ABSTRACT
Background
Bovine milk-based fortifiers (BMBF) have been standard of care for nutrient fortification of feeds for very low birth weight (VLBW) infants, however, there is increasing use of human milk-based fortifiers (HMBF) in neonatal care despite additional costs and limited supporting data. No randomized clinical trial has followed infants fed these fortifiers after initial hospitalization.
Objective
To compare neurodevelopment in infants born weighing <1250 g fed maternal milk with supplemental donor milk and either a HMBF or BMBF.
Methods
This is a follow-up of a completed pragmatic, triple-blind, parallel group randomized clinical trial conducted in Southern Ontario between August 2014 and March 2016 (NCT02137473) with feeding tolerance as the primary outcome. Infants weighing <1250 g at birth were block randomized by an online third-party service to receive either HMBF (n = 64) or BMBF (n = 63) added to maternal milk with supplemental donor milk during hospitalization. Neurodevelopment was assessed at 18-mo corrected age using the Bayley Scales of Infant and Toddler Development, Third Edition. Follow-up was completed in October 2017.
Results
Of the 127 infants randomized, 109 returned for neurodevelopmental assessment. No statistically significant differences between fortifiers were identified for cognitive composite scores [adjusted mean scores 94.7 in the HMBF group and 95.9 in the BMBF group; fully adjusted mean difference, −1.1 (95% CI: −6.5 to 4.4)], language composite scores [adjusted scores 92.4 in the HMBF group and 93.1 in the BMBF; fully adjusted mean difference, −1.2 (−7.5 to 5.1)], or motor composite scores [adjusted scores 95.6 in the HMBF group and 97.7 in the BMBF; fully adjusted mean difference, −1.1 (−6.3 to 4.2)]. There was no difference in the proportion of participants that died or had neurodevelopmental impairment or disability between groups.
Conclusions
Providing HMBF compared with BMBF does not improve neurodevelopmental scores at 18-mo corrected age in infants born <1250 g otherwise fed a human milk diet. This trial was registered at clinicaltrials.gov as NCT02137473.
Keywords: very low birth weight infants, human milk, human milk-based fortifier, bovine milk-based fortifier, neurodevelopment, donor milk
Introduction
Maternal milk is recognized as the best source of nutrition for all infants, particularly those born at a very low birth weight (VLBW, <1500 g) (1–5). In the absence of sufficient maternal milk to meet infant needs, feeding pasteurized donor human milk (donor milk) rather than formula reduces risk of necrotizing enterocolitis (NEC) without any evidence of long-term effects on survival, growth, or neurodevelopment (6, 7, 8).
Due to elevated nutritional needs of infants born at a VLBW, additional nutrient fortification is required to support growth and development (9). Bovine milk-based fortifiers (BMBF) have been the standard of care for VLBW infants; however, human milk-based fortifiers (HMBF) are increasingly used in neonatal intensive care units at substantial additional financial cost. Earlier trials that compared HMBF and BMBF found that infants fed maternal milk or donor milk fortified with HMBF had lower rates of NEC and improved feeding tolerance relative to infants fed maternal milk fortified with BMBF and supplemented with formula as necessary (10–12). Although promising, these trials compared infants fed exclusively human milk products to those who received bovine products as formula and BMBF, so it was unclear whether the absence of BMBF, infant formula, or a combination of the 2 factors explained the better outcomes in the HMBF group. In addition, these trials do not reflect the current standard of care in North America, where it is recommended that VLBW infants be fed maternal milk first with donor milk, and not formula, as a supplement (13–15). To address these issues, our group conducted a randomized clinical trial that compared the use of HMBF and BMBF in VLBW infants fed human milk diets without any formula (16). In contrast to the earlier findings, our trial identified no differences in feeding tolerance or rates of Bell Stage ≥II NEC between the groups.
Follow-up comparing subsequent neurodevelopment in infants fed HMBF or BMBF is important for several reasons. Feeding infants maternal milk rather than bovine milk-derived formula is associated with improved performance on intelligence and cognitive tests in childhood and adolescence, even after adjusting for maternal education or socioeconomic status (5, 17). These differences may be mediated in part by the absence of bovine milk components, or by the presence of bioactive components of human milk that promote brain growth and development, including micronutrients, such as iron and zinc (18), neurotrophic factors, such as brain-derived neurotrophic factor and glial cell-derived neurotrophic factor (19), and cytokines, such as transforming growth factor (TGF)-β and tumor necrosis factor (TNF)-α (20). Many of these components are to some degree resistant to heat (21), and presumably are present in HMBF. The HMBF also provided slightly more protein than the BMBF used in our original trial at what seems to be a sensitive period, as intakes of protein, lipid, and energy in the first weeks following birth have been associated with performance on neurodevelopmental tests at 1 y and 18 mo among infants born at a VLBW (22, 23). Another important consideration is that the HMBF used in our study was provided in liquid form and displaced between 25–67% of other human milk (mother or donor) from the infants’ diets (16). This displacement could lead to unintended neurodevelopmental consequences that require investigation. Here, we present the results of the first randomized clinical trial of HMBF and BMBF with neurodevelopmental follow-up.
