ABSTRACT
The spiny mouse, Acomys spp., is a recently described model organism for regeneration studies. For a mammal, it displays surprising powers of regeneration because it does not fibrose (i.e. scar) in response to tissue injury as most other mammals, including humans, do. In this Primer article, we review these regenerative abilities, highlighting the phylogenetic position of the spiny mouse relative to other rodents. We also briefly describe the Acomys tissues that have been used for regeneration studies and the common features of their regeneration compared with the typical mammalian response. Finally, we discuss the contribution that Acomys has made in understanding the general principles of regeneration and elaborate hypotheses as to why this mammal is successful at regenerating.
KEY WORDS: Spiny mouse, Acomys, Tissue regeneration, Fibrosis, Scarring
Summary: This Primer summarizes the studies using the spiny mouse as a mammalian model for regeneration, its phylogenetic position, historical perspective and what we have learnt about principles of regeneration from this new model.
Introduction
The field of regenerative medicine aims to identify strategies to either engineer or repair human tissues, organs and body parts that cannot naturally be replaced when damaged by trauma or disease. Who would not want to take a drug to stimulate the proliferation of cardiomyocytes and recover from a heart attack? Or, stimulate axonal regrowth across a site of contusion in the spinal cord and recover function of the lower body and extremities? To solve this problem of inducing regeneration in humans, conventional wisdom suggests that we must identify the molecular and cellular signals that guide regeneration in regeneration-competent organisms, such as axolotls and zebrafish, and extrapolate what we learn to induce it in regeneration-incompetent organisms such as rats and mice, and eventually humans.
But is it true that mammals really are regeneration-incompetent organisms? The general impression would surely be yes, mammals cannot regenerate, but a deeper look across the remarkably few species that have been investigated reveals some surprises. For example, human children and mice can regenerate their digit tips (Illingworth, 1974; Yu et al., 2019); mammalian fetuses can heal skin wounds in a regenerative manner (Yates et al., 2012); male deer can annually regenerate their antlers, which are initially covered in skin or velvet which itself has regenerated (Goss, 1983); young C57BL/6J mice can regenerate hair follicles via wound-induced hair follicle neogenesis following large skin wounds (Ito et al., 2007); the neonatal mouse heart can regenerate until postnatal day 7 (Porrello et al., 2011); and some species can regenerate large holes punched through their ears, including rabbits (Gawriluk et al., 2016; Voronstova and Liosner, 1960) and perhaps also chinchillas, cows and pigs (Williams-Boyce and Daniel, 1986). It is also known that several individual mammalian tissues can regenerate, such as skeletal muscle after myotoxin administration (Musarò, 2014) and the liver, which displays prodigious powers of proliferation during compensatory hypertrophy (Fausto et al., 2012). This compensatory hypertrophy is a process which the lungs can also undergo (Hsia, 2017), whereby the tissue remaining after removal of part of the organ expands to compensate for the missing part. Many mammalian epithelial tissues, such as the epidermis or the intestinal lining, also exhibit continuous replacement, although this is a property of all animals and so is not considered an unusual regenerative process. Admittedly, all of these regenerative abilities observed in mammals are limited compared with those seen in axolotls (Joven et al., 2019) or zebrafish (Marques et al., 2019), but this only represents a very narrow sampling of extant mammalian species and there may be some truly regeneration-competent mammals out there that remain undiscovered.
Spiny mice, Acomys spp., are one such example of a regeneration-competent mammal, regenerating several tissues of their body to full functionality after injury – rather than the reduced functionality normally observed after scarring or fibrosis. Here, we provide an overview of the history and regenerative abilities of spiny mice. We propose that if fibrosis can be prevented in humans – as it is in spiny mice – then we may be able to regenerate a surprising array of tissues. Moreover, we highlight how the study of spiny mice can allow us to begin to compare regeneration-competent mammals with regeneration-incompetent mammals to discover the cellular and molecular signals governing regeneration.
General background on Acomys
There are 2050 living species of rodents (which are defined by having upper and lower pairs of continually growing incisors) and two thirds of these belong to the family Muroidea. There are likely 16 subfamilies of Muroidea including the old world rats (Rattus) and mice (Mus), which are placed in the subfamily Muridae (Steppan and Schenk, 2017). Spiny mice of the genus Acomys are placed in one of the other subfamilies called Deomyinae (previously called Acomyinae) along with Rudd's mouse (Uranomys), the Congo forest mouse or link rat (Deomys), and the brush-furred mouse (Lophuromys) (Steppan and Schenk, 2017) (Fig. 1). The closest relatives to Deomyinae are the gerbils of the subfamily Gerbillinae (Chevret et al., 1993; Steppan and Schenk, 2017) from which they separated 17.6-20 million years ago.
Model systems for regeneration.
This article is part of a series entitled ‘Model systems for regeneration’. This series of articles aims to highlight key model systems and species that are currently being used to study tissue and organ regeneration. Each article provides background information about the phylogenetic position of the species, its life-cycle and habitat, the different organs and tissues that regenerate, and the experimental tools and techniques that are available for studying these organisms in a regenerative context. Importantly, these articles also give examples of how the study of these models has increased our understanding of regenerative mechanisms more broadly, and how some of the open questions in the field of regeneration may be answered using these organisms. To see the full collection as it grows, please visit: https://dev.biologists.org/collection/regeneration_models.
Fig. 1.
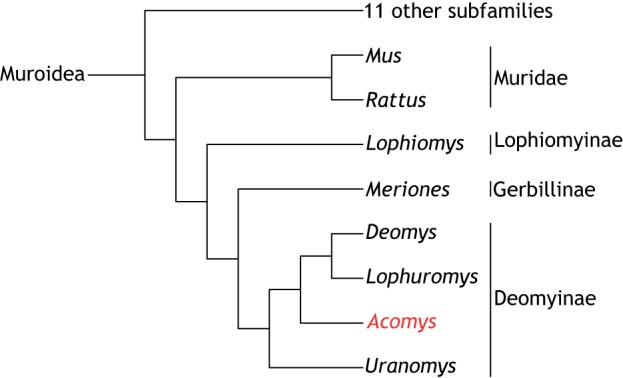
Phylogeny and phenotype of Acomys. Phylogenetic position of the genus Acomys among the 15 sub-families of the family Muridae. There are four genera including Acomys in the sub-family Deomyinae, the closest relatives of which are the gerbils. Adapted from Steppan and Schenk, 2017.
There are at least five major groups in the genus Acomys: subspinosus, spinosissimus, russatus, wilsoni and cahirinus (Aghová et al., 2019). All species share morphological characteristics of dorsal spine-like hairs, which are golden brown, grey or rusty brown depending on the species. Ventral hairs are white. Spiny mice are distributed throughout Africa, the eastern Mediterranean, some Mediterranean islands, the Arabian Peninsula, Iran and Pakistan (Jeremy and Bates, 1994). Only three species have been tested for regenerative ability: Acomys percivali (from the wilsoni group), Acomys kempi (from the cahirinus group) and Acomys cahirinus (Gawriluk et al., 2016; Seifert et al., 2012). All three have been shown to regenerate tissue of the ear following a biopsy punch, although only the regeneration of A. cahirinus has been studied extensively. Thus, we might assume that regeneration is a property of the genus.
The colonies that are currently maintained in Europe, USA and Australia are A. cahirinus, although one colony of Acomys dimidiatus is maintained in Geneva (Montandon et al., 2014). Because of their relatively long history of use as laboratory animals, there are several papers published on the care, maintenance and management of colonies, from early days (Strasser, 1968; Young, 1976) up until the present day (Dickinson and Walker, 2007; Haughton et al., 2016; Pinheiro et al., 2018). From these latter works it appears that their lifespan is usually 3-4 years, but can extend to 6 years.
Spiny mice have a complex social organization and are often housed in groups of up to 20 to permit communal breeding, although keeping three to five females and one male together in a cage allows the parentage of the pups to be determined (Haughton et al., 2016). Gestation lasts 39 days, considerably longer than in other rodents, and one to four pups (usually two or three) are produced. The pups are precocial and born with grey fur, open eyes and unfurled ears, and they are soon mobile and eating solid food. After 6 weeks, the grey coloured pups (Fig. 2) start producing the thick spiny hairs – from posterior to anterior, on the dorsum – and as a result change their coat colour to that of the adult (Fig. 2). They become sexually mature at 3-4 months of age (Dieterlen, 1961). Females have an 11-day oestrus cycle after which they undergo menstruation (Bellofiore and Evans, 2019). Mating does not result in the formation of a vaginal plug, making identification of the day of fertilization impossible, although later stages of gestation have been identified by ultrasound (Dickinson and Walker, 2007).
