Abstract
The anabolic action of “fast” whey protein on the regulation of postprandial muscle protein synthesis has been established to be short-lived in healthy young adults. We assessed the time course of anabolic signaling activation and stimulation of myofibrillar protein synthesis rates (MPS) after ingestion of a food source that represents a more typical meal-induced pattern of aminoacidemia. Seven young men (age: 22 ± 1 y) underwent repeated blood and biopsy sampling during primed, continuous l-[ring-2H5]phenylalanine and l-[1-13C]leucine tracer infusions and ingested 38 g of l-[1-13C]phenylalanine- and l-[1-13C]leucine-labeled milk protein concentrate. A total of ∼27 ± 4 (∼10 g) and ∼31 ± 1% (∼12 g) of dietary protein-derived amino acids were released in circulation between 0 and 120 min and 120–300 min, respectively, of the postprandial period. l-[ring-2H5]phenylalanine-based MPS increased above basal (0.025 ± 0.008%/h) by ∼75% (0.043 ± 0.009%/h; P = 0.05) between 0 and 120 min and by ∼86% (0.046 ± 0.004%/h; P = 0.02) between 120 and 300 min, respectively. l-[1-13C]leucine-based MPS increased above basal (0.027 ± 0.002%/h) by ∼72% (0.051 ± 0.016%/h; P = 0.10) between 0 and 120 min and by ∼62% (0.047 ± 0.004%/h; P = 0.001) between 120 and 300 min, respectively. Myofibrillar protein-bound l-[1-13C]phenylalanine increased over time (P < 0.001) and equaled 0.004 ± 0.001, 0.008 ± 0.002, 0.017 ± 0.004, and 0.020 ± 0.003 mole percent excess at 60, 120, 180, and 300 min, respectively, of the postprandial period. Milk protein ingestion increased mTORC1 phosphorylation at 120, 180, and 300 min of the postprandial period (all P < 0.05). Our results show that ingestion of 38 g of milk protein results in sustained increases in MPS throughout a 5-h postprandial period in healthy young men.
NEW & NOTEWORTHY The stimulation of muscle protein synthesis after whey protein ingestion is short-lived due to its transient systemic appearance of amino acids. Our study characterized the muscle anabolic response to a protein source that results in a more gradual release of amino acids into circulation. Our work demonstrates that a sustained increase in postprandial plasma amino acid availability after milk protein ingestion results in a prolonged stimulation of muscle protein synthesis rates in healthy young men.
Keywords: anabolic signaling, leucine, mammalian target of rapamycin, muscle mass regulation, nutrition
INTRODUCTION
Several studies have shown that protein ingestion elevates circulating amino acid availability to stimulate muscle protein synthesis rates in healthy adults (9, 11, 18, 23, 29, 30). This work defined dietary protein as a main anabolic stimulus to human skeletal muscle tissue. Less attention has been given to the time course of stimulation of muscle protein synthesis rates in response to elevated plasma amino acid availability. It has been shown previously that the ingestion of whey protein isolate stimulates a transient increase (ranging from ∼45 to 120 min) in postprandial muscle protein synthesis rates before rapidly returning to baseline values despite a prolonged elevation in plasma amino acid availability during the ensuing postprandial period (2, 21). This short-lived stimulation of the postprandial muscle protein synthetic response after whey protein ingestion has since been referred to as the “muscle-full” effect (2).
What is noteworthy, however, is that the postprandial plasma amino acid profile after whey protein ingestion (5, 23, 25) is unique when compared with other isolated protein sources such as casein or soy (25) as well as whole food sources of protein such as eggs or beef (9, 28). Specifically, the ingestion of whey, due to its solubility, results in high and transient appearance pattern of amino acids into circulation (2, 21, 23), which likely instigates the muscle-full phenomenon. Hence, it is relevant to define the time-dependent regulation of postprandial muscle protein synthesis rates after the ingestion of other types of protein sources with a more gradual and sustained release of dietary protein-derived amino acids into circulation when compared with “fast” digesting whey protein. Such information can be utilized when developing anabolic feeding strategies in the practice of clinical or performance nutrition.
Therefore, the purpose of this work was to assess the relationship between dietary protein-derived amino acid availability and the subsequent time-dependent regulation of muscle protein synthesis rates after the ingestion of milk protein concentrate containing both fast whey and “slow casein” as part of its protein matrix. To do this, we applied continuous l-[ring-2H5]phenylalanine and l-[1-13C]leucine tracer infusion combined with the oral administration of intrinsically l-[1-13C]phenylalanine and l-[1-13C]leucine-labeled milk protein and repeated muscle biopsy sampling in healthy young men. This approach allowed for determination of the temporal pattern of dietary protein-derived amino acid release in the circulation and the stimulation of postprandial muscle protein synthesis rates as well as the utilization of the dietary protein-derived amino acids for de novo muscle protein synthesis throughout a 5-h postprandial period (8). Milk protein concentrate contains a combination of fast whey and slow casein protein fractions and small amounts of carbohydrate and fats as part of its food matrix. As such, the ingestion of milk protein provides a more gradual release of dietary protein-derived amino acids throughout the postprandial phase when compared with the ingestion of free amino acid or whey protein (5, 23). We hypothesized that the ingestion of 38 g of milk protein would result in a sustained activation of anabolic signaling, a sustained stimulation of postprandial muscle protein synthesis rates, and a progressive accumulation of dietary protein-derived amino acids for de novo muscle protein accretion throughout the 0- to 300-min postprandial period in healthy young men.
METHODS
Participants and ethical approval.
Seven healthy young men (age: 22 ± 1 yr) volunteered to participate in this study. All participants were deemed healthy based on their response to a routine medical screening questionnaire. Volunteers had no history of participating in past stable isotope amino acid tracer experiments. Participants’ characteristics are presented in Table 1. All participants were informed about the experimental procedures to be used, the purpose of the study, and all potential risks before giving written consent. The study conformed to all standards for the use of human participants in research as outlined in the Declaration of Helsinki and was approved by the local Institutional Review Board at the University of Illinois at Urbana-Champaign (IRB no. 14234).
Table 1.
