Abstract
Objectives:
To critically evaluate published systemic estradiol levels during use of low-dose vaginal estrogens considering detection method and estrogen dose; describe challenges with accurately measuring estradiol; and determine the normal estradiol level range in postmenopausal women.
Methods:
PubMed was searched for studies reporting systemic estradiol levels with lower-dose vaginal estrogens (≤25 μg estradiol or 0.3 mg conjugated equine estrogens). Estradiol levels at baseline and during treatment, area under the curve, and maximum estradiol concentrations were summarized by dose within assay type. A proposed range of systemic estradiol in normal, untreated, postmenopausal women was estimated by conservatively pooling means and standard deviations from published studies.
Results:
Mean basal estradiol levels were 3.1 to 4.9 pg/mL using liquid or gas chromatography/mass spectroscopy (LC or GC/MS/MS) with a range of undetectable to 10.5 pg/mL using radioimmunoassay. Systemic estradiol levels with vaginal estrogens reflected their doses as measured with LC or GC/MS/MS in different studies: 7.1 to 9.1 pg/mL and 16.7 to 22.7 pg/mL with a 25-μg softgel capsule insert and a tablet insert, respectively; 4.6 to 7.4 pg/mL and 6.6 to 14.8 pg/mL with a 10-μg softgel capsule and a tablet insert, respectively; and 3.6 to 3.9 pg/mL with a 4-μg softgel capsule insert. A mean systemic estradiol concentration ranging from undetectable to 10.7 pg/mL is proposed as an estimate for basal estradiol levels in normal, untreated, postmenopausal women. Systemic estradiol absorption may be influenced by the placement of estradiol higher (as with an applicator) versus lower (as without an applicator) in the vagina, as estradiol transport to the uterus would be more likely further away than closer to the introitus.
Conclusion:
Serum estradiol concentrations were generally lower when measured with more specific and sensitive assays. Estradiol absorption was dose-dependent, and may be influenced by dose, formulation, and positioning in the vagina. Very low systemic estradiol absorption with low/ultralow-dose vaginal estrogens may potentially decrease any adverse events that may be associated with higher doses of vaginal estrogens used for treating moderate to severe VVA due to less estradiol exposure.
Keywords: Estradiol, Estrogens, Pharmacokinetics, Vaginal
Several vaginal estrogen preparations are approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy (VVA) in postmenopausal women. Guidelines published by relevant medical societies recommend vaginal estrogen use for moderate to severe symptomatic VVA unresponsive to nonprescription therapies, especially in women without other menopausal symptoms such as hot flashes.1-4 Published data indicate that low-dose vaginal estrogens minimally increase plasma estradiol and clearly not to levels observed with oral or transdermal estrogen products. Use of vaginal estrogens has increased since publication of the results from the Women's Health Initiative (WHI),5-8 presumably from concerns about the adverse effects of increased systemic estrogen levels. Notably, local administration of vaginal estrogens minimizes systemic estrogen exposure,9 compared with that of oral or transdermal estrogens. Data from the observation study of WHI showing no increase in cancer or cardiovascular risk with vaginal estrogens are consistent with these ideas.10
Despite existing data on low systemic estradiol levels with vaginal estrogens, some surveyed postmenopausal women are hesitant to use vaginal estrogen products because of the potential of systemic absorption11,12 with possible resultant side effects, universally described in the product packaging of all estrogen products for menopause, as a result of US FDA class labeling. A review of serum levels of estradiol after various doses and formulations of locally administered vaginal estrogens was previously performed.9 Collectively, the reviewed data demonstrated that systemic absorption of estradiol was dose-dependent with lower systemic levels resulting from use of lower vaginal estrogen doses, and that highly specific assays detected lower levels of estradiol because of minimal amounts of cross-reacting substances.9
In this current review, we extend the previous review of estradiol levels of various vaginal estrogen formulations by dose and assay, and describe the challenges associated with measuring serum estradiol levels accurately. This review also allows us to estimate the normal range of circulating estradiol in postmenopausal women not being treated with exogenous estradiol based on measuring estradiol levels with mass spectrometry (MS)—a highly sensitive, and increasingly available, assay methodology.
METHODS
A review of estradiol absorption with vaginal estrogens was published in 2015.9 We have updated the data of that review by searching for relevant studies of lower-dose products reported since that publication. PubMed was searched from November, 2018 for studies published in the past 5 years that examined systemic estradiol levels in postmenopausal women using combinations of keywords that included estradiol, estrogen(s), dehydroepiandrosterone (DHEA), prasterone, vaginal, and pharmacokinetic. General information on estradiol assay development was also sought.
Studies were included in the review if they reported on the pharmacokinetics of vaginal estrogens of ≤25 μg estradiol or 0.3 mg conjugated equine estrogens. Within this review, we distinguish between low-dose vaginal estrogens as 25 μg estradiol or 0.3 mg conjugated equine estrogens and ultralow-dose vaginal estrogens as those with ≤10 μg estradiol. Studies that examined estradiol levels in women prescribed aromatase inhibitors were excluded, as those women would likely have lower systemic estradiol levels during therapy than healthy postmenopausal woman.13
Systemic estradiol and other pharmacokinetic parameters of estradiol, including mean serum levels, area under the curve (AUC), and maximum concentrations (Cmax), were extracted from tables or text of retrieved articles. If actual estradiol values were not reported in the text or tables of the article, values were estimated from figures illustrating the data. All estradiol concentrations reported as pmol/L were converted to pg/mL for comparison purposes. Estradiol levels from the reviewed studies were summarized in Tables 1 and 2 by detection assay type, with primary detection types including gas chromatography tandem mass spectrometry (GC/MS/MS), liquid chromatography tandem mass spectrometry (LC/MS/MS), bioassay, radioimmunoassay (RIA), and enzyme-linked immunosorbent assay (ELISA). Estradiol levels were then summarized by dose within assay type.
TABLE 1.
