Abstract
Adrenocortical carcinoma (ACC) is an uncommon endocrine malignancy with limited treatment options. While the overall 5-year survival rate in patients with ACC is 35%, the disease is often rapidly progressive with long-term survival in only 5% of patients. Although tumor stage, grade, and excess hormonal activity predict unfavorable prognosis, additional biomarkers are needed to identify patients with aggressive disease. A 23-year-old woman presented with rapidly progressing signs and symptoms of Cushing’s syndrome, with associated abdominal pain and fullness. Evaluation revealed a large left adrenal mass which had developed over 8 months. En bloc surgical resection was performed by an endocrine surgeon, and pathology revealed adrenocortical carcinoma with Ki67 of 60%. Despite adjuvant treatment with mitotane and etoposide–doxorubicin–carboplatin chemotherapy, the patient had rapid disease progression with metastatic spread to liver, lung, bone, brain, and leptomeningies, and she died 11 months after the initial diagnosis. Subsequent analysis of the patient’s tumor revealed mutations in TP53 and MEN1. RNA sequencing was compared against the the Cancer Genome Atlas data set and clustered with the high steroid, proliferative subtype, associated with the worst prognosis. The tumor also demonstrated a low BUB1B/PINK1 ratio and G0S2 hypermethylation, both predictive of very aggressive ACC. This case represents a subset of ACC characterized by rapid and fatal progression. Clinically available predictors as well as recently reported molecular signatures and biomarkers correlated with this tumor’s aggressiveness, suggesting that development and validation of combinations of biomarkers may be useful in guiding personalized approaches to patients with ACC.
Keywords: adrenocortical carcinoma, leptomeningeal metastasis
Adrenocortical carcinoma (ACC) is a rare, aggressive malignancy with incidence of 0.7 to 2 cases per million annually [1]. ACC presents across the age spectrum with peaks in children less than 5 years and in adults in their fourth and fifth decade of life [1]. The prognosis is poor, as the majority of patients present with regional and distant metastasis at the time of diagnosis. Long-term survivors, however, are occasionally reported. Clinically, Ki67 immunohistochemistry has been used as the primary prognostic biomarker. Recent global profiling of ACC tumors has demonstrated that comprehensive genomic and genetic signature clusters correlate with ACC tumor aggressiveness, but this clustering has not been used prospectively and may be cumbersome for clinical practice [2].
Common sites of metastasis for ACC include the liver, lungs, and bone [1]. Rarely ACC metastasizes to skin and brain [1]. Leptomeningeal carcinomatous metastases (LM) across all tumor types typically present as multifocal lesions in the leptomeninges in less than 5% of patients with advanced cancer [3]. The most common solid tumors to result in LM include breast, lung, melanoma, and gastrointestinal malignancies [3]. There have been few case reports of ACC resulting in brain metastasis [4–16] and there are only 2 previous ACC cases with leptomeningeal metastases reported in the literature [17, 18].
Here we present the case of a young woman who presented with severe Cushing’s syndrome due to ACC with rapid disease progression and LM despite conventional therapy.
1. Material and Methods
A. Patient Data Collection
The institutional review board was approved at the University of Colorado to perform this retrospective study and the patient consented for data collection. A chart review of the patient’s history was performed. Tumor characteristics, treatment regimens, and clinical outcome data were collected.
B. Tissue Processing, Real-Time qPCR and Methylation-sensitive Restriction Digest/qPCR
Tumor tissues were harvested and RNA and DNA was extracted using Trizol as previously reported [19] and quantitative polymerase chain reaction (qPCR) performed (all supplementary material and figures are located in a digital research materials repository [20]). Genomic DNA extracted from the patient was subjected to methylation-sensitive restriction digestion using Epitect II Methylation enzyme kit (Qiagen # 335452) according to the manufacturer’s protocol [21]. Following digestion, genomic DNA was amplified for G0S2 using a G0S2 primer mix from Qiagen (#EPHS101235-1A) and the percent G0S2 methylation was calculated.
