Abstract
Background
Cannabis usage is increasing with its widespread legalization. Cannabis use by mothers during lactation transfers active cannabinoids to the developing offspring during this critical period and alters postnatal neurodevelopment. A key neurodevelopmental landmark is the excitatory to inhibitory GABA switch caused by reciprocal changes in expression ratios of the K+/Cl− transporters KCC2 and NKCC1.
Methods
Rat dams were treated with THC or a synthetic cannabinoid during the first 10 days of postnatal development and experiments were then conducted in the offspring exposed to these drugs via lactation. The network influence of GABA transmission was analyzed using cell-attached recordings. KCC2 and NKCC1 levels were determined using Western blot and qPCR analyses. USV and homing behavioral experiments were carried out at relevant time-points.
Results
Treating rat dams with cannabinoids during early lactation retards transcriptional upregulation and expression of KCC2, thereby delaying the GABA switch in pups of both sexes. This perturbed trajectory was corrected by the NKCC1 antagonist bumetanide and accompanied by alterations in ultrasonic vocalization without changes in homing behavior. Neurobehavioral deficits were prevented by CB1R antagonism during maternal exposure, showing that CB1R underlie the cannabinoid-induced alterations.
Conclusions
These results reveal how perinatal cannabinoid exposure retards an early milestone of development, delaying the trajectory of GABA’s polarity transition and altering early-life communication.
Keywords: prefrontal cortex, lactation, GABA, maturation, cannabis, perinatal, CB1 receptor, endocannabinoid.
Introduction
Cannabis is the most widely used illicit drug in the world with increasing use in Western nations (1). Its actions are primarily attributed to Δ9-tetrahydrocannabinol (THC), which acts on cannabinoid receptors (CB1R and CB2R), who together with naturally occurring endocannabinoids (eCBs) and their synthesizing/degrading enzymes, comprise the endogenous cannabinoid system (ECS; 2). Cannabis consumption during pregnancy ranges from 1–6% (3, 4) and will likely rise with widespread decriminalization and legalization. Public perception categorizes cannabis usage during pregnancy as low-risk (5). Nonetheless, consequences of infant exposure to cannabinoids remain poorly researched.
The role of the ECS during development is well-established in animals (6, 7) and humans (8–10). Importantly, consumption of cannabis results in significant quantities of THC and active metabolites in breastmilk (11–13), which transfer to offspring in both humans (14) and animals (15, 16). Additionally, THC exposure has adverse impacts on fetal and perinatal neurodevelopment (17–19), with significant consequences throughout life (7, 20, 21). Furthermore, the ECS plays a crucial role in prefrontal cortex development (PFC; 22), a cognitive hub whose developmental perturbation has been linked to a variety of maturational deficits (23–25).
The PFC is the most highly evolved brain region (2, 24), participating in behaviors from working memory and emotion to cognitive flexibility (26, 27). The ECS is a modulatory neurotransmitter system in the PFC (28), highly concentrated at interneuron synapses (10, 29) and more prevalent in deep than superficial layers (30, 31). Importantly, eCBs serve a critical function in the developmental trajectory of GABAergic interneurons (32). Consequently, ECS perturbations during neonatal development have lasting effects on GABAergic transmission (33).
While GABA is the primary adult inhibitory neurotransmitter, in immature brains it exhibits excitatory influence due to high intracellular Cl− caused by low levels of the KCC2 chloride transporter (34). Increasing KCC2 expression and declining NKCC1, subsequently decrease intracellular Cl− (34–37), mediating the inhibitory transition of GABA. Aberrations in this transition’s timing are linked with disorders including autism, Down syndrome, Fragile X and schizophrenia (38–42). Its timing differs between brain regions: from embryonic day 15 in hippocampus to postnatal day (P) 15 in neocortex (43). The development of PFC GABA synapses is maximal between P10–15 (44, 45), though the functional valence of these sites has not been investigated.
The sparse data of the consequences of ECS perturbation during the postnatal period on GABAergic function suggest significant, lasting impacts (46). While it is known that cannabis exposure during PFC development has profound consequences (47), the mechanistic underpinnings remain largely unexplored. Here, we investigated the postnatal impact of cannabinoids via maternal exposure to assess potential risks associated with cannabis use during this period.
Methods
Further information and requests for resources/reagents should be directed to the Lead Contact, Olivier J.J. Manzoni (olivier.manzoni@inserm.fr).
Animals
Animals were treated in compliance with the European Communities Council Directive (86/609/EEC) and the United States NIH Guide for the Care and Use of Laboratory Animals. All rats were group-housed with 12h light/dark cycles with ad libitum access to food and water. All behavioral, biochemical and synaptic plasticity experiments were performed on male and female RjHan:wi-Wistar rats (P09–21) from pregnant females obtained from Janvier Labs. Pregnant dams arrived at E15 and remained undisturbed until delivery. Newborn litters found before 05:00p.m. were considered to be born that day (P0). Male and female electrophysiological and biochemical results exhibited no difference; thus data were pooled (Tables 1–2 for details).
Table 1: Sex-distribution of electrophysiological data (Picrotoxin; PTX).
Samples used in electrophysiological data were collected from both male and female rats at all ages. Values for individual groups are expressed as individual rats. Mean and SEM are given for post-PTX relative (normalized) spike frequency. No significant differences were found between sexes within treatment groups responding to PTX in slice conditions.
| Parameter PND | Treatment | Sex | n | Mean | SEM | P value (unpaired t test) |
|---|---|---|---|---|---|---|
| PTX 10 | Sham | Male | 5 | 67.25 | 9.99 | 0.9699 |
| Sham | Female | 5 | 67.72 | 6.62 | ||
| WIN | Male | 3 | 77.76 | 7.40 | 0.4998 | |
| WIN | Female | 3 | 69.22 | 8.79 | ||
| THC | Male | 4 | 53.44 | 5.32 | 0.3985 | |
| THC | Female | 4 | 46.13 | 6.024 | ||
| AM+WIN | Male | 3 | 23.58 | 8.226 | 0.9770 | |
| AM+WIN | Female | 2 | 23.03 | 14.12 | ||
| Bumetanide + WIN | Male | 3 | 60.18 | 12.91 | 0.8462 | |
| Bumetanide + WIN | Female | 2 | 55.64 | 16.36 | ||
| PTX 15 | Sham | Male | 5 | 158.6 | 27.65 | 0.6290 |
| Sham | Female | 5 | 143.5 | 10.5 | ||
| WIN | Male | 3 | 59.53 | 18.34 | 0.8815 | |
| WIN | Female | 2 | 62.67 | 3.76 | ||
| THC | Male | 3 | 64.36 | 10.58 | 0.4957 | |
| THC | Female | 3 | 54.60 | 7.36 | ||
| AM+WIN | Male | 3 | 131.3 | 9.38 | 0.7676 | |
| AM+WIN | Female | 3 | 140.7 | 27.05 | ||
| Bumetanide + WIN | Male | 3 | 171.0 | 36.38 | 0.8219 | |
| Bumetanide + WIN | Female | 3 | 160.9 | 19.63 | ||
| PTX 20 | Sham | Male | 3 | 148.9 | 40.09 | 0.8878 |
| Sham | Female | 2 | 141.1 | 31.11 | ||
| WIN | Male | 3 | 176.8 | 46.43 | 0.9621 | |
| WIN | Female | 2 | 180.1 | 44.85 | ||
| THC | Male | 3 | 189.8 | 50.37 | 0.7790 | |
| THC | Female | 3 | 173.4 | 12.31 |
Table 2: Sex-distribution of electrophysiological data (Isoguavacine; ISO).
Samples used in electrophysiological data were collected from both male and female rats at all ages. Values for individual groups are expressed as individual rats. Mean and SEM are given for post-ISO relative (normalized) spike frequency. No significant differences were found between sexes within treatment groups responding to ISO in slice conditions.
