Abstract
The tumor suppressor phosphatase and tensin homolog on chromosome 10 (PTEN) is a tightly regulated enzyme responsible for dephosphorylating the progrowth lipid messenger molecule phosphatidylinositol 3,4,5-trisphosphate (PIP3) on the plasma membrane. The carboxy-terminal tail (CTT) of PTEN is key for regulation of the enzyme. When phosphorylated, the unstructured CTT interacts with the phosphatase-C2 superdomain to inactivate the enzyme by preventing membrane association. PTEN mutations associated with cancer also inactivate the enzyme. Alternate translation-initiation sites generate extended isoforms of PTEN, such as PTEN-L that has multiple roles in cells. The extended amino-terminal region bears a signal sequence and a polyarginine sequence to facilitate exit from and entry into cells, respectively, and a membrane-binding helix that activates the enzyme. This amino-terminal region also facilitates mitochondrial and nucleolar localization. This review explores PTEN structure and its impact on localization and regulation.
Phosphatase and tensin homolog on chromosome 10 (PTEN) has been the subject of intense study since its discovery more than 20 years ago as a “candidate tumor suppressor gene” in 1997 (Li 1997), and has frequently surprised researchers during those 20 years. Initially identified as a dual-specificity phosphatase (DUSP) protein (Myers et al. 1997), it was later determined to have phosphatidylinositol 3-phosphatase activity (crucial to its tumor suppressor activity [Myers et al. 1998]), and more recently a potential 5-phosphatase activity also (Malek et al. 2017). The structure of most of PTEN was determined by X-ray crystallography soon after (Lee et al. 1999), and this provided a great deal of insight into PTEN's mechanism, despite a number of the intrinsically disordered regulatory elements of PTEN being removed to facilitate crystallization. The landscape of PTEN's structure and regulation is varied, incorporating dimerization (Papa et al. 2014; Heinrich et al. 2015), membrane binding (Das et al. 2003; Shenoy et al. 2012; Masson et al. 2016), and a litany of posttranslational modifications, including oxidation (Lee et al. 2002), phosphorylation (Torres and Pulido 2001; Vazquez et al. 2001; Rahdar et al. 2009; Cordier et al. 2012), acetylation (Okumura 2006), SUMOlyation (Huang et al. 2012; Bassi et al. 2013), and ubiquitination (Wang et al. 2007). Furthermore, several alternative translation-initiation sites for PTEN have been characterized, such as PTEN-Long (also known as PTEN-L and PTEN-α) (Hopkins et al. 2013; Liang et al. 2014) and PTEN-β (Liang et al. 2017), as well as variants within these variants (Tzani et al. 2016). PTEN also engages in interactions with other proteins that regulate its activity. This complex modulation all takes place within a relatively compact enzyme. One central mechanism of PTEN regulation is controlling plasma membrane binding, as this is where its PIP3 substrate resides. Membrane binding also results in an allosteric regulation of PTEN's activity, and changes in interdomain interaction. In this review, we will explore how the structure of PTEN relates to its crucial phosphatase function, and how this is regulated.
THE STRUCTURE AND REGULATION OF PTEN
PTEN consists primarily of two folded globular domains, a DUSP domain (residues 15–185), and a C2 domain (192–353) (Fig. 1), with three disordered segments of the protein proceeding, linking, and following these domains. These disordered segments are crucial for the membrane binding and allosteric regulation that govern PTEN activity. The overall fold of PTEN is strikingly similar to the cytoskeletal actin-capping protein, tensin, and the clathrin/Hsc70/membrane-interacting protein, auxilin (see Fig. 2; Liaw et al. 1997; Steck et al. 1997; Guan et al. 2010). This similarity includes both the phosphatase and C2 domains.
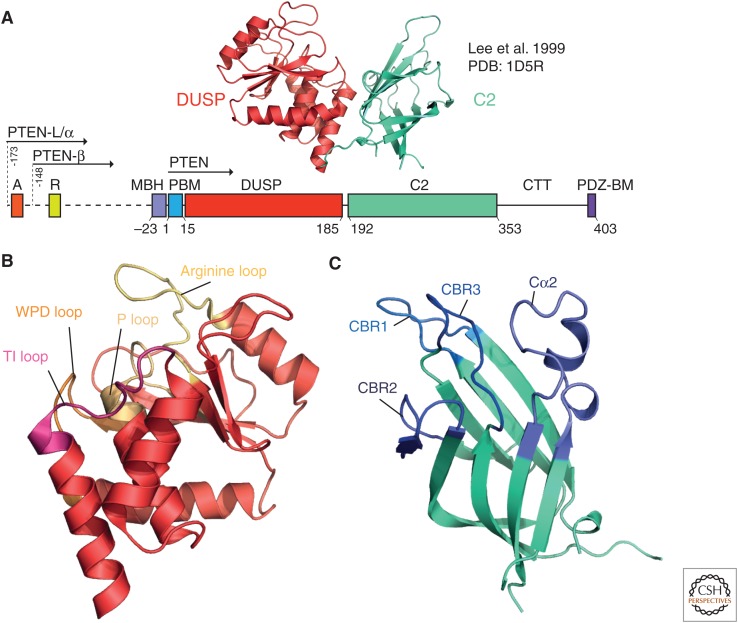
Figure 1.
PTEN crystal structures and variants. (A) Domain organization of PTEN and translational variants. The crystal structure (Lee et al. 1999) is shown above in cartoon-format, colored by domain, with the dual-specificity phosphatase (DUSP) domain in red and the C2 domain in teal. The domain organization of PTEN consists of the PIP2-binding motif (PBM), DUSP, and C2 domains, followed by the carboxy-terminal tail (CTT) and the PDZ-binding motif. Translational variants of PTEN show amino-terminal extensions, with PTEN-L/α having a 173-amino-acid disordered extension, and PTEN-β having a 148-amino-acid extension. The amino-terminal extension contains a polyalanine (domain “A,” 12–17-L) and polyarginine (“R,” 47–52-L), as well as a membrane-binding α-helix ([MBH], 151–174-L). (B) The DUSP domain. The three core loops of the active site of PTEN (the TI, WPD, and P loops) are shown, along with the arginine loop, key for membrane binding. (C) The calcium-independent C2 domain of PTEN, with the calcium-binding region (CBR) loops highlighted, along with the enlarged Cα2 loop.
