Abstract
Recombinant Immunotoxins (RITs) are chimeric proteins containing an Fv that binds to tumor cells, fused to a fragment of Pseudomonas exotoxin (PE) that kills the cell. Their efficacy is limited by their short half-life in the circulation. Chemical modification with polyethylene glycol (PEG) is a well-established method to extend the half-lives of biologics. Our goal was to engineer RITs with an increase in half-life and high cytotoxic activity. We introduced single cysteines at different locations in five anti-mesothelin RITs and employed site-specific PEGylation to conjugate them to 20kD PEG. Because our previous PEGylation method using β-mercapto-ethanol reduction, gave poor yields of PEG-modified protein, we employed a new method using TCEP to reduce the protein, and could PEGylate RITs at ~90% efficiency. The new proteins retained 19-65% of cytotoxic activity. Although all proteins are modified with the same PEG, the radius of hydration varies from 5.2 to 7.1 showing PEG location has a large effect on protein shape. The RIT with the smallest radius of hydration has the highest cytotoxic activity. The PEGylated RITs have a 10-30-fold increase in half-life which is related to the increase in hydrodynamic size. Biodistribution experiments indicate that the long half-life is due to delayed uptake by the kidney. Anti-tumor experiments show that several PEG-RITs are much more active than unmodified RIT and the PEG location greatly affects anti-tumor activity. We conclude that PEGylation is a useful approach to improve the half-life and anti-tumor activity of RITs.
Keywords: Mesothelin, mesothelioma, cancer therapy, immunotherapy, pegylation
Introduction
Small therapeutic proteins have limited efficacy because they are rapidly removed from circulation (1). Two well-known mechanisms of removal are renal filtration and hepatic elimination. Increasing the hydrodynamic volume is a widely adopted strategy to increase half-life. Other strategies include fusion with albumin, albumin binding domain, Fc domain of an antibody, or N-terminal glycosylation (1). Most of these strategies take advantage of the naturally occurring FcRn-mediated, pH-dependent recycling of IgG and albumin that is responsible for their exceptionally long half-life.
RITs are small chimeric proteins consisting of the Fv portion of an antibody that serves as the targeting moiety, fused to a bacterial toxin that kills the target cells (2). Our lab studies RITs that target mesothelin, a cell-surface glycoprotein that is robustly expressed in many common solid tumors (3). The bacterial toxin is Pseudomonas exotoxin A (PE). To make RITs domain I, the cell-targeting domain of PE is replaced by an anti-mesothelin Fv or Fab and unnecessary portions of domain II are deleted. The size of the RITs ranges from 50 to 72kD, and they have half-lives of 10 to 20 minutes in mice. Recently we reported that the addition of an albumin binding domain to a RIT targeting mesothelin greatly increased half-life and anti-tumor activity (4). This finding encouraged us to explore other approaches to increase half-life that do not require the addition of a foreign protein domain, which could increase immunogenicity.
PEGylation is an approach in which polyethylene glycol (PEG) is conjugated to a macromolecule. It is a well-established method for half-life extension and does this by increasing the hydrodynamic volume and reducing renal filtration (5). Currently, there are 14 FDA approved PEGylated drugs on the market and 20 more are in clinical trials. They range from proteins to liposomes, indicating the versatile nature of PEGylation (6). Our initial effort at PEGylation was to modify human transforming growth factor-α fused to the 38kD PE38; this resulted in a protein with enhanced plasma half-life and anti-tumor activity; however, the pegylation was lysine-specific that resulted in heterogenous populations of PEG-modified immunotoxin because there are multiple lysines presented (7). We subsequently showed that PEGylation of the Fv portion of LMB-2, a RIT composed of an Fv that binds to CD25 fused to the PE38 also increased half-life, reduced toxicity and immunogenicity (8). The drawback of this study was that the method involved reduction of the protein with β-mercaptoethanol resulting in variable and poor yields and purity. Therefore, we could only modify a single position in the immunotoxin. Our goal in this study was to make multiple PEG-modified immunotoxins, each with a single PEG site-specifically conjugated at a unique position in order to find a site that retains high cytotoxic activity, a long half-life in the circulation and high anti-tumor activity. In order to do this, we needed to develop a new method of PEGylation using TCEP to reduce the protein (9).
The mechanism by which RITs kill mesothelin expressing cancer cells consists of several steps. First the RIT binds to the cell through recognition of mesothelin, followed by internalization through endocytosis. Then the toxin is cleaved from the Fv by the furin protease and undergoes retrograde transport through the Golgi to the endoplasmic reticulum (ER). Next the toxin is released into the cytosol where it ADP ribosylates and inactivates elongation factor 2 (EF2); this halts protein synthesis causing cell death (3). Given the complex network of interactions, it is important to identify positions on the RIT where PEG can be placed that do not decrease the activity of the RIT by sterically hindering mesothelin binding, furin cleavage, retrograde transport to the ER, transfer to the cytosol from the ER and ADP-ribosylation of EF2 (8).
To overcome these challenges, we adopted approaches that would give us the best chance to produce active protein: First we used a single-chain Fv as the targeting moiety, because it only contains buried disulfide bonds, which cannot be PEGylated. Second, we exploited structural modeling of the mesothelin-RIT-EF2 complex to locate cysteines at positions distant from the mesothelin and EF2-binding sites. Third, we placed cysteines on spatially and functionally distinct domains. These were located on the Fv, next to the furin cleavage site, and on domain III of the toxin. Fourth, we employed the highly efficient maleimide-based site-specific conjugation to prevent random PEGylation.
Materials and Methods
Materials:
methoxy-PEG-maleimide (molecular weight 20 kD) was obtained from JenKem Technology. Other materials were obtained from standard sources.
Bacterial Strains and Plasmids:
Escherichia coli DH5α (High Efficiency) was obtained from New England Biolabs for the propagation of plasmids. E. coli BL21(λDE3), which carries T7 RNA polymerase gene under the control of an inducible promoter on a λ prophage, was used as a host to express RIT. Plasmids that express RIT are under the control of T7 promoter and contain a single-chain Fv that is genetically fused to a 24kD bacterial toxin PE by a flexible GS linker and a furin cleavage site.