| Parameter PND | Treatment | Sex | n | Mean | SEM | P value (unpaired t test) |
|---|---|---|---|---|---|---|
| ISO 10 | Sham | Male | 5 | 113.0 | 7.107 | 0.3383 |
| Sham | Female | 4 | 128.1 | 12.31 | ||
| WIN | Male | 3 | 137.5 | 22.46 | 0.5465 | |
| WIN | Female | 3 | 121.1 | 6.488 | ||
| THC | Male | 4 | 146.7 | 10.04 | 0.9843 | |
| THC | Female | 3 | 146.5 | 6.586 | ||
| AM+WIN | Male | 3 | 140.2 | 3.376 | 0.6531 | |
| AM+WIN | Female | 3 | 170.8 | 58.41 | ||
| Bumetanide + WIN | Male | 3 | 162.2 | 30.79 | 0.4224 | |
| Bumetanide + WIN | Female | 3 | 194.8 | 17.69 | ||
| ISO 15 | Sham | Male | 5 | 58.93 | 7.867 | 0.9168 |
| Sham | Female | 7 | 61.08 | 18.02 | ||
| WIN | Male | 3 | 130.9 | 5.228 | 0.1707 | |
| WIN | Female | 3 | 148.6 | 8.663 | ||
| THC | Male | 3 | 162.0 | 25.94 | 0.9455 | |
| THC | Female | 3 | 159.6 | 20.41 | ||
| AM+WIN | Male | 3 | 49.59 | 21.73 | 0.8974 | |
| AM+WIN | Female | 3 | 46.01 | 14.06 | ||
| Bumetanide + WIN | Male | 4 | 43.03 | 13.16 | 0.8393 | |
| Bumetanide + WIN | Female | 4 | 47.76 | 17.97 | ||
| ISO 20 | Sham | Male | 3 | 57.87 | 11.38 | 0.5154 |
| Sham | Female | 3 | 48.45 | 5.899 | ||
| WIN | Male | 3 | 55.22 | 8.797 | 0.5470 | |
| WIN | Female | 3 | 46.18 | 10.53 | ||
| THC | Male | 3 | 43.04 | 21.91 | 0.7646 | |
| Sthc | Female | 3 | 35.42 | 5.412 |
Maternal behavior was assessed by quantifying time in the nest and nursing time/type (Table 3). Observations were made twice daily (10h/16h) during 1 of every 5 minutes for 20 minutes. No treatments impacted time in the nest (Table 3; F3,4=1.129, p=0.4374, one-way ANOVA) or nursing (F3,44=5.398, p>0.9999, one-way ANOVA).
Table 3: Nursing time did not differ between treatment conditions.
Data were collected from litters for each condition as described in Methods (Sham N=4, WIN N=4, THC N=4, AM+WIN N=3). Values are presented as the percentage of total time during the observation period ± SEM. Total time spent nursing (i.e. the combined percentage of “arched”, “blanket” and “passive” nursing as compared to the percentage of “no nursing” observations) did not differ between groups (F9,44=1.116, P=0.3719. Two-way ANOVA followed by Tukey’s post-hoc analysis).
| Nursing behavior |
Arched nursing |
Blanket nursing |
Passive nursing |
No nursing | Total time in nest |
|---|---|---|---|---|---|
| Sham | 8.33±3.40 | 48.96±12.31 | 26.67±2.55 | 11.46±3.56 | 88.54±1.78 |
| WIN | 9.37±3.56 | 58.33±2.95 | 16.67±8.74 | 16.67±5.38 | 89.58±1.80 |
| THC | 9.37±3.56 | 54.17±4.50 | 19.17±1.99 | 18.75±2.69 | 91.67±0.85 |
| AM+WIN | 8.33±2.08 | 43.06±3.18 | 30.21±6.36 | 16.67±5.51 | 94.44±0.60 |
Pups from WIN- or THC-treated dams exhibited slower growth (significantly lower average weights) from P07–10 (Table 4; F3,5=15.63, p=0.0057). In line with electrophysiological and biochemical data, co-administration of AM-251 prevented the reduced weight gains in pups by P10 (P=0.0970 compared to Sham P10, Tukey’s multiple comparisons test).
Table 4: Pup weights are significantly reduced during the treatment period by perinatal cannabinoid exposure.
Pups weights were collected daily from P01-P10 (Sham N=4, WIN N=4, THC N=4, AM+WIN N=3). Values are expressed as mean (grams) ± SEM. P values are given for each day as compared to pups from Sham-treated dams on the same postnatal day, as determined by Tukey’s post-hoc comparison following a significant Two-way ANOVA (F27,63=14.68, P<0.0001).
| Postnatal day weights |
Sham | WIN | THC | AM+WIN |
|---|---|---|---|---|
| P01 | 8.23±0.24 | 7.55±0.50 P=0.9480 |
7.11±1.25 P=0.7490 |
7.07±1.96 P=0.7243 |
| P02 | 9.38±0.79 | 8.39±0.31 P=0.8620 |
7.86±1.02 P=0.5293 |
8.21±1.72 P=0.7262 |
| P03 | 10.82±0.83 | 9.89±1.53 P=0.8827 |
8.23±0.85 P=0.1036 |
9.48±1.65 P=0.6320 |
| P04 | 12.59±1.48 | 10.48±0.55 P=0.3400 |
9.23±0.94 P=0.0190 |
11.11±2.06 P=0.6451 |
| P05 | 14.30±2.03 | 12.05±0.25 P=0.2824 |
10.88±1.14 P=0.0190 |
12.98±2.41 P=0.5548 |
| P06 | 17.28±2.34 | 13.89±0.38 P=0.0414 |
12.67±1.17 P=0.0006 |
14.87±2.62 P=0.1453 |
| P07 | 20.66±1.55 | 16.39±0.38 P=0.0057 |
14.31±0.55 P=0.0006 |
17.35±1.59 P=0.1453 |
| P08 | 22.95±0.87 | 18.11±0.18 P=0.0057 |
15.59±0.42 P<0.0001 |
19.86±1.88 P=0.0209 |
| P09 | 26.64±0.83 | 20.40±0.69 P<0.0001 |
17.77±0.52 P<0.0001 |
23.53±1.60 P=0.0346 |
| P10 | 29.09±0.85 | 23.36±0.35 P=0.0001 |
19.47±0.46 P<0.0001 |
26.47±1.68 P=0.0970 |
Drug treatments
Dams were injected daily sub-cutaneously (s.c.) from P01–10 with the synthetic cannabimimetic WIN55,212–2 (WIN; 0.5mg/kg/day) alone or with AM-251 (0.5mg/kg/day), or with Δ9-Tetrahydrocannabinol (THC; 2mg/kg/day). WIN55,212–2, THC or AM-251 were suspended in 1:1:18 DMSO, cremophor and saline, and injected at 1ml/kg. Control dams (Sham) received vehicle. Bumetanide (in 0.1% DMSO, 99.9% saline) was injected twice daily (0.2mg/kg/injection, 10μl/g; 09:00a.m. and 05:00p.m.) from P01–15.
Electrophysiology
Coronal slices containing the prelimbic area of the medial prefrontal cortex (mPFC) were prepared as previously described (Lafourcade et al., 2007). Details of slice-preparation and acquisition are in Supplemental Methods.
Spontaneous Spiking Activity
Spontaneous spiking activity was recorded in cell-attached configuration with a patch pipette filled with ACSF. A >500 MOhm seal was obtained in current-clamp configuration before recording in I=D0 mode. Data were filtered at 2 kHz and digitized at 10 kHz. Activity was analyzed in Clampfit 10.5 (Molecular Devices) threshold detection with a trigger threshold of >2x SD of baseline noise. Mean spike activity was calculated as an average of spikes per minute over a 10-minute baseline period. For drug-effects, means represent an average of spikes/min over a 10-minute period following >5 minutes of bath perfusion.
Single channel and whole-cell patch-clamp recordings
Single-channel chloride reversal potential (GABArev) recordings were obtained in cell-attached configuration with a patch pipette containing an internal solution (detailed in Supplemental Methods). A >500 MOhm seal was obtained in current-clamp configuration before recording activity at imposed voltages (−100mV to +40mV). Data were filtered at 1kHz and digitized at 5kHz. Channel openings were analyzed in Clampfit 10.5 (Molecular Devices). Current magnitudes were obtained from >10 openings per holding potential. GABArev was then calculated using the unitary chord conductance (γ) wherein γ = IA – IB/ΔV (IA and IB ,current values with opposite polarity closest to the reversal potential) as previously described (48). Following channel recordings, membrane seals were broken and resting membrane potentials (Em) were confirmed in whole-cell configuration within ~1min to avoid cell dialysis. Imposed values are relative to Vpipette zeroed in cell-attached mode and are thus a function of Em.
Western-blots
Brains were harvested and snap frozen in isobutane on dry ice and stored at −80C. A brain matrix (Braintree Scientific #BS-SS 605C) at −20°C was used to prepare 1mm coronal sections. Brain regions were harvested on a dry ice-chilled glass plate. mPFCs were split at the midline and processed for either Western blot analysis or qRT-PCR. For Westerns, samples were homogenized in RIPA buffer (50mM Tris, pH8.0, 150mM NaCl, 1% Tx-100, 0.1% SDS, 0.5% CHAPS, 1x HALT protease and phosphatase inhibitor (Thermo Fisher Scientific, #78440)) and centrifuged (10,000xg, 10 minutes, 4°C). Supernatants were collected and mixed with 4X sample buffer and incubated (10 minutes at 65°C) and run on 4–12% NuPage gels (Thermo Fisher Scientific, #NP0323B0X). Following protein transfer, blots were stained (Revert Total Protein stain, Li-Cor, # 926–11011), scanned for total protein and blocked in Li-Cor Blocking Buffer (Li-Cor Bioscience #927–40000; 60 minutes, 22°C). They were then incubated with either rabbit or mouse anti-KCC2 or rabbit anti-NKCC1 diluted in a mixture of Li-Cor Blocking Buffer and 1XPBS (1:1). Blots were re-probed for protein content using rabbit anti-GAPDH. Blots were incubated with primary antibodies overnight at 4°C. Next, blots were washed (4×15 minutes, 22°C) in TBST (20 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20), then incubated in the buffer above containing Li-Cor donkey anti-rabbit IR680, donkey anti-rabbit IR800, donkey anti-mouse IR680 or donkey anti-mouse IR800 antibodies (1 hour, 22°C). Finally, blots were washed as above and scanned on a Li-Cor Odyssey near-IR imager. Apparent molecular weights were determined using either Benchmark (Thermo Fisher Scientific, #10748–010) or Chameleon (Li-Cor, #928–60000). Band densities were calculated using FIJI software. GAPDH staining and total protein over development and treatments were highly correlated. Bands corresponding to KCC2 and NKCC1 were normalized to GAPDH density.
qRT-PCR
qRT-PCR was performed on mPFC harvested as above following published procedures (20) Primers and probes described in the Key Resources table. Duplicates were run for each sample, and relative gene expression was determined using the double delta Ct method.
