Abstract
Cognitive and neuroimaging evidence suggests that episodic and semantic memory—memory for autobiographical events and conceptual meaning, respectively—support different facets of creative thinking, with a growing number of studies reporting activation of brain regions within the default network during performance on creative thinking tasks. The present research sought to dissociate neural contributions of these memory processes by inducing episodic or semantic retrieval orientations prior to performance on a divergent thinking task during fMRI. We conducted a representational similarity analysis (RSA) to identify multivoxel patterns of neural activity that were similar across induction (episodic and semantic) and idea generation. At the behavioral level, we found that semantic induction was associated with increased idea originality, assessed via computational estimates of semantic distance between concepts. RSA revealed that multivoxel patterns during semantic induction and subsequent idea generation were more similar (compared to episodic induction) within the left angular gyrus (AG), posterior cingulate cortex (PCC), and left anterior inferior parietal lobe (IPL). Conversely, activity patterns during episodic induction and subsequent generation were more similar within left parahippocampal gyrus and right anterior IPL. Together, the findings point to dissociable contributions of episodic and semantic memory processes to creative cognition and suggest that distinct regions within the default network support specific memory-related processes during divergent thinking.
Keywords: creativity, default network, episodic memory, semantic memory, representational similarity analysis
Creative thinking is a complex process that incorporates components of attention, cognitive control, and memory (Benedek & Fink, 2019). An increasing amount of research has focused on the role of memory, with several studies aiming to characterize contribution of semantic and episodic memory retrieval to creative thinking. Semantic memory refers to the memory system that stores facts and knowledge independent of time and context, whereas episodic memory refers to the memory system that stores autobiographical memories that depend on time and context (Tulving, 1972).
Semantic memory is thought to support creative thinking by providing a knowledge base of facts and concepts that can be combined to solve creative problems and generate novel ideas (Kenett & Faust, 2019; Mednick, 1962). Decades of cognitive and neuroimaging research have demonstrated the importance of semantic memory for creativity (Abraham & Bubic, 2015; Bowden & Jung-Beeman, 1998; Chen et al., 2015; Faust & Lavidor, 2003; Kenett, 2018b). More recently, researchers have begun to explore contributions of episodic memory (Tulving, 2002) to creative task performance, with an increasing number of studies suggesting that constructive episodic retrieval processes support creative idea production (Addis, Pan, Musicaro, & Schacter, 2016; Madore, Addis, & Schacter, 2015; Madore, Jing, & Schacter, 2016). Evidence for the involvement of memory processes in creative cognition has also been demonstrated via functional imaging studies (Beaty, Benedek, Silvia, & Schacter, 2016; Benedek & Fink, 2019). These studies highlight activation within regions of the brain’s default network, a large-scale system that supports aspects of both episodic and semantic memory (Kim, 2016; Marron et al., 2018) in the creative process. Yet because the default network contributes to either or both types of memory retrieval, the extent to which activity of the default network during creative task performance reflects episodic or semantic processing remains unclear.
In the present research, we sought to dissociate episodic and semantic contributions to creative idea production by inducing either an episodic or semantic retrieval orientation prior to performance on a divergent thinking (DT) task (cf. Abraham, Asquith, Ahmed, & Bourisly, 2019; Madore et al., 2015). Previous studies directly comparing neural activation related to semantic and episodic memory have shown how they relate to different brain regions (Ranganath & Ritchey, 2012; Wiggs, Weisberg, & Martin, 1998). Examining the effect of such different induction type on DT—the frequently used method to measure creative thinking requiring the production of novel ideas in response to open-ended prompts (Acar & Runco, 2019; Runco & Acar, 2012)—can advance our understanding of the type of memory source that contributes to creative thinking.
Semantic Memory and Creative Cognition
Several classic and more recent theories on creativity acknowledge the contribution of connecting weakly related or further apart concepts in semantic memory in the creative process (Beaty, Silvia, Nusbaum, Jauk, & Benedek, 2014; Benedek, Könen, & Neubauer, 2012; Mednick, 1962; Schilling, 2005; Smith & Ward, 2012; Volle, 2018). Yet, due to the challenge of representing semantic memory and measuring semantic distance, the relationship between semantic distance and creativity has not been thoroughly investigated (Kenett, 2018a, 2018b). Recent developments in computational network science methodology have shed light on the cognitive architecture of semantic memory (Siew, Wulff, Beckage, & Kenett, 2019), methods that are increasingly being applied in creativity research (Kenett & Faust, 2019).
For example, Marupaka, Iyer, and Minai (2012) propose that novel semantic combinations arise from a ‘small-world’ network structure—a network structure in which concepts are highly connected and ‘closer’ to each other in such a theoretical space (see also Oltețeanu & Schultheis, 2017). Such work provides support from computational modelling to the early theoretical framework of Mednick (1962), who proposed that individual differences in creativity are related to variation in the structure of semantic memory. Along these lines, recent empirical studies have found that higher creative individuals are characterized by a more flexible (higher connectivity and shorter distances between concepts) semantic memory network architecture (Benedek et al., 2017; Kenett, Anaki, & Faust, 2014; Kenett, Beaty, Silvia, Anaki, & Faust, 2016). According to Kenett and colleagues, this architecture may facilitate efficient retrieval of semantically distant concepts within a network, thus increasing the likelihood of establishing remote conceptual combinations when solving creative problems (Hass, 2016, 2017b; Kenett et al., 2014; Kenett & Austerweil, 2016; Kenett et al., 2016).
A critical notion in theories on the role of semantic memory in creative thinking is semantic distance. This is based on the notion that the farther one moves away from a concept in a semantic memory space, the more novel or creative this new concept will be (Kenett, 2018a, 2019). A key feature of DT is moving away from conventional to more distant, weakly related responses (Acar & Runco, 2019; Hass, 2017a, 2017b; Runco & Acar, 2012). In the classic alternate uses task (AUT) of DT, for example, participants are asked to think of unusual uses for common objects; responses are often scored for their degree of divergence, such as uncommonness or cleverness. Beaty et al. (2014) found that DT is related to increased semantic distance between concepts generated during a verbal fluency task, suggesting that more divergent participants may represent concepts differently (i.e., more distantly) in semantic memory. A popular computational method to represent semantic distance in creativity research is through Latent Semantic Analysis (LSA; Landauer & Dumais, 1997; Landauer, Foltz, & Laham, 1998). LSA computes a high-dimensional word co-occurrence space in which the probability of pairwise co-occurrences is used as a proxy for semantic similarity. Recent studies have shown how LSA-distances correlate positively with individual differences in creative ability (such as performing artists) and how explicit instructions to generate creative responses increases the distances of these responses (Gray et al., 2019; Heinen & Johnson, 2018; Kenett, 2019).
Semantic distance effects in creativity have also been examined through tasks that aim to measure ‘conceptual expansion’, i.e., the ability to ‘expand’ a concept’s meaning to include unusual associations (Abraham, 2014). Such tasks involve expanding the meaning of concepts, either passively (evaluating) or actively (generating), by measuring how participants process stimuli that vary with respect to unusualness and appropriateness (Abraham, 2014). At the brain level, passive conceptual expansion has been studied both via EEG (Kröger et al., 2013; Rutter, Kröger, Hill, et al., 2012) and fMRI (Abraham et al., 2012; Kröger et al., 2012; Rutter, Kröger, Stark, et al., 2012). On the other hand, research on active conceptual expansion has assessed neural and cognitive correlates associated with the generation of novel concepts. In a series of experiments, Green and colleagues examined the neural basis of semantic conceptual expansion—the ability to expand acquired conceptual structures to include novel elements (Ward, 1994)—during a creative generation task (Green, 2016; Green, Kraemer, Fugelsang, Gray, & Dunbar, 2010, 2012). Across several studies, the authors found that generating semantically distant concepts (measured via LSA) is associated with activation of the frontopolar cortex (Green, 2016). Together, a growing literature implicates the semantic system and brain regions associated with semantic processing during performance on creative thinking tasks.
Episodic Memory and Creative Cognition
More recently, researchers have begun to investigate potential contributions of episodic memory to creativity. The episodic system is thought to be a constructive system: rather than simply reactivating a memory trace exactly how it was encoded, episodic memory retrieval involves recombining episodic details (e.g., scenes and people) to reconstruct a past event (Schacter & Addis, 2007). These flexible recombinatory processes are also thought to support episodic future thinking—imagining a possible future experience that has not yet occurred—by similarly retrieving and combining elements of past experience.
A series of recent experiments have examined whether the flexibility of the episodic system may also be conducive to generating creative ideas. Madore et al. (2015) assessed links between episodic retrieval and DT using an episodic specificity induction (ESI; for review, see Schacter & Madore, 2016). The ESI is an experimental procedure based on the Cognitive Interview (Fisher & Geiselman, 1992) that induces an episodic retrieval orientation by prompting participants to recall as much episodic information as possible (people, places, and actions) about a recent event (e.g., a short video clip). This method can reveal the extent to which a cognitive process relies on episodic retrieval by comparing performance on a task after receiving the ESI with performance after receiving a control induction that does not involve episodic retrieval (e.g., providing general impressions about the video; Madore et al., 2015; Madore, Jing, et al., 2016; Madore, Thakral, Beaty, Addis, & Schacter, 2019). Madore and colleagues have found that ESI increases the number of internal (episodic) but not external (semantic) details on tasks requiring episodic retrieval and future simulation, indicating that ESI selectively impacts episodic retrieval processes (for detailed discussion of the logic of this approach, see Schacter & Madore, 2016). Regarding creativity, the ESI has been shown to selectively enhance performance on DT tasks by boosting the number (fluency) and conceptual variability (flexibility) of responses (Madore et al., 2015; Madore, Jing, et al., 2016). ESI is thought to affect creative thinking by biasing episodic retrieval mechanisms, constructive processes that facilitate the extraction and flexible recombination of episodic elements in novel and useful ways (Madore & Schacter, 2016).
