Abstract
Aims/hypothesis
We evaluated the secular trend of glycaemic control in individuals with type 2 diabetes in developing countries, where data are limited.
Methods
The International Diabetes Management Practices Study provides real-world evidence of patient profiles and diabetes care practices in developing countries in seven cross-sectional waves (2005–2017). At each wave, each physician collected data from ten consecutive participants with type 2 diabetes during a 2 week period. The primary objective of this analysis was to evaluate trends of glycaemic control over time.
Results
A total of 66,088 individuals with type 2 diabetes were recruited by 6099 physicians from 49 countries. The proportion of participants with HbA1c <53 mmol/mol (<7%) decreased from 36% in wave 1 (2005) to 30.1% in wave 7 (2017) (p < 0.0001). Compared with wave 1, the adjusted ORs of attaining HbA1c ≤64 mmol/mol (≤8%) decreased significantly in waves 2, 5, 6 and 7 (p < 0.05). Over 80% of participants received oral glucose-lowering drugs, with declining use of sulfonylureas. Insulin use increased from 32.8% (wave 1) to 41.2% (wave 7) (p < 0.0001). The corresponding time to insulin initiation (mean ± SD) changed from 8.4 ± 6.9 in wave 1 to 8.3 ± 6.6 years in wave 7, while daily insulin dosage ranged from 0.39 ± 0.21 U/kg (wave 1) to 0.33 ± 0.19 U/kg (wave 7) for basal regimen and 0.70 ± 0.34 U/kg (wave 1) to 0.77 ± 0.33 (wave 7) U/kg for basal–bolus regimen. An increasing proportion of participants had ≥2 HbA1c measurements within 12 months of enrolment (from 61.8% to 92.9%), and the proportion of participants receiving diabetes education (mainly delivered by physicians) also increased from 59.0% to 78.3%.
Conclusions
In developing countries, glycaemic control in individuals with type 2 diabetes remained suboptimal over a 12 year period, indicating a need for system changes and better organisation of care to improve self-management and attainment of treatment goals.
Electronic supplementary material
The online version of this article (10.1007/s00125-019-05078-3) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Clinical diabetes, Education, Epidemiology, Healthcare delivery, Insulin therapy, Prediction and prevention of type 2 diabetes
Introduction
Diabetes affects approximately 463 million people worldwide, of whom 90% have type 2 diabetes, and the prevalence is expected to increase by 51% by 2045. A large number of people with diabetes live in developing regions, with estimates suggesting that 55 million people in the Middle East/North Africa, 32 million in South and Central America, 19 million in sub-Saharan Africa and 88 million in South East Asia have diabetes [1].
Poor glycaemic control can lead to an increased risk of blindness, end-stage renal disease, cardiovascular disease and lower limb amputations [2]. In 2012, 1.5 million deaths worldwide were directly caused by diabetes [2]. A further 2.2 million deaths were due to cardiovascular disease, chronic kidney disease and tuberculosis, associated with high blood glucose levels [2]. Optimal blood glucose control is therefore needed to reduce the risk of complications and premature death in developing regions, which have a high burden of diabetes and possess fewer resources to treat end-stage disease, creating a considerable impact on healthcare systems.
Over the last decade, there have been major advances in diabetes management, resulting in improved outcomes for individuals with type 2 diabetes. These include the introduction of novel technologies, newer oral glucose-lowering drugs (OGLD; e.g. sodium–glucose cotransporter [SGLT]2 inhibitors, dipeptidyl peptidase 4 inhibitors [DPP-4i] and glucagon-like peptide-1 receptor agonists [GLP-1 RA]), insulin therapies and delivery systems/devices.
While the majority of guidelines consider an HbA1c goal of 53 mmol/mol (<7%) to be appropriate in most individuals with diabetes, use of individualised HbA1c goals are recommended, based on patient preferences, characteristics, comorbidities and risk of adverse events [3–9]. These individualised goals can range from <48 mmol/mol (<6.5%) in individuals with low hypoglycaemia risk and <64 mmol/mol (<8%) in high-risk patients, such as those with a history of severe hypoglycaemia, extensive comorbidities or complications, older individuals or those with long-standing diabetes [3–9].
Self-management is a cornerstone in diabetes care and provision of diabetes education can improve self-management behaviours and glycaemic control [10–13]. Most professional bodies recommend the use of structured diabetes education and support programmes delivered by trained healthcare providers (HCPs), such as nurses, to improve self-management, and that these education programmes should be given at the time of diagnosis [3–9]. According to the International Diabetes Federation guidelines, for every primary care facility, at least one HCP should be trained as a diabetes educator, and these facilities should provide regular, structured group education to support individuals when needed [5].
In anticipation of increasing disease awareness, better care standards and technological advancements, the International Diabetes Management Practices Study (IDMPS) was designed to document and track patient profiles and patterns of care across time in developing countries, where data are limited. The IDMPS is the largest international observational study with the participation of over 6000 physicians from 49 countries across Africa, the Middle East, South Asia, Latin America, Asia and Eurasia. Data were collected using structured case report forms in a series of yearly ‘waves’, with each wave recruiting a different cohort of participants. The first wave of data collection began in 2005 and the most recent wave (wave 7) was completed in 2017. Apart from a standard dataset collected in all waves, each wave had a particular theme, such as understanding factors involved in glycaemic control (wave 1), healthcare resource utilisation (wave 2), barriers to insulin therapy (wave 3), hypoglycaemia (wave 4), symptoms of depression (wave 5), self-management (wave 6) and insulin discontinuation (wave 7) [10, 14–17].
Results from previous waves have demonstrated the low attainment of treatment goals for LDL-cholesterol (<2.6 mmol/l), BP (<130/80 mmHg) and HbA1c (53 mmol/mol [<7%]) with only 25% of participants with type 1 diabetes and 36% of participants with type 2 diabetes achieving HbA1c of 53 mmol/mol (<7%); <8% of participants achieved all three goals [14]. Data analysis from other waves has demonstrated the positive associations of good glycaemic control with patient education and self-monitoring of blood glucose (SMBG) [10], in addition to the positive association of diabetes-related complication rates and increased healthcare resource utilisation [15, 17].
The present analysis of real-world data describes glycaemic goal achievement, therapy use and care management practices in people with type 2 diabetes over time, from the first wave of IDMPS data collection (2005) to the most recent wave (2017).
