In addition to providing sensory stimuli, usually taste, smell and sight, olive oil contains a range of minor components, mostly phenolic in nature. These components are endowed with pharmacological or pharma‐nutritional properties that are the subject of active research worldwide. Based on our more than 25 years of experience in this field, we critically focus on what we believe are the most pharmacologically prominent actions of the constituents of olive oil. Most of the effects are due to the phenolic compounds in extra virgin olive oil, such as hydroxytyrosol and oleocanthal (which are often mis‐categorized as in vivo antioxidants) and concern the cardiovascular system. Other potentially beneficial activities are still to be investigated in depth. We conclude that—in the context of a proper diet that includes high‐quality products—the use of high‐quality olive oil contributes to achieving and sustaining overall health.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- 3,4‐DHPEA‐EDA
dialdehydic form of decarboxymethyl elenolic acid linked to hydroxytyrosol
- CVD
cardiovascular disease
- EFSA
European Food Safety Authority
- ER
endoplasmic reticulum
- EVOO
extra virgin olive oil
- HT
hydroxytyrosol
- HVAL
homovanillyl alcohol
- IBD
inflammatory bowel diseases
- iNOS
inducible NOS
- Nrf2
nuclear factor (erythroid‐derived 2)‐like 2
- OC
oleocanthal
- OLE
oleuropein
- OMWW
olive mill waste waters
- OOPC
olive oil phenolic compound
- TAG
triacylglycerol
- VOO
virgin olive oil
1. INTRODUCTION
The term “Mediterranean diet” encompasses the dietary regimens traditional of the Mediterranean basin (Martinez‐Gonzalez, Hershey, Zazpe, & Trichopoulou, 2017). This diet is considered to be among the healthiest ones worldwide and inhabitants of the Mediterranean basin exhibit high longevity and lower incidence of age‐related disease (Capurso, Crepaldi, & Capurso, 2019). The Mediterranean diet's main features have been described and agreed upon (Martinez‐Gonzalez et al., 2017), but, probably, what really sets the Mediterranean diet apart from other plant‐based diets is the use of olive oil as the main source of visible fat (Visioli et al., 2018). Worthy of note, olive oil is a fruit juice in that it is obtained from drupes by physical means. Therefore, it retains most of the components originally present in the olives, including fatty acids, terpenes, chlorophylls, carotenoids, and (poly)phenols including hydroxytyrosol (HT) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6308 (OC) (vide infra; Bonvino et al., 2018). This peculiar amalgam grants olive oil its unique flavour and taste (it is noteworthy that hundreds of olive cultivars exist worldwide, each of which yields distinct oils). In addition to providing sensory stimuli (taste, smell and sight), the minor components of olive oil, most of which phenolic in nature, are endowed with pharmacological/pharma‐nutritional properties (Crespo, Tome‐Carneiro, Davalos, & Visioli, 2018) that we review in this paper.
Given the large amount of literature published on this topic and based on our more than 25 years of experience in this field, we critically focus on what we believe are the most pharmacologically prominent actions of the constituents of olive oil.
2. OLIVE OIL AND ITS MAJOR COMPONENTS
2.1. Fatty acids
Olive oils are—of course—composed of ~98–99% of fatty acids, mainly triacylglycerol (TAG) esters of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1054 (55–83%), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1055 (7.5–20%), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1052 (3.5–21%), and other fatty acids such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3377 (0.5–5%). In terms of stereospecificity, triolein makes up ~40% of the TAGs in olive oil, whereas other less frequent esterification possibilities include (a) one palmitic acid in position sn‐3 and two oleic acids in the sn‐1 and sn‐2 and (b) one molecule of linoleic acid in position sn‐2 bordered by two oleic acids (Karupaiah & Sundram, 2007). The remaining ~1–2% is made of “minor components” such as the (poly)phenols. The saponifiable fraction can be further, conveniently, subdivided into many different families, including aliphatic and triterpenic alcohols, sterols, squalene, volatile compounds, tocopherols, carotenes, and chlorophylls.
2.2. Oleic acid
The contribution of oleic acid (18:1n‐6) to the cardioprotective effect of a Mediterranean diet is still being debated and no firm conclusion has been reached (Voelker, 2019). Indeed, the substitution of saturated fat with monounsaturated and polyunsaturated fatty acids does correlate with lower CVD incidence, but whether this effect is due to some biological activities of oleic acid or to the displacement of saturates is difficult to ascertain. It should be emphasized that oleic acid is not an essential fatty acid, as the body can synthesize it and no clinical signs of deficiency have been described to date. Also, in countries other the Mediterranean ones, for example, the United States and the UK, dietary oleic acid is consumed through meat, namely, pork and chicken (Visioli et al., 2018). Hence, the overall amount of dietary oleic acid consumed does not differ between olive oil users, such as those in the Mediterranean region and subjects in other countries with lower use of olive oil (Dougherty, Galli, Ferro‐Luzzi, & Iacono, 1987). As mentioned, available human evidence from ecological and clinical trials in which blood fatty acid composition was associated with disease incidence, actually indicates that high plasma or phospholipid concentrations of 18:1 fatty acids are associated with higher, not lower, cardiovascular disease (CVD) risk. A notable example was provided by Würtz et al. (2015), who showed—by using metabolomics—that higher serum monounsaturated fatty acid concentrations are associated with increased CVD risk. Polyunsaturated fatty acids exhibited the opposite association. Other data along the same lines have been published by Marangoni et al. (2014) and Block, Harris, Reid, and Spertus (2008), who reported higher monounsaturated fatty acid concentrations in myocardial infarction patients, compared with controls (again, ω 6 fatty acids were associated with better prognosis). In summary, any purported (yet often touted) cardiometabolic benefit of total monounsaturated fat, such as oleic acid, is based on scant data (Voelker, 2019). Limitations in these kinds of studies include the fact that plasma (or blood) concentrations of 18:1 fatty acids are a poor proxy of intake because oleate can be synthesized de novo. In addition, slowly accumulating evidence is suggesting that there might be considerable health differences depending on the source of oleic acids, that is, from vegetables and olive oil or from animal products (Zong et al., 2018). Therefore, even though some authors (Gillingham, Harris‐Janz, & Jones, 2011) do point to the fact that the substitution of saturated fatty acids with oleic acid reduces total and LDL cholesterol and replacement of carbohydrates with oleic acid lowers TAGs and LDL cholesterol (all effects that lead to a reduction of cardiovascular risk), we would like to reiterate that available evidence indicates that 18:1 fatty acids per se are not the olive oil component chiefly responsible for its cardioprotective potential. Finally, several high‐oleic acid seed oils are available in the market and are mostly employed by the snack industry. Further ecological data will eventually prove or disprove our contention, but based on current science, we would like to introduce the reader to the pivotal biological roles of the (poly)phenols in olive oil.
2.3. Phenolic compounds
As mentioned above, olive oil phenolic compounds (OOPCs) are now believed to contribute to the health benefits attributed to extra virgin olive oil (EVOO; Crespo et al., 2018; Robles‐Almazan et al., 2018). Indeed, the soluble fraction of olive oil is mainly composed of OOPC, including phenolic acids, phenolic alcohols (HT and tyrosol), secoiridoids such as oleuropein (OLE), HT linked to the dialdehydic form of elenolic acid (3,4‐EDA), and flavonoids (Rodriguez‐Morato et al., 2016). Upon production, when acidity exceeds 0.8%, olive oil needs to be refined, which dramatically decreases the concentration of its minor components. This creates two commercial categories of olive oils, that is, EVOO and olive oil (European Communities, 2002). As the latter form of olive oil is almost devoid of minor components, the pharma‐nutritional properties generically attributed to olive oil truly only pertain to EVOO.
The concentration and profile of OOPCs in EVOO depends on many factors, such as the variety of olive cultivar, degree of ripening, climatic conditions, soil, irrigation, technical process for oil separation (i.e., temperature, crushing, malaxation, and its water content), and the time and storage conditions (Boskou, 2015). In addition, the correct quantification of OOPCs is extremely complicated by the lack of appropriate standards and a standardized method to analyse (poly)phenols (Boskou, 2015). In summary, as EVOO is an agricultural product, it cannot be standardized and it is, therefore, impossible to provide “pharmacological” advice to consumers with respect to the type (including brand) and amount of EVOO to be consumed for optimal health.
Any type of olive oil is produced by mechanical pressure applied to the olive paste, which is obtained by a process of milling and malaxation, that is, the continuous addition of water to the olive paste. This procedure yields considerable amounts of olive mill waste waters (OMWW; 800,000 m3·year−1 in Italy alone) and water‐soluble (poly)phenols, extracted from the olives by malaxation, according to their partition coefficient, will be found in the OMWW. Several techniques have been developed over the years to selectively extract (poly)phenols from OMWW (Aissa et al., 2017), and some nutraceuticals are available in the market. In addition to their commercial exploitation, OMWW are useful in pharma‐nutritional research because they allow for the administration of well‐characterized raw extracts, in which, for example, OLE or HT is the only bioactive component (Khymenets et al., 2016). Several studies have been published (Visioli & Bernardini, 2011), from basic mechanisms in vitro to in vivo actions, including in human subjects (vide infra). The initial studies focused on the antioxidant activities of OMWW, which, after the debacle of antioxidant therapy, are now being proposed as additives for animal feed, food and cosmetics, to prolong shelf life (Aissa et al., 2017). The major limitation concerns the strong flavour and bitter taste of OMWW, an issue that is currently unresolved. Other studies have targeted the anti‐inflammatory properties of OMWW and their ability to increase GSH concentrations (Visioli, Wolfram, Richard, Abdullah, & Crea, 2009), possibly via nuclear factor (erythroid‐derived 2)‐like 2 (Nrf2)‐mediated pathways (see Visioli & Bernardini, 2011).
3. THE FOREMOST PHENOLIC COMPONENT OF OLIVE OIL: HT
3,4‐Dihydroxyphenylethanol (HT; Figure 1) is the main OOPC and it is mostly found in the fruits and leaves of olive trees, enclosed in complex structures, namely, the secoiridoids, OLE and 3,4‐EDA. OLE in oils is present as an aglycone, due to the action of hydrolytic enzymes released during the preparation of the oil. This hydrolysis also causes partial modification of the aglycone due to keto‐enolic tautomeric equilibrium that involves the ring opening of secoiridoids: During the mechanical process employed to obtain unrefined olive oils, OLE is mostly hydrolysed into aglycone derivatives such as 3,4‐DHPEA‐EDA and 3,4‐DHPEA‐EA, and a small part undergoes hydrolysis to liberate HT. Upon ingestion by humans, the main secoiridoid structures (HT precursors) are rapidly hydrolysed into simple phenolic structures (phenolic acids and alcohols), leading to an increase in free HT in the small intestine (de Bock et al., 2013; López de las Hazas et al., 2016). These simple phenols are then available to be metabolized into Phase I and Phase II metabolites (Liu & Hu, 2007) by the activity of sulphotransferases (Dunn & Klaassen, 1998) and UDL‐glucuronosyltransferases (Shelby, Cherrington, Vansell, & Klaassen, 2003). Indeed, the absorption, distribution, metabolism, and excretion of the phenolics in olive oil have been largely elucidated (Caruso, Visioli, Patelli, Galli, & Galli, 2001; Rodriguez‐Morato et al., 2016). In fact, after the intake of EVOO, VOO, olive leaf extracts, HT, or olive byproducts or precursors, HT metabolites (mainly as sulfated derivatives) are the main compounds found circulating in the blood (de Bock et al., 2013; Rubio et al., 2012) and recovered in the urine (Khymenets et al., 2016). The low bioavailability and low plasma concentration of native forms of phenols are typical of (poly)phenols and such findings are stimulating research based on the hypothesis that the conjugates could be biologically active and produce beneficial effects (Del Rio et al., 2013). Interestingly, 3‐O‐methyl‐hydroxytyrosol, also known as homovanillyl alcohol (HVAL), can be measured in the urine as a marker of EVOO consumption. High urinary HVAL concentrations have been associated with lower risk of CVD and total mortality in elderly individuals (De la Torre et al., 2017). We would point out that the concentrations of HVAL in the urine of rats are much higher than the humans' ones, questioning the [often unavoidable] use of rats to study the metabolism of HT (Visioli et al., 2003). Finally, a recent paper by Terzuoli et al. (2019) demonstrated that HT 3‐O sulfate blunts endothelial‐to‐mesenchymal transition in the inflamed endothelium, emphasizing again the need to study (poly)phenols' metabolites and focus on the root of degenerative diseases, that is, inflammation.
