Abstract
The Epilepsy Therapy Screening Program (ETSP), formerly known as the Anticonvulsant Screening Program (ASP), has played an important role in the preclinical evaluation of many of the antiseizure drugs (ASDs) that have been approved by the FDA and thus made available for the treatment of seizures. Recent changes to the animal models used at the contract site of the ETSP at the University of Utah have been implemented in an attempt to better model the unmet clinical needs of people with pharmacoresistant epilepsy and thus identify improved therapies. In this review, we describe the changes that have occurred over the last several years in the screening approach used at the contract site and, in particular, detail the pharmacology associated with several of the animal models and assays that are either new to the program or hve been recently characterized in more depth. There is optimism that the refined approach used by the ETSP contract site, wherein etiologically relevant models that include those with spontaneous seizures are used, will identify novel, potentially disease modifying therapies for people with pharmacoresistant epilepsy and those at risk for developing epilepsy.
Introduction
The Epilepsy Therapy Screening Program (ETSP), formerly known as the Anticonvulsant Screening Program (ASP), has played an important role for over 44 years in the preclinical evaluation of many of the antiseizure drugs (ASDs) that have been approved by the FDA and thus made available for the treatment of seizures. The National Institute of Neurological Diseases and Stroke (NINDS) of the National Institute of Health (NIH) funds and administers the ETSP and, since 1975, has awarded consecutive 5-year contracts to the University of Utah to perform the blinded screening of compounds in a variety of acute and chronic seizure models as well as, more recently, in animals with epilepsy. The ETSP has helped investigators from academia, industry, and government assemble compelling efficacy packages that serve to facilitate advancement of new compounds toward the clinic for the symptomatic control of seizures (Kehne et al., 2017). While the newer ASDs are better tolerated and can be effective in preventing seizures, it is still widely recognized that nearly a third of all people with epilepsy do not have their seizures adequately controlled with existing ASDs (Chen et al., 2018). Thus, there is a significant unmet clinical need to treat pharmacoresistant epilepsy. In addition, none of the currently available ASDs can modify the disease; they only reduce seizure incidence and seizures will recur if the person stops taking the medication. Finally, there are currently no therapies to prevent those people who are at risk from developing epilepsy. Therefore, in response to reports generated following review of the ETSP by the 2012 and 2015 National Advisory Neurological Disorders and Stroke (NANDS) Council Working groups (Kehne et al., 2017), wherein recommendations regarding testing of novel compounds were made, changes were made to the animal models used at the contract site at the University of Utah in an attempt to better model the unmet clinical needs of people with pharmacoresistant epilepsy.
The new models and screening approaches implemented by the ETSP contract site have dramatically transformed the program, which traditionally evaluated compounds for the ability to prevent evoked seizures in either naïve or kindled animals in a search for anti-seizure drugs (ASDs). A team of experts comprising the External Consultant Board (ECB) has also informed this progress. The ECB regularly reviews work performed by the ETSP and the contract site, and makes recommendations that aid in the development and evaluation of new models. Since the release of the first working group report in 2012, the contract site has introduced more etiologically relevant animal models of chronic seizures and epilepsy. Historically, the contract site has used models consisting of acute evoked seizures induced in naïve animals or kindled animals. While these models are useful in the identification of the anti-seizure potential of novel compounds, they are lacking in key pathophysiological changes that occur in human epilepsy populations. Conversely, spontaneous seizure models recapitulate many of the key clinical findings of epilepsy such as hippocampal sclerosis, network-level changes, neuroinflammation, and behavioral/cognitive dysfunction. Therefore, these models are more etiologically relevant for the evaluation of novel compounds. The ETSP, as well as some of these incorporated changes, has been described in great detail in three recent publications (Barker-Haliski et al., 2017, Kehne et al., 2017, Klein et al., 2017). Therefore, we will focus this review on recent developments within the program that have sought to determine the in-depth pharmacological profiles of some of the newer assays introduced to identify improved treatments for pharmacoresistant epilepsy. In particular, we will review the development and implementation of the 6 Hz rat model of focal seizures, an in vitro brain slice model, the lamotrigine-resistant kindled (LTG-K) rat model of temporal lobe epilepsy (TLE), and a summary of an infection-induced model of TLE. We will also briefly discuss and review changes that have been implemented in the program over the last several years with the assumption that the models of pharmacoresistant acute and chronic evoked seizures (e.g. kindling), as well as those models with chronic epilepsy now included in our testing scheme, are clinically relevant to human pharmacoresistant epilepsy. This should provide an improved screening platform for the identification of novel compounds for the prevention, treatment, and modification of epilepsy, wherein pharmacoresistant seizures pose the greatest challenge for treatment. We propose that this approach will yield novel compounds with improved tolerability and efficacy.
Drug resistance in clinical epilepsy is not fully understood, but several mechanisms have been proposed. These include the “transporter hypothesis”, “target hypothesis”, and poor drug specificity (Kwan et al., 2011). Animal models of pharmacoresistant epilepsy are often described as such because few, if any, prototype ASDs effectively reduce or block seizures at well-tolerated doses (Wilcox et al., 2013). In some cases, molecular or network changes have been described that are consistent with diminished drug sensitivity. For example, in animals resistant to phenobarbital in a model of status epilepticus, receptor characteristics (binding, subunit expression) and cellular damage were observed (Volk et al., 2006). Similarly, in kindling and status epilepticus (SE) models, sodium channel inhibition is diminished, and this finding is consistent with clinical observations in TLE (Bethmann et al., 2008). However, such changes have not been described for all models of pharmacoresistance (Potschka, 2012). Despite the lack of data for some models, the preponderance of evidence for animal models suggests that lack of drug sensitivity is concomitant with cellular/molecular/network changes, and are similar to those found in clinical populations.