Methods
Study design and participants
The IDMPS is an ongoing international, multicentre, non-interventional, observational study, which documents current practices in diabetes management. From 2005 to 2017, data were collected in seven individual waves, each wave enrolling different patients and physicians. At each wave, during a 2 week period, participating physicians were asked to enter details of the first five patients with type 1 diabetes (data not shown) and ten patients with type 2 diabetes who made a routine visit, using structured care report forms. Participating physicians included both general practitioners and specialists, providing they had experience of caring for individuals with diabetes and prescribing insulin therapy. Physicians were randomly selected following stratification for specialty. Study design and reporting format are in accordance with the recommended STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines. Ethics approval was obtained from institutional review boards in each country. The study was conducted in accordance with the Declaration of Helsinki. Data from all seven waves are presented herein.
Inclusion/exclusion criteria
Men and women above the lower legal age limit (country-specific) with a clinical diagnosis of type 2 diabetes who provided informed consent were eligible for inclusion. Exclusion criteria included concomitant participation in another clinical study, participation in a previous IDMPS wave and current receipt of temporary insulin therapy due to gestational diabetes, surgery or pancreatic cancer.
Outcome measures
In this analysis, we examined the secular trend of glycaemic control and use of medications (i.e. insulin and/or OGLD) in individuals with type 2 diabetes over a 12 year period in 49 countries across Africa, the Middle East, South Asia, Latin America, Asia and Eurasia.
Statistical analysis
Data collection
Patient information was recorded by the attending physician using individual case report forms during the 2 week study period.
Baseline variables and demographic characteristics
Evaluation variables were recorded by participating physicians and included the proportion of participants attaining recommended and individualised glycaemic goals according to their characteristics and treatments. Due to the non-interventional nature of the survey, no safety data were collected. Spontaneous adverse drug reactions were reported according to country-specific regulations.
Analysis populations
All individuals who fulfilled eligibility criteria and had no missing data concerning the treatment for diabetes defined as OGLD [yes/no] and insulin [yes/no] were included in the eligible population for analysis. Sample size was estimated assuming that insulin is the least prescribed therapy (in terms of proportion) to give an absolute precision of 20% and a CI of 95%. Data were analysed in the overall type 2 diabetes population and stratified by treatment subgroups including OGLD only, OGLD + insulin and insulin only.
Data analyses
Results presented are based on real-world data from all seven waves. Quantitative variables are expressed as mean ± SD and qualitative variables are expressed as numbers and percentages. No imputation was made for missing data, with the exception of age (missing date of birth was set to ‘15’ and missing month of birth was set to ‘June’); missing data were not counted in the percentages. Trend analyses were conducted on all-wave data to assess the significance of changes over time for key variables including last HbA1c measurement, HbA1c goal attainment, current insulin use, BMI and waist circumference recorded at enrolment. Statistical approaches for trends analyses varied depending on the data sets: categorical variables (e.g. HbA1c <53/≥53 mmol/mol [<7/≥7%] or yes/no) were assessed using a two-sided Cochran–Armitage test to investigate the relationship between study waves and the variables of interest, assuming a no-trend null hypothesis; continuous variables were assessed using a least-squares fit value (R2). We used logistic regression models to explore the trend of attainment of HbA1c <53 mmol/mol (<7%) over time across all waves; data were adjusted for age, sex, treatment regimen, disease duration and diabetes education. While an HbA1c goal of <53 mmol/mol (<7%) is considered appropriate for many people with type 2 diabetes, guidelines state the importance of individualised goals, the highest of which is <64 mmol/mol (<8%) [3–9]. Consequently, achievement of HbA1c <64 mmol/mol (<8%) was also assessed. Owing to the heterogeneity of study populations across regions and differences in regional participation across waves, this further analysis included region as an adjustment, in addition to the adjustments used for analysis of HbA1c <53 mmol/mol (<7%), and analyses were performed comparing waves 2–7 individually using wave 1 as a reference. Statistical analyses were performed using SAS (software version 9.4 [2016], SAS Institute, Cary, NC, USA).
Results
Study population and baseline characteristics
Patient population
We included 66,088 individuals with type 2 diabetes from seven waves of data collection; of these, 42,171 (63.8%) received OGLD only, 14,529 (22.0%) received OGLD + insulin and 7566 (11.4%) received insulin only. Patients treated with meal plan/physical activity alone (n = 1685, 2.5%) and those who did not use OGLD, insulin or diet for their diabetes (n = 137, 0.2%) were excluded from this analysis. A full list of participating countries is listed in electronic supplementary material (ESM) Table 1. A total of 49 countries were included in the seven waves of data collection, grouped into regions with the composition of each region varying by wave depending on logistic availability.
Baseline demographics and characteristics
During the 12 year period, the mean age of participants with type 2 diabetes was ~58 years and ~52% were women. Disease duration increased from 8.3 years in wave 1 to 9.8 years in wave 7, accompanied by a rising trend of BMI in the whole group and in the treatment subgroups (data for therapy subgroups not shown). The proportion of participants with hypertension and dyslipidaemia increased significantly over time by 5.3% and 19.6%, respectively (p < 0.0001 for both) (Table 1).
Table 1.
