Key Points
Question
Pharmacy fill data are increasingly accessible to evaluate medication-taking behavior, yet how often do patient-reported and pharmacy fill–based medication persistence agree?
Findings
Of 8373 patients included in this post hoc hypothesis-driven secondary analysis of the cluster randomized ARTEMIS trial, 50.0% were concordantly persistent by both self-report and pharmacy fills, 36.5% were discordantly persistent, and 13.5% were concordantly nonpersistent. Observed rates of death, myocardial infarction, or stroke were highest for patients who were concordantly nonpersistent.
Meaning
Patient-reported and pharmacy fill–based persistence measures are frequently discordant, but patients who are nonpersistent by both methods have the worst clinical outcomes and should be prioritized for interventions to improve medication-taking behavior.
Abstract
Importance
Pharmacy fill data are increasingly accessible to clinicians and researchers to evaluate longitudinal medication persistence beyond patient self-report.
Objective
To assess the agreement and accuracy of patient-reported and pharmacy fill–based medication persistence.
Design, Setting, and Participants
This post hoc analysis of the cluster randomized clinical trial ARTEMIS (Affordability and Real-world Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) enrolled patients at 287 US hospitals (131 randomized to intervention and 156 to usual care) from June 5, 2015, to September 30, 2016, with 1-year follow-up and blinded adjudication of major adverse cardiovascular events. In total, 8373 patients with myocardial infarction and measurement of P2Y12 inhibitor persistence by both patient self-report and pharmacy data were included. Serum P2Y12 inhibitor drug levels were measured for 944 randomly selected patients. Data were analyzed from May 2018 to November 2019.
Interventions
Patients treated at intervention-arm hospitals received study vouchers to offset copayments at each P2Y12 inhibitor fill for 1 year after myocardial infarction.
Main Outcomes and Measures
Nonpersistence was defined as a gap of 30 days or more in P2Y12 inhibitor use (patient report) or supply (pharmacy fill) and as serum P2Y12 inhibitor levels below the lower limit of quantification (drug level). Among patients in the intervention arm, a “criterion standard” definition of nonpersistence was a gap of 30 days or more in P2Y12 inhibitor use by both voucher use and pharmacy fill. Major adverse cardiovascular events were defined as adjudicated death, recurrent myocardial infarction, or stroke.
Results
Of 8373 patients included in this analysis, the median age was 62 years (interquartile range, 54-70 years), 5664 were men (67.7%), and 990 (11.8%) self-reported as nonwhite race/ethnicity. One-year estimates of medication nonpersistence rates were higher using pharmacy fills (4042 patients [48.3%]) compared with patient self-report (1277 patients [15.3%]). Overall, 4185 patients (50.0%) were persistent by both pharmacy fill data and patient report, 1131 patients (13.5%) were nonpersistent by both, and 3057 patients (36.5%) were discordant. By application of the criterion standard definition, the 1-year nonpersistence rate was 1184 of 3703 patients (32.0%); 892 of 3318 patients (26.9%) in the intervention arm who self-reported persistence were found to be nonpersistent, and 303 of 1487 patients (20.4%) classified as nonpersistent by pharmacy fill data were actually persistent. Agreement between serum P2Y12 inhibitor drug levels and either patient-reported (κ = 0.11-0.23) or fill-based (κ = 0.00-0.19) persistence was poor. Patients who were nonpersistent by both pharmacy fill data and self-report had the highest 1-year major adverse cardiac event rate (18.3%; 95% CI, 16.0%-20.6%) compared with that for discordant patients (9.7%; 8.7%-10.8%) or concordantly persistent patients (8.2%; 95% CI, 7.4%-9.0%).
Conclusions and Relevance
Patient report overestimated medication persistence rates, and pharmacy fill data underestimated medication persistence rates. Patients who are nonpersistent by both methods have the worst clinical outcomes and should be prioritized for interventions that improve medication-taking behavior.
Trial Registration
ClinicalTrials.gov Identifier: NCT02406677
This ad hoc secondary analysis of the cluster randomized ARTEMIS trial assesses the agreement and accuracy of medication persistence rates measured in a 1-year follow-up of usual care by patient report, pharmacy fill, voucher use for medication copayment (intervention arm), and serum P2Y12 inhibitor levels among patients with major adverse cardiovascular events.
Introduction
Many patients with acute coronary syndromes prematurely discontinue guideline-recommended secondary prevention medications, leading to mortality and morbidity that may have been preventable.1,2,3,4 Targeting nonpersistent patients with interventions to improve medication-taking behavior has the potential to improve clinical outcomes.5 However, the best method of assessing medication persistence is unknown.6 Clinicians often rely on patient report, but it is vulnerable to recall and social desirability bias.7 Classifications based on pharmacy fill data are subject to bias from missing data.8 Testing of drug or metabolite levels is generally not feasible for large populations of patients and may be biased if the patient knows the intent of the test and when it is occurring. With evolving interoperability of electronic health data, clinicians are increasingly able to access pharmacy fill data for their patients. Yet, little is known about the agreement and accuracy of patient-reported and fill-based medication persistence or about how pharmacy fill data might assist clinicians in identifying patients most likely to benefit from interventions that improve medication-taking behavior.