Construction, Expression, and Purification of RIT:
Double-stranded gene fragment (gBlock, Integrated DNA Technologies) that contains the designated cysteine was ligated onto our standard laboratory RIT production vector as described before (10) using Gibson Assembly Master Mix (New England Biolabs) according to the manufacturer’s protocol. The correct assembly of plasmids was confirmed by cutting with the appropriate restriction enzymes and sequencing analysis. The plasmid was transformed into E. coli. BL21(λDE3) and RIT was induced with 1mM IPTG at OD.2.5 for 2 hours. All RITs were expressed and purified as inclusion body according to the protocol described before (11). Briefly, inclusion body was dissolved in GTE buffer (6M guanidine-HCl, 100mM Tris-HCl, 2mM EDTA) for 19 hours, followed by refolding in 1000ml 100mM Tris-HCl, 1mM EDTA, 0.5M arginine and 0.5M NDSB-201, pH 10.0 for 31 hours and dialysis against 50 liters of 30mM Tris-HCl, 0.1M urea for 19 hours. The refolded RITs were purified through Q-sepharose and MonoQ ion exchange columns (GE Healthcare)(10).
PEGylation of RIT:
RITs were PEGylated in collaboration with Selecta Bioscience. 4mg RIT was buffer exchanged into 20mM potassium phosphate, pH 8.0, 2mM EDTA using Vivaspin 20 (5kD MWCO, GE healthcare) until the pH of the flow through matches that of the buffer. A 3-molar excess of tris(2-carboxyethyl)phosphine (TCEP) was added to the final 2mg/ml RIT and incubated at room temperature for 1-hour to reduce cysteine. Then a 10-molar excess of methoxy-PEG maleimide was added and left to react for overnight. For purification the PEGylated RIT was buffer exchanged into 10mM Tris-HCl, pH 8.0 using a Vivaspin column and applied to an anion exchange spin column (Pierce). Unreactive methoxy-PEG maleimide was washed away with four 10 ml washes of the same buffer and the RIT was eluted with 1XPBS. Samples collected before and after TCEP treatment, and after the final elution were analyzed on SDS-PAGE gels. For high-performance liquid chromatography (HPLC) analysis, additional samples were collected during column washes to make sure methoxy-PEG maleimide was completely gone.
HPLC Analysis:
Reverse phase HPLC was performed to separate PEGylated RIT from the parental RIT and unreacted PEG. The column was purchased from Water Corporation and features a non-polar C4 stationary phase with 3.5μm particle size and 2.1mm I.D. Mobile phases A and B contain 5% and 90% acetonitrile, respectively and are supplemented with 0.1% trifluoroacetic acid. 5μl of ~1mg/ml samples were injected into the gradient run, starting from 95% buffer A to 100% buffer B in 10min.
Mesothelin Binding:
A 96-well microtiter plate was coated with 50ul of 1μg/ml MSLN-hFc, a fusion protein consisting of human IgG Fc and mesothelin protein and incubated at 4°C overnight. The plate was blocked with blocking buffer (1x PBS supplemented with 25% DMEM, 25mM HEPES, 0.5% BSA, 0.1% Azide, 5% FBS) and 50μl serial dilution of the PEGylated RITs was added in triplicate and 50μl monoclonal anti-PE antibody (IP12) and HRP-labeled goat anti-mouse IgG were added as a primary and secondary antibodies (4). After washing with blocking buffer, the plate was developed with TMB substrate kits (Thermos Scientific) and subsequently read with the plate reader at 640 and 450nm wavelength. Binding curve was generated using GraphPad and 50% binding (IC50) was calculated. Table 1 summarizes average IC50 values of two experiments.
Table 1.
Summary of properties of RITs
| RIT names | IC50 (ng/ml) in KLM 1 |
Half Max Mesothelin Binding |
Half Life (min) | AUC | Rh | ||
|---|---|---|---|---|---|---|---|
| without PEG | with PEG (% Activity) |
(μg/ml) | Fast (α) | Slow (β) | (arbitrary unit, x104) |
(nm) | |
| SS1P | 0.19 | n/a | nt | 19 | n/a | nt | nt |
| LMB-12 | 0.22 | n/a | 0.022 ± 0.001 | 11 | n/a | 0.2 | 3.4 |
| LMB-84 | 0.19 | n/a | nt | nt | n/a | nt | nt |
| LMB-249 | 0.22 ± 0.06 | 1.18 ± 0.03(18%) | 0.027 ± 0.001 | 144 | 74400 | 5.9 | 7.1 |
| LMB-203 | 0.29 ± 0.02 | 1.10 ± 0.19 (26%) | 0.024 ± 0.004 | 59 | 51600 | 5.1 | 6.9 |
| LMB-163 | 0.22 ± 0.03 | 1.13 ± 0.19 (19%) | 0.029 ± 0.006 | 29 | 62284 | 4.3 | 5.6 |
| LMB-179 | 0.21 ± 0.01 | 1.09 ± 0.13 (19%) | 0.023 ± 0.002 | 38 | 26600 | 3.9 | 5.9 |
| LMB-244 | 0.16 ± 0.03 | 0.25 ± 0.05 (64%) | 0.013 ± 0.001 | 25 | 256 | 2.2 | 5.2 |
nt: not tested; n/a: not applicable
Bolded numbers refer to PEGylated RITs
ADP-ribosylation:
A 20μl reaction was set up to contain 4μl buffer (20mM Tris-HCl, pH 7.5, 1mM EDTA), 1ul 1M DTT, 1ul 20ng/ul RIT, 5μg protein lysate (prepared from KLM1 cell) and 1μl 250uM Biotin-NAD (Trevigen) and incubated at room temperature for 1 hour. Samples were analyzed in SDS-PAGE gel and western blot was performed. The PVDF membrane was probed with HRP-streptavidin followed by ECL development to visualize ADP-ribosylated EF2.
Cytotoxicity Assay:
Human cell lines KLM1 (provided by Dr. U. Rudloff, National Cancer Institute; 12), L55 (provided by Dr. T. Yamori, Pharmaceuticals and Medical Device Agency; 13) and A431/H9 (A431 human carcinoma cells transfected with mesothelin cDNA; 14) were described previously and confirmed by short tandem repeat (STR) testing. MTBM test results were all negative. Cells were grown in RPMI media supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C until 70-78% confluency before they were trypsinized. Then 4000 cells were seeded onto each well in a 96-well plate, and serial dilutions of RIT were added in triplicate rows and incubated for 72 hours. Cytotoxicity activity was evaluated with a WST8 assay according to the manufacturer’s protocol (Dojindo Molecular Technologies). Plates were read at 640 and 450nm wavelength, and data were plotted using GraphPad to obtain IC50 values.
Half-Life Assay:
For each protein two mice (6-8 weeks old, 23-25g) were I.V. injected with 25μg RIT. Blood was harvested at 5min, 2, 4, 8 and 24 hours for LMB-203-PEG, LMB-244-PEG, and LMB-249-PEG and 5min, 1, 4, 24 hours for LMB-163-PEG and LMB-179-PEG afterward, and serum was isolated. ELISA was used to determine the amount of RIT at each time point as described in Mesothelin Binding experiment. Half-life and AUC was calculated using GraphPad, with two-phase decay fitting, except LMB-12 which was fitted with one-phase decay. The 5min sample was used as the initial amount of RIT in the blood, and the later time points were normalized against this value.
