Abstract
Angiopoietin-like protein 3 (ANGPTL3) inhibits lipid clearance and is a promising target for managing cardiovascular disease. Here we investigated the effects of a high-sugar (high-fructose) diet on circulating ANGPTL3 concentrations in rhesus macaques. Plasma ANGPTL3 concentrations increased ∼30% to 40% after 1 and 3 months of a high-fructose diet (both P < 0.001 vs. baseline). During fructose-induced metabolic dysregulation, plasma ANGPTL3 concentrations were positively correlated with circulating indices of insulin resistance [assessed with fasting insulin and the homeostatic model assessment of insulin resistance (HOMA-IR)], hypertriglyceridemia, adiposity (assessed as leptin), and systemic inflammation [C-reactive peptide (CRP)] and negatively correlated with plasma levels of the insulin-sensitizing hormone adropin. Multiple regression analyses identified a strong association between circulating APOC3 and ANGPTL3 concentrations. Higher baseline plasma levels of both ANGPTL3 and APOC3 were associated with an increased risk for fructose-induced insulin resistance. Fish oil previously shown to prevent insulin resistance and hypertriglyceridemia in this model prevented increases of ANGPTL3 without affecting systemic inflammation (increased plasma CRP and interleukin-6 concentrations). ANGPTL3 RNAi lowered plasma concentrations of ANGPTL3, triglycerides (TGs), VLDL-C, APOC3, and APOE. These decreases were consistent with a reduced risk of atherosclerosis. In summary, dietary sugar-induced increases of circulating ANGPTL3 concentrations after metabolic dysregulation correlated positively with leptin levels, HOMA-IR, and dyslipidemia. Targeting ANGPTL3 expression with RNAi inhibited dyslipidemia by lowering plasma TGs, VLDL-C, APOC3, and APOE levels in rhesus macaques.
Keywords: triglycerides, insulin resistance, apolipoproteins, lipoproteins, inflammation, C-reactive protein, nonhuman primate, metabolic disorders, RNA interference
Elevated circulating lipids and lipoproteins are the major known modifiable risk factors for CVD, the leading cause of death in the United States (1–4). Severe hypertriglyceridemia (e.g., >800 mg/dl) also increases the risk for acute pancreatitis, which can be life-threatening (5). The prevalence of hyperlipidemias increases with obesity and aging, although less common genetic disorders can also lead to moderate to severe dyslipidemias. Current treatments focus on reducing fasting plasma concentrations of cholesterol packaged in LDLs and triglycerides (TGs). Multiple treatment options are available that lower plasma lipids/lipoproteins. However, monogenic disorders causing familial hypercholesterolemia or hypertriglyceridemia and polygenic disorders causing severe hypertriglyceridemia often require alternative approaches to achieve treatment goals (1, 6). New approaches for increasing lipid clearance from the circulation and reducing residual risk for CVD are needed.
Angiopoietin-like protein 3 (ANGPTL3) is a secretory protein expressed in the liver (a “hepatokine”) and is considered a promising lead target in the development of lipid-lowering therapies (7). ANGPTL3 belongs to a family of secretory proteins (ANGPTL3, ANGPTL4, and ANGPTL8) that affect the clearance of circulating lipids by regulating endothelial lipase and lipoprotein lipase (8). Specifically, ANGPTL3 interacts with ANGPTL8 to suppress lipoprotein lipase activity after feeding (9–11). ANGPTL3 also regulates the clearance of HDLs by binding to and inhibiting the activity of endothelial lipase (12). The clinical potential of ANGPTL3 for reducing plasma lipids is supported by human genetics. Specifically, inactivating ANGPTL3 gene variants associate with lower plasma levels of LDL-C and TGs and a lower risk of CVD (13–19). Moreover, small trials using antisense or monoclonal antibodies to inhibit ANGPTL3 have shown clinically significant reductions in plasma LDL-C and TG concentrations in patients with familial hypercholesterolemia (20–22).
APOC3 has been recently targeted for the treatment of dyslipidemia. APOC3 is a 8.8 kDa glycoprotein released primarily from the liver and is a major structural component of atherogenic VLDL particles; it is also found in HDLs and chylomicron particles (23). APOC3 inhibits the processing of large, TG-rich VLDLs (23) and has important intracellular functions in hepatocytes that facilitate VLDL production (24). Loss-of-function APOC3 gene variants associate with significant (∼40%) reductions in CVD risk and plasma concentrations of TGs and LDL-C (6–9). Preclinical studies in rodent and porcine models indicate that increased APOC3 synthesis is sufficient for producing hypertriglyceridemia (10, 11, 25). Clinical trials using antisense oligonucleotides to suppress APOC3 synthesis (Volanesorsen, Ionis 304801) have demonstrated substantial antihyperlipidemic effects in patients with severe hypertriglyceridemia (12, 13).
Nonhuman primates are an important resource for the translational research of novel therapies against obesity-related metabolic diseases (26, 27). Previous studies have demonstrated that rhesus macaques provided with fructose or high-fructose corn syrup (HFCS) supplements (300–600 kcal/d) rapidly gain weight and develop features of metabolic syndrome (insulin resistance, hypertriglyceridemia, and increased APOC3) (28–30). Using this model, we recently reported that an RNAi construct targeting hepatic APOC3 expression reduces plasma TG concentrations (30). Moreover, elevations of plasma TG concentrations during fructose consumption are positively correlated with increases of circulating APOC3 concentrations (30). However, the impact of dietary composition and diet-induced obesity/metabolic dysfunction on plasma ANGPTL3 concentrations has not been investigated.
The current experiments determined the effects of a high-sugar diet on circulating ANGPTL3 levels and the effects of fish oil supplementation in male rhesus macaques. The results of a large (n = 59) study into the effects of fructose supplements on weight gain and indices of insulin resistance [fasting insulin, fasting glucose, and homeostatic model assessment of insulin resistance (HOMA-IR)] and dyslipidemia have previously been reported (28–31). These studies reported rapid gains in body weight, fasting hyperinsulinemia, fasting hypertriglyceridemia, and elevated plasma APOC3 concentrations with fructose consumption that are largely prevented by fish oil supplementation (29, 30). The current investigation examined dietary effects and relationships between plasma concentrations of ANGPTL3 and indices of insulin resistance, systemic inflammation, and lipoprotein metabolism. We also report on the effects of dietary fish oil supplementation on ANGPTL3 responses to fructose. The plasma samples used for this experiment are from a study whose outcomes were reported previously (29). Finally, we report on the effects of inhibiting hepatic ANGPTL3 expression with RNAi on plasma lipid/lipoprotein profiles. This study includes comparisons with the responses to inhibiting hepatic APOC3 concentrations, which have been reported previously (30).
MATERIALS AND METHODS
Protocols for all animal studies were approved by the University of California, Davis Institutional Animal Care and Use Committee and were conducted in accordance with the U.S. Department of Agriculture Animal Welfare Act and the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Rhesus macaques
The animal studies, feeding protocols, and dietary effects of fructose and fish oil on body weight and blood chemistries have been described previously (29–31). In brief, adult male rhesus macaques (n = 59) (age: 12.0 ± 2.8 years; range: 6.4–17.8 years) maintained at the California National Primate Research Center were provided a standard commercial nonhuman primate diet (5047; LabDiet, St. Louis, MO) and water. This grain-based diet provides 30%/kcal as protein, 11%/kcal as fat, and 59%/kcal as complex carbohydrates.
After determining baseline body weights and collecting fasting blood samples, animals were provided a solution containing 75 g fructose (300 kcal) daily in a total volume of 500 ml flavored Kool-Aid (Kraft Foods, Chicago, IL) beverages. Male rhesus macaques consume on average 800–900 kcal/day. These animals consumed approximately 30% of their daily caloric intake from fructose. Body weight measurements and fasting blood samples were collected after 1 and 3 months of fructose consumption.
Ten additional adult male fructose-fed rhesus monkeys were supplemented with 4 g/day whole fish oil (Jedwards International, Inc., Braintree, MA) for 6 months. These animals were compared with a subset of nine animals from the group of 59 animals that were studied concurrently for 6 months (29). Body weight measurements and fasting blood samples from these animals were collected at baseline and after 1, 3, and 6 months.
