Abstract
Bacterial vaginosis (BV) is associated with HIV acquisition and adverse pregnancy outcomes. Recurrence after metronidazole treatment is high. HIV-negative, non-pregnant Rwandan BV patients were randomized to four groups (n = 17/group) after seven-day oral metronidazole treatment: behavioral counseling only (control), or counseling plus intermittent use of oral metronidazole, Ecologic Femi+ vaginal capsule (containing multiple Lactobacillus and one Bifidobacterium species), or Gynophilus LP vaginal tablet (L. rhamnosus 35) for two months. Vaginal microbiota assessments at all visits included Gram stain Nugent scoring and 16S rRNA gene qPCR and HiSeq sequencing. All interventions were safe. BV (Nugent 7–10) incidence was 10.18 per person-year at risk in the control group, and lower in the metronidazole (1.41/person-year; p = 0.004), Ecologic Femi+ (3.58/person-year; p = 0.043), and Gynophilus LP groups (5.36/person-year; p = 0.220). In mixed effects models adjusted for hormonal contraception/pregnancy, sexual risk-taking, and age, metronidazole and Ecologic Femi+ users, each compared to controls, had higher Lactobacillus and lower BV-anaerobes estimated concentrations and/or relative abundances, and were less likely to have a dysbiotic vaginal microbiota type by sequencing. Inter-individual variability was high and effects disappeared soon after intervention cessation. Lactobacilli-based vaginal probiotics warrant further evaluation because, in contrast to antibiotics, they are not expected to negatively affect gut microbiota or cause antimicrobial resistance.
Subject terms: Infectious diseases, Molecular medicine
Introduction
Most women have a vaginal microbiota (VMB) that consists predominantly of lactobacilli1. The most common type of vaginal dysbiosis is bacterial vaginosis (BV), characterized by a decrease in lactobacilli and increase in fastidious anaerobes2. Other types of bacterial dysbiosis, vulvovaginal candidiasis, and Trichomonas vaginalis (TV) are also common. These conditions are associated with vaginal inflammation, thereby increasing the risk of HIV acquisition3. BV is also associated with pelvic inflammatory disease, infertility, and adverse pregnancy outcomes2.
The majority of women seeking care for vaginal symptoms receive antibiotic or antifungal treatment empirically or syndromically without any diagnostic testing4. In some specialized clinics, women might be offered limited diagnostic testing, such as vaginal pH determination and/or wet mount microscopy. In research settings, BV is typically diagnosed by the Amsel criteria or Nugent scoring5,6, with the latter currently being considered the gold standard: Gram-stained vaginal smears are scored based on microscopic visualization of three bacterial morphotypes with a score of 0–3 considered normal, 4–6 intermediate, and 7–10 BV regardless of symptoms. In the last 15 years, molecular methods have become more widely available, and have been applied to the VMB, although mostly in descriptive studies to date1.
Evidence is mounting that ‘microbiological-BV’ (defined here as the absence of lactobacilli-domination by Nugent scoring or by molecular methods regardless of symptoms) can cause long-term adverse outcomes in the presence or absence of vaginal symptoms2. BV is notoriously difficult to treat7–9. About 60–80% of patients are cured after a course of oral or vaginal metronidazole or clindamycin, but recurrence rates are high7. Therapies to restore and maintain an optimal lactobacilli-dominated VMB after antibiotic treatment are not standard practice, but some clinicians in Europe and North America recommend twice weekly 0.75% metronidazole vaginal gel for 4–6 months to lower the risk of BV recurrence7. This recommendation was tested in a randomized controlled trial in the USA, which showed a statistically significant reduction in BV recurrence (34% by Nugent scoring)10. In addition, two African trials evaluating oral (2 g monthly) and vaginal metronidazole (five nights every three months) for BV prevention also showed significant incidence reductions (45% and 10%, respectively)11,12.
Probiotic lactobacilli may be able to restore and maintain a lactobacilli-dominated VMB, may be better able to prevent or disrupt BV-associated biofilms than antibiotics, and can likely be used safely for long periods without the risk of causing antimicrobial resistance13. Lactobacilli-containing vaginal probiotic clinical trials to date have shown mixed results, but eight of the 12 trials showed sufficiently promising efficacy for BV prevention to warrant further investigation13–24. Some trials used commercially available probiotic strains (mostly derived from the gut or fermented foods) and others vaginal strains isolated from healthy women25, but efficacy signals were similar for the two probiotic strain categories13–24. None of the trials reported major safety concerns or colonization beyond the dosing period. Because ‘natural’ strains do not seem to outperform commercially available strains, and for pragmatic reasons, we chose to evaluate two vaginal probiotics that are currently on the market. Our aim was to assess their safety and impact on the VMB in much more detail than previous trials had done, and to develop data analytic methods to enable use of high-dimensional 16S rRNA gene sequencing data for this purpose. The two probiotics that we evaluated were Ecologic Femi+ vaginal capsule (EF+; Winclove Probiotics, Amsterdam, The Netherlands) and Gynophilus LP vaginal tablet (GynLP; Biose, Arpajon-sur-Cère, France). EF+ contains multiple lyophilized bacteria (Bifidobacterium bifidum W28, Lactobacillus acidophilus W70, L. helveticus W74, L. brevis W63, L. plantarum W21, and L. salivarius W24) in a total dose of 1.5 × 109 colony forming units (CFU). GynLP contains 1.6 × 109 CFU of Lcr Regenerans, a culture of the L. rhamnosus 35 (Lcr35) strain. The tablet disintegrates in the vagina after forming a gel to release Lcr35. Gynophilus (the same active ingredient as GynLP but a different formulation) had shown promise in preventing BV recurrence in a previous trial, but EF+ had not previously been studied for this indication15.
Methods
From June 2015 until February 2016, we conducted a randomized clinical trial in Kigali, Rwanda, to evaluate intermittent use of the above-mentioned vaginal probiotics as well as oral metronidazole (Tricozole, Laboratory & Allied ltd, Nairobi, Kenya) to prevent the recurrence of microbiological-BV after metronidazole treatment. The trial was a pilot trial with a modest sample size (N = 68) at the request of the funder.
Eligibility
Women were eligible for screening if they were aged 18–45, in good overall physical and mental health, and at high urogenital infection risk defined as having had more than one sex partner in the last 12 months or having been treated for a sexually transmitted infection and/or BV in the last 12 months. They were eligible for enrollment if they were confirmed HIV-negative and non-pregnant, were diagnosed with BV (Nugent score 7–10 and/or by modified Amsel criteria, defined as two of the following three criteria positive: vaginal pH > 4.5, positive whiff test, or ≥20% clue cells) and/or TV (by wet mount and/or culture), and were cured after seven days of oral metronidazole treatment (500 mg twice per day)5. Cure was defined as no BV by modified Amsel criteria and no TV by wet mount6. We did not use Nugent scores and TV culture as tests-of-cure to allow for same day enrollment but results became available after enrollment. At enrollment, 51/68 women were BV-negative by modified Amsel and Nugent criteria (score 0–6), 17/68 women were BV-negative by modified Amsel but BV-positive by Nugent criteria (score 7–10), and all women were TV-negative by both wet mount and culture and free of symptomatic vulvo-vaginal candidiasis, urinary tract infection, syphilis, and clinician-observed genital abnormalities or vaginal discharge. We did not exclude women with gonorrhea and/or chlamydia because the local testing turn-around time was slow. Positive herpes simplex type 2 serology was not a reason for exclusion. Additional exclusion criteria applied but these were rare (Fig. 1, Supplement 1).
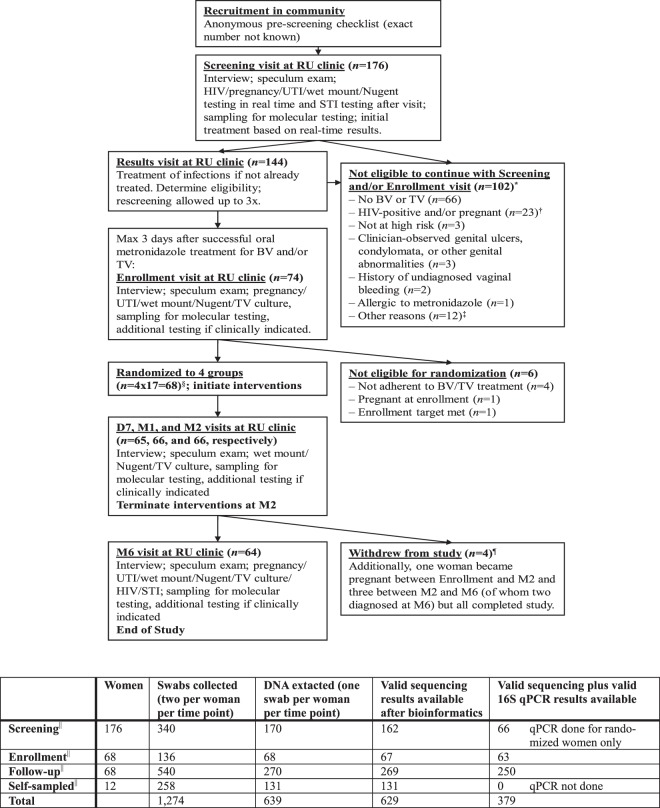
Figure 1.
Flow diagram of numbers of women seen, study procedures, and samples collected. Abbreviations: BV, bacterial vaginosis; D7, day 7 visit; DNA, desoxyribonucleic acid; M1/2/6, month 1/2/6 visit; qPCR, quantitative polymerase chain reaction; RU, Rinda Ubuzima; STI, sexually transmitted infection; TV, Trichomonas vaginalis; UTI, urinary tract infection. *Totals to 110 reasons among 102 women because there could be more than one reason per woman. †No speculum exam performed; molecular testing of self-sampled vaginal swabs. ‡Reasons: outside of metronidazole treatment window (n = 5), enrollment target already met (n = 4), has a mental disorder (n = 1), did not complete screening procedures and was subsequently lost to follow-up (n = 1), withdrew consent during the screening visit because she thought the reimbursement was too low (n = 1). §Three women in each randomization group were selected for self-sampling every other day during the first month of follow-up. ¶Reasons: moved away from Kigali (n = 2), lost interest because symptoms resolved (n = 1), was verbally harassed by partner and sister about study participation (n = 1). ||These were all physician-collected. See Supplement 1 for information about samples that should have been but were not collected or were lost during processing.
