Abstract
Study Objectives:
Treatable sleep-related conditions are frequent in stroke patients, although their prevalence across stroke types and ideal method for screening is not clear. The objectives of this study were to evaluate the prevalence of sleep disturbance across different stroke types and identify approaches to the collection of sleep-related measures in clinical practice.
Methods:
We performed an observational cohort study of 2,213 patients with ischemic stroke, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), or transient ischemic attack seen in a cerebrovascular clinic February 17, 2015 through July 5, 2017 who completed at least one of the following sleep-related questionnaires: Patient-Reported Outcomes Measurement Information System (PROMIS) sleep disturbance, Insomnia Severity Index (ISI), Sleep Apnea Probability Scale (SAPS), and sleep duration. Prevalence of abnormal scores were calculated using the following thresholds: PROMIS sleep disturbance ≥ 55, ISI ≥ 15, SAPS score ≥ 0.50, and sleep duration fewer than 6 or more than 9 hours. Sensitivity, specificity, and positive and negative predictive values of PROMIS sleep disturbance T-score ≥ 55 to identify patients with moderate-severe insomnia (ISI ≥ 15) were computed.
Results:
In the cohort, 28.6% patients (624/2183) had PROMIS sleep disturbance score ≥ 55, 17.6% (142/808) had ISI ≥ 15, and 61.3% (761/1241) had a positive SAPS screen. The frequency of abnormal sleep scale scores was similar across time periods and stroke types. The sensitivity and specificity of PROMIS sleep disturbance T-score ≥ 55 to identify patients with ISI ≥ 15 were 0.89 (95% confidence interval 0.83–0.94) and 0.81 (95% confidence interval 0.78–0.84), respectively.
Conclusions:
The prevalence of sleep-related symptoms in patients with mild stroke are similar across stroke types and time periods after stroke. Potential approaches to screening for sleep disturbance in stroke patients are provided.
Citation:
Katzan IL, Thompson NR, Walia HK, Moul DE, Foldvary-Schaefer N. Sleep-related symptoms in patients with mild stroke. J Clin Sleep Med. 2020;16(1):55–64.
Keywords: patient-reported outcomes, sleep, stroke
BRIEF SUMMARY
Current Knowledge/Study Rationale: Treatable sleep-related conditions are frequent in patients with stroke, although their prevalence across stroke types is not clear. There is general consensus that systematic assessment of sleep disturbance is important in stroke patients, yet the optimal content of sleep-related questions in in this population is not known.
Study Impact: The prevalence of a positive screen for sleep apnea is lower in patients with intracerebral hemorrhage and subarachnoid hemorrhage compared to ischemic stroke and transient ischemic attack, but sleep symptoms are otherwise similar across stroke types, suggesting a uniform method for assessment of sleep disturbance is reasonable. The PROMIS sleep disturbance scale, along with a sleep apnea screen such as the Sleep Apnea Probability Scale, and a question regarding sleep duration may be a reasonable approach to assessment of sleep disturbance in patients with stroke.
INTRODUCTION
Sleep disturbances are common in patients with stroke. Insomnia occurs in up to 38% of patients with recent ischemic stroke1 and is associated with reduced quality of life,2 depression,3 physical disability, cognitive difficulty, and anxiety.1 Up to 27% of patients with recent stroke sleep longer than 10 hours per day,4 which has been associated with worse functional outcomes in stroke patients admitted to acute rehabilitation unit.5 Obstructive sleep apnea (OSA) occurs in more than half of patients with stroke and transient ischemic attack (TIA)6 and is associated with worse functional status7,8 and increased mortality after stroke.9 Less commonly, patients with stroke may have circadian rhythm disturbances, sleep-related movement disorders, or disruptions in sleep architecture resulting in unrestful sleep.10 Importantly, effective treatments exist for these sleep disorders in general.11 Data comparing prevalence of sleep disturbance across stroke types are sparse and this may affect the approach to screening for sleep disorders after stroke.
In addition, the optimal content of sleep-related questions to assess sleep disturbance in patients with stroke is not clear. Ideally, the set of sleep-related assessment questions stroke patients are asked to complete would: (1) provide an overall assessment of sleep symptoms; (2) allow the screening for common treatable sleep disorders; and (3) entail minimal patient burden. The objectives of this analysis were to evaluate the presence of sleep disturbances from sleep questionnaires collected in a real-world setting of patients across different stroke types and identify approaches to the collection of sleep-related patient-reported outcomes measures (PROMs) that fulfill the aforementioned criteria from a selected set of scales.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study design
We performed a retrospective cohort study of patients with ischemic stroke, TIA, intracerebral hemorrhage (ICH), or subarachnoid hemorrhage (SAH) seen in the ambulatory Cerebrovascular Clinic from February 17, 2015 to July 5, 2017. As described previously,12 cerebrovascular patients routinely completed PROMs using the Knowledge Program data collection system13 either on electronic tablets at the time of their ambulatory visit or through the electronic health record patient portal (MyChart, Epic Systems) before their appointment. Clinicians completed the National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) during each visit and recorded the date of the last cerebrovascular event.
Patient population
Patients were included in this analysis if they were 18 years old or older, were seen for an outpatient visit in the Cleveland Clinic Cerebrovascular Center, had a diagnosis of ischemic stroke, TIA, ICH, or SAH, and completed at least one sleep-related scale between February 17, 2015 and July 5, 2017. Diagnosis of stroke was identified either from provider documentation of ischemic stroke, ICH, SAH, or TIA in structured fields of the encounter note or a visit diagnosis of one of these cerebrovascular events as defined by the following 9th and 10th editions of International Classification of Diseases Clinical Modification codes: ischemic stroke – 433.x1, 434.x1, 433.10, 434.00, 436, I63.x; ICH – 431.xx, I61.xx; SAH – 430.xx, I60xx; TIA – 435.xx, G45. For patients completing sleep scales at more than one visit, the first scale score was used in the analysis.