Radius of hydration (Rh):
The Rh of five PEGylated proteins was measured using Dynamic Light Scattering (Wyatt Technology). Proteins in the range of 0.5-1mg/ml were spun down at 13,000rpm for 5 min to pellet aggregates and 20μl was transferred to a quartz cuvette slowly to avoid bubbles. Each sample was measured at least two times, and each time constitutes an average of 30 measurements. Rh values were extracted and plotted against the half-life, cytotoxicity, and anti-tumor activity using GraphPad. Linear regression analysis was performed to obtain a line of best-fit and R2 values.
Biodistribution of RIT:
5.8nmol of LMB-249-PEG and 4.0nmol of SS1P were incubated with 23.1nmol and 15.9 nmol of FNIR-Z-759 in PBS buffer, pH 8.5, at room temperature for 1 hour, respectively. The labeled protein was purified with Sephadex G25 column. 3 mice bearing ~100mm3 A431/H9 tumor were I.V. injected with 41μg (580pmol) of labeled LMB-249-PEG or 25μg (400pmol) of labeled SS1P, followed by serial dorsal and ventral 800-nm fluorescence imaging immediately and 15, 30min, 1, 2, 3, 4, 5, 6, 9, 12, 24, 48hours afterward. Fluorescence accumulation in the kidney, liver, and tumor was monitored. Background fluorescence was also calculated to obtain the target-to-background ratio. Plots were generated using GraphPad from average results of 3 mice.
Anti-tumor Experiment:
All animal experiments were performed in accordance with NIH guidelines and approved by the NCI Animal Care and Use Committee. Female (10 per group) nu/nu mice (6-week-old, 20-25g) were injected s.c. in the flank with 5 x 106 KLM1 cells (12). When the tumor reached 100mm3, mice were grouped into similar weight and tumor size and RIT in PBS supplemented with 0.2% HSA was I.V. injected at 10μg/mouse three times per week for one week. Tumor volume and mice weight were monitored every two to three days. Mice were euthanized if they lost more than 10% of the body weight. The experiment was repeated for LMB-244-PEG and LMB-163-PEG (n = 10) and injected at 20μg/mouse, two times per week for two weeks. Mouse weight was monitored every two to three days and were euthanized if they lost more than 10% of the body weight.
Results
Design of RITs
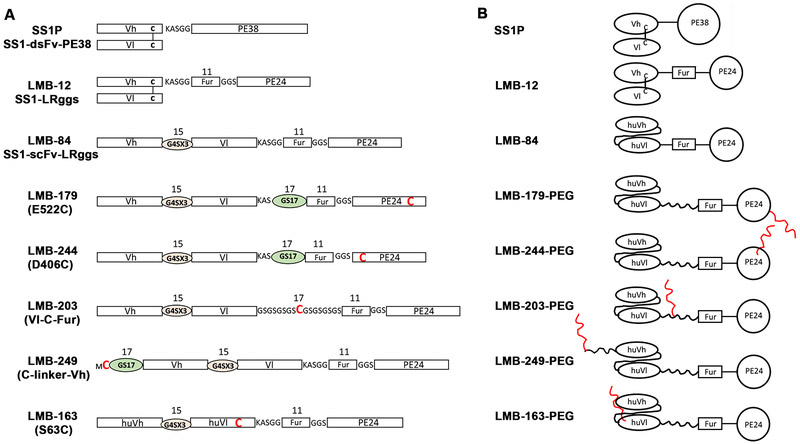
The RITs used in this study are shown in Figure 1A. SS1P is the first immunotoxin made to target mesothelin and contains an anti-mesothelin Fv attached to PE38 that contains domains II and III of PE (15). LMB-12 and LMB-84 both have domain II deleted and contain an 11-amino acids furin cleavage site that connects the Fv to domain III (PE24). In LMB-12, the Vl and Vh are connected by a disulfide bond and in LMB-84 they are connected by a flexible 15-residues (G4S)3 linker. LMB-179, LMB-244, LMB-203, LMB-249, and LMB-163 are all derived from LMB-84, but have cysteines used for PEGylation. They also contain a 17-residue GS linker placed either between the Vl and the furin cleavage site (LMB-179, LMB-244, and LMB-203) or at the N-terminus of the Vh (LMB-249). LMB-163 is similar to LMB-84 but contains mutations that humanize the Fv portion. Figure 1B shows a cartoon representation of the RITs. The 20kD PEG is shown as a red wiggly line.
Figure 1. Schematics of anti-mesothelin RITs.
A. SS1P contains an anti-mesothelin disulfide linked Fv connected to PE38 (domain II and III of PE). LMB-12 is derived from SS1P with domain II deleted and replaced with an 11 amino acid furin cleavge peptide connecting the Fv to domain III. LMB-84 contains a single-chain Fv instead of a dsFv and is the parental RIT from which the cysteine containing PEG-modified proteins were prepared. LMB-179, LMB-244, and LMB-203 have an additional 17-GS linker that separates Vl from the furin cleavage site. LMB-249 has GS linker placed at the N-terminus of Vh. LMB-163 contains mutations that humanize the Fv portion. For each construct, the engineered cysteine for site-specific PEGylation is indicated by a bolded red C. B. Cartoon representations of RITs shown in A. PEGs on cysteines are represented by red lines. Domains are not drawn to scale.
We use site directed mutagenesis to insert cysteine residues at various locations in the RIT. To choose locations that are less likely to interfere with immunotoxin activity, we modeled the RIT using the existing structures of its various components. The model of immunotoxin is shown in Figure 2A. The structural complex of the immunotoxin bound to mesothelin and EF2 is shown in Figure 2B, and it was generated by superposing the templates of mesothelin-mAb (PDB 4F3F (16)), scFV (PDB 3GKZ (17)), PE-EF2 (PDB 1ZM4 (18)) and PE-NAD-AMP (PDB 1DMA (19)) in UCSF Chimera (20). Figure 2C shows a model of 20kD PEG, composed of 454 units of ethylene glycol, conjugated to immunotoxin at D406 of domain III. Five different sites were mutated to cysteine for PEG conjugation as indicated by red balls. For all five locations, we tried to diminish interference with mesothelin binding, processing by furin protease and ADP-ribosylation of EF2.
Figure 2. Ribbon diagrams of anti-mesothelin RITs.
