Abstract
Background:
Bisphenol A (BPA) is one of the highest volume chemicals produced worldwide. It has recognized activity as an endocrine-disrupting chemical and has suspected roles as a neurological and reproductive toxicant. It interferes in steroid signaling, induces oxidative stress, and affects gene expression epigenetically. Gestational, perinatal and neonatal exposures to BPA affect developmental processes, including brain development and gametogenesis, with consequences on brain functions, behavior, and fertility.
Methods:
This review critically analyzes recent findings on the neuro-toxic and reproductive effects of BPA (and its ana-logues), with focus on neuronal differentiation, synaptic plasticity, glia and microglia activity, cognitive functions, and the central and local control of reproduction.
Results:
BPA has potential human health hazard associated with gestational, peri- and neonatal exposure. Beginning with BPA’s disposition, this review summarizes recent findings on the neurotoxicity of BPA and its analogues, on neuronal dif-ferentiation, synaptic plasticity, neuro-inflammation, neuro-degeneration, and impairment of cognitive abilities. Furthermore, it reports the recent findings on the activity of BPA along the HPG axis, effects on the hypothalamic Gonadotropin Releas-ing Hormone (GnRH), and the associated effects on reproduction in both sexes and successful pregnancy.
Conclusion:
BPA and its analogues impair neuronal activity, HPG axis function, reproduction, and fertility. Contrasting re-sults have emerged in animal models and human. Thus, further studies are needed to better define their safety levels. This re-view offers new insights on these issues with the aim to find the “fil rouge”, if any, that characterize BPA’s mechanism of action with outcomes on neuronal function and reproduction.
Keywords: BPA, neuronal differentiation, synaptic plasticity, neuroinflammation, epigenetics, hypothalamus, HPG axis, GnRH, Kiss1, reproduction
1. INTRODUCTION
The main consequence of industrialization is the release of substances capable of interfering in the physiological endocrine function [i.e., endocrine-disrupting chemicals (EDCs)]. These substances include pesticides, dioxins, pharmaceuticals, metals, phytoestrogens, phthalates, plasticizers and
polychlorinated biphenyls, among the others; worldwide, EDCs represent a threat to health and environment [1, 2].
Among EDCs, Bisphenol A (BPA) interferes in steroid signaling and thus affects several biological functions causing reproductive, developmental, and metabolic dysfunction in humans, animals, and plants [3-8]. BPA is widely used as a monomer in the production of epoxy resins and polycarbonate plastics and thus is introduced to humans and the environments via storage containers for food and beverages, medical devices, tableware, lenses, DVDs, electronics, sports equipment, thermal paper, dental sealants, etc. [1, 6]. Skin contact, oral exposure through the ingestion of contaminated foods and drinks, or inhalation are the main exposure routes of BPA [9]. Once in the body, BPA can accumulate in biological tissues, with long-term effects on health [10]; it is released into biological fluids such as urine [11] or maternal milk [12, 13] and has the ability to bypass the placental barrier and access the fetus [14, 15]. As a consequence, widespread exposure to BPA affects many physiological functions depending on dose, exposure routes, exposure time and life stage [1]. Tissue damage and oxidative stress, hormonal imbalance and developmental effects are the main consequences of BPA exposure, particularly during the gestational, neonatal and perinatal timeframe. In addition, transgenerational effects leading to deficiencies in behavior, reproduction and metabolism have been observed [9]. Despite many routes of BPA exposure, the majority of environmental doses to humans are below the current tolerable daily intake (t-TDI) of 4 μg/kg/day established by the European Food Safety Authority (EFSA) [16, 17]; however, BPA exposure warrants further research due to indications from human and experimental models that the current t-TDI is not completely safe [1, 3]. Thus, BPA analogues like bisphenol B (BPB), Bisphenol F (BPF), Bisphenol S (BPS) and Bisphenol AF (BPAF) have been developed with the aim to substitute BPA in routine life [18] Fig. (1); but, the safety of these analogues is a matter of debate [13, 19-21].
Fig. (1).
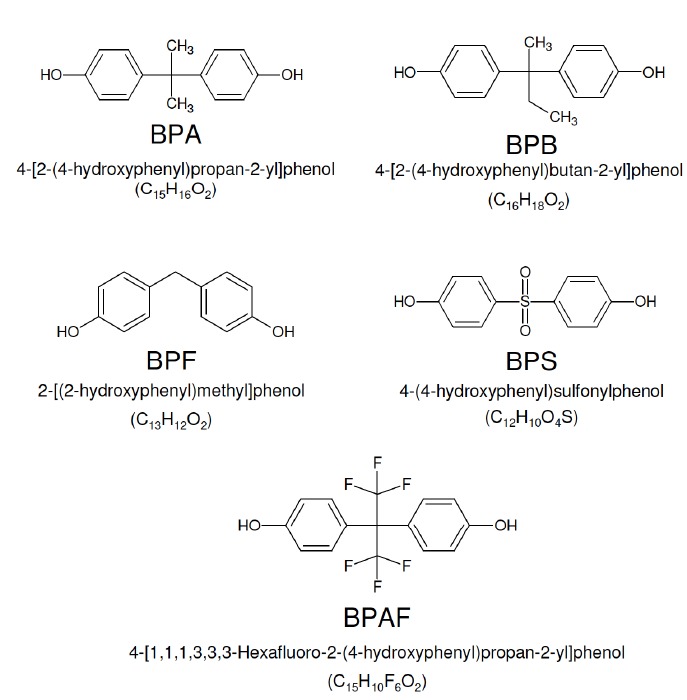
The chemical structure of BPA, BPB, BPF, BPS and BPAF.
The central issues are the acute and chronic effects of BPA (and its analogues) within the brain, the organ that controls all other organs by integrating information and providing rapid and coordinated responses to environmental and physiological change. An intricate neuronal network develops during embryogenesis but only in the postnatal period does the brain fully mature. Hence, the protection of neuronal cells and synaptic plasticity is essential for a healthy brain; conversely, neuronal damage, neuro-inflammation and synapse loss are central to the development of neurological disorders and cognitive decline. Environmental exposure to BPA may interfere in brain development and physiology after bypassing the endogenous defence mechanisms and may require exogenous interventions [22-24].
In this respect, the hypothalamus responds to exogenous and endogenous cues such as energy depletion, stress signals, temperature deviation, and hormonal fluctuations. It stimulates the anterior lobe of the pituitary gland to release tropic and non-tropic hormones [i.e. Follicle Stimulating Hormone (FSH), Luteinizing Hormone (LH), Thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), prolactin (PRL), and melanocyte-stimulating hormone (MSH), growth hormone (GH)] which targets peripheral tissues. Therefore, the hypothalamus was found to be particularly affected by EDCs in sex, time, and exposure dependent manner [25]. Due to the strong dependence from steroid biosynthesis and activity, the effects of BPA along the hypothalamus-pituitary-gonad (HPG) axis were particularly studied with reproductive success and offspring health as main endpoints.
In this review, we summarize the effects of BPA and its analogues within the brain, focusing on neuronal differentiation, synaptic plasticity, inflammation processes, neuro-degeneration, and impairment of cognitive abilities. We then discuss the mediobasal hypothalamus, the brain region involved in the control of reproduction and then summarize the recent findings on the BPA-dependent modulation of the reproductive axis and the effects on reproduction and successful pregnancy.
2. BIOLOGICAL MECHANISMS AND TOXICANT DISPOSITION OF BPA: AN OVERVIEW
2.1. Molecular Mechanisms
According to the World Health Organization (WHO) definition, BPA has been considered an EDC because it induces adverse health effects by an endocrine mechanism of action [26]. BPA has both estrogenic and anti-androgenic activities due to its capability to bind steroid receptors like the nuclear estrogen receptor (ER) α and β (ERα and ERβ), estrogen-related receptor γ (ERRγ), androgen receptor (AR) and the membrane estrogen receptor GPER30 among others [27]. Bisphenol compounds adversely affect endocrine systems through genomic, non-genomic and epigenetic modes of action, especially in sensitive developmental windows [4, 7, 9].
Recently, attention has moved toward the epigenetic effects, which occur without an alteration in the DNA nucleotide sequence with mechanisms including DNA methylation and imprinting, histone modifications and non-coding RNAs [28]. Among bisphenols, BPA is well known to alter DNA methylation, through the modulation of both expression rate and activity of the DNA methyltransferases (DNMTs) [29]. Starting from studies carried out by Dolinoy et al. [30, 31], BPA has been suggested to affect the methylation status of CpG islands in the promoter regions of specific genes or the genome-wide methylation in fetal and adult brain [32, 33] as well as in peripheral organs and cells, such as liver, prostate, and male germ cells [34-37].
Genomic imprinting in the embryo, the epigenetic phenomenon that causes genes to be expressed from a parent of origin-specific manner [38], largely depends on DNA methylation. BPA exposure alters the expression of imprinted genes, especially involved in brain development, in the mouse embryo and placenta, resulting in the loss of genomic imprinting at some genetic loci [39]. This effect is transmitted across generations [40]. The placenta shows more sensitivity to imprinting perturbations than the embryo, probably because it is in direct contact with maternal tissues and, therefore, is more highly exposed to the environment [39].
BPA also influences histone post-translational modifications like histone methylation and acetylation, after in utero and in vivo exposures at low concentration [41-43]; thus, affecting chromatin remodeling in mechanisms possibly mediated by the NAD+ dependent deacetylase sirtuin 1 (SIRT1) [44]. Interestingly, both DNA methylation and histone modifications may be simultaneously altered by BPA and/or BPA analogues [37].
Non-coding RNAs [i.e., microRNA, long non-coding RNA, circRNA] deeply affect brain physiology in health and disease [45-47]. Many small non-coding RNAs are directly involved in the post-transcriptional modification of other RNA species; as a consequence, the spatial and temporal dynamics of gene activation in the brain requires a functional interplay between the activity of small non-coding RNAs and RNA modification [48, 49].
BPA and its analogues target non-coding RNAs to affect epigenetic processes in cells. Several studies attempted to identify miRNA signatures after BPA exposure [50, 51]. In particular, miR-146a-5p is induced by BPA in both cell culture and mouse models, repressing the expression of Mta3, a pivotal chromatin remodeling transcription factor controlling the steroidogenic activity [52]. The miRNA profile after BPA exposure has been also described in mouse Sertoli cell line TM4, with a significant downregulation of 37 miRNA patterns [53].
BPA exposure from gestating mothers - through placenta permeability - influences fetus development [54]; as a consequence, somatic and germline epigenomes of descendants are affected [55]. Additionally, newborns may be exposed to bisphenols during breastfeeding due to accumulation in mother’s milk [56]. Paternal exposure to BPA also alters offspring health [57], suggesting that epigenetic payload in the father influences embryonic health and adult offspring phenotype [9, and references therein]. This is possible because spermatozoa use non-coding RNAs such as long non-coding RNAs, miRNAs, and tRNA-derived RNA fragments as the carrier of paternal hereditary information. As a consequence, “epigenetic crosstalk” has been suggested in spermatozoa [58]. Therefore, spermatozoa have been suggested as a vehicle of “epigenetic memory” and an “environmental message” that may be transmitted through paternal lineage, altering developmental trajectories of the offspring.
