Abstract
Aims
Brain derived neurotrophic factor (BDNF) is essential for cognitive function. It is also found in non-neuronal tissues with various regulatory actions, including metabolic. Physical fitness (PF) is associated with improved synthesis and secretion of BDNF and reduced obesity. However, the importance of PF for the relationship of BDNF with obesity has not been investigated. This study aims at examining the relationship of PF with BDNF and obesity in 174 young (age = 25.30 ± 9.2 years) healthy adults.
Main methods
Serum BDNF was evaluated using ELISA while obesity was determined using body weight (BW), BMI, and waist circumference (WC). Six minute walk distance (6MWD) test was used to estimate PF.
Key findings
Serum BDNF was greater (p = 0.000) in the participants with high (Hi6MWD) versus low (Lw6MWD) PF group. Additionally, 6MWD explained 6.8% of serum BDNF. Obesity measures were greater (p < 0.05) in the participants with low versus high BDNF. In regression analyses, serum BDNF explained 4.7% of BW (p = 0.004), 3.8% of BMI (p = 0.011), and 6.2% of WC (p = 0.001). However, when the participants were divided into Hi6MWD and Lw6MWD, BDNF explained 8.2% of BW (p = 0.009), 6.0% of BMI (p = 0.03), and 7.0% of WC (p = 0.013), only in the Hi6MWD, but not in the Lw6MWD (p > 0.05) groups.
Significance
The finding confirms the relationship of BDNF with obesity. Additionally, it further suggests the importance of PF level to this relationship among young adults. Future studies are needed to confirm these findings.
Keywords: Biological sciences, Proteins, Biochemistry, Molecular biology, Toxicology, Pathophysiology, Health sciences, Public health, Physiology, Six minute walk distance, Fitness, BDNF, Obesity, BMI, Waist circumference
Biological sciences; Proteins; Biochemistry; Molecular biology; Toxicology; Pathophysiology; Health sciences; Public health; Physiology; Six minute walk distance; Fitness; BDNF; Obesity; BMI; Waist circumference.
1. Introduction
Obesity is a proliferating global epidemic. Since 1980 to 2013, the global prevalence has increased from 857 million to 2.1 billion, whereas it is expected to reach ~3.3 billion in 2030 [1,2]. It affects many body functions such as cardiovascular, endocrine, and Musculoskeletal functions, is a risk factor for several diseases including metabolic syndrome, diabetes and hypertension, can results in profound loss in life quality and expectancy, and is a leading cause of morbidity and mortality [3, 4]. In fact, recently obesity has been classified as a disease [5, 6, 7].
The underlining cause of obesity is the positive energy balance (i.e. high consumption versus low expenditure of calories). However, the etiology is multifactorial that involves complex orchestration of peripheral and central mediators. Therefore, the WHO has called for a global surveillance system to identify risk factors, thus strategies, to restrain obesity proliferation by 2025 [8].
Brain derived neutorophic factor (BDNF) is synthesized and functions primarily in the central nervous system (CNS) and is pivotal for neurocognitive performance. However, it is found and functions in various body locations, including the adipose tissue [9]. Additionally, it has been implicated in cardiometabolic diseases, suggesting a metabotrophic role, though the exact function is yet to be clarified [10].
The relationship of exercise with BDNF is well-known. Exercise seems to increase mRNA transcription [11], subsequently BDNF levels [12, 13, 14, 15]. Similarly, moderate exercising, as little as 150 min/week, can contribute to weight control, though additional participation is more effective [16, 17, 18]. Furthermore, it has been suggested that BDNF deficiency is a risk factor for obesity [19] whereas activation of BDNF receptor prevents the progression of diabetes [20]. However, the effect of physical fitness (PF), a reflection of exercise participation [21], on the relationship of BDNF with obesity is not known. Since BDNF [12, 13, 14, 15] and obesity [21] are altered with exercise, the relationship might also be PF-dependent. Therefore, the purpose of the current study is two folds. (i) Verify the relationship of serum BDNF with PF and obesity; (ii) Examine the role of PF on the relationship of BDNF with obesity. Determining this triangular relationship can further enhance our knowledge of the importance of exercise/PF for BDNF and obesity.
2. Material and methods
2.1. Design and recruitment
The study was cross-sectional and observational to examine the role of PF in the relationship of BDNF with obesity. Serum BDNF, obesity, and PF were assessed in apparently healthy young (age: 18–35 years) adults. All participants were randomly selected from the local community in May 2014–April 2016. Participants were invited using flayers, advertisements, and personal contacts. After agreeing to participate, the participants filled a medical history questionnaire before initiation of the study. All eligible participants signed an informed consent approved by the Institutional Review Board with ethical clearance number: MA/OK-155/2014. Volunteers with acute medical conditions, cardiovascular diseases, diabetes mellitus, psychiatric, stress-related, and mood disorders were excluded from the study. Additionally, individuals taking medications were excluded from the study. Data was analyzed by a blinded researcher to ensure impartiality.
2.2. Obesity measurements
Three measures were used to determine obesity, including body weight (BW), body mass index (BMI), and waist circumference (WC). Standard weight scale (Microlife WS 100, Microlife AG, Heerbrugg, Switzerland) and tab measure were used to obtain weight and height, respectively. The WC determined at the umbilicus height [22, 23]. The international standards for BMI ranges were used in the current study. These ranges include height [22, 23]:
Underweight: BMI is less than 18.5
Normal weight: BMI is 18.5–24.9
Overweight: BMI is 25–29.9
Obese: BMI is 30 or more
Additionally, the following ranges were used for the WC in the current study height [22, 23]:
Men: 102 cm or more
Women: 89 cm or more
2.3. Serum BDNF measurement
Fasting blood samples were drawn from antecubital veins of the participants into plain glass tubes while they were in sitting position. After collection, samples were spun for 8–10 min at 1500 xg to obtain serum for BDNF. Serum samples were then divided into several aliquots and immediately stored at -80 °C for future use.
