Summary
Prepregnancy overweight and obesity are associated with higher risk of perinatal complications. However, the effect of weight change prior to pregnancy on perinatal outcome is largely unknown. Therefore, it is aimed to examine the impact on perinatal outcomes of interpregnancy BMI change in women of different BMI categories. The MEDLINE, EMBASE, LILACS, and CINAHL databases were searched (1990‐August 2019). Observational studies on interpregnancy BMI change were selected. Outcomes evaluated were gestational diabetes mellitus (GDM), preeclampsia, gestational hypertension (GH), cesarean section, preterm birth, and newborns being large (LGA) or small (SGA) for gestational age. Meta‐analyses and meta‐regression analyses were executed. Thirty studies were included (n > 1 million). Interpregnancy BMI gain was associated with a higher risk of GDM (for BMI gain ≥3 kg/m2: OR 2.21; [95%CI 1.53‐3.19]), preeclampsia (1.77 [1.53‐2.04]), GH (1.78 [1.61‐1.97]), cesarean section (1.32 [1.24‐1.39]), and LGA (1.54 [1.28‐1.86]). The effects of BMI gain were most pronounced in women with BMI <25 kg/m2 before the first pregnancy regarding GDM, GH, and cesarean section. Except for LGA, interpregnancy BMI loss did not result in a decreased risk of perinatal complications. In this study, women of normal weight who gain weight before pregnancy were identified as a high‐risk population for perinatal complications. This emphasizes that weight management is important for women of all BMI categories and a pregnancy wish.
Keywords: body mass index, cesarean delivery, gestational diabetes, obesity, pregnancy
Abbreviations
- 95%CI
95% confidence interval
- BMI
Body mass index
- GDM
Gestational diabetes mellitus
- GH
Gestational hypertension
- LGA
Large for gestational age
- OR
Odds ratio
- SGA
Small for gestational age
1. BACKGROUND
The prevalence of overweight and obesity in women of reproductive age has reached epidemic proportions and is associated with health risks for both mother and child. Risk factors including maternal overweight and obesity immediately before pregnancy and excessive gestational weight gain have been associated with adverse perinatal outcomes, such as gestational diabetes mellitus (GDM), preeclampsia, cesarean section, large (LGA) and small (SGA) for gestational age, and preterm birth.1, 2, 3 These risk factors are often preceded by a certain lifestyle including unhealthy food and insufficient physical activity.4, 5 In turn, these lifestyle‐related environmental exposures during fetal and neonatal development can lead to epigenetic changes resulting in fetal and metabolic programming.6 This programming influences the risk of cardiometabolic derangements and subsequent noncommunicable diseases in childhood and adult life and contributes therefore to the intergenerational transmission of health risks.6, 7, 8 It is however unknown whether these adverse effects of overweight and obesity are caused merely by the static situation of the body mass index (BMI) that women have at the start of their pregnancy, or that these are the result of an increase in BMI in the period prior to pregnancy. At the same time, the effects of weight reduction in the preconception phase are an unexplored field as well.
Although the World Health Organization emphasizes the need for preconception care,9 there is still a lack of studies regarding the effects of preconception weight change and lifestyle interventions on perinatal outcome. As a substitute, interpregnancy weight change—defined as change in maternal preconception weight from first to second pregnancies—offers a unique opportunity to collect data on weight change before the subsequent pregnancy. Therefore, the aim of this review is to study the impact of interpregnancy weight or BMI change between two consecutive pregnancies on perinatal outcomes in women with underweight, normal weight, overweight, and obesity before the first pregnancy.
2. METHODS
A protocol for this systematic review and meta‐analysis was prospectively registered in the International Prospective register of systematic reviews (PROSPERO; 2016: CRD42016043307).10
2.1. Search strategy
The MEDLINE (Ovid; search strategy in Text S1), EMBASE (Ovid), LILACS, and CINAHL databases were searched for relevant studies from 1990 to August 2019. The following keywords and variations of these terms were included: “body weight,” “weight loss,” “weight gain,” “obesity,” “body mass index,” “change,” “birth intervals,” “consecutive pregnancies,” and “between gestation.” The searches were limited to human studies. The search strategy was verified by an information specialist using the Peer Review of Electronic Search Strategies (PRESS) checklist.11
2.2. Study eligibility criteria
To answer the prognostic research question, observational (longitudinal, cohort, and case‐control) studies were included. An article was considered eligible and was included if it concerned the difference in prepregnancy weight or BMI between two consecutive singleton pregnancies. In order to answer the research question for the general population, studies were excluded in which only women with perinatal complications in the first pregnancy had been included. Studies were excluded if they did not report on any of the following outcomes of interest: GDM, gestational hypertension (GH), preeclampsia, caesarean section, preterm birth, SGA, and LGA. Inclusion of studies was not restricted by language, publication date, country, or duration of the interpregnancy interval.
Titles and abstracts of studies retrieved from the search strategy and, subsequently, full texts of potentially eligible studies were screened independently by two reviewers (YT and EO). Any disagreement regarding eligibility was discussed with a third reviewer until consensus was reached.
