Abstract
BACKGROUND:
Osimertinib (AZD9291), a third generation mutation-selective epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (EGFR-TKI), is an approved drug for non-small cell lung cancer (NSCLC) patients with activating EGFR mutations or those harboring resistant T790M mutation. Unfortunately, all patients eventually relapse and develop resistance to osimertinib. The current study addressed whether ERK inhibition exerts similar effects as MEK inhibition in overcoming acquired resistance to osimertinib.
EXPERIMENTAL DESIGN:
Drug effects on cell and tumor growth were assessed by measuring cell number alterations and colony formation in vitro and with xenografts in nude mice in vivo. Apoptosis was assessed with annexin V/flow cytomentry and protein cleavage. Protein alterations in cells were detected with Western blotting. Gene overexpression and knockout were achieved with lentiviral infection and CRISPR/Cas9, respectively.
RESULTS:
The combination of osimertinib with an ERK inhibitor synergistically decreased the survival of osimertinib-resistant cell lines with enhanced induction of apoptosis and effectively inhibited the growth of osimertinib-resistant xenografts in nude mice. Moreover, the combination of a MEK or ERK inhibitor with a first (e.g., erlotinib) or second (e.g., afatinib) generation EGFR-TKI also very effectively inhibited the growth of osimertinib-resistant cells in vitro and tumors in vivo although these cell lines were cross-resistant to first or second generation EGFR-TKIs.
CONCLUSIONS:
Our findings emphasize the importance of targeting MEK/ERK signaling in maintaining the long-term benefit of osimertinib through overcoming acquired resistance to osimertinib, warranting further investigation of this therapeutic strategy to improve osimertinib therapeutic efficacy in the clinic.
Keywords: MEK/ERK, EGFR, osimertinib, resistance, lung cancer
Precis:
The findings emphasize the importance of targeting MEK/ERK signaling in maintaining the long-term benefit of osimertinib through overcoming acquired resistance to osimertinib, warranting further investigation of this therapeutic strategy to improve osimertinib therapeutic efficacy in the clinic.
INTRODUCTION
The discovery of activating epidermal growth factor receptor (EGFR) mutations that confer sensitivity to EGFR tyrosine kinase inhibitors (EGFR-TKIs) and the development of EGFR-TKIs for the treatment of non-small cell lung cancer (NSCLC) harboring these activating mutations represent major advances in lung cancer biology and therapy. The majority of EGFR activating mutations (90%) present as an exon 19 deletion (Del19; ~60%) or exon 21 point mutation L858R (~30%). The prevalence rates of these mutations in Western and Asian populations with NSCLC are ~15% and ~40%, respectively 1. First generation EGFR-TKIs, such as gefitinib and erlotinib, act as competitive reversible inhibitors of EGFR-TK and have provided significant clinical benefit in patients with these mutations, representing the first successful targeted therapy against lung cancer. However, patients ultimately relapse due to the emergence of acquired resistance, which limits the long-term efficacy of these agents 1-3.
Development of the T790M resistance mutation in exon 20 of the EGFR gene is the major mechanism of acquired resistance, accounting for approximately 60% of treatment failure following the use of first and 2nd generation EGFR-TKI. MET amplification is another important mechanism detected in approximately 5–22% of relapsed patients 1-3. In recognition of this challenge, third generation EGFR-TKIs such as osimertinib (TAGRISSO™ or AZD9291), rociletinib (CO1686), olmutinib (HM61713), nazartinib (EGF816), naquotinib (ASP8273), mavelertinib (PF-0647775) and AC0010 have been developed, which selectively and irreversibly inhibit EGFR carrying the common “sensitive” mutations, 19del and L858R, and the resistant T790M mutation while sparing wild-type (WT) EGFR 4. Among them, osimertinib has successfully progressed to an FDA-approved drug for the treatment of NSCLC patients with activating EGFR mutations (first-line) or those who have developed resistance to 1st generation EGFR-TKIs through the T790M mutation (second-line). Similar to other EGFR-TKIs, the emergence of resistance to osimertinib has also been documented and becomes the major obstacle for long-term control of disease in the clinic 4-6. Hence, understanding the underlying resistance mechanisms and developing effective strategies to overcome resistance to osimertinib is highly desirable and urgently needed in the clinic.
We have recently demonstrated one key mechanism by which osimertinib induces apoptosis through concurrent elevation of Bim and reduction of Mcl-l levels via modulation of MEK-dependent protein degradation. This activity is lost in different cell lines with acquired resistance to osimertinib. Inhibition of MEK with a MEK inhibitor leads to Bim elevation and Mcl-1 reduction in these resistant cell lines and accordingly restores cell sensitivity to osimertinib, achieving impressive effects on overcoming osimertinib resistance both in vitro and in vivo 7. In the current study, we aimed to reinforce this therapeutic strategy to overcome osimertinib resistance through addressing whether ERK inhibition exerts similar effects as MEK inhibition in overcoming acquired resistance to osimertinib. Moreover, we wanted to know whether some osimertinib-resistant cell lines remain responsive to first or second generation EGFR-TKIs.
