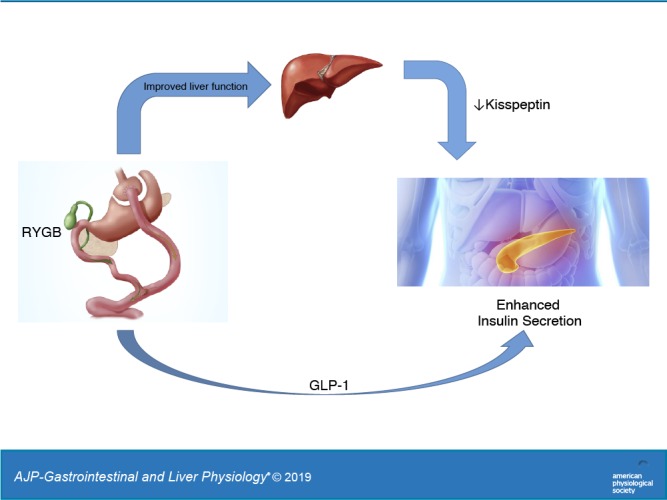
Keywords: glucagon, hepatic glucose metabolism, insulin secretion, weight loss surgery
Abstract
Roux-en-Y gastric bypass surgery (RYGB) is known to improve whole-body glucose metabolism in patients with type 2 diabetes (T2D), although the mechanisms are not entirely clear and are likely multifactorial. The aim of this study was to assess fasting hepatic glucose metabolism and other markers of metabolic activity before and after RYGB in patients with and without T2D. Methods: Metabolic characteristics of patients who are obese with T2D were compared with those without the disease (non-T2D) before and 1 and 6 mo after RYGB. Fasting plasma insulin and the insulin:glucagon ratio were markedly reduced as early as 1 mo after RYGB in both patients with T2D and without T2D. Despite this reduction, endogenous glucose production and fasting plasma glucose levels were lower in both groups after RYGB, with the reductions being much larger in T2D. Plasma kisspeptin, an inhibitor of insulin secretion, was reduced only in T2D after surgery. Improved hepatic glucose metabolism and lower plasma kisspeptin in T2D after RYGB may link improved hepatic function with enhanced insulin responsiveness after surgery.
NEW & NOTEWORTHY Our manuscript is the first, to the best of our knowledge, to present data showing that Roux-en-Y gastric bypass surgery (RYGB) lowers fasting kisspeptin levels in patients who are obese with type 2 diabetes. This lowering of kisspeptin is important because it could link improvements in liver glucose metabolism after RYGB with increased insulin responsiveness also seen after surgery.
INTRODUCTION
Type 2 diabetes mellitus (T2D) is a progressively debilitating metabolic disease that is associated with obesity, insulin resistance, and the dysfunction of a number of key glucoregulatory organs (5). A hallmark characteristic of T2D is impaired whole-body glucose metabolism, which is manifest by elevated fasting glucose levels and/or abnormally high blood glucose levels in response to the ingestion of carbohydrate. In the past, numerous elegant studies in humans with T2D have shown that the elevation in fasting glucose is closely tethered to elevated rates of endogenous glucose production (EGP) (1, 3) and that diminished insulin responsiveness to glucose ingestion plays the primary role in glucose intolerance (12, 16, 17, 24).
Roux-en-Y gastric bypass surgery (RYGB) continues to be the most effective treatment for T2D (18–20). Previous work has documented that EGP and fasting plasma glucose are lower in patients with T2D after RYGB (4, 7, 11, 15) and that insulin responsiveness to an oral glucose challenge or a mixed meal, most evident in the first phase of insulin secretion, is also enhanced (11, 15). Together, these improvements are responsible for gains in whole-body glucose metabolism and the lowering of hemoglobin A1C after surgery (18–20).