Fig. 2.
Hair colour and development in Acomys. Images of the hair colour at three stages of development of Acomys cahirinus viewed from the side (top row) and from above (bottom row). Pups (left column) are born with grey hairs; the golden spiny hairs first begin to appear on the caudal dorsum as sexual maturity approaches (middle column), spreading completely over the dorsum by adulthood (right column). See Jiang et al. (2019) for details on different hair types and their development.
Although spiny mice have recently come to prominence because of their striking ability to regenerate several organs and tissues, as described below, they have been used as research animals and kept in breeding colonies since at least 1911 (Bonhote, 1911). Indeed, it is surprising that their regenerative abilities had not been observed before 2012 (Seifert et al., 2012). Previous research using spiny mice has included studies into diabetes, because these mice show spontaneous degeneration of the islets of Langerhans and are prone to hyperglycaemia and diabetes with obesity but without insulin resistance (Creutzfeldt et al., 1970; Pictet et al., 1967; Shafrir et al., 2006). They have also been used to study renal physiology because they have among the highest recorded urine urea concentration (of 4.7-4.8 M), likely linked to their desert dwelling (Shkolnik and Borut, 1969). Recent research has also described them as the first known menstruating rodent (Bellofiore and Evans, 2019; Bellofiore et al., 2017, 2018), despite early studies on their reproductive physiology (Dewsbury and Hodges, 1987; Peitz, 1981; Peitz et al., 1979).
Studies on the development of spiny mice have been expansive, examining olfactory response during early post-natal life (Janus, 1988, 1993; Porter and Etscorn, 1976; Porter et al., 1978a,b, 1982, 1986, 1989); fetal, parental and social behaviour (Makin and Porter, 1984; Nováková et al., 2008; Porter, 1976; Porter et al., 1977, 1980, 1981, 1983; Robinson and Smotherman, 1992), and the development of the kidney (Dickinson et al., 2005), lung (Oosterhuis et al., 1984), brain (Brunjes, 1989; Brunjes et al., 1989), endocrine system (Lamers et al., 1986; Quinn et al., 2013) and spiny hairs (Montandon et al., 2014). Recent research has also used spiny mice as a model of birth asphyxia (Hutton et al., 2009a,b). Finally, the precocial spiny mouse has been proposed as a better model for human pregnancy and birth compared with the altricial mice and rats normally used because the development of the kidney (Dickinson et al., 2005), liver (Lamers et al., 1986), lung (Oosterhuis et al., 1984) and various brain regions (Brunjes, 1989) is essentially completed by the time of birth, which is more similar to humans than the continued development and maturation during the neonatal period of other commonly used rodents (Dickinson and Walker, 2007).
Tools and techniques for studying Acomys
As the spiny mouse is a very new model organism to enter the field of regeneration, there has been little time to develop tools and techniques that would enable a molecular analysis of its regenerative ability. Furthermore, there are some characteristics that make it a difficult organism for developing such techniques.
As mentioned above, females do not plug following copulation, making the determination of the day of fertilization difficult. An alternative approach to obtaining embryos of precise stages is to coordinate breeding immediately following birth of a litter when the female is fertile in postpartum oestrus, thus providing the day of conception for the subsequent litter (Dickinson and Walker, 2007). This method has been used to obtain two-cell, four-cell and eight-cell embryos for studying gene transcription (Mamrot et al., 2018 preprint). In addition, ultrasound techniques have been developed for pregnant Acomys making it possible to detect fetuses from day 12 of gestation (Dickinson and Walker, 2007). However, female Acomys only produce two to three pups per pregnancy, making the large-scale production of embryos rather difficult, although A. dimidiatus tends to have a larger litter size (Frynta et al., 2011). Superovulation techniques are available though (Pasco et al., 2012), so the development of in vitro systems for Acomys embryo culture should be possible.
Cell culture of Acomys tissues is commonly used, with media composition based on that used to culture mouse tissues (Simkin et al., 2017; Stewart et al., 2018). In theory, the generation of immortalized cell lines, transfected cells for lineage studies and even induced pluripotent stem cells (iPSCs) should therefore now be possible. Likewise, using CRISPR to alter cells is feasible because genetic information is available from three published transcriptomes: one derived from ear regeneration (Gawriluk et al., 2016), one from early embryos (Mamrot et al., 2018 preprint) and one from skin regeneration (Brant et al., 2019). Annotated genomes will soon be available from several groups.
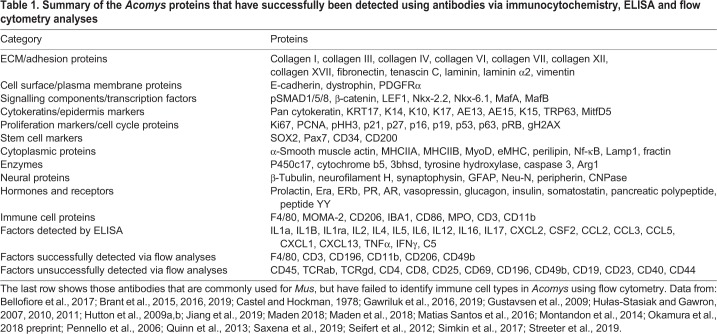
Immunocytochemistry is routinely performed with a range of commercially available antibodies that have been used to label a large variety of proteins in Acomys tissues, ranging from extracellular matrix (ECM) proteins, immune cell markers, cytoplasmic proteins to nuclear transcription factors (Table 1). There are at least 88 such antibodies reported and ELISAs have also been used to detect cytokines, thus there appears to be little problem of cross-reactivity with these antibodies, and where protein homology has been compared between Acomys, Mus and human it is expectedly high (Gawriluk et al., 2019 preprint). In contradiction, however, very few antibodies used for Mus T cell analysis by flow cytometry cross-react with Acomys (Gawriluk et al., 2019 preprint; Pennello et al., 2006), suggesting that an investment in antibody production in this area of research would be highly beneficial.
Table 1.
Summary of the Acomys proteins that have successfully been detected using antibodies via immunocytochemistry, ELISA and flow cytometry analyses
Thus, the typical reagents and techniques used in regeneration research are mostly available for use with Acomys. Once our understanding of the embryology and reproductive physiology of Acomys has advanced, it should be possible to generate transgenic animals, although the small litter size will presumably continue to be a significant drawback to rapid progress.
Tissue regeneration in Acomys
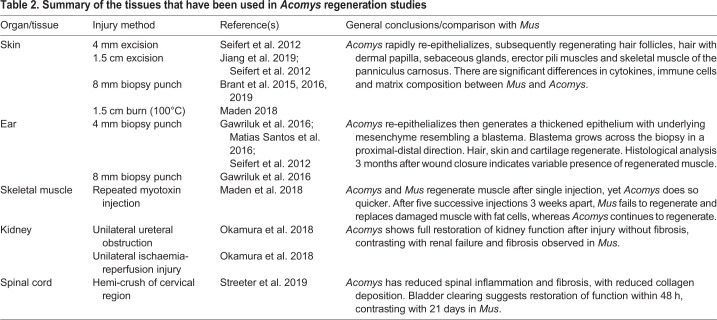
Despite more than a century of keeping colonies, the regenerative abilities of Acomys were not reported until 2012 (Seifert et al., 2012). Then, it was reported that spiny mice captured in the wild with large areas of dorsal skin missing could regenerate their skin successfully; it was also noted that their skin was weak and could tear easily. Their weak skin was previously reported in the context of the care and general biology of spiny mice. These early reports advised to avoid tail-handling, as the spiny mice tail is weak and can deglove (Bate, 1903; Shargal et al., 1999), and suggested that frequent bite-wounds could be treated with antibiotic spray ‘until the fur re-grows’ (Dickinson and Walker, 2007). Since these early reports, research into the regeneration of spiny mouse tissues has expanded from skin and ear punches to include skeletal muscle, kidneys and the spinal cord (Fig. 3, Table 2).
Fig. 3.