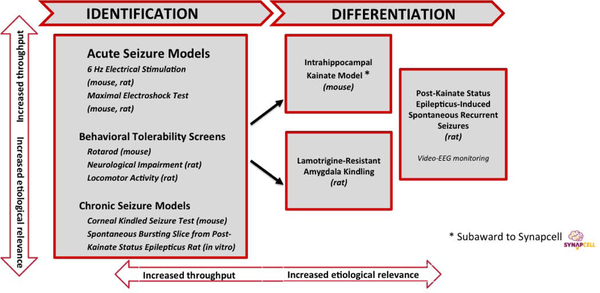
When a compound targeting pharmacoresistant epilepsy enters into the ETSP, the assays initially used are designed to assess both acute and chronic seizure models in a workflow that will ensure the proper evaluation of investigational compounds for the ability to provide improved therapy for pharmacoresistant seizures. This workflow includes animal models that are currently thought to best model temporal lobe epilepsy. Animal models of TLE are important resources for identifying potential therapies, provide insight into novel molecular targets for therapies, and establish useful outcome measures and biomarkers for therapy development (Clossen and Reddy, 2017). The flow chart that has been optimized for this approach is shown in Figure 1 (modified and reprinted with permission from (Barker-Haliski et al., 2017)). The staff at the NINDS office of the ETSP, in consultation with the suppliers of compounds, recommends testing in the assays of the identification and differentiation phase and, to ensure rigor, the contract site is routinely blinded with respect to the compound that is to be tested and the supplier of the compound. Within the identification phase of testing, assays that allow for higher throughput are at the top of the figure and include acute seizure models, both maximal electroshock seizures (MES) and 6 Hz stimulation-induced seizures in both mice and rats. The rat 6 Hz model was recently developed and incorporated into the testing scheme in 2017 by the contract site of the ETSP and is reviewed in detail below. Because the 6 Hz models are pharmacoresistant to numerous ASDs, compounds found to be effective in the 6 Hz models without significant tolerability issues may be moved into the differentiation phase of the testing scheme. While the MES test is sensitive to numerous ASDs and it is not considered a pharmacoresistant test, efficacy in only this model can indicate activity of a compound that, given information from the supplier regarding targets, might then be considered sufficiently novel to move into the differentiation phase. Indeed, the MES model has been very successful in identifying ASDs since Merritt and Putnam first used this approach in cats and identified phenytoin for the treatment of epilepsy (Putnam and Merritt, 1937, Merritt and Putnam, 1938). During the identification phase, compounds are also assessed for the potential to induce impaired activity using a number of assays including a rotarod assay in mice. In rats, motor impairment is assessed in both an open field and a modified Irwin test, which has been recently implemented into the program (Mathiasen and Moser, 2018). Finally, the most etiologically relevant assays during the identification phase include testing in corneal kindled mice and, for those compounds for which brain penetration might prove difficult, in a brain slice preparation that is obtained from kainic acid (KA) treated rats and exhibits recurrent epileptiform discharges (REDs) in the entorhinal cortex. These REDs are pharmacoresistant to ASDs that act on the GABAergic system as well as some ASDs with mixed mechanisms of action and this model is also reviewed below and summarized in Table 1. When compounds are found to be effective in the initial assays of the identification phase, more in-depth studies may be performed to determine time to peak effect (TPE) of the compounds and ED50s.
Figure 1.
Pharmacoresistant epilepsy workflow for the Epilepsy Therapy Screening Program
Table 1.
Performance of antiseizure drugs (ASDs) in selected models of pharmacoresistant epilepsy.
| Drug / Mechanism | 6 Hz Mouse (2xCC97)a | 6 Hz Rat (2xCV97)a | Lamotrigine-Resistant Kindled Ratb | Intra-Hippocampal Kainate (mouse)c | Bursting Slicef |
|---|---|---|---|---|---|
| Na+ Channel Blockers | |||||
| Carbamazepine | + | - | +/− | ++ d | + |
| Eslicarbazepine | + | - | - | + | |
| Lacosamide | ++ | - | - | + | |
| Lamotrigine | - | - | - | - - | + |
| Phenytoin | + | - | - | + | |
| Rufinamide | + | - | + | + | |
| GABAA Modulation | |||||
| Clobazam | ++ | - | + | - | +/− |
| Clonazepam | ++ | - | +/− | ||
| Phenobarbital | + | + | + | ++ | +/− |
| GABA Uptake Inhibition | |||||
| Tiagabine | + | - | + | ++ | + |
| Modulation of T-type Ca2+ Channels | |||||
| Ethosuximide | + | - | - | - | |
| Modulation of K+ Channels | |||||
| Ezogabine | - | ++ | ++ | + | |
| Modulation of α2δ Ca2+ Channel Subunits | |||||
| Gabapentin | - | - | +/− | - | |
| SV2A Modulation | |||||
| Levetiracetam | - | - | - | +/− e | - |
| Mixed Mechanisms | |||||
| Topiramate | +/− | - | - | - | |
| Valproic Acid | + | + | ++ | +/− | - |
6 Hz mouse, 2xCC97 (e.g. mouse 44 mA; rat 80 V / 106 mA)a
• 6 Hz and Lamotrigine-resistant kindling model: (++) effective with protective indices (PI: TD50 / ED50) of 2 or more; (+/−) effective with PI of 1–2; (−) ineffective or PI less than 1
• IHK model efficacy was compared to mouse 6 Hz ED50 values (Metcalf et al. 2017). (++) greater than 50% reduction in HPD duration or number, (+) significant reduction in HPD duration or number, (+/−) significant reduction in HPD duration or number at higher doses only, (−) no reduction in HPD duration or number, (- -) significant increases in HPD duration and/or number.
• Bursting Slice model: (+) significant reduction in burst duration or frequency at therapeutic human plasma concentrations, (+/−) significant reduction in burst duration or frequency at concentrations >3x therapeutic human plasma concentrations, (−) small or no effects on burst duration or frequency.
References and Notes
Levetiracetam is not effective in the mouse 6 Hz assay (for comparative purposes). In the intra-hippocampal kainate model, levetiracetam was only effective (reduced HPD duration and/or number) at the highest doses, 600–800 mg/kg.
As was the case for the identification stage, the staff at the NINDS office of the ETSP, in consultation with the suppliers of compounds, recommends testing in the assays of the differentiation phase. The models in this part of the workflow include the intrahippocampal kainate (IHK) mouse model of TLE and the LTG-K rat. The IHK assay is performed as a subaward to Synapcell in Grenoble France. This model exhibits differential sensitivity to different classes of ASDs, with suppression of hippocampal paroxysmal discharges (HPDs) occurring most readily to compounds that work through the GABAergic system (see Table 1) (Duveau et al., 2016) and is a model of TLE that has been used extensively in epilepsy research (Bouilleret et al., 1999, Langlois et al., 2010, Armstrong et al., 2013, Krook-Magnuson et al., 2013, Twele et al., 2017). In the LTG-K rat model, seizures are pharmacoresistant to not only lamotrigine, but also several additional sodium channel-blocking compounds. Once efficacy of a compound is noted in one of these models, or with significant rationale based on previous data, (e.g., knowledge of the molecular target by NINDS ETSP staff, etc.), a compound could be advanced to the kainic acid-induced, status epilepticus (KA-SE) rat model of spontaneous recurrent seizures. This model of TLE has also been used extensively to understand the basic mechanisms of epilepsy as well as to evaluate potential therapies for pharmacoresistant seizures (Buckmaster and Dudek, 1997, Ben-Ari and Cossart, 2000, Grabenstatter and Dudek, 2005, Grabenstatter et al., 2005, Takahashi et al., 2010, Thomson et al., 2017). In this assay, compounds can be administered in a subchronic dosing regimen to determine their ability to block seizures that are recorded with 24hr/7d/week video EEG; the gold standard of epilepsy research. Recent unpublished data on the pharmacology of this model by the contract site of the ETSP suggests that spontaneous seizures in this model of epilepsy may also be pharmacoresistant to several commonly used ASDs. The ability of a compound to perform well in all of these models is unusual and could very well differentiate a novel compound from many of the commonly used ASDs. The reliance on KA-SE (both rat and mouse) models in the differentiation phase arises from the recognized face validity of these models and the long history associated with its use in epilepsy research (Ben-Ari and Cossart, 2000, Levesque and Avoli, 2013, Levesque et al., 2016). It is our contention that compounds that successfully emerge from this new approach are more likely to be efficacious in a diverse array of people with pharmacoresistant seizures, especially those with TLE.
