Abstract
Background:
Advances in basic and molecular biology have promoted the use of cell cultures in a wide range of areas, including the evaluation of drug efficacy, safety and toxicity.
Objective:
This article aims to provide a general overview of the methodological parameters of cell cultures used to investi-gate therapeutic options for Attention Deficit Hyperactivity Disorder.
Method:
A systematic search was performed in the electronic databases PubMed, Scopus, and DOAJ. In vitro experimental studies using cell cultures were included.
Results:
A total of 328 studies were initially identified, with 16 included for qualitative synthesis. Seven studies used neu-ronal cells (SH-SY5Y neuroblastoma and PC12 cell line) and nine used non-neuronal cells. All the studies described the cul-ture conditions, but most studies were inconsistent with regard to reporting results and raw data. Only one-third of the stud-ies performed cell viability assays, while a further 30% conducted gene expression analysis. Other additional tests included electrophysiological evaluation and transporter activity. More than 50% of the studies evaluated the effects of drugs such as methylphenidate and atomoxetine, while plant extracts were assessed in four studies and polyunsaturated fatty acids in one.
Conclusion:
We suggested a flowchart to guide the planning and execution of studies, and a checklist to be completed by authors to allow the standardized reporting of results. This may guide the elaboration of laboratory protocols and further in vitro studies.
Keywords: Cell model, neuronal cell, ADHD, ADHD treatment, methodological aspect, flowchart, checklist
1. INTRODUCTION
Advances in basic and molecular biology have promoted the use of cell cultures in a wide range of areas, including for the evaluation of drug efficacy, safety and toxicity as well as for the production of vaccines and biopharmaceuticals [1]. This methodology allows the use of immortalized cell lines, primary cell cultures and stem cells-derived cell models in experiments, according to the research objective [2].
In the field of Attention Deficit Hyperactivity Disorder (ADHD), a neurological disorder that affects up to 7.2% of children and adolescents and 2.5% of adults [3-6], cell cultures have been used to investigate new therapeutic alternatives or to test the safety and toxicity of the available drugs
[7-10]. Current therapeutic alternatives include psychostimulant drugs (methylphenidate, dexamphetamine), noradrenergic agents (atomoxetine), antidepressants and others [11, 12].
The most commonly used cell lines to investigate ADHD include neuronal cell lines (neuroblastoma SH-SY5Y) and non-neuronal cell lines such as HEK293rtTA, HEK-293, TsA201 and JAR, cells isolated from patients, and cells isolated from animals [9, 10, 13-21]. However, the effective use of cell cultures requires some fundamental aspects to be met. These include knowledge of the culture conditions (appropriate growth medium, micronutrients, temperature and pH), cell differentiation methods to allow the induction of neuronal dopaminergic and cholinergic phenotypes, cell viability methods to demonstrate cell survival or death in response to exposure to substances, and the evaluation of gene expression for possible cellular reactions due to pharmacological treatments [22-25]. The optimization and standardization of these parameters are essential to develop laboratory protocols, in order to obtain higher quality results and guide further in vitro and in vivo research.
Currently, only a few studies exist about the standardization of cell culture models in the neurological field. Some recent systematic reviews have synthesized information on in vitro research for Parkinson's disease and Bipolar Disorder, but there is still no synthesis for cell culture models in ADHD [26, 27]. Thus, our objective was to perform a systematic review to provide a broader overview of the methodological aspects to the use of cell cultures in the investigation of therapeutic options to ADHD.
2. METHODS
This research was designed according to the recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [28], Cochrane Handbook for Systematic Reviews of Interventions [29], and the Joana Briggs Institute [30]. The steps of screening article titles and abstracts, full-text reading, and data extraction were conducted by two independent reviewers, with a third reviewer consulted in case of discrepancies.
2.1. Systematic Literature Search and Eligibility
A systematic search was performed in the electronic databases PubMed, Scopus, and Directory of Open Access Journals (DOAJ) (September 2017). A manual search was also conducted of the bibliographic references of the included articles.
The inclusion criteria were in vitro experimental studies that used cell culture (any type of cell line) to investigate possible therapeutic options (any form, dosage or regimen) for the treatment of ADHD. Other types of studies (e.g. only in vivo evaluations, studies with humans), and studies not reporting data on cell culture or ADHD were excluded.
2.2. Data Extraction and Reporting Evaluation
A standardized form was used to extract data on the culture conditions and methodological aspects, the differentiation process, cell viability and proliferation methods, other additional tests of gene expression, electrophysiological evaluation and transporter activity.
Additionally, to better visualize the information provided by the studies on the methods used and the result reported, we applied an adapted version of the SYstematic Review Center for Laboratory Animal Experimentation tool (SYRCLE's) to the included studies [31].
3. RESULTS
A total of 328 studies were screened (titles and abstracts were read), after duplicate removal, of which 83 were included for full-text appraisal. Finally, 16 studies were eligible for qualitative synthesis, as shown in Fig. (1) [7-10, 13-21, 32-34].
Fig. (1).
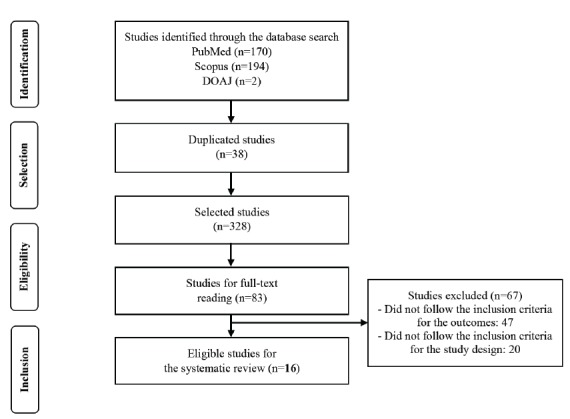
Flowchart of systematic review process.
The main characteristics of the included studies are presented in Table 1. Studies were conducted in different countries, mostly in Germany (50%), and published between 2007 and 2017. Neuronal cell lines (neuroblastoma SH-SY5Y and PC12) and non-neuronal cell lines such as HEK293rtTA, HEK-293, TsA201, and JAR, cells isolated from patients such as human lymphocytes, lymphoblastoid cell lines, peripheral blood mononuclear cells, and cells isolated from animals (L929sA, CHO, MDCK) were reported. The evaluated therapeutic options included drugs (e.g. amphetamine, atomoxetine, methylphenidate, pipamperone), extracts of different plants and polyunsaturated fatty acids. All the studies presented data on the cell culture conditions, while 5 also evaluated cell viability, and 5 assessed gene expression. The results for the conduct and reporting of studies with the SYRCLE tool showed that all the studies described the conditions of cell culture at least. However, studies were imprecise or failed to present complete results for all of the tests. It is unclear whether studies were free of selective data reporting. Half of the studies were also ‘unclear’ about the acknowledgments or conflicts of interest related to the pharmaceutical industry.