Ultrasonic vocalizations
USV induced by maternal isolation were recorded from male and female rats at P09 and P15 as described (49–51). Offspring were left undisturbed in homecages with their biological dams in the test room for habituation (30 minutes). Each pup (2–5 per litter) was tested individually in arbitrary order. USV were recorded over a 3-minute period in a sound attenuating isolation box (37×21×14cm) in another room and equipped with one white-light LED (30 lux). USV were recorded using an ultrasound microphone (Ultravox Noldus) 20 cm above the floor and connected via the Ultravox device (Noldus, Netherlands). Recordings were conducted from 8:00–11:00 a.m. USV were scored for total number of calls and mean dominant frequency. As USV can be influenced by pups’ body temperature (52), box temperature was controlled over the test (35±2°C).
Homing behavior
Homing behavior was tested as previously described (53). P10 and P13 pups of both sexes (2–5/litter) were separated from their mother and placed on a heating pad at 35±2°C. Pups were individually placed into a Plexiglas box (37×21×14cm) with 1/3:2/3 home-cage to fresh bedding. Pups were placed at the clean-bedding side and video recorded (4min). Homing performance was scored for latency to reach the home-cage litter, total time spent in the nest-litter area and number of crossings. Animals failing to reach the nest were eliminated from analysis.
Quantification and statistical analysis
Analyses were conducted using Microsoft Excel 2016 (Redmond, WA) and GraphPad Prism 7 (La Jolla, CA), with significance of 0.05. N values are presented as individual cell or animal (indicated in figure legends). Error bars indicate SEM. Significance was assessed by one-way or two-way analysis of variance (ANOVA; followed by Tukey’s multiple comparisons post hoc analyses), Mann-Whitney U test or Student’s t-test. Grubb’s test (alpha level 0.05) was applied to all datasets to identify outliers which were subsequently excluded from datasets. Statistical details for each experiment are in corresponding figure legends.
Results
No differences were found between sexes throughout this study (Tables 1–2, Supplementary Tables 1–2). Thus, all data were pooled.
In accord with international ethical guidelines to reduce animals used and their treatment/manipulations, once a lack of difference in outcomes in WIN- or THC-expose pups was established (Figures 1, 6), further experiments were carried out only with WIN. All experiments were repeated with a minimum of 2 litters.
Figure 1: Developmental shift from excitation to inhibition by GABA-A receptors in rat medial prefrontal cortex slices is delayed by perinatal cannabinoid exposure.
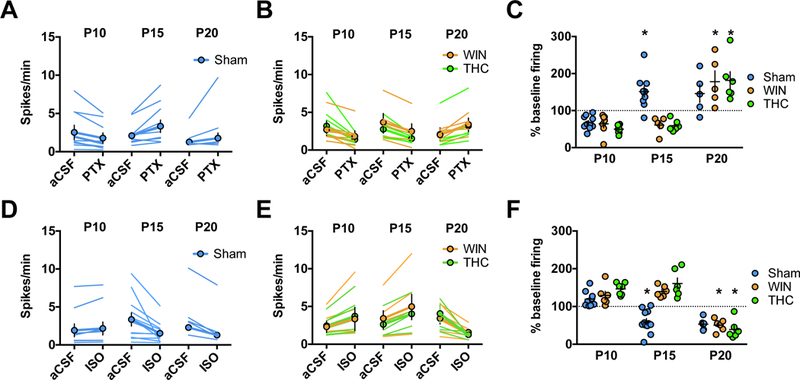
Action potentials were recorded in cell-attached (I=0) layer 5 pyramidal neurons in standard aCSF. After 10 min of baseline recording, picrotoxin (20 μM; GABA-A receptor antagonist, PTX) or isoguvacine (7 μM; GABA-A receptor agonist, ISO) was bath-applied. Spiking activity was calculated as an average of spikes per minute (10 min baseline) compared to the last 10 min of drug application. A-C: GABA-A receptor antagonism is inhibitory in immature P09-P10 mPFC networks in the progeny of Sham-, WIN-, or THC-treated dams but excitatory at P15 in Sham-exposed offspring and P21 in WIN- or THC-exposed offspring. PTX decreased spike frequency in slices obtained from P09–10 rats (Sham: N=8 cells/5 rats, WIN: N=6 cells/4 rats, THC N=7 cells/5 rats). In contrast, PTX increased spike frequency in slices obtained from Sham-treated P15–16 rats (N=9 cells/6 rats) while continuing to decrease spike frequency in slices obtained from WIN- or THC-exposed rats (WIN: N=5 cells/4 rats, THC: N=5 cells/4 rats). At P20–21, PTX application increased spike frequency in slices obtained from either Sham-, WIN-, or THC-exposed rats (Sham: N=5 cells/4 rats, WIN: N=5 cells/4 rats, THC: N=4 cells/4 rats). Two-way ANOVA revealed a significant drug/postnatal day interaction (F4,35 = 6.479, P=0.0003; * indicates P<0.05 as compared to respective P10 normalized post-drug firing rate as determined by Tukey’s post-hoc analysis. Error bars indicate SEM. Example traces shown in Supplementary Figure 4. D-F: GABA-A receptor agonism is excitatory in immature P09–10 mPFC networks in the progeny of Sham-, WIN, or THC-treated dams but inhibitory at P15 in Sham-exposed offspring and P21 in WIN- or THC-exposed offspring. ISO increased spike frequency in slices obtained from P09–10 rats (Sham: N=9 cells/rats, WIN: N=6 cells/4 rats, THC: N=7 cells/4 rats). In contrast, ISO application decreased spike frequency in slices obtained from P15–16 pups from Sham-treated dams (N=11 cells/7 rats) while it continued to increase spike frequency in slices obtained from P15–16 pups from WIN- or THC-treated dams (WIN: N=6 cells/rats, THC: N=6 cells/4 rats). At P20–21, ISO application decreased spike frequency in slices obtained from the offspring of all conditions (Sham: N=6 cells/4 rats, WIN: N=6 cells/4 rats, THC: N=6 cells/4 rats). Two-way ANOVA revealed a significant drug/postnatal day interaction (F4,55 = 12.94, P<0.0001; * indicates P<0.05 as compared to respective P10 normalized post-drug firing rate as determined by Tukey’s post-hoc analysis. Error bars indicate SEM. Example traces shown in Supplementary Figure 5–6.
In the prefrontal cortex, GABA transitions from an excitatory to inhibitory neurotransmitter between P10 and P15
While the developmental GABA trajectory has been characterized in several brain regions (35, 36, 54, 55), it is unknown if it occurs in the medial prefrontal cortex (mPFC). To establish the existence and timing of GABA’s transition in rat mPFC, we used cell-attached recordings in slices containing layer 5 pyramidal neurons to observe spontaneous cell spiking activity before and after the application of either the GABAR antagonist picrotoxin (PTX) or positive allosteric modulator isoguavacine (ISO) as described (36, 44, 56).
At P09-P10, application of PTX significantly decreased spike frequency (Figure 1a,c). Conversely, ISO significantly increased (Figure 1D,F) spike activity. These results are compatible with the idea that GABA serves as an excitatory neurotransmitter at P09–10. Conversely, cells recorded between P15-P16 exhibited increased spiking activity following PTX application (Figure 1a,c), while ISO significantly attenuated (Figure 1D,F) spike frequency. Similarly, at P20 PTX significantly increased spike frequency (Figure 1A,C) while ISO decreased spike frequency (Figure 1D,F). Thus, at or after P15, GABA-A receptor activation exerts an inhibitory influence on mPFC networks, indicating that GABA undergoes a functional “switch” from excitation to inhibition between P10 and P15 which is sustained at P20. Thus, the mPFC GABA switch occurs at a similar time as in other brain regions (36).
Perintal WIN or TCH delays the GABA “switch”
Endocannabinoid signaling during early development, including the first postnatal weeks (57, 6), mediates GABA neuron connectivity (29). Therefore, we investigated the developmental consequences of cannabinoid exposure on GABA’s mPFC trajectory. Dams were treated with either the cannabimimetic CB½R agonist WIN 55,212–2 (WIN; 0.5mg/kg s.c.) or the principle psychoactive component of cannabis, A9-THC (THC; 2mg/kg, s.c.) from P01-P10. Cell-attached recordings were then performed as above from cannabinoid-treated progeny at three time points (Figure 1B–C, E-F).