Serum BDNF level was determined using enzyme linked immunosorbent assay (ELISA) specific for BDNF and as described in the kit manual (Human BDNF Duoset ELISA Kit R&D system, USA). In brief, the samples were added to an anti-human BDNF antibody coated ELISA wells and allowed to incubate for 2 h at room temperature. Subsequently, biotinylated anti-human BDNF antibody was introduced and allowed to bind to the captured BDNF for 1 h at the same temperature. Horse-radish-peroxidase (HRP)-streptavidin conjugate was afterward added to the reaction mixture then incubated at room temperature for 45 min. Finally, tetramethyl benzidine (TMB) was added to each well followed by additional incubation for 30 min at room temperature in the dark. The color developed after the incubation was read at 450 nm immediately and levels of serum BDNF were deducted from the standard provided by the kit [14, 24].
2.4. Physical fitness measurement
Level of aerobic PF was determined using the 6 min walk test (6MWD) [25], according to the American Thoracic Society Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories guidelines [26, 27]. It was conducted in an enclosed corridor and on a flat ground and a circular track of 33 m long [28]. The walking course was demarcated with bright colored cones. During the test, participants were encouraged, at intervals, to make sure that they progress at a constant pace. Number of loops covered by the participants were then multiplied by the loop distance (33 m) [29].
The 6MWD test is widely used in a variety of populations, including healthy and diseased young and elderly. However, norms (i.e. reference values) are scares [30] and vary across populations and countries [31]. Data from Northern Africa [32] and Saudi Arabia [28] were different, though both are from the Middle East region. Thus, given these inconsistencies [30, 33, 34], the participants’ PF in the current study were divided to below (Lw6MWD) and above (Hi6MWD) the 50th percentile of the 6MWD scores.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software for Windows (version 19.0; Chicago, IL). Group data are expressed as means ± SD, and α was set a priori at P < 0.05. All primary study measures (BDNF, 6MWD, and obesity indices) were tested for normality. A measure with skewedness score (Sk) less than |1.0| was considered normally distributed. Additionally, the relative distance of a particular data point from the data mean was used to remove outliers. Subsequent statistical tests (i.e. Student's t-test, ANCOVA, Pearson's correlation, and regression) were performed after transforming skewed variables as well as after removing the outliers.
ANCOVA tests were used to compare BW, BMI, and WC between the participants in the Hi6MWD versus Lw6MWD as well as the 6MWD score in the above (HiBDNF) versus below (LwBDNF) the serum BDNF mean (μ = 26.3 ± 12.8 μg/dl). The ANCOVA was used to covariate for serum cholesterol, glucose, and triglycerides. Cohen's d was used as a measure of effect size; in general, <0.20 is considered to be a small effect, >0.20 to <0.50 a moderate effect, and >0.50 a large effect [35].
The relationships between 6MWD, BDNF, and obesity indices were examined with Pearson's correlations. In general, r values < 0.10 are considered to be a small effect, >0.10 to <0.50 a moderate effect, and >0.50 a large effect [36]. Linear regression was, subsequently, performed to examine the ability of serum BDNF to predict obesity (BW, BMI, and WC) when correlations were found significant between variables. The correlation and regression analyses were performed for the entire sample then in the Hi6MWD and Lw6MWD groups.
3. Results
3.1. Participants
The participant characteristics are shown in Table 1. A total of 174 young adults agreed to participant in the study. No differences were found between the Lw6MWD versus Hi6MWD in height, BW, BMI, and WC. Additionally, the mean 6MWD score and serum BDNF were 563.8 ± 93.7 m and 26.3 ± 12.8 μg/dl, respectively.
Table 1.
Participant characteristics.
| Lw6MWD (n = 86) | Hi6MWD (n = 88) | p-value | |
|---|---|---|---|
| Age (years | 23.5 ± 6.6 | 25.0 ± 6.6 | 0.1 |
| Height (cm) | 166.2 ± 9.6 | 168.8 ± 9.8 | 0.1 |
| Weight (kg) | 67.9 ± 15.0 | 68.5 ± 18.6 | 0.9 |
| BMI (kg/m2) | 24.5 ± 4.6 | 23.9 ± 5.5 | 0.5 |
| Waist circumference (cm) | 83.5 ± 11.8 | 81.8 ± 15.4 | 0.4 |
| Percent body fat (%) | 24.5 ± 8.7 | 23.7 ± 8.9 | 0.6 |
Data presented in mean ± sd.
3.2. Confirming normality
All primary study outcomes, including 6MWD, serum BDNF, and obesity measures were normally distributed (Sk < 1.0).
3.3. Relationship of 6MWD with serum BDNF
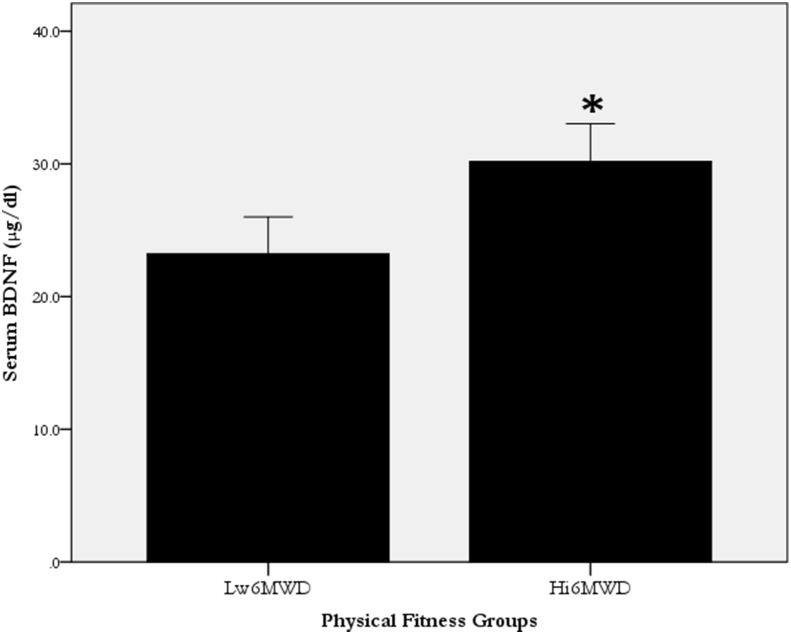
As in Figure 1, the ANCOVA shows that serum BDNF was lower (p = 0.000) in the Lw6MWD versus the Hi6MWD. Pearson's correlation, shown in Table 3, demonstrated a medium relationship (r = 0.3; p = 0.001) of 6MWD test with serum BDNF. Similarly, subsequent simple linear regression analysis revealed that the 6MWD explained 6.8% of BDNF (BDNF = 4.9 + 0.038∗6MWD; p = 0.001).