Randomization groups and visit schedule
Women were randomized to four groups (17 women per group) within three days of completing oral metronidazole treatment: (1) behavioral counseling only (control group); (2) counseling plus 500 mg oral metronidazole twice weekly for two months; (3) counseling plus EF+ vaginal capsule once per day for the first five days followed by thrice weekly for a total of two months; and 4) counseling plus GynLP once every four days for two months. Participants applied the first dose of their intervention under direct observation at the enrollment visit, and returned to the clinic after seven days (D7), one month (M1), two months (M2; cessation of product use), and six months (M6). They were allowed to cease vaginal product use temporarily during menstruation. Product adherence was assessed by review of diary cards, used and unused products, and by a self-rating adherence scale. Symptomatic BV, vulvovaginal candidiasis, and urinary tract infections, and laboratory-confirmed sexually transmitted infections, diagnosed during follow-up were treated using standard oral therapies, and women were urged to continue their interventions during treatment. Visit procedures are summarized in Fig. 1 and described in Supplement 1.
Diagnostic testing
Women were tested for BV, TV, and vulvovaginal candidiasis at each visit. BV was diagnosed by Gram stain Nugent scoring and modified Amsel criteria; the vaginal pH was measured by pressing a pH paper strip (pH range 3.6–6.1 with 0.3 increments; Machery-Nagel, Düren, Germany) against the vaginal wall. TV was diagnosed when motile trichomonads were observed on wet mount and by InPouch culture (Biomed Diagnostics, White City, OR, USA). Vulvovaginal candidiasis was diagnosed when budding yeasts and/or (pseudo)hyphae were observed on wet mount. All other diagnostic tests (Supplement 1) were only done at screening, M6, and when judged clinically necessary by the physician, with the exception of pregnancy and urinalysis tests, which were repeated at enrollment prior to randomization.
16S rRNA gene sequencing and qPCR
We collected vaginal swabs in duplicate but DNA was extracted from one swab per woman only at each time point (639 samples in total): screening (176 screened women and 170 samples), enrollment and four follow-up visits (68 randomized women and 338 samples), and self-sampled swabs from 12 women (three per randomization group) who had been asked to self-sample at home every Monday, Wednesday, and Friday during the first month (12 women and 131 samples) (Fig. 1). Briefly, DNA was extracted using a combination of lysozyme lysis, Qiagen DNeasy Blood and Tissue kit (Qiagen, Manchester, UK), and bead-beating procedures (Supplement 1). Purified DNA underwent two PCR rounds: amplification of the 16S rRNA gene V3-V4 region and dual-index barcoding allowing multiplexing of up to 384 samples. Samples were sequenced on an Illumina HiSeq2500 instrument (Illumina, San Diego, CA, USA), run in rapid mode, 2 × 300 bp using a 250PE and 50PE kit. All samples collected from the same participant were included in the same run. Negative and positive controls (ZymoBiomics Microbial Community DNA standard; Zymo Research Corp, Irvine, USA) were incorporated throughout. The panbacterial 16S rRNA gene copy concentrations of physician-collected samples of enrolled women with valid sequencing results (N = 393) were determined by BactQuant qPCR assay as previously described26.
Molecular data processing
Processing of sequencing reads, taxonomic assignments, and rarefaction are described in Supplement 1. This yielded 401 unique amplicon sequence variants (ASVs) in 629 samples, mapping to species (n = 255; 63.6%), genus (n = 116; 28.9%), or higher taxonomic level (n = 30; 7.5%). Concentrations in cells/μl per ASV per sample were estimated by multiplying the ASV-specific copy-normalized relative abundance by the sample-specific 16S rRNA gene copies concentration (explained in more detail in Supplement 1)27,28. Heatmaps of the 20 most common ASVs by sample are shown in Fig. S1A for relative abundances and Fig. S1B for estimated concentrations. VMB data reduction was required for molecular efficacy analyses, and was done in three different ways. First, the richness (number of ASVs) and Simpson diversity index (1-D) were calculated for each sample. Second, each ASV was assigned to one of four ‘bacterial groups’ based on the published literature (Supplement 2): (1) lactobacilli (all species combined, but with subcategorization into EF+ strains, the GynLP strain, and ‘natural lactobacilli’ in some analyses); (2) BV-anaerobes (fastidious anaerobes that have consistently been associated with BV); (3) pathobionts (bacteria that are considered more pathogenic than BV-anaerobes and that often co-occur with lactobacilli instead of BV-anaerobes, such as streptococci, staphylococci, enterococci, and Enterobacteriaceae)29; and (4) ‘other bacteria’ (a rest group, consisting mostly of skin bacteria and Bifidobacterium species). Within each sample, read counts of ASVs belonging to the same bacterial group were summed. This resulted in four relative abundances (one for each bacterial group) per sample, which sum to 1.0 for each sample. Third, we classified samples into eight mutually exclusive VMB types (each sample was assigned to only one VMB type): (1) L. iners-dominated (>75% lactobacilli of which L. iners was the most common; n = 247 samples); (2) L. crispatus-dominated (>75% lactobacilli of which L. crispatus was the most common; n = 17); (3) other lactobacilli-dominated (>75% lactobacilli of which L. jensenii and L. gasseri were the most common; n = 28); (4) lactobacilli plus BV-anaerobes (25–75% lactobacilli; n = 86); (5) polybacterial with ≥10% Gardnerella vaginalis (<25% lactobacilli and 10–50% G. vaginalis; n = 138), (6) polybacterial with <10% G. vaginalis (<25% lactobacilli and <10% G. vaginalis; n = 23), (7) G. vaginalis-dominated (<25% lactobacilli and >50% G. vaginalis; n = 41); and (8) ≥20% pathobionts (n = 49). The samples assigned to VMB types 1–7 had a maximum of 0–18.2% pathobionts per VMB type. To improve statistical power and to facilitate visualizations, the eight VMB types were condensed further into four ‘pooled VMB types’ as follows: (1) lactobacilli-dominated (original VMB types 1–3 combined; n = 292), (2) lactobacilli plus BV-anaerobes (original VMB type 4; n = 86); (3) BV-like (original VMB types 5–7 combined; n = 202); and ≥20% pathobionts (original VMB type 8; n = 49).
Downstream statistical analysis
Data were analyzed using STATA v13.0 (StataCorp, College Station, TX, USA). The trial safety endpoints were self-reported solicited and unsolicited (serious) adverse events and social harms, and clinician-observed speculum exam findings. Adverse events were coded using the Medical Dictionary for Regulatory Activities (medDRA v19.1, McLean, VA, USA). The primary preliminary efficacy endpoints were incident BV by Nugent score 7–10 or by modified Amsel criteria (binary variables). The secondary preliminary efficacy endpoints were the VMB composition variables derived from the sequencing data: the four bacterial group relative abundances and estimated concentrations (continuous variables) and VMB type or pooled VMB type (categorical variables). For cross-sectional analyses, we used Fisher’s exact test for binary and categorical variables, and Kruskal-Wallis and Mann Whitney U tests for continuous variables. For longitudinal analyses, we used incidence rates and incidence rate ratios (which only included samples collected at study visits; Fig. 1), and mixed effects models (which included all samples, including those that were self-collected in between study visits, but Nugent scores and estimated concentrations were not available for self-collected samples; Fig. 1). Most analyses were conducted on the intent-to-treat (ITT) population (N = 68), but the BV by Nugent score and modified Amsel criteria incidence analyses were conducted on the modified ITT population (which was limited to women who were BV-negative by both modified Amsel and Nugent criteria at the time of randomization; n = 51). We compared each intervention group to the control group for one VMB endpoint at a time at screening, enrollment, and longitudinally over time. All mixed effects models included one VMB endpoint as the outcome, participant identification number as the random effect, and randomization group (an indicator variable with the control group as the reference group) as the main fixed effect, and adjusted for covariates that were associated with at least one VMB endpoint in mixed effects models at p < 0.05 (Supplement 1).
Ethical statement
All participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, sponsored by the University of Liverpool, approved by the Rwanda National Ethics Committee and the University of Liverpool Research Ethics Subcommittee for Physical Interventions, and first posted on ClinicalTrials.gov (NCT02459665) on 2 June 2015.
Results
Participant disposition
Of the 68 randomized women, only four did not complete the trial (Fig. 1), resulting in 29.93 person-years of data. The median age was 31 (interquartile range (IQR) 27–35) (Tables 1 and S1). The majority of women (92.6%) had exchanged sex for money or goods, and had had a median of five (IQR 2–18) sex partners, in the past month. All but three women used condoms, but mostly inconsistently. Two-thirds of the women (61.8%) were using hormonal contraception, and four women became pregnant during the trial. Short course metronidazole/tinidazole use for other indications during the intervention period was evenly distributed among randomization groups (Table S2: n = 1–3 per group; p = 0.688), as was short course use of other oral antibiotics (Table S2: n = 2–4 per group; p = 0.781). Furthermore, these other antibiotics did not impact lactobacilli and BV-anaerobes estimated concentrations significantly (Table S2, Fig. S2). No antifungals were used. Most women adhered with their study product >90% of the time, but this percentage was non-significantly lower in the GynLP group (68.8%) than in the metronidazole (82.4%; Fisher’s exact p = 0.438) and EF+ groups (88.2%, Fisher’s exact p = 0.225; Table S3).
Table 1.
