Abstract
Study Objectives:
To examine the effect of untreated obstructive sleep apnea (OSA) on health care utilization (HCU) and costs among a nationally representative sample of Medicare beneficiaries.
Methods:
Our data source was a random 5% sample of Medicare administrative claims data for years 2006–2013. OSA was operationalized as (1) receipt of one or more International Classification of Disease, Version 9, Clinical Modification diagnostic codes for OSA in combination with (2) initiation of OSA treatment with either continuous positive airway pressure or oral appliance (OA) therapy. First, HCU and costs were assessed during the 12 months prior to treatment initiation. Next, these HCU and costs were compared between beneficiaries with OSA and matched control patients without sleep-disordered breathing using generalized linear models.
Results:
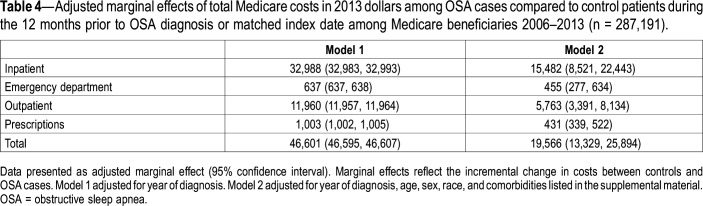
The final sample (n = 287,191) included 10,317 beneficiaries with OSA and 276,874 control patients. In fully adjusted models, during the year prior to OSA diagnosis and relative to matched control patients, beneficiaries with OSA demonstrated increased HCU and higher mean total annual costs ($19,566, 95% confidence interval [CI] $13,239, $25,894) as well as higher mean annual costs across all individual points of service. Inpatient care was associated with the highest incremental costs (ie, greater than control patients; $15,482, 95% CI $8,521, $22,443) and prescriptions were associated with the lowest incremental costs (ie, greater than control patients; $431, 95% CI $339, $522).
Conclusions:
In this randomly selected and nationally representative sample of Medicare beneficiaries and relative to matched control patients, individuals with untreated OSA demonstrated increased HCU and costs across all points of service.
Citation:
Wickwire EM, Tom SE, Vadlamani A, et al. Older adult US Medicare beneficiaries with untreated obstructive sleep apnea are heavier users of health care than matched control patients. J Clin Sleep Med. 2020;16(1):81–89.
Keywords: costs, health care utilization, health economics, Medicare, obstructive sleep apnea, older adults, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is associated with significant economic burden to patients, payers, and society. But most research has been conducted among middle-aged adults, and little is known about the economic effect of OSA among older adults.
Study Impact: Current results are the first to demonstrate the population-level economic effect of OSA among older adults in the US. Relative to a well-characterized control group, older adult Medicare beneficiaries with OSA demonstrated increased health care utilization and higher mean total annual costs across all points of service. Inpatient care was associated with the highest incremental costs. These results demonstrate the substantial costs associated with OSA. Examination of the economic effect and potential economic gain from treating OSA treatments specifically among older adults warrants much greater research attention.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common and costly chronic medical condition with substantial adverse health consequences including increased risk for cardiovascular disease (CVD),1–3 stroke,4,5 metabolic syndromes and type 2 diabetes,6–8 depression,9 reduced quality of life,10 and premature death,11,12 as well as adverse economic outcomes including increased health care utilization (HCU) and costs.13–15 In part due to changes in patency of the upper airway, the prevalence of OSA increases with age and is highest among older adults.16 Among adults aged 30 to 70 years, the prevalence of moderate to severe OSA is approximately 14% among men and 5% among women.17 Further, OSA affects up to 70% of elderly nursing home residents, and these individuals demonstrate increased mortality risk and a greater tendency to die during sleep.18
In addition to these adverse health outcomes, evidence suggests that untreated OSA is associated with increased HCU and costs among older adults. For example, Tarasiuk and colleagues conducted a clinic-based, case control study using linked clinical and claims data.19 During the 2 years prior to OSA diagnosis, elderly patients with OSA (n = 158) demonstrated costs 1.8 times higher than did matched control patients without OSA (n = 158).19 Similarly, Diaz and colleagues performed a retrospective review of inpatient and outpatient Veterans Health Administration data and found that elderly veterans with newly diagnosed OSA (n = 31,287) experienced significantly more emergency department (ED) visits and hospitalizations than did either patients with chronic OSA (n = 50,891) or no OSA (n = 1,785,698).20 Becerra and colleagues21 examined the effect of OSA and obesity on asthma-related hospitalizations in the US Nationwide Inpatient Sample. Among both men and women older than 65 years, OSA was associated with increased asthma-related hospitalization costs. In terms of comorbid OSA, Tuohy and colleagues found that among Medicare beneficiaries older than 67 years and initiating dialysis, untreated OSA was associated with increased nonnephrology outpatient encounters over 1.6 years.22
At the same time, not all studies have found increased HCU among all older adult groups with OSA. Kao and colleagues performed an administrative review in Taiwan and found that among individuals ages 60 to 69 years and relative to matched control patients without OSA (n = 430), patients with OSA (n = 86) demonstrated 1.76× higher total costs as well as increased HCU across all points of service.23 However, no differences in costs or HCU were observed between a smaller sample of older individuals ages 70 to 79 years (n = 33) and matched control patients without OSA (n = 165).23 Similarly, Tarasiuk and colleagues24 found the effect of OSA on HCU to be attenuated among individuals older than 65 years, with few differences between patients with OSA and matched control patients without OSA observed among this age group.
