Abstract
Study Objectives:
The objective of this study was to determine which respiratory and architectural sleep parameters are related to cognitive function and cognitive status (mild cognitive impairment [MCI] versus normal cognitive aging [NCA]) in community-dwelling individuals with hypertension. Additionally, it aimed to determine whether the results changed in the presence or absence of vascular brain lesions (silent brain infarcts and extensive white matter hyperintensities [WMHs]).
Methods:
In a cohort of individuals with hypertension and without previous stroke or dementia, we conducted in-hospital polysomnography including electroencephalography, electro-oculography, electromyography, and magnetic resonance imaging to assess silent brain infarcts and WMHs. Cognitive testing was carried out with a screening test (Dementia Rating Scale version 2 [DRS-2]) and a complete cognitive visit.
Results:
This study included 158 participants with a median age of 65.0 years; 32.3% were females, and the median apnea-hypopnea index was 22.3 events/h. MCI was diagnosed in 24 study participants, and the rest had NCA. Regarding respiratory parameters, total DRS-2 scores (β; 95% CI) 0.121; 0.026, 0.215 were positively associated with mean O2 saturation, whereas total (−0.022; −0.036, −0.009), executive function (−0.016; −0.026, −0.006) and memory (−0.017; −0.029, −0.004) DRS-2 scores were all negatively associated with the percent of time with oxygen saturation < 90% after correcting for education, vascular risk factors, and magnetic resonance imaging lesions. Regarding sleep architecture, Attention DRS-2 scores (0.0153; 0.001, 0.306) were independently associated with total sleep time. Similar results were obtained in the absence of silent brain infarcts or WMHs in the stratified analysis. None of the sleep parameters were associated with cognitive status.
Conclusions:
Low oxygen saturation contributes to cognitive performance, and this effect appears even in the absence of vascular brain lesions in individuals with hypertension.
Citation:
Riba-Llena I, Álvarez-Sabin J, Romero O, et al. Nighttime hypoxia affects global cognition, memory, and executive function in community-dwelling individuals with hypertension. J Clin Sleep Med. 2020;16(2):243–250.
Keywords: cognitive function, sleep parameters, vascular brain lesions
BRIEF SUMMARY
Current Knowledge/Study Rationale: To study the relationship of respiratory and architectural polysomnographic parameters with cognitive performance and mild cognitive impairment in a cohort with hypertension and in the presence or absence of vascular brain lesions measured by brain magnetic resonance imaging.
Study Impact: Sustained nighttime hypoxia as measured by time at oxygen saturation < 90% is associated with poorer global cognitive performance, memory and executive functions, whereas higher oxygen saturation is associated with better memory performance. In addition, attention is higher in those who sleep longer. These results were not affected by the presence of vascular brain lesions, suggesting that other mechanisms may contribute to cognitive impairment in the presence of sustained hypoxia.
INTRODUCTION
Sleep-disordered breathing (SDB) includes several chronic conditions in which partial or complete cessation of breathing and, therefore, intermittent hypoxia occurs during the night. The most frequent form of SDB is obstructive sleep apnea (OSA), a treatable health problem that increases with aging.1 OSA is frequently diagnosed in individuals with hypertension (approximately 50% with essential hypertension2) and among those who have had a stroke (as high as 60%3) or those at risk of stroke or other cardiovascular diseases.4,5
Recently, we showed for the first time that silent lacunar brain lesions, that is, small asymptomatic infarcts generally found during magnetic resonance imaging (MRI) of the brain, were associated with the presence and grade of OSA in Caucasian individuals with hypertension.6 Other MRI lesions, such as extensive white matter hyperintensities (WMHs), have been recently related to OSA or the apnea-hypopnea index (AHI) in community-based studies.7,8 These silent brain infarcts are far more frequent than stroke and are associated with future stroke and dementia,9 whereas extensive WMHs are associated with dementia risk.10 In addition, silent brain infarcts and extensive WMHs are associated with the worst cognitive performance, mainly in executive function but also in memory function.11,12
However, the relationship between SDB and cognitive impairment is conflicting and not completely understood. Although some groups have found relationships between SDB and specific memory impairment,13 others have found a relationship with other cognitive domains (executive function14) or found no relationship at all.15 In addition, some previous studies and a recent meta-analysis also showed an association of SDB with cognitive decline.16 In any case, it is important to note that some previous studies have shown that treating OSA with continuous positive airway pressure (CPAP) may improve cognitive function, especially attention and executive function, although cognitive impairment may persist,17 or the treatment may delay the decline of cognitive function.18 Thus, this intervention seems promising, because no disease-modifying treatment is available for neurodegenerative dementias.
