ABSTRACT
Humans and other mammalian hosts have evolved mechanisms to control the bacteria colonizing their mucosal barriers to prevent invasion. While the breach of barriers by bacteria typically leads to overt infection, increasing evidence supports a role for translocation of commensal bacteria across an impaired gut barrier to extraintestinal sites in the pathogenesis of autoimmune and other chronic, non-infectious diseases. Whether gut commensal translocation is a cause or consequence of the disease is incompletely defined. Here we discuss factors that lead to translocation of live bacteria across the gut barrier. We expand upon our recently published demonstration that translocation of the gut pathobiont Enterococcus gallinarum can induce autoimmunity in susceptible hosts and postulate on the role of Enterococcus species as instigators of chronic, non-infectious diseases.
KEYWORDS: Gut vascular barrier, gut lymphatic barrier, bacterial translocation, autoimmunity, enterococcus, microbiota, intestinal permeability, tight junctions, vancomycin resistance, TLR7, lupus, autoimmune liver disease
Introduction
Only a thin layer of highly specialized epithelium separates our internal organs from trillions of intestinal microbes.1 This gut barrier functions as both a selective gatekeeper that keeps pathogens from invading as well as a site for immune cell education, nutrient absorption, and waste secretion. Although a thick mucus layer, antimicrobial peptides, and IgA serve to maintain the barrier function, perturbations in host defenses and alterations in microbial community composition can lead to pathologic breaches and subsequent disease states.2-4 Our recent demonstration that a gut microbe, Enterococcus gallinarum, crosses the gut barrier in autoimmune-prone hosts to colonize internal organs and incite autoimmunity provides a model for gut commensal translocation leading to pathologic states.5 In this addendum to our published study, we discuss these findings in the context of other investigations of translocation of whole bacteria across the gut barrier.
Epithelial barrier
The intestinal epithelia regulates the entry of micro- and macromolecules from the gut lumen into the host.1 The movement of solutes across the barrier is controlled in part by tight junctions (TJs) that tether together epithelial cells and selectively control solute entry based on their size and charge. Passage of important molecules beneficial to the host usually occurs paracellularly or transcellularly through enterocytes, with larger macromolecules being actively transcytosed. Two major paths of paracellular passage exist: a high capacity pore pathway permeable up to ~10Å molecules and a low capacity leak pathway permeable to up to ~125Å macromolecules.6,7 A third, unrestricted pathway becomes active at sites of erosive and ulcerative damage, such as that induced by dextran-sodium sulfate (DSS), where the loss of cellular integrity enables the passage of ions, metabolites, and even whole bacteria.6,7
Barrier integrity proteins are regulated by internal and external factors and can be direct targets of bacteria. Their expression varies depending on cell type and location. Table 1 summarizes selected barrier proteins-based on cellular and anatomical location as well as their respective contributions to barrier integrity.1,6,8-11 TJs are comprised of multiple transmembrane proteins, including claudins, occludins, and junctional adhesion molecules, which interact with cytoskeletal components through plaque proteins such as zonula occludens (ZOs). Claudins generally regulate the paracellular pore pathway whereas ZOs, occludins, and tricellulins regulate the leak pathway.7 While expression of many of these proteins leads to decreased permeability, up-regulation of some such as claudin-2 lead to increased pore pathway activity. Due to the importance of barrier integrity to the host, there is considerable compensation of barrier proteins if one of them has been disrupted.7 TJ protein formation and disassembly are regulated by multiple signaling pathways including protein kinase C, mitogen-activated protein kinases, myosin light chain kinases, and Rho GTPases.12 The activity of these enzymes is in turn modulated by cytokines and intraluminal molecules including bacterial metabolites and dietary factors.1,12 Some beneficial bacteria exert barrier protective effects by inducing TJ formation and limiting permeability.12 Others take advantage of these TJ regulatory mechanisms for invasion.4
Table 1.
Gut barrier-related molecules & barrier function*.
 |
Vascular barrier and enteric nervous system
Within the lamina propria beneath the epithelial layer lies the gut vascular barrier (GVB) and gut lymphatic barrier (GLB) comprised of endothelial cells that are held together by TJs and adherens junctions (AJs). The GVB barrier is permeable to 4 kD fluorescein isothiocyanate (FITC)-dextran,8 so as with the epithelial barrier, restricts the passage of bacteria. The GVB integrity is influenced by the WNT-β-catenin signaling pathway with β-catenin as a major AJ component.8 When β-catenin is constitutively active in endothelial cells, mice are less susceptible to enhanced GVB permeability and pathogen invasion normally seen with Salmonella typhimurium infection.8
The enteric nervous system is composed of enteric neurons and glial cells.13 Enteric glial cells and pericytes are in immediate contact with the GVB and form the gut vascular unit.8 Cells from the enteric nervous system produce substances that can also modulate barrier permeability. For example, the release of S-nitrosoglutathione by enteric glial cells induces TJ expression at the epithelium, and stimulation of the vagus nerve prevents burn-induced gut permeability.14 Furthermore, mice lacking enteric glial cells are susceptible to epithelial permeability that leads to bacterial invasion and host death.15
Mechanisms of microbial translocation
Bacteria may translocate past the gut epithelial layer through both physiologic and pathologic means depending on the context and type of bacteria.16,17 Bacterial size, virulence factors, defects in host barrier integrity, and uptake by antigen-presenting cells are all factors that influence entry into the host organism.4,8,16,18,19 Inert particles of the size of bacteria are passively taken up in the gastrointestinal tract,20 suggesting that entry of some bacteria may naturally occur because of mass and quantity if they can avoid a sterilizing immune response. Under steady state, M cells, CXCR3R1+ macrophages and CD103+ dendritic cells (DCs) extend protrusions that sample luminal contents and enable some commensals and pathogens to gain access beyond the epithelial layer without causing damage.2,3,21-23 These mechanisms are important for normal induction of IgA and T cell tolerance,18,24 as well as bacterial clearance.22 For example, live commensal E. cloacae are taken in by M cells to Peyer’s patches (PP) and carried by DCs to mesenteric lymph nodes (MLNs) to mount IgA responses.18 Other commensals, such as Alcaligenes, naturally inhabit DCs within host PPs where they induce broadly mucosal IgA in a non-inflammatory context.25,26 These unique bacteria have likely co-evolved for millennia with the host because their levels drop in an IgA-deficient host, suggesting that IgA binding may be needed for their uptake and growth within gut-associated lymphoid tissue.25 Additional commensals within lymphoid tissues promote tolerance by inducing interleukin (IL)-10 and IL-22 by DCs and innate lymphoid cells type 3, respectively, which collectively inhibit Th17 responses and facilitate bacterial colonization.27 In the absence of host defects or virulence and in the presence of intact MLNs, these commensals are prevented from further dissemination, in part due to efficient killing by macrophages.18
On the other hand, pathogens have developed the machinery to exploit these sampling cells and facilitate their invasion, including pili that mediate adhesion and Type III and IV secretion systems that inject effector proteins into host cells. Salmonella typhimurium targets M cells, leading to their destruction and disruption of the intestinal epithelium.2 Shigella flexneri, on the other hand, invades M cells and subsequently reenters enterocytes basolaterally, triggering cell death and inflammation that leads to additional bacterial translocation.3 Mechanisms of invasion by these and other infectious agents including Yersinia enterocolitica and Salmonella enterica have been described in detail previously.2,4
Paracellular passage is achieved by certain pathogens like Entamoeba histolytica, Toxoplasma gondii, Streptococcus agalactiae, and group A Streptococcus, which disrupt TJs and AJs to enhance permeability and facilitate their translocation.2,3,28 Commensals may also gain access to the host paracellularly as a consequence of barrier disruption caused by pathogens or irritants.