Participants’ characteristics
| Variable | Value |
|---|---|
| Age, yr | 22 ± 1.3 |
| Weight, kg | 79.2 ± 4.9 |
| BMI, kg/m2 | 24.8 ± 1.2 |
| Systolic BP, mmHg | 124.7 ± 3.9 |
| Diastolic BP, mmHg | 72.4 ± 3.9 |
| Fat, % | 16.6 ± 1.4 |
| Lean body mass, kg | 64.0 ± 3.7 |
| Appendicular lean mass, kg | 29.0 ± 1.7 |
| Fasting Glucose, mg/dL | 78.3 ± 1.0 |
| Energy intake, MJ/day | 8.74 ± 0.4 |
| Protein intake, g/day | 113.0 ± 4.8 |
| Carbohydrate intake, g/day | 228.7 ± 21.1 |
| Fat intake, g/day | 83.0 ± 1.17 |
Data are mean ± SE; n = 7. BMI, body mass index; BP, blood pressure.
Experimental protocol.
Participants reported to the laboratory at 0700 after an overnight fast and having refrained from strenuous physical activity for ≥3 days before the experimental trial. A Teflon catheter was inserted into a heated dorsal hand vein for repeated arterialized blood sampling and remained patent by a 0.9% saline drip. After a baseline blood sample was taken (t = −180 min), the plasma phenylalanine, tyrosine, and leucine pools were primed with a single dose of l-[ring-2H5]phenylalanine (2.0 µmol/kg), l-[ring-3,5-2H2]tyrosine (0.615 µmol/kg), and l-[1-13C]leucine (4.0 µmol/kg), after which a continuous l-[ring-2H5]phenylalanine (0.05 µmol·kg−1·min−1), l-[ring-3,5-2H2]tyrosine (0.015 µmol·kg−1·min−1), and l-[1-13C]leucine (0.10 µmol·kg−1·min−1) intravenous infusion was initiated (t = −180 min) and maintained over the experimental infusion trial. Muscle biopsy samples were collected before (t = −120 and 0 min) and after (t = 60, 120, 180, and 300 min) the ingestion of 38 g of intrinsically l-[1-13C]phenylalanine- and l-[1-13C]leucine-labeled milk protein concentrate dissolved in 300 mL of water. Biopsies were collected from the middle region of the vastus lateralis (∼15 cm above the patella) with a Bergström needle that was modified for manual suction under local anesthesia (18). Muscle samples were freed from any blood, fat, and visible connective tissue and immediately frozen in liquid nitrogen before storage at −80°C until further analysis. Blood samples were collected in EDTA-containing tubes before (t = −180, −120, −60, and −0 min) and after milk protein ingestion (t = 30, 60, 90, 120, 180, 240, and 300 min). The blood samples were immediately analyzed for whole blood glucose concentrations (2300 Stat Plus; YSI Life Sciences, Springs, OH) and centrifuged at 3000 g for 10 min at 4°C. The plasma samples were subsequently stored at −20°C for future analysis.
Intrinsically labeled milk protein.
Intrinsically l-[1-13C]phenylalanine- and l-[1-13C]leucine-labeled milk protein concentrate was obtained by infusing l-[1-13C]phenylalanine and l-[1-13C]leucine into a lactating Holstein cow, collecting the milk, and purifying the milk protein concentrate, as previously described (10, 24, 27). The l-[1-13C]phenylalanine and l-[1-13C]leucine enrichments in the milk protein concentrate were measured by GC-MS (Agilent 6890N GC coupled with a 5973 inert MDS) and averaged 38.3 and 10.8 mole percent excess (MPE), respectively. The macronutrient composition of the milk protein beverage provided to participants was 38 g of protein (3.46 g of leucine), 4.17 g of carbohydrate, and 1.4 g of fat. The milk protein met all chemical and bacteriological specifications for human consumption.
Plasma analyses.
Plasma insulin concentrations were determined using a commercially available enzyme-linked immunosorbent assays (Alpco Diagnostics, Salem, NH). Plasma amino acid concentrations and enrichments were determined by GC-MS (Agilent 7890A GC/5975C; MSD) as previously described (16).
Muscle analyses.
Myofibrillar proteins were extracted from ∼50 mg of wet muscle by hand-homogenizing in ice-cold homogenization buffer (10 µL·mg) containing phosphatase (Roche PhosSTOP) and protease inhibitors (Roche cOmplete Protease Inhibitor), using a Teflon pestle, as previously described (29). For measurement of muscle protein-bound l-[ring-2H5]-phenylalanine, l-[1-13C]phenylalanine, and l-[1-13C]leucine enrichment, the eluate was dried, and the purified amino acids were derivatized to their N(O,S)-ethoxycarbonyl ethyl esters. The derivatized l-[ring-2H5]phenylalanine samples were measured using a gas chromatography-isotope ratio mass spectrometer (MAT 253; Thermo Fisher Scientific, Bremen, Germany) equipped with a pyrolysis oven (GC-P-IRMS) and a 60 m DB-17MS column and 5-m precolumn (No. 122-4762; Agilent) and GC-Isolink. Ion masses 1 and 2 were monitored to determine the 2H/1H ratios of muscle protein bound phenylalanine. The derivatized l-[1-13C]phenylalanine and l-[1-13C]leucine samples were measured using a gas chromatography-isotope ratio mass spectrometer (Finnigan MAT 252; Thermo Fisher Scientific, Bremen, Germany) equipped with an Ultra I GC-column (no. 19091A-112; Hewlett-Packard, Palo Alto, CA) and combustion interface II (GC-C-IRMS). Ion masses 44, 45, and 46 were monitored for 13C/12C-phenylalanine and leucine, respectively. By establishing the relationship between the enrichment of a series of l-[1-13C]phenylalanine, l-[1-13C]leucine, and l-[ring-2H5]phenylalanine standards of variable enrichment and the enrichment of the N(O,S)-ethoxycarbonyl ethyl esters of these standards, the muscle protein-bound enrichment of phenylalanine and leucine was determined.
