Abstract
Obesity is a serious medical condition worldwide, which needs new approaches and recognized international consensus in treating diseases leading to morbidity. The aim of this review was to examine heterogeneous links among the various phenotypes of obesity in adults. Proteins and associated genes in each group were analysed to differentiate between biomarkers. A variety of terms for classification and characterization within this pathology are currently in use; however, there is no clear consensus in terminology. The most significant groups reviewed include metabolically healthy obese, metabolically abnormal obese, metabolically abnormal, normal weight and sarcopenic obese. These phenotypes do not define particular genotypes or epigenetic gene regulation, or proteins related to inflammation. There are many other genes linked to obesity, though the value of screening all of those for diagnosis has low predictive results, as there are no significant biomarkers. It is important to establish a consensus in the terminology used and the characteristics attributed to obesity subtypes. The identification of specific molecular biomarkers is also required for better diagnosis in subtypes of obesity.
Keywords: Adipose tissue, biomarkers, body fat, genome-wide association studies, heterogeneity, HOMA, obesity, subtypes
Introduction
Over the last few decades, obesity has become an increasing public health problem worldwide, and its related conditions differ by region. For example, in China, Russia and South Africa, obesity is associated with hypertension, angina, diabetes and arthritis, whereas in India, it is associated with hypertension1. Obesity can also lead to a wide variety of other illnesses2,3. Overall, obesity is defined as the excessive accumulation or abnormal distribution of body fat (BF), affecting health4. It is classified, primarily, by body mass index (BMI, kg/m2), which is a very limited criterion5. Obesity is complicated by other diseases such as type 2 diabetes mellitus (T2DM), hepatic steatosis, cardiovascular diseases, stroke, dyslipidaemia, hypertension, gallbladder problems, osteoarthritis, sleep apnoea and other breathing problems and certain types of cancer (endometrial, breast, ovary, prostate, liver, gallbladder, kidney and colon), all of which can lead to an increased risk of mortality6. Cases related to pituitary, thyroid and adrenal gland diseases are considered an independent pathology but may indicate obesity7,8.
Multifactorial polygenic obesity involves several polymorphic genes. This subtype is caused by environmental factors such as diet, lack of physical exercise, ultra-processed foods, fast food, microbiome and the chemical contaminants, which can alter gene expression9. This review was aimed to make a thorough investigation of the heterogeneous links and differences among various phenotypes for diagnosis and treatment of polygenic obesity in adults10 and in the relationship between genes and proteins as possible biomarkers. Table I shows the definitions for the different obesity subtypes11,12,13,14.
Table I.
Definitions used for heterogeneity subtypes in obese individuals
| Obese groups | Definition | Other terminology for this group | Notes |
|---|---|---|---|
| MHO11,12 | Absence of metabolic disorders, including type 2 diabetes mellitus, dyslipidaemia, and hypertension | Metabolically normal obese, metabolically benign obese, metabolically healthy overweight/obese | Definitions vary in different studies, manly based on inflammatory markers and cut-off values |
| MAO11,12 | Defined by 2 main factors, BMI and metabolic status, which is classified as having threeor more points from the NCEP-ATP III, to define MetS | MUO | Several definitions of MetS have been published since 1999, the first was proposed by the WHO |
| MONW11,12,13 | Individuals are characterized by a BMI <25 kg/m2, hyperinsulinaemia and (or) insulin resistance, increase abdominal and visceral adiposity, atherogenic lipid profile, unfavourable adipokine profile, as well as hypertriglyceridaemia and hypertension, and higher levels of oxidative stress | Metabolically obese healthy | Some definitions consider other variables such as BMI, FFM, VAT, HOMA, ATP III |
| Sarcopenic obese14 | BMI <25 kg/m2, low muscle mass and weak muscle strength lack physical exercise | Sarcopenic overweight |
MHO, metabolically healthy obese; MAO, metabolically abnormal obese; MONW, metabolically obese, normal weight; MetS, metabolic syndrome; BMI, body mass index; NCEP-ATP III, National Cholesterol Education Program Adult Treatment Panel III; FFM, fat-free mass; VAT, visceral adipose tissue; HOMA, homeostasis model assessment; ATP III, adult treatment program III; MUO, metabolically unhealthy obese
A selective search of two databases (PubMed and the Cochrane Library) between 1998 and 2017 resulted in the selection of the most commonly reported subtypes of obesity and heterogeneity in adults. The terminology used for searches was as follows: (i) metabolically obese (MO), metabolically unhealthy obese (MUO), metabolically abnormal obese (MAO); (ii) metabolically healthy obese (MHO); (iii) metabolically unhealthy normal weight, metabolically abnormal normal-weight, normal weight obese; (iv) sarcopenic obese (SO); and (v) metabolically healthy normal-weight. All these terms were cross-checked with the words, genes, epigenetic, genome-wide association studies (GWAS), biomarkers and receiver operating characteristic (ROC) analysis. The four most common obesity phenotypes are shown in Table II.
Table II.
