Abstract
Objective:
Klotho is an anti-aging gene that shortens lifespan when disrupted and extends lifespan when overexpressed. This study investigated whether autophagy plays a role in Klotho gene deficiency-induced arterial stiffening and hypertension.
Methods:
Klotho mutant heterozygous (KL+/−) mice and age- and sex-matched wild-type (WT) mice were used. Arteries were examined for autophagy using Western blot assays.
Results:
Pulse wave velocity (PWV), a direct measure of arterial stiffness, and blood pressure (BP) increased significantly in KL (+/−) mice. The autophagy level, as measured by LC3-II expression and autophagy flux, increased in aortas of KL (+/−) mice, indicating that Klotho gene deficiency upregulated autophagy. Chloroquine diminished Klotho gene deficiency-induced increases in PWV and BP and eliminated the upregulation of autophagic flux in KL (+/−) mice. Klotho gene deficiency-induced arterial stiffness was accompanied by upregulation of MMP9, TGFβ−1, TGFβ−3, RUNX2, and ALP, but these changes were effectively mitigated by chloroquine. Chloroquine also halted an increase in scleraxis expression in aortas of Klotho (+/−) mice. In cultured mouse aortic smooth muscle cells, Klotho gene deficiency increased autophagy, leading to upregulation of scleraxis, a key transcription factor of collagen synthesis. Klotho gene deficiency failed to upregulate scleraxis expression when autophagy was inhibited, suggesting that autophagy is a critical mediator of Klotho gene deficiency-induced upregulation of scleraxis.
Conclusion:
Suppression of enhanced autophagy by chloroquine lessens Klotho gene deficiency-induced arterial stiffening and hypertension by stopping upregulation of MMP9 and scleraxis. The enhanced autophagic activity plays a crucial role in Klotho gene deficiency-induced arterial stiffening and hypertension.
Keywords: autophagy, Beclin-1, arterial stiffness, hypertension, chloroquine, scleraxis
CONDENSED ABSTRACT
Haplodeficiency of Klotho gene induces autophagy, which is injurious to vasculature and causes arterial stiffening and hypertension. Our study found that suppressing enhanced autophagy by chloroquine diminished arterial stiffness and hypertension in KL+/− mice. Therefore, we suggest that enhanced autophagic activity plays a critical role in the pathogenesis of Klotho deficiency-induced arterial stiffness. Mechanistically, autophagy selectively regulates MMP9 activity, which is necessary for re-organization of collagen by SMCs. Importantly, Klotho deficiency also upregulated expression of scleraxis, an essential transcription factor for collagen synthesis, in an autophagy-dependent manner. Thus, the role of autophagy in Klotho deficiency-induced arterial stiffening and hypertension is mediated through modulation of MMP9 and scleraxis expression.
INTRODUCTION
Autophagy, a primary cellular survival mechanism for lysosomal degradation and recycling of long-lived proteins and organelles, plays an important role in maintaining cell and organ homeostasis and viability under both basal and various stressful conditions including cell starvation.1–3 However, autophagy can also trigger cell death by impairing cardiovascular function in some instances, underscoring its nature as a “double-edged sword” that could be either protective or harmful depending on the cellular environment, the nature and intensity of the stimulus, and the levels of autophagy.4–6 The functional role of autophagy has to be determined individually within specific contexts.
While autophagy can be measured by microtubule-associated proteins 1A/1B light chain 3B (LC3-II) protein expression levels. However, the accumulation of LC3-II could indicate either autophagic activation or a blockage of downstream steps in autophagy such as inefficient fusion or decreased lysosomal degradation.7,8 The mere detection of LC3’s presence is insufficient for a reliable evaluation of the status of the entire autophagic system. The term “autophagic flux” refers to the dynamic process of autophagy. Autophagic flux is often defined as a measure of autophagic degradation activity, and a number of methods are currently utilized to assess autophagic flux.9 Autophagic flux can be detected by LC3-II turnover using western blot analysis in the presence and absence of lysosomal degradation inhibitors such as bafilomycin A1 (BFA).10 P62/SQSTM1, also known as sequestosome 1, is an ubiquitin binding protein that serves as a link between LC3 and ubiquitinated substrates and is efficiently degraded by autophagy.11 P62 accumulates when autophagy is inhibited and decreases when autophagy is activated. Thus, the level of P62 proteins can be used to monitor autophagic flux.
The Klotho gene was originally identified as a presumed aging-suppressor gene in mice that extended lifespan when overexpressed and caused premature aging when disrupted.12–14 Subsequent studies have reported that Klotho was involved in numerous aging-associated pathologies including chronic kidney disease,15 diabetes,16 cancers,17 and cardiovascular diseases.18 As the Klotho level decreases with age19 the prevalence of arterial stiffness and hypertension increases.20 Kitagawa et al. reported that the serum level of Klotho is significantly decreased in patients with arterial stiffness and chronic kidney diseases,21 and our recent study—which focused on how deficiencies in the Klotho gene caused arterial stiffness—also showed that autophagy increased in KL (+/−) mice aortas.22 Arterial stiffness is an independent predictor of negative cardiovascular outcomes such as hypertension, myocardial infarction, cognitive decline in aging, stroke, and kidney diseases.23–27
Chloroquine, a drug used for treating and preventing malaria, has been used as an autophagy inhibitor.28, 29 By blocking the degradation of autophagy, chloroquine leads to an accumulation of ineffective autophagosomes that inhibit autophagic protein degradation. Thus, we hypothesize that inhibition of autophagy by chloroquine eases arterial stiffening and hypertension associated with Klotho gene deficiency.
