Abstract
West Virginia (WV) is situated at the epicenter of the opioid epidemic with the highest rates of overdose deaths and some of the lowest rates of access to life saving evidence-based medication assisted treatment (MAT) for patients with opioid use disorder (OUD). WV used a modified hub-and-spoke model to build organizational capacity for facilities to use buprenorphine to treat patients with OUD and to provide ongoing case consultation. The purpose of this study is to 1) describe the group-base model of buprenorphine treatment and the model used to build organizational capacity, 2) to describe the preliminary results of buprenorphine expansion in WV and 3) to report preliminary data describing and comparing the characteristics of the patients served across five hubs. A single Coordinating Center uses video conferencing to train hubs and provide ongoing case consultation, as well as clinical support. Hubs were trained to deliver a buprenorphine treatment model that is multi-disciplinary and includes group-based medication management and psychosocial therapy. Five regional hubs independently treat patients and are leading MAT expansion in their local areas by training and mentoring spokes (n = 13). As a result of the WV STR funding, 14 health care facilities have started to use buprenorphine, 56 health professionals were trained and 196 patients with OUD have been treated. There were few sociodemographic characteristic differences across patients treated at the five hubs, while there were differences in self-reported alcohol and drug use in the 30 days prior to intake. Additional research is needed to determine whether the WV modified hub-and-spoke model resulted in statistically significant improvements in buprenorphine treatment capacity; there is a need to address MAT stigma and regulatory barriers in order to ensure the long-term sustainability of the buprenorphine expansion.
1. Introduction
1.1. Opioid epidemic & treatment capacity in rural areas
In 2017, there were 47,600 opioid-involved overdose deaths in the United States (U.S.) (Scholl, Seth, Kariisa, Wilson, & Baldwin, 2018). Drug overdose deaths occur at higher rates in rural areas (Rossen, Khan, & Warner, 2013); the largest relative rate increases in drug overdose deaths between 2016 and 2017 were in counties classified as micropolitan (10,000 to 49,000 inhabitants) (Ingram & Franco, 2014; Scholl et al., 2018). All of the counties in West Virginia (WV) are classified as Appalachian and 62% are classified as rural (Appalachian Regional Commission, 2017; Ingram & Franco, 2014). Over the past decade, WV has had the highest drug overdose mortality rate in the U.S. (Hall et al., 2008; Scholl et al., 2018). In 2017, the WV opioid-involved overdose death rate was 49.6 per 100,000, compared to the national average of 14.9 (Scholl et al., 2018). WV also has high rates of opioid prescribing (Guy et al., 2017), poverty, low education and high unemployment (Meit, Heffernan, Tanenbaum, & Hoffmann, 2017); which independently contribute to the risk of illicit drug use and development of substance use disorders (SUD) (Richman, 1977; Saxe et al., 2001). WV’s ability to effectively respond to the opioid epidemic has been significantly hampered by known health care shortages (HRSA 2018); specifically, SUD prevention and treatment services (Jones, Campopiano, Baldwin, & McCance-Katz, 2015).
WV is estimated to have the highest rate of past year opioid use disorders (OUD) (12.9 per 1000) in the country; in 2012 all nine of WV’s opioid treatment programs (OTPs) were at least 80% or greater capacity and the maximum WV buprenorphine treatment capacity rate (7.0 per 1000) does not meet the demand for services (Jones et al., 2015). According to a Health Resources and Services Administration December 2018 report, nearly half of WV residents are in areas designated as health professional shortage areas; 120 primary care and 129 mental health care professionals are needed in these areas (HRSA, 2018). There are only nine methadone treatment programs in WV and in 2007 a legislative moratorium was passed preventing new methadone programs from opening. Fifty-five percent of WV counties do not have a provider waivered to prescribe buprenorphine, which is slightly better than the national average for rural areas. In 2016, 61% of rural counties nationally did not have a physician who could dispense buprenorphine (Andrilla, Coulthard, & Larson, 2017). WV has no statewide public transportation infrastructure and some patients drive 3 h or more each way for weekly buprenorphine treatment. National research confirms the long distances that rural patients frequently travel to receive treatment for OUD (Rosenblum et al., 2011). The large burden of OUD in the state is compounded by a lack of accessible treatment resources, including both specialty addiction care and primary practice-based addiction care, particularly in the most rural areas.
The purpose of this study was to 1) describe the group-based model of buprenorphine treatment and the model used to build organizational capacity, 2) to describe the preliminary results of buprenorphine expansion in WV and 3) to report preliminary data describing and comparing the characteristics of the patients served across five hubs. There are unique challenges to delivering MAT in rural areas including not only shortages of health care facilities offering medications to treat OUD (Dick et al., 2015; Jones, 2018; Sigmon, 2014), but shortages of health care facilities (HRSA 2018) and waivered prescribers who are willing to treat patients with OUD (Andrilla et al., 2017; Andrilla, Moore, Patterson, & Larson, 2019). It is important to understand whether there are regional/setting differences across the expansion sites that can be addressed by modified programming. A modified hub and spoke model with regional hubs delivering buprenorphine treatment in the context of psychosocial treatment and training local spokes, integrated by a state Coordinating Center, may be promising to address the unmet need for OUD treatment.
