Abstract
The Mediterranean diet (MedDiet) is one of the most widely described and evaluated dietary patterns in scientific literature. It is characterized by high intakes of vegetables, legumes, fruits, nuts, grains, fish, seafood, extra virgin olive oil, and a moderate intake of red wine. A large body of observational and experimental evidence suggests that higher adherence to the MedDiet is associated with lower risk of mortality, cardiovascular disease, metabolic disease, and cancer. Current mechanisms underlying the beneficial effects of the MedDiet include reduction of blood lipids, inflammatory and oxidative stress markers, improvement of insulin sensitivity, enhancement of endothelial function, and antithrombotic function. Most likely, these effects are attributable to bioactive ingredients such as polyphenols, monounsaturated and polyunsaturated fatty acids, or fibre. This review will focus on both established and less established mechanisms of action of biochemical compounds contained in a MedDiet.
Linked Articles
This article is part of a themed section on The Pharmacology of Nutraceuticals. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v177.6/issuetoc
Abbreviations
- CHD
coronary heart disease
- CRC
colorectal cancer
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- EVOO
extra virgin olive oil
- FG
fasting glucose
- FMD
flow‐mediated dilatation
- GI
glycaemic index
- HbA1c
glycosylated haemoglobin
- MedDiet
Mediterranean diet
- MUFAs
monounsaturated fatty acids
- NCDs
non‐communicable disease
- PUFAs
polyunsaturated fatty acids
- RCTs
randomized controlled trials
- SBP
systolic BP
- SCFA
short‐chain fatty acid
- TG
triacyglycerols.
1. INTRODUCTION
The Mediterranean diet (MedDiet) is one of the most widely described and evaluated dietary patterns in scientific literature (Bach‐Faig et al., 2011). A large body of evidence demonstrated an inverse association between adherence to MedDiet and a lower risk of all‐cause mortality, cardiovascular disease (CVD), Type 2 diabetes (T2D), site‐specific cancers, and cognitive disorders (Dinu, Pagliai, Casini, & Sofi, 2018). These observations might be explained by antioxidative, anti‐inflammatory, antithrombotic, anti‐neoplastic, and lipid‐lowering properties of bioactive components of a MedDiet (Martinez‐Gonzalez et al., 2015).
1.1. Defining the MedDiet
A common tool to assess adherence to a MedDiet in epidemiological and randomized controlled trials (RCTs) is based on a priori diet quality indices. These indices are created by scoring fulfilment of predefined criteria, where the summary score reflects high or low adherence to a dietary pattern (Davis, Bryan, Hodgson, & Murphy, 2015). A recent umbrella review of 27 meta‐analyses based on 70 cohort studies identified 34 different scores used to define the MedDiet (Galbete, Schwingshackl, Schwedhelm, Boeing, & Schulze, 2018). The most commonly implemented MedDiet score was proposed by Trichopoulou et al. in 1995 and updated in 2003 (Trichopoulou et al., 1995; Trichopoulou, Costacou, Bamia, & Trichopoulos, 2003). The application of MedDiet indices seems to be heterogeneous. However, a common denominator of all MedDiet scores available to date is the fact that they consider typical food groups to describe the dietary pattern: high amounts of fruits, vegetables, nuts, legumes, fish, cereals including whole grains, and extra virgin olive oil (EVOO); moderate intake of alcohol preferably in the form of red wine; and low amounts of dairy products, as well as red and processed meat (Table 1). This is helpful when it comes to analysing the active ingredients of a MedDiet and their corresponding biological and pharmacological modes of action with respect to the prevention of chronic diseases.
Table 1.
Overview of components of the most often applied Mediterranean diet quality indices
| Component | tMedDiet (Trichopoulou et al., 2003) | aMedDiet (Fung et al., 2006) | Daily antioxidant capacity (μmol trolox equivalents; Saura‐Calixto & Goni, 2009) |
|---|---|---|---|
| Vegetables | + | + | 272 |
| Legumes | + | + | 135 |
| Fruits | + | + | 342 |
| Nuts | + | + | 176 |
| Cereals | + | Whole grains | 33 |
| Fish | + | + | / |
| Meat and meat products | ‐ | Red and processed meat | / |
| Dairy products | ‐ | / | / |
|
Alcohol (red wine) |
o women 5–25 g·day−1, men 10–50 g·day−1 |
o women 5–15 g·day−1, men 10–25 g·day−1 |
617 (Wine) |
|
MUFA:SFA ratio (EVOO) |
+ | + | 13 (Olive oil) |
Note. +, higher intake suggested; ‐, lower intake suggested; and o, moderate intake suggested.
Abbreviations: aMedDiet, alternate Mediterranean diet; EVOO, extra virgin olive oil; tMedDiet, traditional Mediterranean diet.
1.2. MedDiet, risk of chronic diseases, and intermediate disease markers
The main objective of this review is not to give a detailed overview of the available evidence on the MedDiet in the prevention of non‐communicable diseases (NCDs). Thus, we will provide only an overview of the current knowledge on the associations between MedDiet and risk of chronic diseases derived from systematic reviews and meta‐analyses of RCTs and prospective observational studies (Table 2) as well the effect on intermediate disease markers summing up the evidence from RCTs (Table 3). Taking observational evidence into account, adherence to a MedDiet was inversely associated with risk of all‐cause mortality, CVD, coronary heart disease (CHD), stroke, heart failure, cancer mortality, colorectal cancer (CRC), breast cancer, gastric cancer, Type 2 diabetes, obesity, asthma, hip fracture, and dementia (Table 2). Reviewing evidence from RCTs, adhering to a MedDiet resulted in lower risk of CHD, stroke, heart failure, hypertension, Type 2 diabetes, and breast cancer with beneficial effects on anthropometric outcomes, blood lipids, glycaemic control, BP, inflammatory biomarker, adiponectin, and flow‐mediated dilatation (FMD; Table 3).
Table 2.
Overview of meta‐analytical findings investigating the effect/association (RR, HR, OR, MD, SMD, and 95% CI) between Mediterranean dietary pattern and health outcomes
| Outcome | MA of RCTs | Reference | MA of NRS | Reference | ||
|---|---|---|---|---|---|---|
| All‐cause mortality |
↔ |
RR: 1.00 [0.86, 1.15] |
(Liyanage et al., 2016) | ↓ |
RR: 0.79 [0.77, 0.81] |
(Eleftheriou et al., 2018) |
| Cardiovascular disease | ↔ |
RR: 0.99 [0.78, 1.26] |
(Liyanage et al., 2016) | ↓ |
RR: 0.81 [0.74, 0.88] |
(Rosato et al., 2019) |
| Coronary heart disease | ↓ |
RR: 0.65 [0.50, 0.85] |
(Liyanage et al., 2016) | ↓ |
RR: 0.74 [0.66, 0.83] |
(Rosato et al., 2019) |
| Stroke | ↓ |
RR: 0.65 [0.48, 0.88] |
(Liyanage et al., 2016) | ↓ |
RR: 0.84 [0.81, 0.88] |
(Chen et al., 2019) |
| Heart failure | ↓ |
RR: 0.30 [0.17, 0.56] |
(Liyanage et al., 2016) | NA | NA | NA |
|
Cancer |
NA | NA | NA | ↓ |
RR: 0.86 [0.81, 0.91] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) |
| Colorectal cancer | NA | NA | NA | ↓ |
RR: 0.82 [0.75, 0.88] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) |
| Breast cancer | ↓ |
RR: 0.43 [0.21, 0.88] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) | ↓ |
RR: 0.92 [0.89, 0.96] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) |
| Prostate cancer | NA | NA | NA | ↔ |
RR: 0.96 [0.92, 1.00] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) |
| Gastric cancer | NA | NA | NA | ↓ |
RR: 0.72 [0.60, 0.86] |
(Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017) |
| Type 2 diabetes | ↓ |
RR: 0.70 [0.54, 0.91] |
(Schwingshackl, Missbach, et al., 2015) | ↓ |
RR: 0.83 [0.74, 0.92] |
(Schwingshackl, Missbach, et al., 2015) |
| Obesity | NA | NA | NA | ↓ |
RR: 0.82 [0.70, 0.96] |
(Godos et al., 2017) |
| Hypertension | ↓ |
MD (mmHg) SBP: −1.44 [−2.88, 0.01] DBP: −0.70 [−1.34, −0.07] |
(Nissensohn, Roman‐Vinas, Sanchez‐Villegas, Piscopo, & Serra‐Majem, 2016) | NA | NA | NA |
| Chronic inflammatory bowel disease | NA | NA | NA | NA | NA | NA |
| Rheumatoid arthritis | NA | NA | NA | ↔ |
HR: 0.98 [0.80, 1.20] |
(Bloomfield et al., 2016) |
| Chronic obstructive pulmonary disease | NA | NA | NA | NA | NA | NA |
| Asthma | NA | NA | NA | ↓ |
OR: 0.85 [0.75, 0.98] |
(Garcia‐Marcos et al., 2013) |
| Hip fracture | NA | NA | NA | ↓ |
RR: 0.79 [0.72, 0.87] |
(Malmir, Saneei, Larijani, & Esmaillzadeh, 2018) |
| Incident frailty | NA | NA | NA | ↓ | OR: 0.62 [0.47, 0.88] | (Kojima et al., 2018) |
| Eye disease | NA | NA | NA | NA | NA | NA |
| Global cognition/dementia | ↔ | SMD: 0.24 [−0.00, 0.47] | (Radd‐Vagenas et al., 2018) | ↓ |
RR: 0.69 [0.57, 0.84] |
(Cao et al., 2016) |
| Depression | NA | NA | NA | ↔ |
HR: 0.95 [0.79, 1.16] |
(Shafiei et al., 2019) |
Note. ↓, lower risk and ↔, no association.
Abbreviations: HR, hazard ratio; MD, mean difference; NA, not applicable; NRS, non‐randomized studies; OR, odds ratio; RCTs, randomized controlled trials; RR, risk ratio; SMD, standardized mean difference.
Table 3.
Overview of randomized controlled trials investigating the effects of Mediterranean dietary pattern on intermediate disease markers
| Outcome | MA of RCTs | Reference | |
|---|---|---|---|
| Body weight (kg) | ↓ | MD: −1.75 [−2.86, −0.64] | (Dinu et al., 2018) |
| Waist circumference (cm) | ↓ | MD: −0.54 [−0.77, −0.31] | (Dinu et al., 2018) |
| Total cholesterol (mmol·L−1) | ↓ | MD: −0.16 [−0.26, −0.06] | (Dinu et al., 2018) |
| LDL cholesterol (mmol·L−1) | ↓ | MD: −0.11 [−0.24, 0.02] | (Dinu et al., 2018) |
| HDL cholesterol (mmol·L−1) | ↑ | MD: 0.03 [0.01, 0.05] | (Dinu et al., 2018) |
| Triglycerides (mmol·L−1) | ↓ | MD: −0.07 [−0.12, −0.02] | (Dinu et al., 2018) |
| Glucose (mmol·L−1) | ↓ | MD: −0.50 [−0.81, −0.20] | (Dinu et al., 2018) |
| HOMA‐IR | ↓ | MD: −0.45 [−0.74, −0.16] | (Dinu et al., 2018) |
| Insulin (μU·ml−1) | ↓ | MD: −0.55 [−0.81, −0.29] | (Dinu et al., 2018) |
| HbA1c (%) | ↓ | MD: −0.30 [−0.46, −0.14] | (Dinu et al., 2018) |
| Systolic BP (mmHg) | ↓ | MD: −0.72 [−1.03, −0.42] | (Dinu et al., 2018) |
| Diastolic BP (mmHg) | ↓ | MD: −0.94 [−1.45, −0.44] | (Dinu et al., 2018) |
| CRP (mg·L−1) | ↓ | MD: −0.98 [−1.48, −0.49] | (Schwingshackl & Hoffmann, 2014a) |
| Adiponectin (μg·ml−1) | ↑ | MD: 1.69 [0.27, 3.11] | (Schwingshackl & Hoffmann, 2014a) |
| FMD (%) | ↑ | MD: 1.86 [0.23, 3.48] | (Schwingshackl & Hoffmann, 2014a) |
Note. ↓, decreasing effect; ↑, increasing effect; and ↔, no effect.
