I. DETECTION OF SOUNDS BY FISHES
Fishes obtain substantial information about their environment by listening to the sounds around them. Indeed, because sound propagates rapidly and over great distances in water as compared to in air, it provides fishes with information from far greater distances than do other sensory stimuli. Thus, any interference with detection of sounds has the potential of reducing fitness and impacting the lives of fishes (e.g., Popper and Hawkins, 2019).
Although the sounds that fishes hear are confined to low frequencies (often to no more than 800–1000 Hz, but this is very species dependent) in comparison with many terrestrial vertebrates and aquatic mammals, fishes are able to discriminate between sounds of different amplitude and frequency, and between calls that differ in their temporal characteristics (e.g., Fay, 1988; Fay and Megela Simmons, 1999). Fishes are also able to use auditory cues to seek out the location of a sound source (Sand and Bleckmann, 2008; Hawkins and Popper, 2018). Sounds may play a role in navigation, foraging for prey, detection of predators, and communication of reproductive state, and some marine species may use sound for habitat selection. Detailed discussions of the role of sound in the lives of fishes can be found in several recent reviews (e.g., Popper and Hawkins, 2019; Putland et al., 2019).
II. PURPOSE OF THIS PAPER
Because sound is so important to fishes, knowledge of their hearing capabilities is imperative for determining whether human activities, particularly in terms of noise pollution, have an impact on hearing and thus on fish behavior. It is important, therefore, to determine those levels of different sounds that particular species are able to respond to, and those levels that they cannot detect, in order to evaluate the significance of different sounds to fishes and to determine the distances over which sounds can be detected. It is also important to have a far better understanding of how fishes detect and process sounds.
A key point that led to our thinking for this paper derives from the observation that many investigators (including the authors) have measured hearing by fishes using a wide range of techniques and approaches. Most of this work has focused on measuring hearing sensitivity by determining hearing thresholds—defined as the lowest sound levels an animal can detect and respond to at particular frequencies. There have been far fewer studies of other, albeit very important, questions, such as whether, how, and how well, fishes can discriminate between sounds (e.g., frequency, intensity, temporal patterns), detect signals in the presence of sounds that mask them, and determine the direction to a sound source.
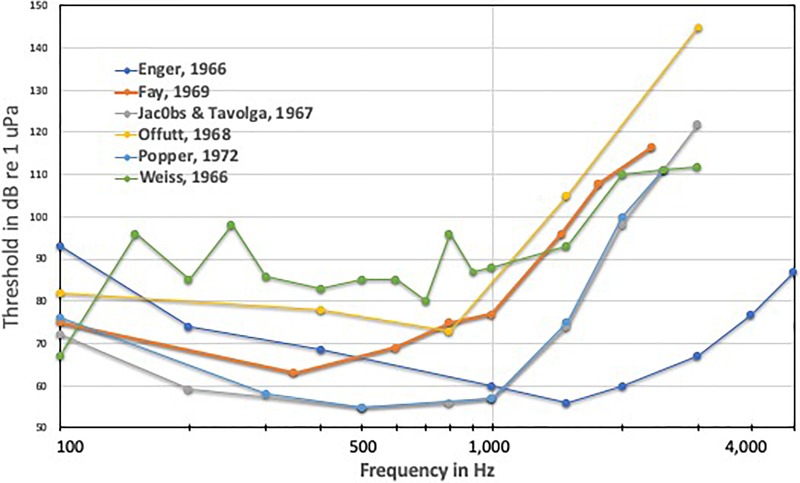
While there are few data for anything but hearing thresholds and bandwidth of hearing, it was recognized a number of years ago that there is very wide variation in thresholds determined for even a single species (Fig. 1). This variation has been attributed to differences in experimental techniques and approach rather than reflecting actual differences in hearing between species (e.g., Hawkins, 1973; Ladich and Fay, 2013; Sisneros et al., 2016). Hence, we are not able to reliably examine and compare the hearing abilities of different species, nor can we even be fully confident of most of the data we have on hearing in species that have been studied in a single lab. This unreliability of data hamper our understanding of what fishes can hear, and thus reduces our understanding of fish bioacoustics. Moreover, uncertain data on fish bioacoustics affects our understanding of the potential impacts of anthropogenic sounds on fishes.
FIG. 1.
(Color online) Variation in auditory thresholds determined behaviorally for the goldfish (Carassius auratus) by various investigators. Similar variation in goldfish thresholds for ABR data can be found in Ladich and Fay (2013).
The purpose of this paper is twofold. First, we discuss the basis for the variation in data, and point out what we see as the major issues resulting from having unreliable data. Second, we present, as investigators who have focused on fish hearing for many decades, some initial thoughts on what and how future scientific work should be carried out to investigate fish hearing for both basic science and applied purposes. Our hope is that these suggestions may provide a basis for future discussions and approaches on how experiments should be done.
We do want to point out that this paper is not meant to be a full or comprehensive review. While we do cite some literature, our intent is to just illustrate points with some basic (and often “historic”) literature and focus on our ideas. Readers interested in more depth on various parts of this paper should refer to the reviews we cite throughout.
III. BACKGROUND
Before getting to the heart of our arguments, it is important to provide a few ideas and terms that help understanding of various issues. In each case, we provide a number of references that will provide more detailed background for those needing additional information.
A. Underwater acoustics
One of the fundamental issues with regard to any experiments on fish hearing is the nature of sound in water, and, in particular, sound in tanks. As a reminder, sound originates as a local mechanical disturbance generated by the movement or vibration of any immersed object, and results from the inherent elasticity of the surrounding medium. Sound consists of a traveling energy wave, within which the component particles of the water are alternately forced together and then apart. The to-and-fro motion that constitutes the sound, referred to as the particle motion, is accompanied by an oscillatory change in pressure above and below the local hydrostatic pressure, defined as the sound pressure. Both the sound pressure and the particle motion are important to fishes, although while all fishes detect and use particle motion, only a subset can detect sound pressure (e.g., Nedelec et al., 2016; Popper and Hawkins, 2018).
The particle motion, which can be measured in terms of displacement, velocity, or acceleration, differs from the sound pressure in that it is inherently directional, and all the motion parameters are vector quantities. Sound pressure, on the other hand, is a scalar quantity, acting in all directions. A more detailed understanding of sound pressure and particle motion can be found at www.dosits.org and in Popper and Hawkins (2018). An early and important discussion of the acoustic near field and far field, and its relevance to fishes, was provided van Bergeijk (1964).
B. Fish sound detection mechanisms
Two sensory systems were initially suggested as detectors of sound in fishes: the paired labyrinth organs of the head (the inner ears), and the lateral line system of the head and trunk. The inner ear includes three semicircular canals as well as three otolithic end organs. The otolithic end organs are involved in hearing in all fishes, through the detection of particle motion (Popper and Hawkins, 2018). In some fishes, sensitivity is also shown to sound pressure through the coupling of a gas-filled body (e.g., the swim bladder) to the inner ear (e.g., Poggendorf, 1952). The gas is more compressible than the surrounding medium, and changes volume in response to the sound pressure, generating greater amplitudes of particle motion at the inner ear (e.g., Sand and Hawkins, 1973).
While the inner ears are sensitive to sound, and to linear and angular acceleration of the fish body, the lateral line is primarily sensitive to local water movements relative to the fish surface (Dijkgraaf, 1963). In this paper, we focus on the role of hearing, but similar methodological issues need to be considered with regard to better understanding of the function of the lateral line. For a comprehensive discussion of the functional overlap and nonoverlap between lateral line and auditory systems, see Braun and Sand (2013).