Most research regarding OSA costs has been conducted on middle-aged adults. However, in light of the rapidly aging US populace,25 understanding the adverse economic effect of OSA among older adults is particularly important to payers, policymakers, and health systems leaders charged with managing population health for the future. In addition, from a US perspective, no study of which we are aware has examined the population-level economic effect of OSA among older adults. Thus, the objective of the current project was to evaluate HCU and costs associated with untreated OSA among a randomly selected, nationally representative sample of Medicare beneficiaries in the United States. We hypothesized that relative to matched control patients without sleep-disordered breathing, beneficiaries with untreated OSA would demonstrate increased HCU and costs.
METHODS
Data source
Data for this study were obtained from a random 5% sample of Medicare administrative claims from the Centers for Medicare and Medicaid Services Chronic Conditions Warehouse for years 2006–2013. Participants were identified within a larger program of sleep research,26,27 including Medicare beneficiaries with sleep disorders (1) in whom any sleep disorder including OSA was diagnosed or (2) who filled a prescription for sleep-related medications, while also maintaining continuous enrollment in Medicare Parts A, B, and D, with no Part C, for 12 months prior to the index date (ie, first date of sleep disorder diagnosis or sleep-related medication fill) and 24 months postindex. These beneficiaries were then matched 1:4 on index date to a control group composed of beneficiaries without sleep disorder diagnoses, diagnostic procedures, or sleep-related medication fills during the entire study period, who also met the same continuous enrollment criteria.
Study design and population
Using the aforementioned dataset, we conducted a case-control study to compare HCU and costs during the 12 months prior to diagnosis between Medicare beneficiaries aged 65 years and older with OSA and control patients without sleep-disordered breathing. Cases were included in this study if they had an OSA diagnosis and at least one fill for a continuous positive airway pressure machine or oral appliance therapy.
OSA cases
OSA was defined by one or more inpatient or outpatient claims including International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes (780.51, 780.53, 780.57, 327.23). The index date was the first date of OSA diagnosis occurring after a 12-month ‘clean’ period where there was no OSA diagnosis.
Control patients
Control patients were identified based on the absence of sleep-related diagnosis (insomnias, non-OSA sleep-related breathing disorders, sleep-related movement disorders, parasomnias, central disorders of hypersomnolence, and other sleep disorders), treatment, or diagnostic procedure during the entire study period (2006–2013).
HCU and costs
HCU was defined as counts of claims over the 12-month period prior to OSA diagnosis and categorized by point of service (inpatient, outpatient, ED, and prescription claims). Mean annual costs were calculated overall and by point of service and reported in 2013 US dollars. In terms of OSA-specific costs, any coding for OSA (eg, provisional diagnosis required for polysomnography or home sleep apnea testing [HSAT]) would have triggered assignment of the index date for OSA cases. Because costs that occur on the index date are excluded from analysis, these and subsequent OSA-specific costs are not considered in the current study. We used the Consumer Price Index inflation calculator available through the US Department of Labor Statistics to convert costs to 2013 dollars.
