Abstract
Objectives:
The surface receptor MET is highly expressed on primary uveal melanoma (UM); MET inhibitors demonstrated early clinical signals of efficacy in slowing UM growth. The primary objective of our study was to compare the progression-free survival rate at 4 months (PFS4) of patients with UM treated with cabozantinib or chemotherapy.
Methods:
Patients with metastatic UM and RECIST measurable disease were randomized 2:1 to receive either cabozantinib (arm 1) versus temozolomide or dacarbazine (arm 2) with restaging imaging every two cycles. Cross-over from arm 2 to cabozantinib after progression was allowed (arm 2X). Available tumor specimens were analyzed by whole exome sequencing and results were correlated with outcome.
Results:
Forty-six eligible patients were accrued with 31, 15 and 9 in arms 1, 2 and 2X, respectively. Median lines of prior therapy, including hepatic embolization, was two. Rates of PFS4 in arm 1 and arm 2 were 32.3% and 26.7% (p=0.35), respectively, with median PFS time of 60 and 59 days (p=0.964; HR=0.99). Median overall survival was 6.4 months and 7.3 months (p=0.580; HR=1.21), respectively. Grade 3–4 CTCAE adverse events were present in 61.3%, 46.7%, and 37.5% in arms 1, 2 and 2X, respectively. Whole exome sequencing demonstrated a mean tumor mutational burden of 1.53 mutations/Mb and did not separate OS ≤ or > 1 year (p=0.14). Known mutations were identified by whole exome sequencing and novel mutations were nominated.
Conclusions:
MET/VEGFR blockade with cabozantinib demonstrated no improvement in PFS but an increase in toxicity relative to temozolomide/dacarbazine in metastatic UM.
Keywords: uveal melanoma, MET, cabozantinib, VEGFR
Introduction
Melanoma arising in the uveal tract (iris, ciliary body, and choroid) is the most common intraocular malignancy though an uncommon type of all melanomas(1). Following management of primary uveal melanoma (UM), patients retain an approximately 50% risk of metastasis. Metastasis has preferential hepatic tropism with median survival of 6–12 months following distant disease(1). To date there are no systemic therapies that have demonstrated a consistent benefit for metastatic UM including immune checkpoint inhibitors(2,3) and targeted therapies(4), and therefore hepatic tumor embolization or alkylating chemotherapy (temozolomide or dacarbazine) remain default approaches(1).
As opposed to cutaneous melanoma(5), UMs lack mutations of B-RAF, N-RAS, and c-KIT; however, the majority carry a mutation in either the G-protein α-subunit q (GNAQ) or 11 (GNA11)(6). GNAQ/GNA11 signaling is thought to drive phospholipase C and other downstream targets to stimulate mitogen-activated protein kinase (MAPK)(7). Beyond the G-α pathway, several other genes have also been identified as recurrently dysregulated or overexpressed. These include the tumor suppressor BAP1, RNA splicing factor SF3B1, the transcription initiation factor EIF1AX, and the phospholipase C regulator PCLB4 in the rare non-GNAQ/GNA11 mutated UM(7).
In addition to recurrently mutated genes, overexpression of the receptor tyrosine kinase MET is described in primary UM in up to 83% of assayed samples(8). MET expression has been associated with a significantly higher risk of death from metastatic disease(9) and MET expression influences melanoma-specific mortality(10). MET being an influencing factor in liver metastasis in UM is logical given that the MET ligand hepatocyte growth factor (HGF) is produced in significant quantities in the liver. The migratory ability of UM cells is promoted by HGF and enhances motility and invasion in UM murine models(11). MET blockade by short hairpin RNA or selective inhibitors demonstrated significant inhibition of tumor cell proliferation, inhibition of cell migration and reduction in metastases(8,12,13).
Cabozantinib is small molecule inhibitor of multiple receptor tyrosine kinases, notably including MET and vascular endothelial growth factor receptor 2 (VEGFR2) as well as additional targets including RET, AXL, KIT, and TIE-2(14). Cabozantinib has been approved for or demonstrated significant activity in medullary thyroid, renal cell as well as hepatocellular carcinoma and has demonstrated bone-centric activity in diseases such as castration-resistant prostate cancer and osteosarcoma(14–16). Cabozantinib was investigated in melanoma via a randomized discontinuation study including 23 patients with UM(17). These patients were described to have substantial tumor burden with median sum of the longest diameter of target lesions of 11.9 cm and hepatic metastases were present in 70%. While no responses were observed in these patients, 61% of patients had stable disease at week 12 with a median progression-free survival (PFS) of 4.8 months. The rate of PFS at 6 months was 41%, six patients stayed on treatment for >10 months and overall survival was 12.6 months. Two patients with bone metastases, who had a baseline bone scan, experienced partial resolution of their bone lesions during treatment with cabozantinib. Here we describe Alliance for Clinical Trials in Oncology A091201, a randomized phase II trial of the multiple TKI cabozantinib that also inhibits MET and VEGFR2 compared with temozolomide (TMZ) or dacarbazine (DTIC) in patients with metastatic UM.
