Abstract
Objective:
There is limited information regarding the tolerability of electroconvulsive therapy (ECT) combined with pharmacotherapy in elderly adults with major depressive disorder (MDD). Addressing this gap, we report acute neurocognitive outcomes from Phase 1 of the Prolonging Remission in Depressed Elderly (PRIDE) study.
Methods:
Elderly adults (age ≥ 60) with MDD received an acute course of six times seizure threshold right unilateral ultrabrief pulse (RUL-UB) ECT. Venlafaxine (VLF) was initiated during the first treatment week and continued throughout the study. A comprehensive neurocognitive battery was administered at baseline and 72 hours following the last ECT session. Statistical significance was defined as a two-sided p-value of less than 0.05.
Results:
240 elderly adults were enrolled. Neurocognitive performance acutely declined post ECT on measures of psychomotor and verbal processing speed, autobiographical memory consistency, short-term verbal recall and recognition of learned words, phonemic fluency, and complex visual scanning / cognitive flexibility. The magnitude of change from baseline to end for most neurocognitive measures was modest.
Conclusion:
This is the first study to characterize the neurocognitive effects of combined RUL-UB ECT and VLF in elderly adults with MDD and provides new evidence for the tolerability of RUL-UB ECT in an elderly sample. Of the cognitive domains assessed, only phonemic fluency, complex visual scanning, and cognitive flexibility qualitatively declined from low average to mildly impaired. While some acute changes in neurocognitive performance were statistically significant, the majority of the indices as based on the effect sizes remained relatively stable.
BRIEF SUMMARY
There is limited information regarding the tolerability of electroconvulsive therapy (ECT) combined with pharmacotherapy in geriatric major depressive disorder. Addressing this gap, we report neurocognitive outcomes from Phase 1 of the Prolonging Remission in Depressed Elderly (PRIDE) study. Elderly adults completed an acute ECT course, and completed neuropsychological assessments before and after the course. The magnitude of change in neurocognitive function from baseline to end for most neurocognitive measures was modest.
ClinicalTrials.gov identifier
.
INTRODUCTION
Consequences of major depressive disorder (MDD) in elderly adults include neurocognitive sequelae (1), suicide (2), and increased all cause morbidity and mortality (3). In many cases, elderly adults receive little to no benefit from antidepressant therapies including pharmacotherapy (4), psychotherapy (5), and repetitive transcranial magnetic stimulation (6), or require rapidly-acting intervention, and thus are prescribed electroconvulsive therapy (ECT) (7).
While ECT has been found to be effective in the elderly with high response and remission rates (8), concern about neurocognitive adverse effects limits its acceptance, particularly for seniors with age-related cognitive define or dementia. Short-term neurocognitive effects of ECT include decreased processing speed, attention, learning and memory, working memory, and executive function (9). A recent systematic review found the neurocognitive adverse effects of ECT in elderly adults to be transient and to occur mainly around the interictal and postictal periods and persist up to one month (10). However, other research suggests that ECT administered with bitemporal electrode configuration and brief pulse stimuli and is associated with retrograde amnesia for autobiographical memory consistency that can persist for up to six months (11). The majority of this research has focused on mixed-age adult populations, with limited information specifically regarding elders (12).
While the mechanisms of the neurocognitive effects of ECT remain unknown (13, 14), ECT parameters including charge, stimulus waveform, and electrode configuration play significant roles. Different combinations of ECT parameters have differential effects on the electric field induced in the brain, and therefore may exert different effects on underlying neurocircuitry (15). The combination of dose-titrated, ultrabrief pulse width, and right unilateral electrode configuration (RUL-UB) relative to the combination of brief pulse width and bitemporal electrode configuration can have fewer neurocognitive adverse effects relative to other ECT dosages and configurations with efficacy that approaches conventional brief pulse bilateral ECT (16, 17).
We present the acute neurocognitive outcomes of elderly adults with MDD treated with combined ECT and venlafaxine in Phase 1 of the Prolonging Remission in Depressed Elderly (PRIDE) study (18). We hypothesized that elderly adults with MDD would tolerate RUL-UB ECT well, and have fewer cognitive adverse effects than typically reported with brief pulse RUL and bitemporal ECT.
METHOD
Study Design Overview
The PRIDE study methods have been described elsewhere (18). Briefly, this was a multicenter, randomized trial of an individualized symptom-titrated algorithm-based longitudinal ECT (STABLE) protocol combined with venlafaxine (VLF) and lithium (Li) to improve long-term outcomes of elderly adults with MDD. In Phase 1, patients received an acute course of RUL-UB ECT 3x weekly combined with VLF. In Phase 2, those who remitted during Phase 1 were randomized to receive pharmacotherapy (VLF and Li) alone or the combined modalities (pharmacotherapy and STABLE) for six months.
The study sites included: Columbia University/New York State Psychiatric Institute, Duke University School of Medicine, Medical College of Georgia/Augusta University, Icahn School of Medicine at Mount Sinai (MSSM, Clinical Coordinating Center), Mayo Clinic, Medical University of South Carolina (data management and statistical coordinating center), New York Presbyterian/Weill Cornell Medical Center, University of Texas Southwestern Medical Center, Wake Forest University Medical Center, Zucker Hillside Hospital/Northwell Health System. The institutional review board at each study site approved the study protocol.
Study Sample
Patients were recruited over a six-year period from clinical referrals for ECT at the enrolling sites. Inclusion criteria included in- or outpatients, age ≥60, DSM-IV-TR diagnosis of unipolar major depressive episode, and 24-item Hamilton Rating Scale for Depression (HRSD24) total score ≥ 21. Exclusion criteria included bipolar disorder, schizoaffective disorder, dementia, substance abuse/dependence in last 6 months, active general medical or neurological conditions that would affect cognitive function or treatment outcome, contraindications to Li or VLF, or failure to respond to an adequate trial of Li + VLF, or ECT in the current episode. All patients provided written informed consent to participate in the study.
Electroconvulsive Therapy and Medication Procedures
Medication Washout and Concomitant Medications.
Patients discontinued psychotropic medications within 1-week of starting Phase 1. Rescue medication (lorazepam up to 3 mg/day or equivalent) was provided as needed for agitation, anxiety, or insomnia.
Electroconvulsive Therapy.
ECT was provided 3 sessions per week with RUL electrode placement using a Somatics Thymatron System IV (Somatics, LLC, Lake Bluff, IL) with an ultrabrief pulse width of 0.25 ms and current of 900 mA or a MECTA SPECTRUM (MECTA Corporation, Portland, OR) device with an ultrabrief pulse width of 0.3 ms and current of 800 mA. Dose titration to determine seizure threshold was conducted at the first ECT session. Subsequent treatments were administered at 6 times individual seizure threshold.
The average seizure threshold during the titration procedures for the overall sample was 30.5 (SD=14.3) millicoulombs. The average number of ECT sessions to produce remission was 7.32 (SD=3.52) for the overall sample, 7.30 (SD=3.03) for remitters, 12.36 (SD=1.04) for non-remitters, and 5.51 (SD=3.31) for drop-outs (see Kellner et al. 2016(18) for comprehensive details on the ECT treatment).
