ABSTRACT
Since the discovery that enhancers can support transcription, the roles of enhancer RNAs have remained largely elusive. We identified that enhancer RNAs interact with and augment bromodomain engagement with acetylated chromatin. Here, we discuss our recent findings and the potential mechanisms underlying the regulation and functions of enhancer RNA-bromodomain associations.
KEYWORDS: Enhancer, eRNA, chromatin, histone modification, bromodomain, BRD4, transcription, gene regulation
Introduction
Enhancers are DNA elements that are bound by transcriptional regulators and determine cell-type and signal-specific gene expression programs [1–6] and enhancer deregulation is implicated in numerous human diseases [7–10]. Active enhancers are commonly identified through the combined assessment of chromatin accessibility [11–13], accumulation of histone modifications that include histone H3 lysine 4 monomethylation (H3K4me1) and histone H3 lysine 27 acetylation (H3K27ac) [13–17], and clustered binding of various transcription factors (TFs) and cofactors [18–21]. Nearly a decade ago, two independent studies discovered that enhancers can function as transcription units [22,23] and the detection of enhancer-derived RNAs (eRNAs) has since become a convenient indexing system to identify active enhancers [16,18,24–28].
While eRNAs have become useful for profiling active enhancers, there remains a key gap in knowledge that relates to whether enhancer transcription, eRNAs, or both are important for enhancer function. In support of the act of enhancer transcription, a seminal study has demonstrated that enhancer transcription plays a key role in maintaining an open chromatin state that is readily accessible to TFs and cofactors [29]. More recently, transcription at intragenic enhancers, versus the eRNA itself, was found to interfere with and attenuate host gene expression during embryonic stem cell differentiation [30]. Also consistent, is the finding that not all active enhancers support eRNA production, suggesting that eRNAs are not functionally important at all enhancers [31]. Yet, the prevalence [25,26] and regulated biogenesis of eRNAs [32–34] underscore the potential for widespread gene regulation by these noncoding transcripts. In support of direct eRNA functions, a growing number of studies have revealed a requirement for specific eRNAs in promoting higher expression of their cognate, protein-coding genes [35–41]. Recent insights into the mechanisms underlying eRNA functions have revealed that eRNAs contribute to gene control by stabilizing enhancer-promoter looping [36,38] and stimulating chromatin remodeling events that provide access to RNA Polymerase II (RNAPII) in cis [37], as well as cohesin loading and maintenance in trans [42]. eRNAs also facilitate the release of paused RNAPII by acting as a decoy for the negative elongation factor (NELF) [43] and through activation of the positive transcription elongation factor b (P-TEFb) complex [44].
The chromatin landscape has also been shown to be regulated by eRNAs. Specifically, histone modifying proteins referred to as “writers” that function by depositing post-translational modifications (PTMs) on the tails of histone proteins were recently found to be regulated by eRNAs [40]. In this study, it was demonstrated that eRNAs increase the histone acetyltransferase (HAT) activity of the CREB-binding protein (CBP) by displacing the activation loop from its active site to yield higher levels of H3K27ac at active enhancer regions [40] (Figure 1). More recently, we identified that a subset of histone effector proteins referred to as “readers”, which recognize and directly bind to various histone marks, are also regulated by eRNAs [41]. In our study, we specifically revealed that eRNAs form interactions with bromodomains (BDs) [41] (Figure 1), which are well-known acetyl lysine recognition domains. Our study demonstrates that BDs are also RNA binding modules and that BD-RNA interactions provide an additional layer of regulation of the epigenome [41].
Figure 1.