Hepatic secretion of kisspeptin is inversely proportional to relative insulin:glucagon signaling in the liver and has been shown to be increased in patients with T2D. Accordingly, Andreozzi and colleagues (2) observed that plasma kisspeptin levels are increased in proportion with body mass index in subjects who are non-T2D and noted that the increase in kisspeptin was associated with diminished insulin secretion. Moreover, Song and colleagues (21) observed in a small sample of human subjects (n = 3 with T2D and n = 3 without T2D) that plasma kisspeptin levels are elevated in people with T2D, while accompanying animal studies supported the hypothesis that this elevation impairs insulin responsiveness to oral glucose in this population. Given the marked impact RYGB has on whole-body glucose metabolism in patients with T2D, we hypothesized that surgery-induced improvements in hepatic glucose metabolism would correspond with lower plasma kisspeptin levels in this population, thereby linking improved hepatic glucose metabolism after surgery with enhanced insulin responsiveness.
RESEARCH DESIGN AND METHODS
Subjects.
All studies adhered to principles of the Declaration of Helsinki and Title 45 of the US Code of Federal Regulations (Part 46, Protection of Human Subjects). Studies were approved by the Vanderbilt Institutional Review Board and conducted in accordance with institutional guidelines. Subjects 18–60 yr of age were recruited from the Vanderbilt Center for Surgical Weight Loss after they were approved to undergo RYGB, and each person provided their informed consent. Potential subjects were disqualified if they had undergone previous gastric operations, had metabolic acidosis, had a positive pregnancy test or were taking medications that impact substrate metabolism (other than T2D medications). None of the subjects were prescribed insulin. Subjects included 15 humans with T2D and 19 controls who were non-T2D (Table 1) who underwent metabolic testing before (10–14 days before surgery) and 1 and 6 mo after RYGB. No significant postoperative complications were observed. All subjects were patients at the same bariatric surgery practice, with standard postoperative clinic visits with both a bariatric dietitian and nurse practitioner at ~1 wk, 3–4 wk, 3 mo, and 6 mo. At these clinic visits, patients were counseled per standard clinic protocol to consume a caloric-restricted bariatric surgery diet appropriate to their postoperative stage (as detailed in a postoperative diet packet) and incorporate physical activity for weight loss.
Table 1.
Patient characteristics before, 1 mo, and 6 mo after RYGB
| Time Point |
||||
|---|---|---|---|---|
| Outcome | Group | Presurgery | 1 Mo Post | 6 Mo Post |
| BMI | CON | 23.3 ± 0.9 | ||
| Non-T2D | 49.4 ± 1.9 | 44.3 ± 1.9* | 36.0 ± 1.8*† | |
| T2D | 47.0 ± 2.2 | 41.7 ± 2.2* | 34.5 ± 2.0*† | |
| HbA1C, % | CON | 5.6 ± 0.1 | ||
| Non-T2D | 5.9 ± 0.2 | 5.5 ± 0.2* | 5.4 ± 0.2* | |
| T2D | 7.1 ± 0.2# | 6.1 ± 0.2* | 5.9 ± 0.2* | |
| HOMA-IR | CON | 0.6 ± 0.1 | ||
| Non-T2D | 3.0 ± 0.3 | 1.9 ± 0.3* | 1.0 ± 0.3*† | |
| T2D | 3.8 ± 0.3 | 1.9 ± 0.3* | 1.3 ± 0.3*† | |
Data are means ± SE. BMI, body mass index; CON, control; HOMA-IR, homeostatic model assessment of insulin resistance; RYGB, Roux-en-Y gastric bypass surgery; T2D, type 2 diabetes.
P ≤ 0.05 compared with presurgery value;
P < 0.05 compared with 1-mo value;
P ≤ 0.05 compared with non-T2D at the same time point.
For the purpose of comparison, we also studied six lean controls that did not undergo RYGB. Portions of these data have been reported previously (9). In the T2D group, the insulin:glucagon ratio has a sample size of n = 13 at each time point. In the non-T2D group, the insulin:glucagon ratio has a sample size of 17, 18, and 16 before, 1 mo after, and 6 mo after RYGB, respectively. Hemoglobin A1C values in the non-T2D group had n = 18 at the 1 mo time point. All other data reflect the entire cohort of subjects in each group.
Metabolic testing procedures.