Mean systemic estradiol levels at baseline and throughout studies of low-dose and ultralow-lose vaginal estrogen therapies measured by radioimmunoassay or another assay
| Assay | Dose | Treatment | n | Study duration | Mean BL (pg/mL) | Mean Cmax (pg/mL) | Mean Cavga (pg/mL) | Mean AUC0-24 (pgh/mL) | Reference |
| RIA | 25 μg | E2 tablet | 59 | 48 wks | 4.1 | NR | Weeks 24 & 48: 9.8 | NR | Weisberg et al, 200515 |
| RIA | 25 μg | E2 tablet | 19 | 12 wks | 7.0 | Day 1: 51 Week 12: 49 | Day 1: 22 Week 12: 23 | Day 1: 538 Week 12: 563 | Notelovitz et al, 200216 |
| RIA | 25 μg | E2 tablet | 6 | 12 wks | 7.5 | 20.4 | Week 2: NR (15.8) Week 12: NR (16.4) 13.6 (after last tablet) | Week 2: 380.0 Week 12: 392.5 | Nilsson and Heimer, 199519 |
| RIA | 25 μg | E2 tablet | 24 | 14 d | 9.5 | Day 1: 42.8 Day 14: 21.8 | Day 14: 16 (24 h) | NR | Nilsson and Heimer, 199220 |
| RIA | 25 μg | E2 tablet (1x/wk) | 17 | 52 wks | 10.5 | NR | Week 2: 10.1 Week 12: 9.9 Week 52: 10.8 | NR | Mettler and Olsen, 199121 |
| RIA | 25 μg | E2 tablet (2x/wk) | 34 | 52 wks | 9.6 | NR | Week 2: 9.3 Week 12: 9.6 Week 52: 11.3 | NR | Mettler and Olsen, 199121 |
| RIA | 25 μg | E2 tablet | 80 | 24 wks | NR (≤30)b | NR | Week 24: 5% of women >49b | NR | Rioux et al, 200075 |
| RIA | 10 μg | E2 tablet | 23 | 12 wks | 7.6 | Day 1: 35 Week 12: 22 | Day 1: 15 Week 12: 11 | Day 1: 349 Week 12: 264 | Notelovitz et al, 200216 |
| RIA | 10 μg | E2 tablet | 24 | 14 d | 9.5 | Day 1: 24.5 Day 14: 15.0 | Day 14: 13, hour 24 | NR | Nilsson and Heimer, 199220 |
| RIA | 10 μg | E2 tablet | 336 | 52 wks | 5.2 | NR | Week 52: 6.1 | NR | Ulrich et al, 201014 |
| RIA | 0.3 mg | CEE cream | 20 | 4 wks | 8 (with placebo) | NR | 13 | NR | Mandel et al, 198322 |
| RIA | 7.5 μg/d | E2 ring; Untreated | 27 27 | 12 mos | 3.7 (E) 4.2 (U) | NR | 4.2 (E2) 4.1 (U) | NR | Naessen and Rodriguez-Macias, 200217 |
| RIA | 7.5 μg/d | E2 ring; Untreated | 20 10 | 6 mos | 4.4 (E) 3.5 (U) | NR | 6 mos: 5.7 (E2); 3.0 (U) | NR | Naessen et al, 199718 |
| RIA | 8.0 μg/d | E2 ring | 126 | 48 wks | 4.4 | NR | Week 24: 13.3 Week 48: 5.5 | NR | Weisberg et al, 200515 |
| RIA | 9.0 μg/d | E2 ring | 222 | 24 wks | <5.5 (UD) | NR | Within postmenopausal rangec | NR | Smith et al, 199323 |
| RIA | 6.6 μg/d | E2 ring | 11 | 84 d | <12.3 (UD) | Median at 2 h: 63.5 (range 29.4-158.8) | Median Day 2: 19.1 Days 7, 14, 28, 84: <12.3 (UD) | NR | Holmgren et al, 198924 |
| RIA | 20.2 μg/d | E2 ring | 11 | 84 d | <12.3 (UD) | Median at 2 h: 96.4 (range 72.7-231.8) | Median Day 2: 51.8 Days 7, 14, 28, 84: <12.3 (UD) | NR | Holmgren et al, 198924 |
| Bioassay | 10 μg | E2 creamd | 7 | 12 wks | 2.0 (E2) 1-3 (U) | Initial: 3.1 Week 3: 3.8 Week 12: 5.7 | NR | NR | Santen et al, 200276 |
| ELISA | 25 μg | E2 tablet | 27 | 12 wks | 7.6 | NR | 8.9 | NR | Manonai et al, 200125 |
| IC | 25 μg | E2 tablet; Placebo | 828 784 | 12 mos | 15.7 (E) 14.2 (P) | NR | 4 mos: 17.3 (E); 15.1 (P) 12 mos: 15.5 (E); 13.8 (P) | NR | Simunic et al, 200377 |
| NR | 25 μg | E2 tablet | 48 | 24 wks | <2.7 | NR | <2.7e | NR | Dugal et al, 200078 |
AUC0-24, area under the curve over 24 hours; BL, baseline; Cavg, average concentration; CEE, conjugated equine estrogens; Cmax, maximum concentration; E2, 17β-estradiol; ELISA, enzyme-linked immunosorbent assay; GC, gas chromatography; IC, immunochemical (Ortho-Clinical Diagnostics); LC, liquid chromatography; MS, mass spectrometry; MS/MS, tandem mass spectrometry; NR, not reported (for average E2 levels with NR, AUC0-24 [if reported] was divided by 24 to calculate the average); P, placebo; RIA, radioimmunoassay; U, untreated; UD, undetectable (below limit of detection).
aOr level reported but specified as Cavg.
bReported percentage of women who had levels > postmenopausal range (>49 pg/mL); basal level of ≤30 pg/mL based on inclusion criteria.
cE2 levels were in postmenopausal range (27.2-68.1 pg/mL) and below limit of detection (5.5 pg/mL) before and during (time not specified) treatment.23
dDiluted Estrace given daily during first 3 weeks then twice weekly for 9 weeks.76
eBlood E2 levels were within the normal range for postmenopausal women (<2.7 pg/mL) after 24 weeks of treatment.78
TABLE 2.