Table 1. Main characteristics of all the studies included in the systematic review.
| Study | Country | Cell Culture | Substance Tested | Parameters Evaluated |
|---|---|---|---|---|
| Schmidt et al., 2010 | Germany | SH-SY5Y, U-937 |
Amphetamine, Atomoxetine, Methylphenidate |
A, C |
| Schmidt et al., 2010a | Germany | SH-SY5Y, U-937 |
Amphetamine, Atomoxetine, Methylphenidate |
A, B |
| Schmidt et al., 2010b | Germany | SH-SY5Y | Hypericum perforatum, Pinus radiata, Pinus pinaster | A, B |
| Nam et al., 2014 | Korea | SH-SY5Y | YY162* | A |
| Feio-Azevedo et al., 2017 | Portugal | SH-SY5Y | Amphetamine e metabolites** | A, C |
| Bartl et al., 2010 | Germany | PC12 | Methylphenidate | A, B, C |
| Bartl et al., 2014 | Switzerland | PC12 | Polyunsaturated fatty acids | A, B |
| Craenenbroeck et al., 2006 | Belgium | HEK293rtTA, L929sA, CHO | Pipamperone | A, C |
| Wakamatsu et al., 2009 | Japan | HEK-293 | Methylphenidate | A |
| Ludolph et al., 2010 | Germany | TsA201 | Atomoxetine | A |
| Knorle et al., 2012 | Germany | JAR | Sideritis scardica | A, B |
| Suter et al., 2006 | Switzerland | Human lymphocytes | Methylphenidate | A |
| Schwarz et al., 2014 | Germany | Lymphoblastoid cell lines | Methylphenidate | A, C |
| Kittel-Schneider et al., 2016 | Germany | Peripheral blood mononuclear cells | Methylphenidate | A |
| Zhao et al., 2007 | China | CHO | Fructus Psoraleae | A |
| Salviano et al., 2015 | Brazil | MDCK | Methylphenidate | A, B |
Abbreviations: SH-SY5Y: neuroblastoma SH-SY5Y; U-937: Human monocytic U-927; PC12: Rat pheochromocytoma; HEK293rtTA cells: Human embryonic kidney cells expressing the tetracycline transactivator; HEK-293: Human embryonic kidney 293; TsA201: Transformed human embryonic kidney; JAR: Human choriocarcinoma; L929sA: pMx5-HT2AR cells mouse fibrosarcoma; CHO: Chinese hamster ovary; MDCK: Madin-darby canine kidney; *YY162: Mixture of “terpenoid-strengthened Ginkgo biloba” and “ginsenoside Rg3”; **4-hydroxyamphetamine e 4-hydroxynorephedrine; A: culture conditions; B: cell viability; C: gene expression.
3.1. Neuronal Cell Culture
From the 16 studies selected in our systematic search, five (31.3%) used the SH-SY5Y neuroblastoma [7, 8, 10, 33, 34] and two (12.5%) used the PC12 cell line [18, 32]. This first strain is a sub-line of the SK-N-SH cell, which was established in culture in 1970 from human metastatic neuroblastoma tissue [35]. One study acquired these cells from the European Collection of Cell Cultures (ECACC) - United Kingdom (UK), and other used the Korean Cell Line Bank - Seoul as a source of cells [10, 34]. The other studies did not report this information. The PC12 cell is a clonal cell line derived from a pheochromocytoma of the rat adrenal medulla [36]. Bartl et al. [18] acquired these cells through donation by researchers from the Technion Faculty of Medicine, Israel.
3.1.1. Culture Conditions for Neuronal Cells
In the seven included studies, the media used for cell growth was Dulbecco's Modified Eagle's Medium (DMEM) or Roswell Park Memorial Institute medium (RPMI), with compositions that varied the concentration and condition of Fetal Bovine Serum (FBS) (varying from 3% heat-inactivated FBS to 15% FBS), the concentration of antibiotics (penicillin, streptomycin or gentamycin), and other supplements (e.g. amino acids). Other information, such as confluence, the use of trypsin to collect the cells, cell density and number of passages was reported only by Feio-Azevedo [10]. In all the studies, cells were maintained in a 5% CO2 incubator. Four studies reported an incubation temperature of 37 °C [10, 18, 32, 34].
3.1.2. Phenotype and Cell Differentiation for Neuronal Cells
The SH-SY5Y cell line of neuroblasts can be differentiated into mature human neurons from methods based on the use of substances that trigger biochemical changes and may induce different neuronal phenotypes. One of the substances used is retinoic acid (RA), which is capable of inducing a cholinergic phenotype when applied in isolation or a dopaminergic phenotype when associated with phorbol ester and 2-O-tetradecanoylphorbol-13-acetate (TPA) [24, 37]. Only one study [10] reported the process of cell differentiation in the dopaminergic phenotype, using the method previously described by Ferreira et al. [38] in which the cells were exposed to RA and TPA in DMEM medium for 6 days. The PC12 cell line can differentiate to resemble sympathetic neurons when cultured in the presence of nerve growth factor (NGF) or several other compounds [36]. Bartl et al. [32] differentiated these cells with 50 ng/mL human NGF-b for 7 days according to the protocol used. The remaining studies did not report this information.
3.1.3. Cell Viability for Neuronal Cells
Three studies [8, 9, 11] quantitatively evaluated cellular viability the neuroblastoma SH-SY5Y using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazoline bromide (MTT) assay, measuring lactate dehydrogenase (LDH) activity, or evaluating the adenosine triphosphate (ATP) content.
