Abstract
Background
Acute kidney injury (AKI) remains a concern in hospitalized children undergoing CT with intravenous iodinated contrast material (ICM). Adult studies have shown frequencies of AKI after CT with intravenous ICM to be similar to propensity score–matched ICM-unexposed patient groups; similar data in pediatric patients are lacking.
Purpose
To evaluate the association between intravenous ICM exposure and AKI in hospitalized pediatric patients with stable kidney function undergoing contrast material–enhanced CT by comparing with a propensity score–matched ICM-unexposed patient sample undergoing abdominal US.
Materials and Methods
In this retrospective observational study, hospitalized patients aged 18 years or younger with stable kidney function and available serum creatinine (SCr) measurement before and after imaging who underwent CT with intravenous ICM or abdominal US (control group) between January 2009 and November 2018 were identified. The 1:1 propensity score matching was performed by using 23 covariates, stratified by estimated glomerular filtration rate (eGFR) before imaging (≥60 mL/min/1.73 m2 or <60 mL/min/1.73 m2). AKI was defined by using Acute Kidney Injury Network SCr-related criteria. Multivariable logistic regression was performed to identify risk factors for AKI after imaging, including the effects of eGFR and intravenous ICM exposure before imaging.
Results
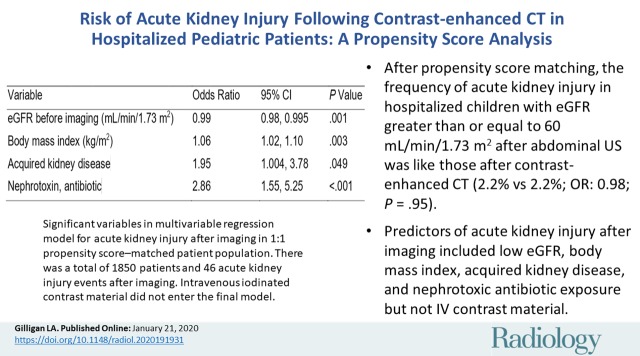
A total of 1850 unique patients were included in the propensity score–matched sample (925 exposed to ICM [mean age ± standard deviation, 8 years ± 6; 484 female patients]; 925 unexposed to ICM [mean age, 7 years ± 6; 484 female patients]). Frequency of AKI with eGFR greater than or equal to 60 mL/min/1.73 m2 was 2.2% (20 of 889) for CT and US (odds ratio [OR]: 0.98; 95% confidence interval [CI]: 0.52, 1.86; adjusted P = .95) and with eGFR less than 60 mL/min/1.73 m2 was 5.6% (two of 36) and 11.1% (four of 36) for CT and US, respectively (OR: 0.75; 95% CI: 0.11, 5.00; adjusted P = .76). Significant multivariable predictors of AKI included eGFR before imaging (OR: 0.99; 95% CI: 0.98, 0.995; P = .001), body mass index (OR: 1.06; 95% CI: 1.02, 1.10; P = .003), acquired kidney disease (OR: 1.95; 95% CI: 1.004, 3.78; P = .049), and nephrotoxic antibiotic exposure (OR: 2.86; 95% CI: 1.55, 5.25; P < .001). Intravenous ICM exposure was not predictive (OR: 0.91; 95% CI: 0.51, 1.64; P > .05).
Conclusion
Hospitalized children with stable kidney function who underwent CT with intravenous iodinated contrast material (ICM) had a similar frequency of acute kidney injury (AKI) compared with a propensity score–matched ICM-unexposed patient group. In pediatric inpatients with estimated glomerular filtration rate greater than or equal to 60 mL/min/1.73 m2, ICM was not independently associated with AKI.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Paltiel in this issue.
Summary
Iodinated contrast media for CT was not an independent risk factor for acute kidney injury in hospitalized pediatric patients with an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or greater.
Key Results
■ After propensity score matching, the frequency of acute kidney injury (AKI) after imaging in hospitalized children with estimated glomerular filtration rate (eGFR) greater than or equal to 60 mL/min/1.73 m2 was the same in patients who underwent contrast material–enhanced CT versus abdominal US (2.2% vs 2.2%; odds ratio [OR]: 0.98; adjusted P = .95).
■ After propensity score matching, the frequency of AKI after imaging in hospitalized children with eGFR less than 60 mL/min/1.73 m2 was no different between the patient groups who underwent contrast-enhanced CT and abdominal US (5.6% compared with 11.1%; OR: 0.75; adjusted P = .76).
■ Significant predictors of AKI after imaging in hospitalized children included eGFR before imaging (OR: 0.99; P = .001), body mass index (OR: 1.06; P = .003), acquired kidney disease (OR: 1.95; P = .049), and nephrotoxic antibiotic exposure (OR: 2.86; P < .001) at multivariable analysis, whereas intravenous contrast material was not an independent risk factor (P > .05).