It is also important to acknowledge that, in addition to sleep respiratory parameters, some sleep architectural parameters are also related to cognitive impairment. However, most architectural sleep changes related to cognitive impairment remain unexplained. A longitudinal community-based study found that changes in sleep duration were negatively associated with decline in all cognitive functions except memory.19 In the case of mild cognitive impairment (MCI), which is the transitional phase between normal cognitive aging and dementia, a recent meta-analysis showed that individuals with MCI had a shorter sleep latency than healthy control patients.20
Therefore, our principal aim was to determine which respiratory and architectural sleep parameters were related to cognitive function in individuals with hypertension from a community. Our second aim was to evaluate the relation of respiratory and architectural sleep parameters with cognitive status (MCI versus normal cognitive aging [NCA]) in these study participants. We also performed a stratified analysis to determine whether differences exist in the results in the presence or absence of silent brain infarcts or extensive WMHs.
METHODS
Patient selection
This analysis was embedded in a nested substudy of the project Investigating Silent Strokes in hYpertensives, a magnetic resonance imaging Study (ISSYS). ISSYS is an observational study conducted with individuals with hypertension aged 50 to 71 years without previous stroke or dementia. At the baseline visit, we collected data related to personal medical history, physical examination, blood sample tests, cognitive assessment and brain MRI. The protocol of the study was published previously.21
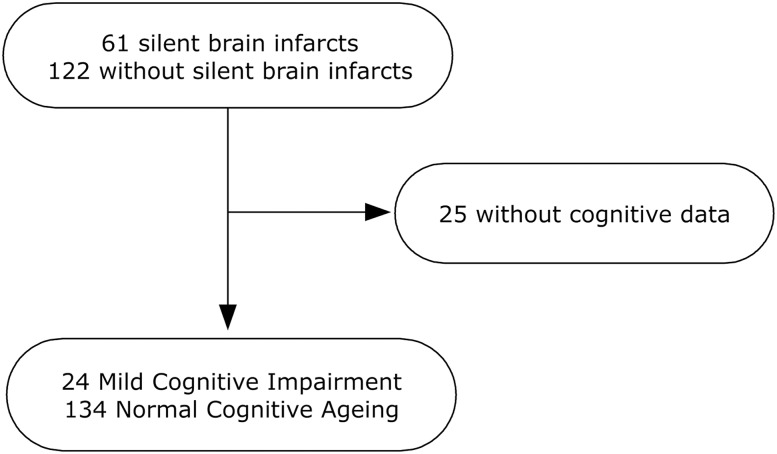
In a previous nested substudy, we included study participants with silent brain infarcts and their counterparts matched by age, sex, body mass index (BMI), and number of antihypertensive drugs who agreed to undergo an in-hospital PSG as described in the previous work.6 In the current analysis, which included 158 study participants, 24 received a diagnosis of MCI and 134 had NCA. Twenty-five study participants from our previous study did not complete the cognitive screening or cognitive diagnostic visit. Those who completed or did not complete the cognitive visit were not significantly different regarding age, sex, diabetes mellitus, blood pressure (except for diastolic blood pressure, median = 76.0; interquartile range [IQR] = 69.0, 84.5 mmHg in those who completed the cognitive visit versus 81.0; 71.5, 88.0 mmHg in those who did not complete the visit, P = .037), number of antihypertensive drugs, dyslipidemia, smoking habit, BMI, Dementia Rating Scale version 2 (DRS-2) scores, silent brain infarcts, AHI, mean O2 saturation, and total time and sleep period. A flow chart of the study is displayed in Figure 1.