Assessment of Tolerability in Rodents
Rodent assessments of tolerability have been reviewed and described elsewhere (Klitgaard et al., 2002, Nampoothiri et al., 2017). While tolerability assessments can be broad-ranging, the ETSP generally limits evaluation to motor impairment. In mice, the ETSP has historically used a fixed speed (6 rpm) rotarod as a primary measure. Similarly, the open field locomotor assay has also been used when needed. In addition, animals are observed by experienced technical staff, and untoward behaviors may also be noted. Quantification of motor impairment (i.e., the number of animals failing the rotarod test within a single treatment group) across several doses for a test compound allows for determination of a median toxic dose (TD50), and when compared to the ED50, allows for calculation of the protective index (therapeutic index, TD50/ED50; see Table 1). In rats both the rotarod and open field locomotor assay can be used. Within the ETSP, a modified behavioral assessment of motor impairment (minimal motor impairment, MMI) has been used (Metcalf et al., 2017). As described above, quantification of the number of animals with notable behavioral impairment across a dose range allows for calculation of a TD50 and a protective index (see Table 1). While these assays are commonly used and well-described, they are limited to assessment of motor impairment. Recently, the ETSP has expanded behavioral observation through implementation of a modified functional observation battery (also referred to as the Irwin assay)(Mathiasen and Moser, 2018). This assay is commonly performed in pre-clinical drug development settings using rats and includes a variety of behavioral assessments (Gauvin et al., 2016). Therefore, these methods provide a rough assessment of tolerability, with emphasis on the potential for candidate drugs to affect motor function. However, tolerability assessment focused primarily on motor function may limit extrapolation of rodent data to humans. Even when the more expanded Irwin test is used, correlation between rodent and human data is low (Mead et al., 2016). Continuing efforts to refine pre-clinical measures of tolerability are therefore needed.
6 Hz Rat Model of Focal Seizures
Focal seizures induced in the mouse with 6 Hz corneal stimulation, at an intensity of two times the convulsive current in 50% of the animals (CC50), are very difficult to prevent with currently available antiseizure drugs (ASDs) and are generally regarded as a pharmacoresistant (Barton et al., 2001, Metcalf et al., 2017). For example in the mouse 6 Hz assay, levetiracetam (as well as sodium channel blockers) block seizures at the 32 mA stimulus intensity whereas this efficacy is lost at the 44 mA stimulus intensity (Barton et al., 2001, Metcalf et al., 2017). This observation, however, is not true for all ASDs. Valproic acid, retigabine, brivaracetam, and perampanel all retain efficacy at the 44 mA stimulus intensity (Barton et al., 2001, Metcalf et al., 2017). Therefore, the mouse 6 Hz seizure model is useful in the differentiation (i.e. based on mechanism of action) of novel ASDs with potential activity against pharmacoresistant seizures(Hanada et al., 2011, Loscher, 2017, Klein et al., 2018). In the testing flow adopted by the ETSP, this mouse model serves as a rapid and inexpensive test and provides information regarding the potential of submitted compounds to be useful for pharmacoresistant seizures. In addition, with a sufficient protective index, the ED50 that is calculated at the time to peak effect (TPE) for a compound is then selected as an initial test dose for the IHK model of TLE.
While the mouse 6 Hz model has been in the testing scheme of the ETSP for over 15 years, a rat 6 Hz model was only recently developed and characterized by the contract site of the ETSP (Metcalf et al., 2017). This was an important model to develop within the ETSP, to help guide dosing for rat assays used in the differentiation phase of the flow chart (Figure 1) for compounds that are not active in the rat MES test. Indeed, the ETSP is very interested in novel compounds that might not necessarily be active in the MES test but are active in the more pharmacoresistant 6 Hz models. Seizures induced by 6 Hz corneal stimulation in the rat are very similar in nature to those observed in mice, with head nodding, jaw clonus, forelimb clonus, twitching of the vibrissae, and Straub tail observed. Like all new animal models, before its introduction into the testing scheme of the ETSP, the rat 6 Hz model was extensively tested with available and clinically validated ASDs. Metcalf et al. 2017 recently published this work (Metcalf et al., 2017) and the primary findings are summarized here and in Table 1.
The effects of numerous ASDs on 6 Hz seizures induced at two different stimulation intensities were evaluated in the rat in this study. After establishing the convulsive voltage required to induce a seizure in 97% of the animals (CV97), the tests were performed at 1.5 and 2 times the CV97. It has been demonstrated in mouse that seizures induced at a stimulus intensity 1.5 times greater than the CC97 (32 mA) are somewhat resistant to many ASDs and that moving to a stimulation intensity of 2 times the CC97 (44 mA) results in a seizure that is very resistant to most ASDs. When we tested a battery of ASDs in the 6 Hz rat model, we found that sodium channel blockers such as phenytoin, carbamazepine, lacosamide, and lamotrigine were less efficacious than in the mouse model when tested at 2 times the CV97 (Metcalf et al., 2017). ASDs targeting the GABAergic system, while fairly effective in the mouse model, only conferred protection in rats at doses that were accompanied with motor impairments. Therefore, the protective index for these compounds was not ideal in the rat model. Of all the compounds evaluated in this model, only three compounds proved to be efficacious at well tolerated doses at both stimulus intensities: ezogabine, sodium valproate, and phenobarbital. Overall, compounds with efficacy in the mouse 6 Hz model that are less effective in the rat 6 Hz model suggest that the rat 6 Hz model is less sensitive to compounds acting on sodium channels, GABAA receptors, or GABA uptake in comparison to the mouse 6 Hz model. This also suggests that the rat 6 Hz model may be helpful in detecting compounds with novel mechanisms of action and potential activity against pharmacoresistant focal seizures.