Clinical profiles, care processes and self-monitoring in participants with type 2 diabetes at enrolment between 2005 and 2017
| Characteristic | Wave 1 (2005) N = 9918 |
Wave 2 (2006) N = 17,232 |
Wave 3 (2008) N = 12,210 |
Wave 4 (2010) N = 5343 |
Wave 5 (2011–12) N = 9603 |
Wave 6 (2013–14) N = 5479 |
Wave 7 (2016–17) N = 6303 |
|---|---|---|---|---|---|---|---|
| Age, years (SD) | 58.1 (11.5) | 58.2 (11.8) | 57.7 (11.8) | 58.4 (11.9) | 57.6 (11.2) | 57.3 (10.7) | 57.2 (11.1) |
| Female sex, n (%) | 5130 (51.9) | 8736 (52.2) | 6458 (54.1) | 2882 (54.3) | 5117 (53.3) | 3048 (55.6) | 3291 (52.2) |
| Weight, kg (SD) | 71.6 (14.9) | 75.4 (16.0) | 76.7 (16.2) | 78.9 (16.5) | 80.6 (16.2) | 82.6 (16.7) | 82.0 (16.7) |
| Disease duration, years | 8.3 (7.1) | 8.6 (7.8) | 8.8 (7.7) | 9.1 (8.1) | 8.7 (7.3) | 9.3 (7.1) | 9.8 (7.4) |
| BMI, kg/m2 (SD) | 27.1 (4.8) | 28.5 (5.3) | 29.0 (5.5) | 29.8 (5.5) | 29.6 (5.5) | 30.2 (5.7) | 29.8 (5.5) |
| Hypertension, n (%) | 6029 (60.9) | 10,681 (62.5) | 7422 (61.0) | 3254 (61.1) | 6315 (66.0) | 3623 (66.4) | 4166 (66.2) |
| Dyslipidaemia, n (%) | 4844 (49.3) | 9609 (60.2) | 7267 (62.5) | 3485 (67.2) | 5855 (63.4) | 3449 (64.8) | 3970 (68.9) |
| Last HbA1c measurement, mmol/mol (SD) | 61.5 (85.0) | 63.2 (86.7) | 62.3 (85.8) | 62.6 (86.1) | 64.4 (87.9) | 64.1 (87.7) | 64.6 (88.1) |
| Last HbA1c measurement, % (SD) | 7.8 (1.8) | 7.9 (1.9) | 7.9 (2.0) | 7.9 (1.9) | 8.0 (1.9) | 8.0 (1.8) | 8.1 (1.9) |
| HbA1c testing, n (%) | 6116 (61.8) | 12,492 (76.5) | 9217 (80.4) | 4401 (85.5) | 8399 (90.0) | 4952 (92.1) | 5719 (92.9) |
| Frequency of testing of HbA1c during past year | 1.6 (1.2) | 2.2 (1.4) | 2.3 (1.4) | 2.2 (1.5) | 2.2 (1.8) | 2.2 (2.0) | 2.2 (1.7) |
Mean values are presented unless otherwise stated
Percentages were calculated for patients with available data; these varied by each category/wave
Hypertension and dyslipidaemia were defined (yes/no) according to the attending physician
Achievement of glycaemic goal
Mean HbA1c varied by therapy type, with lower values in individuals on OGLD only compared with those treated with OGLD + insulin or insulin only (ESM Table 2). Last mean HbA1c measurement increased slightly from 7.8% in wave 1 to 8.1% in wave 7 for the overall population, although this increase was not clinically meaningful. Similar slight increases were shown across the different therapy groups.
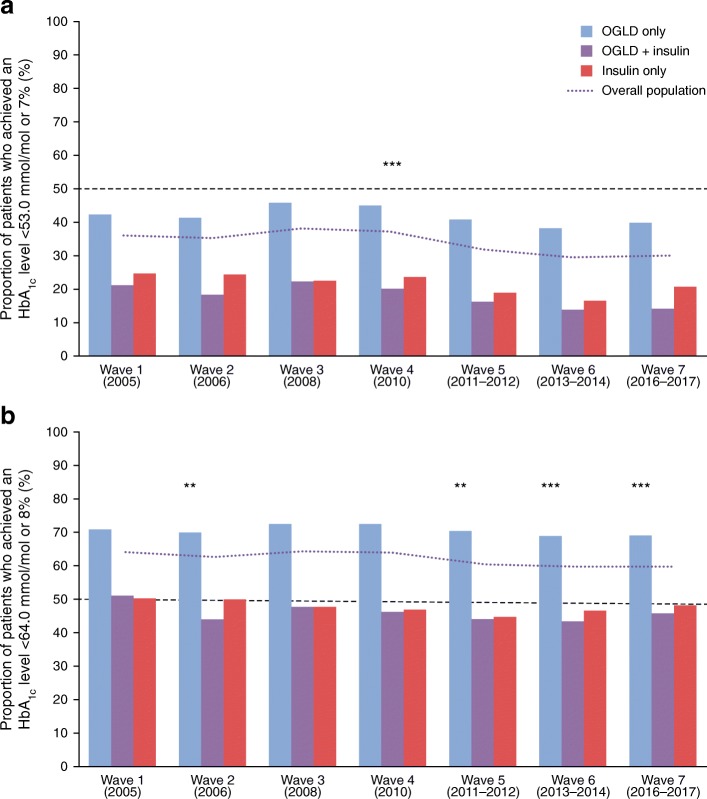
Overall, <50% of participants attained HbA1c goal (<53 mmol/mol [<7%]) in any wave, irrespective of therapy subgroup (Fig. 1); no difference in glycaemic control was seen between patients treated by general practitioners and patients treated by specialists (data not shown). Among participants treated with insulin, <30% achieved HbA1c <53 mmol/mol (<7%), regardless of whether they received insulin alone or in combination with OGLD (Fig. 1a). Trends analyses showed a significant decline in HbA1c <53 mmol/mol (<7%) goal achievement in the overall population (p < 0.0001) and treatment subgroups (p < 0.0001 for all).
Fig. 1.
The proportion of participants attaining HbA1c goal defined as: (a) <53 mmol/mol or <7%; and (b) <64 mmol/mol or <8%, between 2005 and 2017. The p values show test of significance for trend in HbA1c goal achievement in the overall population: (a) over all waves; or (b) waves 2–7 vs reference wave 1. **p < 0.01, ***p < 0.001. A two-sided Cochran–Armitage test was used to investigate the relationship between study waves and the variables of interest, assuming a no-trend null hypothesis: (a) p < 0.0001 for trend over all waves; (b) p = 0.0036 for wave 2 vs wave 1, p = 0.2991 for wave 3 vs wave 1, p = 0.0514 for wave 4 vs wave 1, p = 0.0011 for wave 5 vs wave 1, p = 0.0006 for wave 6 vs wave 1 and p = 0.0017 for wave 7 vs wave 1. HbA1c goal achievement data were missing for 3893 participants in wave 1, 5084 participants in wave 2, 3150 participants in wave 3, 961 participants in wave 4, 1256 participants in wave 5, 548 participants in wave 6 and 608 participants in wave 7
Using HbA1c ≤64 mmol/mol (≤8%) as an alternative goal, the adjusted OR of attaining HbA1c goal (using wave 1 as reference) was significantly lower in waves 2, 5, 6 and 7 (p < 0.05 for all; Fig. 1b). Female participants, those with disease duration >10 years and individuals living in East Europe/Eurasia were less likely to attain HbA1c ≤ 64 mmol/mol (≤8%) (Table 2). Age was also a factor in achievement of this higher glycaemic goal, with individuals aged >40 years being significantly more likely to achieve it compared with participants aged ≤40 years; the likelihood of achieving HbA1c ≤64 mmol/mol (≤8%) increased for each decade over the age of 40 years (Table 2). Proportions of participants achieving various HbA1c goals in each wave are shown in ESM Table 3.
