Abstract
Objective
The aim of this study was to invastigate the effect of body mass index (BMI) on semen parameters and reproductive hormone levels in infertile males.
Material and methods
Overall, 858 infertile male patients, aged between 18 and 55 years, referred to our infertility clinic were included in the study. Patients without risk factors, besides obesity, that could affect semen parameters or reproductive hormones were evaluated. Patients were separated into the following three groups: non-obese (<25 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2). Age, semen parameters, and reproductive hormones were evaluated and compared among the groups. In addition, subgroups based on sperm concentration were compared.
Results
Total testosterone and testosterone-estradiol ratio negatively correlated with BMI (p<0.001). A positive correlation was observed between BMI and age (p<0.001). Even when adjusted for age, the decrease in total testosterone was significant in all groups parallel to the increase in BMI. Although age, prolactin level, and total testosterone had a significant relationship in univariate analysis, the only significant parameters were prolactin and total testosterone according to multivariate analysis. There were no significant differences between BMI and semen parameters. No significant difference related to BMI was observed among the infertile groups [severe oligospermia (34.3%), oligospermia (18.2%), and normospermia (47.6%)].
Conclusion
A significant negative correlation was observed between increasing BMI and total testosterone. No relationship was observed between BMI and semen parameters except progressive motility. Nevertheless, prospective longitudinal clinical trials with larger sample sizes involving weight loss are needed to understand the precise relationship of BMI with reproductive hormones and semen parameters in the same individual.
Keywords: Body mass index, male infertility, obesity, reproductive hormones
Introduction
One of the most common health problems worldwide currently is obesity. The World Health Organization (WHO) determines the limit of body mass index (BMI) for obesity as over 30 kg/m2 and overweight as between 25 and 29.9 kg/m2.[1] According to a prevalent study, almost 2.1 billion people can be classified as overweight or obese.[2] The correlation between obesity and infertility has been investigated. Even though the unfavorable effect of obesity on female fertility is clear, studies regarding male fertility yielded ambiguous outcomes.[3] Several studies have stated that obesity plays a crucial role in decreasing semen quality.[4,5] However, there are is still ambiguity regarding this correlation.[ 6]
Excess white adipose tissue converts testosterone to estrogen through aromatization activity and is responsible for elevated estrogen concentrations in obese men. Increased estrogen has a negative effect on the hypothalamus-pituitary gonadal axis resulting in suppression of gonadotropin secretion. Decreased testosterone and increased estrogen have been associated with subfertility and reduced sperm parameters.[7] Therefore, aromatase inhibitors have been used in the treatment of infertility.[8]
Obesity typically coexists with metabolic disorders, such as hypertension, diabetes, hyperlipidemia, or proinflammatory reactions.[9] Hence, it is challenging to observe only the real effects of obesity on sperm quality and reproductive hormonal changes.
To our knowledge, several recent studies have not mentioned the isolated effect of obesity on fertility in their study population. Therefore, this study aimed to investigate the relationship between obesity and male infertility in a large patient group, after excluding possible confounding variables.
Material and methods
Study population
Our study was designed as a retrospective case control study. The study was approved by the Erciyes University Ethical Committee (approval number: 2018/409). Overall, 3563 consecutive male Turkish patients with complete data aged between 18 and 55 years, who were admitted for infertility evaluation between November 2001 and March 2017, were evaluated. Among these patients, 858 male patients among infertile couples whose lab records and medical histories were documented.