Medication.
Open-label VLF was started 1 – 5 days prior to ECT or up to 2 days after the first treatment at an initial dosage of 37.5 mg po, increased by 37.5 mg every 3 days or as tolerated, with a target dose of 225 mg qD.
Clinical Assessment Procedures
Diagnosis was established using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (study years 1 and 2) or the Mini-International Neuropsychiatric Interview (MINI) (years 3 – 6). The change in diagnostic instrument was made to minimize patient burden. Depressive symptoms were assessed with the HRSD24 and suicidal ideation with the Beck Scale for Suicide Ideation (BSS). Raters were trained to criteria via in-person and video interactive sessions.
Neurocognitive Assessment Procedures
The study assessed multiple cognitive domains including attention and processing speed, verbal fluency, verbal learning and memory, autobiographical memory consistency, and executive functions. The neuropsychological instruments were: Autobiographical Memory Interview-Short Form (AMI-SF)(19), California Verbal Learning Test-II (CVLT-II)(20), Delis-Kaplan Executive Function System (DKEFS) Verbal Fluency (Condition 1: Letter Fluency) Test(21), Dementia Rating Scale-2nd Edition Initiation Perseveration Index (DRS-2 IP)(22), Stroop Color and Word Test(23), and Trail Making Test Parts A and B (TMT A and B). Each neuropsychological instrument, with the exception of the TMT A and AMI-SF, had an alternate form. With the exception of the AMI-SF, all neuropsychological variable raw scores were converted into demographic-adjusted scores. Global cognitive function was assessed with the Mini Mental State Examination (results were previously reported) (18) and premorbid intellectual ability was estimated with the Wechsler Test of Adult Reading (WTAR)(24). Patients completed the neurocognitive battery at baseline and within 72 hours following the last ECT session. Testers at all sites were trained to criteria via in-person and video interactive training sessions following the neuropsychological instrument procedure manual. For longitudinal quality control, the electronic database was range validated to automatically detect errors and alert the study site to correct errors. Every four months, a random sample of data was examined for scoring accuracy.
Statistical Analysis
Descriptive analyses.
Descriptive statistics were used to characterize the demographic, neurocognitive, and clinical features of the study sample (n=240) who met selection criteria and began Phase 1. Means and standard deviations are presented for continuous variables, and frequency distributions are presented for discrete variables. Pearson correlation coefficients and corresponding 95% confidence intervals (CI) around the correlation coefficient were computed to describe the association between baseline neurocognitive variables and initial depression severity (HRSD24 total score).
Missing data.
The percent of missing data for baseline, end of Phase 1 and change from baseline for the neurocognitive outcomes were determined. Clinical and cognitive differences between completers (remitters and nonremitters) and non-completers (dropouts) were described using means and standard deviations or frequency distributions for each outcome category. Multiple imputation to impute missing neurocognitive data was incorporated into the estimation and inferential procedures (see section below)(25).
Estimation and Inferential analyses.
95% CI and paired t-tests obtained using multiple imputation procedure for missing data were used, respectively, to estimate the change from baseline to end of Phase 1 and determine if the change was statistically significant for each neurocognitive outcome.
First, as part of the multiple imputation procedure, logistic regression, with the dichotomous dependent variable missing/not missing, was used to identify the variables predictive of missing values to’ the neurocognitive variables. The multiple imputation process was then carried out using the Markov Chain Monte Carlo (MCMC) method to obtain 100 completed data subsets for the neurocognitive variables. Variables included in the imputation model were outcome status (remitter, nonremitter, dropout), age, baseline and last observed HRSD24 total score, sex, education, baseline and last observed MM SE total raw score, and number of ECT sessions in Phase 1. Next, in the multiple imputation procedure, results were combined across the 100 imputed subsets for final estimation of change from baseline (raw effect size) using 95% CI and inference using paired t-tests. Raw effect sizes were converted to standardized effect sizes (z-scores, Cohen’s d) by dividing by the raw change from baseline standard deviation.
Electric Field Modeling
Following the recommendations of the National Institute of Mental Health (NIMH) to report electric field models for non-invasive neuromodulation therapy dosage (26, 27) and since the study employed ECT stimulators from two different manufacturers that operated at slightly different pulse current amplitudes and pulse widths, we compared the difference in the induced electric field in the brain. An anatomically realistic head model was constructed from magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) data from one of the study subjects (62 years old male at the Zucker Hillside Hospital study site). The subject was scanned in a 3T GE Signa HDxt scanner. Tl-weighted MRI was acquired using a spoiled gradient recalled echo (SPGR) sequence (repetition time = 7.83 ms, echo time = 3.02 ms, voxel size = 0.9375 × 0.9375 × 1 mm3, flip angle = 8°, 216 coronal slices). DTI was acquired using a spin echo planar sequence (repetition time = 14000 ms, echo time = 75.5 ms, voxel size = 0.9375 × 0.9375 × 2.5 mm3, flip angle = 90°). Diffusion sensitizing gradients were applied along 31 noncollinear directions with a b-value of 1000 s/mm2. The finite element head model was constructed using SimNIBS 2.0.1 (28).
The head model consisted of five tissue compartments: skin, skull, cerebrospinal fluid, gray matter, and white matter, with assigned isotropic conductivities of 0.465 S m−1, 0.010 S m−1, 1.654 S m−1, 0.276 S m−1, and 0.126 S m−1, respectively (29). White matter anisotropy was incorporated based on conductivity tensors derived from DTI using a volume normalized mapping approach (30). We simulated the RUL ECT electrode placement with stimulus current amplitude of 800 mA or 900 mA, corresponding to the outputs of the MECTA spECTrum 5000Q and Somatics Thymatron System IV devices, respectively. We computed the distribution of the electric field strength relative to a pulse waveform specific neural activation threshold, E/Eth (31).
RESULTS
Demographic and Baseline Clinical Characteristics
Table 1 shows the demographic and clinical characteristics of the sample (n=240, age range 60–91) who entered and completed Phase 1 of the PRIDE Study. Slightly over half of the participants were female, 95% were White, and the average years of education was 14.5 years (SD=3.3). As typical of patients referred for ECT, most (87.5%) had recurrent MDD and a family history of psychiatric illness (68.7%) and MDD (59.1%). The current major depressive episode was severe (HRSD24 total score = 31.2 (SD=7.3) and most (59%) subjects had melancholic features.
Table 1.