ANGPTL3 RNAi study
The six animals used for this experiment received a modified moderate-fat diet protocol supplemented with HFCS. The rationale for this protocol was to achieve a closer match to a typical human diet and to maximize hypertriglyceridemia. The animals were provided HFCS-sweetened beverages (500 ml; 15% by weight sugar) twice per day, ingesting a total of 150 g (2 servings/day of 300 kcal for a total of 600 kcal/d) of HFCS. HFCS is 55% fructose (330 kcal) and 45% glucose (270 kcal). These animals are likely to have received up to 60% of energy intake in the form of simple sugars. The New World monkey diet (LabDiet) has moderate fat content (23%/kcal vs. 11%/kcal as fat for the 5047 diet).
Fasting blood samples were collected at baseline, after which animals were started on the HFCS/moderate fat diet. After 6 weeks, an RNAi construct targeting ANGPTL3 mRNA (ARO-ANGPTL3; 4 mg/kg) was administered in four animals via subcutaneous injection on day 0 and day 29 (n = 4). Two additional animals received vehicle injections.
ARO-ANGPTL3 is a synthetic double-stranded (both strands contain 21 nucleotides) hepatocyte-targeted N-acetylgalactosamine-conjugated RNAi molecule. The N-acetylgalactosamine moiety targets the RNAi into hepatocytes by acting as a ligand for the highly expressed hepatocyte-specific asialoglycoprotein receptor. The RNAi construct was designed to silence ANGPTL3 mRNA in hepatocytes in humans and nonhuman primates with high specificity. The RNAi was synthesized with 2′-O-methyl/2′-fluoro-modified nucleotides in order to be resistant to nucleases and to abrogate potential immune activation (32). Fasting blood samples were collected at days 8, 15, 21, 29, 36, 43, 50, 57, 71, and 85.
Plasma analyses
Plasma glucose concentrations were measured using a glucose analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma adiponectin, insulin, and leptin concentrations were measured using RIA assays from Millipore (Burlington, MA). Plasma total cholesterol (TC), HDL-C, direct LDL-C, TGs, APOA1, APOB, APOC3, and APOE concentrations were measured using a Polychem chemistry analyzer (Polymedco, Cortlandt Manor, NY); reagents were purchased from MedTest DX (Canton, MI). VLDL-C was calculated by subtracting HDL-C and LDL-C from TC. Plasma ANGPTL3 concentrations were measured using ELISA (DANL30; R&D Systems, Minneapolis, MN) for human ANGPTL3 that cross-reacts with macaque ANGPTL3. Plasma adropin concentrations were measured by ELISA (Peninsula Laboratories, San Carlos, CA).
Plasma concentrations of VLDL and IDL, LDL, and HDL particle subfractions were measured using specific particle-size intervals determined by ion mobility. This approach allows for direct particle quantification as a function of particle diameter (33), following a procedure to remove other plasma proteins (34). The ion mobility instrument uses an electrospray to create an aerosol of particles that then pass through a differential mobility analyzer coupled to a particle counter. Particle concentrations (in nanomoles per liter) are determined for subfractions defined by the following size intervals: VLDL: large (42.40–54.70 nm), medium (33.50–42.39 nm), and small (29.60–33.49 nm); IDL: large (25.00–29.59 nm) and small (23.33–24.99 nm); LDL: large (22.0–23.32 nm), medium (21.41–21.99 nm), small (20.82–21.40 nm), and very small (18.0–20.81 nm); and HDL: large (10.50–14.50 nm) and small (7.65–10.49 nm). Peak LDL diameter (in nanometers) was determined as previously described (33).
Statistical analysis
Data were input and managed using Microsoft Excel. Statistical analyses (repeated-measures ANCOVA, multiple linear regression, calculation of correlation coefficients) using log-transformed data were performed using SPSS Statistics version 24 (IBM, Armonk, NY).
Correlations between plasma ANGPTL3 concentrations with indices of glucose metabolism, lipid metabolism, and inflammation in the larger fructose consumption study (n = 59) were assessed by calculating correlation coefficients, performing regression, or separating animals into tertiles. For simple modeling, we calculated partial correlation coefficients (r) that controlled for variations in age and body weight using two-tailed tests for significance. Multiple linear regression was used to identify factors determining variations in plasma ANGPTL3 concentrations between animals using data collected at baseline or after 1 or 3 months of fructose consumption.
To identify metabolic features defining animals exhibiting small or large fructose-induced changes of plasma ANGPTL3 concentrations, animals were ranked low to high by changes (Δ) in plasma ANGPTL3 concentrations after 3 months of fructose consumption. Differences were assessed using repeated measures (baseline and 1- and 3-month time points) with time on the diet and tertile as fixed variables; age, body weight at baseline (BW), and ΔBW (3 months) were used as covariates for blood chemistries. Pairwise comparisons to determine the level of significance between tertiles used Bonferroni correction to adjust confidence intervals.
For the RNAi experiment, paired t-tests compared baseline data with the mean of the last three measures. For each analysis, P < 0.05 was considered statistically significant between groups.
RESULTS
Effects of fructose consumption on plasma ANGPTL3 concentrations
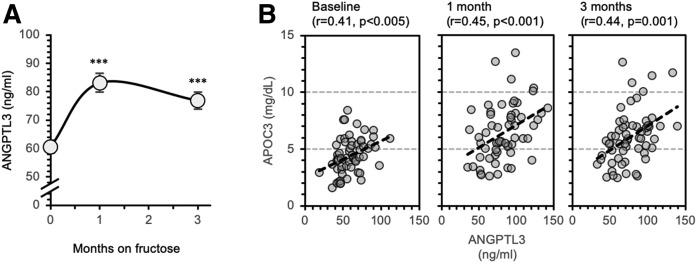
The effects of dietary fructose consumption on weight gain, indices of insulin sensitivity, and lipid/lipoprotein metabolism in rhesus macaques have previously been reported (30). Here we report that fructose consumption increases plasma ANGPTL3 concentrations approximately 30% to 40% after 1 or 3 months (both P < 0.001) (Fig. 1A). Fasting concentrations and absolute changes in values relative to the baseline of plasma ANGPTL3 and TG levels correlated positively during fructose consumption (supplemental Fig. S1A, B). In addition, higher plasma ANGPTL3 concentrations were associated with an accumulation of circulating large TG-rich VLDL particles during fructose consumption (supplemental Tables S1 and S2).
Fig. 1.
Increased plasma ANGPTL3 concentrations during fructose consumption and correlations between plasma ANGPTL3 and APOC3 concentrations. A: Plasma ANGPTL3 concentrations at baseline and after 1 or 3 months of dietary supplementation with a fructose-containing beverage (300 kcal/d). ***P < 0.001 versus baseline. B: Scatterplots showing actual values of plasma ANGPTL3 (x-axes) and APOC3 (y-axes) concentrations at baseline and during fructose consumption. Significantly positively correlations are evident at all time points.
We previously reported that changes in HOMA-IR during fructose consumption are primarily driven by increased fasting plasma insulin concentrations, with fasting glucose concentrations changing only slightly during fructose consumption (28, 29). Here we report an increase (>80%) in plasma concentrations of CRP (mean ± SEM: 1.8 ± 0.2 mg/l at baseline, 3.1 ± 0.3 mg/l at 1 month, and 3.3 ± 0.2 mg/l at 3 months; effect of diet at baseline vs. 1 and 3 months: P < 0.001).
Separating animals into tertiles ranked low to high by increases of plasma ANGPTL3 concentrations at 3 months resulted in animals exhibiting either modest reductions (−7%) or intermediate (+26%) to marked increases (+67%) (Table 1) . The metabolic phenotype of animals in each tertile was related to the effects of fructose on ANGPTL3 responses, specifically with larger increases of indices of insulin resistance (HOMA-IR, fasting insulin), systemic inflammation (CRP), and adiposity (leptin) (Table 1, supplemental Fig. S2A, B). Therefore, increases of ANGPTL3 with fructose consumption in rhesus macaques associate with increases of HOMA-IR, systemic inflammation, and increases of circulating leptin concentrations.
TABLE 1.