2.2. BPA Disposition and Metabolism
In vitro and in vivo studies indicate that BPA can be absorbed through the skin and be a relevant internal exposure. Mielke et al. (2011) found that higher blood concentrations result from a dermal dose compared to the identical oral dose [59]. In contrast, Marquet et al. (2011) tested the absorption and metabolism of BPA with human and rat skin samples. Doses of 50 µl/cm2 and 200 µg/cm2 were applied to the skin samples for 24 hours. Compared to the control group, no significant differences were found [60]. Marquet et al. (2011) found that BPA was very slightly metabolized if not at all metabolized as it passed through the skin.
To facilitate excretion, BPA is conjugated to glucuronide in the liver, forming BPA-GA. This conjugation enables BPA-GA to be excreted with the bile. However, some of the BPA-GA can transfer to the fetus. Nishikawa et al. (2010) tested the metabolism and transfer of BPA through uterine perfusion in which the perfusate, BPA-GA, was pumped into the maternal abdominal aorta where it then circulates through one uterine artery, the placenta, the fetus, and then out of the uterine vein. Nishikawa et al. (2010) subsequently found that the rat fetus de-glucuronidated BPA-GA to the active and harmful parent compound, BPA. This was supported by the discovery that the deconjugating enzyme, beta-glucuronidase (Beta-Gase), is expressed in the fetus. However, it is also possible that BPA-GA is deconjugated in the placenta, and then BPA is passively diffused into the fetus. This is a plausible pathway because, unlike the mother, the fetus has a low ability to glucuronidate BPA [61]. Similarly, Schonfelder et al. (2002) concluded that rodent fetuses are unable to metabolize BPA as quickly as the mother because most uridinediphosphate-glucoronosyltransferase isozymes are not active until after birth, and not fully expressed until 3 months of age [62]. Most studies to date have used rodent models. The human fetus may be more sensitive to BPA and at a higher risk of being exposed in utero to BPA than the rodent fetus [61]. Sexual differentiation of the brain is affected by exposure to a dose of BPA less than 50 µg/kg bw, during the fetal and lactational periods [61].
Takahasi et al. (2000) orally administered BPA at doses 1g/kg and 100-800 mg/kg bw, and found that 20 minutes after administration, maximum concentrations of BPA were found in maternal blood, liver, kidney, and the fetus. This suggests that BPA is rapidly absorbed and distributed throughout maternal tissues and that the placental barrier does not protect the fetus from BPA absorption [63]. Forty minutes after administration, fetal unconjugated BPA concentrations in the blood, liver, and kidney were higher than maternal concentration, leading to the conclusion that the retention time of BPA is greater in fetal blood than in the mother [63].
Teeguarden et al. (2016) studied 30 pregnant women whose average stage of pregnancy was 23.7 weeks. These women were occupationally exposed to an environment with a higher concentration of BPA, working as a cashier, for a thirty-hour period and then they were additionally exposed in a clinical setting [64]. The average daily BPA exposure was 0.037 µg/kg/day. BPA and its metabolites were measured in serum and total BPA was measured in matching urine samples. BPA parent concentration of 0.25-0.51 ng/ml were detected in some serum samples, but there was no significant relationship to total BPA found in corresponding urine samples, or total BPA exposure (work exposure and clinical dose), thus the concentrations found in the urine were concluded to be a consequence of ubiquitous BPA contamination, rather than external exposure [64].
2.3. BPA Compared to BPS
To test the similarities of BPA and BPS materno-fetal transfer, researchers tested concentrations of 61 mother-newborn pairs in China; BPS was found in four maternal (0.03-0.07 ng/ml) and seven cord serum samples (0.03-0.12 ng/ml) showing that BPS does cross the placental-blood barrier [65]. Unlike BPA, the fetus is able to conjugate BPS into BPS glucuronide (BPS-G) in the late stage of pregnancy; however, the BPS-G conjugate remains with the fetus because it is unable to cross through the placenta back to the mother, due to having a higher hydrophilicity than BPA-G [65]. Indeed, the passage of BPS-G from fetus to mother of is almost non-existent; therefore the blood-placental barrier is more effective in limiting the return of BPS-G to the mother than BPA and BPA-G [65]. Therefore, once the compounds have reached the fetus, BPA-G can be deconjugated to BPA, which is harmful to the fetus, but also excretable to the mother. BPS is conjugated to BPS-G by the fetus but then unable to be deconjugated and excreted from the fetus to mother.
2.4. Sex-specific Differences in Disposition and Effects
Schonfelder et al. (2002) tested the disposition of BPA found in rats after various oral administrations of BPA. Parent BPA (10 mg BPA/kg bw) was not detected at any time point in male rats, but the same dose could be detected 22 hours after oral administration in female rats. In 100 mg BPA/kg bw dosages, parent BPA blood concentrations were approximately 10 times greater in female rats as compared to male rats [62]. The same trend was not found in humans. Schonfelder et al. (2002) collected human maternal blood between gestations weeks 32 and 41 and umbilical cord blood after birth. Concentrations of BPA found in maternal plasma ranged from 0.3 ng/ml to 18.9 ng/ml, while the concentration of parent BPA found in fetal plasma ranged from 0.2 ng/ml to 9.2 ng/ml [62]. The concentration found in placental tissue ranged from 1.0 ng/g to 104.9 ng/g [62]. In 12 of 24 cases in males, there was a higher level of parent BPA in fetal plasma (p=0.016) compared to only 2 of 13 cases in females [62].
Roen et al. (2015) examined a group that included 115 boys and 135 girls. The children were assessed at ages 7-9 years old using the child behavior checklist (CBCL). Higher internalizing scores (beta= 0.41, p<0.0001) and externalizing scores (beta=0.40, p<0.0001) were found in the boys in the upper tertile of high prenatal BPA exposure compared to the lower tertiles [66]. In contrast, girls showed a decrease in the internalizing composite score (beta= -0.17, p=-0.04) [66].
Kundakovic et al. (2013) found in juvenile mice that BPA doses to the mother of 2, 20, and 200 μg/kg/day in mice induces sex-specific, dose-dependent (linear and curvilinear), and brain region-specific changes in expression of genes encoding ERs (esr1 and esr2 which encode for ERα and ERβ, respectively) and estrogen-related receptor-γ (errγ). In the cortex and hypothalamus of the juvenile mice, BPA altered the mRNA levels of epigenetic regulators (DNMT1 and DNMT3A). Kundakovic et al. (2013) noted that in the cortex of males that alteration in ERα and DNMT expression was associated with DNA methylation changes in the esr1 gene. Likewise, in the hypothalamus of females, alterations in ERα and DNMT expression was associated with DNA methylation changes in the esr1 gene. Sex-specific effects on social and anxiety-like behavior were influenced by BPA exposure [67].
McCaffrey et al. (2013) exposed Long Evans rats prenatally to 10, 100, 1000, 10,000 μg/kg bw/day BPA through oral administration. In the female hypothalamus, anteroventral periventricular nucleus (AVPV), in all exposure groups, there was a decrease in tyrosine hydroxylase immunoreactive (TH-ir) cell numbers. A decrease in TH-ir cells is an indicator of masculinization. In the male AVPV, TH-ir cell numbers were only reduced in the BPA 10 and BPA 10,000 groups [68]. In females, the sexually dimorphic nucleus of the preoptic area (SND-POA) endpoints was not changed; however, in males, a decrease in SDN-POA volume was observed across all BPA exposure groups [68]. Calbindin (CALB-ir) was observed to be lower in all groups except the BPA 1000 group which is congruous with demasculinization [68]. McCaffrey et al. (2013) determined that oral exposure to BPA below 50 mg/kg/day can alter sex-specific hypothalamic morphology in the rat.