Inclusion and exclusion criteria
All chronic diseases, such as chronic obstructive pulmonary disease, chronic liver disease, hypertension, asthma, diabetes, epilepsy, chronic kidney disease, chronic inflammatory bowel disease, hypothyroidism, hyperthyroidism, cerebrovascular events, and thromboembolism were routinely recorded and considered as possible risk factors affecting fertility. Moreover, patients were questioned regarding all other possible risk factors affecting male infertility, such as varicocele, undescended testis, epididymitis, mumps orchitis, testicular cancer, Klinefelter’s syndrome, cystic fibrosis, immotile cilia syndrome, vasal agenesis, distal ejaculatory duct obstructions, translocation anomalies, hypogonadotropic hypogonadism, history of hydrocelectomy, herniorrhaphy, orchiopexy, vasectomy, and testicular sperm extraction. All the above parameters were accepted as possible risk factors affecting fertility, apart from obesity. Because of the much higher prevalence of azoospermia (28.2%) in our cohort compared with the general infertile population, patients with azoospermia were excluded from the study to exclude patients with obstructive azoospermia from the study group. Overall, 858 male patients among infertile couples who were meticulously selected with exclusion of all possible risk factors were recruited for this study. The study design is summarized in the flowchart (Figure 1).
Figure 1.
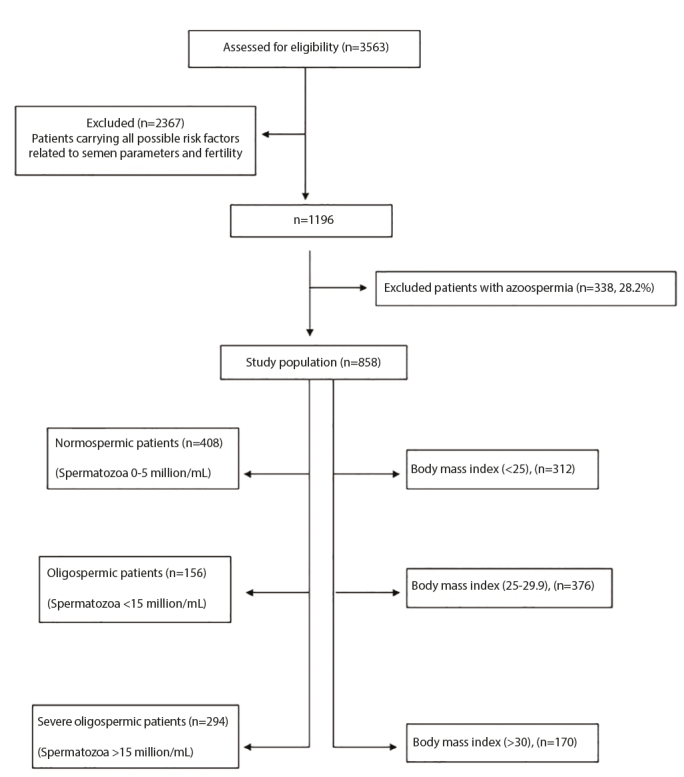
Flowchart of the study
Procedures
Each patient’s weight and height were measured, and BMI was calculated by dividing the weight by the square of the height at their first physical examination. Semen specimens were collected after a sexual abstinence period of three full days. Semen analysis was performed per the WHO 2010 criteria.[10] Semen analysis results before 2010 were adjusted according to WHO 2010 criteria. Reproductive hormones, such as follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, total testosterone (TT), estradiol (E2), and testosterone-estradiol ratio (T/E) were evaluated using electrochemiluminescence immunoassay (ECLIA, Roche® 8000 Diagnostics). Blood samples were routinely collected between 8 and 10 am to assess the reproductive hormones.