Subjects were admitted to Vanderbilt’s Clinical Research Center the evening before each metabolic study, after which they were fed a standard meal, followed by an overnight fast. The following morning, catheters were placed into the forearm or hand vein of each arm for infusions and blood draws. Over a 2.5-h period, 3-3H glucose (Perkin Elmer, Waltham, MA) was infused (33 μCi bolus; 0.14 μCi/min continuous rate) to assess fasting EGP. Blood samples were taken every 5 min during the final 30 min of this basal period to assess EGP, and this was done by dividing the infusion rate of the 3-3H glucose tracer by glucose specific activity (22). Plasma samples were also taken during this 30-min period to assess hormones and substrates, and biochemical assays were performed as described previously (9). Plasma kisspeptin was analyzed using a commercially available assay (Phoenix Pharmaceuticals Inc., Burlingame, CA).
Statistical analysis.
Data were analyzed with repeated measures analysis of variance using SigmaPlot software. All data are expressed as means ± SEM unless noted otherwise.
RESULTS
Subjects included patients who were morbidly obese (Table 1) who did (T2D; n = 15) or did not (non-T2D; n = 19) have type 2 diabetes who underwent testing before and 1 and 6 mo after RYGB. A small group of lean control subjects also participated in the study (CON; n = 6).
Metabolic data prior to RYGB.
Despite similarly elevated homeostatic model assessment of insulin resistance (HOMA-IR) in the two surgery groups compared with CON (Table 1), fasting EGP (Fig. 1B; P < 0.001) and plasma glucose levels (Fig. 1C; P < 0.001) were markedly higher in T2D compared with non-T2D before RYGB, which