Figure 1. Flow chart of the sampling process.
The study was in accordance with the Helsinki Declaration on Human Research. The study protocol was approved by the local ethics committee, and the study participants gave their written informed consent prior to inclusion.
Covariables collected
Office blood pressure was measured with an oscillometric device (Omron M6 Comfort, Omron Corporation, Kyoto, Japan) in the supine position, and the mean of the last two of three measurements after a 5-minute rest was calculated. Diabetes mellitus was defined as self-reported and/or confirmed by clinical records showing a history of diabetes in those taking oral glucose lowering drugs or insulin. Smoking habits were defined as active or inactive. BMI was calculated as the ratio between body weight and the square of height. Personal history of OSA diagnosis, CPAP treatment, and Berlin Questionnaire for OSA risk were recorded. Education was defined as completed years of formal schooling. The Charlson comorbidity index was collected as a comorbidity index measurement. Other covariables that were collected can be found in the protocol for the study.21
Brain MRI
Brain MRI was performed with the same 1.5-T magnetic resonator for the entire cohort.21 Silent cerebrovascular lesions were rated according to standard criteria.22 Silent brain infarcts were defined as tissue loss lesions ≥ 3 mm, with the cerebrospinal fluid signal intensity in all MRI sequences surrounded by a hypertensive rim in the fluid-attenuated inversion recovery (FLAIR) sequence. WMHs were identified as hyperintense FLAIR lesions in deep and periventricular locations and rated on the Fazekas scale.23 For periventricular WMHs, the scores were as follows: grade 0 = no WMH, 1 = caps or pencil-thin lining, 2 = smooth “halo,” and 3 = irregular WMHs extending to the deep white matter. For deep WMHs, the score was 0 = no lesions, 1 = punctuate foci, 2 = beginning confluence of foci, and 3 = large confluent areas. WMHs were considered extensive when rated as two or three in any location. There were three raters for silent brain infarcts and WMH: two neuroradiologists and a neurovascular neurologist. Intrarater agreement was calculated for each rater in a training set before undertaking the present reading and values ranged from 0.60 to 0.75, and interrater agreement was calculated from 0.7 to 0.81 depending on the lesion. Disagreements were sorted by consensus meetings of the raters.
Cognitive assessment
The cognitive assessment was performed in a two-step process, which is a common procedure in population-based studies, and according to our protocol24 (methodology in the supplemental material). At the first visit, study participants were evaluated with a cognitive screening test, the DRS-2. This test has five cognitive subscale scores (attention, memory, initiation-perseveration, conceptualization, and construction) as well as a total score. Raw DRS-2 scores were adjusted for age and education following previously published normative data from our group in which we used common methodology to normalize cognitive scores.25 Those study participants who were suspected of having cognitive impairment (ie, having a total DRS-2 score below 8, adjusted for age and education25) were invited to undergo a second cognitive visit. Briefly, this second visit consisted of a complete cognitive, behavioral, and functional evaluation, and physical examination plus a complete neuropsychological battery after which cognitive diagnosis was established.24
MCI was established according to previous criteria and by consensus of the neurologist and neuropsychologist who evaluated the patients.26 Briefly, a MCI diagnosis was established in study participants in whom DRS-2 score was under 8 adjusted by age and education, had a confirmed impairment in one or more cognitive domains (generally ≤ −1.5 standard deviations on the normative mean in more than one test per domain was needed) and may have cognitive complaints but did not fulfill the dementia criteria.
Other study participants who had a total DRS-2 score > 8 adjusted for age and education were considered to have NCA.
In-hospital nocturnal PSG
Study participants underwent a conventional PSG with a Compumedics E-series digital device (Abbotsford, Australia) as previously described.6 Briefly, the cardiorespiratory parameters collected were air flow with a nasal cannula pressure and thermocouple, thoracoabdominal effort with inductive bands, snoring, oxygen saturation and position. The neurophysiologic parameters recorded were electroencephalography (electrodes positioned at Fp1, Fp2, C3, C4, T3, T4, O1, O2, A1, and A2), submental electromyogram, electrocardiography, and leg movements.