Study variables
Patient-reported outcome measures
Several Patient-Reported Outcomes Measurement Information System (PROMIS) scales, including the sleep disturbance v1.0, fatigue v1.0, and PROMIS Global Health scales, were administered to patients or their proxy if not previously completed within the past 30 days. PROMIS tools measure patient status for different domains of health along a continuous scale in people with a wide range of diseases and symptoms,14 including ischemic stroke and ICH.15–17 Scores are standardized to the general US adult population on the T-scale with mean of 50 and standard deviation (SD) of 10. Higher scores indicate more of the symptom being assessed. A clinically meaningful difference is considered to be 5 or more points.18–20 The PROMIS sleep disturbance scale assesses sleep quality and depth, along with difficulties and satisfaction with sleep. The PROMIS fatigue scale assesses tiredness that decreases the capacity for physical, social, and mental activities. Both the sleep disturbance and fatigue scales were administered using computer adaptive testing, in which the most informative questions are algorithmically selected from an item bank of questions based on the patient’s prior responses. This improves score precision, while minimizing respondent burden and ceiling/floor effects. The PROMIS Global Health scale is composed of 10 global items that each represent a different domain of health and generates mental health and physical health summary scores.21,22 The International Consortium for Health Outcomes Measurement recommends it as an outcome measure for stroke.23
The Insomnia Severity Index (ISI) and the Sleep Apnea Probability Scale (SAPS) were administered at the clinic visit if they have not been previously completed in the past 2 years. Additionally, patients were asked to indicate number of hours they slept in a 24-hour period. The ISI is composed of seven items assessing perceived severity of difficulties initiating sleep, staying asleep, interference with daily functioning, and degree of distress caused by the sleep problems.24 Scores ≥ 15 suggest the presence of moderate-severe insomnia symptoms. The SAPs screening tool is a seven-item scale developed and validated in the stroke population,25 which consists of the four patient-reported yes/no questions derived from the STOP sleep apnea screen26 and the clinical variables: age, sex, body mass index. The SAPS tool estimates the probability that the patient has OSA along a continuous scale when defined as an apnea-hypopnea index ≥ 10 on polysomnography. It has superior performance compared to the STOP screen for identification of stroke patients who have OSA.26 A SAPS score ≥ 0.50 was considered a positive screen and had a sensitivity of 0.87 (95% confidence interval [CI] 0.80–0.93) and specificity of 0.63 (95% CI 0.52–0.73).
The widely used Patient Health Questionnaire-9 (PHQ-9) is a 9-item depression screen used for assessing depression severity in stroke patients within the Cleveland Clinic.27 The PHQ-9 score was cocalibrated to the PROMIS metric providing equivalent PROMIS Depression scores.28
Additional questions assessed whether patients had help completing the PROMs (proxy respondents) and, if so, whether patients could have completed the PROMs on their own.
Clinician-reported data
The NIHSS is the standard scale for measuring neurological impairment. It consists of 15 items with scores ranging from 0 to 42, with higher scores indicating greater impairment.29 The mRS is a one-item measure of global disability with scores ranging from 0 to 6 with 0 representing no symptoms and 6 representing death.30 To optimize the interobserver reliability of mRS in our practice, all providers underwent standardized training and have been certified in the completion of mRS.31 The date of the last cerebrovascular event, if known, was recorded by providers in structured fields. For patients with more than one cerebrovascular event, the date of the last event was used in this analysis.
Additional demographic and visit-based information in the dataset was obtained from the electronic health record, including visit date, race, age, marital status, sex, antidepressant medication use, diagnosis of depression, and ZIP code. Approximate household income was estimated using the ZIP code based on 2010 census data.
Statistical analysis
Baseline clinical characteristics for patients with relevant stroke diagnoses seen in the cerebrovascular clinic during the study period were summarized using descriptive statistics stratified by study inclusion and stroke type. Comparisons were made using two-sample t tests or analysis of variance for continuous variables and chi-square tests for categorical variables. When necessary, nonparametric alternatives were employed (eg, Wilcoxon, Kruskal-Wallis, Fisher exact tests). Pairwise comparisons between stroke types were made when the omnibus value of P ≤ .05. Prevalence of abnormal sleep-related scale scores were determined using the following thresholds: PROMIS sleep disturbance T-score ≥ 55, ISI ≥ 15, SAPs score ≥ 0.50, and sleep duration < 6 hours or > 9 hours. These rates were calculated for the entire cohort and stratified by time from stroke event (stroke < 90 days, 90 to 365 days, and > 365 days).
Correlations between sleep-related scales
Pairwise Spearman correlation coefficients were computed for each sleep scale in the full cohort as well as within each stroke type. We computed 95% CI for each Spearman correlation using the bootstrap bias-corrected and accelerated method. The coefficient of determination (R2), which is the square of the Spearman correlation coefficient, was calculated between the PROMIS sleep disturbance T-score and the other sleep scale scores. In this context, the R2 represents the proportion of variation in the respective sleep scale score rank explained by the PROMIS sleep disturbance T-score rank. Because the relationship between sleep duration and other sleep-related scales was not linear, we constructed boxplots of PROMIS sleep disturbance scores and ISI scores at short (< 6 hours), normal (6 to 9 hours), and long self-reported sleep duration to depict the association between these constructs.