Table 2.
Logistic regression analysis of glycaemic goal achievement defined as HbA1c ≤64 mmol/mol (≤8%)
| Comparison | p value | OR (95% CI) |
|---|---|---|
| Wavea | ||
| Wave 2 vs wave 1 | 0.0036 | 0.90 (0.84, 0.97) |
| Wave 3 vs wave 1 | 0.2991 | 0.96 (0.90, 1.04) |
| Wave 4 vs wave 1 | 0.0514 | 0.92 (0.84, 1.00) |
| Wave 5 vs wave 1 | 0.0011 | 0.89 (0.83, 0.95) |
| Wave 6 vs wave 1 | 0.0006 | 0.87 (0.80, 0.94) |
| Wave 7 vs wave 1 | 0.0017 | 0.89 (0.82, 0.96) |
| Age (years) | ||
| 40–50 vs ≤40 | 0.0161 | 1.11 (1.02, 1.21) |
| 50–60 vs ≤40 | <0.0001 | 1.22 (1.13, 1.33) |
| 60–70 vs ≤40 | <0.0001 | 1.59 (1.46, 1.72) |
| >70 vs ≤40 | <0.0001 | 2.08 (1.89, 2.28) |
| Men vs women | <0.0001 | 1.10 (1.06, 1.14) |
| Time since diagnosis ≤10 vs >10 years | <0.0001 | 1.80 (1.73, 1.88) |
| Region | ||
| Africa/Asia vs Eurasia/East Europe | <0.0001 | 1.26 (1.19, 1.33) |
| Middle East/Latin America vs Eurasia/East Europe | <0.0001 | 1.39 (1.32, 1.47) |
aHbA1c goal achievement data were missing for 3893 participants in wave 1, 5084 participants in wave 2, 3150 participants in wave 3, 961 participants in wave 4, 1256 participants in wave 5, 548 participants in wave 6 and 608 participants in wave 7
OGLD therapy
Overall, >80% of participants were treated with OGLD with or without insulin (Table 3). The use of sulfonylurea monotherapy decreased over time, while use of metformin monotherapy increased. Overall, use of other types of OGLD was low (14.5–20.0% of participants; Table 3). Metformin was the most commonly used monotherapy, while sulfonylureas and DPP-4is were often second- or third-line OGLD (ESM Table 4).
Table 3.
Distribution of use of OGLD in participants with type 2 diabetes between 2005 and 2017
| Characteristic | Wave 1 (2005) N = 9918 |
Wave 2 (2006) N = 17,232 |
Wave 3 (2008) N = 12,210 |
Wave 4 (2010) N = 5343 |
Wave 5 (2011–12) N = 9603 |
Wave 6 (2013–14) N = 5479 |
Wave 7 (2016–17) N = 6303 |
|---|---|---|---|---|---|---|---|
| Proportion of participants treated with OGLD, % | 81.0 | 84.9 | 87.1 | 91.8 | 85.6 | 87.0 | 88.4 |
| Number of OGLD treatments received | |||||||
| 1 OGLD therapy, n (%) | 3527 (35.7) | 6353 (36.9) | 4293 (36.0) | 2304 (43.6) | 4542 (47.3) | 1876 (34.2) | 2125 (34.6) |
| >1 OGLD therapy, n (%) | 4478 (45.3) | 8248 (47.9) | 6062 (50.8) | 2536 (48.0) | 3672 (38.2) | 2890 (52.8) | 3295 (53.6) |
| Type of OGLD treatment receiveda | |||||||
| Metformin alone, n (%) | – | 3258 (18.9) | 2517 (21.1) | 1246 (23.6) | 2342 (24.4) | 1459 (26.6) | 1638 (26.7) |
| Sulfonylureas alone, n (%) | – | 2371 (13.8) | 1331 (11.2) | 307 (5.8) | 714 (7.4) | 306 (5.6) | 257 (4.2) |
| Metformin + sulfonylureas, n (%) | – | 6478 (37.6) | 4726 (39.6) | 2195 (41.6) | 3749 (39.0) | 2199 (40.1) | 2281 (37.2) |
| Other, n (%)b | – | 2494 (14.5) | 1781 (14.9) | 1092 (20.7) | 1409 (14.7) | 802 (14.6) | 1223 (20.0) |
Percentages were calculated for patients with available data; these varied by each category/wave
aData not available for wave 1
bDetailed information on ‘Other’ therapies is available in ESM Table 4
Insulin therapy
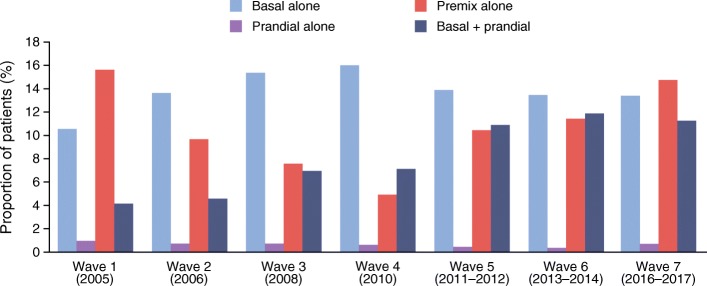
The proportion of participants treated with insulin increased from 32.8% in wave 1 to 41.2% in wave 7 (p < 0.0001; Table 4). Most participants received either basal insulin alone or premix insulin alone in wave 1 with a similar pattern observed in wave 7. The use of premix insulin declined from wave 1 to 4 and increased again from waves 4 to 7, while the use of basal + prandial insulin has increased almost threefold over time (Fig. 2/ESM Fig. 1). Analysis of insulin type (human vs analogue; waves 6 and 7 only; ESM Table 5) revealed that use of long-acting basal insulin analogues increased between waves 6 and 7, but human intermediate-acting insulin was still used by 24.4% of participants receiving basal insulin in wave 7. Approximately 50% of participants using prandial insulin used short-acting analogues in waves 6 and 7 and 61.0% of those using premix insulin received human insulin, although this proportion decreased to 57.8% by wave 7.