Figure 1.
The difference (p < 0.006) in serum BDNF (μg/dl) between the participants with low versus high maximum walked distance groups. Data presented in mean ± SE.
Table 3.
Correlations of BDNF with 6MWD and obesity measures in all participants and the Hi6MWD and Lw6MWD groups.
| Serum BDNF (μg/dl) | |||
|---|---|---|---|
| 6MWD | Weight | BMI | WC |
| All participants | |||
| r = -0.30; p = 0.000 | r = -0.20; 0.004 | r = -0.20; p = 0.011 | r = -0.25; p = 0.001 |
| Hi6MWD group | |||
| r = -0.20; p = 0.023 | r = -0.30; p = 0.009 | r = -0.24; p = 0.030 | r = -0.30; p = 0.013 |
| Lw6MWD group | |||
| r = -0.20; p = 0.135 | r = 0.12; p = 0.400 | r = -0.02; p = 0.850 | r = -0.20; p = 0.170 |
6MWD = maximum walked distance in 6 min; BMI = Body mass index; WC = waist circumference; BDNF = brain derived neurotrophic factor.
3.4. Relationship of BDNF with obesity
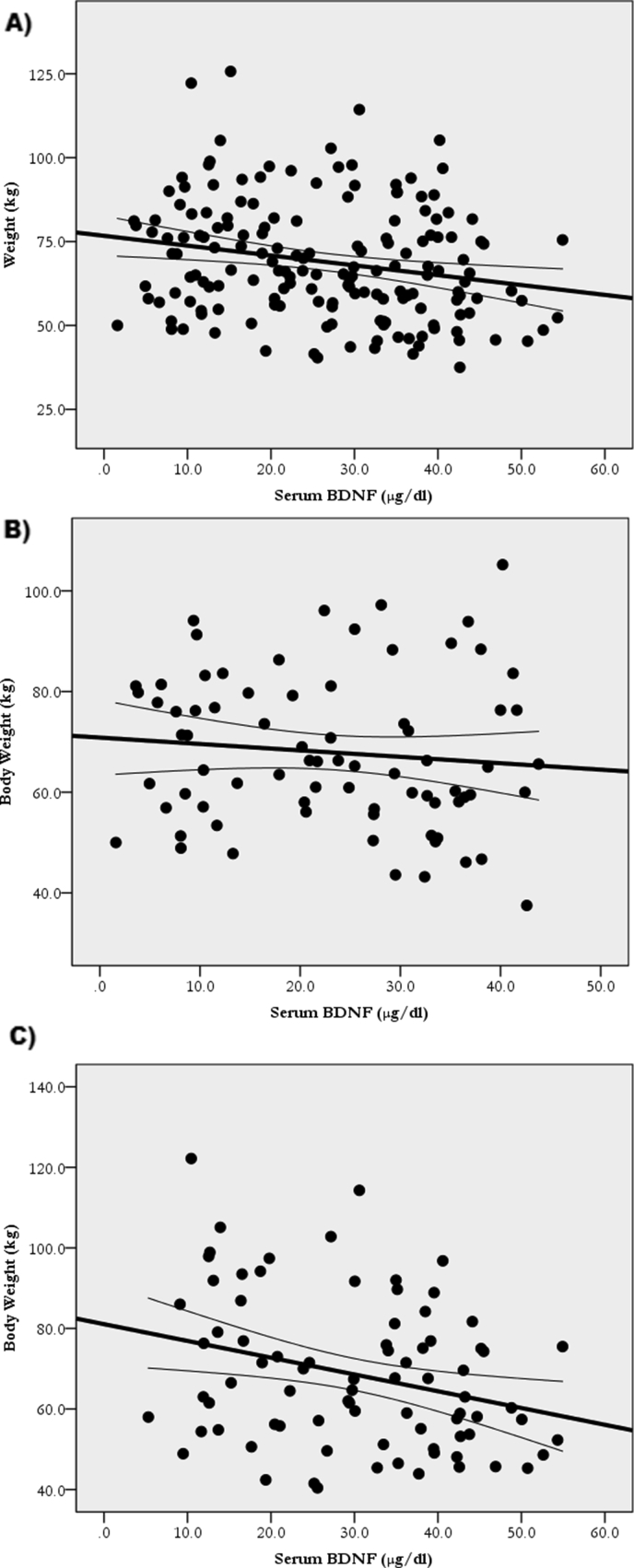
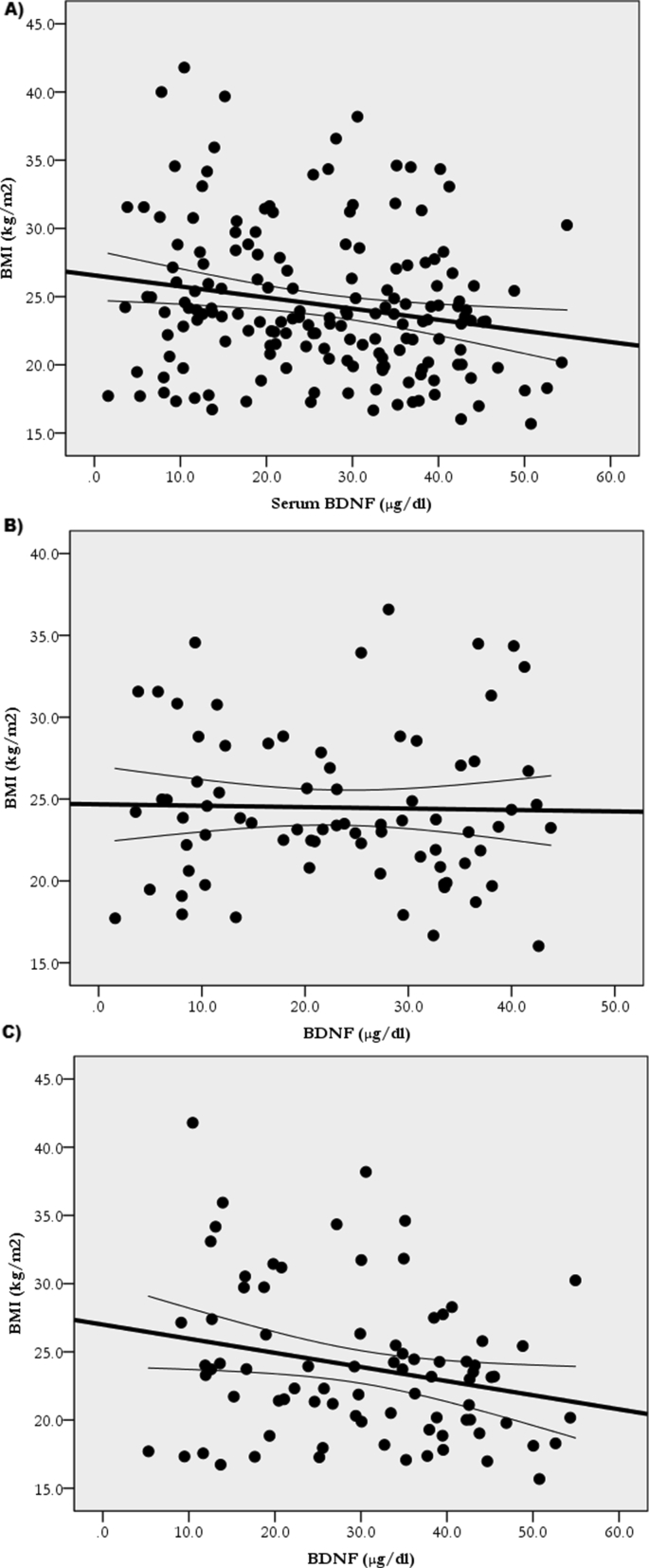
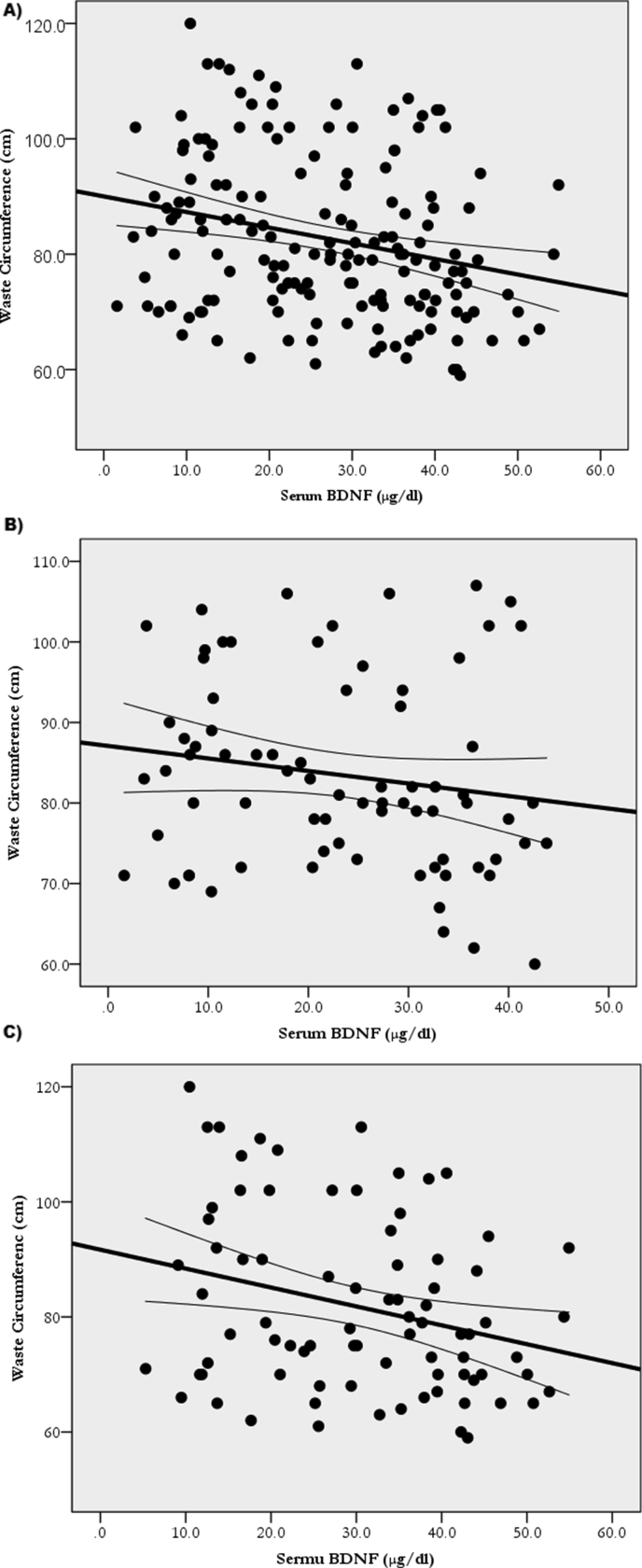
Table 2 shows the ANCOVA revealing greater (p < 0.05) BW, BMI, and WC in the LwBDNF versus HiBDNF groups. Pearson's correlation, in Table 3, revealed that serum BDNF correlated with BW (r = -0.2; p = 0.004), BMI (r = -0.2; p = 0.011), and WC (r = -0.25; p = 0.001). Subsequent simple linear regression analyses revealed that BDNF explained 4.7% of BW (BW = 76.7+(-0.3)∗BDNF; p = 0.004) (Figure 2A), explained 3.8% of BMI (BMI = 26.5+(-0.081)∗BDNF; p = 0.011) (Figure 3A), and explained 6.2% of WC (WC = 90.0+(-0.3)∗BDNF; p = 0.001) (Figure 4A).