Receiver operating characteristic analysis
We performed a receiver operating characteristic analysis where the outcome was abnormal ISI (≥ 15) and PROMIS Sleep Disturbance T-score was the predictor. The area under the curve along with its 95% CI was computed using the DeLong method. Sensitivity, specificity, positive (PPV) and negative predictive values (NPV) for the sensitivity/specificity pair that maximized Youden index (Sensitivity + Specificity – 1) was computed along with their respective 95% CIs. We also computed these accuracy metrics using the hypothesized cutoff of a PROMIS Sleep Disturbance T-score of 55, which represents the clinically meaningful difference from the general population norm. Because PPV and NPV depend on prevalence, we computed PPV and NPV using prevalence values both above and below the observed prevalence of ISI ≥ 15 in our sample. We also calculated the sensitivity and specificity of using both the PROMIS sleep disturbance score ≥ 55 and self-reported sleep duration < 6 hours to predict an ISI ≥ 15. The PPV and NPV of these combined criteria were also determined at different hypothetical prevalence rates.
All computations were done in R, version 3.4.1. All tests were two-sided and values of P < .05 were considered statistically significant. We adjusted for multiple testing using Holm method.32
This study was approved by the Cleveland Clinic Institutional Review Board. Because the dataset consisted of data collected as part of routine care, requirement for informed consent was waived.
RESULTS
Patient population
Between February 17, 2015 and July 5, 2017, a total of 4,149 patients 18 years or older with one of ischemic stroke, TIA, ICH, or SAH visited the Cerebrovascular Center. Of these, 2,213 patients completed at least one sleep-related scale at one or more visits and were thus included in the study. Median mRS score of the study cohort was 1, indicated mild disability. Cohort patients were older, more likely to be white, married, and lived in wealthier ZIP codes. They also had more time elapse since their stroke, and had better NIHSS and mRS scores (Table S1 in the supplemental material).
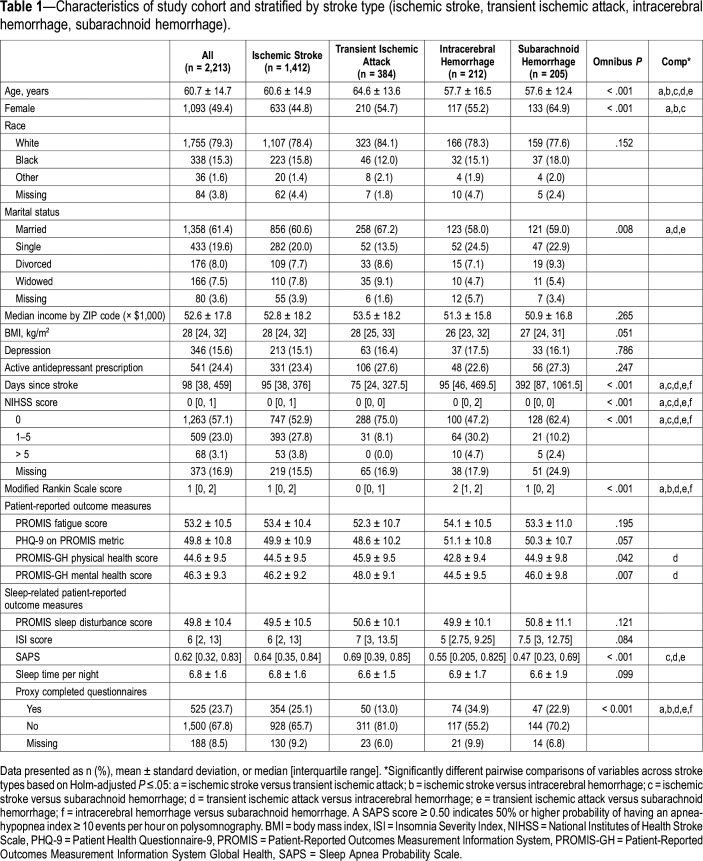
Baseline descriptive statistics of cohort patients stratified by cerebrovascular event type are displayed in Table 1. Statistically significant differences in the four cerebrovascular event types were observed for age, sex, marital status, time since stroke, NIHSS score, mRS score, and PROMIS mental and global health summary scores. Patients with TIA had the best self-reported mental and physical health and best clinician-reported disability of all cerebrovascular event types; patients with ICH had the worst. Patients with SAH were more likely to be female and had longer time since stroke compared to patients with other stroke types. Patients with TIA were less likely to have questionnaires completed by a proxy than the other stroke types; patients with ICH were more likely to have questionnaires completed by a proxy. There was no difference in average sleep-related scale scores, depression, antidepressant use, or self-reported fatigue across stroke types.
Table 1.
Characteristics of study cohort and stratified by stroke type (ischemic stroke, transient ischemic attack, intracerebral hemorrhage, subarachnoid hemorrhage).