1.2. State Targeted Response (STR) funding in West Virginia
In May of 2017, WV was awarded $5.8 M as part of the Substance Abuse and Mental Health Services Administration (SAMHSA) State Targeted Response (STR) grant funding. A portion of these funds were earmarked to support medication assisted treatment (MAT) expansion for patients with OUD and this effort was led by WVU’s Department of Behavioral Medicine and Psychiatry; expansion was modelled on their Comprehensive Opioid Addiction Treatment (COAT) buprenorphine program (Lander, Marshalek, & Sullivan, 2016; Zheng et al., 2016; Zheng et al., 2017; Zullig, Lander, Tuscano, Hobbs, & Faulkenberry, 2018). COAT is a structured outpatient buprenorphine treatment program that includes group-based medication management appointments directly linked with psychosocial therapy groups that are stepped down in frequency as patients progress toward recovery. The COAT buprenorphine treatment model was chosen due to its efficacy and the efficiency of using a group-based approach in treating a large volume of patients with OUD. While COAT is not the only effective model of buprenorphine treatment, there were insufficient resources to support implementation and oversight of multiple buprenorphine treatment models. A modified version of Vermont’s hub and spoke model (Brooklyn & Sigmon, 2017) was used to implement the COAT buprenorphine treatment model across the state. Vermont, a similarly rural state, responded to their limited access to MAT for patients with OUD by creating a hub and spoke model to expand treatment capacity. Over the course of 5 years, Vermont’s hub and spoke model generated a 64% increase in buprenorphine-waivered physicians and a 50% increase in patients served per waivered physician, as well as a robust system for transferring patients between hubs and spokes as appropriate for their treatment needs. In the Vermont Model, buprenorphine treatment is initiated primarily at a single hub (e.g., opioid treatment program) and the goal is to transfer patients to the spokes (e.g., primary care practices) once patients are stabilized on buprenorphine (Brooklyn & Sigmon, 2017). Whereas in WV, the goal was to develop regional hubs that can independently and comprehensively treat patients with OUD in their local community. The regional hubs, in turn, would lead MAT expansion in their local areas by training and mentoring spokes that can independently and comprehensively treat patients with OUD in their local community. Neither the hubs or the spokes in the WV model are federally-certified opioid treatment programs.
1.3. Comprehensive Opioid Addiction Treatment model (COAT)
The COAT program is located on the WVU Morgantown campus in the Chestnut Ridge Center (CRC), which served as the Coordinating Center for the STR-funded MAT expansion. One of the hallmarks of the COAT model is its interdisciplinary team; which includes different clinicians that are waivered to prescribe buprenorphine/naloxone (MD, DO, NP or PA), licensed clinical therapists (MSW, LPC, PhD), case mangers (BA/BS) and medical assistants. Patients are required to sign a treatment agreement at the time of enrollment to ensure that they understand the program rules. As part of the model, patients are required to attend 30-minute group-based medication management visits and 60-minute group-based psychosocial counseling; as well as attend peer-based mutual support group meetings in the community. COAT utilizes a phased approach such that the frequency of treatment sessions progressively declines as patients build consecutive days of abstinence and increased levels of functioning. Abstinence includes alcohol and any illicit drug use which is confirmed by urine drug screening; it excludes appropriate use of prescribed buprenorphine. Random pill counts and call-back urine drug screening are used to confirm medication compliance, detect potential buprenorphine/naloxone diversion, or illicit substance use.
The treatment groups usually have between 8 and 12 patients and include groups that are composed of mixed gender, all female, and pregnant women; as well as groups focused on specific modalities such as mindfulness or yoga. At the medication management group, patients are required to provide written verification that they attended at least 4 h of mutual support group meetings for addiction during the previous week. These meetings mostly consist of traditional 12-step groups such as AA and NA; but also include SMART Recovery, Celebrate Recovery and MAT-specific support groups. Buprenorphine/naloxone prescriptions are provided at the end of the treatment group. The quantity written is sufficient to last until the next medication management appointment which may be weekly, biweekly, or monthly (Table 1). The combination buprenorphine/naloxone products are used almost exclusively, including among pregnant patients, to reduce misuse and diversion (Lowfall & Walsh, 2014). The combination product has been shown to be safe for pregnant women and their neonates (Jumah et al., 2016; Lund et al., 2013; Nguyen et al., 2018). Individual appointments with a therapist are scheduled on a monthly basis or more frequently as necessary.
Table 1.
COAT program levels.
| Medication management | Psycho-educational group therapy | Weekly mutual support groups | Consecutive days AOD abstinence | |
|---|---|---|---|---|
| Level I | Weekly | Weekly | 4a | < 90 days |
| Level II | Biweekly | Biweekly | 4 | 91–364 days |
| Level III | Monthly | Monthly | 1–3 years | |
| Level IV | Bimonthly | N/A | > 3 years | |
| Intensive | 3 per week | 3 per week | Frequent relapses |
Verification of mutual support group meetings required for only level I.
The treatment team meets for 30 min, prior to the consecutive medication management and therapy groups, to discuss treatment planning and patients’ overall progress in the program. This is a critical program component that ensures strong communication across the interdisciplinary team and team agreement on modifications to individual patients’ treatment plans. The medication management group is facilitated by a provider waivered to prescribe buprenorphine. The medical assistant obtains patients’ vital signs and urine drug screens prior to the group; positive urinalysis results are discussed during the group visit. While some may be concerned about privacy and potential of shaming due to the discussion of positive urine toxicology results in a group-based setting, the clinical team uses the group modality to reduce the shame and stigma associated with relapse. The clinical team does not use stigmatizing language (e.g., “dirty” urine) and emphasizes honesty about drug use as part of the recovery process. Further, positive urine toxicology results are opportunities for a broader discussion of stressful events and other triggers that may precede a relapse. Confirmatory urine toxicology results are usually individually conveyed to patients, particularly in circumstances where a higher intensity of clinical care may be needed. The case manager tracks the patient’s days of abstinence and verifies mutual support meeting attendance. The group facilitator queries patients about their challenges to sustaining recovery, discusses any concerns raised during the earlier treatment team meeting and provides positive reinforcement of abstinence. The group therapy utilizes evidence-based practices including cognitive behavioral therapy (CBT), 12 step-facilitation and relapse prevention techniques (Winstanley, Fishman, & Bolon, 2010, chap. 46) and is facilitated by a licensed therapist. The goals of therapy are to 1) help build a solid, sustainable recovery program; 2) develop strategies for relapse prevention; 3) promote peer support and a supportive social network; 4) develop an understanding of the disease of addiction and 5) discuss how buprenorphine works to support recovery. The COAT leadership promotes the use of person-first non-pejorative language (e.g., ‘positive’ urine screen versus ‘dirty’ urine screen) (Wakeman, 2017) and encourages patients to be honest about their relapses, rather than relying solely on urine drug screens.
