/24 h rivastigmine patch in patients with severe Alzheimer's disease. Neurology 2014. [Google Scholar]
- Farlow MR, Doraiswamy PM, Meng X, Cooke K, Somogyi M. The effect of vascular risk factors on the efficacy of rivastigmine patch and capsule treatment in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders Extra 2011; Vol. 1, issue 1:150‐62. [DOI] [PMC free article] [PubMed]
- Farlow MR, Ferris S, Somogyi M, Meng X. Efficacy of 13.3 mg/24 h rivastigmine patch on global functioning and behavior in severe Alzheimer's disease. Annals of neurology 2013;Conference: 138th Annual Meeting of the American Neurological Association, ANA 2013 New Orleans, LA United States. Conference Start: 20131013 Conference End: 20131015. Conference Publication:(var.pagings):S91. [Google Scholar]
- Farlow MR, Grossberg G, Gauthier S, Meng X, Olin JT. The ACTION study: methodology of a trial to evaluate safety and efficacy of a higher dose rivastigmine transdermal patch in severe Alzheimer's disease. Current Medical Research and Opinion 2010; Vol. 26, issue 10:2441‐7. [DOI] [PubMed]
- Farlow MR, Grossberg GT, Sadowsky CH, Meng X, Somogyi M. A 24‐week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6 mg/24 h in severe Alzheimer's dementia. CNS Neuroscience & Therapeutics 2013;19:745‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR, Sadowsky CH, Velting DM, Meng X, Islam MZ. Evaluating response to high‐dose 13.3 mg/24 h rivastigmine patch in patients with severe Alzheimer's disease. CNS Neuroscience & Therapeutics 2015:No pagination specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris S, Isaacson R, Velting D, Meng X. Cognitive efficacy of 13.3 mg/24 h versus 4.6 mg/24 h rivastigmine patch in severe Alzheimer's disease: Severe impairment battery factor analysis. Neurology 2014. [Google Scholar]
- Grossberg G, Cummings J, Frolich L, Bellelli G, Molinuevo JL, Krahnke T. Efficacy of higher dose 13.3 mg/24 h rivastigmine patch on instrumental activities of daily living in patients with mild‐to‐moderate alzheimer's disease. American Journal of Alzheimer's Disease and Other Dementias 2013;28(6):583‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossberg G, Farlow M, Meng X, Somogyi M. Efficacy and safety of high‐dose 13.3 mg/24 h rivastigmine patch in severe Alzheimer's disease with and without concomitant memantine use. Journal of the Neurological Sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication:(var.pagings):e336. [Google Scholar]
- Grossberg G, Farlow M, Meng X, Somogyi M. Efficacy, safety and tolerability of 13.3 mg/24 h rivastigmine patch in patients with severe alzheimer's disease with and without concomitant memantine. Clinical Pharmacology in Drug Development 2013;Conference: 2013 American College of Clinical Pharmacology Annual Meeting Bethesda, MD United States. Conference Start: 20130922 Conference End: 20130924. Conference Publication:(var.pagings):42. [Google Scholar]
- Grossberg G, Farlow M, Meng X, Somogyi M. Efficacy, safety and tolerability of a higher‐dose 13.3 mg/24 h rivastigmine patch in people with severe Alzheimer's disease with and without concomitant memantine use. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P656. [Google Scholar]
- Micca J, Velting D, Meng X. Efficacy of 13.3 mg/24 h versus 4.6 mg/24 h rivastigmine patch on activities of daily living in severe Alzheimer's disease: A factor analysis. Neurology 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky C, Somogyi M, Frolich L, Meng X. Effect of body mass index on the efficacy, safety and tolerability of a higher‐dose 13.3 mg/24 h rivastigmine patch in people with severe Alzheimer's disease. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P654. [Google Scholar]
- Sadowsky CH, Grossberg GT, Somogyi M, Meng X. Predictors of sustained response to rivastigmine in patients with Alzheimer's disease: a retrospective analysis. Primary Care Companion to CNS Disorders 2011:13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Almkvist 2004 {published data only}
- Almkvist O, Darreh Shori T, Stefanova E, Spiegel R, Nordberg A. Preserved cognitive function after 12 months of treatment with rivastigmine in mild Alzheimer's disease in comparison with untreated AD and MCI patients. European Journal of Neurology 2004;11(4):253‐61. [DOI] [PubMed] [Google Scholar]
Auriacombe 2002 {published data only}