Physical characteristics and plasma parameters of animals separated into groups ranked by ΔANGPTL3 after 3 months of fructose consumption
| Tertile | Statistics | |||
| 1st (n = 20) | 2nd (n = 19) | 3rd (n = 20) | ||
| ANGPTL3 (ng/ml) | ||||
| Baseline | 67.5 ± 3.9 | 56.2 ± 4.0 | 57.8 ± 3.9 | Diet × tertile, P < 0.001 |
| 3 months | 63.3 ± 4.2 | 70.7 ± 4.3 | 96.5 ± 4.2 | |
| Δ | −4.3 ± 1.8*** | 14.5 ± 1.9*** | 38.8 ± 1.8*** | ***P < 0.001 between all tertiles |
| Age (y) | 11.7 ± 2.2 (7.8–16.2) | 12.6 ± 3.1 (6.4–16.9) | 11.6 ± 3.2 (6.4–17.8) | |
| Body weight (kg) | ||||
| Baseline | 16.3 ± 0.5 | 15.6 ± 0.5 | 15.8 ± 0.5 | Diet, P < 0.005 |
| 3 months | 18.1 ± 0.5 | 17.1 ± 0.5 | 17.2 ± 0.5 | |
| Δ | +1.9 ± 0.2 | +1.5 ± 0.2 | +1.4 ± 0.2 | |
| HOMA-IR | ||||
| Baseline | 11.2 ± 2.5 | 16.1 ± 2.6 | 12.4 ± 2.5 | |
| 3 months | 15.0 ± 9.5 | 25.0 ± 10.0 | 36.6 ± 9.7 | |
| Δ | +3.8 ± 9.5 | +9.0 ± 9.7 | +23.2 ± 9.4 | |
| Leptin (ng/ml) | ||||
| Baseline | 18.3 ± 2.5 | 18.7 ± 2.5 | 22.2 ± 2.4 | Diet, P = 0.001 |
| 3 months | 21.2 ± 2.6 | 23.7 ± 2.7 | 29.3 ± 2.6* | |
| Δ | 2.9 ± 1.3 | 5.0 ± 1.3 | 7.1 ± 1.3* | *P < 0.05 vs. 1st tertile |
| Adiponectin (mg/dl) | ||||
| Baseline | 8.1 ± 1.7 | 10.0 ± 1.8 | 9.9 ± 1.7 | Diet, P = 0.001 |
| 3 months | 5.6 ± 1.0 | 7.2 ± 1.0 | 5.7 ± 1.0 | Diet × BW, P = 0.001 |
| Δ | −2.4 ± 0.9 | −2.9 ± 0.9 | −4.2 ± 0.9 | Tertile, P = 0.07 |
| CRP (mg/l) | ||||
| Baseline | 1.8 ± 0.3 | 1.9 ± 0.3 | 1.8 ± 0.3 | Diet, P = 0.058 |
| 3 months | 2.7 ± 0.4 | 3.5 ± 0.3 | 3.6 ± 0.3 | Diet × tertile, P < 0.01 |
| Δ | 0.9 ± 0.3 | 1.6 ± 0.3 | 1.8 ± 0.3* | *P < 0.05 vs. 1st tertile |
| TGs (mg/dl) | ||||
| Baseline | 71 ± 8 | 92 ± 8 | 87 ± 8 | |
| 3 months | 140 ± 37 | 170 ± 38 | 245 ± 37 | |
| Δ | 69 ± 32 | 78 ± 33 | 159 ± 32 | Tertile, P = 0.11 |
| LDL-C (mg/dl) | ||||
| Baseline | 66 ± 4 | 69 ± 4 | 64 ± 4 | |
| 3 months | 72 ± 5 | 68 ± 5 | 66 ± 5 | |
| Δ | 6 ± 3 | −1 ± 3 | 2 ± 3 | |
| HDL-C (mg/dl) | ||||
| Baseline | 66 ± 4 | 60 ± 4 | 62 ± 4 | |
| 3 months | 68 ± 4 | 60 ± 4 | 54 ± 4 | |
| Δ | 2 ± 3 | 0 ± 3 | −8 ± 3* | *P < 0.05 vs. 1st tertile |
Age (mean ± SD, range) and age- and body weight-adjusted physical and biochemical parameters (estimated marginal mean ± SEM) are shown at baseline and after 3 months of fructose consumption. The change with fructose after 3 months is also shown. Body weight data are adjusted for age. The statistical analysis used repeated measures (baseline, 1 month, 3 months) to compare the effects of time on the diet and tertile as fixed variables; age, BW, and ΔBW (3 months) were used as covariates for blood chemistries. Asterisks refer to significance determined by post hoc comparisons between tertiles.
High baseline plasma ANGPTL3 and APOC3 levels correlate with increases of HOMA-IR
Multiple linear regression analyses identified a strong relationship between plasma ANGPTL3 and APOC3 concentrations (supplemental Table S3, Fig. 1B). Revising the modeling equation to identify variables predicting plasma APOC3 concentrations identified ANGPTL3 levels as a consistent and highly significant predictor, along with APOB (supplemental Table S4).
ANGPTL3 and APOC3 both have positive effects in increasing circulating lipid concentrations. Elevated baseline activity of either alone or in combination could thus predispose circulating lipids to accumulation during fructose consumption. These hypotheses were assessed in a prospective analysis of the relationship between baseline plasma concentrations of APOC3 or ANGPTL3 alone or in combination on the effects of 3 months of fructose consumption on cardiometabolic risk factors. As plasma concentrations of ANGPTL3 and APOC3 differ by orders of magnitude (ng/ml vs. mg/dl), log-transformed data were converted to z scores (SD from the mean). Age, body weight, and weight gain were controlled for in the analysis.
For baseline [APOC3]z there was a strong inverse correlation with ΔHDL-C (r = −0.53, P < 0.001) and less robust but still significant positive correlations with Δinsulin and ΔHOMA-IR (r = 0.29 and 0.28, P < 0.05). Similar associations were observed for [ANGPTL3]z + [APOC3]z (for ΔHDL-C, r = −0.38 and P < 0.005; for Δinsulin, r = 0.30 and P < 0.05; and for ΔHOMA-IR, r = 0.29 and P < 0.05). However, no significant correlations were observed for [ANGPTL3]z.
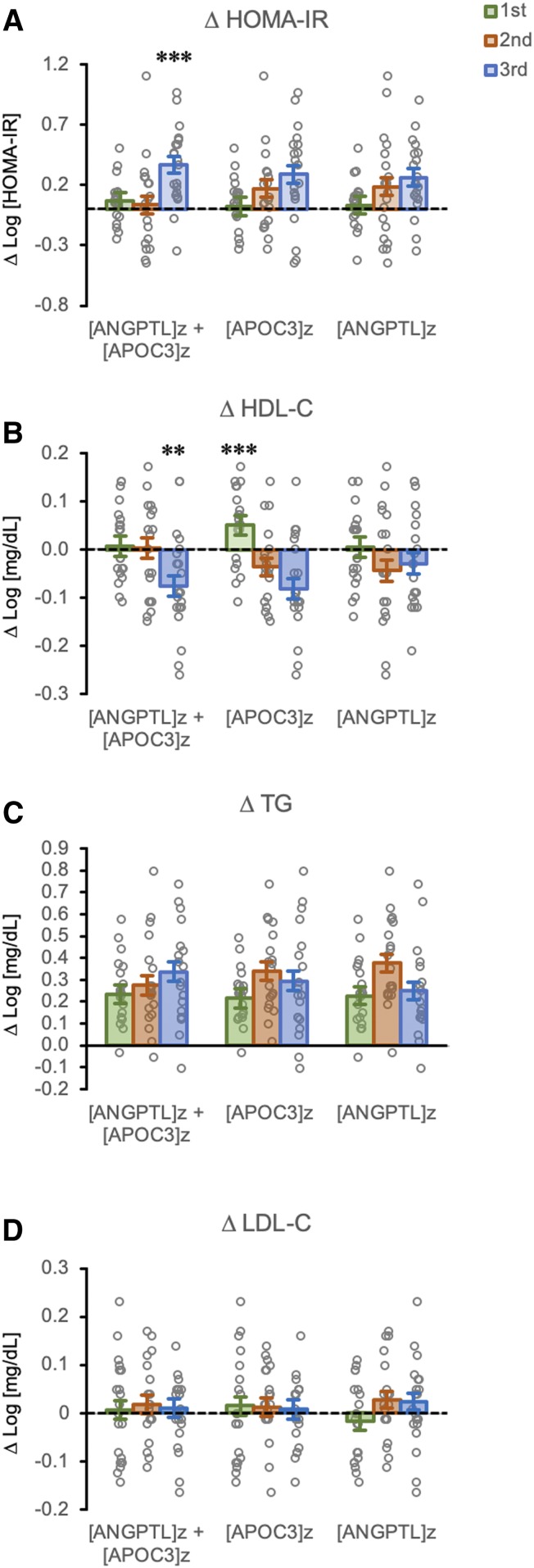
Animals were next ranked (from low to high) into tertiles by baseline [APOC3]z, [ANGPTL3]z, or [ANGPTL3]z + [APOC3]z. Both variables contributed equally to increases of [ANGPTL3]z + [APOC3]z (supplemental Fig. S3). While both baseline [APOC3]z or [ANGPTL3]z correlated with the severity of fructose-induced hyperinsulinemia/increases in HOMA-IR, [ANGPTL3]z + [APOC3]z proved superior for identifying animals exhibiting the largest increases of HOMA-IR (Fig. 2A) and lowering plasma HDL-C concentrations (Fig. 2B). There were no significant differences for TG or LDL-C when baseline [APOC3]z, [ANGPTL3]z, or [ANGPTL3]z + [APOC3]z tertiles were used as the fixed variable (Fig. 2C, D).