At P09–10, PTX significantly reduced spike frequency in slices obtained from pups exposed to either WIN or THC (Figure 1B–C), while application of ISO significantly increased spike frequency in both groups (Figure 1E–F). At P15–16, the effects of both drugs on spike frequency remained consistent PTX still attenuated spike frequency in slices obtained from WIN- or THC-exposed progeny (Figure 1B–C, E-F). Thus, in marked contrast to Shams, GABA remains excitatory at P15-P16 in pups perinatally exposed to cannabinoids.
Considering the delayed GABA switch in a number of disorders (58, 59, 40, 41) as well following alterations to maternal health (60) or behavior (61), we performed recordings on slices from WIN- or THC-exposed pups at P20–21 to ascertain whether the GABA switch had occurred at this age. P20–21 PTX application increased, while ISO application decreased spike frequency in slices from WIN- or THC-exposed pups (Figure 1B–C, E–F). Together with previous results, these findings indicate that in cannabinoid-exposed pups, GABA’s transition from excitatory to inhibitory is delayed, rather than absent. Importantly, co-administration of the CB1R antagonist AM-251 with WIN prevented this delay, indicating a CB1R-dependent locus of effect (Supplementary Figure 1).
The GABA “switch” is correlated with changes in GABArev and EM
Immature cells with high intracellular Cl− due to low levels of KCC2 exhibit relatively depolarized GABA-mediated Cl− reversal potentials (GABArev), driving GABA’s excitatory influence (35, 41, 48, 62, 63). Increased developmental KCC2 expression decreases Cl− levels and hyperpolarizes GABArev, shunting action potentials and inhibiting neuronal activity. This has been well described in other brain regions as cells mature (48, 63, 64), as well as in disease and injury models (65, 66). However, no such measurements have been effectuated in the developing mPFC.
To assess whether the mPFC GABA switch correlates with GABArev hyperpolarization, we performed single-channel recordings of GABA-activated Cl− channels. We observed a progressive hyperpolarization of GABArev between P9-P21 in the offspring of both Sham- and WIN-treated dams (Figure 2A–C). GABArev decreased between P09–10 and P15–16 and remained decreased at P20–21 in Sham-treated offspring, but was unchanged between P09–10 and P15–16 in WIN-exposed offspring. By P20–21, a significant hyperpolarization of GABArev was observed in WIN-exposed offspring. Together, these data identify a delayed developmental GABArev hyperpolarization in the offspring of WIN-versus Sham-treated dams, correlating with the retarded trajectory of GABA’s excitatory-to-inhibitory switch.
Figure 2: Maturational trajectory of the GABA reversal potential (GABArev) and resting membrane potential (EM) are delayed by perinatal cannabinoid exposure.
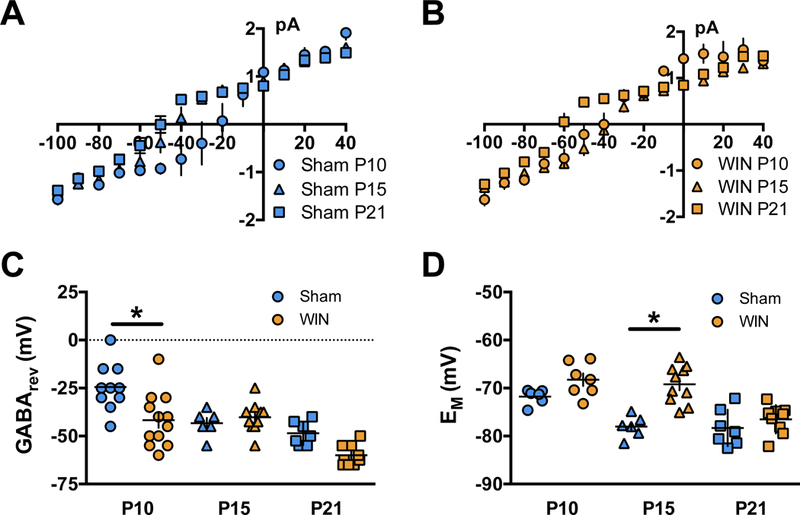
Single-channel recordings were conducted in cell-attached layer 5 PFC pyramidal neurons collected from offspring of either Sham or WIN-treated dams in standard aCSF. Channel opening magnitudes were collected from −100mV to +40mV. GABArev was determined from I-V curves as a reversal potential of the Cl− currents through GABA-activated channels. EM was obtained in a whole-cell patch-clamp configuration. A,B: Current/voltage plots of GABA-activated Cl− channel magnitudes across imposed membrane voltages. Traces inset for +40 and −100mV at each time-point. Error bars indicate 1pa x 1ms. C: GABArev was found to decrease as a function of postnatal age from P09–10 (−24.5 ± 3.9 mV, N=10 cells, 3 rats) to P15–16 (−43.33 ± 2.79 mV, N=6 cells, 3 rats) and remained decreased in slices obtained from the offspring of Sham-treated dams at P20–21 (−48.57 ± 2.3 mV, N=7 cells, 3 rats). Conversely, GABArev remained elevated in slices obtained from the progeny of WIN- or THC-treated dams between P09–10 (−41.67 ± 4.05 mV, N=12 cells, 3 rats) and P15–16 (−40.25 ± 2.49 mV, N=10 cells, 3 rats), but decreased by P20–21 (−60.0 ± 1.82 mV, N=9 cells, 3 rats). As a result, Sidak’s multiple comparisons test following a significant Two-way ANOVA (Treatment, F1,48 = 9.267, P=0.0038) revealed a significant difference at P10 (P=0.0006). D: Em progressively decreased as a function of postnatal age in slices obtained from the progeny of Sham-treated rats. Between P09–10 and P15–16, EM decreased from −71.79 ± 0. 64 mV to −78.03 ± 0.93 mV (N=5 cells, 3 rats and 6 cells, 3 rats, respectively) and remained decreased at P20–21 (−78.31 ± 1.43 mV, 7 cells, 3 rats). Conversely, EM did not change between P09–10 and P15–16 in the offspring of WIN-treated dams (−68 ± 1.3 mV and −69.21 ± 1.25 mV, respectively; N=7 cells, 3 rats and 10 cells, 3 rats, respectively). However, at P20–21 the EM significantly decreased to −76.5 ± 0.99 mV (N=9 cells, 3 rats). As a result, Sidak’s multiple comparisons test following a significant Two-way ANOVA (Treatment, F1,39 = 23.25, P<0.0001) revealed a significant difference between EM at P15, when a decrease was found in cells of slices obtained from the offspring of Sham-, but not WIN-treated dams (P<0.0001). Error bars indicate SEM.
To interpret the influence of GABArev on action potential probability, we measured the resting membrane potential (Em) at these ages in slices of Sham- and WIN-exposed offspring (Figure 2D). Em exhibited a progressive hyperpolarization between P09–10 and P15–16 and remained consistent at P20–21 in Sham-treated animals. No change was observed WIN-exposed offspring between P09–10 and P15–16. However, at P20–21 EM decreased significantly. Thus, in addition to a retarded GABArev, hyperpolarization, the decrease of EM in WIN-exposed offspring was delayed compared to Sham-exposed pups.
KCC2 upregulation is delayed in perinatally cannabinoid-exposed pups
The potassium-chloride transporter 5 (KCC2), together with the sodium-potassium-chloride transporter (NKCC1), regulates intracellular Cl− concentrations thereby determining the ion’s flow during GABA channel opening (54). During early development, KCC2 levels increase while NKCC1 levels decline (37, 54, 67), decreasing intracellular Cl− resulting in a net Cl− influx and cell hyperpolarization. This trajectory thereby mediates GABA’s excitatory to inhibitory transition (34, 68). To determine whether the delayed GABA “switch” (Figure 1–2) was correlated with KCC2/NKCC1 expression changes, Western Blot analyses were performed on mPFC of Sham- or WIN-exposed pups at P10, P15 and P21.
We found a significant KCC2 increase between P10 and P15 in the mPFC of Sham-exposed pups which remain at P21 (Figure 4A). In support of our working hypothesis, KCC2 levels were unchanged between P10–15 in WIN-exposed pups. By P21, levels of KCC2 in WIN-exposed pups significantly increased compared to P10. Interestingly, these levels remain low at P21 compared to Sham offspring. Together, these data indicate that at P15, the lack of an apparent mPFC GABA switch in WIN-exposed pups is correlated with a failure of KCC2 upregulation.
Figure 4: Perinatal THC exposure alters the developmental trajectory of KCC2 mRNA.
