Abstract
The oil yield, fatty acid (FA) composition, physicochemical, quality characteristics and thermal properties were studied in flax, perilla, and basil seed oils cultivated in Iran. Also the similarities and differences among these seed oils were investigated using principal component analysis (PCA). The results indicated that perilla seed oil contained the highest lipid content followed by flax and basil seed oils. The n-6/n-3 FA ratios of these oils had a range of 0.190–0.320, which was notably lower than those of most vegetable oils. Trilinolenin as the predominant triacylglycerol in the studied flax, perilla, and basil seed oils was found at 21.3, 32.0, and 27.5%, respectively. The bioactive compounds, namely tocols, phytosterols, and total phenolics, present in basil and perilla oils were higher than those of flax seed oil. The results of differential scanning calorimeter indicated that the thermal properties of these seed oils were varied, with lower melting and crystallization peak temperature for perilla and basil seed oils. The results of PCA showed that these seed oils could be distinguished using some components however, C14:0, C16:0, C18:3, UFA and ECN 42 could not be used to discriminate among these seed oils. The results were suggestive of the proper nutritional qualities of the studied oils and their possibly being the potential sources of FAs for enriching the diets with α-linolenic acid and other functional compounds.
Electronic supplementary material
The online version of this article (10.1007/s13197-019-04158-x) contains supplementary material, which is available to authorized users.
Keywords: Omega-3 FA, Tocols, Phytosterols, Total phenolic, Oxidative stability, Thermal properties
Introduction
The relationships between functional food ingredients, highly nutritious diets and good health have been evidenced in a variety of studies (Abuzaytoun and Shahidi 2006; Choo et al. 2007; Goyal et al. 2014). Health benefits of n-3 polyunsaturated fatty acids (PUFAs), namely α-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), are well known for their cardio-protection, neurological development, and anti-inflammatory properties (Choo et al. 2007). However, consumption of n-3 fatty acids (FAs) has decreased to one-sixth of traditional intakes while n-6 intake has greatly increased due to greater consumption of processed foods, margarine and vegetable oils (e.g. sunflower, corn oils), which together has resulted in a n-6/n-3 FA ratio of up to 20:l rather than a healthy ratio of 1:1 to 2:1 (Umesha et al. 2015). Studies have indicated that higher intakes of n-6 FAs shift physiological status to prothrombotic and pro-aggregation characterized by increased blood viscosity and vasospasm as well as vasoconstriction and reductions in bleeding time (Abuzaytoun and Shahidi 2006; Choo et al. 2007; Goyal et al. 2014). Therefore, they must be supplied through diet or dietary supplements. Although fish oils are currently the main industrial sources of PUFAs, they are neither suitable for vegetarians; and also non-vegetarians have been found to have limited intakes of n-3 FAs coming from fatty fish due to their religious practices besides the high costs, availabilities, and heavy metal toxicities of the mentioned FAs (Goyal et al. 2014; Kim et al. 2014). Accordingly, many food manufactures and research organizations are interested in alternative plant-based PUFA resources to meet the increased consumer demand of PUFA rich oils.
Currently, flax (Linum usitatissimum, Linaceae family) is cultivated in more than 50 countries, although it is produced mainly in Canada (Choo et al. 2007; Goyal et al. 2014). Due to its health benefits and disease prevention properties, flax seed has a major role in the fields of diets and diseases (Abuzaytoun and Shahidi 2006; Choo et al. 2007; Goyal et al. 2014). This seed consists of nearly 36–40% oil in which ALA contains more than half of its composition (Abuzaytoun and Shahidi 2006; Choo et al. 2007; Goyal et al. 2014; Kim et al. 2014).
Perilla (Perilla frutescens, Lamiaceae family) is a plant native to East Asian countries. This seed is considered as a food supply and natural medicine resource. Perilla seeds contain approximately 30–45% oil and has provided a good source of ALA (50–64%) ingredients (Longvah et al. 2000).
Basil (Ocimum basilicum L.) can be found in the tropical regions of Asia, Africa, and Central and South America (Craig 1999; Liangli et al. 2005). Also, it is one of the pharmaceutical plants that is produced and used in high quantities. Basil seed oil contains almost 57–63% of ALA (Craig 1999; Liangli et al. 2005).
The above-mentioned studies reported the physicochemical properties of flax, perilla and basil seed oils cultivated outside of Iran. Also, the thermal properties of the seed oils are significant parameters for their processing and usage. To the best of our knowledge, the chemical characterization, bioactive compounds and thermal properties of Iranian seed oils, as well as distinguishing between them have never been studied before.
The main objective of this study was to investigate the physicochemical, identity characteristics, as well as thermal properties (such as melting and crystallization behavior) of flax, perilla and basil seed oils and this study also aimed to determine the oxidative stability of these seed oils and correlate it with their compositions, and explore the similarities and differences among these seed oils in terms of FA, ECN, tocols, sterols and TPs contents using PCA.
Materials and methods
Sample collection
Flax (Linum usitatissimum, Linaceae family), perilla (Perilla frutescens, Lamiaceae family), and basil seeds (Ocimum basilicum L.) were collected from a farm in Ardebil Province in Northwest Iran (plantation: March 2016 and harvest: July 2016), forests of Gilan Province in northern Iran (harvest: September 2016) and a farm in Mazandaran Province in northern Iran (plantation: March 2016 and harvest: August 2016) and the seeds verified by Seed and Plant Improvement Institute. The seeds were removed from their pods and dried in a dark place at ambient temperature for 3 days until a constant weight was achieved. The dried seeds were cleaned manually and packed in hermetic plastic bags before being stored in a dark place at 5 °C prior to analyses.
Materials
All HPLC grade solvents, fatty acid methyl ester (FAME), TAG standards, and Folin–Ciocalteu’s reagent were obtained from Sigma Aldrich Co. (St. Louis, MO, USA). Standard tocols were purchased from Calbiochem-Novabiochem Co. (San Diego, CA, USA).
Oil extraction
Seed oils was extracted according to the procedure outlined by Timilsena et al. (2017), with slight modifications. Briefly, the seeds were grinded using a Moulinex grinder (Type DPA1, France) at ambient temperature for 60 s. The powders were subjected to n-hexane extraction (1:4 w/v% of seeds powder to n-hexane) while agitating in a dark place at ambient temperature for 48 h. Then, the solvent was evaporated at 35 °C, using a rotary evaporator (Büchi, Flawil, Switzerland). Finally, the solvent residue was removed under a stream of nitrogen gas before being stored at − 20 °C.