MATERIALS AND METHODS
Animal Study Protocols
This study was performed according to the guidelines of the National Institute of Health on the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee of University of Oklahoma Health Science Center (13–109-I).
Klotho mutant heterozygous mice (KL (+/−) (aged 20–23 months) and age- and sex-matched wild type (WT) mice were used in this study. The KL (+/−) mice were randomly divided into two subgroups. One mouse subgroup received chloroquine (sigma, 50 mg/kg/day, IP, for 2 weeks, n=10) and the other received an equal volume of saline (n=7). Six WT mice receiving saline were used as the control group. Regular Harlan rodent chow was provided for all mice. Blood pressure was measured weekly. Pulse wave velocity (PWV) was measured after two weeks of treatment. All animals were euthanized and perfused transcardially with phosphate buffered saline (PBS) under deep anesthesia (ketamine/xylazine, 90/10 mg, IP). Aortas were then quickly removed, washed, and cut into pieces for subsequent analyses. For details, please see the online Data Supplement.
Cell Culture and Treatment
MOVAS (ATCC®CRL−2797), a continuous mouse vascular aortic smooth muscle cell (VSMC) line, has been demonstrated to retain a VSMC-like phenotype, including a spindle cell morphology and the expression of VSMC-specific markers such as smooth muscle α-actin and SM22-α. For details, please see the online Data Supplement.
Measurement of Pulse Wave Velocity (PWV)
Aortic PWV was measured as described previously.30 For details, please see the online Data Supplement.
Measurement of Blood pressure (BP)
Blood pressure (BP) was measured by the volume-pressure recording (VPR) tail-cuff method with slight warming (28°C) but not heating of the tail using a CODA® non-invasive BP monitoring system (Kent Scientific). This method has been validated by using a telemetry system.31 Animals were gently handled and trained for the VPR tail-cuff measurement to minimize handling stress. No signs of stress were observed during BP measurements. The operator was also rigorously trained on this measurement procedure. At least twenty stable cycle data were obtained for the result analysis for each measurement. We confirmed in our previous studies that this system is a reliable method for monitoring BP.32, 33
Autophagy Assay
For details, please see the online Data Supplement.
Histological and immunohistochemical staining
Histological and immunohistological staining was performed as described previously.34, 35 For details, please see the online Data Supplement.
Western Blot Analysis
The Western blot procedure was described in our previous studies.36 For details, please see the online Data Supplement.
Measurement of MMP2 and MMP9 activity
For details, please see the online Data Supplement.
Statistical Analysis
Quantitative data were presented as means ± SE. Differences between experimental groups were examined by one-way analysis of variance (ANOVA) followed by a Bonferroni post-test using Prism software (GraphPad) for 3 or more groups or t-test for 2 groups. For all analyses, p<0.05 was considered statistically significant. There were 6–10 mice in each group (WT=6, KL+/−-CON=7, KL+/−-CQ=10).
RESULTS
Chloroquine eliminated increases in arterial pulse wave velocity and BP in Klotho-deficient (KL (+/−) mice
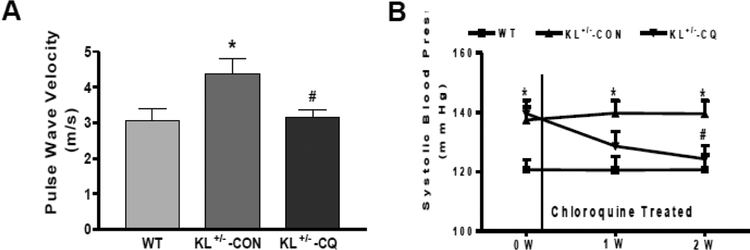
Arterial pulse wave velocity (PWV), a direct measure of arterial stiffness,30 increased significantly (p<0.01) in KL (+/−) mice compared to that of WT mice (Online Fig. 1A), indicating that haplodeficiency of Klotho gene caused arterial stiffening. Autophagic flux, a reliable measure of autophagy, increased significantly (p<0.05) in KL (+/−) mice (Online Fig. 1B), suggesting that Klotho gene deficiency upregulated aortic autophagy.
To investigate whether enhanced autophagy is involved in arterial stiffening, we inhibited autophagy by administering chloroquine (50 mg/kg/day, IP) to KL (+/−) mice. Following a 2-week treatment with chloroquine, we found that PWV decreased significantly in KL (+/−) mice (Fig. 1A). This finding suggests that enhanced autophagy may play an important role in the pathogenesis of Klotho gene deficiency-induced arterial stiffening. Systolic BP in KL (+/−) mice decreased to the level of the WT mice (Fig. 1B) after two weeks of chloroquine treatment. Our results therefore suggest that chloroquine is effective in attenuating Klotho gene deficiency-induced arterial stiffness and hypertension.
Fig. 1. Chloroquine abolished the increase in arterial pulse wave velocity and blood pressure (BP) in Klotho-deficient (KL+/−) mice.
(A) PWV was measured by 10-MHz Doppler probes. (B) Systolic BP was measured by the volume-pressure recording (VPR) tail-cuff method using a CODA 6 BP Monitoring System. Data are expressed as means ± SE and analyzed by one-way ANOVA. n=6–10 (WT=6, KL+/−-CON=7, KL+/−-CQ=10). *p<0.05 vs WT mice; #p<0.05 vs KL (+/−)-CON mice.