In the studies that performed the MTT test, neuronal cells were exposed to amphetamine, atomoxetine, methylphenidate or Pinus radiata D. Don, and Pinus pinaster Aiton extracts, and then incubated at 37 °C (overnight/24 h). Mitochondrial activity was evaluated at different times of exposure and substance concentrations. The final results revealed that none of the substances reduced neuronal cell survival compared with control cells [8, 9, 11]. The activity of LDH enzyme was measured by only one study [11] in which cell death was induced by concentrations of amphetamine (3.50 mM and 5.00 mM). The results showed no correlation between cell death and the exposed substance at the concentration and time analyzed (24 h and 48 h) [11, 37]. Two studies [8, 9] measured the cellular content of ATP, which was performed with a scintillation microplate counter. The cells were exposed to different substances (amphetamine, atomoxetine, methylphenidate and extracts of Hypericum perforatum L, P. radiata and P. pinaster) at different concentrations (500 and 5000 ng/mL) for 24 h at 37°C. There was a significant increase in ATP content only when exposed to the H. perforatum extract at a concentration of 5000 ng/mL.
The studies that evaluated cellular viability with the cells PC12 used XCELLigence and BrdU incorporation tests. Bartl et al. [18] non-invasively evaluated cell viability using an xCELLigence Real-Time Cell Analyzer. In this case, the culture medium of PC12 cells was changed to DMEM containing 4.5 mg/mL glucose, supplemented with 1% FBS and 150 μM fatty acid-free bovine serum albumin (BSA). The results demonstrated that the combination of polyunsaturated fatty acids significantly increased cell viability. The assay of 5-bromo-2-deoxyuridine (bromodeoxyuridine or BrdU) performed by Bartl et al. [32] showed that concentrations of 1, 10, and 100 nM of methylphenidate activated DNA synthesis in PC12 cells compared to the controls, whereas higher concentrations of the drug (1-100 μM) did not influence cell proliferation.
3.1.4. Gene Expression for Neuronal Cells
Among the existing methods to evaluate gene expression, reverse transcription polymerase chain reaction (RT-PCR) is a widely used tool to amplify and detect mRNA [39]. This was the technique used by Schmidt et al. [33] to verify the effect of amphetamine, atomoxetine and methylphenidate in different concentrations (50, 500 and 5000 ng/mL) on the expression of the 8-hydroxyguanine glycosylase 1 (hOGG1). The results revealed that the evaluated drugs decreased the expression of this enzyme, which is directly related to the level of oxidative products of the DNA, suggesting a neuronal protective effect of psychostimulants and atomoxetine. One study [32] evaluated the effect of methylphenidate on gene expression in PC12 cells. Total RNA was extracted by the RNeasy kit and quality was evaluated on an agarose gel.
3.2. Non-neuronal Cell Cultures
Among the 16 studies, nine (56.25%) used non-neuronal cultures or cells isolated from animals . These cell lines were accessed through Cytomyx Ltd. (Cambridge, UK), the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Braunschweig, Germany) [9], and from the Biochemistry Institute of São Paulo University, Brazil [21].
3.2.1. Culture Conditions for Non-neuronal Cells
All of the studies described culture conditions. The media used for cell growth were Minimum Essential Medium (MEM) in 3 of the studies, DMEM in one study or RPMI-1640 in 5 studies; their compositions varied in relation to supplementation of the medium, represented by the addition of FBS (varying from 10 to 20%), different antibiotics and other supplements (e.g. amino acids). Incubation was performed at 37 ºC and 5% CO2.
3.2.2. Main Methodologies for Non-neuronal Cells
The studies were methodologically designed according to the type of cells and their objectives. Most of the studies were carried out to verify the cytotoxic potential of test substances tested or their influence on gene expression, electrophysiological test, levels of transporters and others tests (Table 2). Cell viability and gene expression were evaluated by two (22.2%) and two (22.2%) studies, respectively.
Table 2. Main tests reported in the studies with non-neuronal cells cultures.
| Authors, Year | Cell Culture | Study Objectives |
Cell
Viability |
Gene
Expression |
Others |
|---|---|---|---|---|---|
| Craenenbroeck et al., 2006 |
HEK293rtTA | To evaluate the effect of pipamperone on D4 receptor expression | - | qPCR | Radioligant Western blot |
| Wakamatsu et al., 2009 | HEK 293 | To evalute the effect of methylphenidate on the cardiovascular system | - | - | Electro-physiological |
| Ludolph et al., 2010 | TsA201 | To evaluate the effect of atomoxetine on glutamate receptors | - | - | Electro-physiological |
| Knorle et al., 2012 | JAR | To evaluate the effect of Sideritis scardica on monoamine transporters | LDH | - | Effect on transporters |
| Suter et al., 2006 | Human lymphocytes |
To evaluate the clastogenic effect of methylphenidate | - | - | Chromosome aberration |
| Schwarz et al., 2014 | Lymphoblastoid Cell Lines | To evaluate the effect of methylphenidate on gene expression regulation | - | qRT-PCR Microarray |
- |
| Kittel-Schneider et al., 2016 |
Peripheral blood mononuclear cells | To evaluate the cytogenetic effects of long-term treatment with methylphenidate | - | - | Micronucleus |
| Craenenbroeck et al., 2006 |
L929sA | To evaluate the effect of pipamperone on D4 receptor expression | qPCR | Radioligant Western blot |
|
| Craenenbroeck et al., 2006 |
CHO | To evaluate the effect of pipamperone on D4 receptor expression | - | qPCR | Radioligant Western blot |
| Zhao et al., 2007 | CHO | To evaluate the effect of Fructus Psoraleae on noradrenaline and dopamine transporters | - | - | Effect on transporters |
| Salviano et al., 2015 | MDCK | Evaluate the effect of methylphenidate on the renal system | MTT | - | - |
Abbreviations: HEK293rtTA cells: Human embryonic kidney cells expressing the tetracycline transactivator HEK-293: Human Embryonic Kidney; TsA201: Transformed human embryonic kidney; JAR: Human choriocarcinoma; L929sA: Cells mouse fibrosarcoma; CHO: Chinese Hamster Ovary; MDCK: Madin-Darby Canine Kidney; Receptor D4: Receptor de dopamine 4; 6-OHDA: 6-hydroxydopamine; qRT-PCR: quantitative Real Time PCR; *Eicosapentaenoic acid e docosahexanoic acid e gamma-linolenic acid.
3.2.3. Cell Viability for Non-neuronal Cells
Two studies performed viability and cell proliferation tests using LDH and MTT.
Knorle et al. [9] measured the LDH activity from the exposure of JAR cells to extracts of S. scardica at concentrations of 50 and 500 μg/mL. The cells were incubated at 20 ºC (3 h). There was no significant difference between the cells treated with the extracts and control cells. The studies performing the MTT assay [21] also observed no reduction in cell viability when MDCK cells were treated with methylphenidate at concentrations of 80 to 1.25 μL/mL.