A. The ribbon diagram was generated with UCSF Chimera. Various domains are colored as: N terminal linker = grey, Vh = blue, Vl = cyan, CDR = orange, furin site = green, and PE24 = yellow. Sites that were mutated to cysteine are shown as red spheres, and they were chosen to avoid interfering with functional binding to mesothelin, furin protease, and EF2. B. A structural model of the complex of the RIT in A bound to mesothelin (olive green) and EF2 (dark gray). C. A hypothetical 20kD linear PEG composed of 454 repeating units of ethylene glycol is connected to the cysteine at D406 of PE24 (LMB-244). The structure of PEG is random, and the overall size of the molecule is modeled to approximate the average radius of hydration measured for all five PEGylated RITs.
LMB-179 and LMB-244 have cysteine residues located in domain III at E522 and D406, respectively (Figure 1). The cysteine residues are located on different sides of PE24 and are distant from the EF2 binding site (Figure 2A). LMB-203 has the cysteine placed in the middle of a 17 amino acid linker connecting the Fv to the furin cleavage peptide (Figure 1). It is 8 residues away from the furin cleavage site in order to diminish effects on cleavage or mesothelin binding to the Fv or EF2 binding to domain III. In LMB-249 the cysteine residue is located on the amino terminus of a 17-residue GS linker attached to the first residue of the heavy chain of the Fv (Figure 1 and 2); this location is unlikely to interfere with the binding of mesothelin to the CDRs of the Fv. LMB-163 does not contain an extra 17-residues GS linker like the other four RITs. The cysteine is placed at S63 in the light chain (Figure 1 and 2). We chose S63 to mutate to cysteine because the residue is surface-exposed and distant from the CDRs and the furin cleavage peptide so the mutation is less likely to interfere with binding to mesothelin or cleavage of the RIT by furin protease. All of RITs were expressed in E. coli in the form of inclusion bodies, which were denatured, refolded and purified through Q Sepharose and Mono Q columns.
PEGylation of RITs
Because a free thiol can lead to dimer formation, we stopped the purification after the Mono Q column, where we usually observed a peak of monomer and another of dimer. Figure 3A shows Mono Q fractions of LMB-179, LMB-244, LMB-203 and LMB-249 that are mostly monomer (lanes 2, 5, 6, and 8) or dimer (lanes 3, 4, 7, and 9, respectively). LMB-163 only has monomer fractions (lanes 10 and 11). Monomer runs at ~50kD, and dimer runs slightly above 100kD, consistent with the predicted molecular weight of each species. Since both monomer and dimer can be reduced by TCEP and used for PEGylation, we did not carry out final purification on size exclusion chromatography as used for other RITs.
Figure 3. PEGylation of RITs.
A. RITs eluted from Mono Q anion exchange column. Fractions A, B, and C correspond to separate peaks in the Mono Q chromatogram and were pooled separately. B. Schematic showing a hypothetical site-specific PEGylation reaction, which occurs by the formation of a thioester bond between a free thiol of a surface-exposed cysteine and a maleimide functional group of a PEG. C. Representative HPLC analysis of LMB-203/LMB-203-PEG. D. Non-reducing SDS-PAGE gel analysis of parental, TCEP-reduced, and PEGylated RITs. Gels were visualized by Coomassie Blue staining. The efficiencies of PEGylation were quantified by counting the number of pixels using Image J. PEGylated RIT is indicated by the blue square. White lines indicate the point of merge between two separate gels.
The PEGylation reaction was based on modifying a free thiol group of a surface-exposed cysteine to form a thioester bond with the maleimide functional group of a 20kD linear PEG (Figure 3B). To determine the best conditions for conjugation, we examined different conditions and found that the reaction was most efficient in PBS at pH 8.0. TCEP has been shown to enhance PEGylation efficiency compared to the standard reducing agents such as dithiothreitol and β-mercaptoethanol, used in our previous studies (9). Since TCEP does not contain a free thiol, there is no need to remove it prior to PEGylation, and the yields of reduced protein are much higher than using traditional reducing reagents. A 10-fold molar excess of methoxy PEG-maleimide was added to the TCEP-treated protein for PEGylation overnight. We performed HPLC analysis of the reaction mixture to determine the PEGylation efficiency; a typical HPLC run is shown for LMB-203 and its PEGylated counterpart LMB-203-PEG in Figure 3C. We quantified the peaks and found that the PEGylation was ~90 percent complete. The unreacted methoxy-PEG-maleimide was removed on an anion exchange column and the PEGylated protein was eluted with PBS.
Eluted proteins were analyzed on the SDS-PAGE gel along with pre and post-TCEP treated samples (Figure 3D). For LMB-179 and LMB-244, the starting materials were mostly dimer (lanes 2 and 5). For LMB-203 and LMB-249, there is a mixture of monomer and dimer (lanes 8 and 11). LMB-163 was all monomer (lane 14) perhaps because the SH group in is not readily available for dimer formation. After reduction with TCEP, all became monomers (lanes 3, 6, 9, 12 and 15). After addition of PEG, 90% or more of the TCEP-treated monomer was PEGylated (lanes 4, 7, 10, 13 and 16). The calculated molecular weight of a PEGylated RIT is about 72kD; however, they all run at ~100kD indicating the molecules are asymmetrical. The PEGylated proteins were quantified using Image J. The PEGylation efficiencies were ~90% overall, with LMB-203/LMB-203-PEG being 93-95%, which is consistent with the HPLC analysis. The PEG-modified proteins were frozen at −70°C in small aliquots and thawed as needed for assays.
About 10% of each RIT could not be modified even when we added more methoxy-PEG-maleimide and incubated for an extended period of time. We also tried removing TCEP before the addition of methoxy-PEG-maleimide, but this did not improve the yield. We assume the cysteine is modified during protein purification so that it cannot be reduced to generate a free thiol by TCEP.
