ABSTRACT
Collective cell migration is a critical mechanism involved in cell movement during various physiological and pathological processes such as angiogenesis and metastasis formation. During collective movement, cells remain functionally connected and can coordinate individual cell behaviors to ensure efficient migration. A cell-cell communication process ensures this complex coordination. Although the mechanisms regulating cell-cell communication remain unclear, recent findings indicate that it is based on acto-myosin cytoskeleton tension transmission from cell to cell through adherens junctions. As for single cell migration, small GTPases of the Rho and Rab families have been shown to be critical regulators of collective motion. Here, we discuss our current understanding on how these small GTPases are themselves regulated and how they control cell-cell communication during collective migration. Moreover, we also shed light on the key role of cell-cell communication and RhoGTPases in the physiological context of endothelial cell migration during angiogenesis.
KEYWORDS: collective cell migration, small GTPases, cell-cell communication, Cadherins, actin, angiogenesis
Introduction
Cell migration is essential for many biological phenomena. Most of the research into cell migration has been focused at the individual cell level. However, in the last decade, much evidence has emerged showing that cells can migrate as interconnected groups, both in physiologically normal and pathological processes.1,2 Cohorts of migrating cells have different sizes, and can adopt different organized conformations, such as sheets, strands, non-cohesive groups or clusters.3 To migrate in a cohesive manner, individual cells coordinate their movements by maintaining both physical and functional contacts with their neighbors, allowing them to behave as a unique entity.1,3 During individual cell migration, a cell polarizes, protrudes, adheres to the substratum at the front, then contracts and detaches at the rear of the cell. In addition, extracellular factors such as growth factors, chemokines or ECM components can guide the cell in a particular direction. Each of these steps required for single cell motility must be integrated into the collective movement of the cell group. Indeed, in collective migration, these extracellular guidance cues promote polarization of the group of cells, with cells at the front of the migration, called leader cells, driving the movement.1,3 Leader cells have the ability to detect guidance cues and form protrusions which adhere to the substratum. They also guide the other cells, called follower cells. Leader and follower cells have different morphological shapes, gene expression profiles and actin cytoskeleton organizations and dynamics.4
The intracellular regulation of cell motility is largely dependent on signaling pathways controlling the acto-myosin cytoskeleton and its regulators such as Rho small GTPases (Rac, Cdc42 and RhoA). In single migrating cells, Rac and Cdc42 promote the extension of protrusions at the front, while RhoA promotes retraction of the rear of the cell.5 It is not yet clear how the activities of the Rho GTPases are coordinated during collective cell movement, but recent findings have indicated that this is achieved through a cell-cell contact dependent mechanism called cell-cell communication. Indeed, several studies have revealed that cell-cell communication controls the cohesiveness of the cohort, restrains the protrusive activity at the front region and affects the directionality of the group.6–8 Here we review our current understanding of the roles of the Rho and Rab family small GTPases in the mechanism of cell-cell communication and how this relates to the regulation of endothelial cell function as a physiological example.
Basic principles of cell-cell communication
In order to behave as motile entities, cells migrating as a group need to establish communication between each other. The current model of cell-cell communication proposes that this is achieved, at least in part, through mechanical cues emanating from cell-cell junction complexes.9 Indeed, during migration cell collectives retain their cell-cell junction structures, which involve mostly cadherin but also integrins and members of the immunoglobulin family (N-CAM, L1-CAM, Ephrins/Eph receptors).1,10 They use these junction complexes to transmit polarity cues from cell to cell across the cluster through the tension of the actin cytoskeleton. For instance, in border cells, it has been demonstrated that a positive feedback loop between Rac activation by tyrosine kinase receptors and adhesive receptors favors the formation of stable protrusions in the front cells, increasing tension on the actomyosin cytoskeleton in the leading cell.9 In many systems, the actin cytoskeleton then forms a supracellular actomyosin cable along the inner side of the outer membrane of the cell group.9,11 Thus, the tension generated by the leading cell on these actomyosin cables is transmitted along the group periphery, ensuring migration coordination (Fig. 1).11–13
Figure 1.
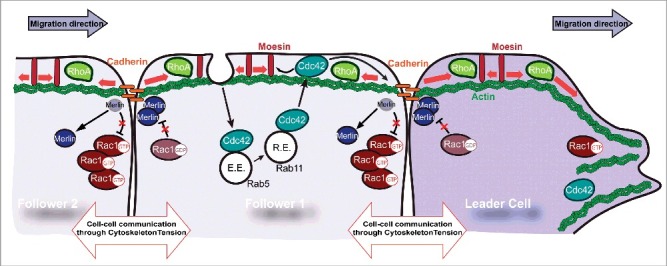
Small GTPase orchestration in cell-cell communication. At the leading edge, RhoGTPases are activated and allows actomyosin formation and contractility. At the interface between the leading cell and the following cell, the actin cytoskeletal tension (red arrows) generated by activation of the RhoA pathway is transmitted from cell to cell via cadherin complexes. In following cells, increased tension at the front induces the delocalization of merlin, allowing discrete Rac activation. Cytoskeletal tension is transmitted to the plasma membrane via the ERM protein Moesin. Endocytic trafficking through Rab5 and Rab11 controls Cdc42 localization. Cdc42 controls Cadherin levels and ERM localization. E.E: Early endosome, R.E: Recycling endosome.