Methods
The participants with follow-up assessments reported here were enrolled in the OptiMoM (Optimizing Mothers’ Milk for Preterm Infants) Fortifier Study (NCT02137473), a pragmatic, multi-center, triple-blind, parallel group, randomized clinical trial designed to compare the efficacy of using HMBF or BMBF in infants born <1250 g fed maternal milk supplemented with donor milk as necessary. The primary outcome of the OptiMoM Fortifier Study was feeding tolerance, with secondary outcomes including growth, gut inflammation, and neonatal mortality and morbidity. Participants were enrolled in the original trial between August 2014 and November 2015 from neonatal intensive care units at Sinai Health System and the Hospital for Sick Children in Toronto, Canada.
Details of the trial methods and results of the original trial have been published (16). Briefly, infants were included if they were born <1250 g and if their parents consented to use supplemental donor milk in the absence of sufficient maternal milk. Infants were excluded if they received infant formula or a BMBF prior to randomization, if enteral feeding was not initiated within 14 d of birth, if they had a chromosomal or congenital anomaly that could affect growth, if they were participants in another study that affected their nutritional management, or if they were likely to be transferred to a neonatal intensive care unit where the study protocol could not be followed.
As part of routine care, each study participant qualified for neonatal neurodevelopmental follow-up at 18-mo corrected age (age taken from expected date of delivery). These assessments occurred between May 2016 and October 2017 at the neonatal follow-up clinics at each recruiting hospital. The protocols for both the original study and this follow-up were approved by the institutional Human Research Ethics Boards at Sinai Health System and the Hospital for Sick Children in Toronto, Canada. Consent was obtained from the parents or guardians of participants. Baseline characteristics for the study participants were collected from patient records and from questionnaires administered by study staff.
Feeding intervention
Full details of the feeding intervention, including the weight-based feeding protocol, have been published previously (16). Infants were randomized by a third-party online service in blocks of 4, stratified by birth weight <1000 g or 1000–1250 g and recruitment center. Briefly, infants were fed maternal milk whenever it was available and donor milk as required; enteral feeds were prepared fresh daily under laminar flow in 1 of 2 designated milk preparation rooms. Tube feeds were prepared in amber syringes, and bottles were wrapped in colored cellophane to maintain blinding of families, healthcare providers, and study staff. Fortification began once enteral feeds reached 100 mL/kg/d, with full enteral feeding considered to be achieved at 160 mL/kg/d. In addition to the study fortifiers, infants were prescribed iron (2–3 mg/kg/d elemental) and vitamins A (375 IU), C (17.5 mg/d), and D (400 IU until body weight reached 2000 g, 200 IU thereafter). The intervention lasted until the first of the following occurred: infants were aged 84 d, were discharged from hospital, or were consistently able to consume 2 oral feeds daily.
Fortification in both groups was initiated when infants reached an enteral tolerance of 100 mL/kg/d. Feeds in the HMBF group were fortified according to the published feeding protocol (16) with Prolact + 4, Prolact + 6, and Prolact + 8 (Prolacta Bioscience City of Industry). Fortification began at 0.81 kcal/mL (2.2 g protein/100 mL prepared feed) and was increased to 0.88 kcal/mL (2.7 g protein/100 mL) when the infant reached intakes of 140 mL/kg/d. In the BMBF group, feeds were fortified with Similac Human Milk Fortifier Powder (Abbott Nutrition) and Similac Neosure powdered formula (Abbott Nutrition); both containing intact protein. Fortification began at 0.72 kcal/mL (1.7 g protein/100 mL prepared feed) and was increased to 0.78 kcal/mL (2.2 g protein/100 mL) once the infant reached intakes of 140 mL/kg/d. An intact protein module (Beneprotein, Nestle Health Sciences) was added to donor milk (0.4 g/100 mL) in the BMBF group once fortification reached 0.78 kcal/mL to better mimic the protein content of maternal milk. No additional bovine protein fortification was added to donor milk in the HMBF group. In both groups, additional fortification was provided to meet growth targets.
Study outcomes
The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III, Pearson Education, Inc.) was offered to all participants at 18-mo corrected age. Briefly, the Bayley-III is a standardized neurodevelopmental test routinely administered to infants born at a VLBW as part of neonatal follow-up. It consists of cognitive, language (receptive, expressive), and motor (gross, fine) subtests that are converted to age-adjusted composite scores standardized to have a mean of 100 and an SD of 15. Scores can be further classified as Very Superior (≥130), Superior (120–129), High Average (110–119), Average (90–109), Low Average (80–89), Borderline (70–79), and Extremely Low (≤69). Families remained blinded to the treatment assignment and tests were administered by trained, blinded assessors, either at the neonatal follow-up clinics at Sinai Health System or the Hospital for Sick Children, or in the participants’ homes if they were unable to attend a follow-up appointment. Interrater reliability between assessors was over 80% in videotaped sessions, and tests were double counted by a second researcher unaware of the feeding assignments. As with our previous study (6) and in line with other experts in the field (24, 25), those who attended the follow-up visit but could not complete the Bayley-III due to disability or who performed below the threshold for individual composite scores were assigned a score of 49.