corresponded with a higher hemoglobin A1C (P < 0.05; Table 1). Both groups that received RYGB exhibited fasting hyperinsulinemia and hyperglucagonemia before surgery (Fig. 2, A and B, respectively), although there was no difference in either hormone between the two surgical groups. As a result, the insulin:glucagon ratio was similar in both T2D and non-T2D subjects but more than threefold higher than CON (Fig. 2C), suggesting a marked impairment in hepatic hormone signaling before surgery in non-T2D (because it required a threefold increase in the insulin:glucagon ratio to normally suppress EGP and prevent fasting hyperglycemia) and an even greater deterioration in T2D (because even with the threefold increase in the insulin:glucagon ratio, EGP was elevated, thereby leading to fasting hyperglycemia). Preoperative fasting plasma kisspeptin levels were elevated by ~25% in T2D compared with subjects who were non-T2D or CON (Fig. 1A).
Fig. 1.
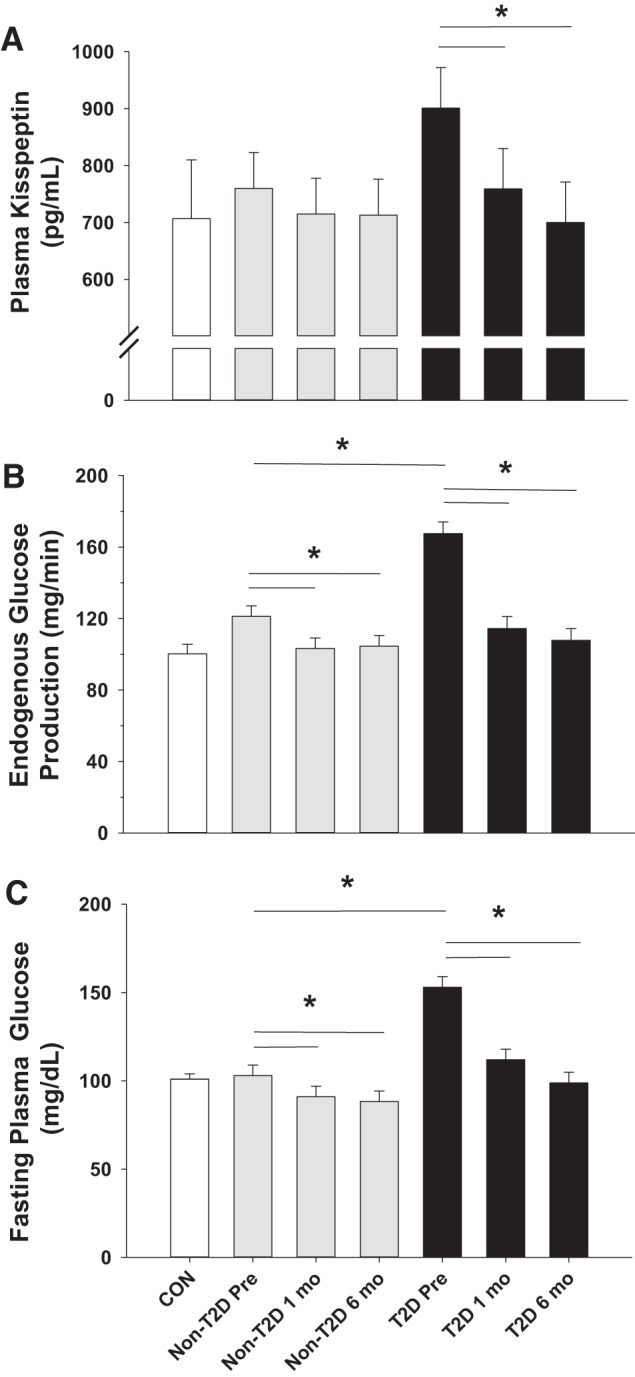
Fasting plasma kisspeptin (A), endogenous glucose production (B), and plasma glucose levels (C) before (pre) and 1 and 6 mo after Roux-en-Y gastric bypass surgery (RYGB) in subjects with and without type 2 diabetes (T2D). Control (CON) subject data are presented as a comparison; they did not undergo RYGB. *P ≤ 0.05.
Fig. 2.
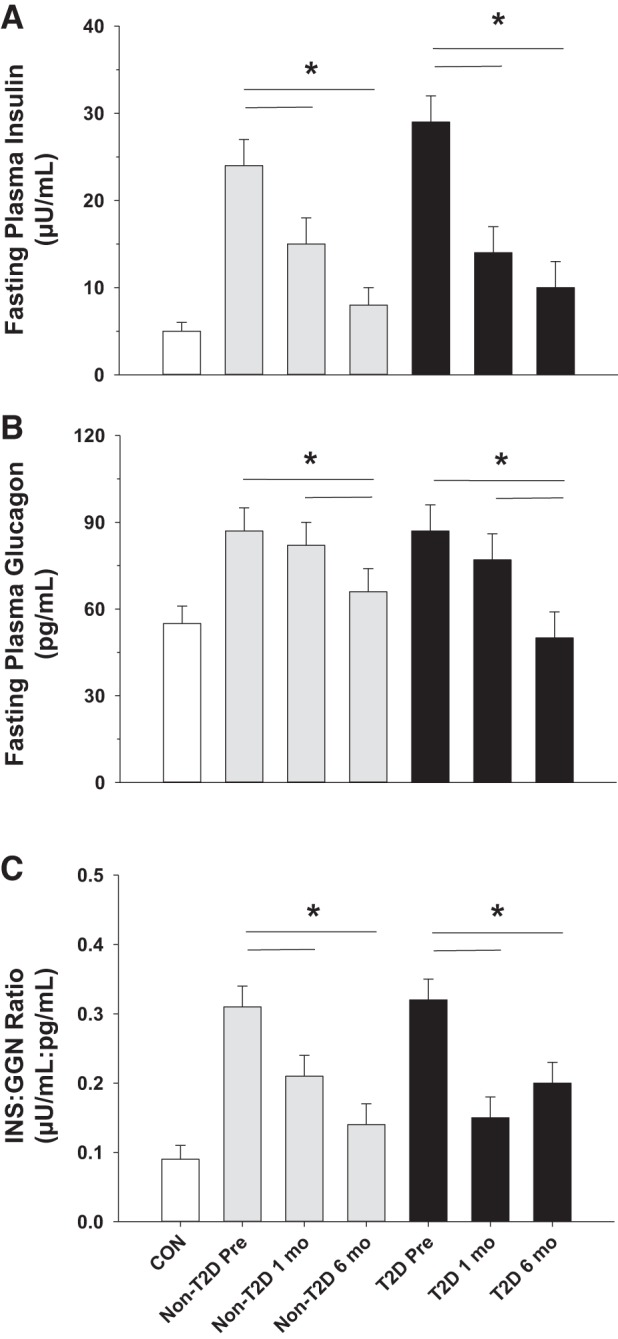
Fasting plasma insulin (A), plasma glucagon (B), and the insulin-to-glucagon ratio (C) before (pre) and 1 and 6 mo after Roux-en-Y gastric bypass surgery (RYGB) in subjects with and without type 2 diabetes (T2D). Control (CON) subject data are presented as a comparison; they did not undergo RYGB. *P ≤ 0.05. GGN, glucagon; INS, insulin.