The mechanisms controlling cell-cell communication are still being characterized but are known to involve RhoGTPases, the JNK signaling pathway8 and Rab11-dependent endocytosis.7 Thus, the correct orchestration of all of these signaling pathways is required for efficient cell-cell communication.
Rac: A coordinated activator of collective cell migration
The study of the Rac GTPase during collective motion first established the concept of cell-cell communication. Indeed, the development of optogenetic tools allowing the spatio-temporal control of Rac signaling by light prompted researchers to reassess cellular dynamics during collective movement.14 A pioneering study in Drosophila border cells using these methods demonstrated that border cell clusters is driven by the cell which presents the highest Rac activity.8 Moreover, inhibition of Rac signaling in response to light in the leading cell induces the formation of protrusions in other cells within the cluster and led to a loss of directionality, demonstrating the existence of a cell-cell communication process.7,8
During collective migration, Rac activity at the leading edge is controlled by classical chemokine and adhesion receptor signaling to promote protrusion formation.2,8 This phenomenon is conserved in others models. Indeed, Rac is activated through Integrin β1 and PI3K signalling at the front of MDCK (Madin-Darby Canine Kidney) epithelial cell groups and it is crucial to drive collective migration.15 The directional movements of Xenopus neural crest cells also require active Rac at the front of the group to promote lamellipodia formation. This is also true in Drosophila trachea, where Rac is active in leader tip cells.16,17
In endothelial sheet migration, Rac synergizes with Cdc42 in leader cells to promote the persistence of motile groups,18 whereas a different signaling program is set up in following cells in order to precisely regulate Rac activation at cell-cell junctions: first, transient Rac activation occurs upon E-cadherin engagement through PI3K signaling and Rac GEFs (guanine exchange factors) such as Vav family proteins or Tiam119,20; then the tumor suppressor merlin (an ERM-like protein) acts as a mechanochemical transducer by allowing discrete Rac activation at the front of following cells upon an increase in tension at the adherens junctions (AJ)21 (Fig. 1). Nonetheless, the signaling pathways involved in Rac regulation at the interface between leader and following cells remain unclear.
Cdc42: A critical regulator of cell-cell communication
In both single and collective migration, constitutive activation or inhibition of Cdc42 disrupts migration, indicating that Cdc42 must be tightly controlled spatiotemporally. In the context of collective cell migration, Cdc42 is recruited to the leading edge during wound healing assays in order to control cell polarization in the direction of migration and that this requires an Arf6-dependent membrane trafficking event.22 In this study both Cdc42 and one of its GEFs, βPIX, were also detected on intracellular vesicles during the collective migration of astrocytes.22 Moreover, Cdc42 has been suggested to act upstream of JNK signaling to control cluster cohesion via polarity and adhesion proteins in drosophila border cells.23 Consistent with this, Cdc42 has been shown to play a critical role in the control of cell-cell interactions in C2C12 myoblasts and in C. elegans hypodermal cells.24,25 Overexpression of a dominant negative form of Cdc42 in drosophila trachea was also shown to induce the formation of ectopic protrusions,17 and we recently demonstrated that Cdc42 controls cell-cell communication in a non-autonomous manner in drosophila border cells.6 In accordance with the mechanical concept of cell communication, we found that inhibition of Cdc42 in one cell altered the behavior of the whole group.6 The molecular mechanisms at work downstream of Cdc42 are still elusive but could rely on the control of actomyosin tension though regulation of moesin (an ERM protein linking actomyosin with the plasma membrane), JNK signaling and cadherin (Fig. 1).6,23 In addition, Cdc42 has been shown to be a critical regulator of apico-basal polarity through the control of the Par6/aPKC module in a wide range of systems. Thus, Cdc42-mediated control of cell-cell communication could also be due to its regulation of apico-basal polarity in migrating cells in coordination with Rac. Consistent with this idea, Rac depletion disrupts apico-basal polarity and the Par complex has been shown to be indispensable for the collective migration of Drosophila border cells.26,27
RhoA: An effector of cell-cell communication?
The role of Rho in cell-cell communication has not yet been addressed per se. However, its function in actomyosin regulation makes Rho a putative critical effector of cell-cell communication. Indeed, as described above, cell-cell communication relies on intercellular mechanocoupling through AJs, thus it is dependent on actomyosin contractility at the outer edge of the moving cluster. This contractility is driven by the local activation of Rho downstream of mature adhesion complexes via the regulated activity of the GTPase activators (RhoGEFs) and inhibitors (RhoGAPs).28 Subsequent activation of the Rho effector module ROCK/myosin II then provides the contractility force along the actin cytoskeleton.11 In Drosophila border cells, non-muscle myosin II activity is concentrated at the periphery and controls the global response of the cluster to environmental pressure.29,30 Moreover, manipulation of the Rho signaling pathway alters protrusion formation and dynamics, inducing border cell migration defects.29,30 Altogether, these data demonstrate that Rho-induced supracellular contractility controls the movement of groups of cells or sheets.
Endocytosis: A master regulator of cell-cell communication?