Cytotoxicity Assays
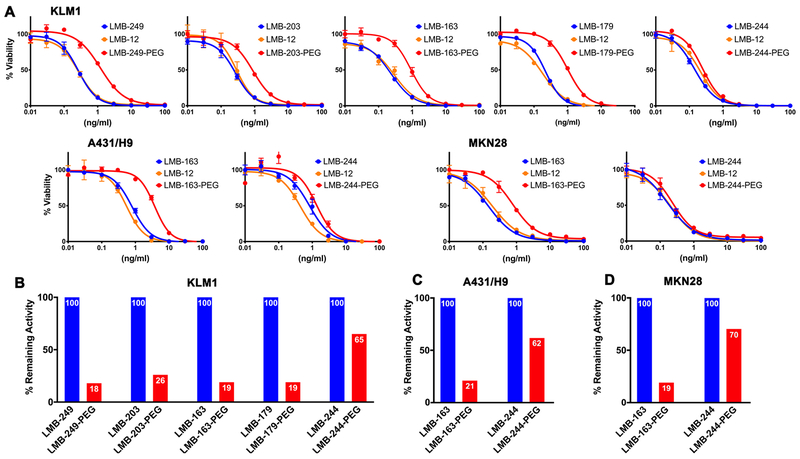
To determine whether PEGylation hampers the activity of the RITs, we performed cytotoxicity assays on three mesothelin-expressing cancer cell lines. LMB-12 and LMB-84 have nearly identical cytotoxic activity (Figure S1A); we picked LMB-12 as un-PEGylated control since it has been widely studied. PEGylated and control RITs were incubated with cancer cells for three days, and WST assays were performed. The results are shown in Figure 4A. The cell-killing data is best fit to a sigmoidal curve. In general, the range of linearity in which the cell-killing activity is dependent on the concentration of RIT and occurs between 0.1-1ng/ml. Using KLM1 cells, all of the cysteine containing proteins are as active as the LMB-12 except for LMB-244 which is somewhat more active (Table 1). For KLM1 (pancreatic) cell line, parental RITs LMB-249, LMB-203, LMB-163 and LMB-179 have IC50 of 0.22 ± 0.06, 0.29 ± 0.02, 0.22 ± 0.03, and 0.21 ± 0.01ng/ml, respectively. LMB-244 has an IC50 of 0.16 ± 0.03 ng/ml. The PEG modified immunotoxins were 4-5-fold less active except for LMB-244-PEG, which lost only 35% activity. After PEGylation the IC50s for LMB-249-PEG, LMB-203-PEG, LMB-163-PEG and LMB-179-PEG were 1.18 ± 0.03, 1.10 ± 0.19, 1.13 ± 0.19, and 1.09 ± 0.13 ng/ml, and LMB-244-PEG was 0.25 ± 0.05 ng/ml. We also tested activities of LMB-244-PEG and LMB-163-PEG on A431/H9 epidermoid carcinoma cells and MKN28 stomach cancer cells. Consistent with KLM1 cell line, LMB-244 was the most active and lost less than 50% of its activity. To show that PEG doesn’t affects mesothelin-specific cell killing, we tested a few of PEG-modified RITs on the A431 epidermoid carcinoma cell line that was not transfected with mesothelin cDNA. The result in Figure S1B shows no specific killing.
Figure 4. WST assays of parental and PEGylated RITs.
A. Representative cytotoxicity assays after 3 days incubation of mesothelin-positive cancer cell lines with different RITs. KLM1, pancreatic cancer cell line; A431/H9, epidermoid carcinoma cell line transfected with mesothelin cDNA; MKN28, gastric cancer cell line. B-D. The IC50 values of PEGylated RITs were normalized against the un-PEGylated controls which were set to 100%. The percent remaining activity is indicated.
Figure 4 B-D shows bar graphs of the activity of PEG-modified RITs relative to the parental control, which is set to 100%. It is evident that LMB-244-PEG is the most active. In three tumor cell lines, KLM1 (Figure 4B), A431/H9 (Figure 4C), and MKN28 (Figure 4D), LMB-244-PEG retains 65, 62 and 70% of the parental activity.
Mesothelin Binding and ADP Ribosylation are not affected by PEG
The first step in the action of RIT is binding of the mesothelin on the cell surface of the cancer cell by the Fv. To test if binding is affected by the PEG, we used serial dilutions of each PEGylated RIT and tested the binding to mesothelin using ELISA. The results are shown in Figure S2 and 50% maximum binding is indicated. The binding of the PEGylated RITs is close to that of un-PEGylated LMB-12. The values are: LMB-12 (0.022 ± 0.001μg/ml), LMB-249-PEG (0.027 ± 0.001μg/ml), LMB-203-PEG (0.024 ± 0.004μg/ml), LMB-163-PEG (0.029 ± 0.006μg/ml), LMB-179-PEG (0.023 ± 0.002μg/ml) and LMB-244-PEG (0.013 ± 0.001μg/ml).
Another crucial step in RIT action is the ADP-ribosylation and inactivation of EF2. This step can be measured by incubating EF2-containing cell lysate with the ADP precursor NAD-biotin and PEG-modified RIT and measuring the amount of biotin incorporated into EF2 using western blots and Strep-HRP antibody. Figure S3 shows an ADP ribosylation assay, in which the PEGylated RIT were incubated with the EF2-containing cell lysate. The resulting blots show a RIT-dependent modification of EF2, with only one major band that is consistent with the molecular weight of EF2 (indicated by arrow). The band is not detected in the negative control where RIT was omitted (lane 16). We quantified the EF2-ADP bands and normalized each to LMB-12 positive control. For accuracy, we loaded 5, 10 and 15 μl of each reaction. Interestingly, all RITs retain ~100% of the original activity. This indicates the loss of cytotoxic activity is not due to the inability of the immunotoxin to inactivate EF2.
Half-life Measurements
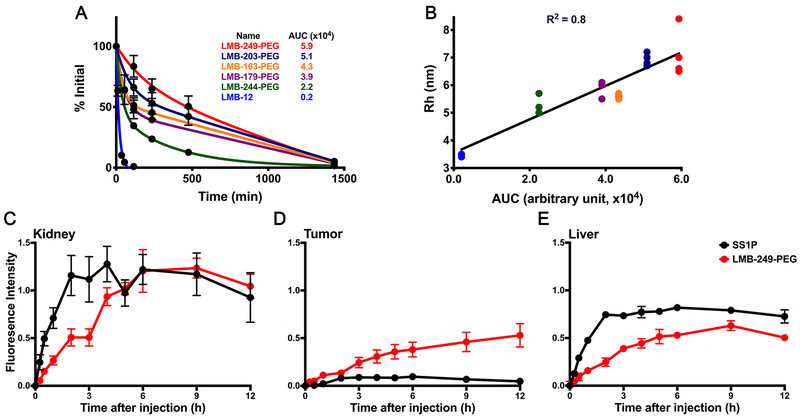
To determine the half-lives of the PEGylated RITs in the circulation, we injected 25 μg of each protein IV into nu/nu mice and collected serum at 5-min and 2, 4, 8 and 24 hours for LMB-203-PEG, LMB-244-PEG, and LMB-249-PEG, and at 5-min and 1, 4 and 24 hours for LMB-163-PEG and LMB-179-PEG. ELISA was used to determine the amount of remaining RIT. To normalize the data, it is plotted relative to the 5-min time point, which is set as 100% (Figure 5A). LMB-12, which has no cysteine added and is not PEGylated was used as an un-PEGylated control. The decay for LMB-12 is rapid and mono-exponential. The curves for the PEG-modified proteins best fit a two-phase decay function; therefore, we calculated two half-life values for each protein, a fast (⍺) and a slow (β) decay (Table 1). We also calculated Area Under Curve (AUC) to represent the amount in the blood present over time so that we have a single value to compare for each protein. Figure 5A shows that all PEGylated proteins have much longer half-lives than LMB-12. The AUC for LMB-12 is 0.2, while the PEGylated proteins have AUCs that are at least 10-fold higher and range from 2.2 for LMB-244-PEG to 5.9 for LMB-249-PEG. LMB-203-PEG has the second largest AUC of 5.1. LMB-163-PEG and LMB-179-PEG have AUCs of 4.3 and 3.9. Based on the AUC values, the order from the longest to the shortest half-life is LMB-249-PEG > LMB-203-PEG > LMB-163-PEG > LMB-179-PEG > LMB-244-PEG. The difference between LMB-244-PEG and LMB-249-PEG is about 3-fold even though the mass of each protein is identical.