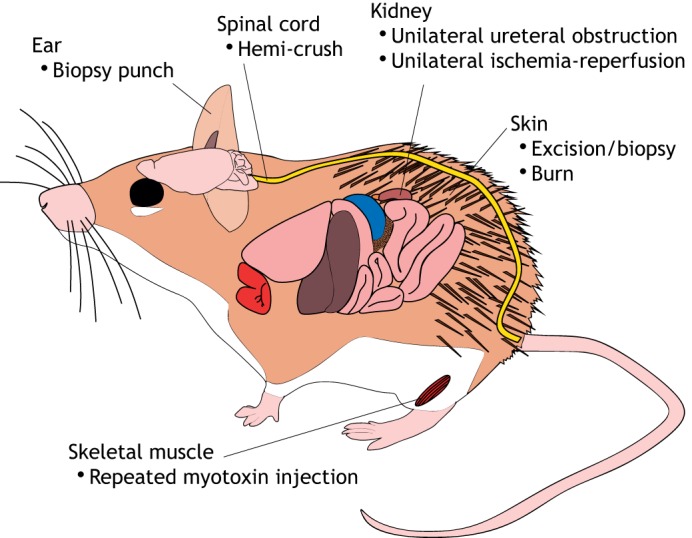
Tissue and organ regeneration in Acomys. Schematic of Acomys cahirinus highlighting the tissue types that can regenerate as well as the methods that have been used to investigate them.
Table 2.
Summary of the tissues that have been used in Acomys regeneration studies
Skin
After full-thickness skin removal or full-thickness burn injury, all the components of the Acomys skin are eventually regenerated, with each component following their own time scale (Brant et al., 2019, 2016, 2015; Maden, 2018; Seifert et al., 2012). Scab formation and haemostasis is rapid, and more than 50% of the wound area can close within 24 h after injury (Seifert et al., 2012). The epidermis – the outermost layer of the skin – responds immediately by inducing proliferation around the wound margin, which thickens as a result and then migrates across the wound. The rate of epithelial migration is notably quicker than that observed in mouse or rat skin (Seifert et al., 2012), and in vitro wound healing experiments reveal that keratinocytes migrate twice as fast in Acomys compared with Mus (Stewart et al., 2018). By the end of the second week, new hair placodes are seen in the wound epithelium and regenerate through defined stages, exhibit high proliferation and reuse molecular pathways from embryonic hair follicle development. The hair follicles arise in response to Wnt signalling as determined by expression of LEF1, a nuclear transcription factor that is a readout of Wnt signalling (Brant et al., 2019; Seifert et al., 2012). Wnt7a, which is known to induce hair follicles in transgenic mice (Ito et al., 2007), is also expressed at this time (Brant et al., 2019), suggesting similar pathways are used in Acomys and Mus for hair follicle induction in the epidermis.
During hair development in Mus, the epidermal placode appears first and is induced by a signal from the dermal mesenchyme, the so-called ‘first dermal signal’, the nature of which is unknown but may be another Wnt signal (Millar, 2002). Fibroblast growth factors (FGFs) and Shh are also involved as mesenchymal signals in hair development and, based on studies of the wound-induced hair follicle neogenesis (WIHN) mouse model, it is known that Fgf9 overexpression increases the number of hairs induced by signalling a feedback loop in dermal fibroblasts involving Wnt2a (Gay et al., 2013). Similarly, ectopic expression of Shh in the WIHN mouse model induces extra hair follicles (Lim et al., 2018). Moving forward, it will be interesting to see whether the same events occur during follicle regeneration in Acomys.
Following hair follicle induction, hair placodes in the Acomys wound epithelium grow and deepen into the newly formed tissue of the wound bed to generate a hair with a dermal papilla. The three types of hair normally present in Acomys skin (guard, awl and zigzag) are regenerated in the same proportions (Jiang et al., 2019). In the fourth week, sebaceous glands develop, along with erector pili muscles that elevate the new hairs, making them fully functional (Brant et al., 2016).
Fibroblasts within the dermis – the layer beneath the epidermis – migrate into the wound bed to replace the missing tissue. These cells generate a new matrix that is lower in cell density than that in the corresponding wound site in Mus. As might be expected, there is a different collagenous organization and composition between scarring Mus wounds and regenerating Acomys wounds, with the wound bed of Acomys being arranged more loosely and being lower in density than that of Mus (Brant et al., 2016). Concerning the composition, Acomys have low levels of collagens compared with Mus, particularly collagen 12a1 (Brant et al., 2015, 2016), which is known to stabilize the ECM and is present in dense connective tissues. The most obvious matrix-associated differences are in the matrix remodelling proteins (MMPs), which are highly induced in Acomys, particularly MMP2 and MMP9 (Brant et al., 2015). By contrast, tissue inhibitors of MMPs (TIMPs), are highly induced in Mus. Considering MMPs and TIMPs in Acomys and Mus, it is possible that ECM degradation is more active in Acomys wound healing. Another noteworthy and highly upregulated matrix-associated gene in Acomys wounds is the collagen triple helix repeat-containing gene, Cthrc1 (Brant et al., 2019). Interestingly, the function of this secreted protein has primarily been investigated in cancer metastasis, which Cthrc1 promotes by enhancing migration, reducing collagen I expression and production (Pyagay et al., 2005) and upregulating Wnt signalling and MMP9 expression (Guo et al., 2017), all highly relevant to skin regeneration. This matrix protein, along with tenascin C, tenascin N, fibronectin, laminin α1, fibrillin 2 and aggrecan, produces a loose ‘regenerative matrix’ in the Acomys wound bed (Brant et al., 2019; Seifert et al., 2012).
There is also a layer of skeletal muscle at the base of the skin, the panniculus carnosus, which is particularly well developed in rodents. Normally, holes punched through skeletal muscle in mice cannot regenerate because the connective tissue component of the muscle driving regeneration has been removed (Ciciliot and Schiaffino, 2010). As such, muscle fails to regenerate in a Mus skin wound and the formed scar is thinner than the original skin. However, the muscle in Acomys can fully regenerate (see Skeletal muscle section). In the second and third weeks after skin wounding, the levels of embryonic myosin (Myh3) rise 450-fold compared with baseline levels, and strong staining of this protein can be seen across the wound bed. After the completion of skin regeneration, at ∼7-8 weeks, mature muscle myosin can be seen in the regenerated muscle and new neuromuscular junctions demonstrate that this regenerated muscle of the panniculus carnosus is functional (Brant et al., 2019, 2016).
Ear punches
Observing the effect of a through-and-through ear punch has been a quick and easy method for assessing the regenerative ability of mammals for many years (cf. Goss, 1987). The ear consists of a plate of cartilage covered by skeletal muscle (with more on the dorsal side), adipocytes, connective tissue, dermis, and epithelium containing hairs with their associated sebaceous glands and erector pili muscles. Thus, full regeneration of the ear punch is clearly a complex phenomenon, requiring the replacement of a considerable number of tissues, and it greatly resembles full-thickness skin regeneration apart from the mode of epithelial healing and the absence of cartilage in skin.
Holes, typically with a diameter of 4 mm (ranging from 2-8 mm), are regenerated in all species of Acomys that have been examined (Gawriluk et al., 2016; Matias Santos et al., 2016; Seifert et al., 2012). Following damage, the epidermis migrates over the wound to cover the internal tissues, which begin to lose their differentiated characteristics as if they were dedifferentiating, as occurs during salamander limb regeneration. Unlike the skin, epithelial closure of the wound occurs more rapidly in Mus than in Acomys (Gawriluk et al., 2016) or at a similar rate (Matias Santos et al., 2016). The epidermis becomes thickened, another characteristic of the amphibian limb, and by the end of the second week a structure resembling a ‘blastema’ (the regenerating tissue mass in salamanders) forms (Gawriluk et al., 2016; Seifert et al., 2012). Proliferation of blastemal cells elongates the structure and, by the end of the third week, re-differentiation of a variety of tissues occurs. New hair follicles differentiate in a proximal to distal spread, whereas new cartilage differentiates abutting the cut end of the old cartilage. By 8 weeks, the 4 mm wound has completely filled in, with new hairs and skin covering the regenerated plate of cartilage and new muscle (Matias Santos et al., 2016).
Interestingly, the hole does not regenerate equally around the circle created by the wound; instead, regeneration occurs from the proximal part (closer to the head), whereas the distal part hardly regenerates at all, making the final closure of the hole displaced from the centre (Matias Santos et al., 2016). This is most likely because, again like the salamander limb, regenerating ear punches depend on a functional nerve supply (Buckley et al., 2011) and the nerve fibres are differentially distributed across the wound (Gawriluk et al., 2016). The proximal semicircle of the punch wound has a plentiful supply of axons from the auricular nerve, but the axons in the distal semicircle of the wound are severed, making the distal part effectively denervated. The role of nerves in axolotl limb regeneration is well established (Stocum, 2019) and acts via the axonal and probably Schwann cell-based synthesis of the growth factor Neuregulin 1, which is released into the blastemal milieu to stimulate blastemal cell proliferation (Farkas et al., 2016). It would be of great interest to determine whether Neuregulin 1 is present in the Acomys auricular nerve and whether its inhibition prevents ear hole regeneration. This would re-ignite the debate of whether the absence of regeneration in various systems is related to an insufficiency of nerves and/or neuregulin growth factors.