3. NEUROTOXICITY OF BPA AND ITS ANALOGUES
3.1. Effects of BPA on Neurogenesis and Synaptic Plasticity
During embryogenesis, brain development starts with the proliferation of neuroepithelial progenitor cells (NPCs) that first expand by symmetric division and then switch to asymmetric division to begin neurogenesis. NPCs transform in radial glia and give rise to both neurons and glia. Committed neuronal cells migrate to the final destination and initiate the production of neurotransmitters and neurotrophic factors, promoting the formation of synaptic contacts and dendritic spines that continue to remodel to establish the complex brain neuronal network [69, 70]. In a mature mammalian brain, neurogenesis persists in the dentate gyrus (DG), hippocampus, and subventricular zone (SVZ) [71-73]. Hippocampal NSCs produce new granule neurons, which functionally incorporate into the existing neuroanatomical circuits, playing an important role in learning and memory [74, 75]. Within this context, several studies indicate that BPA can alter the fine tuning of neurogenesis representing a risk for neurological disorders and cognitive impairments in humans [76]. BPA-induced neurotoxicity occurs in the brain through the reduction of synaptic plasticity, inhibition of neurogenesis, generation of oxidative stress and induction of autophagy and apoptosis. BPA treatment (4, 40 and 400µg/kg/day) of rats from gestational day (GD) 6 to postnatal day (PND) 21 affected NSCs proliferation within the hippocampus and SVZ of rats by targeting the expression/protein levels of Wnt-pathway genes and decreasing β-catenin nuclear translocation at the highest doses [77]. Likewise, Kim et al. [78, 79] showed that BPA treatment suppressed NPCs proliferation also affecting the normal DG formation at 20 mg/kg/day in young mice. Other studies demonstrated that the oral administration (40 and 400 μg/kg/day) of BPA in rats during the early postnatal period (PNDs 14-21) induced apoptotic cell death in the DG, hilus and molecular layer of the hippocampus enhancing the expression of autophagy genes/proteins [80]. The BPA-mediated induction of autophagy in rat hippocampus was also confirmed in hippocampal NSC-derived neurons in vitro. In this case, BPA exposure (100 μM) resulted in the energy sensor AMP kinase (AMPK) – mediated activation of autophagy associated with an increase of raptor and acetyl-CoA carboxylase phosphorylation. In addition, BPA exposure promoted autophagy by down-regulating the mammalian target of rapamycin (mTOR) pathway and decreasing the phosphorylation of ULK1. Notably, ULK1 is not only a key protein playing a crucial role in endocytosis and autophagy but it also participates in other phylogenetically conserved pathways involved in neurite formation and synaptic transmission during CNS development [81, 82]. Therefore, by targeting this protein, BPA could inhibit synapse formation and pruning, triggering neuronal loss and neurodegeneration within the cerebral cortex and hippocampus. BPA-induced effects have been also associated with mitochondrial dysfunction. In hippocampal NSCs BPA is able to increase reactive oxygen species (ROS) generation and enhance PINK and PARKIN proteins levels leading to mitophagy [80]. Mitochondrial function seems to be an important target of BPA. Chronic exposure to BPA of adolescent rats (40 μg/kg/day with a single oral dose) from PND21 to PND90 enhanced oxidative stress and apoptosis instead of autophagy by decreasing superoxide dismutase and catalase levels [83]. The detrimental effect of BPA is not confined to the time of exposure but can impair neurogenesis across generations. It has been documented that the F2 female mice, from pregnant C57BL/6 mice (F0) injected intraperitoneally (i.p.) with 10 mg/kg/day from GD6 to GD17, had a decreasing number of newly generated cells in the hippocampus [84] due to a reduction in phospho-ERK, brain-derived neurotrophic factor (BDNF), and phospho-CREB. Noteworthy, the same authors observed that the effects of BPA on hippocampal neurogenesis correlated with increased DNA methylation of the CREB regulated transcription coactivator 1 (Crtc1) in F2 mice [84]. Epigenetic changes in mouse brain were also found after perinatal exposure (GD7-PND21) to oral BPA (50 µg/kg/day). It was reported that BPA increased histone H3 acetylation in cerebral cortex and hippocampus of postnatal 3 and 8 week male mice and increased or decreased the levels of DNMT1 and DNMT3 depending on the brain region [85]. DNA methyltransferases are key regulators of gene expression required for sustaining DNA methylation and synaptic function in the forebrain and are involved in long-term plasticity and memory formation in the hippocampus [86, 87]. Therefore, by changing the gene expression profile in a spatial and/or temporal manner, BPA can promote cognitive and memory dysfunction both in childhood and adulthood [88, 89]. BPA has been found to target the expression and methylation profile of several genes involved in epigenetic programming within the brain including Bdnf, Fkbp5, and Grin2b. In rat hippocampus, BPA exposure (2,500 μg/kg/day from GD6 to PND21) resulted in hypermethylation of the 5-prime end promoter region of the Bdnf gene in female offspring [90], whereas enhanced DNA methylation of the transcriptional regulators of the glucocorticoid receptors Fkbp5 was found within the hippocampus of BPA-exposed male rats, but not in females, at a low dose (40 mg BPA/kg/d) [91]. These epigenetic changes were all associated with altered spatial learning and memory capabilities. Alavian-Ghavanini et al. [92] observed that in female rat brain, the early-life BPA exposure reduced DNA methylation levels in the promoter region of the Grin2b gene increasing its mRNA expression, even when exposed to doses within the human health risk threshold established by the EFSA (4 μg/kg/day) and the U.S. Food and Drug Administration (FDA) (50 μg/kg/day). Grin2b encodes for a subunit of NMDA-type glutamate receptors which are essential mediators of synaptic transmission and plasticity involved in the regulation of neuronal development, learning, and memory [93]. Interestingly, the same authors found that the maternal exposure to BPA at the dose corresponding to the lower dose administered in the rat study, increased DNA methylation of the Grin2b gene in 7-year old girls [92] suggesting that BPA could induce similar effects in humans. Overall, these results indicate that BPA could induce behavior-related and sex-specific epigenetic modifications mainly targeting the expression pattern of sexually dimorphic genes. However, further studies are needed to assess the exact dose-range and the time of exposure during development by which BPA is able to induce epigenetic modifications. A recent study by Aiba et al. (2018) reported that BPA exposure in the fetal stage (200 μg/kg/day from GD6 to GD17) did not exhibit any significant effect on hippocampal DNA methylation [94].
In spite of its ability to activate the ER signaling pathway in an estrogen-like manner [27], within the CNS, BPA behaves as an antagonist rather than an agonist in the ERs. Leranth (2010) investigated the effects of BPA in steroid-induced synaptogenesis in brain regions that are responsive to changes in circulation gonadal steroid hormones like the hippocampus and prefrontal cortex [95]. They found that the combined subcutaneous injection of BPA (400 μg/kg/day) and 17β -estradiol (60 μg/kg/day) for three days completely inhibited the estrogen-induced synaptic spine formation in ovariectomized adult rats [96]. Notably, spine loss was similar to that found in senescent female animals that did not show hippocampal spine synapse responses to estrogens [97]. These results were supported by evidence in ovariectomized non-human primates [98], suggesting that chronic exposure to BPA, even within safe daily limits, can induce neurodegeneration by promoting synaptic loss in adults.
3.2. Effects of BPA on Glia and Microglia
Neuroglia, including astrocytes, oligondendrocytes and microglia, represent a key component of the mammalian brain-supporting neuronal functions including neuron metabolism, signal conduction, neurogenesis and synaptic plasticity [99]. During embryonic CNS development, multipotent NPCs proliferate and differentiate into “radial glia” which can generate both neurons and glia in response to extrinsic and intrinsic cues. These stimuli represent a local tissue microenvironment maintaining and regulating stem cell capacity to divide or differentiate. Disturbing this environment results in the production of an incorrect cell response leading to improper neuronal differentiation and affects synaptic remodeling and mature glia formation [100, 101]. In addition, erythromyeloid progenitor cells migrate in the CNS early during development in a sexually dimorphic way and colonize the brain forming microglia; they are involved in the removal of pathogens and tissue debris and in synapse pruning [99, 102].
Emerging literature suggests that maternal infection and exposure to environmental pollutants during gestation can activate neuroglia with possible long-term consequences on memory and learning capacities [103, 104]. BPA (400μg/kg/ day) has been found to increase the number of glial cells (and neurons) in the medial prefrontal cortex (mPFC) in male rats exposed to BPA during pregnancy and for the first nine postnatal days. However, authors using Nissl staining were unable to recognize the type of glial cells (astrocytes and oligodendrocytes) specifically targeted by BPA [105]. Further data demonstrated that the oral BPA administration during the period known to be influenced by gonadal hormones (from PND27 to PND46) increased the number of mPFC - microglia in female rats at 40 μg/kg/day while in male rats BPA decreased microglia content at 4 μg/kg/day; instead, no effects were found in astrocytes [106]. Takahashi et al. (2018) provided further insights into the effects of BPA on microglia activation within the dorsal telencephalon and hypothalamus of E15.5 embryos from pregnant mothers treated with 200 μg/kg/day from the presence of a vaginal plug (E0.5) [107]. These authors demonstrated a microglia activation associated with an increased expression of pro-inflammatory factors such as Tumor Necrosis Factor alpha (TNFα) and Interleukin 4 (IL4). Neuroimmune activation, in terms of pro-inflammatory cytokine increase, was also observed within the prefrontal cortex of F1 juvenile mice from mothers treated with BPA in the diet [108]. These observations agree with previous in vitro studies reporting that the treatment of BV2 murine microglial cells with BPA (100μM for 24 and 48 hs) drastically reduced cell proliferation and induced morphological changes typical of microglia activation at non-toxic concentrations (100 nM/L). This effect was mediated by the activation of MAPK, NFkB and JNK pathways leading to enhanced production of TNFα and IL6. Notably, the ER antagonist ICI182780 partially reverted these effects suggesting an ER-mediated action of BPA on microglia [109].
Microglia is hormone-sensitive, and it has been shown that the colonization of the developing brain by microglia elicits some feature of sex dimorphism [102, 110]. Recent studies demonstrated that BPA exposure during the neural development altered social and emotional behaviors in rats and prairie voles (Microtus ochrogaster) [111] by interfering with the sex-specific colonization of microglia within the hippocampus and amygdala [112]. However, there are very few data about the morphological and physiological modification of neuroglia after BPA exposure and data about astrocytes are still scarce and inconclusive. A recent paper reported that early BPA exposure (0.1mg/l in the food, from birth to PND21) caused a significant and persistent ERα-dependent reduction in the number of oligodendrocytes in adult rats. In addition, the loss of oligodendrocytes was associated with myelin basic protein (MBP) and monocarboxylate transporter 1 (MCT1) down-expression in adult hippocampus with considerable reduction in the number of myelinated axons [113]. Taken together, these results suggest that: i) BPA induces sex-specific neurotoxic effects that can vary depending on the neurodevelopmental stage during which BPA contamination occurs and ii) BPA promotes microglia activation and oligodendrocytes injuries possibly contributing to neurodegeneration and neuroinflammation. An overview of BPA-induced neurotoxicity is shown in Fig. (2). In summary, not only the dose and the time of exposure but also the age and sex of exposed subjects are relevant factors for human safety level evaluation. Further studies are required to ascertain the possible contribution of such wide and diffuse contamination in the etiopathogenesis and progression of neurodegenerative diseases.
Fig. (2).