Muscle intracellular free amino acids were extracted from a separate piece of wet muscle (∼30 mg) using a Teflon-coated pestle, as described previously (29). The muscle intracellular leucine and phenylalanine 13C and 2H enrichments were determined by multiple reaction monitoring (MRM) at m/z 132.0 → 86.0 and 133.0 → 87.0 for unlabeled and labeled l-[1-13C]leucine and m/z 166.0 → 103.0, 167.0 → 104.0 and 171.0 → 106.0 for unlabeled and labeled (l-[1-13C] and ring-2H5)phenylalanine, respectively. Software Analyst 1.6.2 was used for data acquisition and analysis.
Western blotting.
A portion of whole muscle homogenates isolated during the myofibrillar protein extractions was used for Western blotting analysis and was described and validated previously (3). Protein content of the homogenates was determined by Bradford Assay (Bio-Rad Laboratories, Hercules, CA), and then equal amounts of protein were separated by SDS-PAGE before being transferred to polyvinyl difluoride membranes for blotting. After blocking, membranes were incubated in primary antibodies overnight at 4°C to determine the phosphorylation status and total protein content of protein kinase B (Akt) at Ser473, mammalian target of rapamycin complex 1 (mTORC1) at Ser2448, 70 kDa S6 protein kinase 1 (p70S6K1) at Thr389 (Cell Signaling Technology, Danvers, MA), and large neutral amino acid transporter (LAT1 SLC7A5) (total protein content only) (Bioss Antibodies, Woburn, MA). Membranes from the respective proteins were then incubated with appropriate secondary antibodies, and protein content was detected using West Femto Maximum Sensitivity substrate (SuperSignal, Thermo Scientific, Waltham, MA) and the ChemiDoc-It2 Imaging System (UVP, Upland, CA). After detection of phosphorylated proteins, membranes were stripped with Western blot stripping buffer (Restore, Thermo Scientific) and re-incubated with antibodies against total protein (Cell Signaling Technology). Western blot data were normalized to an internal control (α-tubulin). Bands were quantified using ImageJ software (National Institutes of Health), normalized to a control sample run on each blot to account for interblot variability, and expressed as fold change from basal.
Calculations.
Ingestion of l-[1-13C]phenylalanine-labeled protein, intravenous infusion of l-[ring-2H5]phenylalanine and l-[ring-3,5-2H2]tyrosine, and arterialized blood sampling were used to assess whole body amino acid kinetics in non-steady-state conditions. Total, exogenous, and endogenous rate of appearance (Ra) and plasma availability of dietary protein-derived phenylalanine (i.e., the fraction of dietary protein-derived phenylalanine that appeared in the systemic circulation, Pheplasma) were calculated using modified Steele’s equations (6, 13), as described previously (19). Furthermore, total rate of phenylalanine disappearance (Rd), utilization of phenylalanine for protein synthesis, and phenylalanine hydroxylation (first step of phenylalanine conversion to tyrosine) were calculated (19). Myofibrillar protein fractional synthetic rates (FSR) were calculated using standard precursor‐product methods by dividing the increment in tracer enrichment in the myofibrillar protein fraction by the enrichment of the plasma or intracellular precursor pools over time, as described previously (8). For basal muscle protein FSR, muscle biopsies at t = −120 and 0 min were used, and for postprandial FSR, biopsies at t = 60, 120, 180, and 300 min were used to calculate FSR.
Statistics.
Differences in plasma and muscle time curves were tested using one-way repeated-measures ANOVA (time). For all analysis, when statistically significant time effects were observed, Fisher’s Least Significant Difference (LSD) tests were performed to locate differences. Differences were considered statistically significant at P < 0.05. All calculations were performed using IBM SPSS Statistics (version 25). All data are expressed as means ± SE.
RESULTS
Plasma parameters.
Plasma parameters are shown in Table 2. Plasma phenylalanine, tyrosine, and leucine concentrations increased rapidly after milk protein ingestion (all time effect: P < 0.01) and remained elevated above basal values (t = 0 min) during the entire postprandial phase (all time points, P < 0.05). Peak concentrations for all three amino acids were observed at 30 min after milk protein ingestion with values of 97 ± 5, 116 ± 10, and 335 ± 32 µmol/L for phenylalanine, tyrosine, and leucine, respectively. Plasma glucose and insulin concentrations increased transiently at 30 min after milk protein ingestion (both time effect: P < 0.01).
Table 2.
Plasm amino acid, glucose, and insulin concentrations in the basal state and after ingestion of milk protein (38 g) in healthy young men
| Time After Drink, min |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 180 | 240 | 300 | |
| Phenylalanine, µm | 60 ± 3 | 97 ± 5* | 83 ± 3* | 79 ± 4* | 76 ± 3* | 70 ± 3* | 70 ± 4* | 69 ± 4* |
| Tyrosine, µm | 55 ± 2 | 116 ± 10* | 99 ± 7* | 93 ± 5* | 90 ± 5* | 80 ± 4* | 79 ± 4* | 74 ± 3* |
| Leucine, µm | 130 ± 4 | 335 ± 32* | 273 ± 24* | 245 ± 15* | 220 ± 8* | 195 ± 8* | 210 ± 9* | 198 ± 11* |
| Glucose, mg/dL | 75 ± 1 | 81 ± 1* | 77 ± 2 | 77 ± 1 | 76 ± 1 | 75 ± 1 | 75 ± 1 | 75 ± 2 |
| Insulin, µIU/mL | 4 ± 1 | 19 ± 4* | 9 ± 3 | 8 ± 3 | 6 ± 2 | 4 ± 1 | 3 ± 1 | |
Data are means ± SE; n = 7. Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. All time effect, P < 0.01.
Different from basal (t = 0 min).
Plasma amino acid enrichments.