Introduction
Low- and iso-osmolality iodinated contrast media (ICM) are commonly administered intravenously during pediatric CT examinations to enhance visualization of the vasculature, viscera, and disease (1). Although generally safe, ICMs have historically been considered to carry a risk of contrast-induced nephropathy, commonly defined as acute kidney injury (AKI) in the 48 hours following, and directly resultant from, intravascular exposure of an ICM (2). However, over the last decade, the phenomenon of contrast-induced nephropathy has been called into question (3). Until recently, the majority of contrast-induced nephropathy investigations have not included a control group of patients unexposed to ICM (2,4,5). Furthermore, association does not imply causation, and there are many risk factors for postcontrast AKI (PC-AKI) related to decreased kidney perfusion, direct nephrotoxic exposures, intrinsic kidney disease, and obstruction of urinary flow that may confound AKI observations (6–8).
Challenges of prospective study designs for PC-AKI include inherent differences in patients who undergo contrast material–enhanced and unenhanced CT examinations, equipoise challenges (eg, conducting a randomized trial assigning patients to undergo contrast-enhanced vs unenhanced imaging), and sample size barriers. Therefore, PC-AKI investigations have used propensity score matching in retrospective observational studies to improve statistical rigor and to uncover the potential nephrotoxic effect of ICM (8–13). Such studies in adult patients have demonstrated either no risk of AKI from intravenous ICM exposure or risk only in patients with severe kidney impairment (ie, estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) (8–13). Thus, contrary to historical belief, most cases of AKI after CT with ICM are likely not caused by the contrast material. Although contrast-induced nephropathy may exist, it is probably uncommon, occurring in patients with substantially impaired kidney function due to underlying chronic kidney disease or acute kidney injury (3).
To date, there is a paucity of literature using propensity score matching to determine the association between ICM exposure and the development of AKI in hospitalized pediatric patients. One recent pediatric study (14) using propensity score matching found no significant difference in the frequencies of AKI in a sample of 305 ICM-exposed and 305 ICM-unexposed patients; however, the authors noted their conclusions were limited given the small sample size. The purpose of our investigation was to further evaluate the association between ICM exposure and PC-AKI in the pediatric population by comparing with a propensity score–matched ICM-unexposed patient sample undergoing abdominal US.
Materials and Methods
Our institutional review board approved this retrospective, single-institution, Health Insurance Portability and Accountability Act–compliant, propensity score–matched observational cohort study. The requirement for written informed consent was waived.
Patients and Data Collection
All patients 0–18 years of age who underwent a CT examination with intravenous ICM while hospitalized at Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio) between January 2009 and November 2018 were eligible for inclusion in the ICM-exposed patient sample. Similarly, hospitalized patients 0–18 years of age who underwent an abdominal US examination during the same period were eligible for inclusion in the ICM-unexposed patient sample. Patients were identified by using electronic billing records held in the department of radiology.
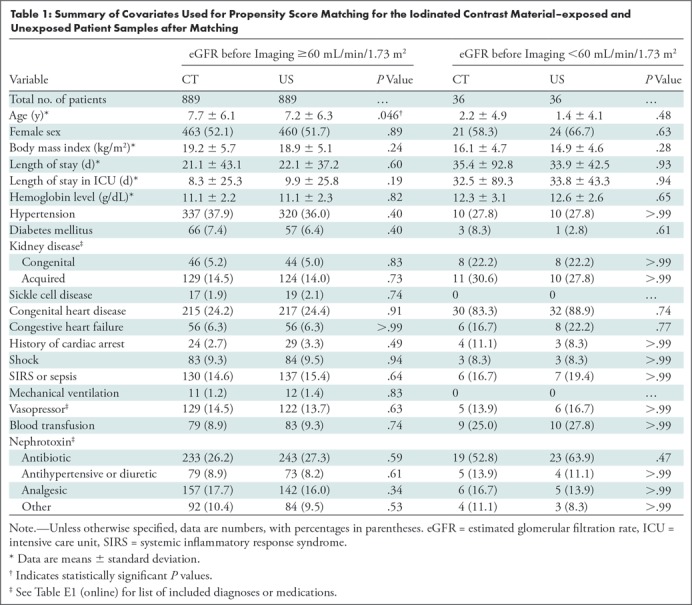
The electronic medical record (Epic Systems, Verona, Wis) of identified patients was queried to derive the following: demographic data, including age, sex, height, and weight obtained closest to and within 1 year of CT or US; clinical data, including length of stay and days in the intensive care unit prior to CT or US, total length of stay, medical diagnoses present before CT or US (using International Classification of Diseases, ninth and 10th revision codes), certain procedures performed before CT or US (using Current Procedural Terminology codes), and medications given from time of admission until CT or US, including nephrotoxins defined by the Nephrotoxic Injury Negated by Just-in-time Action collaborative (15); imaging data, including dates of contrast-enhanced imaging examinations using ICM; and laboratory data, including hemoglobin value (obtained during admission, closest to, and before CT or US) and various serum creatinine (SCr) values (see definitions below). Further details regarding data collection can be found in Table 1 and Table E1 (online).