Baseline Sociodemographic and Clinical Characteristics of Participants in a Study of ECT and Venlafaxine in Geriatric Depression
| Baseline Characteristics | Total Sample (N=240) |
|
|---|---|---|
| Continuous | Mean | SD |
| Age (years) | 69.9 | 7.6 |
| Education (years) a | 14.5 | 3.3 |
| Hamilton Depression Rating Scale (24 item) total score | 31.2 | 7.3 |
| Mini-Mental State Examination total score a | 27.5 | 2.4 |
| Wechsler Test of Adult Reading Full Scale IQ score j | 106.0 | 10.2 |
| Clinical Global Impression severity score a | 5.3 | 0.9 |
| Psychiatric hospitalizations b | 2.4 | 3.4 |
| Cumulative Illness Rating Scale for Geriatrics score c | 8.6 | 4.2 |
| Number of previous antidepressant medications d | 2.4 | 1.6 |
| Categorical | N | % |
| Sex: Female | 138 | 57.5 |
| Race: White | 228 | 95.0 |
| Ethnicity: Hispanic e | 9 | 3.8 |
| Recurrent depressive episode | 210 | 87.5 |
| Depressive Subtype | ||
| Atypical a | 5 | 2.1 |
| Melancholic a | 141 | 59.0 |
| Psychotic | 28 | 11.7 |
| Beck Scale for Suicide Ideation, % with score of 0 f | 105 | 52.0 |
| Family history of psychiatric illness g | 160 | 68.7 |
| Family history of mood disorder h | 143 | 61.9 |
| Family history of major depressive disorder i | 136 | 59.1 |
| Family history of bipolar disorder j | 33 | 14.3 |
Data missing for 1 subject (0.4%).
Data missing for 11 subjects (4.6%).
Data missing for 3 subjects (1.3%).
Data missing for 34 subjects (14.2%).
Data missing for 2 subjects (0.8%).
Data missing for 38 subjects (15.8%).
Data missing for 7 subjects (2.9%).
Data missing for 9 subjects (3.8%).
Data missing for 10 subjects (4.2%).
Data missing for 32 subjects (13.3%).
Baseline Neurocognitive Performance
Baseline neurocognitive values are shown in Table 2. The sample had an estimated premorbid intellectual ability WTAR Full Scale IQ in the average range. Mean global cognitive function MMSE total score was in the intact range. Mean demographic adjusted scores for the neurocognitive variables ranged from average to mildly impaired. Simple visual scanning/psychomotor processing speed (TMT-A) and phonemic fluency (D-KEFS Letter Fluency) were average. Complex visual scanning/psychomotor processing speed and cognitive flexibility (TMT-B) and initiation/perseveration (DRS-2) were low average, but response inhibition (Stroop Color-Word) was mildly impaired. On the CVLT-II, verbal learning, and immediate free recall and recognition of learned words were low average, but delayed free recall was mildly impaired. On average, the participants recalled approximately 83% of information to autobiographical questions.
Table 2.
Neurocognitive Test Results Before and After Treatment with Electroconvulsive Therapy and Venlafaxine Obtained from Paired T-Test a
| Neurocognitive Test |
Baseline | End | Raw effect size (Change from baseline) |
Standardi zed effect size |
T- statist icc |
DFc | P- value c |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | 95% Confidence Interval |
Cohen’s db |
||||
| Autobiographical Memory Interview-Short Form (AMI-SF) | ||||||||||
| Total score | 50.3 | 7.4 | 37.2 | 10.5 | −13.1 | −14.2, − 11.9 | −1.43 | −22.10 | 158.7 | <0.0001 |
| California Verbal Learning Test – II (CVLT-II) | ||||||||||
| Trial 1 −5 total recall T-score | 41.6 | 12.1 | 40.5 | 12.1 | −1.1 | −2.9, 0.6 | −0.08 | −1.28 | 155.0 | 0.20 |
| Short delay free recall z-score | −0.9 | 1.2 | −1.2 | 1.2 | −0.3 | −0.4, −0.1 | −0.20 | −3.17 | 146.9 | 0.002 |
| Long delay free recall z-score | −1.1 | 1.6 | −1.3 | 1.2 | −0.2 | −0.4, 0.0 | −0.10 | −1.58 | 143.3 | 0.12 |
| Recognition discrimination z-score | −0.7 | 1.2 | −1.1 | 1.4 | −0.4 | −0.6, −0.2 | −0.22 | −3.44 | 138.6 | 0.0008 |
| Delis-Kaplan Executive Function System (D-KEFS) | ||||||||||
| Letter fluency scaled score | 8.6 | 4.4 | 7.1 | 3.6 | −1.5 | −2.0, −1.0 | −0.39 | −6.08 | 146.3 | <0.0001 |
| Dementia Rating Scale-2nd Edition (DRS-2) | ||||||||||
| Initiation/Perseveration Index scaled score | 7.8 | 3.3 | 7.3 | 3.5 | −0.4 | −0.9, 0.1 | −0.11 | −1.74 | 165.4 | 0.08 |
| Stroop Color and Word Test (Stroop) | ||||||||||
| Word T-score | 33.7 | 12.3 | 30.8 | 10.9 | −2.9 | −4.4, − 1.4 | −0.24 | −3.73 | 138.1 | 0.0003 |
| Color T-score | 29.5 | 12.3 | 30.3 | 11.6 | 0.7 | −0.8, 2.3 | 0.06 | 0.92 | 142.3 | 0.36 |
| Color-Word T-score | 39.5 | 10.6 | 38.2 | 10.5 | −1.4 | −2.8, − 0.0 | −0.12 | −1.92 | 136.8 | 0.06 |
| Trail Making Test | ||||||||||
| Part A scaled score | 8.1 | 2.7 | 7.1 | 3.0 | −0.9 | −1.4, −0.5 | −0.27 | −4.17 | 114.4 | <0.0001 |
| Part B scaled score | 7.6 | 3.5 | 6.5 | 3.1 | −1.1 | −1.7, −0.5 | −0.23 | −3.63 | 80.1 | 0.0005 |
SD=standard deviation; DF=degrees for freedom
Baseline, end, and change from baseline means and standard deviations (sd) for neurocognitive variables were obtained using a multiple imputation procedure with Markov Chain-Monte Carlo (MCMC) method and 100 imputations for missing data [SAS (v9.4) statistical software (SAS Proc MI and Proc MIAnalyze)]. Variables included in the imputation model were outcome status (remitter, nonremitters, dropout), age, baseline and last observed HRSD24 total score, sex, education, baseline and last observed HRSD24 total raw score, and number of ECT sessions in Phase 1.
Cohen’s d was calculated as the raw change from baseline divided by raw change from baseline standard deviation.
The t-statistic, degrees of freedom (df), and p-value were for paired t-test incorporated into the multiple imputation procedure described in footnote a above.
Relationship Between Baseline Depression Severity and Neurocognitive Performance
At baseline, depression severity as rated on the HRSD24 was negatively associated with global cognitive function (r=−0.14, 95%CI: −0.01 - −0.26, p=0.04), color naming processing speed (r=−0.15, 95%CI: −0.02 - −0.27, p=0.03), autobiographical memory recall (r=−0.29, 95%CI: −0.17 - −0.40, p<0.0001), verbal learning (r=−0.16, 95%CI: −0.03 - −0.28, p=0.02), delayed free recall of learned words (r=−0.18, 95%CI: −0.05 - −0.30, p=0.01), and initiation/perseveration (r=−0.18, 95%CI: −0.05 - −0.30, p=0.01). Though statistically significant, the correlations were relatively weak. Details for the full neurocognitive battery are provided in Table S1 in the online supplemental data.