Figure 1.
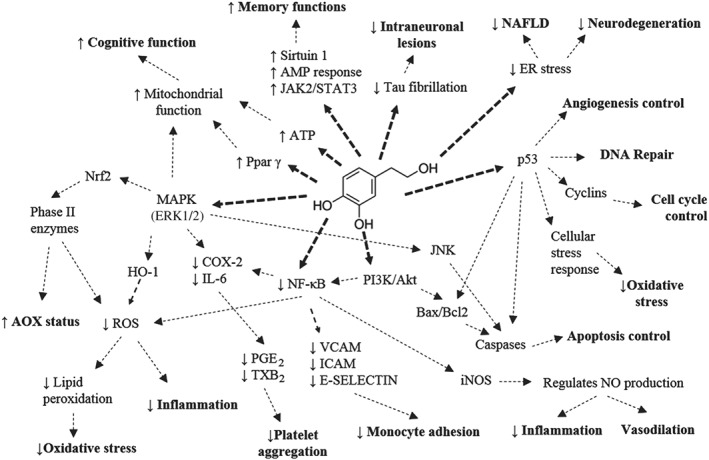
Hydroxytyrosol (HT) and its main biological actions. AOX, antioxidant; BAX, BCL2‐associated X, apoptosis regulator; BCL2, B cell lymphoma 2, apoptosis regulator; ER, endoplasmic reticulum; HO‐1, haem oxygenase; ICAM, intercellular adhesion molecule; iNOS, inducible NOS; NAFLD, non‐alcoholic fatty liver disease; Nrf2, nuclear factor (erythroid‐derived 2)‐like 2; PI3K/Akt, phosphoinositide‐3‐kinase pathway; VCAM, vascular cell adhesion molecule
4. PHARMACOLOGICAL AND NUTRACEUTICAL PROPERTIES OF HT
In the present context, human pathology can be conveniently classified in three major areas: CVD, cancer, and neurological disorders. These macro‐areas comprise most diseases affecting people in the Western world, where the incidence of infection‐based pathologies is low (Afshin et al., 2019). We here summarize the evidence‐to‐date of the effects of olive oil and its components—namely HT—on such illnesses.
4.1. Cardiovascular disease
HT has been first studied in the cardiovascular field, when the atherosclerosis/oxidative stress hypothesis was suggesting a preventive or therapeutic role for antioxidants. Indeed, HT is a strong contributor to the stability of olive oil (Papadopoulos & Boskou, 1991) and is a strong in vitro antioxidant (Visioli, Bellomo, & Galli, 1998). In parallel, other potentially cardiopreventive activities have been explored, namely, anti‐inflammatory (Richard et al., 2011) and hypocholesterolemic effects (for details, see Pedret et al., 2018). In terms of atherosclerosis prevention, some contrasting data have been published following experiments in two different animal models. Whereas HT slowed atherosclerosis progression in the rabbit (Gonzalez‐Santiago et al., 2006; of note, resveratrol promotes atherosclerosis in the same model; Wilson, Knight, Beitz, Lewis, & Engen, 1996), Acín et al. (2006) actually reported increased atherosclerosis in mice‐fed HT. We must highlight how long‐term human experiments are nearly impossible to perform with nutraceuticals (Visioli, 2012). However, the data from the PREDIMED, the preeminent clinical trial that showed the protective activities of the Mediterranean diet and of EVOO (Estruch et al., 2018), are indeed suggestive of a cardioprotective effect of HT, mediated by still largely unexplored mechanisms (vide infra). Of note, HT is the only phenolic compound backed by a [quite controversial] European Food Safety Authority (EFSA) health claim. This states that daily consumption of around 5 mg of HT and its derivatives, that is, OLE complex and tyrosol, protect blood lipids from oxidation (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) 2011). It is important to underline that this health claim is focused in the protection provided to LDL against oxidative damage and that effects on normal lipid metabolism maintenance, blood pressure, or other biological actions are not addressed. Given that the true contribution of LDL oxidation to human pathology is still unclear and that clinical trials of antioxidants failed to demonstrate their usefulness, the fact that HT is capable of protecting LDL from oxidation is—as of today—of dubious clinical relevance.
4.2. Dyslipidemia and metabolic syndrome
The cumulative effects of olive oil on lipid metabolism are—based on current data—quite modest (Visioli et al., 2018).
In terms of pharmacology/pharma‐nutrition of its minor components, most studies performed in humans or animal models, such as mice, rats, rabbits, and zebrafish, have reported small or positive effect of HT consumption on circulating lipids and on lipid peroxidation (Gonzalez‐Santiago et al., 2006; Pirozzi et al., 2016; Santos‐Lopez et al., 2016; Tabernero et al., 2014; Wang, Liu, Ma, & Wen, 2018). Nevertheless, both dyslipidemia and hepatic fat accumulation were reported in rodents after EVOO consumption (Arbones‐Mainar et al., 2007). In one example, an 8‐week supplementation with a dietary attainable amount of HT resulted in hypertriglyceridemia in mice (Tome‐Carneiro et al., 2016). Moreover, after a 10‐week vitamin E‐deficient diet, a 2‐week supplementation with 100‐mg HT·kg−1 diet led to a rise in plasma TAG in rats (Rowett Hooded Lister strain; Rodriguez‐Gutierrez et al., 2012). In Wistar rats, HT supplementation (7.5 mg·kg−1, twice a week, for 30 days) induced elevated TAG and lipid concentrations in cardiac muscle (Faine et al., 2006). Concerning non‐murine models, HT supplementation (1.5‐mg HT·kg−1·day−1), from Day 35 to delivery, resulted in raised TAG in the offspring of Iberian sows with diet‐induced risk of intrauterine growth restriction, compared to controls (Vazquez‐Gomez et al., 2017). In humans, the few available studies reported no effects on the lipid profile after HT consumption. For example, in a study with healthy volunteers, the intake of 0‐, 5‐, or 25‐mg HT·day−1, for 1 week, did not produce any significant effects on the levels of total cholesterol, LDLc, HDLc, TAG, and body weight (Crespo et al., 2015). Also, a 3‐week supplementation with placebo or 15‐mg HT·day−1 did not cause any significant change in total cholesterol, HDLc, and TAG, whereas circulating malondialdehyde concentrations were significantly reduced (Colica et al., 2017). Interestingly, the intake of 5.25 mg of HT (as part of a 30‐g biscuit formulation) decreased the postprandial levels of ox‐LDL (Mateos et al., 2016).
The contribution of dyslipidemia to the metabolic syndrome is quite relevant (Sherling, Perumareddi, & Hennekens, 2017). In this respect, consumption of olive oil as the principal source of visible fat is associated with lower incidence of type 2 diabetes (Visioli et al., 2018). Experimental proof was provided by the PREDIMED study (Salas‐Salvado et al., 2014) and the extent to which EVOO (poly)phenols contribute to this protective effect is being explored by basic science (Hmimed, Belarbi, & Visioli, 2016; Peroulis et al., 2018; Peyrol, Riva, & Amiot, 2017). Even though a correct lifestyle that includes proper dietary habits and physical exercise is of preeminent importance (Sherling et al., 2017), accumulated evidence suggests that EVOO and its minor components, via multi‐targeted actions, might indeed lower the risk of metabolic syndrome and, after proper randomized trials, might be employed as adjunct therapeutic agents (Yubero‐Serrano, Lopez‐Moreno, Gomez‐Delgado, & Lopez‐Miranda, 2018).
4.3. Chemoprevention
Chemopreventive actions have also been proposed for OOPC (see Bernini, Merendino, Romani, & Velotti, 2013; Zhao et al., 2014), based on observational data indicating lower incidence of cancer, for example, breast cancer among olive oil users (Gerber, 1997; Visioli et al., 2018). The PREDIMED also reported a lower incidence of breast cancer after a long‐term consumption of a (poly)phenol‐rich olive oil as part of a Mediterranean diet compared to the low‐fat diet of the control group (Toledo et al., 2015). Machowetz et al. (2007) and Salvini et al. (2006) in healthy males and postmenopausal women, respectively, have reported reduced oxidative DNA damage after short‐term ingestion of phenol‐rich olive oil, which might theoretically mitigate cancer risk.
Basic science is investigating the purported chemopreventive properties of OOPC. The most popular mechanism of action proposed for (poly)phenols is based on their antioxidant properties. However, provision of antioxidants actually increases cancer risk (Jenkins et al., 2018), and the exact role of oxidative stress (now called redox code; Jones & Sies, 2015; and never fully elucidated in humans) in cancer aetiology is, to date, still elusive. Conceivably, modulation of the redox code by (poly)phenols (including OOPC) might play some roles in cancer and progression of cancer stem cells, as well as adjunct therapy (Ritter & Greten, 2019). Such actions are probably not mediated by direct antioxidant activities. Indeed, if OOPC have chemopreventive properties, these are due to a variety of molecular, synergistic activities rather than to a single one. For example, oleocanthal (OC) is cytotoxic to human melanoma cells, but not to normal dermal fibroblasts. This compound inhibits https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 phosphorylation and down‐regulates https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=910 expression (Fogliano & Sacchi, 2006), independent of antioxidant actions. HT and two of its colonic metabolites, that is, phenylacetic acid and hydroxyphenylpropionic acid, can arrest cell cycle and promote apoptosis in HT‐29 and Caco‐2 cells (Lopez de las Hazas, Pinol, Macia, & Motilva, 2017).
The activation of transcription factors such as NF‐κB, STAT3, MAPK, and the hypoxia‐inducible factor 1α contributes to cancer onset and development (Monkkonen & Debnath, 2018). These transcription factors dictate the production of inflammatory molecules such as cytokines and chemokines, in addition to activating https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376. This leads to further activation and recruitment of leukocytes, triggers the inflammasome of tumour cells, and stimulates the production of other inflammatory mediators in a vicious cycle (D'Ignazio, Batie, & Rocha, 2017). (Poly)phenols including OOPC do modulate signal transduction and might potentially block cellular hyperproliferation.
It is relevant to note that exceedingly high concentrations of (poly)phenols that are often employed in in vitro studies. (Poly)phenols are notoriously poorly bioavailable and extrapolation of in vitro data to human physio‐pathology is, therefore, questionable. In this respect, the most physio‐pathologically relevant studies are those carried out using GI tract cells. Other reports should be interpreted in light of these caveats.