Figure 2.
Illustration of the conserved C2 dual-specificity phosphatase (DUSP) superdomain. The C2-phosphatase superdomain is found in PTEN, AUXILIN, and TENSIN and has a similar structure in all three proteins. A cartoon representation is shown for the AUXILIN C2-DUSP (blue) superimposed on PTEN (green).
THE PIP2-BINDING MOTIF
The first 14 residues of PTEN constitute the PI(4,5)P2-binding motif ([PBM], occasionally referred to as the PIP2-binding domain) (Walker et al. 2004), a crucial stretch of basic residues (1-MTAIIKEIVSRNKRR-15) that determines how PTEN binds to anionic lipids and up-regulates PTEN's PIP3-phosphatase activity through an allosteric mechanism (Campbell et al. 2003). A low concentration (∼1%) of PI(4,5)P2 in phosphatidylcholine vesicles stimulated PTEN activity approximately eightfold (Walker et al. 2004), showing that there was potential for a positive-feedback loop for PTEN, with the PI(4,5)P2 product activating the enzyme. The K13E mutation ablates this activation mechanism and significantly reduces PTEN's membrane association in vivo.
Crystallographic studies removed the PBM (Lee et al. 1999), as it is likely that this disordered region would impede crystallization. Molecular modeling, coupled with nuclear magnetic resonance (NMR) measurements have suggested that the PBM may fold on membrane binding, producing an amphipathic, interfacial α-helix that lies partially buried on the membrane surface (Wei et al. 2015). This builds on the observation that when PTEN binds to a membrane containing PI(4,5)P2, there is an associated increase in α-helical character as measured by circular dichroism (Redfern et al. 2008). The binding of PI(4,5)P2 is facilitated by the creation of a distinct lipid-binding pocket, formed by the carboxyl terminus of the newly created aliphatic helix and nearby parts of the phosphatase domain (Wei et al. 2015). The importance of this pocket is made apparent by a number of tumor-derived missense mutations found within this area (S10N, G20E, L42R, and F90S) that disrupt PTEN's tumor-suppressor function by lowering its membrane affinity. Tethering PTEN to the membrane using a myristylation tag recovered PTEN activity, suggesting that there was no direct impact on the catalytic mechanism of PTEN (Nguyen et al. 2015).
THE PHOSPHATASE DOMAIN
The phosphatase domain of PTEN (residues 15–185) consists of a five-stranded β-sheet flanked by two α-helices on one side and four helices on the other, a structure homologous to both auxilin and tensin (see Fig. 1B and Fig. 2). The phosphatase domain of PTEN is also homologous to other DUSP proteins, and this points to PTEN's ability to dephosphorylate highly acidic stretches of protein (Myers et al. 1997). Sitting at the center of this domain, in the region 121–131, is the active site of PTEN, which contains the P loop with the highly conserved CX5R PTP domain motif (HCXXGXXR, in which X is any amino acid) (Patterson et al. 2009). The P loop contains the active site cysteine (C124) and is key to substrate catalysis. Both the cysteine and arginine residues within this loop, C124 and R130, are completely intolerant to mutation (Mighell et al. 2018).
The active site of PTEN is formed from three loops, the WPD loop (spanning residues 88–98 and given the name WPD following PTP1B nomenclature [Lee et al. 1999]), the TI loop, which contains a threonine and isoleucine pair and spans residues 160–171, and the P loop (see Fig. 3). These three loops create an unusually deep and wide, positively charged substrate pocket compared with other DUSPs, allowing PTEN to accommodate the bulky, negatively charged PI(3,4,5)P3 substrate with cysteine-124 sitting at the bottom of the pocket ready for catalysis. The cytosolic region of Ciona intestinalis voltage-sensitive phosphatase (Ci-VSP) is similar to PTEN, and the structure of this enzyme in a complex with inositol 1,4,5-trisphosphate (IP3) provided a glimpse into how the active-site pocket binds the PI(3,4,5) head group (Liu et al. 2012). The three loops are highly conserved (with the P loop the most conserved), and have been the subject of alanine scanning mutagenesis to determine the individual contributions of each amino acid to the phosphatase activity of PTEN (Rodríguez-Escudero et al. 2011; Mighell et al. 2018). These results highlight how sensitive the P loop is to mutation. The crucial catalytic cysteine in the P loop forms a covalent cysteinyl phosphoenzyme intermediate (Xiao et al. 2007), which is subsequently hydrolyzed through the action of D92, within the WPD loop.
Figure 3.
A view of the active site of PTEN, showing how the site is formed at the confluence of the WPD, TI, and P loops.
Within the active site, specific residues can be mutated that alter PTEN's substrate specificity. Although a C124S mutation completely inactivates PTEN (Maehama and Dixon 1998), PTEN can also be mutated to become either a solely lipid or protein phosphatase. The G129E mutant identified from a Cowden syndrome mutation lacks lipid phosphatase activity but remains mostly active with protein substrates (Myers et al. 1998), and the Y138L mutant, an engineered construct used to investigate the role of PTEN's protein phosphatase activity in vivo, does the opposite (i.e., it maintains lipid phosphatase activity while ablating protein phosphatase activity) (Davidson et al. 2010). These two mutations have been crucial in deciphering the contributions that PTEN's lipid and protein phosphatase activity play in PTEN's biological functions (Shi et al. 2014; Shnitsar et al. 2015; Malek et al. 2017), and they highlight the promiscuity of the enzyme and how its activity is mediated by the amino acids found within the three loops comprising the active site of PTEN.
Additionally, within the phosphatase domain, is the “arginine loop,” spanning residues 35–49. This loop forms a positively charged patch on the phosphatase domain that is also vital for membrane binding, and is the main contact for the phosphatase domain's interaction with membranes (Shenoy et al. 2012; Nanda et al. 2015; Masson et al. 2016; Irvine et al. 2019).