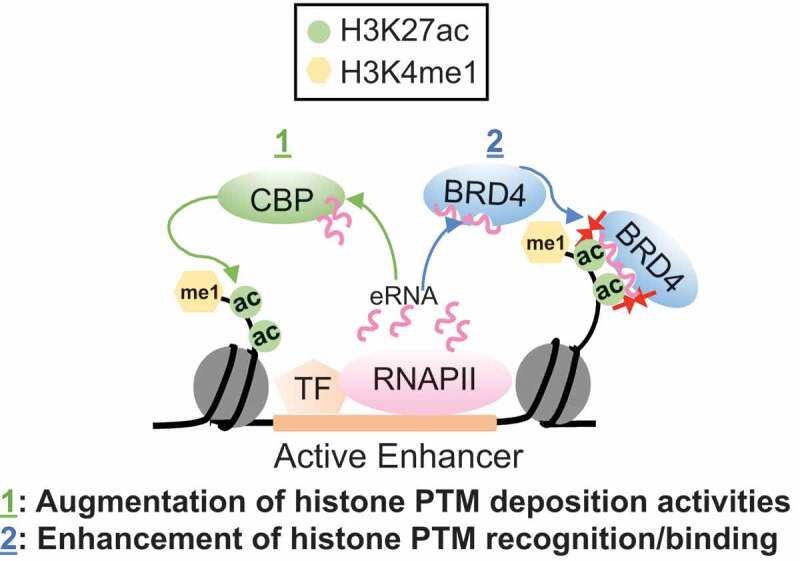
Functional significance of eRNA interactions with chromatin-regulatory factors.
eRNAs associate with CBP to increase its HAT activity and interact with BRD4 BDs to augment its binding to acetylated chromatin at enhancers. These events independently enhance the activity state of the underlying enhancers, which in turn promotes the activation of their cognate genes.
Chromatin reader domains as RNA binding modules
Recent efforts in the global identification of RNA-associating factors have revealed that RNA binding is a common feature of transcriptional regulators and chromatin modifying proteins [45,46]. Among the chromatin regulators that exhibit nucleic acid binding capabilities are several well-studied chromatin reader proteins [47]. To date, 6 of the 23 known histone reader domains have been shown to interact with nucleic acids (DNA and/or RNA) [47]. Notably, it is well-established that chromodomains, which recognize histone methylation, can function as RNA interaction modules [45,48,49]. For example, the chromodomain of reader protein chromobox7 (CBX7), which is a subunit of the Polycomb Repressive Complex 1 (PRC1) has been shown to interact with the ANRIL long noncoding RNA (lncRNA) to regulate senescence in prostate cancer cells [49]. More recently, characterization of the tandem BDs of each BET family member as well as the single BD of BRG1/human BRM (hBRM) revealed the ability of these modules to exhibit DNA binding activity [50,51].
Excitingly, we recently found that the tandem BDs of the bromodomain and extra-terminal motif (BET) protein BRD4 also serve as RNA binding modules [41]. BRD4 has been shown to promote inflammation and tumorigenesis by regulating the transcription initiation, elongation, and activation of key oncogenes such as MYC [52–55]. In addition, BRD4 functions in association with key transcription factors including NFκB [56–58] and p53 [59,60] and assists RNAPII-mediated elongation to regulate the synthesis of coding and noncoding transcripts [61–64]. Toward dissecting the mechanisms that regulate BD binding at active enhancer regions, we recently identified that BRD4 associates with eRNAs derived from a subset of enhancers regulated by gain-of-function p53R273H,P309S mutant in response to chronic immune signaling [41]. Through pulldown and electrophoretic mobility shift assays (EMSAs), we also demonstrated a requirement for the BRD4 BDs in directly interacting with RNAs as revealed by the significant decrease in eRNA binding of a BRD4 mutant that is devoid of both BDs [41].
Our results further demonstrated the commonality of the BD-RNA interactions across all BET family members and the non-BET proteins, BRG1 and BRD7 [41]. This finding raises the intriguing question of whether these RNA-BD interactions, in any capacity underlie differential regulation of various BD-containing proteins. This is of great significance given that the 42 different BD-containing proteins identified to date contribute to gene regulation through various mechanisms (reviewed in [65]). Namely, BD-containing proteins such as BRG1/hBRM, BRD7, and BAF180 are present in multi-subunit SWI/SNF chromatin remodeling complexes that control chromatin compaction and decompaction [65,66]. BD-containing factors also engage in depositing various histone PTMs such as acetylation (CBP and p300) and methylation (MLL complexes and ASH1L) [65]. Importantly, BD-containing proteins can also function together with specific TFs. For example, BD-containing TRIM24 acts as a co-activator to stabilize the chromatin interactions of estrogen receptor-α (ER-α) at ER-response elements in breast cancer cells [67]. Therefore, assessing the role of eRNAs in the differential regulation of various BD-containing factors may uncover novel RNA-mediated gene regulatory mechanisms.