To summarize, olive oil and its (poly)phenols are likely to play important roles in the lower degree of cancer risk observed in the Mediterranean area (Visioli et al., 2018). Basic science is, indeed, suggestive of such effect, but, because of the inherent difficulties of studying cancer in humans and in appropriate animal models, the [causal or casual] association between olive oil consumption and chemoprevention is worth further investigation.
4.4. Neurological disorders and neurodegeneration
The incidence of neurological or neuropsychiatric disorders and age‐related neurodegeneration (dementia, Alzheimer's disease and mild cognitive impairment) is increasing in the Western world (Feigin et al., 2017). Active research is addressing this issue, also in light of its socio‐economic consequences. The Mediterranean diet has long been associated with lower incidence of cognitive disorders, but the precise role of olive oil and its components is difficult to ascertain (Casamenti & Stefani, 2017; Crespo et al., 2018; Rodriguez‐Morato et al., 2015). Of note, better vascular function (an area in which studies on olive oil abound) obviously results in lower neurodegeneration. In terms of OOPC, the first study of Schaffer et al. (2007) used the waste product OMWW (vide infra) and reported improved resilience of mouse brains to external challenge. Indeed, HT accumulates in the brain after the administration of a nutritionally relevant dose (Lopez de las Hazas et al., 2015). Mice receiving EVOO exhibit improved memory and learning as well as a lower rate of Alzheimer's disease (Farr et al., 2012; Qosa, Mohamed, et al., 2015), mediated by largely unexplored mechanisms. Observational studies in humans have been performed in New York City, where olive oil consumption is quite low, which reported lower dementia risk associated with higher adherence of the Mediterranean diet. However, those studies did not discriminate the individual contribution of EVOO from that of other unsaturated fatty acids. In vitro studies are, therefore, slowly evaluating the alleged neuroprotective properties of OOPC, which include restoration of proper insulin signalling (Crespo et al., 2017). In addition, HT and their Phase II metabolites show neuroprotective effects against oxidative stress at physiological concentrations in neuronal cells (López de las Hazas et al., 2018).
As mentioned, the incidence of mental and affective disorders such as depression is rapidly increasing in particular in high‐ and upper‐middle‐income countries (Rehm & Shield, 2019). Adherence to a Mediterranean diet is associated with lower risk of depression and at least one observational study suggests that higher intake of olive oil is related to a lower risk of depression (Psaltopoulou et al., 2013). In addition, VOO consumption seems to protect against loss of age‐related cognitive function (Valls‐Pedret et al., 2015). Again, it is difficult to disentangle the role of EVOO from that of many other contributing factors, most of which social in nature. Several micronutrients have been studied in this respect, with inconclusive results (Garcia‐Blanco, Davalos, & Visioli, 2017). More studies are needed to understand the mechanisms potentially involved in the inverse associations between olive oil consumption and brain resilience. Some such studies have been published. In vitro, tyrosol and HT protect N2a neuroblastoma cells against Aß‐induced toxicity by preventing, at least in part, the NF‐κB activation induced by Aß (St‐Laurent‐Thibault, Arseneault, Longpre, & Ramassamy, 2011). In vitro, release of Ca2+ from endoplasmic reticulum (ER) to cytoplasm for activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1957 occurred after OLE administration in neuronal SH‐SY5Y and RIN‐5F cells, which in turn led to increased https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540 activation and Beclin‐1 and LCII‐mediated lysosomal autophagy for clearance of Aβ deposits (Rigacci et al., 2015). In BV‐2 microglial cells, OLE administration inhibited the production of pro‐inflammatory cytokines via regulation of ERK, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=519 (MAPKs), and NF‐κB activation (Park et al., 2017). In Caenorhabditis elegans strains expressing human Aβ3‐42 gene, OLE significantly reduced Aβ plaque deposition, soluble isomer formation, and ROS levels and improved SOD levels. Of note, decreased paralysis and increased lifespan with respect to untreated animals was also seen (Diomede, Rigacci, Romeo, Stefani, & Salmona, 2013). After supplementation with OLE aglycone, TgCRND8 mice showed improved cognitive performance, presenting a reduction in Aβ formation and deposition in brain regions (Luccarini et al., 2014). This was linked with a reduction of expression levels of glutaminyl cyclase (Luccarini et al., 2015) and to an up‐regulation of autophagy‐related genes, such as Beclin‐1, LC3II, p62, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2343 (Grossi et al., 2013). Although HT mildly benefited cognitive behaviour in APP/PS1 mice, it had no effect on brain Aβ accumulation. In addition, HT was associated with amended mitochondrial dysfunction, increased SOD‐2 expression, and reduced brain inflammatory markers (Y. Peng et al., 2016).
In other in vitro studies, HT impeded the induction of cell death in dopaminergic neurons due to the Parkinson's disease‐related neurotoxin, 6‐hydroxydopamine, and this was linked, at least in part, to the induction of Phase II enzymes (Yu et al., 2016) and to the inhibition of apoptosis via activation of the Nrf2/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1441 axis (Funakohi‐Tago et al., 2018). Furthermore, HT mitigated the increase in spontaneous oxidation of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=940 during MAO inhibition in rat pheochromocytoma PC12 cells (Goldstein et al., 2016).
4.5. Inflammatory bowel diseases
The aetiology of inflammatory bowel diseases (IBD), the second most common inflammatory disease whose incidence is rapidly growing (Ye, Pang, Chen, Ju, & Zhou, 2015), is multi‐component and the precise mechanisms of initiation and progression have not yet been fully unraveled (Balmus, Ciobica, Trifan, & Stanciu, 2016). Albeit poorly characterized, oxidative stress is now regarded as a potential pathogenic and critical factor in the initiation, progression, and severity of IBD (Guan & Lan, 2018). Production of large quantities of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 via up‐regulation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250) can have a variety of effects, which may be detrimental or beneficial depending on the amount, duration, and anatomical site of synthesis (Kolios, Valatas, & Ward, 2004). iNOS‐mediated NO production may occasionally become part of a dysregulated immune response, resulting in chronic inflammatory disorders such as IBD (Guan & Lan, 2018). Transcription factors like NF‐κB mediate the expression of iNOS and other inducible genes such as those for COX‐2, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=821 in immune and inflammatory responses (Xia, Liu, Zhong, & Geng, 2001). A protective effect for EVOO polyphenols against IBD pathogenesis and progression has been suggested at the intestinal level. In a study by Serra and colleagues, a phenolic extract of olive oil polyphenols was able to inhibit H2O2 and NO production triggered by oxysterols and preserve cellular GSH levels (Serra et al., 2018). Furthermore, olive oil's phenolics blocked key inflammatory processes driven by oxysterols such as NF‐κB activation (by hampering the activation of JNK and p38 and the resulting IkB phosphorylation), iNOS induction, and IL‐8 and IL‐6 production (Serra et al., 2018). Other in vitro studies showed the ability of HT to block NF‐κB activation and iNOS and COX‐2 expression (Zhang, Cao, Jiang, & Zhong, 2009; Zhang, Cao, & Zhong, 2009). In vivo studies also indicate an anti‐inflammatory effect of HT and OLE exerted through the modulation of MAPKs signalling (Aparicio‐Soto, Sanchez‐Hidalgo, Rosillo, Castejon, & Alarcon‐de‐la‐Lastra, 2016).
4.6. Cellular and mechanisms of action: A critical overview
The true mechanism(s) of action of (poly)phenols are still elusive as no receptor has been found. The most popular mechanism of action proposed over the years for HT is its antioxidant activity, namely, a direct one. However, accumulated evidence (not limited to HT, but involving all phenolic compounds) is questioning the extent and true nature of free radical scavenging and direct antioxidant activity (Forman, Davies, & Ursini, 2014; Visioli, 2015). This is due to several factors that are emerging along with (poly)phenol research. The most relevant one is the low bioavailability of (poly)phenols in general, including HT. For example, Pastor et al. (2016) recorded a Cmax of HT of 2.8 μM, following ingestion of EVOO, which—albeit low—is even higher than those of most (poly)phenols. The consequence of this low bioavailability is that (poly)phenols and their metabolites contribute little to the endogenous antioxidant pool (Lotito & Frei, 2006), which has been calculated to be in the millimolar range.
If (poly)phenols such as HT act as antioxidants, it might be via indirect mechanisms, namely, the increasingly popular Nrf2 pathway. Some data in support of this hypothesis have, indeed, been published (S. Peng, Zhang, Yao, Duan, & Fang, 2015; Zrelli et al., 2011; Zrelli, Kusunoki, & Miyazaki, 2015), but it should be noted that the authors performed such experiments with supra‐physiological, unattainable concentrations. The human relevance of those data is, therefore, likely to be low. In an attempt to prove the Nrf2 hypothesis in humans, Crespo et al. (2015) administered HT to healthy volunteers and failed to observe any activation of Phase II enzymes. We have to emphasize that the Nrf2 pathway has never been confirmed for any polyphenol, in humans, possibly because of methodological problems, such as the difficulty in obtaining liver biopsies.
Other mechanisms of action have been proposed for HT and olive phenolics (de Pablos, Espinosa‐Oliva, Hornedo‐Ortega, Cano, & Arguelles, 2019). Probably, the most important one concerns inflammation. As first put forward by the late Dr Russel Ross (1999) and recently confirmed by the CANTOS study (Aday & Ridker, 2018), low‐grade, chronic inflammation is at the heart of several degenerative diseases including inflammation, neurodegeneration, and cancer (Calder et al., 2017; see above).
Indeed, olive (poly)phenols have been tested in a variety of in vitro and in vivo models where they exhibited anti‐inflammatory actions, namely, via inhibition of cycloxygenases and lipoxygenases (Visioli & Bernardini, 2011). This might be important in, for example, cancer patients (Diakos, Charles, McMillan, & Clarke, 2014), who exhibit higher inflammatory status. In addition, as inflammation is the major player in age‐related pathologies (a phenomenon termed “inflammaging”; Calder et al., 2017), inhibition of cycloxygenases and lipoxygenases is likely to be the mechanisms of action chiefly responsible for the health benefits of OOPC.
Protein misfolding and ER stress have a central role in several human diseases (Walter & Ron, 2011), including insulin resistance, Type 2 diabetes (Ozcan et al., 2004), inflammatory disease, cancer (Cubillos‐Ruiz et al., 2015), or neurodegeneration (Uehara et al., 2006). As redox status or excessive oxidative stress may contribute to or accompany, ER stress (Kim et al., 2018; Liu et al., 2019), the use of HT or other olive phenolics may influence the unfolded protein response pathway. Indeed, recent evidence suggest that HT (Giordano, Davalos, Nicod, & Visioli, 2014; Wang et al., 2018) or its metabolites (Giordano, Dangles, Rakotomanomana, Baracchini, & Visioli, 2015) reduce ER stress. In short, modulation of ER stress might be an underappreciated yet very relevant mechanism of action of HT and other OOPCs.
A more recent research topic is the role of microRNAs as potential targets of drugs and food components (Davalos & Suarez, 2013; Tome‐Carneiro et al., 2016). There is some in vitro evidence that olive (poly)phenols alter microRNAs in cancer cells. Tomé‐Carneiro et al. also reported modulation of microRNAs, namely, miR‐193a‐5p in rodents and humans after the administration of nutritionally relevant amounts of HT (Tome‐Carneiro et al., 2016), whereas miR‐802‐5p was found to be consistently modulated in mouse liver and intestine after dietary supplementation (Lopez de las Hazas et al., 2019). These observations could in part explain (at the molecular level) some of the biological effects of HT. Yet, although microRNAs are increasingly being indicated as potentially relevant targets for drugs and/or food components (Tome‐Carneiro et al., 2016), we would like to reiterate the need for further ad hoc research.
