Combined chromatin structural data reveals specific chromatin-state transitions that correlate with subsets of functionally distinct rice genes differentially expressed under phosphate starvation.
Abstract
Phosphorus (P) is an essential plant macronutrient vital to fundamental metabolic processes. Plant-available P is low in most soils, making it a frequent limiter of growth. Declining P reserves for fertilizer production exacerbates this agricultural challenge. Plants modulate complex responses to fluctuating P levels via global transcriptional regulatory networks. Although chromatin structure plays a substantial role in controlling gene expression, the chromatin dynamics involved in regulating P homeostasis have not been determined. Here we define distinct chromatin states across the rice (Oryza sativa) genome by integrating multiple chromatin marks, including the H2A.Z histone variant, H3K4me3 modification, and nucleosome positioning. In response to P starvation, 40% of all protein-coding genes exhibit a transition from one chromatin state to another at their transcription start site. Several of these transitions are enriched in subsets of genes differentially expressed under P deficiency. The most prominent subset supports the presence of a coordinated signaling network that targets cell wall structure and is regulated in part via a decrease of H3K4me3 at transcription start sites. The P starvation-induced chromatin dynamics and correlated genes identified here will aid in enhancing P use efficiency in crop plants, benefitting global agriculture.
Phosphorus (P) is among the most limiting essential nutrients for plants because the primary plant-available form of P, inorganic phosphate (Pi), has poor solubility in most soils (Holford, 1997). As a result, P fertilization of soils is required for crop plants to achieve adequate yields. Unfortunately, P fertilization can result in serious environmental concerns due to nutrient run-off, which will worsen in the future due to the nonrenewable nature of P resources (Vance et al., 2003). It is, therefore, necessary to investigate the underlying mechanisms involved in regulating P homeostasis, so as to increase the efficiency of plants to acquire and recycle P. To tolerate low-Pi conditions and maintain optimal P levels, plants have evolved a number of physiological, morphological, and biochemical responses, such as reduced growth, altered root system architecture, and secretion of organic acids, phosphatases, and nucleases to acquire more Pi (Secco et al., 2013). These responses are modulated by large transcriptional networks in which the MYB protein PHOSPHATE STARVATION RESPONSE1 (PHR1) and related transcription factors play key roles (Secco et al., 2013; Sun et al., 2016).
In eukaryotic cells, DNA is complexed with core histones and other chromosomal proteins into chromatin (Luger et al., 1997). Therefore, chromatin structure is a key determinant of gene expression. Despite the fact that a large transcriptional cascade governs responses to low-Pi, relatively little is known regarding the associated chromatin dynamics, although evidence for chromatin-level mechanisms modulating Pi-deficiency responses is emerging. Smith et al. (2010) demonstrated that mutation of the actin-related protein (ARP) gene, ARP6, which encodes a key component of the SWR1 complex that catalyzes H2A.Z deposition (Deal et al., 2007), resulted in decreased H2A.Z localization at a number of Pi deficiency-response genes that were also derepressed. These changes in H2A.Z and expression also occurred in Pi-deficient wild-type plants (Smith et al., 2010). Recently, we demonstrated similar phenomena in rice in which genome-wide H2A.Z distribution was altered by Pi starvation or RNA interference (RNAi) knock-down of ARP6 (Zahraeifard et al., 2018). We also showed that deposition of rice H2A.Z in gene bodies largely resulted in downregulation, whereas H2A.Z at the TSS was positively or negatively correlated with gene expression, depending on the particular Pi deficiency-response genes affected. In a separate study, we revealed that changes in nucleosome occupancy correlated with genes differentially expressed by Pi starvation, implicating nucleosome remodelers in modulating Pi-deficiency responses in rice (Zhang et al., 2018). Finally, two chromatin-related components have been shown to play roles in Pi deficiency-induced root hair growth in Arabidopsis. The ALFIN-LIKE6 gene encodes a plant homeodomain-containing protein that recognizes H3K4 trimethylation and appears to promote enhanced root hair growth during low-Pi conditions by targeting H3K4me3-marked target genes, such as ETC1, which functions in root hair cell patterning (Chandrika et al., 2013). The second factor necessary for normal induction of root hair growth in response to Pi deficiency is Arabidopsis (Arabidopsis thaliana) HDA19, which encodes a histone deacetylase necessary for low-Pi root hair elongation through its role in regulating epidermal cell length (Chen et al., 2015).
Structural components of chromatin, including positioning of nucleosomes, the presence of histone variants, and posttranslational modifications of histones, can be altered by a number of mechanisms (Mariño-Ramírez et al., 2005; Venkatesh and Workman, 2015). In contrast to examining individual chromatin modifications, defining the patterns, or states, of chromatin by examining multiple marks simultaneously in their spatial context is more informative to understanding transcriptional changes in response to stress (Ernst and Kellis, 2012; Pan et al., 2017). In rice (Oryza sativa), two recent studies defined distinct chromatin states (CSs) by combining multiple histone marks and showed various associations between particular CSs and genes differentially expressed by ionizing radiation (Pan et al., 2017) or salinity stress (Zheng et al., 2019). In contrast, no studies have defined CS transitions linked to Pi-deficiency responses in plants. Herein we characterized the impact of Pi starvation on the major histone mark, H3K4me3, as well as on CSs generated from the combined occupancy data of H3K4me3, H2A.Z, and nucleosomes. The data reveal several distinct CS transitions that accompany expression changes of key subsets of Pi-starvation response genes.
RESULTS
H3K4me3 Is Prominent at the 5′ End of Rice Protein-Coding Genes and Colocalizes with the H2A.Z Histone Variant
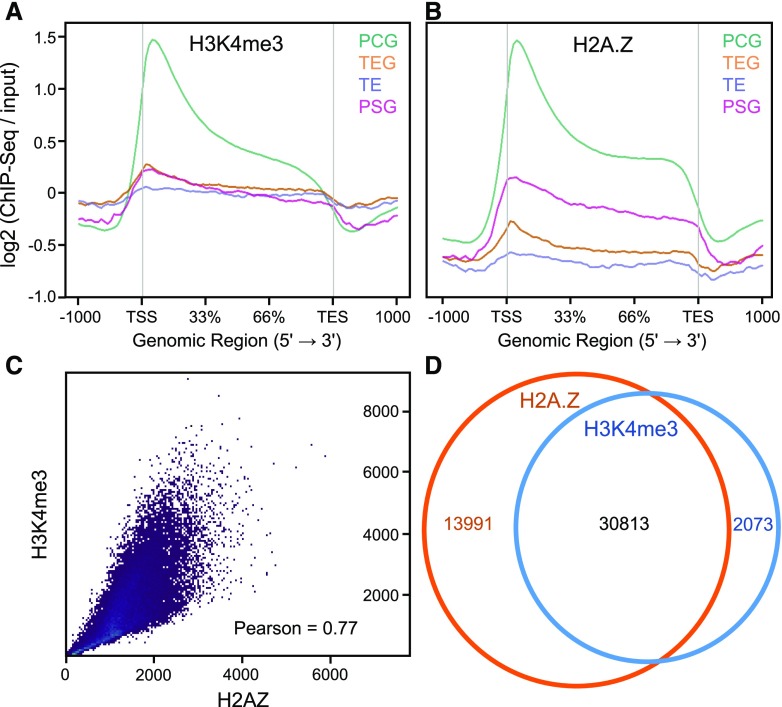
Previously we demonstrated that dynamics of nucleosome occupancy (Zhang et al., 2018) and H2A.Z deposition (Zahraeifard et al., 2018) were linked to genes differentially expressed in response to Pi starvation in rice shoots. The primary goal herein was to evaluate the combined role of nucleosome occupancy, H2A.Z, and a major histone posttranslational modification, H3K4me3, in modulating responses to Pi starvation. We began by determining the genome distribution of H3K4me3 via chromatin immunoprecipitation sequencing (ChIP-seq) on shoots from 36-d–old rice (ssp. japonica ‘Nipponbare’) seedlings (Supplemental Table S1). Genes were categorized into four groups based on the Michigan State University Rice Genome Annotation Release 7.1 (MSU7.1) genome annotation: protein-coding genes (PCGs), “pseudogenes” (PSGs, i.e. annotated genes that are neither expressed nor transposable element-related), transposable element-related genes that are expressed (TEGs), and transposable element-related genes that are not expressed (TEs; Kawahara et al., 2013; Zhang et al., 2018). As shown in Figure 1A, we observed a prominent H3K4me3 peak immediately downstream of the transcription start sites (TSSs) of PCGs, similar to previous studies (Zhang et al., 2009; van Dijk et al., 2010; Du et al., 2013; Zong et al., 2013). In contrast to PCGs, H3K4me3 abundance was relatively low at PSGs, TEGs, and TEs (Fig. 1A). Next we examined whether subgroups of PCGs exhibited different H3K4me3 patterns. Sorting all PCGs according to size revealed a strong correlation between H3K4me3 and gene length (Supplemental Fig. S1, A and B), indicating that the general pattern of H3K4me3 among all PCGs is relatively consistent (i.e. a major peak of H3K4me3 at the TSS). Although a TSS-localized peak of H3K4me3 was observed at virtually all PCGs, the abundance of the peak varied. Clustering analysis at a 100-bp window across the TSS revealed four distinct clusters of H3K4me3 abundance (Supplemental Fig. S1, C and D). Gene ontology (GO) enrichment analysis showed that the clusters with high and moderate abundance were enriched (false discovery rate [FDR] < 0.05) with housekeeping genes, whereas the clusters with relatively low H3K4me3 abundance were enriched in stress-responsive genes (Supplemental Dataset S1).
Figure 1.
H3K4me3 abundance is predominantly associated with the TSS and colocalizes with H2A.Z. Distribution of H3K4me3 (A) and H2A.Z (B) among four gene types in shoots from rice seedlings grown under control conditions. Control input reads were used for ChIP-Seq read normalization. C, Scatter plot of read counts from H3K4me3 and H2A.Z samples (Pearson correlation = 0.77). D, Venn diagram showing the number of H3K4me3- and H2A.Z-enrichment peaks and the overlap.
The H3K4me3 localization patterns at different gene types (Fig. 1A) are similar to those we recently demonstrated for the H2A.Z histone variant (Zahraeifard et al., 2018; Fig. 1B). A key distinction is that the relative difference in abundance of H2A.Z between PCGs and TEGs/TEs is larger than that of H3K4me3. To further examine the apparent association between H3K4me3 and H2A.Z, we first computed a correlation coefficient using the software deepTools (Ramírez et al., 2016), which showed that both chromatin marks were correlated across the rice genome (r = 0.77; Pearson correlation coefficient; Fig. 1C). Next we identified and compared distinct H3K4me3 and H2A.Z peaks using the programs SICER (Zang et al., 2009) and BEDTools (Quinlan and Hall, 2010). This identified 32,886 H3K4me3 peaks and 44,804 H2A.Z peaks, of which 30,813 (93% of H3K4me3 peaks) overlapped (Fig. 1D). Finally, we plotted the average abundance of both marks centered at peak summits of H3K4me3 (Supplemental Fig. S2A) or H2A.Z (Supplemental Fig. S2B). Together, these analyses demonstrate substantial co-occurrence of H3K4me3 and H2A.Z in the rice genome.