Change in Neurocognitive Performance after combined ECT and Venlafaxine
The percent of missing data for the analysis of change from baseline for the neurocognitive outcomes ranged from 27.1 to 50.8%, which is a limitation of the study. The frequency and percent of missing data for each of the variables and the comparison of demographic and baseline clinical and neurocognitive measures for completers (remitters and nonremitters) and noncompleters (dropouts) are presented in Tables S2-S3 in the online supplemental data.
After an acute course of RUL-UB ECT combined with venlafaxine, most neurocognitive variable scores significantly decreased (Table 2). Specifically, performance significantly decreased on neuropsychological measures of simple visual scanning / psychomotor (paired t-test, t=−4.17, df=114.4, p<0.0001) and verbal processing speed (paired t-test, t=−3.73, df= 138. 1, p=0.0003), autobiographical memory consistency (paired t-test, t=−22.1, df=158.7, p<0.0001), short-term free recall (paired t-test, t=−3.17, df=146.9, p=0.002) and recognition (paired t-test, t=−3.44, df=138.6, p=0.0008) of learned words, phonemic fluency (paired t-test, t=−6.08, df=146.3, p<0.0001), and complex visual scanning and cognitive flexibility (paired t-test, t=−3.63, df=80.1, p=0.0005). However, in terms of qualitative changes (as based on the analyzed demographic-adjusted neurocognitive scores) from baseline to end, performance across most neurocognitive variables remained relatively stable as based on the effect sizes and ranged from intact/average to mildly impaired, reflecting little clinical effect on cognitive function. Verbal learning and delayed free recall of learned words, and initiation/perseveration ability showed no significant change from baseline to end of the acute course. The results were similar when analyses were repeated using the observed (non-imputed) data.
Electric Field Model
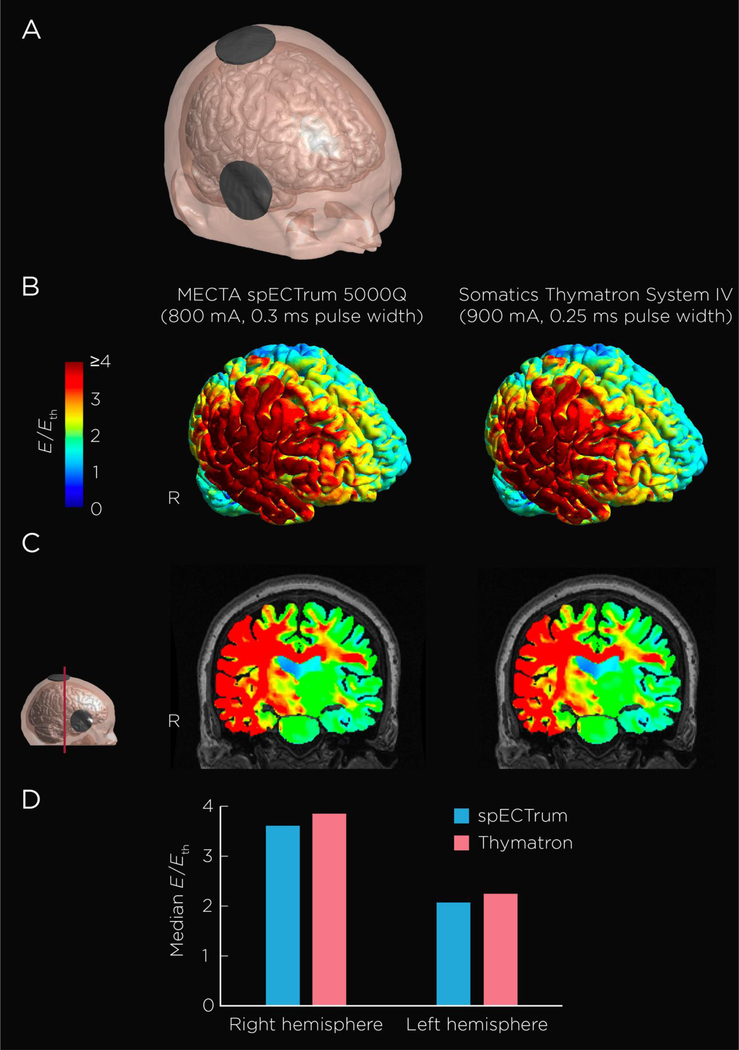
The spECTrum and Thymatron devices deliver rectangular pulses with pulse widths of 0.3 ms and 0.25 ms, respectively. Based on a first-order neuronal response model (31) with a transmembrane time constant of 196 μs (32), the estimated electric field threshold, Eth, for robust neural activation is 28.4 V m−1 for 0.3 ms pulse width and 30.2 V m−1 for 0.25 ms pulse width. The stimulation strength (E/Eth) is shown on the cortical surface (Figure 1 B) and the mid-coronal slice (Figure 1 C) for both the spECTrum and Thymatron devices. The Thymatron, relative to the spectrum, provides stronger stimulation by approximately 5% (Figure 1 D). For both devices, median E/Eth for the right hemisphere was approximately 1.7 times stronger than that in the left hemisphere. Of note, both stimulators deliver comparable electric fields strengths that exceed the threshold for neuronal depolarization by approximately 4-fold for the right hemisphere, and by over 2-fold for the left hemisphere. As shown in Figure 1C, RUL ECT stimulates the right and left hemisphere at field strengths above threshold for neuronal depolarization. Both devices induced electric fields above the threshold for neuronal depolarization in the medial temporal lobe, with the field being greater on the right relative to the left side.
Figure 1. Electroconvulsive Therapy Electric Field Model.
Figure 1 shows an anatomically realistic head model constructed from magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) data of one healthy, 62-year-old, male subject. (A) Head model showing the right unilateral ECT electrode placement. (B) Stimulation strength (electric field magnitude relative to neural activation threshold, E/Eth) on the cortical surface induced the MECTA spECTrum 5000Q and Somatics Thymatron System IV devices. (C) Coronal view of the field distribution. (D) Median E/Eth in the right and left hemispheres.