3.2.4. Gene Expression for Non-neuronal Cells
Two studies [13, 19] evaluated the effect of methylphenidate on gene expression in LCL and CHO cells. Total RNA was extracted by the RNeasy kit and evaluated for quality on an agarose gel. In addition, Schwarz et al. [19] performed microarray hybridization.
The results showed that the effects of long-term methylphenidate treatment were observed on the expression of the ATXN1, HEY1, MAP3K8 and GLUT3 genes, while the effects of acute treatment were observed on the expression of NAV2 and ATXN1 in patients with ADHD, confirmed by quantitative Real-Time PCR (qRT PCR) analysis [19]. Craenenbroeck et al. [13] used qPCR to verify that the drug pipamperone does not increase the expression of dopamine 4 receptor mRNA in the CHO FLAGDRD4 cell line.
3.2.5. Other Tests for Non-neuronal Cells
The electrophysiological tests evaluated the effect of methylphenidate and atomoxetine on cells [16, 17]. The first drug was tested to verify its effect on the cardiovascular system from the delayed rectification potassium current (IKr) analysis in human ether-a-go-related (hERG) human HEK 293 cells. Methylphenidate was applied to the cell culture at different concentrations (0.1, 0.3 and 1 μg/mL) but none of them inhibited IKr, which suggests that this drug does not alter the ventricular repolarization process (prolongation of QT interval) at the recommended therapeutic dosage levels [16].
The effects of atomoxetine on glutaminergic receptors on TsA201 cells transfected with cDNAs, which encode N-methyl-D-aspartate (NMDA) receptors, were also evaluated. Atomoxetine exerted a dose-dependent antagonistic effect on NMDA receptors at lower concentrations, which suggests a relationship between glutaminergic transmission and the development of ADHD [17].
The extracts of the plant species Sideritis scardica Griseb. and Fructus Psoraleae inhibited the monoamine transporters in tests performed with human (JAR) and animal (CHO) cells, respectively [9, 15]. According to the authors, JAR cells were chosen due to the expression of human serotonin transporter (hSERT), while CHO cells were chosen due to the expression of rat dopamine transporter (rDAT), rat serotonin transport (rSERT), mice γ-aminobutyric acid or GABA transporter (mGAT-1) and human noradrenaline transporter (hNET).
Other tests were also performed. Craenenbroeck et al. [13] used radioligand, western blot and tests in HEK293rtTA, L929sA and CHO cells to evaluate that pipamperone acts as a pharmacological chaperone and increases the expression level of the dopamine D4 receptor. This receptor may be involved with the development the ADHD. Chromosomal aberration and micronucleus tests on isolated human lymphocytes were performed to evaluate possible cytotoxic effects of the drug methylphenidate. The results demonstrated the absence of a clastogenic effect in these cells after treatment with D, L-methylphenidate in concentration up to 10 mM [14]. Long-term treatment did not lead to cytogenetic effects [20].
4. DISCUSSION
This is the first systematic review to synthesize information on cell cultures as research models for assessing therapeutic options in ADHD. In addition to contributing further evidence on the safety and toxicity profile of well-established drugs (methylphenidate, amphetamine and atomoxetine), it was possible to verify the use of these models for the investigation of potential therapeutic substances, such as plant extracts and polyunsaturated acids.
The choice of pharmacological treatments for ADHD is mostly based on physiopathological aspects of the disease, such as alterations in the dopaminergic, noradrenergic and serotonergic neurotransmission [40]. Currently, psychostimulants (e.g. amphetamine and methylphenidate) and noradrenaline reuptake inhibitors (e.g. atomoxetine) are considered first and second line treatments, respectively [11]. Although the therapeutic effect (efficacy and safety) of these drugs has been proven in in vivo studies and clinical trials [11], the in vitro studies included in our review also show that these drugs did not interfere with cell viability or gene expression, nor present significant toxicity. Moreover, cell culture assays suggest that psychostimulants and atomoxetine can protect DNA against oxidative stress, because they decrease the expression of the hOGG1 enzyme in cell lines, which has a positive correlation with the level of products of oxidative lesions to the DNA [33]. This is important to guide further investigation into the neuropathophysiology of ADHD, which has still not been fully elucidated.
Our review also found other substances that act at the level of monoamine transporters, making them possible therapeutic options for neurological disorders. Among the extracts of plants and polyunsaturated fatty acids, two substances presented promising results: the extracts S. scardica and Fructus Psoraleae. The results showed that these substances did not alter cell viability, but inhibited dopamine, norepinephrine and serotonin transporters, which are usually altered in patients with ADHD. However, studies to better characterize and isolate the potentially active compounds of the extracts are needed [9, 15]. Moreover, additional tests such as in vitro electrophysiological studies should be performed to investigate cellular mechanisms of action, together with in vivo tests for safety assessment [41].
To obtain reliable results, in vitro studies with cell cultures should be appropriately conducted and reported. Several studies have documented concerns about the lack of reproducibility in scientific studies, particularly preclinical studies involving cells and animals [42-44]. Quality standards and good practices are generally not well defined for in vitro methods and in vivo models, and have not been integrated into preclinical research laboratories [45]. Several factors are commonly attributed to reduce methodological quality, including the poor design of experiments, the lack of training of investigators, and insufficient reporting of results or the withholding of technical details. As a consequence, the literature becomes irreproducible and unreliable, the bench-to-bedside time for new drugs is negatively affected, and the resources needed for clinical development significantly increase [45, 46].
Considering how the in vitro studies included in our review were conducted, and the most appropriate methodological aspects to perform cell cultures for ADHD, we propose the research process depicted in Fig. (2). This flowchart presents the main steps that should be considered by researchers when performing a study aiming to evaluate the effects and toxicity of substances exposed to neuronal and non-neuronal cells, including the selection of the cell culture and test to be performed based on the study’s goals.
Fig. (2).
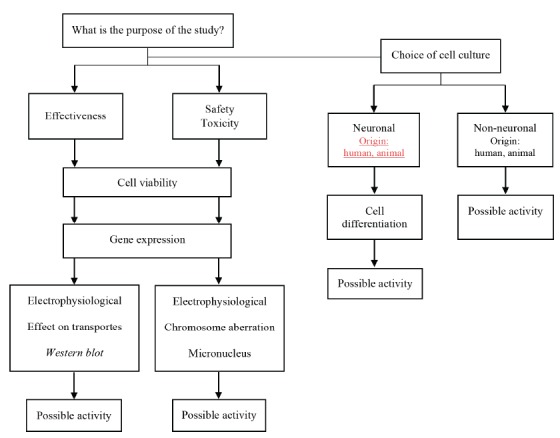
Flowchart with the main methodological steps for in vitro research investigating therapeutic options for ADHD.