H3K4me3 and H2A.Z Abundance Have Distinct Correlations with Gene Expression in Rice
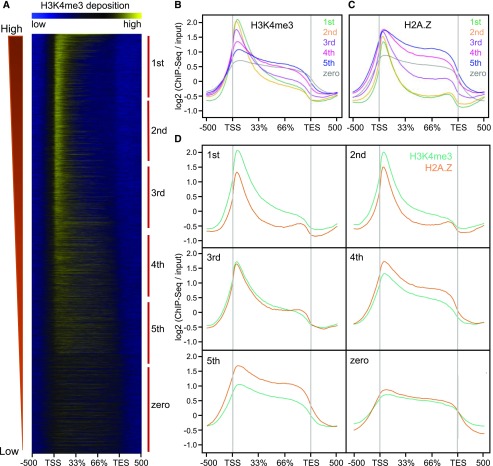
To compare H3K4me3 abundance with gene expression, we analyzed our previously obtained RNA sequencing (RNA-seq) data (Zahraeifard et al., 2018) from shoot tissues of 36-d–old rice seedlings (Supplemental Table S1). PCG were ranked according to fragments per kilobase of transcript per million mapped reads (FPKMs) and divided into five expression quintiles, as well as a sixth group of genes that were not expressed (i.e. FPKM = 0). We found a clear, positive correlation between transcript abundance and H3K4me3 localization around the TSS (Fig. 2, A and B), consistent with studies from a variety of species (Bernstein et al., 2002; Santos-Rosa et al., 2002; Barski et al., 2007; Zhang et al., 2009; van Dijk et al., 2010). In contrast, transcript abundance exhibited a general negative correlation with TES- and gene body-localized H3K4me3 (Fig. 2, A and B). Genes exhibiting no expression were severely depleted in H3K4me3 at the TSS, but had a moderate level of gene-body H3K4me3. Next, we compared the correlation between H3K4me3 abundance and gene expression with that of H2A.Z (Zahraeifard et al., 2018; Fig. 2, C and D). As with H3K4me3, nonexpressed genes were deficient in H2A.Z at the TSS, but contained moderate levels of gene-body H2A.Z. However, for the expression quintiles, H2A.Z exhibited a general negative correlation with expression at both the TSS and, especially, in the gene body. Therefore, although H3K4me3 and H2A.Z are generally colocalized at genic regions, particularly at the TSS of PCG, they have contrasting correlations with gene expression.
Figure 2.
Correlations of H3K4me3 and H2A.Z distribution with gene expression for rice PCGs. A, Heat map of H3K4me3 distribution from 500 bp upstream of the TSS to 500 bp downstream of the TES in control shoot samples for six gene groups ordered based on transcript abundance level (FPKMs), defined as first (highest) to fifth (lowest) or no expression (zero). B, C, and D, Distribution of H3K4me3 (B) or H2AZ (C) or both (D) at the same gene groups as in (A). Control input reads were used for ChIP-Seq read normalization.
Pi-Starvation Impacts H3K4me3 Localization at PCGs
To evaluate a potential role for H3K4me3 in modulating Pi-deficiency responses, we carried out H3K4me3 ChIP-seq on shoots from plants subjected to a 24-h Pi-deficiency treatment (Supplemental Table S1). As shown in Supplemental Figure S3A, Pi-deficiency altered H3K4me3 distribution at PCG, such that the prominent 5′ peak was reduced. Separating PCG into two broad functional categories showed that housekeeping genes exhibited the 5′ peak reduction (Supplemental Fig. S3B), whereas stress-responsive genes exhibited an increase in H3K4me3 at the TSS (Supplemental Fig. S3C). These data along with our prior studies (Zahraeifard et al., 2018; Zhang et al., 2018) indicate that nucleosome occupancy, H2A.Z, and H3K4me3 each exhibit distinct changes in response to Pi starvation.
H3K4me3, H2A.Z, and Nucleosome Occupancy Define Five CSs in the Rice Genome
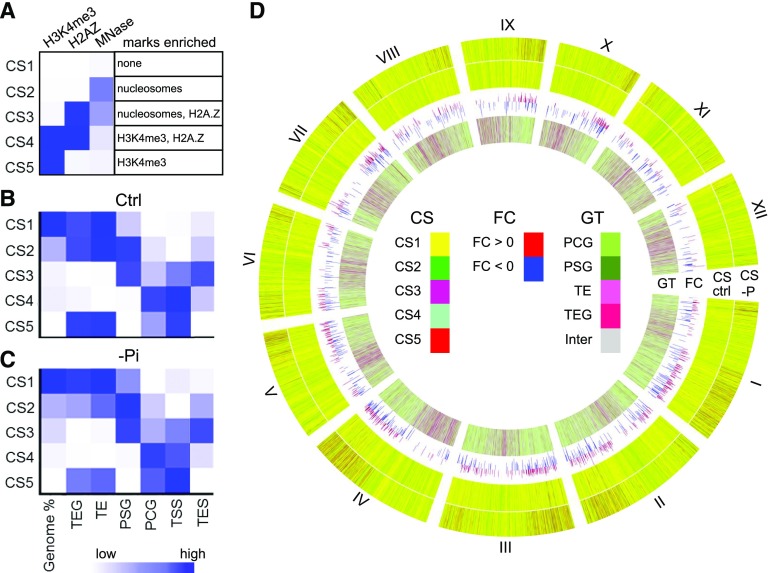
It is becoming increasingly clear that simultaneously examining multiple chromatin marks provides a more robust picture of the dynamic chromatin environment linked to various developmental processes and responses to stimuli (Pan et al., 2017; Yan et al., 2019). Therefore, we integrated our H3K4me3 ChIP-seq, H2A.Z ChIP-seq (Zahraeifard et al., 2018), and MNase sequencing (MNase-seq; Zhang et al., 2018) datasets to define distinct CSs using the program ChromHMM (Ernst and Kellis, 2012). ChromHMM employs a multivariate Hidden Markov model that scores the enrichment of each chromatin mark to determine the major recurring combinatorial and spatial patterns of marks, i.e. CSs. ChromHMM identified five CSs, each distinguishable from the others by differential enrichment of one or more of the marks tested (Fig. 3A). Because the enrichment values are relative, a low score for a particular mark does not indicate a lack of the mark. CS1 and CS2 were each deficient in both H2A.Z and H3K4me3, CS3 was enriched in only H2A.Z, CS4 was enriched in both H2A.Z and H3K4me3, and CS5 was enriched in only H3K4me3. Regarding nucleosome density, CS2 and CS3 had moderately higher nucleosome enrichment compared to the other three states. To support the presence of these five particular CSs in the rice genome, we repeated the ChromHMM analysis by combining our three datasets from control samples with publicly available datasets for two marks recognized as largely repressive—DNA methylation (Secco et al., 2015) and/or H3K27me3 (Zhang et al., 2012) from control shoot samples. As shown in Supplemental Figure S4, the addition of these marks did not change the combinatorial effect of the three marks we used to designate the five CSs, verifying the relevance of these states in our original ChromHMM analysis. The inclusion of DNA methylation or H3K27me3 alone each yielded a sixth CS, whereas including both repressive marks yielded a sixth and seventh state (Supplemental Fig. S4A). In addition, a genome comparison of the five and seven chromatin-state analyses showed similar percentages of the genome represented by each CS except CS1. When the repressive marks were added, some regions of the genome defined as CS1 were changed to CS6 or CS7 (Supplemental Fig. S4, B and C).
Figure 3.
CS predictions for control and phosphate-starved (–Pi) samples defined by H3K4me3, H2A.Z, and nucleosome occupancy. A, Emission parameters for CS1–CS5. The darker-blue color corresponds to a greater probability of observing the mark in the state. Overlap fold enrichment of various genomic regions with five CSs in control (B) and –Pi (C) samples. D, Circos plot showing the CSs (in 5-kb bins) of the whole genome. The first and second rings show the CSs in –Pi (CS –P) and control (CS ctrl) samples, respectively. The third ring shows the log2 fold change (FC) of DEGs, and the last ring represents four gene types (GT). Inter, intergenic region. Roman numerals represent chromosome numbers.
Next, we mapped the distribution of the five CSs across the genome (divided into 200-bp bins), which revealed several biases with genomic features (Fig. 3, B and D). CS1 was the major CS, accounting for 63% of the rice genome, and was enriched at TEs and TEGs. It should be noted that highly repetitive regions of the genome were likely designated CS1 due to low numbers of mappable reads rather than bona fide depletion of the chromatin marks examined. TEs and TEGs were also enriched in CS2 and CS5. This means that the TE-related loci were either deficient in both H2A.Z and H3K4me3 or enriched in H3K4me3 only. In contrast, PSGs were enriched in CS2 and CS3, consistent with depletion of both H2A.Z and H3K4me3 or enrichment of only H2A.Z. Finally, PCGs were enriched in CS4, consistent with enrichment of both H3K4me3 and H2A.Z. To more specifically characterize PCGs, we calculated enrichments at the TSSs and TESs separately (Fig. 3B). Compared to all bins within PCGs, the TSSs were more enriched in CS4, CS5, and CS3, whereas the TESs were more enriched in CS3 and less enriched in CS4. These results indicate for PCGs generally an overall high occupancy of H2A.Z and/or H3K4me3 at the TSSs, but an enrichment of only H2A.Z at the TESs.