Statistical analysis
Shapiro-Wilk test, histogram, and Q-Q plots were applied to assess data normality. Variance homogeneity was assessed using Levene’s test. The Pearson chi-square analysis was employed for categorical variables and Kruskal–Wallis test for continuous variables to compare the differences among the BMI groups. Dunn-Bonferroni test was applied for multiple comparisons. Covariance analyses were conducted to assess age-adjusted differences among the BMI groups. Spearman’s test was performed for correlation analysis. Binary logistic regressions adjusted for baseline age were performed, with results presented as odds ratio and 95% confidence interval. Furthermore, univariate and multivariate logistic regression analyses were used to determine the most significant risk factors. Significant variables at p<0.25 on univariate analysis were taken into multiple model and forward stepwise selection was performed using likelihood ratio statistic at p<0.10 stringency level. Additionally, odds ratio was obtained with 95% confidence interval. Hosmer-Lemeshow test statistics were employed for goodness of fit test for testing model instability. Analyses were conducted using TURCOSA Cloud (Turcosa Analytics Ltd Co, Turkey) software. A p value of less than 5% was considered statistically significant. Power analysis was performed using TURCOSA Cloud software (Turcosa Analytics Ltd. Co., Turkey). Alfa 0.05, Beta 0.20, (1-Beta)=0.900.
Results
Overall, 858 patients were studied. The mean age was 30.8±5.1 years (median 30 years, range: 18–51 years) and the mean BMI was 26.7±4.1 kg/m2 (median 26.1 kg/m2, range: 17–52 kg/m2). Correlations between BMI, reproductive hormone levels, and semen parameters are summarized in Table 1. In the study, TT and T-E ratios negatively correlated with BMI (r=−0.267, p<0.001; r=−0.161, p<0.001, respectively). A positive correlation was observed between BMI and age (r=0.146, p<0.001) (Table 1). The correlation between BMI and TT level is depicted in Figure 2.
Table 1.
Correlations between body mass index and semen parameters and reproductive hormone levels
| n=858 | ||
|---|---|---|
| rho | p* | |
| Age (year) | 0.146 | <0.001 |
| Prolactin (ng/mL) | 0.057 | 0.096 |
| FSH (mIU/mL) | 0.008 | 0.806 |
| LH (mIU/mL) | −0.043 | 0.211 |
| Total testosterone (ng/dL) | −0.267 | <0.001 |
| Estradiol (pg/mL) | −0.032 | 0.358 |
| Testosterone/estradiol ratio | −0.161 | <0.001 |
| Semen volume (ML) | −0.010 | 0.781 |
| Sperm concentration (×106/mL) | −0.011 | 0.752 |
| Total sperm count (×106) | −0.018 | 0.590 |
| Progressive motility (%) | −0.004 | 0.910 |
| Total progressive motile sperm (×106) | −0.008 | 0.810 |
FSH: follicle stimulating hormone; LH: luteinizing hormone; rho: Spearman correlation coefficient; BMI: body mass index; p*: Spearman test.
Figure 2.
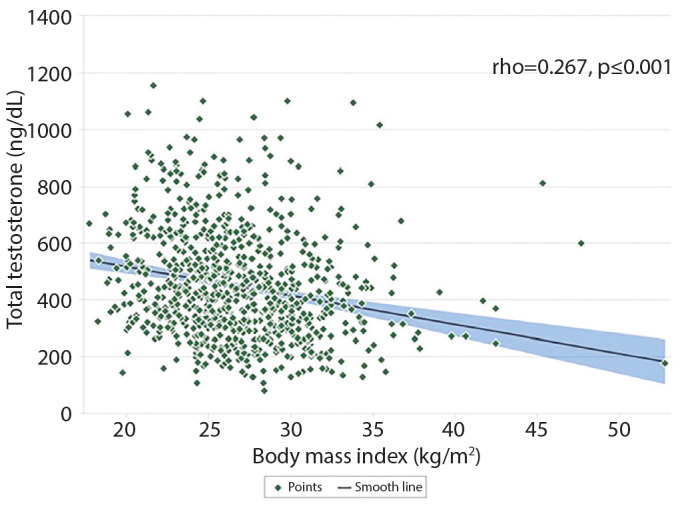
Correlation between total testosterone and body mass
Because of the significant difference in age among the groups, covariance analysis was performed to conduct age-adjusted group comparisons. After adjusting for age, the T-E ratio became insignificant, whereas the TT level was still significantly different among the BMI groups (p<0.001) (Table 2). After reclassification of the BMI as >25 abnormal (n=546, 63.6%) and <25 normal (n=312, 36.4%), logistic regression analysis was performed to determine the most significant risk factors. In univariate logistic regression analysis, parameters like age, prolactin, FSH, TT, testosterone ratio, progressive motility, and total progressive sperm motility were significant (p values of parameters that can be entered into the modelwere p=0.046, p=0.045, p=0.158, p<0.001, p=0.142, p=0.107, and p=0.132, respectively). When the significant variables at p<0.25 on univariate analysis were taken into the multiple model, prolactin (p=0.023) and TT (p≤0.001) were noted to be statistically significant. Univariate analysis revealed that the risk of obesity increased by 1.033 times when the prolactin level increased by one unit. In addition, the risk of obesity increased by 2% (1–0.998) with a decrease of one unit of TT (Table 3). Multivariate analysis observed prolactin and TT to be the only significant parameters (Table 3). Semen volume, sperm concentration, total sperm count, progressive motility, and total progressive motile sperm count were noted to have no significant correlations with BMI on univariate analysis (Table 3).