Fig. 2.
A combination of high baseline plasma concentrations of both APOC3 and ANGPTL3 correlates with the effects of fructose on HOMA-IR and HDL-C. Bar graphs show estimated marginal means and SEMs for changes (Δ) after 3 months of fructose consumption. Animals are grouped into tertiles ranked low (1st) to high (3rd) by baseline values for [APOC3]z + [ANGPTL3]z, [APOC3]z, or [ANGPTL3]z, where z is defined as the SD from the mean. The y-axes are changes in log-transformed values (HOMA-IR, HDL-C, TGs, LDL-C). Individual unadjusted data points are also shown (gray circles). A: Changes in HOMA-IR after 3 months of fructose. A significant effect of tertile was observed only between [APOC3]z + [ANGPTL3]z tertiles (P < 0.005). Increases in HOMA-IR with 3 months of fructose consumption are markedly more frequent in animals with high baseline plasma levels of both APOC3 and ANGPTL3. ***P < 0.005 versus 1st and 2nd tertiles. B: Changes in plasma HDL-C concentrations. Significant effects of tertile were observed for [APOC3]z + [ANGPTL3]z (P < 0.05) and [APOC3]z (P < 0.001). **P = 0.01 versus 1st and 2nd tertiles and ***P < 0.005 versus 2nd and 3rd tertiles. Declining HDL-C concentrations are more frequently observed in animals with high baseline levels of both APOC3 and ANGPTL3 or with high levels of APOC3 only. Changes in plasma concentrations of TG (C) and LDL-C (D) after 3 months of fructose consumption are not significantly different in any of the tertiles.
Low plasma ANGPTL3 levels are associated with higher plasma adropin concentrations
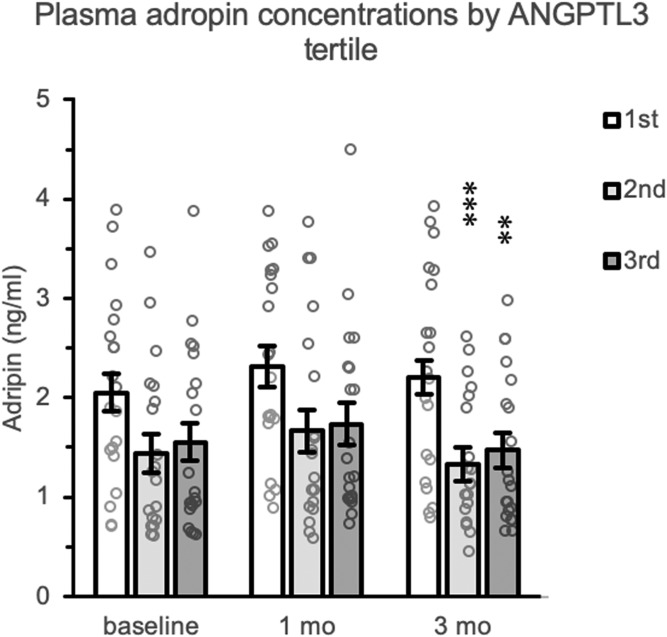
Plasma concentrations of adropin, a secreted peptide with insulin-sensitizing actions in skeletal muscle and liver in mouse models (35–42), were inversely correlated with plasma ANGPTL3 concentrations after 3 months of fructose consumption (Fig. 3). The relationship between plasma adropin and APOC3 concentrations has previously been reported (31). There was no improvement in predicting circulating adropin concentrations by combining APOC3 and ANGPTL3 in the model (data not shown).
Fig. 3.
Low plasma ANGPTL3 concentrations correlate with high plasma adropin concentrations in situations of fructose-induced metabolic dysregulation. The data shown as bar graphs are estimated marginal means for fasting plasma adropin concentrations at baseline and after 1 and 3 months of fructose. Individual unadjusted data points are also shown (gray circles). The animals were separated in tertiles ranked low to high by baseline plasma ANGPTL3 concentrations. There was a significant effect of tertile at the 3-month time point (P = 0.001); animals with low plasma ANGPTL3 concentrations had significantly higher plasma adropin concentrations. **P < 0.005 and ***P = 0.001 compared with the 1st tertile.
Dietary fish oil prevents fructose-induced increases of plasma ANGPTL3
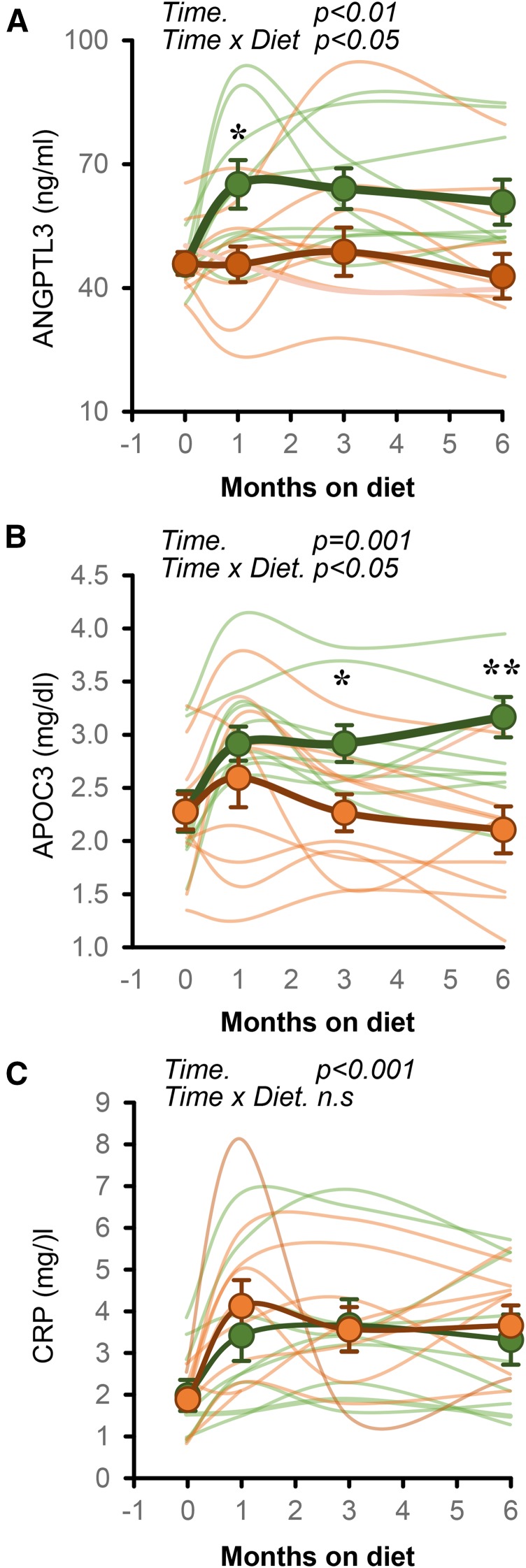
The effects of fish oil supplementation in preventing fructose-induced dyslipidemia and increasing fasting insulin and HOMA-IR have previously been reported (29). Here we report new results on the effects of fish oil on fructose-induced changes of ANGPTL3 and CRP concentrations. There was a significant effect of time and high-fructose diet on plasma concentrations of ANGPTL3 (effect of time on diet in repeated-measures ANOVA: P < 0.01) (Fig. 4A), APOC3 (P = 0.001) (Fig. 4B), and CRP (P < 0.001) (Fig. 4C). Fish oil supplementation prevented the increases of plasma concentrations of ANGPTL3 (Fig. 4A) and APOC3 (Fig. 4B) but had no effect on the increase of plasma CRP levels (Fig. 4C).
Fig. 4.