Fat content
The oils were extracted from the grinded seeds (40 g) using n-hexane in a Soxhlet apparatus at 80 °C for 8 h according to IUPAC Standard Method (IUPAC 1987). The solvents were removed by using a rotary evaporator (Bu¨chi, Flawil, Switzerland) at 40 °C. The contents of the oils were gravimetrically determined and expressed as percent on a dry weight basis (%, d.b.).
FA composition
FA compositions were determined through FA methylation and determination of the resulting methyl esters on an HP-5890 gas chromatography (GC) system (Hewlett-Packard, CA, USA) equipped with a CP-SIL88 capillary column (Supelco, Bellefonte, PA, USA) of fused silica (length 60 m, I.D. 0.22 mm, film thickness 0.2 μm and a flame ionization detector (FID), according to the AOAC method, 963.22). Helium was used as the carrier gas (AOAC 1999).
ECN
AOCS Official Method (Ce 5b-89) was utilized to assess the ECNs of the seed oils. TAG profile was obtained by reverse-phase HPLC (Shimadzu Co., Kyoto, Japan) equipped with a SCL-10AVP system controller, an auto-injector, and a Shimadzu refractive index detector (model RID-6A). For this purpose, 0.5 g oil was dissolved in 10 mL (chloroform/acetone 1/1, v/v). Ten µL of this solution was injected into the HPLC. The TAGs were separated using a commercially packed Hypersil ODS column (150 mm × 4.5 mm). TAGs were eluted from the column with a mixture of acetonitrile/acetone (68/32) at a flow rate of 0.8 mL/min. The total run time in these experiments was 40 min. The chromatograms were processed using a Shimadzu CR4AX-integrator. The peak areas were used to quantify components based on percent area (AOCS 2009).
Identity characteristics
HPLC analysis for tocopherols and tocotrienols
High-performance liquid chromatography (HPLC) system (Alliance system, WATERS, USA) consisting of an intelligent pump, a Rheodyne injector fitted with a 5-lL loop, and a fluorescence detector was employed for tocopherol and tocotrienols analyses. The analyses were performed on a Spherisorb column (25 cm–4 mm I.D.) packed with a 5-μm silica particle in a normal-phase Alliance HPLC system (WATERS, USA). The fluorometric detection of all peaks was performed at 295 nm (excitation) and 330 nm (emission) (ISO 9936 1997). The mobile phase was n-hexane/isopropanol (98.5:0.5 v/v), which was supplied at 1 mL/min. Tocol peaks were identified and quantified against authentic tocopherol external standards. All the results are expressed as mg of tocol per kg of lipids.
GC analysis of sterols
Sterol fraction composition was determined by GC using 5 α-cholestane as an internal standard (ISO 12228 1999). An SE-54 CB column (length 50 m, ID 0.25 mm, and film thickness 0.25 mm, Macherey–Nagel, Duren, Germany) was used to separate the sterols. Column temperature during injection was at 100 °C for 5 min and then programmed to increase to 250 °C at a rate of 25 °C/min for 1 min. Afterwards, the temperature was further increased at a rate of 3 °C/min to reach a final temperature of 290 °C for 20 min. The detector temperature was 350 °C. Hydrogen was used as the carrier gas at 1.5 mL/min.
TPs content
TP contents were determined spectrophotometrically using Folin–Ciocalteu’s reagent, according to the method described by Capannesi et al. (2000), with slight modifications. To 50 mL of each sample (3 replicates), 2.5 mL of a 1 in 10 dilution of Folin–Ciocalteau’s reagent and 2 mL of Na2CO3 (7.5%, w/v) were added and the mixture incubated at ambient temperature for 1 h. Absorbance was measured at 725 nm, using an UV–Vis spectrophotometer (Optizen-212OUV). A calibration curve for a gallic acid–methanol mixture was obtained for the range 0.04–0.40 mg/mL and used to determine sample TP contents.
Chemical and physical characteristics
AOCS Official Methods (AOCS 1998) were applied for iodine number (IN) (Cd 3d-63), saponification value (SV) (Cd 3–25), acid value (AV) (Cd 3d-63), and unsaponifiable matter (USM) (Ca 6a-40). Refractive index (RI) was determined according to the AOAC Official Method (921.08) at 25 ± 0.05 °C using an Abbe refractometer [Carl Zeiss Abbé refractometer (32-G 110e)] (AOAC 1999). The colors of the oils were determined on CIELAB color scales using a MiniScan XE spectrocolorimeter (Hunterlab, Reston, VA, USA).
Oxidative stability
Peroxide, p-anisidine, and totox values
Peroxide value (PV) and p-anisidine (AnV) of the oils were specified, based on AOCS Official Methods for Cd 8–53 and Cd 18–90, respectively. PV is expressed as meq O2/kg of oil. Totox value (TV) is defined as 2 × PV + AnV (AOCS 1998).
Rancimat test
Rancimat apparatus (model 743, Metrohm AG, Herison, Switzerland) were employed for determination of oxidative stability (Timilsena et al. 2017). An air flow of 25 L/h was bubbled through the oils (3 g), which were then heated at 110 °C. Afterwards, the volatile degradation products were trapped in distilled water to increase water conductivity. The induction time was recorded from the inflection point of the conductivity curve that showed an abrupt increase in the slope of the curve.
Determination of thermal characteristics
Thermal characteristics of seed oils were measured according to the procedure outlined by Timilsena et al. (2017), with slight modifications. Briefly, a modulated differential scanning calorimeter (DSC 214 Polyma, NETZSCH, Germany) were used for determination of thermal properties. The instrument was calibrated using indium (156.6 _C, 28.57 J/g). N2 with a flow rate of 40 mL/min and an empty DSC-pan were used as purge gas and inert reference, respectively. The seed oil samples (15–18 mg) were weighed directly into a DSC-pan. Seed oils was allowed to equilibrate at 20 °C for 5 min followed by cooling cycle to − 80 °C at the rate of 5 °C/min. It was maintained for 5 min at this temperature before heating to 80 °C at the same rate. The sample was held for 5 min at 80 °C. The onset, peak, and end temperatures and the enthalpy of the thermal transition (melting and crystallization) were determined from the acquired thermograms.
Statistical analysis
The experiments were performed in triplicate and data were analyzed using ANOVA, which was performed according to SPSS (Ver.20.0). Duncan’s multiple range tests were used to determine the significant differences among the means (p < 0.05). Values are expressed as mean ± standard deviation (x ± SD). PCA was also performed for FA, ECN, tocols, sterols and TPs contents, using SPSS (Ver. 20.0).