Table 1:
Summary of Covariates Used for Propensity Score Matching for the Iodinated Contrast Material–exposed and Unexposed Patient Samples after Matching
Exclusion of Patients
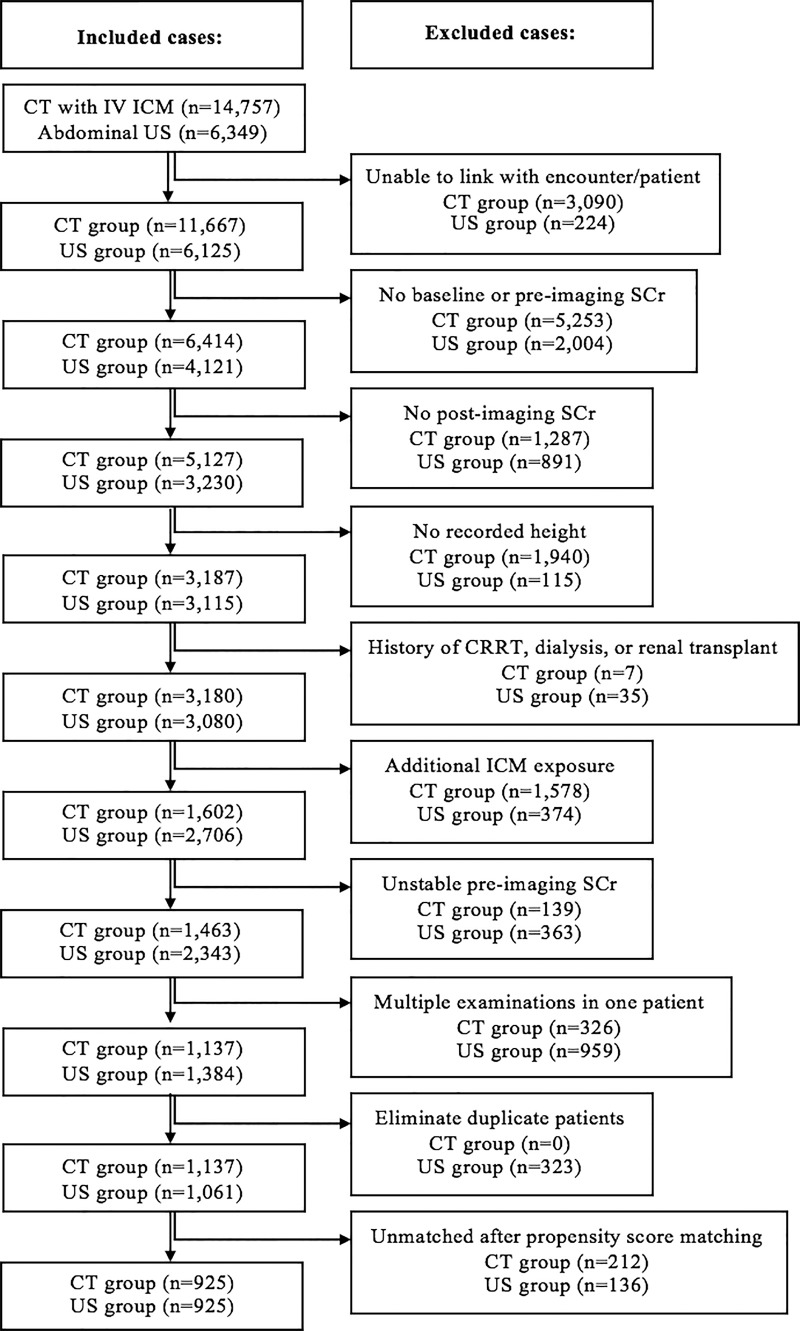
After data were collected from the electronic medical record, imaging encounters with any of the following were excluded: (a) inability to link examination from billing records to an exact patient encounter in the electronic medical record (eg, due to CT technologist failing to electronically document administration of ICM); (b) missing SCr data (baseline, before imaging, or after imaging; defined below); (c) no available height; (d) history of continuous renal replacement therapy, dialysis, or renal transplantation; (e) exposure to ICM (from cardiac catheterization, interventional radiology procedure, or CT examination) within the 5-day period prior to or the 2-day period following the CT or US examination used for this investigation; and (f) unstable kidney function before imaging (defined below). To eliminate repeat measures within an individual, the most recent imaging examination per patient was used, and patients were preferentially assigned to the ICM-exposed patient sample if eligible for inclusion in either sample.