Over the past 15 years the COAT program has grown rapidly with increasing numbers of patients, physicians, therapists, case managers and trainees from multiple disciplines. In the 18 months prior to the STR award, the COAT’s main treatment site at the Chestnut Ridge Center (CRC) had 300+ people on the treatment waiting list for an intake assessment. Even once patients completed an intake assessment, they often had to wait an additional 2–8 weeks to enter treatment due to limited capacity. As of December 2018, over 3000 patients had been treated in the COAT program over the last 15 years, there are approximately 550 patients currently in active treatment and there is no active waitlist.
1.4. Hub and spoke selection and training
Potential hubs were identified from a list of health care facilities that already participated in the WV Project Extensions for Community Healthcare Outcomes (ECHO) Medication Assisted Treatment (MAT) training that began in June 2017. Project ECHO is a video-based hub- and-spoke model of health care education and mentoring that was developed to increase community-based health care providers’ capacity to deliver complex specialty care (Arora et al., 2011). The hubs are usually urban academic medical centers and the spokes are usually rural primary care practices; disease-specific (e.g., addiction, Hepatitis C) Project ECHO models have been developed. New Mexico saw a dramatic increase in the number of buprenorphine-waivered prescribers after the implementation of an integrated addictions and psychiatry Project ECHO (going from 36 to 375) (Komaromy et al., 2016). The WV Project ECHO program is used to provide ongoing training for the hubs and spokes, as well as provide ongoing case consultations with the CRC Coordinating Center. The WV Project ECHO program was developed and implemented through funding and support from the West Virginia Clinical & Translational Science Institute (WVCTSI), Claude Worthington Benedum Foundation, WVU School of Medicine, Cabin Creek Health Systems, Anthem Insurance and the WV Primary Care Association in partnership with Project ECHO®. Typical WV Project ECHO MAT sessions include didactics on topics ranging from substance use disorders, myths and facts about MAT, interpreting lab results, stigma, psychosocial/behavioral treatments and evaluating case severity. Case presentations routinely occur with time allotted for discussion. In year 2 of the STR funding, the newly formed hubs are working to develop their own ECHO-based model to provide video-based supervision and case consultation for their spokes.
Five hubs were recruited from around the state (see Fig. 1) and the sites were chosen based on the following criteria: 1) geographic proximity to serve areas with high demand, 2) having a university affiliation or willingness to train new buprenorphine providers and 3) expressing active interest in MAT and/or already being actively trained by the CRC Coordinating Center.
Fig. 1.
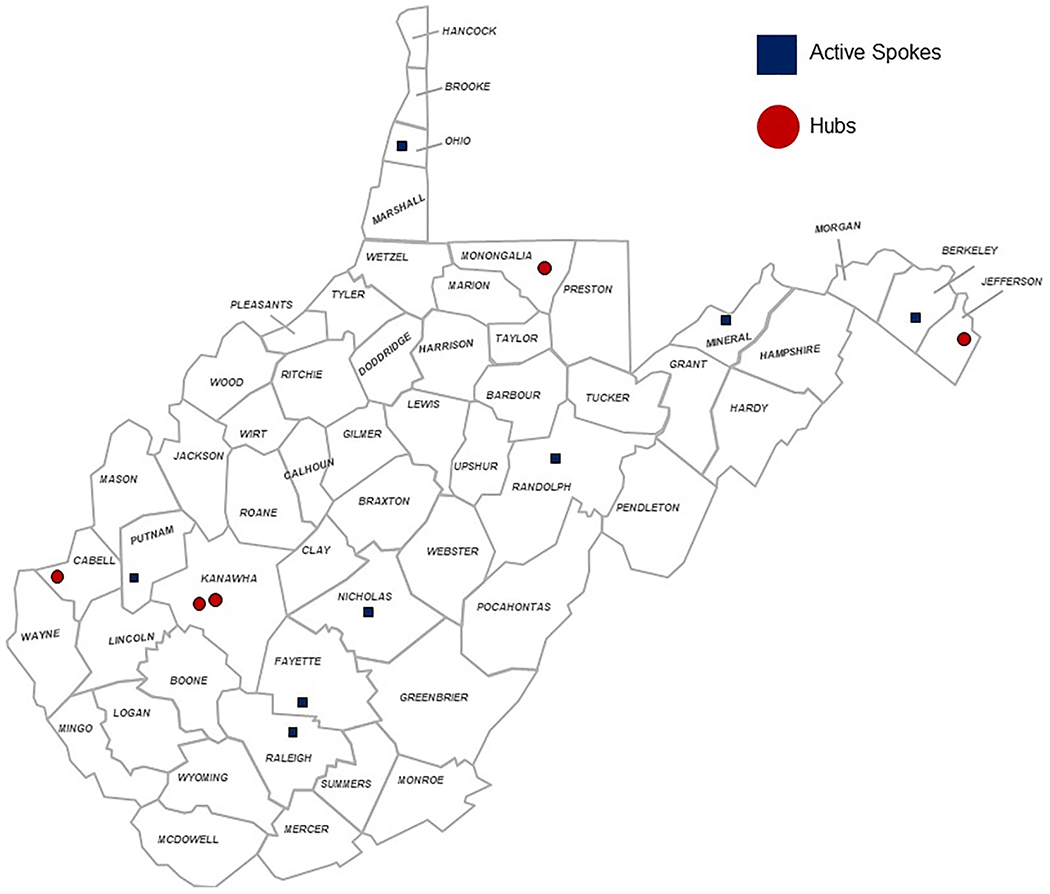
Map of hub and spokes.
The current hubs are WVU Department of Behavioral Medicine and Psychiatry’s Lakewood Clinic (psychiatry, Morgantown), Marshall University’s Recovery Center (psychiatry, Huntington), Charleston Area Medical Center (psychiatry, Charleston), Harpers Ferry Family Medicine (primary care, Harpers Ferry) and Cabin Creek Health Center (Federally Qualified Health Center (FQHC), primary care, Kanawha City) (see Fig. 2). Prescribers at the hubs and spokes are varied in their training and specialization and include addiction psychiatry, general psychiatry, internal medicine, primary care, emergency medicine, obstetrics and gynecology.
Fig. 2.