Obesity subtypes and associated biomarkers
| Obesity subgroup | Study description | Associated or expressed chemical, proteins, cells and index | Related genes |
|---|---|---|---|
| MHO | A cross-sectional sample of 2047 men and women aged 45-74 years15 | Decreased circulating levels of complement C3, hsCRP, TNF-α, IL-6, and plasminogen activator inhibitor-1 and increased adiponectin11 | - |
| MAO | A cross-sectional analysis of 7765 with 3135 overweigh andobese individuals16. A total of 503 individuals with abdominal obesity without cardiovascular diseases were selected17. |
Increase uric acid and visceral adiposity18 | T45T adiponectin genotype is associated with increase of metabolic disorders14 |
| MONW | 3015 individuals with abnormal metabolic phenotype in normal-weight adults in a cross-sectional study19. 1244 individuals in a cross-sectional study included20. 17029 non-diabetic individuals in a cross-sectional study21. 854 individuals non-obese. |
Increase in body fat per cent, uric acid and alanine transaminase, decrease in skeletal muscle per cent, and body water per cent13. Increase in hsCRP, uic acid, cystatin C and leukocytes18. Increase in the production of triglycerides and glucose (TyG index)13. |
Two disparate haplotypes of common FTO gene variants: TCGA and CTAT17 |
| SO | 844 individuals in a cross-sectional study22. 3763 in a study cohort23. | Increased hsCRP in serum24 | PTPRD, CDK14, and IMMP2L14 |
hsCRP, high-sensitivity C-reactive protein; TNF-α, tumour necrosis factor alpha; IL-6, interleukin 6; SO, sarcopenic obese; PTPRD, protein tyrosine phosphatase receptor type D; CDK14, cyclin dependent kinase 14; IMMP2L, inner mitochondrial membrane peptidase subunit 2
Heterogeneity in obese individuals
Among overweight and obese individuals, significant heterogeneity of phenotypes occurs, which is directly related to the participation of molecules, genes and cells, in addition to environmental, social and economic factors. For example, central obesity (also known as visceral obesity) is evident from an apple or android-shaped body, and confers a greater risk of developing metabolic complications. On the other hand, peripheral obesity, or peripheral fat accumulation in the gluteofemoral region, gives a pear-shaped body and has a gynecoid phenotype associated with reduced metabolic risk25.
One of the most commonly accepted diagnoses for obesity in a caucasian population is evidence of a BMI equal to or >30 kg/m26. However, BMIs differ with ethnicity. A study on Dual-energy X-ray absorptiometry (DEXA) indicates that a BMI of 28 kg/m2 in men, and of 24 kg/m2 in women correlates better with adiposity27. It is generally acknowledged that BMI indicates general adiposity, and the waist:height ratio (WHtR) indicates abdominal adiposity28. People with ≥0·5 WHtR are classified as having high abdominal adiposity29, although it may vary in different populations30. A discrepancy also exists, particularly in individuals who have higher muscle mass31.
Metabolically healthy obese (MHO)
MHO group or metabolically normal obese, or metabolically benign obese has been studied extensively32, and, depending on the method of classification, represents 6-40 per cent of the obese population. However, these terms are inconsistent with the pathology, leaving no clear consensus on phenotype. The metabolic spectrum is defined in numerous studies33. The homeostatic model assessment (HOMA) index is also used in MHO classification to identify an increased risk of mortality11. In all MHO individuals, insulin levels and insulin resistance indices for HOMA, quantitative insulin-sensitivity check index (QUICKI), and Mffm/l, high-sensivity C-reactive protein (hsCRP) and interleukin 6 (IL-6) are similar to a healthy population15. In addition, higher or lower HOMA, Quicki or Mffm/l results are not specific to any particular obesity phenotype. However, MHO individuals show increase in other biomarkers, such as, leptin34.
MHO individuals have a higher risk of developing metabolic syndrome when compared to healthy individuals of normal weight35. Over time, there has been a transition from a metabolically healthy overweight/obese phenotype to a metabolically abnormal overweight/obese phenotype. Wang et al36 found that MHO, in particular, was associated with subclinical cardiovascular dysfunction, lower global longitudinal systolic strain, dyssynchrony and early diastolic dysfunction. Chang et al37 reported that MHO individuals had a higher prevalence of subclinical coronary atherosclerosis than metabolically healthy normal-weight individuals; however, later studies suggested that these problems of MHO individuals might be even higher than in the metabolically unhealthy group38.
The inflammatory state is reduced in MHO and may be explained by the fatty acid profile of myristic, palmitic, stearic, oleic and linoleic acids33. MHO is also associated with lower levels of proinflammatory proteins and higher levels of anti-inflammatory molecules39, such as overexpression of fetuin-A (AHSG), histidine-rich glycoprotein (HRG) and retinol-binding histidin-rich protein 4 (RBP4), and downregulation of histamine releasing peptide (HRP), hsCRP, complement factor 4A (C4A), and inter-alpha-trypsin inhibitor heavy chain H4 (ITIH4). Together, these opposing effects counteract each other creating a pro-/anti-inflammatory profile33.
One particular feature of MHO is an abnormality in Bromodomain and extra terminal (BET) proteins. Wang et al40 discovered a connection between Brd2 obesity and T2DM. The Brd2 isoform promotes pancreatic β-cell function and proliferation and is one of the protein factors regulating gene transcription. It binds with acetylated lysines in nucleosomal chromatin and plays a role in energy metabolism41. In MHO, a disruption of the BRD2 gene in the promoter region results in a reduced level of activity. BRD2 knockdown in mice protects them from insulin resistance and pancreatic β-cell dysfunction40. Inhibition of BET proteins may increase insulin production and improve pancreatic β-cell function42.
Metabolically abnormal obese (MAO)
A significant number of individuals in this group are overweight and have central obesity with metabolic syndrome, T2DM, cardiovascular or cerebrovascular disease and are likely to present diastolic or systolic high blood pressure and increased waist-hip circumference. This group differs significantly from the metabolic healthy obese subtype in levels of postprandial blood glucose, high-density lipoprotein cholesterol, triglycerides, insulin and adiponectin. Some of these are measured on the HOMA-IR despite variations43. Certain biomarkers associated with metabolic syndrome, such as alanine aminotransferase, can increase greatly, but are still within the normal range of reference44. In addition, the International Diabetes Federation (IDF), American Heart Association and the National Heart, Lung and Blood Institute (AHA/NHLBI) have published a document on harmonizing the metabolic syndrome45. The consensus criteria for a clinical diagnosis of metabolic syndrome is based on this document.