Figure 5. Biological properties of RITs.
A. Pharmacokinetics of PEGylated RITs. 25μg of LMB-203-PEG, LMB-244-PEG, LMB-163-PEG, LMB-179-PEG, and LMB-249-PEG were injected into nu/nu mice and blood was collected at 5 min, 1 (LMB-163-PEG and LMB-179-PEG), 2, 4, 8 (LMB-203-PEG, LMB-244-PEG, and LMB-249-PEG), and 24 hour time points. ELISA was performed to determine the level of RIT and plotted in two-phase decay using GraphPad. Amount of RIT at 5 min was set to 100% and used to normalize the remaining time points. LMB-12 was data from our previous study and plotted as one-phase decay. The AUC values are summarized. B. Rh of PEGylated protein was measured using Dynamic Light Scattering. 20μl aliquots of proteins (0.5-1mg/ml) were measured at least two times and Rh was plotted against half-life using GraphPad. The same color scheme was used as in A. The line of best fit was generated with R2 value indicated. C-E. Biodistribution of LMB-249-PEG and SS1P in mice. Fluorescently labeled LMB-249-PEG and SS1P was I.V. injected into A431/H9 tumor-bearing mice (n=3, tumor ~100mm3), respectively. Serial dorsal and ventral 800nm fluorescence images were obtained with focus placed on the tumor, kidney, and liver. The fluorescence intensity at each time point was quantified and plotted to show the accumulation in the kidney(C), tumor(D) and liver(E). Plots were generated from the average results of 3 imaged mice.
The PEGylated proteins have much longer β than ⍺ decay. LMB-249-PEG has the longest half-life with an ⍺ of 144-min and a β of 74400-min. LMB-163-PEG has an ⍺ of 29-min and a β of 62200-min. LMB-244-PEG has the shortest half-life, with an ⍺ of 25-min and a β of 256-min. In summary, PEGylation extends the half-life of RITs significantly, particularly the β phase. Because protein removal from the blood is controlled by glomerular filtration with subsequent degradation in the kidney, we postulated that the proteins could have different hydrodynamic radii that affected their rate of filtration by the glomerulus in the kidney.
Hydrodynamic Radius
To determine if the addition of PEG at different locations changed the hydrodynamic radius, we employed the Dynamic Light Scattering. Each sample was measured at least two times and representative measurements are shown in Figure S4 and summarized in Table 1. The %Mass is plotted against the radius. Overall, the results show a monomodal size distribution for LMB-163-PEG, LMB-244-PEG, LMB-12 and LMB-203. For LMB-249-PEG, LMB-203-PEG, and LMB-179-PEG, we observed polymodal size distributions, which is due to the presence of trace amount of aggregate that also diffract laser light. LMB-12 and LMB-203 were included as un-PEGylated controls. LMB-12 has the smallest Rh of 3.4nm. LMB-203 is slightly larger, with Rh of 3.7nm (Figure S4). With addition of 20kD PEG, the Rhs increase substantially to 7.1, 6.9, 5.9, 5.6, and 5.2nm for LMB-249-PEG, LMB203-PEG, LMB-179-PEG, LMB-163-PEG, and LMB-244-PEG, respectively. Comparing LMB-203 and LMB-203-PEG, the increase in Rh is almost 2-fold.
Biodistribution Assay
It is widely-stated that PEG can extend the serum half-life by increasing the hydrodynamic volume of biologics and reducing their renal filtration (5). To determine how quickly the PEGylated RITs were removed by the kidney and liver, we performed biodistribution studies with LMB-249-PEG labelled with FNIR-Z-759, a near-infrared fluorophore that allows external imaging of living mice (21). Labeled LMB-249-PEG (41μg, 580pmol) was injected I.V into tumor-bearing mice and accumulation in the kidney, liver, and tumor was imaged over 12 hours. The images in the upper panel of Figure S5A are from a dorsal view, showing uptake by the kidneys and by tumor that are close to the dorsal side of the mouse. The images in the lower panel of Figure S5A show uptake by liver which is close to the ventral side. To compare the biodistribution with an immunotoxin without PEG, we performed the same experiment with SS1P, which has a MW of 63kD, close to 72kD for the PEG modified RITs. The upper panel of Figure S5B shows accumulation of SS1P in kidney and tumor and the lower panel shows liver uptake.
Quantification of Uptake
To determine the amount of LMB-249-PEG and SS1P taken up by kidney, liver, and tumor, the images were scanned and quantified. Accumulation as a function of time for kidney is plotted in Figure 5 C, for liver in Figure 5D, and for tumor in Figure 5E. The graphs show that uptake of LMB-249-PEG in kidney peaks at 6 hours whereas uptake of SS1P peaks much earlier at 2 hours. Liver uptake of LMB-249-PEG peaks late at 9 hours, while SS1P uptake much earlier at 2 hours. The results for tumor follow a different pattern. Accumulation of LMB-249-PEG by tumor increases steadily over many hours and peaks at 9-12 hours, whereas uptake of SS1P is much smaller and peaks at 2-3 hours.
Anti-tumor experiments
To do an initial assessment of the anti-tumor activity of the various PEGylated RITs, we treated tumor-bearing mice IV with 10μg of PEG-modified RIT given every other day x3, when the tumors reached about ~100 mm3 in size. In the PBS treatment group, the tumors grew rapidly (Figure 6A). All treatment groups responded to PEGylated RITs. LMB-244-PEG and LMB-163-PEG were the most active and the tumors decreased in size to 69 mm3 and 79 mm3 on day 14 and the p-values are both 0.001, respectively. LMB-179-PEG, LMB-203-PEG and LMB-249-PEG slowed tumor growth; on day 14, the tumors reached 259 mm3 in the control group and only 152, 117 and 143 mm3 in LMB-179-PEG, LMB-203-PEG and LMB-249-PEG, respectively. The weights of the treated mice decreased by less than 10% (Figure S6A), but two mice in the LMB-179-PEG group and one in the LMB-249-PEG died.