Further indirect support for the involvement of episodic retrieval in creative cognition comes from neuroimaging research reporting contributions of regions within the default network. The default network shows common engagement during episodic memory retrieval and episodic future thinking, indicating that similar constructive processes support both memory and imagination (Benoit & Schacter, 2015; Schacter, Addis, & Buckner, 2007). Large-scale individual differences research has reported associations between DT and variation in the structure and function of specific default regions (Beaty, Kenett, et al., 2018; Beaty, Seli, & Schacter, 2019; Chen et al., 2019; Jung, Flores, & Hunter, 2016). Neuroimaging experiments also implicate core regions of the default network—such as the mPFC, medial temporal lobe (MTL), and posterior cingulate cortex (PCC)—during creative task performance, including studies of musical improvisation (Limb & Braun, 2008), artistic drawing (Ellamil, Dobson, Beeman, & Christoff, 2012), metaphor production (Beaty, Silvia, & Benedek, 2017; Benedek et al., 2014), and verbal DT (Beaty, Christensen, Benedek, Silvia, & Schacter, 2017; Gonen-Yaacovi et al., 2013; Wu et al., 2015). However, anatomically similar areas have equally been implicated in semantic processing, so it is not clear that these results show the engagement of episodic memory or semantic memory.
Additional evidence for a role of episodic memory in creative cognition comes from studies reporting activation within the MTL, including the hippocampus and parahippocampal gyrus, during a creative thinking task (Ellamil et al., 2012). The MTL shows robust engagement during tasks involving episodic memory retrieval and episodic future thinking (for a recent review, see Schacter, Addis, & Szpunar, 2017). According to the constructive episodic simulation hypothesis (Schacter & Addis, 2007), the hippocampus and adjacent MTL structures support memory and imagination via flexible recombinatory processes involved in the extraction and integration of episodic information to form coherent representations about past and future events. Researchers thus hypothesize that hippocampal activity during creative cognition may reflect similar constructive processes as memory and imagination, consistent with behavioral work linking episodic memory to enhanced creative performance (Madore et al., 2015; Madore, Jing, et al., 2016).
Two recent studies provide more direct evidence for the contribution of MTL/episodic memory to creative thinking (Beaty, Thakral, Madore, Benedek, & Schacter, 2018; Madore, Thakral, et al., 2019). Using the ESI procedure described above to activate episodic retrieval mechanisms prior to DT during fMRI, Madore, Thakral, et al. (2019) replicated the behavioral effect of ESI on DT (i.e., increased fluency and flexibility of AUT responses) and found that this effect corresponded to increased activation in the left anterior hippocampus. Beaty, Thakral et al. (2018) compared neural activity during episodic retrieval, future imagination, and DT, and found that all three tasks activated the bilateral hippocampus, compared to a semantic control condition (i.e., constructing a sentence with semantically-related words). These findings, along with other recent work (Benedek et al., 2018; Marron et al., 2018), have begun to shed light on specific memory-related processes associated with neural activity during creative task performance.
The Present Research
Behavioral and neuroimaging research indicates that both episodic and semantic retrieval play important roles in the generation of creative thought (Beaty & Schacter, 2018; Benedek & Fink, 2019). The involvement of one or both of these memory processes in creative thinking is illustrated in part by fMRI studies reporting activation within regions of the default network, such as recent experiments using induction procedures to activate memory retrieval processes prior to idea generation (Benedek & Fink, 2019; Madore, Thakral, et al., 2019). Importantly, however, regions within the default network have been shown to support either or both episodic and semantic memory retrieval (Kim, 2016). Thus it remains unclear whether default activity during creative thinking tasks reflects the involvement of episodic memory, semantic memory, or both. Although memory induction studies have provided some insight into question (e.g., Madore, Thakral, et al., 2019), such inductions have been conducted prior to fMRI, raising questions about the correspondence between induction- and generation-related brain activity.
In the present research, we aimed to provide greater clarity on how these memory systems support creative cognition by directly comparing neural activity during memory induction and subsequent idea generation. To this end, we employed representational similarity analysis (RSA), a multivariate approach that can quantify the degree of similarity (and dissimilarity) of neural activation patterns between experimental tasks within brain regions of interest (Kriegeskorte, Mur, & Bandettini, 2008). RSA has been successfully applied to study both semantic and episodic memory (Benoit, Paulus, & Schacter, 2019; Clarke & Tyler, 2014; Fairhall & Caramazza, 2013; Xue, 2018). Here, we used a brief induction protocol that was intended to prime either an episodic or semantic retrieval orientation prior to DT on a trial-by-trial basis during fMRI. Specifically, we compared the similarity of neural activation patterns during memory induction and subsequent idea generation, which allowed us to identify neural regions associated with each memory process during performance on a creative thinking task.
Method
Participants
The total sample consisted of 28 young adults from the University of North Carolina at Greensboro (UNCG; 20 females; mean age = 23.36, SD = 6.95). All participants were right-handed, native English speakers, with normal or corrected-to-normal vision, and reported no history of neurological disorder, cognitive disability, or medication that affects the central nervous system. All participants provided informed consent prior to completing study procedures. Five participants were excluded from the fMRI analyses due to excessive head movement (x, y, z > 5mm); behavioral performance data from these participants was retained to increase statistical power of the behavioral analysis. The study was approved by the UNCG Institutional Review Board.
Memory Inductions and Creativity Assessment
The experiment consisted of a series of memory inductions that preceded DT tasks. Half of the trials consisted of “episodic induction” (EI) trials and the other half consisted of “semantic induction” (SI) trials. In an EI trial, participants were presented with a cue word (i.e., concrete objects; e.g., pen) and asked to use the word as a starting point to recall a personal past event, elaborating with as much specificity and detail as possible. This task is analogous to classic assessments of autobiographical memory retrieval, where a cue word is presented and participants are prompted to recall a specific past event (e.g., Addis, Wong, & Schacter, 2007), and it resembles in some respects the ESI procedure used to induce an episodic retrieval orientation in prior work (Madore et al., 2015; Madore, Jing, & Schacter, 2019b; Madore, Szpunar, Addis, & Schacter, 2016). In an SI trial, participants were presented with a cue word, were required to generate a word that was closely associated to it, and then used these two words to construct a short sentence (Addis et al., 2007; Beaty, Thakral, et al., 2018; Binder & Desai, 2011). The task is comparable to the task demands of the EI (retrieving and relating stored information) but requires semantic retrieval and integration. Participants were told to elaborate on the meaning of the cue word if they completed their sentence before the end of the induction phase (Beaty, Thakral, et al., 2018).
Following the induction period for each condition, participants were presented with another cue word (i.e., a common object) and asked to think of a creative and unusual use for it, i.e., the Alternate Uses Task. The goal of the induction procedure was to prime an episodic or semantic retrieval orientation prior to divergent thinking to identify neural activity related to semantic or episodic processing during the subsequent creativity task. Before performing the task in the scanner, participants completed several practice trials of both induction tasks and DT task.
An experimenter recorded participants’ verbal responses, which were subsequently coded for creative quality via LSA. We used the one-to-many comparison (freely available via the LSA website: http://lsa.colorado.edu/) with the default topic space of “General_Reading_up_to_1st_year_college” and “term to term” comparison type to compute LSA values. Previous studies have demonstrated the validity of using LSA in creativity research (Kenett, 2019), including studies reporting correlations with established creativity measures (e.g., creative achievement; Prabhakaran, Green, & Gray, 2014) and human creativity ratings (Gray et al., 2019; Heinen & Johnson, 2018). Following recent guidance on LSA for creativity assessment (Forthmann, Lips, Szardenings, Scharfen, & Holling, 2018), we removed stop words (e.g., “the”) from responses prior to computing LSA-scores. The AUT cue word (e.g., cup) was used as the “main text” in the LSA engine, and the AUT response for each trial was used as the “texts to compare.” Semantic distance was computed by subtracting the LSA values from 1 (i.e., semantic distance = 1-LSA; Prabhakaran et al., 2014). The values were averaged within the respective induction condition (episodic-AUT and semantic-AUT) to form composite variables for analysis; here, greater semantic distance was interpreted as more creative response (Hass, 2017b; Heinen & Johnson, 2018).