The pragmatic clinical trial of P2Y12 inhibitor copayment reduction for patients with recent myocardial infarction (MI) ARTEMIS (Affordability and Real-world Antiplatelet Treatment Effectiveness After Myocardial Infarction Study), measured persistence by patient report and pharmacy fill for most enrolled patients. In the intervention arm of the trial, copayment reductions were administered via study vouchers, and voucher use at the time of P2Y12 inhibitor fill served as a third method of measuring medication persistence. Finally, a randomly selected subset of patients underwent phlebotomy to measure P2Y12 inhibitor drug levels. The present study leveraged this unique data source to describe differences in P2Y12 inhibitor nonpersistence rates between measurement methods, examine the degree of agreement between methods, and compare outcomes associated with concordant and discordant medication persistence when assessed by patient report or pharmacy fills.
Methods
Data Source and Patient Population
Details of the ARTEMIS trial design and primary results have been published previously (trial protocol in Supplement 1).9,10 In brief, ARTEMIS was a pragmatic clinical trial that cluster randomized 301 US hospitals to copayment reduction vs usual care to examine the effect of copayment reduction on the persistent use of P2Y12 inhibitors and on clinical outcomes for 12 months following an acute MI. Patients were enrolled from June 2015 to September 2016. In both arms, P2Y12 inhibitor selection and treatment duration decisions were determined by the clinical care team. Patients enrolled at intervention-arm hospitals were given a voucher card at hospital discharge that could be used at any pharmacy for 1 year to fill a prescription for either a generic (clopidogrel) or brand-name (ticagrelor) P2Y12 inhibitor. Medication refills needed to be initiated by the patient without any study-driven refill reminders.
Eligible patients were 18 years of age or older, hospitalized with acute MI, discharged with a P2Y12 inhibitor prescription, and had US-based health insurance with prescription drug coverage. The ARTEMIS trial enrolled 11 001 patients at 287 hospitals (131 randomized to intervention, 156 randomized to usual care), of whom 10 102 were discharged alive after the index MI hospitalization with a prescription for clopidogrel or ticagrelor. The study protocol was approved by the institutional review board of each participating site, and all patients enrolled in ARTEMIS provided written informed consent. Patients in the usual-care arm received no compensation nor were offered any incentive for participating in the study. Patients in the intervention arm received vouchers to offset their copayment for each P2Y12 inhibitor fill during the 1-year follow-up after MI. The present analysis followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Duke University Medical Center Institutional Review Board approved the use of ARTEMIS data for this post hoc secondary analysis.
Data Collection and Definitions
The distribution of patients with patient-reported, pharmacy fill–based, or serum drug level measurements of P2Y12 inhibitor persistence is shown in Figure 1. Patient-reported nonpersistence was defined as any gap in P2Y12 inhibitor use of 30 days or more, consistent with the primary end point of ARTEMIS. During follow-up interviews conducted 3, 6, 9, and 12 months after MI, all patients were asked to list their current medications. If a P2Y12 inhibitor was added, discontinued, or temporarily stopped since hospital discharge or the last interview, patients reported when that change occurred.
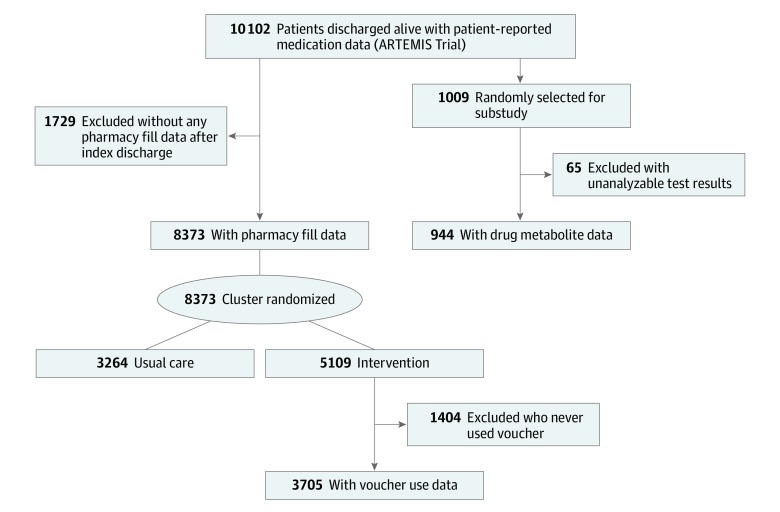
Figure 1. CONSORT Diagram.