THE C2 DOMAIN
PTEN's type II calcium-independent C2 domain (residues 192–353) is a typical β-sandwich structure consisting of two antiparallel β-sheets with two small α-helical segments in the loops connecting β-strands (see Fig. 1C). The C2 domain's structure is unremarkable aside from the Cα2 loop being noticeably longer in PTEN than in homologous structures, and this loop plays a role not only in the regulation of membrane binding (Yasui et al. 2014; Masson et al. 2016) but also in the interactions between the two-folded domains of PTEN (Lee et al. 1999).
The C2 domain interacts nonspecifically with the plasma membrane (Campbell et al. 2003) (although it may preferentially associate with phosphatidylserine [Das et al. 2003] or PI(3)P [Naguib et al. 2015]; see below) and is capable of binding to the membrane when expressed on its own, which the phosphatase domain cannot (Lee et al. 1999). The membrane interaction is largely dependent on the calcium-binding region (CBR) loops of the C2 domain: CBR1 (residues 200–212), CBR2 (226–238), and the key membrane-binding loop, CBR3 (258–268) (Campbell et al. 2003; Nanda et al. 2015; Masson et al. 2016; Irvine et al. 2019). These loops differ from Ca2+-dependent C2 domains as they have largely lost the residues responsible for cation coordination (only Asp268 remains), and instead they have a net +5 positive charge on the CRB3 loop (Lee et al. 1999). Although membrane interaction takes place primarily through these three loops, there may be a possible folding event associated with other loops in the domain, as membrane binding increases the β-sheet content of the domain (Redfern et al. 2008).
One of the key interfaces in the regulation of PTEN is that between the C2 and phosphatase domain, indeed the intricate dialogue between these two domains has led to their description as a “superdomain” (Haynie and Xue 2015). A number of oncogenic mutations in PTEN result in the breakdown of the interdomain interacting interface (Smith et al. 2018), with an increase in structural dynamics observed between the two domains associated with a collapse of the PTEN active site and its subsequent inactivation. Mutations associated with autism spectrum disorder, however, may alter this interface, resulting in an opening of the active site (Smith et al. 2018). There are also mutations found on the interface itself that cause a complete unfolding of the enzyme. Two residues found at this interface, S170 and R173, are among the most commonly mutated residues in cancer (Lee et al. 1999). The interdomain interface spans 1400 A2 and is largely hydrophobic in nature (Haynie and Xue 2015). There is evidence that the relative positions of these two domains are altered on membrane binding by possibly splaying apart from one another (Wei et al. 2015) or turning toward one another by ∼10° (Kalli et al. 2014). Molecular dynamic simulations of the PTP/C2 unit on membranes suggest that PTEN encounters membranes through electrostatic interactions and that the encounter complex undergoes a subsequent reorientation on membranes (Kalli et al. 2014). However, this encounter/reorient sequence is not an intrinsic property of the PTP/C2 superdomain, because the Ci-VSP voltage-sensitive phosphatase (Matsuda et al. 2011), which also contains this superdomain, is predicted to bind membranes without reorientation after the encounter (Kalli et al. 2014).
CARBOXY-TERMINAL TAIL
Following the C2 domain is a 47-residue stretch of PTEN (residues 353–403) that is disordered and, as such, is not present in the crystal structure. Premature stop codons that remove the carboxy-terminal tail (CTT) have been identified as oncogenic (Georgescu et al. 1999), suggesting that the CTT is crucial to the correct functioning of PTEN. Posttranslational modification of the CTT is crucial to the regulation of PTEN (see below) as it forms an autoinhibitory interaction with the two folded domains of PTEN. Additionally, the tail contains PEST (Pro-Glu-Ser-Thr) sequences (Georgescu et al. 2000), a motif associated with a short intracellular half-life caused by it being a target for proteasomal degradation (Rogers et al. 1986). Phosphorylation of the CTT largely regulates PTEN's stability, and the three last residues of PTEN, Thr-Val-Lys, constitute a PDZ domain-binding motif (PDZ-BM). This motif is a target for PDZ domain containing proteins, many of which have scaffolding roles. The removal of the PDZ-BM has no known effect on PTEN's tumor-suppressor activity (Georgescu et al. 1999) nor any effect on PTEN's membrane-binding affinity. There are, however, a large number of proteins that have been found to interact with the PTEN PDZ-BM, such as S-SCAM (synaptic scaffolding molecule, also known as MAGI-2, membrane-associated guanylate kinase inverted 2), which may stabilize and activate PTEN (Adey et al. 2000; Wu et al. 2000; Tolkacheva et al. 2001). The role of the PDZ-BM may be particularly important for PTEN's role in inhibition of neuronal growth (Park et al. 2008), in which the PDZ-BM is necessary for the localization of PTEN (Jurado et al. 2010).
MECHANISM AND REGULATION OF MEMBRANE INTERACTION
PTEN's physiological substrate, PIP3, is found on the inner leaflet of the plasma membrane and, as such, one of the main regulatory nodes in PTEN activity is controlling how and when PTEN interacts with the membrane. The exact orientation of PTEN on the membrane and the degree of penetration into the membrane dictate PTEN's kinetic properties.
The phosphorylation of residues T366, S370, S380, T382, T383, and S385 have been extremely well studied since their discovery (Torres and Pulido 2001) and subsequently confirmed in vivo using mass spectrometry (Miller et al. 2002). PTEN was found to be a phosphoprotein in vivo, and specific phosphorylated residues were crucial to preventing proteasomal degradation of PTEN (Miller et al. 2002). These phosphorylation sites are crucial for keeping PTEN in a stable cytosolic state, and alanine mutation of the sites 380–385 caused PTEN to be found constitutively membrane associated (Odriozola et al. 2007). Although various in vitro studies have shown that CK2 (casein kinase 2) and GSKβ are capable of phosphorylating these sites in vitro (Cordier et al. 2012), with the phosphorylation of the 380–385 cluster preceding the phosphorylation of 370 and 366, inhibition of CK2 and GSKβ in vivo only partially suppressed phosphorylation of these sites, suggesting that there may be other kinases involved, such as Polo-like kinase 3 (Plk) (Xu et al. 2010).