IV. MAJOR METHODOLOGICAL ISSUES
A most important question is why there is great variability in hearing data within the same species determined by different investigators (see Fig. 1). Related to this, and much harder to answer at the moment, is whether the variability is restricted to goldfish (Carassius auratus), the species for which we have the most hearing data, from the widest number of investigators, or whether the same issues would apply to other species if more hearing studies were carried out on them. Our suggestion is that the same methodological issues apparent for goldfish would be found for other species. Thus, hearing data on every species studied to date should be questioned unless one can show that the methods utilized did not impact the results adversely. The only exception to this argument is when different species have been studied in exactly the same acoustic environment (Tavolga and Wodinsky, 1963; Ladich and Fay, 2013; Hawkins, 2014) or when the research questions are related to measuring changes in hearing, such as in studies of temporary threshold shift (e.g., Popper et al., 2007).
The remainder of Sec. IV examines the methodological issues that we consider most critical in affecting what is known about fish hearing capabilities. These include: (a) the acoustic environment (Sec. IV A); (b) behavioral vs electrophysiological methods (Secs. V A and V B); (c) threshold determination (Sec. V C); and (d) other issues (Sec. VI).
A. The acoustic environment
One of the main reasons for differences in the hearing thresholds determined for the same species (Fig. 1) results from the different acoustic conditions under which experiments were conducted. The fundamental issue is the complexity of the sounds in restricted environments, such as aquarium tanks (e.g., Rogers et al., 2016; Campbell, 2019). This issue was actually pointed out decades ago by Griffin (1950) and Parvulescu (1964), who noted that there are pitfalls when carrying out experiments in small tanks. These warnings, however, have rarely been heeded, and most investigators are only “rediscovering” them today, though often still not acting on, or understanding, them.
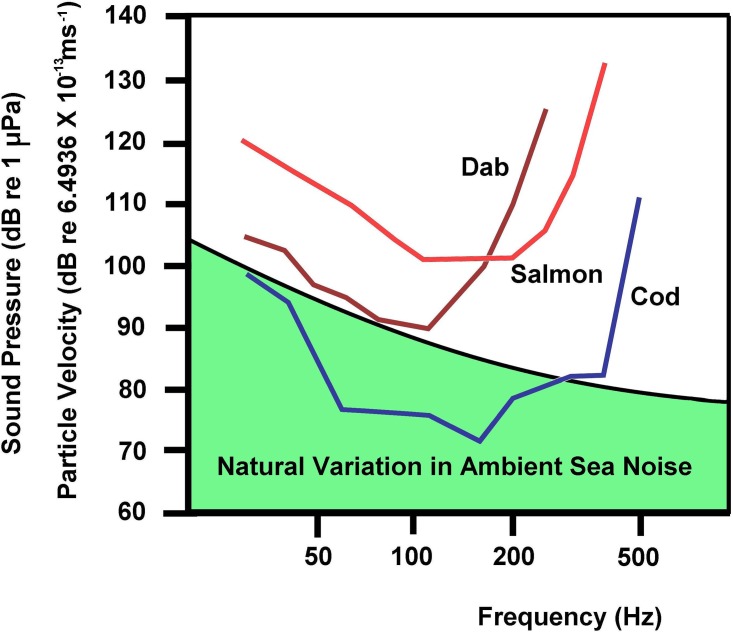
A major contribution to this problem is that it is very hard (if not impossible) to accurately predict or even measure the particle motion components of the sound field in tanks (but see below for exceptions). As a consequence, few fish hearing studies have been carried out under acoustic conditions that allow for accurate determination of the sound field. These include several experiments that have been carried out under free-field conditions in the sea, which provide hearing thresholds for different species, including particle motion thresholds (Fig. 2).
FIG. 2.
(Color online) Fish hearing thresholds obtained in the sea under free-field conditions. The thresholds were determined in response to pure tone stimuli at different frequencies. The absolute thresholds in the Atlantic cod (Gadus morhua) (Chapman and Hawkins, 1973) are below the natural ambient noise levels, especially at the most sensitive frequencies. So, when the fish are in the presence of such noise, the thresholds are raised. The Atlantic cod is sensitive to both sound pressure and particle motion, whereas the dab (Limanda limanda) (Chapman and Sand, 1974) and Atlantic salmon (Salmo salar) (Hawkins and Johnstone, 1978) are only sensitive to particle motion. The reference level for the particle velocity is based on the level that exists in a free sound field for the given sound pressure level.
One of the real difficulties in most fish hearing studies is that they most often express hearing sensitivity in terms of sound pressure alone and ignore particle motion (reviewed in Nedelec et al., 2016; Popper and Hawkins, 2018), partially because sound pressure instrumentation is more readily available. However, since most fishes primarily detect particle motion, presenting hearing sensitivity data in terms of pressure is virtually meaningless.
1. Particle motion and sound pressure in tanks
An issue with measuring particle motion, however, is that it generally has to be measured along three axes because it is a vector quantity and so is far harder to measure than sound pressure (even if one has the right instruments). There are some particle motion hydrophones available commercially, although they can be rather expensive. Many of the particle motion hydrophones used in bioacoustics measurements are custom made, and consist of three accelerometers or velocity transducers, aligned in x, y, and z directions within a watertight housing, and the assembled unit is adjusted to be virtually neutrally buoyant in water. Alternatively, particle motion in a stable sound field may be measured with only one transducer, which is sequentially rotated in order to obtain measurements along three axes.
In contrast to a free-field environment, sounds in a tank or close to any interface (e.g., surface or bottom of a body of water shallower than a wavelength) deviate from ideal conditions because the relationship between sound pressure and particle motion is changed close to interfaces with media of different acoustic properties than water. Standard aquarium tanks are especially deficient in this respect. The sound fields presented to fish within even large tanks are generally very complex and quite unlike natural sound fields that a fish would encounter in a normal aquatic environment, including shallow water (see Duncan et al., 2016; Rogers et al., 2016).
Moreover, the direction of the particle motion may be affected by the presence of hard and soft surfaces. The majority of small aquarium tanks are, in effect, completely surrounded by air, resulting in any loudspeaker immersed in the tank close to the fish producing very high levels of particle motion. The walls of the tank are usually so thin and flexible that they act as pressure release boundaries (Parvulescu, 1964). As pointed out by Rogers et al. (2016), for a tank wall not to be flexible it would have to be >3 cm thick steel. In many tanks, when the sound source is in the water, the sound pressure often falls towards zero at the walls, bottom, and surface, greatly increasing the levels of particle motion. Under such experimental conditions it is virtually impossible to achieve a ratio between sound pressure and particle motion similar to the ratio encountered in a free sound field.
Parvulescu (1964) suggested that sound pressure generated in air outside a small tank could be used to generate a uniform sound pressure field within the tank itself. However, hardly any particle motion is then generated within the water itself, and those species that are sensitive only to particle motion will not respond well to the sounds generated (Popper and Hawkins, 2018).