Skeletal muscle
In mammals, skeletal muscle normally regenerates repeatedly throughout life, owing to the presence of Pax7-positive stem cells, called satellite cells (Chargé and Rudnicki, 2004; Musarò, 2014). This regeneration occurs following physical or toxic insults to skeletal muscle, such as injection of cardiotoxin, physical injury from freezing or crushing, or chemical injury. In general, these insults induce the breakdown of cell membranes, the invasion of immune cells, destruction of actin/myosin filaments, activation of satellite cells, the re-expression of embryonic and developmental myosins and myogenic regulatory factors, and subsequent regeneration of muscle fibres. In mice, a common muscle for investigation is the tibialis anterior, which is found at the front of the lower leg. After myotoxin injection, the regeneration of this muscle is unexpectedly fast and is essentially complete by the third week (14-16 days) after damage (Chargé and Rudnicki, 2004).
Not surprisingly, Acomys can also regenerate the muscle fibres of the tibialis anterior following similar insults, but does so faster – within 10 days – when compared with Mus (Maden et al., 2018). Within 6 days, newly regenerated myofibres are present and the regenerating muscle expresses embryonic myosin and higher levels of dystrophin. Following a consistent theme, there are lower levels of inflammation (see below) and fibrosis as measured by Nf-κB levels, as well as lower levels of collagen I, collagen III and collagen XII, and less necrosis. The damaged muscle becomes hugely infiltrated with M2 pro-regenerative macrophages, which also occurs in Mus, but strikingly lacks MI pro-inflammatory macrophages and has higher levels of the anti-inflammatory chemokine Cxcl12.
A more dramatic difference between Mus and Acomys muscle emerges when the tibialis anterior muscle is subjected to repeated rounds of regeneration (Maden et al., 2018). After five sequential myotoxin injections, spaced 3 weeks apart, the Acomys tibialis anterior continues to regenerate perfectly, as it did after only one round of regeneration. By contrast, the Mus tibialis anterior fails to regenerate and becomes largely composed of fat cells, which replace the muscle fibres and differentiate in the interstitium between the fascicles, a result typical of repeated muscle injury and showing striking resemblance to muscle in advanced cases of Duchenne muscular dystrophy (Uezumi et al., 2010, 2011).
As described above, a hole punched through the panniculus carnosus of Acomys, which is a skeletal muscle layer present at the base of the dermis, regenerates completely in ∼8 weeks and forms new neuromuscular junctions, suggesting it is functional (Brant et al., 2019). This is a striking result because the same damage in Mus does not trigger regeneration (and instead leads to volumetric muscle loss) as there is no connective tissue component remaining to guide regeneration. The fact that Acomys can regenerate this guidance tissue/cue suggests that a special property resides in the connective tissue fibroblasts.
Kidney
Typical fibrosis-inducing models in the kidney involve unilateral ureteral obstruction, whereby one ureter is ligated, and ischaemia reperfusion injury, whereby vascular supply to the kidney is clamped for a period of time and the contralateral kidney removed. After ureter obstruction in Mus, the kidney shows clear hydronephrosis (kidney swelling) after 14 days with a shrinking of the parenchyma and weight loss, which is accompanied by an increase in collagen content and extensive fibrosis (Okamura et al., 2018 preprint). In contrast, the Acomys obstructed kidney preserves its structure and does not lose its weight, increase its collagen content or show fibrosis. In addition, there are reduced numbers of myofibroblasts and F4/80 macrophages in the Acomys obstructed kidney, and tubular integrity is preserved. Thus, the Acomys kidney does not respond to damage by inducing fibrosis and tissue loss.
In the context of the ischaemia reperfusion model, in which ischaemia is performed for 40 min and the contralateral kidney is removed after 24 h, both Mus and Acomys show equivalent levels of elevated blood urea nitrogen and equivalent levels of tubular injury and tissue damage (Okamura et al., 2018 preprint). This indicates that the Acomys kidney suffers significant tissue damage after ischaemia. However, if the contralateral kidney is not removed at the time of ischaemia (thereby preventing death from kidney failure), there is an almost complete absence of fibrosis after 14 days and preservation of renal mass in Acomys compared with the severe fibrosis and 40% loss of renal mass observed in Mus. Thus, after ischaemia, Acomys can almost completely restore its kidney function compared with the progressive renal failure that Mus normally undergoes.
Spinal cord
Damage to the Mus spinal cord typically results in the appearance of a fibrotic glial scar, which is thought to be inhibitory to axonal regrowth across the site of damage and thus prevents any restoration of function. As a lack of fibrosis is characteristic of the response of Acomys to the various damages described above, it is also possible that the same may occur after spinal cord injury. To test this, Mus and Acomys were subjected to a hemi-crush of the spinal cord in the cervical region (Streeter et al., 2019). This study revealed that, in Mus at 3 days post-injury, several pro-inflammatory genes such as Il6, Cxcl3, Ccl12, Ccl7, Il1b and fibrosis genes such as Tgfb1, Serpine1 and Timp1 are induced. In contrast, the majority of upregulated genes in Acomys encode growth factors such as brain-derived neurotrophic factor and glial cell-derived neurotrophic factor, or components of the Wnt pathway, or are genes associated with neural stem cells such as Sox2, Notch1 and Ascl1, or axonal guidance such as Robo1, Efnb1 and Ntn1. Thus, there appears to be a completely different spectrum of genes induced in the two species in response to the same injury. At later sampling times, Acomys shows reduced immunoreactivity for collagen IV and GFAP, which are associated with spinal scarring and fibrosis, and reduced immunoreactivity for IBA1 (also known as AIF1), again suggesting a reduced immune response. These initial studies need to be extended to determine whether the reduced immune and fibrotic response in Acomys results in improved axonal regrowth across the damage site and improved outcomes, as it does in the regenerative situations described above.
Insights into the mechanisms of regeneration
Stem cells
Many regenerative processes are associated with the presence of stem cells, for example the satellite cells of skeletal muscle or the stem cells of the liver, so it is possible that Acomys has more stem cells than non-regenerating mammals or that a stem cell population is present in an Acomys tissue where none exists in other species. In the one tissue in which this has been examined, the skeletal muscle of the tibialis anterior, neither of these situations pertains (Maden et al., 2018). There were more absolute numbers of satellite cells (Pax7+) in the Acomys muscle fibres, but when corrected for an increased size of fibres and quantitated relative to myonuclei, the same value of satellite cells relative to myonuclei was obtained in both species.
Extrapolating from this very limited data, if there is no difference in the number of stem cells present in Acomys it is possible that the special feature that this organism possesses is the ability to regenerate its stem cell niches and repopulate them. This may be why Acomys can regenerate skeletal muscle after the connective tissue has been removed as well as the fibres themselves. The ability to regenerate and repopulate stem cell niches is clearly seen in the case of regenerating hairs, which contain several stem cell populations in the hair bulge, in the dermal papilla and in the sebaceous glands. As functional hairs regenerate in the Acomys skin, this implies these stem cell populations are regenerated from the basal stem cells that re-epithelialize the wound. Thus, the Acomys epithelium may exhibit unique properties or it may receive unique signals compared with the epidermis from other mammals. Some of these signals are beginning to be identified. For example, the overexpression of Wnt7a in Mus epidermis induces hair follicle regeneration after wounding (Ito et al., 2007) and we see high levels of Wnt7a early during Acomys regeneration (Brant et al., 2019). The induction of Shh in Mus wounds also induces hair follicle regeneration (Lim et al., 2018) and we see induction of Shh during Acomys regeneration (Brant et al., 2019). Uncovering these signals and finding ones that are unique to Acomys will be a fruitful avenue for investigation into why Acomys can regenerate but Mus and humans cannot.
Immune-based regulation
The immune system has long been thought to play a role in regeneration, with an immature system correlating with the regenerative ability of lower vertebrates and the skin regenerative abilities of mammalian fetuses (Mescher and Neff, 2005; Seifert and Maden, 2014). In both larval frogs and mammalian fetal skin, the ability to regenerate is lost during development and this loss correlates with the ability of the immune system to mount an inflammatory response in the damaged tissues. We might therefore expect Acomys to generate an immature or embryonic-like immune response to wounding.