Pi Starvation Has a Dramatic Impact on Chromatin Signatures
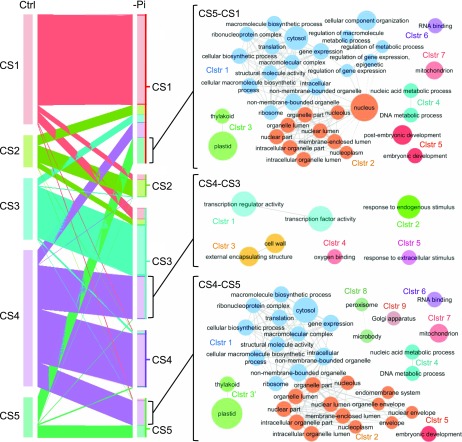
To characterize the impact of Pi starvation on chromatin signatures we compared the distribution of CSs between control and Pi-deficiency conditions. First we analyzed the enrichment of each CS within the four gene types (Fig. 3, C and D). In response to Pi starvation, the genomic bins within TEs, TEGs, and PSGs increased in CS1, but decreased in CS2, CS4, and CS5, consistent with a loss of H3K4me3. On the other hand, PCG bins decreased in CS1, but increased in CS2, CS3, and CS5. At the TSS of PCG, CS2 and CS4 decreased and CS1 increased, whereas at the TES, CS1 and CS4 decreased and CS2 increased (P value < 0.01; Fig. 3, B and C; Supplemental Fig. S5). Because the TSS is an important regulatory region for gene expression, we investigated the effect of Pi starvation at the TSS of PCG in more detail. First we determined the CS at the TSS of each PCG under control conditions (Supplemental Dataset S2). For each CS subgroup, we generated average plots and heatmaps of the relative enrichment of H3K4me3, H2A.Z, and nucleosome occupancy (Supplemental Fig. S6). Next we determined the CS at the TSS of each PCG after Pi starvation (Supplemental Dataset S2). Over 40% of PCG exhibited a CS change, or transition, at their TSS in response to Pi starvation (Supplemental Dataset S2; Fig. 4). The largest groups of transitions were CS4–CS3 (n = 4,088), CS4–CS5 (n = 2,355), and CS5–CS1 (n = 2,496; Fig. 4; Supplemental Fig. S7). GO enrichment analysis showed significantly enriched GO terms (FDR < 0.05) for eight of the transition groups (Supplemental Dataset S3). We then applied Markov Clustering (MCL) to reduce redundancy in enriched GO processes and generated functional clusters (“GOMCL” clusters) to represent the primary functions associated with CS transitions. As shown in Figure 4, the enriched GO terms for CS4-CS3 genes fell into five GOMCL clusters, including transcription factor activity, response to endogenous stimulus, cell wall, oxygen binding, and response to extracellular stimulus. In contrast, CS4-CS5 genes were enriched in GO terms defined by nine GOMCL clusters, which, among other functional categories, were related to translation and gene expression, nuclear functions, plastid functions, nucleic acid metabolism, development, and RNA binding. Interestingly, CS5-CS1 genes shared essentially the same enriched GO terms (Supplemental Dataset S3) and GOMCL clusters (Fig. 4) as CS4-CS5 genes. One explanation for this is that the CS5-CS1 and CS4-CS5 transitions are frequently found together at the TSS. Indeed, examination of the bins that flank the TSS (Supplemental Fig. S8) showed that CS5-CS1 genes were approximately four times more likely than random to exhibit a CS4-CS5 transition in the bin downstream of the TSS (P value < 0.001). Similarly, the CS4-CS5 genes were 3.5-fold more likely to contain a CS5-CS1 transition upstream of the TSS (P value < 0.001). In contrast, CS5-CS1 genes with a CS4-CS5 upstream bin, and CS4-CS5 genes with a CS5-CS1 downstream bin were similar to random or underrepresented, respectively. Thus, the identification of subgroups of functionally similar genes with CS5-CS1 and CS4-CS5 transitions at their TSS is reflective of these genes containing a specific pair of transitions (CS5-CS1 + CS4-CS5, 5′–3′) at the TSS.
Figure 4.
CS transitions of PCGs from control (Ctrl) to phosphate-starved (−Pi) conditions. Left, The size of each segment represents the number of genes in each CS and the width of each ribbon represents the number of genes with a transition to another CS. Right, Networks representing GO MCL terms enriched in CS5-CS1, CS4-CS3, and CS4-CS5 groups. The program Cytoscape was used to visualize enriched GO terms. Node (circle) size represents the number of genes in each node.
CS Transitions Correlate with Differential Expression of Pi Deficiency-Responsive Genes
We analyzed our recent RNA-seq experiments (Zahraeifard et al., 2018; Supplemental Table S1) to investigate the relationship between gene expression and CS transitions in response to Pi starvation. Differential expression analysis with DESeq2 identified 1,385 differentially expressed genes (DEGs) in response to Pi starvation—694 upregulated and 691 downregulated (adjusted P value < 0.001; Supplemental Fig. S9; Supplemental Dataset S4). GO terms enriched for upregulated genes included response to stress, lipid metabolic process, and signal transduction, whereas downregulated genes were enriched in growth, cell–cell signaling, and lipid, carbohydrate, and secondary metabolic processes (Supplemental Table S2; Supplemental Fig. S9, B and C). Although lipid metabolism was overrepresented in both groups of DEGs, genes linked to carotenoid biosynthesis and alpha-Linolenic acid metabolism were among the upregulated DEGs, whereas cutin, suberin, and wax biosynthesis were among the downregulated DEGs. Overall, the functional categories of these DEGs were similar to those from previous transcriptome studies of Pi-deficient plants (Thibaud et al., 2010; Cai et al., 2013; Secco et al., 2013; Zahraeifard et al., 2018).
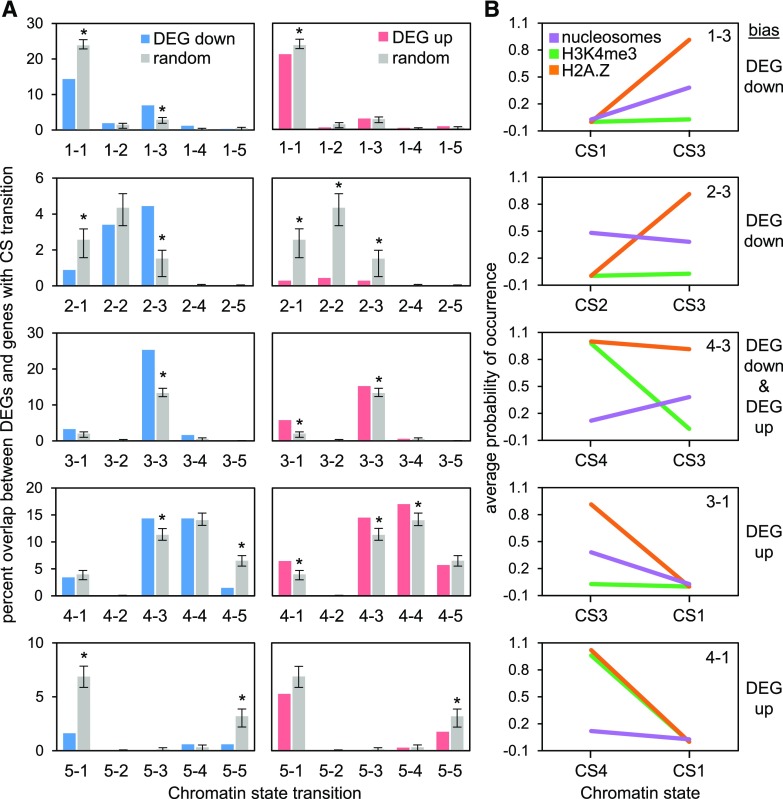
We then investigated whether differential expression in response to Pi starvation correlated with distinct CS transitions. We quantified the overlap between the up- or downregulated DEGs and each CS transition via bootstrapping analyses (1,000 iterations) and carried out binomial tests to identify the over- or under-represented DEGs for each CS transition (Fig. 5). These analyses revealed a number of significant (P value < 0.001) biases between DEGs and CS transitions. First, downregulated DEGs were enriched among CS1-CS3 and CS2-CS3 transitions (i.e. transitions from H2A.Z-deficient CSs to an H2A.Z-enriched state), consistent with downregulation of gene expression being correlated with a gain of H2A.Z. Reciprocally, upregulation of gene expression correlated with a loss of H2A.Z, as indicated by enrichment of upregulated genes among CS3-CS1 transition genes. These observations support a role for H2A.Z as a repressive chromatin mark during Pi starvation, in which some genes are repressed by the deposition of H2A.Z, whereas other genes are induced (derepressed) by loss of H2A.Z. Second, genes containing H2A.Z and H3K4me3 that exhibited decreases in both marks in response to Pi deficiency (i.e. CS4-CS1 transition) were enriched among upregulated genes. This suggests a negative role for not only H2A.Z, but also H3K4me3, in which the loss of both marks from this group of genes results in their derepression. Third, up- and downregulated DEGs were both enriched among CS4-CS3 transition genes (i.e. those with a decrease in H3K4me3 but maintenance of H2A.Z). Interestingly, this suggests a possible dual role of H3K4me3 in Pi-responsive gene modulation. Fourth, the other two prominent groups of transitions, CS5-CS1 and CS4-CS5, which contain many translation-related genes, were underrepresented among downregulated DEGs. This indicates that genes exhibiting these transitions, or pair of transitions (Supplemental Fig. S8), at the TSS are unlikely to be differentially expressed after 24-h Pi deficiency. Because a number of translation-related genes were previously shown to be downregulated by long-term (21-d) Pi deficiency in rice shoots (Secco et al., 2013), we carried out a bootstrapping analysis to test whether our CS5-CS1 and CS4-CS5 genes were enriched among those DEGs. Indeed, both CS5-CS1 and CS4-CS5 groups were enriched (P value < 0.01) among genes downregulated by long-term Pi deficiency (Supplemental Fig. S10). This suggests that the chromatin dynamics observed at these genes after 24 h of Pi starvation is a prelude to decreased transcript abundance not observable until after a longer duration of Pi deficiency.
Figure 5.
CS transitions are associated with DEGs under phosphate-starved (−Pi) conditions. A, Bootstrapping analysis showing the overlap between genes exhibiting CS transitions and down- or upregulated genes in response to phosphate starvation. Randomly selected genes with the same number of DEGs per CS transition were used as a control, and values are means (±sd) for 1,000 iterations. B, Values are the average probability of each chromatin mark at the CS shown. The category of DEG (up- or down-) that is biased to the CS is shown at right.
In addition to the correlations between DEGs and distinct CS transitions, there were also correlations between DEGs and groups of genes that did not transition (Fig. 5). Both up- and downregulated DEGs were significantly enriched among CS3 genes that did not transition (i.e. CS3-CS3), and were underrepresented among CS1-CS1 and CS5-CS5 genes. Furthermore, upregulated DEGs were enriched among CS4-CS4 genes and underrepresented among CS2-CS2 genes. These results show that responsive genes are likely to contain H2A.Z, which is consistent with previous reports (Coleman-Derr and Zilberman, 2012; Zahraeifard et al., 2018). Taken together, these biases demonstrate that specific chromatin dynamics at the TSS are linked to subsets of genes differentially expressed by Pi starvation.