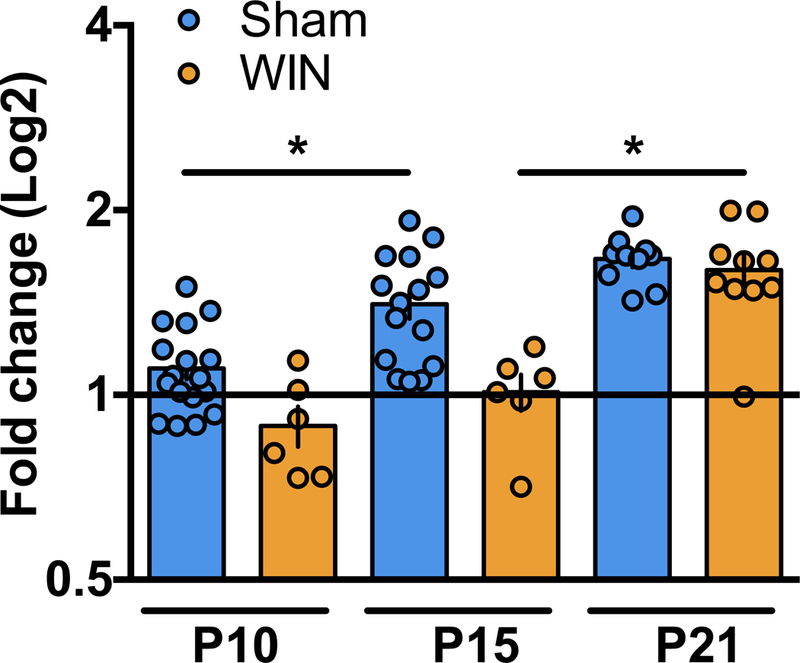
qPCR analysis of KCC2 mRNA reveal altered expression levels between P10 and P15 in progeny of dams exposed to THC during lactation as compared to progeny of Sham-treated dams. A: Levels of KCC2 mRNA are significantly increased between P10 and P15 and remain elevated at P21 in mPFC tissue collected from pups of Sham-treated dams (P10 N=17, P15 N=15, P21 N=10). However, no change in KCC2 mRNA levels was detected in mPFC tissue collected from pups of WIN-treated dams between P10 and P15 (P10 N=6, P15 N=6). At P21, levels of KCC2 mRNA in the mPFC tissue collected from the progeny of WIN-treated dams are significantly elevated compared to P15 (P21 N=10). One-way ANOVA followed by Tukey’s multiple comparisons test, F5,58 = 18.10, p<0.0001. Error bars indicate SEM. *p<0.05
Levels of NKCC1 remain unchanged in both Sham- and WIN-exposed pup mPFC at all three times (Figure 4B). Therefore, the influence of GABA on synaptic transmission appears to be dictated by the KCC2/NKCC1 ratio, in line with previous findings (34). Importantly, the NKCC1 antagonist bumetanide, a previously investigated pharmacotherapeutic treatment targeting GABAergic development in neonatal seizures (69), autism (41) and maternal separation-induced stress (70), corrected the delayed GABA switch when delivered to developing offspring (Supplementary Figure 2). These data confirm the crucial role of Cl− balance in mediating the developmentl GABA transition.
KCC2 mRNA transcriptional upregulation between P10–15 in cannabinoid-exposed pups
To gain mechanistic insight into the delayed KCC2 upregulation in cannabinoid-exposed pups, we performed qPCR on brains from WIN- or THC-exposed pups. We found a delayed developmental upregulation of KCC2 mRNA following perinatal cannabinoid exposure (Figure 5). Specifically, mPFC KCC2 mRNA increased in Sham-, but not WIN-exposed animals between P10–15. By P21, mPFC KCC2 mRNA levels were significantly elevated in WIN-exposed offspring compared to P15. By P21, no difference in mPFC KCC2 mRNA was found WIN- and Sham-exposed offspring. These results support the idea that perinatal cannabinoid exposure attenuates the transcription KCC2 trajectory.
Figure 5. Mean dominant frequency of ultrasonic vocalization is altered in pups exposed to WIN or THC at both P09 and P15.
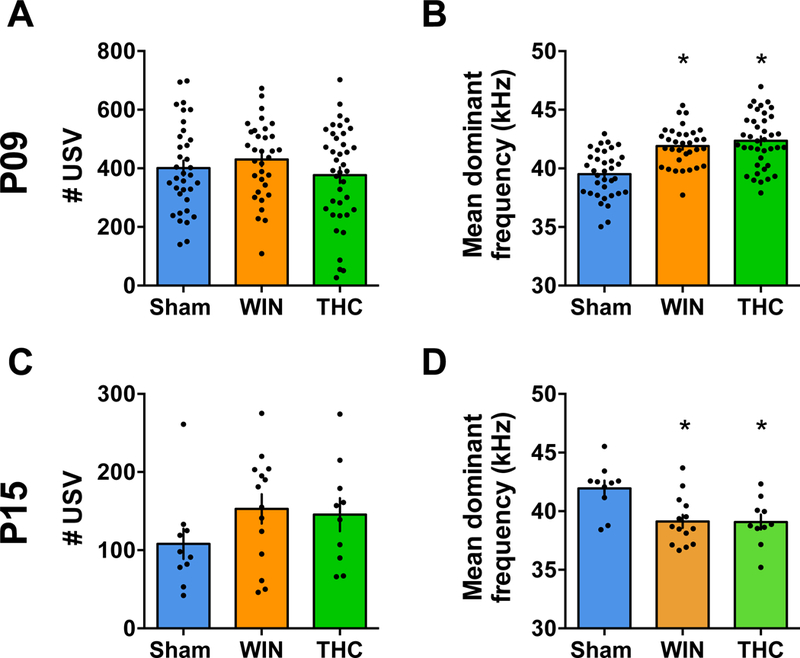
A,C: The number of USV emitted by pups from litters exposed to WIN or THC during the lactation period were not altered (P09, F2,104x = 1.101, P=0.3365; P15, F2,31 = 1.417, P=0.2576; one-way ANOVA). B,D: However, the mean dominant frequency of USV calls made by pups from both WIN- and THC-treated dams was found to be significantly different from the offspring of Sham-treated rats at P09 (F3,88 = 6.239, P=0.0007; one-way ANOVA) and at P15 (F2,31 = 6.656, P=0.0039; one-way ANOVA). P9: Sham, N=21 pups/5 litters; WIN, N=12 pups/4 litters and THC, N=19 pups/5 litters. P15, N= 10 pups/2 litters; WIN, N= 14 pups/2 litters and THC N=10 pups/2 litters). Scatter dot plot represents one animal. Error bars indicate SEM. *p<0.05.
Ultrasonic vocalizations are altered in pups perinatally exposed to WIN
Ultrasonic vocalizations (USV) are emitted by pups separated from their mothers and play an important role in mother-offspring interactions (71, 72), providing an important measure of affect, motivation, and social behavior in pathology models (71, 73, 74). As cannabinoid exposure adversely affects perinatal neurodevelopment (6, 7, 75) and altered USV emission has been associated with a delayed GABA switch (41) and perinatal THC-exposure (50), we evaluated isolation-induced USV in Sham-, WIN- or THC-exposed offspring at P09 and P15 (Figure 5).
Although no changes were observed in the number of USV, the mean dominant frequency was significantly altered (Figure 5A–B). Pups WIN- or THC-exposed pups presented altered USV mean dominant frequency compared to the Sham at both time-points. In line with our previous findings, co-administration of the CB1R antagonist AM251 prevented the alteration in mean dominant frequency at P09 (data not shown).
Discussion
Developmental consequences of perinatal cannabinoid exposure remain woefully under researched despite increasing availability of cannabis and its use during and following pregnancy. Here, we identified consequences of cannabinoid exposure in early development by treating lactating dams with either a synthetic cannabinoid (WIN) or cannabis’s main psychoactive ingredient (THC), followed by electrophysiological and biochemical assessment of GABA maturation in the mPFC. We observed a significant delay in GABA maturation associated with retarded KCC2 upregulation at both a transcriptional and translational level. We also investigated the behavioral consequences of perinatal cannabinoid exposure, as both alterations in GABA signaling and perinatal drug exposure have been associated with early life behavioral aberrations (41, 50, 76). We found a perturbation of USV calls without alterations in motor behavior.
First, our results revealed that GABA exhibits excitatory properties in the mPFC in early development before transitioning to inhibition between P10–15, as ascertained by cell-attached recordings, in line with the timing of this transition in other regions of the developing rat brain, including the hippocampus (37, 41), cerebellum (38) and neocortex (77). This maturational trajectory is mediated by a change in GABArev, ascertained by single-channel recordings of GABA-mediated Cl− currents.
The present results showed that maternal exposure to cannabinoids retards mPFC GABAergic development. WIN- or THC-exposed offspring exhibit a significant delay in the mPFC GABA “switch.” By preventing this effect with maternal co-administration of a CB1R antagonist we confirm its CBlR-mediation. This was associated with similar delays in the hyperpolarizing trajectory of both GABArev and EM, indicating that intracellular Cl− levels and the resulting Cl− reversal through GABA channels determines developing GABA polarity. Additionally, we observed a suppressed trajectory of KCC2 protein and mRNA elevation during this period through Western blot and qPCR analyses, in parallel with findings elsewhere (36). As membrane localization of KCC2 proteins regulates their Cl− balance contribution (78) and certain GABA-development-perturbing treatments such as maternal separation may alter membrane KCC2 levels (61), future experiments must determine whether KCC2 expression changes are similar in the membrane-associated portion.