Respiratory events during sleeping were manually analyzed by sleep clinicians blinded to other data and according to the American Academy of Sleep Medicine (AASM) criteria.27 Obstructive sleep apnea was considered if there was a reduction ≥ 90% or absence of the respiratory flow signal lasting ≥ 10 seconds in the presence of respiratory effort detected by thoracoabdominal bands. Central apnea was recorded as a reduction ≥ 90% or absence of respiratory flow signal lasting ≥ 10 seconds in the absence of respiratory effort assessed by thoracoabdominal bands. Mixed apnea was diagnosed when the apnea started with a central component and finished with an obstructive component lasting ≥ 10 seconds. Hypopnea was recorded as a reduction (≥ 30% and ≤ 90%) of the airflow signal amplitude lasting ≥ 10 seconds and accompanied by oxygen desaturation ≥ 3% and/or an arousal detected by the electroencephalography.
Additionally, the AHI was calculated hour by hour, and the presence and severity of OSA were classified according to AASM as normal (AHI ≤ 5 events/h), mild (AHI > 5 to 15 events/h), moderate (AHI > 15 to 30 events/h) and severe (AHI > 30 events/h).
Other respiratory variables collected were O2 saturation, desaturation indices, % time with oxygen saturation < 90% (CT90), and number of arousals. We also collected several sleep architectural parameters: total sleep time and period; sleep efficiency; wake after sleep onset; sleep and rapid eye movement (REM) sleep latencies; number of REM cycles; percent of total sleep time in stage R, N1/N2, and N3 sleep; and number of arousals.
Statistical analysis
For continuous variables, we first assessed the distribution of the variable according to QQ-plots and a Kolmogorov-Smirnov test. Then, for cognitive function analysis, the total DRS-2 and subscales adjusted for age and the education scores were transformed into Z-scores (individual mean − sample mean / sample standard deviation) to standardize the sample (Z DRS-2 scores).
In univariate analysis, we analyzed the relationship between demographic factors, vascular risk factors, MRI lesions, respiratory and architectural sleep variables with the Z DRS-2 cognitive scores and with cognitive diagnosis (MCI and NCA). Statistical significance for intergroup differences was assessed by Pearson chi-square test for categorical variables and t test or the Mann-Whitney U test for continuous variables, depending on the variable distributions. Correlations between continuous variables were assessed using Pearson or Spearman correlation coefficients.
To study the effect of the architectural and respiratory sleep parameters (independent variables) on Z DRS-2 scores (dependent variables), we performed multiple linear regression analyses for each Z DRS-2 score and sleep parameters adjusted for previously described potential confounders (age, sex, blood pressure, diabetes mellitus, smoking status, silent brain infarcts, and extensive WMHs). We used a similar approach using logistic regression analysis to study the relationship between cognitive status (dependent variable, MCI and NCA) and respiratory and architectural sleep variables (independent variables) adjusted for potential confounders (age, silent brain infarcts, and extensive WMHs).
Finally, in the stratified analysis, we determined whether the results of the previous multivariate analysis changed in the presence or absence of silent brain infarcts or extensive WMHs.
The analysis was performed with SPSS version 20 (IBM Corp, Armonk, New York, United States), and significant P values were set at < .05.