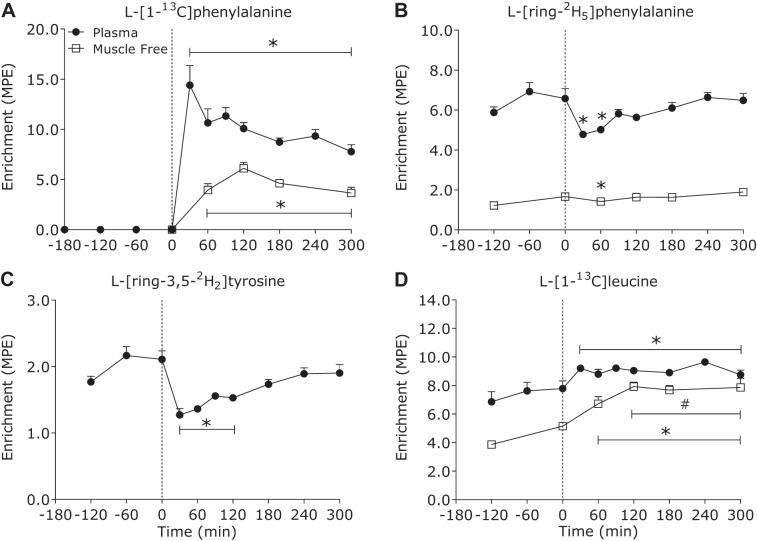
Plasma l-[1-13C]phenylalanine enrichments (Fig. 1A) increased rapidly after milk protein ingestion (time effect: P < 0.01) and remained elevated above basal values (t = 0 min) during the 300-min postprandial phase (all time points, P < 0.05). Plasma l-[ring-2H5]-phenylalanine (infused tracer) enrichments (Fig. 1B) decreased after milk protein ingestion (time effect: P < 0.01) and were reduced below basal values until 60 min of the postprandial period (all time points, P < 0.05). Plasma l-[ring-3,5-2H2]tyrosine (Fig. 1C) decreased after protein ingestion (time effect: P < 0.01) and remained suppressed below basal values (t = 0 min) until 120 min after protein ingestion (all time points, P < 0.05). Plasma l-[1-13C]leucine (Fig. 1D) enrichments increased following protein ingestion (time effect: P < 0.01) at 30 min and remained steady during the remaining postprandial phase.
Fig. 1.
Plasma (●) and muscle free (□) l-[1-13C]phenylalanine (A), l-[ring-2H5]phenylalanine (B), l-[ring-3,5-2H2]tyrosine (C), and l-[1-13C]leucine (D) enrichments [mole percent excess (MPE)] in the basal state and after ingestion of milk protein (38 g) in healthy young men (n = 7). Dashed line refers to protein ingestion. Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. *Different from basal (t = 0 min); #different from 0 to 60 min (P < 0.05). Data are means ± SE.
Muscle free amino acid enrichments.
Muscle tissue free l-[1-13C]-phenylalanine enrichments increased after milk protein ingestion (time effect: P < 0.01), reaching peak values at t = 120 min (6.1 ± 0.6 MPE), and remained elevated above basal values (t = 0 min) during the entire postprandial phase (all time points, P < 0.05) (Fig. 1A). Muscle tissue free l-[ring-2H5]-phenylalanine enrichments decreased after milk protein ingestion (time effect: P < 0.03) and were suppressed below basal values (t = 0 min) at 60 min of the postprandial period (P = 0.02) (Fig. 1B). Muscle tissue free l-[1-13C]leucine enrichments increased after protein ingestion (time effect: P < 0.01) at 60 min after milk protein ingestion (P = 0.04) remained steady during the postprandial phase from 120 min onwards (Fig. 1C).
Plasma amino acid kinetics.
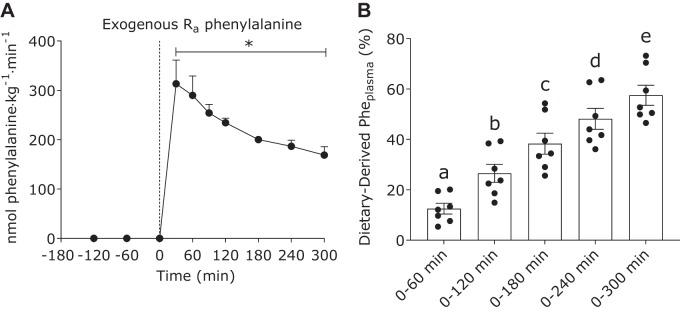
Exogenous phenylalanine rates of appearance (representing the appearance of dietary protein-derived phenylalanine into the circulation; Fig. 2A) increased after milk protein ingestion (time effect: P < 0.01) and remained elevated above basal values (t = 0 min) during the entire postprandial phase (all time points, P < 0.01). Peak plasma exogenous phenylalanine rates of appearance were observed at 30 min after milk protein ingestion and reached a value of 313 ± 48 nmol phenylalanine·kg−1·min−1. The cumulative amount of dietary protein-derived phenylalanine that appeared in circulation during 0–60, 0–120, 0–180, 0–240, and 0–300 min was 13 ± 2, 27 ± 4, 38 ± 4, 48 ± 4, and 58 ± 4%, respectively (Fig. 2B).
Fig. 2.
Exogenous phenylalanine rate of appearance (Ra; A) and cumulative dietary-derived Pheplasma (%; B) in the basal state (not shown for Pheplasma) and after ingestion of milk protein (38 g) in healthy young men (n = 7). Dashed line refers to protein ingestion. Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. Exogenous Ra (A) and cumulative dietary-derived Pheplasma (B): all time effect, P < 0.01. *Different from basal (t = 0 min; P < 0.05). Means without a common letter differ significantly (P < 0.05). Data are means ± SE.
Total plasma phenylalanine appearance and disappearance rates increased after milk protein ingestion (all time effect: P < 0.01) and remained elevated above basal values (t = 0 min) at 180 min of the postprandial period (all time points, P < 0.05). Whole body protein breakdown (represented as endogenous phenylalanine rates of appearance) decreased after milk protein ingestion (time effect: P < 0.01) and remained suppressed below basal values until 300 min of the postprandial phase. Whole body protein oxidation rates (represented as phenylalanine hydroxylation) increased after milk protein ingestion (time effect: P < 0.01) and remained elevated above basal values (t = 0 min) during the entire postprandial phase (all time points, P < 0.05). Whole body protein synthesis rates (represented as total phenylalanine rates of disappearance − phenylalanine hydroxylation) increased after protein ingestion (time effect: P < 0.01). Consequently, whole body net protein balance (represented as synthesis − breakdown) increased after protein ingestion (time effect: P < 0.01) and remained elevated above basal values (t = 0 min) during the entire postprandial phase (all time points, P < 0.01).