The dissemination of bacteria to various organs beyond the intestinal epithelium also depends on whether the GVB or GLB is breached. Some bacteria may breach only lymphatic vessels and are carried to the MLNs. Serratia marcescens, for example, was reported to reside only in lymph and not blood, paralleling translocation of a bacteriophage.29 Group A Streptococcus exhibits tropism for lymphatics as its hyaluronan capsule binds to lymphatic vessel endothelial receptor-1. Disruption of this interaction, interestingly, impairs lymphatic dissemination and leads to blood vessel invasion.30
Bacteria that breach the capillary system invade the enterohepatic circulation and travel to the liver via the portal vein. Thus, the liver represents a firewall after a breach of the GVB whereas the MLNs limit bacteria that break through the GLB.8,31 Particularly pathogenic bacteria, like Salmonella, are able to breach both GVB and GLB.8,18 Additionally, E. gallinarum, a gut pathobiont that induces autoimmunity, sequentially colonizes mesenteric veins, MLN, livers, and spleens of monocolonized mice, suggesting it can breach both lymphatic and blood vessels (Figure 1).5 This sequence of colonization contrasts with direct inoculation of bacteria into the peritoneal cavity, which are recovered in the spleen rather than the MLNs.18
Figure 1.
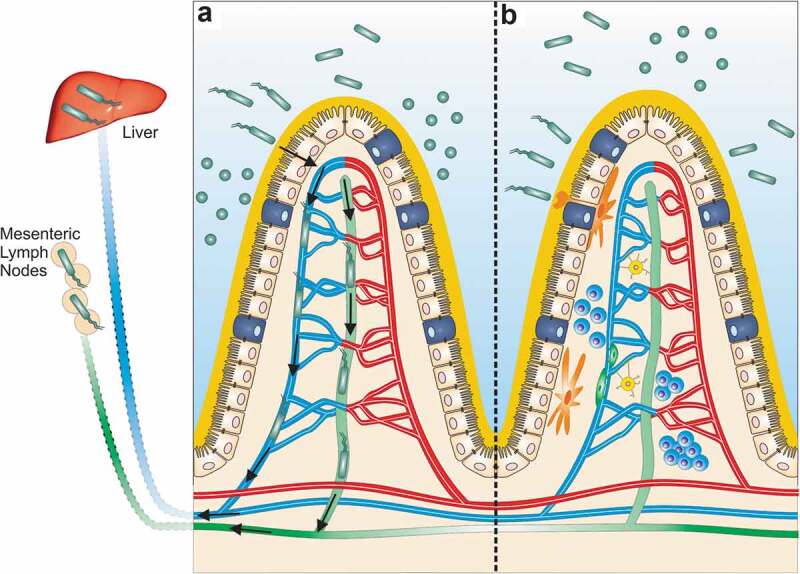
Gut barrier breach by the gut pathobiont E. gallinarum in autoimmune-prone hosts. Beyond the intestinal epithelial barrier (IEB), the gut vascular (GVB) and gut lymphatic barriers (GLB) shield the internal organs from colonization by commensals breaching the inner mucus layer and epithelial lining. (a) In (NZW x BXSB)F1 mice, a leaky gut barrier allows E. gallinarum to translocate beyond the intestinal epithelium. In monocolonized mice, E. gallinarum downregulates barrier molecules related to both the GVB and GLB,5 suggesting that it can breach all barrier components in the gut. Alternatively, it could be carried by host cells into host tissues. Once past the IEB and inside the lamina propria, E. gallinarum travels via the mesenteric veins (blue) and lymphatics (green) to the so-called “firewalls”, the liver and mesenteric lymph nodes, respectively. Within these organs, E. gallinarum interacts with host immune and epithelial cells to promote autoimmunity.5 (b) In a non-autoimmune-prone host, the intestinal epithelia, GVB, and GLB are intact. At steady state, antigen-presenting cells (orange) sample luminal bacteria to induce homeostatic IgA responses. T- and B-lymphocytes (blue) participate in this process leading to T cell-dependent and -independent IgA. The GVB is supported by enteric glial cells (yellow) and pericytes (dark green) surrounding a fenestrated endothelium sealed by tight junctions.8.
Induced gut barrier disruption and translocation
Bacterial translocation can occur after gut barrier disruption due to certain drugs, radiation, epigenetic changes, alcohol, hyperglycemia, ischemia, and autonomic dysfunction after stroke, among other factors.32-39 Chemotherapy and radiation alter the integrity of the gut barrier and allow for the passage of macromolecules and bacteria,38 a process that enhances antitumor immunity.32,34 Treatment of mice with cyclophosphamide enhances gut permeability as shown by the leakage of orally administered FITC-dextran into the blood. Commensals such as Lactobacillus johnsonii, L. murinus, and Enterococcus hirae could be cultured in this setting from lymphoid organs including MLN and spleen.34 Daily injections of low doses of the chemotherapeutic drug streptozosin lead to bacterial translocation to the pancreatic lymph node, innate immune activation, and type I diabetes.33 Bacterial translocation to MLNs, blood, and adipocytes is also seen following DSS-induced colitis as well as indomethacin-induced ileitis.40,41 Furthermore, chronic alcohol consumption, proton pump inhibitors (PPIs), and other non-steroidal anti-inflammatory drugs (NSAIDs) induce intestinal permeability to certain molecules.4,7,36 Interestingly, translocation of Enterococcus spp., in particular, E. faecalis, has been linked to gastric acid suppression in alcoholic liver disease, which leads to liver inflammation and hepatocyte death.36
Besides PPIs and NSAIDs, treatment with antibiotics can enable bacterial translocation.42 Antibiotic-treated mice are more susceptible to DSS-induced epithelial injury, translocation of live bacteria, and inflammasome-mediated damage.43 In addition to eradicating beneficial bacteria that contribute to barrier integrity, antibiotic use can lead to overgrowth of pathogenic bacteria. Ampicillin treatment, for example, promotes colonization with vancomycin-resistant Enterococcus (VRE) in mice and humans prior to bloodstream infections.44 Metronidazole and streptomycin treatment enable E. faecalis overgrowth and approximation to the epithelial border, where it can be visualized inside intestinal epithelial cells, deeper in the lamina propria and beyond the mucosa.45 Similarly, a 2-day course of ceftriaxone enabled E. faecalis and Lactobacillus spp. overgrowth and systemic dissemination to the liver, spleen, and MLN within 3–4 days of exposure, with subsequent clearance by 14 days. Of note, exposure to ceftriaxone did not affect TJ protein expression, fecal albumin, or permeability to FITC-dextran.46 Oral antibiotics were shown to induce colonic goblet cell-associated antigen passages that enabled translocation of live bacteria to MLN, which continued for ~5 days after antibiotic withdrawal.47 Thus, antibiotics may disrupt colonization resistance and physiological homeostatic processes, allowing for transient dissemination of bacteria even without overt intestinal pathology.