In each of the regenerating systems discussed above, there is undoubtedly a blunted cytokine and macrophage response. In skin wounds, several cytokine genes such as Cxcl3, Cxcl5, IL1b, Cxcl1 are massively upregulated in Mus (at least at the gene level) compared with undamaged levels, but this does not happen in Acomys, which expresses far fewer pro-inflammatory cytokines (Brant et al., 2015, 2019). Furthermore, the anti-inflammatory molecule IL10 is strongly upregulated in Acomys wounds, over 100-fold compared with Mus wounds (Brant et al., 2019). The macrophage response is also different. In Mus, the early wound is infiltrated with large numbers of both M1 and M2 macrophages, whereas the regenerating Acomys dermis displays highly reduced numbers of macrophages (Brant et al., 2015). There are plenty of macrophages present in the underlying fascia and at wound margins but, even then, there is a dearth of F4/80 macrophages and reduced numbers of IBA1 macrophages, both of which are pro-inflammatory.
A cytokine analysis in the regenerating ear similarly identified IL6, CCL2 and CXCL1 expressed at higher levels in Mus fibrotic wounds, whereas regenerating Acomys ears exhibit higher levels of IL12 and IL17 (Gawriluk et al., 2019 preprint). There is also a different macrophage profile in the regenerating ear blastema of Acomys compared with Mus (Simkin et al., 2017). It is nearly devoid of classically activated (M1) macrophages, as marked by CD86 staining, but shows plenty of M2 macrophages, as marked by CD206 staining. There is still an inflammatory phase in Acomys (IL12 and IL17), which is also marked by a more robust production of reactive oxygen species from macrophages and there is a strong influx of CD3+ T cells showing the characteristics of activated cytotoxic and regulatory T cells (Gawriluk et al., 2019 preprint).
In the regenerating Acomys skeletal muscle, kidney and spinal cord the story is the same: a reduced inflammatory cytokine response, reduced numbers of pro-inflammatory M1 macrophages and many pro-regenerative M2 macrophages are observed. Importantly, when macrophages are depleted from the regenerating ear, regeneration is blocked, showing that they have a crucial, positive role in regeneration, specifically in histolysis and re-epithelialization during the early phases of regeneration (Simkin et al., 2017). Therefore, it is likely that the macrophage phenotype plays a role in Acomys regeneration, guiding regeneration rather than fibrosis following tissue damage.
The reduced cytokine response and the reduced macrophage response may be responsible for the regeneration versus scarring seen in Acomys versus Mus. If this is the case, then suppression of the inflammatory response in Mus, or the deletion of cytokines, could be used as strategies to generate an improved regenerative response, and there are indeed some good examples of this (Ferreira et al., 2006).
Biomechanics
Another aspect of cell biology that, surprisingly, interacts with the cytokine response concerns the ECM. It was originally observed that Acomys skin is weak and tears easily (Seifert et al., 2012) and we now know that this is a property not only of the skin but of the internal tissues as well (Maden et al., 2018). In addition, the Acomys skin wound ECM has a different composition to that of Mus, supporting the idea that there is a pro-regenerative matrix, a concept which is a guiding principle of attempts to use artificial matrices to induce regeneration: a pro-regenerative matrix ideally generates a pro-regenerative microenvironment that promotes tissue regeneration (He et al., 2018). It is often assumed that a pro-regenerative matrix contains growth factors that can promote regeneration, but it is also possible that the biomechanical properties of the matrix itself are responsible for a reduced inflammatory response and consequent regeneration. There are several examples of the relationship between biomechanical forces and stem cell differentiation, but there is also a relationship between biomechanical forces on a wound and cytokine induction. For example, it has long been known that decreasing the mechanical forces on wounds decreases scar formation and, conversely, that increasing mechanical forces increases scarring (Wong et al., 2012). Mechanical forces from the matrix are transmitted to the cell via cell-surface integrins and relayed to the actin cytoskeleton via a molecule called focal adhesion kinase (FAK; PTK2). Increasing mechanical forces on a wound increases FAK activation. Remarkably, FAK also modulates cytokine and immune signalling; FAK-knockout wounds have reduced levels of macrophage chemotactic protein (MCP-1; Mcpt1) and Ccr2 (the cell surface receptor for MCP-1), and reduced levels of F4/80 macrophages (Wong et al., 2012), precisely the phenotype of Acomys skin wounds.
Thus, it is possible that the evolution of a weak skin phenotype, which is thought to help Acomys escape from predators, may have had unexpected consequences on the regeneration of tissues. Moving forward, we should aim to learn from evolution and apply this knowledge to understand how to induce regeneration in regeneration-incompetent mammals.
Conclusions
A. cahirinus is a mammalian model organism that has been used in research for more than 60 years, but it has only recently been discovered that it shows striking powers of regeneration (Fig. 3, Table 2). The reason for this regenerative potential may be because Acomys does not fibrose in response to damage, as most mammals do. Uncovering the molecular and cellular basis of this lack of fibrosis and learning how to prevent it in other mammals such as humans may lead to the discovery of therapies for the induction of regeneration. But before this can happen, the full power of modern molecular and genetic techniques needs to be applied. Although the Acomys genome has been sequenced and transcriptomes published, techniques for manipulating the genome need to be developed to generate transgenic animals, which have been valuable in unravelling gene function in the laboratory mouse. Moreover, although this new species has the advantages associated with small rodents as laboratory models, it should be noted that the small number of embryos produced per litter and the lengthy gestation time remain as obstacles to rapid progress in understanding regeneration.
Moving forward, it would be fascinating to survey the regenerative ability of the close relatives of Acomys, namely Rudd's mouse (Uranomys), the Congo forest mouse or link rat (Deomys), and the brush-furred mouse (Lophuromys), which are members of the same subfamily of Deomyinae (Fig. 1), to determine whether the regenerative properties described above have only evolved in the genus Acomys or are present throughout the subfamily. In the same regard, the closest subfamily, the Gerbillinae, which includes gerbils, have been used extensively in research, especially in studying the central nervous system, but there is no evidence from the literature that there is any enhanced regeneration potential compared with the typical mammal; indeed there is an anecdotal report that gerbils do not regenerate a hole punched through the ear (Goss, 1980). At present, it thus appears that only the genus Acomys has the ability to regenerate tissues. However, this comparative approach is certainly a valuable one towards understanding the evolution of regenerative ability in mammals and, as emphasized earlier, it may be that there are other previously unrecognized regenerative species out there waiting to be discovered.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Work in the authors’ lab is funded by: National Science Foundation grants 1558017, 1636007; National Institutes of Health grant 1R21 0D023210; W.M. Keck Foundation grant awarded to M.M.; and a Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung Early Postdoc. Mobility Fellowship (P2BEP3_181707) awarded to J.A.V. Deposited in PMC for release after 12 months.