2. Special tanks
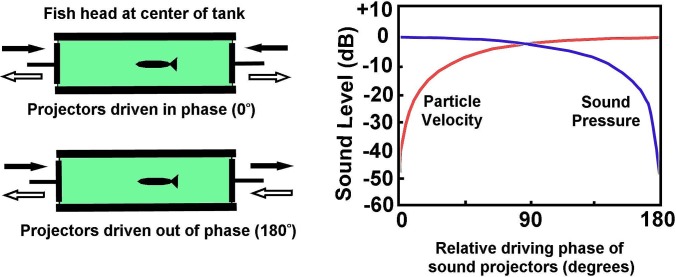
A range of special acoustic tanks have been constructed by different workers. An approach suggested by Parvulescu (1964) was to generate the acoustic field within a tubular tank, shorter than the wavelength of the sounds to be presented, and with the tube be fitted with a sound projector at each end (Fig. 3). By driving the projectors with signals of similar amplitude, but differing phase, it is possible to control the ratio of sound pressure to particle motion at the center of the tube. Poggendorf (1952) (well before Parvulescu) applied these principles in examining the hearing of fishes, although in his case the tube was open at one end. Later, Hawkins and MacLennan (1976) described in detail the characteristics of a standing wave tube that had very thick steel walls (Fig. 3), and applied it in hearing experiments, where they demonstrated sensitivity of a flatfish (plaice, Pleuronectes platessa) to particle motion rather than sound pressure.
FIG. 3.
(Color online) The functioning of a standing wave tube. Two sound projectors opposing one another are driven with the same signal at different phases, thereby varying the ratio of sound pressure to particle motion at the center of the tank, where the head of the fish is placed. (Modified and revised from Hawkins and MacLennan, 1976.)
A related, but more sophisticated, approach is to create standing waves by pairs of opposing sound projectors suspended in large tanks or in the field (Buwalda, 1981). Several researchers have used variants of this technique to alter the amplitude, phase, and direction of the input variables to the ear in a stationary fish (e.g., Buwalda et al., 1983). The most versatile projector configuration includes three projector pairs, which secures complete spatial control of the parameters (Schellart and Buwalda, 1990).
Another, but more difficult, approach is to do studies in midwater in large bodies of water, well away from reflecting boundaries, where measurements of the sound pressure can be used to calculate particle motion levels. The various techniques have been reviewed by Hawkins (2014). These studies include experiments in the sea, where the effects of reflecting boundaries can largely be eliminated and, by changing the distance of the animal from the sound source, the ratio of sound pressure to particle motion can be varied (Chapman and Hawkins, 1973).
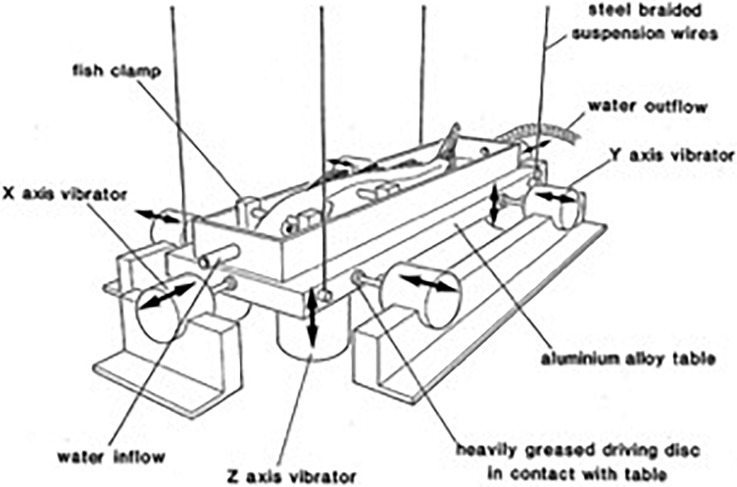
Because a fish in water is nearly acoustically transparent and vibrates with the same phase and amplitude as the surrounding medium, vibration of a fish in air mimics stimulation by the kinetic sound component in water. This technique, such as mounting the fish on a vibrating table in air, was first used by Enger et al. (1973) and later refined and used by others (e.g., Sand, 1974; Hawkins and Horner, 1981; Fay, 1984), and eliminates the problem of making directionally well-defined stimuli in small tanks. Many later neurophysiological studies of hearing in fish have employed variations of this stimulation technique (Fig. 4) (e.g., Lu and Popper, 2001; Meyer et al., 2011).
FIG. 4.
A vibrating table, which allows a fish to be vibrated in different directions. Figure based upon work described by Hawkins and Horner (1981).
3. Sound sources
An additional problem in generating underwater sounds is that there is only a limited range of suitable sound projectors (loudspeakers) available. The generation of underwater sound requires projectors that can operate at quite low frequencies and generate higher sound pressures than is necessary in air. Many of the underwater projectors available commercially have been designed to generate sound for humans to listen to within swimming pools and are not especially effective at producing the low frequency sounds that can effective stimulate the inner ears of fishes.
An additional significant weakness with many studies of fish hearing, and particularly those investigating directional hearing, is the use of monopole sound projectors (projectors that oscillate in volume). In the near field, however, the directional characteristics of the particle motions of monopole sources are greatly different from the characteristics of dipole sources that oscillate with constant volume (van Bergeijk, 1964). Because the majority of natural sound sources are dipoles (except most vocalizing fishes) and many natural, behavioral responses to sounds occur within the near field (due to the low frequencies of relevant sounds), dipole sources may be preferred for studying directional responses in the field. The power of this approach was demonstrated by Zeddies et al. (2012).
4. Background noise
It is also critical when carrying out hearing experiments to ensure that the level of background noise in the experimental tank, or in the sea or freshwater environment, is also monitored. Noise can be a particular problem in aquarium tanks, where pumps, aerators, and the presence of human activities in their vicinity may create high levels of background noise that can affect the sensitivity of fishes to other sounds (e.g., Fig. 2). Even in natural environments, the level of ambient noise from natural sources can also affect the ability of fishes to detect sounds (Chapman and Hawkins, 1973). In many studies, the thresholds reported may well have been masked by background noise, thus resulting in incorrect or underestimated threshold data (see Fig. 2). Thus, the measurement of background noise levels will be important in future studies, to help determine the potential limits of collected hearing threshold data.
5. Summary of the acoustic environment for studying fish hearing
Because of these issues, the design of environments for studying fish hearing is very difficult, and often very expensive. It is likely that most, if not all, of the tanks used by investigators in the past were acoustically messy and gave results that were likely not accurate. Attempts have been made to improve the sound field in tanks using a variety of devices, most often absorbing materials covering the walls and bottoms of the tanks (e.g., foam rubber, horse hair, etc.), but none of these are truly effective in reducing sound reflections (Rogers et al., 2016).
Moreover, where investigators have argued that their tank design is useful, these measures (if they really do work) have only been for sound pressure, and no one, to our knowledge, has ever made a study of how various measures might mitigate the excessive particle motion in a tank. If researchers must use tanks for their fish hearing studies, a suggestion for future studies is to measure the acoustic impedance at different locations within the tank environment (Popper and Fay, 2011) (e.g., the ratio of pressure to particle velocity, p/v) and compare it to the acoustic impedance in water in a free-field.
V. METHODS FOR EXAMINING THE DETECTION OF SOUNDS BY FISHES
Fish hearing capabilities have primarily been studied using either behavioral or electrophysiological approaches to measure behavioral thresholds and to determine the animal's detection bandwidth (that is, the range of sound frequencies that a fish can detect). These approaches have also been used to measure changes in sensitivity, caused by masking sounds and the effects of high intensity sounds on hearing sensitivity. However, only behavioral methods can be used to determine more subtle aspects of hearing, such as discrimination between sounds, determination of sound direction, the ability to detect sounds in the presence of masking noise, and to identify complex signals (e.g., Fay, 2008).
A. Behavioral approach
The behavioral approach involves conditioning fishes to respond to a sound stimulus. This approach was pioneered for fish studies by Karl von Frisch and his students (e.g., von Frisch and Stetter, 1932). The approach was later refined by Tavolga and his colleagues (Tavolga and Wodinsky, 1963; Jacobs and Tavolga, 1967) who applied modern psychoacoustical approaches (Green and Swets, 1966) to “ask” fishes what they could hear.