Data on visual impairment (acuity <20/200 despite amplification), hearing impairment (requiring amplification), or diagnosis of cerebral palsy (diagnosis by doctor blinded to treatment assignment) were collected from the medical records at the 18-mo assessment. Death and neurodevelopmental impairment or death and neurodevelopmental disability were calculated as dichotomous variables by combining infants who either died during the intervention (i.e. after receiving the first fortified feed), or were diagnosed with cerebral palsy or an impairment in hearing or vision, or who scored below cut-offs on the Bayley-III, similar to previous studies (6, 26, 27). The Bayley-III cut-off used for neurodevelopmental impairment was <85, whereas <70 was used for the categorization of neurodevelopmental disability.
Statistical analysis
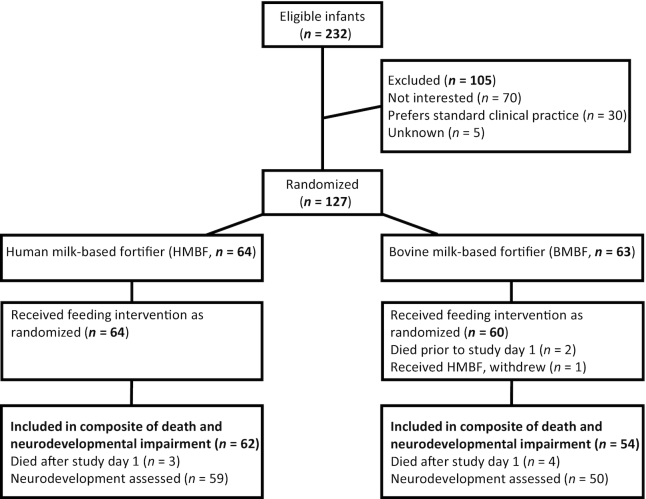
Analysis was carried out in SAS version 9.4 (SAS Institute). Infants who were randomized but who died prior to receiving either study feed (n = 2) and survivors who withdrew from the study (n = 2) or did not attend the 18-mo corrected age follow-up visit and for whom follow-up in their home could not be arranged (n = 7) were not included in the analysis (Figure 1). Participants who died during the study (n = 7) were not included in the analysis of Bayley-III composite scores, but they were included in the analysis of the dichotomous death and neurodevelopmental impairment or neurodevelopmental disability variables. Because of the high rate of follow-up (86% of all subjects, 92% of survivors) and completeness of the dataset, we did not perform any imputation of missing data. Composite scores on the Bayley-III are standardized to a mean of 100 with an SD of 15 (28). Thus, our sample of 109 infants with neurodevelopmental follow-up could provide 80% power at α = 0.05 to detect an 8 point difference in mean composite scores between fortifier groups. Sample size varies slightly by subtest, as a small number of participants did not complete parts of the Bayley-III [language n = 3 (1 in the HMBF group and 2 in the BMBF group), and motor n = 1 (in the HMBF group)] due to behavior. Further, 2 subjects included in the primary analysis had missing data for maternal education or income [n = 2, (both in the HMBF group)], which reduced the available sample size for the fully adjusted model 2.
FIGURE 1.
Participant flow diagram of very low birth weight infants enrolled in the Optimizing Mothers’ Milk for Preterm Infants Fortifier trial. Infants were randomized to receive a human milk-based fortifier or a bovine milk-based fortifier added to an entirely human milk diet (maternal or donor) during initial hospitalization. Participants returned for neurodevelopmental testing with the Bayley Scales of Infant and Toddler Development, Third Edition at 18-mo corrected age per routine clinical follow-up.
Baseline characteristics were compared between the groups using chi-square tests for categorical variables, t-test for normally distributed continuous variables, and the Kruskal–Wallis test for nonnormally distributed continuous variables. Composite scores for each of the 3 Bayley-III domains were analyzed as continuous variables using linear regression models (PROC GLM) adjusting for birth weight strata (<1000 g or 1000–1249 g) in model 1, and for birth weight strata, sex, small for gestational age (y/n), any donor milk intake (y/n), maternal education (high school or less, college or vocational diploma, baccalaureate, postbaccalaureate), and income above or below the poverty line (y/n under the family size-adjusted Ontario poverty line) in model 2. The dichotomous variables of death and neurodevelopmental impairment or death and neurodevelopmental disability were analyzed using logistic regression adjusting for birth weight strata and sex. Interactions between feeding group and the covariates included in each model were tested and removed from the model if nonsignificant. All tests of significance were 2-sided with P < 0.05 after adjustment for multiple comparisons (Tukey–Kramer) considered statistically significant. Data were analyzed using superiority statistics because the substantial additional cost of feeding an infant with HMBF [estimated at >$10,000 in 2012 (29)] and greater potential for displacement of maternal milk from infant diets (up to 67%) necessitate some evidence of benefit to justify a switch from the BMBF standard of care.