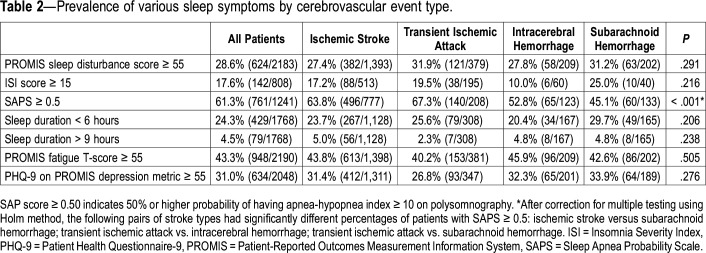
PROMIS sleep disturbance scale scores were meaningfully worse than the general population (≥ 55) in 28.6% patients (624/2183). Moderate-severe insomnia symptoms (ISI ≥ 15) was present in 17.6% (142/808) and a positive SAPs screen, defined as ≥ 0.50 probability that the patient has an AHI ≥ 10 on polysomnography, was present in 61.3% (761/1241). There were no differences in the prevalence of abnormal sleep-related PROMs across cerebrovascular event types except for a positive sleep apnea screen, which occurred less frequently in patients with ICH and SAH (Table 2). Notably, nearly one-quarter reported a habitual sleep duration of less than 6 hours.
Table 2.
Prevalence of various sleep symptoms by cerebrovascular event type.
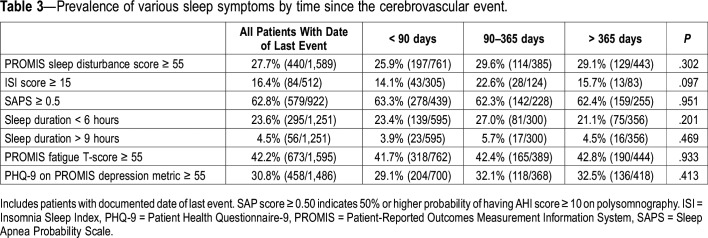
The prevalence of abnormal sleep-related scores among patients with a documented date of last event was very similar to the prevalence of the entire study cohort (data not shown). Among patients with available last-event date, there were no differences in prevalence of patients with abnormal sleep-related PROMs according to time since stroke (Table 3).
Table 3.
Prevalence of various sleep symptoms by time since the cerebrovascular event.
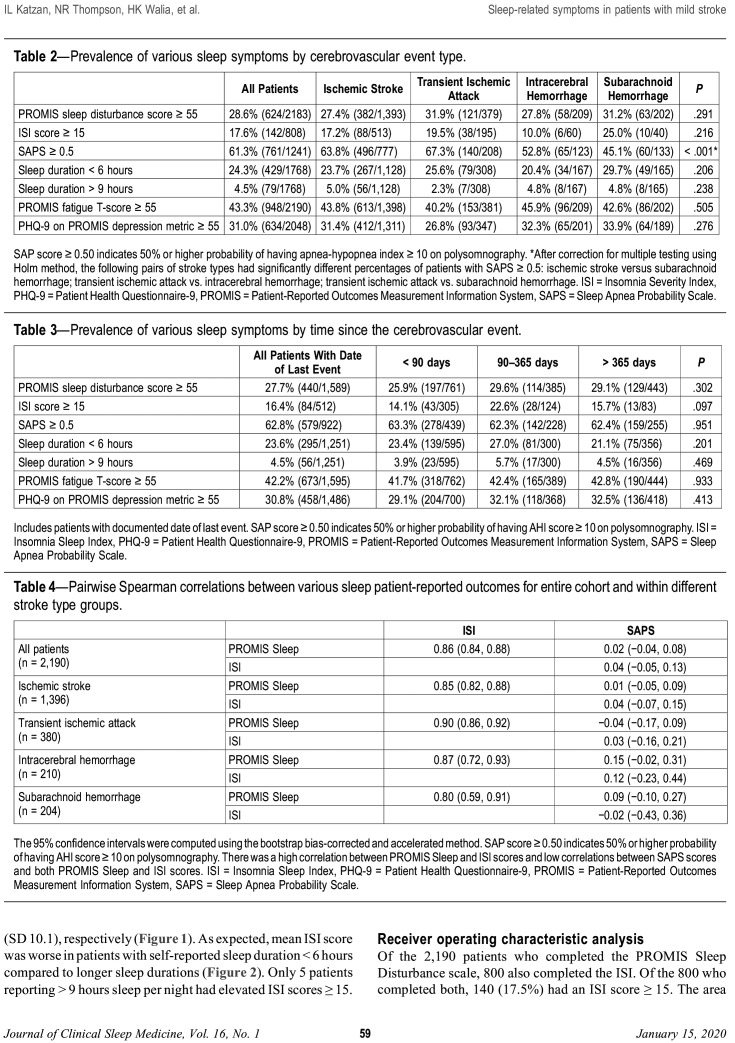
Correlations among sleep scales
Pairwise Spearman correlations between the various sleep PROMs are displayed in Table 4. Both in the full cohort and within each stroke type, the strongest Spearman correlation was between PROMIS Sleep Disturbance and ISI scores, ranging from 0.80 to 0.90. The direction of association between sleep scales was generally the same across stroke types, but the magnitude varied (Table 4). The coefficient of determination (R2), which indicates the amount of variation in the respective score rankings that can be explained by the PROMIS sleep disturbance score, was 0.4% for SAPs score and 74% for ISI score for all patients in the study cohort.
Table 4.
Pairwise Spearman correlations between various sleep patient-reported outcomes for entire cohort and within different stroke type groups.