In Vitro Slice Model of Pharmacoresistant Recurrent Epileptiform Discharges (REDs).
The incorporation of etiologically relevant in vivo models of epilepsy in the early identification stages of compound evaluation within the ETSP has been challenging due to the economic, labor, and time constraints often associated with these models. Therefore, in vitro models that may circumvent these constraints and compliment other early drug discovery efforts have been explored. In particular, models that employ brain slices obtained from animals that have undergone the enduring changes in CNS function that occur during epileptogenesis, such as the kainic acid (KA) models of TLE, are especially intriguing. Brain slices from these rodents often produce spontaneous REDs under otherwise normal conditions (i.e., in the absence of hyperexcitable saline or chemoconvulsants), and they exhibit altered sensitivities to various ASDs and other pharmacologic agents (Smith et al., 2007, Carter et al., 2011, Maslarova et al., 2013, West et al., 2018). Accordingly, an in vitro model of REDs based on brain slices from the rat kainic acid-induced status epilepticus (KA-SE) model of temporal lobe epilepsy in rats was developed and characterized in the ETSP (Smith et al., 2007, West et al., 2018).
The ETSP has evaluated the concentration-dependent actions of 20 ASDs from various mechanistic classes in this in vitro slice model. REDs were recorded from the superficial layers of the medial entorhinal cortex. Although ASDs that target voltage-gated sodium channels (VGSCs) and potassium channels effectively attenuate REDs in this model, our results clearly show that most ASDs targeting GABAergic synaptic transmission and/or other molecular targets incompletely block REDs at concentrations well beyond their expected therapeutic ranges or have no effects at all (West et al., 2018). Thus, these data illustrate a spectrum of ASD-mediated effects on REDs and are expected to serve as a foundation upon which future therapeutics may be identified, differentiated, and assessed for potentially translatable efficacy in people with pharmacoresistant epilepsy.
Taken together, data from the development and characterization of this in vitro assay suggests that epileptiform activity recorded from the superficial layers of the mEC in slices obtained from KA-SE rats is differentially sensitive to existing ASDs. In the context of previously reported data using tissues from naive rodents, the different sensitivities of REDs to ASDs may reflect persistent molecular and/or network changes resulting from disease. Therefore, we propose that in vitro models of spontaneous epileptiform activity that employ tissues from translationally relevant epilepsy models may better reflect the underlying molecular and/or network substrate and, therefore, better predict the efficacy of investigational therapeutics for the treatment of pharmacoresistant epilepsy, particularly those with novel mechanisms of action.
Lamotrigine-resistant, Amygdala Kindled Rat.
The LTG-K model has been a part of the ETSP testing scheme for well over 15 years. However, only recently has a detailed pharmacological profile of this model been published. This model was first described by Postma et al, 2000 (Postma et al., 2000) and soon after was adopted by the ETSP following in-house development (Srivastava et al., 2004, Srivastava and White, 2005). Rats are kindled via electrical stimulation in the amygdala, but during the kindling process are also treated with a low dose of LTG prior to each stimulation session (5 mg/kg). While this treatment does not in any way interfere with the kindling process, the treatment results in kindled rats whose seizures are no longer sensitive to LTG, whereas rats that are fully kindled in the absence of LTG exhibit seizures that are readily blocked by LTG. In addition to being pharmacoresistant to LTG, rats kindled in this manner also are generally no longer sensitive to other ASDs that block sodium channels (e.g. carbamazepine, eslicarbazepine, lacosamide, and rufinamide) (Srivastava and White, 2013, Metcalf et al., 2019). This is in stark contrast to rats kindled in the absence of LTG, as sodium channel blockers often block kindled seizures (Metcalf et al., 2019, Wu et al., 2019). Furthermore, in contrast to sodium channel blockers, compounds targeting either GABAA receptors (clobazam, clonazepam, phenobarbital) or GABA uptake proteins (tiagabine) produced dose-dependent efficacy against behavioral seizures (Metcalf et al., 2019). Furthermore, compounds acting to modulate Ca2+ channels show differential activity: while ethosuximide (T-type Ca2+ channel modulator) was not effective in blocking seizures in this model, gabapentin (α2δ Ca2+ channel subunit modulator) was highly efficacious at well tolerated doses. Finally, while ezogabine and valproate were also highly effective, topiramate and levetiracetam were not effective at the doses tested in this model of pharmacoresistant seizures (Metcalf et al., 2019). Cross-tolerance to compounds with similar mechanisms of action (e.g. sodium channel blockers for the LTG-K rat model) is a limitation of this model, but it is noteworthy that tolerance to drugs with other mechanisms of action may also occur in kindling models (Zhang et al., 2003, Koneval et al., 2018). Beyond the pharmacoresistant nature of the kindled seizures, perhaps one of the most intriguing findings from this comprehensive study was the fact that tolerability of some of the compounds was notably different in the LTG-K rats compared to naïve rats. In particular, whereas rufinamide was found to induce impairment in the minimal motor impairment (MMI) test in naïve rats with a TD50 greater than 350 mg/kg, in LTG-K rats, the TD50 was noted to be less than 40 mg/kg. In contrast, whereas gabapentin is found to impact MMI at approximately 76 mg/kg in naïve rats, it is much better tolerated in LTG-K rats, with a TD50 greater than 300 mg/kg (Metcalf et al., 2019). While traditionally the ETSP has assessed the impact of compounds on the rotarod in naive mice and on a functional observation battery or MMI in naïve rats, the contract site of the ETSP is now moving towards evaluating tolerability of NCEs in LTG-K rats as well. To avoid complications in interpretation due to the use of LTG however, the ETSP may consider using drug-naïve kindled rats. In addition, before any subchronic studies are performed in the KA spontaneous seizing rat model, a compound is administered to a group of KA-treated rats to inform on tolerability. It is hypothesized that tolerability concerns of ASDs that arise in people with epilepsy may be identified at an earlier preclinical step if evaluated in either a kindled animal that has had many seizures and a reduced seizure threshold or a SE model which exhibits spontaneous seizures, rather than a naïve animal.