Mean systemic estradiol levels at baseline and throughout studies of low-dose and ultralow-lose vaginal estrogen therapies measured by mass spectrometry-based assays
| Assay | Dose | Treatment | n | Study duration | Mean BL (pg/mL) | Mean Cmax (pg/mL) | Mean Cavga (pg/mL) | Mean AUC0-24 (pg h/mL) | Reference |
| GC/MS/MS | 25 μg | E2 softgel insert; Placebo | 18 17 | 12 wks | 3.6 (E2) 4.5 (P) | Day 1 29.8 (E2); 6.6 (P) Day 14 15.7 (E2); 5.5 (P) | Day 1 9.1 (E2); 4.9 (P) Day 14 7.1 (E2); 4.3 (P) | Day 1 217.4 (E2); 116.6 (P) Day 14 171.6 (E2); 104.2 (P) | Archer et al, 201726 |
| LC/MS/MS | 25 μg | E2 softgel insert (single dose) | 36 | 1 d | NR | 31.0 | NR (9.1) | 218.7 | Data on file45b |
| E2 tablet (single dose) | 36 | 1 d | NR | 59.8 | NR (22.7) | 545.4 | Data on file45b | ||
| GC/MS | 25 μg | E2 tablet | 27 | 83 d | 4.0 | Day 1: 43 Day 14: 25 Day 83: 18 | Day 1: 19.8 Day 14: 18.3 Day 83: 9.4 | Day 1: 476.1 Day 14: 438.9 Day 83: 225.9 | Eugster-Hausmann et al, 201027 |
| GC/MS | 25 μg | E2 tablet | 10 | 7 d | 3.1 | 21.4 | 16.7 | 401 | Labrie et al, 200928 |
| GC/MS/MS | 10 μg | E2 softgel insert; Placebo | 19 17 | 12 wks | 4.9 | Day 1 10.9 (E2); 6.6 (P) Day 14: 7.3 (E2) 5.5 (P) | Day 1: 5.8 (E2) 4.9 (P) Day 14: 4.6 (E2) 4.3 (P) | Day 1: 138.2 (E2) 116.6 (P) Day 14: 110.1 (E2) 104.2 (P) | Archer et al, 201726 |
| LC/MS/MS | 10 μg | E2 softgel insert (single dose) | 35 | 1 d | NR | 21.2 | NR (7.4) | 178.2 | Data on file45b |
| E2 tablet (single dose) | 35 | 1 d | NR | 32.5 | NR (14.8) | 355.2 | Data on file45b | ||
| GC/MS | 10 μg | E2 tablet | 29 | 83 d | 3.2 | Day 1: 24 Day 14: 8 Day 83: 7.5 | Day 1: 9.4 Day 14: 6.6 Day 83: 4.6 | Day 1: 225.4 Day 14: 157.5 Day 83: 111.4 | Eugster-Hausmann et al, 201027 |
| GC/MS/MS | 4 μg | E2 softgel insert; Placebo | 18 17 | 12 wks | 3.9 | Day 1: 6.5 (E2); 6.6 (P) Day 14: 4.8 (E2); 5.5 (P) | Day 1: 3.9 (E2); 4.9 (P) Day 14: 3.6 (E2); 4.3 (P) | Day 1: 91.7 (E2); 116.6 (P) Day 14: 87.2 (E2); 104.2 (P) | Archer et al, 201726 |
| GC/MS/MS | 0.3 mg | CEE cream | 24 | 7 d | 3.7 | 12.8 | NR (9.6) | 231 | Dorr et al, 201029 |
AUC0-24, area under the curve over 24 hours; BL, baseline; Cavg, average concentration; CEE, conjugated equine estrogens; Cmax, maximum concentration; E2, 17β-estradiol; GC, gas chromatography; LC, liquid chromatography; MS, mass spectrometry; MS/MS, tandem mass spectrometry; NR, not reported (for average E2 levels with NR, AUC0-24 [if reported] was divided by 24 to calculate the average); P, placebo; UD, undetectable (below limit of detection).
aOr level reported but specified as Cavg.
bFrom same study reported by Pickar et al.50
To propose a current, clinically relevant value for systemic estradiol in normal, healthy postmenopausal women, we examined estradiol levels from postmenopausal women in clinical trials administered either vaginal estrogens or vaginal DHEA that reported estradiol levels from the placebo group or at baseline, and untreated, control postmenopausal women in vaginal therapy trials (with or without VVA). Data from postmenopausal women in assay validation studies are also reviewed. Only studies that used detection methods involving gas chromatography (GC) or liquid chromatography (LC) with MS were summarized for this purpose.
RESULTS
Systemic estradiol levels by assay type
As values for estradiol levels vary based on the detection assay used, the best way to compare assays is to contrast mean estradiol levels reported within each assay type. Table 1 shows mean basal estradiol levels measured with RIA ranging from 3.5 pg/mL up to 10.5 pg/mL,14-22 or below the assay detection limit (ie, <5.5 and <12.3).23,24 A study using ELISA—another immunologic method—reported a mean estradiol level of 7.6 pg/mL at baseline in a study of postmenopausal woman before administering a 25-μg estradiol tablet.25 Several more recent clinical studies (published after 2009) reported serum estradiol using LC or GC with MS or tandem MS in postmenopausal women at baseline, those treated with placebo, and those who were untreated or controls with or without VVA (Fig. 1 and Table 2).26-32 These studies reported lower mean levels of serum estradiol than those that utilized RIA, with mean levels ranging from 2.9 to 4.9 pg/mL.26-32
FIG. 1.
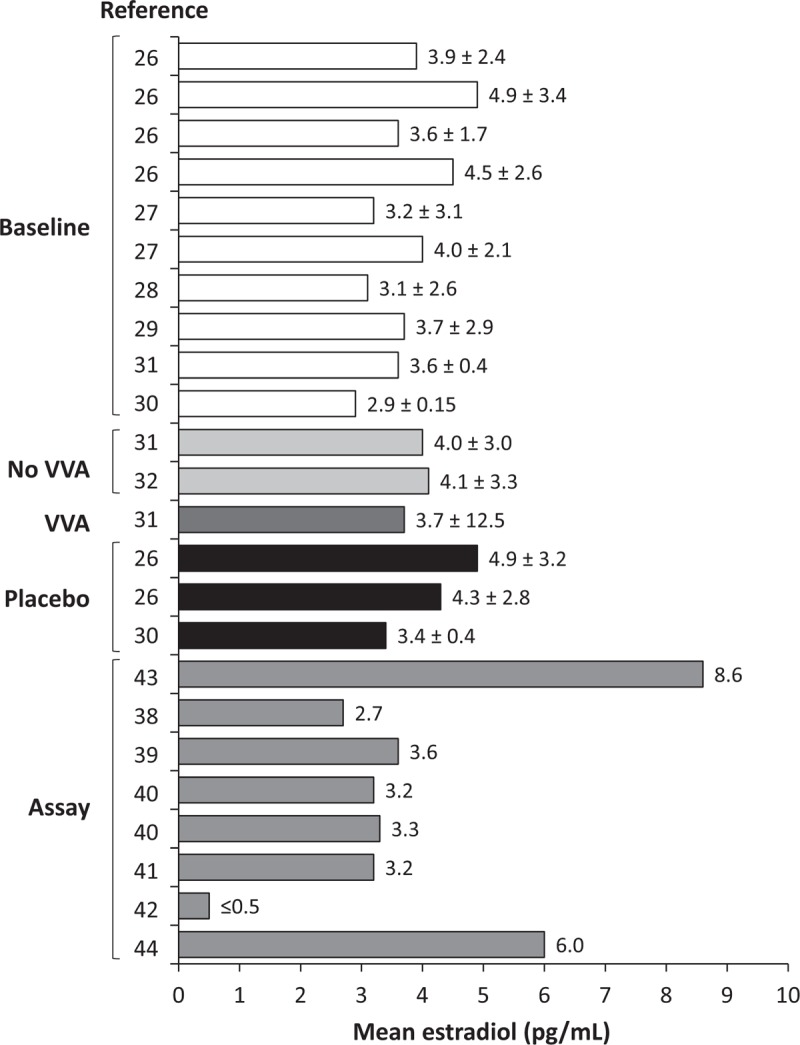
Serum estradiol levels (mean ± SD) in postmenopausal women before vaginal therapy administration or with placebo, or untreated used as a control (without or without VVA), or those from assay validation studies; only studies using MS-based assays were included. Baseline is in PMW with VVA in trials at baseline before vaginal estrogens26-29 or DHEA30,31; No VVA is untreated PMW without VVA in various clinical trials31,32; VVA is untreated PMW with VVA from trials of vaginal DHEA31; Placebo is PMW given placebo in vaginal therapy trials26,30; Assay is PMW whose E2 levels were measured in assay validation studies.37-43 DHEA, dehydroepiandrosterone; E2, 17β-estradiol; MS, mass spectroscopy; PMW, postmenopausal women; VVA, vulvar and vaginal atrophy.