Materials and Methods
Patient Eligibility:
The trial was reviewed and approved by the NCI Central Institutional Review Board (CIRB) or the IRB of each participating site (ClinicalTrials.gov Identifier: NCT01835145). All patients had to meet eligibility criteria including, but not limited to: histologically confirmed metastatic UM, Eastern Cooperative Oncology Group (ECOG) performance status (0–1), response evaluation criteria in solid tumors (RECIST) version 1.0 measurable disease, any number of prior therapies except MET/VEGFR2 inhibitors or alkylating chemotherapy and no increased risk of thrombosis, hemorrhage or pancreatitis as well as standard biochemical parameters including hepatic liver enzymes up to 5 times the upper limit of normal. Each participant signed an IRB-approved, protocol-specific, informed written consent document. This trial was conducted in accordance with Declaration of Helsinki and institutional guidelines.
Trial Design:
A091201 was a randomized phase II trial evaluating cabozantinib (arm 1) versus TMZ or DTIC (arm 2) in patients with metastatic UM. Patients were randomized 2:1 towards arm 1 with stratification factors including prior exposure to MEK inhibitor and site of metastasis (liver versus other). Patients in arm 2 had the potential to cross-over to treatment with cabozantinib (arm 2X) after progression on chemotherapy by RECIST criteria, or resolution of dose-limiting toxicity; these patients were required to meet all eligibility criteria at the time of cross over, as pre-specified in the study protocol. The primary objective was to evaluate whether cabozantinib could improve the 4-month progression-free survival (PFS4) in UM patients from 15%, as previously described for temozolomide(18), to 40% with cabozantinib. A one-sided, two-group, chi-squared test of equal proportions with a 10% type I error was pursued. A total sample size of 66 evaluable patients was proposed with 81% power to detect a difference in PFS4 rates of 0.25 (0.40 versus 0.15). A futility stopping rule was included so that accrual would stop in the event that fewer than 6 of the first 22 patients were progression free at 4 months. Accrual did not stop while assessing the futility stopping rule. The proportion of progression-free patients at the 4-month restaging is presented with a 90% exact binomial confidence interval. Progression-free survival was defined as the time from the start of treatment until disease progression or death from any cause; overall survival (OS) was defined as the time from the start of treatment until death from any cause. The distributions of OS and PFS are presented using the method of Kaplan-Meier with 90% confidence intervals estimated using log(-log(endpoint)) methods. Descriptive statistics (means, standard deviations, medians, ranges, and percentages) are reported for baseline clinical and demographic data.
Secondary endpoints included PFS, OS, RECIST response rate (RR) and safety assessment of each arm. Adverse events were scored based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE v4.0). The trial accrual proceeded from September 18, 2013 to April 21, 2016.
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
This randomized phase II therapeutic trial was monitored at least twice annually by the Alliance Data and Safety Monitoring Board, a standing committee composed of individuals from within and outside of the Alliance.
Whole exome sequencing and analysis:
To inform drug development in mUM, baseline metastatic tumor samples (n=19; 1 lung, 18 liver) were studied by whole exome sequencing (WES) in exploratory fashion following trial completion. Formalin-fixed paraffin-embedded (FFPE) tumor biopsies were collected and reviewed first for diagnostic confirmation and grade tumor percentage by pathologists at the University of Chicago. Tumor DNA were isolated from tumor samples using the QIAGEN AllPrep DNA/RNA FFPE kit (Qiagen, Hilden, Germany), and the integrity and quantification were evaluated on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA) and qubit (Thermo Fisher, Waltham, USA), respectively. 200 ng of DNA was used for whole exome + UTR capture using the Agilent SureSelect Human All Exon V6 plus UTR kit (Agilent Technologies, Santa Clara, USA). 100bp paired-end sequencing reads were generated on an Illumina HiSeq 4000 instrument (Illumina, San Diego, USA) at the University of Chicago Functional Genomics Facility.
The raw sequencing data were analyzed following previously described protocols (19). In brief, the quality of raw reads was assessed by FastQC (v0.11.5), and preprocessed to trim adaptors and merge 3’ overlapping mates using FLASh (v1.2.11). Clean reads were aligned to human reference genome (GRCh38) by BWA-MEM (v0.7.17), followed by duplicate read removal, low mapping quality alignment (<30) removal, and base quality score recalibration by GATK4 (v4.0.10.1). Putative somatic mutations (single nucleotide variants (SNVs) and small insertions/deletions (indels)) were detected by somatic variant caller GATK4-MuTect2. Stringent filters were applied to the variant calls that passed the default setting of MuTect2 to further remove potential germline variants identified as those present at allele frequency (AF) ≥ 0.0001 in 1000 Genomes Project (G1000), the NHLBI Grand Opportunity Exome Sequencing Project (ESP), or the Exome Aggregation Consortium (ExAC) on non-TCGA samples. Variants that passed all filters were carried on for annotation using ANNOVAR (April 2018 release). The tumor mutational mutation burden was calculated by the number of mutations that were predicted to cause protein sequencing change, including non-synonymous/stopgain/stoploss SNVs, frameshift/non-frameshift indels, and variants that modify splicing sites.
Results
Baseline Patient Characteristics
A total of 46 eligible patients were enrolled in this trial, including 31 in arm 1 and 15 in arm 2. One patient in arm one was deemed ineligible after being enrolled because their initial aspartate aminotransferase value was outside the range required by the protocol; this patient was not included in the trial outcome analyses. Nine patients proceeded from arm 2 into arm 2X to receive cabozantinib.