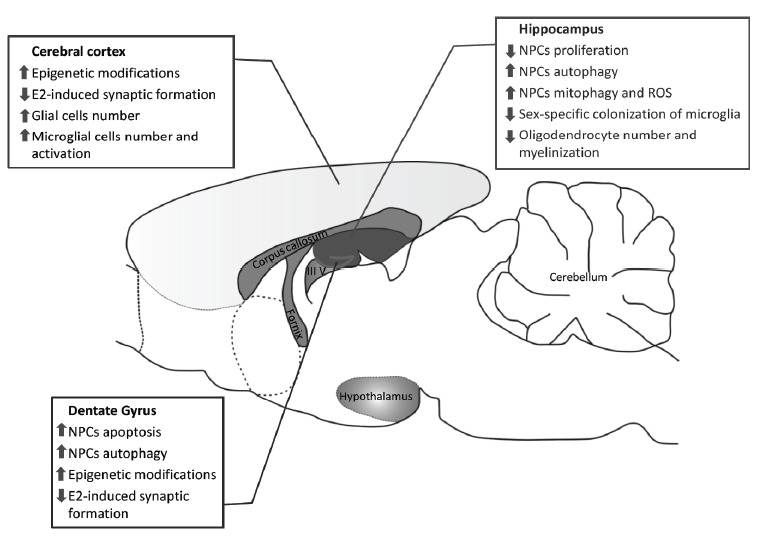
Schematic overview of the main effects of BPA in rodent brain. E2, 17β-estradiol; NPCs, Neuroepithelial Progenitor Cells. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.3. Effects of BPA on Cognitive Functions and Behavior
Because BPA is lipophilic and can cross the blood brain barrier (BBB) [114], it can also affect neuronal functions in different life stages and species. Thus, a number of studies investigated the effects of BPA on cognitive abilities in rats
and mice. Dedicated models were used such as the Barnes maze Y test, the radial arm maze, and the Morris water maze tests. The majority of these studies, especially those performed in animal models at low doses of BPA and close to the current fixed daily limit, reported results suggesting that perinatal, postnatal, prepubertal and pubertal BPA exposure, can negatively affect learning and memory. The oral administration of 600 μg/pup/ day from PND5 to 3 weeks of age induced significant hyperactivity after 4-5 weeks of age, associated to a higher reduction of tyrosine hydroxylase immunoreactivity and apoptotic cell death within the substantia nigra pars compacta of rats [115]. BPA was also able to inhibit the gene expression level of dopamine transporter in the midbrain, indicating neurodegeneration in dopaminergic neurons [115]. Comparable doses of BPA (0.4 mg/kg/day from PND21 to PND49) also induced a significant decline in spatial learning and memory abilities associated with reduced expression of glutamate receptors (NR2 and GluR1) in male rat hippocampus and primary visual cortex [116]. Interestingly, at higher doses (4 mg/kg/day) the BPA-induced impairment in cognitive abilities was accompanied by anxiety-like behaviors in male rats suggesting a dose-dependent and sex-specific effects on juvenile rats [116]. To study the possible effects of BPA exposure on spatial learning and memory during the adolescent period, Zhou et al. (2017) used the Y maze test on mice receiving BPA at concentrations ranging from 0.01 to 1000 μg/kg from 4 to 12 weeks of age. They found a marked decrease in cognitive performance at low doses and an alteration of long term potentiation (LTP) induction in the hippocampus [117]. This LTP alteration was also observed in 4 weeks BPA-treated rats (PND21-PND49), associated with a decrease in presynaptic glutamate production and NMDA-mediated excitatory postsynaptic currents [114]. Juvenile rats exposed to BPA also showed deficits in the classic Morris water maze and Y maze tests [114] indicating that BPA can cause hippocampal-related spatial memory deficits, affecting both pre- and post-synaptic neurons. Changes in neuronal functions and animal behavior following BPA-exposure have been found during the whole life of rats [113,118-121]; however, few data have reported sex differences [122, 123]. Despite the growing literature about BPA-induced effects in rodents, very little is known about the direct consequences of BPA exposure in other species, and epidemiological studies in humans are still inconclusive. One study showed that BPA decreased both working memory accuracy and the number of excitatory synaptic inputs on pyramidal neurons-dendritic spines within the prefrontal cortex and hippocampus of monkeys [124]. Moreover, according to maternal report on the Behavioral Assessment Scale for Children (BASC-2) studies observed that higher maternal urinary BPA concentrations were associated with behavioral disorders in females but not in males, In particular, an increase in hyperactivity, anxiety, and depression behaviors were observed in 3 years old girls [125, 126]. In contrast, another study did not find any significant association between urine BPA concentrations in mothers and behavioral problems in girls but described an increased aggressive behavior and emotional reactivity in boys between 3 and 5 years of age, according to maternal report on the Child Behavior Check List (CBCL) [127]. These studies also investigated the postnatal effects of BPA exposure by analyzing the possible association between urine concentrations of BPA and behavior in children of ages 1 and 4 years. In these studies, no association between urinary BPA concentrations and neurobehavioral alterations were found. However, mid-term pregnancy and postnatal exposure to BPA was associated with an impairment in social communication in 4-year old girls [128]. The fact that BPA exposure could influence negatively social behavior is intriguing since BPA might play a role in the etiology and/or susceptibility to Autism Spectrum Disorders (ASD) [129], a neurodevelopmental syndrome characterized by social interaction and communication impairments. Accordingly, accumulating evidence suggests that some putative ASD genes are selectively targeted by BPA [130] and recent findings reported a significant association between a number of genes differentially expressed in prenatal BPA-exposed male rat hippocampi and ASD-related genes including Auts2 and Foxp2 [131]. The effects of BPA on behavior seems different between males and females and dependent on the time of exposure (prenatal or postnatal). Harley et al. (2013) found increasing internalizing problems, such as anxiety and depression behaviors, in boys at age 7 associated with prenatal urinary BPA concentrations, whereas childhood urinary BPA concentrations were related to enhanced externalizing behavior dysfunctions in girls of 7 years of age [132]. In both sexes, increased attention and hyperactivity behaviors were documented.
In a recent study examining the association between BPA concentrations (together with perfluoalkyl compounds) in newborn dried blood spots and social behaviors, it was reported that there was no relationship between neonatal BPA exposure and behavioral problems in 7 years old children, even though statistical quartile analysis revealed an inverse association between BPA concentrations (median 7.93 ng/ml) and difficulties in prosocial behaviors in the 2nd and 4th quartile [133]. Thus, at the moment it is not possible to state the real consequences of BPA exposure on cognitive functions and behavior in humans, due to other environmental stressors and socio-demographic factors that are not included in above-cited studies. However, animal models strongly suggest a positive correlation between BPA exposure and neurological impairments.
3.4. Neurotoxic Effects of BPA Analogues
Currently BPA analogues are used in several daily-use products (water bottles, food and paper products, thermal receipts, and storage containers; for a review see [18], stimulating new investigations to determine whether the increasing exposure to these BPA analogues could result in neurobehavioral disturbances and induce neurotoxic effects as previously described for BPA. From these very recent studies, it is suggested that some BPA-substitutes have more potent estrogenic activity than BPA. For example, BPAF acts as a full agonist and binds to ERα stronger than its chemical ancestor BPA, but it also acts as an antagonist for ERβ [134, 135]. Moreover, BPS exhibits weaker estrogenic activity than BPA [136] even though it shows higher resistance to degradation [137]. This implies that BPA analogues could represent a potential health risk consequence to their chronic low-dose exposure and accumulation in the body. Some of the BPA analogues (e.g. BPS) are used in “BPA free” thermal printing paper [138], and perhaps in other products that consumers could buy preferentially being uninformed on their possible neurotoxic effects. The emerging literature suggests a detrimental effect of BPA-substitutes on neurological development and behavior [139]. CD-1 mice exposed to BPS (0.2 mg/kg/day in the feeding of pregnant mothers from GD8 until PND21), showed shifted behavior towards increased anxiety and decreased interest in social interactions at 15 weeks of age [140]. Comparable results were obtained by Ohtani et al. (2017) using the open field, the elevated plus maze, and the forced swim tests [141]. They demonstrated enhanced anxiogenic behavior and depressive state in the offspring of mice exposed to BPA or BPF during the fetal period. Interestingly, BPS was also found to alter maternal care either in female mice directly exposed during pregnancy or in their F1 female offspring exposed during the perinatal period. Such altered behavior was associated to hyper expression of ERα within the medial preoptic area (MPOA) which is a brain region involved in maternal behavior [142]. Other studies on animal models investigated the effects of BPA, BPF and BPS on 5α-reductase (5α-R), a key enzyme involved in neuro-steroidogenesis, as well as on dopamine (DA)- and serotonin (5-HT)-related genes, in the PFC of juvenile female rats exposed from PND1 to PND21. The results demonstrated a significant alteration of the DA- and 5-HT-related genes consequent to the treatment with BPA or its analogues. In particular, both BPF and BPS decreased 5α-R3 mRNA levels in PFC at PND21 [143]. In addition, the neurotoxic effects of BPAF were analyzed in a hippocampal cell line (HT-22) and in mouse primary neuronal cells. The treatment with BPAF at 100 to 1000 µM for 24 h induced apoptotic cell death through the induction of oxidative stress and altered MAPK/ERK and JNK pathways [144]. In the same study, the authors also evaluated the effects of BPAF in a microglia/neuroblastoma co-culture model and observed that the BPAF treatment inhibited the microglia activation through the reduction of nitric oxide production.
4. THE EFFECTS ON THE HPG AXIS AND THE OUTCOMES ON FERTILITY
The hypothalamus controls reproduction in both sexes by means of Gonadotropin Releasing Hormone (GnRH) release into the portal capillary system. From here, GnRH reaches the anterior pituitary (adenohypophysis) and mediates the discharge of pituitary gonadotropins (i.e. LH and FSH) into the main circulation; as a consequence, the gonads produce sex steroid hormones, testosterone and estradiol. Such an intricate signaling pathway is further modulated by sex-steroid long-, short- and ultra short- feedback mechanisms and by a large number of centrally and locally produced modulators [22, 145-158]. The success of reproduction strongly depends on the activity of the hypothalamus and gonadic sex steroids. As a consequence, the effects of BPA along the HPG axis were particularly studied with reproductive success and offspring health as the main endpoints. The main targets of BPA along the HPG axis have been depicted in Fig. (3).
Fig. (3).
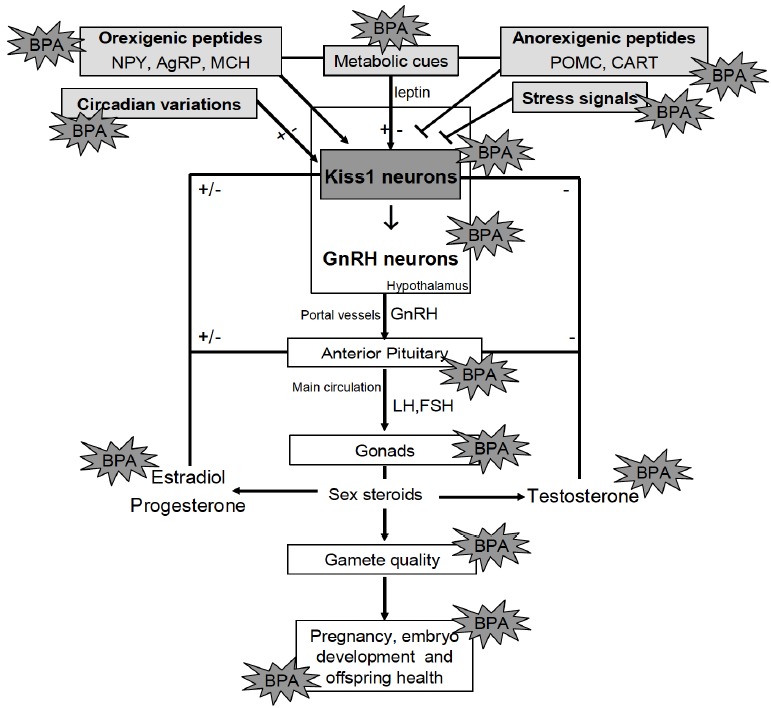
The targets of BPA along the HPG axis and the possible interplay with metabolic and environmental factors. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.1. The Hypothalamic Activity of BPA on Reproduction
The main consequence of early BPA exposure is the advance or the delay in puberty onset of the offspring due to effects on the release of GnRH, impaired production of reproductive hormones in the adult and direct effects on reproductive tissues [1-7, 159, 160]. Therefore, altered levels of circulating pituitary gonadotropins and sex steroids as well as impairment in gamete quality and fertility rate may be observed as discussed in the next paragraphs. In female rats, the effects of neonatal exposure to BPA on HPG axis are opposite at low (25 ng/kg/day) and high (5 mg/kg/day) doses due to the opposite modulation of γ-aminobutyric acid A (GABAA) neurotransmission [161]. In particular, low BPA doses induce a delay in the developmental reduction in GnRH interpulse interval which physiologically precedes sex maturation. In contrast, high doses result in a premature reduction in GnRH interpulse interval and precocious vaginal opening [161].
The activity of HPG axis in male rat offspring is affected by low BPA dose exposure from gestational day 18 to 5PND and causes delayed onset of puberty [162]. The expression rates of hypothalamic GnRH and esr2, pituitary FSH β subunit and AR, testicular FSH receptor and inhibin β subunit are increased in the adult exposed to low BPA doses (0.5 mg/kg), whereas serum T and LH levels are affected at higher doses (5 mg/kg) [162].