Two head-to-head studies33,34 comparing RIA with GC/MS/MS report relatively higher serum estradiol levels with RIA. In 30 postmenopausal women studied by Wang et al,33 mean basal estradiol levels measured by RIA were 11.9 pg/mL, whereas those measured with GC/MS/MS were 7.3 pg/mL. Another study of postmenopausal women (n = 40) by Lee et al34 found mean levels of estradiol to be lower when measured by GC/MS/MS (3.8 pg/mL) than with six of seven RIAs (mean 6.5-13 pg/mL) thought to be highly sensitive and specific.
Estimating a postmenopausal level of systemic estradiol
When attempting to estimate a more accurate basal estradiol level for normal, untreated postmenopausal women based on estradiol levels measured with more sensitive detection assays, several sources of serum estradiol measurements may be appropriate to use for basal levels of estradiol in such women. We collated mean serum estradiol levels from postmenopausal women from vaginal therapy trials that were measured at baseline, from those treated with placebo, or from those with or without VVA who were enrolled in studies as controls (Fig. 1). Because detection methods involving more recent MS methodology are more sensitive than RIA, only studies using GC/MS/MS or LC/MS/MS to measure serum estradiol in the above mentioned women were summarized (Fig. 1). We also summarized mean estradiol values from serum of postmenopausal women that were measured in GC/MS/MS and LC/MS/MS assay validation studies (Fig. 1).
Systemic mean levels of estradiol appear to be consistent across postmenopausal women in these studies (Fig. 1). Mean estradiol levels in postmenopausal women measured at baseline in studies of vaginal estrogens or DHEA (including placebo groups) ranged from 2.9 to 4.9 pg/mL.26-31 Levels of estradiol from untreated postmenopausal women with or without VVA who were used as controls in studies of vaginal DHEA similarly ranged from 3.7 to 4.1 pg/mL.31,32 Two randomized controlled studies of vaginal therapies in which women were treated with placebo also reported estradiol levels (at time-points other than baseline) ranging from 3.4 to 4.5 pg/mL.26,30 Postmenopausal women in these vaginal therapy studies were typically ≤75 years of age with a body mass index (BMI) ≤38 kg/m2 and a most bothersome symptom of moderate to severe vaginal pain associated with sexual activity (dyspareunia).26-29 As women progress through their postmenopausal years, one would expect estradiol levels to diminish further.35 Consistent with this notion, one study in postmenopausal women reported means of 4.9 pg/mL in women <5 years postmenopausal and 1.3 pg/mL in women >5 years postmenopausal using a validated LC/MS/MS assay.36
Mean basal levels of systemic estradiol in normal, generally healthy, untreated, postmenopausal women are approximately 3.9 pg/mL based on the mean estradiol levels reported in published clinical studies26-32 (Fig. 1) using LC or GC with MS to detect serum estradiol. Published standard deviations (SDs) from mean estradiol levels in postmenopausal women ranged from 0.15 to 3.4 for various treatment groups (including placebo) at baseline,26-30 from 3.0 to 12.5 for untreated or control women31 with or without VVA,31,32 and 0.4 to 3.2 from women using placebo (at time-points other than baseline).26,30 One study31 reported an SD of 12.5 in one group of patients, with SDs of 3.03 and 3.29 in the other two groups, suggesting that the SD of 12.5 is an outlier, and as such, was discarded from our estimated range of normal estradiol levels. From the full SD range of 0.15 to 3.4,26-32 conservatively calculating a 95% confidence interval (CI) from the largest SD of ±3.4 (3.4 × 2 = 6.8), and then adding and subtracting 6.8 to and from a mean of 3.9 (average of mean estradiol levels reported in clinical studies of Fig. 1) gives an estimated range of undetectable to 10.7 pg/mL. As discussed below, this normal range would only be a preliminary estimate with the requirement to update it with a large number of samples measured with a state-of-the-art assay, including women without VVA and specifically identifying women having undergone oophorectomy.
In serum from postmenopausal women that was used for validating estradiol assays utilizing GC or LC with MS, mean or median levels of estradiol ranged from <0.5 to 3.6 pg/mL in five of the seven studies shown in Fig. 1.37-41 In the two remaining studies (Fig. 1), estradiol levels were double or more of the five studies (6.0 and 8.6 pg/mL).42,43 One other study only reported a range of estradiol (8-14 pg/mL) in postmenopausal women, also slightly higher than the above five studies.44 The higher concentrations in these three latter studies cannot be explained by lower sensitivity since the limits of detection and/or quantification were 0.1 to 1 pg/mL.42-44 Demographic details of the postmenopausal women for which serum samples were measured were not reported for most of these validation studies, so any reason for the difference in the estradiol concentrations of the three studies reporting higher estradiol concentrations cannot be determined. Thus, mean estradiol levels from these studies were not included in the above calculation for untreated postmenopausal women.
Systemic estradiol levels with ultralow-dose and low-dose vaginal estrogens
Tables 1 and 2 summarize mean estradiol levels in postmenopausal women who used low-dose and ultralow-dose vaginal estrogens in clinical trials. In general, mean estradiol levels resulting from use of vaginal estrogens were generally lower when measured by LC/MS/MS or GC/MS/MS versus RIA or ELISA within the same dose (Table 1). Given the more recent use of more sensitive measures of estradiol with methods involving MS, estradiol levels within each dose measured with MS will be discussed here. The extent of estradiol systemic absorption generally reflected the estradiol doses ranging from 4 to 10 μg (ultralow dose) to 25 μg of estradiol or 0.3 mg conjugated equine estrogens (CEE) (low dose) as demonstrated by the mean values for Cmax, Cavg, and AUC0-24 (Table 2). Estradiol Cavg levels reported from separate studies on day 1 were 9.1 pg/mL with the 25-μg softgel estradiol vaginal insert and 22.7 pg/mL with the 25-μg estradiol tablet vaginal insert (Vagifem; Novo Nordisk, Plainsboro, NJ),26,45 with relatively lower levels being reported with the 10-μg estradiol softgel vaginal insert (5.8-7.4 pg/mL; Imvexxy [TherapeuticsMD, Boca Raton, FL]) and the 10-μg estradiol tablet vaginal insert (14.8 pg/mL; Vagifem),26,45 and increases from the baseline levels (ie, delta changes) around 4 pg/mL for most studies, as described above. In addition, use of a 4-μg estradiol vaginal insert (Imvexxy; now the lowest vaginal estradiol dose) resulted in the lowest mean estradiol levels of 3.9 pg/mL on day 126 (Table 1). Systemic estradiol with 0.3 mg CEE cream (Premarin Vaginal Cream, Wyeth Pharmaceuticals, Philadelphia, PA) was 9.6 pg/mL29 (Table 1), comparable to the higher 25-μg doses of vaginal inserts, although estradiol is a minor component of 0.3 mg CEE. With the approval of vaginally administered prasterone for the treatment of moderate to severe postmenopausal dyspareunia, systemic estradiol levels resulting with its use may be of interest because DHEA is converted intracellularly to estradiol. Published pharmacokinetic studies of vaginal prasterone in postmenopausal women show estradiol levels of around 4 pg/mL after 7 days, 12 weeks, and 12 months of use.46,47 When compared with baseline, increases of serum estradiol were 24% and 27% at day 748 and week 12,49 respectively.