Results
Study infants
Of the 232 participants approached for the original study, 127 were randomized: 64 to receive HMBF added to maternal milk or donor milk, and 63 to receive BMBF (Figure 1). Median duration in the study intervention was 48 d (IQR 30–61) in the HMBF group, and 51 d (39–62) in the BMBF group (P = 0.43) (16). As reported previously, 2 infants in the BMBF group died prior to receiving their first fortified feed and were not included in the analysis of any outcomes, and an additional 2 withdrew from the original study with no further data collection (16). Seven infants died during the original study period, 4 in the BMBF group and 3 in the HMBF group (16). Seven survivors did not complete the 18-mo follow-up visit (2 in the HMBF group and 5 in the BMBF group) due to moving out of the study area (n = 2), lack of interest (n = 2), behavior (n = 1), or loss to follow-up (n = 2). Of the infants who received the fortifier as randomized, 59/64 (92%) of infants in the HMBF and 50/60 (83%) of infants in the BMBF groups attended the 18-mo corrected age visit and had neurodevelopment assessed (P = 0.79).
Neurodevelopment
Neurodevelopment was assessed in 109 of the 118 surviving participants, a response rate of 92%. There were no significant differences in baseline characteristics of the children who attended the 18-mo neurodevelopmental follow-up between the 2 fortifier groups (Table 1). Infants were born at a mean of 897 g (SD 201 g) and 27.8 (SD 2.5) weeks of gestation; 62.4% of infants were born weighing <1000 g and 23.9% were classified as small for gestational age. Females accounted for 57.8% of the study population. There were few differences in baseline characteristics between participants who did and did not complete the 18-mo neurodevelopmental follow-up (Supplemental Table 1).
TABLE 1.
Baseline characteristics of participants who completed neurodevelopmental follow-up at 18-mo corrected age1
| Characteristics | HMBF (n = 59) | BMBF (n = 50) |
|---|---|---|
| Sex, no. (% female) | 35/59 (59.3) | 28/50 (56.0) |
| Birth weight, mean ± SD, g | 893 ± 210 | 901 ± 192 |
| Gestational age at birth,2 mean ± SD, wk | 27.9 ± 2.7 | 27.7 ± 2.2 |
| Multiple birth status, no. (%) | 21/59 (35.6) | 21/50 (42.0) |
| Small for gestational age, no. (%) | 13/59 (22.0) | 13/50 (26.0) |
| Received antenatal steroids, no. (%) | 51/59 (86.4) | 44/50 (88.0) |
| SNAP-II score,3 mean ± SD | 12.7 ± 11.1 | 13.9 ± 11.4 |
| Apgar score at 5 min, mean ± SD | 7.5 ± 2.0 | 7.3 ± 2.3 |
| Any donor milk intake, no. (%) | 27/59 (45.8) | 33/50 (66.0) |
| Mother's age, mean ± SD, y | 33.0 ± 4.6 | 34.1 ± 6.0 |
| Mother's education, no. (%) | ||
| High school or less | 7/58 (12.1) | 8/50 (16.0) |
| College or vocational diploma | 23/58 (39.7) | 18/50 (36.0) |
| Baccalaureate | 20/58 (34.5) | 15/50 (30.0) |
| Postbaccalaureate | 8/58 (13.8) | 9/50 (18.0) |
| Mother's ethnicity,4 no. (%) | ||
| Eastern or Western European | 24/58 (41.4) | 16/50 (32.0) |
| East or Southeast Asian | 14/58 (24.1) | 12/50 (24.0) |
| South or West Asian | 10/58 (17.2) | 10/50 (20.0) |
| Caribbean | 6/58 (10.3) | 8/50 (16.0) |
| Other | 4/58 (6.9) | 4/50 (8.0) |
| Maternal parity, mean ± SD | 1.5 ± 0.9 | 1.6 ± 0.9 |
| Family living below the poverty line,5 No. (%) | 13/58 (22.4) | 13/50 (26.0) |
| Morbidity composite,6 no. (%) | 20/59 (33.9) | 24/50 (48.0) |
| Brain injury,7 no. (%) | 7/59 (11.9) | 7/50 (14.0) |
Data are expressed as mean ± SD or frequency count (%).
Gestational age determined using maternal estimates of her last menstrual period. If early ultrasound prediction differed by 2 wk or more, the gestational age estimate derived from early ultrasound was used.
Score for Neonatal Acute Physiology II. Scores may range from 0 to 100 with higher values indicating higher neonatal risk and newborn illness.
Other includes African (n = 4), Latin American (n = 1), or mixed-race mothers (n = 3).
Based on 2012 Statistics Canada family size-adjusted cut-off values.
Includes late-onset sepsis, chronic lung disease, necrotizing enterocolitis, and retinopathy of prematurity requiring treatment.
Includes echodense intraparenchymal lesions, periventricular leukomalacia, porencephalic cysts, or ventriculomegaly with or without intraventricular hemorrhage.
BMBF, bovine milk-based fortifier group; HMBF, human milk-based fortifier group; SNAP, score for neonatal acute physiology.