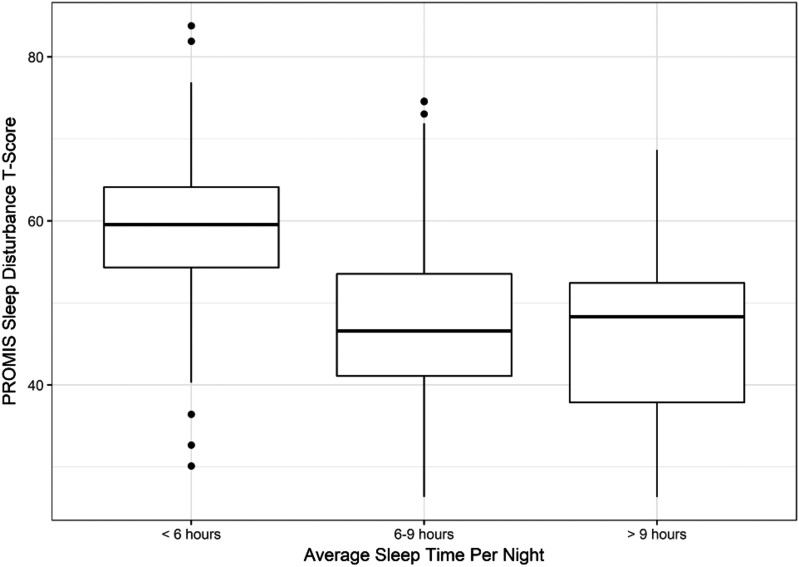
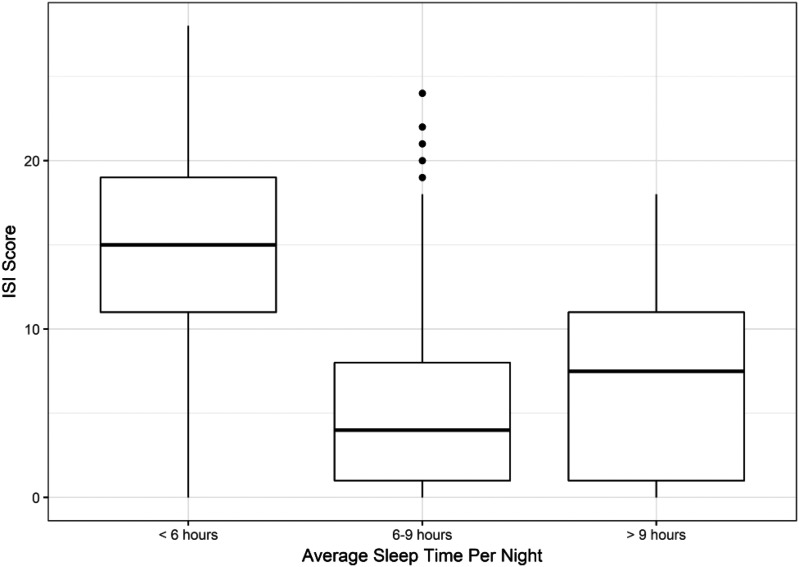
The mean PROMIS sleep disturbance score of patients reporting average sleep duration per night of < 6 hours was 59.5 (SD 8.4), which is meaningfully worse than the general US population. In contrast, mean PROMIS sleep disturbance T-scores of patients reporting 6 to 9 hours and > 9 hours was similar or better than general population; 46.9 (SD 8.8) and 45.9 (SD 10.1), respectively (Figure 1). As expected, mean ISI score was worse in patients with self-reported sleep duration < 6 hours compared to longer sleep durations (Figure 2). Only 5 patients reporting > 9 hours sleep per night had elevated ISI scores ≥ 15.
Figure 1. Severity of sleep disturbance according to sleep duration.
The thick bar represents the median Patient-Reported Outcomes Measurement Information System (PROMIS) sleep disturbance T-score within each category of sleep duration. The ends of the boxes represent the first and third quartiles. The vertical lines represent values that are 1.5 times the interquartile range from each quartile or the min/max if inside 1.5 interquartile range. The small circles represent outliers (more than 1.5 times the interquartile range from a quartile). T-score = 50 represents the mean score of the general population. Higher PROMIS T-scores indicate greater symptoms. T-score ≥ 55 is considered meaningful worse than the general population.
Figure 2. Severity of insomnia symptoms according to sleep duration.
The thick bar represents the median Insomnia Severity Index score within each category of sleep duration. The ends of the boxes represent the first and third quartiles. The vertical lines represent values that are 1.5 times the interquartile range from each quartile or the min/max if inside 1.5 interquartile range. The small circles represent outliers (more than 1.5 times the interquartile range from a quartile). Higher scores indicate greater symptoms. Scores ≥ 15 indicates moderate – severe insomnia symptoms.
Receiver operating characteristic analysis
Of the 2,190 patients who completed the PROMIS Sleep Disturbance scale, 800 also completed the ISI. Of the 800 who completed both, 140 (17.5%) had an ISI score ≥ 15. The area under the curve (95% CI) for PROMIS Sleep Disturbance score predicting ISI ≥ 15 was 0.928 (0.906–0.951).
Assuming sensitivity and specificity carry equal weight, the cutoff score for PROMIS Sleep Disturbance for Youden index was 54.75. The corresponding sensitivity, specificity, PPV, and NPV (95% CI) were 0.90 (0.85–0.95), 0.81 (0.78–0.84), 0.50 (0.46–0.55), and 0.97 (0.96–0.99), respectively.
Using the hypothesized cutoff PROMIS sleep disturbance score of 55, which is considered to represent a clinically meaningful difference from the general population norm, the corresponding values were quite similar; the sensitivity, specificity, PPV, and NPV (95% CI) were 0.89 (0.83–0.94), 0.81 (0.78–0.84), 0.50 (0.46–0.55), and 0.97 (0.96–0.98), respectively.