Table 2.
Patient characteristics
| BMI (<25) n=312 (36.4%) | BMI (25–29.9) n=376 (37.6%) | BMI (≥30) n=170 (19.8%) | p* | p+ | |
|---|---|---|---|---|---|
| Age (year) | 29 (26–33)a | 30 (28–34)b | 31 (28–35)b | <0.001 | NA |
| Prolactin (ng/mL) | 8.2 (5.6–11.6) | 8.3 (6–10.7) | 8.5 (7–11) | 0.140 | 0.063 |
| FSH (mIU/mL) | 4 (2.4–6.8) | 4.2 (2.6–6.8) | 4.2 (2.6–6.5) | 0.793 | 0.321 |
| LH (mIU/mL) | 4.3 (3–5.9) | 3.9 (2.8–5.5) | 4.2 (2.8–5.4) | 0.222 | 0.402 |
| Total testosterone (ng/dL) | 472.1 (353.7–631.7)a | 398 (297.8–522.8)b | 348.1 (261–470)c | <0.001 | <0.001 |
| Estradiol (pg/mL) | 31.6 (22.3–43.2) | 30.3 (21–42.3) | 29.6 (20.9–40) | 0.484 | 0.665 |
| Testosterone/estradiol ratio | 16.2 (9.9–23.9)a | 13.2 (8.6–20)a | 12.1 (8.2–18.7)b | 0.001 | 0.306 |
| Semen volume (mL) | 2.6 (2–3.7) | 2.5 (2–3.4) | 2.4 (1.9–3.5) | 0.635 | 0.422 |
| Sperm concentration (×106/mL) | 12.5 (2.6–30.3) | 15 (3.1–35) | 10.7 (2.4–34) | 0.407 | 0.336 |
| Total sperm count (×106) | 35.5 (6–80) | 33.4 (7.8–89.9) | 25.5 (6.9–79.7) | 0.608 | 0.664 |
| Progressive motility (%) | 27 (16–35) | 28 (20–38) | 27 (17.7–33) | 0.114 | 0.077 |
| Total progressive motile sperm (×106) | 6.8 (0.9–21.7) | 8.6 (1.3–28.5) | 6.2 (1.3–24.5) | 0.247 | 0.239 |
FSH: follicle stimulating hormone; LH: luteinizing hormone; BMI: body mass index (kg/m2); NA: not available; p*: Kruskal–Wallis, Intergroup comparisons; p+, Covariance analyses, Age-adjusted intergroup comparisons. Values are expressed as median (25th–75th percentiles).
Statistically significant groups are shown in different letters.
If the groups labelled by the same letter, it means that there is no statistical difference.
Table 3.