Covariates
Information on beneficiary demographic characteristics was obtained from the claims files. The Chronic Conditions Warehouse contains information on 27 comorbid conditions, with an annual flag for each condition as well as the date of first diagnosis.28 We used the date of first diagnosis to determine whether a condition was present at the date of insomnia diagnosis (ie, index date). Other comorbidities of interest were identified by searching all claim types for relevant ICD-9-CM codes during the study period. Any diagnoses received during the year prior to insomnia diagnosis were assumed to be present at the index date. A comorbidity index based on the Deyo adaptation of the Charlson Comorbidity Index was calculated and included in subsequent analyses.29
Statistical analysis
Bivariate comparisons between OSA cases and healthy sleep control patients were assessed using Pearson chi-square for categorical variables and Student’s t tests for continuous variables. We calculated total unadjusted mean HCU and costs by point of service and present these with their standard deviation (SD).
To accommodate overdispersion of the count data, negative binomial models were used to compare HCU between OSA cases and control patients. All models were run separately for each point of service. Unadjusted models were only adjusted for year of diagnosis. Because of the large number of potential confounders, adjusted models were built using a backward selection procedure, keeping those variables significant at P < .001. We tested effect modification by sex by including an interaction term in each model, with a value of P < .05 indicative of possible effect modification. Mean costs were modeled using a generalized linear model with a gamma distribution and log link. We used a similar process to build unadjusted and adjusted final models.
Because our approach was based on several assumptions, we also conducted multiple sensitivity analyses to test robustness of results. First, we tested a less restrictive definition of OSA that did not require a positive airway pressure fill. Next, we created propensity scores (PS) by modeling case status as a function of baseline comorbidities and demographic characteristics using logistic regression and outputting the probabilities. We used these PS in two ways. First, we included them in our negative binomial and generalized linear models as covariates. Next, we created a 1:1 PS matched set using greedy matching with a caliper distance of 0.2 and reran all between-groups analyses.
All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina, United States) and Stata version 14 (StataCorp LP, College Station, Texas, United States). This study was approved by the University of Maryland, Baltimore Institutional Review Board (Project ID: HCR-HP-00072414-2).
RESULTS
Participants
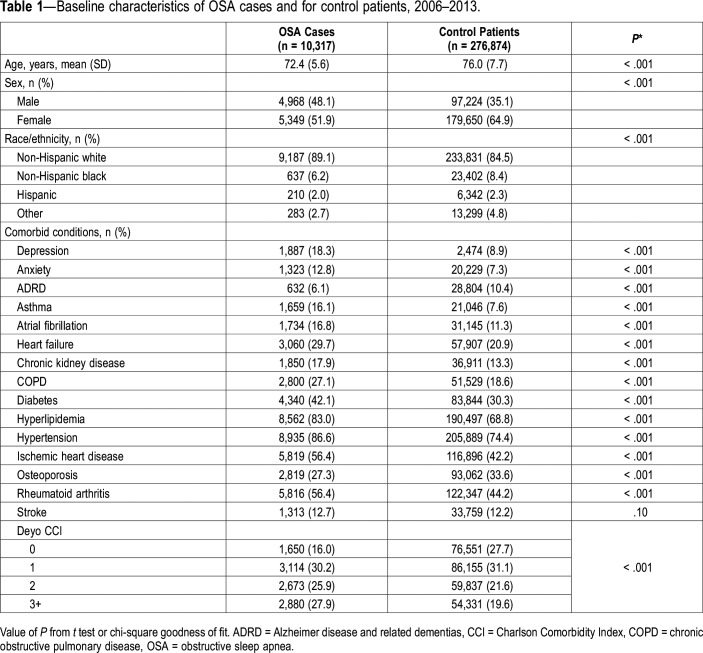
We identified 10,317 OSA cases and 276,874 control patients meeting eligibility criteria between 2006–2013, resulting in a total sample of 287,191 beneficiaries. Participants were predominantly female (64.4%) and non-Hispanic white (84.6%) (Table 1). The mean (SD) age of the sample was 75.9 (7.6) years with OSA cases being younger than control patients (72.4 [5.6] versus 76.0 [7.7] years, P < .001). Despite their younger age, beneficiaries with OSA had a higher comorbidity burden than control patients (almost all comorbidities significantly higher).
Table 1.
Baseline characteristics of OSA cases and for control patients, 2006–2013.