The hypothalamic control of reproduction finds the main gatekeepers in Kisspeptins, the cleavage products of the pre-pro-hormone encoded by Kiss1 gene, and in Kisspeptin receptor GPR54 [163, 164], which is constitutively expressed in GnRH secreting neurons [165]. In female rodents, Kiss neurons are located within the dimorphic AVPV and within the arcuate nucleus (ARC).In male rats, they are localized within the ARC exclusively. Kiss neurons stimulate the release of GnRH and represent the main targets of the hypothalamic feedback mechanisms mediated by sex steroids. In particular, the population of Kiss neurons in the AVPV mediates the positive feedback of estradiol required for GnRH preovulatory surge; conversely, Kiss neurons in the ARC are not sexually dimorphic and mediate the steroid negative feedbacks in both sexes [165].
Steroid hormones direct the sexual differentiation of the hypothalamus and the Kiss-GnRH neurons crosstalk is defined during the neonatal period. Thus, the possibility that gestational/neonatal BPA exposure may lead to lifelong effects has been investigated. Interestingly, the hypothalamic Kisspeptin fiber density, via ERα, is differently impaired by neonatal exposure at estradiol benzoate and phytoestrogens in male and female rats, females being more sensitive than males. BPA, at either high (50 mg/kg bw) or low dose (50 μg/kg bw) had no significant effects on Kiss1 immunoreactivity in the female AVPV, but at high dose significantly reduced Kiss1 immunoreactivity in the ARC [166]. However, neonatal exposure to BPA resulted in altered sexually dimorphic gene expression in the postnatal rat hypothalamus affecting esr1, esr2 and Kiss1 mRNA in dose and sex-dependent manner [167]. Furthermore, the hormone sensitive sexually dimorphic brain region such as AVPV reached enlarged volume following BPA exposure in juvenile rats [168] and gestational exposure to BPA at doses below the no-observed-adverse-effect level induced sex-specific effects on the expression of esr1 and esr2 in the developing brain (1PND) [169]. Thus, both neonatal hypothalamic ERs and Kiss1 expression are sensitive to BPA exposure and Kiss1/ER disrupted signalling may impact the sexually dimorphic hypothalamic organization and underlie adult reproductive difficulties [167].
Two recent studies demonstrated the direct influence of BPA on the activity of Kiss/GnRH secreting neurons. The first study was conducted in female Rhesus monkeys and provided evidence that the direct infusion of BPA 10nM into the stalk-median eminence suppressed both GnRH and Kisspeptin release [170]. Accordingly, by means of calcium imaging, Klenke et al. (2016) demonstrated the direct inhibitory effects of BPA on the activity of GnRH neurons by mechanisms independent from ERβ, GPR30 and ERRγ which are expressed in GnRH neurons, and thus requiring non canonical signaling pathways [171].
Lastly, developing hypothalamic modulators of reproduction seem to be affected by BPA exposure. This is true for phoenixin (PNX), a newly discovered positive regulator of GnRH involved in the hypothalamic coordination of the estrous cycle [172]. In immortalized hypothalamic cell lines, BPA and the fatty acids (i.e. palmitate, DHA and oleate) reduced the expression levels of PNX with palmitate and BPA having a negative effect on the expression rate of PNX receptor, GPR173, mRNA, through the MAPK, p38 [173].
4.1.1. BPA Interference in Energy Intake and Reproduction
Several hormonal and environmental cues such as metabolic status, circadian clock, and stressors act on the reproductive axis modulating the activity of GnRH secreting neurons with outcomes on fertility via hormonal and epigenetic signaling pathways.
BPA has suggested obesogenic effects [174] and raises the risk of obesity and weight gain interfering in adipogenesis, energy homeostasis, liver lipid composition, and insulin signaling within insulin-sensitive organs such as the liver, muscle tissue, and adipose tissues [24, 175]. In mice, prenatal exposure to 5mg/kg/day BPA doses by gavage causes transcriptomic and methylomic alterations in the liver, adipose tissue, and in the hypothalamus of male offspring with cross-tissue perturbation in the lipid metabolism and tissue specific alterations in glucose metabolism, histone proteins, and extracellular matrix [176].
Metabolic and environmental factors regulate reproductive function. This ensures that reproduction proceeds only when metabolic and environmental conditions are favorable. The deep link between energy homeostasis and reproduction has been demonstrated in both animal models and in humans, and leptin- a peptide hormone produced within the white adipose tissue- is the main peripheral biomarker of metabolic status [177]. In accordance, in Ob/Ob mice that lacking leptin gene, are affected by both obesity and hypogonadotropic hypogonadism; low expression rate of Kiss1 gene has been observed within the ARC of this animal model [178]. Both caloric restriction and feeding affect the hypothalamic expression of Kiss1 [179], and the downregulation of hypothalamic Kisspeptin signaling has been observed in diabetes mellitus affected rats [180] and in diet-induced obese (DIO) male rats [181].
BPA affects food intake directly by modulating the activity of metabolic sensors produced in the ARC [182, 183], and thus interferes in the dynamic interplay between GnRH and the neuronal networks involved in the metabolic control of reproduction [22, 184]. In this respect, the orexigenic and anorexigenic neuropeptides produced by neuronal population located within the ARC have significant roles. In particular, the neuropeptides capable of stimulating appetite such as neuropeptide Y (NPY), agouti related protein (AgRP), melanin-concentrating hormone (MCH), and the appetite inhibiting neuropeptides such as proopiomelanocortin (POMC) precursor as well as cocaine- and amphetamine-related transcript (CART) [185]. All affect the central control of reproduction. In fact, orexigenic and anorexigenic neuropeptides exert direct and indirect activity respectively on GnRH secreting neurons. Kiss neurons represent the main intermediate neuronal population in such a communication route [22].
In POMC-expressing cell models, BPA exposure significantly induced pomc mRNA levels in both primary cultures and cell lines (i.e. POMC-expressing cell lines, mHypoA-POMC/GFP-2 and mHypoE-43/5), with mechanisms involving the steroid receptor PPARγ and neuroinflammatory pathways [186]. However, in the same experimental model, BPA differentially modulated also the expression of ERs, without any effects on esr1, but inducing the expression of esrrγ, decreasing those of esr2 and Gpr30 and lowering Esr2/Esr1 ratio [186].
In vivo, perinatal exposure to low BPA doses affected the structural and functional developmental programming of POMC circuitry in male and female mice [187]. Conversely, administration of BPS (25, 50, 100 μg/kg) in drinking water after weaning for 10 weeks altered the orexygenic AgRP neuropeptide but not the anorexygenic neuropeptides POMC and CART [188].
Also, the main endocannabinoid, anandamide (AEA), a lipid derivative of the arachydonic acid which activates type1 cannabinoid receptor (CB1), has a recognized role in the hypothalamic control of food intake and reproduction. In fact, it works as orexigenic factor stimulating food intake, promoting the accumulation of body fat [189] and inhibiting the release of GnRH [190-192] with direct and mediated mechanisms [193-195]. A possible mechanism involving the AEA dependent inactivation of CART has been suggested [196]. Accordingly, in CB1-/- mice that do not respond to the signaling of AEA, the administration of BPA (10 μg/ml in drinking water from 10 day post-coitum to 31 day post-partum) causes anorexigenic effects through the up-regulation of the hypothalamic expression of CART [197].
Lastly, the deleterious effects of BPA on the circadian molecular clock requires the BPA-dependent transcriptional activation of the orexygenic peptide NPY. In fact, in vitro studies suggested that BPA significantly altered the expression rate of the circadian clock genes Bmal1, Per2 and Rev-Erbα in POMC and NPY/ArRP expressing hypothalamic primary culture; conversely, the BPA-dependent physiological positive effect on the transcription rate of NPY, AgRP and POMC mRNA was confirmed for AgRP and POMC mRNA but not for NPY mRNA in cell lines lacking Bmal1 [198].
4.2. Female Reproduction
4.2.1. Effects on Ovary
Studies about BPA effects on oocytes have been performed on zebrafish, mice, rats and primates. Chen et al. (2017) tested the influence of mid-term exposure (20 days) to BPA (10 µM) on sex/gonadal differentiation (20-40 dpf) in zebrafish larvae (20-40 dpf) and found increased female ratios that were accompanied by an increased expression of LH beta subunit (LHβ) and decreased expression of FSH beta subunit (FSHβ). The expression of upstream regulators of gonadotropins was not affected, so the researchers concluded that there was no hypothalamic involvement. Both nuclear and membrane ERs are expressed in the zebrafish gonads, so BPA may promote ovarian differentiation at the gonadal level. Chen et al. (2017) observed a decrease in the expression of LH/choriogonadotropin receptor (LHcgr) at the ovary level of zebrafish exposed to BPA. BPA (10 µM) promoted ovarian differentiation in zebrafish but suppressed the ovarian growth by decreased expression of FSHβ in the pituitary, which resulted in ovarian hypotrophy. The presence of BPA resulted in the up-regulation of proteins Hspa8, Tubb4b, and Eef1a1b and the down-regulation of Gapdh, Eno3, and Mdh2 [199]. When adult zebrafish were exposed to BPA in 5-20 μg/L treatments for three weeks, changes in epigenetic patterns were found [199]. The treatments were associated with follicle atresia and down-regulated genes involved in oocyte maturation [199].
Early exposure to BPA in rodents impairs reproductive functions such as disruption of ovarian growth and folliculogenesis, advanced pubertal onset, and induction of early and persistent estrus [200].
Several studies found that pregnant mice orally exposed to BPA showed inhibition in primordial follicle formation [201], the tendency to chromosomal defects and aneuploidy [202], alterations in both meiotic prophase and follicle formation inhibition [203]. Similar results were found in mice and rats on oocyte formation inhibition and reduction in the primordial follicle pool when postnatally exposed to BPA [204, 205]. Epigenetic mechanisms have also been described about BPA effects on ovary [206-209].
As far as the relationship between BPA exposure and Polycystic Ovary Syndrome (PCOS) is concerned, several studies on humans showed that serum BPA levels correlate with this condition, as well as with serum androgen levels [210-212]. In another study, serum BPA levels were comparable between obese PCOS patients and obese women without PCOS [213], although this result was not confirmed [210], because BPA levels were found to be increased in PCOS patients regardless of body weight, with respect to weight-matched controls. BPA is able to affect ovarian steroidogenesis [214], with critical windows of exposure leading to irreversible changes, as demonstrated by Fernandez et al. (2010) [215], who investigated the effects of neonatal BPA exposure on female Sprague-Dawley rats, which exhibited a PCOS-like syndrome during adulthood. This suggests a possible relationship between the development of a PCOS-like syndrome and an early life BPA exposure.