RESULTS
Description of the sample
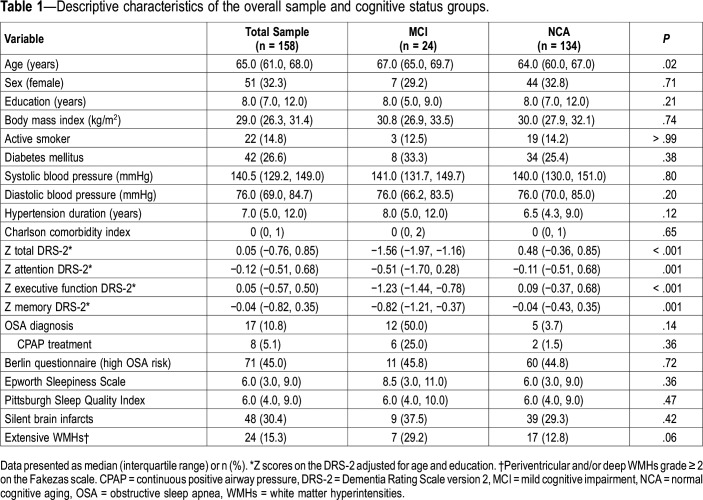
The median age of the study participants was 65.0 years (IQR = 61.0, 68.0), 32.3% were female, and the median education level was 8.0 years (7.0, 12.0). Study participants with MCI were older than those with NCA (median age 67.0 versus 64.0 years, P = .02). As expected, study participants with MCI had worse performance on Z DRS-2 scores (P < .001), especially the total and executive scores (Figure S1 in the supplemental material). Other clinical characteristics were not significantly different between the groups. These results are displayed in Table 1.
Table 1.
Descriptive characteristics of the overall sample and cognitive status groups.
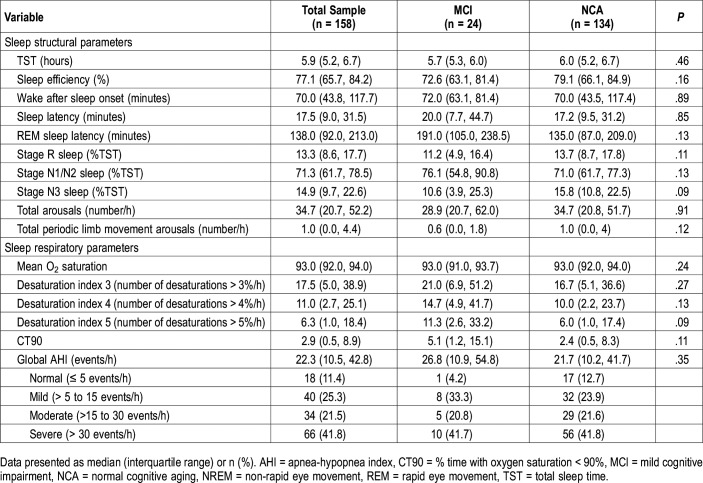
Regarding sleep architectural parameters, total sleep time was 5.9 hours (IQR = 5.2, 6.7), sleep efficiency was 77.1% (65.7, 84.2) and wake after sleep onset was 70.0 minutes (43.8, 117.7). Regarding respiratory parameters, the mean O2 saturation was 93.0% (92.0, 94.0), and 63.3% had global moderate to severe AHI (> 15 events/h). Other polysomnographic characteristics are displayed in Table 2.
Table 2.
Relationship between sleep structure and respiratory events in the overall sample and cognitive status groups (univariate analysis).
Relationship between cognitive status, cognitive function, and polysomnographic measures
Regarding sleep structural parameters, study participants with MCI slept for a shorter time and had lower sleep efficiency than those with NCA, and study participants with MCI spent more time in stage N1/N2 sleep than those with NCA (76.1% versus 70.9%, P > .05) and less time in stage N3 sleep (10.6% versus 15.8%, P = .09). Regarding respiratory parameters, when comparing the MCI and NCA groups, the MCI group had globally higher CT90 than the NCA group (median = 5.1; IQR = 1.2, 15.1 versus 2.4; 0.5, 8.3), but the differences between groups were not statistically significant. Other results are displayed in Table 2.
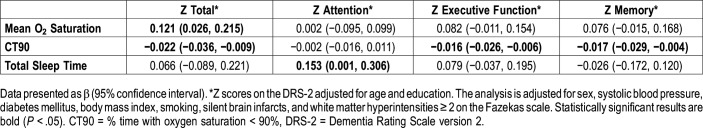
There were some sleep parameters associated with cognitive function in the univariate analysis (data not shown). However, in the case of architectural parameters, only total sleep time was directly associated with Z attention scores (β = 0.153; 95% CI = 0.001, 0.306) after correcting for potential confounders. These results are displayed in Table 3 and are graphically displayed in Figure S1.
Table 3.
Association between cognitive scores and sleep parameters.