Kidney Function Definitions
Three SCr values were queried from the electronic medical record per imaging examination. Baseline kidney function was defined as SCr values obtained between 1 year and 5 days prior to CT or US, using the value closest to CT or US. Kidney function before imaging was defined as SCr values obtained within 5 days prior to CT or US, using the value closest to CT or US. Kidney function after imaging was defined as SCr values obtained within 48 hours after CT or US, using the highest value. AKI after imaging was defined by using stage I Acute Kidney Injury Network SCr-related criteria: an absolute increase in SCr of greater than or equal to 0.3 mg/dL or a percent increase in SCr of greater than or equal to 50% (comparing SCr value after imaging to SCr value before imaging) (16,17).
Unstable kidney function was defined as an absolute increase or decrease of greater than or equal to 0.3 mg/dL, or percent increase or decrease of greater than or equal to 50%, comparing SCr values before imaging to baseline SCr values. To improve the specificity of AKI diagnosis in patients with a baseline SCr value or SCr value before imaging that was less than 0.3 mg/dL, AKI or unstable kidney function was not assigned if the follow-up SCr value was less than 0.5 mg/dL. Otherwise, small and likely clinically irrelevant increases, such as from 0.2 mg/dL to 0.3 mg/dL, would meet the AKI diagnosis (15).
The eGFR before imaging was calculated with the Bedside Schwartz equation by using patient height and SCr values before imaging (18). Patients were grouped into two categories by eGFR before imaging: normal or mildly impaired kidney function (eGFR ≥60 mL/min/1.73 m2) or moderately to severely impaired kidney function (eGFR <60 mL/min/1.73 m2) (19).
Iodinated Contrast Material Administration
All contrast-enhanced CT examinations performed in patients included in this study had been performed with a low-osmolality ICM, ioversol 320 mg I/mL (Optiray; Guerbet, Princeton, NJ). Dosing of contrast material was weight based, administered at a dose of 1.5–2 mL/kg during the study period with a maximum volume of 125 mL. Patients were not universally given intravenous hydration prior to their CT examination. No intravenous contrast media was given during US examinations.
Propensity Score Matching
Twenty-three covariates that have been or may be associated with a risk of developing AKI as a hospitalized inpatient were included in our 1:1 propensity score–matched logistic regression model (Table 1). The 1:1 propensity score matching was performed based on previously published methods by using the “nearest neighbor” matching method. Patients were selected from the ICM-unexposed (US) patient sample that were similar in baseline characteristics and AKI risk factors to the ICM-exposed (CT) patient sample (9–11,20). Matching was stratified into two groups based on eGFR before imaging (normal or mildly decreased kidney function [eGFR ≥60 mL/min/1.73 m2] and moderately to severely decreased kidney function [eGFR <60 mL/min/1.73 m2]).
Statistical Analysis
Continuous variables were summarized by using means and standard deviations, whereas categorical variables were summarized by using counts and percentages. The Student t test (two-tailed) and the χ2 test were performed to assess differences in continuous and categorical variables, respectively, between ICM-exposed and ICM-unexposed patient samples before and after 1:1 propensity score matching. These tests were also used to compare patients who did and patients who did not experience AKI after imaging. After 1:1 propensity score matching, univariable and multivariable logistic regression analyses were performed to assess the association between ICM and other covariates with the development of AKI after imaging. For multivariable modeling, stepwise model selection was used with entry and stay significance levels of .05.
A P value less than .05 was considered to indicate statistical significance for inference testing, and 95% confidence intervals (CIs) were calculated as appropriate. All statistical analyses were performed by using SAS (version 9.4; SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 21 106 imaging examinations were identified based on our inclusion criteria (CT with intravenous ICM [exposed sample], 14 757; abdominal US [unexposed sample], 6349). After applying exclusion criteria and before propensity score matching, a total of 2198 imaging examinations remained (exposed sample, 1137; unexposed sample, 1061). After propensity score matching, 1850 imaging examinations were selected for analysis (ICM-exposed, 925; ICM-unexposed, 925; eGFR ≥60 mL/min/1.73 m2, 889 per sample; eGFR <60 mL/min/1.73 m2, 36 per sample). See the Figure for a flow diagram detailing how the inclusion and exclusion criteria were applied.
Flowchart shows patient selection. CRRT = continuous renal replacement therapy, ICM = iodinated contrast material, IV = intravenous, SCr = serum creatinine.