Baseline characteristics.
| Sociodemographic characteristics at Scr/Enr | Screened (n = 175)* |
Enrolled (n = 68) |
Controls (n = 17) |
Metro (n = 17) |
EF+ (n = 17) |
GynLP (n = 17) |
p† |
|---|---|---|---|---|---|---|---|
| Age in years, median (IQR) | 30 (27 – 34) | 31 (27 – 35) | 29 (24 – 36) | 30 (27 – 34) | 33 (28 – 35) | 30 (27 – 35) | 0.563 |
| Sex partners last mo, median (IQR) | 5 (2 – 16) | 5 (2 – 18) | 5 (3 – 20) | 5 (2 – 10) | 3 (2 – 15) | 3 (2 – 20) | 0.624 |
| Condom during last sex act, n (%)* | 95 (54.6) | 36 (52.9) | 11 (64.7) | 8 (47.1) | 6 (35.3) | 11 (64.7) | 0.279 |
| Currently using contraception, n (%) | 0.750 | ||||||
| None | 69 (40.2) | 24 (35.3) | 6 (35.3) | 6 (35.3) | 4 (23.5) | 8 (47.1) | |
| Hormonal contraception | 99 (57.6) | 42‡ (61.8) | 10 (58.8) | 11 (64.7) | 12 (70.6) | 9 (52.9) | |
| Copper IUD | 4 (2.3) | 2 (2.9) | 1 (5.9) | 0 | 1 (5.9) | 0 | |
| Laboratory results at Scr |
Screened (n = 173) |
Enrolled (n = 68) |
Controls (n = 17) |
Metro (n = 17) |
EF+ (n = 17) |
GynLP (n = 17) |
p† |
| Nugent score, mean (95% CI)* | 4.7 (4.1 – 5.3) | 7.4 (6.8 – 8.0) | 7.6 (6.5 – 8.6) | 6.8 (5.3 – 8.3) | 7.1 (5.5 – 8.6) | 8.2 (7.4 – 9.0) | 0.333 |
| Nugent categories, n (%)* | 0.075 | ||||||
| 0–3 | 55 (38.2)§ | 5 (7.5) | 1 (5.9) | 2 (11.8) | 2 (12.5) | 0 | |
| 4–6 | 20 (13.9) | 6 (9.0) | 0 | 4 (23.5) | 0 | 2 (11.8) | |
| 7–10 | 69 (47.9) | 56 (83.5) | 16 (94.1) | 11 (64.7) | 14 (87.5) | 15 (88.2) | |
| BV by modified Amsel criteria, n (%) | 30 (20.6)‖ | 20 (29.4) | 4 (23.5) | 6 (35.5) | 3 (17.7) | 7 (41.2) | 0.486 |
| Candida on wet mount, n (%) | 14 (9.6)‖ | 6 (8.8) | 1 (5.9) | 2 (11.8) | 3 (17.7) | 0 | 0.493 |
| TV on wet mount, n (%) | 9 (6.2)‖ | 6 (8.8) | 3 (17.7) | 1 (5.9) | 1 (5.9) | 1 (5.9) | 0.707 |
| TV by InPouch culture, n (%) | 17 (11.8)§ | 11 (16.4) | 3 (18.8) | 1 (5.9) | 5 (29.4) | 2 (11.8) | 0.282 |
| UTI by dipstick, n (%) | 33 (19.1) | 17 (25.0) | 4 (23.5) | 6 (35.3) | 4 (23.5) | 3 (17.7) | 0.760 |
| CT by PCR, n (%) | 30 (20.8)§ | 20 (29.4) | 5 (29.4) | 7 (41.2) | 3 (17.7) | 5 (29.4) | 0.560 |
| NG by PCR, n (%) | 18 (12.5)§ | 13 (19.1) | 5 (29.4) | 4 (23.5) | 2 (11.8) | 2 (11.8) | 0.555 |
| HIV serology positive, n (%) | 17 (9.8) | 0 | 0 | 0 | 0 | 0 | NA |
| HSV2 serology positive, n (%) | 117 (67.6) | 44 (64.7) | 9 (52.9) | 12 (70.6) | 11 (64.7) | 12 (70.6) | 0.780 |
| Syphilis serology positive, n (%) | 13 (7.5) | 4 (5.9) | 0 | 1 (5.9) | 0 | 3 (17.7) | 0.182 |
| Pregnancy test positive, n (%) | 7 (4.1) | 0 | 0 | 0 | 0 | 0 | NA |
| Laboratory results at Enr** | Screened |
Enrolled (n = 68) |
Controls (n = 17) |
Metro (n = 17) |
EF+ (n = 17) |
GynLP (n = 17) |
p† |
| Nugent score, mean (95% CI)* | NA | 3.1 (2.2 – 3.9) | 3 (1.2 – 4.8) | 3.3 (1.4 – 5.2) | 1.7 (0.1 – 3.3) | 4.3 (2.6 – 6.0) | 0.187 |
| Nugent categories, n (%)* | |||||||
| 0–3 | NA | 36 (54.6) | 8 (53.3) | 9 (52.9) | 13 (76.5) | 6 (35.3) | 0.149 |
| 4–6 | 13 (19.7) | 5 (33.3) | 2 (11.8) | 1 (5.9) | 5 (29.4) | ||
| 7–10 | 17 (25.8) | 2 (13.3) | 6 (35.3) | 3 (17.7) | 6 (35.3) | ||
| BV by modified Amsel criteria, n (%) | NA | 0 | 0 | 0 | 0 | 0 | NA |
| Candida on wet mount, n (%) | NA | 4 (5.9) | 2 (11.8) | 1 (5.9) | 0 | 1 (5.9) | 0.897 |
| TV by wet mount/culture, n (%) | NA | 0 | 0 | 0 | 0 | 0 | NA |
| VMB outcomes at Enr | Screened |
Enrolled (n = 67)* |
Controls (n = 17) |
Metro (n = 17) |
EF+ (n = 17) |
GynLP (n = 16)* |
p† |
| Richness, mean (95% CI) | NA |
12.8 (10.7 – 14.8) |
17.8 (12.1 – 23.5) |
12.1 (8.7 – 15.5) |
7.5‡‡ (4.9 – 10.2) |
13.7 (10.3 – 17.1) |
0.003 |
| Simpson diversity index, mean (95% CI) | NA |
0.31 (0.25 – 0.38) |
0.38 (0.21 – 0.54) |
0.35 (0.20 – 0.50) |
0.15§§ (0.05 – 0.25) |
0.38 (0.25 – 0.50) |
0.022 |
| Total bacteria estimated conc in log10/μL, mean (95% CI)†† | NA |
5.85 (5.66 – 6.04) |
5.75 (5.30 – 6.20) |
5.79 (5.39 – 6.19) |
5.54 (5.28 – 5.80) |
6.34‡‡ (5.95 – 6.73) |
0.019 |
| Total Lactobacillus estimated conc in log10/μL, mean (95% CI)†† | NA |
5.56 (5.34 – 5.78) |
5.22 (4.63 – 5.82) |
5.59 (5.20 – 5.97) |
5.46 (5.19 – 5.73) |
5.97 (5.44 – 6.49) |
0.092 |
| Total BV-anaerobes estimated conc in log10/μL, mean (95% CI)†† | NA |
4.55 (4.15 – 4.95) |
4.78 (4.17 – 5.39) |
4.50 (3.77 – 5.24) |
3.36‡‡ (2.43 – 4.29) |
5.62‡‡ (4.99 – 6.25) |
0.002 |
| Total pathobionts estimated conc in log10/μL, mean (95% CI)†† | NA |
2.01 (1.48 – 2.54) |
2.36 (1.28 – 3.44) |
2.08 (1.00 – 3.17) |
1.34 (0.29 – 2.39) |
2.30 (0.98 – 3.62) |
0.447 |
| Total other bacteria estimated conc in log10/μL, mean (95% CI)†† | NA |
1.46 (1.01 – 1.92) |
1.80 (0.84 – 2.76) |
1.30 (0.36 – 2.24) |
0.57 (-0.10 – 1.24) |
2.20 (1.07 – 3.34) |
0.066 |
| VMB types, n (%): | NA | 0.048 | |||||
| L. iners-dominated | 35 (52.2) | 7 (41.2) | 9 (52.9) | 14 (82.4) | 5 (31.3) | ||
| L. crispatus-dominated | 0 | 0 | 0 | 0 | 0 | ||
| Other lactobacilli-dominated | 2 (3.0) | 1 (5.9) | 1 (5.9) | 0 | 0 | ||
| Lactobacilli plus BV-anaerobes | 18 (26.9) | 3 (17.7) | 5 (29.4) | 2 (11.8) | 8 (50.0) | ||
| Polybacterial with ≥10% G. vag | 2 (3.0) | 1 (5.9) | 0 | 0 | 1 (5.9) | ||
| Polybacterial with <10% G. vag | 0 | 0 | 0 | 0 | 0 | ||
| G. vaginalis-dominated | 4 (6.0) | 2 (11.8) | 0 | 0 | 2 (12.5) | ||
| ≥20% pathobionts | 6 (9.0) | 3 (17.7) | 2 (11.8) | 1 (5.9) | 0 |
Abbreviations: CI, confidence interval; conc, concentration; CT, Chlamydia trachomatis; EF+, Ecologic Femi+; Enr, enrollment visit; G. vag, G. vaginalis; GynLP, Gynophilus LP; HSV2, herpes simplex virus type 2; IQR, inter-quartile range; Metro, metronidazole; Mo, month; NA, not applicable; NG, Neisseria gonorrhoeae; Scr, screening visit; TV, Trichomonas vaginalis; UTI, urinary tract infection; VMB, vaginal microbiota. *Total numbers are slightly lower due to 1–4 missing values. 176 women were screened. 68 women were randomized but one woman did not have sequencing data at screening and enrollment. †Fisher’s exact test for binary/categorical outcomes and Kruskall Wallis test for continuous outcomes, between randomization groups. ‡‡Seven women reported using oral contraception, 18 hormonal injections, and 17 hormonal implants. §n = 144; women at the screening visit that were HIV-positive and/or pregnant, or were otherwise ineligible, did not undergo testing for this urogenital disease. ||n = 146; women at the screening visit who were HIV-positive and/or pregnant, or were otherwise ineligible, did not undergo testing for this urogenital disease. **All enrolled participants were negative for HIV and pregnancy, and were treated for UTI and syphilis if positive at screening. Twenty-three enrolled participants (six controls, seven in the metronidazole group, four in the EF+ group, and six in the GynLP group) were positive for CT and/or NG at screening and had not received treatment by the enrollment visit. ††Total numbers are slightly lower due to invalid qPCR results: overall enrollment population is n = 63, control group n = 16, and metronidazole group n = 14. ‡‡p < 0.05 by Mann Whitney U test, compared to control group. §§p < 0.01 by Mann Whitney U test, compared to control group.