Initially, the mechanism of autoinhibition was suggested to be caused by the phosphorylated CTT acting as pseudosubstrate, with the phosphorylated flexible CTT entering the active site of PTEN (Miller et al. 2002). Subsequent elegant experiments suggested that the mechanism of inhibition may have been more complex, with an intramolecular interaction being proposed in which the phosphorylated tail would span both the C2 and PTP domains (Odriozola et al. 2007), and this was largely confirmed subsequently, with emphasis on the need for an intact phosphatase domain and active site to maintain the intramolecular interaction with the phosphorylated tail (Rahdar et al. 2009). However, there was conflicting evidence among studies as to whether the positive charge of the CBR3 loop was necessary for the interaction. An alternative method has been used to explore this interaction further, in which a phosphopeptide tail consisting of four phosphorylated residues (380/382/383/385, referred to a “4P” tail) was synthesized and then ligated onto a truncated PTEN (Bolduc et al. 2013; Chen et al. 2016). Through subsequent analysis using chemical cross-linking, alkaline phosphatase sensitivity, small-angle X-ray scattering, and anion-exchange chromatography, they determined that the most likely confirmation of the “4P” phosphorylated tail was interacting with the Cα2 loop and CBR3 loop of the C2 domain and the arginine loop of the phosphatase domain. Rather than inactivating the enzyme by the phosphorylated CTT mimicking a specific substrate, this interaction was proposed to mimic the phospholipid membrane (Chen et al. 2016). This is largely in agreement with hydrogen-deuterium exchange mass-spectrometry (HDX-MS) experiments that mapped the intramolecular interaction (Masson et al. 2016); however, the HDX-MS also explored the role of T366 and S370 phosphorylations.
The T366 and S370 phosphorylation sites are less well characterized. They have been shown to be incapable of blocking the phosphatase activity of PTEN (Tibarewal et al. 2012) and are thought to perhaps occupy the active site of PTEN. HDX-MS analysis pointed to them interacting with the TI loop (Masson et al. 2016), and biochemical analysis has shown that they can be autodephosphorylated (typically in tandem) by PTEN (Tibarewal et al. 2012), suggesting that these two phosphorylated residues may be key to substrate specificity and active-site accessibility rather than membrane binding.
Another possible route for the regulation of membrane binding is via SUMOylation (González-Santamaría et al. 2012; Huang et al. 2012). There are three proposed sites of SUMOlyation that are all found in the C2 domain, K266, K254, and K289, with two of these (K266 and K254) being located in the CBR3 loop—a loop key for membrane binding. The proposed mechanism of driving membrane binding is that the modification of these residues results in a net increase in positive charge on the C2 domain, driving PTEN to associate with the negatively charged inner leaflet of the plasma membrane. The suggested membrane-binding interface of PTEN on SUMOlyation is highly divergent however (Huang et al. 2012), with it being difficult to envision how the phosphatase domain of PTEN would be able to engage with substrate. Additionally, SUMOlyation of K254 has been linked to nuclear localization of PTEN also (Bassi et al. 2013), so it is unclear how exactly SUMOlyation of this residue affects PTEN's localization.
An alternative mechanism for PTEN's membrane binding has also been suggested that proposes PTEN being recruited to endosomal vesicles (Naguib et al. 2015), via the C2 domain specifically binding to PI(3)P, a signature lipid of endosomal membranes. The CBR3 loop was critical for this interaction, and mutations that rendered this loop incapable of binding PI(3)P resulted in dramatic reductions in PTEN's ability to inhibit PI3K-signaling. Additionally, these mutants could be rescued by fusing a FYVE domain (a well-characterized PI(3)P-binding domain) to the loop, reinforcing the necessity of PI(3)P for membrane association. This raised the possibilities of new protein substrates for PTEN, such as Rab7 (Shinde and Maddika 2016).
TRANSLATIONAL VARIANTS OF PTEN
Special note should be given to the translational variants of PTEN known as PTEN-L (Hopkins et al. 2013) (also known as PTEN-α [Liang et al. 2014]), which has a 173-amino-acid amino-terminal extension preceding the “short” PTEN, PTEN-β, which has an 146-amino-acid extension (Liang et al. 2017) and various others (see Fig. 1A; Tzani et al. 2016). Expression of these translational variants involves an eIF2α-dependent alternative initiation codon (CUG) (Liang et al. 2014). Translation requires the formation of an eIF2α/GTP/Met-tRNAi complex, which is dependent on the eIF2α kinase-mediated phosphorylation status of eIF2α (Tzani et al. 2016; Kim et al. 2018). Each translational variant displays unique activities and regulation because of their amino-terminal extension that may preclude them from some of the mechanisms of regulation that are known to affect “short” PTEN.
PTEN-L was first observed and characterized by Hopkins et al. (2013) and followed rapidly by Liang et al. (2014) (who gave it the moniker PTEN-α, although a standardized nomenclature has been agreed on as PTEN-Long [Pulido et al. 2014]), but these two landmark papers observed quite different behaviors of PTEN-L. Hopkins showed that PTEN-L and PTEN had similar phosphatase activity against a soluble lipid-head group substrate (diC8-PIP3, as also observed subsequently by others [Masson et al. 2016]), but PTEN-L had the remarkable property of being secreted and subsequently taken up into neighboring cells. Exit from the cell is achieved by a polyalanine secretion signal sequence (residues 12–17) at the amino-terminus of PTEN-L, and entry into neighboring cells relies on polyarginine protein transduction stretch (residues 47–52).