Muscle anabolic signaling.
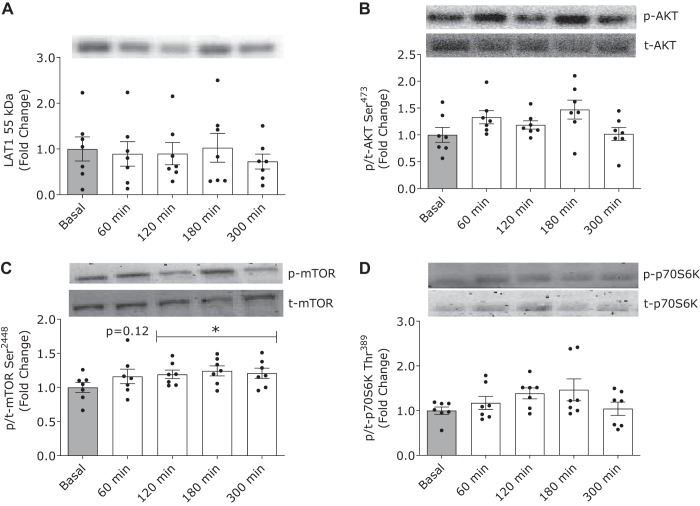
Milk protein ingestion did not modulate relative muscle LAT1 protein content (Fig. 3A) during the postprandial period (time effect: P = 0.53). Akt phosphorylation (Fig. 3B) tended to increase after milk protein ingestion (time effect: P = 0.09). mTORC1 phosphorylation (Fig. 3C) increased after milk protein ingestion (time effect: P = 0.02) and was significantly elevated above basal values (t = 0 min) between 120 and 300 min of the postprandial period (all time points, P < 0.01). There was no difference in p70S6K phosphorylation (Fig. 3D) after milk protein ingestion from basal (time effect: P = 0.11)
Fig. 3.
Phosphorylation status of large neutral amino acid transporter small subunit 1 (LAT1; A), protein kinase B (Akt) Ser473 (B), mammalian target of rapamycin (mTOR) Ser2448 (C), and P70S6K1 Thr389 (D) in the basal state (t = 0 min; gray bars) and after ingestion of milk protein (38 g; open bars) in healthy young men (n = 7). Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. *Different from basal (t = 0 min; P < 0.05). Data are means ± SE.
Myofibrillar protein synthesis.
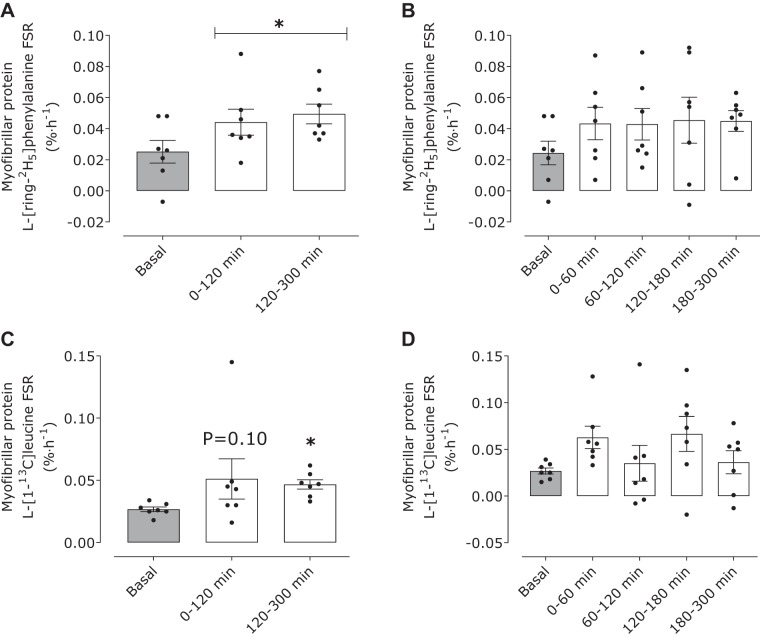
The temporal change in myofibrillar-bound protein enrichments is shown in Table 3. With the use of plasma l-[ring-2H5]phenylalanine enrichments as the precursor, postprandial myofibrillar protein synthesis rates increased above basal values (0.025 ± 0.008%/h) (time effect: P = 0.013) between 0 and 120 (0.043 ± 0.009%/h), 120–300 (0.046 ± 0.004%/h), and 0–300 min (0.045 ± 0.004%/h) (all timepoints, P < 0.05) (Fig. 4A; 0–300 min data not shown). No differences were observed between 0 and 120 and 120–300 min postprandial FSRs (P = 0.76). Postprandial plasma l-[ring-2H5]phenylalanine-based muscle protein FSRs expressed at 0- to 60-, 120- to 180-, 120- to 180-, and 180- to 300-min intervals were 0.043 ± 0.010, 0.043 ± 0.010, 0.045 ± 0.015, and 0.045 ± 0.007%/h, respectively (time effect: P = 0.46; Fig. 4B). FSRs calculated with muscle free l-[ring-2H5]phenylalanine enrichments were 0.105 ± 0.031, 0.154 ± 0.037, 0.150 ± 0.032, 0.162 ± 0.050, and 0.167 ± 0.027%/h at basal and between 0 and 60, 60 and 120, 120 and 180, and 180 and 300 min of the postprandial phase, respectively (time effect: P = 0.64).
Table 3.
Change in myofibrillar protein-bound labeling between 2 muscle biopsies in the basal state and after ingestion of milk protein (38 g) in healthy young men
| Postprandial, min |
|||||
|---|---|---|---|---|---|
| Tracer (ΔMuscle Protein Bound, MPE) | Basal (−120 to 0 min) | 0–60 | 60–120 | 120–180 | 180–300 |
| l-[1-13C]phenylalanine | 0.0042 ± 0.0008 | 0.0041 ± 0.0014 | 0.0085 ± 0.0027 | 0.0033 ± 0.0026 | |
| l-[ring-2H5]phenylalanine | 0.0036 ± 0.0010 | 0.0025 ± 0.0006 | 0.0023 ± 0.0005 | 0.0028 ± 0.0009 | 0.0058 ± 0.0009 |
| l-[1-13C]leucine | 0.0036 ± 0.0003 | 0.0053 ± 0.0014 | 0.0032 ± 0.0016 | 0.0059 ± 0.0016 | 0.0062 ± 0.0021 |
Data are means ± SE; n = 7. MPE, mole percent excess.