Table 2.
Obesity in the LwBDNF versus HiBDNF.
| LwBDNF (n = 85) | HiBDNF (n = 89) | p-value | |
|---|---|---|---|
| Weight (kg) | 74.6 ± 20.1 | 66.5 ± 17.9 | 0.05 |
| BMI (kg/m2) | 26.0 ± 6.3 | 23.8 ± 5.2 | 0.05 |
| Waist circumference (cm) | 86.4 ± 14.8 | 80.5 ± 13.2 | 0.002 |
Data presented in mean ± sd.
Figure 2.
Relationship of BDNF with Weight in all participants A: (BW = 78.9+(-0.3)∗BDNF; R2 = 0.047; p = 0.004); the Hi6MWD group B: (BW = 81.1+(-0.41)∗BDNF; R2 = 0.082; p = 0.009); and the Lw6MWD group C: (BW = 70.8+(-0.1)∗BDNF; R2 = 0.01; p = 0.4).
Figure 3.
Relationship of BDNF with BMI in all participants A: (BMI = 26.5+(-0.081)∗BDNF; R2 = 0.038; p = 0.011); the Hi6MWD B: (BMI = 26.99+(-0.1)∗BDNF; R2 = 0.06; p = 0.03); and the Lw6MWD C: (BMI = 24.7+(-0.009)∗BDNF; R2 = 0.001; p = 0.85).
Figure 4.
Relationship of BDNF with Waist Circumference in all participants A: (WC = 90.0+(-0.3)∗BDNF; R2 = 0.062; p = 0.001), the Hi6MWD group B: (WC = 91.7+(-0.3)∗BDNF; R2 = 0.07; p = 0.013), and the Lw6MWD group C: (WC = 87.1+(-0.2)∗BDNF; R2 = 0.03; p = 0.17).
3.5. Effect of PF level on the relationship of BDNF with obesity
After dividing the participants according to 6MWD scores, as in Table 3, BDNF correlations with obesity measures remained only in the Hi6MWD (BW: r = -0.3; p = 0.009, BMI: r = -0.24; p = 0.03, WC: r = -0.3; p = 0.013) but not in the Lw6MWD (BW: r = -0.12; p = 0.4, BMI: r = -0.02; p = 0.85, WC: r = -0.2; p = 0.17). Additionally, prediction of obesity remained only in the Hi6MWD (BW = 81.1+(-0.41)∗BDNF; R2 = 0.082; p = 0.009 (Figure 2B)), (BMI = 26.99+(-0.1)∗BDNF; R2 = 0.06; p = 0.03 (Figure 3B)), and (WC = 91.7+(-0.3)∗BDNF; R2 = 0.07; p = 0.013 (Figure 4B)) but not in the Lw6MWD (BW = 70.8+(-0.1)∗BDNF; R2 = 0.01; p = 0.4 (Figure 2C)), (BMI = 24.7+(-0.009)∗BDNF; R2 = 0.001; p = 0.85 (Figure 3C)), and (WC = 87.1+(-0.2)∗BDNF; R2 = 0.03; p = 0.17 (Figure 4C)).
4. Discussion
The current study examined the role of PF on the relationship of BDNF with obesity in young adults. A secondary aim was to verify the relationship of BDNF with PF and obesity. Serum BDNF correlated with 6MWD scores and obesity measures including BW, BMI, and WC. Additionally, it was reduced in the participants with greater obesity and lower 6MWD scores. After dividing the participants to Hi6MWD and Lw6MWD groups, the correlations of BDNF with all obesity measures remained only in the Hi6MWD but not in the Lw6MWD group. These findings might suggest that improving PF may enhance the possible favorable contribution of BDNF in obesity control [37, 38].
Regular exercise enhances BDNF in the brain [12, 14, 39], platelets [40, 41] plasma [42], and serum [41, 43]. The effect of PF level on circulating BDNF is however considerably less reported and understood [44, 45]. Data have shown that PF (i.e. estimated VO2max) was associated with lower serum BDNF in healthy males and females [44]. This inverse relationship was attributed to higher circulatory glucocorticoid levels usually found in athletes [46] and individuals with higher PF [47]. However, it was further elaborated that post-exercise glucocorticoid and BDNF levels are not related [48]. Additional speculations indicated that reduced serum BDNF might be due to a more efficient brain uptake of BDNF to promote neural function in trained individuals [44]. However, more experimental evidence to support this speculative hypothesis is needed [44]. Similarly, another study found inverse correlations of serum BDNF with PF, however positively with CV risk factors, including obesity [45]. The study, thus, suggests negative effects of PF on BDNF circulatory level and obesity as a CV risk factor. Thus, increased PF is not always associated with favorable health-related measures [10, 18, 49, 50], including obesity [21].