References
- Aghová T., Palupčíková K., Šumbera R., Frynta D., Lavrenchenko L. A., Meheretu Y., Sádlová J., Votýpka J., Mbau J. S., Modrý D. et al. (2019). Multiple radiations of spiny mice (Rodentia: Acomys) in dry open habitats of Afro-Arabia: evidence from a multi-locus phylogeny. BMC Evol. Biol. 19, 69 10.1186/s12862-019-1380-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate D. M. A. (1903). On the occurrence of Acomys in Cyprus. Ann. Mag. Nat. Hist. 11, 565-567. 10.1080/00222930308678817 [DOI] [Google Scholar]
- Bellofiore N. and Evans J. (2019). Monkeys, mice and menses: the bloody anomaly of the spiny mouse. J. Assist. Reprod. Genet. 36, 811-817. 10.1007/s10815-018-1390-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellofiore N., Ellery S. J., Mamrot J., Walker D. W., Temple-Smith P. and Dickinson H. (2017). First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am. J. Obstet. Gynecol. 216, 40.e1-40.e11. 10.1016/j.ajog.2016.07.041 [DOI] [PubMed] [Google Scholar]
- Bellofiore N., Rana S., Dickinson H., Temple-Smith P. and Evans J. (2018). Characterization of human-like menstruation in the spiny mouse: comparative studies with the human and induced mouse model. Hum. Reprod. 33, 1715-1726. 10.1093/humrep/dey247 [DOI] [PubMed] [Google Scholar]
- Bonhote J. (1911). Exhibition of and remarks upon a young Cairo Spiny Mouse (Acomys cahirinus). Proc. Zool. Soc. Lond. I 5, 5-6. [Google Scholar]
- Brant J. O., Lopez M.-C., Baker H. V., Barbazuk W. B. and Maden M. (2015). A comparative analysis of gene expression profiles during skin regeneration in Mus and Acomys. PLoS ONE 10, e0142931 10.1371/journal.pone.0142931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant J. O., Yoon J. H., Polvadore T., Barbazuk W. B. and Maden M. (2016). Cellular events during scar-free skin regeneration in the spiny mouse, Acomys. Wound Repair. Regen. 24, 75-88. 10.1111/wrr.12385 [DOI] [PubMed] [Google Scholar]
- Brant J. O., Boatwright J. L., Davenport R., Sandoval A. G. W., Maden M. and Barbazuk W. B. (2019). Comparative transcriptomic analysis of dermal wound healing reveals de novo skeletal muscle regeneration in Acomys cahirinus. PLoS ONE 14, e0216228 10.1371/journal.pone.0216228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunjes P. C. (1989). A comparative study of prenatal development in the olfactory bulb, neocortex and hippocampal region of the precocial mouse Acomys cahirinus and rat. Dev. Brain Res. 49, 7-25. 10.1016/0165-3806(89)90055-2 [DOI] [PubMed] [Google Scholar]
- Brunjes P. C., Korol D. L. and Stern K. G. (1989). Prenatal neurogenesis in the telencephalon of the precocial mouse Acomys cahirinus. Neurosci. Lett. 107, 114-119. 10.1016/0304-3940(89)90801-X [DOI] [PubMed] [Google Scholar]
- Buckley G., Metcalfe A. D. and Ferguson M. W. J. (2011). Peripheral nerve regeneration in the MRL/MpJ ear wound model. J. Anat. 218, 163-172. 10.1111/j.1469-7580.2010.01313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M. and Hockman J. (1978). Immunocytochemistry of the hypothalamo-neurohypophyseal system in the common spiny mouse, Acomys cahirinus. Neurosecretion and Neuroendocrine Activity 135-137. 10.1007/978-3-642-66885-2_40 [DOI] [Google Scholar]
- Chargé S. B. P. and Rudnicki M. A. (2004). Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 84, 209-238. 10.1152/physrev.00019.2003 [DOI] [PubMed] [Google Scholar]
- Chevret P., Denys C., Jaeger J. J., Michaux J. and Catzeflis F. M. (1993). Molecular evidence that the spiny mouse (Acomys) is more closely related to gerbils (Gerbillinae) than to true mice (Murinae). Proc. Natl. Acad. Sci. USA 90, 3433-3436. 10.1073/pnas.90.8.3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciciliot S. and Schiaffino S. (2010). Regeneration of mammalian skeletal muscle: basic mechanisms and clinical implications. Curr. Pharm. Des. 16, 906-914. 10.2174/138161210790883453 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Mende D., Willms B. and Soeling H. D. (1970). Vascular basement membrane thickness in muscle of spiny mice and activities of glycolysis and gluconeogenesis in the liver of animals with spontaneous and experimental diabetes and of untreated human diabetics. Diabetologia 6, 356-360. 10.1007/BF01212249 [DOI] [PubMed] [Google Scholar]
- Dewsbury D. A. and Hodges A. W. (1987). Copulatory behavior and related phenomena in spiny mice (Acomys cahirinus) and hopping mice (Notomys alexis). J. Mammal. 68, 49-57. 10.2307/1381044 [DOI] [Google Scholar]
- Dickinson H. and Walker D. W. (2007). Managing a colony of spiny mice (Acomys cahirinus) for perinatal research. ANZCCART News 20, 4-11. [Google Scholar]
- Dickinson H., Walker D. W., Cullen-McEwen L., Wintour E. M. and Moritz K. (2005). The spiny mouse (Acomys cahirinus) completes nephrogenesis before birth. Am. J. Physiol. Renal Physiol. 289, F273-F279. 10.1152/ajprenal.00400.2004 [DOI] [PubMed] [Google Scholar]
- Dieterlen F. (1961). Beitrage zur Biologie der Stachelmaus, Acomys cahirinus dimidiatus Cretzschmar. Zeitschrift für Saugertierkünde 16, 1-13. [Google Scholar]
- Farkas J. E., Freitas P. D., Bryant D. M., Whited J. L. and Monaghan J. R. (2016). Neuregulin-1 signaling is essential for nerve-dependent axolotl limb regeneration. Development 143, 2724-2731. 10.1242/dev.133363 [DOI] [PubMed] [Google Scholar]
- Fausto N., Campbell J. S. and Riehle K. J. (2012). Liver regeneration. J. Hepatol. 57, 692-694. 10.1016/j.jhep.2012.04.016 [DOI] [PubMed] [Google Scholar]
- Ferreira A. M., Takagawa S., Fresco R., Zhu X., Varga J. and DiPietro L. A. (2006). Diminished induction of skin fibrosis in mice with MCP-1 deficiency. J. Investig. Dermatol. 126, 1900-1908. 10.1038/sj.jid.5700302 [DOI] [PubMed] [Google Scholar]
- Frynta D., Fraňková M., Čížková B., Skarlandtová H., Galeštoková K., Průšová K., Šmilauer P. and Šumbera R. (2011). Social and life history correlates of litter size in captive colonies of precocial spiny mice (Acomys). Acta Theriol. 56, 289-295. 10.1007/s13364-011-0024-2 [DOI] [Google Scholar]
- Gawriluk T. R., Simkin J., Thompson K. L., Biswas S. K., Clare-Salzler Z., Kimani J. M., Kiama S. G., Smith J. J., Ezenwa V. O. and Seifert A. W. (2016). Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat. Commun. 7, 11164 10.1038/ncomms11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawriluk T. R., Simkin J., Hacker C. K., Kimani J. M., Kiama S. G., Ezenwa V. O. and Seifert A. W. (2019). Mammalian musculoskeletal regeneration is associated with reduced inflammatory cytokines and an influx of T cells. bioRxiv 723783 10.1101/723783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Kwon O., Zhang Z., Spata M., Plikus M. V., Holler P. D., Ito M., Yang Z., Treffeisen E., Kim C. D. et al. (2013). Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19, 916-923. 10.1038/nm.3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss R. J. (1980). Prospects for regeneration in man. Clin. Orthop. Relat. Res. 151, 270-282. 00003086-198009000-00038 [PubMed] [Google Scholar]
- Goss R. J. (1983). Deer Antlers Regeneration, Function, and Evolution. 1st edn Elsevier. [Google Scholar]
- Goss R. J. (1987). Why mammals don't regenerate—or do they? Physiology 2, 112-115. 10.1152/physiologyonline.1987.2.3.112 [DOI] [Google Scholar]
- Guo B., Yan H., Li L., Yin K., Ji F. and Zhang S. (2017). Collagen triple helix repeat containing 1 (CTHRC1) activates Integrin β3/FAK signaling and promotes metastasis in ovarian cancer. J. Ovarian. Res. 10, 69 10.1186/s13048-017-0358-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsen C. R., Kvicerova C. R., Dickinson H. and Heller R. S. (2009). Acomys, the closest relatives to Gerbils, do express Pdx-1 protein and have similar islet morphology to Gerbils. Islets 1, 191-197. 10.4161/isl.1.3.9557 [DOI] [PubMed] [Google Scholar]