C. RNA Sequencing and Data Analysis
Quantified gene expression data in the HTseq-count [22] format for the Cancer Genome Atlas (TCGA) Adrenocortical Carcinoma cohort [2] was downloaded from the Genomic Data Commons [23]. The patient’s (CU-ACC6) RNA-seq sample was quantified using the GDC mRNA Analysis pipeline [22].
2. Results
A. Case
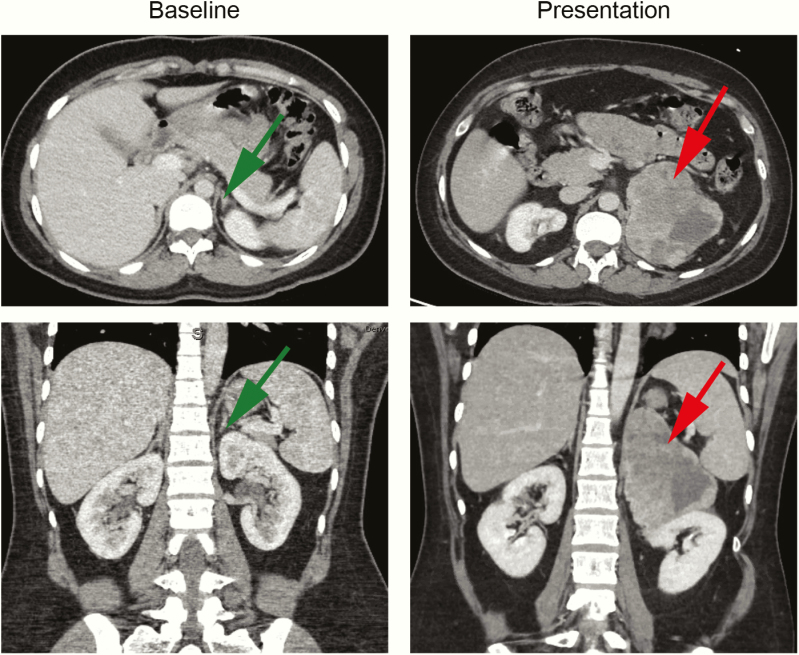
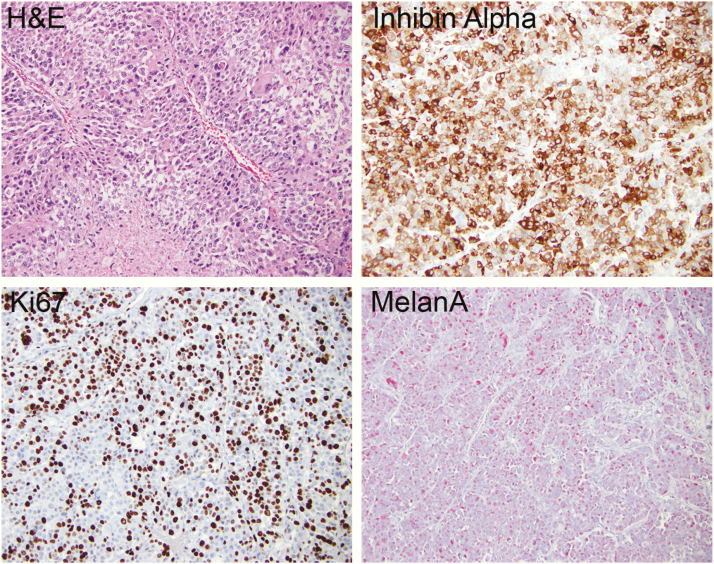
A 23-year-old woman presented with a 50-pound weight gain over 3 months, diffuse swelling, abdominal bloating, and severe hirsutism, with new-onset hypertension and diabetes mellitus and a blood glucose greater than 400 mg/dL. Prior evaluation for abdominal pain, 8 months earlier, revealed normal computed tomography (CT) of the abdomen/pelvis (Fig. 1). On admission, a CT scan revealed a left adrenal mass measuring 10.1 × 7.9 × 10.8 cm without any signs of direct invasion into other structures, no adenopathy, and a normal right adrenal gland (Fig. 1). Laboratory data on admission are outlined in Table 1. Given the rapid onset and size of the adrenal mass and severe cortisol and androgen excess, the patient underwent a left adrenalectomy and nephrectomy. Pathology confirmed ACC Stage 3, pT3pN1Mx with 1/1 lymph node positive with a high Ki67 score of 60% and no detection of abnormalities in mismatch repair proteins (Fig. 2). Postoperatively, the patient was treated with mitotane and adjuvant doxorubicin, etoposide, and carboplatin. The chemotherapy was discontinued after 2 cycles because of neutropenic fever, intra-abdominal abscesses, and pancreatic pseudocysts. Mitotane was continued; however, she was readmitted within a month with recurrence in the left adrenalectomy/nephrectomy bed as well as new liver metastases. Two cycles of palliative carboplatin and paclitaxel were administered. Three months later, the patient presented to the emergency department with a generalized tonic–clonic seizure. Neurological examination at time of presentation was nonfocal. Magnetic resonance imaging of the brain demonstrated leptomeningeal carcinomatosis with parenchymal metastatic disease. She was discharged to hospice care and died shortly after discharge, 11 months from the initial diagnosis.