In the overweight and obese individuals, cardiometabolic risk is one of the main problems for which waist circumference (WC), and WHtR are used for identification46. The other examples of heterogeneity expression are observed in the pro-inflammatory cytokines IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1), regulated on activation, normal T cell expressed, and secreted (RANTES), macrophage inflammatory protein 1 alpha (MIP1α), and plasminogen activator inhibitor-1 (PAI 1) in visceral adipose tissue (VAT), whereas leptin and interferon inducible protein 10 are expressed mainly in subcutaneous AT (SAT)47,48. VAT is related to metabolic disorder and to upregulated activation and expression. Leucine rich repeat containing receptor family pyrin domain containing 3 (NLRP3) gene and IL1b are upregulated in VAT49, which is infiltrated by proinflammatory macrophages in the MUO/MAO subgroup. Marques-Vidal et al50 showed increased levels of hsCRP and also tumor necrosis factor-alpha (TNF-α) in a Swiss population based study which was associated with an increase in WC in men, and BMI in women.
It has been shown that high carbohydrate comsumption and environmental factors among others modulate genotype interactions increases risk of obesity. Therefore, epigenetic mechanisms increase the number of changes in the genome, which may be related to the different phenotypes of obesity51.
All gene variants are related to an increased risk of obesity; for example, the fat mass and obesity associated gene (FTO rs9939609) significantly predisposes an individual to diabetes and increased BMI and hip circumference52,53. However, Veerman54 explained that the predictive power of this gene was attenuated significantly by its incomplete penetrance, suggesting that exploring gene expression in medical practice has limited relevance. Subgroups or subtypes of heterogeneity have also been reported in other studies. A clinical subgroup of MAO is the hypertriglyceridaemic-waist phenotype (HTGW), which is classified by increased WC and increased fasting triglyceride levels, and a cluster of factors related to metabolic syndrome55. An epigenetic mechanism, known as DNA methylation, which is found in the HTGW phenotype in carnitine palmitoyltransferase 1A (CPT1A) and ATP binding cassette subfamily G member 1 (ABCG1) genes, may modify gene function through the addition of methyl to DNA. This process is strongly associated with HTGW in epigenome-wide analysis56.
A number of methylated CpG loci are also associated with obesity. Crujeiras et al57 showed that DNA methylation levels in obese insulin resistant or insulin sensitive patients could be classified by the clamp technique. Through genome-wide epigenetic analysis, 982 differentially methylated CpG sites (DMCpGs) were found in VAT. As proposed by Huang et al58, most of these DMCpGs could be related to the insulin pathway, and some could be used as markers. Pietiläinen et al59 studied SAT in monozygotic twins with different body masses and found 17 obesity-associated genes with differentially methylated 22 CpGs regions.
Metabolically obese normal weight (MONW)
The MONW is also known as metabolically abnormal with no obesity, metabolically abnormal individuals with no obesity (MANO), normal weight dyslipidaemia60, or pre-obesity61. As in other subtypes, MONW has multiple definitions60, most of which are inconsistent. Metabolically abnormal individuals with a normal BMI and no visual signs of obesity are also known as pre-obese individuals61. More than 23 per cent BF is evident in men and 30 per cent in women62 and both may have a visceral fat area (VFA) of ≥100 cm2 with a variable BMI cut off of <23, <25, or <26 kg/m2 62. The abnormal accumulation of BF in MONW10,63 accounts for only a small number of cases but takes into consideration VFA and BF percentage. These individuals may also develop prediabetes or borderline dyslipidaemia with upper-normal WC64.
In studies conducted in the USA, 24 per cent of adults of normal weight (BMI <25 kg/m2) are considered metabolically abnormal and are at a high-risk of chronic diseases11 such as T2DM and cardiovascular disease. These individuals are physically inactive, have a BMI in the range of 20-27 kg/m2 and a fat mass of 2-10 kg, which is more than healthy controls of the same age65.
In MONW, some members of the same family may be hypertensive and have metabolic syndrome or cardiovascular disease, and a small number may be diabetic, although it is notable that the risk of developing diabetes mellitus is not dependent on central obesity, it depends on a number of factors in positive metabolic syndrome66. The adipose mass represents an important source of proinflammatory cytokines in obese individuals, and circulating concentrations of hsCRP, TNF-α, IL-1 α, IL-1β, IL-6 and IL-8 are elevated67,68. HsCRP in adults is strongly associated with a number of factors also seen in metabolic syndrome, central obesity and increased cardiovascular risk; however, it may not be specific to any obesity phenotype69,70. Yaghootkar et al71 reported on monogenic forms of insulin resistance in a subtype of MONW with a ‘lipodystrophy-like’ phenotype linked to 11 genetic variants. It can lead to hypertension, coronary artery disease and diabetes mellitus.
Sarcopenic obesity
Sarcopenic obesity, or sarcopenically obese, is defined as a reduction in lean mass and is associated with predicting factors such as increased age, low socio-economic status, smoking, decreased physical activity, atherosclerosis and pulmonary disease72. These factors are related to an accumulation of BF and a decrease in skeletal muscle mass and muscle strength24. The prevalence of sarcopenic obesity in adults over 65 yr is higher in countries such as Mexico (10.2%), South Africa (10.3%) and Spain (11%)72.
For diagnosis, the under quintile of the skeletal muscle index (muscle skeletal/BMI) is commonly used, along with the measurement for grip strength (<30 kg for men and <20 kg for women)73. BF is measured by skinfold thickness, bioelectrical impedance analysis (BIA), DEXA, or calculation of predictive formulae, among other criteria12. DEXA not only detects adiposity but also shows osteopenia and osteoporosis74. BIA, is quick, inexpensive and non-invasive and is useful in clinical practice75. It measures body composition and is based on resistance and reactance76. Although there is no direct relation between resistance, reactance77 and adiposity, a different BIA prediction equation has been found which gives a positive predictive value for fat-free mass (FFM) in adults, for males and females78.
In particular, in sarcopenia studies with BIA, there are three main issues that need to be considered: (i) lack of standardization in the definition of sarcopenia, (ii) selection of adequate/appropriate equations to calculate FFM or appendicular lean soft tissue, and (iii) selection of population-specific cut-off points79. Sarcopenic obesity can exist in individuals of different ages, not only in the older adult. Kim et al80 showed the prevalence of non-sarcopenic non-obese (53%), sarcopenic non-obese (10%), non-sarcopenic obese (20%) and sarcopenic obese (15%) individuals. They found an increase in the systolic blood pressure in the sarcopenic groups.