This period has also been identified as a crucial time-point in mPFC GABAergic synapse innervation (45), underscoring the relevance of this trajectory with regards to GABA function. We found that the delayed “switch” was prevented by administration of the NKCC1 antagonist bumetanide, which decreases intracellular Cl− to pups. These findings parallel those of others who have treated disorders caused by a delayed GABA “shift” with bumetanide (41, 79, 80). Unfortunately, significant problems accompany in vivo use of bumetanide, including ototoxicity, preclude its use as a pharmacotherapeutic intervention strategy (81, 82). Thus, examination of bumetanide’s effects on behavioral consequences of perinatal cannabinoid exposure were unsuccessful (data not shown).
Pups from WIN-treated dams exhibited numerous developmental alterations. First, weight gain was retarded in pups from cannabinoid-treated dams (Table 4), consistent with the well-established role of eCB signaling in the milk-suckling reflex (6, 8, 83). Exposure to WIN also modified USV call structure, indicated by an increase in the calls’ mean dominant frequency at P09 and a decrease at P15. Along with changes in the number and mean frequency of USV, calls’ structure is altered with age (84, 85), reflecting an evolution from an instinctive behavior elicited by litter separation to social behavior (86). Importantly, at P09, co-administration of the CB1R antagonist AM251 prevented this delay, implicating CBlRs. CB1R activation by exogenous cannabinoids in lactating dams or their offspring during critical periods of development has been demonstrated to trigger USV alterations in progeny associated with later behavioral impairments such as reduced adolescent social interaction and play behavior as well as an anxiogenic-like profile (50). Further, an elevated cry frequency spectrum has been identified in the offspring of cannabis-using mothers (87).
Considering that altered USV may be a harbinger of cognitive impairments, we tested homing behavior in WIN-exposed pups at P10 and P13. Along with intact sensory, olfactory and motor capabilities, homing requires associative and discriminative capabilities that allow the infant rat to recognize and seek its own nest (88). No changes were observed in WIN-exposed offspring, indicating a specific behavioral impairment of altered USV structure that may impair mother- infant interactions (Supplementary Figure 3).
Importantly, while the negative impact of CB1R activation on rodents’ maternal behaviour has been demonstrated (89, 90) we observed no alterations in maternal nursing during WIN or THC administration (Table 3). As USV can be modulated by poor maternal care (86, 91, 92), our finding highlights the direct effect of WIN administration on pups’ vocalizations.
Long-term consequences of delayed GABA development are unknown. However, it has been associated with developmental disorders such as Fragile X syndrome (40), early life epilepsies (93) and autism (41, 94, 95). Additionally, there is precedence for developmental GABA alterations resulting from a maternal insult such as immune activation (60) as well as postnatal exposure to such drugs as caffeine (96). However, we present here the first data suggesting that cannabis exposure delays postnatal GABA development.
GABAergic development has diverse impacts including the regulation of newborn neuron integration and titration of glutamatergic signaling (97) and mediation of neuronal proliferation, migration and synaptogenesis (35). Developmental GABA disturbances in cortical regions also impact glutamatergic transmission, presenting as sensorimotor gating deficits associated with schizophrenia-like behavior (98). The perinatal cannabinoid-exposure induced retardation of the GABA development therefore likely impacts an array of functions in the mPFC and elsewhere, whose consequences later in life remain to be investigated.
Together, our results indicate that perinatal cannabinoid exposure via lactation delays the developmental mPFC GABA trajectory. This exhibits as a delayed GABA “switch” caused by slowed KCC2 upregulation due to suppressed mRNA levels. Furthermore, the normalization of the GABA “switch” by bumetanide treatment of pups confirms the mechanistic role of a KCC2/NKCC1 imbalance. Further analyses of both electrophysiological function and its molecular underpinnings, as well as behavioral consequences associated with this aberrant development may reveal long-term consequences of these early postnatal alterations.
Supplementary Material
Figure 3: Perinatal WIN-exposure alters the developmental trajectory of KCC2 and NKCC1 expression in the mPFC.
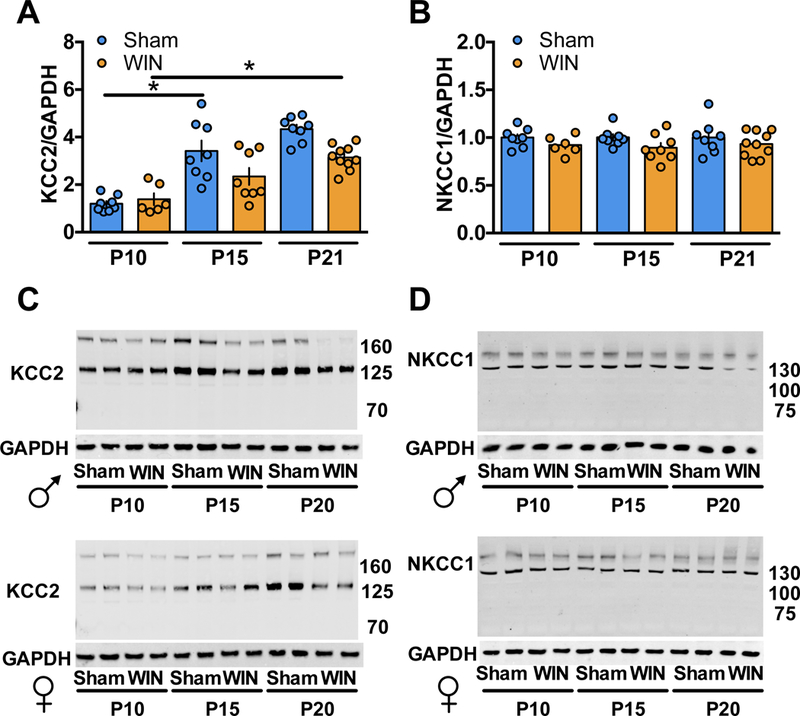
Western-blot analysis of KCC2 and NKCC1 reveal altered expression levels between P10, P15 and P21 in progeny of dams exposed to WIN during lactation as compared to progeny of Sham-treated dams. A: KCC2 levels are significantly increased between P10 and P15 and remain elevated at P21 in the mPFC tissue collected from pups of Sham-treated dams (P10 N=8, P15 N=8, P21 N=8; F5,42=19.38, P<0.0001. One-way ANOVA followed by Tukey’s multiple comparisons test. Sham P10 vs. Sham P15, P<0.0001; Sham P15 vs. Sham P21, P=0.9744). However, no change in KCC2 levels was detected in mPFC tissue collected from pups of WIN-treated dams between P10 and P15 (P10 N=6, P15 N=8). At P21, a significant increase in KCC2 was observed in mPFC tissue from WIN-treated pups as compared to P10 (P21 N=10; P=0.0009, Tukey’s multiple comparisons test). B: No difference in NKCC1 levels was detected in mPFC tissue collected from pups of Sham- or WIN-treated dams at any of the tested time points (Sham P10 N=8, P15 N=8, P21 N=8; WIN P10 N=6, P15 N=8, P21 N=10). One-way ANOVA followed by Tukey’s multiple comparisons test, F5,42 = 1.108, p=0.3707. Error bars indicate SEM. *p<0.05. C,D: Representative Western-blots of KCC2/GAPDH and NKCC1/GAPDH, corresponding to a,b respectively.
Acknowledgments:
The authors are grateful to Dr. C. Pellegrino for his expertise and performing the first series of western blot experiments, Dr. R. Tyzio for his expertise with the GABA single channels recordings, Dr. P. Chavis, Dr. J.L. Gaiarsa and the Chavis-Manzoni team members for helpful discussions and CNPq Brazil for MB support (process 200843/2015–0).
Funding: This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM); the INSERM-NIH exchange program (to A.F.S.); Agence National de la Recherche (ANR « Cannado » » to A.L.P.); Fondation pour la Recherche Médicale (Equipe FRM 2015 to O.M. and M.B.) and the NIH (R01DA043982 to O.M. and K.M. and DA021696 to K.M.).
Footnotes
Declarations of interest: The authors report no biomedical financial interests or potential conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- 1.United Nations Office on Drugs and Crime (2017): World Drug Report 2017. .