Results and discussion
Lipid content
The lipid yields from perilla (42.80%) and basil (22.04%) seeds, respectively, were significantly greater and less than that from flax seed (38.76%) (p < 0.05). The oil yield from Iranian flax seed was less than that of typical Canadian flax seed (44–46%), but it was within the range reported by Lukaszewicz et al. (2004) and slightly higher than amounts reported for North Dakota (32–38%) and Ethiopian (29–36%) cultivars (Ayerza 1995; Lukaszewicz et al. 2004; Wakjira et al. 2004). The oil yield of Iranian perilla was higher than reported values for Japanese (25%) and Korean (38.6%) varieties, but lower than reported values for an Indian cultivar (51.7%) (Longvah and Deosthale 1991; Shin and Kim 1994). The yield of oil from Iranian perilla and flax seeds were greater than those from chia (25–35%) and ground nuts (37.8%) and rapeseed (36.0%) (Ayerza 2010; Ciftci et al. 2012; Timilsena et al. 2017). Differences in oil yield could be attributed to the natures of the seeds, which are affected by genetic and environmental factors, e.g. seeds grown at high temperatures might have lower oil contents compared to those grown at lower temperatures, and values depend on the efficiency and parameters used during extraction including solvent type, temperature and extraction time as well as size of the seeds and moisture contents (Choo et al. 2007; Ayerza 2010).
FA composition
FA compositions for flax, perilla, and basil seed are summarized in Table 1. On average, FAs for flax and perilla oils were in the following order of abundance: α-linolenic acid (C18:3) > oleic acid (C18:1) > linoleic (C18:2) > palmitic acid (C16:0) > stearic acid (C18:0) with the exception of linoleic acid, which exceeded oleic acid in basil seed oils. In agreement with the findings reported in earlier studies (Choo et al. 2007; Sarfraz et al. 2011; Ciftci et al. 2012), ALA was the predominant FA in all the samples studied (53.4–65.6%). The highest ALA content was found in perilla (65.6%), which was significantly (p < 0.05) greater than that for flax (53.4%), but no significant difference was observed between basil (63.8%) and perilla seed oils. Based our results, ALA in flax and perilla seeds was higher than values published by Longvah and Deosthale (1991), Shin and Kim (1994) and Ciftci et al. (2012), which suggests seed oils FA composition depends strongly on factors such as climatic conditions, (plant) nutrient availability, seed maturity and soil conditions. Also, our data corroborate other studies that showed low temperatures, generally, increased concentrations of unsaturated FAs (Ayerza 2010). The ratios of n-6/n-3 FAs in flax and basil seeds were greater than those of perilla (p < 0.05). Although a similar ratio was reported for basil seed oil (0.280–0.630%), we obtained ratios that were greater and less than 0.270 and 0.220 for flax and perilla seed oils, respectively, which were reported by Ciftci et al. (2012). This ratio is comparable to chia but considerably lower than hemp seed (3:1), canola (1:0.45) and walnut oils (1:0.20) (Timilsena et al. 2017). Our results revealed a significant negative correlation between PUFAs and saturated fatty acids (SFAs) (r = 0.985, p < 0.05), based on data reported for the syntheses of both FA types. Thus, the PUFA/SFA ratio was statistically greater for basil seed followed by flax and perilla seeds. PUFA/SFA ratios are usually taken as a measure of susceptibility to autoxidation (Timilsena et al. 2017), meaning higher values imply better residence in a hostile retail or home environment. Also strong negative correlation was observed between monounsaturated fatty acids (MUFA) and PUFAs (r = 0.995, p < 0.05).
Table 1.
Fatty acid composition of flax, perilla and basil seeds (area %)
| Fatty acids | Flax seed oil | Perilla seed oil | Basil seed oil |
|---|---|---|---|
| C14:0 | 0.0370 ± 0.0040a | 0.0150 ± 0.007b | 0.0450 ± 0.0070a |
| C15:0 | 0.0200 ± 0.00a | 0.0100 ± 0.00a | 0.0150 ± 0.0070a |
| C16:0 | 5.85 ± 0.071a | 6.35 ± 0.21a | 4.90 ± 0.00c |
| C16:1 | 0.0500 ± 0.00a | 0.0370 ± 0.0040a | 0.0650 ± 0.0070a |
| C17:0 | 0.0570 ± 0.0040a | 0.0200 ± 0.00a | 0.0200 ± 0.00a |
| C17:1 | 0.0700 ± 0.00a | 0.0100 ± 0.00a | 0.0650 ± 0.0070a |
| C18:0 | 4.15 ± 0.071a | 1.95 ± 0.071c | 2.50 ± ± 0.00a |
| C18:1 | 20.5 ± 0.20a | 13.2 ± 0.14a | 7.55 ± 0.070c |
| C18:2 | 15.0 ± 0.030a | 12.5 ± 0.00c | 20.2 ± 0.00a |
| C18:3 n-6 | 0.180 ± 0.00a | 0.185 ± 0.0070a | 0.250 ± 0.028a |
| C18:3 n-3 | 53.4 ± 1.4a | 65.6 ± 0.00a | 63.8 ± 0.00a |
| C20:0 | 0.175 ± 0.0070a | 0.225 ± 0.0070a | 0.250 ± 0.014a |
| C20:1 | 0.157 ± 0.0040c | 0.300 ± 0.00a | 0.180 ± 0.00a |
| C20:2 | 0.0400 ± 0.00a | 0.0385 ± 0.0020a | 0.0550 ± 0.0070a |
| C22:0 | 0.130 ± 0.00a | 0.0210 ± 0.0010c | 0.0650 ± 0.0070a |
| C24:0 | 0.0600 ± 0.014a | 0.0100 ± 0.00c | 0.0950 ± 0.0070a |
| SFA | 10.5 ± 0.030a | 8.60 ± 0.27a | 7.89 ± 0.00c |
| MUFA | 20.7 ± 0.31a | 13.6 ± 0.14a | 7.86 ± 0.060c |
| PUFA | 68.7 ± 1.4c | 78.3 ± 0.010a | 84.3 ± 0.041a |
| UFA | 89.3 ± 1.1b | 91.9 ± 0.13a | 92.2 ± 0.021a |
| PUFA/SFA | 6.55 ± 0.50c | 9.11 ± 0.17a | 10.7 ± 0.011a |
| n-6/n-3 | 0.290a | 0.190b | 0.320a |
Mean ± SD (standard deviation) within a row with the same lowercase letters are not significantly different at p < 0.05, n = 3. Saturated fatty acids (SFA); monounsaturated fatty acids (MUFA); polyunsaturated fatty acids (PUFA)
ECN
The Composition of the main triacylglycerides and ECNs of the flax, perilla, and basil seed oils are presented in Table 2 and Fig. S1 to Fig. S3 (in Supplementary Material). Due to the high contribution of linolenic acid in all analyzed oils, trilinolenin (LnLnLn) with an ECN of 36 was the predominant TAG in all the studied oils. Its highest concentration was found in perilla oil followed by basil seed oil. The ECN of 36 in flax and perilla oils were slightly higher than the related data published by Ciftci et al. (2012). The LLnLn with an ECN of 38 was determined most amount for basil and perilla, respectively, that this value was lower than the relevant result of Ciftci et al. (2012). Other major TAGs in these seed oils are included OLnLn, LLLn, LnLnP, LLL, StLnLn, OLLn, LnLP, OLnO, OLL, StLLn and PLnP. In analyzed oils, Tri-PUFA TAG’s accounted for 46.9, 46.2, and 34.2% of all TAG’s in basil, perilla and flax seed oils, respectively. Contribution of tri-unsaturated (UUU) TAGs was significantly higher in perilla (71.8%), followed by basil (69.7%), and flax (57.4%) oils and slightly lower than the results of Ciftci et al. (2012). ECNs of commodity oils have been reported to be within a range of 52–54, while those of n-3 FAs oils mainly lie in the range of 36–44 (Ayorinde et al. 2000). These are in accordance with our results, i.e., 86.5, 93.5 and 93.4% of ECN within a range of 36–44 for flax, perilla, and basil seed oils, respectively. Ciftci et al. (2012) studied ECNs for flax, perilla, and chia seed oils, and found values shifted to downwards in response to higher PUFA contents, particularly linolenic acid (Ciftci et al. 2012). The results obtained in this study were in agreement with those reported by Ciftci et al. (2012).