DEGs Exhibiting a CS4-to-CS3 Chromatin Transition Suggest a Coordinated Pi-Deficiency Regulatory Network Targeting the Apoplast
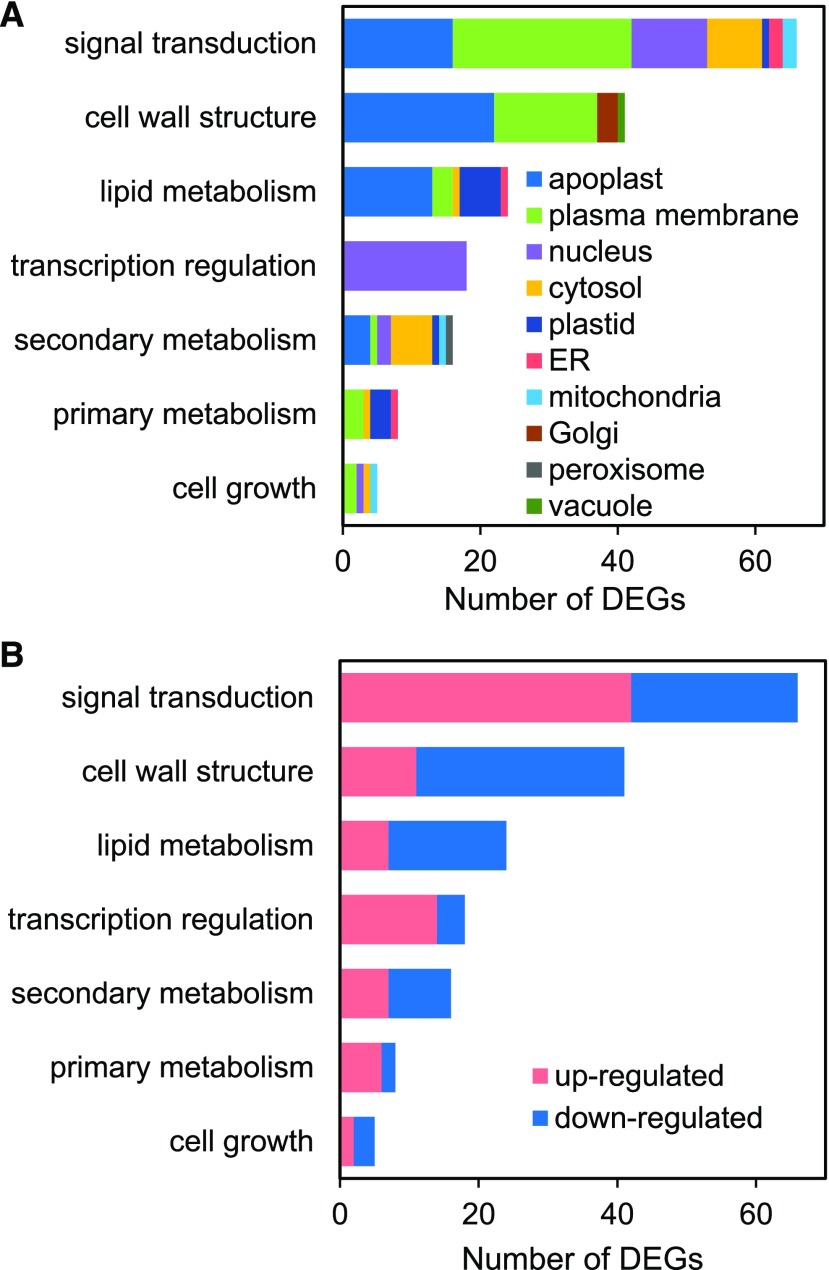
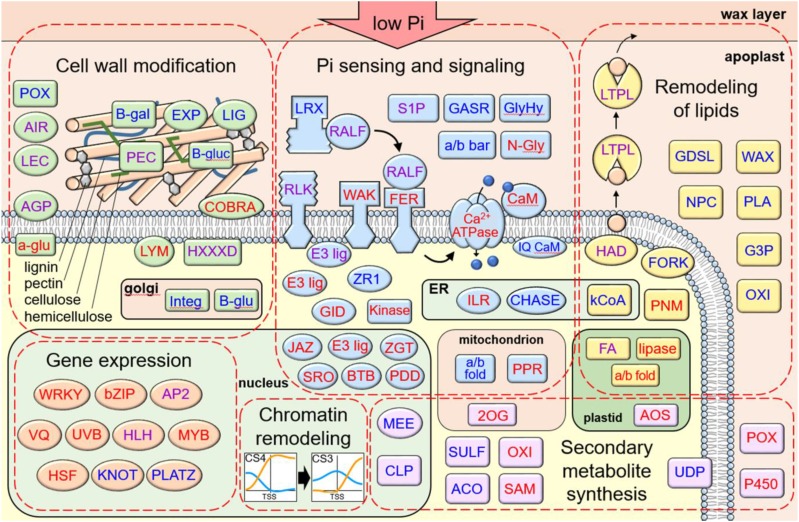
As shown above, the largest group of genes exhibiting a CS shift in response to Pi deficiency was the CS4-CS3 transition group (Fig. 4; Supplemental Dataset S3). These genes were also significantly enriched among both up- and downregulated DEGs (Fig. 5). To gain insight into the predicted functions of the DEGs that exhibited a CS4 to CS3 transition, we carried out GO-term enrichment analysis, which identified four significantly enriched (FDR < 0.05) terms: cell wall, external encapsulating structure, response to biotic stress, and catalytic activity (Supplemental Fig. S11). Due to the relatively limited GO term assignments for rice loci, we carried out extensive data mining on the CS4-CS3 DEGs, which allowed us to assign putative functional and subcellular localization information to over 90% (178 of 196) of the DEGs (Supplemental Dataset S5). These DEGs encode components with putative functions in signal transduction (37%), cell wall structure (23%), lipid composition (13%), transcription regulation (10%), secondary metabolism (9%), primary metabolism, or cell growth (3%), which are mostly targeted to the apoplast (31%), plasma membrane (28%), nucleus (18%), cytosol (10%), or plastid (6%; Fig. 6A). Strikingly, more than half (53%) of the CS4-CS3 DEGs are predicted to encode proteins targeted to the apoplast or plasma membrane, and have functions in signaling or cell wall and lipid composition. Among this group are a number of pectinases, arabinogalactan proteins, and expansins that mostly are downregulated by the 24-h Pi-deficiency treatment (Fig. 6B; Supplemental Dataset S5). A previous study in Arabidopsis identified a similar response of cell wall hydrolytic enzyme-encoding loci in roots subjected to Pi-deficiency treatments of 1, 6, and/or 24 h (Lin et al., 2011). Together, this suggests that modification of the cell wall is an early and prominent response to Pi starvation in roots and shoots across species. In addition to the downregulation of cell wall-related components was a large group of signaling components, including many receptor-like kinases (RLKs) that were predominantly upregulated (Fig. 6B; Supplemental Dataset S5). One of the RLKs is a Catharanthus roseus RLK1-like kinase orthologous to Arabidopsis FERONIA (FER), which has been shown to regulate cell expansion in response to diverse developmental and environmental cues (Liao et al., 2017). For example, during salinity stress, FER maintains cell wall integrity, and is necessary for root growth recovery (Feng et al., 2018). Recently it was demonstrated that FER is one component of a signaling module that transduces cell wall signals during salt stress (Zhao et al., 2018). In the absence of salt stress, a group of apoplastic Leu-rich repeat extensins (LRX) bind to RAPID ALKALINIZATION FACTOR (RALF) peptides. In response to salt stress, LRX and RALF dissociate, and RALF peptides bind FER. This results in FER internalization and, subsequently, inhibition of growth and initiation of stress responses. Calcium transients and SITE-1 PROTEASE activity also play roles in RALF/FER signaling (Stegmann et al., 2017; Feng et al., 2018). Notably, our CS4-CS3 DEG list also contains six genes encoding RALF peptides (out of 14 total in the rice genome; Campbell and Turner, 2017) an LRX, several Ca2+ transport-related components (e.g. Ca2+ ATPase and calmodulin), and two S1P proteases (Supplemental Dataset S5). In addition to the signaling and cell wall components among the CS4-CS3 DEGs were a number of transcription factors, including five AP2 superfamily factors, two HLH factors, and two WRKY transcription factors. These represent families of transcription factors known to be responsive to a number of biotic and abiotic stressors. Interestingly, examining the CS4-CS3 DEGs as a whole suggests an integrated network whereby Pi deficiency initiates reduced cellular growth and low-Pi tolerance mechanisms. A proposed model of this network is shown in Figure 7. In this working model, low-Pi signals entering the apoplast interact with signaling components, including receptor-like and wall-associated kinases. Also, Pi starvation may lead to a transfer of RALF peptides from LRX to the rice FER ortholog and subsequent internalization of FER, which is likely regulated in part by Ca2+. Next, the transduced low-Pi signals lead to differential expression of many regulatory and structural genes including a number of stress-responsive transcription factors. These changes in gene expression, which coincide with chromatin remodeling (i.e. a transition from CS4 to CS3), drive robust metabolic and physiological adaptations, such as cell wall modification, lipid and wax remodeling, and altered secondary metabolite biosynthesis. These modifications, in turn, result in slowed cellular growth and tolerance to sustained Pi starvation. This hypothetical model provides a strong framework for future functional studies aimed at investigating the roles of the specific components in modulating cellular responses to Pi starvation in rice.
Figure 6.
Predicted functions and subcellular locations of DEGs having a CS transition of CS4 to CS3. DEGs are grouped based on the putative functional categories shown. A, The number of DEGs predicted to localize to distinct subcellular locations. B, The number of DEGs that are up- or downregulated in each functional category.
Figure 7.
Predicted interactions and functions of DEGs having a CS transition of CS4 to CS3. Text color indicates upregulation (red), downregulation (blue), or a combination of up- and downregulation (purple). Abbreviations: 2OG, 2OG-Fe oxygenase; a/b bar, A/B barrel; a/b fold, alpha/beta fold hydrolase; ACO, 1-aminocyclopropane-1-carboxylate oxidase; a-glu, heparan-alpha-glucosaminide n-acetyltransferase; AGP, arabinogalactan protein; AIR, auxin response protein; AOS, allene oxide synthase; AP2; B-gal, beta-galactosidase; B-glu, Beta glucan synthase; B-gluc, beta-glucuronidase; BTB, Bric-a-Brac, Tramtrack, Broad Complex protein; bZIP, basic leucine zipper transcription factor; Ca ATPase, calcium-transporting ATPase; CaM, calmodulin-related calcium sensor; CHASE, Cyclases/Histidine kinases Associated Sensory Extracellular domain containing protein; CLP, ATP-dependent caseinolytic protease/crotonase; COBRA, AtCOBRA-like; E3 lig, ubiquitin ligase; ER, endoplasmic reticulum; EXP, expansin; FA, fatty acid hydroxylase; FER, AtFERONIA ortholog; FORK, FORKED1-like; G3P, glycerol-3-phosphate acyltransferase; GASR, GASA/GAST/Snakin; GDSL, GDSL-like lipase/acylhydrolase; GID, gibberellin receptor; GlyHy, glycosyl hydrolase; HAD, HAD phosphoethanolamine/phosphocholine phosphatase; HLH; HLH helix–loop–helix transcription factor; HSF, heat shock factor; HXXXD, HXXXD-type acyl-transferase; ILR, IAA-amino acid hydrolase; Integ, cell wall integrity protein; IQ CaM, IQ calmodulin-binding motif protein; JAZ, ZIM domain-containing JAZ protein; kCoA, 3-ketoacyl-CoA synthase; KNOT, knotted-1-like homeobox protein; LEC, lectin; LIG, lignin dirigent; LTPL, Protease inhibitor/seed storage/LTP protein; LYM, lysM domain-containing GPI-anchored protein; MEE, maternal effect embryo arrest; MYB, MYB domain transcription factor; n-Gly, shiga/ricin-like n-glycosidase; NPC, nonspecific phospholipase; OXI, oxidoreductase; P450, cytochrome P450; PDD, PD-(D/E)XK nuclease superfamily protein; PEC, pectinase; PLA, phospholipase A; PLATZ, plant AT-rich sequence and zinc-binding protein; PNM, phosphoethanolamine n-methyltransferase; POX, peroxidase; PPR, pentatricopeptide repeat protein; S1P, subtilisin site-1 protease; SAM, s-adenosyl-l-Met-dependent methyltransferases; SRO, OsSRO1c; SULF, sulfotransferase; UDP, UDP-glucuronosyl/UDP-glucosyltransferase; UVB, ultraviolet-B-repressible protein; VQ, VQ domain containing protein; WAK, wall-associated kinase; WAX, WAX2-like; WRKY, WRKY domain transcription factor; ZGT, ZGT circadian clock coupling factor; ZR1, FYVE zinc finger domain protein.