Opposite to these findings, the current study found positive correlation between BDNF and PF as measured using 6MWD. This is the first to report a relationship of BDNF with 6MWD, a test mimics popular daily activities (i.e. walking) [25]. Similar to our findings, one study reported greater plasma BDNF in young athletes versus untrained [42]. Additionally, improved PF, following exercise training, correlated with elevations in circulating BDNF in older women [51] and schizophrenics [52].
This increased in circulatory BDNF in individuals with higher PF level/athletes, could be due to increased BDNF brain synthesis and release. Exercise-induced increase in circulating BDNF is most likely came from the brain, as BDNF can easily cross the blood-brain barrier [39]. We think constant muscle contraction during exercise, sends feedback signals to the CNS to manufacture and release BDNF in the brain, which then crosses into the circulation [10]. However, other sources cannot be excluded [53, 54, 55] especially it is stored in platelets in large amounts [54, 55] and synthesized in vascular endothelium cell [53], both are activated during exercise. Certainly more studies are needed to further verify these relationships and to identify BDNF "location(s) of origin".
Levels of BDNF are reduced in obese individuals. The role of BDNF in obesity seems to be primarily centrally mediated but peripheral contribution cannot be ignored. Early studies suggested that BDNF-knockout rats were more prone to obesity as compared to control [37], however when allowed equal amount of food, no differences in weight were observed [56]. However, genetically deleting tropomyosin receptor kinase B (TrkB), a primary BDNF receptor, resulted in hyperphagia and obesity [38]. These results suggested a central role including BDNF reduces appetite, thus promote a negative energy balance. Once activated with BDNF, CNS TrkB triggers a cascade of reactions that inhibits eating urges and weight gains [38]. Though fewer studies claim peripheral role on obesity, BDNF is expressed in the adipose tissue suggesting a metabolic involvement. It seems to communicate a feedback signal between the brain and the adipose tissue-derived adipokines (i.e. leptin), however the exact role is yet to be identified [57]. Still however, peripheral BDNF role in metabolism is unclear and requires further verifications.
The mean 6MWD (μ = 563.8 ± 93.7m) was distinctively different as compared to studies from the Middle East region. It was greater than found in Saudi Arabia (μ = 409 ± 51 m) [28] and lower than found in Northern Africa (μ = 680 ± 70 m) [32]. However similar to that reported from Singaporeans (μ = 560 ± 105 m) [33] and little less than Brazilians (μ = 571 ± 74 m) [58] and Italian (μ = 593 ± 57) [31]. The effect of PA level on the relationship of BDNF with obesity has never been examined. However, one study showed that 30 aerobic exercise sessions resulted in increased serum BDNF level coupled with reduced obesity [41]. Another study, postulated that negative associations of BDNF with CV risk factors, including obesity, might be because of increased PF [45]. The current study is the first to demonstrate that greater 6MWD scores fortified the relationship of BDNF with obesity. These results suggest that the favorable effect of BDNF on obesity might be found in individuals with higher level of PF. Thus enhancing PF is essential for BDNF to reduce obesity in young healthy individuals. Possibly, there is a "PF threshold", above which, circulatory BDNF can stimulate cellular adaptations that impact body metabolism, subsequently obesity. Alternatively, at lower PF, other regulatory mechanisms are more dominates, thus correlations between BDNF and obesity measures were diminished in the Lw6MWD group. In support of this, implementing an exercise program aiming at improving PF seems essential to the relationship of BDNF with obesity [41, 59]. Collectively, the relationships between PF, BDNF, and obesity seem more complex than expected [60], thus, these mere speculations need to be refined with more investigations.
4.1. Clinical implications
Obesity is a proliferating global epidemic, associated with many lethal diseases, and is an independent risk factor of morbidity and mortality. In fact, the recent classification of obesity as a disease has warranted an urgent need for strategies to combat it. Many strategies have been adapted; however, apparently, we are yet to succeed. Therefore, strategies to fight the obesity epidemic, and associated diseases, are still needed. According to the current findings, a strategy aimed at establishing a negative energy balance, enhancing PF, thus increasing BDNF seems appropriate. Obviously, exercise is a strategy that can, potentially, meet all these criteria. Therefore, as recommended by several organizations, moderate exercise on most days of the week should be encouraged for obese or at risk individuals [18].
4.2. Limitations
The current study is not without limitations. First and for most, causality interferences and generalizability of the results are confined to the cross-sectional design and the relatively smaller size. Additionally, we should realize that the 6MWD is not the best measurement for aerobic PF, especially no surrogate measurements were obtained, including heart rate, SpO2, and Borg and breathlessness. Therefore, to confirm the current finings, future studies should consider applying longitudinal/interventional design and recruiting larger sample. Additionally, using more specific test of aerobic PF along with surrogate measurements (i.e. heart rate, SpO2, and Borg and breathlessness scales) are warranted.
5. Conclusion
In the current study, we confirm the importance of BDNF for obesity. Additionally, the relationship of BDNF with obesity was found in individuals with higher, but not lower, fitness level. Therefore, the data suggest that the effect of BDNF might be “fitness-dependent”. However, studies aimed at examining the relationship of BDNF with obesity after improving PA, are warranted.
Declarations
Author contribution statement
M. Alomari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
O. Khabour: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
K. Alawneh: Conceived and designed the experiments; Performed the experiments.
K. Alzoubi: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Maikano: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
This work was supported by the Deanship of Research at the Jordan University of Science and Technology with research ID MA/OK-155/2014.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank the participants.