The behavioral approach involves training fish to do some task, which may include overt movement from one side of a tank to another or hitting a paddle, in an unambiguous behavioral way when the fish detects a particular sound. No matter the task, the fish is trained to respond to sound (the conditioned stimulus) by pairing it with an unconditioned stimulus to which the fish will respond without training, such as food or an electric shock. The results reflect not only detection of sound by the ear, but the processing of the signal by the whole nervous system in order to elicit the response.
In other cases, fish may be trained to change their heart or respiratory rate in anticipation of an aversive stimulus (e.g., electric shock) (e.g., Chapman and Hawkins, 1973; Fay, 1974). In such studies, there is a direct measure of heart or respiratory rate in a restrained fish. The fish is presented with a sound (conditioned stimulus) followed, after a few seconds, by an unconditioned stimulus, a mild electric shock. After several trials, the fish then starts to change its heart or respiratory rate at the onset of the sound, having associated the sound with the shock.
More recently, a different behavioral approach was developed to determine hearing thresholds in larval zebrafish using a modification of the acoustic startle reflex (an innate behavioral response) by pre-pulse tones known as pre-pulse inhibition (Bhandiwad et al., 2013). This very sensitive behavioral testing paradigm has the potential to be adapted to determine hearing thresholds in other larval and adult fishes.
A critically important advantage of the various conditioning paradigms as compared to physiological approaches (discussed below) is that animals tend to be motivated to detect the signal, and so they even respond when the signal is very low, and in all cases the responses are clear and objective—the animal responds, or it does not. And, because this is a behavioral response, the signal is processed within the whole nervous system, thereby enabling the animal to maximize the likelihood of detection and thus respond appropriately.
At the same time, training may take a substantial amount of time (perhaps several days or even weeks) to be reliable. Therefore, this approach is not always suitable for cases where data are needed from a large number of animals. In those cases, and with a more limited number of research questions, an electrophysiological approach may have some advantages.
B. Electrophysiological approach
The electrophysiological approach measures responses to a sound at various levels by placing electrodes close to the ear, nerves, and central nervous system (CNS). This was pioneered for fishes by Enger (e.g., 1963) and later by Kenyon et al. (1998). The simplest application of such techniques involves gross recordings of synchronous neural activity from sensory cells within the ears, the auditory nerves, and/or auditory brainstem activity that is evoked by acoustic stimuli within the CNS. This approach does not require any behavioral response on the part of the animal, and it only indicates detection of sound stimuli potentially up to the level of the auditory brainstem (Sisneros et al., 2016). Such recordings are often referred to as the auditory brainstem response (ABR) or auditory evoked potentials (AEP). Results from such experiments are most often based on the lowest sound level at each frequency that gives a defined repeatable physiological response from some level of the auditory pathway and is based on the subjective analysis of the observer, who decides when the animal has detected the signal. Confounding such studies, however, is that when conducting electrophysiological studies, it is especially important to reduce the level of electrical background noise by careful screening against extraneous sources of electrical noise, since high variability in residual noise can lead to significant inter-observer differences in AEP thresholds (Xiao and Braun, 2008).
Moreover, at least for fishes, there is no good evidence that the electrophysiological results represent the most sensitive hearing or the widest bandwidth that the fish can detect. At the same time, these techniques are ideal for studying a rapid change in auditory sensitivity, such as temporary hearing loss where fishes are compared to themselves before and after exposure to high level sounds (e.g., Popper et al., 2007; Sisneros et al., 2016).
Thus, while AEP and ABR measures have real value, they do not provide the kind of information about the actual hearing capabilities of fishes that can only be gotten in behavioral studies. There is a particular problem in examining the effects of detection and discrimination of complex sounds, including measures of masking and critical bands, where there is likely extensive signal processing of signals in the central nervous system before animals have the information that elicits a response.
C. Determining thresholds
In any sensory study, the lowest level of detectability is generally referred to as the “threshold.” The sensitivity of a fish to sound is expressed as an audiogram, a curve showing the thresholds or minimum sound levels to which the fish will respond over a range of frequencies (e.g., Figs. 1 and 2). In presenting sounds to fishes, to determine auditory thresholds, it is commonplace to use pure tone stimuli that last for a few seconds, and to present them over a range of frequencies. It is important, however, to ensure that there is a gradual rise and fall in the level of each pure tone stimulus, as an abrupt start and stop may result in the generation of a broader band of frequencies from the sound source (e.g., Tavolga and Wodkinsky, 1963).
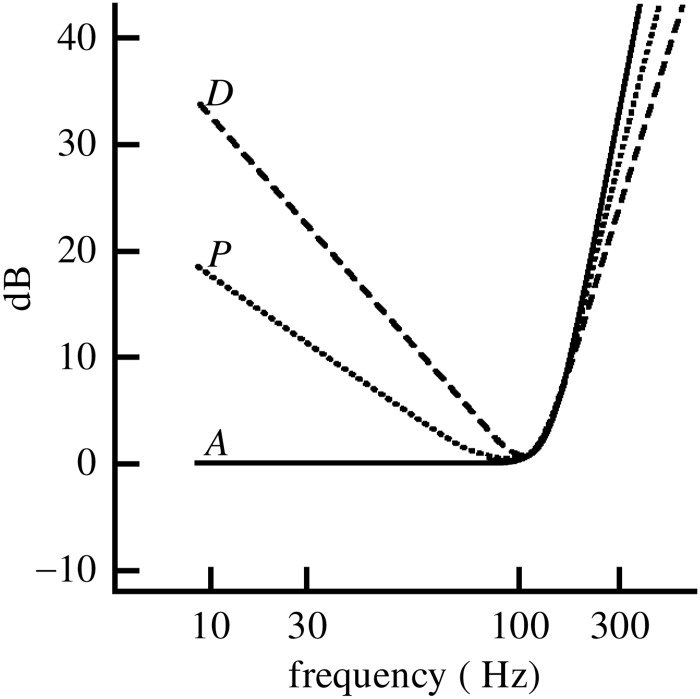
As mentioned earlier, most thresholds for fish have been determined in terms of the measured sound pressures even when they primarily detect particle motion. While it is, as discussed, hard to get a good sound field in a tank, there have been some studies in tubes and in the field where the ratio of sound pressure to particle motion has been varied. In these circumstances, the thresholds for some species follow the sound pressure; but in others the thresholds follow the particle motion. In such cases, the tradition of relating fish audiograms to sound pressure may lead to misinterpretations concerning optimal frequency ranges and hearing capabilities, because the shape of the audiogram greatly depends on the acoustic parameter to which thresholds are related. Figure 5 shows a hypothetical audiogram related to particle displacement, sound pressure (or particle velocity) and particle acceleration. When thresholds are presented in terms of particle acceleration, which is the relevant stimulus parameter for a species sensitive to particle motion rather than to sound pressure, the apparent drop in sensitivity towards low frequencies disappears.
FIG. 5.
Hypothetical fish audiogram related to particle displacement (D), sound pressure or particle velocity (P), and particle acceleration (A) (from Sand and Karlsen, 2000).