- Haughton C. L., Gawriluk T. R. and Seifert A. W. (2016). The biology and husbandry of the african spiny mouse (Acomys cahirinus) and the research uses of a laboratory colony. J. Am. Assoc. Lab. Anim. Sci. 55, 9-17. [PMC free article] [PubMed] [Google Scholar]
- He C., Yang Z., Jin Y., Qi X., Chu J. and Deng X. (2018). ADM scaffolds generate a pro-regenerative microenvironment during full-thickness cutaneous wound healing through M2 macrophage polarization via lamtor1. Front. Physiol. 9, 657 10.3389/fphys.2018.00657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia C. C. W. (2017). Comparative analysis of the mechanical signals in lung development and compensatory growth. Cell Tissue Res. 367, 687-705. 10.1007/s00441-016-2558-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hułas-Stasiak M. and Gawron A. (2007). Immunohistochemical localization of estrogen receptors ERα and ERβ in the spiny mouse (Acomys cahirinus) ovary during postnatal development. J. Mol. Histol. 38, 25-32. 10.1007/s10735-006-9072-3 [DOI] [PubMed] [Google Scholar]
- Hułas-Stasiak M. and Gawron A. (2010). Distribution of androgen and progesterone receptors in the spiny mouse (Acomys cahirinus) ovary during postnatal life. Repro. Biol. 10, 37-51. 10.1016/S1642-431X(12)60036-9 [DOI] [PubMed] [Google Scholar]
- Hułas-Stasiak M. and Gawron A. (2011). Follicular atresia in the prepubertal spiny mouse (Acomys cahirinus) ovary. Apoptosis 16, 967 10.1007/s10495-011-0626-9 [DOI] [PubMed] [Google Scholar]
- Hutton L. C., Shields A. and Walker W. (2009a). Neuropathology and functional deficits in a model of birth asphyxia in the precocial spiny mouse (Acomys cahirinus). Dev. Neurosci. 31, 523-535. 10.1159/000251907 [DOI] [PubMed] [Google Scholar]
- Hutton L. C., Abbass M., Dickinson H., Ireland Z. and Walker D. W. (2009b). Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus). Dev. Neurosci. 31, 437-451. 10.1159/000232562 [DOI] [PubMed] [Google Scholar]
- Illingworth C. M. (1974). Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 9, 853-858. 10.1016/S0022-3468(74)80220-4 [DOI] [PubMed] [Google Scholar]
- Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S. E. and Cotsarelis G. (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316-320. 10.1038/nature05766 [DOI] [PubMed] [Google Scholar]
- Janus C. (1988). The development of responses to naturally occurring odours in spiny mice Acomys cahirinus. Anim. Behav. 36, 1400-1406. 10.1016/S0003-3472(88)80210-0 [DOI] [Google Scholar]
- Janus C. (1993). Stability of preference for odors after short-term exposure in young spiny mice. Dev. Psychobiol. 26, 65-79. 10.1002/dev.420260106 [DOI] [PubMed] [Google Scholar]
- Jeremy P. and Bates J. (1994). The distribution of acomys (rodentia: Muridae) in Africa and Asia. Isr. J. Zool. 40, 199-214. [Google Scholar]
- Jiang T.-X., Harn H. I.-C., Ou K.-L., Lei M. and Chuong C.-M. (2019). Comparative regenerative biology of spiny (Acomys cahirinus) and laboratory (Mus musculus) mouse skin. Exp. Dermatol. 28, 442-449. 10.1111/exd.13899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joven A., Elewa A. and Simon A. (2019). Model systems for regeneration: salamanders. Development 146, dev167700 10.1242/dev.167700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers W. H., Mooren P. G., Griep H., Endert E., Degenhart H. J. and Charles R. (1986). Hormones in perinatal rat and spiny mouse: relation to altricial and precocial timing of birth. Am. J. Physiol. 251, E78-E85. 10.1152/ajpcell.1986.251.1.C78 [DOI] [PubMed] [Google Scholar]
- Lim C. H., Sun Q., Ratti K., Lee S.-H., Zheng Y., Takeo M., Lee W., Rabbani P., Plikus M. V., Cain J. E. et al. (2018). Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 9, 4903 10.1038/s41467-018-07142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M. (2018). Optimal skin regeneration after full thickness thermal burn injury in the spiny mouse, Acomys cahirinus. Burns 44, 1509-1520. 10.1016/j.burns.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Maden M., Brant J. O., Rubiano A., Sandoval A. G. W., Simmons C., Mitchell R., Collin-Hooper H., Jacobson J., Omairi S. and Patel K. (2018). Perfect chronic skeletal muscle regeneration in adult spiny mice, Acomys cahirinus. Sci. Rep. 8, 8920 10.1038/s41598-018-27178-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin J. W. and Porter R. H. (1984). Paternal behavior in the spiny mouse (Acomys cahirinus). Behav. Neural Biol. 41, 135-151. 10.1016/S0163-1047(84)90513-2 [DOI] [PubMed] [Google Scholar]
- Mamrot J., Gardner D. K., Temple-Smith P. and Dickinson H. (2018). Embryonic gene transcription in the spiny mouse (Acomys cahirinus): an investigation into the embryonic genome activation. bioRxiv 280412 10.1101/280412 [DOI] [Google Scholar]
- Marques I. J., Lupi E. and Mercader N. (2019). Model systems for regeneration: zebrafish. Development 146, dev167692 10.1242/dev.167692 [DOI] [PubMed] [Google Scholar]
- Matias Santos D., Rita A. M., Casanellas I., Brito Ova A., Araújo I. M., Power D. and Tiscornia G. (2016). Ear wound regeneration in the African spiny mouse Acomys cahirinus. Regeneration 3, 52-61. 10.1002/reg2.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher A. L. and Neff A. W. (2005). Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 93, 39-66. 10.1007/b99966 [DOI] [PubMed] [Google Scholar]
- Millar S. E. (2002). Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol. 118, 216-225. 10.1046/j.0022-202x.2001.01670.x [DOI] [PubMed] [Google Scholar]
- Montandon S. A., Tzika A. C., Martins A. F., Chopard B. and Milinkovitch M. C. (2014). Two waves of anisotropic growth generate enlarged follicles in the spiny mouse. EvoDevo 5, 33 10.1186/2041-9139-5-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musarò A. (2014). The basis of muscle regeneration. Adv. Biol. 2014, 612471 10.1155/2014/612471 [DOI] [Google Scholar]
- Nováková M., Palme R., Kutalová H., Janský L. and Frynta D. (2008). The effects of sex, age and commensal way of life on levels of fecal glucocorticoid metabolites in spiny mice (Acomys cahirinus). Physiol. Behav. 95, 187-193. 10.1016/j.physbeh.2008.05.017 [DOI] [PubMed] [Google Scholar]
- Okamura D. M., Brewer C. M., Wakenight P., Bahrami N., Bernardi K., Tran A., Olson J., Shi X., Piliponsky A. M., Nelson B. R. et al. (2018). Scarless repair of acute and chronic kidney injury in African Spiny mice (Acomys cahirinus). bioRxiv 315069 10.1101/315069 [DOI] [Google Scholar]
- Oosterhuis W. P., Mooren P. G., Charles R. and Lamers W. H. (1984). Perinatal Development of the lung in rat and spiny mouse: its relation to altricial and precocial timing of birth. Neonatology 45, 236-243. 10.1159/000242011 [DOI] [PubMed] [Google Scholar]
- Pasco R., Gardner D. K., Walker D. W. and Dickinson H. (2012). A superovulation protocol for the spiny mouse (Acomys cahirinus). Reprod. Fertil. Dev. 24, 1117 10.1071/RD12044 [DOI] [PubMed] [Google Scholar]
- Peitz B. (1981). The oestrous cycle of the spiny mouse (Acomys cahirinus). J. Reprod. Fertil. 61, 453-459. 10.1530/jrf.0.0610453 [DOI] [PubMed] [Google Scholar]
- Peitz B., Foreman D. and Schmitt M. (1979). The reproductive tract of the male spiny mouse (Acomys cahirinus) and coagulation studies with other species. J. Reprod. Fertil. 57, 183-188. 10.1530/jrf.0.0570183 [DOI] [PubMed] [Google Scholar]
- Pennello A., Taylor J., Matlack R., Karp J. and Riggs J. (2006). Spiny mice (Acomys cahirinus) do not respond to thymus-independent type 2 antigens. Dev. Comp. Immunol. 30, 1181-1190. 10.1016/j.dci.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Pictet R., Orci L., Gonet A. E., Rouiller C. and Renold A. E. (1967). Ultrastructural studies of the hyperplastic islets of langerhans of spiny mice (Acomys cahirinus) before and during the development of hyperglycemia. Diabetologia 3, 188-211. 10.1007/BF01222197 [DOI] [PubMed] [Google Scholar]