Liang et al. (2014), however, observed a very different behavior with PTEN-L, localizing to the mitochondria and maintaining mitochondrial stability, as well as up-regulating cytochrome c oxidase activity via PINK1. The observation of PTEN-L mitochondrial localization also has been reported by others and further explored (Li et al. 2018; Wang et al. 2018). However, these more recent studies have not been conclusive as to what this localization to the mitochondria achieves, because PTEN-L has been shown to both promote (Li et al. 2018) and prevent (Wang et al. 2018) PINK-1-Parkin-mediated mitophagy. Li et al. found that PTEN-L drove Parkin to the outer mitochondrial membrane, promoting subsequent mitophagy, whereas Wang et al. argue that the major role of PTEN-L is to counter PINK ubiquitin kinase activity via dephosphorylating phosphoubiquitin. This pathway is key to understanding a number of diseases, including neurodegenerative disease, and PTEN-L evidently plays a role in mitochondrial maintenance, although it is not clear what that role is precisely. Additionally, PTEN-β, the 146-amino acid amino-terminal extension variant, has recently been implicated in the regulation of rDNA transcription (Liang et al. 2017). PTEN-β was found to localize primarily in the nucleolus, and it is likely that it inhibits pre-rRNA synthesis by dephosphorylation of nucleolin.
How are these alterations in PTEN's regulation mediated structurally? The amino-terminal extensions of PTEN-L/α and PTEN-β are likely to be largely intrinsically disordered (Malaney et al. 2013a; Masson et al. 2016). It may be that the changes in PTEN's localization and activity are solely because of the secretion signal and protein transduction sequences present within the extension, but the extension also is predicted to be enriched in both posttranslational modifications and protein-binding sites (Malaney et al. 2013b). These interactions and modifications may be key to determining whether PTEN-L is secreted or localized to the mitochondria. These modifications may well provide an explanation also as to why PTEN-β is localized to the nucleolus. Alternatively, Liang et al. suggest that the overall conformation of the extension may differ between PTEN-L and PTEN-β because of the latter's truncation, citing examples in both CK2α and eIF4GI in which truncating intrinsically disordered amino-terminal regions lead to changes in conformation of the remaining protein, although the proposed conformational differences have yet to be shown for PTEN variants of PTEN.
There may be a small section of secondary structure in the amino-terminal extension—a single α-helix. A membrane-binding α-helix (the MBH) may exist between residues K151-L and M174-L (i.e., the 23 residues immediately preceding the start of PTEN), on the basis of solvent exchange rates (Masson et al. 2016), and this helix may also interact with the amino-terminus of the phosphatase domain. This is also supported by unfolding analysis showing there may be some structure to the extension (Johnston and Raines 2015b). The MBH also has been implicated in Parkin binding (Li et al. 2018). The helix was found to be sufficient to alter PTEN's interfacial membrane kinetics in vitro. The MBH switched PTEN catalysis on membranes from a “hopping” to a “scooting” mode of kinetics (i.e., the presence of the MBH drastically prolonged the residence time of PTEN on the membrane). PTEN-L has also been shown to less sensitive to PIP2 as an allosteric activator, and may be in a “constitutively active” state (Johnston and Raines 2015a), which may be attributable to the MBH interacting with the PBM.
DIMERIZATION AS REGULATION
There has been mounting evidence that PTEN can act as a dimer (Papa et al. 2014; Heinrich et al. 2015). Biophysical analyses show that bacterially expressed human PTEN contains both monomers and dimers (Heinrich et al. 2015). However, dimerization is not essential for activity. Monomers purified from human PTEN expressed in insect cells are active and can be regulated by phosphorylation (Masson et al. 2016). Small-angle X-ray scattering and modeling have suggested dimerization of the bacterially expressed protein occurs by association of phosphatase domains and segment swapping (Heinrich et al. 2015). The fraction of PTEN in dimers is not readily apparent, although in vitro analysis suggests that it is likely to be a minor component (Papa et al. 2014). It has been suggested that dimerization leads to activation of PTEN, possibly occurring more readily when PTEN is membrane bound (Papa et al. 2014; Heinrich et al. 2015). A full discussion on PTEN dimerization by Ross (2019) can be found in this collection.
OUTSTANDING QUESTIONS ON PTEN'S STRUCTURE AND REGULATION
The more we research PTEN, increasingly diverse structures are revealed and increasingly complex activities are discovered. The relative prominence of PTEN's lipid phosphatase activity versus protein phosphatase activity, PI(3,4,5)P3 phosphatase activity versus PI(3,4)P3 activity, its localization in the cytoplasm, at the membrane or within the nucleus, the assorted amino-terminal extensions, and the roles of dimer versus monomer all compete with one another for how we see PTEN and its role in the cell. Presently, we believe that the core concern of PTEN in the cell is the desphosphorylation of PI(3,4,5)P3 to PI(4,5)P3 in response to PI3K signaling—PTEN's canonical “tumor suppressor” role. This is not to delegitimize the other activities of PTEN, but cellular and mutagenesis data suggests that on PTEN's dereliction of this particular duty, the dysregulation of the cell is a certainty, rather than a possibility.
Viewed through this prism, three important questions about the mechanism of PTEN's regulation remain unanswered. First, how are the four key phosphorylations on CTT removed to activate the enzyme? Is this PTEN itself? Does it act as a monomer, gradually dephosphorylating itself? If it acts as a dimer, does it work in trans or cis? Is there an extrinsic factor such as another phosphatase, or a regulatory factor that facilitates this in cells? Any additional factor that allows for the activation of PTEN would be expected to be a powerful tumor suppressor, and so identifying whether such an entity exists may be relevant to cancer disease models. If it is an intrinsic property of PTEN, then this increases the importance of PTEN's protein phosphatase activity, and questions would still remain as to how this is achieved in response to PIP3 production, if PTEN is primarily bound by endosomes.
Second, what is exact dimeric structure of PTEN? What residues facilitate this dimerization? How does dimerization alter enzymatic ability? If dimerization is key to PTEN's role as a tumor suppressor in vivo (as has been suggested [Papa et al. 2014; Heinrich et al. 2015]), we could expect that residues that mediate this dimerization would be highly oncogenic. Additionally, where do we find dimers of PTEN? Do we only find dimeric PTEN when membrane bound?