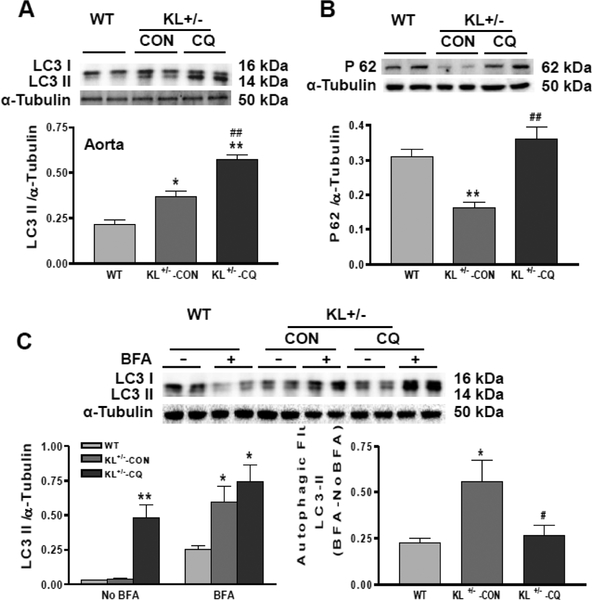
Chloroquine suppressed autophagy in Klotho-deficient mice
Autophagy can be measured by LC3-II expression levels. LC3-II expression levels were increased in KL (+/−) mice aortas (Fig. 2A). This is because chloroquine is a lysosomal degradation inhibitor. Due to inhibition of autophagic degradation, chloroquine led to LC3-II accumulation.
Fig. 2. Chloroquine suppressed autophagy in Klotho-deficient mice (KL+/−).
(A) Western blot analysis of LC3 in the aorta. (B) Western blot analysis of P62 in the aorta. (C) Autophagic flux measured by LC3-II turnover in the presence and absence of lysosomal degradation inhibitor, bafilomycin A1 (BFA) (BFA - NoBFA). Data are expressed as means ± SE and analyzed by one-way ANOVA. n=4. *p<0.05, **p<0.01 vs WT mice; #p<0.05, ##p<0.01 vs KL (+/−)-CON mice.
Autophagy is a highly dynamic, multi-step process. Thus, merely detecting the presence of LC3 is insufficient for evaluation of the entire autophagic system. We therefore assessed autophagic flux by measuring P62 protein levels and LC3-II turnover. The P62 expression level was decreased in the aortas of KL (+/−) mice (Fig. 2B), suggesting that autophagic degradation is increased due to Klotho gene deficiency. Autophagic flux measured by LC3-II turnover in the presence and absence of lysosomal degradation inhibitor bafilomycin A1 was increased in KL (+/−) mice (Fig. 2C). Taken together, our results show that KL (+/−) increases autophagy. However, chloroquine increased P62 expression and decreased autophagic flux measured by LC3-II turnover (Fig. 2B and C) in the aorta of KL (+/−) mice, suggesting that it inhibits autophagy. A limitation of this study is that it did not reveal the localization of changes in LC3-II in the vessel walls.
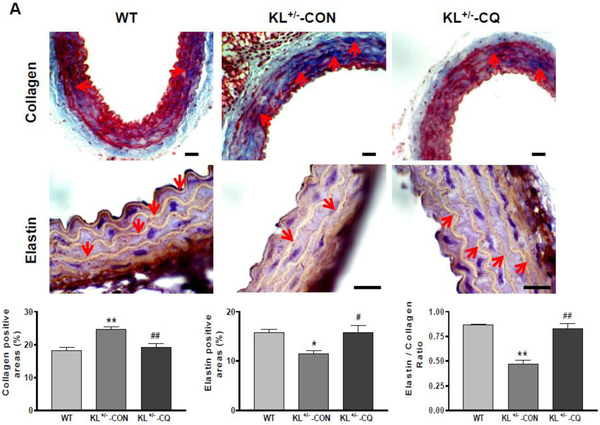
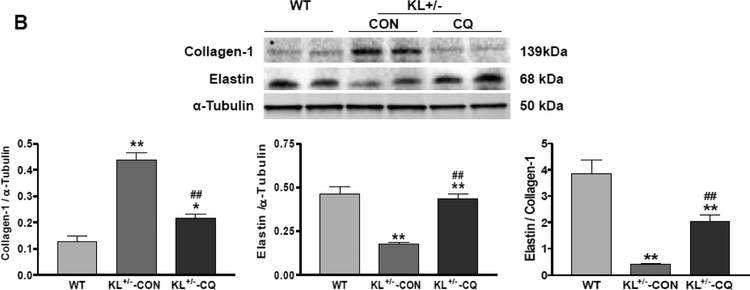
Inhibition of autophagy by chloroquine attenuated the accumulation of collagen and degeneration of elastin in KL (+/−) mice
To investigate the molecular basis of arterial stiffening, we measured arterial collagen and elastin levels through immunostaining and Western blot assays. The immunostaining assay showed that aortic collagen levels increased significantly in KL (+/−) mice (Fig. 3A). Collagen deposition (blue) was mainly found in the medial and adventitial layer of the aorta. On the other hand, aortic elastin levels (brown) decreased significantly in KL (+/−) mice (Fig. 3A). Western blot analysis confirmed that KL (+/−) upregulated collagen I expression but downregulated elastin levels in aortas (Fig. 3B). The ratio of elastin to collagen in aortas was markedly decreased in KL (+/−) mice (Fig. 3A and B), indicating that KL+/− causes arterial remodeling. Due to inhibition of autophagy, chloroquine mitigated the accumulation of collagen and degradation of elastin in aortas, which led to improvements in arterial remodeling in KL (+/−) mice (Fig. 3A and B).