- Auriacombe S, Pere JJ. No donepezil discontinuation effect in patients with Alzheimer's disease who were switched to rivastigmine after failing to benefit from donepezil treatment. Current Medical Research and Opinion 2003;19(8):715‐7. [DOI] [PubMed] [Google Scholar]
- Auriacombe S, Pere JJ, Loria Kanza, Vellas B. Efficacy and safety of rivastigmine in patients with Alzheimer's disease who failed to benefit from treatment with donepezil. Current Medical Research and Opinion 2002;18(3):129‐38. [DOI] [PubMed] [Google Scholar]
B105 {published data only}
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA‐713) in Alzheimer's disease: an overview. Journal of Drug Development and Clinical Practice 1996;8(2):109‐16. [Google Scholar]
- Cutler N, Sramek J, Anand R. Safety and tolerance of ENA 713 in Alzheimer's disease (AD). Proceedings of the 8th European College of Neuropsychopharmacology Congress (ECNP); 1995 Sep 30‐Oct 4, Venice. 1995. [MEDLINE: ]
- Cutler NR, Sramek JJ, Anand R. Safety and tolerance of ENA 713 in patients with Alzheimer's disease. Biological Psychiatry 1995;37(9):643. [Google Scholar]
- Sramek J, Anand R, Wardle T, Irwin P, Hartman R, Cutler N. Safety/tolerability trial of SDZ ENA 713 in patients with probable Alzheimer's disease. Life Sciences 1996;58(15):1201‐7. [DOI] [PubMed] [Google Scholar]
Bilikiewicz 2002 {published data only}
- Bilikiewicz A, Opala G, Podemski R, Puzynski S, Lapin J, Soltys K, et al. An open‐label study to evaluate the safety, tolerability and efficacy of rivastigmine in patients with mild to moderate probable Alzheimer's disease in the community setting. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2002;8(2):PI9‐15. [PubMed] [Google Scholar]
Blesa Gonzalez 2011 {published data only}
Brassen 2003 {published data only}
- Brassen S, Adler G. Short‐term effects of acetylcholinesterase inhibitor treatment on EEG and memory performance in Alzheimer patients: an open, controlled trial. Pharmacopsychiatry 2003;36(6):304‐8. [DOI] [PubMed] [Google Scholar]
Caffarra 2007 {published data only}
- Caffarra P, Vezzadini G, Copelli S, Dieci F, Messa G, Nonis E, Venneri A. Comparing treatment effects in a clinical sample of patients with probable Alzheimer's disease treated with two different cholinesterase inhibitors. Acta Biomedica 2007;78(1):16‐21. [PubMed] [Google Scholar]
Cummings 2000 {published data only}
- Aupperle PM, Koumaras B, Chen M, Rabinowicz A, Mirski D. Long‐term effects of rivastigmine treatment on neuropsychiatric and behavioral disturbances in nursing home residents with moderate to severe Alzheimer's disease: results of a 52‐week open‐label study. Current Medical Research and Opinion 2004;20(10):1605‐12. [DOI] [PubMed] [Google Scholar]
- Cummings J, Anand R, Koumaras, et al. Abstract S79.002. Neurology 2000;54:A468. [Google Scholar]
Cutler 1998 {published data only}
- Cutler NR, Polinsky R, Sramek JJ, Enz A, Jhee S, Mancione L, et al. Dose‐dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurologica Scandinavica 1998;97:244‐50. [DOI] [PubMed] [Google Scholar]
Cutler 2000 {published data only}
- Cutler NR, Hossain M, McDonald C, Pommier F, Sedek G, Jhee SS, Sramek JJ. Pharmacokinetics of rivastigmine and its metabolite in patients with Alzheimer's disease. Biological Psychiatry 2000;47 Suppl 1:S161. [Google Scholar]
- Hossain M, Jhee SS, Shiovitz T, McDonald C, Sedek G, Pommier F, Cutler NR. Non‐blinded bioavailability study of oral and intravenous rivastigmine. Clinical Pharmacokinetics 2002;41(3):225‐34. [DOI] [PubMed] [Google Scholar]
Dantoine 2006 {published data only}
- Dantoine T, Auriacombe S, Sarazin M, Becker H, PereJJ, Bourdeix I. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer's disease who failed to benefit from previous cholinesterase inhibitor treatment. International Journal of Clinical Practice 2006;60(1):110‐8. [DOI] [PubMed] [Google Scholar]
Doraiswamy 2000a {published data only}
- Doraiswamy M. Early intervention with a cholinesterase inhibitor produces long‐term beneficial effects in moderately severe ad patients. Proceedings of the World Alzheimer Congress; 2000 Jul 9‐13, Washington. 2000a.
Edwards 2002 {published data only}
- Edwards K, Goddman W, Anand R, Koumars B, Hartman R. Effect of Alzheimer's disease severity on psychotropic drug use and behavior in nursing home patients treated with rivastigmine. The 8th conference on Alzheimer's disease and related disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:296.
EXCEED {published data only}
- Anon. Double‐blind trial will compare two anti‐Alzheimer's drugs. Journal of Dementia Care 2001c; Vol. 9, issue 5:6.