No significant differences in mean cognitive, language, or motor composite scores were identified between the treatment groups, either in the base or fully adjusted models (Table 2). For model 1, adjusted only for birth weight strata (<1000 g or 1000–1249 g), the adjusted mean cognitive composite scores were 94.7 in the HMBF group compared with 95.9 in the BMBF group, an adjusted mean difference of −1.2 (95% CI: −6.7 to 4.4). Comparing the HMBF and BMBF groups, adjusted mean composite scores were 92.4 versus 93.1 [mean difference −0.7 (−7.4 to 6.1)] for language and 95.6 versus 97.7 [mean difference −2.1 (−7.3 to 3.1)] for motor subtests. The mean differences between the HMBF and BMBF groups in model 2, adjusted for birth weight strata, sex, small for gestational age, maternal education, income above or below the family size-adjusted poverty line, and donor milk intake, were −1.1 (−6.5 to 4.4) for cognition, −1.2 (−7.5 to 5.1) for language, and −1.1 (−6.3 to 4.2) for motor scores. Results were unchanged when an additional model was run including a major in-hospital morbidity composite (late-onset sepsis, chronic lung disease, NEC, or retinopathy of prematurity requiring treatment) and a composite social risk score [adapted from a previously published method (30) including family structure (single or dual parent household), maternal education (less than university educated or university and above), language spoken at home (no English or English), maternal age (under or over 21), and income (below or above the family size-adjusted poverty line)] (data not shown). Findings were also unchanged in sensitivity analyses excluding infants with cerebral palsy (n = 4), a hearing impairment (n = 2), or brain injury (n = 14) (Supplemental Table 2). None of the participants were diagnosed with a visual impairment. Similarly, results were unchanged in sensitivity analyses where participants enrolled at the Hospital for Sick Children (n = 16) were excluded (Supplemental Table 3), or when participants who were assigned scores of 49 were excluded for each of the cognitive, language, and motor composite analyses (Supplemental Table 4).
TABLE 2.
Adjusted neurodevelopment composite scores at 18-mo corrected age assessed by the Bayley Scales of Infant and Toddler Development, Third Edition1
| Adjusted mean (95% CI)2 | Adjusted: model 12 | Adjusted: model 23 | ||||
|---|---|---|---|---|---|---|
| HMBF | BMBF | Effect (95% CI) | P value | Effect (95% CI) | P value | |
| Composite scores4 | ||||||
| Cognitive | 94.7 (90.9, 98.5) | 95.9 (91.7, 100.0) | −1.2 (−6.7, 4.4) | 0.67 | −1.1 (−6.5, 4.4) | 0.70 |
| Language | 92.4 (87.8, 97.1) | 93.1 (88.0, 98.2) | −0.7 (−7.4, 6.1) | 0.85 | −1.2 (−7.5, 5.1) | 0.70 |
| Motor | 95.6 (92.0, 99.2) | 97.7 (93.8, 101.5) | −2.1 (−7.3, 3.1) | 0.43 | −1.1 (−6.3, 4.2) | 0.69 |
Sample sizes for HMBF and BMBF groups, respectively: model 1: cognitive (59, 50), language (58, 48), motor (58, 50), and model 2: cognitive (57, 50), language (57, 48), motor (56, 50).
Adjusted using covariates from model 1 – birth weight strata (<1000 g and 1000–1249 g).
Adjusted using covariates from model 2 – birth weight strata (<1000 g or 1000–1249 g), sex, small for gestational age (y/n), maternal education (high school or less, college or vocational diploma, baccalaureate, postbaccalaureate), income above or below the poverty line (y/n below family size-adjusted poverty line), and any donor milk intake (y/n). Data was missing for maternal education (n = 1) and income above or below the poverty line (n = 1, also missing data for the language subtest) both in the HMBF group.
Standardized mean is 100 (SD 15). Variables were analyzed with linear regression, with adjustments as indicated. All models were tested for treatment interactions. Interaction terms were removed from the model if not statistically significant.
BMBF, bovine milk-based fortifier group; HMBF, human milk-based fortifier group.
Proportions of children were also compared for a dichotomous outcome of death and neurodevelopmental impairment (scores <85) or death and neurodevelopmental disability (scores <70) (Table 3). No statistically significant differences were identified between the fortifier groups for any subtest in either an unadjusted model or a model adjusting for birth weight strata and sex. For the cognitive domain, 24.2% and 22.2% children died or had impaired neurodevelopment in the HMBF and BMBF groups, respectively. For language, the proportions were 36.1% with death or neurodevelopmental impairment in the HMBF group and 40.4% of those in the BMBF group, whereas in the motor domain, 23.0% of those in the HMBF group and 22.2% of those in the BMBF group died or were classified as having a neurodevelopmental impairment. No statistically significant differences were found between groups with respect to the proportion of children who died or had a neurodevelopmental disability for any domain. Similar results were obtained when the analysis was run only for composite scores <85 or <70 excluding those who died, had cerebral palsy, or had an impairment in vision or hearing (Supplemental Table 5), though reliability is limited by the small sample size.
TABLE 3.