Patient baseline characteristics for each cohort are described in Table 1. The median age for entire cohort was 62.5 years (range: 30 to 86) with 56.5% male and median performance status 0. The median disease-free interval (i.e. time between primary diagnosis and metastatic date) for arm 1 was 41.0 months (range 0 to 355.8 months) and arm 2 was 47.8 months (range of 0.2–263.3 months) with overall median of 42.7 months. Liver metastases were present in 44 patients (95.7%); 21 patients (45.7%) had liver only disease. Baseline lactate dehydrogenase (LDH) was above the upper limit of normal in 29 patients (63%). Other common sites of disease included lung (41.3%) and bone (21.7%). All patients had undergone prior surgery or radiation to the primary lesion and median lines of therapy in the metastatic setting was 2. Prior treatment included ipilimumab in 26% of patients, anti-PD1 antibodies in 17% (no patients received ipilimumab plus nivolumab) and hepatic arterial embolization in 13%.
Table 1.
Patient characteristics
| A091201 Patient Characteristics | ||||
|---|---|---|---|---|
| 1 (N=31) | 2 (N=15) | Total (N=46) | p value | |
| Age | 0.2409 | |||
| Median | 60.0 | 67.0 | 62.5 | |
| Gender | 0.7406 | |||
| M | 17(54.8%) | 9 (60.0%) | 26 (56.5%) | |
| Race | ||||
| White | 31 (100.0%) | 15 (100.0%) | 46 (100.0%) | |
| ECOG Performance Status | 0.1571 | |||
| 0 | 23 (74.2%) | 8 (53.3%) | 31 (67.4%) | |
| 1 | 8 (25.8%) | 7 (46.7%) | 15 (32.6%) | |
| Prior treatment with a MEK Inhibitor | 0.4819 | |||
| No | 30 (96.8%) | 15 (100.0%) | 45 (97.8%) | |
| Site of meta static disease | 0.5924 | |||
| Liver (only) | 15 (48.4%) | 6 (40.0%) | 21 (45.7%) | |
| Other site | 16 (51.6%) | 9 (60.0%) | 25 (54.3%) | |
| Elevated LDH | 0.1094 | |||
| Yes | 22 (71.0%) | 7 (46.7%) | 29 (63.0%) | |
| Liver | 0.3145 | |||
| Yes | 29 (93.5%) | 15 (100.0%) | 44 (95.7%) | |
| Bone | 0.8423 | |||
| No | 24 (77.4%) | 12 (80.0%) | 36 (78.3%) | |
| Prior systemic therapy* | 0.7661 | |||
| Yes | 11 (35.5%) | 6 (40.0%) | 17 (37.0%) | |
| Prior hepatic arterial embolization (HAE) | 0.9676 | |||
| Yes | 4 (12.9%) | 2 (13.3%) | 6 (13.0%) | |
Includes three treatments of hepatic immunoembolization not captured in HAE (all three in Arm 1)
Treatment Outcome
Outcomes for response, median PFS, PFS2, PFS4 and OS are described in Table 2. One patient in arms 1 had an unconfirmed RECIST response and best overall response for all patients who underwent a restaging imaging scan after treatment initiation are shown in Supplementary Figure 1.
Table 2.
Clinical outcomes by response, PFS, PFS4, OS
| Arm | Response | Progression-Free Survival (median) | Progression Free at 2 Months | Progression Free at 4 Months | Overall Survival |
|---|---|---|---|---|---|
| 1 | 0/31* | 60 days | 41.9% | 32.3% | 6.4 months |
| 2 | 0/15 | 59 days | 46.7% | 26.7% | 7.3 months |
One unconfirmed response was observed in arm 1 with cabozantinib
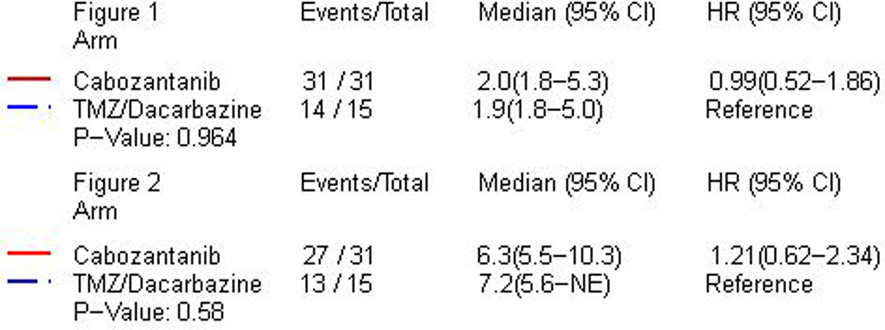
Of the 31 patients randomized to arm 1, 10 met the primary endpoint of PFS4 (32.3%) compared to 4 of 15 randomized to arm 2 (26.7%; p=0.350). Of the first 22 patients treated in arm 1, 5 were progression free at 4 months and the futility stopping rule was triggered though accrual continued during analysis. Progression-free survival for arm 1 and arm 2 are shown in Figure 1. No difference in median PFS was observed between arm 1 at 60 days (95% CI: 56–162 days) compared with arm 2 at 59 days [95% CI: 56–152 days; p=0.964, hazard ratio (HR) 0.99 (95% CI: 0.51–1.86)]. The trial was terminated by the Alliance Data and Safety Monitoring Board for futility after the interim analysis.