4.2.2. Effects on Uterus
BPA is suspected to have endometrial cancer-inducing role. Although this issue has been long debated [216], a recent meta-analysis supports this hypothesis [217]. Surprisingly, BPA concentration was significantly lower in human endometrial fibroblast belonging to oncological samples than in benign ones probably due to a direct action on BPA on the mRNA expression of P450scc, promoting a decidual endometrial phenotype [218]. However, Pollock et al. [219] found that uterine BPA levels increased in mice following exposure to radioactive BPA. After administering BPA and estradiol at the same time, however, the level of uterine BPA decreased significantly with respect to the assumption of BPA alone, probably related to the 1000-fold greater estrogenic affinity for receptors compared to BPA. In fact, under hyperestrogenism conditions, estrogen can saturate the uterine receptor structures preventing the binding of the endocrine disruptors [220]. Another study found increased endometrial proliferation in monkeys exposed to estradiol or estradiol and BPA (but not controls), probably due to an alteration in hormonal balance [221].
Finally, Hiroi et al. [222] evaluated patients with and without endometrial cancer and found a higher level of BPA in serum but a lower concentration at uterine level in the case group than controls, suggesting a complex indirect mode of action of ECDs in the oncogenesis. hypotheses could explain the correlations of BPA and endometrial neoplasia. I) BPA could trigger the vicious cycle of hyperestrogenism acting on the several known risk factors, (obesity, diabetes, hypertension and PCOS) [223, 224]; II) an indirect BPA mechanism of action disrupt the hormonal equilibrium [219, 222, 225, 226]. Indeed, EDCs (such as BPA) could indirectly influence estrogen-dependent tumorigenesis by acting as a trigger on the HPG axis. The excess of circulating BPA may cause central alterations of the gonadotropin secretion feedback.
4.2.3. Effects on the Placenta
During human pregnancy, BPA exposure can lead to increased risk of pregnancy loss, changes in the timing of labor such as longer gestation or preterm birth, and changes in infant birth weights [227]. Evidence supports that BPA targets the mammalian placental epigenome [227]. In mice, BPA affected placental loss-of-imprinting and decreased both global and CpG-specific DNA methylation [227]. In the placental IGF2/H19 domain, loss-of-imprinting and decreased methylation has resulted in the disrupted nutrient allocation and poor fetal growth [227].
Although limited, human studies suggest that there are sex differences in the placental response to BPA and other chemicals [227]. In a study comparing the expression of Kiss1 and leptin receptor in the placentas of women from an electronic-waste recycling town (n=189-192) and from a reference town (n=56-60), the expression of both genes was higher in the placental tissue from the women from the electronic-waste recycling town, and Kiss1 expression was higher in placentas of males [227]. The target genes of miR-146a are responsible for 19 biological functions including cell differentiation and enzymatic activity [227]. MiR-146a expression was higher in the placentas of women who lived in polluted areas [227]. Strakovosky et al. (2018) also found in age-matched placentas without fetal malformations that there were alterations of miR-146a with BPA exposure.
One of the major concern is the placental transfer of BPA from mother to fetus. Newbold et al. (2009) found that in utero development of the ovary and mesonephric duct system is associated with BPA exposure. Mice were treated on days 9-16 of gestation with BPA doses 0.1, 1, 10, 100, or 1000 µg/kg/day [228]. Progressive proliferative lesion (PPL) of the oviduct was observed in all groups of mice exposed to BPA as well as cystic endometrial hyperplasia (CEH), except for the BPA 0.1 μg/kg/day group. Several effects on sexual development were found: adenomatous hyperplasia with CEH, severe lesions of atypical hyperplasia, an increased incidence of stromal polyps, and invasive stromal sarcoma of the cervix [228]. Other female reproductive abnormalities due to perinatal BPA exposure include early onset vaginal opening and puberty, and, altered estrus cyclicity, plasma levels of LH, vaginal and uterine histology, mammary gland and uterus, and ovarian morphology [228].
4.2.4. BPA Effects on Fetus
4.2.4.1. Birth Weight
Many studies have shown a negative correlation between BPA concentrations in amniotic fluid and urine and birth weight [229, 230]. A minority of studies have shown no association between maternal serum and urine concentrations of BPA in early pregnancy and being born small for gestational age [231-233].
Some early studies found that the role of BPA was not clear, and its action on fetuses was unclear: for example, one of the first studies on pregnant rats did not show toxicity or fetal morphometric modifications related to BPA exposure [234]. Miao et al. [235] reported a significant dose-response curve for reduced birth weight after BPA exposure in pregnancy, which is in agreement with other studies done on full-term pregnancies [236].
Scientific attention has been specifically focused on placental BPA concentration. Troisi et al. [237] found a significant negative correlation between calculated birth weight centile and concentrations of placental BPA. Low birth weight and small for gestational age infants had significantly greater placental BPA concentrations as compared to normal weight infants and average/large for gestational age infants.
4.2.4.2. Preterm Birth
Through evaluations of plasma and amniotic fluid, Behnia et al. (2016) [238] showed that mothers with BPA plasma concentrations in the fourth quartile presented a risk of shorter gestation or premature rupture of membranes. Moreover, several studies [239, 240] describe an inverse correlation between maternal urinary BPA concentration and pregnancy duration. Conversely, data from other studies [232, 241] do not confirm any correlation between maternal BPA and pregnancy duration.
Smarr et al. (2015) [242], studied 501 couples and demonstrated an overall linear trend between quartiles of maternal and paternal BPA and gestational age. The lower end of the range of gestational age at birth was higher among female infants (173-290 days) compared to male infants (155-296 days). Despite the lack of statistical significance, the evaluation of father’s BPA exposure is very interesting because of the possible negative effects on sperm DNA or other factors affecting male fertility (as described above).
In summary, there is limited evidence of a relationship between pre-term birth and BPA [239, 240, 242], and this weak correlation is stronger for female newborns than male [242]. The limited evidence indicates that BPA is not strongly related to preterm birth; however, more research is needed.
4.2.4.3. Fetal Malformation
Over 50% of the etiologies of fetal abnormalities are still unknown [243]. To date, there is no evidence of direct alteration of metabolomic profiles in pregnant women exposed to BPA [244]. However, fetuses are sensitive to BPA action; several studies have shown malformations linked to this endocrine disruptor, suggesting that BPA is trans-placentally transferred to the embryo-fetal compartment [244].
Guida et al. [243] investigated total, free and conjugated BPA measured in the blood of 151 pregnant women divided into two groups: one with an established diagnosis of the developmental defect, versus one with the normally developed fetus. Results show that free but not total BPA were higher in the blood of woman carrying a chromosomal and central and peripheral nervous system malformed fetus, compared to the controls. This suggests an increased susceptibility to abnormalities among “poor metabolizers” [243]. However, it is possible that BPA interferes with the progression of meiotic maturation (as seen in vitro) and causes disturbances in the organization of the spindle and the alignment of chromosomes [245].
The effect of BPA on male genital malformations is very well demonstrated in rats and humans [246, 247]. Male fetuses are more susceptible to BPA effects on genitalia [248]. Chen et al. [249] have proposed a specific mechanism of action of BPA: altered patterns of methylation on the genome of testicular cells. In 2016, Fernandez et al. [250] showed an increased risk of male genital malformations due to high BPA placental concentrations. Also, Miao et al. (2011) [235] correlated decreased anogenital distance in male offspring with high BPA parental exposure. Cryptorchidism and hypospadias are among the most frequent neonatal malformations, and many exogenous factors are associated; therefore, correlations between BPA exposure and these malformations are weak.
4.3. Male Reproduction
Numerous in vivo and in vitro studies clearly suggest that BPA and its analogues have toxic effects on reproductive functions and sperm quality. In fact, BPA, BPB, BPF and BPS mimic or antagonize endogenous hormones and interfere in steroid-mediated processes affecting male reproduction. Therefore, several studies revealed controversial results which may be attributed to differences in exposure protocols, duration of the treatment, administered dose, route, and exposure window [1, 3, 5, 7, 9].
In male rats, BPA exposure from fetal period to sexual maturation impairs the cyto-architecture of the seminiferous epithelium thus affecting the expression of the junctional proteins involved in the formation of the blood-testis barrier [251-253]; mechanisms involving oxidative stress and the massive production of ROS have been proposed [253]. As a consequence, the disruption of the functional communications between Sertoli cells and germ cell occurs; spermatogenesis is impaired and cell damage can be observed, especially at post-meiotic stages [253]. The balance between proliferation, differentiation and apoptosis is critical for the progression of spermatogenesis and the production of high quality gametes. BPA increases the number of germ cells entering meiosis [254], creating a state of abnormal proliferation [255] which in turn causes high apoptosis rates of meiotic cells [253, 255]. Accordingly, BPA is able to induce meiotic arrest through the retention of spermatocytes that failed to be differentiated in spermatids [255, 256], inhibits the meiotic double-strand break repair [257, 258], causes chromosomal abnormalities [259], and reduces the enzymatic defences against ROS [253]. As a consequence, antioxidant defenses and repair mechanisms need to be activated to prevent the oxidative damage to DNA, lipids and proteins. In this respect, the NAD+ dependent deacetylase SIRT1 is negatively affected by BPA with a consequent loss in the control of the acetylated target proteins [44, 253]. ROS production inside the testis and DNA damage in post-meiotic spermatids make it plausible that BPA may induce the formation of poor quality spermatozoa, with possible transgenerational effects on the offspring [253, 260]. In accordance, the correlation between BPA exposure and decreased semen quality, evaluated as sperm count, motility and vitality has been demonstrated in human with impact on capacitation and acrosome reaction [261-263]. In particular, BPA modulates the motility of human spermatozoa in vitro, affecting their mitochondrial potential in a pathway involving free Ca2+ as second messenger [264].
In addition to Sertoli and germ cells, Leydig cells are also a BPA target. The main effects of BPA exposure on Leydig cells activity are the disruption of the hormonal micro-environment in testis and the up-regulation of key steroidogenic enzymes with an increase in estrogen production. Thus sex-hormone ratio (testosterone/estradiol) decreases both in mice (in vivo experiments) and in MA-10 cells, a mouse Leydig cell line [265].
The few available studies on BPA analogues in testis demonstrate that they are more harmful than BPA and are not safe alternatives to BPA [266]. In fact, chronic exposure to low dose of BPA analogues (i.e. BPB, BPF and BPS), decreases the steroidogenic activity of Leydig cells, increases their number and reduces their size [267]. This result is in line with a dose-dependent testosterone inhibition found in human testis explants [268] as well as in vivo studies in rat [20]. Conversely, in vitro experiments on rat testis explants do not reveal any change in the biosynthesis of testosterone [20]; in contrast, the stimulation of MA-10 cells with nanomolar dose of BPA analogues strongly induces testosterone secretion [269].