A fish oil supplement prevents increases in plasma ANGPTL3 (A) and APOC3 (B) but does not affect increases in CRP observed with fructose consumption. The data shown are from a subset of the group of 59 animals shown in Fig. 1 (n = 9; green circles), and another group of animals provided the fructose beverage and 4 g/d of fish oil (n = 10; orange circles). *P < 0.05 and **P < 0.01. The data shown in panel B were published in Bremer et al. (29).
We previously reported that supplementing the fructose diet with fish oil attenuates fructose-induced hyperinsulinemia and hypertriglyceridemia (29). The effects of fish oil in protecting against the development of insulin resistance and dyslipidemia in this model thus appear to be independent of the attenuation of systemic inflammatory responses to a high-sugar diet. This relationship was further explored by measuring plasma concentrations of interleukin-6 (IL-6), which is also a circulating biomarker of inflammation. IL-6 concentrations increased nearly 2-fold (baseline concentrations for all animals: 1.1 ± 0.2 and 2.1 ± 0.3 pg/ml after 6 months of fructose; effect of time of diet: P < 0.005). This effect was not attenuated with fish oil, with similar increases in both fructose and fructose + fish oil groups (from 1.2 ± 0.2 to 1.8 ± 0.3 pg/ml in animals provided fructose and from 1.0 ± 0.1 to 2.3 ± 0.5 pg/ml in animals consuming fructose + fish oil in combination).
RNAi suppression of ANGPTL3 improves dyslipidemia induced by high-sugar diet
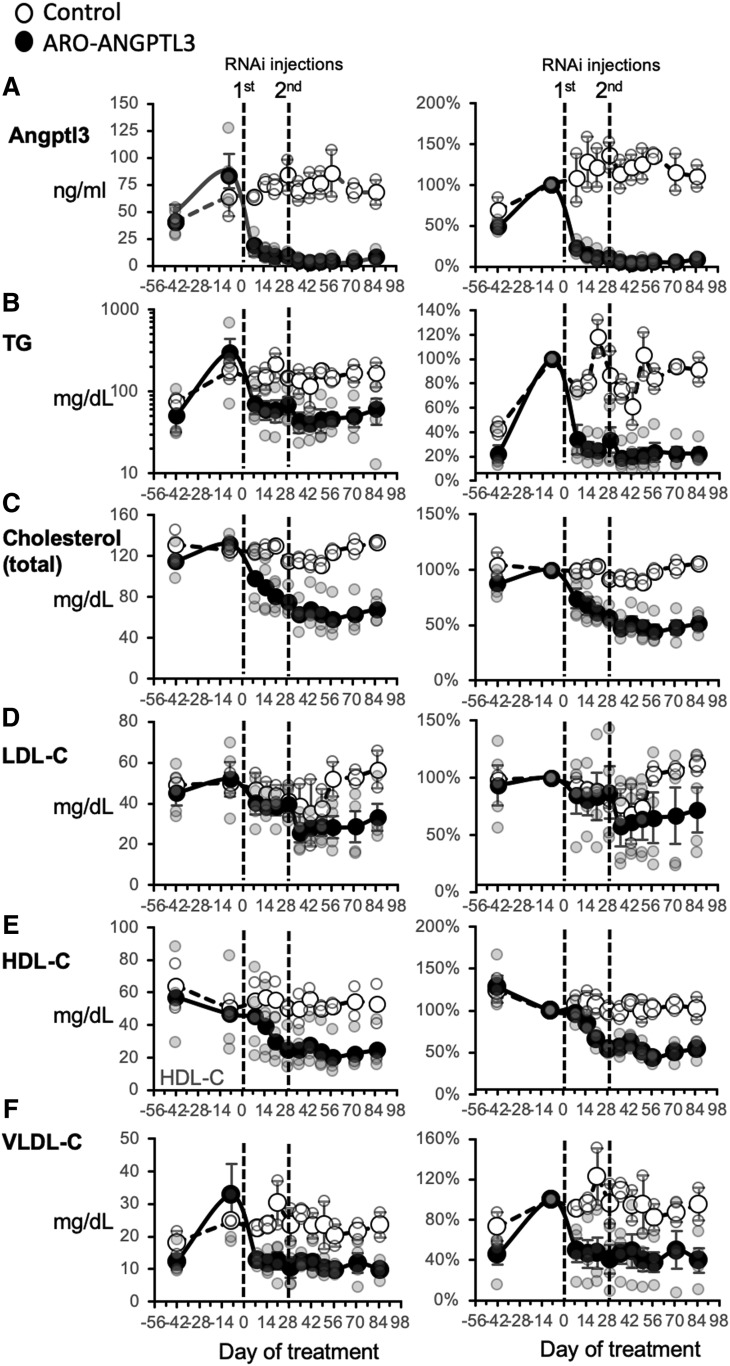
We next determined whether suppressing ANGPTL3 prevents dyslipidemia induced by a high-sugar diet. For these experiments, animals were fed a moderate fat chow and provided 600 kcal/d of an HFCS-sweetened beverage in 2 servings of 300 kcal/d. Treatment of HFCS-fed rhesus macaques with RNAi targeting the Angptl3 gene (ARO-ANGPTL3) resulted in a marked (>90%) reduction of plasma ANGPTL3 concentrations (Fig. 5A).
Fig. 5.
Treatment of rhesus macaques with an RNAi construct targeting liver ANGPTL3 expression markedly suppresses circulating levels of ANGPTL3 and reduces plasma lipid load. The data shown are fasting plasma concentrations of ANGPTL3 (A), TGs (B), TC (C), LDL-C (D), HDL-C (E), and VLDL-C (F). Actual values are shown to the left; data expressed as a percentage of baseline (days 0–8) are shown on the right. Animals were fed a moderately high-fat chow with an HFCS supplement (600 kcal/d). The RNAi construct (n = 4) or control (n = 2) was administered at day 0 and day 29 (indicated by red lines). Values shown are means ± SEMs, with data for individual animals also provided in lighter shading.
The HFCS diet rapidly induced hypertriglyceridemia (Fig. 5). A comparison of the mean of the last 3 days of measurements with baseline levels (days 0–8) indicated the effect of ARO-ANGPTL3 was highly significant (from 84 to 6 ng/ml; P < 0.01). ARO-ANGPTL3 treatment markedly reduced fasting plasma TG concentrations by approximately 80% to levels observed prior to the high-fructose diet (from 301 ± 132 to 53 ± 14 mg/dl; P = 0.06) (Fig. 5B). TC levels also declined by 50% (from 131 ± 5 to 63 ± 8 mg/dl; P < 0.005) (Fig. 5C). Plasma concentrations of LDL-C tended to also be lowered by RNAi treatment (52 ± 8 vs. 30 ± 6 mg/dl; P = 0.10) (Fig. 5D). Reductions were also observed for plasma concentrations of HDL-C (from 47 ± 13 to 22 ± 6 mg/dl; P < 0.05) (Fig. 5E) and VLDL-C (from 33 ± 10 to 11 ± 2 mg/dl; P = 0.07) (Fig. 5F). When normalized to baseline values, the effects of RNAi were significant for TGs (23 ± 5% of baseline; P < 0.001), TC (48 ± 6%; P < 0.005), VLDL-C (43 ± 13%; P < 0.05), and HDL-C (51 ± 5%; P < 0.001).