Death and neurodevelopmental impairment or disability at 18-mo corrected age
| Characteristic | HMBF no./total (%) | BMBF no./total (%) | OR (95% CI)1 | P value |
|---|---|---|---|---|
| Death and neurodevelopmental impairment (death or composite score <85, hearing or visual impairment, or cerebral palsy) | ||||
| Cognitive | 15/62 (24.2) | 12/54 (22.2) | 1.3 (0.5, 3.2) | 0.61 |
| Language | 22/61 (36.1) | 21/52 (40.4) | 0.9 (0.4, 2.0) | 0.75 |
| Motor | 14/61 (23.0) | 12/54 (22.2) | 1.2 (0.5, 3.0) | 0.71 |
| Death and neurodevelopmental disability (death or composite score <70, hearing or visual impairment, or cerebral palsy) | ||||
| Cognitive | 9/62 (14.5) | 9/54 (16.7) | 1.0 (0.3, 2.9) | 0.98 |
| Language | 12/61 (19.7) | 11/52 (21.2) | 1.0 (0.4, 2.8) | 0.92 |
| Motor | 8/61 (13.1) | 10/54 (18.5) | 0.7 (0.3, 2.1) | 0.54 |
Logistic regression analyses of the proportions of participants with scores indicative of neuroimpairment or disability were adjusted only for birth weight strata and sex due to limited sample size.
BMBF, bovine milk-based fortifier group; HMBF, human milk-based fortifier group.
Discussion
In this follow-up study, no neurodevelopmental advantage was identified to providing infants born <1250 g a HMBF rather than a BMBF on top of a human milk background diet which consisted of maternal milk when available and supplemental pasteurized donor milk. Specifically, no statistically significant differences were identified in cognitive, language, or motor composite scores on the Bayley-III between the groups in either unadjusted or adjusted statistical models. The fully adjusted mean differences in composite scores between HMBF and BMBF range between −1.1 and −1.2 (favoring BMBF) with all CIs including 0. This is less than the minimal clinically important difference of 5 points that has been used previously for the Bayley-III in VLBW infants (6, 31). Thus, although not superior to BMBF, the HMBF is also not inferior with respect to neurodevelopment as assessed by the Bayley-III. There were also no significant differences in the proportions of participants who died or had a neurodevelopmental disability or neurodevelopmental impairment between the 2 groups. These results were unchanged in sensitivity analyses in which children with brain injury, hearing impairment, or cerebral palsy were excluded. Thus, the nutritional and bioactive component differences that arose in the 2 arms of the study as a result of differences in the nutrient composition of the HMBF and BMBF, including amount of supplemental donor milk required and displacement of maternal milk, may be insufficient to influence neurodevelopment at 18-mo corrected age among children born <1250 g.
The present study is the first randomized clinical trial to compare neurodevelopment in infants fed a BMBF or a HMBF; however, 1 previous paper addressed this point with a retrospective chart review (32) that compared performance on the Bayley-III at 6-, 12-, and 18-mo corrected age in infants born before or after a hospital introduced the use of a HMBF for infants born <1000 g and <37 weeks gestational age. Infants born prior to the introduction of this program received maternal milk fortified with BMBF and infant formula as a supplement in the absence of sufficient maternal milk, whereas infants born after the introduction of the program received only human milk products, including maternal milk fortified with HMBF and donor milk (without additional protein fortification as was provided in our study) when the maternal milk supply was insufficient, for ≥4 wk and until a weight >1500 g or a postmenstrual age of ≥34 wk was reached. Similar to our study, they identified no differences between the groups in any Bayley domain or in the proportions of infants with neurodevelopmental disability. Because the groups in this study differed both in the type of fortifier they received and in the feeding of supplemental infant formula or donor milk, no firm conclusions on the use of HMBF or BMBF could be drawn. By maintaining all infants on a human milk diet of maternal milk supplemented with donor milk when necessary, our study is the first to directly compare the effects of HMBF and BMBF on neurodevelopment.
Two previous trials reported improvements in feeding tolerance and lower rates of NEC in VLBW infants fed maternal milk with supplemental donor milk and a HMBF compared with those fed maternal milk with bovine milk products, including supplemental infant formula, and BMBF (10–12). This is relevant to the present analysis because early-life morbidity or suboptimal growth can translate into neurodevelopmental delays later in life (33, 34). As with the above-mentioned retrospective chart review (32), limited conclusions can be drawn from these trials about the role of the fortifier type as both fed infants either a human milk diet, with HMBF added to maternal or donor milk, or a diet fortified with BMBF on top of maternal milk or infant formula. Our study's original publication was the first to directly compare BMBF and HMBF on an entirely human milk diet, and in contrast to these previous reports, identified no statistically significant differences in feeding tolerance, growth, or Bell Stage ≥II NEC between the groups, though it did identify a significantly lower proportion of children diagnosed with severe retinopathy of prematurity in the HMBF group (1 of 62) compared with the BMBF group [6 of 59, mean difference −8.6% (−16.9% to −0.02%)] (16). Four of the 7 children diagnosed with severe retinopathy of prematurity returned for follow-up (2 died in hospital, including the only child in the HMBF group, and 1 moved out of the area). None of the 109 children who attended follow-up were diagnosed with a visual impairment (acuity <20/200 despite amplification) by 18-mo corrected age. Our findings with respect to neurodevelopment reported herein are consistent with these earlier findings. Future studies are needed to determine whether there are any long-term effects of fortifier type on eye health.