MATERIALS AND METHODS
Reagents
The resources and preparation of osimertinib, CO1686, erlotinib, AZD6244 (selumetinib), PD0325901, GSK1120212 (trametinib), MG132, actinomycin D (Act D), and cycloheximide (CHX) were the same as described previously 7, 8. Afatinib was obtained from the Pharmacy of Emory University Hospital. Pelitinib, GDC0994 (ravoxertinib) and VRT752271 (ulixertinib or BVD-523) were purchased from MedChem Express (MCE; Monmouth Junction, NJ). All antibodies used in this study were the same as described in our previous studies 7-10.
Cell lines and cell culture
All AZD9291-resistant cell lines used in this study and culture conditions were the same as described previously 7, 8. Bim knockout (KO) cell lines were established with the CRISPR-Cas9 technique as described previously 11. Cell lines with expression of ectopic Mcl-1 were established using lentiviral infection as we did previously 12.
Cell viability assay
Cells seeded in 96-well cell culture plates were exposed to various treatment for 3 days. Cell numbers were then measured by sulforhodamine B (SRB) assay as previously described 13. Combination index (CI) for drug interaction (e.g., synergy) was calculated using CompuSyn software (ComboSyn, Inc.).
Colony formation assays
Low densities of tested cells were seeded in 12-well cell culture plates and were repeatedly treated with the tested agents every three days. After 12 days, cell colonies were stained, pictured and counted as described previously 7, 8.
Detection of apoptosis
Annexin V/7-AAD apoptosis detection kit (BD Biosciences; San Jose, CA) was used to detect apoptosis according to the manufacturer’s instructions. Caspase and PARP cleavage detected by Western blot analysis were also used as additional indicators of apoptosis.
Western blot analysis
Preparation of whole-cell protein lysates and Western blot analysis were the same as described previously 7, 8.
Animal xenograft and treatments
Animal experiments were conducted as described previously 7, 8 with approval by the Institutional Animal Care and Use Committee (IACUC) of Emory University. Treatments in the first experiment included vehicle control, osimertinib (7.5-5 mg/kg/day, og), VRT52271 (50 mg/kg/day; og), and their combination. Treatments in the second experiment included vehicle control, trametinib (3 mg/kg/day; og), erlotinib (25 mg/kg/day, og), afatinib (10-7.5 mg/kg/day, og), trametinib combined with erlotinib or trametinib combined with afatinib. Tumor volumes were measured using caliper measurements and calculated with the formula V ¼ π(length × width2)/6. At the end of treatment, mice were weighed and euthanized with CO2 asphyxia. The tumors were then removed and weighed.
Statistical analysis
The statistical significance of differences between two experimental groups was analyzed with two-sided unpaired Student t tests (for equal variances) or with Welch’s corrected t test (unequal variances) by use of Graphpad InStat 3 software. Data were examined as suggested by the same software to verify that the assumptions for use of the t tests held. Differences among multiple treatments were analyzed with one-way ANOVA. Results were considered to be statistically significant at P < 0.05.
RESULTS
ERK inhibition combined with osimertinib effectively decreases the survival and augments apoptosis of osimertinib-resistant NSCLC cell lines
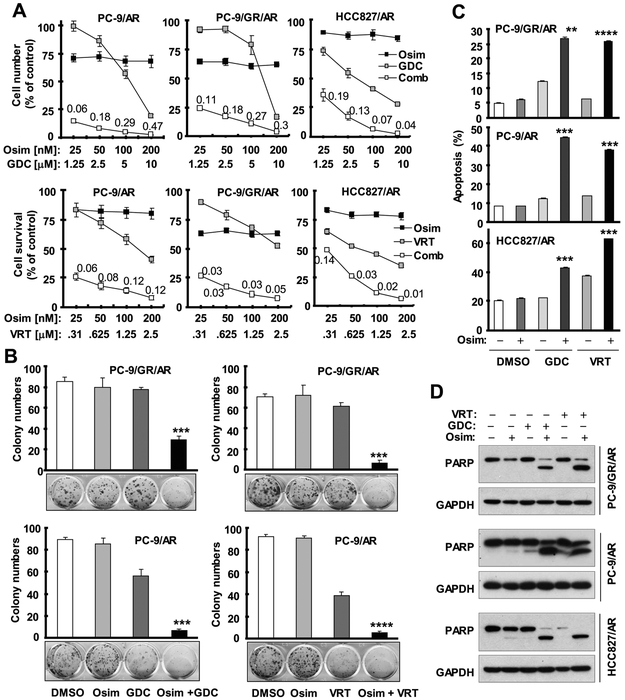
Since ERK functions immediately downstream of MEK and osimertinib modulates ERK-dependent protein alterations of Bim and Mcl-1, we first determined whether directly targeting ERK exerts similar effects as targeting MEK does in sensitizing osimertinib-resistant cells to osimertinib. To this end, we used two ERK small molecule inhibitors, GDC0994 and VRT752271, to inhibit ERK activity. The combination of either inhibitor with osimertinib effectively decreased the survival of three tested osimertinib-resistant cell lines, which had limited responses to each inhibitor alone (Fig. 1A). The CIs in these cell lines were far < 1, indicating highly synergistic effects on decreasing the survival of these cell lines. In a long-term colony formation that allows us to treat cells repeatedly, the combination of osimertinib with either GDC0994 or VRT752271 significantly inhibited the formation and growth of colonies of the tested osimertinib-resistant cancer cell lines, while each single agent almost had no inhibitory effect (Fig. 1B).