Procedure
Participants completed the fMRI task in two runs of a block design. The blocks were counterbalanced across the sample, and all cue words appeared in both inductions such that all participants generated an episodic memory or sentence for the same cue words. Half of the induction trials in each condition (episodic = 30 trials; semantic = 30 trials) presented the same cue word as in the subsequent AUT, and the other half of induction trials presented a different cue word; the same and different trials alternated within a block, and an equal number of same and different trials were presented. The cue words are presented in Supplementary Table 1 (total cue words = 90). The purpose of this procedure was to test for potential behavioral and neural differences when using the same or different cue for induction and generation. Each of the two runs consisted of 30 experimental trials. A block began by presenting the induction condition (i.e., “Memory” for episodic and “Sentence” for semantic; 5 s). Next, a cue was presented on the screen, and participants were asked to use the cue to recall a past experience (i.e., Memory) or to construct a sentence (i.e., Sentence); they were instructed to complete this task silently (14 s). Then, following a brief fixation (3 s), participants were presented with another cue word and asked to generate an alternate use for it (i.e., DT; 12 s). Finally, a green question mark appeared on the screen, and participants were asked to verbally report their idea (5 s) into a MRI-compatible microphone (Optoacoustics; Mazor, Israel; Beaty, Christensen, et al., 2017; Beaty, Silvia, et al., 2017; Benedek et al., 2014). To ensure compliance with the induction task, participants were re-presented with the induction cues during a post-scan behavioral assessment and asked to type brief summaries of their responses (Addis et al., 2007).
MRI Data Acquisition and Preprocessing
Whole-brain imaging was performed on a 3T Siemens Magnetom MRI system (Siemens Medical Systems, Erlangen, Germany) using a 16-channel head coil. BOLD-sensitive T2*-weighted functional images were acquired using a single shot gradient-echo EPI pulse sequence (TR = 2000 ms, TE = 30 ms, flip angle = 78°, 32 axial slices, 3.5 × 3.5 × 4.0 mm, distance factor 0%, FoV = 192×192 mm, interleaved slice ordering) and corrected online for head motion. The first two volumes were discarded to allow for T1 equilibration effects. A high resolution T1 scan was acquired for anatomic normalization. Imaging data were slice-time corrected and realigned using the Statistical Parametric Mapping (SPM) 12 package (Wellcome Institute of Cognitive Neurology, London). Functional volumes were co-registered, normalized to the MNI template brain (Montreal Neurological Institute). Subsequently, these volumes were spatially smoothed with a Gaussian kernel of 8 mm FWHM for univariate analysis.
Univariate Analysis
Before conducting RSA, we performed standard general linear model (GLM) analyses to investigate brain activity associated with two memory inductions. At the subject-level, the onset of two inductions were modeled using a canonical hemodynamic response function, with six motion parameters generated from realignment included as regressors of no interest. Contrast images (EI>SI and SI>EI) from each participant across two blocks were submitted to the second-level random effects for group analysis. The two statistical maps were corrected using multiple comparison correction with the cluster extent threshold (Slotnick, 2017; Slotnick, Moo, Segal, & Hart, 2003). Specifically, we conducted a Monte Carlo simulation, with 10,000 iterations at the whole brain (64×64×32 voxels) and 8-mm full width at half maximum Gaussian kernel, yielding a voxel threshold of p < .001, corrected for multiple comparisons to p < .05 with a cluster extent threshold of 20 voxels. This step was used to generate a combined induction mask which will be used to constrain searchlight analysis to voxels engaged during episodic and semantic processing.
Representational Similarity Analysis (RSA)
RSA was implemented in MATLAB (Mathworks) using the CoSMoMVPA toolbox (https://github.com/CoSMoMVPA; Oosterhof, Connolly, & Haxby, 2016). In RSA, we can test a hypothesized model about the dissimilarity of neural activation patterns elicited by different inductions and their correspondence to activation patterns recorded during subsequent idea generation on the AUT. In this study, RSA includes four steps: 1) constructing a representational dissimilarity matrix (RDM) of conditions, 2) constructing a RDM of neural activity, 3) searchlight analyses, and 4) group-level analyses.
First, an RDM of conditions was constructed by stimulus-wise paired (within each trail) under two inductions across two runs for each subject, which corresponded to the expected dissimilarity between SI and EI, as well as the subsequent AUT conditions (AUT following EI, EI-AUT; AUT following SI, SI-AUT). This process would result in a 120×120 matrix, in which all conditions within one induction (e.g., EI & EI-AUT; SI & SI-AUT) had a similarity coefficient of 0, whereas all conditions in a different induction (e.g., SI & EI; SI-AUT & EI-AUT) had a dissimilarity coefficient of 1. Second, trial-level beta maps were estimated for the two induction conditions (EI and SI) and the two idea generation conditions (EI-AUT and SI-AUT) using a first-level general linear model (GLM) of unsmoothed fMRI volumes. All trials from the two runs were included in the same design matrix, along with six head-motion parameters modeled as nuisance variables. Each trial was estimated by a separate regressor with respective onset time and duration. The RDM of neural activity was calculated by subtracting the Pearson correlation distance from one across two inductions and the following AUT, within spherical searchlights of a 3-voxel radius for each subject. Similar to the RDM of conditions, this process resulted in a 120×120 matrix. Third, a searchlight method was performed to compute similarity between the RDM of conditions and the neural RDM. Here, only unique off-diagonal values of the matrices were entered into the spearman-rank correlation, and then the resulting correlation coefficient was assigned to the voxel at the center of the searchlight. Notably, the searchlight analysis was constrained to a combined mask derived from the second-level contrast activation maps obtained by group-level univariate analyses. This step results in a representational similarity image for each subject. Finally, these images were entered into a second-level (random effects) analysis using one-sample t-tests to obtain the group statistical map. The group statistical map was corrected for multiple comparisons by using a cluster-level family-wise error (FWE, p < .05) correction with a primary voxel-level threshold of p < .001 and a minimum cluster size of at least 20 voxels.
To test the similarity (correlation) between the induction-AUT condition pairs, we extracted the activation signal for each condition in the individual-level beta maps within each region that survived FWE correction in the group statistical map. Then, a paired correlation analysis (e.g., EI&EI-AUT) was used to compute the similarity across the two paired-conditions for each subject. Subsequently, second-level paired-sample t-tests were applied to explore these differences across all regions. To control false-positive, the p-values of multiple pairwise t-tests were corrected for multiple comparisons using a false discovery rate (FDR) with a threshold of p < .05 (Benjamini & Hochberg, 1995). In addition, the same analyses were employed to all possible pairwise conditions (i.e., EI & EI-AUT, SI & EI, SI & EI-AUT, SI & SI-AUT, EI & SI-AUT, and SI-AUT & EI-AUT) to investigate patterns of similarity and dissimilarity across the two inductions and corresponding idea generation periods.
Results
Behavioral Data
To assess performance on the episodic (EI) and semantic (SI) induction tasks, we examined response rates for each induction task during the post-scan behavioral assessment, which asked participants to recall the memories and sentences they produced in the scanner for the episodic and semantic induction conditions, respectively. Consistent with past work (Beaty, Thakral, et al., 2018), participants were able to recall a majority of their responses to the induction prompts during the post-scan session for both the episodic (M = 89.05%, SD = 14.51%) and semantic (M = 83.93%, SD = 17.02%) conditions, with episodic recall performance slightly higher than semantic recall, t(27) = 2.22, p = .04.
Next, we assessed whether EI and SI differentially affected the creative quality of AUT responses by averaging the semantic distance values for the two AUT conditions (i.e., EI-AUT and SI-AUT). Paired-sample t-tests revealed that semantic distance values were significantly larger following semantic (M = .84, SD = .04) than episodic (M = .81, SD = .04) induction, t(27) = 3.11, p = .004, thus indicating that semantic induction increased the original quality of AUT responses. Note that, because participants only generated a single response to each AUT trial, we could not test induction effects on fluency and flexibility, which have been shown to increase following episodic induction (Madore, Thakral, et al., 2019; Schacter & Madore, 2016). We also tested whether using the same cue for induction and generation affected the semantic distance of AUT responses. Paired-sample t-tests revealed no significant difference between AUT trials using the same or different cues, indicating that the effect of induction was specific to activating the process of retrieval—not the content of memory per se. Because no such difference was found at the behavioral level, we collapsed the same and different cue trials across conditions for the following fMRI analyses.
Representational Similarity
First, we report results from the univariate analysis (p < .001, k ≥ 20) of the two induction conditions (EI > SI U SI > EI) which was conducted to form the RSA searchlight mask (see Supplementary Figure 1). Note that this analysis does not test for common activation of these regions (i.e., a conjunction analysis)—it simply provides a combined mask for the subsequent RSA.
As shown in Figure 1 and Table 1, the combined mask included several regions within temporal-parietal cortex, including right inferior parietal lobule (IPL), two clusters within left IPL (anterior and posterior), left angular gyrus (ANG), and left middle temporal gyrus (MTG). The combined mask also included clusters within prefrontal cortex, including bilateral inferior frontal gyrus (IFG), dorsal lateral prefrontal cortex (DLPFC), and right insula, as well as several regions within the core subsystem of the default network, including posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), and left parahippocampal gyrus. In the univariate analyses, we found activity in PCC, mPFC, left AG, left MTG, and left parahippocampal gyrus when contrasting the episodic against the semantic induction. In contrast, semantic induction recruited activity in bilateral IFG, bilateral IPL, bilateral DLPFC, and right Insula compared to episodic induction (see Supplemental Figure 1). Note that these core default regions (e.g., parahippocampal gyrus) were more strongly engaged in the univariate contrast Episodic > Semantic, whereas classic language/executive control regions (e.g., IFG; Fedorenko, Duncan, & Kanwisher, 2013) were more strongly engaged in the contrast Semantic > Episodic, consistent with past studies (Benoit & Schacter, 2015).