- Blesa R, Bullock R, He Y, Bergman H, Gambina G, Meyer J, et al. Effect of butyrylcholinesterase genotype on the response to rivastigmine or donepezil in younger patients with Alzheimer's disease. Pharmacogenetic Genomics 2006;16(11):771‐4. [DOI] [PubMed] [Google Scholar]
- Bullock R, Bergman H, Touchon J, Gasmbina G, He Y, Nagel J, Lane R. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer's disease. Current Medical Research and Opinion 2006;22(3):483‐94. [DOI] [PubMed] [Google Scholar]
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately severe Alzheimer's disease over a 2‐year period. Current Medical Research and Opinion 2005;21(8):1317‐27. [DOI] [PubMed] [Google Scholar]
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, et al. Rivastigmine and donepezil treatment in moderate to moderately‐severe Alzheimer's disease over a 2‐year period. Current Medical Research and Opinion 2005;21(8):1317‐28. [DOI] [PubMed] [Google Scholar]
- Bullock RA, Gambina G, Lane R, Nagel J. Rationale and design of EXCEED, a 24‐month, double‐blind study comparing rivastigmine and donepezil. Annals of Neurology 2002;52(3 Suppl 1):30. [Google Scholar]
- Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Current Medical Research and Opinion 2006;22(1):49‐59. [DOI] [PubMed] [Google Scholar]
Frankfort 2007 {published data only}
- Frankfort SV, Appels BA, Boer A, Tulner LR, Campen JPCM, Koks CHW, et al. Identification of responders and reactive domains to rivastigmine in Alzheimer's disease. Pharmacoepidemiology and Drug Safety 2007;16(5):545‐51. [DOI] [PubMed] [Google Scholar]
Fuschillo 2001 {published data only}
- Fuschillo C, Pia S, Campana F, Pinto A, Simone L. Cognitive deficits in Alzheimer's disease: treatment with acetylcholinesterase inhibitor agents. Archives of Gerontology and Geriatrics 2001;33 Suppl 1:151‐8. [DOI] [PubMed] [Google Scholar]
Holmes 2007 {published data only}
- Holmes C, Wilkinson D, Dean C, Clare C, El‐Okl M, Hensford C, Moghul S. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer's disease: a randomised double blind placebo controlled study. International Journal of Geriatric Psychiatry 2007;22(4):380‐1. [DOI] [PubMed] [Google Scholar]
InDDEx {published data only}
- Feldman H, Scheltens E, Hermann N, Ferris S, Mesenbrink P, Satlin A, Mancione L. Behavioral symptoms in mild cognitive impairment findings from the InDDEx study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:522.
- Feldman H, Scheltens P, Scarpini E, Hermann N, Mesenbrink P, Mancione L, et al. Behavioural symptoms in mild cognitive impairment. Neurology 2004;62:1199‐201. [DOI] [PubMed] [Google Scholar]
- Ferris S. Investigation into Delay to Diagnosis of Alzheimer's Disease with Exelon (InDDEx). Alzheimer's Disease Education and Referral Center (ADEAR) 1999a. [MEDLINE: ]
- Ferris S, Feldman P, Mesenbrink P, Mancione L, Satlin A. Mild Cognitive impairment and Alzheimer's disease are these distinct study populations clinical trials. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:570.
- Rossor MN. A prospective randomised mc double blind placebo controlled parallel group study of the effect of Exelon on the time to clinical diagnosis of Alzheimer's disease in subjects with mild cognitive impairment. Current Controlled Trials 2001.
- Rossor MN. Investigation into delay to diagnosis of Alzheimer's Disease with Exelon (InDDEx). Current Controlled Trials 2000.
- Sharma T. A prospective, randomized, multi‐centre, double‐blind, placebo‐controlled, parallel‐group study of the effect of Exelon on the time to clinical diagnosis of Alzheimer's disease in subjects with mild cognitive impairment. National Research Register 2000.
- Sharma T. Investigating the neural correlates of cognitive enhancement with rivastigmine (Exelon (C)) using functional magnetic resonance imaging in people with mild Alzheimer's disease. National Research Register 2001.
- Sharma T. Structural and functional MRI correlates of memory disorder in people with mild cognitive impairments or Alzheimer's disease. National Research Register 2003.
Kim 2002 {published data only}
- Kim SY. A 24 week trial investigating the safety tolerability and efficacy of rivastigmine in mild to moderately severe Alzheimer's disease patients in Korea. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:360.
Malsch 2001 {published data only}
- Malsch U, Dennler HJ. [Treatment of Alzheimer's disease: Tolerability of rivastigmine during the initial period]. Psychopharmacology 2001;27(6):337‐42. [Google Scholar]
McMillan 1999 {published data only}
Novartis 2005 {published data only}
- Novartis Pharmaceuticals Corporation. An open‐label extension to evaluate the efficacy and safety of the rivastigmine transdermal patch in patients with probable Alzheimer's sisease. ClinicalTrials.gov 2005.