The selection and maintenance of cells is an important first step. Often, cells used in the cultures are acquired by collections of cell biology or donated by research laboratories. Laboratory conditions, including the choice of medium and the type and serum concentrations, should be optimized for each type of cell, since these factors promote cell survival and proliferation, and modulate the biological behavior of cells, influencing the ability of differentiation and metabolic profile [1, 47, 48]. The composition of the growth medium is established differently according to the sources of access [27]. Bovine, adult, newborn or fetal (FBS) serum are the most commonly used [49]. Other substances may be added for the maintenance of culture media. Antibiotics (penicillin, streptomycin, gentamicin, and tylosin) are added to prevent bacterial and fungal contaminants [50], while non-essential amino acid supplementation, as observed in DMEM and RPMI-1640, is used to promote growth and increase cell viability [22]. Moreover, the addition of sodium pyruvate may exhibit a cytoprotective activity against oxidative stress, preventing cell death induced by hydrogen peroxide [51, 52].
As sources of energy for cellular media, glucose is often added in the form of carbohydrates (primary source), and the essential amino acid glutamine (secondary energy source for cellular metabolism). Attention should be paid, however, to the maximum permissible concentrations of these components to maintain normal cell activities [22, 53].
For SH-SY5Y neuroblastoma cells, the process of differentiation into mature human neurons involves the addition of neurotrophic factors, factors secreted by human stem cells (hNSC), extracellular matrix proteins, and the gradual withdrawal of serum, as well as the use of retinoic acid [37]. This combination results in rapid and effective differentiation, with a higher percentage of neurons obtained [54]. The advantage of this methodology is the possibility of obtaining more robust results for comparison with in vivo models [37]. Both neuronal cells and non-neuronal cells can also be tested for cell activity. The substances to be tested, as well as their concentration and time of exposure should also be defined in the study’s protocol. Another critical step in in vitro research is the determination and optimization of methods for cell viability. This enables one or more cellular functions such as mitochondrial activity (MTT), membrane integrity and cellular metabolism (LDH), DNA replication (BrdU incorporation), and ATP production, among others, to be evaluated [25], which may guide the elucidation of molecular pathways. Additionally, gene expression tests may broadly show cellular reactions due to a pathological condition or the effect of pharmacological treatments [23, 54-56]. Since many factors may affect these assays, standardized conditions are needed to obtain accurate results [55-57].
Besides optimized practices for planning and conducting in vitro studies, the reporting of results should also be standardized. In the healthcare area, the concept ‘core outcome sets’ represents a standardized collection of outcomes that should be measured and reported, which facilitate interpretation and generate consistent outcomes across studies [58, 59]. Moreover, well-established checklists and recommendations with a minimum set of items for reporting are required for the publication of clinical and observational studies [28, 29]. For preclinical studies, only a few recommendations such as the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) checklist exist to guide study planning, execution, data analysis and reporting [56]. Thus, we suggest a minimum checklist (Table 3) that should be completed by researchers using cell cultures prior to publication. This checklist was built based on the information reported in the studies included in our review and complemented with published literature on cell-lineage and cell culture methods (Guidance on Good Cell Culture Practice - GCCP) [60]. Together with the flowchart of the research process, the checklist aims to improve laboratory protocols, guide further standardized in vitro and in vivo research, facilitate the interpretation of data by the readers and allow result reproducibility.
Table 3. Checklist for conduct and reporting experimental in vitro studies.
| Checklist Item | Reported on Page Nº |
|---|---|
| CELL CULTURE | |
| Identification of culture type | |
| Identification of cell type | |
| Origin of cells (human, animal) | |
| In case of cells isolated from tissues, report isolation technique and variables involved | |
| Source of access of cells (collection of cell biology, donation by laboratories) | |
| In case of collection of cell biology, report the product/catalog number | |
| Growth medium used | |
| Growth medium supplementation components (serum, antibiotics, micronutrients, others). Report name, concentration, percentage used and brand | |
| Frequency of change of growth medium | |
| pH of the growth medium | |
| Confluence | |
| Use of trypsin or other substance to collect the cells. Report name, concentration, percentage | |
| Cellular density | |
| Number of passages | |
| Incubation temperature (exact 0.0 °C) | |
| Atmosphere conditions (exact 0.0% CO2) | |
| METHODS | |
| Technique name | |
| Report whether the technique has been developed and validated or reproduced | |
| In case of reproduced technique, report if there were adaptations and what were they | |
| In case of use of substance that promotes cellular alteration, report name, function, concentration, percentage, exposure time | |
| Time period of each step | |
| Temperature (exact 0.0 °C) | |
| Atmosphere conditions (exact 0.0% CO2) | |
| Equipment used | |
| Type of statistical analysis and level of significance | |
| Software for statistical analysis | |
Our research has some limitations. Few studies were included in the review, which may limit the generalization of the results to other fields. However, no further studies were added by manual searches. Given the nature of the data and the moderate methodological quality of the studies, other quantitative analyses were not possible.
CONCLUSION
This is the first systematic review to describe the methodological aspects for planning, performing and reporting in vitro studies with cell cultures in ADHD. To date, the SH-SY5Y neuroblastoma cell line is the most widely used for the evaluation of cell proliferation and viability and gene expression. However, non-neuronal cells were shown to be useful for evaluation of the safety and toxicity of drugs and extracts of plants. Given the heterogeneity in the conduct of preclinical studies, we proposed a flowchart of the main methodological steps that should be followed by researchers during the development of laboratory protocols and further in vitro investigation. Additionally, we created a checklist with minimum reporting items that should be used by authors prior to publication.
ACKNOWLEDGEMENTS
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
The study has been financially supported by PRISMA guidelines have been followed in this study.