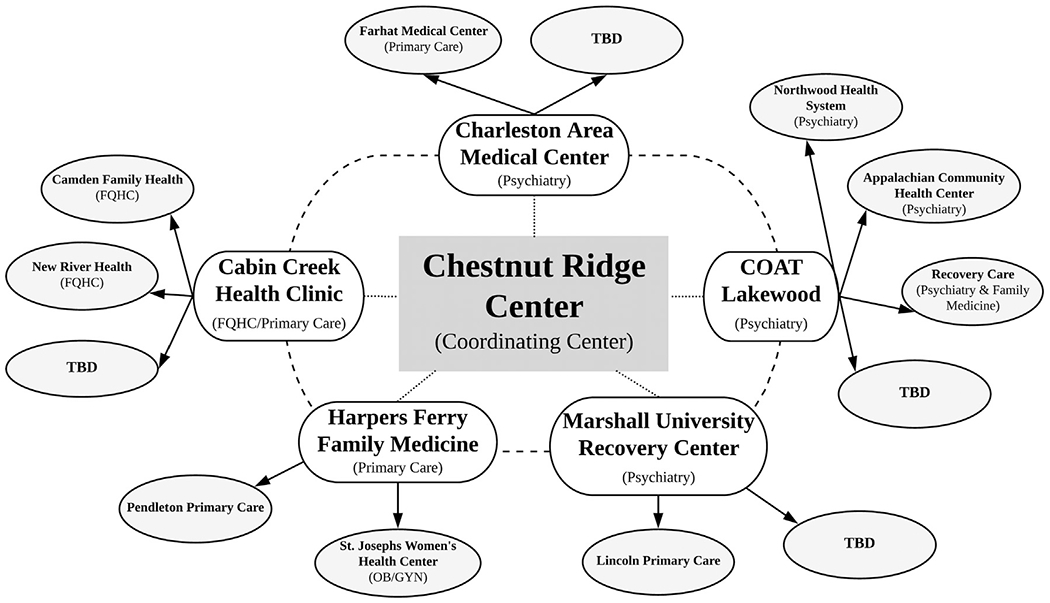
Organizational structure of the hub and spoke model.
Each hub team, which consisted of a prescriber, therapist and case manager, was initially trained on-site at CRC on the COAT buprenorphine treatment model. The hub treatment teams shadowed the COAT program (treatment team meetings, medication management and therapy groups) twice at the CRC and attended at least two days of training at each visit. COAT team members then visited each hub twice, shadowing the hub clinic operations and offering both verbal and written feedback. The hubs participate in bimonthly video conference calls with the CRC Coordinating Center and they are required to attend twice monthly 60-minute WV Project ECHO MAT sessions. Hubs and spokes are provided with written materials detailing policies and procedures of the COAT buprenorphine treatment model, group therapy content resources, a COAT quick tips booklet and a COAT pocket guide. The same training structure was used for training the spokes; each spoke trained at their affiliated hub twice and twice trained at the spoke’s clinic. Hubs can access ongoing support from the CRC Coordinating Center via e-mail and phone consultation as needed. Once trained, hubs and spokes can tailor the buprenorphine treatment model to fit the needs of their clinical organization and their local patients. In Year 2 of the project, each hub has begun developing 2–3 spokes to train and support in the delivery of MAT services using the COAT buprenorphine treatment model. In a rural state this innovative means of training and dissemination of evidence-based treatments is essential to reach as many providers as possible and to increase the comfort level of providers working with this population. Training and ongoing consultation is particularly critical for providers without training in behavioral medicine or addiction treatment (e.g., primary care providers, OB/GYN).
2. Methods
2.1. Participants, measures & data collection
All patients enrolled in treatment at hubs between January and December 2018, and who completed the intake forms, were included in the analysis. As the spokes have only recently begun to treat patients with buprenorphine, these patients (n = 11) were not included in the analysis. The Government Performance and Results Modernization Act (GPRA) data form was collected on all new patients within 10 days of being prescribed buprenorphine/naloxone. The GPRA form collected information on modality of planned services, treatment services (medical, case management, education and aftercare), sociodemographic characteristics of clients served (race, ethnicity, living situation, education, employment), alcohol and drug use (past 30 day use, route of administration), past month health care utilization, mental health and social connectedness. All of the hubs received training sessions in-person and via video conference on how to complete the GPRA data collection forms. The GPRA form was completed either by the therapist conducting the drug and alcohol evaluation or by the case manager. GPRAs were securely electronically sent or mailed to the CRC where data was entered into a REDcap database. GPRA measures were collected at baseline (within 10 days of buprenorphine initiation) and at 6-, 12-month follow-up; and at discharge. Given the limited amount of follow-up data available and that spokes are still being trained to use buprenorphine to treat patients with OUD, only baseline data from the hubs is reported.
2.2. Data analysis
Stata/MP Version 15.1 was used to run descriptive statistics (StataCorp, 2017). Chi-square, fisher’s exact and ANOVA were used to compare patient characteristics across the sites. Statistical significance is defined as a p value ≤ 0.05. The West Virginia University Institutional Review Board approved this project.
3. Results
3.1. Buprenorphine training and expansion
As a direct result of the STR funding, 56 health professionals were trained in the COAT treatment model and this included 19 physicians, 2 nurse practitioners, 1 nurse, 10 therapists, 10 case managers, 3 medical assistants, 10 administrators and 1 scribe. Thirteen spokes have been identified and nine spokes have initiated treatment. Four providers have been newly waivered to prescribe buprenorphine; it is anticipated that additional providers will be waivered as the remaining spokes implement buprenorphine/naloxone treatment (Table 2).
Table 2.