Abbreviations: CRP, C‐reactive protein; FMD, flow‐mediated dilatation; HOMA‐IR, homeostasis model assessment; MD, mean difference; RCTs, randomized controlled trials.
1.3. What is new?
Recent meta‐analyses mostly confirm the favourable associations of adhering to a MedDiet (Chen et al., 2019; Eleftheriou et al., 2018; Kojima, Avgerinou, Iliffe, & Walters, 2018; Rosato et al., 2019; Shafiei, Salari‐Moghaddam, Larijani, & Esmaillzadeh, 2019), although the corresponding data are predominantly based on observational studies. On the one hand, critical summaries of observational studies together with RCTs yielded consistent results with respect to the health benefits of a MedDiet (Martinez‐Gonzalez, Gea, & Ruiz‐Canela, 2019). On the other hand, an updated Cochrane review found only a low to moderate certainty of evidence for interventions with the MedDiet in primary prevention of CVDs (Rees et al., 2019). Availability of RCTs in nutrition research is limited by small sample sizes, high dropout rates, and short follow‐up, while the opposite is needed to observe patient relevant outcomes (Satija, Yu, Willett, & Hu, 2015). Some of the discrepancies are postulated to be caused by the use of different definitions of this dietary pattern (Galbete et al., 2018). Further inconsistencies can be explained by the type of diet used as a comparator. Previous reviews summarizing the mechanisms of the MedDiet focused predominately on individual components of MedDiet or their influence on a specific chronic disease. In the present review, we aimed to present a comprehensive overview of all MedDiet ingredients with a focus on common metabolic pathways rather than on pathophysiological mechanisms of a specific chronic disease.
2. KEY BENEFICIAL COMPONENTS/INGREDIENTS
As shown in Table 1, the MedDiet consists of 10 major components (eight of them are defined as beneficial and therefore described below).
2.1. Vegetables
In a traditional MedDiet, the emphasis is on seasonal, field‐grown vegetables. This includes fresh salads, tomatoes, eggplant, cucumber, cabbage, rocket, radishes, garlic, onion spinach, and lettuce (Hoffman & Gerber, 2013). Vegetables are the most important sources of phenolic compounds (mainly flavonoids) in the MedDiet. In addition, vegetables are characterized by many other nutrients, including dietary fibre, potassium, vitamin A, vitamin C, vitamin K, copper, magnesium, vitamin E, vitamin B6, folate, iron, thiamine, niacin, and choline (Delgado, Vaz‐Almeida, & Parisi, 2017). In several dose–response meta‐analyses of prospective observational studies, a higher consumption of vegetables has been associated with lower risk of all‐cause mortality, CHD, stroke, heart failure, Type 2 diabetes, CRC, and adiposity (Bechthold et al., 2019; Schlesinger et al., 2019; Schwingshackl, Hoffmann, Lampousi, et al., 2017; Schwingshackl, Schwedhelm, et al., 2018; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017).
2.2. Legumes
Commonly consumed legumes in a MedDiet include chickpeas, lentils, and beans. Major ingredients of legumes are protein, fibre, phytosterols, folate, vitamin B6, flavones, and various minerals (Delgado et al., 2017). In dose–response meta‐analyses of prospective observational studies, intake of legumes has been associated with lower risk of all‐cause mortality and CHD (Bechthold et al., 2019; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017). In meta‐analyses of RCTs, intake of legumes showed beneficial effects on body weight, total cholesterol, LDL cholesterol (LDL‐C), systolic BP (SBP), and fasting glucose (FG; Schwingshackl, Schlesinger, et al., 2018).
2.3. Fruits
Frequently consumed fruits in a MedDiet include citrus fruits such as oranges and pomegranates, berries, figs, grapes, and “orange fruits” (e.g., apricots, peaches, nectarines, and cantaloupes). Among many other nutrients, fruits provide dietary fibre, potassium, and vitamin C but also flavonoids and terpenes (Delgado et al., 2017). In dose–response meta‐analyses of prospective observational studies, a higher intake of fruit has been associated with lower risk of all‐cause mortality, CHD, stroke, Type 2 diabetes, CRC, hypertension, and adiposity (Bechthold et al., 2019; Schlesinger et al., 2019; Schwingshackl, Hoffmann, Lampousi, et al., 2017; Schwingshackl, Schwedhelm, Hoffmann, Knuppel, et al., 2017; Schwingshackl, Schwedhelm, et al., 2018; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017).
2.4. Nuts
Commonly consumed nuts include pistachios, almonds, peanuts, hazelnuts, and walnuts. Nuts are a rich source of monounsaturated (MUFAs) and polyunsaturated fatty acids (PUFAs) including http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1052 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1049, phenols, flavonoids, isoflavonoids, phytosterols and phytic acid, vitamin E, vitamin B2, folate, and fibre as well as minerals and trace elements such as magnesium, phosphorus, potassium, copper, and selenium (Delgado et al., 2017). In dose–response meta‐analyses of prospective observational studies, nut consumption has been associated with lower risk of all‐cause mortality, CHD, hypertension, and adiposity (Bechthold et al., 2019; Schlesinger et al., 2019; Schwingshackl, Schwedhelm, Hoffmann, Knuppel, et al., 2017; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017). In meta‐analyses of RCTs, higher nuts intake showed beneficial effects on total cholesterol, LDL‐C, triacylglycerols (TG), SBP, FG, and glycosylated haemoglobin (HbA1c; Schwingshackl, Schlesinger, et al., 2018).
2.5. Grains
This food group includes grains as single foods (e.g., rice, oatmeal, and popcorn), as well as products that use grains as an ingredient (e.g., bread, cereals, crackers, and pasta). Whole grains are a source of nutrients such as dietary fibre, iron, zinc, manganese, folate, magnesium, copper, thiamine, niacin, vitamin B6, phosphorus, selenium, and riboflavin (Delgado et al., 2017). In dose–response meta‐analyses of prospective observational studies, a high consumption of whole grains has been associated with lower risk of all‐cause mortality, CHD, heart failure, Type 2 diabetes, CRC, hypertension, and adiposity (Bechthold et al., 2019; Schlesinger et al., 2019; Schwingshackl, Hoffmann, Lampousi, et al., 2017; Schwingshackl, Schwedhelm, Hoffmann, Knuppel, et al., 2017; Schwingshackl, Schwedhelm, et al., 2018; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017). Meta‐analyses of RCTs have shown a beneficial effect of whole grain intake on total cholesterol, LDL‐C, TG, and FG (Schwingshackl, Schlesinger, et al., 2018).
2.6. Fish/seafood
Typical examples are sardines, mackerel, mussels, octopus, oysters, salmon, sea bass, shrimp, squid, and tuna. The most important bioactive nutrients in fish are generally considered to be the n‐3 long‐chain PUFAs http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3362 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1051; Delgado et al., 2017). In dose–response meta‐analyses of prospective observational studies, fish/seafood intake has been associated with lower risk of all‐cause mortality, CHD, heart failure, and CRC (Bechthold et al., 2019; Schwingshackl, Schwedhelm, et al., 2018; Schwingshackl, Schwedhelm, Hoffmann, Lampousi, et al., 2017). In meta‐analyses of RCTs, higher intakes of fish showed beneficial effects on HDL cholesterol and TG (Schwingshackl, Schlesinger, et al., 2018).
2.7. Alcohol
As a part of the MedDiet, alcohol usually means red wine consumed as part of a meal (Hoffman & Gerber, 2013). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8741 might be a key ingredient in red wine (Delgado et al., 2017). A recent meta‐analysis of 83 prospective studies concluded that the threshold for lowest risk of all‐cause mortality was about 100 g·week−1, whereas for several types of CVD, consuming 100 g·week−1 alcohol showed a detrimental association (Wood et al., 2018).
2.8. Extra virgin olive oil
EVOO is regarded to be a key component of the MedDiet. It is a rich source of both MUFAs and polyphenols or other secondary plant metabolites (e.g., oleuropein, tyrosol, hydroxytyrosol, secoirodoids, and lignans; Lopez‐Miranda et al., 2010). A large meta‐analysis of 32 prospective observational studies has shown that the upper tertile of olive oil consumption was associated with a 20–40% lower risk of stroke and CHD, when compared with the lower tertile (Schwingshackl & Hoffmann, 2014b). In other meta‐analyses including intervention and observational studies, olive oil consumption has been associated with lower diabetes risk and improvements in metabolic and inflammatory biomarkers (Schwingshackl, Christoph, & Hoffmann, 2015; Schwingshackl, Schwedhelm, Galbete, & Hoffmann, 2017). An overview of mechanistic properties of EVOO is presented in Figure 1.
Figure 1.
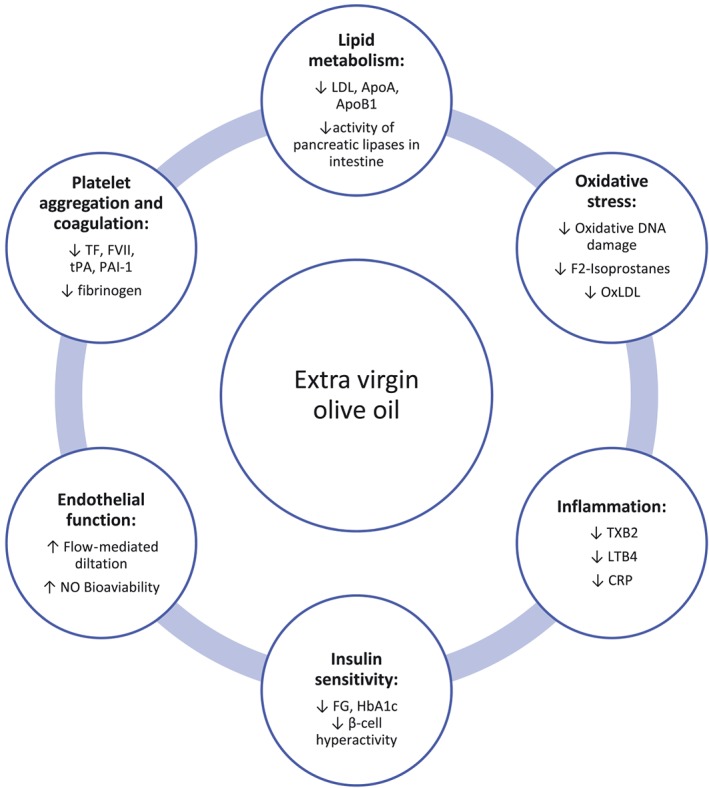
Overview of mechanistic properties of extra virgin oil (EVOO). EVOO may provide a beneficial effect for non‐communicable disease risk by modifying lipid metabolism, decreasing oxidative stress and inflammation, increasing insulin sensitivity, and improving endothelial function and coagulation. ApoA, apolipoprotein A; ApoB1, apolipoprotein B1; CRP, C‐reactive protein; FG, fasting glucose; FVII, coagulation factor VII; OxLDL, oxidized LDL; PAI‐1, plasminogen activator inhibitor‐1; TF, tissue factor; tPA, tissue plasminogen activator
3. PHARMACOLOGICAL MECHANISM—INGREDIENTS
The exact mechanism by which a traditional MedDiet exerts its beneficial effects in lowering the risk of developing CVD, certain cancers, and other metabolic conditions remains to be elucidated. There are various hypotheses about bioactive ingredients and factors, sometimes with interrelated and overlapping modes of action (Figure 2).
Figure 2.