DISCUSSION
We found that the acute neurocognitive effects of F UL-Ultra-Brief ECT in an elderly sample were mild and less severe in magnitude than. those typically reported with brief pulse RUL, bitemporal, or bifrontal (BF) ECT (33). Some neurocognitive measures showed modest changes after ECT (e.g., delayed free recall of learned words, processing speed). For those measures that statistically significantly declined post ECT (autobiographical memory consistency, short-term free recall and recognition of learned words, phonemic fluency, processing speed, and visual scanning and cognitive flexibility), the degree of decline was clinically modest, with small relative differences from baseline as based o. the effect sizes, and some measures fell into the mildly impaired range. It should also be noted that with the autobiographical memory consistency metric (AMI-SF), as opposed to the other neurocognitive measures, research has found that there is typically a decrease in consistency scores over a time period equivalent to an index ECT course even in non-ECT depressed subjects, so that a significant consistency decrease does not necessary imply a neurocognitive impairment(34, 35). Indeed, the AMI-SF has been critiqued due to its sensitivity to time(36), which was substantiated in a recent study that found adult inpatients with severe depression treated with or without ECT showed similar decreased autobiographical recall, which was explained by an effect of time(37). Also, the decrease in autobiographical memory consistency as observed in this acute testing may be less during long-term follow-up as based on prior research (17, 38). As there was no comparator group, the study was unable to clarify the independent effect of time and condition and their interaction on autobiographical memory consistency. In no case were mean post-ECT neurocognitive scores indicative of severe cognitive impairment (as based on a score that is two standard deviations below demographic-matched or population normative data(39)), despite using a comprehensive neurocognitive battery sensitive to ECT-induced cognitive changes. We found weak and clinically nonsignificant associations between baseline depression severity and neurocognitive function, which extend prior findings in a mixed-age adult depressed cohort (40) into the elderly.
The finding that RUL-UB ECT, combined with venlafaxine, is tolerable in elderly patients with MDD is important because depression is a leading cause of disability, all cause morbidity, and suicide among the elderly. While ECT is highly effective in this age group in treating depression, presenting suicide, and improving quality of life (41), concerns regarding its neurocognitive side effects represent a barrier to its effective use, particularly among the elderly who are at increased risk of age-related cognitive decline and dementia. Making ECT more cognitively tolerable can minimize and remove barriers to its effective use in patients who could benefit from the treatment.
Some elderly patients may show no change or even possible improvement in global cognitive function following RUL-UB ECT, as the cognitive impairments associated within the baseline MDD episode resolve (42, 43). However, the mechanism(s) of action underlying changes in neurocognitive function remains unclear and may be moderated by multiple variables including demographic (e.g., age, premorbid intellectual ability) and clinical (e.g., preexisting cognitive impairment) factors (13). There is mixed information whether preexisting cognitive impairment is a risk factor for greater ECT-associated cognitive adverse effects (44), though there is consistent evidence that high intellectual functioning is a protective factor (45). The study cohort had generally intact cognitive abilities and high premorbid intellectual ability at baseline, which could have helped to mitigate the severity of ECT-induced adverse cognitive effects. If depressive relapse risk is increased by cognitive impairment (as has been hypothesized regarding autobiographical memory specificity) (46), then reducing cognitive side effects could benefit long-term outcomes.
Given that age increases seizure threshold, and that ECT was dosed at 6 times above seizure threshold, it was unknown whether these higher dosages needed to effectively treat elderly patients with RUL-UB ECT would show comparable levels of tolerability as seen with RUL-UB ECT in younger patients. It was therefore important to demonstrate that this form of ECT, when given at dosages adequate to ensure efficacy in the elderly, was also tolerable from a neurocognitive perspective. Of note, seizure thresholds with ultrabrief pulse ECT are substantially lower than those seen with brief pulse ECT, enhancing ability to effectively treat patients. In line with this observation, 85% of patients had a generalized tonic-clonic seizure on the first step of the seizure threshold titration protocol, indicating that the initial dose may have been higher than needed to induce a seizure. Despite this limitation, tolerability was excellent and suggests that even with high doses of RUL-UB ECT, adverse cognitive effects were mild (41).
The study design did not allow one to discern the relative contributions of venlafaxine and RUL-UB ECT, or anesthetic agent to the neurocognitive outcomes. For example, the combination of RUL ECT and venlafaxine was found to possibly worsen ECT-associated cognitive adverse effects in other research (47). However, that study was limited by study-site effects. A systematic review suggested that antidepressant agents, including venlafaxine, could have possible benefits in reducing cognitive difficulties in MDD (48). However, there are mixed findings regarding the cognitive effects associated with pharmacotherapeutic agents such as venlafaxine as one large scale study (the International Study to Predict Optimized Treatment-Depression (iSPOT-D) found no cognitive benefits(49), but another large scale study found that duloxetine had positive global cognitive effects in elderly adults(50). Regarding anesthesia regimens, some evidence across the adult lifespan has suggested that unique anesthetic agents may have differential effects on cognitive outcomes after EC I (51–53). Further research is warranted to clarify the cognitive effects of combined ECT and antidepressant medications, as well as specific anesthetic agents.
Unique strengths of this study in comparison with other research on the acute neurocognitive effects of ECT include the exclusive focus on the elderly, a study cohort with high years of education and estimated intellectual ability that is generalizable to other ECT study cohorts ( 54), the use of RUL-UB ECT, the use of standardized neurocognitive measures with demographic-referenced scores, and the use of electric field simulation to demonstrate that the two different ECT devices used were similar in delivered dose. The narrower pulse width used by the Thymatron (0.25 ms, compared to 0.3 ms used by the spECTrum) is compensated by an increase in current pulse amplitude (900 mA, compared to 800 mA used by the spECTrum). The changes in neurocognitive function after RUL-UB ECT plus venlafaxine are consistent with a recent study of RUL-UB ECT in a young to mid-adult (i.e., 40.7 years) aged cohort(54) with demographic-adjusted neurocognitive data, and are relatively less severe than those reported with brief-pulse RUL and BF ECT in elderly adults(33, 55) with non-demographic-adjusted neurocognitive data. Interestingly, our study found that RUL-UB ECT plus venlafaxine had little effect on delayed verbal recall of information. While this finding is in contrast to prior evidence (9), it is consistent with other research studies that have employed a challenging verbal learning and memory measure in which the words are semantically encodable (54).
While RUL-UB ECT reduced the typical cognitive side effect burden seen with ECT, it did not completely eliminate it. Future work to improve ECT technique is warranted to further reduce and optimally eliminate such cognitive side effects. We showed in Figure 1 that even with RUL-UB ECT, both ECT devices induced electric field strengths multiple-fold above the minimum threshold necessary to stimulate neurons. Recent work has begun to explore whether reducing the field strength by lowering ECT pulse amplitude, or via magnetic induction, could lower side effects while retaining efficacy (56, 57). Low amplitude ECT (58) and magnetic seizure therapy (MST) (59) have yet to be systematically examined in the elderly.
Although the head model included in this work was from an ECT patient in the study, a single head model cannot account for interindividual variability in the induced field distribution. A prior modeling study suggested that advanced age-related atrophy can significantly affect the induced electric field characteristics (60); sex differences in head diameter, scalp and skull thicknesses can also contribute to variability. The electric field simulation presented here illustrated the general pattern of electric field distribution induced in the brain by RUL-UB ECT, and demonstrated that both ECT devices produced similar stimulation strength. Future studies should explore the relationships among the electric field distribution, clinical and functional outcomes, and underlying biological and neurophysiological markers(61–63), using patient specific head models from a larger sample.