Baseline demographic & clinical characteristics (n = 196).
| Overall | Morgantown Psychiatry (n = 99) | Kanawha City Primary care (n = 42) | Harpers Ferry Primary care (n = 21) | Charleston Psychiatry (n = 17) | Huntington Psychiatry (n = 17) | p value | |
|---|---|---|---|---|---|---|---|
| Male | 53.6% | 53.5% | 66.7% | 33.3% | 47.1% | 52.9% | 0.16 |
| White | 94.9% | 93.9% | 95.2% | 95.2% | 100.0% | 94.1% | 0.98 |
| Mean age | 35.0 | 34.4 | 37.0 | 31.9 | 38.5 | 34.1 | 0.07 |
| Housed | 97.4% | 95.9% | 100.0% | 100.0% | 100.0% | 93.3% | 0.39 |
| More than HS/GED | 24.5% | 28.6% | 16.7% | 19.1% | 25.0% | 26.7% | 0.61 |
| Employed full or part time | 30.0% | 29.3% | 39.0% | 35.0% | 11.8% | 23.1% | 0.31 |
| Have children | 73.5% | 72.7% | 71.8% | 65.0% | 81.3% | 86.7% | 0.65 |
| Ever experienced serious depression | 63.2% | 71.7% | 50.0% | 44.4% | 71.4% | 54.6% | 0.05 |
| Ever experienced serious anxiety/tension | 72.0% | 79.8% | 52.5% | 58.8% | 92.9% | 66.7% | < 0.00 |
| Ever experienced trouble controlling violent behav. | 7.5% | 6.1% | 7.1% | 10.0% | 14.3% | 9.1% | 0.63 |
| Prescribed psychiatric medication past month | 31.6% | 37.4% | 21.4% | 28.6% | 35.7% | 18.2% | 0.35 |
3.2. Buprenorphine expansion patient characteristics
A total of 196 patients have been served between January to December 2018. The mean patient age was 35.0 years old; patients ranged in age from 20 to 72 years old. A little more than half (53.6%) of the patients were male, the vast majority of the patients were White (94.9%), 3.6% were Black and 2.0% were American Indian. A fourth (26.5%) of patients reported having children and 14.4% of the women reported being currently pregnant.
More than a third (36.1%) of patients reported injection drug use in the past month and among those, 49.2% reported sharing syringes or other injection equipment. Forty-three patients reported injecting heroin; 33 reported injecting methamphetamine and 3 reported injecting buprenorphine. Regarding functional/emotional difficulties related to their drug/alcohol use, 38.5% reported that things were extremely stressful because of their alcohol/drug use and 41.0% reported that their alcohol/drug use has caused them to give up important activities at either a “considerable” or “extreme” level. A little more than half (53.3%) reported that they had considerable or extreme emotional problems because of their alcohol/drug use.
When asked about their health, 50.3% reported that their overall health was “fair” or “poor”. When asked about their cognitive functioning, 39.4% reported trouble understanding, concentrating or remembering. The majority of patients reported having mental health problems, a little less than half (46.4%) reported having been considerably or extremely bothered by mental health problems in the past month and 31.6% had been prescribed psychotropic medications in the last 30 days. More specifically; 63.2% reported experiencing serious depression, 72.0% reported serious anxiety or tension and 4.3% reported experiencing hallucinations. Few (2.1%) patients reported a history of suicide attempt(s) and 7.5% reported trouble controlling violent behavior. While 73.9% of patients reported having family support, 15.2% reported having no one to turn to when they were having trouble. A third of patients (35.3%) reported having attended a self-help group in the past month and 11.8% reported attending a religious or faith-based recovery group in the past month.
3.3. Patient differences across hubs
There were minimal sociodemographic and clinical differences between the patients served across the hubs. Morgantown and Charleston, two of the largest cities in the state, had more patients reporting depression and anxiety symptoms (see Table 2).
There were notable statistically significant differences across the sites in respect to patterns of alcohol and drug use (see Table 3). Patients treated in the Kanawha City site reported higher mean days of alcohol use (12.2 compared to 8.0 overall). Patients treated at the Morgantown site reported (73.7%) higher rates of overall illegal drug use (59.2%) and higher overall alcohol and illegal drug use (24.2% compared to 17.5% overall). Patients treated at the Charleston site reported significantly higher rates of any prescription opioid use (75.0% compared to 24.2%), as well as higher rates of benzodiazepine use (43.8% versus 24.2% overall). Patients treated at the Kanawha City site reported higher rates of methamphetamine use (64.3%), whereas patients at the Harpers Ferry site infrequently reported past month methamphetamine use (4.8%). There were significantly higher rates of cocaine use reported among patients treated at the Huntington site (40.0%) and at the Harpers Ferry site (30.8% versus 15.8% overall). Overall, 47.4% of patients reported illicit buprenorphine use where Morgantown had the highest percentage (69.7%).
Table 3.
Baseline alcohol and drug use characteristics (n = 196).