Table 2.
Composition of the main triacylglycerides and equivalent carbon number of flax, perilla and basil seed oils (area %)
| TAGs | ECN | Flax seed oil | Perilla seed oil | Basil seed oil |
|---|---|---|---|---|
| LnLnLn | 36 | 21.3 ± 0.16Bc | 32.0 ± 0.58Aa | 27.5 ± 0.10Ab |
| LLnLn | 38 | 12.9 ± 0.10Ec | 14.2 ± 0.16Cb | 19.4 ± 0.15Ca |
| OLnLn | 40 | 23.2 ± 0.21Ab | 25.6 ± 0.090Ba | 22.8 ± 0.22Bc |
| LLLn | ||||
| LnLnP | ||||
| LLL | 42 | 14.8 ± 0.19Ca | 11.5 ± 0.23Db | 14.7 ± 0.050Da |
| StLnLn | ||||
| OLLn | ||||
| LnLP | ||||
| OLnO | 44 | 14.3 ± 0.55Da | 10.12 ± 0.54Eb | 9.04 ± 0.54Ec |
| OLL | ||||
| StLLn | ||||
| PLnP | ||||
| OLnP | 46 | 6.79 ± 0.53Fa | 3.12 ± 0.58Fb | 4.15 ± 0.56Fb |
| StLL | ||||
| OLO | ||||
| LOP | 48 | 4.49 ± 0.010Ga | 1.52 ± 0.020Gb | 1.51 ± 0.010Gb |
| OOO | ||||
| StLO | ||||
| OPO | ||||
| StOO | 50 | 1.32 ± 0.10Ha | 0.290 ± 0.010Hc | 0.790 ± 0.010Hb |
| Others | – | 1.55 ± 0.15Ib | 3.25 ± 0.13Fa | 1.38 ± 0.060Gc |
Ln linolenic acid, L linoleic acid, O oleic acid, P palmitic acid, St stearic acid
ND not detected; limit of detection (LOD) < 0.0.05%; n = 3
Mean ± SD within a row with the same lowercase letters are not significantly different at p < 0.05
Mean ± SD within a column with the same uppercase letters are not significantly different at p < 0.05
Identity characteristics
Tocol composition
Oil can be identified based on highly specific information about the compositions of minor components like tocols and phytosterols, especially when direct analysis is performed without derivatization (Ortega-García et al. 2006). Tocol compositions for our Iranian flax, perilla, and basil seed oils are presented in Table 3. As shown, γ-Tocopherol was the main isomer, contributing 85.7, 88.1, and 80.2% of total tocol, respectively. These results are similar to those reported previously by Shin and Kim (1994), Choo et al. (2007), and Ciftci et al. (2012). Similarly, β-tocopherol, β-tocotrienol, and γ-tocotrienol were not detected in any of the oils tested (Shin and Kim 1994; Choo et al. 2007; Ciftci et al. 2012). α-tocopherol concentrations in the oils were low and varied between 3.20 and 23.8 mg/kg, although basil seed oil had significantly higher concentrations of α-tocopherol compared with the others. α-tocotrienol was not detected in flax; whereas in basil seed oil was highest amount. Total tocols contents of flax seed oil was somewhat lower than those published elsewhere (Choo et al. 2007; Ciftci et al. 2012), whereas that of perilla oil was similar to the relevant data reported by Ciftci et al. (2012). There were no published data on the tocol contents and compositions of basil seed oils and hence no comparison was possible. Total tocols of perilla and basil seed oils were higher than those observed in chia seed and hemp seed oils, and cereals like wheat, oat, barley, rye, sorghum (da Silva et al. 2017). A positive relationship between PUFAs and γ-tocopherol content has been reported in some publications (Tuberoso et al. 2007). In fact, this concept was supported by the results of the studied PUFA-rich oils (r = 0.98, p < 0.05).
Table 3.