FUNDING
CAPES/Fundação Araucária scholarship of D.C.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Yao T., Asayama Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017;16(2):99–117. doi: 10.1002/rmb2.12024. [http://dx.doi.org/10.1002/rmb2.12024]. [PMID: 29259457]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter M., Shieh J.C. Guide to Research Techniques in Neuroscience. Cell Culture Techniques; 2010. pp. 281–296. [Google Scholar]
- 3.Simon V., Czobor P., Bálint S., Mészáros A., Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br. J. Psychiatry. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [http://dx.doi.org/10.1192/bjp.bp.107.048827]. [PMID: 19252145]. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatry Association (APA) Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Publishing; 2013. [Google Scholar]
- 5.Polanczyk G.V., Salum G.A., Sugaya L.S., Caye A., Rohde L.A. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child Psychol. Psychiatry. 2015;56(3):345–365. doi: 10.1111/jcpp.12381. [http://dx.doi.org/ 10.1111/jcpp.12381]. [PMID: 25649325]. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R., Sanders S., Doust J., Beller E., Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–e1001. doi: 10.1542/peds.2014-3482. [http://dx.doi.org/10.1542/peds.2014-3482]. [PMID: 25733754]. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt A.J., Krieg J.C., Clement H.W., Gebhardt S., Schulz E., Heiser P. Impact of drugs approved for treating ADHD on the cell survival and energy metabolism: an in-vitro study in human neuronal and immune cells. J. Psychopharmacol. (Oxford) 2010;24(12):1829–1833. doi: 10.1177/0269881109105563. [http://dx.doi.org/10.1177/0269881109105563]. [PMID: 19605603]. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A.J., Krieg J-C., Hemmeter U.M., Kircher T., Schulz E., Clement H-W., Heiser P. Impact of plant extracts tested in attention-deficit/hyperactivity disorder treatment on cell survival and energy metabolism in human neuroblastoma SH-SY5Y cells. Phytother. Res. 2010;24(10):1549–1553. doi: 10.1002/ptr.3198. [b]. [DOI] [PubMed] [Google Scholar]
- 9.Knörle R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J. Neural Transm. (Vienna) 2012;119(12):1477–1482. doi: 10.1007/s00702-012-0824-9. [http://dx.doi.org/10.1007/s00702-012-0824-9]. [PMID: 22622367]. [DOI] [PubMed] [Google Scholar]
- 10.Feio-Azevedo R., Costa V.M., Ferreira L.M., Branco P.S., Pereira F.C., Bastos M.L., Carvalho F., Capela J.P. Toxicity of the amphetamine metabolites 4-hydroxyamphetamine and 4-hydroxynorephedrine in human dopaminergic differentiated SH-SY5Y cells. Toxicol. Lett. 2017;269:65–76. doi: 10.1016/j.toxlet.2017.01.012. [http://dx.doi.org/ 10.1016/j.toxlet.2017.01.012]. [PMID: 28115274]. [DOI] [PubMed] [Google Scholar]
- 11.Thapar A., Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. doi: 10.1016/S0140-6736(15)00238-X. [http://dx.doi.org/10.1016/ S0140-6736(15)00238-X]. [PMID: 26386541]. [DOI] [PubMed] [Google Scholar]
- 12.Li Y., Gao J., He S., Zhang Y., Wang Q. An evaluation on the efficacy and safety of treatments for Attention Deficit Hyperactivity Disorder in children and adolescents: a comparison of multiple treatments. Mol. Neurobiol. 2017;54(9):6655–6669. doi: 10.1007/s12035-016-0179-6. [http://dx. doi.org/10.1007/s12035-016-0179-6]. [PMID: 27738872]. [DOI] [PubMed] [Google Scholar]
- 13.Van Craenenbroeck K., Gellynck E., Lintermans B., Leysen J.E., Van Tol H.H., Haegeman G., Vanhoenacker P. Influence of the antipsychotic drug pipamperone on the expression of the dopamine D4 receptor. Life Sci. 2006;80(1):74–81. doi: 10.1016/j.lfs.2006.08.024. [http://dx.doi. org/10.1016/j.lfs.2006.08.024]. [PMID: 16978659]. [DOI] [PubMed] [Google Scholar]
- 14.Suter W., Martus H-J., Elhajouji A. Methylphenidate is not clastogenic in cultured human lymphocytes and in the mouse bone-marrow micronucleus test. Mutat. Res. 2006;607(2):153–159. doi: 10.1016/j.mrgentox.2006.02.004. [http://dx.doi.org/10.1016/j.mrgentox.2006.02.004]. [PMID: 16829163]. [DOI] [PubMed] [Google Scholar]
- 15.Zhao G., Li S., Qin G-W., Fei J., Guo L-H. Inhibitive effects of Fructus Psoraleae extract on dopamine transporter and noradrenaline transporter. J. Ethnopharmacol. 2007;112(3):498–506. doi: 10.1016/j.jep.2007.04.013. [http://dx.doi.org/10.1016/j.jep.2007.04.013]. [PMID: 17555897]. [DOI] [PubMed] [Google Scholar]
- 16.Wakamatsu A., Nomura S., Tate Y., Shimizu S., Harada Y. Effects of methylphenidate hydrochloride on the cardiovascular system in vivo and in vitro: a safety pharmacology study. J. Pharmacol. Toxicol. Methods. 2009;59(3):128–134. doi: 10.1016/j.vascn.2009.01.003. [http://dx.doi. org/10.1016/j.vascn.2009.01.003]. [PMID: 19281853]. [DOI] [PubMed] [Google Scholar]
- 17.Ludolph A.G., Udvardi P.T., Schaz U., Henes C., Adolph O., Weigt H.U., Fegert J.M., Boeckers T.M., Föhr K.J. Atomoxetine acts as an NMDA receptor blocker in clinically relevant concentrations. Br. J. Pharmacol. 2010;160(2):283–291. doi: 10.1111/j.1476-5381.2010.00707.x. [http://dx.doi. org/10.1111/j.1476-5381.2010.00707.x]. [PMID: 20423340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartl J., Walitza S., Grünblatt E. Enhancement of cell viability after treatment with polyunsaturated fatty acids. Neurosci. Lett. 2014;559:56–60. doi: 10.1016/j.neulet.2013.11.023. [http://dx.doi.org/10.1016/j.neulet.2013.11.023]. [PMID: 24269370]. [DOI] [PubMed] [Google Scholar]