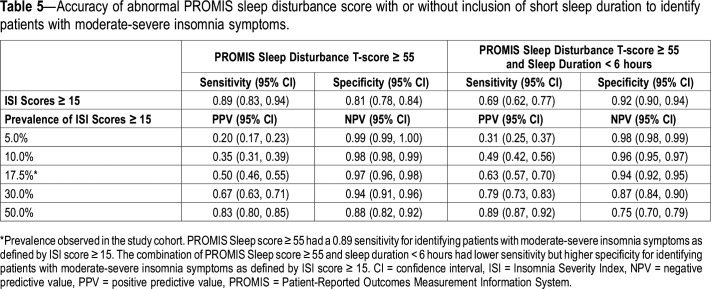
Because PPV and NPV are prevalence dependent, we examined how these values changed when we allowed the prevalence of ISI ≥ 15 to change from the observed value of 17.5% in our sample. We computed these values for prevalence of 5%, 10%, 30% and 50% (Table 5). For instance, using the PROMIS sleep disturbance cutoff score of 55 and assuming a prevalence of 5%, the PPV and NPV (95% CI) were 0.20 (0.17–0.23) and 0.99 (0.99–1.00), respectively. When prevalence was changed to 30%, analogous values were 0.67 (0.63–0.71) and 0.94 (0.91–0.96), respectively.
Table 5.
Accuracy of abnormal PROMIS sleep disturbance score with or without inclusion of short sleep duration to identify patients with moderate-severe insomnia symptoms.
We also calculated the accuracy of two criteria combined—PROMIS sleep disturbance score ≥ 55 and self-reported sleep duration < 6 hours—to identify patients with ISI ≥ 15. The sensitivity (0.69 [0.61–0.77]) was lower than with PROMIS sleep disturbance ≥ 55 alone, but there was a higher specificity (0.92 [0.90–0.94]). Corresponding values for NPV and PPV at different prevalence rates are shown in Table 5.
DISCUSSION
Sleep-related symptoms occurred frequently in patients with mild stroke. Clinically relevant sleep disturbance was present in more than one-fourth of patients. Moderate-severe insomnia symptoms was reported in 17.6% of patients, similar to the 12.6% rate reported in a prior study of patients who had acute ischemic stroke for whom the same definition was used.33 Over half of patients had a positive screening for OSA. These prevalence rates are consistent with what has been described in the literature and support the use of a systematic process to identify these disorders in patients with stroke.34,35
The prevalence of abnormal sleep-related scales was not appreciably different according to time from onset of last stroke event and was similar across cerebrovascular event types with one exception: patients with ICH and SAH were less likely to have positive SAPS screening. A lower rate of sleep apnea in patients with ICH compared to patients with ischemic stroke has been previously reported36 and these findings may be due to differences in rates of preexisting OSA compared with ischemic stroke or TIA. Overall, however, findings from this study suggest that a uniform method of screening for sleep-related conditions is reasonable.
Although we did not specifically evaluate for the presence of excessive daytime sleepiness, our data suggest that it is probably less common than insomnia or sleep apnea. Most patients who reported a degree of sleep disturbance worse than the general population had ISI score consistent with the presence of considerable insomnia symptoms. This is consistent with previous reports that patients with stroke have less daytime sleepiness than people without stroke, even among the subgroup with sleep apnea.37 In addition, relatively few patients in our cohort slept more than 9 hours per day and the severity of sleep disturbance in these patients was only slightly greater than in patients who reported sleeping 6 to 9 hours per night. Long sleep duration in stroke patients may reflect an increased sleep requirement during the recovery process, poststroke fatigue or motor impairment, or comorbid conditions including depression.
Our analyses inform the selection of the patient-reported sleep-related questions for use in clinical care. The PROMIS sleep disturbance scale provides an overall assessment of sleep symptoms and can also be used to screen for patients with clinically relevant insomnia symptoms. A PROMIS sleep disturbance score ≥ 55 has a sensitivity of 89% and a specificity of 81% for the presence of moderate-severe insomnia symptoms (ISI ≥ 15). With a 17% prevalence of ISI scores ≥ 15 as seen in our study, a PROMIS sleep disturbance score ≥ 55 would have failed to identify someone with moderate-severe insomnia symptoms in only 3% of patients (1 − NPV × 100). An elevated PROMIS sleep disturbance score could prompt a clinical exchange with the patient to determine whether he or she has insomnia. This approach would reduce patient questionnaire burden with minimal loss in the ability to detect moderate-severe insomnia. Alternatively, an algorithmic approach to the collection of sleep data could be implemented that entails completion of the ISI only if the sleep disturbance score is ≥ 55. This would improve specificity in the identification of moderate-severe insomnia using patient questionnaires with an increase in patient burden in only a subgroup of patients. The presence of abnormal PROMIS sleep but ISI < 15 should prompt exploration of other disorders such as hypersomnia, sleep-related movement disorders, or circadian rhythm disorders.