In the case of respiratory parameters, the effect of mean O2 saturation was positively associated with Z total score (0.121; 0.026, 0.215) after correcting for previous covariates. CT90 was negatively associated with scores for Z total (−0.022; −0.036, −0.009), Z executive function (−0.016; −0.026, −0.006), and Z memory (−0.017; −0.029, −0.004) after correcting for the same previous covariates (Table 3 and Figure S1). The remaining sleep respiratory parameters, including the global AHI and desaturation indices of 3%, 4%, and 5%, were not associated with cognitive scores after adjusting for covariates (results not shown).
Relationship between sleep parameters, cognitive function, cognitive status and the presence of brain vascular lesions
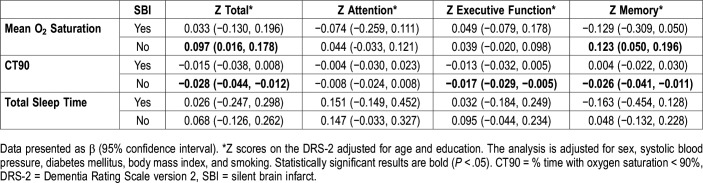
To explore whether associations between sleep parameters, cognitive function and cognitive status were different in the presence or absence of brain vascular lesions, we first stratified the analysis by the presence or absence of silent brain infarcts. We found that in individuals with no silent brain infarcts, the mean O2 saturation was positively associated with scores for Z total (β = 0.097; 95% CI = 0.016, 0.178) and Z memory (0.123; 0.050, 0.196) adjusted for the same covariates as in the whole sample analysis. CT90 was negatively associated with scores for Z total (β = −0.028; 95% CI = −0.044, −0.012), Z executive function (−0.017; −0.029, −0.005) and Z memory (−0.026; −0.041, −0.011) in the same group. These results are displayed in Table 4.
Table 4.
Association between cognitive scores and sleep parameters stratified by the presence or absence of silent brain infarcts.
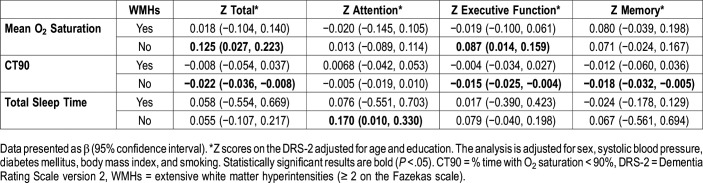
Second, we stratified the analysis by the presence or absence of extensive WMHs. The results for those without extensive WMHs were similar to those in study participants without silent brain infarcts regarding the relationship of CT90 and total sleep period with cognitive scores. For total sleep time the analysis also showed an association with increasing Z attention scores (0.170; 0.010, 0.330). These results are shown in Table 5. We also performed analyses stratified by the presence or absence of periventricular and deep WMH (Table S1 in the supplemental material).
Table 5.
Association between cognitive scores and sleep parameters stratified by the presence or absence of extensive white matter hyperintensities.
Finally, we performed a similar stratified analysis for cognitive status outcomes (MCI/NCA), and we did not find any associated architectural or respiratory parameters.
DISCUSSION
In this sample with hypertension, we found that some respiratory parameters (mean O2 saturation, CT90) were independently associated with global cognition, executive function, and memory scores. Additionally, longer total sleep time was related to better attention in study participants without extensive WMH. However, we did not find any respiratory or architectural parameters independently associated with MCI.
Attention scores were positively associated with total sleep time in individuals with hypertension. This finding was consistent with previous works on sleep deprivation and sleep extension, which showed the worst attention scores in individuals who slept for less time and better in those who were allowed to sleep longer.28,29
In our cohort, lower O2 saturation and spending more time with O2 saturation < 90% were associated with lower total, executive function, and memory scores. This finding is in accordance with a previous extensive review that showed a relationship between executive function and memory scores, in addition to attention, with respiratory parameters (hypoxia, arousals, and AHI depending on the work) in the community.30 Additionally, in a recent systematic review and meta-analysis on community-based studies, including more than four million study participants, it was found that SDB was associated with lower executive performance.14 However, in our case, executive function was correlated with parameters of sustained hypoxia, such as the mean O2 saturation and CT90, and not with parameters of intermittent hypoxia, such as desaturation indices or AHI. Notably, our sample was composed of individuals with hypertension with high median AHI, high oxygen desaturation indices, and a high prevalence of OSA, which might have limited the ability to draw an association with cognitive scores.