Fig. 4.
Myofibrillar protein l-[ring-2H5]phenylalanine (A and B) and l-[1-13C]leucine fractional synthesis rates (FSRs; C and D)as %/h in the basal state (gray bars) and after ingestion of milk protein (38 g; open bars) in healthy young men (n = 7), using the plasma enrichments as the precursor pool. Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. *Different from basal (t = −120–0 min) (P < 0.05). Data are means ± SE.
With the use of plasma l-[1-13C]leucine enrichments as the precursor, postprandial muscle protein FSRs increased above basal values (0.027 ± 0.002%/h; time effect: P = 0.018) between 120 and 300 (0.047 ± 0.004%/h) and 0 and 300 min (0.049 ± 0.007%/h; all time points, P < 0.05) (Fig. 4C). We observed a trend (P = 0.10) for an increase in FSR above basal values between 0 and 120 min (0.051 ± 0.016%/h). No differences were observed between 0 and 120 and 120 and 300 min postprandial muscle protein FSRs (P = 0.85). Postprandial plasma l-[1-13C]leucine enrichment-based muscle protein FSRs expressed at 0–60, 120–180, 120–180, and 180–300 min were 0.065 ± 0.015, 0.035 ± 0.015, 0.067 ± 0.018, and 0.036 ± 0.012%/h, respectively (time effect: P = 0.46; Fig. 4D). Muscle protein FSRs calculated with muscle free l-[1-13C]leucine enrichments were 0.040 ± 0.003, 0.083 ± 0.020, 0.043 ± 0.019, 0.077 ± 0.022, and 0.039 ± 0.014%/h at basal and between 0 and 60, 60 and 120, 120 and 180, and 180 and 300 min of the postprandial stage respectively (time effect: P = 0.25).
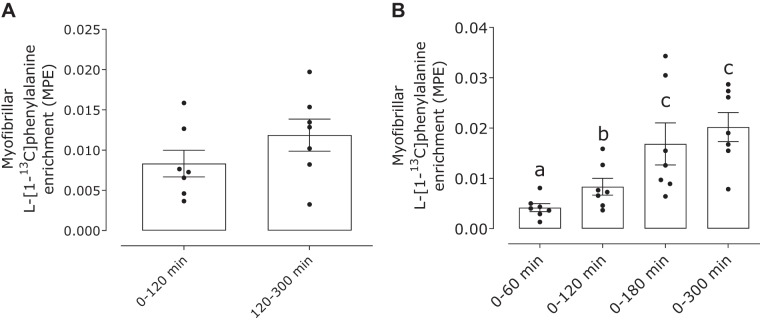
Dietary protein-derived l-[1-13C]phenylalanine enrichment was detected in myofibrillar proteins at 60 min (0.004 ± 0.001 MPE). The muscle protein bound l-[1-13C]phenylalanine enrichment progressively increased at 120 (0.008 ± 0.002 MPE) and 180 min (0.017 ± 0.004 MPE) before a plateau was achieved at 300 min (0.020 ± 0.003 MPE) of the postprandial phase (Fig. 5) (time effect: P < 0.001). No differences were observed in the myofibrillar bound l-[1-13C]phenylalanine enrichments at the early (0–120 min; 0.008 ± 0.002 MPE) or late postprandial stage (120–300 min; 0.012 ± 0.002 MPE; P = 0.17).
Fig. 5.
Myofibrillar protein-bound l-[1-13C]phenylalanine enrichment [mole percent excess (MPE)] after ingestion of milk protein (38 g) in healthy young men (n = 7). Data were analyzed with 1-way repeated-measures ANOVA (time). The least significant difference test was used to locate differences between means when significance was observed. Means without a common letter differ significantly (P < 0.05). Data are means ± SE.
DISCUSSION
Previous studies have used the administration of constant intravenous amino acid infusions or whey protein ingestion to describe a latency and saturable postprandial muscle protein synthetic response after elevated plasma amino acid availability (2, 4, 21). Here, we demonstrated that the ingestion of 38 g of milk protein results in a rapid and sustained release of dietary protein-derived amino acids into circulation, thereby providing a prolonged exposure of exogenous protein-derived amino acids to the muscle during the entire 300-min postprandial phase (2, 21). This pattern of aminoacidemia after milk protein ingestion resulted in a sustained stimulation of muscle protein synthesis rates during the entire 5-h postprandial period. Likewise, dietary protein-derived amino acids are utilized by muscle throughout the early (0–120 min) and late (120–300 min) postprandial period, as evidenced by the progressive increase in l-[1-13C]phenylalanine incorporation into muscle protein.
A noteworthy aspect of our study was the use of the intrinsically labeled protein method (24, 27), which allowed us to quantify the postprandial release of dietary protein-derived amino acids into the circulation and the subsequent time course of stimulation of muscle protein synthesis rates in vivo in humans. We observed rapid protein digestion and amino acid absorption after the ingestion of 38 g of milk protein, which resulted in ∼13 ± 2% (∼4.8 g protein) of dietary protein-derived amino acid becoming available into circulation within the first hour of the postprandial period (Fig. 2B). Plasma amino acid availability peaked between 60 and 120 min, during which time ∼14 ± 1% (~5.3 g) of ingested milk protein was released into circulation, and the milk protein-derived amino acids continued to be released during the later postprandial period (Fig. 2). This is similar to the pattern of aminoacidemia observed after the ingestion of ample amounts of protein contained in eggs, meat, or skim milk, which are other food sources commonly consumed within a Western diet (3, 9, 29). In contrast, whey protein induces a rapid and transient pattern of aminoacidemia (2, 21, 23), with the postprandial stimulation of muscle protein synthesis rates lasting only 60–180 min (2, 21). Overall, the moderate and prolonged dietary protein-derived amino acid release into circulation and the sustained stimulation of muscle protein synthesis rates after the ingestion of 38 g of milk protein is likely more indicative of an anabolic response to whole food ingestion (or mixed meal feeding) in comparison with whey protein ingestion or intravenous amino acid infusions.