Composition of tocols in flax, perilla, and basil seed oils (mg/kg of oils)
| Tocols | Flax seed oil | Perilla seed oil | Basil seed oil |
|---|---|---|---|
| α-Tocopherol | 14.9 ± 0.20Cb | 3.20 ± 2.1^Dc | 23.8 ± 0.30Ea |
| β-Tocopherol | ND | ND | ND |
| γ-Tocopherol | 383 ± 7.5Ac | 617 ± 9.2Ab | 854 ± 7.4Aa |
| δ-Tocopherol | 43.7 ± 5.7Bc | 58.2 ± 2.4Bb | 81.2 ± 1.1Ba |
| α –Tocotrienol | ND | 19.6 ± 0.50Cb | 44.5 ± 1.4Da |
| β-Tocotrienol | ND | ND | ND |
| γ-Tocotrienol | ND | ND | ND |
| δ-Tocotrienol | 5.40 ± 0.40Db | 3.60 ± 0.50Db | 61.0 ± 1.9Ca |
| Total | 447 ± 12c | 701 ± 11b | 1070 ± 10a |
ND not detected; limit of detection (LOD) < 0.1 mg/kg; n = 3
Mean ± SD within a row with the same lowercase letters are not significantly different at p < 0.05
Mean ± SD within a column with the same uppercase letters are not significantly different at p < 0.05
Sterol composition
Table 4 represents the phytosterol compositions of the analyzed oils. Significant differences were found between the sterol composition of all the oils except for their -Avenasterol (p < 0.05). More than 80.0% of the total sterols of all the samples consisted of campesterol, stigmasterol, β-sitosterol, and -Avenasterol. Therefore, β-sitosterol was the predominant constituent of basil, perilla, and flax seed oils. This finding was congruent with that reported by Ciftci et al. (2012). Second major sterols in flax and basil seed oils was campesterol however in perilla was others; also third sterols in flax and perilla was -Avenasterol however in basil seed oil was stigmasterol, respectively; which is inconsistent with the results published by Ciftci et al. (2012). In basil seed oil contained the highest amounts of total sterols followed by those of perilla and flax seeds. The studied basil and perilla seed oils were had higher phytosterol contents as compared to those of extra-virgin olive oil (3280 mg/kg) and safflower oils (3960 mg/kg) (Phillips et al. 2002).
Table 4.
Sterol composition in flax, perilla, and basil seed oils
| Sterols | Flax seed oil | Perilla seed oil | Basil seed oil |
|---|---|---|---|
| Cholesterol (%) | ND | ND | ND |
| Brassicasterol (%) | 1.03 ± 0.030Fa | 0.450 ± 0.010Gb | 0.0600 ± 0.00^Gc |
| Campesterol (%) | 28.7 ± 0.040Ba | 7.90 ± 0.090Dc | 16.2 ± 0.40Bb |
| Stigmasterol (%) | 8.87 ± 0.20Db | 4.74 ± 0.030Ec | 13.1 ± 0.27Ca |
| β-Sitosterol (%) | 44.3 ± 0.71Ac | 59.8 ± 1.0Aa | 53.5 ± 0.65Ab |
| ∆5-Avenasterol (%) | 11.7 ± 0.030Ca | 11.0 ± 0.15Cb | 9.69 ± 0.27Dc |
| ∆7-Stigmasterol (%) | 1.39 ± 0.090Fc | 2.96 ± 0.030Fa | 1.96 ± 0.030Fb |
| ∆7-Avenasterol (%) | 0.410 ± 0.59Ga | 0.350 ± 0.050Ga | 0.470 ± 0.20Ga |
| Others (%) | 4.77 ± 0.46Ec | 12.7 ± 0.12Ba | 5.57 ± 0.38Eb |
| Total (mg/kg) | 3510 ± 2.5c | 4180 ± 1.7b | 5300 ± 0.24a |
ND not detected; limit of detection (LOD) < 0.01%; n = 3
Mean ± SD within a row with the same lowercase letters are not significantly different at p < 0.05
Mean ± SD within a column with the same uppercase letters are not significantly different at p < 0.05
TP content
High concentrations of TP compounds were obtained in the studied basil followed by perilla and flax seed oils (p < 0.05) (Table 5). Similar results for flax seed oil were reported by Abuzaytoun and Shahidi (2006), whereas our results for perilla and basil seed oils were lower than those reported by Wang et al. (2010) and Sarfraz et al. (2011). Changes in TP contents among the different studies can be attributed to different factors such as cultivation techniques and weather conditions as well as methods used for determination of phenolic compounds (Wang et al. 2010).
Table 5.
Chemical, physical, oxidative stability characteristics, and total phenolic of flax, perilla and basil seed oils
| Flax seed oil | Perilla seed oil | Basil seed oil | |
|---|---|---|---|
| RI | 1.4719 ± 0.000c | 1.4760 ± 0.001a | 1.4737 ± 0.000b |
| SV (mgKOH/g) | 191 ± 3.0a | 182 ± ±0.57b | 195 ± 4.0a |
| IN | 193 ± 3.5a | 195 ± 1.8a | 195 ± 5.8a |
| USM (%w/w) | 1.26 ± 0.020c | 1.49 ± 0.080b | 2.03 ± 0.060a |
| AV (mgKOH/g) | 2.16 ± 0.23b | 2.12 ± 0.33b | 3.77 ± 0.10a |
| Color | |||
| L* | 62.0 ± 2.0a | 54.2 ± 0.68b | 55.7 ± 1.0b |
| a* | 7.73 ± 0.49a | − 6.10 ± 0.26c | − 4.77 ± 0.25b |
| b* | 73.8 ± 1.8a | 69.6 ± 2.9a | 64.3 ± 2.3b |
| TPs (mg/kg) | 19.5 ± 0.49c | 34.3 ± 0.58b | 66.8 ± 0.25a |
| PV (meq of O2/kg) | 0.290 ± 0.10b | 0.350 ± 0.050b | 0.730 ± 0.14a |
| AnV (unit) | 0.740 ± 0.080b | 0.780 ± 0.16b | 1.24 ± 0.24a |
| TV (unit) | 1.32 ± 0.23b | 1.48 ± 0.19b | 2.69 ± 0.12a |
| OSI (h) | 1.28 ± ±0.16a | 1.42 ± 0.26a | 1.39 ± 0.15a |
Mean ± SD within a row with the same lowercase letters are not significantly different at p < 0.05; n = 3
RI refractive index, SV saponification value, IN iodine number, USM unsaponifiable matter, AV acid value, TP total phenolic, PV peroxide value, AnV P-anisidine value, TV totox value, OSI oxidative stability index
Chemical and physical characteristics
Table 5 shows the chemical and physical properties of the oils. RI—an index affected by FA composition, carbon chain length, unsaturation degree, and oxidation (AOCS 1998)—differed significantly (p < 0.05) among the oils. RI as a suitable, fast, and low-cost method is a valuable tool that used in optimizing processing and quality control parameters as well as in determining accuracy of oils (Timilsena et al. 2017). RI values for the Iranian seed oils was greater than those reported previously for sunflower (RI = 1.460) and olive oils (RI = 1.470), but less than those for chia seed and fish oils (RI = 1.480) (Timilsena et al. 2017).