Bromodomains demonstrate multivalent interactions with RNAs and histone modifications
It is well-established that BDs typically bind to acetylated histone residues with low affinity [68–70]. However, a number of studies have demonstrated the importance of multivalent BD interactions with various acetylated lysines and other PTMs [68,70,71]. Specifically, it has been shown that the presence of multiple acetyl sites can potentiate higher levels of BD binding to histone peptides [68,70,72]. For example, BRD4 BD1 and BD2 weakly bind to monoacetylated histone H4 lysine 12 (H4K12ac)- and histone H4 lysine 16 (H4K16ac)-modified peptides, but exhibit strong binding to the diacetylated H4K12acK16ac-modified peptide [72]. Moreover, other flanking PTMs have also been shown to influence the binding of BD-containing proteins to acetyl groups. For instance, BRD4 BD2 binding to diacetylated histone H3 lysines 4 and 9 (H3K4acK9ac)-modified peptide is notably enhanced in the presence of histone H3 threonine 3 phosphorylation [68].
Consistent with the importance of multivalency and the additional layers of regulation that impact BD interactions with chromatin, our recent findings report a role for eRNAs in strengthening BRD4 BD binding to monoacetylated H3K27ac- and H4K16ac-modified peptides in vitro [41]. In support of this finding, surface plasmon resonance (SPR) analyses further revealed that the presence of eRNAs significantly increased the maximum binding capacity of the tandem BRD4 BDs to the H3K27ac-modified histone peptide by approximately 2–3 fold [41]. We further confirmed these findings by establishing that eRNAs also augment BRD4 BD binding to histone octamers that were acetylated by the HAT, p300 [41]. We also confirmed the effects of eRNAs on enhanced BRD4 binding at active enhancers. Specifically, following chronic immune signaling in colon cancer cells, eRNA depletion resulted in a notable decrease in BRD4 occupancy at the corresponding enhancer regions [41]. Importantly, the significance of eRNA-BRD4 associations in enhancer and gene activation was demonstrated by the ability of BRD4 to recover target eRNA and mRNA levels in BRD4 single-knockdown, but not BRD4 and eRNA double-knockdown cells [41]. Taken together, our findings establish a new mechanism by which eRNA molecules augment the inherently weak BRD4 BD-acetyl interactions that lead to active maintenance of pro-tumorigenic gene expression in colon cancer cells. In addition, these studies indicate the importance of exploring the interplay between eRNAs and BDs in the context of various chromatin modifications that include, but are not limited to, poly-acetylation and phosphorylation to further underscore the significance of the identified BD-eRNA interactions.
Molecular mechanisms underlying BD-RNA interactions
While our findings reveal that BDs can interact with RNAs, the underlying mechanisms that govern these associations remain to be examined. Interestingly, our in vitro RNA pulldown assays demonstrated that the tandem BDs of BRDT and the single BRG1 BD exhibit lower levels of RNA binding as compared to the tandem BDs of BRD2, BRD3, BRD4 and single BRD7 BD [41]. It is critical to note that while BDs are highly conserved modules, there exists a notable degree of structural diversity among them [65,73,74]. The four α-helices within BDs are connected by variable loops comprised of conserved amino acids that enable the docking of acetylated residues. Importantly, these conserved acetyl binding residues are surrounded by positively charged patches of amino acids [65,73] that have been implicated in facilitating BRDT BD1 [50] and BRG1/hBRM BD [51] interactions with DNA. Thus, it is likely that these basic patches similarly support electrostatic interactions with eRNAs. Notably, however, previous assessment of the electrostatic surface potentials of various BDs alone proved insufficient in accurately predicting BD-DNA interactions [50]. Thus, specific amino acids and/or conformational differences in the BDs may also be contributing to the variability in nucleic acid-BD interactions.