Medication persistence was measured in 4 ways in ARTEMIS, in 4 separate cohorts of patients. Self-reported persistence was measured in the population of patients discharged alive who received clopidogrel or ticagrelor during follow-up interviews (91.4% of patients completed the interview at 3 months, 85.7% at 6 months, 83.1% and 9 months, and 81.1% at 12 months). Persistence by pharmacy fill was measured in the subset of patients who could have their records linked to pharmacy claims data. Among this subset, persistence by combined voucher use and pharmacy fill was measured in the intervention arm for patients who used a copayment assistance voucher at least once. A random subset of the full ARTEMIS population was selected to undergo phlebotomy to measure serum P2Y12 inhibitor drug levels.
Pharmacy fill–based nonpersistence was defined as any gap in P2Y12 inhibitor supply of 30 days or more. Data from enrolled patients were linked to their pharmacy fill records in the Symphony Health (a PRA Health Sciences company) pharmacy claims database, which captures approximately 90% of retail, 60% of mail-order, and 70% of specialty pharmacy fills in the United States.11 Among 8373 patients with at least 1 prescription medication fill in the Symphony database on or after the date of hospital discharge, pharmacy claims data were used to determine, on a daily basis, whether patients had a supply of P2Y12 inhibitors. When patients were hospitalized, it was assumed that they were taking a P2Y12 inhibitor supplied by the hospital, and hospitalized days did not count toward a gap in P2Y12 inhibitor supply. Switches between P2Y12 inhibitor types were considered persistent unless a gap of 30 days or more in supply was observed.
For patients in the intervention arm, voucher use also provided information about the timing and amount of P2Y12 inhibitor filled, and the “criterion standard” for nonpersistence was defined as any gap in P2Y12 inhibitor supply of 30 days or more by both pharmacy fills and copayment voucher use. We combined pharmacy fill and voucher use data because patients in the intervention arm could choose to fill a medication without using the study voucher (ie, if voucher use denoted a gap but pharmacy fill data did not, then the patient would still be categorized as persistent). If pharmacy fill data showed a gap in supply but voucher use during that period did not denote a gap, then the patient was categorized as persistent because this result may reflect missing pharmacy fill data.
In a prespecified substudy, approximately 250 patients were randomly selected 3, 6, 9, and 12 months after MI to undergo phlebotomy to measure serum P2Y12 inhibitor levels. Patients could be selected more than once. Patients were defined as nonpersistent if assay levels were lower than the lower limit of quantification for all P2Y12 inhibitors. Major adverse cardiovascular events (MACEs) within 1 year of hospital discharge, defined as all-cause death, recurrent MI, or stroke, were independently adjudicated according to standard definitions by physicians blinded to randomization status.9
Statistical Analysis
Baseline patient characteristics, including self-reported race/ethnicity, were reported for the overall ARTEMIS population, the cohort with linked pharmacy fill data, and the cohort randomly selected for the phlebotomy substudy. Descriptive statistics are reported as median values (with interquartile ranges) for continuous variables and as frequencies (percentages) for categorical variables.
We report the proportion of patients who persistently used P2Y12 inhibitors by self-report and by pharmacy fill; persistence was assessed both cumulatively (from baseline to the time point of interest; the last observation carried forward method was used for patients who died or missed interviews assessing P2Y12 inhibitor treatment status) and noncumulatively (in the 90 days prior to each time point of interest; patients who did not complete interviews were not included in self-reported persistence metrics for the period covered by the missed interview).10
We classified patients into 4 groups by cumulative medication persistence: (1) persistent by both self-report and pharmacy fill data, (2) persistent by self-report and nonpersistent by pharmacy fill data, (3) nonpersistent by self-report and persistent by pharmacy fill data, and (4) nonpersistent by both self-report and pharmacy fill data. We described the baseline characteristics of patients in each group and produced Kaplan-Meier curves of MACEs for each group. Because switching pharmacies may cause an underestimation of persistence in Symphony fill data, we calculated the proportion of patients who reported changing pharmacies in the 90 days following becoming nonpersistent by pharmacy fill data.
Among patients in the intervention arm who used a copayment assistance voucher at least once, we described cumulative persistence by self-report, pharmacy fill data, and the criterion standard combination of voucher use and pharmacy fill data. We calculated the sensitivity and specificity of self-reported nonpersistence compared with the criterion standard. To quantify how much patient report overestimated medication persistence, we calculated the proportion of patients who self-reported persistence but were nonpersistent by the criterion standard. To quantify how much pharmacy fill data underestimated medication persistence, we calculated the proportion of patients who were nonpersistent by pharmacy fill data but persistent by the criterion standard.
In the subset of patients with measured serum P2Y12 inhibitor levels, the κ statistic was used to assess pairwise agreement beyond that expected by chance at 3, 6, 9, and 12 months after MI, keeping in mind that these levels assessed whether patients were using P2Y12 inhibitors at the time of phlebotomy but did not provide longitudinal medication persistence. All analyses were performed from May 2018 to November 2019 at the Duke Clinical Research Institute using SAS, version 9.4 (SAS Institute Inc), or Stata, version 16.0 (StataCorp).12 We calculated 95% CIs for proportions using a clustered robust standard error to account for within-hospital clustering.