VMB compositions at baseline
By design, all 68 randomized women at screening had BV (by Nugent and/or modified Amsel criteria) and/or TV (by wet mount and/or culture): 56 women (82.4%) had BV alone, 10 women (14.7%) had BV and TV, and two women (2.9%) had TV alone. Therefore, as expected, most women had dysbiotic VMB types by 16S rRNA gene sequencing at screening: polybacterial with ≥10% G. vaginalis (28/67; 41.8%), lactobacilli plus BV-anaerobes (12/67; 17.9%), polybacterial with <10% G. vaginalis (8/67; 11.9%), G. vaginalis-dominated (8/67; 11.9%), and ≥20% pathobionts (1/67; 1.5%). However, 10/67 women (14.9%) had a L. iners-dominated VMB type, of whom six were TV-negative, and these women might not have needed metronidazole treatment. Also by design, all randomized women were BV-negative (by modified Amsel criteria) and TV-negative (by wet mount and culture) at the time of randomization. However, almost half of the women (30/67; 44.8%) were not lactobacilli-dominated by sequencing at the time of randomization, and none of them were L. crispatus-dominated (which is considered the most optimal VMB state): their VMB types were L. iners-dominated (35/67; 52.2%), dominated by other lactobacilli (2/67; 3.0%), lactobacilli plus BV-anaerobes (18/67; 26.9%), ≥20% pathobionts (6/67; 9.0%), G. vaginalis-dominated (6/67; 6.0%), and polybacterial with ≥10% G. vaginalis (2/67; 3.0%) (Table 1).
At randomization, the estimated mean total bacterial concentration ranged from 5.54–6.34 log10 cells/μl in the four randomization groups, and these ranges were 5.22–5.97 for lactobacilli, 3.36–5.62 for BV-anaerobes, 1.34–2.36 for pathobionts, and 0.57–2.20 for ‘other bacteria’ (Table S4; relative abundance data in Table S5). The mean richness ranged from 7.5–17.8, and the mean Simpson diversity from 0.15–0.38. Randomization did not completely balance the baseline VMB compositions of the four groups: the estimated concentrations of total bacteria and BV-anaerobes were higher in the GynLP group than the control group (Mann Whitney U test p < 0.05), and the estimated BV-anaerobes concentration (p < 0.01) and Simpson diversity (p < 0.05) were lower in the EF+ group than the control group (Table 1).
Variables that were associated with at least one VMB endpoint in unadjusted mixed effects models (Table S6) included currently using hormonal contraception or being pregnant (associated with a higher estimated pathobionts concentration), above-average sexual risk taking based on reported numbers of partners and condom use (higher estimated pathobionts concentration), aged 30 years or older (lower estimated BV-anaerobes and pathobionts concentrations), and managing menses with a sanitary pad compared to other methods (higher Nugent score). Reporting current urogenital symptoms was also associated with VMB composition but this likely represents reverse causality. We could not exclude women with ongoing chlamydia and/or gonorrhea infections at randomization because the turn-around time of diagnostic testing was slow, but the VMB compositions after metronidazole treatment of women with and without ongoing chlamydia and/or gonorrhea infection were similar (Fig. S2).
Safety
Two serious adverse events occurred but these were judged not to be related to study participation: one woman in the oral metronidazole group was hospitalized for typhoid fever and one woman in the EF+ group for malaria during pregnancy. Both events occurred after the intervention period and both women recovered completely. Two women reported non-serious social harms that were judged related to study participation. One woman in the control group was beaten by her partner because she engaged in self-sampling; she withdrew from self-sampling but continued participation in the study. One woman in the GynLP group suffered verbal harassment from her partner and her sister for taking part in the study and elected to withdraw.
During the intervention period, urogenital symptoms were reported by 13.4% of participants (genital itching, burning, and pain during sex but no vaginal discharge) with no differences between groups, and only two speculum exam and no bimanual exam findings were reported by the physician (Table 2). After product cessation, urogenital symptom reporting was similar compared to the intervention period (10.8%), but the number of speculum exam findings increased (32.8%), probably reflecting the high urogenital infection incidence in this cohort. A total of 41 adverse events were spontaneously reported between enrollment and M6. All of them were judged definitely not or unlikely to be related to trial participation, and they were evenly distributed among groups.
Table 2.
Safety endpoints.
| Patient-reported symptoms and clinician-observed signs at Screening and Enrollment | Total (n = 68) | Controls (n = 17) | Metro (n = 17) | EF+ (n = 17) | GynLP (n = 17) | p* |
|---|---|---|---|---|---|---|
| Any (current or in last 2 weeks) urogenital symptoms at Scr, n (%) | 49 (72.1)† | 11 (64.7) | 13 (76.5) | 13 (76.5) | 12 (70.6) | 0.933 |
| Any (current) urogenital symptoms at Enr, n (%) | 0 | 0 | 0 | 0 | 0 | NA |
| Any abnormal speculum exam findings at Scr, n (%)‡ | 3 (4.4)§ | 1 (5.9) | 1 (5.9) | 0 | 1 (5.9) | 1 |
| Any abnormal speculum exam findings at Enr, n (%)‡ | 1 (1.5)¶ | 0 | 0 | 0 | 1 (5.9) | 1 |
| AEs – Structurally assessed between Enr and M2 (during product use) | ||||||
| Any current urogenital symptoms, n (%)|| | 9 (13.4)** | 2 (11.8) | 2 (11.8) | 2 (11.8) | 3 (18.8) | 0.894 |
| Any abnormal speculum exam findings, n (%)|| | 2 (3.0)†† | 0 | 0 | 0 | 2 (12.5) | 0.054 |
| Any abnormal bimanual exam findings n (%)|| | 0 | 0 | 0 | 0 | 0 | NA |
| AEs – Structurally assessed between M2 and M6 (after product cessation) | ||||||
| Any current urogenital symptoms, n (%)|| | 7 (10.8)‡‡ | 3 (17.7) | 1 (5.9) | 1 (6.3) | 2 (13.3) | 0.7 |
| Any abnormal speculum exam findings, n (%)|| | 21 (32.8)§§ | 6 (35.3) | 5 (31.3) | 6 (37.5) | 4 (26.7) | 0.951 |
| Any abnormal bimanual exam findings n (%)|| | 1 (1.6)¶¶ | 0 | 0 | 0 | 1 (6.7) | 0.234 |
| AEs – Not structurally assessed | Total (N) | Controls (N) | Metro (N) | EF+ (N) | GynLP (N) | p* |
| Number of women with reported AEs|||| | 27 | 8 | 4 | 8 | 7 | 0.439 |
| Total number reported AEs between Enr-M6 | 41 | 13 | 6 | 9 | 13 | 0.324 |
| Total number reported AEs between Enr-M2 | 32 | 12 | 4 | 5 | 11 | NA |
| Total number reported AEs between M2-M6*** | 9 | 1 | 2 | 4 | 2 | NA |
| AEs – Not structurally assessed | Total AEs (n = 41) | Controls (n = 13) | Metro (n = 6) | EF+ (n = 9) | GynLP (n = 13) | p* |
| Severity of reported AEs, n (%): | NA | |||||
| Mild | 2 (4.9) | 0 | 0 | 1 (11.1) | 1 (7.7) | |
| Moderate | 36 (87.8) | 13 (100) | 5 (83.3) | 6 (66.7) | 12 (92.3) | |
| Severe | 3 (7.3) | 0 | 1 (16.7) | 2 (22.2) | 0 | |
| Life-threatening | 0 | 0 | 0 | 0 | 0 | |
| Deemed related to study by physician, n (%): | NA | |||||
| Definitely not related | 10 (24.4) | 3 (23.1) | 2 (33.3) | 3 (33.3) | 2 (15.4) | |
| Unlikely | 31 (75.6) | 10 (76.9) | 4 (66.7) | 6 (66.7) | 11 (84.6) | |
| Possible/probable/definitely related | 0 | 0 | 0 | 0 | 0 | |
| Outcome of reported AE, n (%): | NA | |||||
| Fully recovered | 40 (97.6) | 12 (92.3) | 6 (100) | 9 (100) | 13 (100) | |
| Not fully recovered, deteriorated, permanent damage, or death | 0 | 0 | 0 | 0 | 0 | |
| Ongoing | 1 (2.4) | 1 (7.7)††† | 0 | 0 | 0 | |
| Action taken by physician, n (%): | NA | |||||
| None | 6 (14.6) | 1 (7.7) | 0 | 2 (22.2) | 3 (23.1) | |
| Medication given | 35 (85.4) | 12 (92.3) | 6 (100) | 7 (77.8) | 10 (76.9) | |
| Study discontinuation | 0 | 0 | 0 | 0 | 0 | |
| Hospitalization‡‡‡ | 0 | 0 | 0 | 0 | 0 | |
Abbreviations: AE, adverse event; EF+, Ecologic Femi+; Enr, enrollment visit; GynLP, Gynophilus LP; M2/6, month 2/6 visit; Metro, metronidazole; NA, not applicable; Scr, screening visit. *Fisher’s exact test for binary outcomes and Kruskall Wallis test for continuous outcomes, between groups. †Most common symptom (89.8%) was genital itching. ‡No abnormal findings observed during bimanual exams. §Includes vaginal discharge (n = 2) and cervical polyps (n = 1). ¶Unusual cervical discharge. ||Total numbers are slightly lower due to loss to follow-up (Fig. 1). No missing values. **Includes genital itching (n = 8) and burning (n = 3), pain during sex (n = 4), and burning when urinating, lower abdominal pain, unusual vaginal discharge, and genital/anal sores (all n = 1). ††One had ulcers/blisters in the vagina, one had lesions on the perineum and labia majora. ‡‡Includes genital itching (n = 5) and burning (n = 3), lower abdominal pain (n = 4), pain during sex, burning when urinating, urinary frequency/urgency (all n = 2), and unusual vaginal discharge (n = 1). §§Includes unusual vaginal (n = 4) and cervical (n = 13) discharge, and cervicitis (n = 10). ¶¶Cervical motion tenderness. ||||Most common AEs according to MedDRA coding were “Infections and infestations” (n = 14), followed by “Reproductive system and breast disorders” (n = 9) and “Gastrointestinal disorders” (n = 7; two in the no-intervention group, none in the metronidazole group, two in the EF+ group, and three in the GynLP group). No headaches were reported. Sexually transmitted infections and vulvovaginal candidiasis were not considered AEs but secondary outcomes. ***No AEs were reported after the M6 visit. †††Case of dental caries. ‡‡‡Both serious AEs involved hospitalizations that were not initiated by the study physician (see manuscript text).