Over the last 10 years, endocytosis has been identified as a critical regulator of collective and single cell migration. Indeed, endocytosis is a major actor in the regulation of the cellular response to extracellular signals through the spatial restriction of signaling pathways during migration.31 For example, the endocytic cycle involving Rab5 and Rab11 is a critical regulator of border cell migration.7,32 Rab5 inhibition in both single cells and cell groups leads to the inhibition of Rac, and this produces dramatic phenotype alterations in border cells.7,33 Rab11 is involved in the regulation of endosomal recycling and its role and regulation in group coordination has been described. Indeed, Rab11 loss of function abolishes the restriction of Rac activity to the leading edge of the migrating group.7 Moreover, in border cells, a positive feedback loop has been described in which guidance receptors promote polarization of endocytic components such as Rab11 and the exocyst complex in leading cells.34 This polarization of the endocytic machinery participates to the polarization of active guidance receptors and Rac activity.32,34 Moreover, components of the multi-vesicular body machinery such as vps25, TSG101, HRS are involved in the maintenance of adherens junctions and thus in epithelial integrity in mouse models.35,36 How endocytosis controls cell-cell communication remains unknown but one possible mechanism is via the regulation of the tension within the supracellular actomyosin network, either directly by allowing the correct spatial positioning of multiple actors such as ERM proteins,6 RhoGTPases and adhesive complexes, or/ and indirectly through the control of epithelial polarity.
Receptor-GTPase pairing in cell-cell communication
RhoGTPases are activated by many different stimuli including G-protein-coupled receptors (GPCR), tyrosine kinase receptors (TKR), adhesive receptors or cytokine receptors leading the Rho-GTPases to reversibly oscillate from their active GTP-bound state and inactive GDP-bound state. For example, in Drosophila border cells and during tracheal development, RhoGTPases signaling is mainly controlled by TKRs PVR, EGFR and FGFR respectively.2,8,17 In endothelial and epithelial cells and in astrocytes, RhoGTPases activation is achieved by β1 integrins.37–39 The spatio-temporal regulation of RhoGTPases in leader cells controls actin dynamics and contractility and allows cell-cell communication through Cadherins. Indeed, at cell-cell contacts, Cadherins controls Rac and Cdc42 via regulation of catenins and recruitment of GEF such as βPIX (PAK-interacting exchange factor beta/ARHGEF7) or TIAM1 (T-cell lymphoma invasion and metastasis 1).40–42 Moreover, at cell-cell junctions of collectively migrating cancer cells, the collagen-activated tyrosine kinase DDR1 (Discoidin Domain Receptor family member 1) fine-tunes actomyosin contractility via the localization of RhoE which antagonizes ROCK-mediated contractility.43
Here we choose to describe the receptor-GTPase pairing in the control of Rho-GTPase activation in the cellular processes that govern EC behavior during angiogenesis, since it is the physiological mechanism we describe below.
Proinflammatory mediators including thrombin, histamine, platelet activating factor (PAF), Vascular Endothelial Growth Factor (VEGF) and Tumor Necrosis Factor α (TNFα) facilitate disassembly of Vascular Endothelial-cadherin (VE-cadherin) in the first steps of angiogenesis.44 Nevertheless, VEGF signaling cascade is the most studied signaling pathway that linkss Rho-GTPases to extracellular signals. In fact, VEGFR2 (VEGF-driven Vascular endothelial Growth Factor Receptor 2) activation leads to the recruitment of different types of proteins on its phosphorylated residues that are dependent of the engaged-cellular process. For instance, to induce vascular permeability, Src is recruited and activates IQGAP1 and Vav which increase Rac activity, itself leading to VE-cadherin cell-cell contact inhibition.45–47 VEGFR2 activation also induces RhoA/ROCK-mediated FAK phosphorylation and focal adhesion contact formation to drive EC migration.48 In this context of migration and vascular sprouting, Cdc42 could also be activated downstream of VEGFR2 via a phospholipase β3 (PLCβ3)-dependent or Nck/Fyn-dependant manner.49–51 Finally, when referring to GTPase-receptor pairing, the concept of mechanosensing induced by hemodynamic forces on EC must be taken into account since it could increase the complexity of the activated signaling pathways.52
Physiological cell-cell communication: The endothelial cell junction
Cell-cell communication and the collective migration of EC are critical events during angiogenesis, a physiological and pathological process through which new blood vessels appear from pre-existing ones. The angiogenic process must be finely balanced during embryonic life in order to allow vascular development, but this process is also essential during adulthood for tissue regeneration and its dysregulation plays a major role in controlling tumor progression.53 In this context, the Rho-GTPases that coordinate the dynamic changes in cell-cell contacts and cytoskeleton remodeling are considered to be fine tuners of vascular morphogenesis and homeostasis.54 In particular, the regulation of VE-cadherin dynamics by Rho-GTPase has been considered as the key event in the control of EC communication and migration during neovessel formation. For the remainder of this section we will discuss the spatio-temporal functions of the Rho-GTPases in tip and stalk cell communication which controls EC sprouting and migration in order to create new vasculature.
During sprouting angiogenesis, EC must orchestrate two essential mechanisms: endothelial junction destabilization, to favor the detachment of the tip cell from the existing vessel, and EC group migration during which protrusion formation at the tip provides directionality to the developing vessel. Cell cohesion at the rear of sprouting cells is also an indispensable requirement in order to facilitate migration, antagonize new angiogenic sprouting and support sprout growth by stabilizing contacts between tip and stalk cells.53 These mechanisms, which are mainly managed by Rho-GTPases, are highly intertwined and dependent on the specific intracellular location of the Rho-GTPase.