Results
Measurement of Medication Persistence
In the ARTEMIS trial, 10 102 enrolled patients were discharged alive after MI, received either ticagrelor or clopidogrel, and were followed up for 1 year to collect data on self-reported P2Y12 inhibitor use. Of these, 8373 patients (82.9%) had linked pharmacy claims to determine pharmacy fill–based persistence. The median age of these patients was 62 years (interquartile range, 54-70 years), most patients (5664) were men (67.7%), and 990 patients (11.8%) self-reported as being of nonwhite race/ethnicity. The characteristics of 1729 patients without a prescription fill in the Symphony database were broadly similar to those of patients with a prescription fill in the Symphony database (eTable 1 in Supplement 2).
In the intervention arm, 3705 patients used the study voucher at least once during the 1 year after hospital discharge and had both self-reported and pharmacy medication persistence data. In the phlebotomy substudy, 944 patients (9.3%) were randomly selected for testing and had analyzable drug metabolite results (Figure 1). The baseline characteristics of these different cohorts were similar (eTable 2 in Supplement 2).
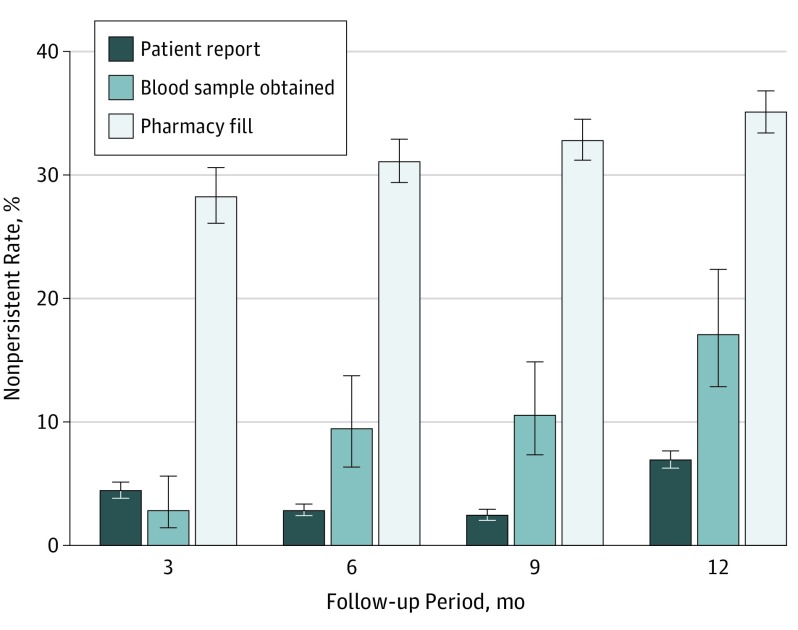
Of the 8373 patients with both patient-reported and pharmacy fill data, 1277 patients (15.3%; 95% CI, 14.0-16.6) self-reported medication nonpersistence, but 4042 patients (48.3%; 95% CI, 46.2-50.4) were nonpersistent using pharmacy claims. As shown in Figure 2, while noncumulative rates of medication nonpersistence increased during the course of follow-up with pharmacy claims and drug metabolite testing, self-reported medication nonpersistence increased only at 12 months of follow-up.
Figure 2. Noncumulative Medication Nonpersistence by Method of Measurement.
Bars show the proportion of patients who were medication nonpersistent by self-report, pharmacy fill, and randomly obtained blood samples from 0 to 3 months, from 3 to 6 months, from 6 to 9 months, and from 9 to 12 months of follow-up; error bars depict 95% CIs. Medication nonpersistence increased with time as measured by pharmacy fill and randomly obtained blood samples, but only increased by self-report at 12 months. Noncumulative persistence indicates no gap for 30 days or more in P2Y12 inhibitor use or supply during the specified 3-month window.
Concordant and Discordant Medication Persistence as Measured by Patient Report and Pharmacy Fill
Of 8373 patients, 5316 patients (63.5%; 95% CI, 61.8-65.1) had concordant persistence results from self-report and pharmacy fill data; 4185 patients (50.0%; 95% CI, 47.9-52.1) were concordantly persistent, and 1131 patients (13.5%; 95% CI, 12.3-14.8) were concordantly nonpersistent (Table). In addition, of 8373 patients, 3057 had discordant results; 2911 (34.8%; 95% CI, 33.1%-36.5%) self-reported persistence but were nonpersistent by pharmacy fills, and 146 (1.7%; 95% CI, 1.5%-2.0%) were nonpersistent by self-report but persistent by pharmacy fill data (eFigure 1 in Supplement 2). Overall, 41.0% of patients who self-reported medication persistence were nonpersistent by pharmacy fill data.