4.7. Safety
Of course, consumption of phenol‐rich EVOO is deemed as safe except when in surplus (Tomé‐Carneiro et al., unpublished experiments). When testing defined compounds or raw mixtures in a nutraceutical setting, we need to make sure that untoward side effects are minimal. Any pharmacological or pharma‐nutritional intervention should in fact follow safety and toxicity protocols, even though the lay public's perception is that “natural equals “safe”. D'Angelo et al. (2001), in the first acute toxicity test in experimental animals, showed that a single dose of 2 g·kg−1 HT did not produce any relevant adverse effect. Subsequent studies evaluating acute toxicity in rats, such as the 90‐day chronic toxicity test, confirmed the absence of toxic effects at doses of 2 g·kg−1·day−1, as well as the absence of teratogenic and mutagenic actions (Christian et al., 2004). In a later study, a no observed adverse effect level of 500‐mg HT·kg−1 day−1 was proposed, which would represent 5‐mg HT·kg−1 day−1 for humans (considering a safety factor of 100) or 300 mg·day−1 for a 60‐kg person (Aunon‐Calles, Canut, & Visioli, 2013). Further, HT was tested for its potential genotoxicity, and the results indicate that it is non‐genotoxic and non‐mutagenic at concentrations that far exceed those attainable after intake (Aunon‐Calles, Giordano, Bohnenberger, & Visioli, 2013). In addition, renal and hepatic function parameters remain unaltered after HT administration, further confirming its safety (Kotronoulas et al., 2013).
A phospholipid conjugate of HT has also been tested for toxicity with no apparent adverse effects (Cornelio et al., 2019). All of these publications led to HT being awarded a Novel Food status by the EFSA (EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) et al., 2017).
5. HUMAN STUDIES
The only two human studies of HT available thus far have been performed by Lopez‐Huertas and collaborators (Gonzalez‐Santiago, Fonolla, & Lopez‐Huertas, 2010; Lopez‐Huertas & Fonolla, 2017). In the former, the authors confirmed human absorption of HT and recorded its transient association with LDL, confirming data by Bonanome et al. (2000), who administered EVOO rather than pure HT. In the latter (not placebo controlled), the authors explored the effects of HT (5 mg·day−1 for 8 weeks to volunteers with mild hyperlipidaemia) on markers of CVD, blood lipids, inflammatory markers, liver or kidney functions, and the electrolyte balance. No significant differences were reported, but plasma concentrations of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4781 increased twofold after 4 and 8 weeks treatment, compared with levels at baseline. The authors propose a physiologically relevant antioxidant function for HT through increasing endogenous vitamin C levels (Afshin et al., 2019). As discussed above, the true contribution of higher antioxidant status to human physio‐pathology is far from being elucidated.
Other human studies have been performed with OMWW (see above). The results include increased GSH plasma concentrations (Visioli et al., 2009), decreased TxB2 production (Leger et al., 2005), amelioration of psoriasis (Herrera Acosta, Alonso Suárez Pérez, Aguilera Arjona, & Visioli, 2016), and reduction of inflammation markers and reported pain in women after breast cancer (Martinez et al., 2019). There are many patents to protect different methods of purification and concentrations of (poly)phenols in OMWW, including ion‐exchange chromatography and reverse osmosis (Visioli & Bernardini, 2011). As mentioned, commercial applications range from nutraceuticals and functional foods to animal feed and cosmetics.
6. OLEOCANTHAL
2‐(4‐Hydroxyphenyl)ethyl(3S,4E)‐4‐formyl‐3‐(2‐oxoethyl)hex‐4‐enoate (OC) is a secoiridoid which is being increasingly investigated (Francisco et al., 2019; Pang & Chin, 2018). It strongly contributes to the sensory properties of olive oils, including bitterness, pungency, and astringency (Francisco et al., 2019). Indeed, intake of OC irritates the upper airways and is often accompanied by throat clearing and coughing. This feature is very similar to that of ibuprofen, and Beauchamp et al. (2005) proposed OC as an anti‐inflammatory compound based on this observation. Because low‐grade, chronic inflammation is being proposed as a major etiopathological trigger of degenerative diseases (Calder et al., 2017), daily consumption of OC in doses lower and, hence, less detrimental to the GI tract, than those of ibuprofen might contribute to the health benefits of EVOO. Basic science data in support of this notion include the ability of OC to lessen some in vitro features of Alzheimer's disease (Pitt et al., 2009), similar to that described for HT (Crespo et al., 2017). The available research on OC is very promising, even though evidence of its metabolism in humans is missing (Fogliano & Sacchi, 2006). However, given the ibuprofen‐like properties of OC, it is conceivable that the frequent intake of OC‐rich EVOOs would lessen the risk of inflammation‐based degenerative conditions such as CVD, cancer (Francisco et al., 2019), and Alzheimer's disease. Concerning the last, in brain endothelial cells, OC significantly increased the levels of transport proteins involved in Aβ load clearance from brain tissues, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768&familyId=152&familyType=TRANSPORTER, and LDL receptor‐related protein‐1 (Abuznait, Qosa, Busnena, El Sayed, & Kaddoumi, 2013; Qosa, Batarseh, et al., 2015). In addition, OC‐treated mouse models showed increased levels of P‐gp and LDL receptor‐related protein‐1, as well as https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595, ApoE, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=756 (Qosa, Batarseh, et al., 2015) and Aβ degrading proteins, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2371, and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=740 (Abuznait et al., 2013). In murine astrocytes, OC ameliorated toxicity of amyloid‐β oligomers, reducing astrocyte activation and IL‐6 and glial fibrillary acidic protein levels, and on neuronal cells increasing the levels of main regulatory proteins in synaptic functions, PSD‐95, and synaptosomal nerve‐associated protein‐25 (Batarseh et al., 2017). It is important to note that there are no human studies with OC, which would be mandatory before pharmacological activities could be claimed.
7. CONCLUSIONS
The accumulated epidemiological observations from the Mediterranean basin consistently correlate consumption of olive oil as the preferential source of visible fat with lower incidence of degenerative diseases and greater longevity. There are very many other factors that could contribute to these correlations from moderate yet constant physical activity by, for example, shepherds and peasants, proper vitamin D synthesis from sunlight exposure, strong family bonds and social interactions, reduced exposure to air pollution, and slower work pace. For over 25 years, however, researchers have been using isolated olive oil components—most of which are (poly)phenolic in nature—in pharmacological rather than nutritional settings (Figure 2). Due to the inherent difficulties of pharma‐nutritional research (small effects; ethical issues from the use of healthy volunteers or patients; choice of appropriate biomarkers, etc.; Andrew & Izzo, 2017; Visioli, 2012), research is progressing less rapidly than in the case of synthetic drugs. However, the data we have critically reviewed in this article allow us to infer that there is indeed a direct correlation between the consumption of high‐quality olive oil and [primary or secondary] cardioprotection, as observed (Rees et al., 2019) and shown (Martinez et al., 2019) in the Mediterranean diet. Many studies with (poly)phenols from OMWW or synthesis are unveiling their manifold mechanisms of actions, which need to be confirmed in humans. Chemoprevention of cancer and avoidance of neurodegeneration by OOPC are yet to be proven and are only suggested by the observational studies.
Figure 2.
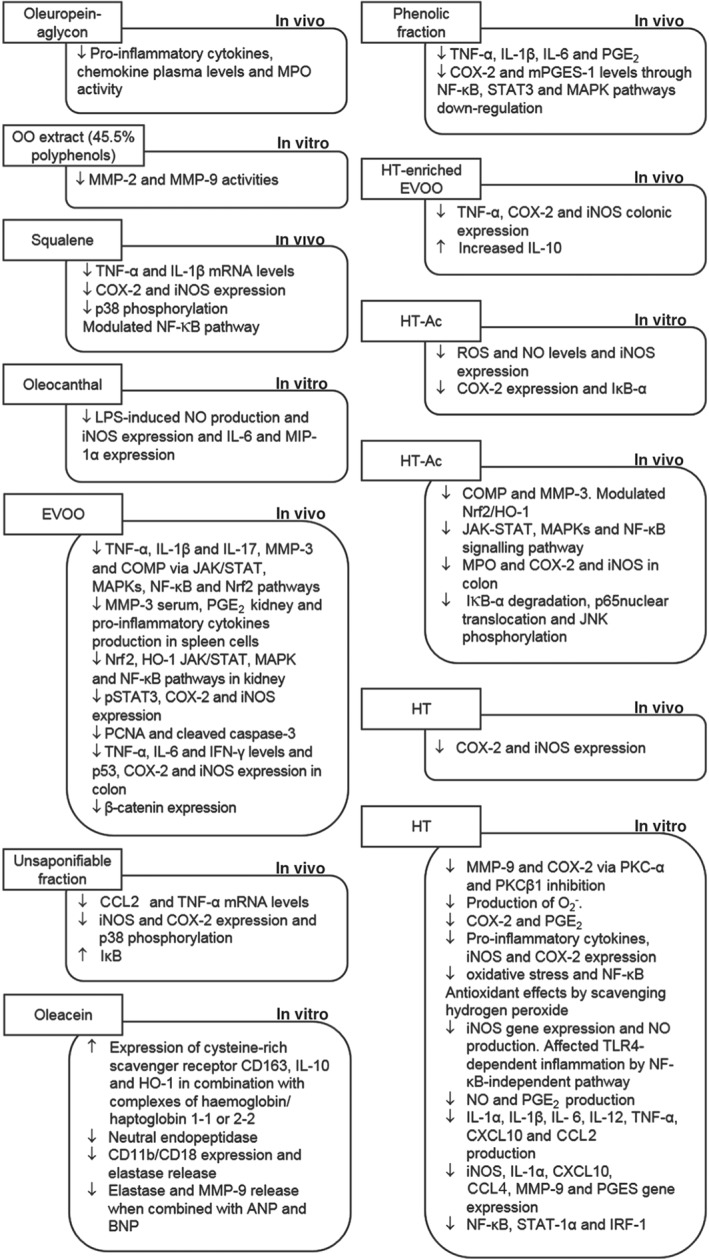
Accumulated in vitro and in vivo evidence of olive oil (poly)phenols activities. ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; EVOO, extra virgin olive oil; HO‐1, haem oxygenase; HT, hydroxytyrosol; HT‐Ac, hydroxytyrosol acetate; iNOS, inducible NOS; IRF‐1, IFN regulatory factor‐1; MPO, myeloperoxidase; Nrf2, nuclear factor (erythroid‐derived 2)‐like 2.
We would like to conclude that—in the context of a proper diet that includes high‐quality products—the use of high‐quality olive oil contributes to achieving and sustaining better overall health.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017a, b).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Our research is supported by grants from the Fundación Ramón Areces (Grant CIVP18A3888) to A.D., J.T.‐C., F.V., and M.C.C.; the Spanish “Agencia Estatal de Investigación”; European FEDER Funds to A.D. (Grant AGL2016‐78922‐R); and by POR FESR 3S4H to F.V. M.‐C.L.H. is supported by a contract from Consejería de Educación, Juventud y Deporte de la Comunidad de Madrid, Fondo Social Europeo and Iniciativa de Empleo Juvenil YEI (Grant PEJD‐2016/BIO‐2781). Tanya Billings edited part of the manuscript.