HCU and costs
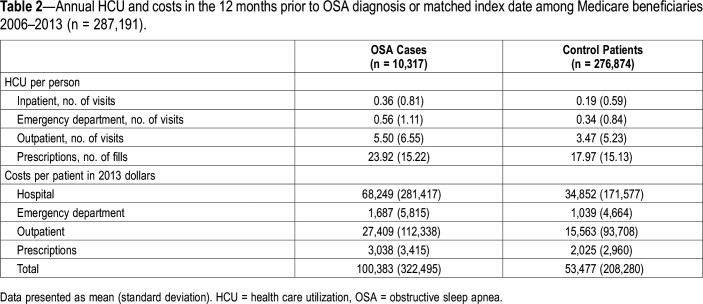
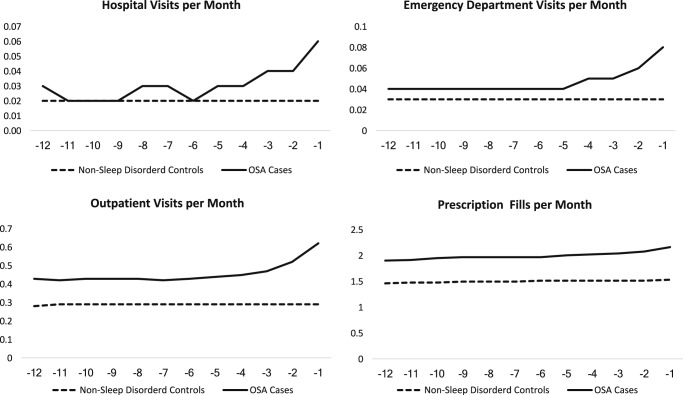
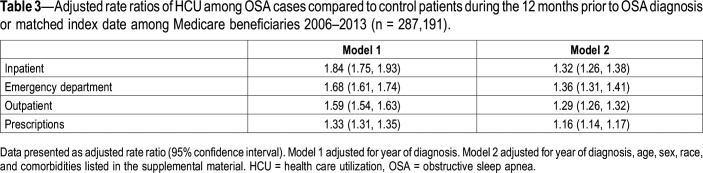
Mean annual HCU during the 12 months prior to OSA diagnosis was higher among beneficiaries with OSA compared to control patients across all points of service (Table 2), with HCU increasing during the 12 months prior to diagnosis (Figure 1). Counts for prescriptions were highest, whereas claims for inpatient HCU were lowest. In fully adjusted (ie, year of diagnosis, age, sex, race, and comorbidities listed in the supplemental material), negative binomial models and relative to control patients, HCU was higher among beneficiaries with OSA across all points of service (Table 3, model 2). No significant effect modification by sex was detected.
Table 2.
Annual HCU and costs in the 12 months prior to OSA diagnosis or matched index date among Medicare beneficiaries 2006–2013 (n = 287,191).
Figure 1. HCU by point-of-service during the year prior to OSA diagnosis or index date for control patients.
Relative to control patients, Medicare beneficiaries with OSA consume more health care resources across all points of service, including hospital visits, emergency department visits, outpatient visits, and prescription fills during the year prior to diagnosis. Visual inspection reveals that among beneficiaries in whom OSA was subsequently diagnosed, HCU increases in the months leading up to OSA diagnosis, with the steepest increases in hospital and ED visits, and the least increase in prescription fills. One possibility is that increases in HCU reflect seeking care for OSA-related symptoms or comorbidities. Conversely, for control patients demonstrate much lower HCU, with less variability, during a similar 12-month period, due to less engagement with the health system. HCU = health care utilization, OSA = obstructive sleep apnea.
Table 3.
Adjusted rate ratios of HCU among OSA cases compared to control patients during the 12 months prior to OSA diagnosis or matched index date among Medicare beneficiaries 2006–2013 (n = 287,191).
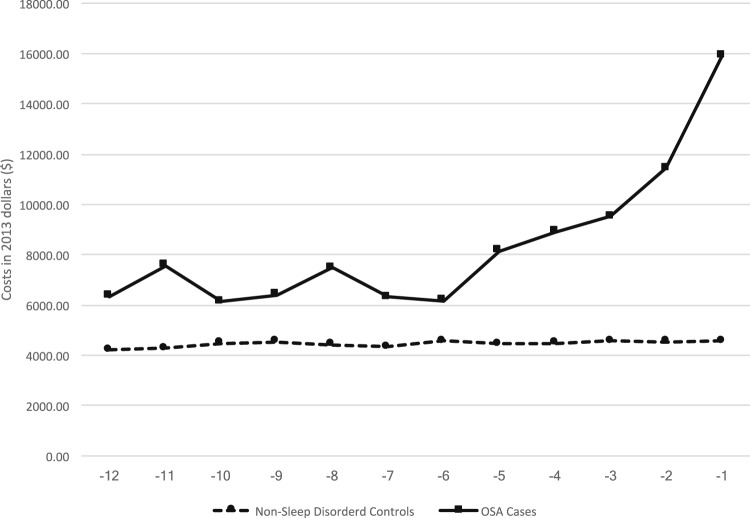
OSA cases demonstrated higher mean annual health care costs than did control patients, including total annual health care costs ($100,383 [SD $322,495] vs $53,477 [SD 208,280], respectively; Table 2), with costs increasing during the 12 months prior to diagnosis (Figure 2). In fully-adjusted (ie, year of diagnosis, age, sex, race, and comorbidities listed in the supplemental material) models and relative to control patients, beneficiaries with OSA demonstrated higher total annual costs ($19,566, 95% CI $13,239, $25,894; Table 4, model 2). Inpatient care was associated with the highest difference in costs between cases and controls ($15,482, 95% CI $8,521, $22,443) and prescriptions were associated with the lowest difference in costs between cases and control patients ($431, 95% CI $339, $522). No effect modification by sex was detected.