Figure 1.
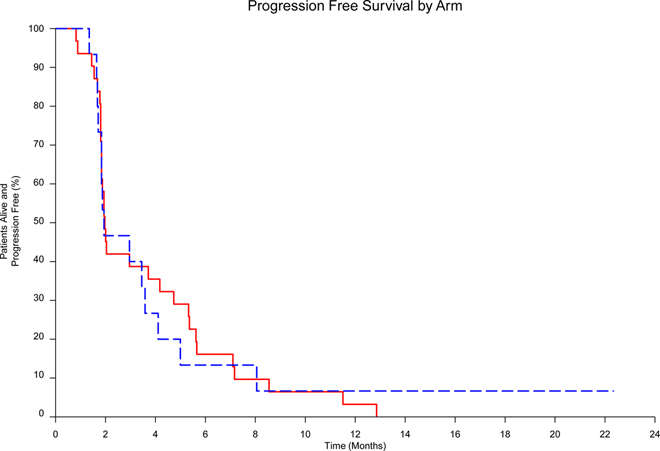
Progression-free survival of Cabozantinib vs TMZ/Dacarbazine)
There remained 6 patients alive at analysis with a median follow-up time of 2.1 years (range 1.9–2.3 years). Four of these patients were randomized to the cabozantinib arm and the other 2 were randomized to the TMZ/DTIC arm. The median OS in arm 1 was 191 days (6.4 months; 95% CI: 168–314) versus 218 days (7.3 months; 95% CI: 170-NA days) in arm 2 with logrank test indicating no difference [p=0.580, HR=1.21 (95% CI: 0.62–2.34)]. Kaplan-Meier analysis of OS is shown in Figure 2.
Figure 2.
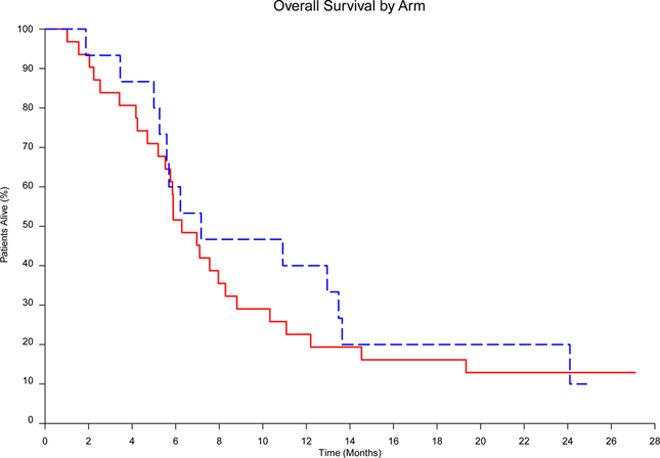
Overall survival of Cabozantinib vs TMZ/Dacarbazine
Of the 9 patients who proceeded from arm 2 to arm 2X, the median PFS, as measured from the time of cross over to progression, was 63.5 days and rate of PFS4 was 33.3%.
Treatment-Related Adverse Events
Adverse event reporting is summarized by high-grade adverse events in Table 3 and all events appear, by arm, in Supplementary Table 1. All patients described adverse events irrespective of attribution; grade 3–4 adverse events were 71.0% and 66.7% in arms 1 and 2, respectively. Common attributable grade 3–4 events included fatigue, increased aspartate aminotransferase (AST) or alanine aminotransferase (ALT) and thromboembolic events. Grade 3 or higher adverse events were present in 51.6% and 20.0% in arms 1 and 2, respectively. Of the 9 patients who proceeded from arm 2 to arm 2X to receive cabozantinib, grade 3 or higher adverse events were present in 33.3% of patients. The median percentage of predicted dose delivered by arm was 87%, 100% and 100% for arm 1, 2 and 2X, respectively. For patients in each arm who experienced Grade 3–4 toxicity the median percentage of dose was 67%, 100% and 65%.
Table 3.
High-grade adverse events
| N | % | ||
|---|---|---|---|
| Patients with at least one: | Arm | 16 | 51.6 |
| Grade 3+ Adverse Event | 1 | ||
| 2 | 3 | 20.0 | |
| 2X | 3 | 33.3 | |
| Grade 4+ Adverse Event | 1 | 1 | 3.2 |
| 2 | 2 | 13.3 | |
| Grade 3+ Hem Adverse Event | 2 | 2 | 13.3 |
| Grade 4+ Hem Adverse Event | 2 | 2 | 13.3 |
| Grade 3+ Non-Hem Adverse Event | 1 | 16 | 51.6 |
| 2 | 1 | 6.7 | |
| 2X | 3 | 33.3 | |
| Grade 4+ Non-Hem Adverse Event | 1 | 1 | 3.2 |
Whole Exome Sequencing Results
Baseline metastatic tumor samples (n=19; 1 lung, 18 liver) were studied by whole exome sequencing (WES) to determine the number and frequency of genetic alterations, as summarized in Figure 3A. Within the G protein–coupled receptor (GPCR) signaling pathway, mutations in GNA11/Q were enriched (20). Other previously described mutations found in UM populations include SF3B1 (37%, n=7) and BAP1 (26%, n=5). Mutations not well described previously were also identified, though without matched normal DNA; these are pending validation and are presented for exploratory purposes (Supplementary Figure 2). This includes mutations in GOLGA6L10 (32%, n=6), PKD1L3 (26%, n=5), and FAM228B (16%, n=3). The total tumor mutational burden (TMB) was also calculated for each sample and demonstrated a TMB of 46±4 (mean±SEM); this did not separate OS ≤ or > 1 year (p=0.14; Figure 3B). Noting that somatic mutations per megabase (mut/Mb) has become a more conventional reporting method for TMB, a mean TMB of 1.53 mut/Mb was calculated using previously-described standardization methods(21).