2.3. Quality assessment and data synthesis
Meta‐analyses were performed where possible: when at least two studies used the same outcome parameters and comparable categories of weight or BMI change. Odds ratios (ORs) adjusted for potential confounders were extracted from the studies and included in the meta‐analysis that reported these data. Where needed, the number of events in each BMI change category was calculated based on the provided adjusted ORs in the studies. In some cases, BMI change categories were merged in order to equalize BMI change categories of different studies, which enabled the combination of these studies in a meta‐analysis. The reference group was defined as BMI change between −1 and 1 kg/m2 or −2 and 2 kg/m2, dependent on the reference groups used in the original studies. Pooled ORs with 95% confidence intervals (95%CI) were calculated by using Comprehensive Meta‐Analysis V 3.0 software (Biostat Inc, Englewood, NJ, USA) from studies that reported raw data. Due to the heterogeneity anticipated, summary statistics were calculated with a random effects model. Statistical heterogeneity was assessed by the I2 statistic, which describes the proportion of variance (from 0% to 100%) that is due to variance in true effect sizes rather than sampling error.12 Meta‐regression was used to determine if differences in effect sizes between various weight or BMI loss and/or gain categories were significant. Specifically, for each pair of weight or BMI gain/loss subgroups used by studies to report on an outcome (eg, BMI maintenance between −1 and 1 kg/m2 vs BMI gain between 1 and 3 kg/m2) we used meta‐regression to determine if the differences in effect size varied significantly across subgroups. A p‐value < 0.05 was considered statistically significant. When studies reported on relevant outcomes but their data could not be aggregated with other studies, these results were presented as a narrative review. A sensitivity analysis was performed to take the quality of the studies into account. The analysis of all studies was compared to the analysis on studies qualified as low or moderate risk of bias to identify the impact of the level of bias on summary effects.
The quality of studies was independently assessed by two reviewers (YT and EO; see Table S2) using the validated Quality In Prognostic Studies (QUIPS) tool.13 Based on the items mentioned in this tool, the risk of bias was determined. For assessment of agreement between the two reviewers, the inter‐rater agreement of the QUIPS tool was evaluated. An online kappa calculator was used to calculate Cohen's kappa with quadratic weighting (http://vassarstats.net/kappa.html) for all domains of the QUIPS tool combined. Disagreements between the review authors were resolved by discussion with a third reviewer (KK) until consensus was reached.
3. RESULTS
3.1. Study selection
A total number of 16,223 articles were retrieved from the search. After removal of duplicates, 12,303 titles and abstracts were eligible for screening. In 122 studies, full text was assessed for eligibility, of which 30 articles were included in this review and eleven articles were included in the meta‐analyses (see PRISMA flow diagram14; Figure 1).
Figure 1.

Flowchart of literature search according to the PRISMA flow diagram. n = number. *Exact breakdown for exclusion not documented
3.2. Study characteristics
The study population, study design, setting, exposure, and outcome measurements are summarized in Tables S1 to S7. All studies were reported between 2002 and 2019 and were executed in European countries, Australia, or the USA with sample sizes ranging from 537 to 465,836 women. The majority of studies were retrospective, and confounders such as smoking status, interpregnancy interval, and BMI at the index pregnancy were controlled for in most studies. In general, the studies included used two different forms of categorization of interpregnancy weight change: (1) change in BMI (in kg/m2) between pregnancies; (2) change in BMI category (underweight, normal weight, overweight, and obesity) between pregnancies.
3.3. Data extraction
In Figures 2 and 3, pooled adjusted ORs are shown for the association between interpregnancy BMI change and adverse perinatal outcomes. Figures S1 to S7 show forest plots for each individual outcome. Table 1 shows the results of the meta‐regression analyses.
Figure 2.

Pooled adjusted odds ratios (aORs) are shown for the association between different categories of BMI change between pregnancies with maternal pregnancy outcomes. Reference group is BMI maintenance (between −1 and 1 kg/m2). BMI, body mass index; GDM, gestational diabetes mellitus; GH, gestational hypertension; 95%CI, 95% confidence interval
Figure 3.
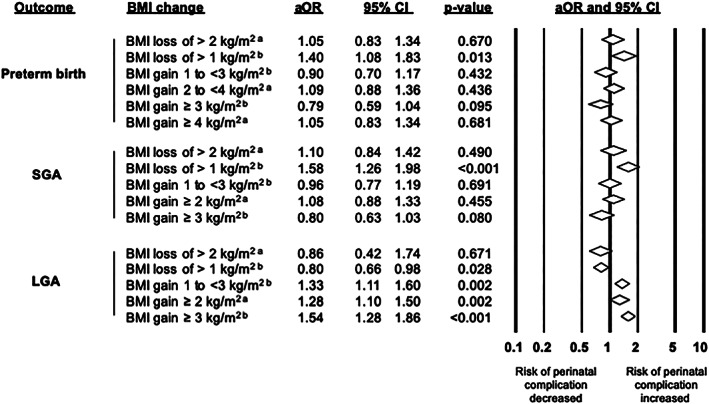
Pooled adjusted odds ratios (aORs) are shown for the association between different categories of BMI change between pregnancies with children's pregnancy outcomes. Reference group is BMI maintenance. aReference group is BMI maintenance between −2 and 2 kg/m2. bReference group is BMI maintenance between −1 and 1 kg/m2. BMI, body mass index; LGA, large for gestational age; SGA, small for gestational age; 95%CI, 95% confidence interval
Table 1.