Result of multiple logistic regression analysis of the possible correlates for body mass index (>25 abnormal, <25 normal)
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p* | OR (95% CI) | p** |
| Age (year) | 1.033 (1.001–1.066) | 0.046 | - | - |
| Prolactin (ng/mL) | 1.033 (1.001–1.067) | 0.045 | 1.034 (1.000–1.070) | 0.048 |
| FSH (mIU/mL) | 0.974 (0.939–1.010) | 0.158 | - | - |
| LH (mIU/mL) | 0.966 (0.899–1.038) | 0.348 | - | - |
| Total testosterone (ng/dL) | 0.998 (0.997–0.999) | <0.001 | 0.998 (0.997–0.999) | <0.001 |
| Estradiol (pg/mL) | 0.996 (0.986–1.006) | 0.429 | - | - |
| Testosterone/estradiol ratio | 0.992 (0.980–1.003) | 0.142 | - | - |
| Semen volume (mL) | 0.988 (0.869–1.123) | 0.852 | - | - |
| Sperm concentration (×106/mL) | 0.998 (0.991–1.004) | 0.523 | - | - |
| Total sperm count (×106) | 0.999 (0.996–1.002) | 0.443 | - | - |
| Progressive motility (%) | 0.990 (0.978–1.002) | 0.107 | - | - |
| Total progressive motile sperm (×106) | 0.995 (0.987–1.002) | 0.132 | - | - |
FSH: follicle stimulating hormone; LH: luteinizing hormone. p* value for comparison between “normal” and “abnormal” groups: Univariate logistic regression test variable. p** value for comparison between “normal” and “abnormal” groups: Multivariate logistic regression test variable
The distribution of patients according to sperm concentration was as follows: severe oligospermia (spermatozoa 0–5 million/ mL) (n=294, 34.3%), oligospermia (spermatozoa <15 million/ mL) (n=156, 18.2%), and normospermia (spermatozoa >15 million/mL) (n=408, 47.6%). No significant difference was observed related to BMI among the groups (Table 4). Furthermore, when patients with azoospermia were included (data not shown) in the study, no significant change was observed in the correlation between semen count and BMI.
Table 4.
Comparisons of sperm concentration among BMI groups
| BMI (<25) | BMI (25–29.9) | BMI (≥30) | Total | p* | |
|---|---|---|---|---|---|
| Severe Oligospermia (<5 million/mL) | 113 (38.4) | 121 (41.2) | 60 (20.4) | 294 (34.3) | 0.652 |
| Oligospermia (5–15 million/mL) | 58(37.2) | 65 (41.7) | 33 (21.2) | 156 (18.2) | |
| Normospermia (>15 million/mL) | 141 (34.6) | 190 (46.6) | 77 (18.9) | 408 (47.6) | |
| Total | 312 (36.4) | 376 (43.8) | 170 (19.8) | 858 (100) |
BMI: body mass index; *, Pearson chi-square; Values are expressed as n, (%)
In addition, results of reproductive hormone levels and semen parameters were compared among the BMI groups in patients with normospermia. Increased prolactin levels were noted to be statistically significant, especially in the obese group (BMI >30 kg/m2). The decrease of TT level was still significantly different in all BMI groups (p<0.001). The progressive sperm motility in the overweight group (BMI 25–29.9 kg/m2) was significantly higher than the obese group (BMI >30 kg/m2) (p=0.039). Comparisons of data of patients with normospermia are summarized in Table 5.
Table 5.