The pathophysiology of cognitive decline and the relationship with sleep parameters is largely unknown. We studied the role of vascular lesions identified in MRI on the relationship of sleep parameters with cognitive function by stratifying our analysis by the presence or absence of these lesions. We found statistically significant associations between some cognitive scores and sleep parameters in the groups that did not display MRI lesions. These results are in contrast with a previous hypothesis suggesting that chronic hypoxia, especially if intermittent, is related to vasculopathy and to ischemic brain damage that primarily affects small vessels of the brain and may ultimately cause cognitive impairment.30 By contrast, we found that in the individuals who did not have silent brain infarcts or extensive WMHs, CT90 was negatively associated with total, memory, and executive function scores, whereas mean O2 saturation was positively associated with total and memory scores. Therefore, other mechanisms not evaluated in this cohort may be involved in this process. Recently, it has also been shown that OSA was associated with increased amyloid markers in the follow-up of individuals with normal cognition.31 In fact, neurodegenerative changes (for instance, accumulation of amyloid and tau in Alzheimer disease) generally begin years before dementia is diagnosed.32 However, our sample came from a community-based cohort with hypertension but free of stroke and dementia, and we do not know if these neurodegenerative changes were present.
However, we did not find any architectural or respiratory parameters to be independently associated with MCI in our cohort. This is probably because our sample of 24 study participants with MCI was too small to show differences with the NCA group and might also be due to the heterogeneity of MCI itself. Of note, we did show in the univariate analysis a longer time on stage N1/N2 sleep and shorter on stage N3 sleep for MCI compared to individuals with NCA. This result is in accordance with what was described previously.33
Our study had several limitations. First, causality could not be established due to the cross-sectional nature of our analysis. Second, we had a small number of study participants with MCI and MRI lesions that may have limited finding some relationship with sleep parameters. Third, because this study has been considered an exploratory analysis, the obtained results have not been corrected by multiple comparisons, so future research in other populations is needed to confirm the conclusions of this study. Finally, some of our patients were on CPAP treatment, which may have improved their cognitive function, but our study was not designed to evaluate this effect in our patients.
In conclusion, our work shows that nighttime hypoxia is related to poor cognitive performance in individuals with hypertension from a community with a high prevalence of moderate to severe AHI. Additionally, attention function is better in individuals who have longer effective sleep times. These results were not found in individuals with silent brain lesions, suggesting that other nonvascular mechanisms may participate in the process.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This research was funded by the Instituto de Salud Carlos III (grant numbers: PI11/01676, PI14/01535, ICI14/307, PI17/02222) and the European Regional Development Fund. Pilar Delgado (CP15/00010) and Iolanda Riba-Llena (JR15/00032) have contracts supported by the Instituto de Salud Carlos III and the European Regional Development Fund. The Neurovascular Research Laboratory receives funds from the Spanish Research Stroke Network (RD/16/0019/0021). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the study participants for their contribution.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- CT90
% of time with oxygen saturation < 90%
- DRS-2
Dementia Rating Scale version 2
- FLAIR
Fluid-Attenuated Inversion Recovery
- IQR
interquartile range
- ISSYS
Investigating Silent Strokes in hYpertensives, a magnetic resonance imaging Study
- MCI
mild cognitive impairment
- MRI
magnetic resonance imaging
- NCA
normal cognitive aging
- OSA
obstructive sleep apnea
- REM
rapid eye movement
- SDB
sleep-disordered breathing
- WMH
white matter hyperintensities
REFERENCES
- 1.Peppard PE, Hagen EW. The last 25 years of obstructive sleep apnea epidemiology-and the next 25? Am J Respir Crit Care Med. 2018;197(3):310–312. doi: 10.1164/rccm.201708-1614PP. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg DS, Oksenberg A. Are sleep-related breathing disorders important contributing factors to the production of essential hypertension? Curr Hypertens Rep. 2001;3(3):209–215. doi: 10.1007/s11906-001-0040-8. [DOI] [PubMed] [Google Scholar]