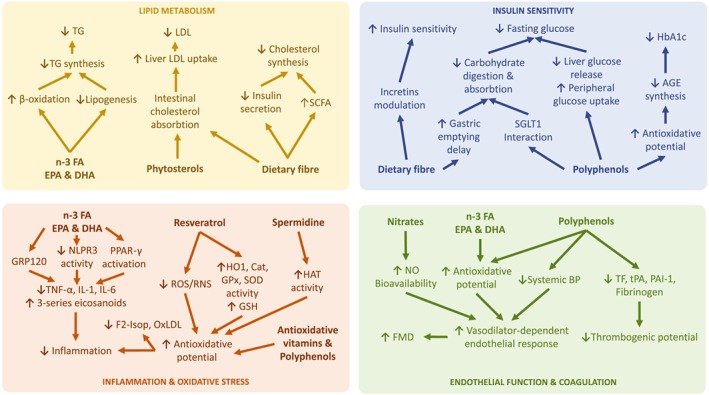
Schematic overview of potential mechanisms involved in the effects of bioactive compounds of ingredients of the Mediterranean diet other than olive oil. Dietary fibre may contribute to lower LDL and cholesterol synthesis, as well as to improve insulin sensitivity and reduce fasting glucose. Polyphenols may increase antioxidative potential, improve endothelial function, and have anti‐inflammatory properties. Moreover, polyphenols may lower fasting glucose and inhibit the synthesis of advanced glycation end products. Additional effects are attributed to the synergistic action of other agents such as ω‐3 fatty acids, phytosterols, resveratrol, spermidine, antioxidative vitamins, and nitrates. AGE, advanced glycation end products; Cat, catalase; DHA, docosahexaenoic acid; EPA, eicosanoid acid; F2‐Isop, F2‐isoprostanes; FMD, flow‐mediated dilation; FVII, coagulation factor VII; GRP120, GPCR 120; GPx, GSH peroxidase; HAT, histone acetyltransferase; HbA1c, glycated haemoglobin A1c; HO1, haem oxygenase 1; n‐3 FA, ω‐3 fatty acids; NLPR3, NACHT, LRR, and PYD domains‐containing protein 3; PAI‐1, plasminogen activator inhibitor‐1; RNS, reactive nitrogen species; SCFA, short‐chain fatty acid; SGLT1, sodium/glucose cotransporter 1; TF, tissue factor; TG, triacylglycerols; tPA, tissue plasminogen activator
3.1. More established pharmacological mechanisms
3.1.1. Lipid metabolism
CVD is the leading cause of death worldwide. Dyslipidaemia, especially elevated LDL‐C levels, are among the most important modifiable risk factors for the development of atherosclerosis, which is an underlying cause of CVD (Eleftheriou et al., 2018).
Walnuts or almonds are rich in linoleic and linolenic acids as well as plant sterols, which might lower LDL‐C (Schwingshackl, Hoffmann, Missbach, Stelmach‐Mardas, & Boeing, 2017). Legumes, nuts, whole grains, vegetables, and fruits are major sources of dietary fibre. Presumably, water‐soluble dietary fibres such as fructans or inulin lower the absorption of cholesterol and bile acids in the small bowel, resulting in an enhanced LDL‐C uptake by the liver (Theuwissen & Mensink, 2008). Foods rich in dietary fibre and low in glycaemic index (LGI) reduced insulin levels and increased the synthesis of short‐chain fatty acids (SCFAs). Both effects inhibit cholesterol production (Theuwissen & Mensink, 2008). The high intake of phytosterols from whole grains, vegetables, fruits, nuts, and seeds may also play an important role in lowering cholesterol levels by competing with intestinal cholesterol absorption (Abumweis, Barake, & Jones, 2008). Moreover, substituting energy from saturated fat with MUFAs yielded reduced levels of LDL, apolipoprotein B and A‐1, and TG (Mensink, 2016). Another explanation for the anti‐hyperlipidaemic effects of EVOO phenolics such as oleuropein might be the delay of postprandial lipaemia via inhibition of pancreatic lipases in the small bowel (Buchholz & Melzig, 2015). Thus, intake of oleuropein extracts exerted lipid‐reducing effects in healthy participants (Lockyer, Rowland, Spencer, Yaqoob, & Stonehouse, 2017). Although the exact mechanisms are not yet clear, there is evidence that some of the lipid‐modulating effects of resveratrol may be mediated through inhibition of the pleiotropic http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1881 and via activation of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1540 (AMPK) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2707 (SIRT1; Voloshyna, Hussaini, & Reiss, 2012).
ω‐3 fatty acids may modulate TG synthesis via reduction of fatty acid availability (lower de novo lipogenesis), decreased activity of triglyceride‐synthesizing enzymes, or increased phospholipid synthesis (Wei & Jacobson, 2011; Harris & Bulchandani, 2006).
AMPK plays a pivotal role in the energy balance of the organism. Malfunctions in AMPK activity are associated with carcinogenesis, diabetes, or inflammation (Garcia & Shaw, 2017). Priore et al. reported a hydroxytyrosol‐induced reduction in lipid synthesis (e.g., fatty acid, cholesterol, and triglycerides) in hepatocytes due to an activation of the AMPK signal transduction pathway. AMPK reduced the activity of different enzymes of lipid metabolism including http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=255#1263 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=639 (Priore, Siculella, & Gnoni, 2014). A comparable effect of hydroxytyrosol was observed in 3T3‐L1 adipocytes (Hao et al., 2010). Transcriptome analysis in peripheral blood mononuclear cells of volunteers consuming olive oil with either high or low polyphenol content revealed an increase in AMPK‐mRNA in the high polyphenol group, which can be interpreted as a dyslipidaemia modulating effect (Camargo et al., 2010).
3.1.2. Oxidative stress
NCDs are associated with an accumulation of oxidative damage to DNA, proteins, and lipids. Oxidized LDL plays a major role in the genesis of atherosclerosis and therefore CVD. The traditional MedDiet is rich in antioxidant vitamins (β‐carotene, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4781, and vitamin E), natural folate, phytochemicals (flavonoids), carotenoids, and selenium.
EVOO is an important source of antioxidant phenolic compounds linked to a reduction in oxidative DNA damage. In an RCT of 182 healthy men, consumption of EVOO resulted in a 13% reduction in urinary 8‐oxo‐deoxyguanosine (Machowetz et al., 2007). Synergistic effects of ß‐sitosterol and polyphenols of EVOO as well as red wine may modulate the effects of oxidized LDL on oxidative stress and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883 synthesis (Vivancos & Moreno, 2008).
An RCT of 86 patients with overweight or obesity reported a significant reduction in urinary 8‐isoprostane following a polyphenol‐rich diet when compared to a control diet (Annuzzi et al., 2014). However, no reduction in F2‐isoprostane levels was observed in the EUROLIVE study (Cicero et al., 2008). The authors suggested that only a long‐term consumption of EVOO is likely to cause changes in isoprostane levels. In a subsample of the PREDIMED study, both MedDiet groups (supplemented with either EVOO or nuts) showed a larger decrease in urine levels of F2‐isoprostane and base 8‐oxo‐7,8‐dihydro‐20‐deoxyguanosine as compared to controls respectively (Mitjavila et al., 2013).
Hydroxytyrosol was shown to increase AMPK activity in vascular endothelial cells. This was associated with higher activities of catalase and forkhead transcription factor 3a, thereby protecting the cells against H2O2‐induced oxidative stress (Zrelli, Matsuoka, Kitazaki, Zarrouk, & Miyazaki, 2011).
Resveratrol was described to increase the resistance to oxidative stress via several signalling pathways including SIRT1, nuclear factor erythroid 2‐related factor 2, and NF‐κB, resistance to oxidative stress could be improved (Truong, Jun, & Jeong, 2018). The sirtuin family consists of seven members (SIRT1–7) with different distribution and functions in the organism. SIRT1 is involved in the regulation of chromatin remodelling, gene expression, and metabolic homeostasis, thereby exerting far‐reaching metabolic effects (Li, 2013). There is accumulating evidence that olive oil‐derived polyphenols (again with a focus on hydroxytyrosol) induce the expression of SIRT1. In an animal model for accelerated aging (senescence accelerated mouse‐prone 8), a diet rich in olive oil polyphenols yielded an increased SIRT1‐induced antioxidative reaction in the heart (Bayram et al., 2012). SIRT1 is known to influence the expression of the antioxidative repertory, such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=270#1381, γ‐glutamyl cysteine synthetase, and NAD(P)H (Méndez‐del Villar, González‐Ortiz, Martínez‐Abundis, Pérez‐Rubio, & Lizárraga‐Valdez, 2014). In addition to hydroxytyrosol, oleuropein was shown to activate SIRT1 and could thus contribute to the antioxidant effect of olive oil (Chung et al., 2010).
3.1.3. Inflammation
Several NCDs including Type 2 diabetes, CVD, obesity, cancer, neurodegenerative disease, or rheumatoid arthritis are related to chronic inflammation (Hunter, 2012). EPA and DHA are substrates for the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=243 (3‐series PG, the LTs, and resolvins). Consumption of ω‐3 fatty acid lowered circulating inflammatory cytokines such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974, and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 (Calder, 2009). A potential target for EPA and DHA mediating altered cytokine gene expression is the transcription factor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595. The activation of PPAR‐γ can lead directly to the production of anti‐inflammatory cytokines and suppress the activation of the pro‐inflammatory transcription factor NF‐κB. Moreover, several ingredients found in whole grains and EVOO may modulate inflammation by inhibiting pro‐inflammatory enzymes or acting as antioxidants. Thus, ferulic acid, alkylresorcinols, apigenin, lignans, and phytic acid have some anti‐inflammatory properties (Price et al., 2012).
In 12 human subjects, levels of the pro‐inflammatory mediators http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4482 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2487 decreased after consuming an EVOO‐rich meal compared to either a refined olive oil or corn oil‐rich meal (Bogani, Galli, Villa, & Visioli, 2007). Similar observations were made in patients with atrial fibrillation (Pignatelli et al., 2015). Furthermore, a walnut‐rich meal high in linolenic acid lowered monocyte mRNA for TNF‐α and IL‐6 (Jimenez‐Gomez et al., 2009).
3.1.4. Insulin sensitivity
Although intervention studies on this topic are inconclusive, there is some experimental evidence that key foods of the MedDiet such as EVOO and red wine may modulate insulin signalling. One proposed mechanism of action is that http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1054 improves cell membrane fluidity inducing beneficial effects on the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1800 (Minich & Bland, 2008). In addition, Lopez and colleagues (Lopez et al., 2011) showed that meals rich in oleic acid buffered beta cell hyperactivity and insulin resistance in patients with hypertriglyceridemia. Supplementing oleuropein and hydroxytyrosol improved insulin secretion and insulin sensitivity (de Bock et al., 2013). Furthermore, olive leaf extracts supplemented as pills reduced FG and HbA1c (Wainstein et al., 2012). Polyphenols can influence glucose metabolism by inhibiting carbohydrate digestion and absorption, reducing glucose release from the liver, or stimulating glucose uptake in peripheral tissues (Hanhineva et al., 2010). Via their antioxidative properties, polyphenols might reduce the production of advanced glycation end products such as HbA1c (Xiao & Hogger, 2015).
An established way to improve glycaemic control is to replace high glycaemic carbohydrates with LGI carbohydrates, that is, to lower the glycaemic load of a specific meal. The additional consumption of dietary fibre as part of a meal can reduce the GI value of a carbohydrate‐rich food and delay gastric emptying. This is the most important determinant for the supply of nutrients to the small intestine and can lead to differences in GI values between rice, bread, and potatoes (Rayner, Samsom, Jones, & Horowitz, 2001). Intestinal motility is slowed down, and effects on hormones in the gut are being manipulated, including ghrelin, incretins, and cholecystokinin (Anderson, 2008). Absorption of glucose in the proximal small intestine is mostly realized via the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=915 at the luminal membrane and via the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=916 at the basolateral membrane. Experimental evidence has shown that several dietary phenolics such as flavonoids and carotenoids interact with sodium–glucose co‐transporter 1 and thus improve glucose uptake (Lin et al., 2016).
Baur et al. demonstrated an AMPK activation by resveratrol in C57BL/6NIA male mice followed by a consecutive improvement in insulin sensitivity (Baur et al., 2006). In addition, Sun et al. observed a resveratrol‐induced SIRT1 activation in C57BL/6J male mice with a subsequent increase in insulin sensitivity (Sun et al., 2007).