A LTG-resistant, corneal kindled mouse model has also been recently described (Koneval et al., 2018). A mouse model of drug resistant kindled seizures is important as it shows that the insensitivity to LTG is not restricted to kindled rats. Additionally, when considering investigations of novel compounds for therapy development, mouse studies require significantly less drug than those in rats and thus this model may be a cost effective alternative to the LTG-K rat model. While there were some differences in efficacy for some compounds between the rats and mice (e.g. ezogabine), the findings that resistance to many currently available ASDs in both models suggests that novel compounds found to be effective in both models may be useful in people with pharmacoresistant epilepsy (Koneval et al., 2018, Metcalf et al., 2019). Future studies in both models will be necessary to determine the mechanisms by which LTG-kindled seizures become resistant to ASDs.
Special Populations and Genetic Epilepsies.
While many of the animal models mentioned above are particularly geared to identifying therapies for the people with difficult to treat TLE, it is very clear that numerous other types of epilepsy, especially the pediatric epileptic encephalopathies, result in pharmacoresistant seizures (Helbig and Tayoun, 2016). Therefore, developing approaches to identify novel therapies for special populations and genetic epilepsies is an exciting new direction for the ETSP contract site. Due to heterogeneity in the etiologies of epilepsy, there is reason to suspect that therapies identified with the models described above may not always be efficacious across different types of epilepsy. Therefore, the 2015 Working Group review recommended that the ETSP should incorporate additional animal models to address pharmacoresistant seizures in different populations of people. Currently two models have been formally incorporated into the testing scheme in this performance area – a benzodiazepine-resistant, pilocarpine-induced status epilepticus (SE) model in rats and a novel model of infection-induced seizures with a distinct and robust inflammatory component (Kirkman et al., 2010, Patel et al., 2017). Of note, the pilocarpine model demonstrated that a unique investigational compound, sec-Butyl-propylacetamide (SPD), was able to block benzodiazepine resistant SE (White et al., 2012) and is available to suppliers with compounds that are thought to be useful in terminating benzodiazepine-resistant SE. As new models of genetic epilepsies are developed by investigators in the field, the ETSP may also consider incorporation of those models into the testing scheme. For example, the ETSP is currently characterizing a mouse model of Dravet Syndrome and this is also briefly addressed below.
Theiler’s Murine Encephalomyelitis Virus (TMEV) Infection Model of TLE.
While there are numerous models of TLE based on acquired insults and chemoconvulsant-induced SE, there has been a notable absence of models of TLE that arise from a CNS infection. The TMEV model is the first animal model of infection-induced TLE and was developed through collaborations in ETSP-participating labs at the University of Utah (Libbey et al., 2008, Stewart et al., 2010b, a, Barker-Haliski et al., 2015, Patel et al., 2017). This model was also recently established in the Lӧscher lab (Broer et al., 2016), which confirmed and replicated key findings. Animals infected with TMEV exhibit both behavioral and focal temporal lobe spontaneous seizures generally between 3 and 8 days following infection and then, several weeks later, seizure thresholds are reduced and a majority of the animals that demonstrated acute seizures develop TLE and behavioral comorbidities (Stewart et al., 2010a, Umpierre et al., 2014). The virus is trophic for hippocampal CA1 and CA2 neurons and these neurons die during the acute infection period, resulting in profound hippocampal sclerosis (Libbey et al., 2008, Loewen et al., 2016). In addition, during the acute infection period, resident microglia in the hippocampus are a significant source of TNFα, whereas infiltrating macrophages are a significant source of IL-6, another cytokine which has been shown to be important for seizure activity following TMEV infection (Kirkman et al., 2010, Cusick et al., 2013, Patel et al., 2017). This is an important new animal model of TLE, as CNS infection dramatically increases the risk of developing epilepsy, especially if seizures occur during the infection period. Indeed, recent work has shown that 1/3 of all pediatric patients with febrile SE have a CNS Human Herpes Virus (HHV) 6b or HHV7 infection that can account for SE and an increased risk for subsequent development of TLE (Epstein et al., 2012). Thus, the TMEV model of TLE provides an etiologically relevant and unique platform for determining therapies for infection-induced seizures and epileptogenesis.
TMEV-infected mice have handling-induced seizures during the acute infection period, thus making this model well-suited to evaluate compounds known to either reduce inflammation or behave as more traditional ASDs. Screening for efficacy of compounds in this model can be done either at a single time point (e.g. during the peak of spontaneous seizures at 5–6 days post infection (dpi)) or sub-chronically, during the entire acute infection/seizure period (Cusick et al., 2013, Barker-Haliski et al., 2015, Patel et al., 2017). Test compounds or vehicle can be administered, and then TMEV-infected mice may be handled to induce a seizure at the time to peak effect (TPE) of the compound. Compounds that confer protection prevent a seizure from being induced from the handling. This approach has been used successfully to date by multiple labs to determine the efficacy of numerous compounds to prevent seizures during the acute infection period. Compounds such as cannabidiol (CBD), valproic acid, and wogonin have all demonstrated activity in this model (Cusick et al., 2013, Barker-Haliski et al., 2015, Patel et al., 2019). As CNS infections are an important cause of acquired epilepsy, it is anticipated that compounds found to be effective in this model may be especially useful in people during periods where seizures occur concomitantly with inflammation and high levels of CNS cytokine activity. It is important to note that the testing performed in this model in the ETSP occurs during the acute infection period, at a time when there is an active infection and a concomitant cytokine storm. Thus the mechanisms underlying seizure generation during the acute infection period are likely different than those at play during the chronic epilepsy period. Handling induced seizures do not occur at the later time points, the virus has cleared, and the extensive temporal lobe damage observed as a consequence of viral infection likely contributes to the development of seizures after the acute period concludes. While it is still early days with use of this model in the ETSP, we anticipate that activity in this model could help differentiate novel ASDs during preclinical development.
New Model Development.
In addition to the presently incorporated models, a genetic model of pediatric epilepsy is currently in development within the program to address the unmet clinical needs of special populations: a mouse model of Dravet Syndrome (DS). This DS mouse model, developed by the Dravet Syndrome Foundation of Spain and Jackson Laboratories, incorporates a human DS-conferring conditional knock-in mutation, A1783V, in the Scn1a gene (Kuo et al., 2019). Both hyperthermia-induced seizures and spontaneous seizures are currently being evaluated in this pediatric genetic model and ASD pharmacological profiles are being assessed for both seizure types. The ETSP contract site will evaluate the collected data and, in conjunction with the ETSP and discussions with the ECB, determine if this model should be adopted by the program and offered to suppliers with an interest in developing compounds for DS.
Development of Disease Modifying Therapies.