Fig. 1. Osimertinib combined with an ERK inhibitor augments the reduction in survival (A), inhibition of colony formation and growth (B) and induction of apoptosis (C and D) of osimertinib-resistant cell lines.
A, The given cell lines plated in 96-well plates were exposed to the indicated concentrations of osimertinib (Osim) alone, GDC0994 (GDC) or VRT752271 (VRT) alone or their respective combinations. After 3 days, cell numbers were estimated using the SRB assay. The data are means ± SDs of four replicate determinations. The numbers inside the graphs are CIs for the given combinations. B, The indicated cell lines were seeded in 12-well cell culture plates. On the second day, the cells were treated with fresh medium containing DMSO, 500 nM GDC or 200 nM VRT alone, 200 nM osimertinib alone and VRT or GDC plus osimertinib and treatments were repeated every 3 days for a total of 12 days. Cell colonies were stained with crystal violet and pictured. The data are means ± SDs of triplicate determinations. C and D, The indicated cell lines were exposed to DMSO, 100 nM osimertinib, 2 μM GDC, 0.5 μM VRT, GDC plus osimertinib or VRT plus osimertinib for 48 h and then harvested for detection of apoptosis with annexin V/flow cytometry (C) and for detection of PARP cleavage with Western blotting (D). The data are means ± SDs of duplicate determinations. ** P < 0.01, *** P < 0.001 and **** P < 0.0001 at least compared with other treatments.
Moreover, we examined the effects of combinations of osimertinib with ERK inhibitors on the induction of apoptosis in these osimertinib-resistant cell lines. We found that combinations of osimertinib with either ERK inhibitor were significantly more active than either agent alone in increasing annexin V-positive cells (Fig. 1C) and in inducing PARP cleavage (Fig. 1D). It is thus clear that ERK inhibition enhances osimertinib-induced apoptosis in osimertinib-resistant cell lines.
ERK inhibition elevates Bim levels and augments the reduction in Mcl-1 levels when combined with osimertinib
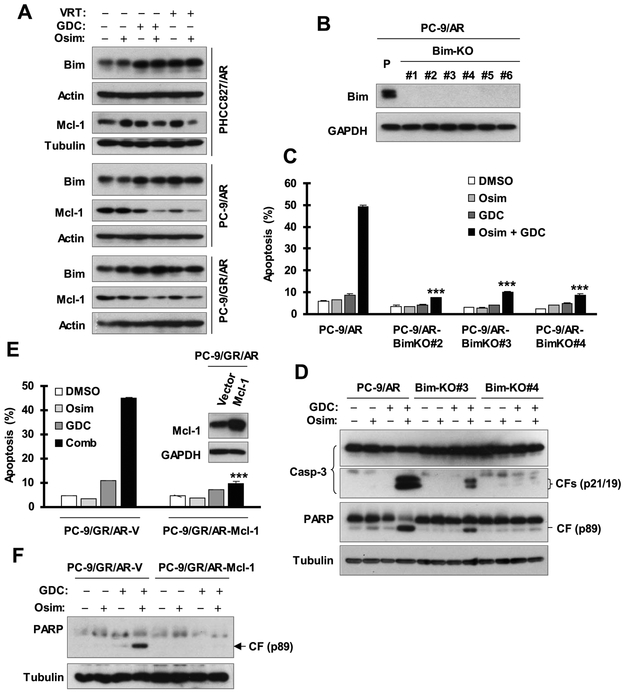
We next determined whether ERK inhibition synergizes with osimertinib in inducing apoptosis of osimertinib-resistant cells through modulation of Bim and Mcl-1 protein levels, as we recently reported for MEK inhibition 7. We analyzed the effects of osimertinib on Bim and Mcl-1 levels in the absence and presence of an ERK inhibitor in three osimertinib-resistant cell lines. Both GDC0994 and VRT752272 alone elevated Bim levels in the three tested cell lines and decreased Mcl-1 levels in PC-9/AR and PC-9/GR/AR cells. Their combination with osimertinib further decreased Mcl-1 levels but did not further enhance Bim elevation in the three cell lines (Fig. 2A). These results together demonstrate that the combination of osimertinib with ERK inhibition enhances the reduction of Mcl-1 levels.
Fig. 2. ERK inhibition combined with osimertinib induces the elevation of Bim levels, enhances Mcl-1 reduction (A) and enhances Bim- and Mcl-1-dependent induction of apoptosis (B-F) in osimertinib-resistant cells.
A, The indicated cell lines were treated with DMSO, 200 nM osimertinib (Osim) alone, GDC0994 (GDC) alone, VRT752271 (VRT) alone or osimertinib combined with GDC or VRT for 16 h. The proteins of interest were detected with Western blotting. B-D, Bim-deficient cell lines derived from PC-9/AR, as confirmed with Western blotting (B), together with PC-9/AR parental cells were exposed to DMSO, 100 nM Osim, 2 μM GDC or their combination for 48 h. Annexin V-positive cells were evaluated with flow cytometry (C) and protein cleavage was detected with Western blotting (D). E and F, The given cell lines were exposed to DMSO, 100 nM Osim, 4 μM GDC or their combination for 48 h. Apoptosis was evaluated by Annexin V flow cytometry (E) and by detection of PARP cleavage with Western blotting (F). The data in C and F are means ± SDs of duplicate determinations. *** P < 0.001 compared with the effect of the combination in control cells.