Figure 2. Total mean monthly costs during year prior to OSA diagnosis or index date for control patients.
Total mean monthly costs for Medicare beneficiaries with OSA increase during the year prior to OSA diagnosis. Dramatic increases occur within 3 months prior to diagnosis and might reflect seeking care for OSA-related symptoms or comorbidities. During the same period, total mean monthly costs for control patients do not increase, reflecting less engagement with the health care system. OSA = obstructive sleep apnea.
Table 4.
Adjusted marginal effects of total Medicare costs in 2013 dollars among OSA cases compared to control patients during the 12 months prior to OSA diagnosis or matched index date among Medicare beneficiaries 2006–2013 (n = 287,191).
In sensitivity analyses, a less specific definition of OSA (ie, requiring an OSA diagnosis but not a positive airway pressure charge requirement) resulted in higher HCU and much higher costs across all categories (eg, greater total incremental costs in control patients of $32,258 vs. $19,566 among those with a positive airway pressure fill, data not shown). Including PS (ie, based on baseline comorbidities and demographic characteristics such as age and sex) in the models as a covariate did not change effect estimates from fully adjusted models. Finally, the PS matching process resulted in 10,317 cases and 10,317 matched control patients, but ultimately did not result in significant changes to effect estimates.
DISCUSSION
The current findings reflect the largest analysis to date of the economic burden of untreated OSA among a randomly selected, national sample of older adult Medicare beneficiaries. Understanding the economic burden of OSA among older adults has been recognized as an understudied and high priority area for research. Overall, results demonstrate both the high burden of comorbid OSA as well as significantly increased HCU and costs associated with the disorder. Relative to matched control patients, older adults with OSA demonstrated higher levels of all medical and psychiatric comorbidities studied, excepting Alzheimer disease and related dementias. Further, relative to matched control patients, beneficiaries with untreated OSA demonstrated greater HCU and costs across all points of service, including inpatient, outpatient, ED, and prescription medications during the 12 months prior to OSA diagnosis. These results are generally consistent with and add a population health perspective to previous literature,19–21 thus highlighting the clinical and economic importance of OSA specifically among older adults. The preponderance of evidence from prior research conducted among mostly middle-aged adults demonstrates that OSA is associated with increased HCU and approximately double the costs.15 However, two of six previous studies23,24 conducted among older adults found no differences in HCU or costs between those with and without OSA. In this vein the current, large national study builds on prior research and supports dramatically elevated costs among older adults with OSA. As health care shifts from volume to value,30 economic aspects of disease will become increasingly important to patients, payers, policymakers, and the public at large.
Older adults represent a particularly important vulnerable population for OSA surveillance and treatment. In the current study, the older adult population is the greatest novel contribution to the literature, as OSA remains largely undiagnosed and untreated in the elderly. For example, in a survey of community-dwelling Medicare beneficiaries age 65 years and older as part of the National Health and Aging Trends Study survey, 56% of participants were estimated to be at high risk of OSA based on self-reported symptoms, but only 8% had undergone diagnostic assessment for OSA. Interestingly, 94% of those tested received a subsequent diagnosis of OSA.31 At the same time, OSA is increasingly recognized as an important comorbidity among the elderly, with a significant increase in sleep testing among Medicare beneficiaries between 2000 to 2014.32 In addition to well-documented health consequences of OSA, current results demonstrate the adverse economic outcomes associated with OSA among older adults. These results are that in terms of cost containment, payers, policymakers, and health systems leaders should consider routine screening for OSA among their older adult patient populations, and especially older adult patients with medical and psychiatric comorbidity.