Fig. 3. Inhibition of autophagy by chloroquine attenuated the accumulation of collagen and degeneration of elastin in the aorta of KL (+/−) mice.
(A) Histological and immunohistochemical staining results of collagen and elastin. Scale bars are 50 μm. (B) Western blot analysis of collagen-1 and elastin. Data are expressed as means ± SE and analyzed by one-way ANOVA. n=4. *p<0.05, **p<0.01 vs WT mice; #p<0.05, ##p<0.01 vs KL (+/−)-CON mice.
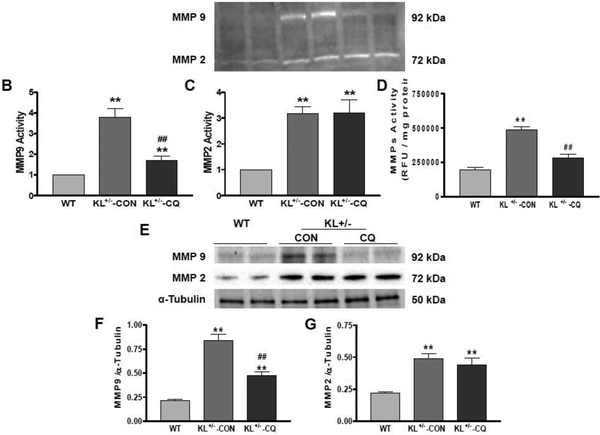
Inhibition of autophagy by chloroquine mitigated the upregulation of arterial MMP activity and expression in KL (+/−) mice
Matrix metalloproteinases (MMPs) are a family of proteases that play important roles in extracellular matrix (ECM) remodeling and degradation. Increased MMP activity could contribute to ECM remodeling and fibrosis. In this study, we measured MMP activity by zymography and found that MMP2 and MMP9 activities increased significantly in aortas of KL (+/−) mice (Fig. 4A–C). Inhibition of autophagy by chloroquine significantly decreased MMP9 activity down to the control level (Fig. 4A and B). However, chloroquine did not affect MMP2 activity (Fig. 4A and C). Zymography results were also supported by ELISA. MMPS activity was increased significantly in aortas of KL (+/−) mice (Fig. 4D). Inhibition of autophagy by chloroquine significantly decreased MMP activity down to the control level (Fig. 4D). Meanwhile, we also measured MMPs expression levels using Western blot analysis and discovered that MMP2 and MMP9 protein expressions increased significantly in aortas of KL (+/−) mice (Fig. 4E–G). Inhibition of autophagy by chloroquine decreased MMP9 expression but did not affect or alter MMP2 expression (Fig. 4E–G). Thus, we concluded that autophagy selectively regulates MMP9 activity and expression.
Fig. 4. Inhibition of autophagy by chloroquine attenuated the upregulation of arterial MMP activity and expression in KL+/− mice.
(A) Representative image of MMPs activity analysis by zymogram PAGE. (B) Quantification of MMP9 activity. (C) Quantification of MMP2 activity. (D) MMPs activity analysis by ELISA. (E) Western blot analysis of MMP9 and MMP2. (F) Quantification of MMP9 protein levels. (G) Quantification of MMP2 protein levels. Data are expressed as means ± SE and analyzed by one-way ANOVA. n=4. **p<0.01 vs WT mice; ##p<0.01 vs KL (+/−)-CON mice.
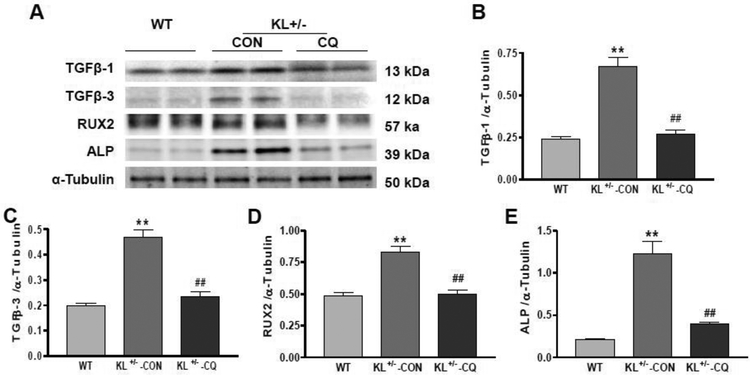
Inhibition of autophagy by chloroquine stopped the upregulation of arterial TGF-β1, TGF-β3, RUNX2 and ALP expression in KL (+/−) mice
Transforming Growth Factor Beta (TGFβ) increases matrix protein synthesis and decreases matrix protein degradation, which results in tissue fibrosis. Runt-related transcription factor 2 (Runx2) and alkaline phosphatase (ALP) are another two markers of fibrosis and arterial stiffening.37, 38 Western blot analysis showed that TGFβ1, TGF-β3, RUNX2 and ALP expressions increased significantly in KL (+/−) mice aortas (Fig. 5A–E). Inhibition of autophagy by chloroquine decreased TGFβ1, TGF-β3, RUNX2 and ALP expressions down to control levels (Fig. 5A–E), suggesting that KL (+/−)-induced upregulation of TGFβ1, TGF-β3, RUNX2 and ALP was abolished by inhibiting autophagy.