Preliminary efficacy: microscopy endpoints
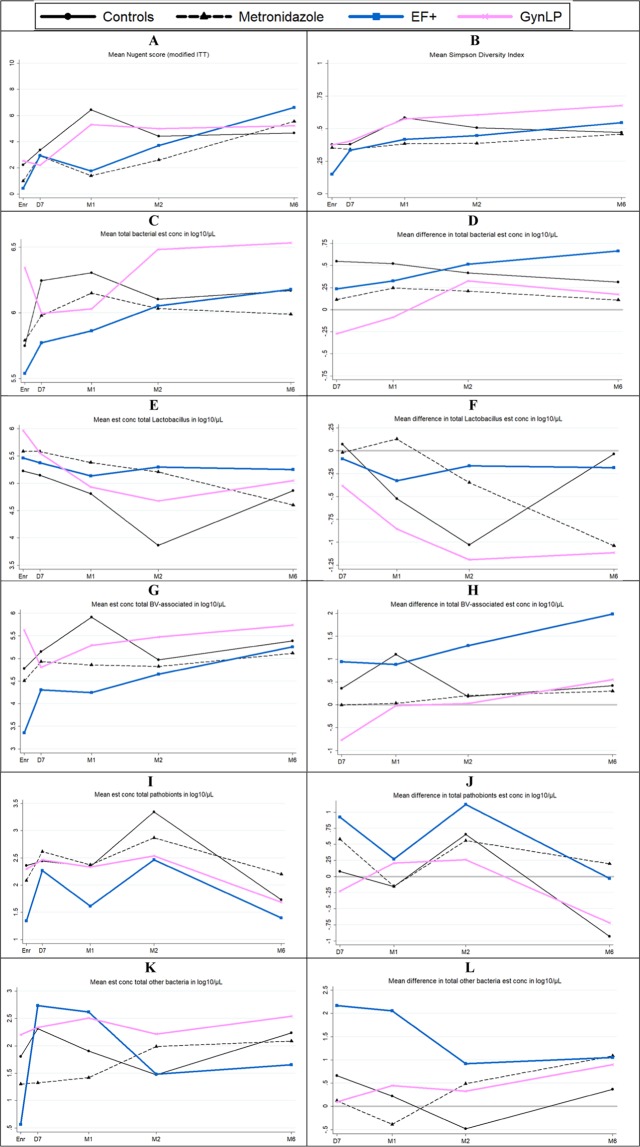
In modified ITT analyses, the BV incidence rate by Nugent score during the intervention period was 10.18 per person-year at risk (PY) in the control group, and lower in the metronidazole (1.41/PY; p = 0.004), EF+ (3.58/PY; p = 0.043), and GynLP groups (5.36/PY; p = 0.220) (Table 3). Mean Nugent scores during the intervention period were highest in the control group, lowest in the metronidazole group, and in-between in the two vaginal probiotics groups (Fig. 2A). By the end of the intervention period, many women had developed microbiological-BV without symptoms. In line with standard practice, they were not treated, but they were also no longer included in the ‘person-years at risk’ denominator because they had already developed the endpoint. BV incidence rates were therefore much lower (1.26/PY overall) after cessation of the intervention, and similar between groups. The results for BV incidence by modified Amsel criteria were similar to those for Nugent scores (Table 3), and no vulvovaginal candidiasis was diagnosed after randomization.
Table 3.
Preliminary efficacy – microscopy endpoints.
| BV IR Enr–M2 | Controls | Metronidazole | EF+ | GynLP | ||||
|---|---|---|---|---|---|---|---|---|
| n/N* | IR (95% CI)† | n/N* | IR (95% CI)† | n/N* | IR (95% CI)† | n/N* | IR (95% CI)† | |
| Nugent 7–10 | 9/11 | 10.18 (5.48–18.92) | 2/10 | 1.41 (0.35–5.62) | 5/12 | 3.58 (1.61–7.96) | 6/10 | 5.36 (2.41–11.93) |
| Modified Amsel¶ | 11/15 | 7.53 (4.28–13.26) | 3/11 | 2.04 (0.66–6.31) | 6/13 | 3.36 (1.51–7.48) | 4/9 | 3.35 (1.26–8.92) |
| BV IR M2–M6 | ||||||||
| Nugent 7–10 | 4/11 | 0.91 (0.34–2.41) | 5/8 | 1.86 (0.78–4.48) | 6/9 | 1.58 (0.71–3.52) | 2/7 | 0.76 (0.19–3.03) |
| Modified Amsel¶ | 3/12 | 2.96 (0.33–3.15) | 5/10 | 2.25 (0.94–5.40) | 6/8 | 3.84 (1.72–8.54) | 3/8 | 1.83 (0.59–5.67) |
| BV IRR Enr–M2 | Controls | Metronidazole vs. Controls | EF+ vs. Controls | GynLP vs. Controls | ||||
| IRR (95% CI)‡ | IRR (95% CI)‡ | IRR (95% CI)‡ | ||||||
| Nugent 7–10 | NA | 0.14 (0.01–0.65) | 0.35 (0.10–1.07) | 0.53 (0.16–1.60) | ||||
| Modified Amsel¶ | NA | 0.27 (0.05–1.00) | 0.45 (0.14–1.28) | 0.44 (0.10–1.47) | ||||
| BV IRR M2–M6 | ||||||||
| Nugent 7–10 | NA | 2.06 (0.44–10.37) | 1.74 (0.41–8.41) | 0.84 (0.08–5.85) | ||||
| Modified Amsel¶ | NA | 2.22 (0.43–14.27) | 3.78 (0.81–23.35) | 1.80 (0.24–13.46) | ||||
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; EF+, Ecologic Femi+; Enr, enrollment visit; GynLP, Gynophilus LP; IR, incidence rate; IRR, incidence rate ratio; ITT, intent to treat; M2/6, month 2/6 visit; NA, not applicable. There were no cases of vulvovaginal candidiasis during follow-up. The table presents modified ITT results: Participants who were BV-negative by modified Amsel but BV-positive by Nugent score 7–10 at the enrollment visit (n = 17) were omitted from modified ITT analyses. *Number of women (n) who developed at least one incident infection during the specified time period as a proportion of the women who had at least one follow-up visit in that time period (N). †Incident infections divided by person-years at risk. Three participants in the Enr–M2 BV by modified Amsel model, seven in the Enr – M2 BV by Nugent model, 10 in the M2–M6 BV by modified Amsel model, and 11 in the M2–M6 BV by Nugent model were omitted due to having been at risk for less than 10 person-days. ¶BV by modified Amsel criteria is defined as at least two of the following three laboratory criteria are positive: vaginal pH > 4.5, whiff test positive, and/or presence of >20% clue cells on wet mount. ‡Compared to the control group.
Figure 2.
Preliminary efficacy by Nugent score, alpha diversity, and bacterial group estimated concentration. Abbreviations: D7, Day 7 visit; EF+, Ecologic Femi+; Enr, enrollment visit; est conc, estimated concentration; GynLP, Gynophilus LP; ITT, intention to treat; M1/2/6, month 1/2/6 visit; Scr, screening visit; VMB, vaginal microbiota. Changes in VMB outcomes over time per randomization group. See Supplement Table S4 for 95% confidence intervals. (A) Mean Nugent scores over time, only including women (n = 51) who were BV-negative by modified Amsel and Nugent criteria (modified ITT analysis). (B) Mean alpha diversity over time. (C) Mean estimated bacterial cell concentration over time. (D) Difference in mean estimated bacterial cell concentration with enrollment, over time. (E) Mean estimated lactobacilli concentration over time. (F) Difference in mean estimated lactobacilli concentration with enrollment, over time. (G) Mean estimated BV-anaerobes concentration over time. (H) Difference in mean estimated BV-anaerobes concentration with enrollment, over time. (I) Mean estimated pathobionts concentration over time. (J) Difference in mean estimated pathobionts concentration with enrollment, over time. (K) Mean estimated other bacteria concentration over time. (L) Difference in mean estimated other bacteria concentration with enrollment, over time.