Varying systemic levels of estradiol were observed based on the type of estradiol vaginal product as noted in head-to-head trials comparing different products. Two randomized, single-dose, open-label, cross-over studies were conducted to compare the pharmacokinetic properties of softgel estradiol vaginal inserts with the same doses of an estradiol tablet vaginal insert.50 In these head-to-head studies, lower estradiol systemic absorption was observed with 10-μg (n = 35; Imvexxy) and 25-μg (n = 36) softgel estradiol vaginal inserts versus 10-μg and 25-μg estradiol tablet inserts (Vagifem) of the same doses, as demonstrated by significantly lower AUC0-24 and Cmax for estradiol with the softgel vaginal inserts (unadjusted data shown in Table 245).50
Factors that may influence estradiol absorption with vaginal estrogens
Thickness of the vaginal wall due to response with estrogen treatment may affect systemic absorption of estradiol when estrogens are used locally in the vagina. Some studies that measured estradiol levels at different times after vaginal estrogen use showed that peak and/or average levels declined over time as vaginal wall thickness likely increased with treatment (Tables 1 and 2).16,20,24,26,27 In the most recent randomized controlled trial of a softgel estradiol vaginal insert, mean Cmax was 47% lower at day 14 than at day 1 with 25 μg estradiol and 33% lower with 10 μg estradiol.26 Similarly, mean Cmax was 42% lower with a tablet vaginal insert of 25 μg estradiol on day 14 versus day 1,27 which was consistent with the trends of two earlier studies of the 10-μg tablet insert.16,20 Another study examining estradiol levels with use of a vaginal ring releasing ∼7.5 μg/d (6.6-20.2 μg/d) found a median of 19.1 pg/mL of estradiol at day 2, but undetectable amounts (<12.3 pg/mL) on days 7, 14, 28, and 84.24 It should be noted that silastic rings exhibit an unusual property of “burst release” of estradiol, presumably from the outer surface of the ring, which lasts for several days and can confound interpretation of results from the first few days. When reported, mean Cavg and AUC0-24 also had similar trends in these studies. Certainly, after longer-term vaginal estrogen treatment (12 weeks, 83 or 84 days, 52 weeks), levels of circulating estradiol were similar to those at baseline or with placebo, and/or at earlier time-points such as after 14 days of treatment.16,21,24,26,27
Systemic estradiol absorption may also be affected by the position in which estradiol is placed in the vagina, whether higher in the vagina as would be with inserting a product with an applicator or lower in the vagina as without an applicator. In the study by Cicinelli et al,51 the effects of estradiol on the blood flow in the uterine and periurethral vessels when estradiol was placed in the inner and outer thirds of the vagina was interpreted as estradiol transport to the uterus. They demonstrated preferential transport depending on placement location in the vagina; uterine artery blood flow significantly increased and blood flow in the periurethral vessels decreased with estradiol administration into the inner vagina, and uterine artery blood flow did not change and the periurethral blood flow significantly increased after administration into the outer vagina.51 From this evidence, the authors recommended placing vaginal estrogens in the outer third of the vagina to reduce the risk of estradiol transport to the uterus.51 In addition, lower estradiol absorption was observed with softgel capsule vaginal inserts placed without an applicator (lower in the vagina) versus tablet vaginal insert of the same doses inserted with an applicator (higher in the vagina) in a head-to-head study.50 Taken together, these data support a difference in estradiol absorption depending on placement of an estrogen product in the vagina.
DISCUSSION
Our updated review of the medical literature found that systemic absorption of estradiol with low-dose and ultralow-dose vaginal estrogen therapies is very low, varies by the product dose, and may be influenced by the product formulation and placement in the vagina. Higher absorption was reported with higher vaginal estradiol doses (25 μg: 7.1-22.7 pg/mL, 10 μg: 4.6-14.8 pg/mL) compared with the lowest available dose of 4 μg (3.6-3.9 pg/mL).26-28,45 We also propose a more updated, although conservative, basal or baseline (untreated/before treatment) range of estradiol levels in normal, untreated postmenopausal women, mostly with VVA, measured in vaginal-therapy clinical trials by highly specific and sensitive LC or GC/MS/MS assays.
Accurately measuring estradiol levels in serum is challenging for many reasons. An Endocrine Society Statement noted that our ability to measure sex steroids properly has not kept pace with their increasing importance in clinical medicine and research.52 Analytical sensitivity and specificity at low concentrations of estradiol are needed for accurate measurement of estradiol levels, especially for postmenopausal women and women taking aromatase inhibitors.52,53 Over the years, methods for measuring estradiol levels from serum have evolved. Early assays involved estradiol extraction, column chromatography, and measurement by RIA and then later, by direct RIAs utilizing buffers that release albumin- and SHBG-bound estradiol. Most recently, extraction followed by chromatography and MS has been used.52 While advantages of immunoassays include eliminating the need for estradiol extraction, reducing costs, and increasing sample throughput, cross-reactivity with immunoassays make them unsuitable for detection of estradiol in patient populations with low estradiol concentrations.53 In addition, RIAs required labor-intensive extractions, handling of radioactive material, and artifact from nonspecific binding to radioactivity.54 Analytical methods such as LC or GC coupled with tandem mass spectrometry (MS/MS) are now being used more frequently for clinical samples as they have overcome some of the sensitivity and specificity limitations with RIAs,52,53 even MS assays have variability between different assays. Varying levels of estradiol are reported when using assays with different cross-reactivities and specificities, particularly when comparing samples measured by RIA and LC/MS or GC/MS. More specific assays (ie, LC or GC with MS) have less cross-reactivity with other steroidal-like molecules and typically yield lower estradiol values than less specific assays with inherently greater cross-reactivities (ie, RIA or ELISA).9,54 Our current review is consistent with previous findings demonstrating that many of the most recently developed highly specific assays can detect or quantify systemic estradiol as low as 0.1 to 2.7 pg/mL using LC/MS/MS or LC/MS,37-41,43,44,55-61 and 1 to 5 pg/mL using GC/MS/MS or GC/MS.26,28,29,42 Indeed, the goal of sensitivity has been to develop assays detecting <5 pg/mL of estradiol for measuring estradiol in serum of postmenopausal women.52,53 Detecting estradiol levels near the lower limit of detection in postmenopausal women may be important in determining their risk for fracture.62
Textbooks report peak normal levels of serum estradiol as ≤20 to 30 pg/mL in postmenopausal women35,63; however, these values were established in older studies with less specific assays, such as RIAs, and should be revised with values from studies using more specific and sensitive assays with less cross-reactivities. Here we reviewed the systemic estradiol levels that we believe would best represent those of normal, untreated, postmenopausal women. Mean estradiol levels from those studies were used to calculate a mean basal level (3.9 pg/mL), with the range of basal estradiol levels for the normal, untreated postmenopausal woman found to be undetectable to 10.7 pg/mL using the 95% CI from the highest published SD (±3.4). Thus, we propose a more current, sensitive mean level of circulating estradiol in normal, untreated women to range from undetectable to 10.7 pg/mL, which we believe to be more accurate than the well-accepted earlier level of ≤20 to 30 pg/mL; yet it is still only an estimate.