- Pinheiro G., Prata D. F., Araújo I. M. and Tiscornia G. (2018). The African spiny mouse (Acomys spp.) as an emerging model for development and regeneration. Lab. Anim. 52, 565-576. 10.1177/0023677218769921 [DOI] [PubMed] [Google Scholar]
- Porrello E. R., Mahmoud A. I., Simpson E., Hill J. A., Richardson J. A., Olson E. N. and Sadek H. A. (2011). Transient regenerative potential of the neonatal mouse heart. Science 331, 1078-1080. 10.1126/science.1200708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. H. (1976). Sex-differences in the agonistic behavior of spiny-mice (Acomys cahirinus). Z. Tierpsychol. 40, 100-108. 10.1111/j.1439-0310.1976.tb00928.x [DOI] [PubMed] [Google Scholar]
- Porter R. H. and Etscorn F. (1976). A sensitive period for the development of olfactory preference in Acomys cahirinus. Physiol. Behav. 17, 127-130. 10.1016/0031-9384(76)90278-X [DOI] [PubMed] [Google Scholar]
- Porter R. H., Deni R. and Doane H. M. (1977). Responses of Acomys cahirinus pups to chemical cues produced by a foster species. Behav. Biol. 20, 244-251. 10.1016/S0091-6773(77)90812-4 [DOI] [PubMed] [Google Scholar]
- Porter R. H., Wyrick M. and Pankey J. (1978a). Sibling recognition in spiny mice (Acomys cahirinus). Behav. Ecol. Sociobiol. 3, 61-68. 10.1007/BF00300046 [DOI] [Google Scholar]
- Porter R. H., Doane H. M. and Cavallaro S. A. (1978b). Temporal parameters of responsiveness to maternal pheromone in Acomys cahirinus. Physiol. Behav. 21, 563-566. 10.1016/0031-9384(78)90131-2 [DOI] [PubMed] [Google Scholar]
- Porter R. H., Cavallaro S. A. and Moore J. D. (1980). Developmental parameters of mother-offspring interactions in Acomys cahirinus. Z. Tierpsychol. 53, 153-170. 10.1111/j.1439-0310.1980.tb01047.x [DOI] [Google Scholar]
- Porter R. H., Moore J. D. and White D. M. (1981). Food sharing by sibling vs nonsibling spiny mice (Acomys cahirinus). Behav. Ecol. Sociobiol. 8, 207-212. 10.1007/BF00299832 [DOI] [Google Scholar]
- Porter R. H., Tepper V. J., Baumeister A. A., Cernoch J. M. and Matochik J. A. (1982). Interactions among unfamiliar spiny mouse (Acomys cahirinus) weanlings. Behav. Neural Biol. 34, 190-200. 10.1016/S0163-1047(82)91574-6 [DOI] [Google Scholar]
- Porter R. H., Cernoch J. M. and Matochik J. A. (1983). Littermate influences on behavioral development in Acomys cahirinus and Mus musculus. Z. Tierpsychol. 62, 93-104. 10.1111/j.1439-0310.1983.tb02144.x [DOI] [Google Scholar]
- Porter R. H., Matochik J. A. and Makin J. W. (1986). Discrimination between full-sibling spiny mice (Acomys cahirinus) by olfactory signatures. Anim. Behav. 34, 1182-1188. 10.1016/S0003-3472(86)80178-6 [DOI] [Google Scholar]
- Porter R. H., McFadyen-Ketchum S. A. and King G. A. (1989). Underlying bases of recognition signatures in spiny mice, Acomys cahirinus. Anim. Behav. 37, 638-644. 10.1016/0003-3472(89)90042-0 [DOI] [Google Scholar]
- Pyagay P., Heroult M., Wang Q., Lehnert W., Belden J., Liaw L., Cramer R. E. and Lindner V. (2005). Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ. Res. 96, 261-268. 10.1161/01.RES.0000154262.07264.12 [DOI] [PubMed] [Google Scholar]
- Quinn T. A., Ratnayake U., Dickinson H., Nguyen T.-H., McIntosh M., Castillo-Melendez M., Conley A. J. and Walker D. W. (2013). Ontogeny of the adrenal gland in the spiny mouse, with particular reference to production of the steroids cortisol and dehydroepiandrosterone. Endocrinology 154, 1190-1201. 10.1210/en.2012-1953 [DOI] [PubMed] [Google Scholar]
- Robinson S. R. and Smotherman W. P. (1992). Behavioral response of altricial and precocial rodent fetuses to acute umbilical cord compression. Behav. Neural Biol. 57, 93-102. 10.1016/0163-1047(92)90581-N [DOI] [PubMed] [Google Scholar]
- Saxena S., Vekaria H., Sullivan P. G., Seifert A. W. (2019). Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Communications 10, 4400 10.1038/s41467-019-12398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert A. W. and Maden M. (2014). New insights into vertebrate skin regeneration. Intl. Rev. Cell Mol. Biol. 310, 129-169. 10.1016/B978-0-12-800180-6.00004-9 [DOI] [PubMed] [Google Scholar]
- Seifert A. W., Kiama S. G., Seifert M. G., Goheen J. R., Palmer T. M. and Maden M. (2012). Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 489, 561-565. 10.1038/nature11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafrir E., Ziv E. and Kalman R. (2006). Nutritionally induced diabetes in desert rodents as models of type 2 diabetes. ILAR J. 47, 212-224. 10.1093/ilar.47.3.212 [DOI] [PubMed] [Google Scholar]
- Shargal E., Rath-Wolfson L., Kronfeld N. and Dayan T. (1999). Ecological and histological aspects of tail loss in spiny mice (Rodentia: Muridae, Acomys) with a review of its occurrence in rodents. J. Zool. 249, 187-193. 10.1111/j.1469-7998.1999.tb00757.x [DOI] [Google Scholar]
- Shkolnik A. and Borut A. (1969). Temperature and water relations in two species of spiny mice (Acomys). J. Mammal. 50, 245-255. 10.2307/1378340 [DOI] [Google Scholar]
- Simkin J., Gawriluk T. R., Gensel J. C. and Seifert A. W. (2017). Macrophages are necessary for epimorphic regeneration in African spiny mice. eLife 6, e24623 10.7554/eLife.24623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan S. J. and Schenk J. J. (2017). Muroid rodent phylogenetics: 900-species tree reveals increasing diversification rates. PLoS ONE 12, e0183070 10.1371/journal.pone.0183070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. C., Serrano P. N., Rubiano A., Yokosawa R., Sandler J., Mukhtar M., Brant J. O., Maden M. and Simmons C. S. (2018). Unique behavior of dermal cells from regenerative mammal, the African Spiny Mouse, in response to substrate stiffness. J. Biomech. 81, 149-154. 10.1016/j.jbiomech.2018.10.005 [DOI] [PubMed] [Google Scholar]
- Stocum D. L. (2019). Nerves and proliferation of progenitor cells in limb regeneration. Dev. Neurobiol. 79, 468-478. 10.1002/dneu.22643 [DOI] [PubMed] [Google Scholar]
- Strasser H. (1968). A breeding program for spontaneously diabetic experimental animals: Psammomys obesus (sand rat) and Acomys cahirinus (spiny mouse). Lab. Anim. Care 18, 328-338. [PubMed] [Google Scholar]
- Streeter K. A., Sunshine M. D., Brant J. O., Sandoval M. A. G. W., Maden M. and Fuller D. D. (2019). Molecular and histologic outcomes following spinal cord injury in spiny mice, Acomys cahirinus. J. Comp. Neurol. [Epub ahead of print]. 10.1002/cne.24836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A., Fukada S., Yamamoto N., Takeda S. and Tsuchida K. (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143-152. 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M. M., Miyagoe-Suzuki Y. et al. (2011). Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell. Sci. 124, 3654-3664. 10.1242/jcs.086629 [DOI] [PubMed] [Google Scholar]
- Voronstova M. and Liosner L. (1960). Asexual Propogation and Regeneration. Oxford: Pergamon Press. [Google Scholar]
- Williams-Boyce P. K. and Daniel J. C. (1986). Comparison of ear tissue regeneration in mammals. J. Anat. 149, 55-63. [PMC free article] [PubMed] [Google Scholar]
- Wong V. W., Rustad K. C., Akaishi S., Sorkin M., Glotzbach J. P., Januszyk M., Nelson E. R., Levi K., Paterno J., Vial I. N. et al. (2012). Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat. Med. 18, 148-152. 10.1038/nm.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates C. C., Hebda P. and Wells A. (2012). Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Res. C Embryo Today 96, 325-333. 10.1002/bdrc.21024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. A. B. (1976). Breeding and fertility of the Egyptian spiny mouse, Acomys cahirinus: effect of different environments. Lab. Anim. 10, 15-24. 10.1258/002367776780948961 [DOI] [PubMed] [Google Scholar]
- Yu L., Dawson L. A., Yan M., Zimmel K., Lin Y.-L., Dolan C. P., Han M. and Muneoka K. (2019). BMP9 stimulates joint regeneration at digit amputation wounds in mice. Nat. Commun. 10, 424 10.1038/s41467-018-08278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]