Importantly, the use of intrinsically l-[1-13C]phenylalanine-labeled milk protein allowed us to determine the utilization of dietary protein-derived amino acids for de novo muscle protein accretion. This approach allowed for the first time the direct assessment of the meal-derived amino acid accretion into muscle proteins in a time-dependent manner (Fig. 5A). We show that dietary-derived amino acids were rapidly used for de novo myofibrillar protein synthesis, as evidenced by the increase in myofibrillar bound l-[1-13C]phenylalanine enrichment at 60 min of the postprandial period. Moreover, dietary protein-derived amino acids are continuously used for postprandial muscle protein accretion into the late phase of the postprandial period (Fig. 5B). Specifically, we show that ∼2.9 ± 0.6% (∼1.1 ± 0.3 g) of dietary protein-derived amino acids were incorporated into de novo muscle protein within 0–120 min, and ∼4.2 ± 0.9% (∼1.6 ± 0.3 g) of the dietary amino acids were incorporated during the subsequent 120- to 300-min postprandial phase.
The mTORC1 pathway has been extensively studied as the nexus for nutrient-related anabolic signals (i.e., elevated dietary amino acids as opposed to plasma insulin) that regulate the postprandial stimulation of muscle protein synthesis rates in humans (2, 15, 21). Previously, we have demonstrated that increases in postprandial mTORC1 phosphorylation events are often modest or undetectable after the ingestion of protein-dense foods (i.e., eggs, pork, or skim milk) (3, 9, 29). We have generally attributed the diminished activation of the mTOR pathway in these prior studies to muscle biopsy timing issues that are often associated with study designs aimed at optimizing the measurement of muscle protein synthesis rates as opposed to capturing static snapshots of protein phosphorylation. We accounted for this issue in the present study by collecting muscle biopsy samples more frequently throughout the postprandial period. With this approach, we show that the ingestion of milk protein increased mTORC1 phosphorylation on Ser2448 at 120 min and that this response remained elevated throughout the subsequent postprandial period (Fig. 3C). This prolonged activation of mTOR supports the notion that the anabolic response to the ingestion of 38 g of milk protein is sustained throughout a 300-min postprandial period. It is worth acknowledging, however, that it has been suggested that mTORC1 phosphorylation on Ser2448 may not be representative of mTORC1 activity and that other targets are likely preferred (e.g., p70S6K) (14). In the present work, p70S6K and other molecular readouts linked to the mTORC1 pathway (p-Akt and total LAT1 protein content) did not change throughout the postprandial period (Fig. 3). The absence of changes in the phosphorylated state of Akt and p70S6K in the current work is perhaps suggesting that modest anabolic signaling activation is sufficient to elicit changes in muscle protein synthesis rates when the postprandial release of dietary amino acids into circulation is more gradual. Past efforts have shown that there is a dose-dependent increase in the phosphorylation of Akt on Ser473 and p70S6K on Thr389 in response to increasing plasma insulin and amino acid concentrations (12, 17). However, the most robust changes in the phosphorylation of anabolic signaling molecules, such as p70S6K, are generally observed after the ingestion of free amino acids (15) or whey protein ingestion (2, 20), which demonstrate more rapid patterns of elevated aminoacidemia when compared with milk protein ingestion. Additionally, we may have been underpowered to detect subtle increases in the phosphorylated state of p70S6K, which was ∼1.5-fold elevated above basal at 120 and 180 min of the postprandial phase, but did not reach statistical significance (time effect P = 0.11).
A question raised by this study is, what is the significance of the sustained increase of muscle protein synthesis rates after the ingestion of protein-dense food? First, we have established that the meal-induced stimulation of postprandial muscle protein synthesis rates is extended beyond the early period (>180 min), which is contrast to earlier observations (2, 21). Like past studies, our experiment was conducted in the morning with participants in the postabsorptive state. Whether this sustained postprandial muscle anabolism may only be pertinent to the first meal of the day (i.e., breakfast) with the anabolic sensitivity of muscle tissue to protein ingestion waning over the course of the day (i.e., breakfast > lunch > dinner) is currently unknown. Indeed, it was previously demonstrated that muscle protein synthesis rates can be maintained above postabsorptive values by the ingestion of whey protein every ∼4 h during a 12-h experimental protocol (1). However, it is not possible to distinguish the anabolic potential of each individual meal from this study design. Future studies are clearly required to assess how the quantity and pattern of dietary protein intake within a mixed meal setting over the course of the day differentially modulates postprandial muscle protein synthesis rates and ultimately influences daily net protein balance to define whether there is a most important protein meal of the day.
From a study design perspective, we applied repeated muscle biopsy sampling to assess the stimulation of muscle protein synthesis rates in an hourly fashion based on the infused tracers (Fig. 4, B and D). Our results, however, show that the postprandial muscle protein synthetic response was not statistically stimulated above basal values based on the hourly analysis regardless of the infused tracer (i.e., labeled leucine or phenylalanine). This finding contrasts with our assessment of muscle protein synthesis over the early (0–120 min) and late (120–300 min) postprandial phase. The disagreement between findings likely relates to the heterogeneity of the response between participants and analytical challenges in detecting changes in protein-bound enrichments when successive muscle biopsy samples are collected during short infusion time intervals (7, 22). Hence, longer incorporation times (>1 h) are likely warranted when assessing the stimulation of postprandial muscle protein synthesis rates to protein-dense food ingestion, especially when using smaller sample sizes.