| Overall | Morgantown Psychiatry (n = 99) | Kanawha City Primary care (n = 42) | Harpers Ferry Primary care (n = 21) | Charleston Psychiatry (n = 17) | Huntington Psychiatry (n = 17) | p value | |
|---|---|---|---|---|---|---|---|
| Any alcohol use past 30 days | 20.4% | 28.3% | 14.3% | 9.5% | 11.8% | 11.8% | 0.14 |
| Mean days alcohol use past 30 Days | 8.0 | 6.3 | 12.2 | 30 | 2.0 | 5.5 | 0.01 |
| Mean days drinking ale. to intox. past mo. | 3.9 | 3.8 | 6.0 | 0 | 2.0 | 5.0 | 0.93 |
| Any illegal drug use past 30 days | 59.2% | 73.7% | 33.3% | 57.1% | 58.8% | 41.2% | < 0.00 |
| Injected drugs past 30 days | 36.1% | 31.1% | 52.4% | 33.3% | 21.4% | 40.0% | 0.13 |
| Any alcohol & illegal drug use past 30 days | 15.8% | 24.2% | 7.1% | 0% | 11.8% | 11.8% | 0.01 |
| Any Rx opioid use past 30 days | 24.2% | 19.2% | 16.7% | 20.0% | 75.0% | 30.8% | < 0.00 |
| Any illicit buprenorphine use | 47.4% | 69.7% | 45.2% | 4.8% | 0.0% | 23.5% | < 0.00 |
| Marijuana | |||||||
| Any use past 30 days | 51.1% | 49.0% | 69.1% | 33.3% | 43.8% | 46.2% | 0.07 |
| Mean days of use past 30 days | 13.9 | 12.1 | 19.5 | 16.3 | 5.7 | 7.6 | 0.01 |
| Methamphetamines | |||||||
| Any use past 30 days | 45.5% | 44.4% | 64.3% | 4.8% | 50.0% | 54.6% | < 0.00 |
| Mean days of use past 30 days | 9.0 | 10.1 | 9.0 | 1.0 | 6.0 | 5.2 | 0.48 |
| Any injection drug use in past 30 days | 41.3% | 47.6% | 40.7% | 0.0% | 25.0% | 16.7% | 0.58 |
| Heroin | |||||||
| Any use past 30 days | 38.6% | 34.3% | 47.6% | 35.0% | 37.5% | 50.0% | 0.56 |
| Mean days of use past 30 days | 14.4 | 13.0 | 15.3 | 23.1 | 10.6 | 11.8 | 0.13 |
| Any IV use in past 30 days | 64.2% | 54.6% | 70.0% | 100.0% | 100.0% | 50.0% | 0.12 |
| Benzodiazepines | |||||||
| Any use past 30 days | 24.2% | 22.2% | 26.2% | 5.0% | 43.8% | 38.5% | 0.05 |
| Mean days of use past 30 days | 7.3 | 6.0 | 5.9 | 2.0 | 13.6 | 9.5 | 0.15 |
| Any injection drug use in past 30 days | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cocaine/crack | |||||||
| Any use past 30 days | 15.8% | 15.2% | 2.4% | 40.0% | 12.5% | 30.8% | < 0.00 |
| Mean days of use past 30 days | 5.6 | 6.3 | 1.0 | 6.4 | 1.0 | 2.0 | 0.81 |
| Any injection drug use in past 30 days | 10.7% | 6.7% | 0.0% | 25.0% | 0.0% | 0.0% | 0.61 |
| OxyCotin/OxyCodone | |||||||
| Any use past 30 days | 10.6% | 5.1% | 14.3% | 10.0% | 31.3% | 16.7% | 0.02 |
| Mean days of use past 30 days | 8.7 | 2.4 | 6.0 | 7.0 | 20.0 | 15.0 | 0.02 |
| Any injection drug use in past 30 days | 16.7% | 20.0% | 16.7% | 50.0% | 0.0% | 0.0% | 0.76 |
4. Discussion
4.1. General discussion
WV’s overdose death rate in 2017 was more than triple the national average and for the past decade the number of overdose deaths in WV has increased annually (Scholl et al., 2018). The ability of the state to effectively respond to the opioid epidemic is constrained by not only a shortage of opioid treatment programs, but also by an overall shortage of health care professionals (HRSA, 2018). Despite the passage of the Drug Addiction Treatment Act of 2000 (DATA, 2000), which allowed for office-based prescribing of buprenorphine, national research has found that few primary care physicians are willing to be trained to prescribe buprenorphine and even those trained may not treat as many patients as allowed (Walley et al., 2008). Clinicians working in HRSA-designated health professional shortage areas are already overwhelmed and treating patients with OUD may be particularly daunting.
The WV STR was successful in training five hubs and 56 health professionals to use the COAT buprenorphine/naloxone treatment model; and the hubs have treated 196 patients with buprenorphine from January to December 2018. The five hubs serve geographically diverse regions of the state and are able to serve as local MAT champions by training and mentoring health clinics (‘spokes’) in their region. The hubs have identified a total of 13 spokes; 8 of which are beginning to deliver buprenorphine treatment as part of a comprehensive MAT program that includes psychosocial treatment. The hub and spokes represent different health care settings including psychiatry (n = 6), primary care (n = 8) and a women’s health clinic (n = 1). The WV MAT expansion was successful in involving a diversity of health professionals trained, from a variety the health care settings, because of the strong ongoing training framework provided by the WV Project ECHO MAT. Further, involvement in the WV Project ECHO MAT may be associated with the rate of buprenorphine adoption. It is certainly possible that WV Project ECHO MAT serves not only as a strategy to train providers to prescribe buprenorphine and treat patients with OUD, but also serves to provide ongoing mentorship and to build trust between the hubs and spokes.
4.2. MAT stigma
There are significant challenges and barriers to continued MAT expansion, which may threaten long-term sustainability. For example, stigma toward patients with OUD, as in many states, continues to be problematic in the recruitment of health clinics as potential spokes. Patients may also encounter stigma when attending mutual support groups (Hadland, Park, & Bagley, 2018) as required by the COAT buprenorphine treatment model. Many mutual support groups in WV are abstinence-based and they define any opioid use, including buprenorphine, to be inconsistent with their philosophy. Patients have reported that even their family members have stigmatizing views of MAT, perceiving it as drug substitution. Problems with MAT-related stigma are not unique to WV (Olsen & Sharfstein, 2014); however, rural areas make anonymity difficult and its consequences may be felt more deeply in Appalachian culture that values familism (Batteau, 1980).
4.3. Organizational and regulatory barriers
To date, only one buprenorphine waivered primary care doctor has discontinued treating patients; however, it is unknown whether preserver retention will become a larger problem in the future and whether anything can be done to prevent prescriber attrition. One major concern raised by the health professionals involved in the MAT expansion is the sustainability of case manager funding. The case managers, currently supported through the STR-funding, are a key component to the clinic structure and are desperately needed to support prescribers working with large numbers of patients. Unfortunately, none of the hubs are licensed behavioral health centers and therefore they are unable to bill for targeted case management services once STR-funding ends. Reimbursement for case management services is critical to the long-term sustainability of MAT expansion in WV, particularly in non-psychiatric settings.
The WV Project ECHO MAT provides an excellent mechanism to deliver ongoing clinical training; however, there is a need for more in-depth training for treating patients with OUD with high acuity and/or patients with complex co-occurring psychiatric and medical conditions. Whereas urban areas may be able to simply refer patients to other specialists (e.g., infectious disease or mental health clinics), in Appalachian rural counties specialty care is sparse. Therefore hubs and spokes have taken on not only buprenorphine treatment, but also treating the psychiatric and medical consequences associated with long-term opioid addiction. Finally and not surprisingly, the logistical challenges commonly found in rural economically-disadvantaged areas remain, such as transportation to treatment, childcare for patients, and availability of mutual support groups, especially those supportive of MAT.