Figure 6. Anti-tumor activity of parental and PEGylated RITs.
A. Antitumor activity of PEGylated RITs in nude mice (n = 10). Mice were implanted with mesothelin-expressing KLM1 tumor cells. When tumors reached ~100mm3, mice were I.V. injected with 10μg/mouse PEGylated RITs qod x3 as indicated by arrows. Tumor burden was monitored over 2 weeks. B. Comparison of anti-tumor activities of LMB-12, LMB-163-PEG and LMB-244-PEG. Groups of 10 mice were treated with 20 μg of each agent as shown by arrows and tumor burden was monitored over 2 weeks.
Since LMB-244-PEG and LMB-163-PEG are the most active, we performed additional studies with them using a dose and schedule that did not cause weight loss. We compared their activity with that of LMB-12, which is very similar to LMB-84 in structure and activity and is not PEG modified. Figure 6B shows that tumors in mice receiving five 20μg doses given over 2 weeks of PEG modified RIT regressed to 46mm3 and 48mm3, whereas tumors in mice treated with LMB-12 increased in size but grew more slowly than tumors treated with PBS. The p-values for LMB-244-PEG and LMB-163-PEG compared to LMB-12 are 0.0049 and 0.0048, respectively. At this schedule the weight of the mice decreased by less than 10% and no mice died (Figure S6B). This data establishes that increasing half-life greatly increases anti-tumor activity.
Discussion
We have developed a method to perform site specific PEGylation at cysteine residues present at different locations in an anti-mesothelin RIT and identified 2 locations that produced PEG modified RITs with high cytotoxic and anti-tumor activity and very prolonged half-lives in the circulation. Our strategy utilizes structural information to identify PEGylation sites that are least likely to interfere with binding to mesothelin or to EF2 or to affect furin cleavage. Since there are many lysine residues in the RIT, we could not achieve specificity using lysine-specific PEGylation and chose the highly efficient site-specific PEGylation of cysteine. Although there are two cysteine residues in Fv, they are buried and not available for reduction. Therefore, we used site-directed mutagenesis to introduce cysteines at locations exposed and distant from known functional sites in the RIT. We were able to PEGylate these mutant RITs at high efficiency. The key to this success is the use of TCEP to reduce the protein followed by treating with a 20 kDa maleimide derivative of PEG at pH 8.0. This that had not been achievable with β-mercaptoethanol in our previous study (8).
The most active of the PEG modified RITs is LMB-244-PEG, which has a PEG attached to residue 406C in domain III of the toxin (Figure 1A). We tested LMB-244-PEG on 3 cell lines and found it retained more than 50% of its cytotoxic activity on all lines. When tested in mice, it had an 11-fold increase in residence time (AUC) in the circulation compared with an un-PEGylated protein LMB-12 and this change resulted in a very large increase in anti-tumor activity (Figure 6). To account for differences in activity, we measured its radius of hydration and found that LMB-244-PEG had the smallest Rh. This finding suggests that one or more steps in immunotoxin action are negatively affected by larger Rh of the protein and a smaller Rh is better. We also observed that LMB-244-PEG has a shorter half-life and AUC than the other PEGylated RITs indicating it is more efficiently filtered by the kidney glomerulus than RITs with higher Rh values.
Because we used the same 20 kDa PEG to modify all the RITs, we were surprised to observe that the Rh values varied widely from 5.2 to 7.1 indicating that the derivatized proteins adopted different conformations. Since the Rh values correlate with the half-life in the circulation (Figure 5B), and since kidney is the major organ responsible for removal of RITs, we assume that the glomerulus in the kidney is sensitive to the size and shape of the proteins and filters them at different rates. In addition, the PEG-modified proteins with the smallest Rh (LMB-244-PEG) had excellent anti-tumor activity, suggesting that entry into tumors is also affected by Rh. We plan to use external tumor imaging to analyze the entry into tumors of all 5 PEGylated RITs in a future study.
The mechanism by which RITs kill cells is complex, beginning with binding to mesothelin on the cell surface and ending with inactivation of EF2 in the cytosol. We are unable to assess each step in the pathway but did assess two crucial steps and found that binding to mesothelin was similar for all PEG modified RITs, although there was a small loss of binding to mesothelin by the two that had PEG added to the Fv (Figure S2). We also found no change in the ability of the modified proteins to ADP-ribosylate EF2 (Figure S3). We are currently unable to measure how efficiently RITs are processed by other steps in the pathway.
The half-life data of PEGylated RITs fits well to biexponential decay curves (Figure 5A). We think it is likely that the ⍺ phase is due to rapid equilibration with the extracellular compartment, and the β phase is due to the metabolism by the kidney and liver (22). We used external imaging to determine which organs in mice take up SS1P, a small un-PEGylated RIT with a half-life of 19 min (AUC 0.2) and LMB-249-PEG with a long half-life and an AUC of 5.9. The data in Figure 5C and 5E show that SS1P is rapidly taken up by kidney and by liver, whereas the uptake of LMB-249-PEG is delayed. This delay leads to the greatly increased half-life and AUC. The initial uptake by tumors is the same for both RITs, but the uptake of SS1P is arrested as blood levels fall (Figure 5D), whereas the uptake of LMB-249-PEG increases for many hours and must be responsible for the enhanced anti-tumor activity of PEGylated RITs.
It is widely-stated that PEG increases the half-life by increasing the hydrodynamic volume, and it has been shown increasing the size of PEG attached to mmTrail increases serum half-life (23). Because a change in PEG size does not account for the different biological properties of our proteins, we employed dynamic light scattering to assess the hydrodynamic volume of the proteins and found for the first time in our knowledge that the hydrodynamic size of protein can be fine-tuned depending on the position of the PEG. In Figure 5B, the Rh values are plotted against AUC. The result shows a good correlation between AUC and Rh with the R2 value of 0.8. In general, larger Rh corresponds to longer half-life. The un-PEGylated LMB-12 is smallest protein and has a Rh of ~3.4. LMB-203 is slightly larger (RH 3.7) having a 15 amino acid linker connecting the light and heavy chains and 17 amino acid linker connecting the Fv to the furin cleavage site (Figure S4). The PEGylated proteins are much larger, with average Rhs of 7.1, 6.9, 5.6, 5.9, 5.2 nm for LMB-249-PEG, LMB-203-PEG, LMB-163-PEG, LMB-179-PEG, and LMB-244-PEG, respectively. Therefore, our results indicate that the PEGylated RITs adopt distinct conformations governed by the position of the PEG, and such conformation influences the half-life. Since Rh is proportional to the hydrodynamic volume, we also concluded that PEG increases the hydrodynamic volume, which leads to slower renal filtration and longer half-life, and this is consistent with the reported properties of PEG (5).