While numerous ASDs are available for the symptomatic treatment of seizures for the people with epilepsy, there are currently no available compounds that can either prevent the development of epilepsy in those people at risk or that can modify the course of the disease and provide either a cure or reduce the severity of the seizure burden in people. Thus, there continues to be an unmet clinical need for those types of medications and treatment approaches. Therefore, the ETSP contract site has, for the first time, adopted a strategy to explore the potential of novel compounds to prevent epilepsy or to be disease modifying. Currently, the contract site of the ETSP utilizes the KA-SE rat model to identify compounds that might be disease modifying. This assay is performed as a subcontract from the University of Utah to the University of Washington and is an approach that has been used in numerous other studies evaluating the potential of compounds to prevent epilepsy (Zeng et al., 2009, Tchekalarova et al., 2014, Liu et al., 2016). Following rat KA-SE, treatment strategies with various compounds are tested and the outcomes on the development of spontaneous seizures are captured with video-EEG for several weeks after the initial insult and treatment. In addition, the IHK model employed by Synapcell, can also be used to evaluate treatment approaches that might prevent the development of seizures or modify the seizure burden following the KA insult. Furthermore, while not currently employed, assays using chronically seizing rats and fully kindled rodents that are used in within the program may also be adapted to provide information regarding the potential of a drug to be disease modifying. Finally, the TMEV model may also be a candidate assay to assess disease modifying therapies, although the low seizure frequency in animals that go on to develop epilepsy in this model complicates study design by requiring large numbers of animals in each study. Nevertheless, for the first time in its long history, the ETSP is now well-positioned to help the field in identifying disease modifying therapies, especially given the more etiologically relevant assays now employed.
While these numerous models of epilepsy provide a framework for investigating the disease modifying potential of novel compounds within the ETSP, this remains a difficult task. For example, there are currently no known clinically available compounds that could serve as a positive control for preventing acquired epilepsy or for disease modification. Therefore, there are numerous unanswered questions with respect to the proper experimental design for use in the screening assays that would serve to rapidly and inexpensively identify compounds that would be effective. For example, following a CNS insult such as SE, traumatic brain injury, or infection, it is unclear what the time window for treatment is for the prevention of the development of epilepsy. In addition, significant methodological considerations such as how to determine the timing, dose and treatment regimen for compounds, the duration of treatment, and even the duration of time one must monitor animals subsequent to treatment to be able to conclude that the course of the disease has been permanently altered, remain. Therefore, to help inform the research community and the ETSP with respect to these and a host of related issues, the NINDS sponsored a two-day workshop in August of 2018 entitled “Accelerating Therapies for Anti-Epileptogenesis and Disease Modification.” This workshop brought together numerous experts in the field to discuss the role of biomarkers in therapy discovery, preclinical and clinical experimental design considerations, as well as regulatory issues that might inform the development of such a novel class of compounds. The workshop also identified major impediments and obstacles to therapy development. The recommendations that emerged from the workshop are being developed into a series of white papers that are well beyond the scope of the present manuscript. However, it is anticipated that continued efforts on the part of NINDS and the greater epilepsy research community would continue to inform the ETSP in this important research area and provide concrete recommendations to pursue in this new performance area.
Summary and conclusions
With the beginning of the new ETSP contract in 2016, numerous changes that were recommended by the NANDS Council working groups in 2012 and 2015 and the External Consultant Board (ECB) resulted in significant changes in the scope of work for the program (https://www.ninds.nih.gov/Current-Research/Focus-Research/Focus-Epilepsy/ETSP). These changes included a renewed emphasis in finding therapies for pharmacoresistant epilepsy as well as new efforts to identify agents that could confer disease modification or even prevention of epilepsy. Since 2012, five etiologically relevant animal models have been introduced into the testing scheme of the ETSP and several new models are also currently in development and being considered for implementation into the screening paradigm. As a consequence, there is considerable optimism that the unmet clinical needs of people with epilepsy will be more readily addressed by the continued success of the ETSP. Furthermore, the Division of Translational Research (DTR) at NINDS has put into place several important new funding mechanisms that can help the supplier move forward with successful compounds that emerge with a complete data package from the ETSP (Klein et al., 2017). Funding mechanisms such as the IGNITE, CREATE, small business programs, and the Blueprint Neurotherapeutics for Small Molecules, can help facilitate further development of efficacious compounds and provide resources to ensure that the compounds have a competitive chance of getting into human clinical trials (Kehne et al., 2017). Indeed, several suppliers to the ETSP have now taken advantage of these new programs for the continued development of their compounds. Thus, there is optimism that the refined approach used by the ETSP contract site, wherein etiologically relevant models that include those with spontaneous seizures are used, will identify novel, potentially disease modifying therapies for people with pharmacoresistant epilepsy and those at risk for developing epilepsy. Finally, while it is perhaps too early to determine if the strategies adopted by the ETSP contract site will be successful in these new endeavors, the fact that many of the validated assays that have been successful in the past in the program, (e.g., 6 Hz and kindled animals) are still employed, suggests that novel ASDs that are perhaps better tolerated and more efficacious in people with pharmacoresistant epilepsy may ultimately be brought to the clinic.
Highlights.
The contract site of the Epilepsy Therapy Screening Program (ETSP) has revised its screening approach to focus on identifying therapies for pharmacoresistant epilepsy
The pharmacological profile of antiseizure drugs (ASDs) in several models incorporated into the refined workflow are described
Etiologically relevant models that include those with spontaneous seizures may identify novel, potentially disease modifying therapies for people with pharmacoresistant epilepsy
Acknowledgements
The authors thank the Epilepsy Therapy Screening Program at the National Institutes of Neurological Diseases and Stroke for their helpful discussions, review, and comments on this manuscript. This project has been funded by Federal funds from the National Institute of Neurological Disorders and Stroke, Epilepsy Therapy Screening Program, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN271201600048C (KSW).
Footnotes
Declaration of Interests
KSW is a consultant to Xenon Pharmaceuticals and CSM is a consultant to SEA Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Karen S. Wilcox, Anticonvulsant Drug Development (ADD) Program, Department of Pharmacology & Toxicology, University of Utah.
Peter J. West, Anticonvulsant Drug Development (ADD) Program, Department of Pharmacology & Toxicology, University of Utah
Cameron S. Metcalf, Anticonvulsant Drug Development (ADD) Program, Department of Pharmacology & Toxicology, University of Utah
References Cited.