Visioli F, Davalos A, López de las Hazas M‐C, Crespo MC, Tomé‐Carneiro J. An overview of the pharmacology of olive oil and its active ingredients. Br J Pharmacol. 2020;177:1316–1330. 10.1111/bph.14782
REFERENCES
- Abuznait, A. H. , Qosa, H. , Busnena, B. A. , El Sayed, K. A. , & Kaddoumi, A. (2013). Olive‐oil‐derived oleocanthal enhances β‐amyloid clearance as a potential neuroprotective mechanism against Alzheimer's disease: In vitro and in vivo studies. ACS Chemical Neuroscience, 4, 973–982. 10.1021/cn400024q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acin, S. , Navarro, M. A. , Arbones‐Mainar, J. M. , Guillen, N. , Sarria, A. J. , Carnicer, R. , … Osada, J. (2006). Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. Journal of Biochemistry, 140(3), 383–391. 10.1093/jb/mvj1166 [DOI] [PubMed] [Google Scholar]
- Aday, A. W. , & Ridker, P. M. (2018). Antiinflammatory therapy in clinical care: The CANTOS trial and beyond. Frontiers in Cardiovascular Medicine, 5, 62 10.3389/fcvm.2018.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshin, A. , Sur, P. J. , Fay, K. A. , Cornaby, L. , Ferrara, G. , Salama, J. S. , … Murray, C. J. L. (2019). Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet., 393, 1958–1972. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aissa, I. , Kharrat, N. , Aloui, F. , Sellami, M. , Bouaziz, M. , & Gargouri, Y. (2017). Valorization of antioxidants extracted from olive mill wastewater. Biotechnology and Applied Biochemistry, 64, 579–589. 10.1002/bab.1509 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017a). The concise guide to PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017b). The concise guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew, R. , & Izzo, A. A. (2017). Principles of pharmacological research of nutraceuticals. British Journal of Pharmacology, 174(11), 1177–1194. 10.1111/bph.13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio‐Soto, M. , Sanchez‐Hidalgo, M. , Rosillo, M. A. , Castejon, M. L. , & Alarcon‐de‐la‐Lastra, C. (2016). Extra virgin olive oil: A key functional food for prevention of immune‐inflammatory diseases. Food & Function, 7, 4492–4505. 10.1039/c4496fo01094f [DOI] [PubMed] [Google Scholar]
- Arbones‐Mainar, J. M. , Ross, K. , Rucklidge, G. J. , Reid, M. , Duncan, G. , Arthur, J. R. , … de Roos, B. (2007). Extra virgin olive oils increase hepatic fat accumulation and hepatic antioxidant protein levels in APOE−/− mice. Journal of Proteome Research, 6, 4041–4054. 10.1021/pr070321a [DOI] [PubMed] [Google Scholar]
- Aunon‐Calles, D. , Canut, L. , & Visioli, F. (2013). Toxicological evaluation of pure hydroxytyrosol. Food and Chemical Toxicology, 55, 498–504. 10.1016/j.fct.2013.01.030 [DOI] [PubMed] [Google Scholar]
- Aunon‐Calles, D. , Giordano, E. , Bohnenberger, S. , & Visioli, F. (2013). Hydroxytyrosol is not genotoxic in vitro. Pharmacological Research, 74, 87–93. 10.1016/j.phrs.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Balmus, I. M. , Ciobica, A. , Trifan, A. , & Stanciu, C. (2016). The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: Clinical aspects and animal models. Saudi Journal of Gastroenterology, 22, 3–17. 10.4103/1319-3767.173753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batarseh, Y. S. , Mohamed, L. A. , Al Rihani, S. B. , Mousa, Y. M. , Siddique, A. B. , El Sayed, K. A. , & Kaddoumi, A. (2017). Oleocanthal ameliorates amyloid‐β oligomers' toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience, 352, 204–215. 10.1016/j.neuroscience.2017.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp, G. K. , Keast, R. S. , Morel, D. , Lin, J. , Pika, J. , Han, Q. , … Breslin, P. A. (2005). Phytochemistry: Ibuprofen‐like activity in extra‐virgin olive oil. Nature, 437, 45–46. 10.1038/437045a [DOI] [PubMed] [Google Scholar]
- Bernini, R. , Merendino, N. , Romani, A. , & Velotti, F. (2013). Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Current Medicinal Chemistry, 20, 655–670. 10.2174/092986713804999367 [DOI] [PubMed] [Google Scholar]
- Block, R. C. , Harris, W. S. , Reid, K. J. , & Spertus, J. A. (2008). ω‐6 and trans fatty acids in blood cell membranes: A risk factor for acute coronary syndromes? American Heart Journal, 156, 1117–1123. 10.1016/j.ahj.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bock, M. , Thorstensen, E. B. , Derraik, J. G. , Henderson, H. V. , Hofman, P. L. , & Cutfield, W. S. (2013). Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Molecular Nutrition & Food Research, 57, 2079–2085. 10.1002/mnfr.201200795 [DOI] [PubMed] [Google Scholar]
- Bonanome, A. , Pagnan, A. , Caruso, D. , Toia, A. , Xamin, A. , Fedeli, E. , … Galli, G. (2000). Evidence of postprandial absorption of olive oil phenols in humans. Nutrition, Metabolism, and Cardiovascular Diseases, 10, 111–120. [PubMed] [Google Scholar]
- Bonvino, N. P. , Liang, J. , McCord, E. D. , Zafiris, E. , Benetti, N. , Ray, N. B. , … Karagiannis, T. C. (2018). OliveNet: A comprehensive library of compounds from Olea europaea. Database: The Journal of Biological Databases and Curation, 2018, bay016 10.1093/database/bay016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskou, D. (2015). Dimitrios Boskou. Olive oil: Properties and processing for use in food, pp 3–33.
- Calder, P. C. , Bosco, N. , Bourdet‐Sicard, R. , Capuron, L. , Delzenne, N. , Dore, J. , … Visioli, F. (2017). Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Research Reviews, 40, 95–119. 10.1016/j.arr.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Capurso, A. , Crepaldi, G. , & Capurso, C. (2019). The Mediterranean diet: A pathway to successful aging. Aging Clinical and Experimental Research. 10.1007/s40520-019-01160-3 [DOI] [PubMed] [Google Scholar]
- Caruso, D. , Visioli, F. , Patelli, R. , Galli, C. , & Galli, G. (2001). Urinary excretion of olive oil phenols and their metabolites in humans. Metabolism, 50, 1426–1428. 10.1053/meta.2001.28073 [DOI] [PubMed] [Google Scholar]
- Casamenti, F. , & Stefani, M. (2017). Olive polyphenols: New promising agents to combat aging‐associated neurodegeneration. Expert Review of Neurotherapeutics, 17, 345–358. 10.1080/14737175.2017.1245617 [DOI] [PubMed] [Google Scholar]
- Christian, M. S. , Sharper, V. A. , Hoberman, A. M. , Seng, J. E. , Fu, L. , Covell, D. , … Crea, R. (2004). The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug and Chemical Toxicology, 27, 309–330. 10.1081/DCT-200039714 [DOI] [PubMed] [Google Scholar]
- Colica, C. , Di Renzo, L. , Trombetta, D. , Smeriglio, A. , Bernardini, S. , Cioccoloni, G. , … De Lorenzo, A. (2017). Antioxidant effects of a hydroxytyrosol‐based pharmaceutical formulation on body composition, metabolic state, and gene expression: A randomized double‐blinded, placebo‐controlled crossover trial. Oxidative Medicine and Cellular Longevity, 2017, 2473495 10.1155/2017/2473495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelio, K. , Espiritu, R. A. , Hanashima, S. , Todokoro, Y. , Malabed, R. , Kinoshita, M. , … Matsunaga, S. (2019). Theonellamide A, a marine‐sponge‐derived bicyclic peptide, binds to cholesterol in aqueous DMSO: Solution NMR‐based analysis of peptide‐sterol interactions using hydroxylated sterol. Biochimica et Biophysica Acta ‐ Biomembranes, 1861, 228–235. 10.1016/j.bbamem.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Crespo, M. C. , Tome‐Carneiro, J. , Burgos‐Ramos, E. , Loria Kohen, V. , Espinosa, M. I. , Herranz, J. , & Visioli, F. (2015). One‐week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacological Research, 95–96, 132–137. 10.1016/j.phrs.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Crespo, M. C. , Tome‐Carneiro, J. , Davalos, A. , & Visioli, F. (2018). Pharma‐nutritional properties of olive oil phenols. Transfer of new findings to human nutrition. Food, 7(6), E90 10.3390/foods7060090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo, M. C. , Tome‐Carneiro, J. , Pintado, C. , Davalos, A. , Visioli, F. , & Burgos‐Ramos, E. (2017). Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer's disease. BioFactors, 43, 540–548. 10.1002/biof.1356 [DOI] [PubMed] [Google Scholar]
- Cubillos‐Ruiz, J. R. , Silberman, P. C. , Rutkowski, M. R. , Chopra, S. , Perales‐Puchalt, A. , Song, M. , … Glimcher, L. H. (2015). ER stress sensor XBP1 controls anti‐tumor immunity by disrupting dendritic cell homeostasis. Cell, 161, 1527–1538. 10.1016/j.cell.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo, S. , Manna, C. , Migliardi, V. , Mazzoni, O. , Morrica, P. , Capasso, G. , … Zappia, V. (2001). Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metabolism and Disposition, 29, 1492–1498. [PubMed] [Google Scholar]
- Davalos, A. , & Suarez, Y. (2013). MiRNA‐based therapy: From bench to bedside. Pharmacological Research, 75, 1–2. 10.1016/j.phrs.2013.06.010 [DOI] [PubMed] [Google Scholar]
- De la Torre, R. , Corella, D. , Castaner, O. , Martinez‐Gonzalez, M. A. , Salas‐Salvador, J. , Vila, J. , … Fitó, M. (2017). Protective effect of homovanillyl alcohol on cardiovascular disease and total mortality: Virgin olive oil, wine, and catechol‐methylathion. The American Journal of Clinical Nutrition, 105, 1297–1304. 10.3945/ajcn.116.145813 [DOI] [PubMed] [Google Scholar]
- Del Rio, D. , Rodriguez‐Mateos, A. , Spencer, J. P. , Tognolini, M. , Borges, G. , & Crozier, A. (2013). Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants & Redox Signaling, 18, 1818–1892. 10.1089/ars.2012.4581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakos, C. I. , Charles, K. A. , McMillan, D. C. , & Clarke, S. J. (2014). Cancer‐related inflammation and treatment effectiveness. The Lancet. Oncology, 15, e493–e503. 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- D'Ignazio, L. , Batie, M. , & Rocha, S. (2017). Hypoxia and inflammation in cancer, focus on HIF and NF‐κB. Biomedicine, 5(2), 21 10.3390/biomedicines5020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede, L. , Rigacci, S. , Romeo, M. , Stefani, M. , & Salmona, M. (2013). Oleuropein aglycone protects transgenic C. elegans strains expressing aβ42 by reducing plaque load and motor deficit. PLoS ONE, 8, e58893 10.1371/journal.pone.0058893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty, R. M. , Galli, C. , Ferro‐Luzzi, A. , & Iacono, J. M. (1987). Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: A study of normal subjects from Italy, Finland, and the USA. The American Journal of Clinical Nutrition, 45, 443–455. 10.1093/ajcn/45.2.443 [DOI] [PubMed] [Google Scholar]
- Dunn, R. T. 2nd , & Klaassen, C. D. (1998). Tissue‐specific expression of rat sulfotransferase messenger RNAs. Drug Metabolism and Disposition, 26, 598–604. [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti‐inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 9: 2033. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) , Turck, D. , Bresson, J.‐L. , Burlingame, B. , Dean, T. , Fairweather‐Tait, S. , … van Loveren, H . (2017). Safety of hydroxytyrosol as a novel food pursuant to regulation (EC) no 258/97. EFSA Journal, 15, e04728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch, R. , Ros, E. , Salas‐Salvadó, J. , Covas, M.‐I. , Corella, D. , Arós, F. , … PREDIMED Study Investigators (2018). Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. New England Journal of Medicine, 378, e34 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- European Communities Commission (2002). Commission regulation (EC) no 1019/2002ed. Community E. Official Journal of the European Communities: Brussels, pp 27–31.