3.1.5. Endothelial function
Endothelial dysfunction plays a key role in the development of atherosclerosis and subsequently CVD (Deanfield John, Halcox Julian, & Rabelink Ton, 2007). Oxidative stress and chronic inflammatory processes interfere with endothelial homeostasis and can promote endothelial dysfunction (Ross, 1999). A non‐invasive technique to measure endothelial function of the brachial artery is called FMD and reflects the local bioavailability of vasodilators derived from endothelium, in particular http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 (Deanfield John et al., 2007). In the CORDIOPREV study, following a MedDiet rich in EVOO significantly improved FMD when compared to a low‐fat diet in patients with Type 2 diabetes (Torres‐Pena et al., 2018). Moreover, two meta‐analyses of RCTs showed an increase in FMD by approximately 2% when comparing a MedDiet with a control diet (Schwingshackl & Hoffmann, 2014a) or when comparing trials high in olive oil with trials mainly supplementing with ω‐3 fatty acids (Schwingshackl, Christoph, & Hoffmann, 2015).
The phenols contained in EVOO are able to antagonize oxidative stress by reducing the activation of inflammatory mediators and increasing the bioavailability of NO, which improves the vasodilatory response of the endothelium (Ruano et al., 2005). The consumption of fatty fish and vegetables may act synergistically on endothelial homeostasis (Harris, Park, & Isley, 2003). Furthermore, a decrease in SBP as well as in lipid oxidation markers was shown in patients with stable hypertension following intake of virgin olive oil (Fito et al., 2005). In two randomized crossover studies, a 50‐ml intake of phenol‐rich olive oil decreased the postprandial status of TXA2 and LTB4 levels, in comparison to refined olive oil in healthy (Bogani et al., 2007) and mildly dyslipidaemic patients (Visioli et al., 2005).
Resveratrol exerted protective effects in human coronary arterial endothelial cells via activation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3057/antioxidant response element pathway (Ungvari et al., 2010). The authors stressed the fact that the required resveratrol concentrations can only be achieved using supplements.
3.1.6. Haemostasis, platelet aggregation, and coagulation
Consumption of EVOO enhanced the production of coagulation factors and intermediate disease markers for platelet aggregation, such as activated http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2607 and plasminogen activator inhibitor‐1 (Ruano et al., 2007). Moreover, a MUFA‐rich diet seems to reduce coagulation factor VIIc in the postprandial status (Capurso, Massaro, Scoditti, Vendemiale, & Capurso, 2014). Two short‐term RCTs investigating the effects of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1054 or phenolic rich EVOO reported an attenuation of the pro‐thrombotic state in the postprandial phase (Delgado‐Lista, Garcia‐Rios, Perez‐Martinez, Lopez‐Miranda, & Perez‐Jimenez, 2011; Pacheco, Lopez, Bermudez, Abia, & Muriana, 2006).
3.2. Less established pharmacological mechanisms
3.2.1. Metagenomics
The microbiome of the human gut is regarded to be a crucial link between nutritional habits and our health (De Angelis et al., 2017). A number of favourable effects of the MedDiet on the microbiota were reported (De Filippis et al., 2016; Pastori et al., 2017). Specific ingredients such as proteins and fibre affect the composition of the microbiome, including synthesis and release of metabolites modulating the immune system and inflammation (Clemente, Ursell, Parfrey, & Knight, 2012; Richards, Yap, McLeod, Mackay, & Mariño, 2016). In this context, the presence as well as the absence of effectors can be of importance. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4551 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4780 are metabolized by bacteria of the gut yielding trimethylamine N‐oxide. Elevated trimethylamine N‐oxide levels are associated with the risk of developing NCDs such as obesity, Type 2 diabetes, or CVD. It activates inflammatory events in blood vessels and exerts direct pro‐thrombotic effects (Schugar et al., 2017). In a MedDiet, choline and L‐carnitine content are considerably lower as compared to a Western diet (with major sources being red meat, eggs, and cheese). Moreover, the MedDiet is characterized by a high content of dietary fibre. This promotes the colonization of the intestine SCFA‐producing bacteria, for example, Faecalibacterium prausnitzii (De Filippis et al., 2016). SCFAs (acetate, propionate, and butyrate) are known to inhibit inflammatory, allergic, and autoimmune events (Thorburn, Macia, & Mackay, 2014) and to support the structure and persistence of the intestinal epithelial barrier (Kau, Ahern, Griffin, Goodman, & Gordon, 2011). Regarding the micro‐components of the MedDiet, a 4‐week intake of polyphenols from red wine resulted in a significant increase of Enterococcus, Prevotella, Bacteroides, Bifidobacterium, Bacteroidesuniformis, Eggerthella lenta, and Blautia coccoides‐Eubacterium rectale in healthy male volunteers (Queipo‐Ortuño et al., 2012).
3.2.2. Autophagy
Autophagy is an important cellular process involving the breakdown and recycling of cellular waste in the lysosome (Mizushima & Komatsu, 2011). A malfunction of autophagy processes was associated with the onset and development of NCDs, for example, CVDs (Menzies et al., 2017). Secondary plant metabolites in food groups of a MedDiet might modulate autophagy. Thus, resveratrol was found to induce autophagy (Morselli et al., 2011) by interfering with signal transduction pathways within the cell (e.g., via activating deacetylase SIRT1 activity; Knutson & Leeuwenburgh, 2008). Similar effects are attributed to polyphenols from olive oil, esp. oleuropein, or oleocanthal (Rigacci, 2015). In a mouse model of 2,6,10,14‐tetramethylpentadecan‐induced systemic lupus erythematosus, application of phenol‐enriched EVOO led to a decrease in kidney damage along with reduced renal levels of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1630 and PGE2 (Menicacci, Cipriani, Margheri, Mocali, & Giovannelli, 2017). Furthermore, incubation of peripheral blood mononuclear cells harvested from systemic lupus erythematosus patients with a phenolic fraction of EVOO resulted in a reduced synthesis of the pro‐inflammatory cytokines TNF‐α, IL‐1β, and IL‐6 with concomitant increase in http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975 production (Aparicio‐Soto et al., 2017).
An autophagy‐promoting effect of hydroxytyrosol was observed in TNF‐α‐stimulated vascular adventitial fibroblasts (Wang et al., 2018). The authors attributed the effect of the polyphenol to an enhanced SIRT1 expression with a subsequent down‐regulation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2109 signalling pathway. A SIRT1‐mediated induction of autophagy by hydroxytyrosol has also been described in H2O2‐treated primary chondrocytes (D'Adamo, Cetrullo, Guidotti, Borzì, & Flamigni, 2017).
3.2.3. Telomerase activity
Telomeres are protective cap structures of DNA and protein located at the end of the chromosomes. Telomere length and therefore efficiency has been associated with numerous diseases (Calado & Young, 2009; Haycock et al., 2014). Although the effects of dietary patterns on telomere length are discussed controversially (Pérez et al., 2017; Vidacek et al., 2017), one may speculate that affecting telomere length might represent another mode of action in the health‐promoting effects of MedDiet. This effect could be achieved by influencing the telomerase activity. In a culture model using endothelial progenitor cells, incubation with oleuropein prior to http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504 treatment was found to increase telomerase activity, to pronounce the rate of proliferation, and to reduce cellular senescence (Parzonko, Czerwińska, Kiss, & Naruszewicz, 2013).
3.2.4. Nutrigenomics
Investigating the interactions between NCDs and dietary patterns or their active ingredients on the different levels of the omics cascade is rather complex. Most of the available data refer to effects on the genome or the epigenome.
The genome is exposed to numerous detrimental influences which can accumulate, and in the long term, this may promote the onset of chronic diseases (López‐Otín, Blasco, Partridge, Serrano, & Kroemer, 2013). A potential protective mechanism of EVOO‐derived polyphenols was reported by Fabiani et al. (2008). They found that these substances inhibited H2O2‐induced DNA damage in human peripheral blood mononuclear cells and promyelotic leukaemia cells. Comparable effects were observed in Hela cells following incubation with phenol‐rich EVOO extracts (Erol, Arda, & Erdem, 2012). This is attributable to previously described mechanisms such as their antioxidative effects. Also discussed is the effect on the chelation of metal ions or the specific protection of genes for DNA repair (Erol et al., 2012).
Epigenomics involve all modifications of the genome that are not associated with a change in the DNA sequence (Bird, 2007). The influence of the “epigenotype” on the pathogenesis and progression of NCDs is widely regarded as an established fact (Keating, Plutzky, & El‐Osta, 2016). The same applies to the influence of the lifestyle on these epigenetic profiles (Di Francesco et al., 2015). In essence, there are three levels of epigenetic regulation: DNA methylation, histone modification, and noncoding RNAs. These processes are predominantly controlled by enzymes, for example, histone‐acetylases, ‐deacetylases, ‐methylases, ‐demethylases, DNA methyltransferases, or DNA demethylases.
3.2.5. DNA methylation
The term DNA methylation refers to the attachment of a methyl group to the 5′ position of cytosine, predominantly in regions known to as CpG islands. This modification usually results in gene silencing, which may last for a long time, even for life (Lillycrop & Burdge, 2012). An influence on the DNA methylation pattern was observed for both the MedDiet and some of its characteristic food groups (Di Francesco et al., 2015). In an animal model using cancer stem cells in athymic nude mice, decarboxymethyl oleuropein aglycone inhibited the tumorigenic phenotype of the cells, due to changes in the DNA methylation profile (Corominas‐Faja et al., 2018). This observation might be explained by synergistic interactions of the polyphenol with both mTOR and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2605&familyId=843&familyType=ENZYME. In addition, variations in methylation patterns were observed in peripheral blood mononuclear cells harvested from patients with kidney disease following supplementation with n‐3 fatty acids (Hoile et al., 2014). A positive effect of MUFAs and PUFAs on the methylation profile of genes associated with the onset of obesity and the metabolic syndrome was reported by Milagro et al. (2012). Moreover, fibre‐induced production of SCFAs can affect DNA methylation, thereby exerting preventive measures with respect to CRC, for example, via reactivating pro‐apoptotic signalling (Zhang et al., 2012).
3.2.6. Histone modifications
The reversible modification of histone proteins via acetylation, methylation, ubiquitination, SUMOylation, and phosphorylation will result in activation or deactivation of chromatin and thus the accessibility of the DNA, for example, for gene expression. In a mouse model of neurodegenerative diseases, oleuropein elicited histone modifications (increased acetylation of H3 and H4 and reduced expression of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2616). This was accompanied by improved synaptic function and increased neuronal autophagy (Luccarini et al., 2015). In an in vitro breast cancer cell study, an EVOO extract rich in oleuropein favoured a hyperacetylated status in H3, which could be associated with a reduced frequency of cellular mitosis (Oliveras‐Ferraros et al., 2011). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1059 was described to inhibit histone deacetylases (Kobayashi, Tan, & Fleming, 2004). Especially in cancer cells with impaired glucose metabolism and reduced mitochondrial oxidation rate, the accumulation of butyrate might mediate a growth‐inhibiting and apoptosis‐promoting effect (Saldanha, Kala & Tollefsbol, 2014). Moreover, it has been suggested that http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2390 inhibits histone acetyltransferases. This could increase resistance to oxidative stress, lower inflammation, and cell necrosis and stimulate autophagy during the aging process (Eisenberg et al., 2016).
3.2.7. Non‐coding RNAs
There is a large number of non‐coding RNAs, the best studied variants belonging to the 20‐ to 25‐bp microRNAs. MicroRNAs interfere with mRNA and are usually associated with negative regulation of gene expression, which might also explain their possible role in the pathogenesis of NCDs (Ding, Sun, & Shan, 2017). Although synthesis of microRNAs is mostly tissue specific, they are also released into circulation and can communicate between different tissues (Huang, 2017). Furthermore, microRNAs of plant origin are comparatively stable and resistant to digestion via human RNases. Thus, they can enter the human organism via food and establish some sort of inter‐kingdom communication including regulatory functions (Micó, Martín, Lasunción, Ordovás, & Daimiel, 2016). Polyphenol‐rich variants of EVOO exerted effects on microRNA expression in animal and human studies (Di Francesco et al., 2015). Hydroxytyrosol modulated the synthesis of different microRNAs in a human colonic adenocarcinoma and a human primary epithelial intestinal cell line. Comparable results were found in an intervention study following a 1‐week supplementation (Tomé‐Carneiro et al., 2016). Moreover, other phytochemicals present in a MedDiet such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5346 and resveratrol were described as modulators of microRNA expression (Martinez‐Gonzalez et al., 2015). Levels of microRNA associated with the regulation of apoptosis were found to be affected by butyrates in different CRC cell lines (Hu, Liu, Chang, Wang, & Raufman, 2015). D'Adamo et al. reported a hydroxytyrosol‐induced activation of SIRT1 in chondrocytes, which could be suppressed by microRNA‐9. Excessive microRNA‐9 content resulted in decreased SIRT1 expression and cell death (D'Adamo et al., 2017).