Preliminary efficacy: sequencing endpoints
We took the baseline VMB composition imbalances into account by not only showing bacterial group means at each visit but also mean differences with enrollment (Fig. 2 and Table S4 for estimated concentrations, and Fig. S3 and Table S5 for relative abundances), and by using mixed effects models including participant identification number as the random effect to determine if any changes were statistically significant (Table 4). Immediately after BV treatment completion, the VMBs of most women gradually worsened (lactobacilli declined and BV-anaerobes expanded), probably due to the high-risk nature of the cohort. The mean estimated lactobacilli concentration declined to a low of 3.86 log10 cells/μl at M2 in the control group, but less so in the intervention groups (ranging from 4.60–5.58 log10 cells/μl at follow-up visits). In unadjusted mixed effects models using data from the intervention period only, metronidazole users had a higher estimated lactobacilli concentration (p = 0.043) and relative abundance (p = 0.006) than controls, EF+ users had a higher relative abundance (p = 0.014) but not estimated concentration than controls, and GynLP users had trends in the same directions that were not statistically significant. The expansion of BV-anaerobes was significantly lower in oral metronidazole users (relative abundance; p = 0.023), and in EF+ users (estimated concentration; p = 0.041), compared to controls. Mean estimated pathobionts concentrations were low in all groups throughout, ranging from 1.61–3.35 log10 cells/μl at follow-up visits. Mixed effects models did not identify any significant associations between randomization groups and estimated pathobionts concentrations or relative abundances, but showed trends (0.05 < p < 0.1) towards lower pathobionts relative abundances in the two vaginal probiotics groups compared to controls (Table 4). Mean estimated concentrations of ‘other bacteria’ were also low throughout, but highest in the EF+ group at the D7 and M1 visits (EF+ contains a Bifidobacterium strain). This was significant in unadjusted mixed effects models for relative abundances (p = 0.023) but not estimated concentrations.
Table 4.
Preliminary efficacy – mixed effects models.
| Unadjusted mixed effects models | Metro | EF+ | GynLP | |||
|---|---|---|---|---|---|---|
| OR (95% CI)* | p* | OR (95% CI)* | p* | OR (95% CI)* | p* | |
| Nugent score† | 0.16 (0.02–1.09) | 0.062 | 0.25 (0.04–1.66) | 0.151 | 1.68 (0.24–11.56) | 0.599 |
| Nugent score categories† | ||||||
| 4–6 vs. 0–3 | 1.04 (0.20–5.50) | 0.960 | 0.36 (0.05–2.43) | 0.293 | 2.16 (0.38–12.31) | 0.387 |
| 7–10 vs. 0–3 | 0.08 (0.01–0.80) | 0.032 | 2.16 (0.38–12.31) | 0.148 | 1.12 (0.15–8.28) | 0.913 |
| Total bacterial est conc†‡ | 0.85 (0.53–1.36) | 0.497 | 0.72 (0.45–1.14) | 0.160 | 0.96 (0.60–1.55) | 0.877 |
| Total Lactobacillus est conc†‡ | 2.14 (1.02–4.49) | 0.043 | 1.86 (0.90–3.86) | 0.095 | 1.43 (0.67–3.04) | 0.352 |
| Total BV-anaerobes est conc†‡ | 0.58 (0.23–1.49) | 0.260 | 0.38 (0.15–0.96) | 0.041 | 0.89 (0.34–2.31) | 0.812 |
| Total pathobionts est conc†‡ | 0.91 (0.30–2.75) | 0.865 | 0.57 (0.19–1.71) | 0.318 | 0.79 (0.25–2.43) | 0.676 |
| Total other bacteria est conc†‡ | 0.72 (0.27–1.90) | 0.507 | 1.42 (0.55–3.70) | 0.471 | 1.60 (0.60–4.29) | 0.349 |
| Total Lactobacillus RA§¶ | 1.36 (1.09–1.70) | 0.006 | 1.32 (1.06–1.64) | 0.014 | 1.10 (0.88–1.38) | 0.408 |
| Total BV-anaerobes RA§¶ | 0.79 (0.65–0.97) | 0.023 | 0.83 (0.68–1.01) | 0.067 | 1.00 (0.81–1.22) | 0.973 |
| Total pathobionts RA§¶ | 0.93 (0.84–1.03) | 0.159 | 0.92 (0.83–1.01) | 0.093 | 0.92 (0.83–1.02) | 0.100 |
| Total other bacteria RA§¶ | 1.00 (1.00–1.01) | 0.739 | 1.01 (1.00–1.01) | 0.023 | 1.00 (1.00–1.01) | 0.671 |
| Pooled VMB type§ | ||||||
| LA vs. LD | 1.17 (0.24–5.78) | 0.849 | 1.20 (0.24–5.92) | 0.823 | 1.72 (0.33–9.04) | 0.520 |
| BV vs. LD | 0.02 (0.00–0.43) | 0.012 | 0.04 (0.00–0.73) | 0.029 | 0.39 (0.03–5.50) | 0.487 |
| PB vs. LD | 0.06 (0.00–1.54) | 0.090 | 0.08 (0.00–1.77) | 0.109 | 0.14 (0.01–3.34) | 0.225 |
| Simpson diversity§ | 0.90 (0.77–1.06) | 0.200 | 0.94 (0.80–1.10) | 0.454 | 1.07 (0.91–1.26) | 0.429 |
| Adjusted mixed effects models** | ||||||
| Nugent score† | 0.19 (0.03–1.31) | 0.092 | 0.26 (0.04–1.85) | 0.178 | 2.05 (0.30–13.92) | 0.464 |
| Nugent score categories† | ||||||
| 4–6 vs. 0–3 | 1.24 (0.20–7.82) | 0.821 | 0.36 (0.04–3.13) | 0.353 | 2.42 (0.36–16.26) | 0.362 |
| 7–10 vs. 0-3 | 0.06 (0.00–0.77) | 0.031 | 0.19 (0.02–1.90) | 0.156 | 1.42 (0.17–11.81) | 0.747 |
| Total bacterial est conc†‡ | 0.75 (0.47–1.18) | 0.208 | 0.64 (0.40–1.02) | 0.061 | 0.90 (0.57–1.43) | 0.666 |
| Total Lactobacillus est conc†‡ | 1.74 (0.83–3.67) | 0.142 | 1.47 (0.69–3.16) | 0.319 | 1.26 (0.60–2.68) | 0.541 |
| Total BV-anaerobes est conc†‡ | 0.57 (0.22–1.47) | 0.243 | 0.37 (0.14–0.98) | 0.046 | 0.91 (0.35–2.36) | 0.848 |
| Total pathobionts est conc†‡ | 0.99 (0.35–2.77) | 0.980 | 0.66 (0.23–1.81) | 0.445 | 0.98 (0.36–2.90) | 0.965 |
| Total other bacteria est conc†‡ | 0.65 (0.25–1.69) | 0.374 | 1.31 (0.49–3.50) | 0.591 | 1.79 (0.68–4.71) | 0.239 |
| Total Lactobacillus RA§¶ | 1.32 (1.06–1.65) | 0.014 | 1.30 (1.03–1.64) | 0.025 | 1.06 (0.85–1.33) | 0.601 |
| Total BV-anaerobes RA§¶ | 0.81 (0.66–1.00) | 0.049 | 0.83 (0.67–1.03) | 0.098 | 1.02 (0.83–1.26) | 0.855 |
| Total pathobionts RA§¶ | 0.93 (0.84–1.03) | 0.180 | 0.92 (0.83–1.02) | 0.120 | 0.93 (0.84–1.03) | 0.147 |
| Total other bacteria RA§¶ | 1.00 (1.00–1.01) | 0.436 | 1.01 (1.00–1.01) | 0.009 | 1.00 (1.00–1.01) | 0.439 |
| Pooled VMB type§ | ||||||
| LA vs. LD | 1.46 (0.26–8.11) | 0.665 | 1.59 (0.27–9.55) | 0.610 | 2.11 (0.37–12.15) | 0.401 |
| BV vs. LD | 0.02 (0.00–0.48) | 0.017 | 0.02 (0.00–0.64) | 0.027 | 0.44 (0.02–7.84) | 0.575 |
| PB vs. LD | 0.08 (0.00–1.49) | 0.090 | 0.11 (0.01–2.02) | 0.136 | 0.21 (0.01–3.71) | 0.284 |
| Simpson diversity§ | 0.93 (0.78–1.09) | 0.359 | 0.96 (0.81–1.13) | 0.597 | 1.10 (0.93–1.30) | 0.272 |
Abbreviations: BV, bacterial vaginosis; CI, confidence interval; EF+, Ecologic Femi+; Enr, enrollment visit; est conc, estimated concentration; GynLP, Gynophilus LP; LA, lactobacilli plus BV-anaerobes VMB type; LD, Lactobacillus-dominated VMB type; Metro, metronidazole group; OR, odds ratio; PB, pathobionts VMB type; VMB, vaginal microbiota. *Compared to the control group. †Including all valid samples during product use (D7, M1, and M2 visits). Self-sampled samples were also taken during product use, but were not Gram stained nor tested by 16S rRNA gene qPCR (see Methods). ‡Estimated concentrations in log10 copies/µl, entered into the models as continuous variables. §Including all valid samples during product use (D7, M1, and M2 visits, and self-sampled samples). ¶Relative abundances, entered into the models as continuous variables constrained between 0 and 1. **Model adjusted for hormonal/pregnancy status (longitudinal assessments), sexual risk taking (longitudinal assessments; a composite variable including condom use consistency and number of sexual partners: women were considered low risk when they reported fewer than five sexual partners in the past month plus consistent condom use), and age (see Methods).
The proportions of women in each randomization group at each visit having a particular VMB type corresponded with the estimated concentration and relative abundance data, and additionally showed that – in lactobacilli-dominated women – the L. iners-dominated VMB type was far more common than the L. crispatus-dominated and other lactobacilli-dominated VMB types throughout (Fig. S4). Among women with dysbiosis, the lactobacilli plus BV-anaerobes and polybacterial with ≥10% G. vaginalis VMB types continued to be the most common dysbiosis types during follow-up. In unadjusted mixed effects models using intervention period data only, metronidazole users and EF+ users, each compared to controls, were significantly less likely to have dysbiotic VMB types (p = 0.012 and p = 0.029, respectively) (Table 4).