- Faine, L. A. , Rodrigues, H. G. , Galhardi, C. M. , Ebaid, G. M. , Diniz, Y. S. , Padovani, C. R. , & Novelli, E. L. (2006). Effects of olive oil and its minor constituents on serum lipids, oxidative stress, and energy metabolism in cardiac muscle. Canadian Journal of Physiology and Pharmacology, 84, 239–245. 10.1139/y05-124 [DOI] [PubMed] [Google Scholar]
- Farr, S. A. , Price, T. O. , Dominguez, L. J. , Motisi, A. , Saiano, F. , Niehoff, M. L. , … Barbagallo, M. (2012). Extra virgin olive oil improves learning and memory in SAMP8 mice. Journal of Alzheimer's Disease, 28, 81–92. 10.3233/JAD-2011-110662 [DOI] [PubMed] [Google Scholar]
- Feigin, V. L. , Abajobir, A. A. , Abate, K. H. , Abd‐Allah, F. , Abdulle, A. M. , Abera, S. F. , … Vos, T. (2017). Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology, 16, 877–897. 10.1016/S1474-4422(17)30299-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogliano, V. , & Sacchi, R. (2006). Oleocanthal in olive oil: Between myth and reality. Molecular Nutrition & Food Research, 50, 5–6. 10.1002/mnfr.200690002 [DOI] [PubMed] [Google Scholar]
- Forman, H. J. , Davies, K. J. , & Ursini, F. (2014). How do nutritional antioxidants really work: Nucleophilic tone and para‐hormesis versus free radical scavenging in vivo. Free Radical Biology and Medicine, 66, 24–35. 10.1016/j.freeradbiomed.2013.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco, V. , Ruiz‐Fernandez, C. , Lahera, V. , Lago, F. , Pino, J. , Skaltsounis, L. , … Gualillo, O. (2019). Natural molecules for healthy lifestyles: Oleocanthal from extra virgin olive oil. Journal of Agricultural and Food Chemistry, 67, 3845–3853. 10.1021/acs.jafc.8b06723 [DOI] [PubMed] [Google Scholar]
- Funakohi‐Tago, M. , Sakata, T. , Fujiwara, S. , Sakakura, A. , Sugai, T. , Tago, K. , & Tamura, H. (2018). Hydroxytyrosol butyrate inhibits 6‐OHDA‐induced apoptosis through activation of the Nrf2/HO‐1 axis in SH‐SY5Y cells. European Journal of Pharmacology, 834, 246–256. 10.1016/j.ejphar.2018.07.043 [DOI] [PubMed] [Google Scholar]
- Garcia‐Blanco, T. , Davalos, A. , & Visioli, F. (2017). Tea, cocoa, coffee, and affective disorders: Vicious or virtuous cycle? Journal of Affective Disorders, 224, 61–68. 10.1016/j.jad.2016.11.033 [DOI] [PubMed] [Google Scholar]
- Gerber, M. (1997). Olive oil, monounsaturated fatty acids and cancer. Cancer Letters, 114, 91–92. 10.1016/S0304-3835(97)04632-6 [DOI] [PubMed] [Google Scholar]
- Gillingham, L. G. , Harris‐Janz, S. , & Jones, P. J. (2011). Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids, 46, 209–228. 10.1007/s11745-010-3524-y [DOI] [PubMed] [Google Scholar]
- Giordano, E. , Dangles, O. , Rakotomanomana, N. , Baracchini, S. , & Visioli, F. (2015). 3‐O‐Hydroxytyrosol glucuronide and 4‐O‐hydroxytyrosol glucuronide reduce endoplasmic reticulum stress in vitro. Food & Function, 6, 3275–3281. 10.1039/c5fo00562k [DOI] [PubMed] [Google Scholar]
- Giordano, E. , Davalos, A. , Nicod, N. , & Visioli, F. (2014). Hydroxytyrosol attenuates tunicamycin‐induced endoplasmic reticulum stress in human hepatocarcinoma cells. Molecular Nutrition & Food Research, 58, 954–962. 10.1002/mnfr.201300465 [DOI] [PubMed] [Google Scholar]
- Goldstein, D. S. , Jinsmaa, Y. , Sullivan, P. , Holmes, C. , Kopin, I. J. , & Sharabi, Y. (2016). 3,4‐Dihydroxyphenylethanol (hydroxytyrosol) mitigates the increase in spontaneous oxidation of dopamine during monoamine oxidase inhibition in PC12 cells. Neurochemical Research, 41, 2173–2178. 10.1007/s11064-016-1959-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Santiago, M. , Fonolla, J. , & Lopez‐Huertas, E. (2010). Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low‐density lipoproteins. Pharmacological Research, 61, 364–370. 10.1016/j.phrs.2009.12.016 [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Santiago, M. , Martin‐Bautista, E. , Carrero, J. J. , Fonolla, J. , Baro, L. , Bartolome, M. V. , … López‐Huertas, E. (2006). One‐month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis, 188, 35–42. 10.1016/j.atherosclerosis.2005.10.022 [DOI] [PubMed] [Google Scholar]
- Grossi, C. , Rigacci, S. , Ambrosini, S. , Ed Dami, T. , Luccarini, I. , Traini, C. , … Stefani, M. (2013). The polyphenol oleuropein aglycone protects TgCRND8 mice against Aß plaque pathology. PLoS ONE, 8, e71702 10.1371/journal.pone.0071702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, G. , & Lan, S. (2018). Implications of antioxidant systems in inflammatory bowel disease. BioMed Research International, 2018, 1290179 10.1155/2018/1290179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera Acosta, E. , Alonso Suárez Pérez, J. , Aguilera Arjona, J. , & Visioli, F. (2016). An olive polyphenol‐based nutraceutical improves cutaneous manifestations of psoriasis in humans. PharmaNutrition, 4, 151–153. 10.1016/j.phanu.2016.10.002 [DOI] [Google Scholar]
- Hmimed, S. , Belarbi, M. , & Visioli, F. (2016). Hydroxytyrosol augments the redox status of high fat diet‐fed rats. PharmaNutrition, 4, 139–142. 10.1016/j.phanu.2016.09.001 [DOI] [Google Scholar]
- Jenkins, D. J. A. , Spence, J. D. , Giovannucci, E. L. , Kim, Y. I. , Josse, R. , Vieth, R. , … Sievenpiper, J. L. (2018). Supplemental vitamins and minerals for CVD prevention and treatment. Journal of the American College of Cardiology, 71, 2570–2584. 10.1016/j.jacc.2018.04.020 [DOI] [PubMed] [Google Scholar]
- Jones, D. P. , & Sies, H. (2015). The redox code. Antioxidants & Redox Signaling, 23, 734–746. 10.1089/ars.2015.6247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupaiah, T. , & Sundram, K. (2007). Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: A review of their nutritional implications. Nutrition & Metabolism (London), 4, 16 10.1186/1743-7075-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khymenets, O. , Crespo, M. C. , Dangles, O. , Rakotomanomana, N. , Andres‐Lacueva, C. , & Visioli, F. (2016). Human hydroxytyrosol's absorption and excretion from a nutraceutical. Journal of Functional Foods, 23, 278–282. 10.1016/j.jff.2016.02.046 [DOI] [Google Scholar]
- Kim, S. H. , Kwon, D. Y. , Kwak, J. H. , Lee, S. , Lee, Y. H. , Yun, J. , … Jung, Y. S. (2018). Tunicamycin‐induced ER stress is accompanied with oxidative stress via abrogation of sulfur amino acids metabolism in the liver. International Journal of Molecular Sciences, 19(12), E4114 10.3390/ijms19124114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolios, G. , Valatas, V. , & Ward, S. G. (2004). Nitric oxide in inflammatory bowel disease: A universal messenger in an unsolved puzzle. Immunology, 113, 427–437. 10.1111/j.1365-2567.2004.01984.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotronoulas, A. , Pizarro, N. , Serra, A. , Robledo, P. , Joglar, J. , Rubio, L. , … de la Torre, R. (2013). Dose‐dependent metabolic disposition of hydroxytyrosol and formation of mercapturates in rats. Pharmacological Research, 77, 47–56. 10.1016/j.phrs.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Leger, C. L. , Carbonneau, M. A. , Michel, F. , Mas, E. , Monnier, L. , Cristol, J. P. , & Descomps, B. (2005). A thromboxane effect of a hydroxytyrosol‐rich olive oil wastewater extract in patients with uncomplicated type I diabetes. European Journal of Clinical Nutrition, 59, 727–730. 10.1038/sj.ejcn.1602133 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Zhang, R. , Huang, L. , Zheng, Z. , Vlassara, H. , Striker, G. , … Zheng, F. (2019). Excessive oxidative stress contributes to increased acute ER stress kidney injury in aged mice. Oxidative Medicine and Cellular Longevity, 2019, 2746521 10.1155/2019/2746521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , & Hu, M. (2007). Natural polyphenol disposition via coupled metabolic pathways. Expert Opinion on Drug Metabolism & Toxicology, 3, 389–406. 10.1517/17425255.3.3.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de las Hazas, M.‐C. , Godinho‐Pereira, J. , Macià, A. , Almeida, A. F. , Ventura, M. R. , Motilva, M.‐J. , & Santos, C. N. (2018). Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. Journal of Functional Foods, 46, 110–117. [Google Scholar]
- López de Las Hazas, M. C. , Martin‐Hernández, R. , Crespo, M. C. , Tomé‐Carneiro, J. , Del Pozo‐Acebo, L. , Ruiz‐Roso, M. B. , … Dávalos, A . (2019). Identification and validation of common molecular targets of hydroxytyrosol. Food Funct. 10.1039/c9fo01159e [DOI] [PubMed] [Google Scholar]
- Lopez de las Hazas, M. C. , Pinol, C. , Macia, A. , & Motilva, M. J. (2017). Hydroxytyrosol and the colonic metabolites derived from virgin olive oil intake induce cell cycle arrest and apoptosis in colon cancer cells. Journal of Agricultural and Food Chemistry, 65, 6467–6476. 10.1021/acs.jafc.6b04933 [DOI] [PubMed] [Google Scholar]
- López de las Hazas, M.‐C. , Piñol, C. , Macià, A. , Romero, M.‐P. , Pedret, A. , Solà, R. , … Motilva, M.‐J. (2016). Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. Journal of Functional Foods, 22, 52–63. [Google Scholar]
- Lopez de las Hazas, M. C. , Rubio, L. , Kotronoulas, A. , de la Torre, R. , Sola, R. , & Motilva, M. J. (2015). Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Molecular Nutrition & Food Research, 59, 1395–1399. 10.1002/mnfr.201500048 [DOI] [PubMed] [Google Scholar]
- Lopez‐Huertas, E. , & Fonolla, J. (2017). Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biology, 11, 384–389. 10.1016/j.redox.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotito, S. B. , & Frei, B. (2006). Consumption of flavonoid‐rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radical Biology and Medicine, 41, 1727–1746. 10.1016/j.freeradbiomed.2006.04.033 [DOI] [PubMed] [Google Scholar]
- Luccarini, I. , Ed Dami, T. , Grossi, C. , Rigacci, S. , Stefani, M. , & Casamenti, F. (2014). Oleuropein aglycone counteracts Aβ42 toxicity in the rat brain. Neuroscience Letters, 558, 67–72. 10.1016/j.neulet.2013.10.062 [DOI] [PubMed] [Google Scholar]