Figure 3. Somatic mutations in UM cases by whole exome sequencing.
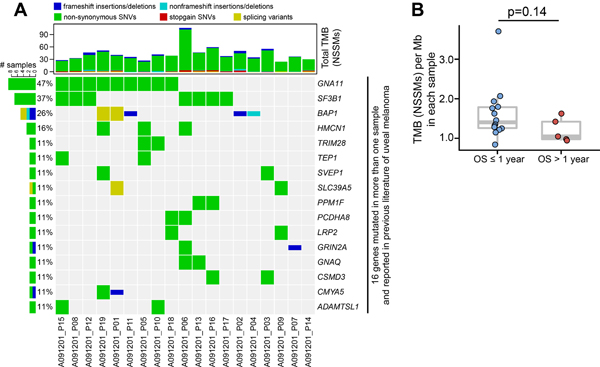
(A) Profiles of recurrent non-synonymous somatic mutations (NSSMs) in UM tumor including previously literature identified genes. Each column represents a separate case. Above each column is the mutational burden of each case as assessed by the total number of NSSMs per tumor. (B) Tumor mutational burden (TMB) in patients with OS ≤ or > one year. Mann-Whitney U test was used in B.
Discussion
Outcomes for patients with metastatic UM are poor and no systemic therapies are clearly associated with a benefit(1). Based on the observation of high expression of the MET receptor in UM(12), we performed a randomized phase II study investigating the clinical activity of the MET inhibitor cabozantinib as compared with chemotherapy. We observed no confirmed RECIST quality responses to either cabozantinib or chemotherapy and noted no difference in PFS or OS, with the clinical trial being discontinued at interim futility analysis.
These results come as somewhat of a surprise given previous early phase trial experience evaluating cabozantinib in patients with UM, where a median PFS of greater than 4 months was described(17). The results are in line with previously published PFS for chemotherapy in metastatic UM(18). These results also may call into question whether pursuing therapeutic targets based on data from the primary setting (i.e., MET) should be prioritized in trials of metastatic disease. Noting the randomized discontinuation design of the previous trial, a major point of difference may concern patient selection, with A091201 predominately drawing from the community practice setting as opposed to referral center phase I programs in the prior study. The baseline patient characteristics in A091201 suggested a poor risk group with nearly all patients having liver metastases and high levels of LDH. In addition, rates of adverse events with cabozantinib may have been higher in the community practice setting, where at the time of the trial there was less experience using cabozantinib. In the study, patients treated in Arm 1 experienced more toxicity (measured by grade 3–4 events) relative to Arm 2. It appears that patients treated in Arm 1 received lower drug exposure than anticipated and this could have affected the efficacy results.
Prior to and during the study accrual period, data emerged suggesting MEK inhibition as a useful therapeutic modality in UM(22),and it was therefore deemed necessary to stratify patients within the study by previous MEK inhibitor therapy. This turned out not to be necessary as only one patient had previous MEK inhibitor treatment and subsequent studies have called into question the broad applicability of MEK inhibition for metastatic UM(4). Additionally, we observed that rates of liver directed treatments, such as hepatic arterial embolization, appeared to be lower than would generally be expected. These points raise the peculiarities of designing clinical trials in rare patient populations where treatment at academic centers may follow different practice patterns relative to the community setting. Accrual to the study was slower than expected due to the rise of immune-checkpoint blockade in melanoma, though it is noted that any possible benefit of checkpoint immunotherapy in UM is quite modest(2,3).
Relative to future trials in UM, a general cross-study comparison to note is the similarity of PFS for therapies that have been deemed to be clinically ineffective. In A091201 the median PFS was nearly identical between experimental and control groups at approximately 2 months (n=46 patients). In the SUMIT trial of selumetinib plus DTIC versus DTIC plus placebo, a median PFS was observed to be 2.8 (n=97 patients) versus 1.8 months (n=32 patients), respectively (HR 0.78; 95% CI, 0.48–1.27; P = .32) (4). Therefore, a reasonable historical comparison from randomized clinical trials of metastatic UM could be considered to be a weighted median of 2.4 months (175 patients A091201 and SUMIT) or 1.9 months (78 patients A091201 plus patients treated with DTIC plus placebo in SUMIT). As mentioned above, accrual to A091201 was slow as it became unclear to the field during the study period (and remains so) what an appropriate control arm therapy should be for metastatic UM. Chemotherapy is historically ineffective in UM(1) and there was reticence among some investigators surrounding the DTIC/TMZ treatment arm, yet checkpoint immunotherapy is only modestly effective. While combined checkpoint therapy has, to date, provided a modest benefit with response rates ranging from 10–17% in single-arm phase II studies, it is more toxic than single agent checkpoint blockade(23). Given the number of patients treated across A091201 and SUMIT with consistent PFS outcomes, perhaps single arm designs for future studies could be explored to limit the number of patients accrued to treatments that the community deems as ineffective.