Table. Baseline Characteristics by Patient-Reported Medication Persistence and by Pharmacy Fill Persistence.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Both Persistent (n = 4185) | Both Nonpersistent (n = 1131) | Patient Report Persistent, Pharmacy Fill Nonpersistent (n = 2911) | Patient Report Nonpersistent, Pharmacy Fill Persistent (n = 146) | |
| Demographic | ||||
| Age, median, (IQR), y | 62 (54-69) | 64 (54-73) | 62 (54-70) | 66 (59-73) |
| Male sex | 2838 (67.8) | 707 (62.5) | 2022 (69.5) | 97 (66.5) |
| Nonwhite race/ethnicity | 400 (9.6) | 175 (15.5) | 390 (13.4) | 25 (17.1) |
| Medicare/Medicaid | 1365 (32.6) | 515 (45.5) | 1089 (37.4) | 54 (37.0) |
| Unemployed | 2077 (49.6) | 707 (62.5) | 1538 (52.8) | 75 (51.4) |
| High school graduate | 3597 (85.9) | 901 (79.7) | 2490 (85.6) | 122 (83.6) |
| Medical history | ||||
| Prior MI | 709 (16.9) | 281 (24.9) | 692 (23.8) | 31 (21.2) |
| Prior PCI | 862 (20.6) | 355 (31.4) | 866 (29.8) | 40 (27.4) |
| Prior CABG | 381 (9.1) | 174 (15.4) | 349 (12.0) | 21 (14.4) |
| Prior stroke or TIA | 217 (5.2) | 96 (8.5) | 223 (7.7) | 14 (9.6) |
| Peripheral arterial disease | 211 (5.0) | 100 (8.8) | 195 (6.7) | 13 (8.9) |
| Prior HF | 237 (5.7) | 149 (13.2) | 244 (8.4) | 19 (13.0) |
| Diabetes | 1230 (29.4) | 420 (37.1) | 1035 (35.6) | 49 (33.6) |
| Hypertension | 2762 (66.0) | 820 (72.5) | 2070 (71.1) | 111 (76.0) |
| Depression | 415 (9.9) | 175 (15.5) | 360 (12.4) | 11 (7.5) |
| Previously missed medication | ||||
| Never | 2245 (53.6) | 541 (47.8) | 1359 (46.7) | 83 (56.9) |
| 1-3 Times/mo | 1471 (35.1) | 394 (34.8) | 1119 (38.4) | 48 (32.8) |
| ≥1 Time/wk | 322 (7.7) | 162 (14.3) | 326 (11.2) | 11 (7.5) |
| Index hospitalization | ||||
| STEMI | 2037 (48.7) | 458 (40.5) | 1300 (44.7) | 52 (35.6) |
| Multivessel CAD | 1901 (45.4) | 526 (46.5) | 1374 (47.2) | 67 (45.9) |
| PCI performed | 3854 (92.1) | 856 (75.7) | 2621 (90.0) | 136 (93.2) |
| CABG | 41 (1.0) | 49 (4.3) | 34 (1.2) | 1 (0.7) |
| Length of stay, median (IQR), d | 2 (2-3) | 3 (2-4) | 2 (2-4) | 3 (2-4) |
Abbreviations: CABG, coronary artery bypass grafting; CAD, coronary artery disease; HF, heart failure; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; TIA, transient ischemic attack.
Compared with patients who were concordantly medication nonpersistent and those with discordant persistence metrics, concordantly persistent patients had the lowest prevalence of cardiovascular comorbidities and risk factors at baseline—including prior MI, prior stroke or transient ischemic attack, heart failure, peripheral arterial disease, hypertension, and diabetes (Table). Compared with patients who were concordantly medication nonpersistent, patients who self-reported persistence but were found to be nonpersistent by pharmacy claims were younger (median age, 64 years [interquartile range, 54-73 years] vs 62 years [interquartile range, 54-70 years]), more often male (707 [62.5%] vs 2022 [69.5%]), currently employed (1373 [47.2%] vs 424 [32.3%]), and high school graduates (901 [79.7%] vs 2490 [85.6%]), and they more often underwent percutaneous coronary intervention during the index hospitalization (856 [75.7%] vs 2621 [90.0%]).
Among 2911 patients who reported medication persistence but were nonpersistent by pharmacy fill data, 508 (17.5%) had no record of a P2Y12 inhibitor pharmacy fill on or after the index discharge date even though they had records of other medications being filled. In addition, 1289 of 2911 patients (44.3%) reported filling P2Y12 inhibitor prescriptions at more than 1 pharmacy during the course of follow-up; in 798 patients (61.9%), the timing of pharmacy switch coincided with the interval during which they became nonpersistent by pharmacy fill.