References
- 1.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly T., Yang W., Chen C.S., Reynolds K., He J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Younis N., Soran H., Farook S. The prevention of type 2 diabetes mellitus: recent advances. QJM. 2004;97:451–455. doi: 10.1093/qjmed/hch077. [DOI] [PubMed] [Google Scholar]
- 4.Eiselein L., Schwartz H.J., Rutledge J.C. The challenge of type 1 diabetes mellitus. ILAR J. 2004;45:231–236. doi: 10.1093/ilar.45.3.231. [DOI] [PubMed] [Google Scholar]
- 5.Malik V.S., Willett W.C., Hu F.B. Global obesity: trends, risk factors and policy implications. Nat. Rev. Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell S., Shaw D. The worldwide epidemic of female obesity. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29(3):289–299. doi: 10.1016/j.bpobgyn.2014.10.002. In this issue. [DOI] [PubMed] [Google Scholar]
- 7.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metrics. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gortmaker S.L., Swinburn B.A., Levy D., Carter R., Mabry P.L., Finegood D.T. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–847. doi: 10.1016/S0140-6736(11)60815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaldakov G., Tonchev A., Aloe L. NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Riv. Psichiatr. 2009;44:79. [PubMed] [Google Scholar]
- 10.Gomez-Pinilla F., Vaynman S., Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. Eur. J. Neurosci. 2008;28:2278–2287. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neeper S.A., Gomez-Pinilla F., Choi J., Cotman C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 12.Alomari M.A., Khabour O.F., Alzoubi K.H., Alzubi M.A. Combining restricted diet with forced or voluntary exercises improves hippocampal BDNF and cognitive function in rats. Int. J. Neurosci. 2015:1–8. doi: 10.3109/00207454.2015.1012587. [DOI] [PubMed] [Google Scholar]
- 13.Alomari M.A., Khabour O.F., Maikano A., Alawneh K. Vascular function and brain-derived neurotrophic factor: the functional capacity factor. Vasc. Med. 2015;20(6):518–526. doi: 10.1177/1358863X15598390. 26285588. [DOI] [PubMed] [Google Scholar]
- 14.Alomari M.A., Khabour O.F., Alzoubi K.H., Alzubi M.A. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav. Brain Res. 2013;247:34–39. doi: 10.1016/j.bbr.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Ang E.T., Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr. Med. Chem. 2007;14:2564–2571. doi: 10.2174/092986707782023280. [DOI] [PubMed] [Google Scholar]
- 16.Ross R., Dagnone D., Jones P.J., Smith H., Paddags A., Hudson R. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 17.Lee S., Kuk J.L., Davidson L.E., Hudson R., Kilpatrick K., Graham T.E. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J. Appl. Physiol. 2005;99:1220–1225. doi: 10.1152/japplphysiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 18.Haskell W.L., Lee I.M., Pate R.R., Powell K.E., Blair S.N., Franklin B.A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 19.Singh M., Meyer E.M., Simpkins J.W. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–2324. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- 20.Chan C.B., Tse M.C., Liu X., Zhang S., Schmidt R., Otten R. Activation of muscular TrkB by its small molecular agonist 7,8-dihydroxyflavone sex-dependently regulates energy metabolism in diet-induced obese mice. Chem. Biol. 2015;22(3):355–368. doi: 10.1016/j.chembiol.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair S.N., Church T.S. The fitness, obesity, and health equation: is physical activity the common denominator? J. Am. Med. Assoc. 2004;292:1232–1234. doi: 10.1001/jama.292.10.1232. [DOI] [PubMed] [Google Scholar]
- 22.Alomari M.A., Keewan E.F., Qhatan R., Amer A., Khabour O.F., Maayah M.F. Blood pressure and circulatory relationships with physical activity level in young normotensive individuals: IPAQ validity and reliability considerations. Clin. Exp. Hypertens. 2011;33:345–353. doi: 10.3109/10641963.2010.531848. [DOI] [PubMed] [Google Scholar]
- 23.Adams G.M., Beam W.C. fifth ed. McGraw-Hill; Boston: 2008. Exercise Physiology : Laboratory Manual. [Google Scholar]
- 24.Khabour O.F., Alzoubi K.H., Alomari M.A., Alzubi M.A. Changes in spatial memory and BDNF expression to simultaneous dietary restriction and forced exercise. Brain Res. Bull. 2013;90:19–24. doi: 10.1016/j.brainresbull.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G.H., Sullivan M.J., Thompson P.J., Fallen E.L., Pugsley S.O., Taylor D.W. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can. Med. Assoc. J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 26.ATS statement: guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 27.Wu G., Sanderson B., Bittner V. The 6-minute walk test: how important is the learning effect? Am. Heart J. 2003;146:129–133. doi: 10.1016/S0002-8703(03)00119-4. [DOI] [PubMed] [Google Scholar]
- 28.Alameri H., Al-Majed S., Al-Howaikan A. Six-min walk test in a healthy adult Arab population. Respir. Med. 2009;103:1041–1046. doi: 10.1016/j.rmed.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Alomari M.A., Shqair D.M., Khabour O.F., Alawneh K., Nazzal M.I., Keewan E.F. The clinical and nonclinical values of nonexercise estimation of cardiovascular endurance in young asymptomatic individuals. TheScientificWorldJOURNAL. 2012;2012:958752. doi: 10.1100/2012/958752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salbach N.M., O’Brien K.K., Brooks D., Irvin E., Martino R., Takhar P. Reference values for standardized tests of walking speed and distance: a systematic review. Gait Posture. 2015;41(2):341–360. doi: 10.1016/j.gaitpost.2014.10.002. 25542397. [DOI] [PubMed] [Google Scholar]
- 31.Chetta A., Zanini A., Pisi G., Aiello M., Tzani P., Neri M. Reference values for the 6-min walk test in healthy subjects 20-50 years old. Respir. Med. 2006;100:1573–1578. doi: 10.1016/j.rmed.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Bourahli M.-K., Bougrida M., Martani M., Mehdioui H., Saad H.B. 6-Min walk-test data in healthy North-African subjects aged 16–40years. Egypt. J. Chest Dis. Tuberc. 2015 [Google Scholar]