DISCUSSION
H3K4me3 and H2A.Z Exhibit Overlapping and Divergent Localization Patterns
Despite being widely recognized as marks of active transcription, assigning specific roles for H3K4me3 and H2A.Z in regulating transcription has been challenging. For instance, H3K4me3 is often assumed to promote transcription, but loss or severe depletion of H3K4me3 levels results in relatively few gene expression changes (Clouaire et al., 2012; Margaritis et al., 2012). Also, whereas loss of H3K4me3 at most genes has no impact on expression, H3K4me3 has been linked to both activation and repression of subsets of genes (Weiner et al., 2015; Cano-Rodriguez et al., 2016). Like H3K4me3, H2A.Z is often associated with gene activity, but plays a complex role in modulating gene expression. Evidence indicates that H2A.Z acts to both promote and repress gene expression, depending on the environmental or developmental context, genic location, and relevant loci (Deal et al., 2007; Zilberman et al., 2008; March-Díaz and Reyes, 2009; Kumar and Wigge, 2010; Smith et al., 2010; Berriri et al., 2016; Sura et al., 2017; Zahraeifard et al., 2018). Interactions among multiple chromatin modifications add complexity to identifying specific chromatin-level mechanisms that modulate gene expression, particularly in light of contradictory findings. For example, Arabidopsis H2A.Z has been proposed to facilitate H3K4 trimethylation at miR156 loci (Xu et al., 2018) but antagonize H3K4me3 abundance at anthocyanin biosynthetic genes (Cai et al., 2019). Thus, there is a need to investigate multiple aspects of the chromatin environment to gain insight into chromatin-level mechanisms that impact gene expression.
Herein we used ChromHMM to combine our H3K4me3 ChIP-seq data from this study with our previous H2A.Z ChIP-seq (Zahraeifard et al., 2018) and MNase-seq (Zhang et al., 2018) data to define five CSs (CS1–CS5) in rice shoots. Genic regions were enriched in CS4, which is characterized by moderate nucleosome occupancy and relatively high levels of H2A.Z and H3K4me3. The TSSs of PCGs were also enriched in CS4, as well as CS3 and CS5, which contain only H2A.Z or H3K4me3, respectively. In contrast, the TES of PCGs was only enriched in CS3. This suggests that H3K4me3 functions mostly at the TSS, whereas H2A.Z functions across the gene. This is generally consistent with previous reports on the functions of H3K4me3 and H2A.Z. Studies in a number of organisms have shown that H3K4me3 localizes near the TSS of active PCGs (Santos-Rosa et al., 2002; Liu et al., 2005; Bernstein et al., 2006; Barski et al., 2007; Zhang et al., 2009; van Dijk et al., 2010; Du et al., 2013; Zong et al., 2013). Our data further support this by showing a prominent peak of H3K4me3 at the TSS of rice PCG (Fig. 1A) that is positively correlated with basal gene expression (Fig. 2). H2A.Z is also abundant at the TSS of PCG, but appears to play roles in gene expression by localizing to gene bodies and the TES as well (Coleman-Derr and Zilberman, 2012; Sura et al., 2017; Zahraeifard et al., 2018). In contrast to H3K4me3, TSS-localized H2A.Z is negatively correlated with basal expression (Fig. 2; Zilberman et al., 2008; Coleman-Derr and Zilberman, 2012; Yelagandula et al., 2014; Dai et al., 2017; Zhang et al., 2017; Zahraeifard et al., 2018). Interestingly, our data show that abundance of H2A.Z and H3K4me3 downstream of the TSS region is negatively correlated with expression. Previous studies have reported this phenomenon for H2A.Z (Zilberman et al., 2008; Coleman-Derr and Zilberman, 2012; Sura et al., 2017), but Arabidopsis H3K4me3 was shown to be positively regulated with expression (van Dijk et al., 2010). This may reflect a difference in the role of H3K4me3 at the 3′ genic region in different plant species. On the other hand, a H3K4me3 profile of genes from an allotetraploid cotton genotype generally showed a negative correlation with expression, whereas a diploid cotton genotype in the same study exhibited a positive correlation (You et al., 2017). H3K4me3 at the TES was reported to play a role in modulating antisense transcription, thereby repressing sense transcription (Ponting et al., 2009; Cui et al., 2012). Therefore, genotypic or cell type-dependent differences in antisense transcription may contribute to the correlation of TES-localized H3K4me3 with sense transcription. Further investigation is required to understand the nature of the differences in 3′ H3K4me3-dependent regulation of gene expression across samples and species.
Pi-Starvation–Induced Chromatin Dynamics Correlate with Gene Repression and Induction
Often, the disruption of histone modifiers, such as H3K4 methyltransferases, through mutagenesis do not have substantial impacts on global steady-state transcription (Guo et al., 2010; Chen et al., 2017; Howe et al., 2017). On the other hand, a number of studies have identified significant roles for particular histone or chromatin modifiers in differential expression in response to environmental stimuli (Ding et al., 2011, 2012; Weiner et al., 2015). Our data support this by revealing that >40% of all rice PCG in shoots exhibit a CS transition at their TSS in response to a 24-h Pi-deficiency treatment, and that several specific transitions correlate with subgroups of genes differentially expressed by Pi starvation. It is noteworthy that although we found significant correlations between chromatin dynamics and subsets of DEGs, there were many genes exhibiting a CS transition that were not differentially expressed (Fig. 4). This lack of a strong global correlation between chromatin dynamics and changes in gene expression has been reported in Zong et al. (2013) and Fiziev et al. (2017), and likely reflects regulation of steady-state transcript levels by other, unobserved, molecular processes, such as the presence of appropriate transcription factors and other chromatin modifications. Also, the duration of our Pi-starvation treatment was likely not adequate to detect changes in transcript abundance of some genes despite the detection of CS changes (Secco et al., 2013; Supplemental Fig. S10). Nevertheless, the correlations between changes in chromatin signatures and gene expression observed herein highlight the importance of chromatin dynamics during differential gene expression in response to Pi starvation.
Genes with CS1-CS3 or CS2-CS3 transitions exhibit increases in nucleosome occupancy and H2A.Z deposition in response to Pi starvation, and are enriched in downregulated genes, whereas CS3-to-CS1 genes, which exhibit decreases in nucleosome occupancy and H2A.Z, are enriched in upregulated genes. These correlations are consistent with a repressive role for H2A.Z at the TSS in modulating Pi deficiency-response genes. This is consistent with our recent work in rice (Zahraeifard et al., 2018) and previous reports in Arabidopsis (Dai et al., 2017; Sura et al., 2017), which all provide evidence for H2A.Z acting as a repressor of expression when localized at gene bodies or the TSS. Work in Arabidopsis also showed general colocalization of H2A.Z and H3K4me3 in promoter regions, but a negative correlation of the two marks at the TSS of genes exhibiting relatively high H2A.Z (Dai et al., 2017). The same study also showed a positive correlation between nucleosome occupancy and H2A.Z at the +1 nucleosome, suggesting that H2A.Z deposition at the +1 nucleosome is linked to high nucleosome occupancy, low H3K4me3 abundance, and low gene accessibility (Dai et al., 2017). Our data bolster support for a model where H2A.Z at the TSS, likely the +1 nucleosome, regulates a subset of Pi deficiency-response genes that contain low levels of H3K4me3 and relatively low basal expression. In response to Pi starvation, H2A.Z is either removed or deposited, resulting in derepression (CS3-CS1) or repression (CS1-CS3/CS2-CS3), respectively. Similar to CS3-CS1, genes with a CS4-CS1 transition, which exhibit a loss of both H2A.Z and H3K4me3, are enriched in upregulated genes (Fig. 6). These genes tend to be more highly expressed during control conditions than CS3 genes, and therefore have a stronger requirement for H3K4me3 for basal expression. In response to Pi starvation, the combined loss of H2A.Z and H3K4me3 may reflect some dependence of H3K4me3 on H2A.Z at these genes, similar to how H2A.Z was suggested to facilitate H3K4me3 deposition at two miR156-encoding genes in Arabidopsis (Xu et al., 2018).