OPTIMA {published data only}
- Alva G, Isaacson R, Sadowsky C, Grossberg G, Meng X, Somogyi M. Efficacy of higher‐dose 13.3 mg/24 h (15cm2) rivastigmine patch on the Alzheimer's Disease Assessment Scale‐cognitive subscale: domain and individual item analysis. International Journal of Geriatric Psychiatry 2014;29(9):920‐7. [DOI] [PubMed] [Google Scholar]
- Black S, Bakchine S, Bellelli G, Molinuevo JL, Downs P, Caputo A, et al. Efficacy of the 13.3 MG/24 h rivastigmine patchoninstrumentalactivitiesofdaily living in the optimising transdermal exelon in mild‐to‐moderate Alzheimer's disease (optima) study: Prospective subgroup analysis by disease severity and time‐to‐meet decline. Alzheimer's and Dementia 2012; Vol. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. Conference Start: 20120714 Conference End: 20120719. Conference Publication:, issue var.pagings.
- Blesa R, Martinez‐Lage P, Monsch AU, Downs P, Strohmaier C. Caregiver preference for rivastigmine patch in the OPtimising Transdermal exelon In Mild‐to‐moderate Alzheimer's disease (OPTIMA) Study. European Journal of Neurology 2012; Vol. Conference: 16th Congress of the European Federation of Neurological Societies, EFNS Stockholm Sweden. Conference Start: 20120908 Conference End: 20120911. Conference Publication:, issue var.pagings.
- Cummings J, Bellelli G, Black S, Bakchine S, Krahnke T, Strohmaier C. The rivastigmine high‐dose, 13 mg/24h (15cm2), transdermal patch provides daily living benefits to people with severe Alzheimer's disease: Retrospective analyses of the optimising transdermal exelon in mild‐to‐moderate Alzheimer's disease (OPTIMA) study. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P658. [Google Scholar]
- Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo J, et al. Randomized, double‐blind, parallel‐group, 48‐week study for efficacy and safety of a higher‐dose rivastigmine patch (15 vs. 10 cm) in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2012; Vol. 33, issue 5:341‐53. [DOI] [PubMed]
- Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo JL, et al. Randomized, double‐blind, parallel‐group, 48‐week study for efficacy and safety of a higher‐dose rivastigmine patch (15 vs. 10 cm2) in Alzheimer's disease. Dementia & Geriatric Cognitive Disorders 2012; Vol. 33, issue 5:341‐53. [DOI] [PubMed]
- Cummings J, Froelich L, Black SE, Bakchine S, Bellelli G, Molinuevo JL, et al. Randomized, double‐blind, parallel‐group, 48‐week study for efficacy and safety of a higher‐dose rivastigmine patch (15 vs. 10 cm2) in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2012;33(5):341‐53. [DOI] [PubMed] [Google Scholar]
- Cummings J, Frolich L, Black S, Bakchine S, Bellelli G, Molinuevo J, et al. Managing functional and cognitive decline in patients with mild‐to‐moderate Alzheimer's disease: a 48‐week, randomized, double‐blind evaluation of 13.3 mg/24 h (15 cm2) versus 9.5 mg/24 H (10 cm2) rivastigmine patch. Neurology 2012; Vol. Conference: 64th American Academy of Neurology Annual Meeting New Orleans, LA United States. Conference Start: 20120421 Conference End: 20120428. Conference Publication:, issue var.pagings.
- Cummings J, Grossberg G, Alva G, Caputo A, Downs P, Strohmaier C. High‐dose 13.3 MG/24 h rivastigmine transdermal patch demonstrates efficacy on instrumental activities of daily living: Individual item analysis. Alzheimer's and Dementia 2012; Vol. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. Conference Start: 20120714 Conference End: 20120719. Conference Publication:, issue var.pagings.
- Frampton JE. Rivastigmine transdermal patch 13.3 mg/24 h: a review of Its use in the management of mild to moderate Alzheimer's dementia. Drugs & Aging 2014;31(8):639‐49. [DOI] [PubMed] [Google Scholar]
- Frolich L. High‐dose rivastigmine patch: Results from the optima study. Neurobiology of Aging 2012; Vol. Conference: 12th International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy Stockholm Sweden. Conference Start: 20120509 Conference End: 20120512. Conference Publication:, issue var.pagings.