Spontaneous gut commensal dissemination
Spontaneous translocation of commensal bacteria beyond physiologic uptake by host cells can occur as a result of inherent alterations in the mucosal barrier, the immune system, or the microbial community structure. Mice grown in germ-free facilities exhibit greater translocation due to an immature immune system, a permeable mucus layer, and the absence of an intact commensal community.48,49 Germ-free mice are impaired in forming PPs and MLNs, and in secreting IgA, which is reversible by colonization with commensal bacteria.50-52 Viable E. coli and L. acidophilus could be recovered from MLNs, spleens, and livers of germ-free mice inoculated with whole cecal content from specific pathogen-free (SPF) mice, but could not be cultured from those organs in SPF mice with intact microbiota.48 Similarly, our laboratory found bacterial translocation and colonization of mesenteric veins, followed by MLNs, livers, and spleens of C57BL/6 mice with E. gallinarum in the monocolonized setting, but not under normal housing conditions, suggesting that the resident microbiota of a healthy host prevent either colonization or translocation of this Enterococcus species. We observed consistent translocation of E. gallinarum to these organs under SPF conditions only in an autoimmune-prone host, the male (NZW x BXSB)F1 mouse, which carries a toll-like receptor 7 (tlr7) gene duplication.5 In addition, lactobacilli translocate in a host predisposed to excessive TLR7 signaling induced by transgenic overexpression of tlr7.53 Specifically, L. reuteri was shown to drive innate inflammation in TLR7 transgenic mice kept under SPF conditions. Translocation of L. reuteri to the MLN or liver was observed in non-transgenic C57BL/6 wild-type (WT) mice only after stimulation with the TLR7 agonist imiquimod. TLR7 signals were needed both under SPF conditions and in L. reuteri-monocolonized C57BL/6 mice for translocation to occur. WT but not TLR7 KO C57BL/6 mice displayed increased gut leakiness to FITC-dextran when exposed to TLR7 transgenic mouse-derived microbiota. In addition, C57BL/6 mice treated with imiquimod have increased permeability to FITC-dextran, further supporting a role for TLR7 in barrier integrity.53 The mechanisms of how TLR7 signals regulate barrier integrity remain to be determined, as the receptor is not expressed in the gut epithelium itself.53,54 In addition, factors that regulate the translocation of lactobacilli across the gut barrier are incompletely understood. Interestingly, deletion of tet2, an enzyme involved in DNA demethylation, also leads to translocation of L. reuteri and related species to the MLN and spleen.39 Overall, enterococci and lactobacilli appear to be predominant genera translocating under pathologic conditions.46
Functional consequences of spontaneous bacterial translocation
A functional consequence of barrier breach and bacterial dissemination, whether induced or spontaneous, is a systemic immune response with the production of systemic IgG in addition to mucosal IgA.41 In genetically susceptible hosts, this systemic immune response, perpetuated by continuous bacterial translocation, may propagate autoimmunity and other non-infectious chronic diseases (Figure 2). Our laboratory has recently shown that spontaneous bacterial translocation of E. gallinarum plays a causative role in the induction of autoimmune disease.5 In the (NZW x BXSB)F1 model, male mice carry a duplication of the tlr7 gene on the BXSB background and additional autoimmune genes on the NZW background, which lead to heightened levels of type I interferon and spontaneous features of systemic autoimmunity. These mice develop impairment of the intestinal barrier starting in adolescence as measured by FITC-dextran, and exhibit translocation of several organisms, among them predominantly and most consistently E. gallinarum. Monocolonization of non-autoimmune-prone C57BL/6 mice with E. gallinarum induced gut permeability to FITC-dextran, suggesting that both microbial, as well as host-related factors (e.g., TLR7-mediated signals as above), contribute to loss of barrier integrity. E. gallinarum reduced gut barrier-tightening molecules (such as those listed in Table 1) in (NZW x BXSB)F1 mice and in monocolonized non-autoimmune C57BL/6 mice.5 In addition, E. gallinarum downregulates RNA of claudin-3 and -5 in gut organoids derived from small intestines of (NZW x BXSB)F1 mice (Figure 3). In long-term-monocolonized C57BL/6 mice, claudin-3 and -5 proteins were also reduced, as assessed by confocal imaging, whereas short-term exposure led to claudin-3 RNA upregulation. These findings likely reflect dynamic kinetics following epithelial cell interactions as seen also for claudin-5 in (NZW x BXSB)F1 ileum-derived organoids (Figure 3).5 Importantly, E. gallinarum functionally breached the gut barrier and migrated to MLNs, liver, and spleen. This process was linked to the progression of autoimmunity, including liver inflammation, systemic autoantibody production (against dsDNA, RNA, β2-glycoprotein I, and endogenous retroviral proteins), signs of lupus nephritis, and widespread thrombi formation as seen in lupus-related antiphospholipid syndrome.5 Notably, the portal inflammation in the liver is reminiscent of autoimmune hepatitis (AIH), an organ-specific autoimmune disease also characterized by anti-dsDNA antibodies in a subset of AIH patients.55 Continuous oral vancomycin treatment depleted E. gallinarum in the gut microbiome of (NZW x BXSB)F1 mice and prevented autoimmune manifestations.5 Ongoing chronic treatment with vancomycin in our animal facility, however, created a niche for the development of vancomycin-resistant strains of Enterococcus (VRE). E. gallinarum is naturally resistant to low-dose vancomycin due to a vanC gene cassette.56 When E. gallinarum acquires the vanB operon via horizontal transfer, however, it becomes VRE with high-level resistance that overcomes the high oral doses we applied to prevent autoimmunity and normally suppress the growth of E. gallinarum (Figure 4). Remarkably, the mouse colonized with the VRE strain of E. gallinarum progressed to full-blown autoimmunity with VRE growing in internal organs. Along with the host-microbial interaction studies we performed, this observation supports that autoimmunity was induced by E. gallinarum and not any of the other diverse bacteria sensitive to vancomycin in the gut of (NZW x BXSB)F1 mice. In addition to monocolonization studies, vaccination against E. gallinarum provides evidence that this bacterium alone was the main driver of organ-specific and systemic autoimmunity. Intramuscular vaccination against E. gallinarum, but not against phylogenetically related E. faecalis or other less related gut bacteria, prevented autoimmunity and associated mortality.5 When introduced into germ-free non-autoimmune-prone C57BL/6 mice by oral gavage, E. gallinarum induced Th17 responses not only in the lamina propria, as has been seen with gut resident segmented filamentous bacteria,57 but also in MLNs, supporting immunologic effects beyond the gut. This phenomenon was linked to E. gallinarum translocation to MLNs, followed by liver and spleen, and induction of serum autoantibodies directed against dsDNA and RNA, which are hallmarks of human SLE.
Figure 2.