An important principle is that assay methods for serum estradiol assessment need to be standardized to be able to accurately, systematically, and consistently measure the low levels of estradiol in postmenopausal women. A single estradiol standard that can be traceable for each biological fluid in which estradiol is measured is needed,52,53 although some of the larger commercial laboratories have made strides to overcoming this obstacle. The Centers for Disease Control and Prevention (CDC) Laboratory/Manufacturer Hormone Standardization (HoSt) Program, endorsed by 14 medical societies, was initiated in 2014 to help provide more accurate and precise hormone measurements used in patient care and research.53,64 The program assesses laboratory methodology and performance by comparing laboratory's specimen measurements with those of their reference LC/MS/MS method.53,64 Progress towards developing accurate, sensitive, and specific assays to measure the very low levels of estradiol in postmenopausal women continues.
Despite several pharmacokinetic studies demonstrating low to negligible systemic absorption of estradiol with low-dose and ultralow-dose vaginal estrogen use, the boxed warning included in the class labeling of systemically administered estrogens is required for low-dose vaginal estrogens, even though experts affiliated with several medical societies have been advocating for its removal.65 While low-dose vaginal estrogens are contraindicated in breast cancer survivors, The North American Menopause Society, Endocrine Society, and the American College of Obstetricians and Gynecologists have recommended a shared decision-making process, which includes an oncologist, to determine low-dose vaginal estrogen use in women with GSM and breast cancer1 or a history of breast cancer,4,66 when they do not respond to nonhormonal therapies. Further support of minimal absorption is shown by the mean estradiol levels with 4-μg and 10-μg estradiol softgel inserts being similar to those at baseline or with placebo in a phase 3, pharmacokinetic substudy.26 This 4-μg estradiol vaginal insert was approved in May, 2018 by the US FDA as Imvexxy,67 is now the lowest vaginal estradiol dose available, and results in mean circulating levels of estradiol <4 pg/mL utilizing LC MS/MS.26
We also found that absorption of estradiol was higher with initial administration (when the vaginal lining was atrophic) than at later treatment periods (when the vaginal lining was thickened) in some studies.16,20,24,26,27 The reason for this higher early absorption has been hypothesized to be due to the thin, atrophied vaginal lining that undergoes “estrogenization” and thickening with continued use of vaginal estrogens over time. The physiological changes and symptoms association with VVA1,68 or the genitourinary syndrome of menopause69 are well known to be associated with the diminishing levels of circulating estrogens at the time of menopause. For example, the ratio of superficial and parabasal cells in the tissue lining the vagina dramatically changes with the loss of superficial cells at menopause.1,68,70 Several clinical studies show that vaginal estrogen therapies effectively increase the percentage of vaginal superficial cells,71-74 essentially “estrogenizing” and thickening the vaginal wall that had atrophied with lower levels of systemic estrogens. Thinner vaginal walls through atrophy are believed to more readily absorb estradiol than thicker, well-rugated, vaginal walls resulting from estrogen therapy; however, because not all studies measuring estradiol over time show the difference early versus later in the study, this hypothesis needs further testing.9 Any biological significance of this acute absorption of estradiol early in treatment is unknown, but not very likely. Additionally, estradiol levels with use for up to 84 days indicated no accumulation of estradiol with lower-dose products, also suggesting that estradiol levels remain steady and within the postmenopausal range with continued therapy with lower-dose products.
CONCLUSIONS
Our review of systemic absorption of estradiol with use of low-dose and ultralow-dose vaginal estrogen therapies found low to negligible amounts of circulating estradiol that may be influenced by product formulation and vaginal placement. This minimal systemic absorption of estradiol with proven efficacy of lower-dose products may be a relevant point when counseling postmenopausal women for the treatment of moderate to severe VVA symptoms. We also propose a more updated basal level of estradiol in normal, untreated postmenopausal women based on levels found in postmenopausal women who are not treated with any hormone therapy. A more accurate level will likely be established with the further standardization of estradiol assays designed to detect minimal amounts of estradiol such as those found in postmenopausal women.
Acknowledgments
The authors acknowledge the medical writing assistance provided by Kathleen Ohleth, PhD, CMPP of Precise Publications, LLC (Bedminster, NJ).
Footnotes
Data presentation: Presented at The North American Menopause Society annual meeting, October 3-6, 2018, San Diego, CA.
Funding/support: TherapeuticsMD supported the medical writing assistance of this review article.
Financial disclosure/conflicts of interest: Dr Santen consults for Sermonix and TherapeuticsMD; and has received research grants from Panterhie Oncology. Dr Constantine consults for multiple pharmaceutical companies, including but not limited to TherapeuticsMD and has stock options with TherapeuticsMD. Drs Mirkin and Bernick are employees of TherapeuticsMD with stock/stock options. Dr Bernick is also a Board member of TherapeuticsMD.