In conclusion, using well-standardized neurocognitive measures, we demonstrated a high degree of tolerability and low degree of acute neurocognitive adverse effects in elderly adults with MDD. Our results support the tolerability and antidepressant efficacy of RUL-UB ECT in combination with venlafaxine in late-life depression.
Supplementary Material
HIGHLIGHTS.
What is the primary question addressed by this study?
What are the acute neurocognitive effects of ultra-brief pulse, dose titrated, right unilateral ECT and venlafaxine in elderly adults with major depressive disorder?
What is the main finding of the study?
There were statistically significant declines in performance across neurocognitive measures. However, the magnitude of decline was clinically modest, with small differences relative to baseline, and some cognitive performance only fell into the mildly impaired range.
What is the meaning of the finding?
The combination of RUL-UB ECT with venlafaxine is a relatively cognitively safe treatment in late-life depression.
Acknowledgements
CORE/PRIDE Work Group: Icah School of Medicine at Mount Sinai: Gabriella Ahle, Amy S. Aloysi, M.D., Ethan Bryson, M.D., Kate Farber, Matthew Majeske, M.D., Elizabeth Muller, Roya Nazarian Rosa Pasculli; New York Presbyterian/Weill Cornell Medical Center: Ashly Cochran, M.S., Laura D. Evans, M.S., David Friedman, Nabil Kotbi, M.D., Bryony Lucas, Arielle Rogers; Augusta University: Brittany Gubosh, Chelsea Hodges, M.S., Laryssa McCloud, Ph.D., Mary Anne Riley, M.S.; Zucker Hillside Hospital/ Northwell Health Sysem: Raphael Braga, M.D., Ingrid Fuentes, Ketan Hiranpara, M.D., Muhammad Khan, M.D., Carmel Powers, Susan Ray, Gail Reiter, Sohag Sanghani, M.D., Elina Shrestha, M.D.; Duke University School of Medicine: Julie L. Adams, M.D., M.P.H, Grace Falcone, N.P., D.N.P., Mehul V. Mankad, M.D., Charles P. McCormick, M.D., M.P.H., Scott D. Moore, M.D., Ph.D., Kristen G. Shirey, M.D., Chris Sikes-Keilp, David C. Steffens, M.D., M.H.S., Nagy Youssef, M.D.; University of Texas Southwestern Medical Center: Enisa Arslanagic, M.D., Matthieu Chansard, Melita Gonzalez, Katalin Martits, Michelle Nichols, M.D., Najeeb Ranginwala, M.D.; Medical University of South Carolina: Hiya Banergee, Catherine Dillon, Andre Thornhill, Wenle Zhao; Mayo Clinic: Allison Hanson, Simon Kung, M.D., Maria Lapid, M.D., Lisa Seymour, Chris Sola, D.O., Cynthia Stoppel; Wake Forest University Medical Center: Niki Boggs, James Kimball, M.D.; NIMH: Galia Siegel, Ph.D., Elizabeth Zachariah, M.S.
Research Support
Supported by National Institute of Mental Health (NIMH) grants U01 MH055495, U01 MH081362, U01 MH086127, U01 MH086127, U01 MH086130, U01 MH08612005, U01 MH084241, and U01 MH086122. Drs. Deng and Lisanby are supported by the NIMH Intramural Research Program.
Acknowledgments (listed in alphabetical order)
Mustafa M. Husain. Dr. Husain reports research support from the National Institute of Mental Health, National Institute of Neurological Disorders and Stroke, Stanley Medical Research Foundation, Neuronetics, Inc., MagStim (equipment only), Brainsway, Inc., NeoSync and consulting income from Cerebain Inc. Speaker bureau for Acadia Pharmaceutical. Consultant to the Neurological Devices Panel of the Medical Devices Advisory Committee, Center for Devices and Radiological Health, Food and Drug Administration (FDA). Editorial Board Member of the Journal of ECT.
Sarah H. Lisanby. Dr. Lisanby has received grant support from the Brain and Behavior Research Foundation, the Stanley Medical Research Foundation, Neosync, Nexstim, NIH, and Brainsway. This manuscript was prepared while Dr. Sarah H. Lisanby was employed at Duke University. The opinions expressed in this article are the author’s own and do not reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
William V. McCall. Dr. McCall has served as a scientific adviser for Multiple Energy Technologies, and he has received research support from the American Foundation for the Prevention of Suicide, NIMH, MECTA Corp, Merck, and Vistagen. He has received royalties from Wolters Kluwer, and honoraria from Anthem, Inc., CME Outfitters, Global Medical Education, and Merck.
Shawn M. McClintock. Dr. McClintock reports research support from the National Institutes of Health.
Georgios Petrides. Dr. Petrides has received research support from Amgen, AstraZeneca, Corcept, Eli Lilly, Proteus, St. Jude Medical, and Sunovion, and he has served on an advisory panel for Corcept.
Robert C. Young. Dr. Young has received research support from NIMH.
Previous Presentation
Parts of this manuscript have previously been presented at the 2016 International Society for ECT and Neurostimulation (ISEN) Annual Meeting and the 2016 American College of Neuropsychopharmacology (ACNP) Annual Meeting.
Footnotes
Disclosures
George Alexopoulos. Dr. Alexopoulos reports having served in the Speakers Bureau of Otsuka, Lundbeck, Takeda, Allergan, Sunovion, and Astra Zeneca.
Samuel H. Bailine. Dr. Bailine reports no conflicts of interest.
Elisabeth Bernhardt. Ms. Bernhardt reports no conflicts of interest.
Mimi C. Briggs. Dr. Briggs reports no conflicts of interest.
C. Munro Cullum. Dr. Cullum reports no conflicts of interest.
Zhi-De Deng. Dr. Deng reports no conflicts of interest.
Mary Dooley. Ms. Dooley reports no conflicts of interest.
Emma T. Geduldig. Ms. Geduldig reports no conflicts of interest.
Robert M. Greenberg. Dr. Greenberg reports no conflicts of interest.
Styliani Kaliora. Dr. Kaliora reports no conflicts of interest.
Charles H. Kellner. Dr. Kellner receives honoraria from UpToDate, Psychiatric Times, and Northwell Health and royalties from Cambridge University Press
Rebecca G. Knapp. Dr. Knapp reports no conflicts of interest.
Vassilios Latoussakis. Dr. Latoussakis reports no conflicts of interest.
Lauren S. Liebman. Ms. Liebman reports no conflicts of interest.
Martina Mueller. Dr. Mueller reports no conflicts of interest.
Joan Prudic. Dr. Prudic reports no conflicts of interest.
Peter B. Rosenquist. Dr. Rosenquist reports no conflicts of interest.
Matthew V. Rudorfer. Dr. Rudorfer reports no conflicts of interest.
Shirlene Sampson. Dr. Sampson reports no conflicts of interest.
Abeba Teklehaimanot. Ms. Teklehaimanot reports no conflicts of interest.
Kristen G. Tobias. Ms. Tobias reports no conflicts of interest.
Richard D. Weiner. Dr. Weiner reports no conflicts of interest.