In the first step of angiogenesis, it is widely acknowledged that RhoA signaling mainly contributes towards destabilizing AJs in order to promote EC sprouting.55,56 In fact, RhoA activation by permeability-inducing factors (VEGF, TNFα, etc.) promotes actomyosin contraction through Rho-kinase (ROCK), leading to stress fiber formation and an increase of intracellular tension, thereby destabilizing endothelial VE-cadherin AJs.55,57,58 This has been validated in vivo, where it was clearly identified that RhoA activation is a prerequisite for EC assembly in new vessels, reinforcing the selectivity of this Rho-GTPase isoform in successful angiogenesis59 (Fig. 2). Other studies have also reported that Rac1 signaling causes the disruption of the endothelial barrier, however the specific involvement of Rac1 seems to be highly dependent on the intracellular EC context.60,61
Figure 2.
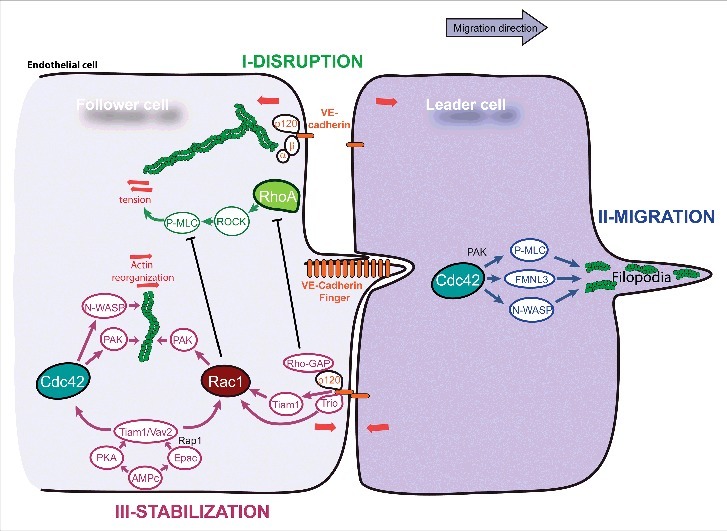
Mechanisms underlying VE-cadherin regulation by small GTPases in angiogenesis process. Successful angiogenesis requires 3 major steps: EC junction disruption (I), collective EC migration (II) and EC junction stabilization to support neovessel growth (III). I: Endothelial junction destabilization is predominently mediated by RhoA activation which promotes MLC-dependent actomyosin contraction via its effector ROCK. II: Collective migration and cell polarization are driven by VE-cadherin finger formation at the interface between tip and stalk cells and by filopodia formation at the tip cell front via Cdc42 activation. III: Stabilization of junctional complexes are mediated by Rac1 and Cdc42. Local Rac1 activity by VE-cadherin signaling at the nascent junction supports reorganization of junctional actin cytoskeleton via PAK and inhibits actomyosin contraction. RhoA activity is locally inhibited, releasing junctional tension and thereby promoting EC junction stabilization. Elevated cAMP level promotes Rac1 and Cdc42 activation via GEFs Tiam1 and Vav2, a process which is mediated by PKA and Epac/Rap1 signalling and resulting in EC junction stabilization. VE-cadherin: vascular-endothelial cadherin, p120: p120-catenin, α: α-catenin, β: β-catenin, ROCK: Rho-associated protein kinase, P-MLC: phosphorylated-myosin light chain, PAK: p21-activated kinase, N-WASP: neural Wiskott-Aldrich syndrome protein, Tiam1: T-lymphoma invasion and metastasis 1, Vav2: vav guanine nucleotide exchange factor 2, PKA: protein kinase A, Epac: exchange protein directly activated by cAMP, cAMP: cyclic adenosine monophosphate, FMNL3: formin-like 3.
AJ disruption leads to EC detachment from the existing vessel and allows the emergence of a leading cell. Very interestingly, studies using different in vivo models appear to agree that Cdc42 coordinates filopodia formation at the front of this leading EC62,63 (Fig. 2). By using a model of angiogenic sprouting of the zebrafish caudal vein plexus, Wakayama et al. demonstrated that, after its activation by Arghef9b, Cdc42 binds to the actin regulatory protein formin-like 3 and stimulates the assembly of actin filaments for filopodia formation.63 Filopodia formation drives collective endothelial cell migration that requires cell-cell contact strengthening at the rear of stalk cells. An elegant piece of work by Hayer and coworkers demonstrated that collectively migrating EC have polarized VE-cadherin-rich membrane protrusions that organize cell guidance64 (Fig. 2). Using an optogenetic model of local RhoA activation, these authors clearly demonstrated that increased contractility at the rear of the leader EC initiated cadherin protrusion engulfment by a neighboring cell which then acquired follower cell behavior.13 Altogether, these results strongly support the idea that cell-cell VE-cadherin junctions remain critical for maintaining cell cohesion, not only to avoid blood perfusion during angiogenesis, but also to provide the directionality of EC collective migration.