SV is an index of average FA molecular weight. The value for perilla oil was significantly lower than the other two oils (p < 0.05), indicating high molecular weight FAs (Tables 1, 5).
IN represented degrees of unsaturation, but there were no significant differences between the oils (Table 5).
USM fractions of vegetable oils contain sterols, tocopherols, terpene alcohols, and hydrocarbons, which typically comprise 0.500–2.50% although some are much higher (5–6%) (Malecka 2002). Basil seed oil had the highest USM followed by perilla and flax oils (Table 5). These results were statistically significant and correlated mostly with tocols and sterol contents (Tables 3, 4). The AV of basil seed oil was highest indicating the presence of free FAs as a result of hydrolysis (Table 5).
The L*, a*, and b* values of the studied flax, perilla, and basil seed oils are compared in Table 5. As exhibited, significant differences exist between a* and b* values of the oils (p < 0.05). The lowest L* values reflecting more brightness were observed in perilla and basil seed oils, while indicating more darkness compared to that of flax seed oil. Significant differences (p < 0.05) were found between the degrees of redness (a*) among all the samples. A lower b* value was found in basil seed oil, whereas no significant difference was observed between flax and perilla oils in this regard (p > 0.05). The higher b* value in these seed oils indicates a more intense yellow color and hence the presence of increased amounts of carotenoids (Timilsena et al. 2017). These values had significantly different than other most vegetable oils, whose L*, a*, and b* values range between 63.4–69.5, 3.8–4.4 and 9.2–10.4, respectively (Timilsena et al. 2017).
Oxidative stability
PV, AnV, and TV
Oxidative stability is a major parameter for assessing the qualities of oils and fats since providing a good reckoning of their susceptibilities to oxidative degeneration, which is the main cause of their changes (Aparicio et al. 1999). PV, AnV, and TV have been frequently utilized as the most important parameters to monitor the qualities of edible oils (Choo et al. 2007). In this study, PVs of all the seed oils were well within the limit of 10 milliequivalents of active oxygen/kg, based on the Codex Alimentarius Commission (1999) standard for refined oils (Table 5).
AnV is applied for detecting any secondary oxidation products and a good-quality oil should have a value of < 2 (Subramanian et al. 2000). AnV values for the three Iranian oil seeds varied between 0.740 and 1.24 (Table 5), which might reflect good handling, extraction, and/or storage conditions of the oils as well as early analysis of the oils in the shelf-life (Farhoosh and Pazhouhanmehr 2009).
TV is a non-volatile carbonyl precursors used for processed oils, allowing the products of oxidation during storage to be measured empirically. According to Frankel (2005), the maximum acceptable TV for a good quality oil is four. TVs for the three Iranian seed oils were less than 3 (Table 5). The higher TV of basil seed oil was related to higher PV and AnV values compared with the other two oils.
Rancimat test
The Rancimat test is a technique designed to speed up oxidation by exposing oils to higher temperatures in the presence of excess air or oxygen. Oxidative stability index (OSI) represents the time needed for decomposition of hydroperoxides produced by oxidation (Decker 1998). Oxidative stability depends on the composition, concentration, and activities of reactive substrates, pro-oxidants as well as an oxidation control system including naturally occurring antioxidants (Decker 1998). The oxidative stabilities of our seed oils (Table 5) were less than those published for other vegetable oils (i.e., canola 12–17 h, olive 6–11 h, coconut 33 h), probably because of their higher PUFA contents. However, our OSI values were greater than those for chia (0.5–2 h) and citrus oils (0.5 h) (Aparicio et al. 1999; Timilsena et al. 2017). In spite of the high amounts of PUFAs in basil seed oil compared to those of flax and perilla oils, their OSIs values were not significantly different (p > 0.05). This contradiction could be attributed to the higher amounts of tocols and TPs in basil seed oil. Regardless, these seed oils is needed to protect from autoxidation during processing, transportation and storage.
Determination of thermal characteristics
Characteristics DSC thermogram of seed oils are shown in Fig. S4 to Fig. S6 (Supplementary Material). The thermograms of all seed oils showed some small differences. The melting curve of the flax seed oil was as follows: the onset temperature of − 34.2 °C, the melting peak temperature of − 29.8 °C and the end temperature of − 28.0 °C with corresponding melting enthalpy of 19.2 J/g (Fig. S4 in Supplementary Material). The melting curve of the perilla seed oil was as follows: the onset temperature of − 34.9 °C, the melting peak temperature of − 31.7 °C and the end temperature of − 25.2 °C with corresponding melting enthalpy of 15.5 J/g (Fig. S5 in Supplementary Material). The melting curve of the basil seed oil was as follows: the onset temperature of − 37.0 °C, the melting peak temperature of − 30.2 °C and the end temperature of − 28.1 °C with corresponding melting enthalpy of 44.5 J/g (Fig. S6 in Supplementary Material). As well as, two shoulder peaks around the temperature of − 15 °C and − 40 °C on the melting curve for these seed oils were also visible (Fig. S4 to Fig. S6 in Supplementary Material). These shoulders may be attributed to the presence of the transformation of the metastable phase to the more stable polymorphic forms or higher-melting triacylglycerols (Timilsena et al. 2017). These curves were approximately flat above 0 °C, indicating that seed oils are purely liquid without any noticeable solid crystals at ambient temperature.
For crystallization curves of seed oils, basil seed oil had the lowest transition point at − 72.2 °C (onset temperature of − 79.4 °C, end temperature of − 64.1 °C and enthalpy of 7.8 J/g), followed by perilla seed oil, − 69.0 °C (onset temperature of − 78.5 °C, end temperature of − 62.2 °C and enthalpy of 7.2 J/g), and flax seed oil, − 68.1 °C (onset temperature of − 76.3 °C, end temperature of − 61.6 °C and enthalpy of 9.8 J/g) (Fig. S4 to Fig. S6 in Supplementary Material).
Generally, the results of both melting and crystallization profiles showed that the DSC profiles of the flax, perilla and basil seed oil are likely influenced by unsaturation of the constituent fatty acids, triglyceride composition and crystal structure (Timilsena et al. 2017).