- Luccarini, I. , Grossi, C. , Rigacci, S. , Coppi, E. , Pugliese, A. M. , Pantano, D. , … Casamenti, F. (2015). Oleuropein aglycone protects against pyroglutamylated‐3 amyloid‐ß toxicity: Biochemical, epigenetic and functional correlates. Neurobiology of Aging, 36, 648–663. 10.1016/j.neurobiolaging.2014.08.029 [DOI] [PubMed] [Google Scholar]
- Machowetz, A. , Poulsen, H. E. , Gruendel, S. , Weimann, A. , Fito, M. , Marrugat, J. , … Koebnick, C. (2007). Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. The FASEB Journal, 21, 45–52. 10.1096/fj.06-6328com [DOI] [PubMed] [Google Scholar]
- Marangoni, F. , Novo, G. , Perna, G. , Perrone Filardi, P. , Pirelli, S. , Ceroti, M. , … Poli, A. (2014). ω‐6 and ω‐3 polyunsaturated fatty acid levels are reduced in whole blood of Italian patients with a recent myocardial infarction: The AGE‐IM study. Atherosclerosis, 232, 334–338. 10.1016/j.atherosclerosis.2013.11.048 [DOI] [PubMed] [Google Scholar]
- Martinez, N. , Herrera, M. , Frias, L. , Provencio, M. , Perez‐Carrion, R. , Diaz, V. , … Crespo, M. C. (2019). A combination of hydroxytyrosol, ω‐3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: Results of a pilot study. Clinical and Translational Oncology, 21, 489–498. 10.1007/s12094-018-1950-0 [DOI] [PubMed] [Google Scholar]
- Martinez‐Gonzalez, M. A. , Hershey, M. S. , Zazpe, I. , & Trichopoulou, A. (2017). Transferability of the Mediterranean diet to non‐Mediterranean countries. What is and what is not the Mediterranean diet. Nutrients, 9(11), E1226 10.3390/nu9111226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos, R. , Martinez‐Lopez, S. , Baeza Arevalo, G. , Amigo‐Benavent, M. , Sarria, B. , & Bravo‐Clemente, L. (2016). Hydroxytyrosol in functional hydroxytyrosol‐enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chemistry, 205, 248–256. 10.1016/j.foodchem.2016.03.011 [DOI] [PubMed] [Google Scholar]
- Monkkonen, T. , & Debnath, J. (2018). Inflammatory signaling cascades and autophagy in cancer. Autophagy, 14, 190–198. 10.1080/15548627.2017.1345412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan, U. , Cao, Q. , Yilmaz, E. , Lee, A. H. , Iwakoshi, N. N. , Ozdelen, E. , … Hotamisligil, G. S. (2004). Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science, 306, 457–461. 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- de Pablos, R. M. , Espinosa‐Oliva, A. M. , Hornedo‐Ortega, R. , Cano, M. , & Arguelles, S. (2019). Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune‐mediated and neurodegenerative diseases. Pharmacological Research, 143, 58–72. 10.1016/j.phrs.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Pang, K. L. , & Chin, K. Y. (2018). The biological activities of oleocanthal from a molecular perspective. Nutrients, 10(5), E570 10.3390/nu10050570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos, G. , & Boskou, D. (1991). Antioxidant effect of natural phenols on olive oil. Journal of the American Oil Chemists Society, 68, 669–671. 10.1007/BF02662292 [DOI] [Google Scholar]
- Park, J. , Min, J. S. , Chae, U. , Lee, J. Y. , Song, K. S. , Lee, H. S. , … Lee, D. S. (2017). Anti‐inflammatory effect of oleuropein on microglia through regulation of Drp1‐dependent mitochondrial fission. Journal of Neuroimmunology, 306, 46–52. 10.1016/j.jneuroim.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Pastor, A. , Rodriguez‐Morato, J. , Olesti, E. , Pujadas, M. , Perez‐Mana, C. , Khymenets, O. , … de la Torre, R. (2016). Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. Journal of Chromatography a, 1437, 183–190. 10.1016/j.chroma.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Pedret, A. , Fernandez‐Castillejo, S. , Valls, R. M. , Catalan, U. , Rubio, L. , Romeu, M. , et al. (2018). Cardiovascular benefits of phenol‐enriched virgin olive oils: New insights from the virgin olive oil and HDL functionality (VOHF) study. Molecular Nutrition & Food Research, 62, e1800456 10.1002/mnfr.201800456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, S. , Zhang, B. , Yao, J. , Duan, D. , & Fang, J. (2015). Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food & Function, 6, 2091–2100. 10.1039/c5fo00097a [DOI] [PubMed] [Google Scholar]
- Peng, Y. , Hou, C. , Yang, Z. , Li, C. , Jia, L. , Liu, J. , … Liu, J. (2016). Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Molecular Nutrition & Food Research, 60, 2331–2342. 10.1002/mnfr.201600332 [DOI] [PubMed] [Google Scholar]
- Peroulis, N. , Androutsopoulos, V. P. , Notas, G. , Koinaki, S. , Giakoumaki, E. , Spyros, A. , … Kampa, M. (2018). Significant metabolic improvement by a water extract of olives: Animal and human evidence. European Journal of Nutrition. 10.1007/s00394-018-1807-x [DOI] [PubMed] [Google Scholar]
- Peyrol, J. , Riva, C. , & Amiot, M. J. (2017). Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients, 9(3), E306 10.3390/nu9030306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzi, C. , Lama, A. , Simeoli, R. , Paciello, O. , Pagano, T. B. , Mollica, M. P. , … Meli, R. (2016). Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. The Journal of Nutritional Biochemistry, 30, 108–115. 10.1016/j.jnutbio.2015.12.004 [DOI] [PubMed] [Google Scholar]
- Pitt, J. , Roth, W. , Lacor, P. , Smith, A. B. 3rd , Blankenship, M. , Velasco, P. , … Klein, W. L. (2009). Alzheimer's‐associated Aβ oligomers show altered structure, immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicology and Applied Pharmacology, 240, 189–197. 10.1016/j.taap.2009.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaltopoulou, T. , Sergentanis, T. N. , Panagiotakos, D. B. , Sergentanis, I. N. , Kosti, R. , & Scarmeas, N. (2013). Mediterranean diet, stroke, cognitive impairment, and depression: A meta‐analysis. Annals of Neurology, 74, 580–591. 10.1002/ana.23944 [DOI] [PubMed] [Google Scholar]
- Qosa, H. , Batarseh, Y. S. , Mohyeldin, M. M. , El Sayed, K. A. , Keller, J. N. , & Kaddoumi, A. (2015). Oleocanthal enhances amyloid‐β clearance from the brains of TgSwDI mice and in vitro across a human blood‐brain barrier model. ACS Chemical Neuroscience, 6, 1849–1859. 10.1021/acschemneuro.5b00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qosa, H. , Mohamed, L. A. , Batarseh, Y. S. , Alqahtani, S. , Ibrahim, B. , LeVine, H. 3rd , … Kaddoumi, A. (2015). Extra‐virgin olive oil attenuates amyloid‐β and τ pathologies in the brains of TgSwDI mice. Journal of Nutritional Biochemistry, 26, 1479–1490. 10.1016/j.jnutbio.2015.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, K. , Takeda, A. , Martin, N. , Ellis, L. , Wijesekara, D. , Vepa, A. , … Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews, 3, CD009825 10.1002/14651858.CD009825.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm, J. , & Shield, K. D. (2019). Global burden of disease and the impact of mental and addictive disorders. Current Psychiatry Reports, 21, 10 10.1007/s11920-019-0997-0 [DOI] [PubMed] [Google Scholar]
- Richard, N. , Arnold, S. , Hoeller, U. , Kilpert, C. , Wertz, K. , & Schwager, J. (2011). Hydroxytyrosol is the major anti‐inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Medica, 77, 1890–1897. 10.1055/s-0031-1280022 [DOI] [PubMed] [Google Scholar]
- Rigacci, S. , Miceli, C. , Nediani, C. , Berti, A. , Cascella, R. , Pantano, D. , … Stefani, M. (2015). Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget, 6, 35344–35357. 10.18632/oncotarget.6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter, B. , & Greten, F. R. (2019). Modulating inflammation for cancer therapy. The Journal of Experimental Medicine, 216(6), 1234–1243. 10.1084/jem.20181739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles‐Almazan, M. , Pulido‐Moran, M. , Moreno‐Fernandez, J. , Ramirez‐Tortosa, C. , Rodriguez‐Garcia, C. , Quiles, J. L. , & Ramirez‐Tortosa, M. (2018). Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Research International, 105, 654–667. 10.1016/j.foodres.2017.11.053 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Gutierrez, G. , Duthie, G. G. , Wood, S. , Morrice, P. , Nicol, F. , Reid, M. , … de Roos, B. (2012). Alperujo extract, hydroxytyrosol, and 3,4‐dihydroxyphenylglycol are bioavailable and have antioxidant properties in vitamin E‐deficient rats—A proteomics and network analysis approach. Molecular Nutrition & Food Research, 56, 1137–1147. 10.1002/mnfr.201100808 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Morato, J. , Boronat, A. , Kotronoulas, A. , Pujadas, M. , Pastor, A. , Olesti, E. , … de la Torre, R. (2016). Metabolic disposition and biological significance of simple phenols of dietary origin: hydroxytyrosol and tyrosol. Drug Metabolism Reviews, 48, 218–236. 10.1080/03602532.2016.1179754 [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Morato, J. , Xicota, L. , Fito, M. , Farre, M. , Dierssen, M. , & de la Torre, R. (2015). Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules, 20, 4655–4680. 10.3390/molecules20034655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, R. (1999). Atherosclerosis—An inflammatory disease. The New England Journal of Medicine, 340, 115–126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- Rubio, L. , Valls, R. M. , Macia, A. , Pedret, A. , Giralt, M. , Romero, M. P. , … Motilva, M. J. (2012). Impact of olive oil phenolic concentration on human plasmatic phenolic metabolites. Food Chemistry, 135, 2922–2929. 10.1016/j.foodchem.2012.07.085 [DOI] [PubMed] [Google Scholar]
- Salas‐Salvado, J. , Bullo, M. , Estruch, R. , Ros, E. , Covas, M. I. , Ibarrola‐Jurado, N. , … Martínez‐González, M. A. (2014). Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Annals of Internal Medicine, 160, 1–10. 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- Salvini, S. , Sera, F. , Caruso, D. , Giovannelli, L. , Visioli, F. , Saieva, C. , … Palli, D. (2006). Daily consumption of a high‐phenol extra‐virgin olive oil reduces oxidative DNA damage in postmenopausal women. The British Journal of Nutrition, 95, 742–751. 10.1079/BJN20051674 [DOI] [PubMed] [Google Scholar]