Even though the precise mechanisms of action still are not always clear, these findings suggest that the ingredients of a MedDiet can influence the epigenetic profile.
4. AGING
One major advantage of the MedDiet is its protective associations against a number of pathophysiological conditions. A recently published umbrella review by Dinu et al. (2018) synthesizing data from numerous meta‐analyses of both observational studies and RCTs covering a wide range of diseases confirmed this point of view. Moreover, a meta‐analysis by Loughrey, Lavecchia, Brennan, Lawlor, and Kelly (2017) demonstrated beneficial effects of a MedDiet on global cognitive functions in older adults. Thus, the MedDiet appears to be a valuable diet for “healthy aging” (defining this process as the longest possible absence of pathological phenomena, including decreased cognitive performance). Many of the previously described effects of MedDiet ingredients can also be considered from the perspective of healthy aging, for example, its effects on AMPK, SIRT1, or mTOR (de Pablos, Espinosa‐Oliva, Hornedo‐Ortega, Cano, & Arguelles, 2019; Johnson, Sangesland, Kaeberlein, & Rabinovitch, 2015; Moreno‐Blas, Gorostieta‐Salas, & Castro‐Obregón, 2018).
Damages to mitochondrial DNA (mtDNA) play a significant role in terms of the aging process. mtDNA is particularly vulnerable due to its proximity to enzymes of the respiratory chain, it is not covered by histone proteins, and the repair mechanisms are not as efficient as those in the nucleus (Richter, Park, & Ames, 1988). Tissue‐specific mtDNA lesions are observed in the aging organism, often affecting the brain, skeletal muscle, and the heart (Zapico & Ubelaker, 2016). In experiments with older animals, the administration of VOO was associated with a reduction of mtDNA damage (Quiles, Ochoa, Ramirez‐Tortosa, Huertas, & Mataix, 2006). Based on results from cell culture studies, the protective effects of olive oil are due to antioxidant ingredients, for example, hydroxytyrosol, oleuropein, tyrosol, caffeic acid, and verbascoside (Fabiani et al., 2008). It should be noted that the concentrations used were in a range that can be realized by consuming EVOO in reasonable amounts (about 50 g·day−1).
Among other mechanisms of action, mitochondrial dysfunction accelerates the aging process via an accumulation of oxygen radicals (López‐Otín et al., 2013). Various studies have shown that hydroxytyrosol counteracts mitochondrial dysfunction by promoting antioxidative enzyme activities or by inhibiting pro‐inflammatory cytokines (Calabriso et al., 2018; Soni, Prakash, Sehwag, & Kumar, 2017). In human skin fibroblasts, it could be shown that these protective effects of hydroxytyrosol were associated with an extended cellular lifespan (Sarsour et al., 2012). A mitochondria‐stabilizing effect could also be attributed to resveratrol, this effect was cross‐linked with the activation of SIRT1 (Wang et al., 2014).
5. LIMITATIONS AND FUTURE PERSPECTIVES
A major limitation in the interpretation of the present findings is the fact that most of the data were established in cell culture studies and animal experiments. This complicates the transfer of these results to human beings. Most often, single ingredients were applied in concentrations that will not be delivered by a conventional MedDiet and its included food groups. In addition, the studies designs were heterogeneous, for example, with respect to the active ingredient, its range of concentrations, the types of cells, or the animal model chosen for the experiments. This explains the narrative approach of the present review. A systematic review would have been subjected to considerable restrictions in terms of the number of studies suitable for inclusion. In addition, choosing individual components from complex diets for the development of nutraceuticals in the context of chronic diseases or the aging process is associated with other restrictions as well. A dietary pattern may exert synergistic effects that could be lost at the level of a single substance. This further stresses the importance of intervention trials to clarify the previously observed effects. Long‐term human studies are needed to clarify biotransformatory changes and pharmacodynamics of the active ingredients of a MedDiet, as well as to evaluate possible side effects of these substances.
6. CONCLUSIONS
In our opinion, despite these limitations, the findings summarized in the present review are supplementing previous epidemiological studies, which by design hardly allow statements on the mechanisms of action of a MedDiet. Based on existing evidence, the MedDiet is a recommended dietary approach in the prevention of various NCDs including CVD, Type 2 diabetes, and certain types of cancer. The current state of knowledge attributes plausible effects of characteristic ingredients of a MedDiet with respect to its lipid‐lowering, insulin‐sensitizing, antioxidative, anti‐inflammatory, and antithrombotic properties. Thus, phenolic compounds derived from EVOO were shown to reduce LDL and its oxidation, decrease DNA oxidative damage and production of pro‐inflammatory cytokines, increase insulin sensitivity by cell membrane modification, and improve endothelial function by higher bioavailability of vasodilatory agents. Additional benefits are attributable to the effects of fibre, phytosterols, and polyphenols such as resveratrol, MUFAs as well as PUFAs, vitamins, and minerals. With respect to less well‐established modes of action, further research is needed to identify how these bioactive ingredients can influence gut microbiota and molecular pathways including telomerase activity, the process of autophagy, and gene expression to better understand the link between the MedDiet and pathophysiological mechanism of diseases.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Schwingshackl L, Morze J, Hoffmann G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br J Pharmacol. 2020;177:1241–1257. 10.1111/bph.14778
REFERENCES
- Abumweis, S. S. , Barake, R. , & Jones, P. J. (2008). Plant sterols/stanols as cholesterol lowering agents: A meta‐analysis of randomized controlled trials. Food & Nutrition Research, 52 10.3402/fnr.v52i0.1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174(Suppl 1), S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators . (2017). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. British Journal of Pharmacology, 174, S360–S446. 10.1111/bph.13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. W. (2008). Dietary fiber and associated phytochemicals in prevention and reversal of diabetes In Pasupuleti V. K., & Anderson J. W. (Eds.), Nutraceuticals, glycemic health and type 2 diabetes (p. xvii). Oxford: Wiley‐Blackwell; 10.1002/9780813804149.ch7 [DOI] [Google Scholar]
- Annuzzi, G. , Bozzetto, L. , Costabile, G. , Giacco, R. , Mangione, A. , Anniballi, G. , … Rivellese, A. A. (2014). Diets naturally rich in polyphenols improve fasting and postprandial dyslipidemia and reduce oxidative stress: A randomized controlled trial. The American Journal of Clinical Nutrition, 99, 463–471. [DOI] [PubMed] [Google Scholar]
- Aparicio‐Soto, M. , Sánchéz‐Hidalgo, M. , Cárdeno, A. , Lucena, J. M. , Gonzáléz‐Escribano, F. , Castillo, M. J. , & Alarcón‐de‐la‐Lastra, C. (2017). The phenolic fraction of extra virgin olive oil modulates the activation and the inflammatory response of T cells from patients with systemic lupus erythematosus and healthy donors. Molecular Nutrition & Food Research, 61(8). 10.1002/mnfr.201601080 [DOI] [PubMed] [Google Scholar]
- Bach‐Faig, A. , Berry, E. M. , Lairon, D. , Reguant, J. , Trichopoulou, A. , Dernini, S. , … Mediterranean Diet Foundation Expert Group (2011). Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutrition, 14, 2274–2284. [DOI] [PubMed] [Google Scholar]
- Baur, J. A. , Pearson, K. J. , Price, N. L. , Jamieson, H. A. , Lerin, C. , Kalra, A. , … Sinclair, D. A. (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature, 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram, B. , Ozcelik, B. , Grimm, S. , Roeder, T. , Schrader, C. , Ernst, I. M. , … Rimbach, G. (2012). A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2‐dependent gene expression. Rejuvenation Research, 15, 71–81. 10.1089/rej.2011.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechthold, A. , Boeing, H. , Schwedhelm, C. , Hoffmann, G. , Knuppel, S. , Iqbal, K. , … Schwingshackl, L. (2019). Food groups and risk of coronary heart disease, stroke and heart failure: A systematic review and dose‐response meta‐analysis of prospective studies. Critical Reviews in Food Science and Nutrition, 59(7), 1071–1090. [DOI] [PubMed] [Google Scholar]
- Bird, A. (2007). Perceptions of epigenetics. Nature, 447, 396–398. 10.1038/nature05913 [DOI] [PubMed] [Google Scholar]
- Bloomfield, H. E. , Koeller, E. , Greer, N. , MacDonald, R. , Kane, R. , & Wilt, T. J. (2016). Effects on health outcomes of a Mediterranean diet with no restriction on fat intake: A systematic review and meta‐analysis. Annals of Internal Medicine, 165, 491–500. 10.7326/M16-0361 [DOI] [PubMed] [Google Scholar]
- Bogani, P. , Galli, C. , Villa, M. , & Visioli, F. (2007). Postprandial anti‐inflammatory and antioxidant effects of extra virgin olive oil. Atherosclerosis, 190, 181–186. 10.1016/j.atherosclerosis.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Buchholz, T. , & Melzig, M. F. (2015). Polyphenolic compounds as pancreatic lipase inhibitors. Planta Medica, 81, 771–783. [DOI] [PubMed] [Google Scholar]
- Calabriso, N. , Gnoni, A. , Stanca, E. , Cavallo, A. , Damiano, F. , Siculella, L. , & Carluccio, M. A. (2018). Hydroxytyrosol ameliorates endothelial function under inflammatory conditions by preventing mitochondrial dysfunction. Oxidative Medicine and Cellular Longevity, 2018, 9086947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado, R. T. , & Young, N. S. (2009). Telomere diseases. The New England Journal of Medicine, 361, 2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder, P. C. (2009). Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie, 91, 791–795. 10.1016/j.biochi.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Camargo, A. , Ruano, J. , Fernandez, J. M. , Parnell, L. D. , Jimenez, A. , Santos‐Gonzalez, M. , … Perez‐Jimenez, F. (2010). Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol‐rich virgin olive oil. BMC Genomics, 11, 253 10.1186/1471-2164-11-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, L. , Tan, L. , Wang, H. F. , Jiang, T. , Zhu, X. C. , Lu, H. , … Yu, J. T. (2016). Dietary patterns and risk of dementia: A systematic review and meta‐analysis of cohort studies. Molecular Neurobiology, 53, 6144–6154. 10.1007/s12035-015-9516-4 [DOI] [PubMed] [Google Scholar]
- Capurso, C. , Massaro, M. , Scoditti, E. , Vendemiale, G. , & Capurso, A. (2014). Vascular effects of the Mediterranean diet part I: Anti‐hypertensive and anti‐thrombotic effects. Vascular Pharmacology, 63, 118–126. [DOI] [PubMed] [Google Scholar]
- Chen, G.‐C. , Neelakantan, N. , Martín‐Calvo, N. , Koh, W.‐P. , Yuan, J.‐M. , Bonaccio, M. , … van Dam, R. M. (2019). Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. European Journal of Epidemiology, 34, 337–349. 10.1007/s10654-019-00504-7 [DOI] [PubMed] [Google Scholar]
- Chung, S. , Yao, H. , Caito, S. , Hwang, J. W. , Arunachalam, G. , & Rahman, I. (2010). Regulation of SIRT1 in cellular functions: Role of polyphenols. Archives of Biochemistry and Biophysics, 501, 79–90. 10.1016/j.abb.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero, A. F. , Nascetti, S. , Lopez‐Sabater, M. C. , Elosua, R. , Salonen, J. T. , Nyyssonen, K. , … EUROLIVE Study Group (2008). Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. Journal of the American College of Nutrition, 27, 314–320. [DOI] [PubMed] [Google Scholar]
- Clemente, J. C. , Ursell, L. K. , Parfrey, L. W. , & Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell, 148, 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corominas‐Faja, B. , Cuyàs, E. , Lozano‐Sánchez, J. , Cufí, S. , Verdura, S. , Fernández‐Arroyo, S. , … Menendez, J. A. (2018). Extra‐virgin olive oil contains a metabolo‐epigenetic inhibitor of cancer stem cells. Carcinogenesis, 39, 601–613. 10.1093/carcin/bgy023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo, S. , Cetrullo, S. , Guidotti, S. , Borzì, R. M. , & Flamigni, F. (2017). Hydroxytyrosol modulates the levels of microRNA‐9 and its target sirtuin‐1 thereby counteracting oxidative stress‐induced chondrocyte death. Osteoarthritis and Cartilage, 25, 600–610. 10.1016/j.joca.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Davis, C. , Bryan, J. , Hodgson, J. , & Murphy, K. (2015). Definition of the Mediterranean diet; a literature review. Nutrients, 7, 9139–9153. 10.3390/nu7115459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis, M. , Garruti, G. , Minervini, F. , Bonfrate, L. , Portincasa, P. , & Gobbetti, M. (2017). The food‐gut human axis: The effects of diet on gut microbiota and metabolome. Current Medicinal Chemistry. 10.2174/0929867324666170428103848 [DOI] [PubMed] [Google Scholar]