Metabolic data after RYGB.
Fasting EGP and plasma glucose levels were both significantly lower in T2D and non-T2D after RYGB (Fig. 1, B and C, respectively), although the absolute reductions in these measures were much larger for T2D compared with non-T2D. The decrement in plasma glucose levels was associated with lower HOMA-IR and hemoglobin A1C in both groups at the 1- and 6-mo time points compared with presurgical values (Table 1; P < 0.05 for each). Because of the greater surgery-induced decrease in fasting glucose in T2D, the percent decrease in HOMA-IR was greater in T2D than it was in non-T2D (52 ± 4% vs. 37 ± 5% in each group, respectively; P = 0.04). Although fasting insulin levels decreased significantly within 1 mo of RYGB in both T2D and non-T2D (P < 0.001; Fig. 2A), a change in glucagon was not observed in either group until 6 mo after RYGB (P < 0.05 at 6 mo compared with presurgery and 1-mo values for both groups; Fig. 2B). Nevertheless, the insulin:glucagon ratio was lower in both surgical groups after RYGB (Fig. 2C; P < 0.01 for both groups compared with their respective presurgical values). One month after RYGB, previously elevated kisspeptin levels were significantly reduced in subjects who were T2D (Fig. 1A; P = 0.05) and continued to fall up to the 6-mo time point (P = 0.02).
DISCUSSION
Type 2 diabetes is a metabolic disease characterized by insulin resistance, impaired function of several key glucoregulatory organs, and diminished insulin responsiveness to oral glucose (5). The liver is among the first organs adversely impacted in the development of diabetes, as it produces glucose at an elevated rate, thereby leading to fasting hyperglycemia (1, 3). On the other hand, it is also one of the first organs to show improved function after RYGB in patients with T2D, manifest by a lowering of EGP and fasting glucose within 1 mo of surgery (4, 7, 11, 15). Our data are in agreement with these previous observations, demonstrating marked early reductions in EGP and fasting plasma glucose levels (both of which were greatest in patients with T2D), along with lower insulin levels and lower whole-body insulin resistance (HOMA-IR) early in the surgical recovery period. Moreover, we report for the first time to our knowledge that RYGB also normalizes plasma kisspeptin levels in patients with T2D, a finding that could link their improved liver function with well-known enhancements in insulin responsiveness to glucose that occur in this population.
The insulin:glucagon ratio at the liver is thought to be the primary determinant of EGP during the fasted state, and previous work has shown that it is diminished in diseases such as T2D (8, 13, 14, 23). Interestingly, improved whole-body insulin sensitivity that accompanies RYGB caused a paradoxical reduction in the fasting insulin:glucagon ratio in both surgical groups as a result of a significant reduction in fasting insulin. Despite the similar reduction of fasting insulin between T2D and non-T2D groups, EGP was reduced to a much greater extent in the patients with T2D. Although we were unable to measure hormone signaling in the livers of these human subjects, the much higher EGP in T2D before surgery makes it evident that the permissive signal of glucagon on EGP exceeded the restraining signal of insulin, thereby resulting in fasting hyperglycemia. This T2D phenotype is in contrast with the non-T2D cohort, which exhibited an insulin:glucagon ratio that was similarly elevated before surgery, but which remained sufficient to adequately suppress EGP so that fasting hyperglycemia did not occur. This baseline discrepancy makes it predictable that subjects with T2D would show the greatest lowering of EGP after RYGB, and that this event was most likely caused by a restoration of hormonal signaling in the liver to a level not different from the non-T2D cohort.
Another interesting observation is that 1 mo after RYGB, the time point at which EGP showed the greatest improvement in subjects with T2D, there was no change in plasma glucagon levels. Although glucagon sensitivity may have been diminished after RYGB, the lowering of HOMA-IR that was observed during the first month after RYGB in patients with T2D makes gains in hepatic insulin action the most likely cause of lowered EGP, with diminished glucagon action likely playing a secondary role. On the other hand, glucagon levels at the 6-mo time point were reduced in both subjects who were T2D and subjects who were non-T2D compared with presurgery, thereby indicating that significant weight loss may be required to lower levels of glucagon and that a reduction in the hormone plays an important role in the long-term sustenance of reduced EGP.