Cabozantinib is an inhibitor of a broad spectrum of tyrosine kinases, including but not limited to MET, VEGFR2, RET, AXL, KIT, and TIE-2(14) and this kinome spectrum may provide insight on UM tumor dependencies and combination strategies to prioritize. Clinical trials have reported promising outcomes of patients treated on single arm phase II studies of angiogenesis-targeting approaches such as sunitinib(24). However, a randomized phase II study of sunitinib versus chemotherapy did not demonstrate significant clinical activity(25). As cabozantinib is a more potent inhibitor of VEGFR2, the results of A091201 would suggest de-prioritization of VEGF(R) blockade in UM as monotherapy. Recent studies of cabozantinib in combination with anti-PD1 immunotherapy in genitourinary malignancies have suggested a benefit, even in non-T cell-inflamed tumor types such as penile cancer(26,27). The proposed immunologic mechanisms of this additive benefit could include impact on T cell trafficking via inhibition of VEGF(28), innate immunity via TAM (Tyro3, Axl, Mer) kinases(29) and others. The majority of UM have been described as non-T cell-inflamed(7,30) and based on these arguments the combination of cabozantinib with immunotherapy might be considered in metastatic UM as well. Particularly it is of interest to consider combination strategies beyond checkpoint blockade with novel immunotherapeutics such as engineered T cell receptor anti-CD3 anti-gp100 bispecific molecules(31) or tumor-infiltrating lymphocytes(32).
This study represents one of few cohorts of metastatic UM samples to be characterized by WES. The mutational patterns are in part consistent with prior descriptions of primary UM tumor samples. For example, mutations in the GPCR signaling pathway were most common: GNA11 occurred more frequently than GNAQ, and in a mutually exclusive pattern(20). Mutations in SF3B1 and BAP1 were also common; BAP1 mutations occurred exclusively in the presence of SF3B1 wild-type tumors as has previously been reported in the analyses of primary UM samples(33). Other mutations that have not been well described in UM were also observed in exploratory fashion and will be of interest as larger data sets of metastatic UM are characterized.
Whereas cutaneous melanoma is known to have a high rate of somatic mutations with a total TMB > 400, the TMB for this study cohort was low at 46±4 (mean±SEM)(27). As a biomarker, TMB has also been explored in cutaneous melanoma where, when combined with interferon-gamma gene expression signatures, a high mutational burden increased the prognostic power to predict a prolonged relapse-free survival in stage III melanoma. However, on its own TMB failed to distinguish responders and non-responders to BRAF/MEK targeted therapies(34,35). Similarly, in this study cohort, TMB failed to differentiate OS > 1 year to OS ≤1, suggesting that TMB as a singular biomarker may not accurately predict survival in UM.
In summary, this randomized phase II study demonstrated no improvement of PFS or OS for cabozantinib as compared with chemotherapy in patients with metastatic UM. Toxicities were substantial, although they might be less now that cabozantinib has obtained broad usage in thyroid, kidney and liver malignancies. Clinical outcomes were similar to other recent randomized studies in UM, potentially suggesting a historical reference point for patients treated outside of major referral centers. Though little future seems likely as monotherapy, exploratory studies in genitourinary cancers (36) suggest a possible utility for cabozantinib in combination with immunotherapy even in non-T cell-inflamed tumors potentially including UM.
Supplementary Material
Translational Relevance.
Uveal melanoma is a rare subset of all melanomas with particularly poor outcomes in the metastatic setting. There is no clear standard of care therapy for metastatic disease as these melanomas lack BRAF mutations and only rarely respond to immune checkpoint blockade. Primary uveal melanoma overexpresses MET kinase, with preclinical studies suggesting inhibition of proliferation with MET blockade. Cabozantinib is a MET/VEGFR2 kinase inhibitor and a randomized discontinuation phase I study of cabozantinib suggested a preliminary benefit in metastatic uveal melanoma. We performed a national, rare tumor, randomized phase II study comparing cabozantinib with chemotherapy, observing no confirmed objective responses or differences in progression-free or overall survival between treatment arms. Whole-exome sequencing of available tumor tissue demonstrated known mutations such as GNAQ/11, SF3B1, BAP1 similar to what has been observed in primary disease
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882 and U24CA196171 (to the Alliance for Clinical Trials in Oncology), U10CA180836, UG1CA189960, and U10CA180863 (CCTG). JJL acknowledges Department of Defense Career Development Award (W81XWH-17-1-0265), National Cancer Institute Cancer Clinical Investigator Team Leadership Award (P30 CA014599), the Arthur J Schreiner Family Melanoma Research Fund, the J. Edward Mahoney Foundation Research Fund, Brush Family Immunotherapy Research Fund and Buffet Fund for Cancer Immunotherapy. DJO acknowledges the Clinical Therapeutics Training Grant (NIH/NIGMS T32GM007019). Also supported in part by funds from Exelixis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. https://acknowledgments.alliancefound.org
Footnotes
co-first authors
Conflict of Interest Disclosures: JJL declares Data and Safety Monitoring Board: TTC Oncology, Scientific Advisory Board: 7 Hills, Actym, Alphamab Oncology, Array, BeneVir, Mavu, Tempest, Consultancy: Aduro, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Castle, CheckMate, Compugen, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Leap, Merck, Mersana, NewLink, Novartis, RefleXion, Spring Bank, Syndax, Tempest, Vividion, WntRx, Research Support: (all to institution for clinical trials unless noted) AbbVie, Array (Scientific Research Agreement; SRA), Boston Biomedical, Bristol-Myers Squibb, Celldex, CheckMate (SRA), Compugen, Corvus, EMD Serono, Evelo (SRA), Delcath, Five Prime, FLX Bio, Genentech, Immunocore, Incyte, Leap, MedImmune, Macrogenics, Novartis, Pharmacyclics, Palleon (SRA), Merck, Tesaro, Xencor, Travel: Array, AstraZeneca, Bayer, BeneVir, Bristol-Myers Squibb, Castle, CheckMate, EMD Serono, IDEAYA, Immunocore, Janssen, Jounce, Merck, Mersana, NewLink, Novartis, RefleXion, Patents: (both provisional) Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti-PD-1/PD-L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof).