- de Bock, M. , Derraik, J. G. , Brennan, C. M. , Biggs, J. B. , Morgan, P. E. , Hodgkinson, S. C. , … Cutfield, W. S. (2013). Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle‐aged overweight men: A randomized, placebo‐controlled, crossover trial. PLoS ONE, 8, e57622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis, F. , Pellegrini, N. , Vannini, L. , Jeffery, I. B. , La Storia, A. , Laghi, L. , … Ercolini, D. (2016). High‐level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut, 65, 1812–1821. 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- de Pablos, R. M. , Espinosa‐Oliva, A. M. , Hornedo‐Ortega, R. , Cano, M. , & Arguelles, S. (2019). Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune‐mediated and neurodegenerative diseases. Pharmacological Research: The Official Journal of the Italian Pharmacological Society, 143, 58–72. 10.1016/j.phrs.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Deanfield John, E. , Halcox Julian, P. , & Rabelink Ton, J. (2007). Endothelial function and dysfunction. Circulation, 115, 1285–1295. 10.1161/CIRCULATIONAHA.106.652859 [DOI] [PubMed] [Google Scholar]
- Delgado, A. , Vaz‐Almeida, M. , & Parisi, S. (2017). Chemistry of the Mediterranean diet. Switzerland: Springer International Publishing. [Google Scholar]
- Delgado‐Lista, J. , Garcia‐Rios, A. , Perez‐Martinez, P. , Lopez‐Miranda, J. , & Perez‐Jimenez, F. (2011). Olive oil and haemostasis: Platelet function, thrombogenesis and fibrinolysis. Current Pharmaceutical Design, 17, 778–785. 10.2174/138161211795428876 [DOI] [PubMed] [Google Scholar]
- Di Francesco, A. , Falconi, A. , Di Germanio, C. , Micioni Di Bonaventura, M. V. , Costa, A. , Caramuta, S. , … D'Addario, C. (2015). Extravirgin olive oil up‐regulates CB₁ tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. The Journal of Nutritional Biochemistry, 26, 250–258. 10.1016/j.jnutbio.2014.10.013 [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Sun, X. , & Shan, P. F. (2017). MicroRNAs and cardiovascular disease in diabetes mellitus. BioMed Research International, 2017, 4080364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinu, M. , Pagliai, G. , Casini, A. , & Sofi, F. (2018). Mediterranean diet and multiple health outcomes: An umbrella review of meta‐analyses of observational studies and randomised trials. European Journal of Clinical Nutrition, 72, 30–43. 10.1038/ejcn.2017.58 [DOI] [PubMed] [Google Scholar]
- Eisenberg, T. , Abdellatif, M. , Schroeder, S. , Primessnig, U. , Stekovic, S. , Pendl, T. , … Madeo, F. (2016). Cardioprotection and lifespan extension by the natural polyamine spermidine. Nature Medicine, 22, 1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleftheriou, D. , Benetou, V. , Trichopoulou, A. , La Vecchia, C. , & Bamia, C. (2018). Mediterranean diet and its components in relation to all‐cause mortality: Meta‐analysis. The British Journal of Nutrition, 120, 1081–1097. [DOI] [PubMed] [Google Scholar]
- Erol, Ö. , Arda, N. , & Erdem, G. (2012). Phenols of virgin olive oil protects nuclear DNA against oxidative damage in HeLa cells. Food and Chemical Toxicology, 50, 3475–3479. 10.1016/j.fct.2012.07.048 [DOI] [PubMed] [Google Scholar]
- Fabiani, R. , Rosignoli, P. , De Bartolomeo, A. , Fuccelli, R. , Servili, M. , Montedoro, G. F. , & Morozzi, G. (2008). Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. The Journal of Nutrition, 138, 1411–1416. [DOI] [PubMed] [Google Scholar]
- Fito, M. , Cladellas, M. , de la Torre, R. , Marti, J. , Alcantara, M. , Pujadas‐Bastardes, M. , … members of the SOLOS Investigators (2005). Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis, 181, 149–158. 10.1016/j.atherosclerosis.2004.12.036 [DOI] [PubMed] [Google Scholar]
- Fung, T. T. , Hu, F. B. , McCullough, M. L. , Newby, P. K. , Willett, W. C. , & Holmes, M. D. (2006). Diet quality is associated with the risk of estrogen receptor‐negative breast cancer in postmenopausal women. The Journal of Nutrition, 136, 466–472. 10.1093/jn/136.2.466 [DOI] [PubMed] [Google Scholar]
- Galbete, C. , Schwingshackl, L. , Schwedhelm, C. , Boeing, H. , & Schulze, M. B. (2018). Evaluating Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta‐analyses. European Journal of Epidemiology, 33, 909–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, D. , & Shaw, R. J. (2017). AMPK: Mechanisms of cellular energy sensing and restoration of metabolic balance. Molecular Cell, 66, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Marcos, L. , Castro‐Rodriguez, J. A. , Weinmayr, G. , Panagiotakos, D. B. , Priftis, K. N. , & Nagel, G. (2013). Influence of Mediterranean diet on asthma in children: A systematic review and meta‐analysis. Pediatric Allergy and Immunology, 24, 330–338. [DOI] [PubMed] [Google Scholar]
- Godos, J. , Zappala, G. , Bernardini, S. , Giambini, I. , Bes‐Rastrollo, M. , & Martinez‐Gonzalez, M. (2017). Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta‐analysis of observational studies. International Journal of Food Sciences and Nutrition, 68, 138–148. 10.1080/09637486.2016.1221900 [DOI] [PubMed] [Google Scholar]
- Hanhineva, K. , Torronen, R. , Bondia‐Pons, I. , Pekkinen, J. , Kolehmainen, M. , Mykkanen, H. , & Poutanen, K. (2010). Impact of dietary polyphenols on carbohydrate metabolism. International Journal of Molecular Sciences, 11, 1365–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, J. , Shen, W. , Yu, G. , Jia, H. , Li, X. , Feng, Z. , … Sharman, E. (2010). Hydroxytyrosol promotes mitochondrial biogenesis and mitochondrial function in 3T3‐L1 adipocytes. The Journal of Nutritional Biochemistry, 21, 634–644. 10.1016/j.jnutbio.2009.03.012 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–d1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, W. S. , & Bulchandani, D. (2006). Why do omega‐3 fatty acids lower serum triglycerides? Current Opinion in Lipidology, 17, 387–393. [DOI] [PubMed] [Google Scholar]
- Harris, W. S. , Park, Y. , & Isley, W. L. (2003). Cardiovascular disease and long‐chain omega‐3 fatty acids. Current Opinion in Lipidology, 14, 9–14. 10.1097/00041433-200302000-00003 [DOI] [PubMed] [Google Scholar]
- Haycock, P. C. , Heydon, E. E. , Kaptoge, S. , Butterworth, A. S. , Thompson, A. , & Willeit, P. (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta‐analysis. BMJ, 349, g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, R. , & Gerber, M. (2013). Evaluating and adapting the Mediterranean diet for non‐Mediterranean populations: A critical appraisal. Nutrition Reviews, 71, 573–584. [DOI] [PubMed] [Google Scholar]
- Hoile, S. P. , Clarke‐Harris, R. , Huang, R. C. , Calder, P. C. , Mori, T. A. , Beilin, L. J. , … Burdge, G. C. (2014). Supplementation with N‐3 long‐chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE, 9, e109896 10.1371/journal.pone.0109896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, S. , Liu, L. , Chang, E. B. , Wang, J. Y. , & Raufman, J. P. (2015). Butyrate inhibits pro‐proliferative miR‐92a by diminishing c‐Myc‐induced miR‐17‐92a cluster transcription in human colon cancer cells. Molecular Cancer, 14, 180 10.1186/s12943-015-0450-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, W. (2017). MicroRNAs: Biomarkers, diagnostics, and therapeutics. Methods in Molecular Biology, 1617, 57–67. 10.1007/978-1-4939-7046-9_4 [DOI] [PubMed] [Google Scholar]
- Hunter, P. (2012). The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Reports, 13, 968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Gomez, Y. , Lopez‐Miranda, J. , Blanco‐Colio, L. M. , Marin, C. , Perez‐Martinez, P. , Ruano, J. , … Pérez‐Jiménez, F. (2009). Olive oil and walnut breakfasts reduce the postprandial inflammatory response in mononuclear cells compared with a butter breakfast in healthy men. Atherosclerosis, 204, e70–e76. 10.1016/j.atherosclerosis.2008.09.011 [DOI] [PubMed] [Google Scholar]
- Johnson, S. C. , Sangesland, M. , Kaeberlein, M. , & Rabinovitch, P. S. (2015). Modulating mTOR in aging and health. Interdisciplinary Topics in Gerontology, 40, 107–127. [DOI] [PubMed] [Google Scholar]
- Kau, A. L. , Ahern, P. P. , Griffin, N. W. , Goodman, A. L. , & Gordon, J. I. (2011). Human nutrition, the gut microbiome and the immune system. Nature, 474, 327–336. 10.1038/nature10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating, S. T. , Plutzky, J. , & El‐Osta, A. (2016). Epigenetic changes in diabetes and cardiovascular risk. Circulation Research, 118, 1706–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson, M. D. , & Leeuwenburgh, C. (2008). Resveratrol and novel potent activators of SIRT1: Effects on aging and age‐related diseases. Nutrition Reviews, 66, 591–596. [DOI] [PubMed] [Google Scholar]
- Kobayashi, H. , Tan, E. M. , & Fleming, S. E. (2004). Acetylation of histones associated with the p21WAF1/CIP1 gene by butyrate is not sufficient for p21WAF1/CIP1 gene transcription in human colorectal adenocarcinoma cells. International Journal of Cancer, 109, 207–213. 10.1002/ijc.11697 [DOI] [PubMed] [Google Scholar]
- Kojima, G. , Avgerinou, C. , Iliffe, S. , & Walters, K. (2018). Adherence to Mediterranean diet reduces incident frailty risk: Systematic review and meta‐analysis. Journal of the American Geriatrics Society, 66, 783–788. 10.1111/jgs.15251 [DOI] [PubMed] [Google Scholar]
- Li, X. (2013). SIRT1 and energy metabolism. Acta Biochim Biophys Sin (Shanghai), 45, 51–60. 10.1093/abbs/gms108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop, K. A. , & Burdge, G. C. (2012). Epigenetic mechanisms linking early nutrition to long term health. Best Practice & Research. Clinical Endocrinology & Metabolism, 26, 667–676. 10.1016/j.beem.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Lin, D. , Xiao, M. , Zhao, J. , Li, Z. , Xing, B. , Li, X. , … Chen, S. (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules, 21 10.3390/molecules21101374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage, T. , Ninomiya, T. , Wang, A. , Neal, B. , Jun, M. , Wong, M. G. , … Perkovic, V. (2016). Effects of the Mediterranean diet on cardiovascular outcomes—A systematic review and meta‐analysis. PLoS ONE, 11, e0159252 10.1371/journal.pone.0159252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer, S. , Rowland, I. , Spencer, J. P. E. , Yaqoob, P. , & Stonehouse, W. (2017). Impact of phenolic‐rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. European Journal of Nutrition, 56, 1421–1432. 10.1007/s00394-016-1188-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez, S. , Bermudez, B. , Ortega, A. , Varela, L. M. , Pacheco, Y. M. , Villar, J. , … Muriana, F. J. (2011). Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. The American Journal of Clinical Nutrition, 93, 494–499. 10.3945/ajcn.110.003251 [DOI] [PubMed] [Google Scholar]
- Lopez‐Miranda, J. , Perez‐Jimenez, F. , Ros, E. , De Caterina, R. , Badimon, L. , Covas, M. I. , … Yiannakouris, N. (2010). Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaen and Cordoba (Spain) 2008. Nutrition, Metabolism, and Cardiovascular Diseases, 20, 284–294. 10.1016/j.numecd.2009.12.007 [DOI] [PubMed] [Google Scholar]
- López‐Otín, C. , Blasco, M. A. , Partridge, L. , Serrano, M. , & Kroemer, G. (2013). The hallmarks of aging. Cell, 153, 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrey, D. G. , Lavecchia, S. , Brennan, S. , Lawlor, B. A. , & Kelly, M. E. (2017). The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: A systematic review and meta‐analysis. Advances in Nutrition, 8, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luccarini, I. , Grossi, C. , Rigacci, S. , Coppi, E. , Pugliese, A. M. , Pantano, D. , … Casamenti, F. (2015). Oleuropein aglycone protects against pyroglutamylated‐3 amyloid‐ß toxicity: Biochemical, epigenetic and functional correlates. Neurobiology of Aging, 36, 648–663. [DOI] [PubMed] [Google Scholar]
- Machowetz, A. , Poulsen, H. E. , Gruendel, S. , Weimann, A. , Fito, M. , Marrugat, J. , … Koebnick, C. (2007). Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. The FASEB Journal, 21, 45–52. [DOI] [PubMed] [Google Scholar]
- Malmir, H. , Saneei, P. , Larijani, B. , & Esmaillzadeh, A. (2018). Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta‐analysis of observational studies. European Journal of Nutrition, 57, 2147–2160. 10.1007/s00394-017-1490-3 [DOI] [PubMed] [Google Scholar]
- Martinez‐Gonzalez, M. A. , Gea, A. , & Ruiz‐Canela, M. (2019). The Mediterranean diet and cardiovascular health. Circulation Research, 124, 779–798. [DOI] [PubMed] [Google Scholar]
- Martinez‐Gonzalez, M. A. , Salas‐Salvado, J. , Estruch, R. , Corella, D. , Fito, M. , & Ros, E. (2015). Benefits of the Mediterranean diet: Insights from the PREDIMED study. Progress in Cardiovascular Diseases, 58, 50–60. 10.1016/j.pcad.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Méndez‐del Villar, M. , González‐Ortiz, M. , Martínez‐Abundis, E. , Pérez‐Rubio, K. G. , & Lizárraga‐Valdez, R. (2014). Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metabolic Syndrome and Related Disorders, 12, 497–501. 10.1089/met.2014.0082 [DOI] [PubMed] [Google Scholar]
- Menicacci, B. , Cipriani, C. , Margheri, F. , Mocali, A. , & Giovannelli, L. (2017). Modulation of the senescence‐associated inflammatory phenotype in human fibroblasts by olive phenols. International Journal of Molecular Sciences, 18, E2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mensink, R. (2016). Effects of saturated fatty acids on serum lipids and lipoproteins: A systematic review and regression analysis. World Health Organization.