Meta‐regression to determine differences between subgroups
| Coefficient | 95%CI | p‐value | ||
|---|---|---|---|---|
| GDM | ||||
| BMI gain of 1 to 3 kg/m2 vs BMI loss of >1 kg/m2 | −0.58 | −0.97 to −0.18 | 0.004 | |
| BMI gain of 1 to 3 kg/m2 vs BMI gain of ≥3 kg/m2 | 0.37 | −0.04 to 0.77 | 0.074 | |
| BMI loss of >1 kg/m2 vs BMI gain of ≥3 kg/m2 | 0.94 | 0.55 to 1.35 | <0.0001 | |
| GH | ||||
| BMI gain of 1 to 3 kg/m2 vs BMI loss of > 1 kg/m2 | −0.34 | −0.60 to −0.09 | 0.008 | |
| BMI gain of 1 to 3 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.29 | 0.05 to 0.54 | 0.019 | |
| BMI loss of >1 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.64 | 0.38 to 0.90 | <0.0001 | |
| Preeclampsia | ||||
| BMI gain of 1 to 3 kg/m2 vs BMI loss of > 1 kg/m2 | −0.32 | −0.54 to −0.10 | 0.005 | |
| BMI gain of 1 to 3 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.32 | 0.12 to 0.52 | 0.002 | |
| BMI loss of >1 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.64 | 0.41 to 0.87 | <0.0001 | |
| Cesarean section | ||||
| BMI gain of 1 to 3 kg/m2 vs BMI loss of > 1 kg/m2 | −0.11 | −0.21 to −0.01 | 0.027 | |
| BMI gain of 1 to 3 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.15 | 0.06 to 0.25 | 0.001 | |
| BMI loss of >1 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.27 | 0.16 to 0.37 | <0.0001 | |
| Preterm birth | ||||
| BMI gain of 2 to 4 kg/m2 vs BMI loss of > 2 kg/m2 | −0.03 | −0.41 to 0.36 | 0.880 | |
| BMI gain of 2 to 4 kg/m2 vs BMI gain of ≥ 4 kg/m2 | −0.07 | −0.45 to 0.32 | 0.727 | |
| BMI loss of >2 kg/m2 vs BMI gain of ≥ 4 kg/m2 | −0.04 | −0.43 to 0.36 | 0.849 | |
| SGA | ||||
| BMI loss of >2 kg/m2 vs BMI gain of ≥ 2 kg/m2 | −0.03 | −0.28 to 0.22 | 0.802 | |
| LGA | ||||
| BMI loss of >2 kg/m2 vs BMI gain of 1 to 3 kg/m2 | 0.59 | 0.27 to 0.92 | 0.0003 | |
| BMI loss of >2 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.86 | 0.53 to 1.19 | <0.0001 | |
| BMI gain of 1 to 3 kg/m2 vs BMI loss of > 1 kg/m2 | v0.63 | −0.93 to −0.33 | <0.0001 | |
| BMI gain of 1 to 3 kg/m2 vs BMI gain of ≥ 3 kg/m2 | 0.27 | −0.02 to 0.56 | 0.072 | |
Note: Meta‐regression was used to determine if subgroups were significantly different in effect size. The coefficient is the difference in odds ratio between subgroups. Significant probability values indicate that the two groups compared were significantly different in the outcome studied. The first subgroup that is mentioned is used as reference group.
Abbreviations: BMI, body mass index; 95%CI, 95% confidence interval; GDM, gestational diabetes mellitus; GH, gestational hypertension; LGA, large for gestational age; SGA, small for gestational age.
3.3.1. Gestational diabetes mellitus
Eleven studies assessed GDM,15, 16, 17, 18, 19, 20 of which five studies21, 22, 23, 24, 25 were eligible for the meta‐analysis. The meta‐analysis found a significant, positive association between interpregnancy BMI gain and GDM development during the second pregnancy (Figure S1: OR 1.64 [95%CI 1.28‐2.11]; I2 = 78% for BMI gain of 1 to 3 kg/m2; OR 2.42 [1.62‐3.62]; I2 = 89% for BMI gain of ≥3 kg/m2). In contrast, BMI loss of ≥1 kg/m2 was not significantly associated with the risk of GDM (Figure S1: OR 0.77 [0.48‐1.21]; I2 = 79%). Meta‐regression showed that the effect size on GDM in women who lost ≥1 kg/m2 between pregnancies was significantly lower than women who gained 1 to 3 kg/m2 (p = 0.004) or ≥3 kg/m2 (p < 0.0001). No difference in effect size was found between women with BMI gain of 1 to 3 kg/m2 and women with BMI gain of ≥3 kg/m2 (p = 0.074; Table 1). In the studies of Glazer et al15 Knight‐Agarwal et al16 McBain et al17 Lu et al18 Whiteman et al19 and Bender et al20 (not included in the meta‐analysis), the results were comparable to the meta‐analysis. It was demonstrated that the effects of interpregnancy BMI gain on the risk for developing GDM were highest in women with normal weight before the first pregnancy (Table S8).17, 21, 22, 23 For mothers with overweight or obesity before the first pregnancy, interpregnancy BMI loss decreased the risk of GDM.17, 22 Changing to a lower BMI category decreased the risk of developing GDM.19
3.3.2. Gestational hypertension
In six studies, GH was assessed.20, 21, 23, 24, 26, 27 Four studies were included in the meta‐analysis,21, 23, 24, 27 which resulted in a nonsignificant effect for BMI loss of ≥1 kg/m2 (Figure S2: OR 1.07 [0.85‐1.34]; I2 = 42%) on the development of GH. Increasing interpregnancy BMI gain was associated with a significantly increased risk of GH development (Figure S2: OR 1.49 [1.34‐1.66]; I2 = 0% for BMI gain of 1 to 3 kg/m2; OR 1.98 [1.56‐2.50]; I2 = 62% for BMI gain of ≥3 kg/m2). Meta‐regression found that effect sizes were significantly different between subgroups (Table 1). The lowest effect size was found for the subgroup of BMI loss of ≥1 kg/m2 and the highest effect size for the subgroup of BMI gain of ≥3 kg/m2 (Figure 2). The studies of Hoff et al26 and Bender et al20 which were not included in the meta‐analysis, found no effect of weight change between pregnancies on GH. The studies of Villamor and Cnattingius,23 Lynes et al24 and Wallace et al27 showed that, in women who started with normal weight, interpregnancy BMI gain resulted in a higher risk of developing GH during the second pregnancy compared to women who started with overweight or obesity (Table S9).