Consistent with the hypothesis that insulin signaling was improved in T2D after RYGB, plasma kisspeptin levels were also reduced only in this group. In previous work, Izzi-Engbeaya and colleagues (10) observed that an acute intravenous infusion of kisspeptin had no effect on insulin after a mixed meal in young, lean (body mass index < 25) men. In contrast, Andreozzi et al. (2) observed that kisspeptin levels in healthy humans are inversely associated with insulin responses to oral glucose, while Song et al. (21) demonstrated in more mechanistic rodent studies that hepatic kisspeptin secretion increases in response to diminished insulin and/or excessive glucagon signaling, the same metabolic conditions that favor excessive HGP in T2D. Accordingly, postsurgical improvements in hepatic insulin signaling relative to glucagon may have the additional benefit of lowering hepatic kisspeptin production in patients who are morbidly obese with T2D, and it raises the possibility that diminished plasma kisspeptin levels after RYGB may improve insulin responsiveness to a meal by acting either independently or in association with glucagon-like peptide-1.
A limitation of this study is that we did not measure insulin secretion, thereby making our findings correlational. However, the evidence is overwhelming that insulin responsiveness to glucose, primarily during the first phase of the hormone’s secretion, is dramatically increased in patients with T2D after RYGB (for review, see Ref. 6). Taken together, these data point toward the need for future experiments to examine kisspeptin physiology more closely before and after RYGB, including the significance of acute changes in the hormone’s availability on glucose stimulated insulin secretion and the function of other glucoregulatory organs (e.g., liver, small intestine, etc.). Moreover, neutralizing kisspeptin signaling in the β-cells of patients with T2D may prove capable of restoring insulin responsiveness, similar to RYGB. Currently, such a relationship has only been established in rodents via studies that did not investigate the effect of weight loss surgery (21).
In summary, our data support the conclusion that improved hepatic glucose metabolism is among the earliest and most important changes that occur in the early post-RYGB period. In addition, we show that plasma kisspeptin levels are substantially reduced by RYGB in patients with T2D, thereby making this a potential link between improved hepatic glucose metabolism and enhanced insulin responsiveness to a meal. Future studies will be required to determine the mechanistic underpinnings of this hepatopancreatic axis in improving whole-body glucose metabolism after RYGB, as this may illuminate ways by which treatment strategies for patients with T2D can be improved.
GRANTS
This work was funded by the National Institutes of Diabetes and Digestive and Kidney Diseases (DK-070860 to Naji Abumrad of Vanderbilt University) and by the Vanderbilt Institute for Clinical and Translational Research (UL1-TR-000445). J. J. Winnick was supported by a career development award (DK-093799) and is the guarantor of the work. J. M. Gregory was funded by F32-DK-1000114-01A1 and K12-HD-087023.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J.W. conceived and designed research; C.R.F., R.A.T., A.B., and R.M.S. performed experiments; C.R.F., V.L.A., R.A.T., A.B., R.M.S., and J.J.W. analyzed data; V.L.A., A.B., and J.J.W. interpreted results of experiments; A.B., R.M.S., and J.J.W. prepared figures; J.J.W. drafted manuscript; C.R.F., V.L.A., R.A.T., J.M.G., A.B., R.M.S., and J.J.W. edited and revised manuscript; C.R.F., V.L.A., R.A.T., J.M.G., A.B., R.M.S., and J.J.W. approved final version of manuscript.