REFERENCES
- 1.The North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. [DOI] [PubMed] [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 2014; 123:202–216. [DOI] [PubMed] [Google Scholar]
- 3.de Villiers TJ, Pines A, Panay N, et al. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013; 16:316–337. [DOI] [PubMed] [Google Scholar]
- 4.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol 2015; 100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 5.Weissfeld JL, Liu W, Woods C, et al. Trends in oral and vaginally administered estrogen use among US women 50 years of age or older with commercial health insurance. Menopause 2018; 25:611–614. [DOI] [PubMed] [Google Scholar]
- 6.Meaidi A, Goukasian I, Lidegaard O. Use of vaginal estrogen in Danish women: a nationwide cross-sectional study. Acta Obstet Gynecol Scand 2016; 95:280–284. [DOI] [PubMed] [Google Scholar]
- 7.Holm E, Aaltonen K, Heikkinen AM, Tiihonen M. From systemic hormone therapy to vaginal estrogen: a nationwide register study in Finland, 2003-2012. Maturitas 2014; 78:293–297. [DOI] [PubMed] [Google Scholar]
- 8.Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women's Health Initiative. Menopause 2012; 19:616–621. [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18:121–134. [DOI] [PubMed] [Google Scholar]
- 10.Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women's Health Initiative Observational Study. Menopause 2018; 25:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kingsberg S, Krychman M, Graham S, Bernick B, Mirkin S. The Women's EMPOWER Survey: identifying women's perceptions on vulvar and vaginal atrophy (VVA) and its treatment. J Sex Med 2017; 14:413–424. [DOI] [PubMed] [Google Scholar]
- 12.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women's VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med 2013; 10:1790–1799. [DOI] [PubMed] [Google Scholar]
- 13.Ingle JN, Kalari KR, Buzdar AU, et al. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids 2015; 99:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrich LS, Naessen T, Elia D, Goldstein JA, Eugster-Hausmann M. Endometrial safety of ultra-low-dose Vagifem 10 microg in postmenopausal women with vaginal atrophy. Climacteric 2010; 13:228–237. [DOI] [PubMed] [Google Scholar]
- 15.Weisberg E, Ayton R, Darling G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric 2005; 8:83–92. [DOI] [PubMed] [Google Scholar]
- 16.Notelovitz M, Funk S, Nanavati N, Mazzeo M. Estradiol absorption from vaginal tablets in postmenopausal women. Obstet Gynecol 2002; 99:556–562. [DOI] [PubMed] [Google Scholar]
- 17.Naessen T, Rodriguez-Macias K. Endometrial thickness and uterine diameter not affected by ultralow doses of 17beta-estradiol in elderly women. Am J Obstet Gynecol 2002; 186:944–947. [DOI] [PubMed] [Google Scholar]
- 18.Naessen T, Berglund L, Ulmsten U. Bone loss in elderly women prevented by ultralow doses of parenteral 17beta-estradiol. Am J Obstet Gynecol 1997; 177:115–119. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson K, Heimer G. Low-dose 17 beta-oestradiol during maintenance therapy: a pharmacokinetic and pharmacodynamic study. Maturitas 1995; 21:33–38. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson K, Heimer G. Low-dose oestradiol in the treatment of urogenital oestrogen deficiency: a pharmacokinetic and pharmacodynamic study. Maturitas 1992; 15:121–127. [DOI] [PubMed] [Google Scholar]
- 21.Mettler L, Olsen PG. Long-term treatment of atrophic vaginitis with low-dose oestradiol vaginal tablets. Maturitas 1991; 14:23–31. [DOI] [PubMed] [Google Scholar]
- 22.Mandel FP, Geola FL, Meldrum DR, et al. Biological effects of various doses of vaginally administered conjugated equine estrogens in postmenopausal women. J Clin Endocrinol Metab 1983; 57:133–139. [DOI] [PubMed] [Google Scholar]
- 23.Smith P, Heimer G, Lindskog M, Ulmsten U. Oestradiol-releasing vaginal ring for treatment of postmenopausal urogenital atrophy. Maturitas 1993; 16:145–154. [DOI] [PubMed] [Google Scholar]
- 24.Holmgren PA, Lindskog M, von Schoultz B. Vaginal rings for continuous low-dose release of oestradiol in the treatment of urogenital atrophy. Maturitas 1989; 11:55–63. [DOI] [PubMed] [Google Scholar]
- 25.Manonai J, Theppisai U, Suthutvoravut S, Udomsubpayakul U, Chittacharoen A. The effect of estradiol vaginal tablet and conjugated estrogen cream on urogenital symptoms in postmenopausal women: a comparative study. J Obstet Gynaecol Res 2001; 27:255–260. [DOI] [PubMed] [Google Scholar]
- 26.Archer DF, Constantine GD, Simon J, et al. TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. Menopause 2017; 24:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric 2010; 13:219–227. [DOI] [PubMed] [Google Scholar]
- 28.Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause 2009; 16:30–36. [DOI] [PubMed] [Google Scholar]
- 29.Dorr MB, Nelson AL, Mayer PR, et al. Plasma estrogen concentrations after oral and vaginal estrogen administration in women with atrophic vaginitis. Fertil Steril 2010; 94:2365–2368. [DOI] [PubMed] [Google Scholar]
- 30.Martel C, Labrie F, Archer DF, et al. Serum steroid concentrations remain within normal postmenopausal values in women receiving daily 6.5 mg intravaginal prasterone for 12 weeks. J Steroid Biochem Mol Biol 2016; 159:142–153. [DOI] [PubMed] [Google Scholar]
- 31.Ke Y, Belanger A, Simard JN, et al. Concentration range of serum sex steroids in normal postmenopausal women and those with diagnosis of vulvovaginal atrophy. Menopause 2018; 25:293–300. [DOI] [PubMed] [Google Scholar]
- 32.Labrie F, Belanger A, Belanger P, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 2006; 99:182–188. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Paris F, Sultan CS, et al. Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. J Clin Endocrinol Metab 2005; 90:1407–1413. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Ettinger B, Stanczyk FZ, et al. Comparison of methods to measure low serum estradiol levels in postmenopausal women. J Clin Endocrinol Metab 2006; 91:3791–3797. [DOI] [PubMed] [Google Scholar]
- 35.Fritz MA, Speroff L. Menopause and the perimenopausal transition. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia, PA: Wolters Kluwer; 2011. p. 673–748. [Google Scholar]
- 36.Rothman MS, Carlson NE, Xu M, et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography-tandem mass spectrometry. Steroids 2011; 76:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Rangiah K, Mesaros C, et al. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids 2015; 96:140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wooding KM, Hankin JA, Johnson CA, et al. Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization. Steroids 2015; 96:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke Y, Bertin J, Gonthier R, Simard JN, Labrie F. A sensitive, simple and robust LC-MS/MS method for the simultaneous quantification of seven androgen- and estrogen-related steroids in postmenopausal serum. J Steroid Biochem Mol Biol 2014; 144 (Pt B):523–534. [DOI] [PubMed] [Google Scholar]