VE-cadherin accumulation at cell junctions also favors vessel quiescence in order to avoid new vessel sprouting, and the maturation of VE-cadherin interactions supports neovessel growth.65 In contrast to RhoA, which mediates the destabilization of AJ, Rac1 and Cdc42 tightly control the maturation of VE-cadherin interactions to stabilize the growing vessel. By using a photoactivatable Rac1 probe, a recent study showed that Rac1 reduced the rate of VE-cadherin dissociation by counteracting the actomyosin tension.66 In fact, Rac1 activation induces an inhibition of RhoA signaling and promotes AJ stabilization. The recruitment of p190RhoGAP, which interacts with p120-catenin at AJ, explains the control of RhoA activity and the reciprocal elevation of Rac1 signaling.67 Another possible molecular mechanism involved in Rac1-stabilized VE-cadherin junctions is the implication of the Rac-GEF Trio, which binds to VE-cadherin and mediates Rac1-induced actin cytoskeleton rearrangements to improve the stability of nascent cell-cell junctions.68 Concerning the specific role of Cdc42, in vivo deletion of this RhoGTPase in EC led to disorganized cell-cell junctions, resulting in an angiogenic tubulogenesis blockade.62 The authors proposed that Cdc42 controls the assembly and maturation of AJ through a decrease in the phosphorylation of Pak2/4 and pMLC; this controls actin contractility and leads to a defect in N-WASP recruitment to VE-cadherin junctions to regulate actin polymerization. The role of Rac1 and Cdc42 in barrier reinforcement is also attributed to cAMP signaling induced by physiological mediators such as PGE2/I2 (prostaglandins) or ANP (atrial natriuretic peptide). Indeed, increased cAMP has been shown to lead to Epac/Rap1-mediated activation of Rac1 and Cdc42 via the GEFs Tiam1 and Vav2, resulting in endothelial barrier enhancement69 (Fig. 2).
Finally, it is important to note that other less-studied Rho GTPases like RhoG, a Rac-GTPase related protein, or RhoJ, related to the cdc42-GTPase, are also expressed in endothelial vascular beds and participate in blood vessel morphogenesis. Indeed, RhoG was identified as an upstream protein of the cdc42-signalling module that controls filopodia formation and enhances endothelial tubule lengthening.70 Moreover, RhoJ is also important for EC migration and lumen formation by negatively regulating Rho-induced actin contractility.71-73 Very interestingly, targeting RhoJ was proposed as an adjuvant of conventional antitumor therapies since its blockade induces a vascular shutdown of tumor vessels with minimal side effects.74 Nevertheless, both these pro angiogenic GTPases need further investigations to clearly decipher their non-redundant EC fonctions compared to the more classically studied Rho GTPases.
Altogether, these studies clearly illustrate the fundamental role of small Rho-GTPases in regulating the plasticity of EC junctions, upon which the success of angiogenesis is dependent. The predominant effect of each Rho-GTPase on the control of EC behavior is described above, but their net-effect on the stabilization/destabilization of EC junctions is more complex and depends on the stimulus, their possible combinatorial effects and the endothelial cellular background (microvascular or macrovascular EC).75 A large amount of literature is available that describes how Rho-GTPases also play crucial roles in other cellular processes that govern angiogenesis, such as extracellular matrix remodeling, proliferation and morphogenesis.76 Future studies to precisely follow these activated Rho-GTPases in real time in vivo will be an interesting challenge but will go a long way to deciphering how these proteins coordinate these dynamic mechanisms. Collectively, these results emphasize the complexity of the spatio-temporal regulation of Rho GTPase activity that controls angiogenesis from its onset to completion, and demonstrate the challenges involved in developing anti-angiogenic therapies for diseases of aberrant angiogenesis.
Concluding remarks
Cell-cell communication can be considered as teamwork: it allows cell collectives to coordinate their behavior and achieve their functions. Thus, cell-cell communication is a process common to a wide range of different types of cell groups (whatever their final fate) that relies on cell-cell adhesion regulation. For example, during wound healing epithelial cells use the same global molecular mechanism, involving adhesion complexes and actin tension regulation, as that used by endothelial cells during angiogenesis. Thus, understanding the molecular mechanisms that control cell-cell communication is critical to improve our basic comprehension of collective cell migration.
The coordination of collective migration is achieved via the mechanical coupling of individual cells through their actin cytoskeleton network and cell-cell junction complexes. Even though many of the molecular players involved in this process are known, the initial signals and the mechanisms that determine the leading cells remain unclear. Environmental cues play a major role in determining the position of the leading cells, for example chemoattractants play a crucial role in establishing the front-back polarity of the leading cells and thus in the establishment of tension across the moving group of cells. Rho GTPases are key players in all of these processes but, although their general mode of action is well known, their precise orchestration in collectively migrating groups is still poorly understood. However, a hierarchy can be established based on recent findings. Cdc42 and Rac activation downstream of guidance cues promotes protrusion formation at the leading edge and the establishment of an actin cytoskeleton tension at the rear of the leading cell. Then, Rho driven actomyosin contractility is induced and transmitted to other cells through adherens junctions. In following cells, this force induces discrete Rac and Rho activation that generates cryptic protrusion formation and cytoskeletal tension respectively. In following cells, Cdc42 could be considered as a master regulator of Rac and Rho signaling as it controls the correct positioning of adherens junction complexes (Fig. 1). The emergence of new toolkits, such as optogenetic probes which allow the acute control of RhoGTPase signaling,8,13,77 together with FRET-based activity probes, will help to precisely define the spatiotemporal regulation of RhoGTPase signaling. This strategy has already been successfully applied to determine the function and regulation of RhoA at the cell group level in addition to investigating its sub-cellular resolution using traction force microscopy.78 These interesting studies should soon provide key information on how cell-cell and cell-matrix adhesive proteins coordinate with Rho and Rab GTPase dynamics in collectively moving groups.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
We thank K. Thornber for critical reading of the manuscript. A.C. was supported by grants from the Ministère de la Recherche et de la Technologie and from Fondation pour la Recherche sur le Cancer (ARC). N.C. is supported by a grant from Agence Nationale de la Recherche (ANR). Fondation de France and INSERM support M.L., S.G and D.R.