Fig. 5. Inhibition of autophagy by chloroquine abolished the upregulation of arterial TGF-β1, TGF-β3, RUNX2 and ALP expression in KL+/− mice.
(A) Western blots analysis of TGF-β1, TGF-β3, RUNX2 and ALP protein expression. (B) Quantification of TGF-β1 protein levels. (C) Quantification of TGF-β3 protein levels. (D) Quantification of RUX2 protein levels. (E) Quantification of ALP1 protein levels. Data are expressed as means ± SE and analyzed by one-way ANOVA. n=4. **p<0.01 vs WT mice; ##p<0.01 vs KL (+/−)-CON mice.
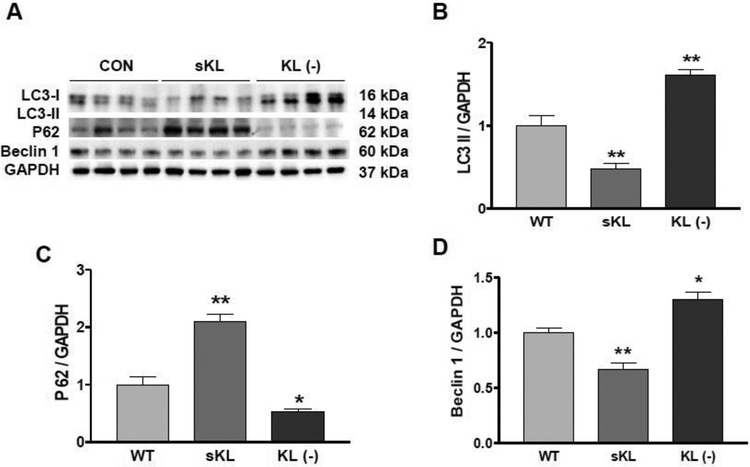
Klotho regulated autophagy and Beclin-1 expression in mouse vascular aortic smooth muscle cells (MOVAS)
One of the most interesting findings of our study is that autophagy was induced in KL (+/−) mice (Fig. 2). However, it is still unclear whether Klotho gene deficiency directly induces autophagy. In MOVAS, we found that recombinant secreted Klotho (sKL) protein inhibited autophagy, as evidenced by the decrease of LC3-II expression and increase of P62 expression (Fig. 6 A–C). By contrast, Klotho-deficient medium (KL (−)) increased LC3-II expression and decreased P62 expression (Fig. 6 A–C), suggesting that Klotho gene deficiency induces autophagy. To explore this underlying mechanism, we measured how Beclin-1, an autophagy-related gene, was regulated in MOVAS and found that secreted Klotho (sKL) decreased Beclin-1 expression, while KL (−) increased Beclin-1 expression (Fig. 6A and D). This suggests that Klotho’s regulation of autophagy may involve Beclin-1.
Fig. 6. Klotho regulated autophagy and Beclin-1 expression in mouse vascular aortic smooth muscle cells (MOVAS).
(A) Western blots analysis of LC3, P62, and Beclin-1. (B) Quantification of LC3-II expression. (C) Quantification of P62 expression. (D) Quantification of Beclin-1 expression. Data are expressed as means ± SE and analyzed by one-way ANOVA. n=4 independent experiments. *p<0.05, **p<0.01 vs Control (CON).
Beclin-1 mediated Klotho gene deficiency–induced autophagy, which is essential for scleraxis upregulation in MOVAS
Scleraxis is a transcription factor linked to regulating collagen synthesis in arterial cells.22 Scleraxis, a member of the basic helix-loop-helix (bHLH) family of transcription factors, is specifically expressed in tendons and ligaments, where it can be detected from early progenitor cells to mature fibroblasts.39–41 Scleraxis is also sufficient to upregulate expression of the collagen 1α2 gene in primary cardiac fibroblasts.42, 43 Scleraxis was upregulated in the aortas of KL (+/−) mice (Online Fig. 2A and B). Inhibition of autophagy by chloroquine decreased scleraxis expression in KL (+/−) mice down to the control level (Online Fig. 2A and B), suggesting that autophagy mediated Klotho gene deficiency–induced upregulation of scleraxis.
To further determine what role autophagy plays in Klotho gene deficiency–induced increase of scleraxis, we silenced Beclin-1 by knocking it down with Beclin-1 siRNA in MOVAS. The Beclin-1 protein was knock down 55% by siRNA (Online Fig. 2C and D). This led to autophagy being inhibited. LC3-II level was increased and P62 level was decreased in KL(−) medium-treated MOVAS which were prevented by siRNA knockdown of Beclin-1 (Online Fig. 2C, E and F). Moreover, Klotho gene deficiency upregulated scleraxis expression in MOVAS (Online Fig. 2C and G), which was abolished by siRNA knockdown of Beclin-1 (Online Fig. 2C and G). This result suggests that induction of autophagy is essential for Klotho gene deficiency–induced increases in scleraxis.
DISCUSSION
The present study demonstrated that Klotho gene deficiency increased autophagy, as evidenced by increased expression of LC3B-II and autophagy flux in the aorta. Interestingly, upregulation of autophagy may plan an important role in the pathogenesis of Klotho deficiency-induced arterial stiffening which can be effectively attenuated by inhibition of autophagy by chloroquine in KL (+/−) mice (Fig. 1 and 2). Recent studies showed that chloroquine inhibits autophagic protein degradation (e.g., P62).28, 29 By blocking the last step of the autophagy pathway, chloroquine leads to the accumulation of ineffective autophagosomes. Other research has reported that chloroquine prevents the development of monocrotaline-induced pulmonary hypertension and attenuates the progression of pulmonary hypertension.29 Our study, however, is the first to show that suppression of autophagy by chloroquine mitigated Klotho gene deficiency-induced arterial stiffening and hypertension.