- Frolich L, Monsch A, Kressig R, Downs P, Caputo A, Strohmaier C. Comparisons of patient characteristics in subpopulations of the optimising transdermal Exelon in mild‐to‐moderate Alzheimer's disease (OPTIMA) study. Alzheimer's and Dementia 2012; Vol. Conference: Alzheimer's Association International Conference 2012 Vancouver, BC Canada. Conference Start: 20120714 Conference End: 20120719. Conference Publication:, issue var.pagings.
- Frolich L, Touchon J, Massaia M, Callegari F, Strohmaier C. Safety and tolerability of 9.5mg/24h(10cm2) and 13.3mg/24h (15cm2) rivastigmine patches: results from the optimising transdermal Exelon in mild‐to‐moderate Alzheimer's disease (OPTIMA) study. European Journal of Neurology 2012; Vol. Conference: 16th Congress of the European Federation of Neurological Societies, EFNS Stockholm Sweden. Conference Start: 20120908 Conference End: 20120911. Conference Publication:, issue var.pagings.
- Molinuevo JL, Cummings J, Frolich L, Galvin J, Krahnke T, Strohmaier C. High‐dose 13.3 mg/24 h rivastigmine patch efficacy and safety in mild‐to‐moderate Alzheimer's disease with and without concomitant memantine use. Journal of the Neurological Sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication:(var.pagings):e348. [Google Scholar]
- Molinuevo JL, Grossberg G, Frolich L, Galvin J, Krahnke T, Strohmaier C. Incidence and predictors of response to the 13.3 (15 cm2) and 9.5 mg/24h (10 cm2) rivastigmine patch in the optimising transdermal Exelon in mild‐to‐moderate Alzheimer's disease (OPTIMA) study. Alzheimer's & Dementia 2013;Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. Conference Start: 20130713 Conference End: 20130718. Conference Publication:(var.pagings):P656‐7. [Google Scholar]
- Molinuevo JL, Grossberg G, Frolich L, Galvin J, Krahnke T, Strohmaier C. Predictors of response to the 13.3 and 9.5 mg/24 h rivastigmine patch: The optimizing transdermal Exelon in mild‐to‐moderate Alzheimer's disease (optima) study. Journal of the neurological sciences 2013;Conference: 21st World Congress of Neurology Vienna Austria. Conference Start: 20130921 Conference End: 20130926. Conference Publication:(var.pagings):e336. [Google Scholar]
- Sadowsky C, Frolich L, Meng X, Somogyi M. Effect of body mass index on the efficacy, safety and tolerability of higher‐dose 13.3 mg/24 h rivastigmine patch in severe Alzheimer's disease. Clinical Pharmacology in Drug Development 2013;Conference: 2013 American College of Clinical Pharmacology Annual Meeting Bethesda, MD United States. Conference Start: 20130922 Conference End: 20130924. Conference Publication:(var.pagings):42‐3. [Google Scholar]
Potkin 1999a {published data only}
- Potkin SG, Anand R, Fleming K, Alva G, Keator D, Carreon D, et al. Brain metabolic and clinical effects of rivastigmine in Alzheimer's disease. International Journal of Neuropsychopharmacology 2001;4(3):223‐30. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Wu JC, Messina J, Fleming K, Keator D, Bunney WE, An R. Neuroimaging techniques and applications. Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; 1999 May 15‐20, Washington DC. 1999. [MEDLINE: ]
Riepe 2005 {published data only}
- Riepe MW, Adler G, Ibach B, Weinkauf B, Tracik F. Adding memantine to therapy with rivastigmine in patients with mild to moderate Alzheimer's disease: results of a 12‐week pilot study. 57th Annual Meeting of the American Academy of Neurology, Miami Beach, April 2005. 2005, issue P06.081.
Rozzini 2002 {published data only}
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzani S, Leonardi R, et al. Acetylcholinesterase inhibitors are effective in real world patients with mild to moderate Alzheimer disease evidence from a large population treated with rivastigmine or donepezil. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:329.
- Rozzini L, Bargnani C, Bosio A, Chia F, Franzoni S, Leonardi R, et al. Comparison of efficacy and safety of rivastigmine and donepezil in patients with mild to moderate Alzheimer disease: results from a multicentre randomised trial. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:240.
Schmidt 2002 {published data only}
- Schmidt R, Lechner A, Petrovic K. Rivastigmine in outpatient services: experience of 114 neurologists in Austria. International Clinical Psychopharmacology 2002;17(2):81‐5. [DOI] [PubMed] [Google Scholar]
Shanks 2001 {published data only}
- Shanks MF, Venneri A, Staff RT, Pestell SJ, Forbes KE, Gemmell HG, Murray AD. Cerebral blood flow and cognition enhanced by rivastigmine in a controlled study of Alzheimer's disease. Alzheimer's Disease International, 17th International Conference, Christchurch, New Zealand, 25‐27 October 2001. 2001:37.