References
- 1.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. PMID:19546857 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Espartero CH, Ramel D, Farago M, Malartre M, Luque CM, Limanovich S, Katzav S, Emery G, Martín-Bermudo MD. GTP exchange factor Vav regulates guided cell migration by coupling guidance receptor signalling to local Rac activation. J Cell Sci. 2013;126:2285–2293. doi: 10.1242/jcs.124438. PMID:23525006 [DOI] [PubMed] [Google Scholar]
- 3.Rorth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–429. doi: 10.1146/annurev.cellbio.042308.113231. PMID:19575657 [DOI] [PubMed] [Google Scholar]
- 4.Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17:97–109. doi: 10.1038/nrm.2015.14. PMID:26726037 [DOI] [PubMed] [Google Scholar]
- 5.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases. 2014;5:e28997 doi: 10.4161/sgtp.28997. PMID:25054920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombie N, Choesmel-Cadamuro V, Series J, Emery G, Wang X, Ramel D. Non-autonomous role of Cdc42 in cell-cell communication during collective migration. Dev Biol. 2017;423:12–18. doi: 10.1016/j.ydbio.2017.01.018. PMID:28143705 [DOI] [PubMed] [Google Scholar]
- 7.Ramel D, Wang X, Laflamme C, Montell DJ, Emery G. Rab11 regulates cell-cell communication during collective cell movements. Nat Cell Biol. 2013. doi: 10.1038/ncb2681. PMID:23376974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–597. doi: 10.1038/ncb2061. PMID:20473296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical Feedback through E-Cadherin Promotes Direction Sensing during Collective Cell Migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. PMID:24855950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedl P, Mayor R. Tuning Collective Cell Migration by Cell-Cell Junction Regulation. Cold Spring Harbor Perspectives Biol. 2017;9. doi: 10.1101/cshperspect.a029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reffay M, Parrini MC, Cochet-Escartin O, Ladoux B, Buguin A, Coscoy S, Amblard F, Camonis J, Silberzan P. Interplay of RhoA and mechanical forces in collective cell migration driven by leader cells. Nat Cell Biol. 2014;16:217–223. doi: 10.1038/ncb2917. PMID:24561621 [DOI] [PubMed] [Google Scholar]
- 12.Sunyer, R, Conte V, Escribano J, Elosegui-Artola A, Labernadie A, Valon L, Navajas D, García-Aznar JM, Muñoz JJ, Roca-Cusachs P, et al. . Collective cell durotaxis emerges from long-range intercellular force transmission. Science. 2016;353:1157–1161. doi: 10.1126/science.aaf7119. PMID:27609894 [DOI] [PubMed] [Google Scholar]
- 13.Valon L, Marin-Llaurado A, Wyatt T, Charras G, Trepat X. Optogenetic control of cellular forces and mechanotransduction. Nat Communications. 2017;8:14396. doi: 10.1038/ncomms14396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 2009;461:104–108. doi: 10.1038/nature08241. PMID:19693014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi N, Mizutani T, Kawabata K, Haga H. Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin beta1 and PI3K. Scientific Reports. 2015;5:7656. doi: 10.1038/srep07656. PMID:25563751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Dev Cell. 2010;19:39–53. doi: 10.1016/j.devcel.2010.06.012. PMID:20643349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebreton G, Casanova J. Specification of leading and trailing cell features during collective migration in the Drosophila trachea. J Cell Sci. 2014;127:465–474. doi: 10.1242/jcs.142737. PMID:24213534 [DOI] [PubMed] [Google Scholar]
- 18.Vitorino P, Meyer T. Modular control of endothelial sheet migration. Genes Dev. 2008;22:3268–3281. doi: 10.1101/gad.1725808. PMID:19056882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25:8–19. PMID:16170364 [DOI] [PubMed] [Google Scholar]
- 20.Malliri A, van Es S, Huveneers S, Collard JG. The Rac exchange factor Tiam1 is required for the establishment and maintenance of cadherin-based adhesions. J Biol Chem. 2004;279:30092–30098. doi: 10.1074/jbc.M401192200. PMID:15138270 [DOI] [PubMed] [Google Scholar]
- 21.Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol. 2015;17:276–287. doi: 10.1038/ncb3115. PMID:25706233 [DOI] [PubMed] [Google Scholar]
- 22.Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191, 1261–1269. doi: 10.1083/jcb.201003091. PMID:21173111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llense F, Martin-Blanco E. JNK signaling controls border cell cluster integrity and collective cell migration. Curr Biol. 2008;18:538–544. doi: 10.1016/j.cub.2008.03.029. PMID:18394890 [DOI] [PubMed] [Google Scholar]
- 24.Plutoni C, Bazellieres E, Le Borgne-Rochet M, Comunale F, Brugues A, Séveno M, Planchon D, Thuault S, Morin N, Bodin S, et al. . P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol. 2016;212:199–217. doi: 10.1083/jcb.201505105. PMID:26783302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouellette MH, Martin E, Lacoste-Caron G, Hamiche K, Jenna S. Spatial control of active CDC-42 during collective migration of hypodermal cells in Caenorhabditis elegans. J Mol Cell Biol. 2015. PMID:26578656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felix M, Chayengia M, Ghosh R, Sharma A, Prasad M. Pak3 regulates apical-basal polarity in migrating border cells during Drosophila oogenesis. Development. 2015;142:3692–3703. doi: 10.1242/dev.125682. PMID:26395489 [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro EM, Montell DJ. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development. 2004;131:5243–5251. doi: 10.1242/dev.01412. PMID:15456726 [DOI] [PubMed] [Google Scholar]