- Menzies, F. M. , Fleming, A. , Caricasole, A. , Bento, C. F. , Andrews, S. P. , Ashkenazi, A. , … Rubinsztein, D. C. (2017). Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron, 93, 1015–1034. 10.1016/j.neuron.2017.01.022 [DOI] [PubMed] [Google Scholar]
- Micó, V. , Martín, R. , Lasunción, M. A. , Ordovás, J. M. , & Daimiel, L. (2016). Unsuccessful detection of plant microRNAs in beer, extra virgin olive oil and human plasma after an acute ingestion of extra virgin olive oil. Plant Foods for Human Nutrition, 71, 102–108. 10.1007/s11130-016-0534-9 [DOI] [PubMed] [Google Scholar]
- Milagro, F. I. , Gómez‐Abellán, P. , Campión, J. , Martínez, J. A. , Ordovás, J. M. , & Garaulet, M. (2012). CLOCK, PER2 and BMAL1 DNA methylation: Association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiology International, 29, 1180–1194. [DOI] [PubMed] [Google Scholar]
- Minich, D. M. , & Bland, J. S. (2008). Dietary management of the metabolic syndrome beyond macronutrients. Nutrition Reviews, 66, 429–444. 10.1111/j.1753-4887.2008.00075.x [DOI] [PubMed] [Google Scholar]
- Mitjavila, M. T. , Fandos, M. , Salas‐Salvadó, J. , Covas, M.‐I. , Borrego, S. , Estruch, R. , … Sáez, G. T. (2013). The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clinical Nutrition, 32, 172–178. 10.1016/j.clnu.2012.08.002 [DOI] [PubMed] [Google Scholar]
- Mizushima, N. , & Komatsu, M. (2011). Autophagy: Renovation of cells and tissues. Cell, 147, 728–741. [DOI] [PubMed] [Google Scholar]
- Moreno‐Blas, D. , Gorostieta‐Salas, E. , & Castro‐Obregón, S. (2018). Connecting chaperone‐mediated autophagy dysfunction to cellular senescence. Ageing Research Reviews, 41, 34–41. [DOI] [PubMed] [Google Scholar]
- Morselli, E. , Mariño, G. , Bennetzen, M. V. , Eisenberg, T. , Megalou, E. , Schroeder, S. , … Kroemer, G. (2011). Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. The Journal of Cell Biology, 192, 615–629. 10.1083/jcb.201008167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissensohn, M. , Roman‐Vinas, B. , Sanchez‐Villegas, A. , Piscopo, S. , & Serra‐Majem, L. (2016). The effect of the Mediterranean diet on hypertension: A systematic review and meta‐analysis. Journal of Nutrition Education and Behavior, 48, 42–53.e41. [DOI] [PubMed] [Google Scholar]
- Oliveras‐Ferraros, C. , Fernández‐Arroyo, S. , Vazquez‐Martin, A. , Lozano‐Sánchez, J. , Cufí, S. , Joven, J. , … Menendez, J. A. (2011). Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2‐targeting drugs by inducing GADD45‐sensed cellular stress, G2/M arrest and hyperacetylation of Histone H3. International Journal of Oncology, 38, 1533–1547. 10.3892/ijo.2011.993 [DOI] [PubMed] [Google Scholar]
- Pacheco, Y. M. , Lopez, S. , Bermudez, B. , Abia, R. , & Muriana, F. J. (2006). Extra‐virgin vs. refined olive oil on postprandial hemostatic markers in healthy subjects. Journal of Thrombosis and Haemostasis, 4, 1421–1422. 10.1111/j.1538-7836.2006.01963.x [DOI] [PubMed] [Google Scholar]
- Parzonko, A. , Czerwińska, M. E. , Kiss, A. K. , & Naruszewicz, M. (2013). Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase‐1 pathway. Phytomedicine, 20, 1088–1094. 10.1016/j.phymed.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Pastori, D. , Carnevale, R. , Nocella, C. , Novo, M. , Santulli, M. , Cammisotto, V. , … Violi, F. (2017). Gut‐derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: Effect of adherence to mediterranean diet. Journal of the American Heart Association, 6, e005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez, L. M. , Amaral, M. A. , Mundstock, E. , Barbé‐Tuana, F. M. , Guma, F. T. C. R. , Jones, M. H. , … Mattiello, R. (2017). Effects of diet on telomere length: Systematic review and meta‐analysis. Public Health Genomics, 20, 286–292. 10.1159/000486586 [DOI] [PubMed] [Google Scholar]
- Pignatelli, P. , Pastori, D. , Farcomeni, A. , Nocella, C. , Bartimoccia, S. , Vicario, T. , … Violi, F. (2015). Mediterranean diet reduces thromboxane A2 production in atrial fibrillation patients. Clinical Nutrition, 34, 899–903. [DOI] [PubMed] [Google Scholar]
- Price, R. K. , Wallace, J. M. , Hamill, L. L. , Keaveney, E. M. , Strain, J. J. , Parker, M. J. , & Welch, R. W. (2012). Evaluation of the effect of wheat aleurone‐rich foods on markers of antioxidant status, inflammation and endothelial function in apparently healthy men and women. The British Journal of Nutrition, 108, 1644–1651. [DOI] [PubMed] [Google Scholar]
- Priore, P. , Siculella, L. , & Gnoni, G. V. (2014). Extra virgin olive oil phenols down‐regulate lipid synthesis in primary‐cultured rat‐hepatocytes. The Journal of Nutritional Biochemistry, 25, 683–691. 10.1016/j.jnutbio.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Queipo‐Ortuño, M. I. , Boto‐Ordóñez, M. , Murri, M. , Gomez‐Zumaquero, J. M. , Clemente‐Postigo, M. , Estruch, R. , … Tinahones, F. J. (2012). Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. The American Journal of Clinical Nutrition, 95, 1323–1334. 10.3945/ajcn.111.027847 [DOI] [PubMed] [Google Scholar]
- Quiles, J. L. , Ochoa, J. J. , Ramirez‐Tortosa, M. C. , Huertas, J. R. , & Mataix, J. (2006). Age‐related mitochondrial DNA deletion in rat liver depends on dietary fat unsaturation. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 61, 107–114. [DOI] [PubMed] [Google Scholar]
- Radd‐Vagenas, S. , Duffy, S. L. , Naismith, S. L. , Brew, B. J. , Flood, V. M. , & Fiatarone Singh, M. A. (2018). Effect of the Mediterranean diet on cognition and brain morphology and function: A systematic review of randomized controlled trials. The American Journal of Clinical Nutrition, 107, 389–404. 10.1093/ajcn/nqx070 [DOI] [PubMed] [Google Scholar]
- Rayner, C. K. , Samsom, M. , Jones, K. L. , & Horowitz, M. (2001). Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care, 24, 371–381. [DOI] [PubMed] [Google Scholar]
- Rees, K. , Takeda, A. , Martin, N. , Ellis, L. , Wijesekara, D. , Vepa, A. , … Stranges, S. (2019). Mediterranean‐style diet for the primary and secondary prevention of cardiovascular disease. The Cochrane Database of Systematic Reviews, 3, Cd009825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards, J. L. , Yap, Y. A. , McLeod, K. H. , Mackay, C. R. , & Mariño, E. (2016). Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clinical & Translational Immunology, 5, e82 10.1038/cti.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, C. , Park, J. W. , & Ames, B. N. (1988). Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proceedings of the National Academy of Sciences of the United States of America, 85, 6465–6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigacci, S. (2015). Olive oil phenols as promising multi‐targeting agents against Alzheimer's disease. Advances in Experimental Medicine and Biology, 863, 1–20. 10.1007/978-3-319-18365-7_1 [DOI] [PubMed] [Google Scholar]
- Rosato, V. , Temple, N. J. , La Vecchia, C. , Castellan, G. , Tavani, A. , & Guercio, V. (2019). Mediterranean diet and cardiovascular disease: A systematic review and meta‐analysis of observational studies. European Journal of Nutrition, 58, 173–191. [DOI] [PubMed] [Google Scholar]
- Ross, R. (1999). Atherosclerosis—An inflammatory disease. The New England Journal of Medicine, 340, 115–126. 10.1056/NEJM199901143400207 [DOI] [PubMed] [Google Scholar]
- Ruano, J. , Lopez‐Miranda, J. , de la Torre, R. , Delgado‐Lista, J. , Fernandez, J. , Caballero, J. , … Pérez‐Jiménez, F. (2007). Intake of phenol‐rich virgin olive oil improves the postprandial prothrombotic profile in hypercholesterolemic patients. The American Journal of Clinical Nutrition, 86, 341–346. 10.1093/ajcn/86.2.341 [DOI] [PubMed] [Google Scholar]
- Ruano, J. , Lopez‐Miranda, J. , Fuentes, F. , Moreno, J. A. , Bellido, C. , Perez‐Martinez, P. , … Pérez Jiménez, F. (2005). Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. Journal of the American College of Cardiology, 46, 1864–1868. [DOI] [PubMed] [Google Scholar]
- Saldanha, S. N. , Kala, R. , & Tollefsbol, T. O. (2014). Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic‐modulating epigallocatechin gallate and sodium butyrate. Experimental Cell Research, 324, 40–53. 10.1016/j.yexcr.2014.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarsour, E. H. , Kumar, M. G. , Kalen, A. L. , Goswami, M. , Buettner, G. R. , & Goswami, P. C. (2012). MnSOD activity regulates hydroxytyrosol‐induced extension of chronological lifespan. Age, 34, 95–109. 10.1007/s11357-011-9223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija, A. , Yu, E. , Willett, W. C. , & Hu, F. B. (2015). Understanding nutritional epidemiology and its role in policy. Advances in Nutrition, 6, 5–18. 10.3945/an.114.007492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura‐Calixto, F. , & Goni, I. (2009). Definition of the Mediterranean diet based on bioactive compounds. Critical Reviews in Food Science and Nutrition, 49, 145–152. [DOI] [PubMed] [Google Scholar]