The strengths of this article include the randomized design, use of a consistent human milk diet between the treatment groups, a high rate of follow-up, and a population representing a range of ethnicities and income levels. This is the first study to compare the neurodevelopmental effects of feeding HMBF or BMBF on a diet containing only human milk without any infant formula, which is the current standard of care in North America (13, 14). Thus, these findings are extremely relevant to hospitals and care providers evaluating the adoption of HMBF as opposed to BMBF, especially given the additional financial cost of providing HMBF.
A limitation of the neurodevelopment analyses reported here is that the sample size was established to estimate treatment differences in in-hospital feeding tolerance, and not scores on the Bayley-III. A posthoc power calculation of the cognitive, language, and motor composites suggest we were powered (80%, α-level of 0.05) to detect a 0.48–0.55 SD difference or 7–9.5 point difference between treatment groups. Although a 0.5 SD difference is generally considered a moderate effect size in psychological research (35), including in previous studies using the Bayley-III in children born preterm or at a VLBW (36–38), it is greater than the minimal clinically important difference of 5 points we and others have used previously (6, 31). This being said, in the present analyses, the mean differences in composite scores were generally 2 points or less, which is unlikely to be clinically relevant. A further limitation of this work is that scores on the Bayley-III at 18-mo corrected age may not be predictive of other important neurodevelopmental outcomes, such as performance at school age or IQ, particularly in studies with nutritional interventions (3, 39). Future studies that use more sensitive measurements and/or later time points would be useful for the further evaluation of the use of HMBF.
In conclusion, we identified no neurodevelopmental benefit or detriment at 18-mo corrected age of feeding a HMBF compared with a BMBF during initial hospitalization in infants born at <1250 g. In infants being fed a human milk diet (maternal milk supplemented with donor milk where necessary), the evidence does not support use of HMBF rather than BMBF for improved neurodevelopment.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the study families for their participation. We also acknowledge the members of the OptiMoM Feeding group at the Hospital for Sick Children, Sinai Health System, Trillium Health Partners, St. Michael's Hospital, Humber River Hospital, Lakeridge Health, Markham Stouffville Hospital, North York General Hospital, Scarborough and Rouge Hospital, Mackenzie Health, Michael Garron Hospital, St. Joseph's Health Centre, Southlake Regional Health Centre, and the William Osler Health System for their contribution to study design, oversight and data collection, as well as the Rogers Hixon Ontario Human Milk Bank for providing the donor milk for the study. We also thank Sara Shama, Jane Francis, Lauren LeMay-Nedjelski, Meghan McGee, Aneta Plaga, and staff at the Neonatal Developmental Follow-Up Program at the Hospital for Sick Children and the Neonatal Follow-Up Clinic at Sinai Health System for their assistance.
The authors’ contributions were as follows—DLO, AK, and SU: designed the research; all authors: participated in conducting the research; KEH: analyzed the data with the input of all co-authors; KEH: drafted the manuscript; and all authors contributed to the revision and production of the final version, and have read and approved the manuscript prior to submission; SU and DLO; had primary responsibility for the final content.
Notes
Supported by (includes grants, fellowships, gifts of materials): Canadian Institutes of Health Research Programmatic Grants in Food and Health (Optimizing Mothers’ Milk for Preterm Infants (OptiMoM), FHG 129919). KEH was supported by a Canadian Institutes of Health Research Fellowship. Feeding supplies used as part of routine clinical care (e.g. donor milk, bovine milk-based fortifiers) were provided by participating centers. Prolacta Bioscience provided the human milk-based fortifiers at manufacturing cost. The sources of support had no role in the design or conduct of the research study, statistical analysis, data interpretation, or writing of the manuscript.
Author disclosures: The authors have no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: BMBF, bovine milk-based fortifier; HMBF, human milk-based fortifier; NEC, necrotizing enterocolitis; OptiMoM, Optimizing Mothers’ Milk for Preterm Infants; VLBW, very low birth weight.