Figure 1.
The abdominal computed tomography (CT) of the patient at baseline and at presentation only 8 months later revealing a large (10.1 x 7.9 x 10.8 cm) heterogeneous left adrenal mass. Green arrow shows normal adrenal gland; Red arrow shows large ACC mass.
Table 1.
ACC006 patient laboratory values.
| Labs | Baseline | Postsurgery | Recurrence | Reference range |
|---|---|---|---|---|
| TSH | 0.85 | 1.96 | 0.1 | 0.34–5.50 mIU/L |
| Free T4 | 0.91 | 1.61 | 0.68 | 0.89–1.76 ng/dL |
| Cortisol | 65 | 14 | – | 4–22 µg/dL |
| ACTH | 13 | 20 | Not checked | 6–58 pg/mL |
| DHEAS | 949 | 29 | 391 | 65–380 µg/dL |
| Testosterone | 809 | <20 | 820 | 14–76 ng/dL |
| Estradiol | – | <20 | – | 56–214 pg/mL (midcycle) |
| FSH | – | 3 | – | 3–33 mIU/mL (midcycle) |
Figure 2.
Immunohistochemistry of ACC006 tumor tissue showing hematoxylin and eosin (H&E), Inhibin alpha, Ki67, and MelanA staining.
B. Genetic and Genomic Profiling
To further examine the molecular mechanisms of this aggressive ACC tumor, transcriptome and whole-exome sequencing were performed. In contrast to the majority of ACC tumors, IGF2 was downregulated in the ACC006 tumor [20]. Hierarchical clustering of this tumor with TCGA ACC samples was performed, and demonstrated that ACC006 clustered with the most aggressive subtype—high proliferative secretory tumors [20]. Whole-exome sequencing revealed only two somatic mutations predicted to be damaging: TP53 p.V14G and MEN1 p.E368X, both unfortunately, nonactionable.
C. BUB1B–PINK Score and G0S2 Methylation
To test the usefulness of the recently reported ACC molecular biomarkers, we retrospectively analyzed the expression of BUB1B (BUB1 Mitotic Checkpoint Serine/Threonine Kinase B) and PINK1 (PTEN induced kinase 1) in tumor samples. The BUB1B–PINK1 score was 3.47, supportive of the approach here a score <5.2 has been associated with the risk for a poor outcome [24,25]. Analysis of methylation status of G0S2, another predictive biomarker, by methylation-sensitive restriction digest/qPCR demonstrated high 97% methylation (20.9 % hypermethylated and 76.9 % intermediate methylation). RNA expression analysis of the G0S2 by qPCR generated a delta Ct value that was undetectable, consistent with the highly methylated status of the gene.
3. Discussion
ACC carries a poor prognosis, especially in patients who have distant metastasis at the time of presentation. However, a large variation in overall survival has been reported [1]. Our patient initially presented without metastasis, yet had a very aggressive course, unresponsive to standard therapy. Predicting an aggressive tumor and identifying possible biomarkers can be challenging and is an ongoing area of study.