Characteristics of normospermic patients according to BMI groups
| BMI (<25) n=141 (34.5%) | BMI (25–29.9) n=190 (46.6%) | BMI (≥30) n=77 (18.9%) | p* | |
|---|---|---|---|---|
| Age (year) | 29 (27–34.5) | 31 (27.7–34) | 31 (28–35) | 0.133 |
| Prolactin (ng/mL) | 7.9 (5.5–11.1)a | 8.2 (5.7–10.1)a | 8.9 (7.2–11.2)b | 0.016 |
| FSH (mIU/mL) | 3.3 (2.2–5.1) | 3.4 (2.4–4.9) | 3.5 (2.3–4.5) | 0.689 |
| LH (mIU/mL) | 3.7 (2.7–5) | 3.7 (2.7–5.1) | 3.7 (2.6–4.7) | 0.739 |
| Total testosterone (ng/dL) | 472 (349.9–659)a | 406 (304–524.1)b | 337 (249.7–469)c | <0.001 |
| Estradiol (pg/mL) | 32.5 (22.8–44.5) | 32.1 (22.2–44.2) | 28.7 (21.1–28.3) | 0.220 |
| Testosterone/estradiol ratio | 15.9 (9.6–23.6)a | 12.9 (8.1–18.8)b | 11.8 (7.4–17.7)b | 0.004 |
| Semen volume (mL) | 2.4 (2–3.4) | 2.6 (1.9–3.4) | 2.3 (1.8–3.3) | 0.481 |
| Sperm concentration (×106/mL) | 34 (23.7–51) | 35 (24–52) | 35 (24–54) | 0.811 |
| Total sperm count (×106) | 83.7 (60.4–122.8) | 88.9 (58.2–126) | 90 (54–134.1) | 0.917 |
| Progressive motility (%) | 31 (22–38.5)ab | 32 (25–41)a | 30 (21–34.5)b | 0.039 |
| Total progressive motile sperm (×106) | 24.6 (15.4–41.6) | 28.1 (15.7–48.3) | 27.2 (11.7–37.7) | 0.339 |
FSH: follicle stimulating hormone; LH: Luteinizing hormone; BMI: body mass index (kg/m2); p*: Kruskal–Wallis, Intergroup comparisons; Values are expressed as median (25th–75th percentiles).
Statistically significant groups are shown in different letters.
If the groups labelled by the same letter, it means that there is no statistical difference.
Discussion
Several studies have analyzed the effect of obesity on male infertility with different numbers of patient groups. Considering the new information, this effect consists of multiple factors and several different pathophysiological mechanisms.[5,11] The generally accepted mechanism is defined as aromatization of testosterone to estradiol by peripheral adipose tissue and the suppression of high levels of estradiol of the hypothalamus-pituitary gonadal axis via negative feedback inhibition.[7,12,13] Consequently, the decreased testosterone and increased estrogen may reduce sperm parameters and may result in subfertility. Thereby, obesity may cause hypogonadotropic hypogonadism and hyperestrogenism. Obesity may lead to impaired sperm quality, negative effects on sperm mitochondrial activity, increased sperm DNA damage, and increased seminal oxidative stress.[14] All these factors have been investigated in several studies, albeit with conflicting results.
In a meta-analysis reported by MacDonald et al.[6], no strong evidence was detected between semen parameters and obesity. In this meta-analysis, there was strong evidence of a negative relationship of TT, sex hormone binding globulin (SHBG), and free testosterone with increased BMI. The major limitation was that 26 of 31 studies were unsuitable for the meta-analysis. The common limitation of the studies reported in the meta-analysis was that weight and height measurements were self-reported and recruited participants did not have the same inclusion criteria. Wu et al.[15] observed significant negative relationships of testosterone, free testosterone, and SHBG with BMI, and noted no relationship of LH in 3200 participants recruited from the general population. Furthermore, Aggerholm et al.[16] observed a significant negative relationship between testosterone and SHBG but they did not find any relationship between E2, LH, and FSH in 1989 men recruited from the general population. In our study, the decrease in TT level was significant in all BMI groups when the age factor was eliminated.