Inflammatory markers, such as hsCRP, increase in males with sarcopenic obesity22. Further, an increase in MCP-1 in serum marks the proinflammatory state. Several loci are associated with sarcopenic obesity, such as those located in PTPRD, CDK14 and IMMP2L genes23. Similarly, single nucleotide polymorphism (SNPs), such as the TP53 polymorphism, predict the risk of sarcopenia, contrasting with other kinds of obesity81. An association between −308 G/A TNF-α polymorphism and sarcopenic obesity was also established82.
Adipose tissue, biomarkers and heterogeneity
There are three varieties of adipocytes: brown, white and beige. In humans, brown adipocytes are found in the neck, interscapular and supraclavicular areas83. White adipocytes are found in subcutaneous and visceral regions, while beige are found in the supraclavicular region, inguinal canal and near the carotid sheath and the long muscle of the neck (musculus longus colli)84. White adipose tissue (WAT) has an intrinsic heterogeneity with depot-specific differences85. Subcutaneous depot expresses higher levels of TBx15 gene (T-Box transcription factor 15) and adiponectin in visceral WAT than other markers86. Percentages of arachidonic acid and docosahexaenoic acid are higher in subcutaneous WAT and have an upregulation of 5-lipoxygenase in T2DM in women, in contrast to VAT (vWAT)87.
Other methods providing quantitative non-invasive biomarkers include magnetic resonance imaging88, near-infrared-based optical spectroscopy and nuclear magnetic resonance (NMR), the last two of which have been validated by determining hepatic fat content through a minimally invasive needle-like probe89. In addition, high-resolution pulsed field gradient diffusion NMR spectroscopy might delineate WAT and brown AT90.
The adipocytes produce a number of cytokines including adiponectin, leptin, interleukin (IL-6), PAI-1, adipsin, TNF-α, resistin, angiotensinogen, aromatase and CRP91. These are related to obesity, hypertension, atherosclerosis, diabetes and thrombosis48, and some have a strong association with eating behaviours, chronic inflammation and metabolic disease.
Abdominal obesity is associated with an increase in IL-6, while BMI and WC relate to TNF-α levels50. Lim et al92 found that BMI was a poor indicator of excess adiposity in the elderly and showed that WC was a better marker. They also associated MCP-1 with the proinflammatory state, in accordance with studies by Yang et al22 in which they found an increase in hsCRP in elderly males with sarcopenic obesity.
Accuracy and limitations in terminology and biomarkers
When considering the main group classifications for, monogenic, polygenic, multifactorial obesity and mixed cases9, monogenic is proved to be the most useful in confirming the specific type by molecular methods, and subsequently, implementing strategies for personalized medicine93. In cases linked to multiple genes or polygenic phenotypes, the study of genetic markers is not beneficial in clinical diagnosis. This takes into consideration that genetic predisposition is not equal to inevitability of disease in wider concept94. A wide spectrum of disease susceptibility may be evident from the genes found in polygenic obesity (for example, in genes LEPR, MC4R, PCK1, POMC and PPARG), and is also significant in monogenic obesity95. This indicates that highly penetrant rare variants may be related to severe obesity, and genes with common variants could be related to more common obesity. In addition, FTO, the gene most strongly associated with obesity, only explains 0.34 per cent of phenotypic variance, which increases to 1.45 per cent with 32 GWAS96. Several of studies claimed that parental BMI, birth weight, maternal occupation, maternal gestational weight and gestational smoking gave a better predictive risk of obesity than GWAS97. Therefore, genetic studies should be endorsed only in individuals with early-onset obesity if they have intellectual disabilities or exhibit developmental delays, or in syndromic types.
Without agreed terminology, at present, no research or clinical diagnoses define the different phenotypes sufficiently. Paradoxically, if the individual has normal biochemical blood parameters, they are considered healthy. The question, originally raised by Scully98, still remains, as to how to properly distinguish between a real disease and merely disturbing risk factors, defects or deficits. One other concern of MHO diagnosis is the doctor´s bias towards, or perception of a patient99. Other obesity subgroups related to diet, physical activity chemical compounds and endocrine disruptors100 (dichloro-diphenyl-dichloro-ethylene, bisphenol A, polychlorinated biphenols, phthalates, phytoestrogens, glycyrrhetinic acid and tricyclic antidepressants among others), have not been taken into consideration, that will very likely be participating.
Perspectives
Despite a lack of clear definitions to classify obesity subgroups, there are markers or indeces that are useful to make basic differentiations such as VAT, and fat mass, that together with BMI, WC and WHtR, all related with intra-abdominal adiposity could help in the subgroups classification18 (Figure).
Figure.
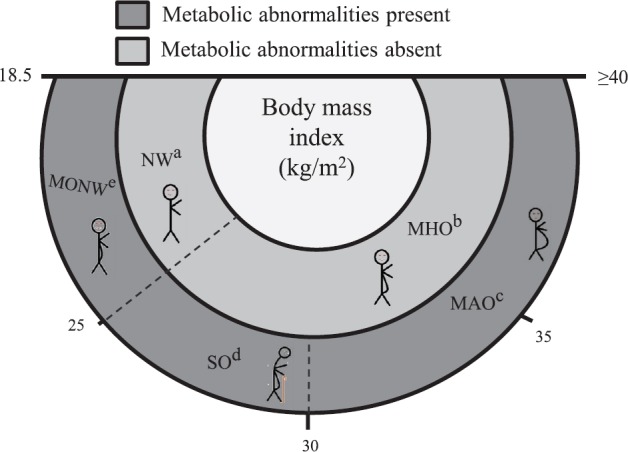
Differences between phenotypes of obesity. aNormal weight (NW) metabolically healthy and normal visceral adipose tissue (VAT) and normal BMI. bMetabolically healthy obese (MHO) individuals have high body mass index (BMI) and healthy metabolic profile, characterized by having excessive body fat, high insulin sensitivity, low VAT/total body fat mass index and low VAT. cMetabolically abnormal obese (MAO) individuals present high BMI, are associated with abnormal metabolic profile, high VAT and increased uric acid. dSarcopenically obese (SO) are characterized by loss of skeletal muscle mass and function, increases risk of metabolic alterations mainly in older individuals and have high VAT with BMI between 25 and 30 kg/m2. eMetabolically obese normal weight (MONW) individuals are characterized by high VAT and a normal BMI. Source: Ref. 17.