- 40.Pauwels S, Antonio L, Jans I, et al. Sensitive routine liquid chromatography-tandem mass spectrometry method for serum estradiol and estrone without derivatization. Anal Bioanal Chem 2013; 405:8569–8577. [DOI] [PubMed] [Google Scholar]
- 41.Ray JA, Kushnir MM, Bunker A, Rockwood AL, Meikle AW. Direct measurement of free estradiol in human serum by equilibrium dialysis-liquid chromatography-tandem mass spectrometry and reference intervals of free estradiol in women. Clin Chim Acta 2012; 413:1008–1014. [DOI] [PubMed] [Google Scholar]
- 42.Caron P, Turcotte V, Guillemette C. A chromatography/tandem mass spectrometry method for the simultaneous profiling of ten endogenous steroids, including progesterone, adrenal precursors, androgens and estrogens, using low serum volume. Steroids 2015; 104:16–24. [DOI] [PubMed] [Google Scholar]
- 43.Kushnir MM, Rockwood AL, Bergquist J, et al. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am J Clin Pathol 2008; 129:530–539. [DOI] [PubMed] [Google Scholar]
- 44.Faqehi AMM, Cobice DF, Naredo G, et al. Derivatization of estrogens enhances specificity and sensitivity of analysis of human plasma and serum by liquid chromatography tandem mass spectrometry. Talanta 2016; 151:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.TherapeuticsMD. Data on file. 2019. [Google Scholar]
- 46.Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111:178–194. [DOI] [PubMed] [Google Scholar]
- 47.Ke Y, Gonthier R, Simard JN, et al. Serum steroids remain within the same normal postmenopausal values during 12-month intravaginal 0.50% DHEA. Horm Mol Biol Clin Investig 2015; 24:117–129. [DOI] [PubMed] [Google Scholar]
- 48.Labrie F, Martel C. A low dose (6.5 mg) of intravaginal DHEA permits a strictly local action while maintaining all serum estrogens or androgens as well as their metabolites within normal values. Horm Mol Biol Clin Investig 2017; 29:39–60. [DOI] [PubMed] [Google Scholar]
- 49.Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 2009; 16:907–922. [DOI] [PubMed] [Google Scholar]
- 50.Pickar JH, Amadio JM, Bernick BA, Mirkin S. Pharmacokinetic studies of solubilized estradiol given vaginally in a novel softgel capsule. Climacteric 2016; 19:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cicinelli E, Di NE, De ZD, et al. Placement of the vaginal 17beta-estradiol tablets in the inner or outer one third of the vagina affects the preferential delivery of 17beta-estradiol toward the uterus or periurethral areas, thereby modifying efficacy and endometrial safety. Am J Obstet Gynecol 2003; 189:55–58. [DOI] [PubMed] [Google Scholar]
- 52.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 2013; 98:1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smy L, Straseski JA. Measuring estrogens in women, men, and children: recent advances 2012-2017. Clin Biochem 2018; 62:11–23. [DOI] [PubMed] [Google Scholar]
- 54.Ketha H, Girtman A, Singh RJ. Estradiol assays: the path ahead. Steroids 2015; 99:39–44. [DOI] [PubMed] [Google Scholar]
- 55.Botelho JC, Ribera A, Cooper HC, Vesper HW. Evaluation of an isotope dilution HPLC tandem mass spectrometry candidate reference measurement procedure for total 17-beta estradiol in human serum. Anal Chem 2016; 88:11123–11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi X, Leung E, Bridgman R, Koo S, Yeo K-T. High-sensitivity micro LC-MS/MS assay for serum estradiol without derivatization. J Appl Lab Med 2016; 1:14–24. [DOI] [PubMed] [Google Scholar]
- 57.Keski-Rahkonen P, Desai R, Jimenez M, Harwood DT, Handelsman DJ. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem 2015; 87:7180–7186. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Franke AA. Improved profiling of estrogen metabolites by orbitrap LC/MS. Steroids 2015; 99:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khedr A, Alahdal AM. Liquid chromatography-tandem mass spectrometric analysis of ten estrogen metabolites at sub-picogram levels in breast cancer women. J Chromatogr B 2016; 1031:181–188. [DOI] [PubMed] [Google Scholar]
- 60.Owen LJ, Wu FC, Keevil BG. A rapid direct assay for the routine measurement of oestradiol and oestrone by liquid chromatography tandem mass spectrometry. Ann Clin Biochem 2014; 51:360–367. [DOI] [PubMed] [Google Scholar]
- 61.Fiers T, Casetta B, Bernaert B, Vandersypt E, Debock M, Kaufman JM. Development of a highly sensitive method for the quantification of estrone and estradiol in serum by liquid chromatography tandem mass spectrometry without derivatization. J Chromatogr B 2012; 893-894:57–62. [DOI] [PubMed] [Google Scholar]
- 62.Cauley J, LaCroix A, Robbins J, et al. Baseline serum estradiol and fracture reduction during treatment with hormone therapy: the Women's Health Initiative randomized trial. Osteoporos Int 2010; 21:167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lobo RA. Strauss JF, Barbieri RL. Menopause and aging. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 6th ed.Philadelphia, PA: Saunders Elsevier; 2009. 325–355. [Google Scholar]
- 64.Centers for Disease Control and Prevention. HoSt/VDSCP: hormone and vitamin D standardization programs. Available at: https://www.cdc.gov/labstandards/hs.html Accessed December 1, 2018. [Google Scholar]
- 65.Manson JE, Goldstein SR, Kagan R, et al. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause 2014; 21:911–916. [DOI] [PubMed] [Google Scholar]
- 66.American College of Obstetricians and Gynecologists. The use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Committee Opinion No. 659. Obstet Gynecol 2016; 127:e93–96. [DOI] [PubMed] [Google Scholar]
- 67.Imvexxy (estradiol vaginal inserts) Prescribing Information. Boca Raton, FL: TherapeuticsMD; 2018. [Google Scholar]
- 68.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarrel PM, Portman D, Lefebvre P, et al. Incremental direct and indirect costs of untreated vasomotor symptoms. Menopause 2014; 22:260–266. [DOI] [PubMed] [Google Scholar]
- 70.Bercovici B, Gron S, Pisanty S. Vaginal and oral cytology of the menopause. A comparative study. Acta Cytol 1985; 29:805–809. [PubMed] [Google Scholar]
- 71.Constantine G, Simon JA, Pickar JH, et al. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2017; 24:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol 2008; 112:1053–1060. [DOI] [PubMed] [Google Scholar]
- 73.Bachmann G, Bouchard C, Hoppe D, et al. Efficacy and safety of low-dose regimens of conjugated estrogens cream administered vaginally. Menopause 2009; 16:719–727. [DOI] [PubMed] [Google Scholar]
- 74.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 2008; 111:67–76. [DOI] [PubMed] [Google Scholar]
- 75.Rioux JE, Devlin C, Gelfand MM, Steinberg WM, Hepburn DS. 17β-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause 2000; 7:156–161. [DOI] [PubMed] [Google Scholar]
- 76.Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause 2002; 9:179–187. [DOI] [PubMed] [Google Scholar]
- 77.Simunic V, Banovic I, Ciglar S, Jeren L, Pavicic BD, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet 2003; 82:187–197. [DOI] [PubMed] [Google Scholar]
- 78.Dugal R, Hesla K, Sordal T, Aase KH, Lilleeidet O, Wickstrom E. Comparison of usefulness of estradiol vaginal tablets and estriol vagitories for treatment of vaginal atrophy. Acta Obstet Gynecol Scand 2000; 79:293–297. [PubMed] [Google Scholar]