ERK inhibition combined with osimertinib enhances Bim- and Mcl-1-dependent apoptosis in osimertinib-resistant cells
Next, we determined the impact of Bim/Mcl-1 modulation on the enhanced induction of apoptosis by the combination of osimertinib with ERK inhibition. We established Bim-KO cell lines from the PC-9/AR cell line using the CRISPR/Cas9 technology as shown in Fig. 2B. The combination of osimertinib and GDC0994 enhanced the induction of apoptosis in PC-9/AR control cells, but had only minimal effects in three Bim-KO cell lines (Fig. 2C). In agreement, we detected greatly reduced amounts of cleaved forms of caspase-3 and PARP in both PC-9/AR-BimKO#3 and PC-9/AR-BimKO#4 cells in comparison with PC-9/AR cells (Fig. 2D). These results together indicate that the combination of ERK inhibition with osimertinib enhances Bim-dependent apoptosis. Moreover, we enforced expression of ectopic Mcl-1 in PC-9/GR/AR cells and examined its impact on enhanced induction of apoptosis by the combination of GDC0994 and osimertinib. Similar to Bim knockout, enhanced induction of apoptosis was detected in PC-9/GR/AR-V cells, but not in PC-9/GR/AR-Mcl-1 cells, as evaluated by measuring annexin V positive cells (Fig. 2E) and detecting PARP cleavage (Fig. 2F). This finding suggests that Mcl-1 reduction is also a critical mechanism for the enhanced induction of apoptosis by ERK inhibition in combination with osimertinib.
ERK inhibition combined with osimertinib effectively inhibits the growth of osimertinib-resistant tumors in vivo
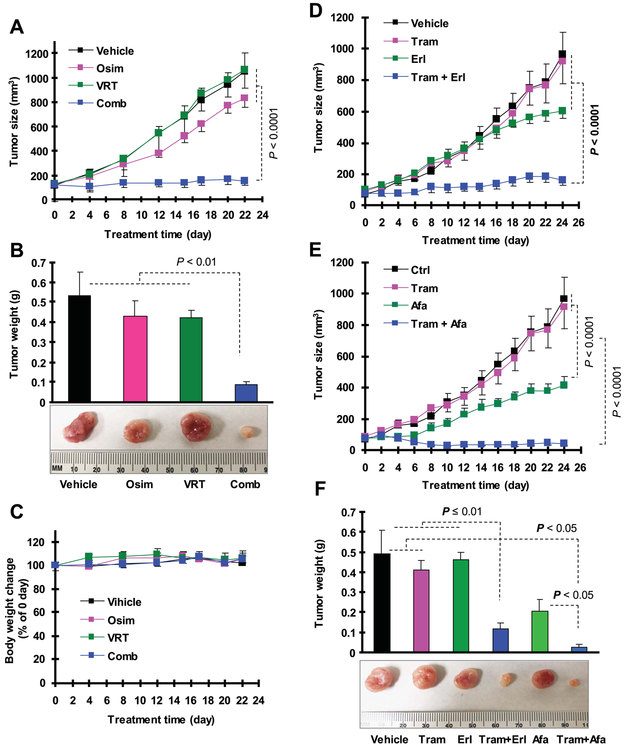
We then tested the effect of osimertinib combined with an ERK inhibitor on the growth of osimertinib-resistant tumors in nude mice. Treatment with either single agent did not significantly inhibit the growth of PC-9/AR xenografts while the combination of VRT752271 and osimertinib was significantly more potent than either agent alone in suppressing growth (Figs. 3A and 3B). Treatment with the combination did not decrease mouse body weights (Fig. 3C), suggesting that it is well tolerated by mice.
Fig. 3. The combination of an ERK inhibitor with osimertinib (A-C) or MEK inhibitor with erlotinib (D and F) or afatinib (E and F) effectively inhibits the growth of PC-9/AR xenografts in vivo.
A-C, PC-9/AR xenografts were treated with vehicle control, osimertinib (Osim), VRT752271 (VRT) or their combination starting on the same day after grouping for 22 consecutive days. D-F, PC-9/AR xenografts were treated with vehicle control, trametinib (Tram), erlotinib (Erl), afatinib (Afa), trametinib combined with erlotinib or trametinib plus afatinib starting on the same day after grouping for 24 consecutive days. Tumor sizes (A, D and E) were measured as indicated. Each measurement is mean ± SE (n = 6). At the end of treatment, mice were sacrificed to remove tumors, which were weighed (B and F). Mouse body weights were also compared (C).