Figure 1.
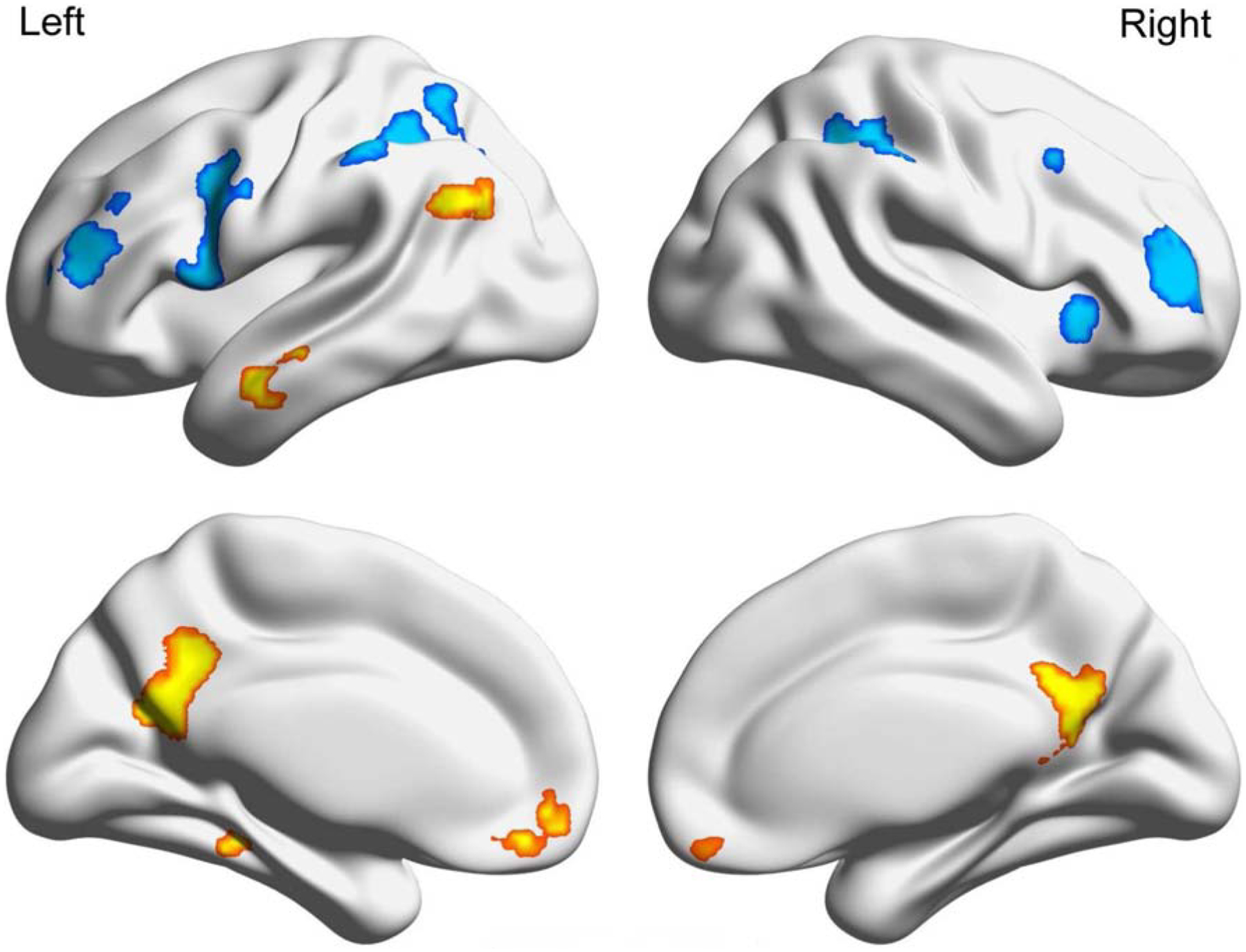
Combined induction mask used to constrain searchlight analysis to voxels engaged during episodic and semantic processing
Notes. The above mask was created by binarizing and combining the statistical maps for episodic > semantic (yellow) and semantic > episodic (blue). The two statistical maps were thresholded at p < .001 cluster extent threshold with a minimum of 20 voxels.
Table 1.
RSA results for the two AUT conditions (EI-AUT and SI-AUT) conducted within the searchlight mask (EI>SI U SI>EI)
| Regions | Hem | MNI coordinates | T | cluster size | ||
|---|---|---|---|---|---|---|
| X | y | z | ||||
| Middle Temporal Gyrus | L | −54 | −8 | −26 | 7.11 | 53 |
| Medial Prefrontal Cortex | R | 2 | 42 | −12 | 8.66 | 259 |
| Insula | R | 36 | 20 | 0 | 6.59 | 112 |
| Inferior Frontal Gyrus | R | 34 | 50 | 12 | 6.96 | 267 |
| Dorsolateral Prefrontal Cortex | L | −48 | 4 | 22 | 9.43 | 402 |
| Posterior Cingulate Cortex/Precuneus | R | 14 | −58 | 22 | 7.73 | 968 |
| Inferior Frontal Gyrus | L | −40 | 40 | 20 | 6.43 | 155 |
| Angular Gyrus | L | −44 | −64 | 26 | 7.43 | 114 |
| Inferior Parietal Lobule (anterior) | L | −26 | −56 | 38 | 6.94 | 329 |
| Inferior Parietal Lobule (posterior) | L | −38 | −40 | 42 | 7.36 | 63 |
| Inferior Parietal Lobule | R | 32 | −46 | 42 | 11.33 | 370 |
| Dorsolateral Prefrontal Cortex | R | 50 | 14 | 42 | 6.51 | 41 |
| Parahippocampal Cortex | L | −28 | −34 | −14 | 5.7 | 49 |
Notes. Results are reported at a statistical threshold of p < .05, FWE-corrected for multiple comparisons at voxel level with cluster threshold of 5 voxels. AUT = alternate uses task; EI = episodic induction; Hem = hemisphere; L = left; R = right; SI = semantic induction.
We then employed searchlight analysis within the combined mask to identify regions that were most informative in distinguishing stimulus-wise similarity across the two induction conditions (see Table 1 and Figure 2). Results revealed several smaller clusters within the larger combined mask, primarily within lateral parietal (e.g., bilateral IPL, left AG), midline (mPFC, PCC), lateral frontal (IFG, DLPFC), and subcortical regions (left parahippocampal gyrus).
Figure 2.
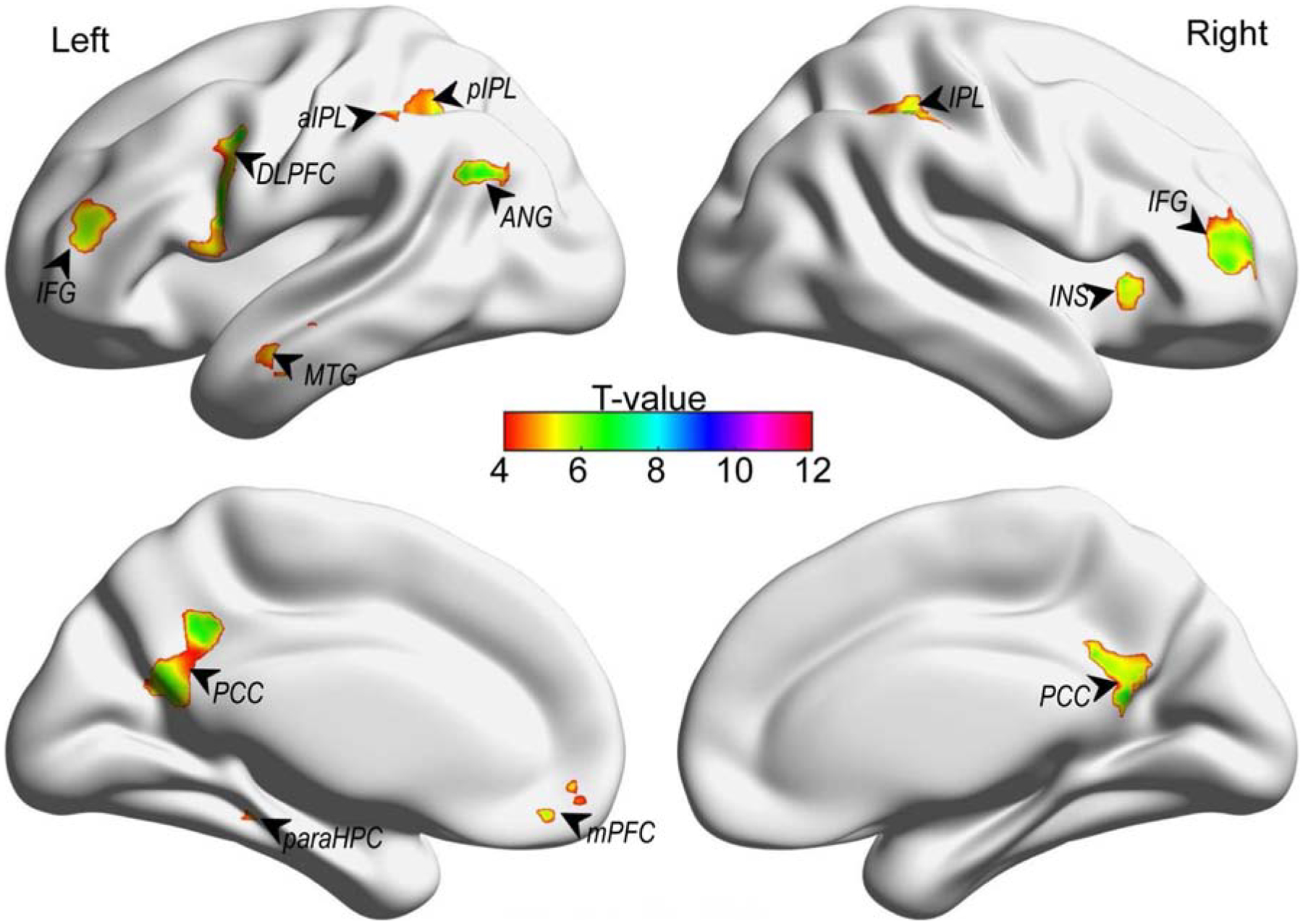
Searchlight analysis within the combined induction mask during the two divergent thinking conditions
Notes. aIPL = anterior inferior parietal lobe; ANG = angular gyrus; DLPFC = dorsolateral prefrontal cortex; IFG = inferior frontal gyrus; INS = insula; paraHPC = parahippocampal cortex; PCC = posterior cingulate cortex. This map was corrected for multiple comparisons using a family-wise error (FWE) correction at a corrected threshold of p < .05 and with a minimum of 20 voxels.