Bibliography
- 1.Luke JJ, Triozzi PL, McKenna KC, Van Meir EG, Gershenwald JE, Bastian BC, et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res 2015;28(2):135–47 doi 10.1111/pcmr.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke JJ, Callahan MK, Postow MA, Romano E, Ramaiya N, Bluth M, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013;119(20):3687–95 doi 10.1002/cncr.28282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122(21):3344–53 doi 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, et al. Selumetinib in Combination With Dacarbazine in Patients With Metastatic Uveal Melanoma: A Phase III, Multicenter, Randomized Trial (SUMIT). J Clin Oncol 2018;36(12):1232–9 doi 10.1200/JCO.2017.74.1090. [DOI] [PubMed] [Google Scholar]
- 5.Luke JJ, FLaherty KT, Ribas A, GV L Optimizing clinical outcomes in advanced-stage melanoma with targeted agents and immunotherapies Nature Reviews Clinical Oncology 2017. [DOI] [PubMed] [Google Scholar]
- 6.Zuidervaart W, van Nieuwpoort F, Stark M, Dijkman R, Packer L, Borgstein AM, et al. Activation of the MAPK pathway is a common event in uveal melanomas although it rarely occurs through mutation of BRAF or RAS. British journal of cancer 2005;92(11):2032–8 doi 10.1038/sj.bjc.6602598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017;32(2):204–20 e15 doi 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdel-Rahman MH, Boru G, Massengill J, Salem MM, Davidorf FH. MET oncogene inhibition as a potential target of therapy for uveal melanomas. Invest Ophthalmol Vis Sci 2010;51(7):3333–9 doi iovs.09–4801 [pii] 10.1167/iovs.09-4801. [DOI] [PubMed] [Google Scholar]
- 9.Mallikarjuna K, Pushparaj V, Biswas J, Krishnakumar S. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: an immunohistochemical study. Curr Eye Res 2007;32(3):281–90 doi 773375054 [pii] 10.1080/02713680601161220. [DOI] [PubMed] [Google Scholar]
- 10.Economou MA, All-Ericsson C, Bykov V, Girnita L, Bartolazzi A, Larsson O, et al. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: intercorrelation and prognostic implications. Acta ophthalmologica 2008;86 Thesis 4:20–5 doi 10.1111/j.1755-3768.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 11.Rusciano D, Lorenzoni P, Burger MM. Expression of constitutively activated hepatocyte growth factor/scatter factor receptor (c-met) in B16 melanoma cells selected for enhanced liver colonization. Oncogene 1995;11(10):1979–87. [PubMed] [Google Scholar]
- 12.Wu X, Zhou J, Rogers AM, Janne PA, Benedettini E, Loda M, et al. c-Met, epidermal growth factor receptor, and insulin-like growth factor-1 receptor are important for growth in uveal melanoma and independently contribute to migration and metastatic potential. Melanoma Res 2012. doi 10.1097/CMR.0b013e3283507ffd. [DOI] [PubMed] [Google Scholar]
- 13.Surriga O, Rajasekhar VK, Ambrosini G, Dogan Y, Huang R, Schwartz GK. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol Cancer Ther 2013;12(12):2817–26 doi 10.1158/1535-7163.MCT-13-0499. [DOI] [PubMed] [Google Scholar]
- 14.Markowitz JN, Fancher KM. Cabozantinib: A Multitargeted Oral Tyrosine Kinase Inhibitor. Pharmacotherapy 2018;38(3):357–69 doi 10.1002/phar.2076. [DOI] [PubMed] [Google Scholar]
- 15.Fioramonti M, Fausti V, Pantano F, Iuliani M, Ribelli G, Lotti F, et al. Cabozantinib Affects Osteosarcoma Growth Through A Direct Effect On Tumor Cells and Modifications In Bone Microenvironment. Scientific reports 2018;8(1):4177 doi 10.1038/s41598-018-22469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fioramonti M, Santini D, Iuliani M, Ribelli G, Manca P, Papapietro N, et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget 2017;8(12):20113–21 doi 10.18632/oncotarget.15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daud A, Kluger HM, Kurzrock R, Schimmoller F, Weitzman AL, Samuel TA, et al. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. British journal of cancer 2017;116(4):432–40 doi 10.1038/bjc.2016.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedikian AY, Papadopoulos N, Plager C, Eton O, Ring S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res 2003;13(3):303–6 doi 10.1097/01.cmr.0000056231.78713.e2. [DOI] [PubMed] [Google Scholar]