The associations in unadjusted mixed effects models persisted after adjustment for hormonal contraception use/pregnancy, sexual risk taking, and age, except for the association with estimated Lactobacillus concentration among metronidazole users. Metronidazole users compared to controls had a significantly higher Lactobacillus relative abundance (p = 0.014), a significantly lower BV-anaerobes relative abundance (p = 0.049), and were significantly less likely to have BV by Nugent scoring (p = 0.031) or by VMB types (the two polybacterial and G. vaginalis-dominated VMB-types combined; p = 0.017) (Table 4). EF+ users compared to controls had a significantly higher Lactobacillus relative abundance (p = 0.025), a significantly lower estimated BV-anaerobes concentration (p = 0.046), a significantly higher relative abundance of ‘other bacteria’ (p = 0.009), and were significantly less likely to have BV-like VMB types (p = 0.027).
Detection of probiotic strains
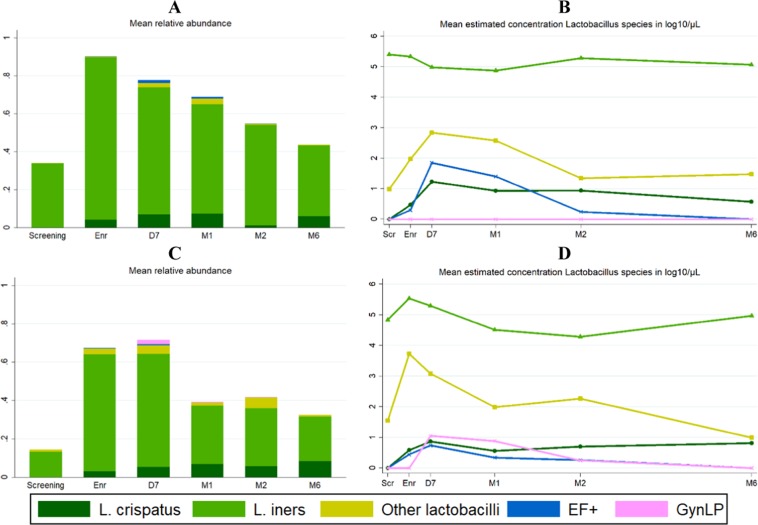
During the intervention period, relevant probiotic strains were detected in 39% of samples from EF+ users and 20% of samples from GynLP users (all swabs combined, including self-sampled swabs). The detection percentages were 58% and 31%, respectively, in sensitivity analyses using non-rarefied data. Some of the EF+ strains cannot be differentiated from naturally occurring strains, and EF+-like strains were therefore detected (at low levels) in all groups at most time points (Fig. 3, Table S4). However, the mean estimated concentrations were highest in the EF+ group during the intervention period (mean estimated concentrations 0.48–1.92 log10 cells/μl per visit for all women combined, and 3.62–4.28 log10 cells/μl per visit for women who did have EF+ strains detected using rarefied data). The GynLP strain was only detected in the GynLP group during the intervention period (mean estimated concentrations 0.25–1.05 log10 cells/μl per visit for all women combined, and 3.72–4.55 log10 cells/μl per visit for women who did have GynLP detected using rarefied data). Inter- and intra-individual differences between participants were high: the highest estimated vaginal probiotic concentration detected in an individual EF+ user was 5.51 log10 cells/μl, and in an individual GynLP user was 6.17 log10 cells/μl. During the intervention period, the mean relative abundances of the probiotic strains were 0.03 in both EF+ and GynLP users, and 0.08 and 0.15, respectively, if only samples in which any strains were detected were included.
Figure 3.
Detection of probiotic strains during the trial. Abbreviations: D7, Day 7 visit; EF+, Ecologic Femi+; Enr, enrollment visit; GynLP, Gynophilus LP; M1/2/6, month 1/2/6 visit; Scr, screening visit. Mean relative abundance (A) and mean estimated concentration (B) of Lactobacillus species over time in the EF+ group; mean relative abundance (C) and mean estimated concentration (D) of Lactobacillus species over time in the GynLP group. The length of bars in (A,C) depicts total relative abundance of all Lactobacillus species combined.
VMB transitions
The stacked graph and alluvial diagrams (Fig. S4) show that transitions from one VMB type to another were common. As expected, most transitions during the intervention period were from Lactobacillus-dominated states to dysbiotic states, but the reverse also occurred. Transitions between VMB types were common in all randomization groups. The percentage of actual transitions divided by potential transitions between D7 and M6 were 29/66 (43.9%) in the control group, 32/64 (50.0%) in the metronidazole group, 28/67 (41.8%) in the EF+ group and 24/56 (42.9%) in the GynLP group (Fisher’s exact p = 0.792).
Incidence of sexually transmitted and urinary tract infections
As expected, the incidences of HIV (n = 2), herpes simplex type 2 (n = 1), syphilis (n = 1), gonorrhea (n = 5), chlamydia (n = 6), TV (n = 5) and urinary tract infection (n = 7) were too low to determine differences between randomization groups (Table S7).
Discussion
This trial showed that all three interventions were safe. Our preliminary efficacy results confirm that intermittent use of metronidazole reduces BV recurrence10–12, and suggest that intermittent use of lactobacilli-containing vaginal probiotics may also reduce BV recurrence. These findings are important because some women and clinicians may prefer safe and efficacious vaginal probiotics to metronidazole since they are not expected to negatively affect other body niche microbiomes or cause antimicrobial resistance.
Our trial was funded as a pilot study and therefore had a modest sample size. Despite this, many of the preliminary efficacy associations for intermittent metronidazole and EF+ use reached statistical significance. While this was not the case for intermittent GynLP use, all of the trends in this group were in the same directions. We believe that the GynLP group suffered a few disadvantages compared to the other randomization groups, which may explain the lack of statistical significance. Randomization imbalances commonly occur in small trials30, and in our trial, this led to women in the GynLP group being more dysbiotic at baseline than controls and EF+ users. We ameliorated this disadvantage in our analysis strategy, but we may not have been able to eradicate it. In addition, GynLP users were less adherent on average than metronidazole and EF+ users. Differences in adherence using triangulated data did not reach statistical significance, but probiotic strain detection rates (58% for EF+ samples and 31% for GynLP samples using non-rarefied data) support this claim. Biose has since simplified the dosing regimen of next generation Gynophilus products to two fixed days a week to boost adherence. Finally, GynLP dosing (once every four days) was similar to metronidazole dosing (twice weekly) but less frequent than EF+ dosing (once per day for the first five days followed by thrice weekly for the remainder of the intervention period).
Probiotic detection rates were 58% for EF+ samples and 31% for GynLP samples, and inter- and intra-individual variabilities were high (with estimated probiotic concentrations ranging from zero to 6.17 log10 cells/μl). This detection variability is consistent with most other vaginal probiotic studies that used sampling at non-daily intervals and molecular assessment methods21,31–33. A major drawback of all of those studies, including ours, is that product use was not directly observed but self-reported, and precise information about the time period between last product insertion and sample collection was lacking. The average total bacterial load of a healthy vagina is currently not known34, but we estimate it to be in the order of 2 × 1010 bacteria (Supplement 1). The vaginal probiotic strain(s) in our trial were both applied at about 1.5 × 109 CFU per dose, which would be about 7.5% of the total vaginal bacterial load after application if all probiotic bacteria were to remain in the vagina. We detected mean relative abundances of 7.7% for EF+ strains and 15.1% for GynLP when only samples with any relevant probiotic strains detected during product use were included (thereby eliminating any potential non-adherence). A recently published study of Gynophilus Slow Release tablet (which is almost identical to GynLP) in which women self-sampled every day showed that mean estimated vaginal concentrations of Lcr35 by qPCR were between 104 and 106 CFU/μl in women who used the tablet once every four or five days35. Using our estimated concentration data, we detected a similar concentration range in samples with any GynLP detected. This consistency is reassuring, but the question remains whether probiotic concentrations of this order of magnitude optimally prevent BV recurrence in the long-term. Furthermore, all studies referenced in this paragraph, including ours, have shown that probiotic strains do not persist in the vagina after dosing has ceased. The second question then is whether the colonization capacity of probiotic bacteria should be improved. Our data suggest that probiotic lactobacilli may boost ‘natural’ lactobacilli indirectly, which may be sufficient. Indirect effects may include increased localized lactic acid production, modulation of cervicovaginal mucosal immune responses, and/or inhibition of biofilm formation, by probiotic bacteria3,36,37.
Additional limitations of our study include lack of proof that the probiotic contents (strains and doses) were in agreement with the product labels, the high urogenital infection risk of this cohort (which makes prevention more challenging), and our inability to fully control for potential confounders. However, the mixed effects models were controlled for some of the best known VMB determinants (hormonal contraception, pregnancy, sexual risk taking, and age)1,38–40. We were not able to exclude women with gonorrhea and/or chlamydia infection at the time of randomization due to the slow laboratory turn-around time, but the VMB compositions of women with and without infection were similar, and we therefore think that this did not negatively affect our results.
With the development of better genomic and culturing methods, we are now on the cusp of a new era in vaginal probiotic research. Past vaginal probiotic studies have shown mixed results13–24, but almost all of these studies used imprecise VMB assessments based on clinical symptoms and microscopy. The addition of sequencing methods showed that many more women than previously thought are not lactobacilli-dominated after standard antibiotic BV treatment, that host responses to antibiotic and probiotic treatment are highly variable, and that it is possible to differentiate between probiotic strains and ‘natural’ lactobacilli. Furthermore, others have shown that quantifying relative abundance data in the same manner as we have done in this study correlates well with species-specific quantitative PCRs of non-minority species27,28. This then allows for microbiota data reduction into quantitative variables that can be analyzed in mixed effects models that adjust for repeated measures and confounding.
We conclude that lactobacilli-based vaginal probiotics warrant further development to improve long-term beneficial effects. We recommend that future trials incorporate quantitative molecular methods, optimize dosing and timing of product insertion versus sample collection, and enroll women with various urogenital risk profiles. Ideally, these trials would also evaluate the effects of interventions on vaginal biofilm formation, and – eventually – the impact on pregnancy complications, HIV epidemics, and other adverse outcomes.