Having identified relevant clusters, we then conducted an ROI-based activation similarity analysis for the two induction conditions (i.e., EI and SI) and two generation conditions (i.e., EI-AUT and SI-AUT) using a paired-sample t-test corrected for multiple comparisons (p < .05, FDR; Table 2). Compared to episodic induction and subsequent generation, we found that neural activity patterns during semantic induction and subsequent generation were more similar within two core default regions—left AG (t = 2.59, p = .017) and PCC (t = 3.34, p = .005)—as well as the left anterior IPL (t = 2.46, p = .022). Compared to semantic induction and subsequent generation, we found that neural activity patterns during episodic induction and subsequent generation were more similar within the left parahippocampal gyrus (t = 2.79, p = .009) and right IPL (t = 2.46, p = .023). For completeness, we report all other comparisons across inductions and generation in the Supplemental Information (see Fig. S2).
Table 2.
Paired sample t-test on the ROI-based RSA activation similarity contrasting SI-AUT and EI AUT (SI-AUT>EI-AUT) conducted within the searchlight mask
| ROI | t-value | p-value |
|---|---|---|
| Medial Prefrontal Cortex | 1.91 | .069 |
| Posterior Cingulate Cortex | 3.34 | .005 |
| L Angular Gyrus | 2.59 | .017 |
| L Dorsolateral Prefrontal Cortex | 1.84 | .08 |
| L Inferior Frontal Gyrus | 1.88 | .073 |
| L Posterior Inferior Parietal Lobe | 0.26 | .795 |
| L Anterior Inferior Parietal Lobe | 2.46 | .022 |
| L Middle Temporal Gyrus | 1.85 | .078 |
| L Parahippocampal Cortex | −2.79 | .009 |
| R Inferior Frontal Gyrus | −0.56 | .581 |
| R Insula | 1.29 | .212 |
| R Inferior Parietal Lobe | −2.46 | .023 |
| R Dorsolateral Prefrontal Cortex | 0.71 | .487 |
Notes. Results were corrected for multiple comparisons using a false discovery rate (FDR) at a corrected threshold of p < .05. AUT = alternate uses task; EI = episodic induction; L = left; R = right; SI = semantic induction.
Discussion
The present research aimed to identify neural activity associated with semantic and episodic processing during creative cognition. To this end, we induced a semantic or episodic retrieval orientation prior to performance on an alternate uses task. Using RSA, we identified multivoxel patterns of neural activity associated with semantic and episodic processing during DT. Compared to episodic induction, we found that semantic induction and subsequent generation were characterized by increased pattern similarity within the left AG, left IPL, and PCC, indicating that these regions contributed to semantic processing during the AUT. Conversely, compared to semantic induction, episodic induction and generation were marked by increased pattern similarity within the left parahippocampal gyrus and right IPL, further suggesting common cognitive processing during these two conditions related to episodic processing. Taken together, these findings shed light on the neural basis of semantic and episodic processing during DT by revealing contributions of specific regions within the default network and other memory-relevant brain regions to creative idea production.
Our study extends neuroimaging research on the roles of semantic and episodic memory in creative cognition. Although several studies have previously reported activation within brain regions associated with memory retrieval—for example, within regions of the default network—the extent to which these activations reflected semantic or episodic processing remained unclear because the same regions can support both types of memory retrieval (Kim, 2016). On the one hand, the PCC, for instance, shows robust engagement during episodic retrieval of autobiographical memories (Benoit & Schacter, 2015). However, meta-analyses of fMRI studies on semantic processing find the PCC among the most consistently activated brain regions (Binder & Desai, 2011). The present findings provide some clarity on the role of PCC in creative cognition by demonstrating greater neural pattern similarity during DT and a canonical semantic task (i.e., sentence construction) compared to a task involving episodic memory (i.e., autobiographical retrieval). Given the PCC’s role as an associational hub involved in complex semantic integration—and the demands of the induction task to generate and integrate semantic information—the PCC may support similar processes known to be relevant for creative cognition (Abraham, 2018). Likewise, we also found that neural activity within left AG—which is associated with both episodic and semantic processing (Binder & Desai, 2011; Kim, 2016)—was more similar during semantic induction and subsequent generation than compared to episodic counterparts. The left AG has been consistently implicated in both activation (Gonen-Yaacovi et al., 2013) and connectivity analyses (Beaty et al., 2019) of DT, likely due to its role in complex semantic processing (Binder & Desai, 2011).
Regarding episodic effects, we found that episodic induction and subsequent generation shared common activity patterns within the left parahippocampal gyrus. Activation of the parahippocampal gyrus has been reported across several tasks requiring creative performance, including DT (Benedek et al., 2018), novel metaphor production (Benedek et al., 2014), and creative drawing (Ellamil et al., 2012). Moreover, a recent study contrasting neural activity during episodic retrieval, future imagination, and DT found common engagement within the parahippocampal gyrus (Beaty, Thakral, et al., 2018). As a region within the medial temporal system of the default network (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010), the parahippocampal gyrus contributes to constructive processes relevant to the flexible retrieval and integration of episodic representations (Schacter & Addis, 2007). Thus, common engagement of the right parahippocampal gyrus during episodic induction and subsequent idea generation may reflect common cognitive processes relevant for episodic simulation, such as scene construction (Hassabis & Maguire, 2009).
The current work extends behavioral and neuroimaging research on episodic induction and DT. Madore et al. (2015) provided the first behavioral evidence that episodic induction can improve the fluency and flexibility of responses generated on the AUT, a finding that was subsequently replicated in a sample of older and younger adults (Madore, Jing, et al., 2016). A recent fMRI study replicated and extended these behavioral effects, finding that enhanced DT performance corresponded to increased activation within the left anterior hippocampus (Madore, Thakral, et al., 2019). Here, we extend this line of research by directly comparing neural activity during induction and generation, demonstrating neural pattern similarity during induction and subsequent generation within the right IPL and left parahippocampal gyrus. Notably, the left parahippocampal gyrus cluster peak found in the present study (x = −28, y = −34, z = −14) is anatomically close to a previous study of future imagination by Madore, Szpunar et al. (2016), who found that episodic induction increased activity within left parahippocampal gyrus (x = −24, y = −34, z = −14).
It is important to note that the episodic induction employed in the current study differed in some respects from the ESI used in a majority of past work (Madore et al., 2015; Madore, Thakral, et al., 2019). Whereas our study presented memory cues and prompted participants to recall a personal past event—a task that is widely used in the autobiographical memory literature—prior ESI/DT studies (e.g., Madore et al., 2015; Madore, Jing, et al., 2016) used a more extensive procedure that involved experimenter-guided recall of a recently-experienced event (e.g., watching a video), a protocol that is based on the classic cognitive interview developed to promote the accuracy and detail of eye-witness testimony (but see Madore, Jing, et al., 2019b for a modified induction comparable to the current approach). Despite these procedural differences, the current study provides clarity on the contribution of brain regions that are known to support episodic retrieval with those previously activated in neuroimaging studies of DT (e.g., parahippocampal gyrus). We suspect that activation of such regions within then medial temporal lobe may reflect the involvement of constructive episodic retrieval processes, such as those involved in scene construction (Madore, Jing, & Schacter, 2019a). Recently, Madore et al. (2019a) found that episodic induction impacts the amount of detail generated on a scene construction task, including spatial, sensory, and action details. Taken in the context of the current study and related recent work, one possibility is that engaging the episodic system prior to DT facilitates mental imagery processes, consistent with evidence implicating mental imagery in creativity (Palmiero et al., 2016).