An unexpected finding in the current analysis is that more women than men were included in our OSA sample. This finding might simply because of the age of the OSA group, and life expectancy in the United States. Or, based on our operational definition of OSA (new diagnosis and treatment initiation), another possible explanation for this result could be age-related trajectories of OSA. It is well documented that through middle age, the prevalence of OSA is higher among men than among women,33 perhaps due to differences in clinical manifestation that make OSA among women less likely to be diagnosed.34,35 However, after menopause the prevalence of OSA increases among women, such that the prevalence of OSA among older adult men and women is approximately equal, likely because of changes in upper airway patency among women.33 Thus, it is possible that OSA in older women is more likely to be newly diagnosed, whereas men are more likely to have preexisting OSA (which would have resulted in exclusion from this study). Regardless of the mechanism underlying the number of men and women who participated in this study, the association between OSA and HCU and costs was not modified by sex.
Results from this study suggest important directions for future research. Most importantly, researchers should seek to determine the mechanisms by which untreated OSA so dramatically increases HCU and costs among older adults. Given the high rates of comorbidity associated with OSA and observed in this study, we propose at least two methodologic approaches will be helpful to identify OSA-specific costs. First, researchers should examine the clinical and economic effect of OSA among subsets of patients with high-cost comorbidities, such as CVD, chronic pain, depression, and type II diabetes mellitus.36 This will help reduce confounding and reduce methodological variance.
Second, given the highly heterogeneous nature of OSA itself, future research should seek to identify empirically derived clusters of patients with OSA based on demographic, comorbid disease, daytime symptoms, medication adherence, and cost profiles, among other factors.37,38 Such phenotyping efforts are likely to lead to advanced understanding of OSA disease processes, clarify OSA clinical and economic trajectories, stratify patients with OSA into meaningful clusters based on disease and economic risk, and identify patients likely to benefit from aggressive treatment efforts. Related to understanding OSA costs and comorbidities, greater insight is needed into triggers and clinical pathways that lead to OSA diagnosis.39 For example, consistent with earlier literature, we found that HCU and costs increased during the 12 months prior to OSA diagnosis.39,40 It will be important to understand the effect of undiagnosed OSA on the medical conditions that led to contact with the health system in the 12 months prior to diagnosis. Related to this, although all participants in the current study were insured Medicare beneficiaries, the role of health-seeking behavior and provider referrals warrants research attention. Finally, there is dramatic need to examine economic aspects of OSA following diagnosis, including the potential economic benefit from OSA treatments among older adults. Wickwire and colleagues41 recently performed a systematic review of peer-reviewed literature regarding economic effect of OSA treatments, yet only 2 of 17 included studies42,43 were conducted among older adults.
This study has strengths. First, our results represent the largest analysis to date of the economic burden associated with untreated OSA among Medicare beneficiaries. Second, in addition to being large, our sample was randomly selected from a nationally representative sample of actual Medicare administrative claims. Medicare beneficiaries represent a largely affected population as Medicare is the largest health payer for older adults in the United States, and a leading developer of health policy. Third, we employed a highly specific operational definition of OSA, requiring not only a physician-assigned OSA diagnosis but also initiation of a Medicare-approved first-line OSA therapy (continuous positive airway pressure or oral appliance). Fourth, we captured a very broad range of health expenditures across multiple points of service, including HCU and costs associated with outpatient, inpatient, ED, and medication prescriptions, thus ensuring comprehensive assessment of costs from the payer perspective.