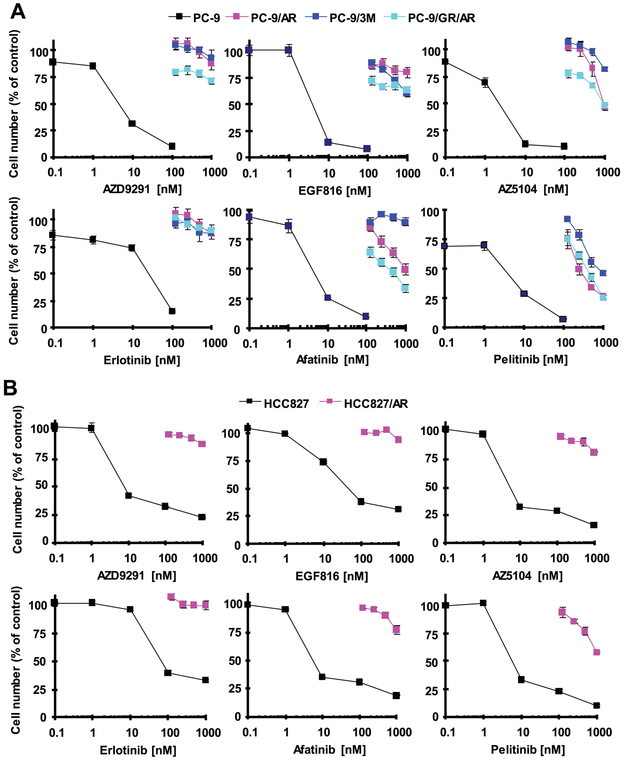
Osimertinib-resistant EGFR-mutant NSCLC cell lines are cross-resistant to other EGFR-TKIs including first and second generation EGFR-TKIs
It has been suggested that some osimertinib-resistant cell lines or tumors may be responsive to first generation EGFR-TKIs such as erlotinib 6. We thus determined how our osimertinib-resistant EGFR-mutant NSCLC cell lines respond to other EGFR-TKIs. The resistant cell lines derived from PC-9 cells including PC-9/AR, PC-9/GR/AR and PC-9/3M were all insensitive to EGF816 (a third generation EGFR-TKI), AZD5104 (an active osimertinib metabolite), erlotinib (a first generation EGFR-TKI), afatinib (a second generation EGFR-TKI) and pelitinib (a second generation EGFR-TKI) (Fig. 4A). Similar results were also generated in HCC827/AR cells derived from the HCC827 EGFR-mutant NSCLC cell line (Fig. 4B). Relatively, afatinib and particularly pelitinib were more active than other tested EGFR-TKIs in decreasing the survival of these resistant cell lines, with approximately 50% decrease in cell numbers at 1 μM.
Fig. 4. Osimertinib-resistant cell lines are cross-resistant to other EGFR-TKIs.
Osimertinib-resistant cell lines derived from PC-9 (A) or HCC827 (B) as indicated were seeded in 96-well plates and, on the second day, treated with different concentrations of the given EGFR-TKIs. After 3 days, cell numbers were estimated with the SRB assay. The data are means ± SDs of four replicate determinations.
MEK or ERK inhibition combined with a first or second generation EGFR-TKI effectively decreases cell survival and enhances apoptosis of osimertinib-resistant cell lines
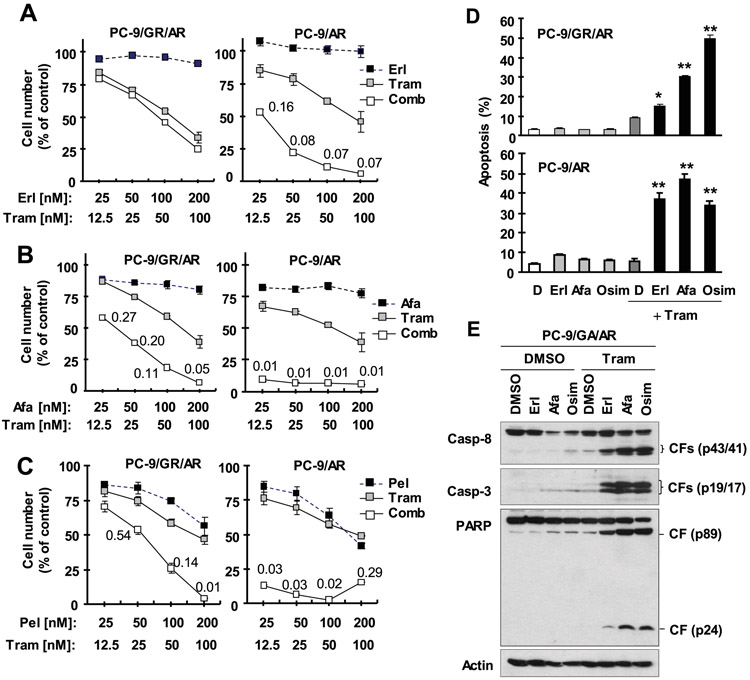
Our previous study has demonstrated that MEK inhibition effectively overcomes acquired resistance to osimertinib both in vitro and in vivo 7. We were interested in the effect of MEK/ERK inhibition combined with a first or second generation EGFR-TKI in overcoming osimertinib resistance in the current study. PC-9/GR/AR, an osimertinib-resistant cell line derived from gefitinib-resistant PC-9 cells due to T790M mutation 7, was insensitive to the combination of trametinib and erlotinib, but sensitive to the combination of trametinib with afatinib or pelitinib. However, PC-9/AR cells were very sensitive to the combination of trametinib with any of the tested EGFR-TKIs, erlotinib, afatinib and pelitinib, with CIs far smaller than 1 indicating synergistic reduction of the survival of PC-9/AR cells (Figs. 5A-C). Similar results were seen using a different MEK inhibitor, PD0325901 (Fig. S1).