- 19.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018;359(6371):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363(23):2191–9 doi 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang H, Sasson A, Srinivasan S, Golhar R, Greenawalt DM, Geese WJ, et al. Bioinformatic Methods and Bridging of Assay Results for Reliable Tumor Mutational Burden Assessment in Non-Small-Cell Lung Cancer. Mol Diagn Ther 2019. doi 10.1007/s40291-019-00408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014;311(23):2397–405 doi 10.1001/jama.2014.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piulats Rodriguez JM, De La Cruz Merino L, Espinosa E, Alonso Carrión L, Martin Algarra S, López-Castro R, et al. 1247PDPhase II multicenter, single arm, open label study of nivolumab in combination with ipilimumab in untreated patients with metastatic uveal melanoma (GEM1402.NCT02626962). Annals of Oncology 2018;29(suppl_8) doi 10.1093/annonc/mdy289.003. [DOI] [Google Scholar]
- 24.Mahipal A, Tijani L, Chan K, Laudadio M, Mastrangelo MJ, Sato T. A pilot study of sunitinib malate in patients with metastatic uveal melanoma. Melanoma Res 2012;22(6):440–6 doi 10.1097/CMR.0b013e328358b373. [DOI] [PubMed] [Google Scholar]
- 25.Sacco JJ, Nathan PD, Danson S, Lorigan P, Nicholson S, Ottensmeier C, et al. Sunitinib versus dacarbazine as first-line treatment in patients with metastatic uveal melanoma. Journal of Clinical Oncology 2013;31(15_suppl):9031- doi 10.1200/jco.2013.31.15_suppl.9031. [DOI] [Google Scholar]
- 26.Nadal R, Mortazavi A, Stein MN, Pal SK, Lee DK, Parnes HL, et al. Clinical efficacy of cabozantinib plus nivolumab (CaboNivo) and CaboNivo plus ipilimumab (CaboNivoIpi) in patients (pts) with chemotherapy-refractory metastatic urothelial carcinoma (mUC) either naïve (n) or refractory (r) to checkpoint inhibitor (CPI). Journal of Clinical Oncology 2018;36(15_suppl):4528- doi 10.1200/JCO.2018.36.15_suppl.4528. [DOI] [Google Scholar]
- 27.Spranger S, Luke JJ, Bao R, Zha Y, Hernandez KM, Li Y, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci U S A 2016;113(48):E7759-E68 doi 10.1073/pnas.1609376113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018;15(5):325–40 doi 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akalu YT, Rothlin CV, Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev 2017;276(1):165–77 doi 10.1111/imr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson DB, Bao R, Ancell KK, Daniels AB, Wallace DE, Sosman JA, et al. Response to Anti-PD1 in Uveal Melanoma Without High Volume Liver Metastasis. Journal of the National Comprehensive Cancer Network 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T, Nathan PD, Hernandez-Aya LF, Sacco JJ, Orloff MM, Truscello J, et al. Intra-patient escalation dosing strategy with IMCgp100 results in mitigation of T-cell based toxicity and preliminary efficacy in advanced uveal melanoma. Journal of Clinical Oncology 2017;35(15_suppl):9531- doi 10.1200/JCO.2017.35.15_suppl.9531. [DOI] [Google Scholar]
- 32.Chandran SS, Somerville RPT, Yang JC, Sherry RM, Klebanoff CA, Goff SL, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol 2017;18(6):792–802 doi 10.1016/S1470-2045(17)30251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, et al. Driver Mutations in Uveal Melanoma: Associations With Gene Expression Profile and Patient Outcomes. JAMA ophthalmology 2016;134(7):728–33 doi 10.1001/jamaophthalmol.2016.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribas A, Dreno B, Garbe C, Robert C, McArthur G, Caro I, et al. Genomic features of complete responders (CR) versus fast progressors (PD) in patients with BRAFV600-mutated metastatic melanoma treated with cobimetinib + vemurafenib or vemurafenib alone. Annals of Oncology 2016;27(suppl_6) doi 10.1093/annonc/mdw379.03. [DOI] [Google Scholar]
- 35.G.V. L, A. H, M. S, V.G. A, M. M, V. C-S, et al. Updated relapse-free survival (RFS) and biomarker analysis in the COMBI-AD trial of adjuvant dabrafenib + trametinib (D + T) in patients (pts) with resected BRAF V600–mutant stage III melanoma. LBA 43. 2018. ESMO Congress. [Google Scholar]
- 36.Apolo AB, Mortazavi A, Stein MN, Pal SK, Davarpanah NN, Parnes HL, et al. A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. Journal of Clinical Oncology 2017;35(6_suppl):293- doi 10.1200/JCO.2017.35.6_suppl.293. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