Klotho homozygous mice suffer extensive premature aging phenotypes and die early (<8 weeks). Klotho homozygous mice also develop severe hyperphosphatemia and soft tissue calcification27, 44. As a result, Klotho homozygous mice were not used in this study. Klotho is predominantly expressed in the distal tubular epithelial cells of the kidneys and secreted to the blood44. The Klotho levels in heterozygous KL+/− deficient mice were reduced by 50% in the kidney and serum.22 Young KL+/− mice may not show obvious elevation of systolic blood pressure. However, systolic BP of the KL+/− mice begins to elevate progressively after 4 months of age and remained elevated thereafter.34 In this study, we used 20–23 months old KL+/− mice which showed accelerated aging phenotypes including obvious arterial stiffness and systolic hypertension.
We found that β-gal and p53 levels in aortas were increased in KL (+/−) mice (Online Fig. 3), indicating that Klotho deficiency causes vascular aging. Interestingly, inhibition of autophagy by chloroquine abolished upregulation of β-gal and p53 levels in KL (+/−) mice (Online Fig. 3), suggesting that chloroquine prevents vascular aging.
LC3-II expression levels may be insufficient for reliably assessing autophagic activity. However, autophagic flux measured by the level of P62 protein and LC3-II turnover in the presence and absence of lysosomal degradation inhibitor can serve as a marker of autophagic activity. Accumulating evidence shows that, in the brain of Klotho mutant mice, cathepsin D and LC-3 expression were enhanced, and lysosomes and autophagy-related structures in neurons also increased.45 The autophagic-lysosomal pathway was activated in the masseter and tongue muscles of the Klotho mutant mice.46 These findings suggest that autophagy is increased in Klotho-deficient mice. However, whether Klotho deficiency directly induces autophagy is not clear. In cultured mouse aortic vascular smooth cells (MOVAS), we found that KL-deficient medium (KL (−)) increased LC3-II expression and decreased P62 expression, suggesting that Klotho deficiency may induce autophagy. By contrast, adding recombinant secreted Klotho (sKL) protein to the culture medium inhibited autophagy, as evidenced by a decrease in LC3-II expression and an increase in P62 expression. These results support the conclusion that Klotho directly regulates autophagy. It is noticed that sKL decreased Beclin-1 expression and KL (−) increased Beclin-1 expression (Fig. 6) which suggests that Klotho likely regulates autophagy through its effect on Beclin-1 expression. Beclin-1 interacts with BCL-2 or PI3K class III, thus playing an important role in autophagy regulation.
Despite the protective nature of autophagy in human physiology and disease, its role in vascular function and disorders is poorly understood, probably because of the multifarious actions involved in the autophagic process.47 Undoubtedly, overactivation of autophagy is not always protective, as it may cause cell death with unique morphological features distinct from apoptosis and necrosis, sometimes referred to as autosis or autophagic cell death. In this study, we showed that enhanced autophagy in KL (+/−) mice may be injurious to vasculature by causing arterial stiffening and hypertension.
Chloroquine, as a well-known autophagy inhibitor, may have other autophagy-irrelevant effects. The limitation of this study is that it cannot exclude the possibility that the ameliorating effects of chloroquine on arterial stiffness and hypertension may be partly attributed to its autophagy-irrelevant effects. Although we found that Klotho directly regulates autophagy in MOVAS cells, the effects of direct inhibition of autophagy by knockdown of key autophagy genes in Klotho gene deficient mice should be evaluated in the future study.
One important finding in our study is that autophagy selectively regulates MMP9 activity but not MMP2 activity (Fig. 4). MMPs are calcium-dependent zinc-containing endopeptidases involved in matrix degradation. These enzymes share similar characteristics including common modes of activation, conserved amino acid sequences in the putative metal binding active site region, and inhibition by specific proteinase inhibitors known as tissue inhibitors of metalloproteinases (TIMPs).48 Smooth muscle cells (SMCs) produce MMP2 in a physiological condition and other MMPs, including MMP9, in response to cytokine stimulation.49 MMP2 and MMP9 are largely thought to have similar functions based on sharing substrate affinity (namely short collagens), degradation products of interstitial collagen, and elastin.49 Although Klotho gene deficiency increased both MMP2 and MMP9 activity, autophagy inhibition by chloroquine only eased MMP9 activity. Galis et al. reported that MMP9, but not MMP2, was necessary for organization of collagen by SMCs.49 Thus, autophagy may regulate the collagen content in the aorta partly through its effect on MMP9 activity. Conversely, autophagy may not be involved in Klotho gene deficiency-induced upregulation of MMP2 activity, which was not affected by chloroquine-induced autophagy inhibition.
We recently showed that scleraxis is expressed in MOVAS and may also be involved in collagen synthesis.22 In cultured aortic MOVAS, Klotho gene deficiency failed to increase scleraxis expression under the inhibition of autophagy, but we found that Klotho gene deficiency (KL+/−) upregulated scleraxis in aortas, which was abolished by inhibition of autophagy (Online Fig. 2). Our results thus provide the first evidence that autophagy may regulate scleraxis expression and that induction of autophagy is essential for Klotho gene deficiency–induced upregulation of scleraxis, an important transcription factor for collagen synthesis. Increased collagen synthesis in the extracellular matrix is associated with hypertension-related pathogenesis of cardiovascular stiffness and remodeling. Arterial stiffness has been significantly correlated with markers of collagen metabolism independent of age, sex, BP levels, and pulse rate in hypertensive patients.50 Thus, haplodeficiency of Klotho gene upregulated scleraxis in an autophagy-dependent manner, which may contribute to overproduction of collagen and arterial remodeling.