The relationship of obesity with semen parameters and reproductive hormone levels were not significant in other similar studies.[16,17] On the contrary, another meta-analysis determined a negative relationship between BMI and semen parameters.[5] In a comprehensive cohort study comprising 10,665 patients, a statistically significant negative relationship was detected between BMI and semen volume, concentration, and motility, but no relationship with morphology was detected.[18] Nonetheless, heterogeneity of the study population and no information regarding any exclusion criteria for the selection of patients were major limitations of this study. In this context, the elimination of all possible risk factors that may affect BMI makes our study more reasonable. Bieniek et al.[19] reported that the strongest correlations were observed between increasing BMI and sperm concentration (r=−0.08, p<0.001) and motility (r=−0.07, p<0.001) in a multi-institutional cohort study (n=4440). However, when we looked at the relationship reported as strong, the correlation coefficient of the study was noted to be weak. Their study groups consisted of men who were referred for male infertility evaluation from two centers and semen analyses were performed using two different methods (computer-assisted and manual). This statistically significant correlation could be due to the large sample size. Conversely, we did not observe any relationship between BMI and semen parameters in all analyses of our study group.
Nevertheless, research investigating the effect of weight loss, through bariatric surgery or aerobic exercise program, on sperm parameters is valuable in terms of evaluation of semen parameters and reproductive hormone levels in the same patients [20–22]. Increased testosterone levels and improved semen parameters were observed following weight loss in these studies. Likewise, our study observed that increased weight correlated with decreased TT level in all groups.
We performed a subgroup analysis of severe oligospermia, oligospermia, and normospermia in men without risk factors affecting male infertility. We observed that an increase in BMI did not affect sperm count adversely. According to our results, the decrease in TT and T-E ratios are because of increased BMI and indicate peripheral aromatization and negative feedback. Decreased TT levels were observed in all BMI groups, even with age-adjusted analysis.
Generally, several studies in the literature have suggested a weak, albeit significant relationship of semen parameters and reproductive hormone levels with increased BMI. This result is probably because of the large sample size. In our study, this weak relationship was observed despite careful patient selection. Moreover, it is thought that even with exclusion of several parameters that affect semen parameters or hormone values, no clear relationship is observed.
Nonetheless, our study had few limitations. First, this study had a retrospective case control design and was not a population-based study. This limitation prevented us to conduct a longitudinal study for investigating the effects of weight loss on semen parameters. Second, percentages of normal sperm morphology were not considered owing to missing data. Third, the inclusion of only infertile males might have prevented us from drawing comparisons with the general population. Moreover, we excluded men with azoospermia because of the impossibility of evaluating the relationship between semen parameters and BMI.
In conclusion, in this study, we evaluated the relationship of semen parameters and reproductive hormones with BMI in a large patient group after excluding possible risk factors that may affect infertility. We observed a significant negative correlation between increasing BMI and TT levels. Although no relationship was observed between BMI and semen parameters upon the overall group comparison, progressive motility had a negative association in normospermic obese patients. Nevertheless, prospective longitudinal clinical trials with larger sample sizes involving weight loss are needed to accurately understand the relationship of reproductive hormones and semen parameters with BMI in the same individual.
Main Points.
Decreased testosterone and increased estrogen have been associated with subfertility and reduced sperm parameters.
The generally accepted mechanism is defined as aromatization of testosterone to estradiol by peripheral adipose tissue and the suppression of high levels of estradiol of the hypothalamuspituitary gonadal axis via negative feedback inhibition.
It is challenging to observe only the real effects of obesity on sperm quality and reproductive hormonal changes.
Generally, several studies in the literature have suggested a weak, albeit significant relationship of semen parameters and reproductive hormone levels with increased BMI. This result is probably because of the large sample size.
Prospective longitudinal clinical trials with larger sample sizes involving weight loss are needed to accurately understand the relationship of reproductive hormones and semen parameters with BMI in the same individual.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Erciyes University (Approval No: 2018/409).