REFERENCES
- 1.Anderwald C, Bernroider E, Krssak M, Stingl H, Brehm A, Bischof MG, Nowotny P, Roden M, Waldhäusl W. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes 51: 3025–3032, 2002. doi: 10.2337/diabetes.51.10.3025. [DOI] [PubMed] [Google Scholar]
- 2.Andreozzi F, Mannino GC, Mancuso E, Spiga R, Perticone F, Sesti G. Plasma kisspeptin levels are associated with insulin secretion in nondiabetic individuals. PLoS One 12: e0179834, 2017. doi: 10.1371/journal.pone.0179834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden G, Chen X, Stein TP. Gluconeogenesis in moderately and severely hyperglycemic patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 280: E23–E30, 2001. doi: 10.1152/ajpendo.2001.280.1.E23. [DOI] [PubMed] [Google Scholar]
- 4.Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63: 1725–1737, 2014. doi: 10.2337/db13-1307. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D, Holst JJ, Madsbad S. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 55: 1890–1901, 2012. doi: 10.1007/s00125-012-2556-7. [DOI] [PubMed] [Google Scholar]
- 7.Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K, Feurer ID, Tamboli RA. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care 35: 137–142, 2012. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD. Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 50: 1872–1882, 2001. doi: 10.2337/diabetes.50.8.1872. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrini E, Tamboli RA, Magkos F, Marks-Shulman PA, Eckhauser AW, Richards WO, Klein S, Abumrad NN. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 139: 448–455, 2010. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izzi-Engbeaya C, Comninos AN, Clarke SA, Jomard A, Yang L, Jones S, Abbara A, Narayanaswamy S, Eng PC, Papadopoulou D, Prague JK, Bech P, Godsland IF, Bassett P, Sands C, Camuzeaux S, Gomez-Romero M, Pearce JTM, Lewis MR, Holmes E, Nicholson JK, Tan T, Ratnasabapathy R, Hu M, Carrat G, Piemonti L, Bugliani M, Marchetti P, Johnson PR, Hughes SJ, James Shapiro AM, Rutter GA, Dhillo WS. The effects of kisspeptin on β-cell function, serum metabolites and appetite in humans. Diabetes Obes Metab 20: 2800–2810, 2018. doi: 10.1111/dom.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen NB, Jacobsen SH, Dirksen C, Bojsen-Møller KN, Naver L, Hvolris L, Clausen TR, Wulff BS, Worm D, Lindqvist Hansen D, Madsbad S, Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 303: E122–E131, 2012. doi: 10.1152/ajpendo.00073.2012. [DOI] [PubMed] [Google Scholar]
- 12.Kelley D, Mokan M, Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism 43: 1549–1557, 1994. doi: 10.1016/0026-0495(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W, Roden M. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53: 3048–3056, 2004. doi: 10.2337/diabetes.53.12.3048. [DOI] [PubMed] [Google Scholar]
- 14.Liljenquist JE, Mueller GL, Cherrington AD, Keller U, Chiasson J-L, Perry JM, Lacy WW, Rabinowitz D. Evidence for an important role of glucagon in the regulation of hepatic glucose production in normal man. J Clin Invest 59: 369–374, 1977. doi: 10.1172/JCI108649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinussen C, Bojsen-Møller KN, Dirksen C, Jacobsen SH, Jørgensen NB, Kristiansen VB, Holst JJ, Madsbad S. Immediate enhancement of first-phase insulin secretion and unchanged glucose effectiveness in patients with type 2 diabetes after Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 308: E535–E544, 2015. doi: 10.1152/ajpendo.00506.2014. [DOI] [PubMed] [Google Scholar]
- 16.Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 326: 22–29, 1992. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 17.Mitrakou A, Kelley D, Veneman T, Jenssen T, Pangburn T, Reilly J, Gerich J. Contribution of abnormal muscle and liver glucose metabolism to postprandial hyperglycemia in NIDDM. Diabetes 39: 1381–1390, 1990. doi: 10.2337/diab.39.11.1381. [DOI] [PubMed] [Google Scholar]
- 18.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 376: 641–651, 2017. doi: 10.1056/NEJMoa1600869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med 370: 2002–2013, 2014. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366: 1567–1576, 2012. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab 19: 667–681, 2014. doi: 10.1016/j.cmet.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol 187: 15–24, 1956. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- 23.Taylor R, Magnusson I, Rothman DL, Cline GW, Caumo A, Cobelli C, Shulman GI. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J Clin Invest 97: 126–132, 1996. doi: 10.1172/JCI118379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest 95: 690–698, 1995. doi: 10.1172/JCI117715. [DOI] [PMC free article] [PubMed] [Google Scholar]