- 2.Araque A, Castillo PE, Manzoni OJ, Tonini R (2017): Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology, A New Dawn in Cannabinoid Neurobiology. 124: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fergusson DM, Horwood LJ, Northstone K, ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood (2002): Maternal use of cannabis and pregnancy outcome. BJOG. 109: 21–27. [DOI] [PubMed] [Google Scholar]
- 4.Metz TD, Stickrath EH (2015): Marijuana use in pregnancy and lactation: a review of the evidence. American Journal of Obstetrics and Gynecology. 213: 761–778. [DOI] [PubMed] [Google Scholar]
- 5.Jarlenski M, Tarr JA, Holland CL, Farrell D, Chang JC (2016): Pregnant women’s access to information about perinatal marijuana use: A qualitative study. Womens Health Issues. 26: 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fride E (2004): The endocannabinoid-CB1 receptor system in pre- and postnatal life. European Journal of Pharmacology, SPECIAL CELEBRATORY VOLUME 500 Dedicated to Professor David de Wied Honorary and Founding Editor 500: 289–297. [DOI] [PubMed] [Google Scholar]
- 7.Trezza V, Campolongo P, Manduca A, Morena M, Palmery M, Vanderschuren LJMJ, Cuomo V (2012): Altering endocannabinoid neurotransmission at critical developmental ages: impact on rodent emotionality and cognitive performance. Front Behav Neurosci. 6. doi: 10.3389/fnbeh.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fride E (2002): Endocannabinoids in the central nervous system--an overview. Prostaglandins Leukot Essent Fatty Acids. 66: 221–233. [DOI] [PubMed] [Google Scholar]
- 9.Mato S, Del Olmo E, Pazos A (2003): Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience. 17: 1747–1754. [DOI] [PubMed] [Google Scholar]
- 10.Mulder J, Aguado T, Keimpema E, Barabás K, Ballester Rosado CJ, Nguyen L, et al. (2008): Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc Natl Acad Sci U S A. 105: 8760–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker T, Datta P, Rewers-Felkins K, Thompson H, Kallem RR, Hale TW (2018): Transfer of Inhaled Cannabis Into Human Breast Milk. Obstet Gynecol. 131: 783–788. [DOI] [PubMed] [Google Scholar]
- 12.Marchei E, Escuder D, Pallas CR, Garcia-Algar O, Gómez A, Friguls B, et al. (2011): Simultaneous analysis of frequently used licit and illicit psychoactive drugs in breast milk by liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 55: 309–316. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Reyes M, Wall ME (1982): Presence of delta9-tetrahydrocannabinol in human milk. N Engl J Med. 307: 819–820. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad GR, Ahmad N (1990): Passive consumption of marijuana through milk: a low level chronic exposure to delta-9-tetrahydrocannabinol(THC). J Toxicol Clin Toxicol. 28: 255–260. [DOI] [PubMed] [Google Scholar]
- 15.Jakubovic A, Hattori T, McGeer PL (1973): Radioactivity in suckled rats after giving 14 C-tetrahydrocannabinol to the mother. Eur J Pharmacol. 22: 221–223. [DOI] [PubMed] [Google Scholar]
- 16.Jakubovic A, Tait RM, McGeer PL (1974): Excretion of THC and its metabolites in ewes’ milk. Toxicol Appl Pharmacol. 28: 38–43. [DOI] [PubMed] [Google Scholar]
- 17.Astley SJ, Little RE (1990): Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol. 12: 161–168. [DOI] [PubMed] [Google Scholar]
- 18.Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, Ehiri JE (2016): Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 6. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson KA, Hester AK, McLemore GL (2016): Prenatal cannabis exposure - The “first hit” to the endocannabinoid system Neurotoxicology and Teratology, SI: Developmental Marijuana; 58: 5–14. [DOI] [PubMed] [Google Scholar]
- 20.Bara A, Manduca A, Bernabeu A, Borsoi M, Serviado M, Lassalle O, et al. (2018): Sex-dependent effects of in utero cannabinoid exposure on cortical function. Elife. 7. doi: 10.7554/eLife.36234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V (2011): Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology. 214: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dow-Edwards D, Silva L (2017): Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res. 1654: 157–164. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein RZ, Volkow ND (2011): Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12: 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheyer AF, Martin HGS, Manzoni OJ (2017): The Endocannabinoid System in Prefrontal Synaptopathies In: Melis M, editor. Endocannabinoids and Lipid Mediators in Brain Functions. Cham: Springer International Publishing, pp 171–210. [Google Scholar]
- 25.Schubert D, Martens GJM, Kolk SM (2015): Molecular underpinnings of prefrontal cortex development in rodents provide insights into the etiology of neurodevelopmental disorders. Mol Psychiatry. 20: 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Euston DR, Gruber AJ, McNaughton BL (2012): The role of medial prefrontal cortex in memory and decision making. Neuron. 76: 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman-Rakic PS (1991): Chapter 16 Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates In: Uylings HBM, Van Eden CG, De Bruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research, The Prefrontal Its Structure, Function and Cortex Pathology. (Vol. 85), Elsevier, pp 325–336. [DOI] [PubMed] [Google Scholar]
- 28.Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ (2007): Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2: e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, et al. (2007): Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 316: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 30.Fortin DA, Levine ES (2007): Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 17: 163–174. [DOI] [PubMed] [Google Scholar]
- 31.Heng L, Beverley JA, Steiner H, Tseng KY (2011): Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 65: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berghuis P, Dobszay MB, Wang X, Spano S, Ledda F, Sousa KM, et al. (2005): Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 102: 19115–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Gil L, De Miguel R, Romero J, Perez A, Ramos JA, Fernández-Ruiz JJ (1999): Perinatal Δ9-Tetrahydrocannabinol Exposure Augmented the Magnitude of Motor Inhibition Caused by GABAB, but not GABAA, Receptor Agonists in Adult Rats. Neurotoxicology and Teratology. 21: 277–283. [DOI] [PubMed] [Google Scholar]
- 34.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014): Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 15: 637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Ari Y (2002): Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 3: 728. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Ari Y, Gaiarsa J, Tyzio R, Khazipov R (2007): GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 87: 1215–1284. [DOI] [PubMed] [Google Scholar]
- 37.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. (1999): The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 397: 251. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ari Y (2008): Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends in Neurosciences. 31: 626–636. [DOI] [PubMed] [Google Scholar]
- 39.Garber K (2007): Autism’s Cause May Reside in Abnormalities at the Synapse. Science. 317: 190–191. [DOI] [PubMed] [Google Scholar]
- 40.He Q, Nomura T, Xu J, Contractor A (2014): The Developmental Switch in GABA Polarity Is Delayed in Fragile X Mice. J Neurosci. 34: 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hübner CA, Represa A, et al. (2006): Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 314: 1788–1792. [DOI] [PubMed] [Google Scholar]
- 42.Zoghbi HY (2003): Postnatal Neurodevelopmental Disorders: Meeting at the Synapse? Science. 302: 826–830. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe M, Fukuda A (2015): Development and regulation of chloride homeostasis in the central nervous system. Front Cell Neurosci. 9: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouamrane L, Scheyer AF, Lassalle O, Iafrati J, Thomazeau A, Chavis P (2016): Reelin-Haploinsufficiency Disrupts the Developmental Trajectory of the E/I Balance in the Prefrontal Cortex. Front Cell Neurosci. 10: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virtanen MA, Lacoh CM, Fiumelli H, Kosel M, Tyagarajan S, de Roo M, Vutskits L (2018): Development of inhibitory synaptic inputs on layer 2/3 pyramidal neurons in the rat medial prefrontal cortex. Brain Struct Funct . doi: 10.1007/s00429-017-1602-0. [DOI] [PubMed] [Google Scholar]
- 46.Schneider M (2009): Cannabis use in pregnancy and early life and its consequences: animal models. Eur Arch Psychiatry Clin Neurosci. 259: 383–393. [DOI] [PubMed] [Google Scholar]
- 47.Realini N, Rubino T, Parolaro D (2009): Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 60: 132–138. [DOI] [PubMed] [Google Scholar]
- 48.Serafini R, Valeyev AY, Barker JL, Poulter MO (1995): Depolarizing GABA-activated Cl− channels in embryonic rat spinal and olfactory bulb cells. J Physiol. 488: 371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR (2005): Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 35: 67–72. [DOI] [PubMed] [Google Scholar]
- 50.Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratù MR, et al. (2008): Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl). 198: 529–537. [DOI] [PubMed] [Google Scholar]
- 51.Willey AR, Varlinskaya EI, Spear LP (2009): Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 202: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sokoloff G, Blumberg MS (1997): Thermogenic, respiratory, and ultrasonic responses of week-old rats across the transition from moderate to extreme cold exposure. Dev Psychobiol. 30: 181–194. [DOI] [PubMed] [Google Scholar]