Oxidative stress in testicular tissue causes its poor development with a consequent reduction in daily sperm production [267, 270]. This is the final effect of a precocious arrest in spermatogonial cell differentiation. Immortalized C18-4 germline - established from type A spermatogonia isolated from 6-day-old mouse testes - exhibits typical morphological features of spermatogonial cells [271]; it constitutes a valuable in vitro cell model to evaluate testicular toxicity of BPA selected analogues. BPA and its analogues induce dose- and time-dependent alterations of cell cycle, significant change in nuclear shape, DNA damage and cytoskeleton disorganization with aberrant F-actin distribution in the cytoplasm of C18-4 cells [272]. Significant effects of BPA analogues have also been observed in GC-2 cell line, derived from mouse spermatocytes. Environmentally relevant concentrations of these compounds affect cell viability of GC-2 cells and trigger their apoptosis via mitochondria-mediated pathways [273]. Similarly to BPA, BPF and BPS affect global DNA methylation in GC-2 cells, increasing the content of 5-methylcytosines [36, 273].
Therefore, BPA analogue-induced damage follows all stages of spermatogenesis, starting from spermatogonia to spermatozoa. Both prenatal and postnatal exposure of mice to BPA analogues increase testosterone levels in mice serum, cause meiotic failure and delay the transition stages before spermiation, thus reducing sperm count, motility and quality [274, 275]. Lastly, spermatozoa release strongly depends on Sertoli cell integrity and ability to contact spermatozoa through ectoplasmic specializations [276]. Autophagy in Sertoli cells is essential for such a function [277], but the process is inhibited by BPA analogues [273]. Additionally, both in vivo and in vitro experiments suggest that BPA analogues cause lipid peroxidation and DNA fragmentation in spermatozoa [278].
Therefore, the effects of BPA analogues on reproduction are similar to those of the BPA itself, if not worse.
CONCLUSION
BPA interferes in steroid signaling, impairs developmental processes, and causes tissue damage through the induction of oxidative stress. It exerts deleterious activity on brain development and functions, affecting neurogenesis, synaptic plasticity and postnatal brain maturation, inducing neuro-inflammation and neuro-degeneration. As a consequence of the decrease in synaptic plasticity, impairment of cognitive abilities occurs, including learning and memory. The few available studies in humans suggest the possibility that BPA can increase hyperactivity, anxiety, and depression in a sex- and age-specific manner. BPA may also play a role in the development and progression of behavioral diseases such as ASD, functioning as a “dysregulator” of the expression of ASD-related genes. BPA significantly affects reproduction in both sexes by interfering with the physiology of the HPG axis with reproductive success and offspring health as main endpoints. In our opinion, due to the variety of BPA targets and its capacity to interfere with the ER signaling, which is an almost ubiquitous cell signaling system with pleiotropic effects at central and peripheral levels, it is very difficult to establish the tolerable limits of BPA exposure. In addition, the development of “safe” BPA alternatives and exposure concentrations is not realistic. Because, despite a large number of scientific articles on BPA-adverse effects on health, very few data address the effects of BPA analogues. Hence, further studies are necessary in this field because the growing and widespread distribution of BPA and of these new-generation xenoestrogens could represent a novel human health risk.
ACKNOWLEDGEMENTS
RM coordinated the organization of the manuscript. Manuscript draft: Par1 RM; par2 RC, SR, EP, and RM; par3 AS, SLN, AV; par 4 RM, RC, JT, MG, SF, RP, RS and EP; par 5 RM, AS, AV. AS, AS, AV and RM designed and prepared figures and graphical abstract; SF, RP, MG and RM critically revised the manuscript; SR and EP carried out language and editing revision of the manuscript; all the authors approved the final version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Rubin B.S. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127(1-2):27–34. doi: 10.1016/j.jsbmb.2011.05.002. [http://dx.doi.org/10.1016/j.jsbmb.2011.05.002]. [PMID: 21605673]. [DOI] [PubMed] [Google Scholar]
- 2.Frye C.A., Bo E., Calamandrei G., Calzà L., Dessì-Fulgheri F., Fernández M., Fusani L., Kah O., Kajta M., Le Page Y., Patisaul H.B., Venerosi A., Wojtowicz A.K., Panzica G.C. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012;24(1):144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [http://dx.doi.org/10.1111/j.1365-2826.2011.02229.x]. [PMID: 21951193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter C.A., Birnbaum L.S., Farabollini F., Newbold R.R., Rubin B.S., Talsness C.E., Vandenbergh J.G., Walser-Kuntz D.R., vom Saal F.S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24(2):199–224. doi: 10.1016/j.reprotox.2007.06.004. [http://dx.doi.org/10.1016/j.reprotox.2007.06.004]. [PMID: 17683900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares R.S., Escada-Rebelo S., Correia M., Mota P.C., Ramalho-Santos J. The non-genomic effects of endocrine-disrupting chemicals on mammalian sperm. Reproduction. 2016;151(1):R1–R13. doi: 10.1530/REP-15-0355. [http://dx.doi.org/10.1530/REP-15-0355]. [PMID: 26585413]. [DOI] [PubMed] [Google Scholar]
- 5.Peretz J., Vrooman L., Ricke W.A., Hunt P.A., Ehrlich S. [Google Scholar]; Hauser R., Padmanabhan V., Taylor H.S., Swan S.H., Vande V.C.A., Flaws J.A. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007-2013. Environ. Health Perspect. 2014;122(8):775–786. doi: 10.1289/ehp.1307728. [http://dx.doi.org/10.1289/ehp.1307728]. [PMID: 24896072]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrales J., Kristofco L.A., Steele W.B., Yates B.S., Breed C.S., Williams E.S., Brooks B.W. Global assessment of bisphenol a in the environment: Review and analysis of its occurrence and bioaccumulation. Dose-Response. An. Int. J. 2015;13:1–29. doi: 10.1177/1559325815598308. [http://dx.doi.org/10.1177/1559325815598308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenberg L.N., Ehrlich S., Belcher S.M., Ben-Jonathan N., Dolinoy D.C., Hugo E.R., Hunt P.A., Newbold R.R., Rubin B.S., Saili K.S., Soto A.M., Wang H.S., vom Saal F.S. Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocr. Disrupt. 2013 doi: 10.4161/endo.26490. 1e25078. [DOI] [Google Scholar]
- 8.Le Magueresse-Battistoni B., Multigner L., Beausoleil C., Rousselle C. Effects of bisphenol A on metabolism and evidences of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018;475:74–91. doi: 10.1016/j.mce.2018.02.009. [http://dx.doi.org/10.1016/j.mce.2018.02.009]. [PMID: 29481862]. [DOI] [PubMed] [Google Scholar]
- 9.Chianese R., Troisi J., Richards S., Scafuro M., Fasano S., Guida M., Pierantoni R., Meccariello R. Bisphenol A in reproduction: epigenetic effects. Curr. Med. Chem. 2018;25(6):748–770. doi: 10.2174/0929867324666171009121001. [PMID: 28990514]. [DOI] [PubMed] [Google Scholar]
- 10.Nunez A.A., Kannan K., Giesy J.P., Fang J., Clemens L.G. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42(8):917–922. doi: 10.1016/s0045-6535(00)00196-x. [http://dx.doi.org/10.1016/S0045-6535(00)00196-X]. [PMID: 11272914]. [DOI] [PubMed] [Google Scholar]
- 11.Calafat A.M., Ye X., Wong L.Y., Reidy J.A., Needham L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ. Health Perspect. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [http://dx.doi.org/10.1289/ehp.10753]. [PMID: 18197297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mercogliano R., Santonicola S. Investigation on bisphenol A levels in human milk and dairy supply chain: A review. Food Chem. Toxicol. 2018;114:98–107. doi: 10.1016/j.fct.2018.02.021. [http://dx.doi.org/10.1016/j.fct.2018.02.021]. [PMID: 29448092]. [DOI] [PubMed] [Google Scholar]
- 13.Dualde P., Pardo O., Corpas-Burgos F., Kuligowski J., Gormaz M., Vento M., Pastor A., Yusà V. Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed infants. Sci. Total Environ. 2019;668:797–805. doi: 10.1016/j.scitotenv.2019.03.024. [http://dx.doi.org/10.1016/j.scitotenv.2019.03.024]. [PMID: 30870748]. [DOI] [PubMed] [Google Scholar]
- 14.Mørck T.J., Sorda G., Bechi N., Rasmussen B.S., Nielsen J.B., Ietta F., Rytting E., Mathiesen L., Paulesu L., Knudsen L.E. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010;30(1):131–137. doi: 10.1016/j.reprotox.2010.02.007. [http://dx.doi.org/10.1016/j.reprotox.2010.02.007]. [PMID: 20214975]. [DOI] [PubMed] [Google Scholar]
- 15.Corbel T., Gayrard V., Puel S., Lacroix M.Z., Berrebi A., Gil S., Viguié C., Toutain P.L., Picard-Hagen N. Bidirectional placental transfer of Bisphenol A and its main metabolite, Bisphenol A-Glucuronide, in the isolated perfused human placenta. Reprod. Toxicol. 2014;47:51–58. doi: 10.1016/j.reprotox.2014.06.001. [http://dx.doi.org/10.1016/j.reprotox.2014.06.001]. [PMID: 24933518]. [DOI] [PubMed] [Google Scholar]
- 16.EFSA Panel on Food Contact Materials. Enzymes, Flavourings and Processing Aids (CEF). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015;13(1):3978. [http://dx.doi.org/10.2903/j.efsa.2015.3978]. [Google Scholar]
- 17.EFSA A statement on the developmental immunotoxicity of bisphenol A (BPA): answer to the question from the Dutch Ministry of Health, Welfare and Sport. EFSA J. 2016;14(10):4580. doi: 10.2903/j.efsa.2016.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenfeld C.S. Neuroendocrine disruption in animal models due to exposure to bisphenol A analogues. Front. Neuroendocrinol. 2017;47:123–133. doi: 10.1016/j.yfrne.2017.08.001. [http://dx.doi.org/10.1016/j.yfrne.2017.08.001]. [PMID: 28801100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andra S.S., Charisiadis P., Arora M., van Vliet-Ostaptchouk J.V., Makris K.C. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ. Int. 2015;85:352–379. doi: 10.1016/j.envint.2015.09.011. [http://dx.doi.org/10.1016/j.envint.2015.09.011]. [PMID: 26521216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullah A., Pirzada M., Jahan S., Ullah H., Shaheen G., Rehman H., Siddiqui M.F., Butt M.A. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere. 2018;209:508–516. doi: 10.1016/j.chemosphere.2018.06.089. [http://dx.doi.org/10.1016/j.chemosphere.2018.06.089]. [PMID: 29940534]. [DOI] [PubMed] [Google Scholar]