Among the gene groups that exhibit CS transitions, the CS4-CS3 group contains the largest number of genes, and is characterized by a loss of H3K4me3, but maintenance of H2A.Z, during Pi starvation. Interestingly, these genes are enriched among both up- and downregulated genes, indicating that loss of H3K4me3 is linked to gene activation and repression during Pi deficiency. In contrast to H2A.Z, H3K4me3 is generally not recognized as playing a negative role in gene expression. Studies in a variety of plant species and tissues have examined the change in genic levels of H3K4me3 in response to environmental stressors (Tsuji et al., 2006; Sokol et al., 2007; Kim et al., 2008; van Dijk et al., 2010; Jaskiewicz et al., 2011; Zeng et al., 2019). These studies generally reported increases in H3K4me3 at genes upregulated by stress. However, most of the studies examined relatively small numbers of genes, and the genome-level studies that compared average H3K4me3 genic profiles between control and stressed samples found substantial decreases in 5′ localization of H3K4me3 in response to stress (Zong et al., 2013; Zeng et al., 2019). We observed a similar effect when comparing the H3K4me3 profiles for all PCGs between control and Pi-deficiency conditions (Supplemental Fig. S3A). One explanation for our CS4-CS3 genes being linked to both induction and repression is that the TSSs of the corresponding genes contain bivalent histone modifications. Bivalent domains are characterized by containing both active and repressive histone modifications. First described in mouse embryonic stem cells were bivalent domains containing H3K4me3 and H3K27me3, in which H3K4me3 is proposed to poise genes for activation, whereas H3K27me3 maintains the genes in a repressed state (Bernstein et al., 2006). A recent study in potato (Solanum tuberosum) found an association between genes containing the bivalent H3K4me3 and H3K27me3 marks and differential expression in response to cold stress (Zeng et al., 2019). Interestingly, the bivalent mark was enriched among upregulated genes linked to stress responses, as well as downregulated genes linked to developmental processes. The authors proposed that the bivalent H3K4me3-H3K27me3 domain confers greater accessibility to regulatory proteins that can induce or repress genes in response to cold stress. A similar phenomenon might explain our observed correlations between the CS4-CS3 transition and both up- or downregulated genes in response to Pi starvation. A decrease in H3K4me3 at the TSS may reflect a switch from nucleosomes modified with only H3K4me3 to nucleosomes containing both H3K4me3 and H3K27me3. This would favor enhanced DNA accessibility, which could facilitate the targeting of transcriptional machinery for induction or repression. Recently, an interaction between H2A.Z deposition and H3K27 trimethylation was reported in Arabidopsis, in which H2A.Z deposition promotes H3K27 trimethylation (Carter et al., 2018). It is possible that the maintenance of H2A.Z at the CS4-CS3 genes is required for proper H3K27me3 deposition at the bivalent marks. Future experiments that examine H3K27me3 localization would shed light on this hypothesis.
Differential Expression of Cell Wall-Related Genes Correlates with Decreased H3K4me3 and Maintenance of H2A.Z
Cell walls provide rigidity to plant cells but are also restrictive to cell expansion. Thus, cells must simultaneously weaken cell wall structure and maintain turgor and cell integrity to achieve growth (Voxeur and Hofte, 2016). Correspondingly, plants must employ signaling mechanisms aimed at regulating cell wall structure in response to developmental and environmental cues. Several plasma membrane-localized RLKs, such as FER, have been implicated in cell wall integrity sensing in response to a variety of environmental stressors (Liao et al., 2017). The majority of our CS4-CS3 DEGs encode putative apoplastic or plasma membrane proteins with predicted roles in signaling and cell wall composition (Fig. 6A). The signaling genes were mostly upregulated, whereas the cell wall-related genes were largely downregulated (Fig. 6B). Comparing the transcriptomic profile of the CS4-CS3 DEGs to public transcriptome studies using the software Genevestigator (https://genevestigator.com; Hruz et al., 2008) revealed substantial overlap with several pairwise comparisons from a previous study on rice lamina joint development (Zhou et al., 2017). Comparisons between older stages of development (maturation or postmaturation) with a younger stage showed similar expression profiles as our CS4-CS3 Pi-deficiency DEGs (not shown). Interestingly, cell wall thickening is a prominent feature during younger stages of lamina joint development, and this declines over time. This suggests that Pi starvation results in decreased cell wall thickening, or more generally, a decrease in cell elongation. Transcriptomic profiles of several biotic and abiotic (e.g. salinity and heat) stressors also showed high similarity to our CS4-CS3 DEG profile, suggesting the apparent apoplastic signaling network overlaps with multiple stressors. Our CS4-CS3 DEG list contains many orthologs of Arabidopsis components involved in salinity stress responses, including FER, LRX, and RALF peptides (Zhao et al., 2018). It is of interest to evaluate whether the rice orthologs exhibit similar functions in response to stressors including salinity and Pi starvation.
A Distinct Pair of CS Transitions May Poise Translation-Related Genes for Repression
After the CS4-CS3 gene group, the transitions with the most genes were the CS5-CS1 and CS4-CS5 groups, which were enriched with similar functional categories of genes (Fig. 4) including those related to translation, particularly a number of ribosomal protein genes. Examination of the two bins adjacent to the TSS revealed that a number of these genes contained both transitions with the CS5-CS1 transition immediately upstream of the CS4-CS5 transition. Our bootstrapping results showed that these genes are not enriched among our DEGs. On the contrary, members of the CS4-CS5 subgroup are underrepresented among downregulated DEGs (Fig. 6). Interestingly, a group of genes shown in a previous study (Secco et al., 2013) to be downregulated after 21 d of Pi deficiency were enriched among our CS5-CS1/CS4-CS5 genes (Supplemental Fig. S10). This might indicate that 24 h of Pi deficiency is sufficient to observe chromatin dynamics at these genes without observing a corresponding detectable decline in transcript abundance. We propose that the sequential CS5-CS1 and CS4-CS5 transitions observed at the TSS reflect genes under control conditions that contain low H2A.Z and high H3K4me3 in the −1 nucleosome and high levels of both marks in the +1 nucleosome. Pi starvation, then, results in a moderate loss of nucleosome occupancy at both the +1 and −1 nucleosomes, and specific removal of H3K4me3 from the −1 nucleosome and H2A.Z from the +1 nucleosome. In yeast, Spp1 promotes the H3K4 trimethylase activity of the Set1 complex (Morillon et al., 2005). As a result, deletion of Spp1 results in substantial loss of global H3K4me3 levels, but the remaining H3K4me3 (∼20%) is not evenly distributed among genes. Genes that retain the highest levels of H3K4me3 in Δspp1 mutants are enriched in ribosomal protein genes and other translation-related genes, whereas genes exhibiting the most severe H3K4me3 depletion are enriched in stress-related genes (Howe et al., 2014). Also, the Spp1-independent genes tend to be more highly expressed during control conditions, and repressed during environmental stress, whereas the Spp1-dependent genes generally exhibit low expression during control conditions and induced expression during stress. Finally, in response to diamide stress, many yeast ribosomal protein genes are downregulated and exhibit a decrease in H3K4me3 (Weiner et al., 2015). Our data suggest that rice employs different mechanisms to modulate H3K4me3 levels at distinct gene groups, similar to yeast. This is consistent with our CS4-CS3 and CS5-CS1 gene groups undergoing decreases in H3K4me3 via different chromatin remodeling complexes. Future studies on the roles of H3K4me3 and H2A.Z, in conjunction with additional marks such as H3K27me3, in the Pi deficiency-dependent regulation of gene expression will provide valuable information on the chromatin dynamics that impact low-Pi adaptation mechanisms.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Sterilization and pregermination (1 d at 37°C followed by 2 d at 28°C) were carried out on rice cultivar ‘Nipponbare’ (Oryza sativa ssp. japonica) seeds. Seeds were transferred to 12-h light/12-h dark at 30°C/22°C conditions to germinate for 14 d. Seedlings were grown hydroponically in modified Yoshida Rice culture media as described by Yoshida et al. (1971) and Secco et al. (2013). The solution was replaced every 7 d. After 21 d, seedlings were used for a 24-h Pi-deficiency treatment (modified Yoshida Rice solution without NaH2PO4).
ChIP-Seq
Four grams of frozen shoot tissue from 24-h Pi-deficiency or control treatment was used to perform ChIP as described by Zahraeifard et al. (2018) using an antibody (lot no. 2648189; Millipore) against H3K4me3, a custom polyclonal antibody against rice H2A.Z (Zahraeifard et al., 2018), and input genomic DNA as a control. Three biological replicates were used for both input and antibody treatments. Purification of ChIP DNA was carried out with a DNA Clean & Concentrator Kit (Zymo Research). Libraries were constructed using a 1:20 diluted adaptor from a Hyper Library Construction Kit (Kapa Biosystems) and 10 cycles of DNA amplification. Libraries were quantitated (using quantitative PCR) and multiplexed, and single-end sequencing was completed with a HiSeq2500 (Illumina) using a HiSeq SBS Sequencing Kit (version 4) for 101 cycles at the University of Illinois’s Roy J. Carver Biotechnology Center. Approximately 147 million ChIP-seq reads were quality-checked and cleaned using the tools FastQC and Trimmomatic-0.33 (Andrews, 2010; Bolger et al., 2014). Using the software Bowtie (http://bowtie-bio.sourceforge.net/index.shtml), the reads were aligned to MSU7.1 (http://rice.plantbiology.msu.edu/) with one mismatch allowed to retain uniquely mapped reads. The age SICER (Zang et al., 2009) was used to define the H3K4me3 enrichment regions with the following parameters: W = 200, G = 200, FDR < 1.00e-02. The input genomic DNA was used as a background control. Differential H3K4me3 enrichment peaks between control and Pi-deficiency samples were determined using a SICER-df.sh shell script (W = 200, G = 200, FDR < 1.00e-02). We defined the existence of peaks with PCGs if 50% of peaks overlapped with PCGs (including 250 bp upstream and downstream) using the program BEDTools’ “intersect” function (Quinlan and Hall, 2010). The genome-wide distribution pattern of H3K4me3 and the published profile of H2A.Z (Zahraeifard et al., 2018) were visualized using the program ngs.plot (Shen et al., 2014). K-means clustering within ngs.plot was used to find different patterns of H3K4me3. GO terms enriched among clusters were analyzed with the tool BiNGO (https://www.psb.ugent.be/cbd/papers/BiNGO/Home.html) and visualized with the software Cytoscape (Maere et al., 2005).
RNA-Seq Analysis
RNA-seq reads were generated by Zahraeifard et al. (2018). A minimum of 58 million high-quality RNA-seq reads (100-bp single end) per sample were mapped to the MSU7.1 using the software BowTie2 (Langmead and Salzberg, 2012). FPKMs were calculated with the tool Cuffdiff (Trapnell et al., 2012). The program DESeq2 was applied to identify DEGs (Love et al., 2014). The cutoff (adjusted P value < 0.001) recommended for a small-sample RNA-seq experiment was used (Soneson and Delorenzi, 2013). GO terms enriched among DEGs were analyzed with the tool BiNGO and visualized with the software Cytoscape (Maere et al., 2005).