Shua‐Haim 2002a {published data only}
- Shua‐Haim J, Smith J, Potel S. A head to study of donepezil (Aricept), rivastigmine (Exelon) and galantamine (Reminyl) for the treatment of Alzheimer's disease safety tolerability clinical and caregiver impression after 4‐5 months of treatment: a prospective study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:286.
Shua‐Haim 2002b {published data only}
- Shua‐Haim J, Smith J, Amin S, Shua‐Haim V. Comparison of combination therapy with rivastigmine exelon and donepezil aricept versus rivastigmine alone for treatment of Alzheimer's disease safety tolerability and clinical experience after one year of treatment a cross section study. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:292.
Shua‐Haim 2002c {published data only}
- Shua‐Haim JR, Smith JM, Amin S. Slow dose escalation of rivastigmine (Exelon®) treatment of agitation in patients with alzheimer disease: an eight month prospective study. The International Symposium on Advances in Alzheimer Therapy, 2002, Geneva. 2002:242.
Small 2005 {published data only}
- Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R. Cognitive performance in Alzheimer's disease patients receiving rivastigmine for up to 5 years. International Journal of Clinical Practice 2005;59(4):473‐7. [DOI] [PubMed] [Google Scholar]
Sobow 2002 {published data only}
- Sobow T. Cholinesterase inhibitors in the real world: donepezil vs rivastigmine tolerability study. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:244.
Stefanova 2002 {published data only}
- Stefanova E, Blennow K, Darreh‐Shori T, Hellstrom‐Lindhal E, Wall A, Almkvist O, et al. Evaluation of cerebral glucose metabolism, CSF‐TAU and CSF‐A342 after long‐term rivastigmine and tacrine treatments in Alzheimer disease patients. Proceedings of the 7th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy, 2002 Apr 3‐6, Geneva. 2002:246.
Thomas 2001 {published data only}
- Thomas A, Iacono D, Bonanni L, D' Andreamatteo G, Onofrj M. Donepezil, rivastigmine,and vitamin E in Alzheimer disease: A combined P300 event‐related potentials/neuropsychologic evaluation over 6 months. Clinical Neuropharmacology 2001;24(1):31‐42. [DOI] [PubMed] [Google Scholar]
Tsolaki 2002 {published data only}
- Tsolaki M, Gerothanassis D, Aristotle CP. Efficacy and safety of cholinesterase inhibitors a longitudinal comparative study between donepezil and rivastigmine. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:2038.
Venneri 2002 {published data only}
- Venneri A, Shanks MF. Charting patterns of progression in treated and untreated patients with Alzheimer's disease using SPECT. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:474.
Wang 2001 {published data only}
- Wang Y, Chen Q, Zhang Z, et al. The treatment by using rivastigmine for patients with alzheimer disease: results of a multicenter, randomized, open‐labeled, controlled clinical trial. Chinese Journal of Neurology 2001;34(4):210‐3. [Google Scholar]
Wang 2003 {published data only}
- Wang QH, Zhang ZX, Xu XH. [Efficacy of rivastigmine in treating mild to severe alzheimer's disease with different hachinski ischemia index]. Chinese Journal of Neurology 2003;36(1):44‐7. [Google Scholar]
Weiser 2002 {published data only}
- Weiser M, Davidson M, Rotmensch H, Korczyn A, Hartman R, Cicin‐Sain A, Anand R. A pilot randomised open label trial assessing safety and pharmacokinetic parameters of co administration of rivastigmine with risperidone in dementia patients with behavioral disturbances. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:633. [DOI] [PubMed]
- Weiser M, Rotmensch HH, Korczyn AD, Hartman R, Cicin Sain A, Anand R, Rivastigmine Risperidone Study Group. A pilot, randomized, open‐label trial assessing safety and pharmacokinetic parameters of co‐administration of rivastigmine with risperidone in dementia patients with behavioral disturbances. International Journal of Geriatric Psychiatry 2002;17(4):343‐6. [DOI] [PubMed] [Google Scholar]
Werber 2002 {published data only}
- Werber EA, Klein C, Rabey MJ. Evaluation of cholinergic treatment in demented by p300 evoked related potentials. The 8th conference on Alzheimer's Disease and Related Disorders, July 20‐25, 2002, Stockholm, Sweden. 2002:442.
Wilkinson 2002 {published data only}
- Bullock R, Passmore F, Potocnik F, Hock C. The tolerability, ease of use and efficacy of donepezil and rivastigmine in Alzheimer's disease patients: a 12‐week, multinational, comparative study. Journal of the American Geriatrics Society 2001;49(4):S19. [Google Scholar]
- Potocnik FC, Smith R, Passmore P, Hock C, Wilkinson D, Maud CM, Hopker S. Tolerability, ease of use, and efficacy of donepezil and rivastigmine in Alzheimer's disease patients. Proceedings of the Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans. 2001.