In summary, we are the first to provide insight into the postprandial release of dietary amino acids into circulation and the subsequent regulation of postprandial muscle protein synthesis rates to a more slowly digested protein source than whey. We show that the ingestion of 38 g of milk protein results in a persistent “anabolic drive” to skeletal muscle tissue, as shown by the sustained increase in plasma amino acid availability, increased mTORC1 phosphorylation, and the stimulation of postprandial muscle protein synthesis rates during the early (0–120 min) and late (120–300 min) postprandial periods. Similarly, the utilization of dietary protein-derived amino acids for de novo muscle protein accretion is rapid and persists into the late postprandial period in healthy young men. Future studies are required to determine whether our results are relevant to all meals consumed in 1 day (e.g., lunch or dinner).
GRANTS
This project was funded by the University of Illinois Campus Research Board.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.J.v.L. and N.A.B. conceived and designed research; S.v.V., J.W.B., A.M.H., S.A.P., M.D.L., L.J.v.L., and N.A.B. performed experiments; S.v.V., A.M.H., R.S.E., J.P.G., M.D.L., and L.J.v.L. analyzed data; S.v.V., J.W.B., A.M.H., and N.A.B. interpreted results of experiments; S.v.V. prepared figures; S.v.V. and N.A.B. drafted manuscript; S.v.V., J.W.B., A.M.H., R.S.E., J.P.G., S.A.P., M.D.L., L.J.v.L., and N.A.B. edited and revised manuscript; S.v.V., J.W.B., A.M.H., R.S.E., J.P.G., S.A.P., M.D.L., L.J.v.L., and N.A.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the participants who volunteered for this study. We also thank Joan M. Senden, Annemie P. Gijsen, Justin T. Parel, Alexander V. Ulanov, and Zhong Li for their technical assistance.
REFERENCES
- 1.Areta JL, Burke LM, Ross ML, Camera DM, West DWD, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591: 2319–2331, 2013. doi: 10.1113/jphysiol.2012.244897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- 3.Beals JW, Sukiennik RA, Nallabelli J, Emmons RS, van Vliet S, Young JR, Ulanov AV, Li Z, Paluska SA, De Lisio M, Burd NA. Anabolic sensitivity of postprandial muscle protein synthesis to the ingestion of a protein-dense food is reduced in overweight and obese young adults. Am J Clin Nutr 104: 1014–1022, 2016. doi: 10.3945/ajcn.116.130385. [DOI] [PubMed] [Google Scholar]
- 4.Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94: 14930–14935, 1997. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrère B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol Endocrinol Metab 271: E1083–E1091, 1996. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- 7.Bornø A, Hulston CJ, van Hall G. Determination of human muscle protein fractional synthesis rate: an evaluation of different mass spectrometry techniques and considerations for tracer choice. J Mass Spectrom 49: 674–680, 2014. doi: 10.1002/jms.3387. [DOI] [PubMed] [Google Scholar]
- 8.Burd NA, Cermak NM, Kouw IW, Gorissen SH, Gijsen AP, van Loon LJ. The use of doubly labeled milk protein to measure postprandial muscle protein synthesis rates in vivo in humans. J Appl Physiol (1985) 117: 1363–1370, 2014. doi: 10.1152/japplphysiol.00411.2014. [DOI] [PubMed] [Google Scholar]
- 9.Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr 102: 828–836, 2015. doi: 10.3945/ajcn.114.103184. [DOI] [PubMed] [Google Scholar]
- 10.Burd NA, Hamer HM, Pennings B, Pellikaan WF, Senden JMG, Gijsen AP, van Loon LJC. Substantial differences between organ and muscle specific tracer incorporation rates in a lactating dairy cow. PLoS One 8: e68109, 2013. doi: 10.1371/journal.pone.0068109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 108: 958–962, 2012. doi: 10.1017/S0007114511006271. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza RF, Markworth JF, Figueiredo VC, Della Gatta PA, Petersen AC, Mitchell CJ, Cameron-Smith D. Dose-dependent increases in p70S6K phosphorylation and intramuscular branched-chain amino acids in older men following resistance exercise and protein intake. Physiol Rep 2: e12112, 2014. doi: 10.14814/phy2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballèvre O, Beaufrère B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol 549: 635–644, 2003. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figueiredo VC, Dungan CM, Peterson CA, McCarthy JJ. On the appropriateness of antibody selection to estimate mTORC1 activity. Acta Physiol (Oxf) 31: e13354, 2019. doi: 10.1111/apha.13354. [DOI] [PubMed] [Google Scholar]
- 15.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582: 813–823, 2007. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorissen SH, Burd NA, Hamer HM, Gijsen AP, Groen BB, van Loon LJ. Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. J Clin Endocrinol Metab 99: 2250–2258, 2014. doi: 10.1210/jc.2013-3970. [DOI] [PubMed] [Google Scholar]
- 17.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groen BBL, Horstman AM, Hamer HM, de Haan M, van Kranenburg J, Bierau J, Poeze M, Wodzig WKWH, Rasmussen BB, van Loon LJC. Post-Prandial Protein Handling: You Are What You Just Ate. PLoS One 10: e0141582, 2015. doi: 10.1371/journal.pone.0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr 90: 106–115, 2009. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- 20.Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 201: 365–372, 2011. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. J Physiol 587: 897–904, 2009. doi: 10.1113/jphysiol.2008.164087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 46: 943–948, 1997. doi: 10.1016/S0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 23.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 93: 997–1005, 2011. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 24.Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJ. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci 94: 4366–4373, 2011. doi: 10.3168/jds.2011-4451. [DOI] [PubMed] [Google Scholar]
- 25.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985) 107: 987–992, 2009. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 27.van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci 92: 4812–4822, 2009. doi: 10.3168/jds.2009-2317. [DOI] [PubMed] [Google Scholar]
- 28.van Vliet S, Beals JW, Parel JT, Hanna CD, Utterback PL, Dilger AC, Ulanov AV, Li Z, Paluska SA, Moore DR, Parsons CM, Burd NA. Development of intrinsically labeled eggs and poultry meat for use in human metabolic research. J Nutr 146: 1428–1433, 2016. doi: 10.3945/jn.115.228338. [DOI] [PubMed] [Google Scholar]
- 29.van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR, Burd NA. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr 106: 1401–1412, 2017. doi: 10.3945/ajcn.117.159855. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 9: 57, 2012. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]