Third, clarity needs to be brought to the roles of translational variants of PTEN, especially PTEN-L. PTEN-L appears to have two roles, both of which may have critical therapeutic implications: as a tumor-suppressor capable of entering neighboring cells or as a mediator of mitophagy via Parkin. Within the amino-terminal extension, there is potentially a very large number of interactions and posttranslational modifications that may be responsible for PTEN-L's dual roles (Malaney et al. 2013b). Under what conditions these translational variants are expressed is an unexplored area. The production of CUG translational variants is linked to eIF2α phosphorylation status, and this is an underexplored lynchpin that might bias cells toward greater production of PTEN-L/-β. Understanding when, where, and how these variants are produced and how they engage with their targets requires further exploration to fully exploit this potential therapeutic target.
CONCLUDING REMARKS
PTEN has been intensively studied, but not exhaustively. New discoveries are still being made concerning this enzyme that merits so much attention. The simplicity of its structure belies the complexity of its regulation. The past 5 years of research on PTEN have enormously expanded the scope of our research, and the questions and challenges ahead are numerous and varied.
ACKNOWLEDGMENTS
Glenn Masson is supported by an AstraZeneca/Laboratory of Molecular Biology Blue Skies Fund Grant (BSF33). R.L.W. thanks the MRC and Cancer Research UK for funding (Grants MC_U105184308 and C14801/A21211, respectively).
Footnotes
Editors: Charis Eng, Joanne Ngeow, and Vuk Stambolic
Additional Perspectives on The PTEN Family available at www.perspectivesinmedicine.org
REFERENCES
- Adey NB, Huang L, Ormonde PA, Baumgard ML, Pero R, Byreddy DV, Tavtigian SV, Bartel PL. 2000. Threonine phosphorylation of the MMAC1/PTEN PDZ binding domain both inhibits and stimulates PDZ binding. Cancer Res 60: 35–37. [PubMed] [Google Scholar]
- Bassi C, Ho J, Srikumar T, Dowling RJO, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. 2013. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341: 395–399. 10.1126/science.1236188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc D, Rahdar M, Tu-Sekine B, Sivakumaren SC, Raben D, Amzel LM, Devreotes P, Gabelli SB, Cole P. 2013. Phosphorylation-mediated PTEN conformational closure and deactivation revealed with protein semisynthesis. eLife 2: e00691 10.7554/eLife.00691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RB, Liu F, Ross AH. 2003. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 278: 33617–33620. 10.1074/jbc.C300296200 [DOI] [PubMed] [Google Scholar]
- Chen Z, Dempsey DR, Thomas SN, Hayward D, Bolduc DM, Cole PA. 2016. Molecular features of phosphatase and tensin homolog (PTEN) regulation by C-terminal phosphorylation. J Biol Chem 291: 14160–14169. 10.1074/jbc.M116.728980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier F, Chaffotte A, Terrien E, Préhaud C, Theillet F-X, Delepierre M, Lafon M, Buc H, Wolff N. 2012. Ordered phosphorylation events in two independent cascades of the PTEN C-tail revealed by NMR. J Am Chem Soc 134: 20533–20543. 10.1021/ja310214g [DOI] [PubMed] [Google Scholar]
- Das S, Dixon JE, Cho W. 2003. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci 100: 7491–7496. 10.1073/pnas.0932835100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson L, Maccario H, Perera NM, Yang X, Spinelli L, Tibarewal P, Glancy B, Gray A, Weijer CJ, Downes CP, et al. 2010. Suppression of cellular proliferation and invasion by the concerted lipid and protein phosphatase activities of PTEN. Oncogene 29: 687–697. 10.1038/onc.2009.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. 1999. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci 96: 10182–10187. 10.1073/pnas.96.18.10182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. 2000. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 60: 7033–7038. [PubMed] [Google Scholar]
- González-Santamaría J, Campagna M, Ortega-Molina A, Marcos-Villar L, la Cruz-Herrera de CF, González D, Gallego P, Lopitz-Otsoa F, Esteban M, Rodríguez MS, et al. 2012. Regulation of the tumor suppressor PTEN by SUMO. Cell Death Dis 3: e393 10.1038/cddis.2012.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Han D, Harrison SC, Kirchhausen T. 2010. Structure of the PTEN-like region of auxilin, a detector of clathrin-coated vesicle budding. Structure 18: 1191–1198. 10.1016/j.str.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynie DT, Xue B. 2015. Superdomains in the protein structure hierarchy: the case of PTP-C2. Protein Sci 24: 874–882. 10.1002/pro.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich F, Chakravarthy S, Nanda H, Papa A, Pandolfi PP, Ross AH, Harishchandra RK, Gericke A, Lösche M. 2015. The PTEN tumor suppressor forms homodimers in solution. Structure 23: 1952–1957. 10.1016/j.str.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins BD, Fine B, Steinbach N, Dendy M, Rapp Z, Shaw J, Pappas K, Yu JS, Hodakoski C, Mense S, et al. 2013. A secreted PTEN phosphatase that enters cells to alter signaling and survival. Science 341: 399–402. 10.1126/science.1234907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yan J, Zhang J, Zhu S, Wang Y, Shi T, Zhu C, Chen C, Liu X, Cheng J, et al. 2012. SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane. Nat Commun 3: 911 10.1038/ncomms1919 [DOI] [PubMed] [Google Scholar]
- Irvine WA, Flanagan JU, Allison JR. 2019. Computational prediction of amino acids governing protein-membrane interaction for the PIP3 cell signaling system. Structure 27: 371–380.e3. 10.1016/j.str.2018.10.014 [DOI] [PubMed] [Google Scholar]