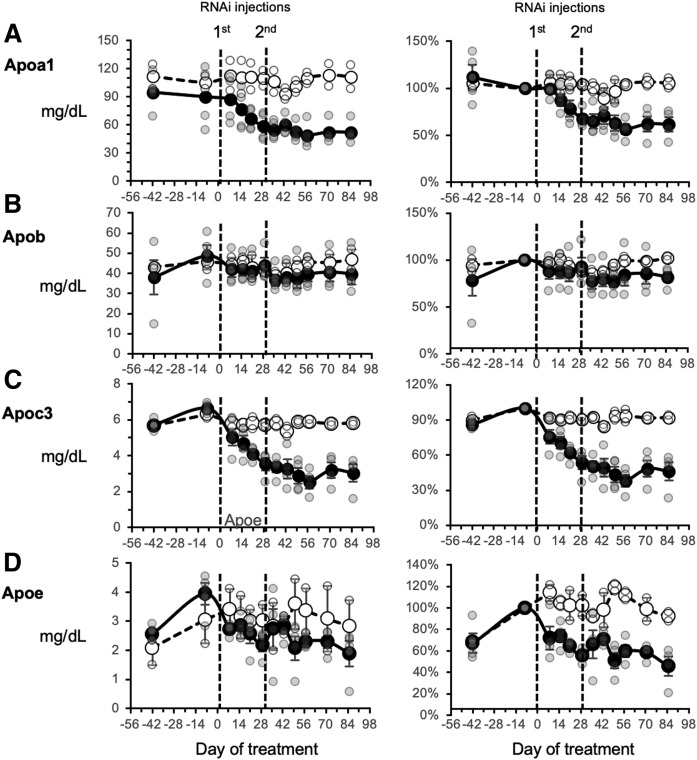
With the exception of APOB, animals treated with ARO-ANGPTL3 exhibited decreases in the plasma concentrations of the major apolipoproteins (A1, C3, and E) (Fig. 6). The effects of ARO-ANGPTL3 on plasma concentrations of specific apolipoproteins were related to the changes of the lipoprotein classes. APOA1 is a major component of HDL-C, and plasma APOA1 concentrations decreased by 40% (from 90 ± 16 to 51 ± 6 mg/dl; P = 0.05) (Fig. 6A). Plasma APOB concentrations also tended to decrease (49 ± 6 vs. 40 ± 4 mg/dl; P = 0.07) (Fig. 6B). Plasma APOC3 concentrations decreased by 60% (from 6.6 ± 0.1 to 2.9 ± 0.4 mg/dl; P < 0.005) (Fig. 6C), while APOE levels declined by nearly 50% (3.9 ± 0.4 to 2.2 ± 0.2 mg/dl; P < 0.005) (Fig. 6D). Although there was a trend for treatment with ARO-ANGPTL3 to attenuate the insulin resistance as assessed with an intravenous glucose tolerance test, this did not achieve statistical significance (P = 0.09) (supplemental Fig. S4).
Fig. 6.
Fasting plasma concentrations of APOA1 (A), APOB (B), APOC3 (C), and APOE (D) before and after treating rhesus macaques with an RNAi construct targeting hepatic ANGPTL3 expression. Refer to the Fig. 4 legend for a description of the study.
DISCUSSION
These results provide important new insights into the role of ANGPTL3 in metabolic homeostasis using a nonhuman primate model of diet-induced obesity. Increases of plasma concentrations of ANGPTL3 are related to fructose-induced increases of insulin and HOMA-IR values and to markers of systemic inflammation (CRP) and adiposity (leptin). The responses of ANGPTL3 to a high-sugar diet are significantly correlated with changes in the plasma lipid profile (increases of TGs and lowering of HDL-C levels) that predispose humans to CVD. This study defines for the first time significant positive relationships between circulating levels of ANGPTL3 and APOC3. Measuring APOC3 and ANGPTL3 in plasma may be informative, as animals with high plasma levels of both were more susceptible to the adverse effects of fructose consumption on circulating insulin and HOMA-IR. It is also interesting to note that the inflammatory responses to fructose consumption (increased plasma levels of CRP and IL-6) are dissociated from dyslipidemia and fasting hyperinsulinemia by fish oil supplementation. The beneficial effects of marine-derived fatty acids therefore appear to be independent of their known anti-inflammatory actions (43).
The mechanism explaining the strength of the relationship between ANGPTL3 and APOC3 is unclear at this time. However, one explanation is ANGPTL3 inhibiting the clearance of TG-rich VLDL particles containing APOC3 from the circulation. In fact, strong correlations were also observed between plasma ANGPTL3 concentrations and the levels of IDL and VLDL particles.
During fructose consumption negative correlations developed between ANGPTL3 and HDL-C levels. APOA1 is a component of HDL-C and also correlated inversely with ANGPTL3 levels. The inverse relationships between ANGPTL3, HDL-C, and APOA1 are anticipated because ANGPTL3 influences HDL-C levels by suppressing the activity of endothelial lipase (12).
Experiments performed in obese mice resulting from leptin receptor mutations or with streptozotocin-induced diabetes indicate increased ANGPTL3 mRNA and protein levels in liver (44, 45). Insulin also acts directly on hepatocytes to suppress ANGPTL3 expression (45). The positive correlation between fructose-induced hyperinsulinemia and elevated plasma ANGPTL3 concentration might therefore be anticipated if the animals develop hyperinsulinemia.
The design of the current study allowed for a prospective analysis of variables that predict plasma ANGPTL3 concentrations both in the baseline state (low-sugar/chow-fed) and in animals challenged with a high-sugar diet. Our data suggest plasma concentrations of ANGPTL3 and APOC3 may identify animals most at risk for developing fasting hyperinsulinemia and atherogenic dyslipidemia during fructose consumption. Baseline plasma concentrations of ANGPTL3 and APOC3 appear to be weak predictors of the effects on fructose on fasting hyperinsulinemia. However, modeling that uses both proteins together was superior in identifying animals at greater risk for increasing fasting insulin concentrations and HOMA-IR during fructose consumption. Surprisingly, no relationship was evident for fructose-induced hypertriglyceridemia. However, animals with high APOC3 levels, or APOC3 and ANGPTL3 levels combined, exhibited large decreases of HDL-C concentrations after 3 months of fructose consumption.
Previous reports have suggested that certain gene variants that lower TG concentrations and reduce CVD risk can also influence the risk of type 2 diabetes (15, 46–50). Previous studies have also implicated a relationship between ANGPTL3 levels and risk of type 2 diabetes (15, 46). ANGPTL3 deficiency enhances insulin sensitivity in mice and humans (15, 22, 51), while loss-of-function ANGPTL4 gene variants are associated with improved glucose homeostasis and reduced risk of type 2 diabetes (52). Mechanistically, these associations may relate to differences in the uptake of free fatty acids into insulin-responsive tissues. Factors that regulate lipase activity will alter the flux of free fatty acids into target tissues, thereby affecting the response of these tissues to insulin (51).
Plasma adropin concentrations are related to indices of hepatic lipid metabolism in nonhuman primates and humans (31, 53–56). Adropin is a secreted peptide that has insulin-sensitizing actions in mouse models of insulin resistance (35–42). Lower adropin levels observed in animals with high ANGPTL3 could thus contribute to poor glycemic control.
Elevated levels of both IL-6 and CRP are associated with an elevated risk of type 2 diabetes (57). Plasma CRP has been implicated as an important independent predictor of CVD risk (58). Increases of both CRP and IL-6 concentrations in fructose-fed animals indicate rapid development of a proinflammatory response. However, fish oil supplementation appears to disassociate the relationship between systemic inflammation and metabolic dysregulation. Fish oil prevented fructose-induced fasting hyperinsulinemia, insulin resistance assessed by the intravenous glucose tolerance test, and dyslipidemia (increased TGs and APOC3) (29). Here we report that fish oil also prevents fructose-induced increases of ANGPTL3 concentrations. However, increases of circulating CRP or IL-6 levels in response to fructose consumption are not affected by fish oil supplementation. The impact of fructose in insulin sensitivity and dyslipidemia thus appears to be independent of inflammation. Alternatively, fish oil may limit the impact of inflammation on metabolic dysregulation.
The RNAi construct targeting ANGPTL3 synthesis is highly effective in decreasing ANGPTL3 expression, as reflected by the marked decrease of plasma ANGPTL3 as well as lowering circulating levels of most lipids/lipoproteins that we measured (plasma TGs, VLDL-C, APOC3, and APOE) that are implicated as risk factors for CVD in humans. Plasma HDL-C concentrations were also lowered by RNAi, which could be considered to increase CVD risk. However, subjects with homozygous null ANGPTL3 mutations that result in lower CVD risk have been reported to also exhibit lowered HDL-C levels (14). Interestingly, the administration of an RNAi construct directed at hepatic APOC3 expression that we previously reported lowers TG levels in rhesus macaques (30) does not influence circulating ANGPTL3 concentrations (data not shown). This suggests circulating ANGPTL3 levels are not regulated by pathways involving APOC3. The strong correlations observed between plasma APOC3 and ANGPTL3 concentrations are primarily driven by actions of the latter on the clearance of lipoproteins from the circulation.
CONCLUSIONS
Increased circulating ANGPTL3 concentrations in response to fructose consumption may be best explained by the development of fasting hyperinsulinemia and increases in HOMA-IR, which are indicators of insulin resistance. This response is prevented in animals concurrently receiving supplementation with fish oil. The inhibition of ANGPTL3 expression with RNAi reduces circulating ANGPTL3, as well as plasma concentrations of TGs, APOC3, and APOE in rhesus macaques. This is potentially clinically significant, as APOC3 is also an important determinant of TG-rich lipoprotein clearance from the circulation. Finally, this is the first study to demonstrate an effect of diet (fructose and n-3 fatty acids) on ANGPTL3 and suggests that ANGPTL3 is a promising therapeutic target for hypertriglyceridemia and related disorders of lipid metabolism.