At the behavioral level, we found that semantic induction increased the semantic distance of AUT responses (assessed via LSA) relative to episodic induction. Related research has shown mixed effects of semantic induction on creative cognition by priming related or unrelated concepts prior to creative task performance. For example, Beaty, Christensen et al. (2017) found that studying noun-verb pairs yielded decreased semantic distance for the studied nouns on a subsequent verb generation task, compared to generating verbs to nouns that had not been previously studied. Likewise, when participants are shown prototypical examples prior to a drawing task, past work has shown that they tend to conform to the examples and produce less original drawings (Ward, Smith, & Finke, 1995). On the other hand, Fink and colleagues found that exposing participants to common ideas of others prior to subsequent idea generation led to more original responses (compared to exposure to meaningless words; (Fink et al., 2010; Fink et al., 2012). Notably, the extent to which activating prior knowledge constrains or facilitates creative production appears to rely in part on stimulus modality, with visual stimuli found to be more constraining than verbal (Chrysikou, Motyka, Nigro, Yang, & Thompson-Schill, 2016).
Although activating existing knowledge and salient exemplars can constrain creativity, the biasing of task-relevant retrieval mechanisms has been shown to facilitate aspects of creative performance. In the episodic domain, an increasing number of studies have found that biasing episodic retrieval mechanisms via experimental induction increases the fluency and flexibility of responses on the AUT (Madore et al., 2015; Madore, Szpunar, et al., 2016; Madore, Thakral, et al., 2019). This work suggests that activating relevant retrieval mechanisms—and not the content of episodic memory, per se—can improve performance on tasks that require these memory processes. Our study provides further evidence that memory inductions can activate relevant memory retrieval processes without necessarily priming memory content and influencing subsequent idea generation. Specifically, we found that using the same cue across induction and generation did not affect behavioral performance (i.e., semantic distance of subsequent AUT responses) nor corresponding neural activity, suggesting that priming the same cue word—across episodic and semantic conditions—may not impact the quality of ideas. This null effect should be interpreted tentatively, however, until further work has been conducted to examine whether cue effects impact other aspects of creative performance, such as fluency, which could not be assessed in our single-response design.
Importantly, the current study used a modified version of the episodic induction employed in past work, which involves a semi-structured interview guided by an experimenter to encourage maximal episodic retrieval. Moreover, past work has found benefits of episodic induction on fluency and flexibility—AUT metrics that could not be assessed due to the single-response aspect of our experimental design. Regarding semantic induction, however, we do provide novel evidence that activating the semantic system via a seemingly unrelated task (i.e., sentence construction) can benefit subsequent creative performance by increasing the semantic distance of AUT responses. Considered in the context of recent work on episodic induction (Madore et al., 2015, 2016), these findings provide preliminary evidence for a dissociation of episodic and semantic processing in DT: episodic induction may promote idea quantity—based on the work of Madore and colleagues showing increases in idea quantity (but not quality) following episodic induction—while semantic induction may promote idea quality (originality or semantic distance), based on the current findings. Another possibility is that LSA scoring is more sensitive to effects of semantic induction compared to episodic induction, given the algorithm’s intended purpose to detect semantic similarities. These interpretations remain tentative, however; future work is required to determine how and when semantic retrieval facilitates creative cognition.
Summary, Limitations, and Future Directions
The present study identified neural activity uniquely associated with episodic and semantic processing during DT, providing some insight into the cognitive contributions of brain regions within the default network to creative idea production. Future research could extend this work by determining the specific features of the episodic and semantic processes that support different aspects of creative cognition—such as scene construction or semantic integration—and the corresponding neural systems that underpin these cognitive operations. This approach can address open questions regarding how and when different memory processes benefit and constrain creative thought processes. It is worth noting, however, that there are various differences between the episodic and semantic inductions. Specifically, the episodic induction may also evoke more sensory retrieval, because it instructed participants to recall more details. The semantic induction may include additional syntactic demands by asking for sentence production. Future research should consider these differences in task characteristics when designing experimental induction procedures.
Notably, our induction procedure relied on brief post-scan reports of responses generated during the induction phases in the scanner. The brevity of such responses, and the potential loss of episodic/semantic detail from delayed recall, limits our ability to draw conclusions about the correspondence between memory content and idea generation. Relatedly, we found that the episodic condition showed better post-scan recall, while the semantic condition led to more divergent responses. It is possible that these aspects of the tasks explain some of the differences in their neural loci. We encourage future work to take a closer look at how memory content and retrieval processes differentially impact the quantity and quality of generated ideas.
In a similar vein, future research should continue to explore the potential of memory inductions to augment different aspects of creative performance. Although we found that semantic induction yielded increased originality of DT responses (as indexed via LSA) and prior work reported benefits of episodic induction for ideational fluency (Madore et al., 2015; Madore, Thakral, et al., 2019), the specific features of memory inductions that support creative cognition require further clarification, particularly in light of recent work showing that not all inductions produce beneficial effects on creative performance (Abraham et al., 2019). Finally, further research is needed to examine how other relevant brain systems, namely the executive control network and the salience network, interact with the default network (and related episodic and semantic processes) during DT—a pattern of functional connectivity that is commonly associated with creative performance (Beaty, Benedek, Kaufman, & Silvia, 2015; Beaty, Kenett, et al., 2018). Overall, we believe that such questions can be addressed with the tools of cognitive neuroscience by combining nuanced experimental designs with powerful analytical approaches (e.g., network science) to probe the complex neural dynamics that give rise to the production of creative thoughts.
Supplementary Material
Figure 3.
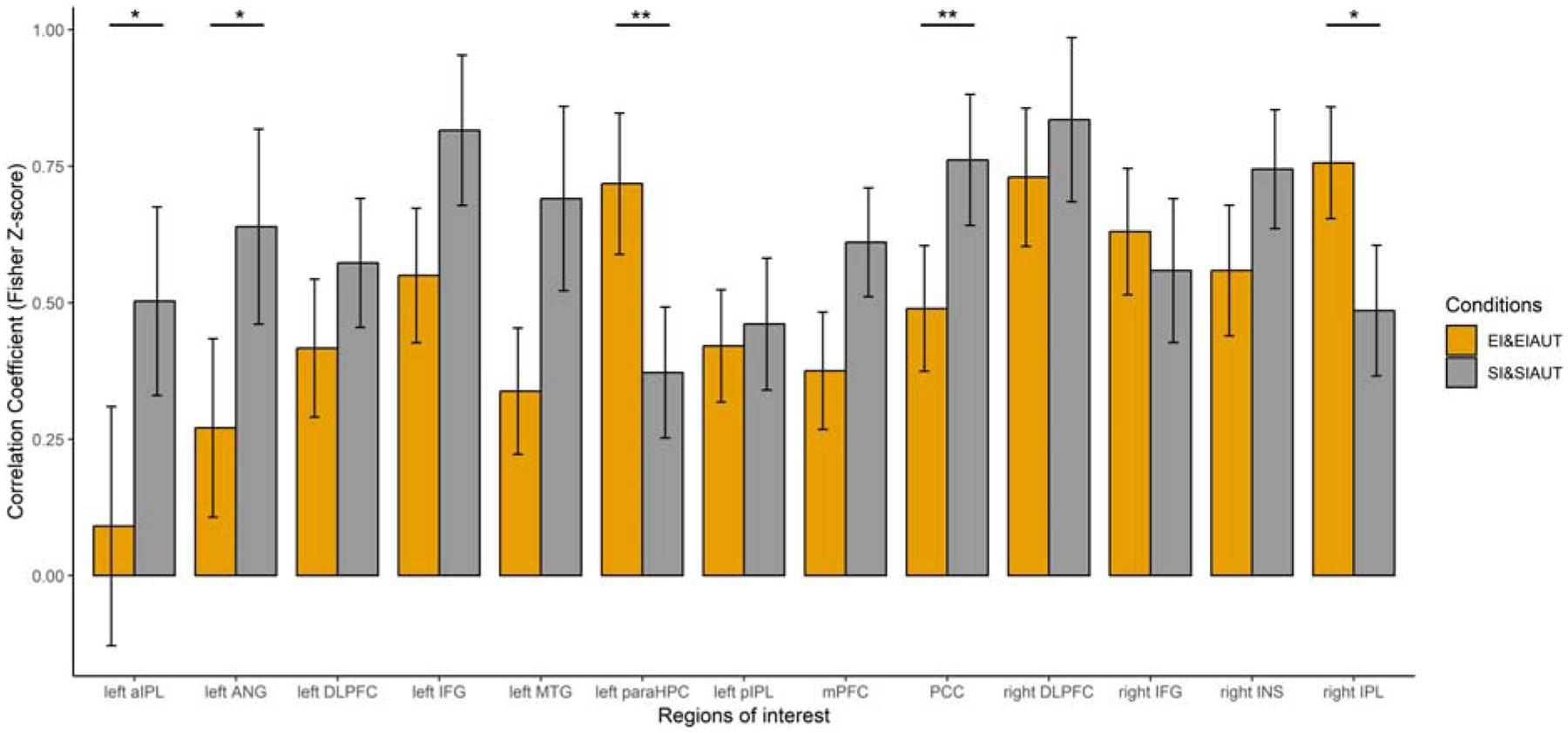
ROI-based similarities between divergent thinking following semantic induction and episodic induction
Notes. aIPL = anterior inferior parietal lobe; ANG = angular gyrus; AUT = alternate uses task; DLPFC = dorsolateral prefrontal cortex; EI = episodic induction; IFG = inferior frontal gyrus; INS = insula; paraHPC = parahippocampal cortex; PCC = posterior cingulate cortex; SI = semantic induction. The p-values of multiple pairwise t-tests were corrected for multiple comparisons using a false discovery rate (FDR) with a threshold of p < .05. * - p < .05. ** - p < .01.