- Wilkinson D, Passmore P, Potocnik F, Maud C, Hock C. Donepezil compared to rivastigmine in Alzheimer's disease: similar efficacy but better tolerability and physician and caregiver satisfaction in a multinational randomized trial. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26, San Francisco. 2001.
- Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FCV, et al. A multinational, randomised, 12‐week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. International Journal of Clinical Practice 2002;56(6):441‐6. [PubMed] [Google Scholar]
Additional references
Benton 1974
- Benton AL. Revised visual retention test. 4th Edition. San Antonio, Texas: The Psychological Association, 1974. [Google Scholar]
Birks 2006
- Birks JS, Harvey R. Donepezil for dementia due to Alzheimer's disease. Cochrane Database of Systematic Reviews 2006, Issue 1. [Art. No.: CD001190. DOI: 10.1002/14651858.CD001190] [DOI] [PubMed] [Google Scholar]
Birks 2008
- Birks J. The evidence for the efficacy of cholinesterase inhibitors in the treatment of Alzheimer's disease is convincing. International Psychogeriatrics 2008;20(2):279‐86. [DOI] [PubMed] [Google Scholar]
Blessed 1968
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry 1968;114:797‐811. [DOI] [PubMed] [Google Scholar]
Brunner 1990
- Brunner C, Spiegel R. Eine Validierungsstudie mit der NOSGER (Nurses' Observation Scale for Geriatric Patients), einem neuen Beurteilungsinstrument für die Psychogeriatrie [Eine Validierungsstudie mit der NOSGER (Nurses' Observation Scale for Geriatric Patients), einem neuen Beurteilungsinstrument für die Psychogeriatrie]. Zeitschr für Psychologie 1990;XIX(3):211‐9. [Google Scholar]
Burns 2004
- Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. International Journal of Geriatric Psychiatry 2004;19(3):243‐9. [DOI] [PubMed] [Google Scholar]
Clegg 2002
- Clegg A, Bryant J, Nicholson T, McIntyre L, Broe S, Gerard K, Waugh N. Clinical and cost‐effectiveness of donepezil, rivastigmine, and galantamine for Alzheimer's disease: a systematic review. International Journal of Technology Assessment in Health Care 2002;18(3):497‐507. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1995
- Cohen‐Mansfield J. Assessment of disruptive behaviour/agitation in the elderly: function, methods and difficulties. Journal of Geriatric Psychiatry Neurology 1995;8:52‐60. [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenburg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐13. [DOI] [PubMed] [Google Scholar]
DeJong 1989
- DeJong R, Osterlund OW, Roy GW. Measurement of Quality‐of‐Life changes in patients with Alzheimer's Disease. Clinical Therapeutics 1989;11(4):545‐54. [PubMed] [Google Scholar]
DSM IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press, 1994. [Google Scholar]
Erkinjuntti 2002
- Erkinjuntti T, Skoog I, Lane R, Andrews C. Rivastigmine in patients with Alzheimer's disease and concurrent hypertension. International Journal of Clinical Practice 2002;56:791‐6. [PubMed] [Google Scholar]
Farlow 2003a
- Farlow M, Potkin S, Koumaras B, Veach J, Mirski D. Analysis of outcome in retrieved dropout patients in a rivastigmine vs placebo, 26‐week, Alzheimer disease trial. Archives of Neurology 2003a;60(6):843‐8. [DOI] [PubMed] [Google Scholar]
Fillit 2004
- Fillit H, Hill J. The economic benefits of acetylcholinesterase inhibitors for patients with alzheimer disease and associated dementias. Alzheimer Disease and Associated Disorders 2004;18 Suppl 1:S24‐9. [DOI] [PubMed] [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. ‘Mini‐Mental State’: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Fuld 1981
- Fuld PA. The Fuld object memory evaluation. Chicago: Steeling Instrument Co., 1981. [Google Scholar]
Galasko 1997
- Galasko D, Schmitt FA, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11:33‐9. [PubMed] [Google Scholar]
Gelinas 1999
- Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. American Journal of Occupational Therapy 1999;53:471‐81. [DOI] [PubMed] [Google Scholar]
Grossberg 2000
- Grossberg GT, Stahelin HB, Messina JC, Anand R, Veach J. Lack of adverse pharmacodynamic drug interactions with rivastigmine and twenty‐two classes of medications. International Journal of Geriatric Psychiatry 2000;15:242‐7. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy W (editor). ECDEU Assessment manual for psychopharmacology. Rockville: National Institute of Mental Health, 1976. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauber 2000
- Hauber AB, Gnanasakthy A, Mauskopf JA. Savings in the cost of caring for patients with Alzheimer's disease in Canada: an analysis of treatment with rivastigmine. Clinical Therapeutics 2000;22(4):439‐51. [DOI] [PubMed] [Google Scholar]
Helsinki declaration
- Declaration of Helsinki. http://www.faseb.org/arvo/helsinki.htm.