Supplementary Material
Acknowledgments
The authors thank Vanessa Bakula, Ross Allen, Marinelle Nunez, Sarah Davis, Jenny Short, and the staff and administration of the California National Primate Research Center for their technical and logistical contributions to this study.
Footnotes
Abbreviations:
- ANGPTL3
- angiopoietin-like protein 3
- BW
- body weight at baseline
- HFCS
- high-fructose corn syrup
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IL-6
- interleukin 6
- TC
- total cholesterol
- TG
- triglyceride
This work was supported by National Institutes of Health Grants AT250099, AT003645, UL1 RR024146, HL121324, DK095960, and U24 DK092993; the American Diabetes Association; California National Primate Research Center Grant OD-0111107; and University of California Office of the President Award 142691. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.H. and S.W. are employed by Arrowhead Pharmaceuticals, which is developing RNAi technology for targeting APOC3 and ANGPTL3 for the management of hypertriglyceridemia. P.J.H. has received funding from Arrowhead Pharmaceuticals for studies investigating the effects of targeting ANGPTL3 and APOC3 for hypertriglyceridemia in the fructose/high-fructose corn syrup rhesus macaque model developed by his laboratory.
The online version of this article (available at https://www.jlr.org) contains a supplement.
REFERENCES
- 1.Chait A., and Subramanian S.. 2000. Hypertriglyceridemia: pathophysiology, role of genetics, consequences, and treatment In Endotext [Internet]. Feingold K. R., Anawalt B., Boyce A., et al., editors. MDText.com, Inc., South Dartmouth, MA. [Google Scholar]
- 2.Baigent C., Blackwell L., Emberson J., Holland L. E., Reith C., Bhala N., Peto R., Barnes E. H., Keech A., Simes J., et al. 2010. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 376: 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarese E. P., Robinson J. G., Kowalewski M., Kolodziejczak M., Andreotti F., Bliden K., Tantry U., Kubica J., Raggi P., and Gurbel P. A.. 2018. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 319: 1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siri-Tarino P. W., and Krauss R. M.. 2016. The early years of lipoprotein research: from discovery to clinical application. J. Lipid Res. 57: 1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegele R. A., Ginsberg H. N., Chapman M. J., Nordestgaard B. G., Kuivenhoven J. A., Averna M., Boren J., Bruckert E., Catapano A. L., Descamps O. S., et al. 2014. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2: 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berberich A. J., and Hegele R. A.. 2019. The complex molecular genetics of familial hypercholesterolaemia. Nat. Rev. Cardiol. 16: 9–20. [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard B. G., Nicholls S. J., Langsted A., Ray K. K., and Tybjaerg-Hansen A.. 2018. Advances in lipid-lowering therapy through gene-silencing technologies. Nat. Rev. Cardiol. 15: 261–272. [DOI] [PubMed] [Google Scholar]
- 8.Kersten S. 2017. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 13: 731–739. [DOI] [PubMed] [Google Scholar]
- 9.Quagliarini F., Wang Y., Kozlitina J., Grishin N. V., Hyde R., Boerwinkle E., Valenzuela D. M., Murphy A. J., Cohen J. C., and Hobbs H. H.. 2012. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 109: 19751–19756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haller J. F., Mintah I. J., Shihanian L. M., Stevis P., Buckler D., Alexa-Braun C. A., Kleiner S., Banfi S., Cohen J. C., Hobbs H. H., et al. 2017. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J. Lipid Res. 58: 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi X., Britt E. C., Shows H. W., Hjelmaas A. J., Shetty S. K., Cushing E. M., Li W., Dou A., Zhang R., and Davies B. S. J.. 2017. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol. Metab. 6: 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimamura M., Matsuda M., Yasumo H., Okazaki M., Fujimoto K., Kono K., Shimizugawa T., Ando Y., Koishi R., Kohama T., et al. 2007. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler. Thromb. Vasc. Biol. 27: 366–372. [DOI] [PubMed] [Google Scholar]
- 13.Musunuru K., Pirruccello J. P., Do R., Peloso G. M., Guiducci C., Sougnez C., Garimella K. V., Fisher S., Abreu J., Barry A. J., et al. 2010. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N. Engl. J. Med. 363: 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minicocci I., Montali A., Robciuc M. R., Quagliarini F., Censi V., Labbadia G., Gabiati C., Pigna G., Sepe M. L., Pannozzo F., et al. 2012. Mutations in the ANGPTL3 gene and familial combined hypolipidemia: a clinical and biochemical characterization. J. Clin. Endocrinol. Metab. 97: E1266–E1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robciuc M. R., Maranghi M., Lahikainen A., Rader D., Bensadoun A., Oorni K., Metso J., Minicocci I., Ciociola E., Ceci F., et al. 2013. Angptl3 deficiency is associated with increased insulin sensitivity, lipoprotein lipase activity, and decreased serum free fatty acids. Arterioscler. Thromb. Vasc. Biol. 33: 1706–1713. [Erratum. 2013. Arterioscler. Thromb. Vasc. Biol. 33: e124. ] [DOI] [PubMed] [Google Scholar]
- 16.Pisciotta L., Favari E., Magnolo L., Simonelli S., Adorni M. P., Sallo R., Fancello T., Zavaroni I., Ardigo D., Bernini F., et al. 2012. Characterization of three kindreds with familial combined hypolipidemia caused by loss-of-function mutations of ANGPTL3. Circ Cardiovasc Genet. 5: 42–50. [DOI] [PubMed] [Google Scholar]
- 17.Martín-Campos J. M., Roig R., Mayoral C., Martinez S., Marti G., Arroyo J. A., Julve J., and Blanco-Vaca F.. 2012. Identification of a novel mutation in the ANGPTL3 gene in two families diagnosed of familial hypobetalipoproteinemia without APOB mutation. Clin. Chim. Acta. 413: 552–555. [DOI] [PubMed] [Google Scholar]
- 18.Noto D., Cefalu A. B., Valenti V., Fayer F., Pinotti E., Ditta M., Spina R., Vigna G., Yue P., Kathiresan S., et al. 2012. Prevalence of ANGPTL3 and APOB gene mutations in subjects with combined hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 32: 805–809. [DOI] [PubMed] [Google Scholar]
- 19.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J. A., Marti G., Pisciotta L., Noto D., Cefalu A. B., et al. 2013. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. J. Lipid Res. 54: 3481–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudet D., Gipe D. A., Pordy R., Ahmad Z., Cuchel M., Shah P. K., Chyu K. Y., Sasiela W. J., Chan K. C., Brisson D., et al. 2017. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 377: 296–297. [DOI] [PubMed] [Google Scholar]
- 21.Dewey F. E., Gusarova V., Dunbar R. L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C. V., Bruse S., Dansky H. M., et al. 2017. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 377: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham M. J., Lee R. G., Brandt T. A., Tai L. J., Fu W., Peralta R., Yu R., Hurh E., Paz E., McEvoy B. W., et al. 2017. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 377: 222–232. [DOI] [PubMed] [Google Scholar]
- 23.Ramms B., and Gordts P.. 2018. Apolipoprotein C-III in triglyceride-rich lipoprotein metabolism. Curr. Opin. Lipidol. 29: 171–179. [DOI] [PubMed] [Google Scholar]
- 24.Sundaram M., Zhong S., Bou Khalil M., Links P. H., Zhao Y., Iqbal J., Hussain M. M., Parks R. J., Wang Y., and Yao Z.. 2010. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J. Lipid Res. 51: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J., Ouyang H., Wang Y., Pang D., Cong N. X., Wang T., Leng B., Li D., Li X., Wu R., et al. 2012. Characterization of a hypertriglyceridemic transgenic miniature pig model expressing human apolipoprotein CIII. FEBS J. 279: 91–99. [DOI] [PubMed] [Google Scholar]