Sleep duration had modest correlations with PROMIS sleep and ISI and is insufficient alone as a screen for clinically relevant sleep disturbance. However, collection of self-reported sleep duration may aid in the interpretation of an elevated PROMIS sleep score and help distinguish insomnia from other sleep-related conditions. In our patient cohort, the combined use of PROMIS sleep disturbance and sleep duration increased the specificity for the identification of moderate-severe insomnia symptoms but with a loss of sensitivity. Clinical practices may choose to use information from both sleep duration and PROMIS sleep disturbance to identify patients with insomnia if they wish to optimize specificity while streamlining the patient data collection process. Apart from its utility in the assessment of sleep patterns and sleep-related conditions, sleep duration—like insomnia38 and OSA39—can help predict the risk of future stroke.40 An analysis of more than 150 publications including more than 15 million participants found strong associations between short sleep duration and stroke-related health outcomes including hypertension, diabetes, cardiovascular disease, coronary artery disease, obesity, and mortality, with measurable but less robust relationships with stroke.41 Proposed biological and behavioral mechanistic underpinnings for the relationship between short sleep duration and stroke include increased sympathetic nervous system activity producing increased blood pressure and heart rate, proinflammatory markers, and mental distress.42
Not surprisingly, SAPS score had minimal correlation with symptoms of sleep disturbance. Patients with stroke do not experience the same degree of symptoms typically seen in others with sleep apnea and the performance of symptom-based screening tools to identify stroke patients at high risk for sleep apnea on polysomnography is poor.43 Tools that combine clinical information with patient symptoms, such as the SAPS tool25 or the new SLEEP Inventory44 improve the ability to predict sleep apnea compared to symptoms alone.
This study has several limitations, which should be considered when interpreting its findings. Formal clinical assessment of sleep disturbance by sleep specialist and sleep studies were not performed and the accuracy of ISI or PROMIS sleep disturbance was not formally assessed. Scales for several specific sleep-related disorders, such as daytime sleepiness or sleep-related movement disorders were not collected. In addition, there are other scales that measure the same constructs measured in our study, such as the Pittsburgh Sleep Quality Index,45 which assesses overall sleep quality similar to the PROMIS sleep disturbance, or the Brief Insomnia Questionnaire,46 which like the ISI, assesses insomnia symptoms. Thus, we cannot make conclusions about the usefulness of other scales in comparison with, or in addition to, the sleep scales analyzed in our study. Finally, our cohort study consisted of a convenience sample of patients from a single tertiary care center with overall mild disability (median mRS score = 1). The proportion of proxy completions of questionnaires differed across stroke types. All of these factors may all have introduced selection bias. Although depression and antidepressant use were similar between patients in the study cohort and those who were excluded because they did not complete sleep-related questions, there were differences in several other characteristics. The generalizability of our findings to other stroke populations, especially those patients who have had more severe stroke is unclear. It should be noted, however, that many of the management strategies found to be effective for sleep disorders have been studied primarily in patients with nonsevere functional status.47
In summary, our analysis of sleep symptoms in an ambulatory cerebrovascular clinic confirm a high prevalence in patients with mild stroke. The prevalence of a positive screen for sleep apnea is lower in patients with ICH and SAH compared to ischemic stroke and TIA, but sleep symptoms are otherwise similar across stroke types. There is no appreciable difference according to time from stroke. A uniform method of screening for sleep-related conditions, therefore, is reasonable. We provide potential approaches to screening for sleep disturbance in patients with stroke that reduce the patient burden while providing information to guide next steps in the evaluation of these treatable conditions in stroke. This information will be valuable to other organizations implementing patient-reported data collection related to sleep disturbance in patients with stroke.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- CI
confidence interval
- ICH
intracerebral hemorrhage
- ISI
Insomnia Severity Index
- mRS
modified Rankin Scale
- NPV
negative predictive value
- NIHSS
National Institutes of Health Stroke Scale
- OSA
obstructive sleep apnea
- PHQ-9
Patient Health Questionnaire-9
- PPV
positive predictive value
- PROMIS
Patient-Reported Outcomes Measurement Information System
- PROMs
patient-reported outcome measures
- R2
coefficient of determination
- SAH
subarachnoid hemorrhage
- SAPS
sleep apnea probability scale
- SD
standard deviation
REFERENCES
- 1.Leppävuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T. Insomnia in ischemic stroke patients. Cerebrovasc Dis. 2002;14(2):90–97. doi: 10.1159/000064737. [DOI] [PubMed] [Google Scholar]
- 2.Tang WK, Grace Lau C, Mok V, Ungvari GS, Wong KS. Insomnia and health-related quality of life in stroke. Top Stroke Rehabil. 2015;22(3):201–207. doi: 10.1179/1074935714Z.0000000026. [DOI] [PubMed] [Google Scholar]
- 3.Hermann DM, Bassetti CL. Sleep-disordered breathing and stroke. Curr Opin Neurol. 2003;16(1):87–90. doi: 10.1097/01.wco.0000053587.70044.be. [DOI] [PubMed] [Google Scholar]
- 4.Hermann DM, Bassetti CL. Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313–1322. doi: 10.1212/WNL.0b013e3181bd137c. [DOI] [PubMed] [Google Scholar]
- 5.Harris AL, Elder J, Schiff ND, Victor JD, Goldfine AM. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res. 2014;5(2):292–300. doi: 10.1007/s12975-013-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- 7.Aaronson JA, van Bennekom CA, Hofman WF, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38(9):1431–1437. doi: 10.5665/sleep.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26(3):293–297. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 9.Birkbak J, Clark AJ, Rod NH. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med. 2014;10(1):103–108. doi: 10.5664/jcsm.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baglioni C, Nissen C, Schweinoch A, et al. Polysomnographic characteristics of sleep in stroke: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0148496. doi: 10.1371/journal.pone.0148496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mims KN, Kirsch D. Sleep and stroke. Sleep Med Clin. 2016;11(1):39–51. doi: 10.1016/j.jsmc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Katzan IL, Thompson NR, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018;90(16):e1364–e1371. doi: 10.1212/WNL.0000000000005327. [DOI] [PubMed] [Google Scholar]
- 13.Katzan I, Speck M, Dopler C, et al. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011;2011:683–692. [PMC free article] [PubMed] [Google Scholar]
- 14. PROMIS Network. PROMIS - Dynamic Tools to Measure Health Outcomes from the Patient Perspective. www.nihpromis.org. Accessed June 29, 2018.