To differentiate the presence or absence of the metabolic component, VAT is useful because it is mechanistically related and strongest predictor to insulin resistance, T2DM, hypertension dyslipidaemia and cardiovascular disease101. The drawback of measuring VAT is the high cost and difficulty in carrying out these procedures. It may not be not accurate, but is useful if factors such as age, race, ethnicity and gender are taken into consideration. For examples, WC has a good correlation with DEXA measures of trunk fat mass percentage and metabolic syndrome102. To predict estimated per cent BF in older Caucasian American females and males, use of Siri-Brozeck equations is recommended26. A simple index, which was evaluated in a cross-sectional study with 17,029 non-diabetic individuals from the Korea National Health and Nutrition Examination Survey, discriminated individuals with MONW from MHO is the triglyceride glucose (TyG) index21. Others markers of visceral obesity: the visceral adiposity index and the lipid accumulation product (LAP) are good to identify MONW phenotype; these were evaluated in 3552 normal-weight individuals from the China Health and Nutrition Survey 2009 and identified people predisposed to develop metabolic diseases13.
Conclusion
Although all obese individuals have excess BF, there are important heterogenic differences between the subtypes. After reviewing various clinical, biochemical and genetic reports it is found that important progress has been made by the different groups in identifying specific differences in types of obesity, and the present criteria can help in diagnosis and treatment of obesity. Ideally, we need progress in two ways, first, to find better markers to distinguish each subtype of obesity more accurately for improvements in treatment, and second, to have an international consensus on terminology.
Acknowledgment
Authors thank Ms Charlotte Grundy for her editorial assistance.
Footnotes
Financial support & sponsorship: This work was supported by the Clinical Pathology Laboratory ‘Dr. Eduardo Perez Ortega’, Oaxaca, Mexico, and the Department of Biochemistry and Immunology, National Institute of Technology of Mexico
Conflicts of Interest: None.
References
- 1.Shukla A, Kumar K, Singh A. Association between obesity and selected morbidities: A study of BRICS countries. PLoS One. 2014;9:e94433. doi: 10.1371/journal.pone.0094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabrera-Fuentes HA, Aragones J, Bernhagen J, Boening A, Boisvert WA, Bøtker HE, et al. From basic mechanisms to clinical applications in heart protection, new players in cardiovascular diseases and cardiac theranostics: Meeting report from the third international symposium on ‘New frontiers in cardiovascular research’. Basic Res Cardiol. 2016;111:69. doi: 10.1007/s00395-016-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Fuentes HA, Alba-Alba C, Aragones J, Bernhagen J, Boisvert WA, Bøtker HE, et al. Meeting report from the 2nd International Symposium on New Frontiers in Cardiovascular Research. Protecting the cardiovascular system from ischemia: Between bench and bedside. Basic Res Cardiol. 2016;111:7. doi: 10.1007/s00395-015-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA. Evaluation of obesity. Who are the obese? Postgrad Med. 2003;114:19–27, 38. doi: 10.3810/pgm.2003.12.1544. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 2008;32:959–66. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purnamasari D, Badarsono S, Moersadik N, Sukardji K, Tahapary DL. Identification, evaluation and treatment of overweight and obesity in adults: Clinical practice guidelines of the obesity clinic, Wellness Cluster Cipto Mangunkusumo Hospital, Jakarta, Indonesia. JAFES. 2011;26:117–21. [Google Scholar]
- 7.Sidhu S, Parikh T, Burman KD. Endocrine Changes in Obesity. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Endotext. South Dartmouth (MA): MDText.com; [Google Scholar]
- 8.Álvarez-Castro P, Sangiao-Alvarellos S, Brandón-Sandá I, Cordido F. [Endocrine function in obesity] Endocrinol Nutr. 2011;58:422–32. doi: 10.1016/j.endonu.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz Yáñez C, García Vargas GG, Pérez-Morales R. Monogenic, polygenic and multifactorial obesity in children: Genetic and environmental factors. Austin J Nutr Metab. 2017;4:1052. [Google Scholar]
- 10.Zhang YP, Zhang YY, Duan DD. From genome-wide association study to phenome-wide association study: New paradigms in obesity research. Prog Mol Biol Transl Sci. 2016;140:185–231. doi: 10.1016/bs.pmbts.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 12.Du T, Yu X, Zhang J, Sun X. Lipid accumulation product and visceral adiposity index are effective markers for identifying the metabolically obese normal-weight phenotype. Acta Diabetol. 2015;52:855–63. doi: 10.1007/s00592-015-0715-2. [DOI] [PubMed] [Google Scholar]
- 13.Conus F, Rabasa-Lhoret R, Péronnet F. Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab. 2007;32:4–12. doi: 10.1139/h06-092. [DOI] [PubMed] [Google Scholar]
- 14.Lee DC, Shook RP, Drenowatz C, Blair SN. Physical activity and sarcopenic obesity: Definition, assessment, prevalence and mechanism. Future Sci OA. 2016;2:FSO127. doi: 10.4155/fsoa-2016-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–9. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 16.Du T, Zhang J, Yuan G, Zhang M, Zhou X, Liu Z, et al. Nontraditional risk factors for cardiovascular disease and visceral adiposity index among different body size phenotypes. Nutr Metab Cardiovasc Dis. 2015;25:100–7. doi: 10.1016/j.numecd.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berezina A, Belyaeva O, Berkovich O, Baranova E, Karonova T, Bazhenova E, et al. Prevalence, risk factors, and genetic traits in metabolically healthy and unhealthy obese individuals. Biomed Res Int. 2015;2015:548734. doi: 10.1155/2015/548734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjaer IG, Kolle E, Hansen BH, Anderssen SA, Torstveit MK. Obesity prevalence in Norwegian adults assessed by body mass index, waist circumference and fat mass percentage. Clin Obes. 2015;5:211–8. doi: 10.1111/cob.12100. [DOI] [PubMed] [Google Scholar]