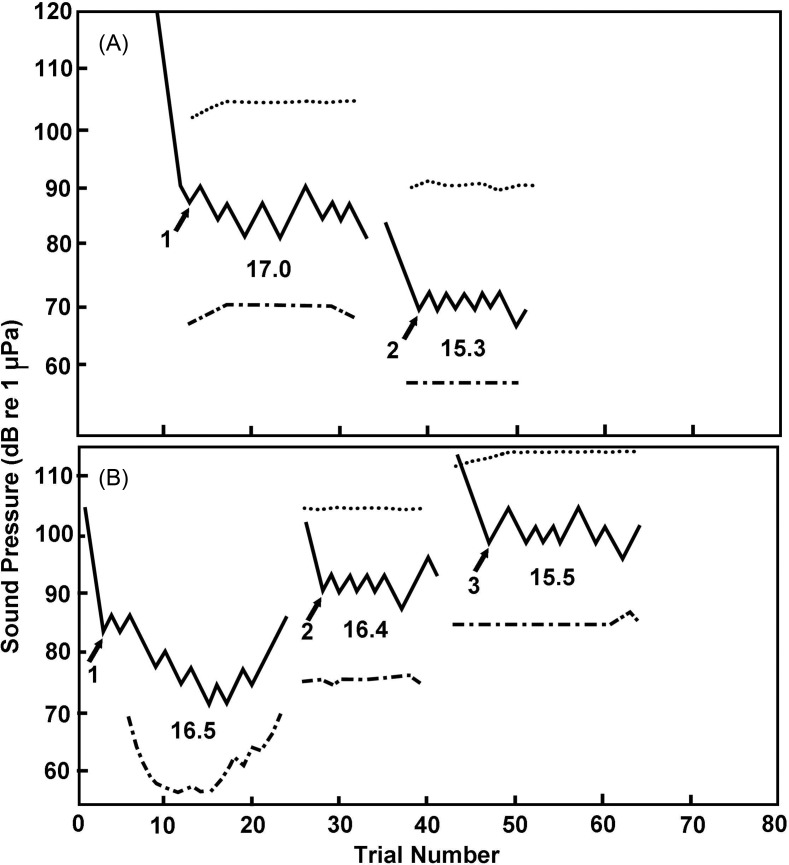
It must also be recognized that thresholds may vary not only between animals, but also within animals from moment to moment. As a consequence, thresholds are considered to be a statistical measure (e.g., Green and Swets, 1966). With regard to fishes, a number of different threshold criteria have been used (e.g., two standard deviations above background noise level, a given sound pressure or particle acceleration level), but the most commonly used approach is to define the threshold as the signal level that is detected in 50% of the presentations. A variety of different psychophysical approaches have been used to determine thresholds. One of the most frequently used with fishes has been the “staircase method,” where the sound is successively raised and lowered by several dB to bracket the 50% level (Fig. 6) (Cornsweet, 1962; Tavolga and Wodinsky, 1963).
FIG. 6.
Auditory thresholds determined for the Atlantic cod, showing the staircase method for determining thresholds. In this method, the sound level is lowered in increments (e.g., 3 or 6 dB) until the animal no longer responds. The sound is then raised until a response occurs. The “threshold” at that point in time is midway between the non-detection and detection level. A full threshold determination involves multiple lowering and raising of sound levels and the individual thresholds are then averaged to get the overall threshold for that session. A: shows threshold determination to a 160 Hz pure tone when the sea was hit by a natural rain squall (1), where the threshold was higher. A 2 shows the threshold determination after the squall was over. B: shows changes in the 160 Hz thresholds as the background noise was raised artificially. B 1 was obtained in the presence of natural ambient noise, whereas 2 and 3 involved the presence of artificial noise. The dotted lines above the thresholds show the noise levels measured over the frequency band 20–1000 Hz, whereas the dot-dashed lines below show the spectrum level of noise. The numbers provide the threshold/noise ratios (Chapman and Hawkins, 1973).
These methods are not just applied to hearing sensitivity per se. They have also been used to determine thresholds for the detection of signals in the presence of noise (Fig. 6) (e.g., Chapman and Hawkins, 1973). A variant, referred to as the just noticeable difference (JND) has been used to measure the ability to discriminate between sounds (e.g., Jacobs and Tavolga, 1967) as well as to determine the ability to discriminate sound directions (e.g., Chapman and Johnstone, 1974). The JND is often determined by conditioning the fish to respond to a stimulus in which sound pulses of different frequency, amplitude, or direction alternate with one another.
Rather than determining the AEP or ABR thresholds, it is possible instead to examine the relative sensitivity of the animal to different frequencies by monitoring the sound levels that generate similar AEP or ABR responses at those frequencies (see, for example, Hawkins and MacLennan, 1976). However, as pointed out above, these methods only measure responses at lower levels of the auditory system and do not reflect the full hearing capabilities of an animal.
VI. CRITICAL ISSUES FOR UNDERSTANDING FISH HEARING
To this point in the paper we have discussed the most widely used approaches for studies examining the auditory sensitivity and hearing of fish, with the intention of showing that there is substantial inconsistency in how studies have been performed. We have also made suggestions about approaches that might be explored in future studies, often using examples based on the older literature, with the goal of developing a body of data that are the most useful for understanding fish hearing and effects of anthropogenic sound on fishes.
In this section, we briefly discuss a number of specific issues which are important to address in the future. In each case, getting useful data requires carefully designed experiments not only from the perspective of acoustics and other methodology, but also from how the questions are actually asked. Moreover, great care will be needed to ensure that the most useful approaches are taken in order to get useful data that are comparable between species and laboratories.
Moreover, future studies should consider several variables that impact hearing directly, but are beyond threshold determination per se. Among a wide range of interesting questions in need of clarification are potential impacts on hearing capabilities of fish size, season, age, etc. Considering that fishes continue to grow throughout their lives, that they continue to add sensory hair cells to the ear, and that the physical relationship between structures such as the swim bladder and inner ear may change, there is reason to ask whether hearing changes in fishes with age. It is also an open question as to how hearing in fishes possessing a swim bladder may be affected by changes in depth. Indeed, changes in the acoustic properties of the swim bladder with depth have, for example, been examined for the Atlantic cod (e.g., Sand and Hawkins, 1973).
Several aspects of experimental design have not been considered in many studies done to date. Most investigators do not discuss the importance of the temperature of the water in which their animals are held, though this has been shown to have a significant impact on hearing sensitivity in several studies (e.g., Wysocki et al., 2009; Maiditsch and Ladich, 2014). And while it has been shown that drug treatment, including the use of anesthetics, during hearing studies have the potential to impact hearing and the lateral line (e.g., Cordova and Braun, 2007), this has yet to be systematically examined in fishes.
A. Beyond hearing sensitivity
Most earlier studies have focused on determining thresholds. Yet, there is greater need for more practical or “real world” data on detection of more natural sounds as well as the detection of sound in the presence of natural and anthropogenic sound (masking); discrimination between sounds of different frequencies, intensities, temporal characteristics, direction and distance; and the extent of hearing loss in the presence of high level sounds.
Moreover, the majority of studies to date have focused on use of pure tones or simple band-limited noise. However, “real world” sounds to which fish are exposed every day are often very complex signals that change in amplitude, spectrum, and temporal pattern. Little is known about detection and processing of such real-world signals by fishes.
Another problem with current knowledge is that the only comprehensive studies of complex processing of sound by fishes have been done on goldfish (reviewed in Fay and Megela Simmons, 1999). While having some of the aforementioned problems with tank acoustics, these studies provide very important insight into how fishes can deal with varying sound sources. The goldfish (and it is now popular relative, the zebrafish, Danio rerio) is from a group of mostly freshwater fishes (Otophysi) that is highly specialized for hearing and not representative of the vast majority of teleost fishes, and certainly not the species of most economic importance, or for which there is greatest concern about potential effects of anthropogenic sound. Therefore, while recognizing that the extent of Fay's goldfish studies would be almost impossible to replicate for other species today, a select sub-set of these studies, those that would give greatest insight into overall hearing capabilities, should be done for some other species.