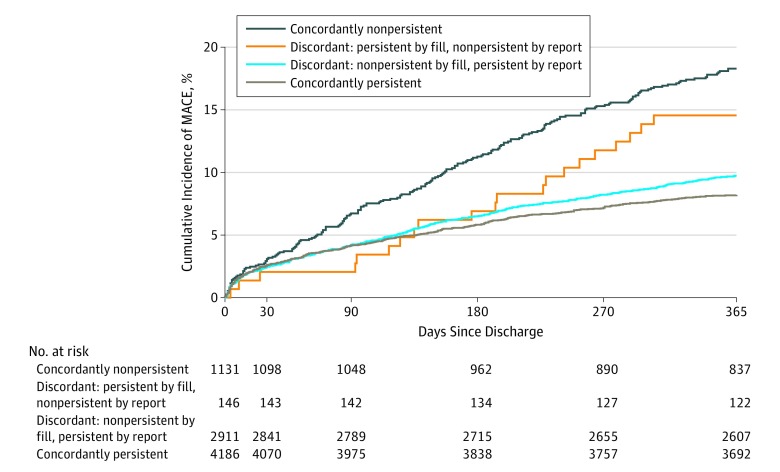
Major adverse cardiac events occurred in 842 of 8373 patients (10.1%) over the 12-month follow-up period. Kaplan-Meier event rates were highest (18.3%; 95% CI, 16.0%-20.6%) in the concordantly nonpersistent group, lowest (8.2%; 95% CI, 7.4%-9.0%) in the concordantly persistent group, and intermediate (9.7%; 95% CI, 8.7%-10.8%) in the discordant group that was persistent by patient report but nonpersistent by pharmacy fill. Of the 146 patients who reported medication nonpersistence but were persistent by pharmacy fill data, 21 (14.5%; 95% CI, 8.8%-20.3%) had MACEs (Figure 3; eTable 3 in Supplement 2).
Figure 3. Major Adverse Cardiovascular Events (MACEs) by Self-reported and Pharmacy Fill Persistence.
Cumulative incidence curves show MACEs during a 1-year follow-up. Rates of MACEs were highest for patients concordantly medication nonpersistent, lowest in patients concordantly persistent, and intermediate for both groups of discordant patients.
Accuracy of Medication Persistence Measured by Self-report vs Pharmacy Fill
In total, 3703 patients were treated at hospitals randomized to the copayment voucher intervention, used their voucher at least once after hospital discharge, and had pharmacy fill data. Medication nonpersistence, defined as any gap in supply of 30 days or more when using both voucher and Symphony data, was detected in 1184 patients (32.0%; 95% CI, 29.9%-34.2%). Compared with this criterion standard, medication nonpersistence rates were 10.4% (95% CI, 8.9%-12.1%) by patient report alone and 40.2% (95% CI, 36.5%-43.9%) by pharmacy fills alone in this population. Among the 3318 patients who self-reported persistence, 892 (26.9%; 95% CI, 25.1%-28.7%) were nonpersistent by both pharmacy fill data and voucher use. Patient report had a sensitivity of 24.7% and a specificity of 96.3% for identifying medication nonpersistence. The positive predictive value was 75.8%, and the negative predictive value was 73.1%. Of the 1487 patients nonpersistent by pharmacy fill data alone, 303 (20.4%; 95% CI, 15.7%-25.9%) were found to be persistent when voucher data were considered (eFigure 2 in Supplement 2).
Of 944 patients randomly selected for serum P2Y12 inhibitor level testing, 93 (9.6%; 95% CI, 8.0%-11.6%) had levels below the detectable range. The κ values between patient report and serum P2Y12 inhibitor level results ranged from 0.11 to 0.23 at 3, 6, 9, and 12 months, indicating poor agreement beyond chance. The κ values between pharmacy fill and serum P2Y12 inhibitor level results ranged from 0.00 to 0.19.
Discussion
The ARTEMIS trial included extensive measurements of longitudinal medication use based on a combination of patient self-report, pharmacy fills, copayment voucher use, and random cross-sectional serum testing of P2Y12 inhibitor drug levels. Overall, there was imperfect agreement between these measurements of persistence. Persistence rates were substantially higher when assessed by patient report than when assessed by pharmacy fill data, likely owing to overestimation by patient report and underestimation by pharmacy data. Patients with medication nonpersistence identified by both patient report and pharmacy fill had the highest rate of adverse cardiovascular event rates. These results show the lack of a reliable single standard for measuring medication persistence in clinical care or pragmatic clinical trials.
Patient report is subject to a number of biases that can lead to incorrect estimation of persistence.13 Social desirability bias, in which patients misrepresent medication-taking behavior to avoid disappointing their care team,14 can lead patients to overestimate persistence. Recall bias, when patients do not remember which medications they are taking, affects persistence estimates unpredictably. The net effect, as we showed in this analysis, is an overestimation of medication persistence; approximately 25% of patients reporting persistence with P2Y12 inhibitors were found to be nonpersistent, with a sensitivity of 24% for detecting nonpersistence compared with the criterion standard.