Fig. 5. Trametinib combined with erlotinib (A), afatinib (B) or pelitinib (C) synergistically decreases the survival of osimertinib-resistant cell lines (A-C) through enhanced induction of apoptosis (D and E).
A-C, The given cell lines plated in 96-well plates were exposed to the indicated concentrations of trametinib (Tram) alone, a tested EGFR-TKI alone or their respective combinations. After 3 days, cell numbers were estimated using the SRB assay. The data are means ± SDs of four replicate determinations. The numbers inside the graphs are CIs for the given combinations. D and E, The indicated cell lines were exposed to DMSO, 50 nM trametinib, 100 nM EGFR-TKI or their respective combination for 48 h and then harvested for detection of apoptosis with annexin V/flow cytometry (D) and for detection of protein cleavage with Western blotting (E). The data in D are means ± SDs of duplicate determinations. * P < 0.05 and ** P < 0.01 at least compared with DMSO, trametinib and each corresponding EGFR-TKI alone.
In agreement with cell survival data, the combination of trametinib with erlotinib or afatinib was as effective as the combination of trametinib and osimertinib in inducing apoptosis in PC-9/AR cells, evidenced by increased annexin V-positive cell populations (Fig. 5D). In PC-9/GA/AR cells, the combination of trametinib and afatinib was as effective in inducing apoptosis as the combination of trametinib and osimertinib, as measured by annexin V-positive cell populations (Fig. 5D) and cleavage of caspase and PARP (Fig. 5E). In fact, the combination of trametinib with erlotinib also showed enhanced increase in annexin V-positive cells (Fig. 5D) and cleavage of caspase-3, capase-8 and PARP (Fig. 5E), albeit to a limited degree.
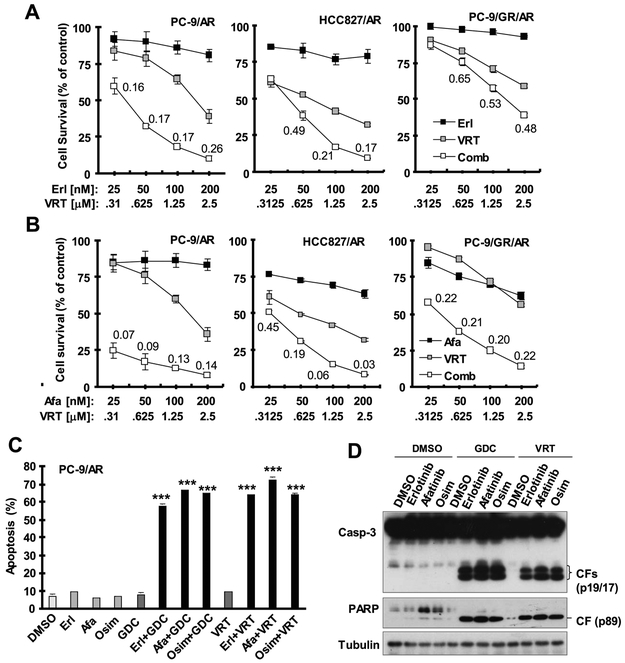
We also examined the impact of ERK inhibition combined with a first or second generation EGFR-TKI on the growth of osimertinib-resistant cells. Similar to MEK inhibition, the combination of VRT752271 with either erlotinib or particularly afatinib synergistically decreased the survival of three tested osimertinib-resistant cell lines, despite the weak activity of VRT752271 and erlotinib combination in PC-9/GR/AR cells (Fig. 6A). Similar results were also generated with GDC0994 combined with either erlotinib or afatinib (Fig. S2). When specifically measuring the effects of these combinations on the induction of apoptosis, the combinations of GDC0994 or VRT752271 with any of the tested EGFR-TKIs, erlotinib, afatinib and osimertinib, strongly increased the percentages of annexin V-positive cells (Fig. 6B) and induced high levels of cleaved caspase-3 and PARP (Fig. 6C) in PC-9/AR cells, whereas each single agent alone did not possess these activities under the tested conditions. Thus, inhibition of ERK in combination with erlotinib or afatinib, as in combination with osimertinib, strongly enhances induction of apoptosis in osimertinib-resistant cells.
Fig. 6. The ERK inhibitor VRT52271 combined with erlotinib or afatinib synergistically decreases the survival of osimertinib-resistant cell lines (A and B) through enhanced induction of apoptosis (C and D).
A and B, The given cell lines plated in 96-well plates were exposed to the indicated concentrations of VRT752271 (VRT) alone, erlotinib (Erl) or afatinib (Afa) alone or their respective combinations. After 3 days, cell numbers were estimated using the SRB assay. The data are means ± SDs of four replicate determinations. The numbers inside the graphs are CIs for the given combinations. C and D, PC-9/AR cells were exposed to DMSO, 0.5 μM VRT or 2 μM GDC, 100 nM the tested EGFR-TKI or their respective combination for 48 h and then harvested for detection of apoptosis with annexin V/flow cytometry (C) and for detection of protein cleavage with Western blotting (D). The data in C are means ± SDs of duplicate determinations. *** P < 0.001 at least compared with DMSO, each ERK inhibitor alone and each corresponding EGFR-TKI alone.