At the same time, our administrative approach has limitations. First, although our operational definition of OSA was highly specific, requiring confirmed OSA and initiation of treatment, OSA is underdiagnosed among older adults. Thus, it is possible that our control group contained undiagnosed OSA cases. Of course, this confound would have biased results toward the null, suggesting that the true difference in HCU and costs is likely higher than reported here. Interestingly, sensitivity analyses suggested higher HCU and costs when OSA cases who did not initiate treatment were included. Second, ICD-9-CM codes and administrative claims data do not provide needed insight into OSA disease severity, sleep characteristics, daytime symptoms, or other important patient-centered, clinical information such as body mass index that is related both to OSA as well as many comorbid conditions. Third, HSAT (“portable monitoring”) was approved during the study period, via Centers for Medicare and Medicaid Services National Coverage Determination (NCD) 240.4 that was implemented on August 1, 2008. Prior to NCD 240.4, all OSA diagnostic testing was performed via more expensive in-laboratory polysomnography. In the current study, sleep apnea diagnostic testing costs are reflected in outpatient costs and total costs, but OSA testing costs do not affect ED, inpatient, or prescription costs. Although not assessed as part of the current study, differences in outpatient costs and total costs between beneficiaries with OSA and control patients were presumably greater prior to NCD 240.4. Even so, OSA cases demonstrated greater outpatient and total HCU and costs during the overall study period, which includes the implementation of HSAT. Fourth, our administrative design precludes determination of causality. Specifically, we are unable to determine whether OSA leads to costly comorbid conditions, or whether conditions such as CVD, type 2 diabetes mellitus, and others lead to OSA, which then increases costs. Fifth, OSA cases differed significantly from control patients and were more likely to experience almost all comorbid conditions studied. To minimize the effects of this potential confounding, we controlled for all comorbid conditions and employed PS matching to optimize exchangeability between OSA cases and control patients in sensitivity analyses. Even so, despite our best efforts, residual confounding might have been present. Sixth and finally, although our administrative review enabled a comprehensive assessment of HCU and costs from the payer perspective, we were unable to assess economic outcomes from the patient, employer, or societal perspectives. Understanding the economic effect of OSA from the perspective of these varied stakeholders is vitally needed.
In conclusion, the current study represents the largest analysis to date of the economic burden associated with untreated OSA among Medicare beneficiaries in the United States. Relative to control patients without sleep-disordered breathing, beneficiaries with untreated OSA demonstrated markedly increased HCU and costs across all points of service, including outpatient encounters, inpatient stays, ED visits, and prescription medications. Future research should seek to understand the effect of comorbid OSA as well as evaluate the economic effect of OSA treatments among older adults.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. This research was supported by an investigator-initiated grant awarded from ResMed to The University of Maryland, Baltimore (PI: EMW). EMW, JSA, and SMS’s institution has received research funding from the AASM Foundation, Department of Defense, Merck, and ResMed. EMW has served as a scientific consultant to DayZz, Eisai, Merck, and Purdue and is an equity shareholder in WellTap. SET is supported by National Institute on Aging grant 1K01AG050723. AV is supported by National Institutes of Health grant T32AG000262. LMC is a full-time employee and equity shareholder in ResMed. JSA is supported by Agency for Healthcare Research and Quality grant K01HS024560. The other authors report no conflicts of interest.
ABBREVIATIONS
- ANOVA
analysis of variance
- CI
confidence interval
- CVD
cardiovascular disease
- ED
emergency department
- GBP
Great Britain pounds
- HCPCS
Healthcare Common Procedure Coding System
- HCU
health care utilization
- HSAT
home sleep apnea testing
- ICD-9-CM
International Classification of Disease, Version 9, Clinical Modification
- NCD
National Coverage Determination
- OSA
obstructive sleep apnea
- RR
rate ratio
- SD
Standard deviation
REFERENCES
- 1.Konecny T, Kuniyoshi FHS, Orban M, et al. Under-diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol. 2010;56(9):742–743. doi: 10.1016/j.jacc.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 5.Chan W, Coutts SB, Hanly P. Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke. 2010;41(12):2973–2975. doi: 10.1161/STROKEAHA.110.596759. [DOI] [PubMed] [Google Scholar]
- 6.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 7.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5(8):e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 10.Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21(7):701–706. [PubMed] [Google Scholar]
- 11.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kryger MH, Roos L, Delaive K, Walld R, Horrocks J. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19(9 Suppl):S111–S116. doi: 10.1093/sleep/19.suppl_9.s111. [DOI] [PubMed] [Google Scholar]
- 14.Banno K, Ramsey C, Walld R, Kryger MH. Expenditure on health care in obese women with and without sleep apnea. Sleep. 2009;32(2):247–252. doi: 10.1093/sleep/32.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost & Sullivan; American Academy of Sleep Medicine Hidden Health Crisis Costing America Billions. https://aasm.org/advocacy/initiatives/economic-impact-obstructive-sleep-apnea/. Published August 2016. Accessed November 21, 2019.