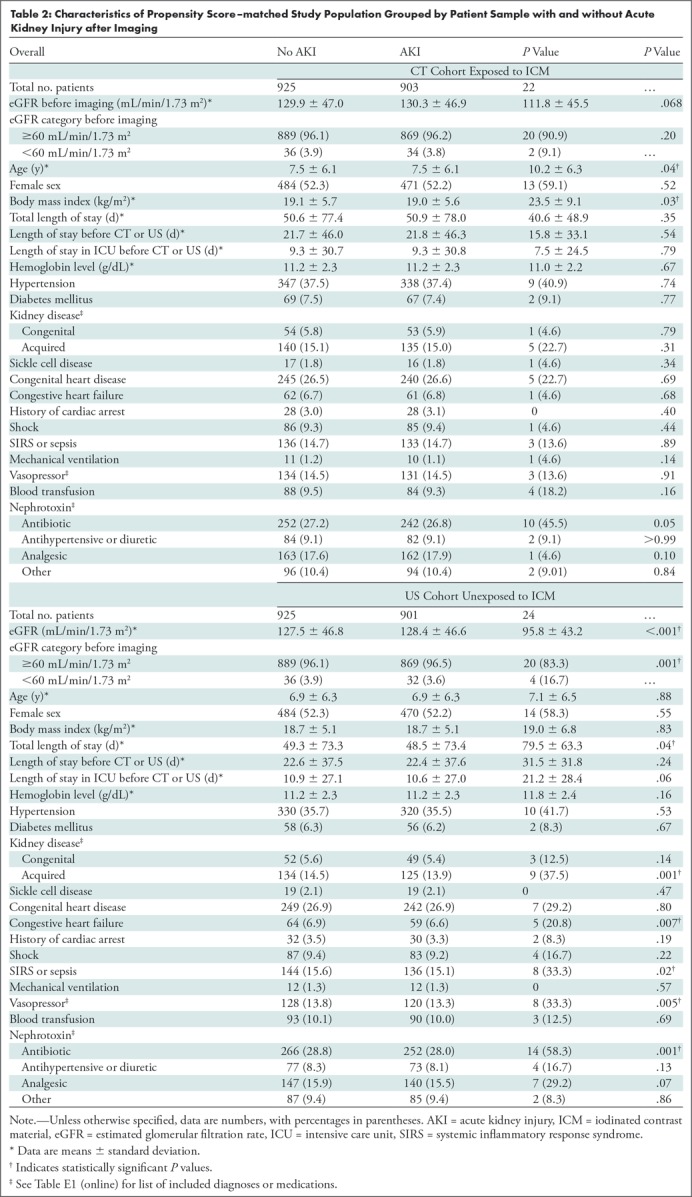
Table 1 and Table E2 (online) summarizes the covariates used for propensity score matching of the exposed and unexposed samples, stratified by eGFR before imaging. After propensity score matching stratified by eGFR before imaging, only a single variable (age) remained different between patient samples for the eGFR greater than or equal to 60 mL/min/1.73 m2 stratum (CT vs US samples: 7.7 years ± 6.1 [standard deviation] vs 7.2 years ± 6.3; P = .046). Table 2 summarize these covariates for the entire 1:1 propensity score–matched study population, grouped by patient sample and by presence or absence of AKI after imaging.
Table 2:
Characteristics of Propensity Score–matched Study Population Grouped by Patient Sample with and without Acute Kidney Injury after Imaging
Univariable Logistic Regression Analyses
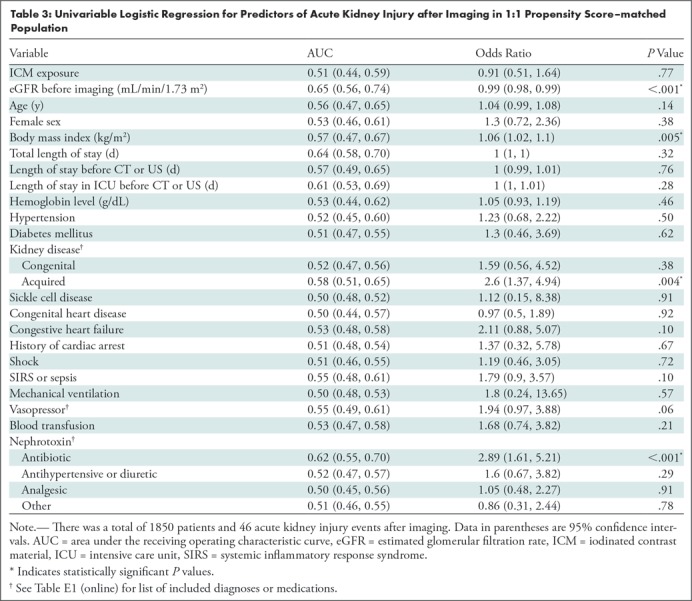
Results from univariable logistic regression analyses testing the association between covariates and the development of AKI after imaging in the 1:1 propensity score–matched population are presented in Table 3. Of note, significant univariable predictors for AKI after imaging included eGFR before imaging (odds ratio [OR]: 0.99 per unit [95% CI: 0.98, 0.99]; P < .001), body mass index (OR: 1.06 per unit [95% CI: 1.02, 1.10]; P = .005), presence of an acquired kidney disease (OR: 2.60 [95% CI: 1.37, 4.94]; P = .004), and nephrotoxic antibiotic exposure (OR: 2.89 [95% CI: 1.61, 5.21]; P < .001). ICM exposure was not a significant predictor of AKI after imaging (OR: 0.91 [95% CI: 0.51, 1.64]; P = .77).