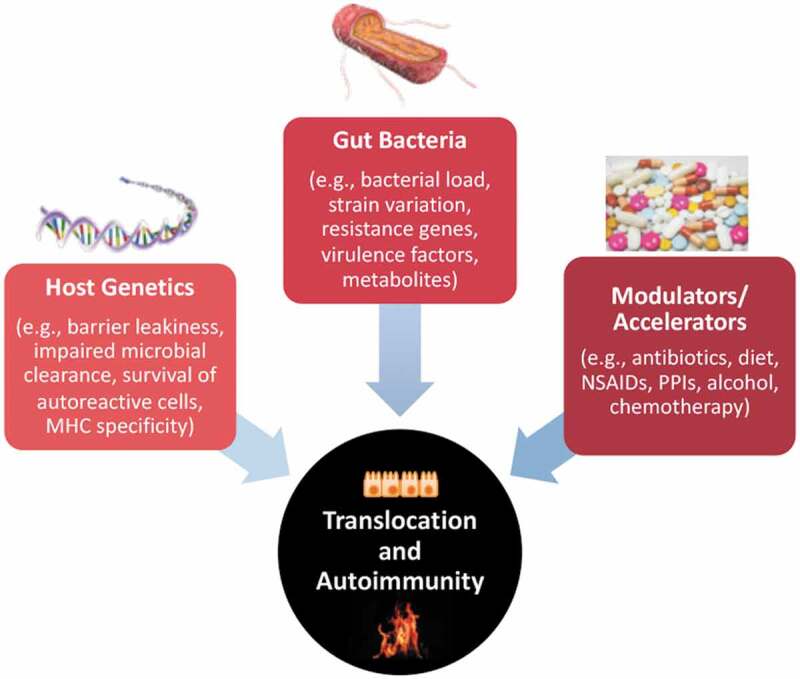
Factors influencing translocation of bacteria and extraintestinal autoimmunity. Autoimmunity arises in genetically susceptible hosts that are exposed to environmental stimuli that accelerate loss of self-tolerance. Barrier leakiness can either be intrinsic to the host or induced by external factors such as diet or medications. Bacteria that are normally contained in the gut lumen can gain access to extraintestinal tissues in response to enhanced intestinal permeability and via intrinsic bacterial mechanisms. The capability of bacteria to translocate depends on virulence factors, their abundance and proximity to the epithelial barrier, and their ability to compete within the gastrointestinal niche and evade immune defenses. Once translocated, bacteria such as E. gallinarum colonizing internal organs can directly induce autoimmunity by interacting with host tissues or indirectly via metabolites and their influence on the innate and adaptive immune system. The examples listed for each factor (host genetics, gut bacteria, modulators/accelerators) can impact translocation, autoimmunity or both (for instance, diet can influence barrier function as well as autoimmune responses). MHC, major histocompatibility complex; NSAIDs, non-steroidal anti-inflammatory drugs; PPIs, proton pump inhibitors.
Figure 3.
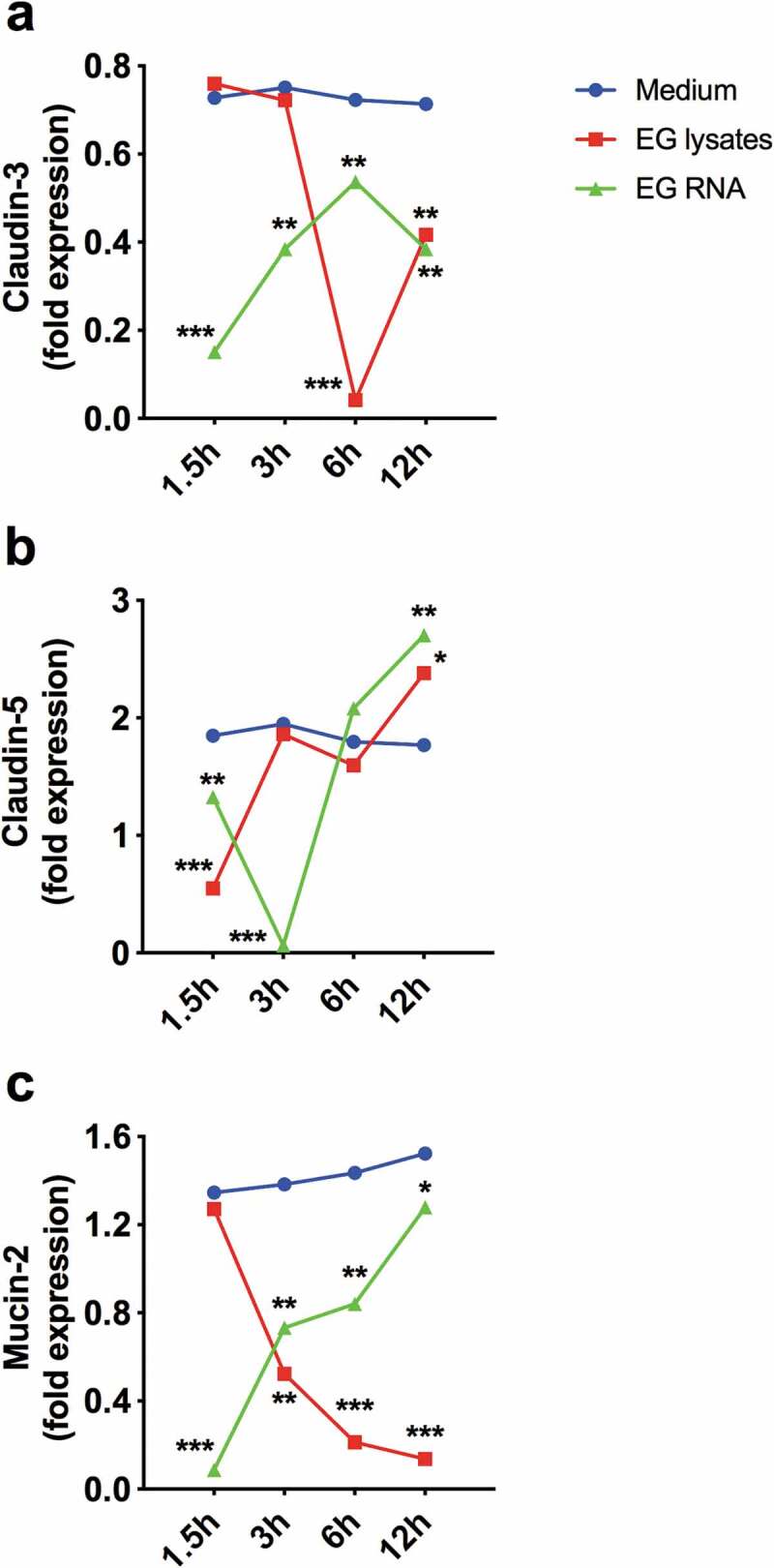
Small intestinal organoids from autoimmune-prone mice downregulate gut barrier molecules upon exposure to E. gallinarum. Ileum tissue was dissected from 12-week-old (NZW x BXSB)F1 mice. Crypts were isolated and cultured for 7 days in IntestiCult Organoid Growth Medium (STEMCELL Technologies) and Matrigel Matrix (Corning). On day 7, heat-killed E. gallinarum (EG) or EG RNA, as prepared in ref. 5, or medium was added to the organoid cultures (n = 3) for 1.5, 3, 6, and 12 h. RNA was extracted and RT-qPCR performed as described in ref. 5. RNA expression of the barrier molecules claudin-3 and claudin-5, as well as the mucus protein mucin-2, were quantified in relation to actin. Blue lines, media alone; red lines, EG lysate; green lines, EG RNA. Data are presented as mean ± SD in (a) to (c); *P < 0.05, **P < 0.01, ***P < 0.001; ANOVA followed by Bonferroni multiple-comparisons test.
Figure 4.