Although there are some data on how well fishes can detect signals in the presence of maskers, the mechanisms by which fishes do this detection is still not understood. In mammals, detection of signals in noise depends on the presence of the cochlea where the only sounds that interfere with detection of a signal are those in the same frequency band (Green and Swets, 1966). Studies of critical bands (or the related critical ratios) in fishes have been done a few times (Tavolga, 1974; Hawkins and Chapman, 1975; Coombs and Fay, 1989), but these are difficult and time-consuming experiments that need repetition. They can only be done behaviorally and must be designed with utmost care in order to ensure useful data.
There are many other issues that need to be considered, such as the most appropriate way to measure thresholds, and how to measure hearing in free swimming vs restrained animals, etc. Moreover, while we have focused on hearing in the “sonic” range for fishes, it is important to keep in mind that a related issue is that low frequency hearing in fishes extends into the infrasonic (<20 Hz) range (e.g., Sand and Karlsen, 2000), while fishes in the Clupeid family Alosinae can detect sounds within the ultrasonic (>10 000 Hz) range (e.g., Mann et al., 2001).
VII. CONCLUSIONS AND SUGGESTIONS FOR CONSIDERATION
Determining hearing capabilities of fishes is clearly a complex problem that requires dealing not only with the animal, but the acoustic environment in which determinations are made, and the approach taken in making those determinations. As implied above, the vast majority of studies to date have been done in ways, and under conditions, that result in data that may be less than useful in helping to understand what (and how) fishes hear, and the potential effects of anthropogenic sound on fish hearing and behavior.
There is a wealth of information about fish hearing capabilities in the literature, but because of the limitations we have discussed, the value of most of the studies must be questioned in relation to the methodological caveats we have examined. Of particular concern are the data on hearing in fishes that are primarily detectors of particle motion (most species), and the uncertainty of data collected for these particle motion sensitive fish that characterize hearing sensitivity in terms of sound pressure.
A. What to do now
The ideal situation would be some kind of agreement as to the best approaches to adopt in studying fish hearing, along with the development of appropriate tools, so that data from different labs and for different species will be consistent and comparable. The problem, however, is twofold.
First, it will take time to develop the approaches to gain consistency. There are also numerous gaps in what we know about fish bioacoustics (e.g., Hawkins et al., 2015), and even if we can get better data on fish hearing per se, as discussed in this paper, these other gaps need to be filled as well.
Second, there are too few investigators today, and far too little funding, to encourage more studies of fish hearing, and to carry out the kinds of studies needed. This is a paradoxical situation in light of the growing concern about potential impacts of anthropogenic sound on fishes, particularly because fishes make up such a major component of the human (and marine mammal) food chain.
Our view is that there needs to be a renewed focus on doing studies of fish hearing in order to really know what fish can hear and how well they hear. To do this, we make the following recommendations:
-
•
More studies should be done behaviorally in order to gain better understanding of what fish actually can actually hear and respond to. Behavioral measures of fish hearing are often the most appropriate, because acoustic stimuli must be processed by higher order processing areas in the auditory CNS in order to elicit the measured behavioral response. This is particularly the case when studying abilities that require complex analysis of signals, such as directional hearing, discrimination, and detection of masked signals.
-
•
All hearing studies must be done in terms of both sound pressure and particle motion. We also suggest that the impedance (ratio of sound pressure to velocity for the given stimulus frequencies) of the testing environment should be reported.
-
•
Focus on select species that may best reflect hearing over broad groups defined by hearing type rather than taxonomically (see Popper and Fay, 2011). Perhaps use the designations described by Popper et al. (2014).
-
•
Carry out a detailed analysis of hearing in several selected species, not only to determine hearing sensitivity to pure tones, but also to examine hearing in the presence of anthropogenic maskers, frequency and intensity discrimination, temporal discrimination, directional discrimination, and other more complex processes used by fishes in sound analysis (discussed by Fay and Megela Simmons, 1999).
-
•
Regarding anthropogenic sound, it is still unclear which components of such sounds may evoke avoidance responses in fishes as well as other types of sound (e.g., continuous vs pulsatile). Focus has been on sonic frequencies, but it has been suggested that infrasonic particle acceleration due to water motion caused by the sound source (e.g., a moving ship hull) is more important (Sand et al., 2008). This question should be clarified.
-
•
Hearing studies conducted in the laboratory should focus on the development of some version of the standing wave technique either using acoustic tubes or opposing pairs of suspended sound projectors, thus allowing variation of the ratio of sound pressure to particle motion.
-
•
Where feasible, it would be sensible to revert to doing studies in the natural environment where the fish are found (Hawkins, 2014), and where the sound field is far easier to define and monitor.
B. What must be done in the future
While our recommendations above are for immediate work, future studies would also benefit greatly from even greater standardization to enable comparison and use of data obtained in all labs. Standardization can take several forms. One is to set up a site that is designed for studies of fish hearing—where the acoustic conditions are well understood and can be controlled, particularly with regard to sound pressure and particle motion. With such a site, investigators would not have to spend a great deal of time in designing the correct acoustic conditions and then constructing, testing, and calibrating complex sound fields.
Of course, the downside of this is that investigators, and their research animals, would have to travel to the research site (and a particular species may not be allowed at such sites if they could be seen as potentially invasive), making long-term studies difficult. Furthermore, such sites still may not have the ability to work in both fresh and salt water, etc. Finally, any site designed to provide a fully understood acoustic field is likely to be complex and expensive to build and maintain, and perhaps outside of the funds available to individual investigators.
A second approach is to develop not only standards for how to carry out studies, but also provide designs for experimental chambers and other apparatus, as well as experimental approaches and conditions, that would allow investigators to build their own setups, adapted for their specific needs and laboratory conditions. It is particularly important to design efficient dipole sound sources and infrasound sources that generate high level near field particle motion.
Thus, our final recommendation is that the community of scholars interested in, and needing, an understanding of fish hearing should work together to develop common, experimental standards.
ACKNOWLEDGMENTS
A.N.P. and A.D.H. contributed equally to this work.
This paper is part of a special issue on The Effects of Noise on Aquatic Life.