- 19.Xia L, Dong F, Gong H, Xu G, Wang K, Liu F, et al. Association between indices of body composition and abnormal metabolic phenotype in normal-weight chinese adults. Int J Environ Res Public Health. 2017:14. doi: 10.3390/ijerph14040391. pii: E391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermans MP, Amoussou-Guenou KD, Bouenizabila E, Sadikot SS, Ahn SA, Rousseau MF. The normal-weight type 2 diabetes phenotype revisited. Diabetes Metab Syndr. 2016;10:S82–8. doi: 10.1016/j.dsx.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS, et al. Identifying subgroups of obesity using the product of triglycerides and glucose: The Korea National Health and Nutrition Examination Survey, 2008-2010. Clin Endocrinol (Oxf) 2015;82:213–20. doi: 10.1111/cen.12502. [DOI] [PubMed] [Google Scholar]
- 22.Yang CW, Li CI, Li TC, Liu CS, Lin CH, Lin WY, et al. Association of sarcopenic obesity with higher serum high-sensitivity c-reactive protein levels in chinese older males - A community-based study (taichung community health study-elderly, TCHS-E) PLoS One. 2015;10:e0132908. doi: 10.1371/journal.pone.0132908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu Y, Newton E, McLean R, Kiel D. Genome-wide association study of sarcopenic-obesity, identifies novel candidate genes The Framingham study adiposity & its sequelae (clinical/translational) Houston: The Endocrine Society's 94th Annual Meeting and Expo; 2012. [Google Scholar]
- 24.Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. doi: 10.1155/2013/204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34:1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers AJ, Parise E, McCrory JL, Cham R. A comparison of prediction equations for the estimation of body fat percentage in non-obese and obese older Caucasian adults in the United States. J Nutr Health Aging. 2014;18:586–90. doi: 10.1007/s12603-014-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah NR, Braverman ER. Measuring adiposity in patients: The utility of body mass index (BMI), percent body fat, and leptin. PLoS One. 2012;7:e33308. doi: 10.1371/journal.pone.0033308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowalkowska J, Poínhos R, Franchini B, Afonso C, Correia F, Pinhão S, et al. General and abdominal adiposity in a representative sample of Portuguese adults: Dependency of measures and socio-demographic factors’ influence. Br J Nutr. 2016;115:185–92. doi: 10.1017/S0007114515004055. [DOI] [PubMed] [Google Scholar]
- 29.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes Rev. 2012;13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoo EG. Waist-to-height ratio as a screening tool for obesity and cardiometabolic risk. Korean J Pediatr. 2016;59:425–31. doi: 10.3345/kjp.2016.59.11.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traissac P, Pradeilles R, El Ati J, Aounallah-Skhiri H, Gartner A, Delpeuch F. Within-subject non-concordance of abdominal v. general high adiposity: Definition and analysis issues. Br J Nutr. 2016;116:567–8. doi: 10.1017/S0007114516002154. [DOI] [PubMed] [Google Scholar]
- 32.Muñoz-Garach A, Cornejo-Pareja I, Tinahones FJ. Does Metabolically Healthy Obesity Exist? Nutrients. 2016:8. doi: 10.3390/nu8060320. pii: E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perreault M, Zulyniak MA, Badoud F, Stephenson S, Badawi A, Buchholz A, et al. A distinct fatty acid profile underlies the reduced inflammatory state of metabolically healthy obese individuals. PLoS One. 2014;9:e88539. doi: 10.1371/journal.pone.0088539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrer R, Pardina E, Rossell J, Oller L, Viñas A, Baena-Fustegueras JA, et al. Morbidly ‘healthy’ obese are not metabolically healthy but less metabolically imbalanced than those with type 2 diabetes or dyslipidemia. Obes Surg. 2015;25:1380–91. doi: 10.1007/s11695-014-1528-z. [DOI] [PubMed] [Google Scholar]
- 35.Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: The Atherosclerosis Risk in Communities Study. Obesity (Silver Spring) 2013;21:203–9. doi: 10.1002/oby.20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YC, Liang CS, Gopal DM, Ayalon N, Donohue C, Santhanakrishnan R, et al. Preclinical systolic and diastolic dysfunctions in metabolically healthy and unhealthy obese individuals. Circ Heart Fail. 2015;8:897–904. doi: 10.1161/CIRCHEARTFAILURE.114.002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang Y, Kim BK, Yun KE, Cho J, Zhang Y, Rampal S, et al. Metabolically-healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–86. doi: 10.1016/j.jacc.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 38.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann Intern Med. 2015;163:827–35. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doumatey AP, Zhou J, Zhou M, Prieto D, Rotimi CN, Adeyemo A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: A proteomics study. Obesity (Silver Spring) 2016;24:1257–65. doi: 10.1002/oby.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Liu H, Blanton WP, Belkina A, Lebrasseur NK, Denis GV. Brd2 disruption in mice causes severe obesity without Type 2 diabetes. Biochem J. 2009;425:71–83. doi: 10.1042/BJ20090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Deeney JT, Denis GV. Brd2 gene disruption causes ‘metabolically healthy’ obesity: Epigenetic and chromatin-based mechanisms that uncouple obesity from type 2 diabetes. Vitam Horm. 2013;91:49–75. doi: 10.1016/B978-0-12-407766-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deeney JT, Belkina AC, Shirihai OS, Corkey BE, Denis GV. BET bromodomain Proteins Brd2, Brd3 and Brd4 selectively regulate metabolic pathways in the pancreatic β-Cell. PLoS One. 2016;11:e0151329. doi: 10.1371/journal.pone.0151329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salgado AL, Carvalho Ld, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47:165–9. doi: 10.1590/s0004-28032010000200009. [DOI] [PubMed] [Google Scholar]