Table 3:
Univariable Logistic Regression for Predictors of Acute Kidney Injury after Imaging in 1:1 Propensity Score–matched Population
Multivariable Logistic Regression Analyses
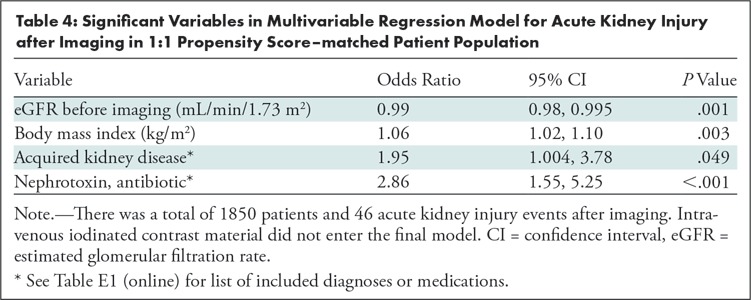
Table 4 demonstrates the results of multivariable logistic regression for AKI after imaging. ICM exposure was not an independent predictor of AKI after imaging and therefore was not included in the final multivariable model following the stepwise variable selection process. The following variables were found to be significant independent predictors of AKI in the multivariable model: eGFR before imaging (OR: 0.99 per unit [95% CI: 0.98, 0.995]; P = .001), body mass index (OR: 1.06 per unit [95% CI: 1.02, 1.10]; P = .003), presence of an acquired kidney disease (OR: 1.95 [95% CI: 1.004, 3.78]; P = .049), and nephrotoxic antibiotic exposure (OR: 2.86 [95% CI: 1.55, 5.25]; P < .001).
Table 4:
Significant Variables in Multivariable Regression Model for Acute Kidney Injury after Imaging in 1:1 Propensity Score–matched Patient Population
Incidence of AKI after Imaging
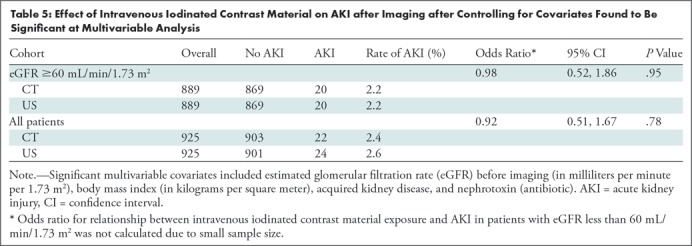
The incidence of AKI after imaging in patients with an eGFR before imaging of greater than or equal to 60 mL/min/1.73 m2 was 2.2% (20 of 889) in both the exposed and unexposed propensity score–matched samples (OR: 0.98 [95% CI: 0.52, 1.86]; adjusted P = .95). In patients with an eGFR before imaging of less than 60 mL/min/1.73 m2, AKI after imaging occurred at a higher absolute frequency in the ICM-unexposed propensity score–matched sample (11.1%, four of 36) than in the ICM-exposed propensity score–matched sample (5.6%, two of 36), although this difference was not statistically significant and underpowered due to small sample size (OR: 0.75 [95% CI: 0.11, 5.00]; adjusted P = .76). The overall frequency of PC-AKI was 2.4% (22 of 925) when including patients from both eGFR strata. Table 5 shows the association between ICM exposure and AKI after imaging after controlling for covariates that were significant in the multivariable logistic model for hospitalized pediatric patients, with an eGFR before imaging of greater than or equal to 60 mL/min/1.73 m2.
Table 5:
Effect of Intravenous Iodinated Contrast Material on AKI after Imaging after Controlling for Covariates Found to Be Significant at Multivariable Analysis
Discussion
There is a lack of propensity score–matched studies documenting the risk of acute kidney injury (AKI) after intravenous iodinated contrast material (ICM) exposure in the pediatric population. Similarly, there is a paucity of published literature assessing specific risk factors for AKI after imaging, including ICM exposure. In this propensity score–matched study, we observed identical frequencies of stage I AKI (2.2%; adjusted P = .95) in hospitalized pediatric patients with estimated glomerular filtration rate (eGFR) greater than or equal to 60 mL/min/1.73 m2 who underwent contrast material–enhanced CT and abdominal US (ICM-unexposed sample), respectively. We found an incidence of stage I postcontrast AKI (PC-AKI) of 2.4% for the entire contrast-exposed study sample, although pediatric patients with eGFR less than 60 mL/min/1.73 m2 are generally underrepresented, thereby limiting conclusions in this group. Multivariable logistic regression showed intravenous ICM exposure was not independently associated with AKI after imaging (P > .05; ICM exposure did not enter the final model).
We observed that intravenous ICM exposure was not a significant predictor of AKI in univariable or multivariable logistic regression analyses in the 1:1 propensity score–matched study population. Our study provides evidence in a pediatric population supporting the growing body of literature in adults calling into question the nephrotoxicity of intravenous ICM for patients with mild kidney impairment. Our study is one of a very small number of pediatric investigations to use propensity score matching to study PC-AKI and follows published adult data: no propensity score–matched study in adults has found an increased risk of AKI in ICM-exposed patients with eGFR greater than or equal to 60 mL/min/1.73 m2 (11). We believe our results should reassure medical care providers who order clinically indicated contrast-enhanced CT examinations in pediatric patients with normal or mildly impaired renal function.