Intracranial ACC metastasis is very rare, with few cases reported in the literature (roughly 13 cases in adults and 9 cases in children). Tables 2 and 3 shows the variability in patient presentation with intracranial metastasis. Many of the patients had concomitant lung and liver metastases, the more common sites of metastases in ACC [1]. Our patient was unique in that she had leptomeningeal metastasis, an extremely rare presentation, with only 2 cases previously reported [17,18]. Similar to the patients included in Tables 2 and 3, she also had liver and lung metastases at the time of the CNS spread, a mutation in TP53 and her post-treatment survival was poor. Compared to previous ACC cases with LM, our patient presented with overt Cushing’s and similar survival as the pediatric case, whereas the previously reported adult patient had a nonfunctioning tumor and longer survival.
Table 2.
Adult patients with ACC and intracranial metastases.
| Author | Age (years) | Gender | Other metastases | Intracranial location | Survival (months) | Functional status | Brain metastasis from initial diagnosis |
|---|---|---|---|---|---|---|---|
| Seabold et al. (1977) [12] | 35 | F | Liver and lung | Brain | − | − | - |
| Seabold et al. (1977) [12] | 54 | M | Liver | Brain | − | Non-functioning | 17 months |
| Tartour et al. (1993) [13] | 60 | F | Liver, bone | Brain | − | − | - |
| Kubota et al. (1997) [6] | 47 | M | Lung and liver | Brain | 3 | − | 9 months |
| Bartley et al. (2001) [5] | 24 | M | Lung and vertebrae | Orbit/brain | 15 | Nonfunctioning | 2 years |
| Ohwada et al. (2006) [8] | 43 | F | Lung, live, bone | Brain | 23.7 | Cushingoid | ~2 years |
| Capone et al. (2009) [18] | 45 | M | Liver and lung | Brain, skull, meninges | 24 | Nonfunctioning | 6 months |
| Velarde et al. (2015) [14] | 29 | F | Liver and lung | Brain | 2 | − | 3 years |
| Velarde et al. (2015) [14] | 37 | M | Liver, lungs, lymph nodes | Brain | 8 | − | 3 years |
| Velarde et al. (2015) [14] | 38 | F | Liver and lung | Brain | 12 | − | 6 years |
| Velarde et al. (2015) [14] | 44 | M | Liver, bone, pleura | Brain | 7 | − | 6 years |
| Velarde et al. (2015) [14] | 48 | F | Lung | Brain | − | − | 11 years |
| Velarde et al. (2015) [14] | 60 | M | Lung | Brain | Lost to follow-up | − | 2 years |
Table 3.
Pediatric patients with ACC and intracranial metastases.
| Author | Age | Gender | Other metastases | Intracranial Location | Survival (months) | Functional Status | Brain metastasis from initial diagnosis |
|---|---|---|---|---|---|---|---|
| Lefvre et al. (1983) [16] | 17 months | M | Lung | Brain | − | − | − |
| Lefvre et al. (1983) [16] | 4 years | − | Brain | − | 3 | − | − |
| Saracco et al., (1988) [11] | 1 day | M | Skin | Brain | − | − | 4 months |
| Ayass, (1991) [4] | 17 months | M | Lung, paraspinal | Brain | − | − | − |
| Lack et al. (1992) [7] | 10 years | M | Lung, liver, kidneys | Brain | − | − | − |
| Piniella (2000) [9] | 9 years | F | Brain | − | >4 | − | − |
| Romaguera et al. (2001) [10] | 9 years | F | Lung | Brain | 24 | Cushingoid | 5 years |
| Hertel et al. (2003) [17] | 14 years | F | Lung and thigh | Meninges | 12 | Cushingoid | 8 years |
| Wagner et al. (2005) [15] | 10 years | M | Liver and lung | Brain | 9 | − | 3 years |
We and others have previously shown that cell cycle activation is a predominant part of the ACC transcriptome, especially for aggressive ACCs [2,19,26], and that a score based on mRNA expression of mitotic regulators BUB1B and PINK1 predicts prognosis and overall survival [24, 25]. More recently, hypermethylation of another cell cycle regulator G0S2 (G0/G1 switch 2) was shown to be an independent predictor of recurrence-free survival and overall survival in ACC patients [21]. Moreover, patients with hypermethylation of G0S2 (>4.69% detected by the Epitect Methylation kit) combined with a BUB1B–PINK1 score less than 5.2 resulted in intermediate or poor outcomes [21]. Our patient had a BUB1B–PINK1 score of 3.42 and demonstrated 97% methylation of G0S2. From the time of the discovery of her tumor to her death was slightly less than a year—a dismal prognosis, which correlated with these predictors.