- 26.Havel P. J., Kievit P., Comuzzie A. G., and Bremer A. A.. 2017. Use and importance of nonhuman primates in metabolic disease research: current state of the field. ILAR J. 58: 251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox L. A., Olivier M., Spradling-Reeves K., Karere G. M., Comuzzie A. G., and VandeBerg J. L.. 2017. Nonhuman primates and translational research-cardiovascular disease. ILAR J. 58: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremer A. A., Stanhope K. L., Graham J. L., Cummings B. P., Wang W., Saville B. R., and Havel P. J.. 2011. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin. Transl. Sci. 4: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremer A. A., Stanhope K. L., Graham J. L., Cummings B. P., Ampah S. B., Saville B. R., and Havel P. J.. 2014. Fish oil supplementation ameliorates fructose-induced hypertriglyceridemia and insulin resistance in adult male rhesus macaques. J. Nutr. 144: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butler A. A., Price C. A., Graham J. L., Stanhope K. L., King S., Hung Y. H., Sethupathy P., Wong S., Hamilton J., Krauss R. M., et al. 2019. Fructose-induced hypertriglyceridemia in rhesus macaques is attenuated with fish oil or ApoC3 RNA interference. J. Lipid Res. 60: 805–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler A. A., Zhang J., Price C. A., Stevens J. R., Graham J. L., Stanhope K. L., King S., Krauss R. M., Bremer A. A., and Havel P. J.. 2019. Low plasma adropin concentrations increase risks of weight gain and metabolic dysregulation in response to a high-sugar diet in male nonhuman primates. J. Biol. Chem. 294: 9706–9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robbins M., Judge A., and MacLachlan I.. 2009. siRNA and innate immunity. Oligonucleotides. 19: 89–102. [DOI] [PubMed] [Google Scholar]
- 33.Caulfield M. P., Li S., Lee G., Blanche P. J., Salameh W. A., Benner W. H., Reitz R. E., and Krauss R. M.. 2008. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin. Chem. 54: 1307–1316. [DOI] [PubMed] [Google Scholar]
- 34.Mora S., Caulfield M. P., Wohlgemuth J., Chen Z., Superko H. R., Rowland C. M., Glynn R. J., Ridker P. M., and Krauss R. M.. 2015. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Trial. Circulation. 132: 2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar K. G., Trevaskis J. L., Lam D. D., Sutton G. M., Koza R. A., Chouljenko V. N., Kousoulas K. G., Rogers P. M., Kesterson R. A., Thearle M., et al. 2008. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab. 8: 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganesh Kumar K., Zhang J., Gao S., Rossi J., McGuinness O. P., Halem H. H., Culler M. D., Mynatt R. L., and Butler A. A.. 2012. Adropin deficiency is associated with increased adiposity and insulin resistance. Obesity (Silver Spring). 20: 1394–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao S., McMillan R. P., Jacas J., Zhu Q., Li X., Kumar G. K., Casals N., Hegardt F. G., Robbins P. D., Lopaschuk G. D., et al. 2014. Regulation of substrate oxidation preferences in muscle by the peptide hormone adropin. Diabetes. 63: 3242–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S., McMillan R. P., Zhu Q., Lopaschuk G. D., Hulver M. W., and Butler A. A.. 2015. Therapeutic effects of adropin on glucose tolerance and substrate utilization in diet-induced obese mice with insulin resistance. Mol. Metab. 4: 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao S., Ghoshal S., Zhang L., Stevens J. R., McCommis K. S., Finck B. N., Lopaschuk G. D., and Butler A. A.. 2019. The peptide hormone adropin regulates signal transduction pathways controlling hepatic glucose metabolism in a mouse model of diet-induced obesity. J. Biol. Chem. 294: 13366–13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thapa D., Xie B., Zhang M., Stoner M. W., Manning J. R., Huckestein B. R., Edmunds L. R., Mullett S. J., McTiernan C. F., Wendell S. G., et al. 2019. Adropin treatment restores cardiac glucose oxidation in pre-diabetic obese mice. J. Mol. Cell. Cardiol. 129: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thapa D., Xie B., Manning J. R., Zhang M., Stoner M. W., Huckestein B. R., Edmunds L. R., Zhang X., Dedousis N. L., O’Doherty R. M., et al. 2019. Adropin reduces blood glucose levels in mice by limiting hepatic glucose production. Physiol. Rep. 7: e14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altamimi T. R., Gao S., Karwi Q. G., Fukushima A., Rawat S., Wagg C. S., Zhang L., and Lopaschuk G. D.. 2019. Adropin regulates cardiac energy metabolism and improves cardiac function and efficiency. Metabolism. 98: 37–48. [DOI] [PubMed] [Google Scholar]
- 43.Schunck W. H., Konkel A., Fischer R., and Weylandt K. H.. 2018. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 183: 177–204. [DOI] [PubMed] [Google Scholar]
- 44.Inukai K., Nakashima Y., Watanabe M., Kurihara S., Awata T., Katagiri H., Oka Y., and Katayama S.. 2004. ANGPTL3 is increased in both insulin-deficient and -resistant diabetic states. Biochem. Biophys. Res. Commun. 317: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 45.Shimamura M., Matsuda M., Ando Y., Koishi R., Yasumo H., Furukawa H., and Shimomura I.. 2004. Leptin and insulin down-regulate angiopoietin-like protein 3, a plasma triglyceride-increasing factor. Biochem. Biophys. Res. Commun. 322: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 46.Park C. Y., Moon J., Jo G., Lee J., Kim O. Y., Oh H., Lim H., and Shin M. J.. 2019. The association between genetic variants of angiopoietin-like 3 and risk of diabetes mellitus is modified by dietary factors in Koreans. Sci. Rep. 9: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lotta L. A., Stewart I. D., Sharp S. J., Day F. R., Burgess S., Luan J., Bowker N., Cai L., Li C., Wittemans L. B. L., et al. 2018. Association of genetically enhanced lipoprotein lipase-mediated lipolysis and low-density lipoprotein cholesterol-lowering alleles with risk of coronary disease and type 2 diabetes. JAMA Cardiol. 3: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lotta L. A., Gulati P., Day F. R., Payne F., Ongen H., van de Bunt M., Gaulton K. J., Eicher J. D., Sharp S. J., Luan J., et al. 2017. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 49: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu D. J., Peloso G. M., Yu H., Butterworth A. S., Wang X., Mahajan A., Saleheen D., Emdin C., Alam D., Alves A. C., et al. 2017. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 49: 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahajan A., Wessel J., Willems S. M., Zhao W., Robertson N. R., Chu A. Y., Gan W., Kitajima H., Taliun D., Rayner N. W., et al. 2018. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat. Genet. 50: 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., McNutt M. C., Banfi S., Levin M. G., Holland W. L., Gusarova V., Gromada J., Cohen J. C., and Hobbs H. H.. 2015. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl. Acad. Sci. USA. 112: 11630–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gusarova V., O’Dushlaine C., Teslovich T. M., Benotti P. N., Mirshahi T., Gottesman O., Van Hout C. V., Murray M. F., Mahajan A., Nielsen J. B., et al. 2018. Genetic inactivation of ANGPTL4 improves glucose homeostasis and is associated with reduced risk of diabetes. Nat. Commun. 9: 2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghoshal S., Stevens J. R., Billon C., Girardet C., Sitaula S., Leon A. S., Rao D. C., Skinner J. S., Rankinen T., Bouchard C., et al. 2018. Adropin: an endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 8: 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao S., Stevens J. R., and Butler A. A.. 2016. Adropin - a circulating factor in metabolic control or a drop in the ocean? Expert Rev. Endocrinol. Metab. 11: 239–241. [DOI] [PubMed] [Google Scholar]
- 55.Butler A. A., St-Onge M. P., Siebert E. A., Medici V., Stanhope K. L., and Havel P. J.. 2015. Differential responses of plasma adropin concentrations to dietary glucose or fructose consumption in humans. Sci. Rep. 5: 14691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler A. A., Tam C. S., Stanhope K. L., Wolfe B. M., Ali M. R., O’Keeffe M., St-Onge M. P., Ravussin E., and Havel P. J.. 2012. Low circulating adropin concentrations with obesity and aging correlate with risk factors for metabolic disease and increase after gastric bypass surgery in humans. J. Clin. Endocrinol. Metab. 97: 3783–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X., Bao W., Liu J., Ouyang Y. Y., Wang D., Rong S., Xiao X., Shan Z. L., Zhang Y., Yao P., et al. 2013. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 36: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaptoge S., Di Angelantonio E., Pennells L., Wood A. M., White I. R., Gao P., Walker M., Thompson A., Sarwar N., et al. 2012. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 367: 1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.