Supplementary information
Acknowledgements
We thank the study participants, the Rinda Ubuzima team, the National Reference Laboratory in Kigali, the Centre for Genomic Research and research support staff at the University of Liverpool, Christina Gill, Robert Meester, Mike Humphreys, Jessica Younes, Vicky Jespers, Tania Crucitti, and Anna Maria Geretti. This work was funded by the DFID/MRC/Wellcome Trust Joint Global Health Trials Scheme as a Development Project (grant reference MR/M017443/1; grant title: “Preparing for a clinical trial of interventions to maintain normal vaginal microbiota for preventing adverse reproductive health outcomes in Africa”) and the University of Liverpool (Technology Directorate Voucher). Vaginal probiotics for use in the trial were donated free of charge by Winclove Probiotics (Amsterdam, The Netherlands) and Biose (formerly Probionov, Arpajon-sur-Cère, France). The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the authors’ institutions or companies, or the funders.
Author contributions
J.v.d.W. obtained the research funding and wrote the study protocol and data collection documents. J.v.d.W., A.N., E.L., and J.R. were members of the Trial Steering Committee. S.A., L.M., V.M., M.U., and J.v.d.W. collected the primary data. L.M. performed the diagnostic testing. M.V., C.B., A.D., and J.R. conducted or oversaw the 16S rRNA gene sequencing and qPCRs. J.v.d.W. and M.V. developed the analytical approach and performed the statistical analyses. J.v.d.W. and M.V. wrote the manuscript. All authors commented on and approved the final manuscript. JvdW had full access to the data and had final responsibility for the decision to submit for publication.
Data availability
Participants were not explicitly asked for consent related to use of their data by external parties or use that does not address the research questions described in the approved study protocol (publicly available at https://datacat.liverpool.ac.uk/). Data were therefore deposited in a controlled access repository at the University of Liverpool. Data can be requested by emailing the Research Data Management team (rdm@liverpool.ac.uk) and the data steward (Professor Janneke van de Wijgert; j.vandewijgert@liverpool.ac.uk). Requests will be submitted to the University of Liverpool ethics committee (ethics@liverpool.ac.uk) for approval.
Competing interests
AN is employed by Biose (owner of trial product GynLP) and EL by Winclove Probiotics BV (owner of trial product EF+). AN and JR have financial and/or intellectual investments in competing products. The other authors report no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Janneke H. H. M. van de Wijgert and Marijn C. Verwijs.
Supplementary information
is available for this paper at 10.1038/s41598-020-60671-6.
References
- 1.van de Wijgert JHHM, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9:e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Wijgert JHHM, Jespers V. The global health impact of vaginal dysbiosis. Res. Microbiol. 2017;168:859–864. doi: 10.1016/j.resmic.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Borgdorff H, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016;9:621–633. doi: 10.1038/mi.2015.86. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidelines for the management of sexually transmitted infections, http://applications.emro.who.int/aiecf/web79.pdf (2003). [PubMed]
- 5.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991;29:297–301. doi: 10.1128/JCM.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amsel R, et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 7.Hay P. Recurrent bacterial vaginosis. Curr. Opin. Infect. Dis. 2009;22:82–86. doi: 10.1097/QCO.0b013e32832180c6. [DOI] [PubMed] [Google Scholar]
- 8.Verstraelen H, Verhelst R. Bacterial vaginosis: an update on diagnosis and treatment. Expert Rev. Anti Infect. Ther. 2009;7:1109–1124. doi: 10.1586/eri.09.87. [DOI] [PubMed] [Google Scholar]
- 9.Donders G. Diagnosis and management of bacterial vaginosis and other types of abnormal vaginal bacterial flora: a review. Obstet. Gynecol. Surv. 2010;65:462. doi: 10.1097/OGX.0b013e3181e09621. [DOI] [PubMed] [Google Scholar]
- 10.Sobel JD, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am. J. Obstet. Gynecol. 2006;194:1283–1289. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Taha Taha E, Kumwenda Newton I, Kafulafula George, Makanani Bonus, Nkhoma Chiwawa, Chen Shu, Tsui Amy, Hoover Donald R. Intermittent Intravaginal Antibiotic Treatment of Bacterial Vaginosis in HIV-Uninfected and -Infected Women: A Randomized Clinical Trial. PLoS Clinical Trials. 2007;2(2):e10. doi: 10.1371/journal.pctr.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland RS, et al. Improvement of vaginal health for Kenyan women at risk for acquisition of Human Immunodeficiency Virus type 1: results of a randomized trial. J. Infect. Dis. 2008;197:1361–1368. doi: 10.1086/587490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastromarino P, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 14.Larsson, P.-G., Stray-Pedersen, B., Ryttig, K. R. & Larsen, S. Human lactobacilli as supplementation of clindamycin to patients with bacterial vaginosis reduce the recurrence rate; a 6-month, double-blind, randomized, placebo-controlled study. BMC Womens Health8 (2008). [DOI] [PMC free article] [PubMed]
- 15.Petricevic L, Witt A. The role of Lactobacillus casei rhamnosus Lcr35 in restoring the normal vaginal flora after antibiotic treatment of bacterial vaginosis. Br. J. Obstet. Gynaecol. 2008;115:1369–1374. doi: 10.1111/j.1471-0528.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw CS, et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLoS One. 2012;7:e34540. doi: 10.1371/journal.pone.0034540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson K, Carlsson B, Forsum U, Larsson P-G. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta Derm. Venereol. 2005;85:42–46. doi: 10.1080/00015550410022249. [DOI] [PubMed] [Google Scholar]
- 18.Anukam KC, et al. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 2006;8:2772–2776. doi: 10.1016/j.micinf.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Hemalatha R, Mastromarino P, Ramalaxmi BA, Balakrishna NV, Sesikeran B. Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:3097–3105. doi: 10.1007/s10096-012-1671-1. [DOI] [PubMed] [Google Scholar]
- 20.Ling Z, et al. The restoration of the vaginal microbiota after treatment for bacterial vaginosis with metronidazole or probiotics. Microb. Ecol. 2013;65:773–780. doi: 10.1007/s00248-012-0154-3. [DOI] [PubMed] [Google Scholar]
- 21.Bisanz JE, et al. A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women. PLoS One. 2014;9:e104511. doi: 10.1371/journal.pone.0104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdenelli MC, et al. Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr. Microbiol. 2016;73:483–490. doi: 10.1007/s00284-016-1085-x. [DOI] [PubMed] [Google Scholar]
- 23.Bohbot JM, et al. Efficacy and safety of vaginally administered lyophilized Lactobacillus crispatus IP 174178 in the prevention of bacterial vaginosis recurrence. J. Gynecol. Obstet. Hum. Reprod. 2018;47:81–86. doi: 10.1016/j.jogoh.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Rapisarda AMC, et al. Efficacy of vaginal preparation containing Lactobacillus acidophilus, lactic acid and deodorized garlic extract in treatment and prevention of symptomatic bacterial vaginitis: result from a single-arm pilot study. Ital. J. Gynaecol. Obstet. 2018;30:21–31. [Google Scholar]
- 25.O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 26.Liu CM, et al. BactQuant: an enhanced broad-coverage bacterial quantitative real-time PCR assay. BMC Microbiol. 2012;12:56. doi: 10.1186/1471-2180-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keystone Symposia | Scientific Conferences on Biomedical and Life Science Topics, https://www.keystonesymposia.org/18S6.
- 28.Jian, C., Luukkonen, P., Yki-Jarvinen, H., Salonen, A. & Korpela, K. Quantitative PCR provides a simple and accessible method for quantitative microbiome profiling: bioRxiv, 10.1101/478685 (2018). [DOI] [PMC free article] [PubMed]
- 29.van de Wijgert JHHM. The vaginal microbiome and sexually transmitted infections are interlinked: consequences for treatment and prevention. PLoS Med. 2017;14:e1002478. doi: 10.1371/journal.pmed.1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- 31.Antonio MAD, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J. Clin. Microbiol. 2003;41:1881–1887. doi: 10.1128/JCM.41.5.1881-1887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonio MAD, Meyn LA, Murray PJ, Busse B, Hillier SL. Vaginal colonization by probiotic Lactobacillus crispatus CTV‐05 is decreased by sexual activity and endogenous lactobacilli. J. Infect. Dis. 2009;199:1506–1513. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 33.Tomusiak, A. et al. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: a multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des. Devel. Ther. 5345, 10.2147/DDDT.S89214 (2015). [DOI] [PMC free article] [PubMed]
- 34.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dausset C, et al. Comparative phase I randomized open-label pilot clinical trial of Gynophilus® (Lcr regenerans®) immediate release capsules versus slow release muco-adhesive tablets. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1869–1880. doi: 10.1007/s10096-018-3321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrova Mariya I., Imholz Nicole C. E., Verhoeven Tine L. A., Balzarini Jan, Van Damme Els J. M., Schols Dominique, Vanderleyden Jos, Lebeer Sarah. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLOS ONE. 2016;11(8):e0161337. doi: 10.1371/journal.pone.0161337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Gajer P, et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012;4:132ra52–132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borgdorff H, et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS One. 2017;12:e0181135. doi: 10.1371/journal.pone.0181135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noyes, N., Cho, K.-C., Ravel, J., Forney, L. J. & Abdo, Z. Associations between sexual habits, menstrual hygiene practices, demographics and the vaginal microbiome as revealed by Bayesian network analysis. PLoS One13 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Participants were not explicitly asked for consent related to use of their data by external parties or use that does not address the research questions described in the approved study protocol (publicly available at https://datacat.liverpool.ac.uk/). Data were therefore deposited in a controlled access repository at the University of Liverpool. Data can be requested by emailing the Research Data Management team (rdm@liverpool.ac.uk) and the data steward (Professor Janneke van de Wijgert; j.vandewijgert@liverpool.ac.uk). Requests will be submitted to the University of Liverpool ethics committee (ethics@liverpool.ac.uk) for approval.