Acknowledgments
R.E.B. is supported by a grant from the National Science Foundation [DRL-1920653]. This research was supported by grant RFP-15-12 to R.E.B., M.B., and P.J.S. from the Imagination Institute (www.imagination-institute.org), funded by the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the view of the Imagination Institute or the John Templeton Foundation. D.L.S was supported by National Institute of Mental Health R01 MH060941.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham A (2014). Creative thinking as orchestrated by semantic processing versus cognitive control brain networks. Frontiers in Human Neuroscience, 8, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A (2018). The neuroscience of creativity. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Abraham A, Asquith S, Ahmed H, & Bourisly AK (2019). Comparing the efficacy of four brief inductions in boosting short-term creativity. Journal of Cognitive Enhancement, 3(1), 85–93. [Google Scholar]
- Abraham A, & Bubic A (2015). Semantic memory as the root of imagination. Frontiers in Psychology, 6, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham A, Pieritz K, Thybusch K, Rutter B, Kröger S, Schweckendiek J, Stark R, Windmann S, & Hermann C (2012). Creativity and the brain: Uncovering the neural signature of conceptual expansion. Neuropsychologia, 50(8), 1906–1917. [DOI] [PubMed] [Google Scholar]
- Acar S, & Runco MA (2019). Divergent thinking: New methods, recent research, and extended theory. Psychology of Aesthetics, Creativity, and the Arts, 13(2), 153–158. [Google Scholar]
- Addis DR, Pan L, Musicaro R, & Schacter DL (2016). Divergent thinking and constructing episodic simulations. Memory, 24(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, & Schacter DL (2007). Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia, 45(7), 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Kaufman SB, & Silvia PJ (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5, 10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Benedek M, Silvia PJ, & Schacter DL (2016). Creative cognition and brain network dynamics. Trends in Cognitive Sciences, 20(2), 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Christensen AP, Benedek M, Silvia PJ, & Schacter DL (2017). Creative constraints: Brain activity and network dynamics underlying semantic interference during idea production. NeuroImage, 148, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Kenett YN, Christensen AP, Rosenberg MD, Benedek M, Chen Q, Fink A, Qiu J, Kwapil TR, Kane MJ, & Silvia PJ (2018). Robust prediction of individual creative ability from brain functional connectivity. Proceedings of the National Academy of Sciences, 115(5), 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, & Schacter DL (2018). Episodic memory and cognitive control: Contributions to creative idea production In Vartanian O & Jung RE (Eds.), The Cambridge Handbook of the Neuroscience of Creativity (pp. 249). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Beaty RE, Seli P, & Schacter DL (2019). Network neuroscience of creative cognition: mapping cognitive mechanisms and individual differences in the creative brain. Current Opinion in Behavioral Sciences, 27, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ, & Benedek M (2017). Brain networks underlying novel metaphor production. Brain and Cognition, 111, 163–170. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Silvia PJ, Nusbaum EC, Jauk E, & Benedek M (2014). The roles of associative and executive processes in creative cognition. Memory & Cognition, 42(7), 1–12. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Thakral PP, Madore KP, Benedek M, & Schacter DL (2018). Core network contributions to remembering the past, imagining the future, and thinking creatively. Journal of Cognitive Neuroscience, 30(12), 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, Beaty R, Jauk E, Koschutnig K, Fink A, Silvia PJ, Dunst B, & Neubauer AC (2014). Creating metaphors: The neural basis of figurative language production. NeuroImage, 90(0), 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek M, & Fink A (2019). Toward a neurocognitive framework of creative cognition: the role of memory, attention, and cognitive control. Current Opinion in Behavioral Sciences, 27, I116–122. [Google Scholar]
- Benedek M, Kenett YN, Umdasch K, Anaki D, Faust M, & Neubauer AC (2017). How semantic memory structure and intelligence contribute to creative thought: a network science approach. Thinking & Reasoning, 23(2), 158–183. [Google Scholar]
- Benedek M, Könen T, & Neubauer AC (2012). Associative abilities underlying creativity. Psychology of Aesthetics, Creativity and the Arts, 6(3), 273–281. [Google Scholar]
- Benedek M, Schües T, Beaty RE, Jauk E, Koschutnig K, Fink A, & Neubauer AC (2018). To create or to recall original ideas: Brain processes associated with the imagination of novel object uses. Cortex, 99, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B, 57(1), 289–300. [Google Scholar]
- Benoit RG, Paulus PC, & Schacter DL (2019). Forming attitudes via neural activity supporting affective episodic simulations. Nature Communications, 10(1), 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, & Schacter DL (2015). Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia, 75, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden EM, & Jung-Beeman M (1998). Getting the right idea: Semantic activation in the right hemisphere may help solve insight problems. Psychological Science, 9(6), 435–440. [Google Scholar]
- Chen Q, Beaty RE, Wei D, Yang J, Sun J, Liu W, Yang W, Zhang Q, & Qiu J (2019). Longitudinal alterations of frontoparietal and frontotemporal networks predict future creative cognitive ability. Cerebral Cortex, 28(1), 103–115. [DOI] [PubMed] [Google Scholar]
- Chen Q-L, Xu T, Yang W-J, Li Y-D, Sun J-Z, Wang K-C, Beaty RE, Zhang Q-L, Zuo X-N, & Qiu J (2015). Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia, 75, 441–449. [DOI] [PubMed] [Google Scholar]
- Chrysikou EG, Motyka K, Nigro C, Yang S-I, & Thompson-Schill SL (2016). Functional fixedness in creative thinking tasks depends on stimulus modality. Psychology of Aesthetics, Creativity, and the Arts, 10(4), 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, & Tyler LK (2014). Object-Specific Semantic Coding in Human Perirhinal Cortex. The Journal of Neuroscience, 34(14), 4766–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, & Christoff K (2012). Evaluative and generative modes of thought during the creative process. NeuroImage, 59(2), 1783–1794. [DOI] [PubMed] [Google Scholar]
- Fairhall SL, & Caramazza A (2013). Brain Regions That Represent Amodal Conceptual Knowledge. The Journal of Neuroscience, 33(25), 10552–10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, & Lavidor M (2003). Convergent and divergent priming in the two cerebral hemispheres: Lexical decision and semantic judgment. Cognitive Brain Research, 17, 585–597. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink A, Grabner RH, Gebauer D, Reishofer G, Koschutnig K, & Ebner F (2010). Enhancing creativity by means of cognitive stimulation: Evidence from an fMRI study. Neuroimage, 52(4), 1687–1695. [DOI] [PubMed] [Google Scholar]
- Fink A, Koschutnig K, Benedek M, Reishofer G, Ischebeck A, Weiss EM, & Ebner F (2012). Stimulating creativity via the exposure to other people’s ideas. Human Brain Mapping, 33(11), 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RP, & Geiselman RE (1992). Memory enhancing techniques for investigative interviewing: The cognitive interview: Charles C Thomas Publisher. [Google Scholar]
- Forthmann B, Lips C, Szardenings C, Scharfen J, & Holling H (2018). Are speedy brains needed when divergent thinking is speeded—or unspeeded? The Journal of Creative Behavior. [Google Scholar]
- Gonen-Yaacovi G, De Souza LC, Levy R, Urbanski M, Josse G, & Volle E (2013). Rostral and caudal prefrontal contribution to creativity: A meta-analysis of functional imaging data. Frontiers in Human Neuroscience, 7, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray K, Anderson S, Chen EE, Kelly JM, Christian MS, Patrick J, Huang L, Kenett YN, & Lewis K (2019). “Forward flow”: A new measure to quantify free thought and predict creativity. American Psychologist, 74(5), 539–554. [DOI] [PubMed] [Google Scholar]
- Green AE (2016). Creativity, within reason: Semantic distance and dynamic state creativity in relational thinking and reasoning. Current Directions in Psychological Science, 25(1), 28–35. [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, & Dunbar KN (2010). Connecting long distance: Semantic distance in analogical reasoning modulates frontopolar cortex activity. Cerebral Cortex, 20(1), 70–76. [DOI] [PubMed] [Google Scholar]
- Green AE, Kraemer DJM, Fugelsang JA, Gray JR, & Dunbar KN (2012). Neural correlates of creativity in analogical reasoning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 38(2), 264–272. [DOI] [PubMed] [Google Scholar]
- Hass RW (2016). Conceptual expansion during divergent thinking In Papafragou A & Grodner D & Mirman D & Trueswell JC (Eds.), Proceedings of the 38th Annual Meeting of the Cognitive Science Society (pp. 996–1001). Philadelphia, PA: Cognitive Science Society. [Google Scholar]
- Hass RW (2017a). Semantic search during divergent thinking. Cognition, 166, 344–357. [DOI] [PubMed] [Google Scholar]
- Hass RW (2017b). Tracking the dynamics of divergent thinking via semantic distance: Analytic methods and theoretical implications. Memory & Cognition, 45(2), 233–244. [DOI] [PubMed] [Google Scholar]
- Hassabis D, & Maguire EA (2009). The construction system of the brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen DJP, & Johnson DR (2018). Semantic distance: An automated measure of creativity that is novel and appropriate. Psychology of Aesthetics, Creativity, and the Arts, 12(2), 144–156. [Google Scholar]