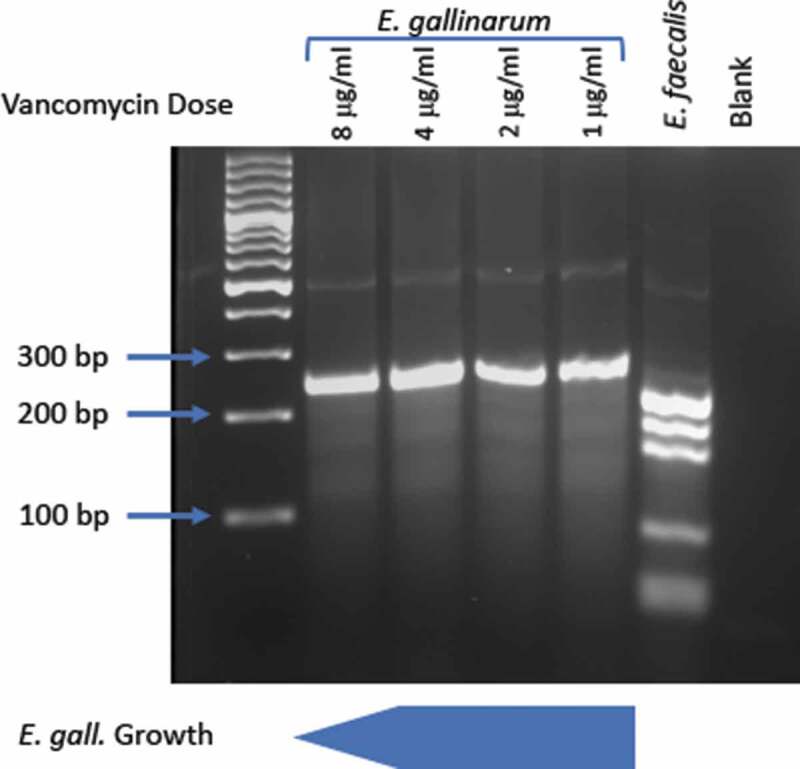
Vancomycin resistance gene restriction fragment length patterns for E. gallinarum and E. faecalis. E. gallinarum was grown in increasing concentrations from 1 μg/ml to 8 μg/ml of vancomycin. Restriction digest with MspI and multiplex PCR was performed as described in ref. 56 to determine vancomycin resistance genes. E. gallinarum normally carries the low-level vancomycin resistance vanC-1 operon (fragment sizes at 230/237 bp). E. faecalis carries the high-level vancomycin resistance vanB operon (fragment sizes at 136, 160, 188/189 bp). Horizontal transfer of this operon to E. gallinarum can occur within gut microbiomes (not shown). Depicted below the gel is the in vitro growth of E. gallinarum without the vanB operon. Normal growth at concentrations between 1 and 4 μg/ml is noted with diminishing growth starting at 8 μg/ml and little to no growth at 20 μg/ml and beyond (not shown).
These findings have relevance to human disease as E. gallinarum DNA can be detected by PCR in liver biopsy samples from patients with AIH and SLE, but not from healthy controls.5 We found that healthy livers contained other Enterococcus spp., suggesting that bacteria of this genus are prone to gut barrier translocation like lactobacilli, which were also present in human liver samples.5,53 Sera from patients with AIH and SLE have greater antibody titers to E. gallinarum RNA in comparison to RNA from E. faecalis or B. thetaiotaomicron, whereas antibody titers to these three bacterial RNAs occur at similar levels in healthy controls.5 Of note, E. gallinarum RNA antibodies correlated highly with autoantibodies against human RNA, possibly due to shared immunogenic sequences.
Strain comparisons and further gnotobiotic studies should help elucidate if only particular E. gallinarum strains promote autoimmunity or if genetic predisposition of the host is needed for penetrance of autoimmune manifestations independently from the E. gallinarum strain. In addition, the duration of translocation and microbial burden in tissues likely matters in eliciting disease phenotypes. In the autoimmune-prone model, we noted persistent and progressive translocation overtime but could not detect naturally colonizing E. gallinarum in feces compared to very high fecal levels after exogenous reconstitution in a pancreatic sepsis model.5,58 In that model, E. gallinarum translocation to blood, pancreas, and spleen was linked to sepsis that was partly TLR2 dependent.58 Another open question is how enterococci travel and persist within the host. It remains to be determined if E. gallinarum migrates freely in the extracellular space or hijacks antigen-presenting cells or other host cells to reach internal organs and persist in host tissues. E. faecalis is well known to persist within macrophages, which is one of many possibilities for E. gallinarum persistence within its host that need to be tested.59
Similarly, it would be important to know if the closely related E. casseliflavus is also capable of inducing autoimmunity in susceptible models, as E. faecalis is not capable of doing so but is more distantly related to E. gallinarum. Although E. faecalis induced barrier leakiness and translocation similar to E. gallinarum, it did not induce signs of systemic autoimmunity. In contrast to monocolonization of C57BL/6 mice with E. gallinarum, E. faecalis did not induce Th17 cells or systemic autoantibodies.5 Biosynthetic gene clusters are markedly different in E. faecalis compared to E. gallinarum/E. casseliflavus (Figure 5),60 suggesting different virulence factors may contribute to autoimmunity versus translocation. Clearly, different translocating species within the Enterococcus genus promote diverse host phenotypes depending on distinct pathogenicity and host factors, respectively (Table 2 and Figure 2). The commonality of translocating into host tissues may be related to its evolutionary history.
Figure 5.
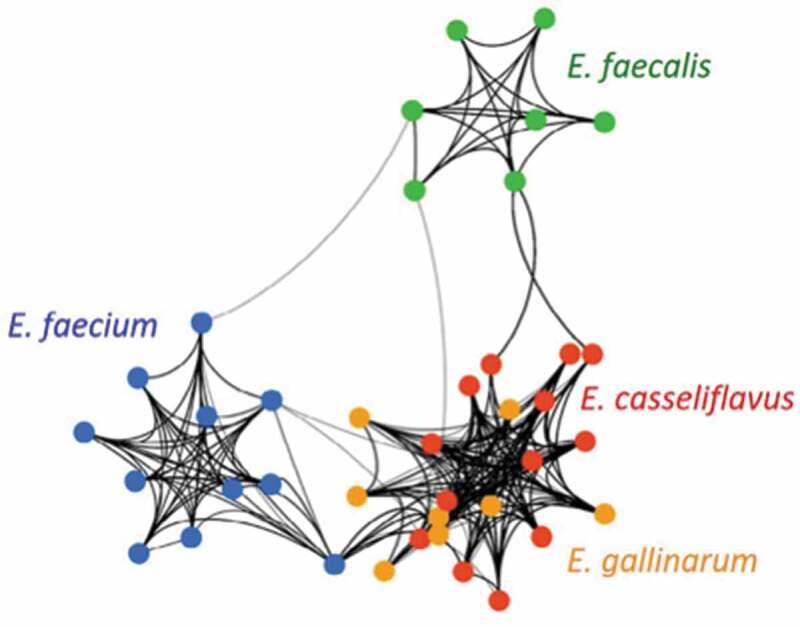
Network of predicted biosynthetic gene clusters (BGCs) from E. faecium, E. faecalis, E. gallinarum, and E. casseliflavus genomes according to Rashidi et al. Figure reproduced with permission from ref. 60. Unique strains are represented by colored nodes. Connecting lines indicate at least 75% similarity of BGCs between two strains. The weight of each line is proportional to the number and strength of BGC connections between two strains. Thicker lines represent more frequent higher-similarity pathways. Secondary metabolite profile predictions revealed distinct clusters. Intrinsically vancomycin-resistant E. gallinarum (orange) and E. casseliflavus (red) cluster together whereas E. faecalis (green) and E. faecium (blue) cluster independently from each other.
Table 2.