Higgins 2011
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. In: Higgins JPT, Green S editor(s). [updated March 2011]. Available from www.cochrane‐handbook.org.: The Cochrane Collaboration, 2011. [Google Scholar]
Homma 1991
- Homma A, Niina R, Ishii T, Hasegawa K. Development of a new rating scale for dementia in the elderly: Mental Function Impairment Scale (MENFIS). Japanese Journal of Geriatric Psychiatry 1991;2:1217‐22. [Google Scholar]
Howard 2011
- Howard R, Phillips P, Johnson T, O’Brien J, Sheehan B, Lindesay J, et al. Determining the minimum clinically important differences for outcomes in the DOMINO trial. International Journal of Geriatric Psychiatry 2011;26(8):812‐7. [DOI] [PubMed] [Google Scholar]
Jann 2000
- Jann MW. Rivastigmine, a new‐generation cholinesterase inhibitor for the treatment of Alzheimer's disease. Pharmacotherapy 2000;20:1‐12. [DOI] [PubMed] [Google Scholar]
Kennedy 1999
- Kennedy JS, Polinsky RJ, Johnson B, Loosen P, Enz A, Laplanche R, et al. Preferential cerebrospinal fluid acetylcholinesterase inhibition by rivastigmine in humans. Journal of Clinical Psychopharmacology 1999;19:513‐21. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical Diagnosis of Alzheimer's Disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939‐44. [DOI] [PubMed] [Google Scholar]
Novartis 1998
- Novartis Pharmaceutical (UK) Limited. EXELON (rivastigmine) Beyond cognition: prolonging functional ability. Product monograph. EXELON (rivastigmine) Beyond cognition: prolonging functional ability. Product monograph. Frimley, 1998.
Panisset 1994
- Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery: a neurological test for severely demented patients. Archives of Neurology 1994;51:41‐5. [DOI] [PubMed] [Google Scholar]
Polinsky 1998
- Polinsky RJ. Clinical pharmacology of rivastigmine: A new‐generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clinical Therapeutics 1998;20(4):634‐46. [DOI] [PubMed] [Google Scholar]
Reisberg 1982
- Reisberg B, Ferris SH, Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry 1982;139:1136‐9. [DOI] [PubMed] [Google Scholar]
Reisberg 1989
- Reisberg B, Franssen E. Stage specific incidence of potentially remediable behavioral symptoms in aging and AD: a study of 120 patients using the BEHAVE‐AD. Bulletin of Clinical Neuroscience 1989;54:95‐112. [Google Scholar]
Reisberg 1994
- Reisberg B, Ferris SH. CIBIC‐plus interview guide. Unknown, 1994. [Google Scholar]
Reitan 1958
- Reitan RM. The validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271‐6. [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry 1984;41:356‐64. [DOI] [PubMed] [Google Scholar]
Saxton 1990
- Saxton J, McGonigle‐Gibson K, Swihart A, Miller M, Boller F. Assessment of severely impaired patients: description and validation of a new neuropsychological test battery. Psychological Assessment 1990;2:298‐303. [Google Scholar]
Schneider 1997
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:S22‐S32. [DOI] [PubMed] [Google Scholar]
Schneider 1998
- Schneider LS, Anand R, Farlow MR. Systematic review of the efficacy of rivastigmine for patients with Alzheimer's disease. International Journal of Geriatric Psychopharmacology 1998;1:S26‐S34. [Google Scholar]
Schrag 2012
- Schrag A, Schott JM, Alzheimer's Disease Neuroimaging Initiative. Research paper: What is the clinically relevant change on the ADAS‐Cog?. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83(2):171‐3. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Spencer 1998
- Spencer CM, Noble S. Rivastigmine. A review of its use in Alzheimer's disease. Drugs & Aging 1998;13(5):391‐411. [DOI] [PubMed] [Google Scholar]
Watson 1993
- Watson YI, Arfken CL, Birge SJ. Clock completion: an objective screening test for dementia. Journal of the American Geriatric Society 1993;41:1235‐40. [DOI] [PubMed] [Google Scholar]
Wechsler 1987
- Wechsler D. Wechsler Memory Scale‐Revised (WMS‐R). San Antonio: Psychological Corporation Harcourt Brace Jovanovich Inc, 1987. [Google Scholar]
Williams 2003
- Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clinical Therapeutics 2003;25(6):1634‐53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Birks 2000
- Birks J, Grimley Evans J, Iakovidou V, Tsolaki M. Rivastigmine for Alzheimer's disease. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001191] [DOI] [PubMed] [Google Scholar]