Moreover, our analyses revealed that the specific BRD4-associating eRNAs are produced from genomic sites that support high levels of enhancer transcription and BRD4 occupancy [41]. This observation was consistent with findings that report CBP specifically associates with RNAs synthesized from genomic regions where CBP is found to accumulate [40]. Furthermore, our in vitro binding data demonstrates that BRD4 BDs interact with multiple RNA species and independently of nucleic acid sequence. Therefore, these findings suggest that BRD4-RNA interactions may be determined by the proximity between the RNA and BRD4 rather than RNA sequence specificity. However, while our analysis includes an assessment of a few target RNAs, global interrogation of BD-interacting transcripts is needed to advance our understanding of whether BDs preferentially associate with defined RNA sequences or structural motifs.
Lastly, promotion of liquid-liquid phase separation through RNA interactions with proteins that contain intrinsically disordered regions (IDRs) such as BRD4 provides preface for an additional mechanism that may further modulate BRD4-RNA interactions [75–77]. More specifically, BRD4 has been shown to form distinct puncta within transcriptional condensates at superenhancers [76]. In addition, it was recently established that eRNA-dependent ribonucleoprotein complexes that exhibit properties of phase-separated condensates are indispensable for the cooperative activation of ligand-dependent enhancers [78]. Thus, the link between BRD4, eRNAs, and phase separated condensates may add yet another layer of complexity to the regulation of BRD4-eRNA interactions. Future investigations into the molecular characteristics underlying RNA interactions with BD-containing factors are pivotal to advance our understanding of such RNA-protein complexes that can play critical roles in the regulation of gene expression.
Conclusion
Despite the identification of eRNAs nearly a decade ago, the functions of these noncoding RNAs have remained largely mysterious. Our recent efforts have provided new insights into eRNA functions by revealing an exciting convergence between the chromatin reader BDs and eRNAs. These findings significantly extend the conventional understanding of BD functions in regulating the chromatin landscape. Future studies focused on identifying additional eRNA binding partners and the significance of eRNA sequence and structure promise to further unveil novel mechanisms through which eRNAs contribute to gene regulatory events.
Funding Statement
S.M.L is supported by NIH grant R35 GM128900-01 . H.R. is supported by National Science Foundation Graduate Research Fellowship (NSF-GRFP) and the NIH T32 GM007240).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barish GD, Yu RT, Karunasiri M, et al. Bcl-6 and NF-kappaB cistromes mediate opposing regulation of the innate immune response. Genes Dev. 2010;24:2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lefterova MI, Steger DJ, Zhuo D, et al. Cell-specific determinants of peroxisome proliferator-activated receptor gamma function in adipocytes and macrophages. Mol Cell Biol. 2010;30:2078–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mullen AC, Orlando D, Newman J, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell. 2011;147:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nielsen R, Pedersen TA, Hagenbeek D, et al. Genome-wide profiling of PPARgamma: rXRand RNA polymerase II occupancy reveals temporal activation of distinct metabolic pathways and changes in RXR dimer composition during adipogenesis. Genes Dev. 2008;22:2953–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Trompouki E, Bowman T, Lawton L, et al. Lineage regulators direct BMP and Wnt pathways to cell-specific programs during differentiation and regeneration. Cell. 2011;147:577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maurano MT, Humbert R, Rynes E, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hnisz D, Abraham BJ, Lee TI, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155:934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lovén J, Hoke H, Lin C, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herz HM. Enhancer deregulation in cancer and other diseases. Bioessays. 2016;38:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thurman RE, Rynes E, Humbert R, et al. The accessible chromatin landscape of the human genome. Nature. 2012;489:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rivera CM, Ren B. Mapping human epigenomes. Cell. 2013;155:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Calo E, Wysocka J. Modification of enhancer chromatin: what, how, and why? Mol Cell. 2013;49:825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Heintzman ND, Hon GC, Hawkins RD, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chepelev I, Wei G, Wangsa D, et al. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 2012;22:490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Creyghton MP, Cheng AW, Welstead GG, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107:21931–21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rada-Iglesias A, Bajpai R, Swigut T, et al. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang D, Garcia-Bassets I, Benner C, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature. 2011;474:390–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Spitz F, Furlong EEM. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. [DOI] [PubMed] [Google Scholar]