REFERENCES
- 1.Butters MA, Young JB, Lopez O, et al. : Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience 2008; 10:345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sachs-Ericsson N, Hames JL, Joiner TE, et al. : Differences Between Suicide Attempters and Nonattempters in Depressed Older Patients: Depression Severity, White-Matter Lesions, and Cognitive Functioning. The American Journal of Geriatric Psychiatry 2014; 22:75–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P, Smit H: Excess mortality in depression: a me .a-analysis of community studies. Journal of Affective Disorders 2002; 72:227–236 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds CFI, Dew MA, Pollock BG, et al. : Maintenance Treatment of Major Depression in Old Age. New England Journal of Medicine 2006; 354:1130–1138 [DOI] [PubMed] [Google Scholar]
- 5.Huang AX, Delucchi K, Dunn LB, et al. : A Systematic Review and Meta-analysis of Psychotherapy for Late-Life Depression. The American Journal of Geriatric Psychiatry 2015; 23:261–273 [DOI] [PubMed] [Google Scholar]
- 6.Sabesan P, Lankappa S, Khalifa N, et al. : Transcranial magnetic stimulation for geriatric depression: Promises and pitfalls. World Journal of Psychiatry 2015; 5:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisanby SH. Electroconvulsive Therapy for Depression. New England Journal of Medicine 2007; 357:1939–1945 [DOI] [PubMed] [Google Scholar]
- 8.O’Connor MK, Knapp R, Husain M, et al. The Influence of Age on the Response of Major Depression to Electroconvulsive Therapy. A C.O.R.E. Report. The American Journal of Geriatric Psychiatry 2001; 9:382–390 [PubMed] [Google Scholar]
- 9.Semkovska M, McLoughlin DM. Objective Cognitive Performance Associated with Electroconvulsive Therapy for Depression. A Systematic Review and Meta-Analysis. Biological Psychiatry 2010; 68:568–577 [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Mulsant BH, Liu AY, et al. : Systematic Review of Cognitive Effects of Electroconvulsive Therapy in Late-Life Depression. The American Journal of Geriatric Psychiatry 2016; 24:547–565 [DOI] [PubMed] [Google Scholar]
- 11.Lisanby SH, Maddox JH, Prudic J, et al. : The effects of electroconvulsive therapy on memory of autobiographical and public events. Archives of Genral Psychiatry 2000; 57:581–590 [DOI] [PubMed] [Google Scholar]
- 12.Antosik-Wojcinska A, Swiecicki L: The efficacy and safety of ECT in population before and after 60 years of age. Psychiatria Polska 2016; 50:1015–1026 [DOI] [PubMed] [Google Scholar]
- 13.McClintock SM, Choi J, Deng ZD, et al. : Multifactorial determinants of the neurocognitive effecdts of electroconvulsive therapy. Journal of ECT 2014; 30:165–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng ZD, McClintock SM, Lisanby SH: Brain, network properties in depressed patients receiving seizure therapy: A graph theoretical analysis of peri-treatment resting EEG. 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015; 2203–2206 [DOI] [PubMed] [Google Scholar]
- 15.Lee WH, Deng Z- D, Kim T- S, et al. : Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: Influence of white matter anisotropic conductivity. Neuroimage 2012; 59:2110–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loo CK, Katalinic N, Smith DJ, et al. : A randomised controlled trial of brief and ultrabrief right unilateral electroconvulsive therapy. International Journal of Neuropsychopharmacology 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semkovska M, Landau S, Dunne R, et al. : Bitemporal versus high-dose unilateral twice-weekly electroconvulsive therapy for depression (EFFECT-Dep): A pragmatic, randomized, non-inferiority trial. American Journal of Psychiatry 2016; 173:408–417 [DOI] [PubMed] [Google Scholar]
- 18.Kellner CH, Husain MM, Knapp RG, et al. : Right unilateral ultrabrief pulse ECT in geriatric depression: Phase 1 of the PRIDE Study. American Journal of Psychiatry 2016; 173:1101–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElhiney MC, Moody BJ, Sackeim HA: The Autobiographical Memory Interview Short Form: Manual for Administration and Scoring, New York, Department of Psychiatry, New York State Psychiatric Institute, 2001 [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, et al. : California Verbal Learning Test-Second Edition Adult Version. Manual, San Antonio, TX, Psychological Corporation, 2000 [Google Scholar]
- 21.Delis DC, Kaplan E,J K: Delis Kaplan Executive Function System, San Antonio, TX, The Psychological Corporation, 2001 [Google Scholar]
- 22.Jurica PJ, Leitten CL,Mattis S: DRS-2: Dementia Rating Scale-2 Professional Manual, Lutz, FL, Psychological Assessment Resources, Inc., 2001 [Google Scholar]
- 23.Golden CJ,Freshwater SM: Stroop Color and Word Test: A manual for clincal and experimental use, Wood Dale, Il, Stoelting Co., 2002 [Google Scholar]
- 24.Wechsler D: The Wechsler Test of Adult Reading (WTAR), San Antonio, TX, The Psychological Corporation, 2001 [Google Scholar]
- 25.O’Kelly M,Ratitch B: Clinical Trials with Missing Data: A Guide for Practitioners, Chichester, West Sussex, John Wiley and Sons, 2014 [Google Scholar]
- 26.McMullen DP: Where to target? The precision medicine approach to brain stimulation. Biologic Psychiatry 2018; 84:e1–e2 [DOI] [PubMed] [Google Scholar]
- 27.Bikson M, Brunoni AR, Charvet LE, et al. : Rigor and reproducibility in research with tranŝra/ial electrical stimulation: An NIMH-sponsored workshop. Brain Stimulation 2018; 11:465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thielscher A, Antunes A,Saturnino G: Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS?, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Thielscher A, Opitz A,Windhoff M: Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 2011; 54:234–243 [DOI] [PubMed] [Google Scholar]
- 30.Güllmar D, Haueisen J,Reichenbach JR: Influence of anisotropic electrical conductivity in white matter tissue on the EEG/MEG forward and inverse solution. A high-resolution whole head simulation study. Neuroimage 2010; 51:145–163 [DOI] [PubMed] [Google Scholar]
- 31.Deng Z- D, Lisanby SH,Peterchev AV: Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J Neural Eng 2011; 8:016007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterchev AV, Goetz SM, Westin GG, et al. : Pulse width dependence of motor threshold and input-output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol 2013; 124:1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dybedal GS, Tanum L, Sundet K, et al. : Cognitive side-effects of electroconvulsive therapy in elderly depressed patients. The Clinical Neuropsychologist 2014; 28:1071–1090 [DOI] [PubMed] [Google Scholar]
- 34.Weiner RD, Rogers HJ, Davidson JRT, et al. : Effects of stimulus parameters on cognitive side effects. Annals of the New York Academy of Sciences 1986; 462:315–325 [DOI] [PubMed] [Google Scholar]