Finally, autophagy inhibition by chloroquine abolished upregulation of collage deposition and degradation of elastin, both of which contribute to attenuation of arterial stiffening and hypertension. The beneficial effects of chloroquine on arterial remodeling are therefore likely to be mediated through reversing the upregulation of scleraxis and MMP9, respectively.
CONCLUSION
Our study demonstrated that haplodeficiency of Klotho gene induces autophagy, which is injurious to vasculature and causes arterial stiffening and hypertension. Suppression of enhanced autophagy by chloroquine attenuates arterial stiffness and hypertension in KL (+/−) mice. Therefore, enhanced autophagic activity may play a critical role in the pathogenesis of Klotho gene deficiency-induced arterial stiffness. Mechanistically, autophagy selectively regulates MMP9 activity, which is necessary for reorganizing collagen by SMCs. Importantly, Klotho gene deficiency upregulated expression of scleraxis, an essential transcription factor for collagen synthesis, in an autophagy-dependent manner. Thus, we conclude that suppression of autophagy by chloroquine attenuates Klotho gene deficiency-induced arterial stiffening and hypertension through modulating MMP9 and scleraxis expression.
Supplementary Material
Key Messages.
Klotho gene deficiency upregulates autophagy.
Upregulation of autophagy plays a role in the pathogenesis of arterial stiffening.
Autophagy regulates MMP9 activity and scleraxis expression.
ACKNOWLEDGMENTS
We would like to thank Dr. Nathan Tipton for his assistance in editing the manuscript.
Support and Funding: This work was supported by NIH R01 HL118558, AG049780, HL122166, HL116863, DK093403, AG062375, HL102074, HL105302.
ABBREVIATIONS:
- ALP
alkaline phosphatase
- BFA
bafilomycin A1
- bHLH
basic helix-loop-helix
- KL (−))
Klotho free medium
- KL (+/−)
Klotho mutant heterozygous
- LC3-II
microtubule-associated proteins 1A/1B light chain 3B
- MMP
matrix metalloproteinases
- MOVAS
mouse vascular aortic smooth muscle cell
- P62/SQSTM1
sequestosome 1
- PBS
phosphate buffered saline
- PWV
pulse wave velocity
- Runx2
Runt-related transcription factor 2
- SCX
scleraxis
- sKL
recombinant secreted Klotho
- TGFβ
transforming growth factor beta
- TIMP
tissue inhibitors of metalloproteinases
- VSMC
vascular smooth muscle cells
- WT
wild type
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Electronic supplementary material
ESM 1 (DOCX 461 kb)
Conflicts of Interest: NONE.
REFERENCES
- 1.Kobayashi S and Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochimica et biophysica acta. 2015;1852:252–61. [DOI] [PubMed] [Google Scholar]
- 2.Poillet-Perez L, Xie X, Zhan L, Yang Y, Sharp DW, Hu ZS, Su X, Maganti A, Jiang C, Lu W, Zheng H, Bosenberg MW, Mehnert JM, Guo JY, Lattime E, Rabinowitz JD and White E. Autophagy maintains tumour growth through circulating arginine. Nature. 2018;563:569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito T, Kuma A, Sugiura Y, Ichimura Y, Obata M, Kitamura H, Okuda S, Lee HC, Ikeda K, Kanegae Y, Saito I, Auwerx J, Motohashi H, Suematsu M, Soga T, Yokomizo T, Waguri S, Mizushima N and Komatsu M. Autophagy regulates lipid metabolism through selective turnover of NCoR1. Nat Commun. 2019;10:1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang C and Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez AF, Sebti S, Wei Y, Zou Z, Shi M, McMillan KL, He C, Ting T, Liu Y, Chiang WC, Marciano DK, Schiattarella GG, Bhagat G, Moe OW, Hu MC and Levine B. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558:136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savini M and Wang MC. Does Autophagy Promote Longevity? It Depends. Cell. 2019;177:221–222. [DOI] [PubMed] [Google Scholar]
- 7.Klionsky DJ, Abdelmohsen K, Abe A and Abedin MJ. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeganeh B, Lee J, Ermini L, Lok I, Ackerley C and Post M. Autophagy is required for lung development and morphogenesis. J Clin Invest. 2019;130:2904–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loos B, du Toit A and Hofmeyr JH. Defining and measuring autophagosome flux-concept and reality. Autophagy. 2014;10:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klionsky DJ, Elazar Z, Seglen PO and Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–50. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H and Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. The Journal of cell biology. 2005;171:603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuro-o M. Klotho in health and disease. Current opinion in nephrology and hypertension. 2012;21:362–8. [DOI] [PubMed] [Google Scholar]
- 13.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R and Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. [DOI] [PubMed] [Google Scholar]
- 14.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP and Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Li Y, Zhou D, Tan RJ and Liu Y. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. Journal of the American Society of Nephrology : JASN. 2013;24:771–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Y and Sun Z. In Vivo Pancreatic beta-Cell-Specific Expression of Antiaging Gene Klotho: A Novel Approach for Preserving beta-Cells in Type 2 Diabetes. Diabetes. 2015;64:1444–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, Takahashi M, Boothman DA and Kuro-o M. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. The Journal of biological chemistry. 2011;286:8655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moe SM. Klotho: a master regulator of cardiovascular disease? Circulation. 2012;125:2181–3. [DOI] [PubMed] [Google Scholar]
- 19.Xiao NM, Zhang YM, Zheng Q and Gu J. Klotho is a serum factor related to human aging. Chin Med J (Engl). 2004;117:742–7. [PubMed] [Google Scholar]