- Schlesinger, S. , Neuenschwander, M. , Schwedhelm, C. , Hoffmann, G. , Bechthold, A. , Boeing, H. , & Schwingshackl, L. (2019). Food groups and risk of overweight, obesity, and weight gain: A systematic review and dose‐response meta‐analysis of prospective studies. Advances in Nutrition, 10(2), 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schugar, R. C. , Shih, D. M. , Warrier, M. , Helsley, R. N. , Burrows, A. , Ferguson, D. , … Brown, J. M. (2017). The TMAO‐producing enzyme flavin‐containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Reports, 19, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Christoph, M. , & Hoffmann, G. (2015). Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta‐analysis. Nutrients, 7, 7651–7675. 10.3390/nu7095356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , & Hoffmann, G. (2014a). Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta‐analysis of intervention trials. Nutrition, Metabolism, and Cardiovascular Diseases, 24, 929–939. [DOI] [PubMed] [Google Scholar]
- Schwingshackl, L. , & Hoffmann, G. (2014b). Monounsaturated fatty acids, olive oil and health status: A systematic review and meta‐analysis of cohort studies. Lipids in Health and Disease, 13, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Hoffmann, G. , Lampousi, A. M. , Knuppel, S. , Iqbal, K. , Schwedhelm, C. , … Boeing, H. (2017). Food groups and risk of type 2 diabetes mellitus: A systematic review and meta‐analysis of prospective studies. European Journal of Epidemiology, 32, 363–375. 10.1007/s10654-017-0246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Hoffmann, G. , Missbach, B. , Stelmach‐Mardas, M. , & Boeing, H. (2017). An umbrella review of nuts intake and risk of cardiovascular disease. Current Pharmaceutical Design, 23, 1016–1027. [DOI] [PubMed] [Google Scholar]
- Schwingshackl, L. , Missbach, B. , Konig, J. , & Hoffmann, G. (2015). Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta‐analysis. Public Health Nutrition, 18, 1292–1299. 10.1017/S1368980014001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Schlesinger, S. , Devleesschauwer, B. , Hoffmann, G. , Bechthold, A. , Schwedhelm, C. , … Boeing, H. (2018). Generating the evidence for risk reduction: A contribution to the future of food‐based dietary guidelines. The Proceedings of the Nutrition Society, 77, 432–444. [DOI] [PubMed] [Google Scholar]
- Schwingshackl, L. , Schwedhelm, C. , Galbete, C. , & Hoffmann, G. (2017). Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta‐analysis. Nutrients, 9, E1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Schwedhelm, C. , Hoffmann, G. , Knuppel, S. , Iqbal, K. , Andriolo, V. , … Boeing, H. (2017). Food groups and risk of hypertension: A systematic review and dose‐response meta‐analysis of prospective studies. Advances in Nutrition, 8, 793–803. 10.3945/an.117.017178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl, L. , Schwedhelm, C. , Hoffmann, G. , Knuppel, S. , Laure Preterre, A. , Iqbal, K. , … Schlesinger, S. (2018). Food groups and risk of colorectal cancer. International Journal of Cancer, 142, 1748–1758. [DOI] [PubMed] [Google Scholar]
- Schwingshackl, L. , Schwedhelm, C. , Hoffmann, G. , Lampousi, A. M. , Knuppel, S. , Iqbal, K. , … Boeing, H. (2017). Food groups and risk of all‐cause mortality: A systematic review and meta‐analysis of prospective studies. The American Journal of Clinical Nutrition, 105, 1462–1473. 10.3945/ajcn.117.153148 [DOI] [PubMed] [Google Scholar]
- Shafiei, F. , Salari‐Moghaddam, A. , Larijani, B. , & Esmaillzadeh, A. (2019). Adherence to the Mediterranean diet and risk of depression: A systematic review and updated meta‐analysis of observational studies. Nutrition Reviews, 77, 230–239. 10.1093/nutrit/nuy070 [DOI] [PubMed] [Google Scholar]
- Soni, M. , Prakash, C. , Sehwag, S. , & Kumar, V. (2017). Protective effect of hydroxytyrosol in arsenic‐induced mitochondrial dysfunction in rat brain. Journal of Biochemical and Molecular Toxicology, 31(7). 10.1002/jbt.21906 [DOI] [PubMed] [Google Scholar]
- Sun, C. , Zhang, F. , Ge, X. , Yan, T. , Chen, X. , Shi, X. , & Zhai, Q. (2007). SIRT1 improves insulin sensitivity under insulin‐resistant conditions by repressing PTP1B. Cell Metabolism, 6, 307–319. [DOI] [PubMed] [Google Scholar]
- Theuwissen, E. , & Mensink, R. P. (2008). Water‐soluble dietary fibers and cardiovascular disease. Physiology & Behavior, 94, 285–292. 10.1016/j.physbeh.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Thorburn, A. N. , Macia, L. , & Mackay, C. R. (2014). Diet, metabolites, and “western‐lifestyle” inflammatory diseases. Immunity, 40, 833–842. [DOI] [PubMed] [Google Scholar]
- Tomé‐Carneiro, J. , Crespo, M. C. , Iglesias‐Gutierrez, E. , Martín, R. , Gil‐Zamorano, J. , Tomas‐Zapico, C. , … Dávalos, A. (2016). Hydroxytyrosol supplementation modulates the expression of miRNAs in rodents and in humans. The Journal of Nutritional Biochemistry, 34, 146–155. 10.1016/j.jnutbio.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Torres‐Pena, J. D. , Garcia‐Rios, A. , Delgado‐Casado, N. , Gomez‐Luna, P. , Alcala‐Diaz, J. F. , Yubero‐Serrano, E. M. , … Lopez‐Miranda, J. (2018). Mediterranean diet improves endothelial function in patients with diabetes and prediabetes: A report from the CORDIOPREV study. Atherosclerosis, 269, 50–56. 10.1016/j.atherosclerosis.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Trichopoulou, A. , Costacou, T. , Bamia, C. , & Trichopoulos, D. (2003). Adherence to a Mediterranean diet and survival in a Greek population. The New England Journal of Medicine, 348, 2599–2608. [DOI] [PubMed] [Google Scholar]
- Trichopoulou, A. , Kouris‐Blazos, A. , Wahlqvist, M. L. , Gnardellis, C. , Lagiou, P. , Polychronopoulos, E. , … Trichopoulos, D. (1995). Diet and overall survival in elderly people. BMJ, 311, 1457–1460. 10.1136/bmj.311.7018.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong, V. L. , Jun, M. , & Jeong, W. S. (2018). Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors, 44, 36–49. [DOI] [PubMed] [Google Scholar]
- Ungvari, Z. , Bagi, Z. , Feher, A. , Recchia, F. A. , Sonntag, W. E. , Pearson, K. , … Csiszar, A. (2010). Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. American Journal of Physiology Heart and Circulatory Physiology, 299, H18–H24. 10.1152/ajpheart.00260.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidacek, N. , Nanic, L. , Ravlic, S. , Sopta, M. , Geric, M. , Gajski, G. , … Rubelj, I. (2017). Telomeres, nutrition, and longevity: Can we really navigate our aging? The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 73, 39–47. [DOI] [PubMed] [Google Scholar]
- Visioli, F. , Caruso, D. , Grande, S. , Bosisio, R. , Villa, M. , Galli, G. , … Galli, C. (2005). Virgin olive oil study (VOLOS): Vasoprotective potential of extra virgin olive oil in mildly dyslipidemic patients. European Journal of Nutrition, 44, 121–127. 10.1007/s00394-004-0504-0 [DOI] [PubMed] [Google Scholar]
- Vivancos, M. , & Moreno, J. J. (2008). Effect of resveratrol, tyrosol and β‐sitosterol on oxidised low‐density lipoprotein‐stimulated oxidative stress, arachidonic acid release and prostaglandin E2 synthesis by RAW 264.7 macrophages. The British Journal of Nutrition, 99, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Voloshyna, I. , Hussaini, S. M. , & Reiss, A. B. (2012). Resveratrol in cholesterol metabolism and atherosclerosis. Journal of Medicinal Food, 15, 763–773. [DOI] [PubMed] [Google Scholar]
- Wainstein, J. , Ganz, T. , Boaz, M. , Bar Dayan, Y. , Dolev, E. , Kerem, Z. , & Madar, Z. (2012). Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. Journal of Medicinal Food, 15, 605–610. 10.1089/jmf.2011.0243 [DOI] [PubMed] [Google Scholar]
- Wang, B. , Sun, J. , Ma, Y. , Wu, G. , Tian, Y. , Shi, Y. , & le, G. (2014). Resveratrol preserves mitochondrial function, stimulates mitochondrial biogenesis, and attenuates oxidative stress in regulatory T cells of mice fed a high‐fat diet. Journal of Food Science, 79, H1823–H1831. 10.1111/1750-3841.12555 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Jing, T. , Yang, X. , He, Y. , Wang, B. , Xiao, Y. , … Lin, R. (2018). Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1‐mediated signaling pathway. Canadian Journal of Physiology and Pharmacology, 96, 88–96. 10.1139/cjpp-2016-0676 [DOI] [PubMed] [Google Scholar]
- Wei, M. Y. , & Jacobson, T. A. (2011). Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta‐analysis. Current Atherosclerosis Reports, 13, 474–483. 10.1007/s11883-011-0210-3 [DOI] [PubMed] [Google Scholar]
- Wood, A. M. , Kaptoge, S. , Butterworth, A. S. , Willeit, P. , Warnakula, S. , Bolton, T. , … Emerging Risk Factors Collaboration/EPIC‐CVD/UK Biobank Alcohol Study Group (2018). Risk thresholds for alcohol consumption: Combined analysis of individual‐participant data for 599 912 current drinkers in 83 prospective studies. The Lancet, 391, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, J. B. , & Hogger, P. (2015). Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Current Medicinal Chemistry, 22, 23–38. [DOI] [PubMed] [Google Scholar]
- Zapico, S. C. , & Ubelaker, D. H. (2016). Relationship between mitochondrial DNA mutations and aging. Estimation of age‐at‐death. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 71, 445–450. 10.1093/gerona/glv115 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Bai, J. , Ren, S. , Wang, R. , Zhang, L. , & Zuo, Y. (2012). Sodium butyrate restores ASC expression and induces apoptosis in LS174T cells. International Journal of Molecular Medicine, 30, 1431–1437. 10.3892/ijmm.2012.1156 [DOI] [PubMed] [Google Scholar]
- Zrelli, H. , Matsuoka, M. , Kitazaki, S. , Zarrouk, M. , & Miyazaki, H. (2011). Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK‐FOXO3a pathway. European Journal of Pharmacology, 660, 275–282. 10.1016/j.ejphar.2011.03.045 [DOI] [PubMed] [Google Scholar]