- Santos‐Lopez, J. A. , Garcimartin, A. , Merino, P. , Lopez‐Oliva, M. E. , Bastida, S. , Benedi, J. , & Sánchez‐Muniz, F. J. (2016). Effects of silicon vs. hydroxytyrosol‐enriched restructured pork on liver oxidation status of aged rats fed high‐saturated/high‐cholesterol diets. PLoS ONE, 11, e0147469 10.1371/journal.pone.0147469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer, S. , Podstawa, M. , Visioli, F. , Bogani, P. , Muller, W. E. , & Eckert, G. P. (2007). Hydroxytyrosol‐rich olive mill wastewater extract protects brain cells in vitro and ex vivo. Journal of Agricultural and Food Chemistry, 55, 5043–5049. 10.1021/jf0703710 [DOI] [PubMed] [Google Scholar]
- Serra, G. , Incani, A. , Serreli, G. , Porru, L. , Melis, M. P. , Tuberoso, C. I. G. , … Deiana, M. (2018). Olive oil polyphenols reduce oxysterols‐induced redox imbalance and pro‐inflammatory response in intestinal cells. Redox Biology, 17, 348–354. 10.1016/j.redox.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby, M. K. , Cherrington, N. J. , Vansell, N. R. , & Klaassen, C. D. (2003). Tissue mRNA expression of the rat UDP‐glucuronosyltransferase gene family. Drug Metabolism and Disposition, 31, 326–333. 10.1124/dmd.31.3.326 [DOI] [PubMed] [Google Scholar]
- Sherling, D. H. , Perumareddi, P. , & Hennekens, C. H. (2017). Metabolic syndrome. Journal of Cardiovascular Pharmacology and Therapeutics, 22, 365–367. 10.1177/1074248416686187 [DOI] [PubMed] [Google Scholar]
- St‐Laurent‐Thibault, C. , Arseneault, M. , Longpre, F. , & Ramassamy, C. (2011). Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid‐β‐induced toxicity. Involvement of the NF‐κB signaling. Current Alzheimer Research, 8, 543–551. [DOI] [PubMed] [Google Scholar]
- Tabernero, M. , Sarria, B. , Largo, C. , Martinez‐Lopez, S. , Madrona, A. , Espartero, J. L. , … Mateos, R. (2014). Comparative evaluation of the metabolic effects of hydroxytyrosol and its lipophilic derivatives (hydroxytyrosyl acetate and ethyl hydroxytyrosyl ether) in hypercholesterolemic rats. Food & Function, 5, 1556–1563. 10.1039/c3fo60677e [DOI] [PubMed] [Google Scholar]
- Terzuoli, E. , Nannelli, G. , Giachetti, A. , Morbidelli, L. , Ziche, M. , & Donnini, S. (2019). Targeting endothelial‐to‐mesenchymal transition: The protective role of hydroxytyrosol sulfate metabolite. European Journal of Nutrition. 10.1007/s00394-019-01920-x [DOI] [PubMed] [Google Scholar]
- Toledo, E. , Salas‐Salvado, J. , Donat‐Vargas, C. , Buil‐Cosiales, P. , Estruch, R. , Ros, E. , … Martínez‐González, M. A. (2015). Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: A randomized clinical trial. JAMA Internal Medicine, 175, 1752–1760. 10.1001/jamainternmed.2015.4838 [DOI] [PubMed] [Google Scholar]
- Tome‐Carneiro, J. , Crespo, M. C. , Iglesias‐Gutierrez, E. , Martin, R. , Gil‐Zamorano, J. , Tomas‐Zapico, C. , … Dávalos, A. (2016). Hydroxytyrosol supplementation modulates the expression of miRNAs in rodents and in humans. The Journal of Nutritional Biochemistry, 34, 146–155. 10.1016/j.jnutbio.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Uehara, T. , Nakamura, T. , Yao, D. , Shi, Z. Q. , Gu, Z. , Ma, Y. , … Lipton, S. A. (2006). S‐nitrosylated protein‐disulphide isomerase links protein misfolding to neurodegeneration. Nature, 441, 513–517. 10.1038/nature04782 [DOI] [PubMed] [Google Scholar]
- Valls‐Pedret, C. , Sala‐Vila, A. , Serra‐Mir, M. , Corella, D. , de la Torre, R. , Martinez‐Gonzalez, M. A. , … Ros, E. (2015). Mediterranean diet and age‐related cognitive decline: A randomized clinical trial. JAMA Internal Medicine, 175, 1094–1103. 10.1001/jamainternmed.2015.1668 [DOI] [PubMed] [Google Scholar]
- Vazquez‐Gomez, M. , Garcia‐Contreras, C. , Torres‐Rovira, L. , Pesantez, J. L. , Gonzalez‐Anover, P. , Gomez‐Fidalgo, E. , … Gonzalez‐Bulnes, A. (2017). Polyphenols and IUGR pregnancies: Maternal hydroxytyrosol supplementation improves prenatal and early‐postnatal growth and metabolism of the offspring. PLoS ONE, 12, e0177593 10.1371/journal.pone.0177593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visioli, F. (2012). Can experimental pharmacology be always applied to human nutrition? International Journal of Food Sciences and Nutrition, 63, 10–13. 10.3109/09637486.2012.665439 [DOI] [PubMed] [Google Scholar]
- Visioli, F. (2015). Xenobiotics and human health: A new view of their pharma‐nutritional role. PharmaNutrition, 3, 60–64. 10.1016/j.phanu.2015.04.001 [DOI] [Google Scholar]
- Visioli, F. , Bellomo, G. , & Galli, C. (1998). Free radical‐scavenging properties of olive oil polyphenols. Biochemical and Biophysical Research Communications, 247, 60–64. 10.1006/bbrc.1998.8735 [DOI] [PubMed] [Google Scholar]
- Visioli, F. , & Bernardini, E. (2011). Extra virgin olive oil's polyphenols: Biological activities. Current Pharmaceutical Design, 17, 786–804. 10.2174/138161211795428885 [DOI] [PubMed] [Google Scholar]
- Visioli, F. , Franco, M. , Toledo, E. , Luchsinger, J. , Willett, W. C. , Hu, F. B. , & Martinez‐Gonzalez, M. A. (2018). Olive oil and prevention of chronic diseases: Summary of an international conference. Nutrition, Metabolism, and Cardiovascular Diseases, 28, 649–656. 10.1016/j.numecd.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Visioli, F. , Galli, C. , Grande, S. , Colonnelli, K. , Patelli, C. , Galli, G. , & Caruso, D. (2003). Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. The Journal of Nutrition, 133, 2612–2615. 10.1093/jn/133.8.2612 [DOI] [PubMed] [Google Scholar]
- Visioli, F. , Wolfram, R. , Richard, D. , Abdullah, M. I. , & Crea, R. (2009). Olive phenolics increase glutathione levels in healthy volunteers. Journal of Agricultural and Food Chemistry, 57, 1793–1796. 10.1021/jf8034429 [DOI] [PubMed] [Google Scholar]
- Voelker, R. (2019). Oleic acid can make heart claim without hard evidence. JAMA, 321, 23 10.1001/jama.2018.19747 [DOI] [PubMed] [Google Scholar]
- Walter, P. , & Ron, D. (2011). The unfolded protein response: From stress pathway to homeostatic regulation. Science, 334, 1081–1086. 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- Wang, N. , Liu, Y. , Ma, Y. , & Wen, D. (2018). Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet‐induced obesity mice. The Journal of Nutritional Biochemistry, 57, 180–188. 10.1016/j.jnutbio.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Wilson, T. , Knight, T. J. , Beitz, D. C. , Lewis, D. S. , & Engen, R. L. (1996). Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sciences, 59, PL15–PL21. 10.1016/0024-3205(96)00260-3 [DOI] [PubMed] [Google Scholar]
- Wurtz, P. , Havulinna, A. S. , Soininen, P. , Tynkkynen, T. , Prieto‐Merino, D. , Tillin, T. , … Salomaa, V. (2015). Metabolite profiling and cardiovascular event risk: A prospective study of 3 population‐based cohorts. Circulation, 131, 774–785. 10.1161/CIRCULATIONAHA.114.013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y. F. , Liu, L. P. , Zhong, C. P. , & Geng, J. G. (2001). NF‐κB activation for constitutive expression of VCAM‐1 and ICAM‐1 on B lymphocytes and plasma cells. Biochemical and Biophysical Research Communications, 289, 851–856. 10.1006/bbrc.2001.6067 [DOI] [PubMed] [Google Scholar]
- Ye, Y. , Pang, Z. , Chen, W. , Ju, S. , & Zhou, C. (2015). The epidemiology and risk factors of inflammatory bowel disease. International Journal of Clinical and Experimental Medicine, 8, 22529–22542. [PMC free article] [PubMed] [Google Scholar]
- Yu, G. , Deng, A. , Tang, W. , Ma, J. , Yuan, C. , & Ma, J. (2016). Hydroxytyrosol induces phase II detoxifying enzyme expression and effectively protects dopaminergic cells against dopamine‐ and 6‐hydroxydopamine induced cytotoxicity. Neurochemistry International, 96, 113–120. 10.1016/j.neuint.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Yubero‐Serrano, E. M. , Lopez‐Moreno, J. , Gomez‐Delgado, F. , & Lopez‐Miranda, J. (2018). Extra virgin olive oil: More than a healthy fat. European Journal of Clinical Nutrition. 10.1038/s41430-018-0304-x [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Cao, J. , Jiang, L. , & Zhong, L. (2009). Suppressive effects of hydroxytyrosol on oxidative stress and nuclear factor‐κB activation in THP‐1 cells. Biological & Pharmaceutical Bulletin, 32, 578–582. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Cao, J. , & Zhong, L. (2009). Hydroxytyrosol inhibits pro‐inflammatory cytokines, iNOS, and COX‐2 expression in human monocytic cells. Naunyn‐Schmiedeberg's Archives of Pharmacology, 379, 581–586. 10.1007/s00210-009-0399-7 [DOI] [PubMed] [Google Scholar]
- Zhao, B. , Ma, Y. , Xu, Z. , Wang, J. , Wang, F. , Wang, D. , … Jiang, H. (2014). Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor‐κ B pathways. Cancer Letters, 347, 79–87. 10.1016/j.canlet.2014.01.028 [DOI] [PubMed] [Google Scholar]
- Zong, G. , Li, Y. , Sampson, L. , Dougherty, L. W. , Willett, W. C. , Wanders, A. J. , … Sun, Q. (2018). Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. The American Journal of Clinical Nutrition, 107, 445–453. 10.1093/ajcn/nqx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrelli, H. , Kusunoki, M. , & Miyazaki, H. (2015). Role of hydroxytyrosol‐dependent regulation of HO‐1 expression in promoting wound healing of vascular endothelial cells via Nrf2 de novo synthesis and stabilization. Phytotherapy Research, 29, 1011–1018. 10.1002/ptr.5339 [DOI] [PubMed] [Google Scholar]
- Zrelli, H. , Matsuoka, M. , Kitazaki, S. , Araki, M. , Kusunoki, M. , Zarrouk, M. , & Miyazaki, H. (2011). Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO‐1 induction. Journal of Agricultural and Food Chemistry, 59, 4473–4482. 10.1021/jf104151d [DOI] [PubMed] [Google Scholar]