- 28.Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. Centralspindlin and alpha-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol. 2012;14:818–828. doi: 10.1038/ncb2532. PMID:22750944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranjuez G, Burtscher A, Sawant K, Majumder P, McDonald JA. Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue. Mol Biol Cell. 2016;27:1898–1910. doi: 10.1091/mbc.E15-10-0744. PMID:27122602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combedazou A, Choesmel-Cadamuro V, Gay G, Liu J, Dupré L, Ramel D, Wang X. Myosin II governs collective cell migration behaviour downstream of guidance receptor signalling. J Cell Sci. 2017;130:97–103. doi: 10.1242/jcs.179952. PMID:27034137 [DOI] [PubMed] [Google Scholar]
- 31.Scita G, Di Fiore PP. The endocytic matrix. Nature 2010;463, 464–473. doi: 10.1038/nature08910. PMID:20110990 [DOI] [PubMed] [Google Scholar]
- 32.Assaker G, Ramel D, Wculek SK, Gonzalez-Gaitan M, Emery, G. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc Natl Acad Sci USA. 2010;107:22558–22563. doi: 10.1073/pnas.1010795108. PMID:21149700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. PMID:18614017 [DOI] [PubMed] [Google Scholar]
- 34.Wan P, Wang D, Luo J, Chu D, Wang H, Zhang L, Chen J. Guidance receptor promotes the asymmetric distribution of exocyst and recycling endosome during collective cell migration. Development. 2013;140:4797–4806. doi: 10.1242/dev.094979. PMID:24198275 [DOI] [PubMed] [Google Scholar]
- 35.Buchanan SM, Schalm SS, Maniatis T. Proteolytic processing of protocadherin proteins requires endocytosis. Proc Natl Acad Sci U S A. 2010;107:17774–17779. doi: 10.1073/pnas.1013105107. PMID:20876099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. PMID:16256743 [DOI] [PubMed] [Google Scholar]
- 37.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. PMID:17395884 [DOI] [PubMed] [Google Scholar]
- 38.Peng HOng YM, Shah WA, Holland PC, Carbonetto S. Integrins regulate centrosome integrity and astrocyte polarization following a wound. Dev Neurobiol. 2013;73:333–353. doi: 10.1002/dneu.22055. PMID:22949126 [DOI] [PubMed] [Google Scholar]
- 39.Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. PMID:11929834 [PubMed] [Google Scholar]
- 40.Bazellieres E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Muñoz JJ, Sales-Pardo M, Guimerà R, Trepat X. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol. 2015;17:409–420. doi: 10.1038/ncb3135. PMID:25812522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellenbroek SI, Iden S, Collard JG. The Rac activator Tiam1 is required for polarized protrusional outgrowth of primary astrocytes by affecting the organization of the microtubule network. Small GTPases. 2012;3:4–14. doi: 10.4161/sgtp.19379. PMID:22710731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol. 2006;16:2395–2405. doi: 10.1016/j.cub.2006.10.026. PMID:17081755 [DOI] [PubMed] [Google Scholar]
- 43.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. PMID:21170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckers CM, van Hinsbergh VW, van Nieuw Amerongen GP. Driving Rho GTPase activity in endothelial cells regulates barrier integrity. Thrombosis Haemostasis. 2010;103:40–55. doi: 10.1160/TH09-06-0403. PMID:20062930 [DOI] [PubMed] [Google Scholar]
- 45.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. PMID:17060906 [DOI] [PubMed] [Google Scholar]
- 46.Meyer RD, Sacks DB, Rahimi N. IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS One. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. PMID:19050761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. PMID:16763158 [DOI] [PubMed] [Google Scholar]
- 48.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. PMID:15247219 [DOI] [PubMed] [Google Scholar]
- 49.Bhattacharya R, Kwon J, Li X, Wang E, Patra S, Bida JP, Bajzer Z, Claesson-Welsh L, Mukhopadhyay D. Distinct role of PLCbeta3 in VEGF-mediated directional migration and vascular sprouting. J Cell Sci. 2009;122:1025–1034. doi: 10.1242/jcs.041913. PMID:19295129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamalice L, Houle F, Huot J. Phosphorylation of Tyr1214 within VEGFR-2 triggers the recruitment of Nck and activation of Fyn leading to SAPK2/p38 activation and endothelial cell migration in response to VEGF. J Biol Chem. 2006;281:34009–34020. doi: 10.1074/jbc.M603928200. PMID:16966330 [DOI] [PubMed] [Google Scholar]
- 51.Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene 2004;23:434–445. doi: 10.1038/sj.onc.1207034. PMID:14724572 [DOI] [PubMed] [Google Scholar]
- 52.Komarova YA, Kruse K, Mehta D, Malik AB. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ Res. 2017;120:179–206. doi: 10.1161/CIRCRESAHA.116.306534. PMID:28057793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. PMID:21925313 [DOI] [PubMed] [Google Scholar]
- 54.Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi: 10.1016/j.ceb.2015.08.005. PMID:26363959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.RES.87.4.335. PMID:10948069 [DOI] [PubMed] [Google Scholar]