The anti-tumor activity of the PEGylated-RITs varied widely. Two of them, LMB-244-PEG and LMB-163-PEG, produced substantial tumor regressions, but the others were less active (Figure 6A). The high anti-tumor activity of LMB-244-PEG reflects several factors. The starting protein, LMB-244, is almost 2-fold more active than the other proteins and PEG addition caused less than a 2-fold loss of activity. Also, LMB-164-PEG has the smallest Rh indicating it is more compact and probably enters tumors better than the other PEG modified proteins. However, the small Rh probably contributes to its low AUC of 2.2. LMB-163-PEG also has good tumor activity despite being 3-fold less cytotoxic than LMB-264-PEG. The basis of the variable anti-tumor activity is not understood and will be the subject of further studies.
Conclusion
We have developed a new approach to carry out site specific modification of RITs with PEG and identified two RITs that have a long half-life and high anti-tumor activity. We have shown that the location of the PEG affects the hydrodynamic size of the protein and contributes to anti-tumor activity. PEG modified RITs merit further preclinical development for cancer therapy.
Supplementary Material
Acknowledgements
Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081). We thank the Biophysics Resource in the Structural Biophysics Laboratory, Center for Cancer Research, NCI at Frederick for assistance with light-scattering studies. This research was supported by the Intramural Research Program of the NIH, the National Cancer Institute, and the Center for Cancer Research (I.P.).
Footnotes
Conflict of Interest Statement:
I.P. is an inventor on several patents on immunotoxins that have all been assigned to the NIH.
References
- 1.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol 2011;22:868–76 [DOI] [PubMed] [Google Scholar]
- 2.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer 2006;6:559–65 [DOI] [PubMed] [Google Scholar]
- 3.Alewine C, Hassan R, Pastan I. Advances in anticancer immunotoxin therapy. Oncologist 2015;20:176–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei J, Bera TK, Liu XF, Zhou Q, Onda M, Ho M, et al. Recombinant immunotoxins with albumin-binding domains have long half-lives and high antitumor activity. Proc Natl Acad Sci U S A 2018;115:E3501–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damodaran V, Fee JC Protein PEGylation: An overview of chemistry and process considerations. 2010. 18–26 p. [Google Scholar]
- 6.Swierczewska M, Lee KC, Lee S. What is the future of PEGylated therapies? Expert Opinion on Emerging Drugs 2015;20:531–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang QC, Pai LH, Debinski W, FitzGerald DJ, Pastan I. Polyethylene glycol-modified chimeric toxin composed of transforming growth factor alpha and Pseudomonas exotoxin. Cancer Res 1993;53:4588–94 [PubMed] [Google Scholar]
- 8.Tsutsumi Y, Onda M, Nagata S, Lee B, Kreitman RJ, Pastan I. Site-specific chemical modification with polyethylene glycol of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) improves antitumor activity and reduces animal toxicity and immunogenicity. Proc Natl Acad Sci U S A 2000;97:8548–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphreys DP, Heywood SP, Henry A, Ait-Lhadj L, Antoniw P, Palframan R, et al. Alternative antibody Fab’ fragment PEGylation strategies: combination of strong reducing agents, disruption of the interchain disulphide bond and disulphide engineering. Protein Eng Des Sel 2007;20:227–34 [DOI] [PubMed] [Google Scholar]
- 10.Pastan I, Beers R, Bera TK. Recombinant immunotoxins in the treatment of cancer. Methods Mol Biol 2004;248:503–18 [DOI] [PubMed] [Google Scholar]
- 11.Mazor R, Eberle JA, Hu X, Vassall AN, Onda M, Beers R, et al. Recombinant immunotoxin for cancer treatment with low immunogenicity by identification and silencing of human T-cell epitopes. Proc Natl Acad Sci U S A 2014;111:8571–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollevoet K, Mason-Osann E, Liu XF, Imhof-Jung S, Niederfellner G, Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther 2014;13:2040–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu XF, Zhou Q, Hassan R, Pastan I. Panbinostat decreases cFLIP and enhances killing of cancer cells by immunotoxin LMB-100 by stimulating the extrinsic apoptotic pathway. Oncotarget 2017;8:87307–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho M, Hassan R, Zhang J, Wang QC, Onda M, Bera T, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res 2005;11:3814–20 [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury PS, Pastan I. Improving antibody affinity by mimicking somatic hypermutation in vitro. Nat Biotechnol 1999;17:568–72 [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Tang WK, Esser L, Pastan I, Xia D. Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights. J Biol Chem 2012;287:33123–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celikel R, Peterson EC, Owens SM, Varughese KI. Crystal structures of a therapeutic single chain antibody in complex with two drugs of abuse-Methamphetamine and 3,4-methylenedioxymethamphetamine. Protein Sci 2009;18:2336–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen R, Merrill AR, Yates SP, Marquez VE, Schwan AL, Boesen T, et al. Exotoxin A-eEF2 complex structure indicates ADP ribosylation by ribosome mimicry. Nature 2005;436:979–84 [DOI] [PubMed] [Google Scholar]
- 19.Li M, Dyda F, Benhar I, Pastan I, Davies DR. The crystal structure of Pseudomonas aeruginosa exotoxin domain III with nicotinamide and AMP: conformational differences with the intact exotoxin. Proc Natl Acad Sci U S A 1995;92:9308–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 2004;25:1605–12 [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Gorka AP, Nagaya T, Michie MS, Nani RR, Nakamura Y, et al. Role of Fluorophore Charge on the In Vivo Optical Imaging Properties of Near-Infrared Cyanine Dye/Monoclonal Antibody Conjugates. Bioconjug Chem 2016;27:404–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shancer Z, Liu XF, Nagata S, Zhou Q, Bera TK, Pastan I. Anti-BCMA immunotoxins produce durable complete remissions in two mouse myeloma models. Proc Natl Acad Sci U S A 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nie Q, Jia D, Yang H, Feng Y, Fan Q, Shi Q, et al. Conjugation to 10 kDa Linear PEG Extends Serum Half-Life and Preserves the Receptor-Binding Ability of mmTRAIL with Minimal Stimulation of PEG-Specific Antibodies. Mol Pharm 2017;14:502–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.