- 19.Schwarz R., Reif A., Scholz C-J., Weissflog L., Schmidt B., Lesch K-P., Jacob C., Reichert S., Heupel J., Volkert J., Kopf J., Hilscher M., Weber H., Kittel-Schneider S. A preliminary study on methylphenidate-regulated gene expression in lymphoblastoid cells of ADHD patients. World J. Biol. Psychiatry. 2015;16(3):180–189. doi: 10.3109/15622975.2014.948064. [http://dx.doi.org/10.3109/15622975.2014. 948064]. [PMID: 25162476]. [DOI] [PubMed] [Google Scholar]
- 20.Kittel-Schneider S., Spiegel S., Renner T., Romanos M., Reif A., Reichert S., Heupel J., Schnetzler L., Stopper H., Jacob C. Cytogenetic effects of chronic methylphenidate treatment and chronic social stress in adults with Attention-Deficit/Hyperactivity Disorder. Pharmacopsychiatry. 2016;49(4):146–154. doi: 10.1055/s-0035-1569361. [http://dx. doi.org/10.1055/s-0035-1569361]. [PMID: 26926233]. [DOI] [PubMed] [Google Scholar]
- 21.Salviano L.H.M.S., Linhares M.I., de Lima K.A., de Souza A.G., Lima D.B., Jorge A.R.C., da Costa M.F., Filho A.J.M.C., Martins A.M.C., Monteiro H.S.A., de Jesus Ponte Carvalho T.M., de França Fonteles M.M. Study of the safety of methylphenidate: Focus on nephrotoxicity aspects. Life Sci. 2015;141:137–142. doi: 10.1016/j.lfs.2015.09.014. [http://dx.doi.org/10.1016/j.lfs.2015.09.014]. [PMID: 26407472]. [DOI] [PubMed] [Google Scholar]
- 22.Arora M. Cell culture media: a review. 2013 https://www.labome.com/method/Cell-Culture-Media-A-Review. html
- 23.Larson D.R., Singer R.H., Zenklusen D. A single molecule view of gene expression. Trends Cell Biol. 2009;19(11):630–637. doi: 10.1016/j.tcb.2009.08.008. [http://dx.doi.org/10.1016/j.tcb.2009.08.008]. [PMID: 19819144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie H.R., Hu L.S., Li G.Y. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. (Engl.) 2010;123(8):1086–1092. [PMID: 20497720]. [PubMed] [Google Scholar]
- 25.Adan A., Kiraz Y., Baran Y. Cell proliferation and cytotoxicity assays. Curr. Pharm. Biotechnol. 2016;17(14):1213–1221. doi: 10.2174/1389201017666160808160513. [http://dx.doi.org/10.2174/1389201017666160808160513]. [PMID: 27604355]. [DOI] [PubMed] [Google Scholar]
- 26.Viswanath B., Jose S.P., Squassina A., Thirthalli J., Purushottam M., Mukherjee O., Vladimirov V., Patrinos G.P., Del Zompo M., Jain S. Cellular models to study bipolar disorder: A systematic review. J. Affect. Disord. 2015;184:36–50. doi: 10.1016/j.jad.2015.05.037. [http://dx.doi.org/10.1016/j.jad.2015.05.037]. [PMID: 26070045]. [DOI] [PubMed] [Google Scholar]
- 27.Xicoy H., Wieringa B., Martens G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: a systematic review. Mol. Neurodegener. 2017;12(1):10. doi: 10.1186/s13024-017-0149-0. [http://dx.doi.org/10.1186/s13024-017-0149-0]. [PMID: 28118852]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ. 2007:339. [Google Scholar]
- 29.Higgins J.P.T., Green S. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. 2010 http://handbook.cochrane.org/
- 30.Joanna Briggs Institute. Methodology for JBI scoping reviews Joanna Briggs Institute. Joanna Briggs Institute Reviewers’ Manual: 2015 edition. 2018 https://joannabriggs.org/assets/ docs/sumari/Reviewers-Manual_Methodology-for-JBI-Scoping-Reviews_2015_v2.pdf
- 31.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [http://dx.doi.org/10.1186/1471-2288-14-43]. [PMID: 24667063]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartl J., Link P., Schlosser C., Gerlach M., Schmitt A., Walitza S., Riederer P., Grünblatt E. Effects of methylphenidate: the cellular point of view. Atten. Defic. Hyperact. Disord. 2010;2(4):225–232. doi: 10.1007/s12402-010-0039-6. [http://dx.doi.org/10.1007/s12402-010-0039-6]. [PMID: 21432609]. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt A.J., Clement H-W., Gebhardt S., Hemmeter U.M., Schulz E., Krieg J-C., Kircher T., Heiser P. Impact of psychostimulants and atomoxetine on the expression of 8-hydroxyguanine glycosylase 1 in human cells. J. Neural Transm. (Vienna) 2010;117(6):793–797. doi: 10.1007/s00702-010-0408-5. [http://dx.doi.org/10.1007/s00702-010-0408-5]. [PMID: 20467764]. [DOI] [PubMed] [Google Scholar]
- 34.Nam Y., Shin E-J., Shin S.W., Lim Y.K., Jung J.H., Lee J.H., Ha J.R., Chae J.S., Ko S.K., Jeong J.H., Jang C-G., Kim H-C. YY162 prevents ADHD-like behavioral side effects and cytotoxicity induced by Aroclor1254 via interactive signaling between antioxidant potential, BDNF/TrkB, DAT and NET. Food Chem. Toxicol. 2014;65:280–292. doi: 10.1016/j.fct.2013.12.046. [http://dx.doi.org/10.1016/j.fct.2013.12.046]. [PMID: 24394491]. [DOI] [PubMed] [Google Scholar]
- 35.Biedler J.L., Helson L., Spengler B.A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33(11):2643–2652. [PMID: 4748425]. [PubMed] [Google Scholar]
- 36.Shafer T.J., Atchison W.D. Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: a model for neurotoxicological studies. Neurotoxicology. 1991;12(3):473–492. [PMID: 1720882]. [PubMed] [Google Scholar]
- 37.Shipley M.M., Mangold C.A., Szpara M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. 2016;108(108):53193. doi: 10.3791/53193. [PMID: 26967710]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira P.S., Nogueira T.B., Costa V.M., Branco P.S., Ferreira L.M., Fernandes E., Bastos M.L., Meisel A., Carvalho F., Capela J.P. Neurotoxicity of “ecstasy” and its metabolites in human dopaminergic differentiated SH-SY5Y cells. Toxicol. Lett. 2013;216(2-3):159–170. doi: 10.1016/j.toxlet.2012.11.015. [http://dx.doi.org/10.1016/j.toxlet.2012. 11.015]. [PMID: 23194825]. [DOI] [PubMed] [Google Scholar]