- 44.Mojiminiyi OA, Abdella NA, Al Mohammedi H. Higher levels of alanine aminotransferase within the reference range predict unhealthy metabolic phenotypes of obesity in normoglycemic first-degree relatives of patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich) 2010;12:301–8. doi: 10.1111/j.1751-7176.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 46.Vikram NK, Latifi AN, Misra A, Luthra K, Bhatt SP, Guleria R, et al. Waist-to-height ratio compared to standard obesity measures as predictor of cardiometabolic risk factors in Asian Indians in North India. Metab Syndr Relat Disord. 2016;14:492–9. doi: 10.1089/met.2016.0041. [DOI] [PubMed] [Google Scholar]
- 47.Lee MJ, Fried SK. Depot-specific biology of adipose tissues: Links to fat distribution and metabolic risk. In: Leff T, James G, Granneman JG, editors. Adipose tissue in health and disease, Ch. 15. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2010. pp. 283–306. [Google Scholar]
- 48.Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes Dev. 2008;22:1269–75. doi: 10.1101/gad.1681308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esser N, L’homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–97. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 50.Marques-Vidal P, Bochud M, Bastardot F, Lüscher T, Ferrero F, Gaspoz JM, et al. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus Study) Obes Facts. 2012;5:734–44. doi: 10.1159/000345045. [DOI] [PubMed] [Google Scholar]
- 51.Huang T, Hu FB. Gene-environment interactions and obesity: Recent developments and future directions. BMC Med Genomics. 2015;8(Suppl 1):S2. doi: 10.1186/1755-8794-8-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veerman JL. On the futility of screening for genes that make you fat. PLoS Med. 2011;8:e1001114. doi: 10.1371/journal.pmed.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemieux I, Poirier P, Bergeron J, Alméras N, Lamarche B, Cantin B, et al. Hypertriglyceridemic waist: A useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23(Suppl B):23B–31B. doi: 10.1016/s0828-282x(07)71007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mamtani M, Kulkarni H, Dyer TD, Göring HH, Neary JL, Cole SA, et al. Genome- and epigenome-wide association study of hypertriglyceridemic waist in Mexican American families. Clin Epigenetics. 2016;8:6. doi: 10.1186/s13148-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crujeiras AB, Diaz-Lagares A, Moreno-Navarrete JM, Sandoval J, Hervas D, Gomez A, et al. Genome-wide DNA methylation pattern in visceral adipose tissue differentiates insulin-resistant from insulin-sensitive obese subjects. Transl Res. 2016;178:13–24e5. doi: 10.1016/j.trsl.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Huang RC, Garratt ES, Pan H, Wu Y, Davis EA, Barton SJ, et al. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics. 2015;10:995–1005. doi: 10.1080/15592294.2015.1080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pietiläinen KH, Ismail K, Järvinen E, Heinonen S, Tummers M, Bollepalli S, et al. DNA methylation and gene expression patterns in adipose tissue differ significantly within young adult monozygotic BMI-discordant twin pairs. Int J Obes (Lond) 2016;40:654–61. doi: 10.1038/ijo.2015.221. [DOI] [PubMed] [Google Scholar]
- 60.Højland Ipsen D, Tveden-Nyborg P, Lykkesfeldt J. Normal weight dyslipidemia: Is it all about the liver? Obesity (Silver Spring) 2016;24:556–67. doi: 10.1002/oby.21443. [DOI] [PubMed] [Google Scholar]
- 61.Nagatomo A, Nishida N, Fukuhara I, Noro A, Kozai Y, Sato H, et al. Daily intake of rosehip extract decreases abdominal visceral fat in preobese subjects: A randomized, double-blind, placebo-controlled clinical trial. Diabetes Metab Syndr Obes. 2015;8:147–56. doi: 10.2147/DMSO.S78623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS One. 2013;8:e60673. doi: 10.1371/journal.pone.0060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: A risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J. 2010;31:737–46. doi: 10.1093/eurheartj/ehp487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Upper-normal waist circumference is a risk marker for metabolic syndrome in normal-weight subjects. Nutr Metab Cardiovasc Dis. 2016;26:67–76. doi: 10.1016/j.numecd.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 66.Lee IT, Chiu YF, Hwu CM, He CT, Chiang FT, Lin YC, et al. Central obesity is important but not essential component of the metabolic syndrome for predicting diabetes mellitus in a hypertensive family-based cohort. Results from the Stanford Asia-pacific program for hypertension and insulin resistance (SAPPHIRe) Taiwan follow-up study. Cardiovasc Diabetol. 2012;11:43. doi: 10.1186/1475-2840-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 68.De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: Early inflammation? Am J Clin Nutr. 2007;85:40–5. doi: 10.1093/ajcn/85.1.40. [DOI] [PubMed] [Google Scholar]
- 69.Bennett NR, Ferguson TS, Bennett FI, Tulloch-Reid MK, Younger-Coleman NO, Jackson MD, et al. High-sensitivity C-reactive protein is related to central Obesity and the number of metabolic syndrome components in Jamaican young adults. Front Cardiovasc Med. 2014;1:12. doi: 10.3389/fcvm.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshikane H, Yamamoto T, Ozaki M, Matsuzaki M. Clinical significance of high-sensitivity C-reactive protein in lifestyle-related disease and metabolic syndrome. J Cardiol. 2007;50:175–82. [PubMed] [Google Scholar]