References
- 1. Bhandiwad, A. A. , Zeddies, D. G. , Raible, D. W. , Rubel, E. W. , and Sisneros, J. A. (2013). “ Auditory sensitivity of larval zebrafish (Danio rerio) measured using a behavioral prepulse inhibition assay,” J. Exp. Biol. 216, 3504–3513. 10.1242/jeb.087635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braun, C. B. , and Sand, O. (2013). “ Functional overlap and nonoverlap between lateral line and auditory systems,” in The Lateral Line System, edited by Coombs C., Bleckmann H., Fay R. R., and Popper A. N. ( Springer, New York: ), pp. 281–312. [Google Scholar]
- 3. Buwalda, R. J. (1981). “ Segregation of directional and nondirectional acoustic information in the cod,” in Hearing and Sound Communication in Fishes, edited by Tavolga W. A., Popper A. N., and Fay R. R. ( Springer, New York: ), pp. 139–171. [Google Scholar]
- 4. Buwalda, R. , Schuijf, A. , and Hawkins, A. (1983). “ Discrimination by the cod of sounds from opposing directions,” J. Comp. Physiol. 150, 175–184. 10.1007/BF00606367 [DOI] [Google Scholar]
- 5. Campbell, J. (2019). “ Particle motion and sound pressure in fish tanks: A behavioural exploration of acoustic sensitivity in the zebrafish,” Behav. Process. 164, 38–47. 10.1016/j.beproc.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 6. Chapman, C. J. , and Hawkins, A. (1973). “ A field study of hearing in the cod, Gadus morhua L,” J. Comp. Physiol. 85, 147–167. 10.1007/BF00696473 [DOI] [Google Scholar]
- 7. Chapman, C. , and Johnstone, A. (1974). “ Some auditory discrimination experiments on marine fish,” J. Exp. Biol. 61, 521–528. [DOI] [PubMed] [Google Scholar]
- 8. Chapman, C. , and Sand, O. (1974). “ Field studies of hearing in two species of flatfish Pleuronectes platessa (L.) and Limanda limanda (L.) (Family Pleuronectidae),” Comp. Biochem. Physiol. Part A: Physiology 47, 371–385. 10.1016/0300-9629(74)90082-6 [DOI] [PubMed] [Google Scholar]
- 9. Coombs, S. , and Fay, R. R. (1989). “ The temporal evolution of masking and frequency selectivity in the goldfish (Carassius auratus),” J. Acoust. Soc. Am. 86, 925–933. 10.1121/1.398727 [DOI] [PubMed] [Google Scholar]
- 10. Cordova, M. S. , and Braun, C. B. (2007). “ The use of anesthesia during evoked potential audiometry in goldfish (Carassius auratus),” Brain Res. 1153, 78–83. 10.1016/j.brainres.2007.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornsweet, T. N. (1962). “ The staircase-method in psychophysics,” Am. J. Psychol. 75, 485–491. 10.2307/1419876 [DOI] [PubMed] [Google Scholar]
- 12. Dijkgraaf, S. (1963). “ The functioning and significance of the lateral-line organs,” Biol. Rev. 38, 51–105. 10.1111/j.1469-185X.1963.tb00654.x [DOI] [PubMed] [Google Scholar]
- 13. Duncan, A. J. , Lucke, K. , Erbe, C. , and McCauley, R. D. (2016). “ Issues associated with sound exposure experiments in tanks,” Proc. Mtgs. Acoust. 27, 070008. 10.1121/2.0000280 [DOI] [Google Scholar]
- 14. Enger, P. S. (1963). “ Single unit activity in peripheral auditory system of a teleost fish,” Acta Physiol. Scand. 59 (Suppl. 210), 1–48.14065851 [Google Scholar]
- 15. Enger, P. , Hawkins, A. , Sand, O. , and Chapman, C. (1973). “ Directional sensitivity of saccular microphonic potentials in the haddock,” J. Exp. Biol. 59, 425–433. [DOI] [PubMed] [Google Scholar]
- 16. Fay, R. R. (1974). “ Masking of tones by noise for the goldfish (Carassius auratus),” J. Comp. Physiol. Psych. 87, 708–716. 10.1037/h0037002 [DOI] [PubMed] [Google Scholar]
- 17. Fay, R. R. (1984). “ The goldfish ear codes the axis of acoustic particle motion in three dimensions,” Science 225, 951–954. 10.1126/science.6474161 [DOI] [PubMed] [Google Scholar]
- 18. Fay, R. R. (1988). Hearing in Vertebrates: A Psychophysics Databook ( Hill-Fay Associates, Winnetka, IL: ). [Google Scholar]
- 19. Fay, R. R. (2008). “ Sound source perception and stream segregation in nonhuman vertebrate animals,” in Auditory Perception of Sound Sources, edited by Yost W., Popper A. N., and Fay R. R. ( Springer-Verlag, New York: ), pp. 307–323. [Google Scholar]
- 20. Fay, R. R. , and Megela Simmons, A. (1999). “ The sense of hearing in fishes and amphibians,” in Comparative Hearing: Fish and Amphibians, edited by Fay R. R. and Popper A. N. ( Springer-Verlag, New York: ), pp. 269–318. [Google Scholar]
- 21. Green, D. M. , and Swets, J. A. (1966). Signal Detection Theory and Psychophysics ( Wiley, New York: ). [Google Scholar]
- 22. Griffin, D. R. (1950). “ Underwater sounds and the orientation of marine animals, a preliminary survey” (DTIC document).
- 23. Hawkins, A. D. (1973). “ The sensitivity of fish to sounds,” Oceanogr. Mar. Biol. Annu. Rev 11, 291–340. [Google Scholar]
- 24. Hawkins, A. D. (2014). “ Examining fish in the sea: A European perspective on fish hearing experiments,” in Perspectives on Auditory Research, edited by Popper A. N. and Fay R. R. ( Springer, New York: ), pp. 247–267. [Google Scholar]
- 25. Hawkins, A. D. , and Chapman, C. J. (1975). “ Masked auditory thresholds in the cod, Gadus morhua L,” J. Comp. Physiol. 103, 209–226. 10.1007/BF00617122 [DOI] [Google Scholar]
- 26. Hawkins, A. , and Horner, K. (1981). “ Directional characteristics of primary auditory neurons from the cod ear,” in Hearing and Sound Communication in Fishes, edited by Tavolga W. N., Popper A. N., and Fay R. R. ( Springer, New York: ), pp. 311–328. [Google Scholar]
- 27. Hawkins, A. D. , and Johnstone, A. D. F. (1978). “ The hearing of the Atlantic salmon, Salmo salar,” J. Fish. Biol. 13, 655–673. 10.1111/j.1095-8649.1978.tb03480.x [DOI] [Google Scholar]
- 28. Hawkins, A. D. , and MacLennan, D. N. (1976). “ An acoustic tank for hearing studies on fish,” in Sound Reception in Fish, edited by Schuijf A. and Hawkins A. D. ( Elsevier, Amsterdam: ), pp. 149–170. [Google Scholar]
- 29. Hawkins, A. D. , Pembroke, A. , and Popper, A. (2015). “ Information gaps in understanding the effects of noise on fishes and invertebrates,” Rev. Fish Biol. Fisheries 25, 39–64. 10.1007/s11160-014-9369-3 [DOI] [Google Scholar]
- 30. Hawkins, A. D. , and Popper, A. N. (2018). “ Directional hearing and sound source localization by fishes,” J. Acoust. Soc. Am. 144, 3329–3350. 10.1121/1.5082306 [DOI] [PubMed] [Google Scholar]
- 31. Jacobs, D. W. , and Tavolga, W. N. (1967). “ Acoustic intensity limens in the goldfish,” Anim. Behav. 15, 324–335. 10.1016/0003-3472(67)90019-X [DOI] [PubMed] [Google Scholar]
- 32. Kenyon, T. N. , Ladich, F. , and Yan, H. Y. (1998). “ A comparative study of hearing ability in fishes: The auditory brainstem response approach,” J. Comp. Physiol. A 182, 307–318. 10.1007/s003590050181 [DOI] [PubMed] [Google Scholar]