References
- 1. O'Connor DL, Jacobs J, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, Connor SL, Fitzgerald K, Groh-Wargo S et al.. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J Pediatr Gastroenterol Nutr. 2003;37:437–46. [DOI] [PubMed] [Google Scholar]
- 2. Patel AL, Johnson TJ, Engstrom JL, Fogg LF, Jegier BJ, Bigger HR, Meier PP. Impact of early human milk on sepsis and health-care costs in very low birth weight infants. J Perinatol. 2013;33:514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belfort MB, Anderson PJ, Nowak VA, Lee KJ, Molesworth C, Thompson DK, Doyle LW, Inder TE. Breast milk feeding, brain development, and neurocognitive outcomes: a 7-year longitudinal study in infants born at less than 30 weeks' gestation. J Pediatr. 2016;177:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Higgins RD, Langer JC, Poole WK, National Institute of Child Health and Human Development National Research Network. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120:e953–9. [DOI] [PubMed] [Google Scholar]
- 5. Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC et al.. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–90. [DOI] [PubMed] [Google Scholar]
- 6. O'Connor DL, Gibbins S, Kiss A, Bando N, Brennan-Donnan J, Ng E, Campbell DM, Vaz S, Fusch C, Asztalos E et al.. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA. 2016;316:1897–905. [DOI] [PubMed] [Google Scholar]
- 7. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;7:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2018;6:CD002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JV, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Database Syst Rev. 2016;5:CD000343. [DOI] [PubMed] [Google Scholar]
- 10. Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM et al.. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7. [DOI] [PubMed] [Google Scholar]
- 11. Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, Dudell G, Rechtman DJ, Lee ML, Lucas A et al.. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5. [DOI] [PubMed] [Google Scholar]
- 12. Ghandehari H, Lee ML, Rechtman DJ. H2MF Study Group. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Res Notes. 2012;5:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim J, Unger S. Human milk banking. Paediatr Child Health. 2010;15:595–602. [PMC free article] [PubMed] [Google Scholar]
- 14. Committee On Nutrition, Section On Breastfeeding, Committee On Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics. 2017;139(1):e20163440. [DOI] [PubMed] [Google Scholar]
- 15. Patra K, Hamilton M, Johnson TJ, Greene M, Dabrowski E, Meier PP, Patel AL. NICU human milk dose and 20-month neurodevelopmental outcome in very low birth weight infants. Neonatology. 2017;112:330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Connor DLO, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, Daneman A, Francis J, Kotsopoulos K, Shah PS et al.. Human milk-based compared to a bovine milk-based fortifier to nutrient-enrich human milk for infants born <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108:108–16. [DOI] [PubMed] [Google Scholar]
- 17. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14–9. [DOI] [PubMed] [Google Scholar]
- 18. Cusick SE, Georgieff MK.. The role of nutrition in brain development: the golden opportunity of the “First 1000 Days”. J Pediatr. 2016;175:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garay PA, McAllister AK.. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Connor DL, Ewaschuk JB, Unger S. Human milk pasteurization: benefits and risks. Curr Opin Clin Nutr Metab Care. 2015;18:269–75. [DOI] [PubMed] [Google Scholar]
- 22. Stephens BE, Walden RV, Gargus RA, Tucker R, McKinley L, Mance M, Nye J, Vohr BR. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics. 2009;123:1337–43. [DOI] [PubMed] [Google Scholar]
- 23. dit Trolli SE, Kermorvant-Duchemin E, Huon C, Bremond-Gignac D, Lapillonne A. Early lipid supply and neurological development at one year in very low birth weight (VLBW) preterm infants. Early Hum Dev. 2012;88:(Suppl 1):S25–9. [DOI] [PubMed] [Google Scholar]
- 24. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–65. [DOI] [PubMed] [Google Scholar]
- 25. Wadhawan R, Oh W, Perritt RL, McDonald SA, Das A, Poole WK, Vohr BR, Higgins RD. Twin gestation and neurodevelopmental outcome in extremely low birth weight infants. Pediatrics. 2009;123:e220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Trial of Indomethacin Prophylaxis in Preterms Investigators. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, Bell EF, Rysavy MA, Duncan AF, Vohr BR et al.. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. 2017;376:617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bayley N. Bayley Scales of Infant and Toddler Development. 3rd ed San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- 29. Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. 2012;7:29–37. [DOI] [PubMed] [Google Scholar]
- 30. Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44:276–80. [DOI] [PubMed] [Google Scholar]
- 31. Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, Poole WK, Network NNR. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118:e115–23. [DOI] [PubMed] [Google Scholar]
- 32. Colacci M, Murthy K, DeRegnier RO, Khan JY, Robinson DT. Growth and development in extremely low birth weight infants after the introduction of exclusive human milk feedings. Am J Perinatol. 2017;34:130–7. [DOI] [PubMed] [Google Scholar]
- 33. Martin CR, Dammann O, Allred EN, Patel S, O'Shea TM, Kuban KC, Leviton A. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr. 2010;157:751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vinall J, Grunau RE, Brant R, Chau V, Poskitt KJ, Synnes AR, Miller SP. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med. 2013;5:168ra8. [DOI] [PubMed] [Google Scholar]
- 35. Sullivan GM, Feinn R.. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Colditz P, Sanders MR, Boyd R, Pritchard M, Gray P, O'Callaghan MJ, Slaughter V, Whittingham K, O'Rourke P, Winter L et al.. Prem Baby Triple P: a randomised controlled trial of enhanced parenting capacity to improve developmental outcomes in preterm infants. BMC Pediatr. 2015;15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Hus JW, Jeukens-Visser M, Koldewijn K, Van Sonderen L, Kok JH, Nollet F, Van Wassenaer-Leemhuis AG. Comparing two motor assessment tools to evaluate neurobehavioral intervention effects in infants with very low birth weight at 1 year. Phys Ther. 2013;93:1475–83. [DOI] [PubMed] [Google Scholar]
- 38. Wild KT, Betancourt LM, Brodsky NL, Hurt H. The effect of socioeconomic status on the language outcome of preterm infants at toddler age. Early Hum Dev. 2013;89:743–6. [DOI] [PubMed] [Google Scholar]
- 39. Colombo J, Carlson SE.. Is the measure the message: the BSID and nutritional interventions. Pediatrics. 2012;129:1166–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.