- 20.Kotsis V and Stabouli S. Arterial stiffness, vascular aging, and intracranial large artery disease. American journal of hypertension. 2011;24:252. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, Yamanari T, Kikumoto Y, Uchida HA, Kitamura S, Maeshima Y, Nakamura K, Ito H and Makino H. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e56695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen K, Zhou X and Sun Z. Haplodeficiency of Klotho Gene Causes Arterial Stiffening via Upregulation of Scleraxis Expression and Induction of Autophagy. Hypertension. 2015;66:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto J and Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–46. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto J and Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62:542–9. [DOI] [PubMed] [Google Scholar]
- 25.Karras A, Haymann JP, Bozec E, Metzger M, Jacquot C, Maruani G, Houillier P, Froissart M, Stengel B, Guardiola P, Laurent S, Boutouyrie P, Briet M and Nephro Test Study G. Large artery stiffening and remodeling are independently associated with all-cause mortality and cardiovascular events in chronic kidney disease. Hypertension. 2012;60:1451–7. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Herrington DM, Brubaker PH, Moore JB, Eggebeen J and Haykowsky MJ. Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension. 2013;61:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aging Sun Z., arterial stiffness, and hypertension. Hypertension. 2015;65:252–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E. Principles and current strategies for targeting autophagy for cancer treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ and Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circulation research. 2013;112:1159–70. [DOI] [PubMed] [Google Scholar]
- 30.Hartley CJ, Taffet GE, Michael LH, Pham TT and Entman ML. Noninvasive determination of pulse-wave velocity in mice. The American journal of physiology. 1997;273:H494–500. [DOI] [PubMed] [Google Scholar]
- 31.Whitesall SE, Hoff JB, Vollmer AP and D’Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. American journal of physiology Heart and circulatory physiology. 2004;286:H2408–15. [DOI] [PubMed] [Google Scholar]
- 32.Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H and Sun Z. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension. 2016;68:1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Y, Chen J and Sun Z. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension. 2016;67:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X, Chen K, Lei H and Sun Z. Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol. 2015;26:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen GF and Sun Z. Effects of chronic cold exposure on the endothelin system. J Appl Physiol (1985). 2006;100:1719–26. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Kobayashi S, Xu X, Viollet B and Liang Q. AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PloS one. 2013;8:e59682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capelli A, Lusuardi M, Cerutti CG and Donner CF. Lung alkaline phosphatase as a marker of fibrosis in chronic interstitial disorders. American journal of respiratory and critical care medicine. 1997;155:249–53. [DOI] [PubMed] [Google Scholar]
- 38.Lin ME, Chen T, Leaf EM, Speer MY and Giachelli CM. Runx2 Expression in Smooth Muscle Cells Is Required for Arterial Medial Calcification in Mice. The American journal of pathology. 2015;185:1958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA and Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–110. [DOI] [PubMed] [Google Scholar]
- 40.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A and Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–66. [DOI] [PubMed] [Google Scholar]
- 41.Brent AE, Schweitzer R and Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–48. [DOI] [PubMed] [Google Scholar]
- 42.Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM and Czubryt MP. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. Journal of molecular and cellular cardiology. 2009;47:188–95. [DOI] [PubMed] [Google Scholar]
- 43.Bagchi RA and Czubryt MP. Synergistic roles of scleraxis and Smads in the regulation of collagen 1alpha2 gene expression. Biochimica et biophysica acta. 2012;1823:1936–44. [DOI] [PubMed] [Google Scholar]
- 44.Xu Y and Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev. 2015;36:174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiozaki M, Yoshimura K, Shibata M, Koike M, Matsuura N, Uchiyama Y and Gotow T. Morphological and biochemical signs of age-related neurodegenerative changes in klotho mutant mice. Neuroscience. 2008;152:924–41. [DOI] [PubMed] [Google Scholar]
- 46.Iida RH, Kanko S, Suga T, Morito M and Yamane A. Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Molecular and cellular biochemistry. 2011;348:89–98. [DOI] [PubMed] [Google Scholar]
- 47.De Meyer GR, Grootaert MO, Michiels CF, Kurdi A, Schrijvers DM and Martinet W. Autophagy in vascular disease. Circulation research. 2015;116:468–79. [DOI] [PubMed] [Google Scholar]
- 48.Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M and Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Developmental biology. 1999;205:158–70. [DOI] [PubMed] [Google Scholar]
- 49.Johnson C and Galis ZS. Matrix metalloproteinase-2 and −9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:54–60. [DOI] [PubMed] [Google Scholar]
- 50.Ishikawa J, Kario K, Matsui Y, Shibasaki S, Morinari M, Kaneda R, Hoshide S, Eguchi K, Hojo Y and Shimada K. Collagen metabolism in extracellular matrix may be involved in arterial stiffness in older hypertensive patients with left ventricular hypertrophy. Hypertension research : official journal of the Japanese Society of Hypertension. 2005;28:995–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.