Due to the variability in prognosis in patients with ACC, it can be often difficult to provide a clear treatment plan following radical surgery [24]. While this patient presented with a hormonally active tumor and high Ki67%, both prognosticators of aggressive disease, she did not have widely metastatic disease at presentation. With substantial variability reported in Ki67 scoring even between expert laboratories [27], additional predictive biomarkers such as the BUB1B–PINK1 score and hypermethylation of G0S2 could be useful prognostic tools to estimate disease aggressiveness. While these biomarkers did not have an impact on our patient, identifying additional prognosticators will be helpful to further stratify prognosis in patients with aggressive ACC, and guide treatment as additional therapies become available in the future.
Acknowledgments
Financial Support: This work was supported by NIH K08CA222620 (to K.K.V.), Cancer League of Colorado Award (to K.K.V. and S.L.), Veterans Affairs Merit Review Award 001 (to M.E.W.). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Glossary
Abbreviation
- ACC
adrenocortical carcinoma
- CT
computed tomography
- LM
leptomeningeal carcinomatous metastases
- qPCR
quantitative polymerase chain reaction
Additonal Information
Disclosure Summary: The authors declare no potential conflicts of interest
References
- 1. Else T, Kim AC, Sabolch A, et al. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng S, Cherniack AD, Dewal N, et al. ; Cancer Genome Atlas Research Network Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell. 2016;29(5):723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le Rhun E, Taillibert S, Chamberlain MC. Carcinomatous meningitis: Leptomeningeal metastases in solid tumors. Surg Neurol Int. 2013;4(Suppl 4):S265–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ayass M, Gross S, Harper J. High-dose carboplatinum and VP-16 in treatment of metastatic adrenal carcinoma. Am J Pediatr Hematol Oncol. 1991;13(4):470–472. [DOI] [PubMed] [Google Scholar]
- 5. Bartley GB, Campbell RJ, Salomão DR, Bradley EA, Marsh WR, Bite U. Adrenocortical carcinoma metastatic to the orbit. Ophthalmic Plast Reconstr Surg. 2001;17(3):215–220. [DOI] [PubMed] [Google Scholar]
- 6. Kubota Y, Iwai T, Nakatani K, Sakai N, Hara A. [Central nervous system metastasis from non-functioning adrenocortical carcinoma: report of a case]. No Shinkei Geka. 1997;25(11): 1039–1042. [PubMed] [Google Scholar]
- 7. Lack EE, Mulvihill JJ, Travis WD, Kozakewich HP. Adrenal cortical neoplasms in the pediatric and adolescent age group. Clinicopathologic study of 30 cases with emphasis on epidemiological and prognostic factors. Pathol Annu. 1992;27(Pt 1):1–53. [PubMed] [Google Scholar]
- 8. Ohwada S, Izumi M, Kawate S, et al. Surgical outcome of stage III and IV adrenocortical carcinoma. Jpn J Clin Oncol. 2007;37(2):108–113. [DOI] [PubMed] [Google Scholar]
- 9. Piniella AM, Siatkowski RM. Adrenal cortical carcinoma metastatic to the brain in a child. J Neuroophthalmol. 2000;20(1):35–37. [DOI] [PubMed] [Google Scholar]
- 10. Romaguera RL, Minagar A, Bruce JH, et al. Adrenocortical carcinoma with cerebral metastasis in a child: case report and review of the literature. Clin Neurol Neurosurg. 2001;103(1):46–50. [DOI] [PubMed] [Google Scholar]
- 11. Saracco S, Abramowsky C, Taylor S, Silverman RA, Berman BW. Spontaneously regressing adrenocortical carcinoma in a newborn. A case report with DNA ploidy analysis. Cancer. 1988;62(3):507–511. [DOI] [PubMed] [Google Scholar]