Translocation of enterococci in non-infectious diseases*.
| Disease | Genus/spp. | Main Study Findings | Translocation | Reference |
|---|---|---|---|---|
| Systemic and Organ-Specific Autoimmunity | ||||
| • Monocolonized C57BL/6 mice | E. gallinarum | E. gallinarum translocates and induces autoimmunity in a spontaneous model of lupus and non-autoimmune prone monocolonized mice. Features of SLE, antiphospholipid syndrome, and AIH are induced by E. gallinarum. Vaccination against E. gallinarum prevents translocation and autoimmunity. | Mesenteric veins, MLN, Liver, Spleen | Manfredo Vieira et al. (5) |
| • (NZW x BXSB)F1 mice | E. gallinarum detected in liver biopsies of SLE and AIH subjects but not healthy controls. | |||
| • SLE and AIH patients | SLE and AIH patients have greater IgG response to E. gallinarum RNA compared to controls. | |||
| • PSC patients | E. gallinarum | E. gallinarum among other species (Klebsiella pneumoniae, Proteus mirabalis) is enriched in feces of patients with PSC and shown to translocate to MLN and promote Th17 responses related to PSC in a humanized gnotobiotic model. | MLN | Nakamoto et al. (75) |
| Viral and Alcoholic Liver Disease | ||||
| • Cirrhosis patients (mainly due to viral and alcoholic liver disease) | Enterococcus | Enterococcus spp. among others detected in MLN of patients with cirrhosis. Bacterial translocation increased with increasing Child-Pugh Score. | MLN | Cirera et al. (73) |
| • Gastric acid suppression (Atp4aSl/Sl) in ethanol or high fat diet-treated mice • Patients with alcoholic hepatitis |
E. faecalis | E. faecalis intestinal expansion linked to translocation, hepatic inflammation and hepatocyte death in a TLR2-dependent manner. | MLN, Liver | Llorente et al. (36) |
| Hematopoietic Stem Cell Transplantation | ||||
| • Allogeneic stem cell transplant patients | VRE | VRE colonization is a surrogate marker but not independent predictor of worse outcomes after HCT. | Blood | Hefazi et al. (81) |
| Stroke | ||||
| • Stroke patients | Enterococcus | Enterococcus spp. most commonly detected in bloodstream post-stroke in patients. | Blood, Lung | Stanley et al. (37) |
| • Induced stroke mouse model | E. faecalis translocation after stroke in monocolonized setting. | |||
| Pancreatitis | ||||
| • Acute pancreatitis in mice with bile duct obstruction pretreated with meropenem | E. gallinarum | E. gallinarum translocation linked to sepsis and mortality in mice with acute pancreatitis in a TLR2-dependent process. | Blood, Pancreas, Spleen | Soares et al. (58) |
| Inflammatory Bowel Disease | ||||
| • Crohn’s disease patients | E. faecalis | E. faecalis detected in mesenteric fat and omentum of patients with Crohn’s disease. E. faecalis linked to adipocyte proliferation and reduction in lipid content in vitro. | Mesenteric fat, Omentum | Zulian et al. (69) |
| • IL-10-/- monocolonized mice | E. faecalis | E. faecalis-monoassociated IL-10-/- mice develop distal colitis at 10–12 weeks of age and later duodenal inflammation and obstruction. E. faecalis induces IFN-γ- and IL-4-producing CD4+ T cells, which precedes histological inflammation in colon. | MLN | Kim et al. (71) |
| Cancer and Cancer Therapy | ||||
| • Oral cancer patients | E. faecalis | E. faecalis, among other microbes, detected in lymph nodes excised from patients with oral cancer, most commonly in metastatic lymph nodes. | LN | Sakamoto et al. (65) |
| • Nod2-/- mice with sarcoma treated with cychlophosphamide | E. hirae | NOD2-dependent translocation of E. hirae to secondary lymphoid organs increased intratumoral CD8+ T cell/Treg ratio, thereby facilitating chemotherapeutic effects mediated by cyclophosphamide. | MLN, spleen | Daillere et al. (34) |
* Summary of studies demonstrating enterococcal translocation either alone or together with other bacteria (not addressed here) in chronic, non-infectious diseases or their treatment. Abbreviations: SLE – systemic lupus erythematosus; AIH – autoimmune hepatitis; MLN – mesenteric lymph node; LN – lymph node; TLR – toll-like receptor-2; VRE – vancomycin resistent Enterococcus; PSC – primary sclerosing cholangitis; HCT – hematopoietic cell transplantation
Evolution of enterococci as instigators of chronic human diseases
In the antibiotic era, enterococci have evolved from commensals to hospital-endemic human pathogens, now becoming a major cause of nosocomial infection.61 By nature, enterococci are ubiquitous bacterial residents of the gastrointestinal tracts of mainly land animals, and are highly adapted to terrestrialization and the selective pressures of desiccation, starvation, and isolation. Their intrinsic-hardened outer cell walls and ability to exist in an environmentally persistent state naturally confer resistance to many antibiotics and disinfectants.61 The introduction of antibiotics over the last 80 years selected for lineages of E. faecalis and E. faecium that lack CRISPR protection of their genomes.62 This leads to a destabilization of the coevolved relationship with the human host. As a result of the easier entry of mobile genetic elements (MGE), hospital-adapted lineages possess “swollen genomes” replete with MGEs including a pathogenicity island, lysogenic phages, and many plasmids and transposons that confer new metabolic abilities and resistance to even last-line antibiotics.62-64 This leads to a destabilization of the coevolved relationship with the human host. The combination of long evolved and recently acquired traits make them well suited for persistent residence in hospitals and transmission among antibiotic-treated individuals. These processes result in nosocomial infections, especially bacteremia, surgical site, and urinary tract infections.
Even more so, enterococci are becoming recognized as contributors of diseases not classically considered infectious in origin (Table 2).65 Their broad living conditions may make them particularly suitable to detect in disease states, especially compared to bacteria that are harder to culture or propagate. Within the intestine, Enterococcus has been considered a driver of both inflammatory bowel disease and neoplasm.66-71 Beyond the intestine, Enterococcus expansion is linked to hepatic inflammation in hepatitis B virus-related cirrhosis and E. faecalis enhances inflammation in mouse models of alcoholic cirrhosis with gastric acid suppression.36,72 Enterococcus spp. among other species were also recovered from MLNs of cirrhotic patients and this genus is enriched in the gut mucosa of encephalopathic patients with cirrhosis.73,74 Furthermore, Enterococcus is enriched in the fecal microbiome of patients with primary sclerosing cholangitis (PSC),75 another liver autoimmune disease related to AIH and associated with bacterial RNA in the portal tracts of the liver.76 Consistent with these findings, Enterococcus abundance in the stool of PSC patients correlates with alkaline phosphatase, a marker of PSC progression.75 In addition, very recent work from Japan found specifically E. gallinarum translocated to the MLN in humanized gnotobiotic mice along with Klebsiella pneumoniae and Proteus mirabilis, which together promoted Th17 responses in PSC.77 Similarly, another recent study showed anti-tumor properties of an E. gallinarum strain mediated by its flagellin,78 following human cancer microbiome studies which revealed E. hirae, E. durans, and E. gallinarum among the most enriched species in the stool of patients responding to checkpoint immunotherapy.79 In a study with transplant patients, Enterococcus spp. including E. faecium and E. gilvis expanded after allogeneic stem cell transplantation, especially in those patients with graft-versus-host disease.80 Patients with the outgrowth of these bacteria also have lower urinary 3-indoxyl sulfate, which correlates with poor early and long-term outcomes.80,81 Finally, E. gallinarum translocates to internal organs in autoimmune-prone hosts and interacts with host cells in tissues, contributing to autoimmunity related to SLE, AIH, and PSC as detailed above. Further research is needed to fully assess E. gallinarum as a target in human autoimmune diseases, but the evidence for Enterococcus spp. in promoting non-infectious, chronic disease states is growing. More broadly, disease-promoting candidates across different genera will likely be discovered as the “dark matter” within the human microbiome is being revealed.82,83
Funding Statement
This work was supported by National Institutes of Health (NIH) grants K08AI095318, R01AI118855, T32AI07019, and T32DK007017-39; the Yale Rheumatic Diseases Research Core (NIH grant P30 AR053495); the Yale Liver Center (NIH grant P30 DK34989); Women’s Health Research at Yale; the O’Brien Center at Yale (NIH grant P30DK079310); the Arthritis National Research Foundation; the Arthritis Foundation; and the Lupus Research Institute.