- [20].Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. [DOI] [PubMed] [Google Scholar]
- [21].Visel A, Blow MJ, Li Z, et al. ChIP-seq accurately predicts tissue-specific activity of enhancers. Nature. 2009;457:854–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].De Santa F, Barozzi I, Mietton F, et al. A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 2010;8:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim T-K, Hemberg M, Gray JM, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Djebali S, Davis CA, Merkel A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Andersson R, Gebhard C, Miguel-Escalada I, et al. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arner E, Daub CO, Vitting-Seerup K, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347:1010–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hah N, Danko C, Core L, et al. A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell. 2011;145:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li W, Notani D, Rosenfeld MG. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 2016;17:207–223. [DOI] [PubMed] [Google Scholar]
- [29].Kaikkonen MU, Spann N, Heinz S, et al. Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol Cell. 2013;51:310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cinghu S, Yang P, Kosak JP, et al. Intragenic enhancers attenuate host gene expression. Mol Cell. 2017;68:104–117 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mikhaylichenko O, Bondarenko V, Harnett D, et al. The degree of enhancer or promoter activity is reflected by the levels and directionality of eRNA transcription. Genes Dev. 2018;32:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Koch F, Fenouil R, Gut M, et al. Transcription initiation platforms and GTF recruitment at tissue-specific enhancers and promoters. Nat Struct Mol Biol. 2011;18:956–963. [DOI] [PubMed] [Google Scholar]
- [33].Natoli G, Andrau JC. Noncoding transcription at enhancers: general principles and functional models. Annu Rev Genet. 2012;46:1–19. [DOI] [PubMed] [Google Scholar]
- [34].Lai F, Gardini A, Zhang A, et al. Integrator mediates the biogenesis of enhancer RNAs. Nature. 2015;525:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lam MTY, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li W, Notani D, Ma Q, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mousavi K, Zare H, Dell’orso S, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hsieh C-L, Fei T, Chen Y, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci U S A. 2014;111:7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ilott NE, Heward JA, Roux B, et al. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun. 2014;5:3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bose DA, Donahue G, Reinberg D, et al. RNA binding to CBP stimulates histone acetylation and transcription. Cell. 2017;168:135–149 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rahnamoun H, Lee J, Sun Z, et al. RNAs interact with BRD4 to promote enhanced chromatin engagement and transcription activation. Nat Struct Mol Biol. 2018;25:687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tsai P-F, Dell’Orso S, Rodriguez J, et al. A muscle-specific enhancer RNA mediates cohesin recruitment and regulates transcription in trans. Mol Cell. 2018;71:129–141 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schaukowitch K, Joo J-Y, Liu X, et al. Enhancer RNA facilitates NELF release from immediate early genes. Mol Cell. 2014;56:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhao Y, Wang L, Ren S, et al. Activation of P-TEFb by androgen receptor-regulated enhancer RNAs in castration-resistant prostate cancer. Cell Rep. 2016;15:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].He C, Sidoli S, Warneford-Thomson R, et al. High-resolution mapping of RNA-binding regions in the nuclear proteome of embryonic stem cells. Mol Cell. 2016;64:416–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Caudron-Herger M, Rusin SF, Adamo ME, et al. R-deep: proteome-wide and quantitative identification of RNA-dependent proteins by density gradient ultracentrifugation. Mol Cell. 2019;75:184–199.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Weaver TM, Morrison EA, Musselman CA. Reading more than histones: the prevalence of nucleic acid binding among reader domains. Molecules. 2018;23:2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Akhtar A, Zink D, Becker PB. Chromodomains are protein-RNA interaction modules. Nature. 2000;407:405–409. [DOI] [PubMed] [Google Scholar]