Pharmacy fill data also have limitations. In the United States, medications are variably dispensed based on health system, payer, insurance plan, pharmacy, or pharmacy benefit manager selections without a centralized data repository.11,15 Our study showed that, even with large pharmacy claims databases, not all pharmacy transactions may be captured. Among patients who reported persistence but were deemed nonpersistent by pharmacy fills, almost half reported switching pharmacies, with the majority of those switches coinciding with their transition to nonpersistent status. Patients often need to switch pharmacies (eg, to mail-order fills as mandated by some insurers or pharmacy benefit managers, or for convenience and cost reasons), and such patients may be incorrectly classified as nonpersistent if subsequent fills are incompletely represented. Similar ascertainment bias may be present in payer claims (eg, Medicare Part D) or integrated health systems (eg, US Department of Veteran Affairs) in which data are linked to the patient rather than to the pharmacy. Patients who fill prescriptions without going through their insurance may be deemed medication nonpersistent. Pharmacy fill records, regardless of source, can also misclassify patients who get medications from the hospital, clinic, or another source.
The accuracy of each measurement method is difficult to ascertain on a broad scale. The ARTEMIS trial design was unique in being able to compare patient-reported and pharmacy fill–based persistence first against voucher use and then against serum drug levels. Recognizing that patients may forget or choose not to use the voucher, we defined a criterion standard based on both voucher use and pharmacy fills so that patients would not be miscategorized if they filled without using the voucher. This approach, while imperfect, leveraged the study intervention to allow us to quantify the potential gaps in ascertainment associated with either patient report or pharmacy fills alone. The ARTEMIS trial was also unique in directly measuring serum drug levels, and it showed poor correlation between serum testing results and either patient report or pharmacy fill. A phlebotomy-based medication assessment is not scalable and provides only a single snapshot in time. A patient who is medication nonpersistent may have taken a dose prior to providing a scheduled blood sample and may be classified as persistent even if a gap in use existed prior to that last dose.
Similar to prior analyses, the present study showed that patient self-report overestimated medication persistence compared with other more “objective” measures of persistence.16,17,18 New compared with prior studies, the present study quantified the underestimation of medication persistence by pharmacy fill data and discordance when compared with serum drug metabolite levels and copayment voucher use. Lack of concordance across the methods highlights the lack of a true criterion standard for measurement of medication persistence and the potential benefits of measuring persistence with multiple methods.19
In clinical care, medication nonpersistence leads to worse outcomes, and identifying nonpersistent patients is critical to implementing strategies, such as remote monitoring, pharmacist intervention, and intensive patient education effort, to improve medication persistence and downstream outcomes.20,21 Physicians have historically measured medication-taking behavior by asking patients, which is self-report. Increasingly, electronic health records have provided physicians and other health care professionals access to pharmacy fill data, thus providing an alternative or complementary measure of medication-taking behavior. The present study showed that neither patient report alone nor pharmacy fill data alone represents the “truth” about medication persistence. However, patients who are nonpersistent by both methods have the worst clinical outcomes; these are patients who should be prioritized for interventions that can improve medication-taking behavior.
Limitations
As a clinical trial studying medication persistence, ARTEMIS may have limitations in generalizability owing to the Hawthorne effect. Self-reported persistence was measured only for patients who enrolled in and completed follow-up for the clinical trial; rates of medication persistence may already be higher for these patients compared with those treated in routine clinical practice. In addition, eligible patients had to have US-based health insurance with a prescription drug benefit, and the results may not be generalizable to patients without health insurance or prescription coverage. Although ARTEMIS linked to a pharmacy fill database that encompasses most US pharmacies, P2Y12 inhibitor persistence may be underestimated if it is filled at a nonlinked pharmacy. For analyzing MACEs by persistence status, patients were categorized based on persistence during the entire study period, and it is possible that recurrent MI or stroke could affect persistence following the event. Timing of phlebotomy relative to the last P2Y12 inhibitor dose was not captured. Direct observed therapy or pill counts could not be feasibly implemented in this large, pragmatic, multicenter trial; thus, the criterion standard for persistence was defined in the intervention arm, in which persistence could be corroborated through multiple features, recognizing that this definition remains vulnerable to a disconnect between prescription fill and actual medication consumption.
Conclusions
Patient-reported and pharmacy fill–based persistence measures were frequently discordant. Patient report overestimated medication persistence, and pharmacy fill data underestimated medication persistence. Patients with nonpersistence identified by both patient report and pharmacy fill had the highest rate of adverse cardiovascular event rates, and such patients should be prioritized for interventions that improve medication-taking behavior.