- 3.Ferre A, Ribó M, Rodriguez-Luna D, et al. Strokes and their relationship with sleep and sleep disorders. Neurologia. 2013;28(2):103–118. doi: 10.1016/j.nrl.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37(9):2317–2321. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 5.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126(12):1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Sabin J, Romero O, Delgado P, et al. Obstructive sleep apnea and silent cerebral infarction in hypertensive individuals. J Sleep Res. 2018;27(2):232–239. doi: 10.1111/jsr.12571. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Mera RM, Zambrano M, Castillo PR. Relationship between obstructive sleep apnea and neuroimaging signatures of cerebral small vessel disease in community-dwelling older adults. The Atahualpa Project. Sleep Med. 2017;37:10–12. doi: 10.1016/j.sleep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Song TJ, Park JH, Choi KH, et al. Moderate-to-severe obstructive sleep apnea is associated with cerebral small vessel disease. Sleep Med. 2017;30:36–42. doi: 10.1016/j.sleep.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med. 2014;12(1):119. doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 11.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348(13):1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 13.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203–220. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review meta-analysis. JAMA Neurol. 2017;74(10):1237–1245. doi: 10.1001/jamaneurol.2017.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutsey PL, Bengtson LG, Punjabi NM, et al. Obstructive sleep apnea and 15-year cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Sleep. 2016;39(2):309–316. doi: 10.5665/sleep.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Zhao Y. Sleep-disordered breathing and the risk of cognitive decline: a meta-analysis of 19,940 participants. Sleep Breath. 2017;22(1):165–173. doi: 10.1007/s11325-017-1562-x. [DOI] [PubMed] [Google Scholar]
- 17.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297–1305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu M, Zhang P, Li C, et al. Sleep disturbance in mild cognitive impairment: a systematic review of objective measures. Neurol Sci. 2017;38(8):1363–1371. doi: 10.1007/s10072-017-2975-9. [DOI] [PubMed] [Google Scholar]
- 21.Riba-Llena I, Jarca CI, Mundet X, et al. Investigating silent strokes in hypertensives: a magnetic resonance imaging study (ISSYS): rationale and protocol design. BMC Neurol. 2013;13:130. doi: 10.1186/1471-2377-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 24.Riba I, Jarca CI, Mundet X, et al. Cognitive assessment protocol design in the ISSYS (Investigating Silent Strokes in hYpertensives: a magnetic resonance imaging Study) J Neurol Sci. 2012;322(1-2):79–81. doi: 10.1016/j.jns.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Riba-Llena I, Nafria C, Giralt D, et al. Dementia Rating Scale-2 normative data for middle-and older-aged Castilian speaking Spaniards. Clin Neuropsychol. 2016;30(sup1):1443–1456. doi: 10.1080/13854046.2016.1174307. [DOI] [PubMed] [Google Scholar]
- 26.Espinosa A, Alegret M, Valero S, et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. JAD. 2013;34(3):769–780. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- 27.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010;185:91–103. doi: 10.1016/B978-0-444-53702-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 29.Arnal PJ, Sauvet F, Leger D, et al. Benefits of sleep extension on sustained attention and sleep pressure before and during total sleep deprivation and recovery. Sleep. 2015;38(12):1935–1943. doi: 10.5665/sleep.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmerman ME, Aloia MS. Sleep-disordered breathing and cognition in older adults. Curr Neurol Neurosci Rep. 2012;12(5):537–546. doi: 10.1007/s11910-012-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma RA, Varga AW, Bubu OM, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly: a longitudinal study. Am J Respir Crit Care Med. 2018;197(7):933–943. doi: 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva RA. Sleep disturbances and mild cognitive impairment: a review. Sleep Sci. 2015;8(1):36–41. doi: 10.1016/j.slsci.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.