- [49].Yap KL, Li S, Muñoz-Cabello AM, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell. 2010;38:662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Miller TC, Simon B, Rybin V, et al. A bromodomain-DNA interaction facilitates acetylation-dependent bivalent nucleosome recognition by the BET protein BRDT. Nat Commun. 2016;7:13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Morrison EA, Sanchez JC, Ronan JL, et al. DNA binding drives the association of BRG1/hBRM bromodomains with nucleosomes. Nat Commun. 2017;8:16080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dawson MA, Prinjha RK, Dittmann A, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zuber J, Shi J, Wang E, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang B, Yang XD, Zhou MM, et al. Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol Cell Biol. 2009;29:1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zou Z, Huang B, Wu X, et al. Brd4 maintains constitutively active NF-kappaB in cancer cells by binding to acetylated RelA. Oncogene. 2014;33:2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Brown JD, Lin CY, Duan Q, et al. NF-kappaB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell. 2014;56:219–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wu SY, Lee AY, Lai HT, et al. Phospho switch triggers Brd4 chromatin binding and activator recruitment for gene-specific targeting. Mol Cell. 2013;49:843–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Stewart HJ, Horne GA, Bastow S, et al. BRD4 associates with p53 in DNMT3A-mutated leukemia cells and is implicated in apoptosis by the bromodomain inhibitor JQ1. Cancer Med. 2013;2:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jang MK, Mochizuki K, Zhou M, et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell. 2005;19:523–534. [DOI] [PubMed] [Google Scholar]
- [62].Winter GE, Mayer A, Buckley DL, et al. BET bromodomain proteins function as master transcription elongation factors independent of CDK9 recruitment. Mol Cell. 2017;67:5–18 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kanno T, Kanno Y, LeRoy G, et al. BRD4 assists elongation of both coding and enhancer RNAs by interacting with acetylated histones. Nat Struct Mol Biol. 2014;21:1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nagarajan S, Hossan T, Alawi M, et al. Bromodomain protein BRD4 is required for estrogen receptor-dependent enhancer activation and gene transcription. Cell Rep. 2014;8:460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fujisawa T, Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol. 2017;18:246–262. [DOI] [PubMed] [Google Scholar]
- [66].Tang L, Nogales E, Ciferri C. Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription. Prog Biophys Mol Biol. 2010;102:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tsai WW, Wang Z, Yiu TT, et al. TRIM24 links a non-canonical histone signature to breast cancer. Nature. 2010;468:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Filippakopoulos P, Picaud S, Mangos M, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Moriniere J, Rousseaux S, Steuerwald U, et al. Cooperative binding of two acetylation marks on a histone tail by a single bromodomain. Nature. 2009;461:664–668. [DOI] [PubMed] [Google Scholar]
- [71].Ruthenburg AJ, Li H, Patel DJ, et al. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol. 2007;8:983–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jung M, Philpott M, Müller S, et al. Affinity map of bromodomain protein 4 (BRD4) interactions with the histone H4 tail and the small molecule inhibitor JQ1. J Biol Chem. 2014;289:9304–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Dhalluin C, Carlson JE, Zeng L, et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. [DOI] [PubMed] [Google Scholar]
- [74].Romero FA, Taylor AM, Crawford TD, et al. Disrupting acetyl-lysine recognition: progress in the development of bromodomain inhibitors. J Med Chem. 2016;59:1271–1298. [DOI] [PubMed] [Google Scholar]
- [75].Lin Y, Protter DS, Rosen MK, et al. Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell. 2015;60:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sabari BR, Dall’Agnese A, Boija A, et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boeynaems S, Alberti S, Fawzi NL, et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nair SJ, Yang L, Meluzzi D, et al. Phase separation of ligand-activated enhancers licenses cooperative chromosomal enhancer assembly. Nat Struct Mol Biol. 2019;26:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]