- 53.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN (2008): Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 1: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009): Cation-chloride cotransporters and neuronal function. Neuron. 61: 820–838. [DOI] [PubMed] [Google Scholar]
- 55.Payne JA, Rivera C, Voipio J, Kaila K (2003): Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26: 199–206. [DOI] [PubMed] [Google Scholar]
- 56.Lagostena L, Rosato-Siri M, D’Onofrio M, Brandi R, Arisi I, Capsoni S, et al. (2010): In the adult hippocampus, chronic nerve growth factor deprivation shifts GABAergic signaling from the hyperpolarizing to the depolarizing direction. J Neurosci. 30: 885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan H (2005): Altering cannabinoid signaling during development disrupts neuronal activity. Proc Natl Acad Sci U S A. 102: 9388–9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang X, Kim J, Zhou L, Wengert E, Zhang L, Wu Z, et al. (2016): KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci U S A. 113: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amin H, Marinaro F, De Pietri Tonelli D, Berdondini L (2017): Developmental excitatory-to-inhibitory GABA-polarity switch is disrupted in 22q11.2 deletion syndrome: a potential target for clinical therapeutics. Sci Rep. 7. doi: 10.1038/s41598-017-15793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corradini I, Focchi E, Rasile M, Morini R, Desiato G, Tomasoni R, et al. (2017): Maternal Immune Activation Delays Excitatory-to-Inhibitory Gamma-Aminobutyric Acid Switch in Offspring. Biol Psychiatry . doi: 10.1016/j.biopsych.2017.09.030. [DOI] [PubMed] [Google Scholar]
- 61.Furukawa M, Tsukahara T, Tomita K, Iwai H, Sonomura T, Miyawaki S, Sato T (2017): Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys Res Commun. 493: 1243–1249. [DOI] [PubMed] [Google Scholar]
- 62.Owens DF, Boyce LH, Davis MBE, Kriegstein AR (1996): Excitatory GABA Responses in Embryonic and Neonatal Cortical Slices Demonstrated by Gramicidin Perforated-Patch Recordings and Calcium Imaging. J Neurosci. 16: 6414–6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tyzio R, Holmes GL, Ben-Ari Y, Khazipov R (2007): Timing of the Developmental Switch in GABAA Mediated Signaling from Excitation to Inhibition in CA3 Rat Hippocampus Using Gramicidin Perforated Patch and Extracellular Recordings. Epilepsia. 48: 96–105. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Ari Y (2002): Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 3: 728. [DOI] [PubMed] [Google Scholar]
- 65.Dzhala VI, Kuchibhotla KV, Glykys JC, Kahle KT, Swiercz WB, Feng G, et al. (2010): Progressive NKCC1-Dependent Neuronal Chloride Accumulation during Neonatal Seizures. J Neurosci. 30: 11745–11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nardou R, Ben-Ari Y, Khalilov I (2009): Bumetanide, an NKCC1 Antagonist, Does Not Prevent Formation of Epileptogenic Focus but Blocks Epileptic Focus Seizures in Immature Rat Hippocampus. Journal of Neurophysiology. 101: 2878–2888. [DOI] [PubMed] [Google Scholar]
- 67.Hübner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ (2001): Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 30: 515–524. [DOI] [PubMed] [Google Scholar]
- 68.Valeeva G, Valiullina F, Khazipov R (2013): Excitatory actions of GABA in the intact neonatal rodent hippocampus in vitro. Front Cell Neurosci. 7. doi: 10.3389/fncel.2013.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Löscher W, Puskarjov M, Kaila K (2013): Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments. Neuropharmacology. 69: 62–74. [DOI] [PubMed] [Google Scholar]
- 70.Hu D, Yu Z-L, Zhang Y, Han Y, Zhang W, Lu L, Shi J (2017): Bumetanide treatment during early development rescues maternal separation-induced susceptibility to stress. Sci Rep. 7: 11878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Branchi I, Santucci D, Alleva E (2006): Analysis of ultrasonic vocalizations emitted by infant rodents. Curr Protoc Toxicol. Chapter 13: Unit13.12. [DOI] [PubMed] [Google Scholar]
- 72.Simola N, Granon S (2018): Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology. . doi: 10.1016/j.neuropharm.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 73.Branchi I, Santucci D, Alleva E (2001): Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 125: 49–56. [DOI] [PubMed] [Google Scholar]
- 74.Wöhr M, Silverman JL, Scattoni ML, Turner SM, Harris MJ, Saxena R, Crawley JN (2013): Developmental delays and reduced pup ultrasonic vocalizations but normal sociability in mice lacking the postsynaptic cell adhesion protein neuroligin2. Behav Brain Res. 251: 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K (2007): The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 28: 83–92. [DOI] [PubMed] [Google Scholar]
- 76.Kabir ZD, Kennedy B, Katzman A, Lahvis GP, Kosofsky BE (2014): Effects of prenatal cocaine exposure on social development in mice. Dev Neurosci. 36: 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luhmann HJ, Prince DA (1991): Postnatal maturation of the GABAergic system in rat neocortex. J Neurophysiol. 65: 247–263. [DOI] [PubMed] [Google Scholar]
- 78.Mahadevan V, Pressey JC, Acton BA, Uvarov P, Huang MY, Chevrier J, et al. (2014): Kainate Receptors Coexist in a Functional Complex with KCC2 and Regulate Chloride Homeostasis in Hippocampal Neurons. Cell Rep. 7: 1762–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marguet SL, Le-Schulte VTQ, Merseburg A, Neu A, Eichler R, Jakovcevski I, et al. (2015): Treatment during a vulnerable developmental period rescues a genetic epilepsy. Nature Medicine. 21: 1436–1444. [DOI] [PubMed] [Google Scholar]
- 80.Reid KH, Guo SZ, Iyer VG (2000): Agents which block potassium-chloride cotransport prevent sound-triggered seizures in post-ischemic audiogenic seizure-prone rats. Brain Res. 864: 134–137. [DOI] [PubMed] [Google Scholar]
- 81.Ben-Ari Y, Damier P, Lemonnier E (2016): Failure of the Nemo Trial: Bumetanide Is a Promising Agent to Treat Many Brain Disorders but Not Newborn Seizures. Front Cell Neurosci. 10. doi: 10.3389/fncel.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delpire E, Lu J, England R, Dull C, Thorne T (1999): Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet. 22: 192–195. [DOI] [PubMed] [Google Scholar]
- 83.Fride E, Gobshtis N, Dahan H, Weller A, Giuffrida A, Ben-Shabat S (2009): Chapter 6 The Endocannabinoid System During Development: Emphasis on Perinatal Events and Delayed Effects Vitamins & Hormones, Vitamins and Hormones. (Vol. 81), Academic Press, pp 139–158. [DOI] [PubMed] [Google Scholar]
- 84.Brudzynski SM (2005): Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 35: 85–92. [DOI] [PubMed] [Google Scholar]
- 85.Brudzynski SM, Kehoe P, Callahan M (1999): Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 34: 195–204. [DOI] [PubMed] [Google Scholar]
- 86.Boulanger-Bertolus J, Rincón-Cortés M, Sullivan RM, Mouly A-M (2017): Understanding pup affective state through ethologically significant ultrasonic vocalization frequency. Sci Rep. 7. doi: 10.1038/s41598-017-13518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lester BM, Dreher M (1989): Effects of marijuana use during pregnancy on newborn cry. Child Dev. 60: 765–771. [PubMed] [Google Scholar]
- 88.Bignami G (1996): Economical test methods for developmental neurobehavioral toxicity. Environ Health Perspect. 104 Suppl 2: 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schechter M, Pinhasov A, Weller A, Fride E (2012): Blocking the postpartum mouse dam’s CB1 receptors impairs maternal behavior as well as offspring development and their adult social-emotional behavior. Behav Brain Res. 226: 481–492. [DOI] [PubMed] [Google Scholar]
- 90.Schechter M, Weller A, Pittel Z, Gross M, Zimmer A, Pinhasov A (2013): Endocannabinoid receptor deficiency affects maternal care and alters the dam’s hippocampal oxytocin receptor and brain-derived neurotrophic factor expression. J Neuroendocrinol. 25: 898–909. [DOI] [PubMed] [Google Scholar]
- 91.Panksepp J, Burgdorf J, Beinfeld MC, Kroes RA, Moskal JR (2004): Regional brain cholecystokinin changes as a function of friendly and aggressive social interactions in rats. Brain Res. 1025: 75–84. [DOI] [PubMed] [Google Scholar]
- 92.Pryce CR, Bettschen D, Feldon J (2001): Comparison of the effects of early handling and early deprivation on maternal care in the rat. DevPsychobiol. 38: 239–251. [DOI] [PubMed] [Google Scholar]
- 93.Briggs SW, Galanopoulou AS (2011): Altered GABA Signaling in Early Life Epilepsies. Neural Plasticity. Research article. doi: 10.1155/2011/527605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cellot G, Cherubini E (2014): GABAergic Signaling as Therapeutic Target for Autism Spectrum Disorders. FrontPediatr. 2. doi: 10.3389/fped.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sgadò P, Dunleavy M, Genovesi S, Provenzano G, Bozzi Y (2011): The role of GABAergic system in neurodevelopmental disorders: a focus on autism and epilepsy. Int J Physiol Pathophysiol Pharmacol. 3: 223–235. [PMC free article] [PubMed] [Google Scholar]
- 96.Silva CG, Métin C, Fazeli W, Machado NJ, Darmopil S, Launay P-S, et al. (2013): Adenosine receptor antagonists including caffeine alter fetal brain development in mice. Sci Transl Med. 5: 197ra104. [DOI] [PubMed] [Google Scholar]
- 97.Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G, Song H (2006): GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 439: 589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang DD, Kriegstein AR (2011): Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb Cortex. 21: 574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