There are WV-specific treatment delivery regulations that further confound the provision of services. Regulatory requirement 69 CSR 12, which was initiated in June 2017 for office based opioid treatment (OBOT), stipulates that OBOT settings be licensed by the WV Department of Health and Human Resources (DHHR) and subject to inspection by the Office of Health Facility Licensure and Certification (OHFLAC). The regulation outlines specific requirements including that patients be seen with certain frequency per month, with specified number if urine drugs screens monthly and required number of psychosocial interventions, which make it difficult for health care settings that do not have the capacity or resources to meet these regulatory requirements. These regulations also contribute to lower retention rates for patients who struggle with transportation or low motivation to participate in psychosocial treatment. Regulatory barriers have been documented as buprenorphine adoption barriers in other rural states (DeFlavio, Rolin, Nordstrom, & Kazal, 2015).
4.4. Limitations
Despite the strengths of the current report, there are some limitations which warrant discussion. First, providers who were trained were not formally tracked as part of the evaluation nor was a formal implementation study conducted. Second, the GPRA data collection forms do not include a question to identify patients that transferred from another MAT program nor a question on illicit buprenorphine use. The hubs were trained to track illicit buprenorphine in the ‘other’ drug category; however, it is probable that this was inconsistently captured and it is notable given that 47% of patients did report illicit buprenorphine use in the 30 days prior to intake. And finally, the discrepancies in the percent of patients reporting any illicit days of drug use suggest data collection errors may have occurred and that retraining is necessary.
4.5. Future work
Future research, once all spokes have been trained and initiated buprenorphine treatment, can be conducted to determine whether the WV modified hub-and-spoke model was effective in creating a statically significant increase in MAT capacity. According to the SAMHSA buprenorphine treatment locator, there are currently 345 buprenorphine-waivered prescribers at 225 unique sites in WV. Unlike publicly-funded substance abuse treatment programs, data on office-based buprenorphine treatment is not routinely collected and hence the unique unduplicated number of patients treated is unknown. Previous research has found that few prescribers treat the maximum number of patients (Walley et al., 2008) and therefore it would important for future research to capture new prescribers and increased capacity per existing prescribers. An implementation study is needed to further understand barriers to buprenorphine treatment from both a provider and patient perspective, and longer-term barriers to sustainability. The COAT leadership team is already engaged in work to reduce stigma associated with MAT in the state, this includes addressing stigma in educational training for both providers and community members, and working face-to-face with key stakeholders.
5. Conclusions
The STR-funded buprenorphine expansion in WV was successful in increasing buprenorphine treatment capacity and the number of patients served. While this is only a modest step in addressing the estimated 12,600 WV residents needing OUD treatment, it is an essential step forward that has built the foundation for continued expansion and provided a blueprint for ongoing clinical training for health professionals. WV’s modified hub-and-spoke model may be a mechanism to expand buprenorphine/naloxone treatment in rural areas, particularly in areas with transportation barriers and that lack geographic proximity to opioid treatment programs. Interventions to reduce stigma and efforts to reduce regulatory barriers are essential to the long-term success of the buprenorphine expansion.
Acknowledgments
Funding
This publication was supported by grants from the Substance Abuse and Mental Health Services Administration (TI080256) through a subcontract from West Virginia Department of Health and Human Resources Bureau for Behavioral Health, and from the National Institute of General Medical Sciences of the National Institutes of Health (2U54GM104942-02).
References
- Andrilla CHA, Coulthard C, & Larson EH (2017). Barriers rural physicians face prescribing buprenorphine for opioid use disorders. Annals of Family Medicine, 15, 359–362. 10.1370/afm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrilla CHA, Moore TE, Patterson DG, & Larson EH (2019). Geographic distribution of providers with a DEA waiver to prescribe buprenorphine for the treatment of opioid use disorder: A 5-year update. The Journal of Rural Health, 35, 108–112. [DOI] [PubMed] [Google Scholar]
- Appalachian Regional Commission (ARC). The Appalachian Region. (2017). https://www.arc.gov/appalachian_region/TheAppalachianRegion.asp (Accessed October 8, 2017).
- Arora S, Kalishman S, Dion D, Som D, Thornton K, Bankjurst A, … Yutzy S (2011). Partnering urban academic medical centers and rural primary care clinicians to provide complex chronic disease care. Health Affairs (Millwood), 30(6), 10.1377/hlthaff.2011.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteau A (1980). Appalachia and the concept of culture: A theory of shared misunderstandings. Appalachian Journal, 7(1/2), 9–31. [Google Scholar]
- Brooklyn JR, & Sigmon SC (2017). Vermont hub-and-spoke model of care for opioid use disorder: Development, implementation, and impact. Journal of Addiction Medicine, 11(4), 286–292. 10.1097/ADM.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFlavio JR, Rolin SA, Nordstrom BR, & Kazal LA (2015). Analysis of barriers to adoption of buprenorphine maintenance therapy by family physicians. Rural Remote Health, 15(15), 3019 (Epub 2015 Feb 4). [PubMed] [Google Scholar]
- Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie DL, & Stein BD (2015). Increasing potential access to opioid agonist treatment in U.S. treatment shortage areas. Health Affairs (Millwood), 34(6), 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, … Dowell D (2017). Vital signs: Changes in opioid prescribing in the United States, 2006–2015. Morbidity and Mortality Weekly Report, 66(26), 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Park TW, & Bagley SM (2018). Stigma associated with medication treatment for young adults with opioid use disorder: A case series. Addiction Science & Clinical Practice, 13, 15 10.1186/s13722-018-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, … Paulozzi LJ (2008). Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA, 300(22), 2613–2620. 10.1001/jama.2008.802. [DOI] [PubMed] [Google Scholar]
- Health Resource and Services Administration (HRSA) (2018). Designated health professional shortage areas. Retrieved 30 January 2019 from https://ersrs.hrsa.gov/ReportServer?/HGDW_Reports/BCD_HPSA/BCD_HPSA_SCR50_Qtr_Smry_HTML&rc:Toolbar=false.