- 35.Martin DM, Galvez V,Loo CK: Predicting retrograde autobiographical memory changes following electroconvulsive therapy: Relationships between individual, treatment, and early clinical factors. International Journal of Neuropsychopharmacology 2015;18:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semkovska M, McLoughlin DM: Retrograde Autobiographical Amnesia After Electroconvulsive Therapy: On the Difficulty of Finding the Baby and Clearing Murky Bathwater. The Journal of ECT 2014; 30:187–188 [DOI] [PubMed] [Google Scholar]
- 37.Semkovska M,O’Grady T: Unravelling autobiographical retrograde amnesia following bitemporal electroconvulsive therapy: Effect of treatment versus effect of time. Psychology 2017; 8:611–626 [Google Scholar]
- 38.Semkovska M, Keane D, Babalola O, et al. : Unilateral brief-pulse electroconvulsive therapy and cognition: Effects of electrode placement, stimulus dosage and time. Journal of Psychiatric Research 2011; 45:770–780 [DOI] [PubMed] [Google Scholar]
- 39.Brooks BL, Iverson GL: Comparing actual to estimated base rates of “abnormal” scores on neuropsychological test batteries: Implications for interpretation. Archives of Clinical Neuropsychology 2010; 25:14–21 [DOI] [PubMed] [Google Scholar]
- 40.McClintock SM, Cullum CM, Husain MM, et al. : Evaluation of the effects of severe depression on global cognitive function and memory. CNS Spectrums 2010; 15:304–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCall WV, Lisanby SH, Rosenquist PB, et al. : Effects of a right unilateral ultrabrief pulse electroconvulsive therapy course on health related quality of life in elderly depressed patients. Journal of Affective Disorders 2017; 209:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner GS, McClintock SM, Rosenquist PB, et al. : Major depressive disorder with psychotic features may lead to misdiagnosis of dementia: A case report and review of the literature. Journal of Psychiatric Practice 2011; 17:432–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pier KS, Briggs MC, Pasculli RM, et al. : Successful electroconvulsive therapy for major depression misdiagnosed as Alzheimer dementia. American Journal of Geriatric Psychiatry 2012; 20:909–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hausner L, Damian M, Sartorius A, et al. : Efficacy and cognitive side effects of electroconvulsive therapy (ECT) in depressed elderly inpatients wtih coexisting mild cognitive impairment or dementia. Journal of Clinical Psychiatry 2011; 72:91–97 [DOI] [PubMed] [Google Scholar]
- 45.Sackeim HA, Prudic J, Fuller R, et al. : The Cognitive Effects of Electroconvulsive Therapy in Community Settings. Neuropsychopharmacology 2006; 32:244–254 [DOI] [PubMed] [Google Scholar]
- 46.Raes F, Hermans D, Williams JMG, et al. : Reduced autobiographical memory specificity and rumination in predicting the course of depression. Journal of Abnormal Psychology 2006; 115:699–704 [DOI] [PubMed] [Google Scholar]
- 47.Sackeim HA, Dillingham EM, Prudic J, et al. : Effect of concomitant pharmacotherapy on electrocovulsive therapy outcomes: Short-term efficacy and adverse effects. Archives of Genral Psychiatry 2009; 66:729–737 [DOI] [PubMed] [Google Scholar]
- 48.Keefe RSE, McClintock SM, Roth RM, et al. : Cognitive effects of pharmacotherapy for major depressive disorder: A systematic review. Journal of Clinical Psychiatry 2014; 75:864–876 [DOI] [PubMed] [Google Scholar]
- 49.Shilyansky C, Williams LM, Gyurak A, et al. : Effect of antiepressant treatment on cognitive impariments associated with depression: A randomised longitudinal study. The Lancet Psychiatry 2016; 3:425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raskin J, Wiltse CG, Siegal A, et al. : Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: An 8-week, double-blind, placebo-controlled trial. American Journal of Psychiatry 2007; 164:900–909 [DOI] [PubMed] [Google Scholar]
- 51.Geretsegger C, Nickel M, Judendorfer B, et al. : Propofol and Methohexital as Anesthetic Agents for Electroconvulsive Therapy: A Randomized, Double-Blind Comparison of Electroconvulsive Therapy Seizure Quality, Therapeutic Efficacy, and Cognitive Performance. The Journal of ECT 2007; 23:239–243 [DOI] [PubMed] [Google Scholar]
- 52.Ding Z,White PF: Anesthesia for Electroconvulsive Therapy. Anesthesia & Analgesia 2002; 94:1351–1364 [DOI] [PubMed] [Google Scholar]
- 53.Bryson EO, Aloysi AS, Farber KG, et al. : Individualized Anesthetic Management for Patients Undergoing Electroconvulsive Therapy: A Review of Current Practice. Anesthesia & Analgesia 2017; 124:1943–1956 [DOI] [PubMed] [Google Scholar]
- 54.Vasavada MM, Leaver AM, Njau S, et al. : Short- and long-term cognitive outcomes in patients wtih major depression treated wtih electroconvulsive therapy. Journal of ECT 2017; 33:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dybedal GS, Bjolseth TM, Benth JS, et al. : Cognitive effects of bifrontal versus right unilateral electrconvulsive therapy in the treatment of major depression in elderly adults: A randomized, controlled trial. Journal of ECT 2016; 32:151–158 [DOI] [PubMed] [Google Scholar]
- 56.Peterchev AV, Krystal AD, Rosa MA, et al. : Individualized low-amplitude seizure therapy: Minimizing current for electroconvulsive therapy and magnetic seizure therapy. Neuropsychopharmacology 2015; 40:2076–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daskalakis ZJ, Dimitrova J, McClintock SM, et al. : Magnetic seizure therapy (MST) for major depressive disorder. Neuropsychopharmacology 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosa MA, Abdo GL, Lisanby SH, et al. : Seizure induction with low-amplitude- current (0.5 A) electroconvulsive therapy. Journal of ECT 2011; 27:342. [DOI] [PubMed] [Google Scholar]
- 59.Lisanby SH, Luber B, Schlaepfer TE, et al. : Safety and feasibility of magnetic seizure therapy (MST) in major depression: Randomized within-subject comparison with electroconvulsive therapy. Neuropsychopharmacology 2003; 28:1852–1865 [DOI] [PubMed] [Google Scholar]
- 60.Deng ZD, Lisanby SH,Peterchev AV: Effect of anatomical variability on electric field characteristics of electroconvulsive therapy and magnetic seizure therapy: A parametric modeling study. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2015; 23:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farzan F Atluri S, Mei Y, et al. : Brain temporal complexity in explaining the therapeutic and cognitive effects of seizure therapy. Brain 2017; 140:1011–1025 [DOI] [PubMed] [Google Scholar]
- 62.Jiang B, Abbott CC, T J, et al. : SMRI biomarkers predict electroconvulsive therapy outcomes: Accuracy with independent data sets. Neuropsychopharmacology 2018; 43:1078–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Njau S, Joshi SH, Espinoza R, et al. : Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. Journal of Psychiatry and Neuroscience 2017; 42:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.