Table 4.
Distribution of insulin regimens and insulin dose in participants with type 2 diabetes between 2005 and 2017
| Characteristic | Wave 1 (2005) N = 9918 |
Wave 2 (2006) N = 17,232 |
Wave 3 (2008) N = 12,210 |
Wave 4 (2010) N = 5343 |
Wave 5 (2011–12) N = 9603 |
Wave 6 (2013–14) N = 5479 |
Wave 7 (2016–17) N = 6303 |
|---|---|---|---|---|---|---|---|
| Proportion of participants treated with insulin, % | 32.8 | 29.8 | 31.5 | 31.8 | 36.7 | 37.9 | 41.2 |
| Time to initiation of insulin treatment, years | 8.4 (6.9) | 9.3 (7.5) | 9.6 (7.6) | 10.0 (7.8) | 8.4 (6.8) | 8.4 (6.4) | 8.3 (6.6) |
| Time on insulin treatment, years | 5.8 (5.1) | 5.0 (4.7) | 3.5 (4.4) | 3.5 (4.3) | 3.8 (4.4) | 4.5 (4.7) | 4.7 (4.8) |
| Daily insulin dose, U | |||||||
| Basal alone | 26.6 (14.9) | 28.6 (16.9) | 28.2 (16.1) | 26.3 (13.7) | 25.5 (13.4) | 26.7 (13.8) | 26.4 (15.6) |
| Prandial alone | 35.6 (19.4) | 36.1 (23.8) | 30.0 (17.4) | 33.6 (21.8) | 28.8 (17.7) | 24.2 (16.0) | 38.9 (35.8) |
| Premix alone | 36.6 (17.0) | 42.1 (20.2) | 44.1 (20.8) | 48.8 (21.8) | 42.4 (19.9) | 44.6 (22.4) | 44.9 (24.7) |
| Basal + prandial | 49.9 (23.1) | 56.6 (27.4) | 56.2 (27.8) | 57.2 (27.3) | 56.9 (28.6) | 62.2 (25.8) | 64.0 (28.9) |
| Daily insulin dose (weight-adjusted), U/kg | |||||||
| Basal alone | 0.39 (0.21) | 0.39 (0.23) | 0.38 (0.21) | 0.36 (0.19) | 0.32 (0.16) | 0.33 (0.16) | 0.33 (0.19) |
| Prandial alone | 0.54 (0.29) | 0.50 (0.31) | 0.40 (0.22) | 0.40 (0.22) | 0.40 (0.27) | 0.31 (0.25) | 0.47 (0.32) |
| Premix alone | 0.53 (0.24) | 0.56 (0.25) | 0.59 (0.27) | 0.62 (0.26) | 0.54 (0.25) | 0.55 (0.24) | 0.56 (0.28) |
| Basal + prandial | 0.70 (0.34) | 0.73 (0.34) | 0.73 (0.33) | 0.72 (0.34) | 0.69 (0.32) | 0.74 (0.30) | 0.77 (0.33) |
Values are presented as mean (SD) unless otherwise stated. Percentages were calculated for patients with available data; these varied by each category/wave
Fig. 2.
Changes in use of insulin regimens in type 2 diabetes between 2005 and 2017
The mean time to insulin initiation in the overall type 2 diabetes population was >8 years across all waves and remained stable over time (Table 4). Similar results were seen when assessing the insulin only and insulin + OGLD therapy subgroups (data not shown). The time on insulin treatment prior to study inclusion declined over time in all individuals treated with insulin (Table 4), especially in the OGLD + insulin subgroup (data not shown). The mean daily dose of insulin increased in all insulin regimens except for basal. The mean daily dose for the overall insulin-treated population, adjusted for body weight (U/kg), increased for premix insulin alone and basal + prandial but decreased for basal alone and prandial alone (Table 4).
Blood glucose monitoring and diabetes education
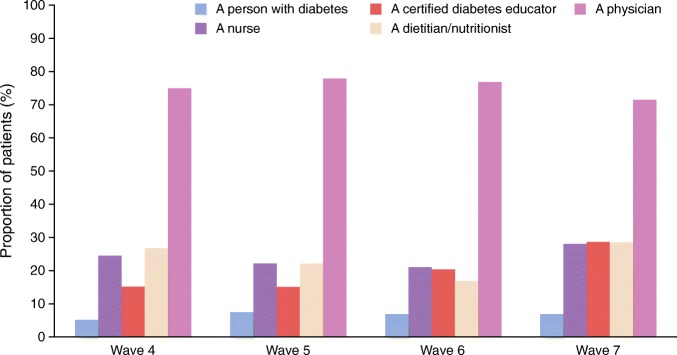
The proportion of individuals who had HbA1c testing increased from wave 1 (61.8%) to wave 7 (92.9%), with screening typically occurring twice a year (Table 1/ESM Table 2). Participants treated with insulin were increasingly likely over time to own a glucose meter, but this was not reflected in the performance of SMBG; cost was increasingly cited as a limiting factor for regular SMBG testing (ESM Table 6). The overall proportion of participants receiving diabetes education increased over time, irrespective of therapy subgroup (ESM Table 7). However, based on data from waves 4–7, education was mainly provided on an individual basis by physicians with very few individuals attending structured diabetes education courses (Fig. 3).
Fig. 3.
Distribution of sources of education in participants who received diabetes-related education. Data on the provider of diabetes education were not captured in waves 1–3
Discussion
Glycaemic control in developing countries has been persistently poor over the past 12 years and is growing steadily worse, based on real-world data captured in this large international observational study involving over 66,000 individuals with type 2 diabetes. This situation has developed despite multiple advances in the field of diabetes management, including the development of new medications proven to improve diabetes control and clinical outcomes [18].