- 39.Bustin S.A., Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J. Biomol. Tech. 2004;15(3):155–166. [PMID: 15331581]. [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp S.I., McQuillin A., Gurling H.M.D. Genetics of attention-deficit hyperactivity disorder (ADHD). Neuropharmacology. 2009;57(7-8):590–600. doi: 10.1016/j.neuropharm.2009.08.011. [http://dx.doi.org/10.1016/j.neuropharm.2009.08. 011]. [PMID: 19715710]. [DOI] [PubMed] [Google Scholar]
- 41.Food and Drug Administration (FDA) S7B Nonclinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by Human Pharmaceuticals. Guidance for Industry; 2005. [PubMed] [Google Scholar]
- 42.Ioannidis J.P. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [http://dx.doi.org/10.1371/journal.pmed. 0020124]. [PMID: 16060722]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins F.S., Tabak L.A. Policy: NIH plans to enhance reproducibility. Nature. 2014;505(7485):612–613. doi: 10.1038/505612a. [http://dx.doi.org/10. 1038/505612a]. [PMID: 24482835]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiala C., Diamandis E.P. How to reduce scientific irreproducibility: the 5-year reflection. Clin. Chem. Lab. Med. 2017;55(12):1845–1848. doi: 10.1515/cclm-2017-0759. [http://dx.doi.org/10.1515/cclm-2017-0759]. [PMID: 29031017]. [DOI] [PubMed] [Google Scholar]
- 45.Begley C.G., Ioannidis J.P. Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 2015;116(1):116–126. doi: 10.1161/CIRCRESAHA.114.303819. [http://dx.doi.org/10.1161/CIRCRESAHA.114. 303819]. [PMID: 25552691]. [DOI] [PubMed] [Google Scholar]
- 46.Lieu C.H., Tan A.C., Leong S., Diamond J.R., Eckhardt S.G. From bench to bedside: lessons learned in translating preclinical studies in cancer drug development. J. Natl. Cancer Inst. 2013;105(19):1441–1456. doi: 10.1093/jnci/djt209. [http://dx.doi.org/10.1093/jnci/djt209]. [PMID: 24052618]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X., Lin M., Li Y., Zhao X., Yan F. Effects of DMEM and RPMI 1640 on the biological behavior of dog periosteum-derived cells. Cytotechnology. 2009;59(2):103–111. doi: 10.1007/s10616-009-9200-5. [http://dx.doi.org/10. 1007/s10616-009-9200-5]. [PMID: 19496017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Z., Shao W., Gu J., Hu X., Shi Y., Xu W., Huang C., Lin D. Effects of culture media on metabolic profiling of the human gastric cancer cell line SGC7901. Mol. Biosyst. 2015;11(7):1832–1840. doi: 10.1039/c5mb00019j. [http://dx.doi.org/10.1039/C5MB00019J]. [PMID: 25925870]. [DOI] [PubMed] [Google Scholar]
- 49.Brunner D., Frank J., Appl H., Schöffl H., Pfaller W., Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27(1):53–62. doi: 10.14573/altex.2010.1.53. [http://dx.doi.org/ 10.14573/altex.2010.1.53]. [PMID: 20390239]. [DOI] [PubMed] [Google Scholar]
- 50.Perlman D. Use of antibiotics in cell culture media. Methods Enzymol. 1979;58:110–116. doi: 10.1016/s0076-6879(79)58128-2. [http://dx.doi.org/10.1016/S0076-6879 (79)58128-2]. [PMID: 423753]. [DOI] [PubMed] [Google Scholar]
- 51.Mazzio E., Soliman K.F.A. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci. Lett. 2003;337(2):77–80. doi: 10.1016/s0304-3940(02)01327-7. [http://dx.doi.org/10.1016/S0304-3940(02)01327-7]. [PMID: 12527392]. [DOI] [PubMed] [Google Scholar]
- 52.Wang X., Perez E., Liu R., Yan L-J., Mallet R.T., Yang S-H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132(1):1–9. doi: 10.1016/j.brainres.2006.11.032. [http://dx.doi.org/10.1016/j.brainres.2006.11.032]. [PMID: 17174285]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasieka A.E., Morgan J.F. Glutamine metabolism of normal and malignant cells cultivated in synthetic media. Nature. 1959;183(4669):1201–1202. doi: 10.1038/1831201a0. [http://dx.doi.org/10.1038/1831201a0]. [PMID: 13657053]. [DOI] [PubMed] [Google Scholar]
- 54.Yang H., Wang J., Sun J., Liu X., Duan W-M., Qu T. A new method to effectively and rapidly generate neurons from SH-SY5Y cells. Neurosci. Lett. 2016;610(610):43–47. doi: 10.1016/j.neulet.2015.10.047. [http://dx.doi.org/ 10.1016/j.neulet.2015.10.047]. [PMID: 26497914]. [DOI] [PubMed] [Google Scholar]
- 55.Bustin S.A. Real-time reverse transcription PCR. Ncyclopedia of Diagnostic Genomics and Proteomics. 2005;1131:1135. [Google Scholar]
- 56.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [http://dx.doi.org/10. 1373/clinchem.2008.112797]. [PMID: 19246619]. [DOI] [PubMed] [Google Scholar]
- 57.Taylor S., Wakem M., Dijkman G., Alsarraj M., Nguyen M. A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines. Methods. 2010;50(4):S1–S5. doi: 10.1016/j.ymeth.2010.01.005. [http://dx. doi.org/10.1016/j.ymeth.2010.01.005]. [PMID: 20215014]. [DOI] [PubMed] [Google Scholar]
- 58.Chiarotto A., Ostelo R.W., Turk D.C., Buchbinder R., Boers M. Core outcome sets for research and clinical practice. Braz. J. Phys. Ther. 2017;21(2):77–84. doi: 10.1016/j.bjpt.2017.03.001. [http://dx.doi.org/10.1016/j.bjpt.2017.03. 001]. [PMID: 28460714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke M., Williamson P.R. Core outcome sets and systematic reviews. Syst. Rev. 2016;5:11. doi: 10.1186/s13643-016-0188-6. [http://dx.doi.org/10.1186/s13643-016-0188-6]. [PMID: 26792080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coecke S., Balls M., Bowe G., Davis J., Gstraunthaler G., Hartung T., Hay R., Merten O-W., Price A., Schechtman L., Stacey G., Stokes W. Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern. Lab. Anim. 2005;33(3):261–287. doi: 10.1177/026119290503300313. [PMID: 16180980]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.