3.3.3. Preeclampsia
Six studies assessed preeclampsia as an outcome.23, 24, 27, 28, 29, 30 Three studies were included in the meta‐analysis,23, 24, 27 which found no effect for preeclampsia after loss of >1 kg/m2 between pregnancies (Figure S3: OR 0.84 [0.69‐1.01]; I2 = 0%). An increased risk for developing preeclampsia during the second pregnancy was found for interpregnancy BMI gain of 1 to 3 kg/m2 (Figure S3: OR 1.36 [1.22‐1.53]; I2 = 0%) and an even higher risk was found for BMI gain ≥3 kg/m2 (OR 1.77 [1.53‐2.04]; I2 = 0%). Meta‐regression found that the subgroup of BMI loss of >1 kg/m2 between pregnancies had a significantly lower effect size compared to both subgroups of BMI gain of 1 to 3 kg/m2 (p = 0.005) and BMI gain of ≥3 kg/m2 (p < 0.0001). The difference in effect size of the subgroup of BMI gain of 1 to 3 kg/m2 and the subgroup of BMI gain of ≥3 kg/m2 (p = 0.002) was significant as well (Table 1). In the study of Jain et al28 (not included in the meta‐analysis), significantly different prevalence rates of preeclampsia between women with BMI loss of >2 kg/m2 (5.2%), BMI maintenance (6.1%), and BMI gain of ≥2 kg/m2 (7.1%) were shown. Getahun et al29 demonstrated that overweight or obesity in either the first or the second pregnancy was associated with an increased risk for preeclampsia, independent of BMI gain or loss between pregnancies (Table S10). The effect of interpregnancy BMI gain was strongest in women with overweight or obesity.27
3.3.4. Cesarean section
Ten studies included cesarean section as an outcome,16, 20, 24, 26, 31, 32, 33 of which four studies were eligible for meta‐analysis.21, 23, 24, 27 The meta‐analysis showed no effect of BMI loss ≥1 kg/m2 (Figure S4: OR 1.01 [0.91‐1.11]; I2 = 40%) on the prevalence of a cesarean section. Higher interpregnancy BMI gain was associated with a higher risk of a cesarean section at the end of the second pregnancy (OR 1.14 [1.05‐1.24]; I2 = 51% for BMI gain of 1 to 3 kg/m2; OR 1.33 [1.19‐1.48]; I2 = 50% for BMI gain of ≥3 kg/m2). Meta‐regression showed that effect sizes varied significantly per subgroup (Table 1). The lowest effect size was found for BMI loss of ≥1 kg/m2 and the highest effect size for BMI gain of ≥3 kg/m2 (Figure 2). In the study of Dude et al31 (not included in the meta‐analysis), a decreased risk of a cesarean section after BMI loss of >2 kg/m2 between pregnancies was shown. Furthermore, interpregnancy weight gain resulted in an increased risk of a cesarean section.31 The studies of Knight‐Agarwal et al16 Hoff et al26 and Bender et al20 which were not included in the meta‐analysis as well, showed no significant effects of BMI change and BMI category shift between two pregnancies on the risk of cesarean section in the second pregnancy. On the other hand, Getahun et al32 and Whiteman et al33 did show a significant effect of a BMI shift between pregnancies from all BMI categories before the first pregnancy to becoming affected by overweight or obesity before the second pregnancy on the risk of a cesarean section. Women of normal weight before the first pregnancy who changed BMI in any direction between the pregnancies had a significantly higher risk of an emergency cesarean section in the second pregnancy compared to women with overweight or obesity before the first pregnancy (Table S11).27
3.3.5. Preterm birth
Eleven studies analyzed preterm birth as an outcome.17, 20, 26, 27, 34, 35, 36, 37, 38, 39, 40 Four of these studies were included in the meta‐analysis.17, 27, 39, 40 Meta‐analysis demonstrated that interpregnancy BMI loss of >1 kg/m2 was associated with a higher risk of preterm birth (Figure S5: OR 1.41 [1.06‐1.89]; I2 = 72%). Other BMI change categories were not associated with the risk of preterm birth. Studies not included in the meta‐analysis demonstrated no effect of BMI change between pregnancies on preterm birth.20, 26, 27, 34, 35, 36, 37, 38 Women with underweight or normal weight before the first pregnancy, were at highest risk for preterm birth when losing weight between pregnancies (Table S12).27, 40 A lower risk for spontaneous preterm birth, though a higher risk for medically indicated preterm birth, was found in women becoming affected by overweight or obesity before the second pregnancy or gaining weight between pregnancies.36 Interestingly, women with overweight or obesity before the first pregnancy who lost weight were at higher risk for preterm birth between 32 and 36 weeks of gestational age.39
3.3.6. Small for gestational age
In six studies, SGA was assessed.17, 26, 27, 28, 41, 42 Two of the studies were included in the meta‐analysis.17, 28 In this analysis, it was shown that BMI loss of >1 kg/m2 was related to a higher risk of SGA (Figure S6: OR 1.58 [1.26‐1.98]; I2 = 84%). BMI loss of >2 kg/m2 and none of the BMI gain categories were associated with SGA. When considering the studies not included in the meta‐analysis, both studies of Wallace et al originated from the same data source.27, 42 Therefore, it was decided to exclude the data of Wallace et al42 (2017) from the data extraction because of a higher sample size in the other study of Wallace et al27 (2014) while both studies were of equal quality. Hoff et al26 and Cheng et al41 (not included in the meta‐analysis) showed similar findings as the results of the meta‐analysis. Wallace et al27 found that women with decreasing interpregnancy BMI had a higher risk of SGA, whereas women with increasing BMI had a lower risk of SGA. Sensitivity analyses resulted in an increased risk for SGA after weight loss between pregnancies in the case of women who had an initial BMI <25 kg/m2 (Table S13).17, 27, 40 Contrasting results on risks of SGA were found for women who had overweight or obesity before the first pregnancy and lost weight between pregnancies.17, 27
3.3.7. Large for gestational age