Most participants in this study were aged 40–65 with 8–9 years of diabetes duration, with over 80% of participants receiving OGLD. While the use of sulfonylurea monotherapy declined over time, use of metformin monotherapy increased over time and metformin was the most commonly used monotherapy, in line with guideline recommendations [3–9]. Few participants received newer agents, such as SGLT2 inhibitors, although it should be noted that these therapies were approved relatively recently in many developing countries; as such, data on their use are only available for wave 7. The proportion of individuals receiving insulin also increased over time, with use of basal + prandial insulin showing a marked increase; despite this rise, however, there was no improvement in glycaemic control. Newer insulin analogue therapies can provide clinical benefits in terms of reduced glycaemic variability or hypoglycaemia risk [19], which may facilitate individuals to achieve glycaemic control; however, in developing countries, these analogues may not be readily available due to issues of access or cost. Data from wave 7 highlighted that a substantial proportion of participants still received human intermediate-acting insulins (24.4%), although long-acting analogue use increased between waves 6 and 7. Participants receiving prandial insulin were mainly using human regular insulins or premix insulins.
Overall, <50% of participants achieved a glycaemic goal of HbA1c <53 mmol/mol (<7%), and <70% achieved HbA1c <64 mmol/mol (8%). Furthermore, only 15–25% or 45–50% of participants treated with insulin achieved either the HbA1c <53 mmol/mol (<7%) goal or the HbA1c <64 mmol/mol (8%) goals, respectively. These results might be ascribed to a combination of delayed and inappropriate insulin regimen prescription. Logistic regression analysis was used to confirm the declining trend of HbA1c <64 mmol/mol (<8%) goal attainment over time. The odds of goal attainment compared with wave 1 were significantly lower for waves 2, 5, 6 and 7 (p < 0.05 for all). Other patient groups (including younger individuals [≤40 years], women, those with longer disease duration [>10 years] and those from Europe/Eurasia) were also less likely to achieve the HbA1c goal compared with their counterparts. Visits to general practitioners or specialists did not seem to influence achievement of glycaemic goal; any potential difference may have been attenuated by study selection criteria requiring all physicians to have prior experience in prescribing insulin. Additionally, individuals with poor glycaemic control or advanced disease progression might tend to seek specialist care.
Although there was an increase in the proportion of individuals receiving diabetes education from physicians, very few received structured diabetes education courses delivered by nurses, dietitians or certified diabetes instructors. This may potentially result in a lack of sufficient contact time to help individuals deal with day-to-day concerns.
These original findings from developing countries concur with other reports based on IDMPS data [14, 20], and are in line with data from developed countries indicating poor rates of glycaemic goal attainment (<53 mmol/mol [<7%]; ~20–40%) [21, 22], indicating that the challenge of attaining good glycaemic control is universal. It is concerning that glycaemic control remains poor, given the increased use of insulin over time; it should be noted that the insulin dose of 0.3–0.7 U/kg was comparable if not higher than that used in clinical trial settings [23–25]. In this survey, the mean time to insulin initiation was 8 years, similar to that reported in developed countries [26, 27].
Considering that >50% of participants in this study receiving OGLD and/or insulin treatment displayed an HbA1c value >53 mmol/mol (>7%), this is a clear indicator that the initiation and intensification of insulin remain a major barrier in real-world practice. The discrepancy between doses prescribed and glycaemic control suggests possible patient non-adherence, although this was not formally tested. In this regard, quality improvement programmes implemented at a system level have been shown to improve control of cardiometabolic risk factors and clinical outcomes in community settings; such programmes include multidisciplinary care (with training programmes for physicians and nurses), risk-stratified care planning and regular structured/scheduled assessment of metabolic control and vascular complications [28–31]. The provision of professional diabetes education for HCPs starting in medical colleges, together with postgraduate training, is critically important to build capacity to help HCPs in educating patients and improving care. An alternative (ideally complementary) approach would be to make changes to the practice environment to provide more integrated multidisciplinary care, to ultimately provide sustained improvements in care outcomes. Such an approach would be conducive to promoting trustworthy relationships and communication between patients and HCPs, and should encompass empathetic listening, tuition of self-management skills and ongoing support [32].
According to professional guidelines, individuals with diabetes should receive structured diabetes education and support programmes [3, 5]; such programmes have been shown to improve glycaemic control, as reported in previous waves of the IDMPS [10]. In this study, over 70% of physicians reported having offered education to their patients; however, physicians are often time-poor in comparison with other HCPs (e.g. nurses, dietitians or certified diabetes instructors), and time is a major factor for a successful diabetes education programme. A meta-analysis of the efficacy of self-management education on glycaemic control in individuals with type 2 diabetes reported that approximately 24 h of contact time (e.g. face to face visits, phone calls) with HCPs are needed to sustain a 1% reduction in HbA1c over a 12 month period [33]. There are also grounds to argue that regular follow-ups are needed, as results from a recent meta-review indicate that post-intervention improvements in HbA1c persist until 6 months but tend to attenuate after 12 or 24 months [34]. Consultation times may also be inadequate, perhaps partly due to the pressures of the increasing global population of people with diabetes which may increase the number of patients each physician treats. A recent meta-analysis of primary care physician consultation time (spanning developed and developing countries) reported that consultation time was less than 5 min in 18 of the included countries, accounting for approximately 50% of the global population, with consultation length proportional to per capita health spending [35]. Such data as these indicate a strong need for additional support to engage patients and emphasise the importance of time spent talking to individuals about their needs and concerns. However, in our survey, <20% of participants received a structured educational diabetes programme from any source, which could be a contributing factor to the poor glycaemic goal achievement observed herein. We suggest that promising methods to improve glycaemic control would include training of nurses and dietitians in the provision of diabetes education, a general increase in the number of certified diabetes instructors and the delivery of such education through structured programmes.
The affordability and ownership of blood glucose monitoring accessories (e.g. blood glucose monitors and test strips) are important factors for improving self-management. In this study, an increasing proportion of participants possessed SMBG monitors over time, but there was also a rise in the number of individuals citing cost as a limiting factor for regular SMBG; in those treated with insulin, where a greater frequency of SMBG is needed, the high cost of strips may be a deterrent. Considered together, clinical inertia, insufficient access to structured diabetes education, infrequent SMBG and high cost of monitoring accessories/medications may all contribute to persistently poor glycaemic control in individuals with type 2 diabetes. A combination of factors ascribed to patients (e.g. fear of hypoglycaemia/injections, complex treatment regimen, polypharmacy), physicians (e.g. poor/ineffective communication, insufficient knowledge and support) or healthcare systems (e.g. lack of time/resource for physicians, lack of tools for patient/physician to monitor insulin titration, lack of medical coverage), may be particularly relevant in developing countries where development of infrastructure and capacity cannot cope with the rapid rate of increase in diabetes [36, 37]. Other patient-related factors, such as older age, higher education level and short disease duration, have also been reported to be associated with improved glycaemic control [38, 39]; it is possible that lifestyle factors, acceptance of diagnosis and adherence may all contribute in these cases.