In the sample of patients with moderate to severe kidney impairment (eGFR <60 mL/min/1.73 m2), a higher absolute frequency of AKI was observed after abdominal US (11.1%) than after contrast-enhanced CT (5.6%). However, the frequencies of AKI were not different between the two groups (underpowered assessment; 36 per cohort). Data in the adult literature are conflicting regarding differential AKI risk in intravenous ICM-exposed patients with moderate to severe chronic kidney disease (11). One large study using propensity score matching showed a lower risk of AKI after imaging in ICM exposed versus unexposed adult patients (13). Conversely, a similar propensity-matched study in adult inpatients found a greater risk of AKI in patients with severe kidney impairment (eGFR <30 mL/min/1.73 m2) who were exposed to intravenous ICM (36% vs 19%) (8). Because of small sample size, we are unable to make any strong conclusions about the association between AKI after imaging and intravenous ICM exposure in pediatric patients with moderately to severely impaired kidney function. We believe further research in this population would be of particular interest.
The observed rate of AKI after imaging in our propensity score–matched study population is lower than the observed rates in other pediatric studies. In a study by Sutherland et al (21), the incidence of AKI, defined by Acute Kidney Injury Network SCr-related criteria, in hospitalized children was 37%. However, the Sutherland et al study aimed to elicit differences in AKI rates between available standardized definitions, did not apply exclusion criteria similar to our study, and observed patients for AKI over an entire admission. It is noteworthy that our study intentionally excluded patients with unstable baseline creatinine or history of renal transplant, continuous renal replacement therapy, or dialysis and observed for AKI only over a 48-hour period following imaging (per established guidelines). In a pediatric investigation similar to ours, McDonald et al (14) demonstrated a frequency of PC-AKI in an ICM-exposed group of 3.3%.
We elected to use abdominal US for our control group for several reasons. First, unenhanced abdominal CT examinations are infrequently performed at our pediatric hospital, yielding a small number of potential study participants and possible selection bias. Second, abdominal US examinations are frequently performed at our hospital, and these inpatients may be ill and/or unable to travel to CT examinations, yielding a relatively large number of potential unexposed study participants with comorbidities for propensity score matching. Prior to our investigation and the recent pediatric study by McDonald et al, conclusions regarding the association between intravenous ICM exposure and PC-AKI in pediatric patients could only be extrapolated from adult studies (8–10,12–14). Although there remains only a small body of pediatric literature on this topic to date, the results of these studies have mirrored those observed in adults.
Our study had limitations. Propensity score matching in an observational study can only control known confounders. Therefore, our study may be biased by unknown confounders that enrich the control group with greater risk. We relied on our hospital’s electronic medical record to identify pediatric patients exposed and patients unexposed to ICM as well as associated comorbidities, medications, anthropomorphic data, et cetera. This methodology is vulnerable to biases related to record accuracy and completeness. For example, we were unable to identify which patients received renoprotective measures prior to CT, such as intravenous hydration. Although our matched population included more than 1800 pediatric patients, our study is still relatively small when compared with similar adult investigations, because CT is less commonly performed in children. Our study included only 72 patients with moderate or severe kidney impairment, which prevented assessment of the nephrotoxic risk of ICM in that cohort. Furthermore, those patients were, on average, much younger than were those with an eGFR greater than or equal to 60 mL/min/1.73 m2. Finally, we did not compare the total volume of ICM injected with the risk of AKI in this study, as the volume administered is standardized based on patient size.
In conclusion, hospitalized pediatric patients with stable kidney function who underwent contrast material–enhanced CT imaging had a similar frequency of acute kidney injury (AKI) compared with a propensity score–matched patient sample unexposed to iodinated contrast material (ICM) who underwent abdominal US. We found the overall frequency of postcontrast AKI (PC-AKI) to be 2.4%. In patients with normal or mildly decreased kidney function (estimated glomerular filtration rate ≥60 mL/min/1.73 m2), intravenous ICM exposure was not independently associated with PC-AKI.
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgments
We would like to acknowledge the Division of Biomedical Informatics at Cincinnati Children’s Hospital Medical Center, which provided in-kind support in the form of data collection. Research data services provided by the Division of Biomedical Informatics are partially supported by the Center for Clinical & Translational Science & Training (CCTST).
Supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (5UL1TR001425–03). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures of Conflicts of Interest: L.A.G. disclosed no relevant relationships. M.S.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: receives royalties from UpToDate.com and Wolters Kluwer. Other relationships: disclosed no relevant relationships. A.T.T. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Guerbet. Other relationships: disclosed no relevant relationships. W.S. disclosed no relevant relationships. B.Z. disclosed no relevant relationships. S.L.G. disclosed no relevant relationships. J.R.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from Bracco Diagnostics. Other relationships: disclosed no relevant relationships.