CSs Analysis
We used the program ChromHMM (Ernst and Kellis, 2012) with default parameters to characterize the CS maps for control and Pi-deficiency samples. We used the published profiles of H2A.Z ChIP-seq (Zahraeifard et al., 2018) and nucleosome occupancy (Zhang et al., 2018) of MNase-seq, as well as the H3K4me3 profile generated in this study. All input data were binarized with the tool BinarizedBam, included in the program ChromHMM (Ernst and Kellis, 2012), and input genomic DNA was used to adjust binarization thresholds locally. The common model of CSs in both control and Pi-deficiency samples was developed by concatenating the marks using a hidden Markov model. Five CSs were generated based on the learned model parameters as described in the program ChromHMM (Ernst and Kellis, 2012). CS changes were analyzed using a method described by Fiziev et al. (2017). Briefly, the control and Pi-deficiency genomes were divided into 200-bp bins, each occupied by one CS, and the CS annotations of control and Pi-deficiency genomes were overlapped. The number of bins in each possible CS were counted and called the “observed” number. The expected number was calculated by multiplying the number of bins in the two CSs involved in each transition (a change in transition from control to Pi-deficiency sample) and divided by total bins in the genome to calculate enrichment scores. Similarity between each pair of CSs was controlled by dividing the enrichment scores of each state transition to the enrichment scores of the reverse state transition. The distribution of CSs were identified using the software CEAS (Shin et al., 2009). Each PCG was assigned to one CS based on the state of the 200-bp bin encompassing the TSS. For bootstrapping analysis, we used a custom FORTRAN script (Zahraeifard et al., 2018) to obtain the same number of randomly selected genes and estimate the percentage of overlap between these genes and each group of state transitions (1,000 iterations). Binomial distribution tests were carried out with the software R (pbinom, P value < 1.00e-03). For the CS transition plot (Fig. 4), CSs in control samples were differentially color-coded. Genes in each control CS were sorted based on their positions within each chromosome. Chromatin transitions for each gene were connected with lines of colors matching those used for control CSs. Genes in each chromatin transition were positioned according to their expression changes upon Pi-deficiency treatment, with upregulated genes on the top and downregulated on the bottom. These transition connections were plotted with the program ggplot2 (Wickham, 2009). For the Circos plot (Fig. 3; Krzywinski et al., 2009), each rice chromosome was partitioned into bins of 5 kb. CSs were merged from 200-bp bins to 5-kb bins in both control and Pi-deficiency samples. The most dominant CS in each merged bin, or the CS of the previous bin if most dominant CS could not be determined, was selected as the CS for that bin. For gene type partitioning, the most dominant gene type, in base pairs, was used as the bin type for each bin. CSs, differential expression status, and bin types for the merged bins were determined using customized scripts and visualized with the R package “circlize” (Gu et al., 2014).
GO Clustering
To identify enriched gene functions associated with the selected CS transitions, we followed a two-step process. We searched for enriched GO terms in gene clusters assigned to each CS using the enrichment tool BiNGO (Maere et al., 2005). However, a single gene can be assigned to multiple GO terms and there is inherent redundancy in processes reported on functional lists generated by GO enrichment analysis tools that cannot be manually assessed, especially when large lists representing thousands of genes are used. Therefore, we used the MCL Algorithm (van Dongen and Abreu-Goodger, 2012) on these primary lists of enriched functions to identify nonredundant functional clusters. Briefly, each GO term was represented by a node in a network, and an edge connecting two nodes in the network represents how many genes are shared between each node. We computed a similarity value for each edge based on how many genes were shared between the nodes for each edge to cluster closely related groups. We used an Overlap Coefficient set to a 0.5 cutoff and a granularity value set to 2 for MCL. The source code written in the software language Python for clustering is available at https://github.com/Guannan-Wang/GOMCL. We identified the clusters with the largest number of genes, the hub nodes with the largest number of connections to other nodes, and the most significant clusters with high enrichment for further assessment of these representative functions associated with each CS.
Accession Numbers
H3K4me3 ChIP-seq and RNA-seq datasets from this article were submitted to the National Center for Biotechnology Information Sequence Read Archive Database (ncbi.nlm.nih.gov/sra) under the accession number SRP102668.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. H3K4me3 abundance is strongly correlated with gene length but varies in abundance at the TSS.
Supplemental Figure S2. Peaks of H3K4me3 and H2A.Z are correlated in the rice genome.
Supplemental Figure S3. 24-h Pi deficiency alters the H3K4me3 enrichment pattern across rice PCGs.
Supplemental Figure S4. Comparison of defined CS predictions.
Supplemental Figure S5. Fold changes of CS enrichments between the Pi-deficiency and control samples (–Pi/ctrl).
Supplemental Figure S6. The H3K4me3, H2AZ, and MNase densities of each CS.
Supplemental Figure S7. The average H3K4me3, H2AZ, and MNase density plots of the genes in the three largest CS transitions in control and Pi-deficiency (–Pi) samples.
Supplemental Figure S8. CS5-CS1 and CS4-CS5 transitions occur in sequence.
Supplemental Figure S9. Identification of DEGs in response to 24 h of Pi deficiency.
Supplemental Figure S10. Bootstrapping analysis showing the overlap between genes exhibiting CS transitions (CS5-CS1, CS4-CS5) and DEGs that are downregulated in shoots after a 21-d Pi-deficiency treatment.
Supplemental Figure S11. GO terms enriched in DEGs that have a CS transition of CS4 to CS3.
Supplemental Table S1. Summary of ChIP-seq and RNA-seq libraries (short reads).
Supplemental Table S2. Summary of GO analysis of genes differentially expressed under phosphate deficiency (–Pi).
Supplemental Dataset S1. Significantly enriched GO terms for four subgroups of PCGs displaying different H3K4me3 abundance levels at the TSS.
Supplemental Dataset S2. CSs of all PCGs at their TSS in control and phosphate-deficiency samples.
Supplemental Dataset S3. Significantly enriched GO terms for eight gene groups exhibiting specific chromatin transitions.
Supplemental Dataset S4. Up- and downregulated genes in response to Pi starvation.
Supplemental Dataset S5. DEGs showing a CS4-CS3 chromatin transition.
Acknowledgments
The authors thank High Performance Computing at Louisiana State University (HPC@LSU) for providing computer resources. We also thank Aliasghar Sepehri for sharing the custom FORTRAN script to perform bootstrapping analyses.
Footnotes
This work was supported by the United States Department of Agriculture, National Institute of Food and Agriculture (USDA-NIFA grant no. 2016-10070), the National Science Foundation, Division of Integrative Organismal Systems and Division of Molecular and Cellular Biosciences (NSF-IOS grant no. 1127051 and NSF-MCB grant no. 1616827), and the Next Generation BioGreen21 Program of the Rural Development Administration, Ministry of Education of the Republic of Korea (grant No. PJ01317301).
Articles can be viewed without a subscription.
References
- Andrews S. (2010) FastQC: A quality control tool for high throughput sequence data. www.bioinformatics.babraham.ac.uk/projects/fastqc/
- Barski A, Cuddapah S, Cui K, Roh T-Y, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K (2007) High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837 [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL (2002) Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci USA 99: 8695–8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, et al. (2006) A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326 [DOI] [PubMed] [Google Scholar]
- Berriri S, Gangappa SN, Kumar SV (2016) SWR1 chromatin-remodeling complex subunits and H2A.Z have non-overlapping functions in immunity and gene regulation in Arabidopsis. Mol Plant 9: 1051–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Xie W, Lian X (2013) Comparative analysis of differentially expressed genes in rice under nitrogen and phosphorus starvation stress conditions. Plant Mol Biol Report 31: 160–173 [Google Scholar]
- Cai H, Zhang M, Chai M, He Q, Huang X, Zhao L, Qin Y (2019) Epigenetic regulation of anthocyanin biosynthesis by an antagonistic interaction between H2A.Z and H3K4me3. New Phytol 221: 295–308 [DOI] [PubMed] [Google Scholar]
- Campbell L, Turner SR (2017) A comprehensive analysis of RALF proteins in green plants suggests there are two distinct functional groups. Front Plant Sci 8: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Rodriguez D, Gjaltema RA, Jilderda LJ, Jellema P, Dokter-Fokkens J, Ruiters MHJ, Rots MG (2016) Writing of H3K4Me3 overcomes epigenetic silencing in a sustained but context-dependent manner. Nat Commun 7: 12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B, Bishop B, Ho KK, Huang R, Jia W, Zhang H, Pascuzzi PE, Deal RB, Ogas J (2018) The chromatin remodelers PKL and PIE1 act in an epigenetic pathway that determines H3K27me3 homeostasis in Arabidopsis. Plant Cell 30: 1337–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrika NNP, Sundaravelpandian K, Yu SM, Schmidt W (2013) ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis. New Phytol 198: 709–720 [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Wu K, Schmidt W (2015) The histone deacetylase HDA19 controls root cell elongation and modulates a subset of phosphate starvation responses in Arabidopsis. Sci Rep 5: 15708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-Q, Luo J-H, Cui Z-H, Xue M, Wang L, Zhang X-Y, Pawlowski WP, He Y (2017) ATX3, ATX4, and ATX5 encode putative H3K4 methyltransferases and are critical for plant development. Plant Physiol 174: 1795–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouaire T, Webb S, Skene P, Illingworth R, Kerr A, Andrews R, Lee J-H, Skalnik D, Bird A (2012) Cfp1 integrates both CpG content and gene activity for accurate H3K4me3 deposition in embryonic stem cells. Genes Dev 26: 1714–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman-Derr D, Zilberman D (2012) Deposition of histone variant H2A.Z within gene bodies regulates responsive genes. PLoS Genet 8: e1002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui P, Liu W, Zhao Y, Lin Q, Ding F, Xin C, Geng J, Song S, Sun F, Hu S, et al. (2012) The association between H3K4me3 and antisense transcription. Genomics Proteomics Bioinformatics 10: 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Bai Y, Zhao L, Dou X, Liu Y, Wang L, Li Y, Li W, Hui Y, Huang X, et al. (2017) H2A.Z represses gene expression by modulating promoter nucleosome structure and enhancer histone modifications in Arabidopsis. Mol Plant 10: 1274–1292 [DOI] [PubMed] [Google Scholar]
- Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Avramova Z, Fromm M (2011) The Arabidopsis trithorax-like factor ATX1 functions in dehydration stress responses via ABA-dependent and ABA-independent pathways. Plant J 66: 735–744 [DOI] [PubMed] [Google Scholar]
- Ding Y, Fromm M, Avramova Z (2012) Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat Commun 3: 740. [DOI] [PubMed] [Google Scholar]
- Du Z, Li H, Wei Q, Zhao X, Wang C, Zhu Q, Yi X, Xu W, Liu XS, Jin W, et al. (2013) Genome-wide analysis of histone modifications: H3K4me2, H3K4me3, H3K9ac, and H3K27ac in Oryza sativa L. Japonica. Mol Plant 6: 1463–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Kellis M (2012) ChromHMM: Automating chromatin-state discovery and characterization. Nat Methods 9: 215–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Kita D, Peaucelle A, Cartwright HN, Doan V, Duan Q, Liu M-C, Maman J, Steinhorst L, Schmitz-Thom I (2018) The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr Biol 28: 666–675 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiziev P, Akdemir KC, Miller JP, Keung EZ, Samant NS, Sharma S, Natale CA, Terranova CJ, Maitituoheti M, Amin SB, et al. (2017) Systematic epigenomic analysis reveals chromatin states associated with melanoma progression. Cell Reports 19: 875–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Gu L, Eils R, Schlesner M, Brors B (2014) Circlize implements and enhances circular visualization in R. Bioinformatics 30: 2811–2812 [DOI] [PubMed] [Google Scholar]