The present study has some limitations. The cross-sectional nature of the survey provides a ‘snapshot’ of practice at any one time; therefore, these observations only allow us to form hypotheses, and cannot infer causality. The self-selecting nature of the patient population should be considered, as those with the poorest control are most likely to visit the clinic; therefore, glycaemic control in the general population may be higher than that shown here. All data were completed by the attending physicians with potential bias in interpretation and recall. Due to the pragmatic nature of the survey, no adjudication was conducted for the reported complications. The varying degrees of local support in implementing the survey also mean that different countries/regions were included/excluded in different waves. These variables add to the heterogeneity of practice, although we have adjusted for major variables (notably age, sex, disease duration and region) when analysing secular trends. Despite these limitations, we believe that aspects of this study such as its large, global population, long duration (12 years) and the practice of structured data collection using predefined variables have provided valuable real-world evidence from areas hit hardest by the diabetes epidemic, where data are lacking to inform practice and policies.
In conclusion, in this 12 year study, poor and worsening glycaemic control was observed in individuals with type 2 diabetes. These real-world data highlight an urgent need for improvement in practice environments, workflow and team structure; such amendments would allow early assessment of patients and identification of unmet needs, thus empowering individuals to improve self-management and consequently glycaemic control. These changes will need to be supplemented by institutional support through capacity building, and policies that promote good diabetes care; these would ideally encompass improved accessibility to and affordability of medications and monitoring accessories, in both developing countries and subpopulations of patients with poor literacy/low incomes in developed countries [5, 40]. Altogether, these could lead to a successful improvement in diabetes care and clinical outcomes in individuals with type 2 diabetes living in developing countries.
Electronic supplementary material
(PDF 277 kb)
Acknowledgements
The authors received editorial/writing support in the preparation of this manuscript provided by H. Mourad-Agha of Fishawack Communications Ltd., funded by Sanofi, Paris, France. Coordination of the development of this manuscript and assistance with the revision was provided by C. Baradez, at Sanofi. We would like to acknowledge all the participants and physicians who have participated in this study over the past 14 years.
Abbreviations
- DPP-4i
Dipeptidyl peptidase 4 inhibitor
- HCP
Healthcare provider
- IDMPS
International Diabetes Management Practices Study
- OGLD
Oral glucose-lowering drugs
- SGLT
Sodium–glucose cotransporter
- SMBG
Self-monitoring of blood glucose
Contribution statement
All authors contributed to the study concept and design, data analysis and interpretation, drafting and critical revision of the manuscript. PA (lead author and guarantor) accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish. All authors approved the final version of the manuscript for publication. The manuscript was drafted by H. Mourad-Agha. Coordination of the development of this manuscript and assistance with the revision was provided by C. Baradez.
Funding
This observational study was sponsored by Sanofi, as was the editorial/writing support detailed above. The study funder (Sanofi) was involved in study design and data collection and provided funding for data analysis and medical writing assistance.
Data availability
Qualified researchers may request access to related study documents, which will be redacted to protect the privacy of study participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
Duality of interest
JMC is an employee of Sanofi. All other authors are members of the IDMPS Steering Committee and have received honoraria and travelling sponsorships in relation to the IDMPS.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/6/2020
Unfortunately, the standard deviations for ‘last HbA<Subscript>1c</Subscript> measurement’ in mmol/mol were miscalculated in Table 1 of this paper.
Change history
3/6/2020
Unfortunately, the standard deviations for ���last HbA1c measurement��� in mmol/mol were miscalculated in Table 1 of this paper.
References
- 1.International Diabetes Federation (2019) IDF Diabetes Atlas, 9th edition 2017. Available from https://www.diabetesatlas.org/en/
- 2.World Health Organization (2016) Global Report on Diabetes. Available from https://www.who.int/diabetes/global-report/en/
- 3.American Diabetes Association Standards of Medical Care in Diabetes–2019. Diabetes Care. 2019;42(Suppl 1):S1–S193. [Google Scholar]
- 4.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61(12):2461–2498. doi: 10.1007/s00125-018-4729-5. [DOI] [PubMed] [Google Scholar]
- 5.International Diabetes Federation (2017) Clinical practice recommendations for managing type 2 diabetes in primary care. Available from https://www.idf.org/component/attachments/attachments.html?id=1270&task=download. Accessed 30 July 2019
- 6.Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38(Suppl 1):1–115. doi: 10.1007/s13410-018-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SEMDSA Type 2 Diabetes Guidelines Expert Committee SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. J Endocrinol Metab Diabetes S Afr. 2017;22(1):S1–S196. [Google Scholar]
- 8.Guzman JR, Lyra R, Aguilar-Salinas CA, et al. Treatment of type 2 diabetes in Latin America: a Consensus statement by the medical associations of 17 Latin American countries. Latin American Diabetes Association. Rev Panam Salud Publica. 2010;28(6):463–471. doi: 10.1590/s1020-49892010001200008. [DOI] [PubMed] [Google Scholar]
- 9.Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32(5):442–458. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gagliardino JJ, Aschner P, Baik SH, et al. Patients’ education, and its impact on care outcomes, resource consumption and working conditions: data from the International Diabetes Management Practices Study (IDMPS) Diabetes Metab. 2012;38(2):128–134. doi: 10.1016/j.diabet.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Gagliardino JJ, Lapertosa S, Pfirter G, et al. Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR) Diabet Med. 2013;30(9):1102–1111. doi: 10.1111/dme.12230. [DOI] [PubMed] [Google Scholar]