- Jung RE, Flores RA, & Hunter D (2016). A new measure of imagination ability: Anatomical brain imaging correlates. Frontiers in Psychology , 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenett YN (2018a). Going the extra creative mile: The role of semantic distance in creativity – theory, research, and measurement In Jung RE & Vartanian O (Eds.), The Cambridge Handbook of the Neuroscience of Creativity (pp. 233–248). New York, NY: Cambridge University Press. [Google Scholar]
- Kenett YN (2018b). Investigating creativity from a semantic network perspective In Kapoula Z & Volle E & Renoult J & Andreatta M (Eds.), Exploring Transdisciplinarity in Art and Sciences (pp. 49–75). Cham: Springer International Publishing. [Google Scholar]
- Kenett YN (2019). What can quantitative measures of semantic distance tell us about creativity? Current Opinion in Behavioral Sciences, 27, 11–16. [Google Scholar]
- Kenett YN, Anaki D, & Faust M (2014). Investigating the structure of semantic networks in low and high creative persons. Frontiers in Human Neuroscience, 8(407), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenett YN, & Austerweil JL (2016). Examining search processes in low and high creative individuals with random walks In Papafragou A & Grodner D & Mirman D & Trueswell JC (Eds.), Proceedings of the 38th Annual Meeting of the Cognitive Science Society (pp. 313–318). Austin, TX: Cognitive Science Society. [Google Scholar]
- Kenett YN, Beaty RE, Silvia PJ, Anaki D, & Faust M (2016). Structure and flexibility: Investigating the relation between the structure of the mental lexicon, fluid intelligence, and creative achievement. Psychology of Aesthetics, Creativity, and the Arts, 10(4), 377–388. [Google Scholar]
- Kenett YN, & Faust M (2019). A semantic network cartography of the creative mind. Trends in Cognitive Sciences, 23(4), 271–274. [DOI] [PubMed] [Google Scholar]
- Kim H (2016). Default network activation during episodic and semantic memory retrieval: A selective meta-analytic comparison. Neuropsychologia, 80, 35–46. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, & Bandettini PA (2008). Representational similarity analysis - connecting the branches of systems neuroscience. Frontiers in Systems Neuroscience, 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger S, Rutter B, Hill H, Windmann S, Hermann C, & Abraham A (2013). An ERP study of passive creative conceptual expansion using a modified alternate uses task. Brain Research, 1527, 189–198. [DOI] [PubMed] [Google Scholar]
- Kröger S, Rutter B, Stark R, Windmann S, Hermann C, & Abraham A (2012). Using a shoe as a plant pot: Neural correlates of passive conceptual expansion. Brain Research, 1430, 52–61. [DOI] [PubMed] [Google Scholar]
- Landauer TK, & Dumais ST (1997). A solution to Plato’s problem: The latent semantic analysis theory of acquisition, induction, and representation of knowledge. Psychological Review, 104(2), 211–240. [Google Scholar]
- Landauer TK, Foltz PW, & Laham D (1998). An introduction to latent semantic analysis. Discourse Processes, 25(2–3), 259–284. [Google Scholar]
- Limb CJ, & Braun AR (2008). Neural substrates of spontaneous musical performance: an FMRI study of jazz improvisation. PLoS One, 3(2), e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Addis DR, & Schacter DL (2015). Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychological Science, 26(9), 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Jing HG, & Schacter DL (2016). Divergent creative thinking in young and older adults: Extending the effects of an episodic specificity induction. Memory & Cognition, 44(6), 974–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Jing HG, & Schacter DL (2019a). Episodic specificity induction and scene construction: Evidence for an event construction account. Consciousness and Cognition, 68, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Jing HG, & Schacter DL (2019b). Selective effects of specificity inductions on episodic details: evidence for an event construction account. Memory, 27(2), 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, & Schacter DL (2016). Remembering the past and imagining the future: Selective effects of an episodic specificity induction on detail generation. The Quarterly Journal of Experimental Psychology, 69(2), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Szpunar KK, Addis DR, & Schacter DL (2016). Episodic specificity induction impacts activity in a core brain network during construction of imagined future experiences. Proceedings of the National Academy of Sciences, 113(38), 10696–10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore KP, Thakral PP, Beaty RE, Addis DR, & Schacter DL (2019). Neural mechanisms of episodic retrieval support divergent creative thinking. Cerebral Cortex, 29(1), 150–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron TR, Lerner Y, Berant E, Kinreich S, Shapira-Lichter I, Hendler T, & Faust M (2018). Chain free association, creativity, and the default mode network. Neuropsychologia, 118, 40–58. [DOI] [PubMed] [Google Scholar]
- Marupaka N, Iyer LR, & Minai AA (2012). Connectivity and thought: The influence of semantic network structure in a neurodynamical model of thinking. Neural Networks, 32, 147–158. [DOI] [PubMed] [Google Scholar]
- Mednick S,A (1962). The associative basis of the creative process. Psychological Review, 69(3), 220–232. [DOI] [PubMed] [Google Scholar]
- Oltețeanu A-M, & Schultheis H (2017). What determines creative association? Revealing two factors which separately influence the creative process when solving the Remote Associates Test. The Journal of Creative Behavior. [Google Scholar]
- Oosterhof NN, Connolly AC, & Haxby JV (2016). CoSMoMVPA: multi-modal multivariate pattern analysis of neuroimaging data in Matlab/GNU Octave. Frontiers in Neuroinformatics, 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiero M, Piccardi L, Nori R, Palermo L, Salvi C, & Guariglia C (2016). Creativity and mental imagery. Frontiers in Psychology, 7, 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakaran R, Green AE, & Gray JR (2014). Thin slices of creativity: Using single-word utterances to assess creative cognition. Behavior Research Methods, 46(3), 641–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nat Rev Neurosci, 13(10), 713–726. [DOI] [PubMed] [Google Scholar]
- Runco MA, & Acar S (2012). Divergent thinking as an indicator of creative potential. Creativity Research Journal, 24(1), 66–75. [Google Scholar]
- Rutter B, Kröger S, Hill H, Windmann S, Hermann C, & Abraham A (2012). Can clouds dance? Part 2: An ERP investigation of passive conceptual expansion. Brain and Cognition, 80(3), 301–310. [DOI] [PubMed] [Google Scholar]
- Rutter B, Kröger S, Stark R, Schweckendiek J, Windmann S, Hermann C, & Abraham A (2012). Can clouds dance? Neural correlates of passive conceptual expansion using a metaphor processing task: implications for creative cognition. Brain and cognition, 78(2), 114–122. [DOI] [PubMed] [Google Scholar]
- Schacter DL, & Addis DR (2007). The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1481), 773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Buckner RL (2007). Remembering the past to imagine the future: the prospective brain. Nature Reviews Neuroscience, 8(9), 657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, & Szpunar KK (2017). Escaping the past: Contributions of the hippocampus to future thinking and imagination, The hippocampus from cells to systems (pp. 439–465): Springer. [Google Scholar]
- Schacter DL, & Madore KP (2016). Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Memory Studies, 9(3), 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling MA (2005). A “small-world” network model of cognitive insight. Creativity Research Journal, 17(2–3), 131–154. [Google Scholar]
- Siew CSQ, Wulff DU, Beckage NM, & Kenett YN (2019). Cognitive network science: A review of research on cognition through the lens of network representations, processes, and dynamics. Complexity, 2019, 24. [Google Scholar]
- Slotnick SD (2017). Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cognitive Neuroscience, 8(3), 150–155. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, & Hart J (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Smith SM, & Ward TB (2012). Cognition and the creation of ideas In Holyoak KJ & Morrison RG (Eds.), Oxford Handbook of Thinking and Reasoning (pp. 456–474). Oxford, UK: Oxford University Press. [Google Scholar]
- Tulving E (1972). Episodic and semantic memory. Organization of memory, 1, 381–403. [Google Scholar]
- Tulving E (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53(1), 1–25. [DOI] [PubMed] [Google Scholar]
- Volle E (2018). Associative and controlled cognition in divergent thinking: Theoretical, experimental, neuroimaging evidence, and new directions In Jung RE & Vartanian O (Eds.), The Cambridge handbook of the neuroscience of creativity (pp. 333–362). New York, NY: Cambridge University Press. [Google Scholar]
- Ward TB (1994). Structured imagination: The role of category structure in exemplar generation. Cognitive Psychology, 27(1), 1–40. [Google Scholar]
- Ward TB, Smith SM, & Finke RA (1995). The creative cognition approach: MIT press. [Google Scholar]
- Wiggs CL, Weisberg J, & Martin A (1998). Neural correlates of semantic and episodic memory retrieval. Neuropsychologia, 37(1), 103–118. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang W, Tong D, Sun J, Chen Q, Wei D, Zhang Q, Zhang M, & Qiu J (2015). A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Human Brain Mapping, 36(7), 2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G (2018). The neural representations underlying human episodic memory. Trends in Cognitive Sciences, 22(6), 544–561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