- Guo L, Yu Y, Law JA, Zhang X (2010) SET DOMAIN GROUP2 is the major histone H3 lysine 4 trimethyltransferase in Arabidopsis. Proc Natl Acad Sci USA 107: 18557–18562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford I. (1997) Soil phosphorus: Its measurement, and its uptake by plants. Soil Res 35: 227–240 [Google Scholar]
- Howe FS, Boubriak I, Sale MJ, Nair A, Clynes D, Grijzenhout A, Murray SC, Woloszczuk R, Mellor J (2014) Lysine acetylation controls local protein conformation by influencing proline isomerization. Mol Cell 55: 733–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe FS, Fischl H, Murray SC, Mellor J (2017) Is H3K4me3 instructive for transcription activation? BioEssays 39: 1–12 [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator V3: A Reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskiewicz M, Conrath U, Peterhänsel C (2011) Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 12: 50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, et al. (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice (N Y) 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-M, To TK, Ishida J, Morosawa T, Kawashima M, Matsui A, Toyoda T, Kimura H, Shinozaki K, Seki M (2008) Alterations of lysine modifications on the histone H3 N-tail under drought stress conditions in Arabidopsis thaliana. Plant Cell Physiol 49: 1580–1588 [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA (2009) Circos: An information aesthetic for comparative genomics. Genome Res 19: 1639–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA (2010) H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140: 136–147 [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with BowTie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Tang R, Zhang X, Luan S, Yu F (2017) FERONIA receptor kinase at the crossroads of hormone signaling and stress responses. Plant Cell Physiol 58: 1143–1150 [DOI] [PubMed] [Google Scholar]
- Lin W-D, Liao Y-Y, Yang TJ, Pan C-Y, Buckhout TJ, Schmidt W (2011) Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol 155: 1383–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Kaplan T, Kim M, Buratowski S, Schreiber SL, Friedman N, Rando OJ (2005) Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol 3: e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature 389: 251–260 [DOI] [PubMed] [Google Scholar]
- Maere S, Heymans K, Kuiper M (2005) BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449 [DOI] [PubMed] [Google Scholar]
- March-Díaz R, Reyes JC (2009) The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Mol Plant 2: 565–577 [DOI] [PubMed] [Google Scholar]
- Margaritis T, Oreal V, Brabers N, Maestroni L, Vitaliano-Prunier A, Benschop JJ, van Hooff S, van Leenen D, Dargemont C, Géli V, et al. (2012) Two distinct repressive mechanisms for histone 3 lysine 4 methylation through promoting 3′-end antisense transcription. PLoS Genet 8: e1002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño-Ramírez L, Kann MG, Shoemaker BA, Landsman D (2005) Histone structure and nucleosome stability. Expert Rev Proteomics 2: 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillon A, Karabetsou N, Nair A, Mellor J (2005) Dynamic lysine methylation on histone H3 defines the regulatory phase of gene transcription. Mol Cell 18: 723–734 [DOI] [PubMed] [Google Scholar]
- Pan X, Fang Y, Yang X, Zheng D, Chen L, Wang L, Xiao J, Wang X-E, Wang K, Cheng Z, et al. (2017) Chromatin states responsible for the regulation of differentially expressed genes under 60Co-γ ray radiation in rice. BMC Genomics 18: 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting CP, Oliver PL, Reik W (2009) Evolution and functions of long noncoding RNAs. Cell 136: 629–641 [DOI] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dündar F, Manke T (2016) deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44(W1): W160–W165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T (2002) Active genes are tri-methylated at K4 of histone H3. Nature 419: 407–411 [DOI] [PubMed] [Google Scholar]
- Secco D, Jabnoune M, Walker H, Shou H, Wu P, Poirier Y, Whelan J (2013) Spatio-temporal transcript profiling of rice roots and shoots in response to phosphate starvation and recovery. Plant Cell 25: 4285–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secco D, Wang C, Shou H, Schultz MD, Chiarenza S, Nussaume L, Ecker JR, Whelan J, Lister R (2015) Stress induced gene expression drives transient DNA methylation changes at adjacent repetitive elements. eLife 4: e09343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Shao N, Liu X, Nestler E (2014) ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics 15: 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Liu T, Manrai AK, Liu XS (2009) CEAS: Cis-regulatory element annotation system. Bioinformatics 25: 2605–2606 [DOI] [PubMed] [Google Scholar]
- Smith AP, Jain A, Deal RB, Nagarajan VK, Poling MD, Raghothama KG, Meagher RB (2010) Histone H2A.Z regulates the expression of several classes of phosphate starvation response genes but not as a transcriptional activator. Plant Physiol 152: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol A, Kwiatkowska A, Jerzmanowski A, Prymakowska-Bosak M (2007) Up-regulation of stress-inducible genes in tobacco and Arabidopsis cells in response to abiotic stresses and ABA treatment correlates with dynamic changes in histone H3 and H4 modifications. Planta 227: 245–254 [DOI] [PubMed] [Google Scholar]
- Soneson C, Delorenzi M (2013) A comparison of methods for differential expression analysis of RNA-seq data. BMC Bioinformatics 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann M, Monaghan J, Smakowska-Luzan E, Rovenich H, Lehner A, Holton N, Belkhadir Y, Zipfel C (2017) The receptor kinase FER is a RALF-regulated scaffold controlling plant immune signaling. Science 355: 287–289 [DOI] [PubMed] [Google Scholar]
- Sun L, Song L, Zhang Y, Zheng Z, Liu D (2016) Arabidopsis PHL2 and PHR1 act redundantly as the key components of the central regulatory system controlling transcriptional responses to phosphate starvation. Plant Physiol 170: 499–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sura W, Kabza M, Karlowski WM, Bieluszewski T, Kus-Slowinska M, Pawełoszek Ł, Sadowski J, Ziolkowski PA (2017) Dual role of the histone variant H2A.Z in transcriptional regulation of stress-response genes. Plant Cell 29: 791–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, Chiarenza S, Creff A, Bustos R, Paz-Ares J, Poirier Y, Nussaume L (2010) Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. Plant J 64: 775–789 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Saika H, Tsutsumi N, Hirai A, Nakazono M (2006) Dynamic and reversible changes in histone H3-Lys4 methylation and H3 acetylation occurring at submergence-inducible genes in rice. Plant Cell Physiol 47: 995–1003 [DOI] [PubMed] [Google Scholar]
- Vance CP, Uhde‐Stone C, Allan DL (2003) Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- van Dijk K, Ding Y, Malkaram S, Riethoven J-JM, Liu R, Yang J, Laczko P, Chen H, Xia Y, Ladunga I, et al. (2010) Dynamic changes in genome-wide histone H3 lysine 4 methylation patterns in response to dehydration stress in Arabidopsis thaliana. BMC Plant Biol 10: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen S, Abreu-Goodger C (2012) Using MCL to extract clusters from networks. Methods Mol Biol 204: 281–295 [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Workman JL (2015) Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol 16: 178–189 [DOI] [PubMed] [Google Scholar]
- Voxeur A, Hofte H (2016) Cell wall integrity signaling in plants: “To grow or not to grow that’s the question”. Glycobiology 26: 950–960 [DOI] [PubMed] [Google Scholar]
- Weiner A, Hsieh T-HS, Appleboim A, Chen HV, Rahat A, Amit I, Rando OJ, Friedman N (2015) High-resolution chromatin dynamics during a yeast stress response. Mol Cell 58: 371–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009) ggplot2: Elegant Graphics for Data Analysis. Springer, New York [Google Scholar]
- Xu M, Leichty AR, Hu T, Poethig RS (2018) H2A.Z promotes the transcription of MIR156A and MIR156C in Arabidopsis by facilitating the deposition of H3K4me3. Development 145: dev152868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Liu Y, Zhang K, Song J, Xu W, Su Z (2019) Chromatin state-based analysis of epigenetic H3K4me3 marks of Arabidopsis in response to dark stress. Front Genet 10: 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelagandula R, Stroud H, Holec S, Zhou K, Feng S, Zhong X, Muthurajan UM, Nie X, Kawashima T, Groth M, et al. (2014) The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell 158: 98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cock JH (1971) Laboratory Manual for Physiological Studies of Rice International Rice Research Institute, University of the Philippines Los Baños, Laguna, Phillipines [Google Scholar]
- You Q, Yi X, Zhang K, Wang C, Ma X, Zhang X, Xu W, Li F, Su Z (2017) Genome-wide comparative analysis of H3K4me3 profiles between diploid and allotetraploid cotton to refine genome annotation. Sci Rep 7: 9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahraeifard S, Foroozani M, Sepehri A, Oh D-H, Wang G, Mangu V, Chen B, Baisakh N, Dassanayake M, Smith AP (2018) Rice H2A.Z negatively regulates genes responsive to nutrient starvation but promotes expression of key housekeeping genes. J Exper Botany 69: 4907–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang C, Schones DE, Zeng C, Cui K, Zhao K, Peng W (2009) A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25: 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Zhang W, Marand AP, Zhu B, Buell CR, Jiang J (2019) Cold stress induces enhanced chromatin accessibility and bivalent histone modifications H3K4me3 and H3K27me3 of active genes in potato. Genome Biol 20: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Xu W, Wang C, Yi X, Zhang W, Su Z (2017) Differential deposition of H2A.Z in combination with histone modifications within related genes in Oryza sativa callus and seedling. Plant J 89: 264–277 [DOI] [PubMed] [Google Scholar]
- Zhang L, Cheng Z, Qin R, Qiu Y, Wang J-L, Cui X, Gu L, Zhang X, Guo X, Wang D, et al. (2012) Identification and characterization of an epi-allele of FIE1 reveals a regulatory linkage between two epigenetic marks in rice. Plant Cell 24: 4407–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Oh D-H, DiTusa SF, RamanaRao MV, Baisakh N, Dassanayake M, Smith AP (2018) Rice nucleosome patterns undergo remodeling coincident with stress-induced gene expression. BMC Genomics 19: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bernatavichute YV, Cokus S, Pellegrini M, Jacobsen SE (2009) Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 10: R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Zayed O, Yu Z, Jiang W, Zhu P, Hsu C-C, Zhang L, Tao WA, Lozano-Durán R, Zhu J-K (2018) Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc Natl Acad Sci USA 115: 13123–13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, Wang L, Chen L, Pan X, Lin K, Fang Y, Wang X-E, Zhang W (2019) Salt-responsive genes are differentially regulated at the chromatin levels between seedlings and roots in rice. Plant Cell Physiol 60: 1790–1803 [DOI] [PubMed] [Google Scholar]
- Zhou L-J, Xiao L-T, Xue H-W (2017) Dynamic cytology and transcriptional regulation of rice lamina joint development. Plant Physiol 174: 1728–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456: 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong W, Zhong X, You J, Xiong L (2013) Genome-wide profiling of histone H3K4-tri-methylation and gene expression in rice under drought stress. Plant Mol Biol 81: 175–188 [DOI] [PubMed] [Google Scholar]