Eight studies were included in the data extraction for LGA26, 43 of which six studies were included in meta‐analysis.17, 23, 27, 28, 40, 44 BMI loss of >2 kg/m2 did not result in a significant effect on LGA (BMI change between −2 and 2 kg/m2 was used as reference) while BMI loss of >1 kg/m2 did result in a significant decreased effect (Figure S7: OR 0.80 [0.66‐0.98]; I2 = 95%; BMI change between −1 and 1 kg/m2 was used as reference). This can potentially be caused by a lack of power due to a low number of events in the meta‐analysis for BMI loss of >2 kg/m2. In all cases, BMI gain resulted in an increased risk for LGA (OR 1.33 [1.11‐1.60]; I2 = 0% for BMI gain of 1 to 3 kg/m2; OR 1.28 [1.10‐1.50]; I2 = 58% for BMI gain of ≥2 kg/m2; OR 1.54 [1.28‐1.86]; I2 = 0% for BMI gain of ≥3 kg/m2). Both the effect sizes of the subgroup with BMI loss of >2 kg/m2 (p = 0.003) and the subgroup with BMI loss >1 kg/m2 (p < 0.0001) were significantly different from the subgroup with BMI gain of 1 to 3 kg/m2. No significant difference in effect size was found between subgroups of BMI gain of 1 to 3 kg/m2and BMI gain ≥3 kg/m2 (p = 0.072; Table 1). When considering the results of the study of Getahun et al43 (not included in the meta‐analysis), the risk of LGA was reduced in women with underweight or normal weight before the first and second pregnancy, while an increased risk was found in most other BMI category shift options. Hoff et al26 found no differences between women who were overweight before the first pregnancy and shifted to other BMI categories before the second pregnancy. A decreased risk was found for LGA after weight loss between pregnancies and an increased risk after weight increase both for women with a BMI < 25 kg/m2 and women with a BMI ≥25 kg/m2 before the first pregnancy (Table S14).23, 27, 40, 44 McBain et al did not find significant results in these sensitivity analyses.17
3.4. Risk of bias
Cohen's kappa coefficient of all domains of the QUIPS tool was 0.75 (95%CI 0.61‐0.89). Seven studies included in the meta‐analysis and three studies not included in the meta‐analysis were assessed as having a low risk of bias. Eleven studies were assessed as having a moderate risk of bias of which four studies were included in the meta‐analysis. Nine studies, all not included in the meta‐analysis, had a high risk of bias (Table S15). Most studies properly described the process of the databases and statistical models used. The most commonly found risk of bias was related to self‐reported prepregnancy weight and height and was thus not based on objective measures. Furthermore, most of the studies with a high risk of bias had insufficiently adjusted for possible confounders. Excluding the studies with a high risk of bias did not affect the results for GDM, preeclampsia, cesarean section, preterm birth, and SGA. For GH and LGA, no studies had to be excluded from the meta‐analyses. By excluding the insignificant results of the studies with a high risk of bias, the increased risk for GH and LGA became stronger.20, 26
4. DISCUSSION
In this systematic review and meta‐analysis, data is pooled of over 1 million women covering the entire BMI spectrum and various geographical regions. In all BMI categories, interpregnancy BMI gain resulted in a higher incidence rate of GDM, GH, preeclampsia, cesarean section, and LGA. Women with underweight and normal weight were more affected by weight gain compared to women with overweight or obesity in terms of risk for GDM, GH, and cesarean section. Interpregnancy BMI loss was associated with a decreased risk of LGA independently of BMI category, and with a decreased risk of GDM in women with overweight or obesity before the first pregnancy. No protective effect of weight loss was found for the other perinatal outcomes, whereas an adverse effect of weight loss—demonstrated by higher SGA and preterm birth incidence—was found.
This review is innovative in the approach to study weight change between pregnancies. It is demonstrated that weight gain between pregnancies increases the risk for perinatal complications. Interestingly, the effects of interpregnancy weight change were dependent on the initial BMI of women before conception. Previous studies have already demonstrated that a higher BMI before conception is associated with a higher risk of GDM, GH, preeclampsia, cesarean section, and LGA.3, 45, 46 Furthermore, it has been demonstrated that excessive gestational weight gain is associated with an increased risk of perinatal complications.1, 47, 48 The results from the current review provide the insight that it is not merely the static weight status before conception and gestational weight gain that negatively influence perinatal outcomes. Weight change in the period immediately prior to pregnancy also has a significant impact on perinatal outcomes. The findings of the current review are in line with the results of the review of Oteng‐Ntim et al who considered the effect of interpregnancy weight change.49 Both reviews found that interpregnancy weight gain is related to an increased risk of GDM, cesarean section, and LGA. Moreover, the current systematic review provides new insights on additional perinatal outcomes including GH, preeclampsia, and preterm birth. Furthermore, a meta‐regression is included in this review that showed differences in effects between subgroups indicating a dose‐dependent effect of interpregnancy weight gain. Contrasting results were found regarding the effect of interpregnancy weight loss on GDM. Oteng‐Ntim et al described a decreased risk of GDM when losing weight between pregnancies whereas the current review only demonstrated this effect in women with overweight or obesity before the first pregnancy. This difference might be explained by the inclusion of a larger number of studies in the current review.