PCA analysis
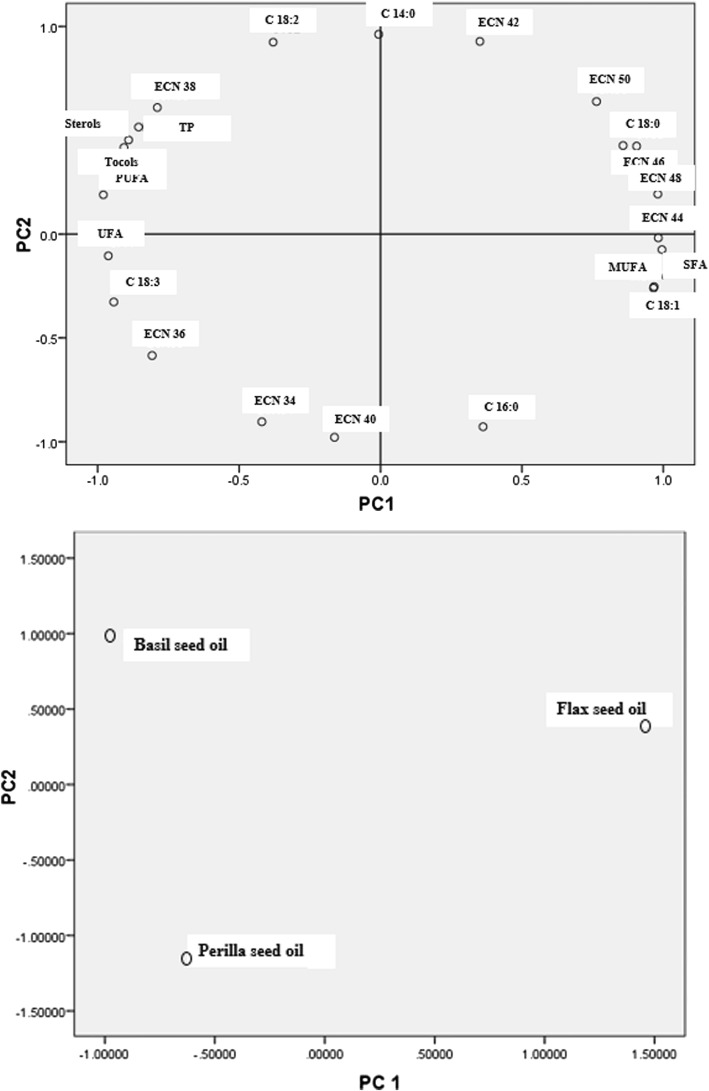
In order to found relationship the compounds (FA composition, ECN, tocols, sterols and TPs), PCA was applied, reveling separation and classification between two groups of compounds. PCA can explain a new set of variable data in order to find how many components are necessary to show highest variance with a minimum loss of information. According to data, KMO index was 0.700, exceeding the recommended value of 0.600, whereas the significance of the Bartlett’s sphericity test was 0.000, i.e., lower than 0.05. These results confirmed the validity of extracted factors. Factor selection procedures based on eigenvalues carried out by the Kaiser–Guttmann rule (Kaiser Criterion) and the scree test. In this method the component with eigenvalues greater than 1 is selected as PC. According to the data, the first two principal components represented 98.428% of the variance. As shown in Fig. 1, the first PC accounted for 63.280% of the total variance and positively correlated with C18:0 (0.905), C18:1 (0.967), SFA (0.995), MUFA (0.965), ECN 44 (0.982), ECN 46 (0.858), ECN 48 (0.980), ECN 50 (0.764), which were predominant in flax seed oil and negatively correlated with PUFA (− 0.980), ECN 36 (− 0.808) and ECN 38 (− 0.789), tocols (− 0.907), sterols (− 0.891), TPs (− 0.855), which were predominant in perilla and basil seed oils, respectively; as well as the second PC accounted for 35.148% of the total variance and positively correlated with C18:2 (0.924) and ECN 38 (0.610), that predominant in basil seed oil and negatively correlated with ECN 34 (− 0.904) and ECN 40 (− 0.979), which were predominant in perilla seed oil. Furthermore, C18:3 and UFA from PC1 and C14:0, C16:0 and ECN 42 from PC2 were not appropriate for distinguish between these seed oils because predominant FA in these seed oils was ALA.
Fig. 1.
Loading plot and score plot after principle components analysis of various compounds by the two principle components (PC1 and PC2)
Conclusion
We demonstrated that flax, perilla and basil, grown in Iran, gave oils with different physicochemical characteristics that might be important in distinguishing them with respect to future potential uses and authenticity. These seed oils can be used to enrich food products with linolenic acid and improve the n-6:n-3 fatty acids ratio in the diet. However, these oils were more susceptible to oxidation and, consequently, production of undesirable flavors as well as potentially harmful compounds. Thus, there would be a need to protect these oils during handling, processing, and storage. Each seed oil exhibited different transitions of melting and crystallization profiles due to its fatty acid and triglyceride composition as well as crystal structure. PCA analysis showed good separation of samples based on the contents of particular groups of compounds, but C18:3 and UFA from PC1 and C14:0, C16:0 and ECN 42 from PC2 were not appropriate for distinguishing between these seed oils because the predominant FA in these seed oils was ALA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abuzaytoun R, Shahidi F. Oxidative stability of flax and hemp oils. J Am Oil Chem Soc. 2006;83(10):855–861. doi: 10.1007/s11746-006-5037-7. [DOI] [Google Scholar]
- Alimentarius C. Codex standard for named vegetable oils. Codex Stand. 1999;210:1–13. [Google Scholar]
- American Oil Chemists’ Society (AOCS) Official methods and practices of the AOCS. 5. Champaign: AOCS Press; 1998. [Google Scholar]
- American Oil Chemists’ Society (AOCS) Official methods and practices of the AOCS. 5. Champaign: AOCS Press; 2009. [Google Scholar]
- AOAC . Official methods of analysis. 16. Gaithersburg: AOAC International; 1999. [Google Scholar]
- Aparicio R, Roda L, Albi MA, Gutiérrez F. Effect of various compounds on virgin olive oil stability measured by Rancimat. J Agric Food Chem. 1999;47:4150–4155. doi: 10.1021/jf9812230. [DOI] [PubMed] [Google Scholar]
- Ayerza R. Oil content and fatty acid composition of chia (Salvia hispanica L.) from five northwestern locations in Argentina. J Am Oil Chem Soc. 1995;72:1079–1081. doi: 10.1007/BF02660727. [DOI] [Google Scholar]
- Ayerza R. Effects of seed color and growing locations on fatty acid content and composition of two chia (Salvia hispanica L.) genotypes. J Am Oil Chem Soc. 2010;87(10):1161–1165. doi: 10.1007/s11746-010-1597-7. [DOI] [Google Scholar]
- Ayorinde FO, Garvin K, Saeed K. Determination of the fatty acid composition of saponified vegetable oils using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2000;14(7):608–615. doi: 10.1002/(SICI)1097-0231(20000415)14:7<608::AID-RCM918>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Capannesi C, Palchetti I, Mascini M, Parenti A. Electrochemical sensor and biosensor for polyphenols detection in olive oils. Food Chem. 2000;71:553–562. doi: 10.1016/S0308-8146(00)00211-9. [DOI] [Google Scholar]
- Choo W, Birch J, Dufour J. Physicochemical and quality characteristics of cold pressed flax seed oils. J Food Compos Anal. 2007;20:201–211. doi: 10.1016/j.jfca.2006.12.002. [DOI] [Google Scholar]