- 33.Poh H., Eastwood P.R., Cecins N.M., Ho K.T., Jenkins S.C. Six-minute walk distance in healthy Singaporean adults cannot be predicted using reference equations derived from Caucasian populations. Respirology. 2006;11:211–216. doi: 10.1111/j.1440-1843.2006.00820.x. [DOI] [PubMed] [Google Scholar]
- 34.Graham J.E., Ostir G.V., Kuo Y.F., Fisher S.R., Ottenbacher K.J. Relationship between test methodology and mean velocity in timed walk tests: a review. Arch. Phys. Med. Rehabil. 2008;89:865–872. doi: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen J. second ed. L. Erlbaum Associates; Hillsdale, N.J.: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 36.Fleiss J.L. Wiley classics library ed. Wiley; New York: 1999. The Design and Analysis of Clinical Experiments. [Google Scholar]
- 37.Kernie S.G., Liebl D.J., Parada L.F. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu B., Goulding E.H., Zang K., Cepoi D., Cone R.D., Jones K.R. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seifert T., Brassard P., Wissenberg M., Rasmussen P., Nordby P., Stallknecht B. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 40.Cho H.C., Kim J., Kim S., Son Y.H., Lee N., Jung S.H. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO(2)max performance in healthy college men. Neurosci. Lett. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Araya A.V., Orellana X., Godoy D., Soto L., Fiedler J. Effect of exercise on circulating levels of brain-derived neurotrophic factor (BDNF) in overweight and obese subjects. Horm. Metab. Res. 2013;45:541–544. doi: 10.1055/s-0032-1333237. [DOI] [PubMed] [Google Scholar]
- 42.Zoladz J.A., Pilc A., Majerczak J., Grandys M., Zapart-Bukowska J., Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J. Physiol. Pharmacol. 2008;59(Suppl 7):119–132. [PubMed] [Google Scholar]
- 43.Babaei P., Azali Alamdari K., Soltani Tehrani B., Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum brain derived neurotrophic factor and memory performance in middle aged males with metabolic syndrome. J. Sports Med. Phys. Fit. 2013;53:437–443. [PubMed] [Google Scholar]
- 44.Currie J., Ramsbottom R., Ludlow H., Nevill A., Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci. Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 45.Jung S.H., Kim J., Davis J.M., Blair S.N., Cho H.C. Association among basal serum BDNF, cardiorespiratory fitness and cardiovascular disease risk factors in untrained healthy Korean men. Eur. J. Appl. Physiol. 2011;111:303–311. doi: 10.1007/s00421-010-1658-5. [DOI] [PubMed] [Google Scholar]
- 46.Minetto M.A., Lanfranco F., Baldi M., Termine A., Kuipers H., Ghigo E. Corticotroph axis sensitivity after exercise: comparison between elite athletes and sedentary subjects. J. Endocrinol. Invest. 2007;30:215–223. doi: 10.1007/BF03347428. [DOI] [PubMed] [Google Scholar]
- 47.Luger A., Deuster P.A., Kyle S.B., Gallucci W.T., Montgomery L.C., Gold P.W. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N. Engl. J. Med. 1987;316:1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- 48.Rojas Vega S., Struder H.K., Vera Wahrmann B., Schmidt A., Bloch W., Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121:59–65. doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 49.Jurca R., Lamonte M.J., Church T.S., Earnest C.P., Fitzgerald S.J., Barlow C.E. Associations of muscle strength and fitness with metabolic syndrome in men. Med. Sci. Sports Exerc. 2004;36:1301–1307. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 50.Dupuy O., Gauthier C.J., Fraser S.A., Desjardins-Crepeau L., Desjardins M., Mekary S. Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front. Hum. Neurosci. 2015;9:66. doi: 10.3389/fnhum.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaughan S., Wallis M., Polit D., Steele M., Shum D., Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014;43:623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- 52.Kim H.J., Song B.K., So B., Lee O., Song W., Kim Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: a pilot study. Psychiatr. Res. 2014;220:792–796. doi: 10.1016/j.psychres.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Nakahashi T., Fujimura H., Altar C.A., Li J., Kambayashi J., Tandon N.N. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 54.Fujimura H., Altar C.A., Chen R., Nakamura T., Nakahashi T., Kambayashi J. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemostasis. 2002;87:728–734. [PubMed] [Google Scholar]
- 55.Matthews V.B., Astrom M.B., Chan M.H., Bruce C.R., Krabbe K.S., Prelovsek O. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 56.Unger T.J., Calderon G.A., Bradley L.C., Sena-Esteves M., Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothman S.M., Griffioen K.J., Wan R., Mattson M.P. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012;1264:49–63. doi: 10.1111/j.1749-6632.2012.06525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwama A.M., Andrade G.N., Shima P., Tanni S.E., Godoy I., Dourado V.Z. The six-minute walk test and body weight-walk distance product in healthy Brazilian subjects. Braz. J. Med. Biol. Res. 2009;42:1080–1085. doi: 10.1590/s0100-879x2009005000032. [DOI] [PubMed] [Google Scholar]
- 59.Lee S.S., Yoo J.H., Kang S., Woo J.H., Shin K.O., Kim K.B. The effects of 12 Weeks regular aerobic exercise on brain-derived neurotrophic factor and inflammatory factors in juvenile obesity and type 2 diabetes mellitus. J. Phys. Ther. Sci. 2014;26:1199–1204. doi: 10.1589/jpts.26.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang T., Larsen K.T., Ried-Larsen M., Moller N.C., Andersen L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand. J. Med. Sci. Sports. 2014;24:1–10. doi: 10.1111/sms.12069. [DOI] [PubMed] [Google Scholar]