The results of this systematic review provide evidence for the impact of weight gain between pregnancies, but might also be relevant for primigravidae. When the adverse effects of interpregnancy weight gain are extrapolated to weight gain in the years before the first pregnancy, the change from normal weight to overweight and obesity frequently found in this specific life phase is worrisome. Data derived from population surveys in the USA show that obesity prevalence almost doubles, from 21% in female adolescents (12‐19 years of age) to 37% in women of 20 to 39 years of age. This underscores the high risk for gaining weight at reproductive age.50, 51 This distinct increase in overweight and obesity prevalence from childhood to adolescence is also evident in Europe, Asia, and South America.52, 53, 54, 55
The prevalence of BMI gain in the studies included in this systematic review indicates that a significant number of women are at risk for interpregnancy BMI gain and thus for an increase in perinatal complications, as 32% to 53% of women had a BMI gain of at least 1 kg/m2.16, 21, 22, 23, 24, 27 Determinants for interpregnancy BMI gain in the studies included in this review were a younger maternal age, longer interpregnancy interval, a lower educational level, and preeclampsia, GH, and a cesarean section during the first pregnancy.21, 23, 24, 27 Regarding other factors with a potential impact, consensus was not reached on risk factors such as no breastfeeding, smoking, parity, lack of sleeping time, lack of exercise in the postpartum period, and higher prepregnancy BMI.56, 57, 58
While interpregnancy weight gain is associated with an increased risk for several adverse perinatal outcomes, weight loss between pregnancies only resulted in a reduced risk of limited perinatal complications. Since several studies found evidence for the relationship between overweight/obesity and GH, preeclampsia, cesarean section, and preterm birth,3, 45, 48 the finding that weight loss only resulted in limited protective effects on these perinatal health risks was unexpected. A possible explanation might be that although BMI reduced in women classified in the “weight loss” group in the studies, the total BMI loss of >1 kg/m2 without further stratification was too small to have an effect on complications. A larger degree of weight loss, a shift in BMI category from overweight or obesity to normal weight, was not found to decrease the risk of preeclampsia, cesarean section and preterm birth, either.19, 29, 36 From the studies included in this systematic review, it is unclear which factors contributed to the weight loss that was observed.
This comprehensive systematic review merged data from more than 1 million women globally of which 745,993 women could be included in the meta‐analysis. This large sample size allows strong conclusions to be drawn from the results. However, some critical remarks need to be considered. First, only a subset of studies could be included in the meta‐analyses. Those studies were all classified as having a low or moderate risk of bias and a large part of the total number of participants could be included in the meta‐analyses, which underscores the ability to draw strong conclusions from it. Second, definitions of several outcome measures were heterogeneous. Apart from the differences in inclusion criteria between studies, this may have contributed to the statistical and between‐study heterogeneity. Furthermore, the broad time frame (1959‐2017) in which included studies collected their data might have contributed to the heterogeneity between studies due to changes in society and public health practices. On the other hand, our limited exclusion criteria enhance the generalizability of our findings. Second, although several confounders were considered in most studies, potential other confounders such as lifestyle behaviors could have influenced the risk on perinatal complications. In addition, the adjustment for confounders was variable across studies.
In summary, the findings of this meta‐analysis imply that, in current clinical and research practice on prevention of adverse perinatal outcomes, the focus on specific target groups could be extended. First, our findings indicate that limiting gestational weight gain should not be an exclusive target, but that prevention of weight gain in the interpregnancy period should also be emphasized. In the light of the growing prevalence rates of obesity in women of childbearing age, it is important to start targeting women in the years before conception. As gestational weight gain is part of the interpregnancy weight change, limiting gestational weight gain should of course not be neglected in prevention strategies.56 Second, women with normal weight currently tend to escape our attention.59 Data aggregated in this systematic review stresses the importance of targeting prevention strategies not only on women with overweight and obesity but also on women of normal weight. Third, further research should focus on defining determinants that predict the benefits to be derived from prevention of weight gain, thus allowing targeting specific prevention strategies at those women most at risk. Finally, it was found that prevention strategies should aim at prevention of weight gain in the years before conception and between pregnancies rather than at stimulating weight loss. Due to the lack of studies regarding the effects of these targets, it is recommended to prospectively examine the effects of the prevention of prepregnancy weight gain in women of all BMI categories on perinatal outcomes by a randomized controlled trial, taken into account the potential determinants of weight gain. These studies might be the way forward in the prevention of adverse perinatal outcomes.
DECLARATION OF INTERESTS
Jos Kleijnen declares Kleijnen Systematic Reviews Ltd has received project funding from various pharmaceutical companies for work in areas unrelated to this manuscript. For the remaining authors, there are no declarations of interest.