- Armstrong C, Krook-Magnuson E, Oijala M, Soltesz I (2013) Closed-loop optogenetic intervention in mice. Nat Protoc 8:1475–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Haliski ML, Dahle EJ, Heck TD, Pruess TH, Vanegas F, Wilcox KS, White HS (2015) Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler’s murine encephalomyelitis virus mouse model. J Pharmacol Exp Ther 353:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker-Haliski ML, Johnson K, Billingsley P, Huff J, Handy LJ, Khaleel R, Lu Z, Mau MJ, Pruess TH, Rueda C, Saunders G, Underwood TK, Vanegas F, Smith MD, West PJ, Wilcox KS (2017) Validation of a Preclinical Drug Screening Platform for Pharmacoresistant Epilepsy. Neurochem Res 42:1904–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS (2001) Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 47:217–227. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R (2000) Kainate, a double agent that generates seizures: two decades of progress. Trends in Neuroscience 23:580–587. [DOI] [PubMed] [Google Scholar]
- Bethmann K, Fritschy JM, Brandt C, Loscher W (2008) Antiepileptic drug resistant rats differ from drug responsive rats in GABA A receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis 31:169–187. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G (1999) Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience 89:717–729. [DOI] [PubMed] [Google Scholar]
- Broer S, Kaufer C, Haist V, Li L, Gerhauser I, Anjum M, Bankstahl M, Baumgartner W, Loscher W (2016) Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp Neurol 279:57–74. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE (1997) Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol 385:385–404. [PubMed] [Google Scholar]
- Carter DS, Deshpande LS, Rafiq A, Sombati S, DeLorenzo RJ (2011) Characterization of spontaneous recurrent epileptiform discharges in hippocampal-entorhinal cortical slices prepared from chronic epileptic animals. Seizure 20:218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Brodie MJ, Liew D, Kwan P (2018) Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol 75:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clossen BL, Reddy DS (2017) Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochimica et biophysica acta Molecular basis of disease 1863:1519–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS (2013) Infiltrating macrophages are key to the development of seizures following virus infection. Journal of virology 87:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duveau V, Pouyatos B, Bressand K, Bouyssieres C, Chabrol T, Roche Y, Depaulis A, Roucard C (2016) Differential Effects of Antiepileptic Drugs on Focal Seizures in the Intrahippocampal Kainate Mouse Model of Mesial Temporal Lobe Epilepsy. CNS Neurosci Ther 22:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LG, Shinnar S, Hesdorffer DC, Nordli DR, Hamidullah A, Benn EK, Pellock JM, Frank LM, Lewis DV, Moshe SL, Shinnar RC, Sun S, team Fs (2012) Human herpesvirus 6 and 7 in febrile status epilepticus: the FEBSTAT study. Epilepsia 53:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin DV, Yoder JD, Holdsworth DL, Harter ML, May JR, Cotey N, Dalton JA, Baird TJ (2016) The standardized functional observational battery: Its intrinsic value remains in the instrument of measure: The rat. Journal of pharmacological and toxicological methods 82:90–108. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Dudek FE (2005) The effect of carbamazepine on spontaneous seizures in freely-behaving rats with kainate-induced epilepsy. Epilepsia 46:287. [DOI] [PubMed] [Google Scholar]
- Grabenstatter HL, Ferraro DJ, Williams PA, Chapman PL, Dudek FE (2005) Use of chronic epilepsy models in antiepileptic drug discovery: the effect of topiramate on spontaneous motor seizures in rats with kainate-induced epilepsy. Epilepsia 46:8–14. [DOI] [PubMed] [Google Scholar]
- Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, Hatakeyama S, Ohgoh M, Ueno M, Nishizawa Y (2011) Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia 52:1331–1340. [DOI] [PubMed] [Google Scholar]
- Helbig I, Tayoun AA (2016) Understanding Genotypes and Phenotypes in Epileptic Encephalopathies. Mol Syndromol 7:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehne JH, Klein BD, Raeissi S, Sharma S (2017) The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem Res 42:1894–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS (2010) Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 51:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein BD, Jacobson CA, Metcalf CS, Smith MD, Wilcox KS, Hampson AJ, Kehne JH (2017) Evaluation of Cannabidiol in Animal Seizure Models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res 42:1939–1948. [DOI] [PubMed] [Google Scholar]
- Klein P, Diaz A, Gasalla T, Whitesides J (2018) A review of the pharmacology and clinical efficacy of brivaracetam. Clinical pharmacology : advances and applications 10:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Lamberty Y (2002) Use of epileptic animals for adverse effect testing. Epilepsy Res 50:55–65. [DOI] [PubMed] [Google Scholar]
- Koneval Z, Knox KM, White HS, Barker-Haliski M (2018) Lamotrigine-resistant corneal-kindled mice: A model of pharmacoresistant partial epilepsy for moderate-throughput drug discovery. Epilepsia 59:1245–1256. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I (2013) On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature communications 4:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo FS, Cleary CM, LoTurco JJ, Chen X, Mulkey DK (2019) Disordered breathing in a mouse model of Dravet syndrome. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P, Schachter SC, Brodie MJ (2011) Drug-resistant epilepsy. The New England journal of medicine 365:919–926. [DOI] [PubMed] [Google Scholar]
- Langlois M, Polack PO, Bernard H, David O, Charpier S, Depaulis A, Deransart C (2010) Involvement of the thalamic parafascicular nucleus in mesial temporal lobe epilepsy. J Neurosci 30:16523–16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Avoli M (2013) The kainic acid model of temporal lobe epilepsy. Neuroscience and biobehavioral reviews 37:2887–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Avoli M, Bernard C (2016) Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J Neurosci Methods 260:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, Fujinami RS (2008) Seizures following picornavirus infection. Epilepsia 49:1066–1074. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zheng P, Wright DK, Dezsi G, Braine E, Nguyen T, Corcoran NM, Johnston LA, Hovens CM, Mayo JN, Hudson M, Shultz SR, Jones NC, O’Brien TJ (2016) Sodium selenate retards epileptogenesis in acquired epilepsy models reversing changes in protein phosphatase 2A and hyperphosphorylated tau. Brain 139:1919–1938. [DOI] [PubMed] [Google Scholar]
- Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, Wilcox KS (2016) Neuronal Injury, Gliosis, and Glial Proliferation in Two Models of Temporal Lobe Epilepsy. J Neuropathol Exp Neurol 75:366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W (2017) The Search for New Screening Models of Pharmacoresistant Epilepsy: Is Induction of Acute Seizures in Epileptic Rodents a Suitable Approach? Neurochem Res 42:1926–1938. [DOI] [PubMed] [Google Scholar]
- Maslarova A, Salar S, Lapilover E, Friedman A, Veh RW, Heinemann U (2013) Increased susceptibility to acetylcholine in the entorhinal cortex of pilocarpine-treated rats involves alterations in KCNQ channels. Neurobiol Dis 56:14–24. [DOI] [PubMed] [Google Scholar]
- Mathiasen JR, Moser VC (2018) The Irwin Test and Functional Observational Battery (FOB) for Assessing the Effects of Compounds on Behavior, Physiology, and Safety Pharmacology in Rodents. Curr Protoc Pharmacol 83:e43. [DOI] [PubMed] [Google Scholar]
- Mead AN, Amouzadeh HR, Chapman K, Ewart L, Giarola A, Jackson SJ, Jarvis P, Jordaan P, Redfern W, Traebert M, Valentin JP, Vargas HM (2016) Assessing the predictive value of the rodent neurofunctional assessment for commonly reported adverse events in phase I clinical trials. Regulatory toxicology and pharmacology : RTP 80:348–357. [DOI] [PubMed] [Google Scholar]
- Merritt HH, Putnam TJ (1938) A new series of anticonvulsant drugs tested by experiments on animals. Arch Neurol Psychiat 39:1003–1015. [Google Scholar]
- Metcalf CS, Huff J, Thomson KE, Johnson K, Edwards SF, Wilcox KS (2019) Evaluation of Antiseizure Drug Efficacy and Tolerability in the Rat Lamotrigine-Resistant Amygdala Kindling Model. Epilepsia Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf CS, West PJ, Thomson KE, Edwards SF, Smith MD, White HS, Wilcox KS (2017) Development and pharmacologic characterization of the rat 6 Hz model of partial seizures. Epilepsia 58:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nampoothiri SS, Potluri T, Subramanian H, Krishnamurthy RG (2017) Rodent Gymnastics: Neurobehavioral Assays in Ischemic Stroke. Molecular neurobiology 54:6750–6761. [DOI] [PubMed] [Google Scholar]
- Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, Szymkowski DE, West PJ, Smeal RM, Patel M, Fujinami RS, White HS, Wilcox KS (2017) Hippocampal TNFalpha Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, Wallis G, Fujinami RS, Wilcox KS, Smith MD (2019) Cannabidiol Reduces Seizures Following CNS Infection with Theiler’s Murine Encephalomyelitis Virus. Epilepsia Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma T, Krupp E, Li XL, Post RM, Weiss SR (2000) Lamotrigine treatment during amygdala-kindled seizure development fails to inhibit seizures and diminishes subsequent anticonvulsant efficacy. Epilepsia 41:1514–1521. [DOI] [PubMed] [Google Scholar]
- Potschka H (2012) Animal models of drug-resistant epilepsy. Epileptic disorders : international epilepsy journal with videotape 14:226–234. [DOI] [PubMed] [Google Scholar]
- Putnam TJ, Merritt HH (1937) Experimental determination of the anticonvulsant properties of some phenyl derivatives. Science 85:525–526. [DOI] [PubMed] [Google Scholar]
- Smith MD, Adams AC, Saunders GW, White HS, Wilcox KS (2007) Phenytoin- and carbamazepine-resistant spontaneous bursting in rat entorhinal cortex is blocked by retigabine in vitro. Epilepsy Res 74:97–106. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Franklin MR, Palmer BS, White HS (2004) Carbamazepine, but not valproate, displays pharmaco-resistance in lamotrigine-resistant amygdala kindled rats. Epilepsia 45:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, White HS (2005) Retigabine decreases behavioral and electrographic seizures in the lamotrigine-resistant amygdala kindled rat model of pharmacoresistant epilepsy. Epilepsia 46:217–218.15679502 [Google Scholar]
- Srivastava AK, White HS (2013) Carbamazepine, but not valproate, displays pharmacoresistance in lamotrigine-resistant amygdala kindled rats. Epilepsy Res 104:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS (2010a) Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. Journal of neuropathology and experimental neurology 69:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS (2010b) Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia 51:1418–1428. [DOI] [PubMed] [Google Scholar]
- Takahashi DK, Vargas JR, Wilcox KS (2010) Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis 40:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchekalarova JD, Ivanova NM, Pechlivanova DM, Atanasova D, Lazarov N, Kortenska L, Mitreva R, Lozanov V, Stoynev A (2014) Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol Biochem Behav 127:27–36. [DOI] [PubMed] [Google Scholar]
- Thomson KE, Modi AC, Glauser TA, Rausch JR, Steve White H (2017) The impact of nonadherence to antiseizure drugs on seizure outcomes in an animal model of epilepsy. Epilepsia 58:1054–1062. [DOI] [PubMed] [Google Scholar]
- Twele F, Schidlitzki A, Tollner K, Loscher W (2017) The intrahippocampal kainate mouse model of mesial temporal lobe epilepsy: Lack of electrographic seizure-like events in sham controls. Epilepsia Open 2:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, West PJ, White HS, Wilcox KS (2014) Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol Dis 64:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HA, Arabadzisz D, Fritschy JM, Brandt C, Bethmann K, Loscher W (2006) Antiepileptic drug-resistant rats differ from drug-responsive rats in hippocampal neurodegeneration and GABA(A) receptor ligand binding in a model of temporal lobe epilepsy. Neurobiol Dis 21:633–646. [DOI] [PubMed] [Google Scholar]
- West PJ, Saunders GW, Billingsley P, Smith MD, White HS, Metcalf CS, Wilcox KS (2018) Recurrent epileptiform discharges in the medial entorhinal cortex of kainate-treated rats are differentially sensitive to antiseizure drugs. Epilepsia 59:2035–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Alex AB, Pollock A, Hen N, Shekh-Ahmad T, Wilcox KS, McDonough JH, Stables JP, Kaufmann D, Yagen B, Bialer M (2012) A new derivative of valproic acid amide possesses a broad-spectrum antiseizure profile and unique activity against status epilepticus and organophosphate neuronal damage. Epilepsia 53:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KS, Dixon-Salazar T, Sills GJ, Ben-Menachem E, White HS, Porter RJ, Dichter MA, Moshe SL, Noebels JL, Privitera MD, Rogawski MA (2013) Issues related to development of new antiseizure treatments. Epilepsia 54 Suppl 4:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ido K, Ohgoh M, Hanada T (2019) Mode of seizure inhibition by sodium channel blockers, an SV2A ligand, and an AMPA receptor antagonist in a rat amygdala kindling model. Epilepsy Res 154:42–49. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci 29:6964–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Xing GQ, Russell S, Obeng K, Post RM (2003) Unidirectional cross-tolerance from levetiracetam to carbamazepine in amygdala-kindled seizures. Epilepsia 44:1487–1493. [DOI] [PubMed] [Google Scholar]