- Johnston SB, Raines RT. 2015a. Catalysis by the tumor-suppressor enzymes PTEN and PTEN-L. PLoS ONE 10: e0116898 10.1371/journal.pone.0116898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SB, Raines RT. 2015b. Conformational stability and catalytic activity of PTEN variants linked to cancers and autism spectrum disorders. Biochemistry 54: 1576–1582. 10.1021/acs.biochem.5b00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S, Benoist M, Lario A, Knafo S, Petrok CN, Esteban JA. 2010. PTEN is recruited to the postsynaptic terminal for NMDA receptor-dependent long-term depression. EMBO J 29: 2827–2840. 10.1038/emboj.2010.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli AC, Devaney I, Sansom MSP. 2014. Interactions of phosphatase and tensin homologue (PTEN) proteins with phosphatidylinositol phosphates: insights from molecular dynamics simulations of PTEN and voltage sensitive phosphatase. Biochemistry 53: 1724–1732. 10.1021/bi5000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Kim JH, Seo K, Hong KY, An SWA, Kwon J, Lee SJV, Jang SK. 2018. eIF2A, an initiator tRNA carrier refractory to eIF2α kinases, functions synergistically with eIF5B. Cell Mol Life Sci 75: 4287–4300. 10.1007/s00018-018-2870-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-O, Yang H, Georgescu M-M, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP. 1999. Crystal structure of the PTEN tumor suppressor. Cell 99: 323–334. 10.1016/S0092-8674(00)81663-3 [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. 2002. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277: 20336–20342. 10.1074/jbc.M111899200 [DOI] [PubMed] [Google Scholar]
- Li J. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947. 10.1126/science.275.5308.1943 [DOI] [PubMed] [Google Scholar]
- Li G, Yang J, Yang C, Zhu M, Jin Y, McNutt MA, Yin Y. 2018. PTENα regulates mitophagy and maintains mitochondrial quality control. Autophagy 14: 1742–1760. 10.1080/15548627.2018.1489477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, He S, Yang J, Jia X, Wang P, Chen X, Zhang Z, Zou X, McNutt MA, Shen WH, et al. 2014. PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism. Cell Metab 19: 836–848. 10.1016/j.cmet.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Chen X, Yin Q, Ruan D, Zhao X, Zhang C, McNutt MA, Yin Y. 2017. PTENβ is an alternatively translated isoform of PTEN that regulates rDNA transcription. Nat Commun 8: 14771 10.1038/ncomms14771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw D, Marsh DJ, Li J, Dahia PLM, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacoke M, et al. 1997. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16: 64–67. 10.1038/ng0597-64 [DOI] [PubMed] [Google Scholar]
- Liu L, Kohout SC, Xu Q, Müller S, Kimberlin CR, Isacoff EY, Minor DL. 2012. A glutamate switch controls voltage-sensitive phosphatase function. Nat Struct Mol Biol 19: 633–641. 10.1038/nsmb.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378. 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- Malaney P, Pathak RR, Xue B, Uversky VN, Davé V. 2013a. Intrinsic disorder in PTEN and its interactome confers structural plasticity and functional versatility. Sci Rep 3: 2035 10.1038/srep02035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaney P, Uversky VN, Davé V. 2013b. The PTEN long N-tail is intrinsically disordered: increased viability for PTEN therapy. Mol BioSyst 9: 2877 10.1039/c3mb70267g [DOI] [PubMed] [Google Scholar]
- Malek M, Kielkowska A, Chessa T, Anderson KE, Barneda D, Pir P, Nakanishi H, Eguchi S, Koizumi A, Sasaki J, et al. 2017. PTEN regulates PI(3,4)P2 signaling downstream of class I PI3K. Mol Cell 68: 566–580.e10. 10.1016/j.molcel.2017.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson GR, Perisic O, Burke JE, Williams RL. 2016. The intrinsically disordered tails of PTEN and PTEN-L have distinct roles in regulating substrate specificity and membrane activity. Biochem J 473: 135–144. 10.1042/BJ20150931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Takeshita K, Kurokawa T, Sakata S, Suzuki M, Yamashita E, Okamura Y, Nakagawa A. 2011. Crystal structure of the cytoplasmic phosphatase and tensin homolog (PTEN)-like region of Ciona intestinalis voltage-sensing phosphatase provides insight into substrate specificity and redox regulation of the phosphoinositide phosphatase activity. J Biol Chem 286: 23368–23377. 10.1074/jbc.M110.214361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mighell TL, Evans-Dutson S, O'Roak BJ. 2018. A saturation mutagenesis approach to understanding PTEN lipid phosphatase activity and genotype-phenotype relationships. Am J Hum Genet 102: 943–955. 10.1016/j.ajhg.2018.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. 2002. Direct identification of PTEN phosphorylation sites. FEBS Lett 528: 145–153. 10.1016/S0014-5793(02)03274-X [DOI] [PubMed] [Google Scholar]
- Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. 1997. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci 94: 9052–9057. 10.1073/pnas.94.17.9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MP, Pass I, Batty IH, Van der Kaay J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP, Tonks NK. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc Natl Acad Sci 95: 13513–13518. 10.1073/pnas.95.23.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib A, Bencze G, Cho H, Zheng W, Tocilj A, Elkayam E, Faehnle CR, Jaber N, Pratt CP, Chen M, et al. 2015. PTEN functions by recruitment to cytoplasmic vesicles. Mol Cell 58: 255–268. 10.1016/j.molcel.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda H, Heinrich F, Lösche M. 2015. Membrane association of the PTEN tumor suppressor: neutron scattering and MD simulations reveal the structure of protein–membrane complexes. Methods 77–78: 136–146. 10.1016/j.ymeth.2014.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Yang JM Jr, Rahdar M, Keniry M, Swaney KF, Parsons R, Park BH, Sesaki H, Devreotes PN, Iijima M. 2015. A new class of cancer-associated PTEN mutations defined by membrane translocation defects. Oncogene 34: 3737–3743. 10.1038/onc.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola L, Singh G, Hoang T, Chan AM. 2007. Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem 282: 23306–23315. 10.1074/jbc.M611240200 [DOI] [PubMed] [Google Scholar]
- Okumura K. 2006. PCAF modulates PTEN activity. J Biol Chem 281: 26562–26568. 10.1074/jbc.M605391200 [DOI] [PubMed] [Google Scholar]
- Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, et al. 2014. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell 157: 595–610. 10.1016/j.cell.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322: 963–966. 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. 2009. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 418: 475–489. 10.1042/BJ20082234 [DOI] [PubMed] [Google Scholar]
- Pulido R, Baker SJ, Barata JT, Carracedo A, Cid VJ, Chin-Sang ID, Dave V, Hertog den J, Devreotes P, Eickholt BJ, et al. 2014. A unified nomenclature and amino acid numbering for human PTEN. Sci Signal 7: pe15–pe15. 10.1126/scisignal.2005560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. 2009. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci 106: 480–485. 10.1073/pnas.0811212106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RE, Redfern D, Furgason MLM, Munson M, Ross AH, Gericke A. 2008. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry 47: 2162–2171. 10.1021/bi702114w [DOI] [PubMed] [Google Scholar]
- Rodríguez-Escudero I, Oliver MD, Andrés-Pons A, Molina M, Cid VJ, Pulido R. 2011. A comprehensive functional analysis of PTEN mutations: implications in tumor- and autism-related syndromes. Hum Mol Genet 20: 4132–4142. 10.1093/hmg/ddr337 [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. 1986. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234: 364–368. 10.1126/science.2876518 [DOI] [PubMed] [Google Scholar]
- Ross A. 2019. Structure with an emphasis on the dimerization of PTEN. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a036145 [DOI] [Google Scholar]
- Shenoy SS, Nanda H, Lösche M. 2012. Membrane association of the PTEN tumor suppressor: electrostatic interaction with phosphatidylserine-containing bilayers and regulatory role of the C-terminal tail. J Struct Biol 180: 394–408. 10.1016/j.jsb.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang J, Chandarlapaty S, Cross J, Thompson C, Rosen N, Jiang X. 2014. PTEN is a protein tyrosine phosphatase for IRS1. Nat Struct Mol Biol 21: 522–527. 10.1038/nsmb.2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde SR, Maddika S. 2016. PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7. Nat Commun 7: 10689 10.1038/ncomms10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitsar I, Bashkurov M, Masson GR, Ogunjimi AA, Mosessian S, Cabeza EA, Hirsch CL, Trcka D, Gish G, Jiao J, et al. 2015. PTEN regulates cilia through Dishevelled. Nat Commun 6: 8388 10.1038/ncomms9388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IN, Thacker S, Jaini R, Eng C. 2018. Dynamics and structural stability effects of germline PTEN mutations associated with cancer versus autism phenotypes. J Biomol Struct Dyn 60: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck PA, Pershouse MA, Jasser SA, Yung WKA, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, et al. 1997. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15: 356–362. 10.1038/ng0497-356 [DOI] [PubMed] [Google Scholar]
- Tibarewal P, Zilidis G, Spinelli L, Schurch N, Maccario H, Gray A, Perera NM, Davidson L, Barton GJ, Leslie NR. 2012. PTEN protein phosphatase activity correlates with control of gene expression and invasion, a tumor-suppressing phenotype, but not with AKT activity. Sci Signal 5: ra18 10.1126/scisignal.2002138 [DOI] [PubMed] [Google Scholar]
- Tolkacheva T, Boddapati M, Sanfiz A, Tsuchida K, Kimmelman AC, Chan AM. 2001. Regulation of PTEN binding to MAGI-2 by two putative phosphorylation sites at threonine 382 and 383. Cancer Res 61: 4985–4989. [PubMed] [Google Scholar]
- Torres J, Pulido R. 2001. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus: implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 276: 993–998. 10.1074/jbc.M009134200 [DOI] [PubMed] [Google Scholar]
- Tzani I, Ivanov IP, Andreev DE, Dmitriev RI, Dean KA, Baranov PV, Atkins JF, Loughran G. 2016. Systematic analysis of the PTEN 5′ leader identifies a major AUU initiated proteoform. Open Biol 6: 150203 10.1098/rsob.150203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR. 2001. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem 276: 48627–48630. 10.1074/jbc.C100556200 [DOI] [PubMed] [Google Scholar]
- Walker SM, Leslie NR, Perera NM, Batty IH, Downes CP. 2004. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J 379: 301–307. 10.1042/bj20031839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al. 2007. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128: 129–139. 10.1016/j.cell.2006.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cho YL, Tang Y, Wang J, Park JE, Wu Y, Wang C, Tong Y, Chawla R, Zhang J, et al. 2018. PTEN-L is a novel protein phosphatase for ubiquitin dephosphorylation to inhibit PINK1-Parkin-mediated mitophagy. Cell Res 28: 787–802. 10.1038/s41422-018-0056-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Stec B, Redfield AG, Weerapana E, Roberts MF. 2015. Phospholipid-binding sites of phosphatase and tensin homolog (PTEN). J Biol Chem 290: 1592–1606. 10.1074/jbc.M114.588590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, Wood J, Ross C, Sawyers CL, Whang YE. 2000. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci 97: 4233–4238. 10.1073/pnas.97.8.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Yeong Chit Chia J, Gajewski JE, Sio Seng Lio D, Mulhern TD, Zhu HJ, Nandurkar H, Cheng HC. 2007. PTEN catalysis of phospholipid dephosphorylation reaction follows a two-step mechanism in which the conserved aspartate-92 does not function as the general acid—mechanistic analysis of a familial Cowden disease-associated PTEN mutation. Cell Signal 19: 1434–1445. 10.1016/j.cellsig.2007.01.021 [DOI] [PubMed] [Google Scholar]
- Xu D, Yao Y, Jiang X, Lu L, Dai W. 2010. Regulation of PTEN stability and activity by Plk3. J Biol Chem 285: 39935–39942. 10.1074/jbc.M110.166462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui M, Matsuoka S, Ueda M. 2014. PTEN hopping on the cell membrane is regulated via a positively charged C2 domain. PLoS Comput Biol 10: e1003817 10.1371/journal.pcbi.1003817 [DOI] [PMC free article] [PubMed] [Google Scholar]