Acknowledgments
We would like to thank Francois Lebreton (Harvard Medical School) for discussions on the evolution of enterococci. We also would like to thank all members of the Kriegel lab for insightful discussions on host-microbe interactions in autoimmunity.
Disclosure of Potential Conflicts of Interest
M.A.K. received salary, consulting fees, honoraria and research funds from Roche, Bristol-Meyers Squibb, AbbVie, and Cell Applications. M.A.K. and S.M.V. hold an international patent on Enterococcus as a therapeutic target in autoimmunity and received royalties.
References
- 1.Luissint AC, Parkos CA, Nusrat A.. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151:616–632. doi: 10.1053/j.gastro.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doran KS, Banerjee A, Disson O, Lecuit M. Concepts and mechanisms: crossing host barriers. Cold Spring Harb Perspect Med. 2013:3. doi: 10.1101/cshperspect.a010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015;17:173–183. doi: 10.1016/j.micinf.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7:e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.France MM, Turner JR. The mucosal barrier at a glance. J Cell Sci. 2017;130:307–314. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odenwald MA, Turner JR. The intestinal epithelial barrier: A therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 9.Spadoni I, Pietrelli A, Pesole G, Rescigno M. Gene expression profile of endothelial cells during perturbation of the gut vascular barrier. Gut Microbes. 2016;7:540–548. doi: 10.1080/19490976.2016.1239681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B. Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol. 2008;38:2142–2155. doi: 10.1002/eji.200838140. [DOI] [PubMed] [Google Scholar]
- 12.Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr. 2011;141:769–776. doi: 10.3945/jn.110.135657. [DOI] [PubMed] [Google Scholar]
- 13.Yoo BB, Mazmanian SK. The enteric network: interactions between the immune and nervous systems of the gut. Immunity. 2017;46:910–926. doi: 10.1016/j.immuni.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costantini TW, Krzyzaniak M, Cheadle GA, Putnam JG, Hageny AM, Lopez N, Eliceiri BP, Bansal V, Coimbra R. Targeting alpha-7 nicotinic acetylcholine receptor in the enteric nervous system: A cholinergic agonist prevents gut barrier failure after severe burn injury. Am J Pathol. 2012;181:478–486. doi: 10.1016/j.ajpath.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. [DOI] [PubMed] [Google Scholar]
- 16.Mainous MR, Tso P, Berg RD, Deitch EA. Studies of the route, magnitude, and time course of bacterial translocation in a model of systemic inflammation. Arch Surg. 1991;126:33–37. [DOI] [PubMed] [Google Scholar]
- 17.Gautreaux MD, Deitch EA, Berg RD. Bacterial translocation from the gastrointestinal-tract to various segments of the mesenteric lymph-node complex. Infect Immun. 1994;62:2132–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macpherson AJ, Uhr T. Induction of protective iga by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 19.Wolochow H, Hildebrand GJ, Lamanna C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J Infect Dis. 1966;116:523–528. doi: 10.1093/infdis/116.4.523. [DOI] [PubMed] [Google Scholar]
- 20.Payne JM, Sansom BF, Garner RJ, Thomson AR, Miles BJ. Uptake of small resin particles (1–5µ diameter) by the alimentary canal of the calf. Nature. 1960;188:586–587. doi: 10.1038/188586a0. [DOI] [PubMed] [Google Scholar]
- 21.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell tlr engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. Cx3cr1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 23.Farache J, Koren I, Milo I, Gurevich I, Kim KW, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal bacteria recruit cd103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from cx3cr1(+) macrophages to cd103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata N, Kunisawa J, Hosomi K, Fujimoto Y, Mizote K, Kitayama N, Shimoyama A, Mimuro H, Sato S, Kishishita N, et al. Lymphoid tissue-resident alcaligenes lps induces iga production without excessive inflammatory responses via weak tlr4 agonist activity. Mucosal Immunol. 2018;11:693–702. doi: 10.1038/mi.2017.103. [DOI] [PubMed] [Google Scholar]
- 27.Fung TC, Bessman NJ, Hepworth MR, Kumar N, Shibata N, Kobuley D, Wang K, Ziegler CGK, Goc J, Shima T, et al. Lymphoid-tissue-resident commensal bacteria promote members of the il-10 cytokine family to establish mutualism. Immunity. 2016;44:634–646. doi: 10.1016/j.immuni.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cywes C, Wessels MR. Group a streptococcus tissue invasion by cd44-mediated cell signalling. Nature. 2001;414:648–652. doi: 10.1038/414648a. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand GJ, Wolochow H. Translocation of bacteriophage across the intestinal wall of the rat. Proc Soc Exp Biol Med. 1962;109:183–185. [DOI] [PubMed] [Google Scholar]
- 30.Lynskey NN, Banerji S, Johnson LA, Holder KA, Reglinski M, Wing PA, Rigby D, Jackson DG, Sriskandan S. Rapid lymphatic dissemination of encapsulated group a streptococci via lymphatic vessel endothelial receptor-1 interaction. PLoS Pathog. 2015;11:e1005137. doi: 10.1371/journal.ppat.1005137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balmer ML, Slack E, de Gottardi A, Lawson MAE, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med. 2014;6. doi: 10.1126/scitranslmed.3008618 [DOI] [PubMed] [Google Scholar]
- 32.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa FR, Francozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Camara NO, de Zoete MR, Palm NW, et al. Gut microbiota translocation to the pancreatic lymph nodes triggers nod2 activation and contributes to t1d onset. J Exp Med. 2016;213:1223–1239. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, et al. Enterococcus hirae and barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376–1383. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 36.Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, Wang HJ, Loomba R, Bajaj JS, Schubert ML, Sikaroodi M, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal enterococcus. Nat Commun. 2017;8:837. doi: 10.1038/s41467-017-00796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. doi: 10.1038/nm.4194. [DOI] [PubMed] [Google Scholar]
- 38.Guzman-Stein G, Bonsack M, Liberty J, Delaney JP. Abdominal radiation causes bacterial translocation. J Surg Res. 1989;46:104–107. [DOI] [PubMed] [Google Scholar]
- 39.Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, Pierre JF, Ernest JD, Galipeau HJ, Thuille N, et al. Microbial signals drive pre-leukaemic myeloproliferation in a tet2-deficient host. Nature. 2018;557:580–584. doi: 10.1038/s41586-018-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Beclin E, Odou MF, et al. Mesenteric fat as a source of c reactive protein and as a target for bacterial translocation in crohn’s disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G. Gut microbiota-induced immunoglobulin g controls systemic infection by symbiotic bacteria and pathogens. Immunity. 2016;44:647–658. doi: 10.1016/j.immuni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg RD. Inhibition of escherichia coli translocation from the gastrointestinal tract by normal cecal flora in gnotobiotic or antibiotic-decontaminated mice. Infect Immun. 1980;29:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayres JS, Trinidad NJ, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med. 2012;18:799–806. doi: 10.1038/nm.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van Den Brink MRM, Kamboj M, et al. Vancomycin-resistant enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–4341. doi: 10.1172/Jci43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakraborty R, Lam V, Kommineni S, Stromich J, Hayward M, Kristich CJ, Salzman NH. Ceftriaxone administration disrupts intestinal homeostasis, mediating noninflammatory proliferation and dissemination of commensal enterococci. Infect Immun. 