- Ingram DD, & Franco SJ (2014). 2013 NCHS urban-rural classification scheme for counties. National Center for Health Statistics. Vital and Health Statistics, 2(166). [PubMed] [Google Scholar]
- Jones CM, Campopiano M, Baldwin G, & McCance-Katz E (2015). National and state treatment need and capacity for opioid agonist medication-assisted treatment. American Journal of Public Health, 105, e55–e63. 10.2105/AJPH.2015.302664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EB (2018). Medication-assisted opioid treatment prescribers in federally qualified health centers: Capacity laps in rural areas. The Journal of Rural Health, 34, 14–22. [DOI] [PubMed] [Google Scholar]
- Jumah NA, Edwards C, Balfour-Boehm J, Loewn K, Dooley J, Gerber FL, & Kelly L (2016). Observational study of the safety of buprenorphine/naloxone in pregnancy in a rural and remote population. BMJ Open, 6(10), e011774 10.1136/bmjopen-2016-011774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaromy M, Duhigg D, Metcalf A, Carlson C, Kalishman S, Hayes L, … Arora S (2016). Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care about treatment of substance use disorders. Substance Abuse, 37(1), 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander LR, Marshalek P, & Sullivan CR (2016). Medication-assisted treatment for pregnant women: An interdisciplinary group based model. Journal of Groups in Addiction & Recovery, 11(3), 182–193. 10.1080/1556035X.2016.1185987. [DOI] [Google Scholar]
- Lowfall M, & Walsh S (2014). A review of buprenorphine diversion and misuse: The current evidence base and experiences around the world. Journal of Addiction Medicine, 3(5), 315–326. 10.1097/ADM.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IO, Fischer G, Welle-Strand GK, Debelak K, Morrone WR, & Jones HE (2013). A comparison of buprenorphine/naloxone to buprenorphine and methadone in the treatment of opioid dependence during pregnancy: Maternal and neonatal outcomes. Substance Abuse: Research and Treatment, 2013(7), 61 10.4137/SART.S10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meit M, Heffernan M, Tanenbaum E, & Hoffmann T (2017). Appalachian diseases of despair PREPARED FOR: Appalachian Regional Commission. The Walsh Center for Rural Health Analysis, Bethesda, MD: Retrieved 30 January 2019 from https://www.arc.gov/assets/research_reports/AppalachianDiseasesofDespairAugust2017.pdf. [Google Scholar]
- Nguyen L, Lander LR, O’Grady KE, Marshalek PJ, Schmidt A, Kelly AK, & Jones HE (2018). Treating women with opioid use disorder during pregnancy in Appalachia: Initial neonatal outcomes following buprenorphine + naloxone exposure. American Journal on Addictions, 27(2), 92–96. 10.1111/ajad.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen Y, & Sharfstein JM (2014). Confronting the stigma of opioid use disorder – And its treatment. JAMA, 311(14), 1393–1394. 10.1001/jama.2014.2147. [DOI] [PubMed] [Google Scholar]
- Richman A (1977). The epidemiology of drug abuse: Current issues. Ecological studies of narcotic addiction, National Institute on Drug Abuse Research Monograph. 173–196. [PubMed] [Google Scholar]
- Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, & Parrion M (2011). Distance traveled and cross-state commuting to opioid treatment programs in the United States. Journal of Environmental and Public Health 948789. 10.1155/2011/948789 (10 pp.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen LM, Khan D, & Warner M (2013). Trends and geographic patterns in drugpoisoning death rates in the US, 1999–2009. American Journal of Preventative Medicine, 45(6), e19–e25. 10.1016/j.amepre.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe L, Kadushin C, Beveridge A, Livert D, Tighe E, Rindskopf D, … Brodsky A (2001). The visibility of illicit drugs: Implications for community-based drug control strategies. American Journal of Public Health, 91, 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L, Seth P, Kariisa M, Wilson N, & Baldwin G (2018). Drug and opioid-involved overdose deaths — United States, 2013–2017. Morbidity and Mortality Weekly Report, 67(5152), 1419–1427. 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC (2014). Access to treatment for opioid dependence in rural America: Challenges and future directions. JAMA Psychiatry, 71 (4), 359–360. [DOI] [PubMed] [Google Scholar]
- StataCorp (2017). Stata statistical software: Release 15. College Station, TX: StataCorp LLC. [Google Scholar]
- Wakeman SE (2017). Medications for addiction treatment: Changing language to improve care Journal of Addiction Medicine, 11, 1–2. 10.1097/ADM.0000000000000275. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK,, Cheng DM, Botticelli M, Castro-Donlan C, … Alford DP (2008). Journal of General Internal Medicine, 23(9), 1393–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley EL, Fishman M, & Bolon SK (2010). Treatment outcomes for addiction. In Miller NS, & Gold MS (Eds.). Addictions in medicine: Principles and practices. Wiley, Inc.. [Google Scholar]
- Zheng W, Nickasch M, Lander L, Wen S, Xiao M, Marshalek P, … Sullivan C (2017). Treatment outcome comparison between telepsychiatry and face-to-face buprenorphine medication-assisted treatment for opioid use disorder: A 2-year retrospective data analysis. Journal of Addiction Medicine, 11(2), 138–144. 10.1097/ADM.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng WH, Wakim RJ, Geary RC, Lander LR, Wen SJ, Xiao MC, & Sullivan CR (2016). Self-reported sleep improvement in buprenorphine MAT (medication assisted treatment) population. Austin Journal of Drug Abuse and Addiction, 3(1), 1–7. [PMC free article] [PubMed] [Google Scholar]
- Zullig KJ, Lander LR, Tuscano M, Hobbs GR, & Faulkenberry L (2018). Incorporating mindfulness-based relapse prevention into outpatient therapy for treatment of opioid use disorder with medication-assisted treatment. Annals of Community Medicine & Practice, 4(1), 1032. [Google Scholar]