Abbreviations:
- AKI
- acute kidney injury
- CI
- confidence interval
- eGFR
- estimated glomerular filtration rate
- ICM
- iodinated contrast material
- OR
- odds ratio
- PC-AKI
- postcontrast AKI
- SCr
- serum creatinine
References
- 1. Trout AT, Dillman JR, Ellis JH, Cohan RH, Strouse PJ. . Patterns of intravenous contrast material use and corticosteroid premedication in children--a survey of Society of Chairs of Radiology in Children’s Hospitals (SCORCH) member institutions . Pediatr Radiol 2011. ; 41 ( 10 ): 1272 – 1283 . [DOI] [PubMed] [Google Scholar]
- 2. Nash K, Hafeez A, Hou S. . Hospital-acquired renal insufficiency . Am J Kidney Dis 2002. ; 39 ( 5 ): 930 – 936 . [DOI] [PubMed] [Google Scholar]
- 3. American College of Radiology . ACR Manual on Contrast Media . Reston, Va: : American College of Radiology; , 2018. . [Google Scholar]
- 4. Cochran ST, Wong WS, Roe DJ. . Predicting angiography-induced acute renal function impairment: clinical risk model . AJR Am J Roentgenol 1983. ; 141 ( 5 ): 1027 – 1033 . [DOI] [PubMed] [Google Scholar]
- 5. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. . Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality . Am J Med 1997. ; 103 ( 5 ): 368 – 375 . [DOI] [PubMed] [Google Scholar]
- 6. Rose BD. . Pathophysiology of renal disease . 2nd ed. New York, NY: : McGraw-Hill; , 1987. . [Google Scholar]
- 7. Moore A, Dickerson E, Dillman JR, et al. Incidence of nonconfounded post-computed tomography acute kidney injury in hospitalized patients with stable renal function receiving intravenous iodinated contrast material . Curr Probl Diagn Radiol 2014. ; 43 ( 5 ): 237 – 241 . [DOI] [PubMed] [Google Scholar]
- 8. Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. . Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate . Radiology 2013. ; 268 ( 3 ): 719 – 728 . [DOI] [PubMed] [Google Scholar]
- 9. Davenport MS, Khalatbari S, Cohan RH, Ellis JH. . Contrast medium-induced nephrotoxicity risk assessment in adult inpatients: a comparison of serum creatinine level- and estimated glomerular filtration rate-based screening methods . Radiology 2013. ; 269 ( 1 ): 92 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. . Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material . Radiology 2013. ; 267 ( 1 ): 94 – 105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dekkers IA, van der Molen AJ. . Propensity Score Matching as a Substitute for Randomized Controlled Trials on Acute Kidney Injury After Contrast Media Administration: A Systematic Review . AJR Am J Roentgenol 2018. ; 211 ( 4 ): 822 – 826 . [DOI] [PubMed] [Google Scholar]
- 12. McDonald JS, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. . Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate . Radiology 2014. ; 271 ( 1 ): 65 – 73 . [DOI] [PubMed] [Google Scholar]
- 13. McDonald RJ, McDonald JS, Bida JP, et al. Intravenous contrast material-induced nephropathy: causal or coincident phenomenon? Radiology 2013. ; 267 ( 1 ): 106 – 118 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonald JS, McDonald RJ, Tran CL, Kolbe AB, Williamson EE, Kallmes DF. . Postcontrast Acute Kidney Injury in Pediatric Patients: A Cohort Study . Am J Kidney Dis 2018. ; 72 ( 6 ): 811 – 818 . [DOI] [PubMed] [Google Scholar]
- 15. Menon S, Kirkendall ES, Nguyen H, Goldstein SL. . Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months . J Pediatr 2014. ; 165 ( 3 ): 522 – 7 . e2, e522 . [DOI] [PubMed] [Google Scholar]
- 16. Lopes JA, Jorge S. . The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review . Clin Kidney J 2013. ; 6 ( 1 ): 8 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury . Crit Care 2007. ; 11 ( 2 ): R31 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwartz GJ, Work DF. . Measurement and estimation of GFR in children and adolescents . Clin J Am Soc Nephrol 2009. ; 4 ( 11 ): 1832 – 1843 . [DOI] [PubMed] [Google Scholar]
- 19. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification . Am J Kidney Dis 2002. ; 39 ( 2 Suppl 1 ): S1 – S266 . [PubMed] [Google Scholar]
- 20. Austin PC. . An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies . Multivariate Behav Res 2011. ; 46 ( 3 ): 399 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sutherland SM, Byrnes JJ, Kothari M, et al. AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions . Clin J Am Soc Nephrol 2015. ; 10 ( 4 ): 554 – 561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.