- 16.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 17.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Klauber MR, Kripke DF, Parker L, Cobarrubias M. Sleep apnea in female patients in a nursing home. Increased risk of mortality. Chest. 1989;96(5):1054–1058. doi: 10.1378/chest.96.5.1054. [DOI] [PubMed] [Google Scholar]
- 19.Tarasiuk A, Greenberg-Dotan S, Simon-Tuval T, Oksenberg A, Reuveni H. The effect of obstructive sleep apnea on morbidity and health care utilization of middle-aged and older adults. J Am Geriatr Soc. 2008;56(2):247–254. doi: 10.1111/j.1532-5415.2007.01544.x. [DOI] [PubMed] [Google Scholar]
- 20.Diaz K, Faverio P, Hospenthal A, Restrepo MI, Amuan ME, Pugh MJ. Obstructive sleep apnea is associated with higher healthcare utilization in elderly patients. Ann Thorac Med. 2014;9(2):92–98. doi: 10.4103/1817-1737.128854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becerra MB, Becerra BJ, Teodorescu M. Healthcare burden of obstructive sleep apnea and obesity among asthma hospitalizations: results from the U.S.-based Nationwide Inpatient Sample. Respir Med. 2016;117:230–236. doi: 10.1016/j.rmed.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Tuohy CV, Montez-Rath ME, Turakhia M, Chang TI, Winkelman JW, Winkelmayer WC. Sleep disordered breathing and cardiovascular risk in older patients initiating dialysis in the United States: a retrospective observational study using medicare data. BMC Nephrol. 2016;17(1):16. doi: 10.1186/s12882-016-0229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kao LT, Lee HC, Lin HC, Tsai MC, Chung SD. Healthcare service utilization by patients with obstructive sleep apnea: a population-based study. PLoS One. 2015;10(9):e0137459. doi: 10.1371/journal.pone.0137459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarasiuk A, Greenberg-Dotan S, Brin YS, Simon T, Tal A, Reuveni H. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128(3):1310–1314. doi: 10.1378/chest.128.3.1310. [DOI] [PubMed] [Google Scholar]
- 25.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. https://www.census.gov/library/publications/2014/demo/p25-1140.html. Published May 2014. Accessed November 21, 2019.
- 26.Wickwire EM, Tom SE, Scharf SM, Vadlamani A, Bulatao IG, Albrecht JS. Untreated insomnia increases all-cause health care utilization and costs among Medicare beneficiaries. Sleep. 2019;42(4) doi: 10.1093/sleep/zsz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrecht JS, Wickwire EM, Vadlamani A, Scharf SM, Tom SE. Trends in insomnia diagnosis and treatment among Medicare beneficiaries, 2006-2013. Am J Geriatr Psychiatry. 2019;27(3):301–309. doi: 10.1016/j.jagp.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finan PH, Buenaver LF, Bounds SC. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65(2):363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.Wickwire EM, Verma T. Value and payment in sleep medicine. J Clin Sleep Med. 2018;14(5):881–884. doi: 10.5664/jcsm.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braley TJ, Dunietz GL, Chervin RD, Lisabeth LD, Skolarus LE, Burke JF. Recognition and diagnosis of obstructive sleep apnea in older Americans. J Am Geriatr Soc. 2018;66(7):1296–1302. doi: 10.1111/jgs.15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiao W, Durr ML. Trends in sleep studies performed for Medicare beneficiaries. Laryngoscope. 2017;127(12):2891–2896. doi: 10.1002/lary.26736. [DOI] [PubMed] [Google Scholar]
- 33.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28(3):309–314. [PubMed] [Google Scholar]
- 35.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383–389. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 36.Wickwire EM. Making dollars and sense of SAVE. J Clin Sleep Med. 2017;13(5):765–766. doi: 10.5664/jcsm.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailly S, Destors M, Grillet Y, et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS One. 2016;11(6):e0157318. doi: 10.1371/journal.pone.0157318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pien GW, Ye L, Keenan BT, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort. Sleep. 2018;41(3) doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith R, Ronald J, Delaive K, Walld R, Manfreda J, Kryger MH. What are obstructive sleep apnea patients being treated for prior to this diagnosis? Chest. 2002;121(1):164–172. doi: 10.1378/chest.121.1.164. [DOI] [PubMed] [Google Scholar]
- 40.Wittmann V, Rodenstein DO. Health care costs and the sleep apnea syndrome. Sleep Med Rev. 2004;8(4):269–279. doi: 10.1016/j.smrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Wickwire EM, Albrecht JS, Towe MM, et al. The impact of treatments for OSA on monetized health economic outcomes. Chest. 2019;155(5):947–961. doi: 10.1016/j.chest.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 42.McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2(10):804–812. doi: 10.1016/S2213-2600(14)70172-9. [DOI] [PubMed] [Google Scholar]
- 43.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011;183(4):539–546. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.