- 56.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascular Pharmacol. 2002;39:187–199. doi: 10.1016/S1537-1891(03)00008-9. [DOI] [PubMed] [Google Scholar]
- 57.Beckers CM, Knezevic N, Valent ET, Tauseef M, Krishnan R, Rajendran K, Hardin CC, Aman J, van Bezu J, Sweetnam P, et al. . ROCK2 primes the endothelium for vascular hyperpermeability responses by raising baseline junctional tension. Vascular Pharmacol. 2015;70:45–54. doi: 10.1016/j.vph.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. PMID:9705325 [DOI] [PubMed] [Google Scholar]
- 59.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. PMID:14769914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrett TA, Van Buul JD, Burridge K. VEGF-induced Rac1 activation in endothelial cells is regulated by the guanine nucleotide exchange factor Vav2. Exp Cell Res. 2007;313:3285–3297. doi: 10.1016/j.yexcr.2007.05.027. PMID:17686471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naikawadi RP, Cheng N, Vogel SM, Qian F, Wu D, Malik AB, Ye RD. A critical role for phosphatidylinositol (3,4,5)-trisphosphate-dependent Rac exchanger 1 in endothelial junction disruption and vascular hyperpermeability. Circ Res. 2012;111:1517–1527. doi: 10.1161/CIRCRESAHA.112.273078. PMID:22965143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barry DM, Xu K, Meadows SM, Zheng Y, Norden PR, Davis GE, Cleaver O. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development. 2015;142:3058–3070. doi: 10.1242/dev.125260. PMID:26253403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakayama Y, Fukuhara S, Ando K, Matsuda M, Mochizuki N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev Cell. 2015;32:109–122. doi: 10.1016/j.devcel.2014.11.024. PMID:25584797 [DOI] [PubMed] [Google Scholar]
- 64.Hayer, A, Shao L, Chung M, Joubert LM, Yang HW, Tsai FC, Bisaria A, Betzig E, Meyer T. Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol. 2016;18:1311–1323. doi: 10.1038/ncb3438. PMID:27842057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. VE-Cadherin-mediated cell-cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol. 2009;19:668–674. doi: 10.1016/j.cub.2009.02.057. PMID:19345098 [DOI] [PubMed] [Google Scholar]
- 66.Daneshjou, N, Sieracki N, van Nieuw Amerongen GP, Conway DE, Schwartz MA, Komarova YA, Malik AB. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J Cell Biol. 2015;208:23–32. doi: 10.1083/jcb.201409108. PMID:25559184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zebda N, Tian Y, Tian X, Gawlak G, Higginbotham K, Reynolds AB, Birukova AA, Birukov KG. Interaction of p190RhoGAP with C-terminal domain of p120-catenin modulates endothelial cytoskeleton and permeability. J Biol Chem. 2013;288:18290–18299. doi: 10.1074/jbc.M112.432757. PMID:23653363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TW Jr, Yin T, et al. . A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci. 2015;128:3041–3054. doi: 10.1242/jcs.168674. PMID:26116572 [DOI] [PubMed] [Google Scholar]
- 69.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov, KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715–724. doi: 10.1002/jcp.21354. PMID:18064650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham S, Scarcia M, Bagshaw RD, McMahon K, Grant G, Harvey T, Yeo M, Esteves FO, Thygesen HH, Jones PF, et al. . A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis. Nat Commun. 2015;6:7286. doi: 10.1038/ncomms8286. PMID:26129894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fukushima, Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. PMID:21505259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur, S, Leszczynska K, Abraham S, Scarcia M, Hiltbrunner S, Marshall CJ, Mavria G, Bicknell R, Heath VL. RhoJ/TCL regulates endothelial motility and tube formation and modulates actomyosin contractility and focal adhesion numbers. Arterioscler Thromb Vasc Biol. 2011;31:657–664. doi: 10.1161/ATVBAHA.110.216341. PMID:21148427 [DOI] [PubMed] [Google Scholar]
- 73.Yuan L, Sacharidou A, Stratman AN, Le Bras A, Zwiers PJ, Spokes K, Bhasin M, Shih SC, Nagy JA, Molema G, et al. . RhoJ is an endothelial cell-restricted Rho GTPase that mediates vascular morphogenesis and is regulated by the transcription factor ERG. Blood. 2011;118:1145–1153. doi: 10.1182/blood-2010-10-315275. PMID:21628409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, Park I, Jung J, Kataoka H, Lee D, et al. . Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell. 2014;25, 102–117. doi: 10.1016/j.ccr.2013.12.010. PMID:24434213 [DOI] [PubMed] [Google Scholar]
- 75.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovascular Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 76.Bryan BA, D'Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–2065. doi: 10.1007/s00018-007-7008-z. PMID:17530172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O'Neill PR, Kalyanaraman V, Gautam N. Subcellular optogenetic activation of Cdc42 controls local and distal signaling to drive immune cell migration. Mol Biol Cell. 2016;27:1442–1450. doi: 10.1091/mbc.E15-12-0832. PMID:26941336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valon L, Etoc F, Remorino A, di Pietro F, Morin X, Dahan M, Coppey M. Predictive spatiotemporal manipulation of signaling perturbations using optogenetics. Biophys J. 2015;109:1785–1797. doi: 10.1016/j.bpj.2015.08.042. PMID:26536256 [DOI] [PMC free article] [PubMed] [Google Scholar]