- 15.Katzan IL, Fan Y, Uchino K, Griffith SD. The PROMIS physical function scale: a promising scale for use in patients with ischemic stroke. Neurology. 2016;86(19):1801–1807. doi: 10.1212/WNL.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 16.Katzan IL, Schuster A, Newey C, Uchino K, Lapin B. Patient-reported outcomes across cerebrovascular event types: More similar than different. Neurology. 2018;91(23):e2182–e2191. doi: 10.1212/WNL.0000000000006626. [DOI] [PubMed] [Google Scholar]
- 17.Sangha RS, Caprio FZ, Askew R, et al. Quality of life in patients with TIA and minor ischemic stroke. Neurology. 2015;85(22):1957–1963. doi: 10.1212/WNL.0000000000002164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapin B, Thompson NR, Schuster A, Katzan IL. Clinical utility of patient-reported outcome measurement information system domain scales. Circ Cardiovasc Qual Outcomes. 2019;12(1):e004753. doi: 10.1161/CIRCOUTCOMES.118.004753. [DOI] [PubMed] [Google Scholar]
- 19.Yost KJ, Eton DT, Garcia SF, Cella D. Minimally important differences were estimated for six Patient-Reported Outcomes Measurement Information System-Cancer scales in advanced-stage cancer patients. J Clin Epidemiol. 2011;64(5):507–516. doi: 10.1016/j.jclinepi.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. The truly remarkable universality of half a standard deviation: confirmation through another look. Expert Rev Pharmacoecon Outcomes Res. 2004;4(5):581–585. doi: 10.1586/14737167.4.5.581. [DOI] [PubMed] [Google Scholar]
- 21.Katzan IL, Lapin B. PROMIS GH (Patient-Reported Outcomes Measurement Information Global Health) scale in stroke - a validation study. Stroke. 2018;49(1):147–154. doi: 10.1161/STROKEAHA.117.018766. [DOI] [PubMed] [Google Scholar]
- 22.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–880. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47(1):180–186. doi: 10.1161/STROKEAHA.115.010898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 25.Katzan IL, Thompson NR, Uchino K, Foldvary-Schaefer N. A screening tool for obstructive sleep apnea in cerebrovascular patients. Sleep Med. 2016;21:70–76. doi: 10.1016/j.sleep.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI-II, CES-D, and PHQ-9 to PROMIS depression. Psychol Assess. 2014;26(2):513–527. doi: 10.1037/a0035768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 30.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 31.Quinn TJ, Lees KR, Hardemark HG, Dawson J, Walters MR. Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials. Stroke. 2007;38(8):2257–2261. doi: 10.1161/STROKEAHA.106.480723. [DOI] [PubMed] [Google Scholar]
- 32.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 33.Kim KT, Moon HJ, Yang JG, et al. The prevalence and clinical significance of sleep disorders in acute ischemic stroke patients-a questionnaire study. Sleep Breath. 2017;21(3):759–765. doi: 10.1007/s11325-016-1454-5. [DOI] [PubMed] [Google Scholar]
- 34.Hermann DM, Bassetti CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. 2016;87(13):1407–1416. doi: 10.1212/WNL.0000000000003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duss SB, Brill AK, Bargiotas P, et al. Sleep-wake disorders in stroke-increased stroke risk and deteriorated recovery? an evaluation on the necessity for prevention and treatment. Curr Neurol Neurosci Rep. 2018;18(10):72. doi: 10.1007/s11910-018-0879-6. [DOI] [PubMed] [Google Scholar]
- 36.Szücs A, Vitrai J, Janszky J, et al. Pathological sleep apnoea frequency remains permanent in ischaemic stroke and it is transient in haemorrhagic stroke. Eur Neurol. 2002;47(1):15–19. doi: 10.1159/000047941. [DOI] [PubMed] [Google Scholar]
- 37.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41(3):e129–e134. doi: 10.1161/STROKEAHA.109.566463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chien KL, Chen PC, Hsu HC, et al. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33(2):177–184. doi: 10.1093/sleep/33.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 40.Leng Y, Cappuccio FP, Wainwright NW, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84(11):1072–1079. doi: 10.1212/WNL.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Wang D, Cao S, et al. Sleep duration and risk of stroke events and stroke mortality: A systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;223:870–876. doi: 10.1016/j.ijcard.2016.08.302. [DOI] [PubMed] [Google Scholar]
- 43.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 44.Sico JJ, Yaggi HK, Ofner S, et al. Development, validation, and assessment of an ischemic stroke or transient ischemic attack-specific prediction tool for obstructive sleep apnea. J Stroke Cerebrovasc Dis. 2017;26(8):1745–1754. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 46.Kessler RC, Coulouvrat C, Hajak G, et al. Reliability and validity of the brief insomnia questionnaire in the America insomnia survey. Sleep. 2010;33(11):1539–1549. [PMC free article] [PubMed] [Google Scholar]
- 47.Sterr A, Kuhn M, Nissen C, et al. Post-stroke insomnia in community-dwelling patients with chronic motor stroke: Physiological evidence and implications for stroke care. Sci Rep. 2018;8(1):8409. doi: 10.1038/s41598-018-26630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.