- 33. Ladich, F. , and Fay, R. R. (2013). “ Auditory evoked potential audiometry in fish,” Rev. Fish Biol. Fisheries 23, 317–364. 10.1007/s11160-012-9297-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu, Z. , and Popper, A. N. (2001). “ Neural response directionality correlates of hair cell orientation in a teleost fish,” J. Comp. Physiol. A 187, 453–465. 10.1007/s003590100218 [DOI] [PubMed] [Google Scholar]
- 35. Maiditsch, I. P. , and Ladich, F. (2014). “ Effects of temperature on auditory sensitivity in eurythermal fishes: Common carp Cyprinus carpio (Family Cyprinidae) versus Wels Catfish Silurus glanis (Family Siluridae),” PLoS One 9, e108583. 10.1371/journal.pone.0108583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mann, D. A. , Higgs, D. M. , Tavolga, W. N. , Souza, M. J. , and Popper, A. N. (2001). “ Ultrasound detection by clupeiform fishes,” J. Acoust. Soc. Am. 109, 3048–3054. 10.1121/1.1368406 [DOI] [PubMed] [Google Scholar]
- 37. Meyer, M. , Popper, A. N. , and Fay, R. R. (2011). “ Coding of sound direction in the auditory periphery of the lake sturgeon, Acipenser fulvescens,” J. Neurophysiol. 107, 658–665. 10.1152/jn.00390.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nedelec, S. L. , Campbell, J. , Radford, A. N. , Simpson, S. D. , and Merchant, N. D. (2016). “ Particle motion: The missing link in underwater acoustic ecology,” Method Ecol. Evol. 7, 836–842. 10.1111/2041-210X.12544 [DOI] [Google Scholar]
- 39. Parvulescu, A. (1964). “ Problems of propagation and processing,” in Marine Bio-Acoustics, edited by Tavolga W. N. ( Pergamon, Oxford: ), pp. 87–100. [Google Scholar]
- 40. Poggendorf, D. (1952). “ Die absoluten Hörschwellen des Zwergwelses (Amiurus nebulosus) und Beiträge zur Physik des Weberschen Apparates der Ostariophysen” (“The absolute threshold of hearing of the bullhead (Amiurus nebulosus) and contributions to the physics of the Weberian apparatus of the Ostariophysi”), Z. Verg. Physiol. 34, 222–257. 10.1007/BF00298202 [DOI] [Google Scholar]
- 41. Popper, A. N. , and Fay, R. R. (2011). “ Rethinking sound detection by fishes,” Hear. Res. 273, 25–36. 10.1016/j.heares.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 42. Popper, A. N. , Halvorsen, M. B. , Kane, A. S. , Miller, D. L. , Smith, M. E. , Song, J. , Stein, P. , and Wysocki, L. E. (2007). “ The effects of high-intensity, low-frequency active sonar on rainbow trout,” J. Acoust. Soc. Am. 122, 623–635. 10.1121/1.2735115 [DOI] [PubMed] [Google Scholar]
- 43. Popper, A. N. , and Hawkins, A. D. (2018). “ The importance of particle motion to fishes and invertebrates,” J. Acoust. Soc. Am. 143, 470–486. 10.1121/1.5021594 [DOI] [PubMed] [Google Scholar]
- 44. Popper, A. N. , and Hawkins, A. D. (2019). “ An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes,” J. Fish. Biol. 94, 692–713. 10.1111/jfb.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Popper, A. N. , Hawkins, A. D. , Fay, R. R. , Mann, D. A. , Bartol, S. , Carlson, T. J. , Coombs, S. , Ellison, W. T. , Gentry, R. L. , Halvorsen, M. B. , Lokkeborg, S. , Rogers, P. H. , Southall, B. , Zeddies, D. , and Tavolga, W. A. (2014). ASA S3/SC1. 4 TR-2014 Sound Exposure Guidelines for Fishes and Sea Turtles: A Technical Report prepared by ANSI-Accredited Standards Committee S3/SC1 and registered with ANSI ( Springer, New York: ). [Google Scholar]
- 46. Putland, R. L. , Montgomery, J. C. , and Radford, C. A. (2019). “ Ecology of fish hearing,” J. Fish. Biol. 95, 39–52. 10.1111/jfb.13867 [DOI] [PubMed] [Google Scholar]
- 47. Rogers, P. H. , Hawkins, A. D. , Popper, A. N. , Fay, R. R. , and Gray, M. D. (2016). “ Parvulescu revisited: Small tank acoustics for bioacousticians,” in The Effects of Noise on Aquatic Life II, edited by Popper A. N. and Hawkins A. D. ( Springer Science+Business Media, New York: ), pp. 933–941. [DOI] [PubMed] [Google Scholar]
- 48. Sand, O. (1974). “ Directional sensitivity of microphonic potentials form the perch ear,” J. Exp. Biol. 60, 881–899. [DOI] [PubMed] [Google Scholar]
- 49. Sand, O. , and Bleckmann, H. (2008). “ Orientation to auditory and lateral line stimuli,” in Fish Bioacoustics, edited by Webb J. F., Fay R. R., and Popper A. N. ( Springer Science+Business Media, LLC, New York: ), pp. 183–222. [Google Scholar]
- 50. Sand, O. , and Hawkins, A. D. (1973). “ Acoustic properties of the cod swim bladder,” J. Exp. Biol. 58, 797–820. [Google Scholar]
- 51. Sand, O. , and Karlsen, H. E. (2000). “ Detection of infrasound and linear acceleration in fishes,” Philos. Trans. R. Soc. London B 355, 1295–1298. 10.1098/rstb.2000.0687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sand, O. , Karlsen, H. E. , and Knudsen, F. R. (2008). “ Comment on ‘Silent research vessels are not quiet’ [J. Acoust. Soc. Am. 121, EL145–EL150],” J. Acoust. Soc. Am. 123, 1831–1833. 10.1121/1.2839134 [DOI] [PubMed] [Google Scholar]
- 53. Schellart, N. A. , and Buwalda, R. J. (1990). “ Directional variant and invariant hearing thresholds in the rainbow trout (Salmo gairdneri),” J. Exp. Biol. 149, 113–131. [Google Scholar]
- 54. Sisneros, J. A. , Popper, A. N. , Hawkins, A. D. , and Fay, R. R. (2016). “ Auditory evoked potential audiograms compared to behavioral audiograms in aquatic animals,” in The Effects of Noise on Aquatic Life II, edited by Popper A. N. and Hawkins A. D. ( Springer Science+Business Media, New York: ), pp. 1049–1056. [Google Scholar]
- 55. Tavolga, W. N. (1974). “ Signal-noise ratio and the critical band in fishes,” J. Acoust. Soc. Am. 55, 1323–1333. 10.1121/1.1914704 [DOI] [PubMed] [Google Scholar]
- 56. Tavolga, W. N. , and Wodinsky, J. (1963). “ Auditory capacities in fishes: Pure tone thresholds in nine species of marine teleosts.,” Bull. Amer. Mus. Nat. Hist. 126, 177–240. [Google Scholar]
- 57. van Bergeijk, W. A. (1964). “ Directional and nondirectional hearing in fish,” in Marine Bio-Acoustics, edited by Tavolga W. A. ( Pergamon, New York: ), pp. 281–299. [Google Scholar]
- 58. von Frisch, K. , and Stetter, H. (1932). “ Untersuchungen über den Sitz des Géhörsinnes bei der Elritze,” Z. vergl Physiol. 17, 686–801. 10.1007/BF00339067 [DOI] [Google Scholar]
- 59. Wysocki, L. E. , Montey, K. , and Popper, A. N. (2009). “ The influence of ambient temperature and thermal acclimation on hearing in a eurythermal and a stenothermal otophysan fish,” J. Exp. Biol. 212, 3091–3099. 10.1242/jeb.033274 [DOI] [PubMed] [Google Scholar]
- 60. Xiao, J. , and Braun, C. B. (2008). “ Objective threshold estimation and measurement of the residual background noise in auditory evoked potentials of goldfish,” J. Acoust. Soc. Am. 124, 3053–3063. 10.1121/1.2982366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeddies, D. G. , Fay, R. R. , Gray, M. D. , Alderks, P. W. , Acob, A. , and Sisneros, J. A. (2012). “ Local acoustic particle motion guides sound-source localization behavior in the plainfin midshipman fish, Porichthys notatus,” J. Exp. Biol. 215, 152–160. 10.1242/jeb.064998 [DOI] [PubMed] [Google Scholar]