- Ciftci ON, Przybylski R, Rudzińska M. Lipid components of flax, perilla, and chia seeds. Eur J Lipid Sci Technol. 2012;114:794–800. doi: 10.1002/ejlt.201100207. [DOI] [Google Scholar]
- Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70(3):491s–499s. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- da Silva BP, Anunciação PC, da Silva Matyelka JC, Della Lucia CM, Martino HSD, Pinheiro-Sant’Ana HM. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017;221:1709–1716. doi: 10.1016/j.foodchem.2016.10.115. [DOI] [PubMed] [Google Scholar]
- Decker EA. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci Technol. 1998;9:241–248. doi: 10.1016/S0924-2244(98)00045-4. [DOI] [Google Scholar]
- Farhoosh R, Pazhouhanmehr S. Relative contribution of compositional parameters to the primary and secondary oxidation of canola oil. Food Chem. 2009;114:1002–1006. doi: 10.1016/j.foodchem.2008.10.054. [DOI] [Google Scholar]
- Frankel EN. Lipid oxidation. Bridgwater: The Oily Press; 2005. [Google Scholar]
- Goyal A, Sharma V, Upadhyay N, Gill S, Sihag M. Flax and flaxseed oil: an ancient medicine & modern functional food. J Food Sci Technol. 2014;51(9):1633–1653. doi: 10.1007/s13197-013-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO 12228 (1999) Animal and vegetable fats and oils—determination of individual and total sterol contents—method using gas chromatography
- ISO 9936 (1997) Animal and vegetable fats and oils—determination of tocopherols and tocotrienols contents—method using high performance liquid chromatography
- IUPAC . Standard methods for the analysis of oils, fats and derivatives. 7. Oxford: Blackwell Scientific Pub. Ltd; 1987. [Google Scholar]
- Kim KB, Nam YA, Kim HS, Hayes AW, Lee BM. α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem Toxicol. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Liangli Y, Parry JW, Zhou K (2005) Oils from herbs, spices, and fruit seeds. In: Shahidi F, Bailey AE (eds) Bailey’s industrial oil and fat product, vol 3, pp 233–258
- Longvah T, Deosthale YG. Chemical and nutritional studies on Hanshi (Perilla frutescens), a traditional oilseed from Northeast India. J Am Oil Chem Soc. 1991;68:781–784. doi: 10.1007/BF02662172. [DOI] [Google Scholar]
- Longvah T, Deosthale YG, Kumar PU. Nutritional and short term toxicological evaluation of Perilla seed oil. Food Chem. 2000;70(1):13–16. doi: 10.1016/S0308-8146(99)00263-0. [DOI] [Google Scholar]
- Lukaszewicz M, Szopa J, Krasowska A. Susceptibility of lipids from different flax cultivars to peroxidation and its lowering by added antioxidants. J Food Chem. 2004;88:225–231. doi: 10.1016/j.foodchem.2003.12.042. [DOI] [Google Scholar]
- Malecka M. Antioxidant properties of the unsaponifiable matter isolated from tomato seeds, oat grains and wheat germ oil. Food Chem. 2002;79:327–330. doi: 10.1016/S0308-8146(02)00152-8. [DOI] [Google Scholar]
- Ortega-García J, Gámez-Meza N, Noriega-Rodriguez JA, Dennis-Quiñonez O, García-Galindo HS, Angulo-Guerrero JO, Medina-Juárez LA. Refining of high oleic safflower oil: effect on the sterols and tocopherols content. Eur Food Res Technol. 2006;223(6):775–779. doi: 10.1007/s00217-006-0267-3. [DOI] [Google Scholar]
- Phillips KM, Ruggio DM, Toivo JI, Swank MA, Simpkins AH. Free and esterified sterol composition of edible oils and fats. J Food Compos Anal. 2002;15:123–142. doi: 10.1006/jfca.2001.1044. [DOI] [Google Scholar]
- Sarfraz Z, Anjum FM, Khan MI, Arshad MS, Nadeem M. Characterization of basil (Ocimum basilicum L.) parts for antioxidant potential. Afr J Food Sci Technol. 2011;2(9):204–213. [Google Scholar]
- Shin HS, Kim SW. Lipid composition of perilla seed. J Am Oil Chem Soc. 1994;71:619–622. doi: 10.1007/BF02540589. [DOI] [Google Scholar]
- Subramanian R, Nandini KE, Sheila PM, Gopalakrishna AG, Raghavarao KSMS, Nakajima M, Kimura T, Maekawa T. Membrane processing of used frying oils. J Am Oil Chem Soc. 2000;77:323–328. doi: 10.1007/s11746-000-0052-2. [DOI] [Google Scholar]
- Timilsena YP, Vongsvivut J, Adhikari R, Adhikari B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017;228:394–402. doi: 10.1016/j.foodchem.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Tuberoso C, Kowalczyk A, Sarritzu E, Cabras P. Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem. 2007;103:1494–1501. doi: 10.1016/j.foodchem.2006.08.014. [DOI] [Google Scholar]
- Umesha SS, Manohar RS, Indiramma AR, Akshitha S, Naidu KA. Enrichment of biscuits with microencapsulated omega-3 fatty acid (Alpha-linolenic acid) rich Garden cress (Lepidium sativum) seed oil: physical, sensory and storage quality characteristics of biscuits. LWT Food Sci Technol. 2015;62(1):654–661. doi: 10.1016/j.lwt.2014.02.018. [DOI] [Google Scholar]
- Wakjira A, Labuschagne MT, Hugo A. Variability in oil content and fatty acid composition of Ethiopian and introduced cultivars of linseed. J Sci Food Agric. 2004;84(6):601–607. doi: 10.1002/jsfa.1698. [DOI] [Google Scholar]
- Wang S, Hwang H, Yoon S, Choe E. Temperature dependence of autoxidation of perilla oil and tocopherol degradation. J Food Sci. 2010;75(6):C498–C505. doi: 10.1111/j.1750-3841.2010.01681.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.