2018:86. doi: 10.1128/IAI.00674-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knoop KA, McDonald KG, Kulkarni DH, Newberry RD. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut. 2016;65:1100–U60. doi: 10.1136/gutjnl-2014-309059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson ME, Jakobsson HE, Holmen-Larsson J, Schutte A, Ermund A, Rodriguez-Pineiro AM, Arike L, Wising C, Svensson F, Backhed F, et al. Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 2015;18:582–592. doi: 10.1016/j.chom.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–51S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 51.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zegarra-Ruiz DF, El Beidaq A, Iniguez AJ, Lubrano Di Ricco M, Manfredo Vieira S, Ruff WE, Mubiru D, Fine RL, Sterpka J, Greiling TM, et al. A diet-sensitive commensal lactobacillus strain mediates tlr7-dependent systemic autoimmunity. Cell Host Microbe. 2019;25:113–27e6. doi: 10.1016/j.chom.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price AE, Shamardani K, Lugo KA, Deguine J, Roberts AW, Lee BL, Barton GM. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49:560–75e6. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Czaja AJ, Morshed SA, Parveen S, Nishioka M. Antibodies to single-stranded and double-stranded DNA in antinuclear antibody-positive type 1-autoimmune hepatitis. Hepatology. 1997;26:567–572. doi: 10.1002/hep.510260306. [DOI] [PubMed] [Google Scholar]
- 56.Patel R, Uhl JR, Kohner P, Hopkins MK, Cockerill FR 3rd.. Multiplex pcr detection of vana, vanb, vanc-1, and vanc-2/3 genes in enterococci. J Clin Microbiol. 1997;35:703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares FS, Amaral FC, Silva NLC, Valente MR, Santos LKR, Yamashiro LH, Scheffer MC, Castanheira F, Ferreira RG, Gehrke L, et al. Antibiotic-induced pathobiont dissemination accelerates mortality in severe experimental pancreatitis. Front Immunol. 2017;8:1890. doi: 10.3389/fimmu.2017.01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gentry-Weeks CR, Karkhoff-Schweizer R, Pikis A, Estay M, Keith JM. Survival of enterococcus faecalis in mouse peritoneal macrophages. Infect Immun. 1999;67:2160–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rashidi A, Ebadi M, Shields-Cutler RR, DeFor TE, Al-Ghalith GA, Ferrieri P, Young JH, Dunny GM, Knights D, Weisdorf DJ. Pretransplant gut colonization with intrinsically vancomycin-resistant enterococci (E. gallinarum and E. casseliflavus) and outcomes of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24:1260–1263. doi: 10.1016/j.bbmt.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS. Tracing the enterococci from paleozoic origins to the hospital. Cell. 2017;169:849–61 e13. doi: 10.1016/j.cell.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palmer KL, Gilmore MS. Multidrug-resistant enterococci lack crispr-cas. MBio. 2010:1. doi: 10.1128/mBio.00227-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shankar N, Baghdayan AS, Gilmore MS. Modulation of virulence within a pathogenicity island in vancomycin-resistant enterococcus faecalis. Nature. 2002;417:746–750. doi: 10.1038/nature00802. [DOI] [PubMed] [Google Scholar]
- 64.Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, et al. Role of mobile DNA in the evolution of vancomycin-resistant enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. [DOI] [PubMed] [Google Scholar]
- 65.Sakamoto H, Naito H, Ohta Y, Tanakna R, Maeda N, Sasaki J, Nord CE. Isolation of bacteria from cervical lymph nodes in patients with oral cancer. Arch Oral Biol. 1999;44:789–793. doi: 10.1016/s0003-9969(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 66.Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, Yang L, Liu ZJ, Yuan YZ, Liu F, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97:1044–1052. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 67.Balish E, Warner T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am J Pathol. 2002;160:2253–2257. doi: 10.1016/S0002-9440(10)61172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barnett MP, McNabb WC, Cookson AL, Zhu S, Davy M, Knoch B, Nones K, Hodgkinson AJ, Roy NC. Changes in colon gene expression associated with increased colon inflammation in interleukin-10 gene-deficient mice inoculated with enterococcus species. BMC Immunol. 2010;11:39. doi: 10.1186/1471-2172-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zulian A, Cancello R, Ruocco C, Gentilini D, Di Blasio AM, Danelli P, Micheletto G, Cesana E, Invitti C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and crohn’s disease. An in vivo and in vitro study. PLoS One. 2013;8. doi: 10.1371/journal.pone.0078495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onderdonk AB, Richardson JA, Hammer RE, Taurog JD. Correlation of cecal microflora of hla-b27 transgenic rats with inflammatory bowel disease. Infect Immun. 1998;66:6022–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim SC, Tonkonogy SL, Albright CA, Tsang J, Balish EJ, Braun J, Huycke MM, Sartor RB. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128:891–906. [DOI] [PubMed] [Google Scholar]
- 72.Mou H, Yang F, Zhou J, Bao C. Correlation of liver function with intestinal flora, vitamin deficiency and il-17a in patients with liver cirrhosis. Exp Ther Med. 2018;16:4082–4088. doi: 10.3892/etm.2018.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taura P, Fuster J, Garcia-Valdecasas JC, Lacy A, Suarez MJ, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–37. [DOI] [PubMed] [Google Scholar]
- 74.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der Merwe S, Vermeire S, et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from ibd. Gut. 2016;65:1681–1689. doi: 10.1136/gutjnl-2015-311004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, Becker C, Neurath MF, Lohse AW, Herkel J, et al. Increased t helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 2013;58:1084–1093. doi: 10.1002/hep.26447. [DOI] [PubMed] [Google Scholar]
- 77.Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, Suzuki T, Koda Y, Chu PS, Taniki N, et al. Gut pathobionts underlie intestinal barrier dysfunction and liver t helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019. doi: 10.1038/s41564-018-0333-1. [DOI] [PubMed] [Google Scholar]
- 78.Laute-Caly DL, Raftis EJ, Cowie P, Hennessy E, Holt A, Panzica DA, Sparre C, Minter B, Stroobach E, Mulder IE. The flagellin of candidate live biotherapeutic enterococcus gallinarum mrx0518 is a potent immunostimulant. Sci Rep. 2019;9:801. doi: 10.1038/s41598-018-36926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of pd-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 80.Weber D, Oefner PJ, Hiergeist A, Koestler J, Gessner A, Weber M, Hahn J, Wolff D, Stammler F, Spang R, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015;126:1723–1728. doi: 10.1182/blood-2015-04-638858. [DOI] [PubMed] [Google Scholar]
- 81.Hefazi M, Damlaj M, Alkhateeb HB, Partain DK, Patel R, Razonable RR, Gastineau DA, Al-Kali A, Hashmi SK, Hogan WJ, et al. Vancomycin-resistant enterococcus colonization and bloodstream infection: prevalence, risk factors, and the impact on early outcomes after allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. Transpl Infect Dis. 2016;18:913–920. doi: 10.1111/tid.12612. [DOI] [PubMed] [Google Scholar]
- 82.Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P, et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176:649–62 e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, Lawley TD, Finn RD. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