Trial Protocol
eTable 1. Baseline Characteristics of the Population With and Without a Prescription Fill in the Symphony Health Database
eTable 2. Baseline Patient Characteristics
eTable 3. Twelve-Month Kaplan-Meier Event Rates by Patient Reported and Pharmacy Fill Persistence
eFigure 1. Agreement Between Patient Report and Pharmacy Fill
eFigure 2. Agreement Between Patient Report, Pharmacy Fill, and Voucher Use in Intervention Arm Patients Who Used the Voucher at Least Once
References
- 1.Chen H-Y, Saczynski JS, Lapane KL, Kiefe CI, Goldberg RJ. Adherence to evidence-based secondary prevention pharmacotherapy in patients after an acute coronary syndrome: a systematic review. Heart Lung. 2015;44(4):299-308. doi: 10.1016/j.hrtlng.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177-186. doi: 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]
- 3.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521-530. doi: 10.1097/01.mlr.0000163641.86870.af [DOI] [PubMed] [Google Scholar]
- 4.Benjamin RM. Medication adherence: helping patients take their medicines as directed. Public Health Rep. 2012;127(1):2-3. doi: 10.1177/003335491212700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier D, Gupta R, Kamath D, et al. Community health worker-based intervention for adherence to drugs and lifestyle change after acute coronary syndrome: a multicentre, open, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4(3):244-253. doi: 10.1016/S2213-8587(15)00480-5 [DOI] [PubMed] [Google Scholar]
- 6.Lavsa SM, Holzworth A, Ansani NT. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc (2003). 2011;51(1):90-94. doi: 10.1331/JAPhA.2011.09154 [DOI] [PubMed] [Google Scholar]
- 7.Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi: 10.1155/2015/217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther. 1999;21(6):1074-1090. doi: 10.1016/S0149-2918(99)80026-5 [DOI] [PubMed] [Google Scholar]
- 9.Doll JA, Wang TY, Choudhry NK, et al. Rationale and design of the Affordability and Real-world Antiplatelet Treatment Effectiveness after Myocardial Infarction Study (ARTEMIS): a multicenter, cluster-randomized trial of P2Y12 receptor inhibitor copayment reduction after myocardial infarction. Am Heart J. 2016;177:33-41. doi: 10.1016/j.ahj.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Wang TY, Kaltenbach LA, Cannon CP, et al. Effect of medication co-payment vouchers on P2Y12 inhibitor use and major adverse cardiovascular events among patients with myocardial infarction: the ARTEMIS randomized clinical trial. JAMA. 2019;321(1):44-55. doi: 10.1001/jama.2018.19791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navar AM, Taylor B, Mulder H, et al. Association of prior authorization and out-of-pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2(11):1217-1225. doi: 10.1001/jamacardio.2017.3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.StataCorp Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 13.Shi L, Liu J, Fonseca V, Walker P, Kalsekar A, Pawaskar M. Correlation between adherence rates measured by MEMS and self-reported questionnaires: a meta-analysis. Health Qual Life Outcomes. 2010;8(1):99. doi: 10.1186/1477-7525-8-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnette A, Zhang Y, Shao H, Shi L. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices: an updated review. Pharmacoeconomics. 2018;36(1):17-27. doi: 10.1007/s40273-017-0570-9 [DOI] [PubMed] [Google Scholar]
- 15.Wang SM, Aranda GA Jr, Gao S, Patel BV. Benefit restrictions and gout treatment. J Manag Care Pharm. 2013;19(9):773-782. doi: 10.18553/jmcp.2013.19.9.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42(7):649-652. doi: 10.1097/01.mlr.0000129496.05898.02 [DOI] [PubMed] [Google Scholar]
- 17.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413-422. doi: 10.1345/aph.1L496 [DOI] [PubMed] [Google Scholar]
- 18.Kubica A, Kosobucka A, Fabiszak T, Gorog DA, Siller-Matula JM. Assessment of adherence to medication in patients after myocardial infarction treated with percutaneous coronary intervention: is there a place for new self-reported questionnaires? Curr Med Res Opin. 2019;35(2):341-349. doi: 10.1080/03007995.2018.1510385 [DOI] [PubMed] [Google Scholar]
- 19.Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590-600. doi: 10.1016/j.pcad.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785-795. doi: 10.7326/0003-4819-157-11-201212040-00538 [DOI] [PubMed] [Google Scholar]
- 21.Walker PC, Bernstein SJ, Jones JNT, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009;169(21):2003-2010. doi: 10.1001/archinternmed.2009.398 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Baseline Characteristics of the Population With and Without a Prescription Fill in the Symphony Health Database
eTable 2. Baseline Patient Characteristics
eTable 3. Twelve-Month Kaplan-Meier Event Rates by Patient Reported and Pharmacy Fill Persistence
eFigure 1. Agreement Between Patient Report and Pharmacy Fill
eFigure 2. Agreement Between Patient Report, Pharmacy Fill, and Voucher Use in Intervention Arm Patients Who Used the Voucher at Least Once