- 12. Seabold JE, Haynie TP, DeAsis DN, Samaan NA, Glenn HJ, Jahns MF. Detection of metastatic adrenal carcinoma using 131I-6-beta-iodomethyl-19-norcholesterol total body scans. J Clin Endocrinol Metab. 1977;45(4):788–797. [DOI] [PubMed] [Google Scholar]
- 13. Tartour E, Caillou B, Tenenbaum F, et al. Immunohistochemical study of adrenocortical carcinoma. Predictive value of the D11 monoclonal antibody. Cancer. 1993;72(11):3296–3303. [DOI] [PubMed] [Google Scholar]
- 14. Velarde M, Edgerly M, Tageja N, et al. Brain metastasis in patients with adrenocortical carcinoma: a clinical series. J Clin Endocrinol Metab. 2015;100(2):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner AS, Fleitz JM, Kleinschmidt-Demasters BK. Pediatric adrenal cortical carcinoma: brain metastases and relationship to NF-1, case reports and review of the literature. J Neurooncol. 2005;75(2):127–133. [DOI] [PubMed] [Google Scholar]
- 16. Lefevre M G-MR, Gubler JP, Chaussain JL, Lemerle J. Adrenal cortical carcinoma in children: 42 patients treated from 1958 to 1980 at Villejuif. In: Adrenal and Endocrine Tumors in Children: Adrenal Cortical Carcinoma and Multiple Endocrine Neoplasia. Boston, MA: Springer US; 1983: 265–276. [Google Scholar]
- 17. Hertel NT, Carlsen N, Kerndrup G, et al. Late relapse of adrenocortical carcinoma in Beckwith-Wiedemann syndrome. Clinical, endocrinological and genetic aspects. Acta Paediatr. 2003;92(4):439–443. [DOI] [PubMed] [Google Scholar]
- 18. Capone G, Della Pepa GM, Sabatino G, et al. A rare bone-leptomeningeal metastasis from an adrenal cortical carcinoma. J Clin Neurosci. 2009;16(7):977–980. [DOI] [PubMed] [Google Scholar]
- 19. Kiseljak-Vassiliades K, Zhang Y, Kar A, et al. Elucidating the role of the maternal embryonic leucine zipper kinase in adrenocortical carcinoma. Endocrinology. 2018;159(7):2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreiber AR, Kar A, Goodspeed AE, et al. Leptomeningeal metastasis from adrenocortical carcinoma: a case report. v2, Dryad Digital Repository Dataset. 10.5061/dryad.tqjq2bvvh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohan DR, Lerario AM, Else T, et al. Targeted assessment of G0S2 methylation identifies a rapidly recurrent, routinely fatal molecular subtype of adrenocortical carcinoma. Clin Cancer Res. 2019;25(11):3276–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grossman RL, Heath AP, Ferretti V, et al. Toward a shared vision for cancer genomic data. N Engl J Med. 2016;375(12):1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Reyniès A, Assié G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27(7):1108–1115. [DOI] [PubMed] [Google Scholar]
- 25. Fragoso MC, Almeida MQ, Mazzuco TL, et al. Combined expression of BUB1B, DLGAP5, and PINK1 as predictors of poor outcome in adrenocortical tumors: validation in a Brazilian cohort of adult and pediatric patients. Eur J Endocrinol. 2012;166(1):61–67. [DOI] [PubMed] [Google Scholar]
- 26. Mohan DR, Lerario AM, Hammer GD. Therapeutic targets for adrenocortical carcinoma in the genomics era. J Endocr Soc. 2018;2(11):1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Polley MY, Leung SC, McShane LM, et al. ; International Ki67 in Breast Cancer Working Group of the Breast International Group and North American Breast Cancer Group An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105(24):1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]