- 71.Yaghootkar H, Scott RA, White CC, Zhang W, Speliotes E, Munroe PB, et al. Genetic evidence for a normal-weight ‘metabolically obese’ phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63:4369–77. doi: 10.2337/db14-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyrovolas S, Koyanagi A, Olaya B, Ayuso-Mateos JL, Miret M, Chatterji S, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: A multi-continent study. J Cachexia Sarcopenia Muscle. 2016;7:312–21. doi: 10.1002/jcsm.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abellan van Kan G, Houles M, Vellas B. Identifying sarcopenia. Curr Opin Clin Nutr Metab Care. 2012;15:436–41. doi: 10.1097/MCO.0b013e328356bbf4. [DOI] [PubMed] [Google Scholar]
- 74.Peppa M, Stefanaki C, Papaefstathiou A, Boschiero D, Dimitriadis G, Chrousos GP. Bioimpedance analysis vs. DEXA as a screening tool for osteosarcopenia in lean, overweight and obese Caucasian postmenopausal females. Hormones (Athens) 2017;16:181–93. doi: 10.14310/horm.2002.1732. [DOI] [PubMed] [Google Scholar]
- 75.Ricciardi R, Talbot LA. Use of bioelectrical impedance analysis in the evaluation, treatment, and prevention of overweight and obesity. J Am Acad Nurse Pract. 2007;19:235–41. doi: 10.1111/j.1745-7599.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 76.Barbosa-Silva MC, Barros AJ, Wang J, Heymsfield SB, Pierson RN., Jr Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am J Clin Nutr. 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- 77.Kumar S, Dutt A, Hemraj S, Bhat S, Manipadybhima B. Phase angle measurement in healthy human subjects through bio-impedance analysis. Iran J Basic Med Sci. 2012;15:1180–4. [PMC free article] [PubMed] [Google Scholar]
- 78.Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition. 2001;17:248–53. doi: 10.1016/s0899-9007(00)00553-0. [DOI] [PubMed] [Google Scholar]
- 79.Gonzalez MC, Barbosa-Silva TG, Heymsfield SB. Bioelectrical impedance analysis in the assessment of sarcopenia. Curr Opin Clin Nutr Metab Care. 2018;21:366–74. doi: 10.1097/MCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 80.Kim JH, Cho JJ, Park YS. Relationship between sarcopenic obesity and cardiovascular disease risk as estimated by the Framingham risk score. J KoreanMed Sci. 2015;30:264–71. doi: 10.3346/jkms.2015.30.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Renzo L, Gratteri S, Sarlo F, Cabibbo A, Colica C, De Lorenzo A. Individually tailored screening of susceptibility to sarcopenia using p53 codon 72 polymorphism, phenotypes, and conventional risk factors. Dis Markers. 2014;2014:743634. doi: 10.1155/2014/743634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Di Renzo L, Sarlo F, Petramala L, Iacopino L, Monteleone G, Colica C, et al. Association between -308 G/A TNF-α polymorphism and appendicular skeletal muscle mass index as a marker of sarcopenia in normal weight obese syndrome. Dis Markers. 2013;35:615–23. doi: 10.1155/2013/983424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 84.Cedikova M, Kripnerová M, Dvorakova J, Pitule P, Grundmanova M, Babuska V, et al. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016;2016:6067349. doi: 10.1155/2016/6067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: Molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–81. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heemskerk MM, Giera M, Bouazzaoui FE, Lips MA, Pijl H, van Dijk KW, et al. Increased PUFA content and 5-lipoxygenase pathway expression are associated with subcutaneous adipose tissue inflammation in obese women with type 2 diabetes. Nutrients. 2015;7:7676–90. doi: 10.3390/nu7095362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–49. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nachabé R, van der Hoorn JW, van de Molengraaf R, Lamerichs R, Pikkemaat J, Sio CF, et al. Validation of interventional fiber optic spectroscopy with MR spectroscopy, MAS-NMR spectroscopy, high-performance thin-layer chromatography, and histopathology for accurate hepatic fat quantification. Invest Radiol. 2012;47:209–16. doi: 10.1097/RLI.0b013e318237527b. [DOI] [PubMed] [Google Scholar]
- 90.Verma SK, Nagashima K, Yaligar J, Michael N, Lee SS, Xianfeng T, et al. Differentiating brown and white adipose tissues by high-resolution diffusion NMR spectroscopy. J Lipid Res. 2017;58:289–98. doi: 10.1194/jlr.D072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sethi JK, Vidal-Puig AJ. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lim JP, Leung BP, Ding YY, Tay L, Ismail NH, Yeo A, et al. Monocyte chemoattractant protein-1: A proinflammatory cytokine elevated in sarcopenic obesity. Clin Interv Aging. 2015;10:605–9. doi: 10.2147/CIA.S78901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 2016;130:943–86. doi: 10.1042/CS20160136. [DOI] [PubMed] [Google Scholar]
- 94.Turksen K. End of inevitability: Programming and reprogramming. Stem CellRev Rep. 2013;9:385–7. doi: 10.1007/s12015-013-9459-y. [DOI] [PubMed] [Google Scholar]
- 95.Ng MC, Bowden DW. Is genetic testing of value in predicting and treating obesity? N C Med J. 2013;74:530–3. [PMC free article] [PubMed] [Google Scholar]
- 96.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morandi A, Meyre D, Lobbens S, Kleinman K, Kaakinen M, Rifas-Shiman SL, et al. Estimation of newborn risk for child or adolescent obesity: Lessons from longitudinal birth cohorts. PLoS One. 2012;7:e49919. doi: 10.1371/journal.pone.0049919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scully JL. What is a disease? EMBO Rep. 2004;5:650–3. doi: 10.1038/sj.embor.7400195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Young ME, Norman GR, Humphreys KR. The role of medical language in changing public perceptions of illness. PLoS One. 2008;3:e3875. doi: 10.1371/journal.pone.0003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Munoz Yanez C, Garcia Vargas GG, Perez-Morales R. Monogenic, polygenic and multifactorial obesity in children: Genetic and Environmental Factors. Austin J Nutr Metab. 2017;4:1052. [Google Scholar]
- 101.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442–54. doi: 10.1093/aje/kwq306. [DOI] [PMC free article] [PubMed] [Google Scholar]