AUTHORS' CONTRIBUTION
All authors contributed to the study design. Y.T, E.O, and E.V were involved in data collection and analysis. KK and AV supervised data analysis. Y.T, K.K, E.O, and A.V drafted the manuscript. All authors contributed to interpretation of the results and to the discussion, critically reviewed the paper, and approved the final version of the article. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DATA ACCESSIBILITY
Not applicable; all data used in the meta‐analyses are extracted from the original articles included in the meta‐analyses. No individual data has been used.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGEMENTS
We gratefully acknowledge Steven Duffy (Kleijnen Systematic Reviews [KSR] Ltd, York, UK) for checking the search strategy. No funding sources were available for this work.
Timmermans YEG, van de Kant KDG, Oosterman EO, et al. The impact of interpregnancy weight change on perinatal outcomes in women and their children: A systematic review and meta‐analysis. Obesity Reviews. 2020;21:e12974 10.1111/obr.12974
REFERENCES
- 1. Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta‐analysis. JAMA. 2017;317(21):2207‐2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175‐1182. [DOI] [PubMed] [Google Scholar]
- 3. Abenhaim HA, Kinch RA, Morin L, Benjamin A, Usher R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch Gynecol Obstet. 2007;275:39‐43. [DOI] [PubMed] [Google Scholar]
- 4. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non‐communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Ludwig DS. Dietary guidelines in the 21st century—a time for food. JAMA. 2010;304(6):681‐682. [DOI] [PubMed] [Google Scholar]
- 6. Hales CN, Barker DJ. The thrifty phenotype hypothesis: Type 2 diabetes. Br Med Bull. 2001;60:5‐20. [DOI] [PubMed] [Google Scholar]
- 7. Catalano PM, Ehrenberg HM. The short‐ and long‐term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126‐1133. [DOI] [PubMed] [Google Scholar]
- 8. Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long‐term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46(6):434‐438. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization . Preconception care to reduce maternal and childhood mortality and morbidity. Switzerland: World Health Organization: Geneva; 2013. [Google Scholar]
- 10. Timmermans YEG, Oosterman EO, Spaanderman MEA, Kleijnen JMP, Vreugdenhil ACE, van de Kant KDG. Systematic review of the effect of interpregnancy weight change on perinatal outcomes in women and their children. PROSPERO 2016:CRD42016043307 2016. http://www.crd.york.ac.uk/PROSPERO/display_record.asp? ID=CRD42016043307 [DOI] [PMC free article] [PubMed]
- 11. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 12. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. United Kingdom: John Wiley & Sons Ltd; 2009. [Google Scholar]
- 13. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280‐286. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733‐737. [DOI] [PubMed] [Google Scholar]
- 16. Knight‐Agarwal CR, Williams LT, Davis D, et al. Association of BMI and interpregnancy BMI change with birth outcomes in an Australian obstetric population: a retrospective cohort study. BMJ Open. 2016;6:e010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McBain RD, Dekker GA, Clifton VL, Mol BW, Grzeskowiak LE. Impact of inter‐pregnancy BMI change on perinatal outcomes: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2016;205:98‐104. [DOI] [PubMed] [Google Scholar]
- 18. Lu GC, Luchesse A, Chapman V, Cliver S, Rouse DJ. Screening for gestational diabetes mellitus in the subsequent pregnancy: is it worthwhile? Am J Obstet Gynecol. 2002;187(4):918‐921. [DOI] [PubMed] [Google Scholar]
- 19. Whiteman VE, Aliyu MH, August EM, et al. Changes in prepregnancy body mass index between pregnancies and risk of gestational and type 2 diabetes. Arch Gynecol Obstet. 2011;284(1):235‐240. [DOI] [PubMed] [Google Scholar]
- 20. Bender W, Hirshberg A, Levine LD. Interpregnancy body mass index changes: distribution and impact on adverse pregnancy outcomes in the subsequent pregnancy. American Journal of Perinatology. 2019;36(5):517‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bogaerts A, Van Den Bergh BR, Ameye L, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol. 2013;122(5):999‐1009. [DOI] [PubMed] [Google Scholar]
- 22. Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011;117(6):1323‐1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population‐based study. Lancet. 2006;368:1164‐1170. [DOI] [PubMed] [Google Scholar]
- 24. Lynes C, McLain AC, Yeung EH, Albert P, Liu J, Boghossian NS. Interpregnancy weight change and adverse maternal outcomes: a retrospective cohort study. Ann Epidemiol. 2017;27:632‐637. e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sorbye LM, Skjaerven R, Klungsoyr K, Morken NH. Gestational diabetes mellitus and interpregnancy weight change: a population‐based cohort study. PLoS Med. 2017;14:e1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hoff GL, Cai J, Okah FA, Dew PC. Pre‐pregnancy overweight status between successive pregnancies and pregnancy outcomes. J Womens Health. 2009;18:1413‐1417. [DOI] [PubMed] [Google Scholar]
- 27. Wallace JM, Bhattacharya S, Campbell DM, Horgan GW. Inter‐pregnancy weight change impacts placental weight and is associated with the risk of adverse pregnancy outcomes in the second pregnancy. BMC Pregnancy Childbirth. 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jain AP, Gavard JA, Rice JJ, Catanzaro RB, Artal R, Hopkins SA. The impact of interpregnancy weight change on birthweight in obese women. Am J Obstet Gynecol. 2013;208:205 e1‐205 e7. [DOI] [PubMed] [Google Scholar]
- 29. Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol. 2007;110(6):1319‐1325. [DOI] [PubMed] [Google Scholar]