MEK inhibition combined with a first or second generation EGFR-TKI effectively inhibits the growth of osimertinib-resistant xenografts in vivo
To confirm our above in vitro findings, we conducted an in vivo study with PC-9/AR xenografts in nude mice to test the effects of these combinations on the growth of osimertinib-resistant xenografts. As demonstrated in vitro, afatinib alone showed some activity in inhibiting the growth of PC-9/AR xenografts while erlotinib alone had limited activity against the growth of this tumor. However, the presence of trametinib, which itself did not exert an inhibitory effect, greatly increased the growth inhibitory effects of erlotinib and afatinib in PC-9/AR xenografts (Figs. 3D-3F); the combined effects were significantly more potent than those of erlotinib or afatinib alone. Under the same conditions, the combination of trametinib with either erlotinib or afatinib did not decrease mouse body weights (Fig. S3), indicating that these combinations did not accordingly increase toxicity while enhancing anticancer activity against osimertinib-resistant tumors.
DISCUSSION
Our previous study has suggested that targeting MEK is an effective strategy for overcoming acquired resistance to osimertinib 7. The scientific rationale is that inhibition of MEK induces Bim elevation and Mcl-1 reduction in osimertinib-resistant cells, which are resistant to the modulation of Bim and Mcl-1 and induction of apoptosis by osimertinib, thus restoring the sensitivity of these osimertinib-resistant cells to osimertinib and to undergoing Bim-dependent apoptosis. In this process, ERK phosphorylation of Bim and Mcl-1 is the key mechanism underlying the modulation of their degradation. ERK is the immediate downstream protein kinase of MEK. Thus, it is reasonable to speculate that targeting ERK should have similar outcome as targeting MEK in overcoming osimertinib acquired resistance. Indeed, the current study clearly shows that inhibition of ERK with two different ERK small molecule inhibitors, when combined with osimertinib, effectively decreased the survival of different osimertinib-resistant cell lines and augmented the induction of apoptosis in these resistant cell lines. Moreover, this combinatorial strategy was very effective in suppressing the growth of osimertinib-resistant tumors (e.g., PC9/AR) in nude mice, validating its activity in overcoming osimertinib resistance in vivo. Therefore, targeting ERK is as effective as MEK inhibition in overcoming acquired resistance to osimertinib, reinforcing our notion that targeting MEK/ERK signaling is a very effective therapeutic strategy for overcoming osimertinib acquired resistance. There are several ERK small molecule inhibitors including the ones used in this study that have been tested or being tested in the clinical Phase I/II trails, among which both GDC0994 and VRT752271 have demonstrated the preliminary antitumor activity 14, 15. Our findings warrant future clinical testing of these ERK inhibitors in combination with osimertinib or other third generation EGFR-TKIs for overcoming acquired resistance to osimertinib or other third generation EGFR-TKIs.
ERK inhibition, as MEK inhibition did in our previous report 7, elevated Bim levels concurrently with enhanced reduction of Mcl-1 levels when combined with osimertinib in osimertinib-resistant cell lines. Both Bim elevation and enhanced Mc-1 reduction are critical for the enhanced induction of apoptosis by the combination of ERK inhibition and osimertinib in osimertinib-resistant cells since either Bim knockout or Mcl-1 overexpression significantly attenuated or abolished the ability of the combination to augment apoptosis in these cell lines. Mcl-1 is known to exert its anti-apoptotic function through sequestering Bim 16. Hence, enhanced Mcl-1 degradation induced by the combination of osimertinib and an ERK inhibitor in osimertinib-resistant cells will free up Bim and consequently facilitates Bim-dependent apoptosis.
One possible strategy for managing osimertinib resistant lung cancer patients is to re-expose them to a first generation EGFR-TKI based on anecdotal report of clinical response in carefully selected patients. In this study, we found that all the osimertinib-resistant cell lines established in our lab were resistant not only to other third generation EGFR-TKIs (e.g., EGF518), but also to first (erlotinib) and second (afatinib and pelitinib) generation EGFR-TKIs to varying degrees despite although these resistant cell lines may have different underlying resistance mechanisms. This finding clearly suggests that osimertinib-resistant cells are cross-resistant to other EGFR-TKIs. Therefore, it may not be an effective strategy to treat osimertinib-resistant patients with first or second generation EGFR-TKIs in the absence of a clear and biologically rational mechanism. Interestingly, the presence of a MEK inhibitor rendered both erlotinib and afatinib very effective in decreasing the survival of these osimertinib-resistant cell lines and inducing apoptosis. Consistently, these combinations were also very active in suppressing the growth of osimertinib-resistant tumors (e.g., PC-9/AR) in vivo. Identical results were also generated when combining an ERK inhibitor with a first or second generation EGFR-TKI. These findings again support the critical role of targeting the MEK/ERK signaling in overcoming acquired resistance to osimertinib. We noted that the combinations were particularly active in PC-9/AR cells without T790M mutation in comparison with PC-9/GR/AR cells harboring T790M mutation. This may imply that this strategy, i.e., the combination of a MEK or ERK inhibitor with a first or second generation EGFR-TKI, has high potential in treating patients relapsed from first-line treatment with osimertinib in the clinic.