Informed Consent: Written informed consent could not be obtained from patients who participated in this study because of the retrospective design.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – O.E.; Design – O.E., N.B.; Supervision – O.E., E.C.A.; Resources – N.B., E.C.A.; Materials – N.B., O.E.; Data Collection and/or Processing – İ.S., O.E., N.B.; Analysis and/or Interpretation – N.B., İ.S., O.E., G.E.Z.; Literature Search – İ.S., N.B.; Writing Manuscript – İ.S., N.B., G.E.Z.; Critical Review – E.C.A., O.E.; Other – G.E.Z.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Chung F. Morbidly obese patients: a clinical challenge. Curr Opin Anaesthesiol. 2016;29:101–2. doi: 10.1097/ACO.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaraju GA, Teppala S, Prathigudupu K, Kalagara M, Thota S, Kota M, et al. Association between obesity and sperm quality. Andrologia. 2018;50 doi: 10.1111/and.12888. doi: 10.1111/and.12888. [DOI] [PubMed] [Google Scholar]
- 4.Engin-Ustun Y, Yilmaz N, Akgun N, Aktulay A, Tuzluoglu AD, Bakirarar B. Body Mass Index Effects Kruger’s Criteria in Infertile Men. Int J Fertil Steril. 2018;11:258–62. doi: 10.22074/ijfs.2018.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–31. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–6. doi: 10.1210/jc.76.5.1140. [DOI] [PubMed] [Google Scholar]
- 8.Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril. 2011;95:809–11. doi: 10.1016/j.fertnstert.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–87. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 10.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmq020. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, Louis GM. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Human Reprod. 2015;30:493–4. doi: 10.1093/humrep/deu322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jc.79.4.997. [DOI] [PubMed] [Google Scholar]
- 13.Kley HK, Deselaers T, Peerenboom H, Kruskemper HL. Enhanced conversion of androstenedione to estrogens in obese males. J Clin Endocrinol Metab. 1980;51:1128–32. doi: 10.1210/jcem-51-5-1128. [DOI] [PubMed] [Google Scholar]
- 14.Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. 2017;27:441–5. doi: 10.1097/MOU.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 15.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. Hypothalamic- pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–45. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 16.Aggerholm AS, Thulstrup AM, Toft G, Ramlau-Hansen CH, Bonde JP. Is overweight a risk factor for reduced semen quality and altered serum sex hormone profile? Fertil Steril. 2008;90:619–26. doi: 10.1016/j.fertnstert.2007.07.1292. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Lin H, Ma M, Li L, Cai M, Zhou N, et al. Semen quality of 1346 healthy men, results from the Chongqing area of southwest China. Human Reprod. 2009;24:459–69. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 18.Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, Dalleac A, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. 2014;102:1268–73. doi: 10.1016/j.fertnstert.2014.07.1212. [DOI] [PubMed] [Google Scholar]
- 19.Bieniek JM, Kashanian JA, Deibert CM, Grober ED, Lo KC, Brannigan RE, et al. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil Steril. 2016;106:1070–5. doi: 10.1016/j.fertnstert.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 20.Andersen JM, Herning H, Aschim EL, Hjelmesaeth J, Mala T, Hanevik HI, et al. Body Mass Index Is Associated with Impaired Semen Characteristics and Reduced Levels of Anti-Mullerian Hormone across a Wide Weight Range. PloS One. 2015;10:e0130210. doi: 10.1371/journal.pone.0130210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis LO, Zani EL, Saad RD, Chaim EA, de Oliveira LC, Fregonesi A. Bariatric surgery does not interfere with sperm quality--a preliminary long-term study. Reprod Sci. 2012;19:1057–62. doi: 10.1177/1933719112440747. [DOI] [PubMed] [Google Scholar]
- 22.Rosety MA, Diaz AJ, Rosety JM, Pery MT, Brenes-Martin F, Bernardi M, et al. Exercise improved semen quality and reproductive hormone levels in sedentary obese adults. Nutr Hosp. 2017;34:603–7. doi: 10.20960/nh.549. [DOI] [PubMed] [Google Scholar]