- 12.Trento M, Gamba S, Gentile L, et al. Rethink Organization to iMprove Education and Outcomes (ROMEO): a multicenter randomized trial of lifestyle intervention by group care to manage type 2 diabetes. Diabetes Care. 2010;33(4):745–747. doi: 10.2337/dc09-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samann A, Muhlhauser I, Bender R, Kloos C, Muller UA. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia. 2005;48(10):1965–1970. doi: 10.1007/s00125-005-1905-1. [DOI] [PubMed] [Google Scholar]
- 14.Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS) Diabetes Care. 2009;32(2):227–233. doi: 10.2337/dc08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringborg A, Cropet C, Jonsson B, Gagliardino JJ, Ramachandran A, Lindgren P. Resource use associated with type 2 diabetes in Asia, Latin America, the Middle East and Africa: results from the International Diabetes Management Practices Study (IDMPS) Int J Clin Pract. 2009;63(7):997–1007. doi: 10.1111/j.1742-1241.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- 16.Casciano R, Malangone E, Ramachandran A, Gagliardino JJ. A quantitative assessment of patient barriers to insulin. Int J Clin Pract. 2011;65(4):408–414. doi: 10.1111/j.1742-1241.2010.02590.x. [DOI] [PubMed] [Google Scholar]
- 17.Gagliardino JJ, Atanasov PK, Chan JC, et al. Resource use associated with type 2 diabetes in Africa, the Middle East, South Asia, Eurasia and Turkey: results from the International Diabetes Management Practice Study (IDMPS) BMJ Open Diabetes Res Care. 2017;5(1):e000297. doi: 10.1136/bmjdrc-2016-000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 19.Tibaldi JM. Evolution of insulin: from human to analog. Am J Med. 2014;127(10):S25–S38. doi: 10.1016/j.amjmed.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Gagliardino JJ, Elgart J, Lujan F, Querzoli I, Chantelot JM. Type 2 diabetes: prescription patterns and treatment outcomes of IDMPS survey in Argentina. Diabetes Res Clin Pract. 2019;153:86–92. doi: 10.1016/j.diabres.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 22.Mannucci E, Monami M, Dicembrini I, Piselli A, Porta M. Achieving HbA1c targets in clinical trials and in the real world: a systematic review and meta-analysis. J Endocrinol Investig. 2014;37(5):477–495. doi: 10.1007/s40618-014-0069-6. [DOI] [PubMed] [Google Scholar]
- 23.Aschner P, Chan J, Owens DR, et al. Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet. 2012;379(9833):2262–2269. doi: 10.1016/S0140-6736(12)60439-5. [DOI] [PubMed] [Google Scholar]
- 24.Owens DR, Traylor L, Dain M-P, Landgraf W. Efficacy and safety of basal insulin glargine 12 and 24 weeks after initiation in persons with type 2 diabetes: a pooled analysis of data from treatment arms of 15 treat-to-target randomised controlled trials. Diabetes Res Clin Pract. 2014;106(2):264–274. doi: 10.1016/j.diabres.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Yki-Järvinen H, Kotronen A. Is there evidence to support use of premixed or prandial insulin regimens in insulin-naive or previously insulin-treated type 2 diabetic patients? Diabetes Care. 2013;36(Suppl 2):S205–S211. doi: 10.2337/dcS13-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khunti K, Damci T, Meneghini L, Pan CY, Yale JF, Solve Study Group Study of Once Daily Levemir (SOLVE): insights into the timing of insulin initiation in people with poorly controlled type 2 diabetes in routine clinical practice. Diabetes Obes Metab. 2012;14(7):654–661. doi: 10.1111/j.1463-1326.2012.01602.x. [DOI] [PubMed] [Google Scholar]
- 27.Khunti K, Nikolajsen A, Thorsted BL, Andersen M, Davies MJ, Paul SK. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409. doi: 10.1111/dom.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luk AOY, Hui EMT, Sin MC, et al. Declining trends of cardiovascular-renal complications and mortality in type 2 diabetes: the Hong Kong Diabetes Database. Diabetes Care. 2017;40(7):928–935. doi: 10.2337/dc16-2354. [DOI] [PubMed] [Google Scholar]
- 29.Prestes M, Gayarre MA, Elgart JF, et al. Multistrategic approach to improve quality of care of people with diabetes at the primary care level: study design and baseline data. Prim Care Diabetes. 2017;11(2):193–200. doi: 10.1016/j.pcd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Prestes M, Gayarre MA, Elgart JF, et al. Improving diabetes care at primary care level with a multistrategic approach: results of the DIAPREM programme. Acta Diabetol. 2017;54(9):853–861. doi: 10.1007/s00592-017-1016-8. [DOI] [PubMed] [Google Scholar]
- 31.Chan Juliana C.N., Lim Lee-Ling, Luk Andrea O.Y., Ozaki Risa, Kong Alice P.S., Ma Ronald C.W., So Wing-Yee, Lo Su-Vui. From Hong Kong Diabetes Register to JADE Program to RAMP-DM for Data-Driven Actions. Diabetes Care. 2019;42(11):2022–2031. doi: 10.2337/dci19-0003. [DOI] [PubMed] [Google Scholar]
- 32.Lim LL, Lau ESH, Kong APS, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care. 2018;41(6):1312–1320. doi: 10.2337/dc17-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25(7):1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 34.Captieux M, Pearce G, Parke HL, et al. Supported self-management for people with type 2 diabetes: a meta-review of quantitative systematic reviews. BMJ Open. 2018;8(12):e024262. doi: 10.1136/bmjopen-2018-024262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irving G, Neves AL, Dambha-Miller H, et al. International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open. 2017;7(10):e017902. doi: 10.1136/bmjopen-2017-017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488–496. doi: 10.1111/dom.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: results of a quantitative survey. Diabetes Obes Metab. 2018;20(2):301–308. doi: 10.1111/dom.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardino JJ, Elgart J, Forti L, Guaita MS, Chantelot JM. Treat-to-target HbA1c and lipid profile to prolong β-cell mass/function and optimize treatment goal attainment. Diabetes Metab Res Rev. 2019;35(6):e3166. doi: 10.1002/dmrr.3166. [DOI] [PubMed] [Google Scholar]
- 39.Kazemian Pooyan, Shebl Fatma M., McCann Nicole, Walensky Rochelle P., Wexler Deborah J. Evaluation of the Cascade of Diabetes Care in the United States, 2005-2016. JAMA Internal Medicine. 2019;179(10):1376. doi: 10.1001/jamainternmed.2019.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saydah S, Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep. 2010;125(3):377–388. doi: 10.1177/003335491012500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 277 kb)
Data Availability Statement
Qualified researchers may request access to related study documents, which will be redacted to protect the privacy of study participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.