Since osimertinib inhibits MEK/ERK signaling, as MEK or ERK inhibitors do, a concern is whether their combination may accordingly increase toxicity while enhancing anticancer activity against osimertinib-resistant cells. Consistent with our previous findings 7, the combination of osimertinib with an ERK inhibitor or the combination of a MEK inhibitor with either erlotinib or afatinib did not apparently decrease mouse body weights, suggesting favorable tolerability of these combinations in mice. Hence, targeting MEK/ERK signaling is a safe and very effective strategy for overcoming acquired resistance to osimertinib, at least in preclinical settings. Although the combination of MERK or ERK inhibition with an EGFR-TKI effectively overcomes acquired resistance to osimertinib in EGFR-mutant NSCLC cells, we found that the same combinations did not accordingly enhance the killing of NSCLC cell lines with WT EGFR, which are insensitive to EGFR-TKIs, based on our preliminary study. This finding may in part explain why this combinatorial strategy may not accordingly increase toxicity in tissues with WT EGFR.
In summary, the current study has provided strong evidence to support targeting MEK/ERK signaling as an effective strategy for overcoming acquired resistance to osimertinib regardless whether the acquired resistance arises from first-line or second-line treatment with osimertinib. This can be achieved by using either a MEK or ERK inhibitor in combination with osimertinib or even a first or second generation EGFR-TKI (e.g., erlotinib or afatinib). The promising preclinical activities of the combinations and the availability of several MEK and ERK inhibitors either as approved anticancer drugs or as clinically tested agents warrant the validation of this strategy for overcoming acquired resistance to osimertinib and even to other third generation EGFR-TKIs in the clinic.
Supplementary Material
ACKNOWLEDGEMENT
We are grateful to Dr. Dongsheng Wang in our department for help with animal experiments and to Dr. Anthea Hammond in our department for editing the manuscript.
Funding support: NIH/NCI R01 CA223220 (to SYS) and UG1 CA233259 (to SSR and SYS), Emory Winship Cancer Institute lung cancer research pilot funds (to SYS) and Lee Foundation Award to the Winship Lung Cancer Program for supporting the pilot project
Footnotes
Conflicts of interest disclosures: SSR is on consulting/advisory board for AstraZeneca, BMS, Merck, Roche, Tesaro and Amgen. TKO is on consulting/advisory board for Novartis, Celgene, Lilly, Sandoz, Abbvie, Eisai, Takeda, Bristol-Myers Squibb, MedImmune, Amgen, AstraZeneca and Boehringer Ingelheim. No potential conflicts of interest were disclosed for other authors.
REFERENCES
- 1.Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Ther Adv Respir Dis. 2015;9: 242–250. [DOI] [PubMed] [Google Scholar]
- 2.Juchum M, Gunther M, Laufer SA. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist Updat. 2015;20: 10–28. [DOI] [PubMed] [Google Scholar]
- 3.Remon J, Moran T, Majem M, et al. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: A new era begins. Cancer Treat Rev. 2014;40: 93–101. [DOI] [PubMed] [Google Scholar]
- 4.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65: 1–10. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2017: JCO2017747576. [DOI] [PubMed] [Google Scholar]
- 6.Ricordel C, Friboulet L, Facchinetti F, Soria JC. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann Oncol. 2018;29: i28–i37. [DOI] [PubMed] [Google Scholar]
- 7.Shi P, Oh YT, Deng L, et al. Overcoming Acquired Resistance to AZD9291, A Third-Generation EGFR Inhibitor, through Modulation of MEK/ERK-Dependent Bim and Mcl-1 Degradation. Clin Cancer Res. 2017;23: 6567–6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi P, Oh YT, Zhang G, et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett. 2016;380: 494–504. [DOI] [PubMed] [Google Scholar]
- 9.Yao W, Oh YT, Deng J, et al. Expression of death receptor 4 is positively regulated by MEK/ERK/AP-1 signaling and suppressed upon MEK inhibition. J Biol Chem. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh YT, Liu X, Yue P, et al. ERK/ribosomal S6 kinase (RSK) signaling positively regulates death receptor 5 expression through co-activation of CHOP and Elk1. J Biol Chem. 2010;285: 41310–41319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian G, Yao W, Zhang S, et al. Co-inhibition of BET and proteasome enhances ER stress and Bim-dependent apoptosis with augmented cancer therapeutic efficacy. Cancer Lett. 2018;435: 44–54. [DOI] [PubMed] [Google Scholar]
- 12.Ren H, Koo J, Guan B, et al. The E3 ubiquitin ligases beta-TrCP and FBXW7 cooperatively mediates GSK3-dependent Mcl-1 degradation induced by the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer. 2013;12: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SY, Yue P, Dawson MI, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57: 4931–4939. [PubMed] [Google Scholar]
- 14.Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. 2018;8: 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roskoski R Jr., Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol Res. 2019;142: 151–168. [DOI] [PubMed] [Google Scholar]
- 16.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584: 2981–2989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.