- 30. Bhattacharya S, Campbell DM, Smith NC. Pre‐eclampsia in the second pregnancy: does previous outcome matter? European Journal of Obstetrics, Gynecology, & Reproductive Biology. 2009;144:130‐134. [DOI] [PubMed] [Google Scholar]
- 31. Dude AM, Lane‐Cordova AD, Grobman WA. Interdelivery weight gain and risk of cesarean delivery following a prior vaginal delivery. Am J Obstet Gynecol. 2017;217:373 e1‐373 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Getahun D, Kaminsky LM, Elsasser DA, Kirby RS, Ananth CV, Vintzileos AM. Changes in prepregnancy body mass index between pregnancies and risk of primary cesarean delivery. Am J Obstet Gynecol. 2007;197(376):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 33. Whiteman VE, McIntosh C, Rao K, Mbah AK, Salihu HM. Interpregnancy BMI change and risk of primary caesarean delivery. J Obstet Gynaecol. 2011;31:589‐593. [DOI] [PubMed] [Google Scholar]
- 34. Chen A, Klebanoff MA, Basso O. Pre‐pregnancy body mass index change between pregnancies and preterm birth in the following pregnancy. Paediatr Perinat Epidemiol. 2009;23(3):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riley KL, Carmichael SL, Mayo JA, et al. Body mass index change between pregnancies and risk of spontaneous preterm birth. Am J Perinatol. 2016;33(10):1017‐1022. [DOI] [PubMed] [Google Scholar]
- 36. Whiteman VE, Rao K, Duan J, Alio A, Marty PJ, Salihu HM. Changes in prepregnancy body mass index between pregnancies and risk of preterm phenotypes. Am J Perinatol. 2011;28:67‐74. [DOI] [PubMed] [Google Scholar]
- 37. Girsen AI, Mayo JA, Wallenstein MB, et al. What factors are related to recurrent preterm birth among underweight women? J Matern Fetal Neonatal Med. 2018;31:560‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Merlino A, Laffineuse L, Collin M, Mercer B. Impact of weight loss between pregnancies on recurrent preterm birth. Am J Obstet Gynecol. 2006;195(3):818‐821. [DOI] [PubMed] [Google Scholar]
- 39. Villamor E, Cnattingius S. Interpregnancy weight change and risk of preterm delivery. Obesity. 2016;24(3):727‐734. [DOI] [PubMed] [Google Scholar]
- 40. Benjamin RH, Littlejohn S, Canfield MA, Ethen MK, Hua F, Mitchell LE. Interpregnancy change in body mass index and infant outcomes in Texas: a population‐based study. BMC Pregnancy and Childbirth. 2019;19:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheng CJ, Bommarito K, Noguchi A, Holcomb W, Leet T. Body mass index change between pregnancies and small for gestational age births. Obstet Gynecol. 2004;104(2):286‐292. [DOI] [PubMed] [Google Scholar]
- 42. Wallace JM, Bhattacharya S, Horgan GW. Weight change across the start of three consecutive pregnancies and the risk of maternal morbidity and SGA birth at the second and third pregnancy. PLoS One. 2017;12:e0179589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Getahun D, Ananth CV, Peltier MR, Salihu HM, Scorza WE. Changes in prepregnancy body mass index between the first and second pregnancies and risk of large‐for‐gestational‐age birth. Am J Obstet Gynecol. 2007;196(530):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 44. Ziauddeen N, Wilding S, Roderick PJ, Macklon NS, Alwan NA. Is maternal weight gain between pregnancies associated with risk of large‐for‐gestational age birth? Analysis of a UK population‐based cohort. BMJ Open. 2019;9:e026220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bhattacharya S, Campbell DM, Liston WA, Bhattacharya S. Effect of Body Mass Index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zaballa K, Liu A, Peek MJ, Mongelli M, Nanan R. Association between World Health Organization categories of body mass index and relative risks for weight‐related pregnancy outcomes: a retrospective cohort study. Obstet Med. 2012;5(3):112‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Macdonald‐Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2013;209(327):e1‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou A, Xiong C, Hu R, et al. Pre‐pregnancy BMI, gestational weight gain, and the risk of hypertensive disorders of pregnancy: a cohort study in Wuhan, China. PLoS One. 2015;10:e0136291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oteng‐Ntim E, Mononen S, Sawicki O, Seed PT, Bick D, Poston L. Interpregnancy weight change and adverse pregnancy outcomes: a systematic review and meta‐analysis. BMJ Open. 2018;8:e018778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flegal KM, Kruszon‐Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284‐2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988‐1994 through 2013‐2014. JAMA. 2016;315(21):2292‐2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berghofer A, Pischon T, Reinhold T, Apovian C, Sharma A, Willich SN. Obesity prevalence from a European perspective: a systematic review. BMC Public Health. 2008;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wijnhoven TM, van Raaij JM, Spinelli A, et al. WHO European Childhood Obesity Surveillance Initiative 2008: weight, height and body mass index in 6‐9‐year‐old children. BMC Public Health. 2014;14:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Endres LK, Straub H, McKinney C, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstet Gynecol. 2015;125(1):144‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord. 2000;24(12):1660‐1668. [DOI] [PubMed] [Google Scholar]
- 58. Schauberger CW, Rooney BL, Brimer LM. Factors that influence weight loss in the puerperium. Obstet Gynecol. 1992;79(3):424‐429. [DOI] [PubMed] [Google Scholar]
- 59. Oteng‐Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta‐analysis. BMC Med. 2012;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Data Availability Statement
Not applicable; all data used in the meta‐analyses are extracted from the original articles included in the meta‐analyses. No individual data has been used.