- 21.Rochester J.R., Bolden A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015;123(7):643–650. doi: 10.1289/ehp.1408989. [http://dx.doi.org/10.1289/ehp.1408989]. [PMID: 25775505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chianese R., Coccurello R., Viggiano A., Scafuro M., Fiore M., Coppola G., Operto F.F., Fasano S., Layé S., Pierantoni R., Meccariello R. Impact of dietary fats on brain functions. Curr. Neuropharmacol. 2018;16(7):1059–1085. doi: 10.2174/1570159X15666171017102547. [http://dx.doi.org/10.2174/1570159X15666171017102547]. [PMID: 29046155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motti M.L., Angelo D. S.; Meccariello, R. MicroRNAs, cancer and diet: Facts and new exciting perspectives. Curr. Mol. Pharmacol. 2018;11(2):90–96. doi: 10.2174/1874467210666171013123733. [http://dx.doi.org/10.2174/1874467210666171013123733]. [PMID: 29034844]. [DOI] [PubMed] [Google Scholar]
- 24.D’Angelo S., Scafuro M., Meccariello R. BPA and nutraceuticals, simultaneous effects on endocrine functions. Endocr. Metab. Immune Disord. Drug Targets. 2019;19(5):594–604. doi: 10.2174/1871530319666190101120119. Epub ahead of print [http://dx.doi.org/10.2174/1871530319666190101120119]. [PMID: 30621569]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebuli M.E., Patisaul H.B. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J. Steroid Biochem. Mol. Biol. 2016;160:148–159. doi: 10.1016/j.jsbmb.2015.08.021. [http://dx.doi.org/10.1016/j.jsbmb.2015.08.021]. [PMID: 26307491]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mhaouty-Kodja S., Belzunces L.P., Canivenc M.C., Schroeder H., Chevrier C., Pasquier E. Impairment of learning and memory performances induced by BPA: Evidences from the literature of a MoA mediated through an ED. Mol. Cell. Endocrinol. 2018;475:54–73. doi: 10.1016/j.mce.2018.03.017. [http://dx.doi.org/10.1016/j.mce.2018.03.017]. [PMID: 29605460]. [DOI] [PubMed] [Google Scholar]
- 27.Murata M., Kang J.H., Bisphenol A., Bisphenol A. BPA) and cell signaling pathways. Biotechnol. Adv. 2018;36(1):311–327. doi: 10.1016/j.biotechadv.2017.12.002. [http://dx.doi.org/10.1016/j.biotechadv.2017.12.002]. [PMID: 29229539]. [DOI] [PubMed] [Google Scholar]
- 28.Barouki R., Melén E., Herceg Z., Beckers J., Chen J., Karagas M., Puga A., Xia Y., Chadwick L., Yan W., Audouze K., Slama R., Heindel J., Grandjean P., Kawamoto T., Nohara K. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ. Int. 2018;114:77–86. doi: 10.1016/j.envint.2018.02.014. [http://dx.doi.org/10.1016/j.envint.2018.02.014]. [PMID: 29499450]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doshi T., Mehta S.S., Dighe V., Balasinor N., Vanage G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology. 2011;289(2-3):74–82. doi: 10.1016/j.tox.2011.07.011. [http://dx.doi.org/10.1016/j.tox.2011.07.011]. [PMID: 21827818]. [DOI] [PubMed] [Google Scholar]
- 30.Dolinoy D.C. The agouti mouse model: An epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008;66(Suppl. 1):S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [http://dx.doi.org/10.1111/j.1753-4887.2008.00056.x]. [PMID: 18673496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolinoy D.C., Huang D., Jirtle R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [http://dx.doi.org/10.1073/pnas.0703739104]. [PMID: 17670942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaoi T., Itoh K., Nakamura K., Ogi H., Fujiwara Y., Fushiki S. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [http://dx.doi.org/10.1016/j.bbrc.2008.09.028]. [PMID: 18804091]. [DOI] [PubMed] [Google Scholar]
- 33.Wolstenholme J.T., Rissman E.F., Connelly J.J. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011;59(3):296–305. doi: 10.1016/j.yhbeh.2010.10.001. [http://dx.doi.org/10.1016/j.yhbeh.2010.10.001]. [PMID: 21029734]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J.H., Sartor M.A., Rozek L.S., Faulk C., Anderson O.S., Jones T.R., Nahar M.S., Dolinoy D.C. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15:30. doi: 10.1186/1471-2164-15-30. [http://dx.doi.org/10.1186/1471-2164-15-30]. [PMID: 24433282]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tse L.A., Lee P.M.Y., Ho W.M., Lam A.T., Lee M.K., Ng S.S.M., He Y., Leung K.S., Hartle J.C., Hu H., Kan H., Wang F., Ng C.F. Bisphenol A and other environmental risk factors for prostate cancer in Hong Kong. Environ. Int. 2017;107:1–7. doi: 10.1016/j.envint.2017.06.012. [http://dx.doi.org/10.1016/j.envint.2017.06.012]. [PMID: 28644961]. [DOI] [PubMed] [Google Scholar]
- 36.Yin L., Dai Y., Jiang X., Liu Y., Chen H., Han F., Cao J., Liu J. Role of DNA methylation in bisphenol A exposed mouse spermatocyte. Environ. Toxicol. Pharmacol. 2016;48:265–271. doi: 10.1016/j.etap.2016.11.003. [http://dx.doi.org/10.1016/j.etap.2016.11.003]. [PMID: 27855348]. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H., Zhou X., Li D.K., Yang F., Pan H., Li T., Miao M., Li R., Yuan W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS One. 2017;12(6):e0178535. doi: 10.1371/journal.pone.0178535. [http://dx.doi.org/10.1371/journal.pone.0178535]. [PMID: 28582417]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson-Smith A.C. Genomic imprinting: the emergence of an epigenetic paradigm. Nat. Rev. Genet. 2011;12(8):565–575. doi: 10.1038/nrg3032. [http://dx.doi.org/10.1038/nrg3032]. [PMID: 21765458]. [DOI] [PubMed] [Google Scholar]
- 39.Susiarjo M., Sasson I., Mesaros C., Bartolomei M.S. Bisphenol a exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9(4):e1003401. doi: 10.1371/journal.pgen.1003401. [http://dx.doi.org/10.1371/journal.pgen.1003401]. [PMID: 23593014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drobná Z., Henriksen A.D., Wolstenholme J.T., Montiel C., Lambeth P.S., Shang S., Harris E.P., Zhou C., Flaws J.A., Adli M., Rissman E.F. Transgenerational effects of bisphenol a on gene expression and DNA methylation of imprinted genes in brain. Endocrinology. 2018;159(1):132–144. doi: 10.1210/en.2017-00730. [http://dx.doi.org/10.1210/en.2017-00730]. [PMID: 29165653]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichenlaub-Ritter U., Pacchierotti F., Bisphenol A. Bisphenol a effects on mammalian oogenesis and epigenetic integrity of oocytes: A case study exploring risks of endocrine disrupting chemicals. BioMed Res. Int. 2015:2015698795. doi: 10.1155/2015/698795. [http://dx.doi.org/10.1155/2015/698795]. [PMID: 26339634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doherty L.F., Bromer J.G., Zhou Y., Aldad T.S., Taylor H.S. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer. 2010;1(3):146–155. doi: 10.1007/s12672-010-0015-9. [http://dx.doi.org/10.1007/s12672-010-0015-9]. [PMID: 21761357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viré E., Brenner C., Deplus R., Blanchon L., Fraga M., Didelot C., Morey L., Van Eynde A., Bernard D., Vanderwinden J.M., Bollen M., Esteller M., Di Croce L., de Launoit Y., Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439(7078):871–874. doi: 10.1038/nature04431. [http://dx.doi.org/10.1038/nature04431]. [PMID: 16357870]. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z., Zuo X., He D., Ding S., Xu F., Yang H., Jin X., Fan Y., Ying L., Tian C., Ying C. Long-term exposure to a 'safe' dose of bisphenol A reduced protein acetylation in adult rat testes. Sci. Rep, 2017;9(7):40337. doi: 10.1038/srep40337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godlewski J., Lenart J., Salinska E. MicroRNA in brain pathology: Neurodegeneration the other side of the brain cancer. Noncoding RNA. 2019;5(1):E20. doi: 10.3390/ncrna5010020. [http://dx.doi.org/10.3390/ncrna5010020]. [PMID: 30813461]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi C., Zhang L., Qin C. Long non-coding RNAs in brain development, synaptic biology, and Alzheimer’s disease. Brain Res. Bull. 2017;132:160–169. doi: 10.1016/j.brainresbull.2017.03.010. [http://dx.doi.org/10.1016/j.brainresbull.2017.03.010]. [PMID: 28347717]. [DOI] [PubMed] [Google Scholar]
- 47.Sekar S., Liang W.S. Circular RNA expression and function in the brain. Noncoding RNA Res. 2019;4(1):23–29. doi: 10.1016/j.ncrna.2019.01.001. [http://dx.doi.org/10.1016/j.ncrna.2019.01.001]. [PMID: 30891534]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leighton L.J., Bredy T.W. Functional interplay between small non-coding RNAs and RNA modification in the brain. Noncoding RNA. 2018;4(2):E15. doi: 10.3390/ncrna4020015. [http://dx.doi.org/10.3390/ncrna4020015]. [PMID: 29880782]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noack F., Calegari F. Epitranscriptomics: A New Regulatory Mechanism of Brain Development and Function. Front. Neurosci. 2018;12:85. doi: 10.3389/fnins.2018.00085. [http://dx.doi.org/10.3389/fnins.2018.00085]. [PMID: 29515357]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avissar-Whiting M., Veiga K.R., Uhl K.M., Maccani M.A., Gagne L.A., Moen E.L., Marsit C.J. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010;29(4):401–406. doi: 10.1016/j.reprotox.2010.04.004. [http://dx.doi.org/10.1016/j.reprotox.2010.04.004]. [PMID: 20417706]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derghal A., Djelloul M., Trouslard J., Mounien L. An emerging role of micro-RNA in the effect of the endocrine disruptors. Front. Neurosci. 2016;10:318. doi: 10.3389/fnins.2016.00318. [http://dx.doi.org/10.3389/fnins.2016.00318]. [PMID: 27445682]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao G.Z., Zhao Y., Li H.X., Li W. Bisphenol A-elicited miR-146a-5p impairs murine testicular steroidogenesis through negative regulation of Mta3 signaling. Biochem. Biophys. Res. Commun. 2018;501(2):478–485. doi: 10.1016/j.bbrc.2018.05.017. [http://dx.doi.org/10.1016/j.bbrc.2018.05.017]. [PMID: 29746863]. [DOI] [PubMed] [Google Scholar]
- 53.Cho H., Kim S.J., Park H.W., Oh M.J., Yu S.Y., Lee S.Y., Park C., Han G.R., Oh J.H., Hwang S.Y., Yoon S.J. A relationship between miRNA and gene expression in the mouse Sertoli cell line after exposure to bisphenol A. Biochip J. 2010;4:75–81. [http://dx.doi.org/10.1007/s13206-010-4112-1]. [Google Scholar]
- 54.Kuruto-Niwa R., Tateoka Y., Usuki Y., Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66(6):1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [http://dx.doi.org/10.1016/j.chemosphere.2006.06.073]. [PMID: 16904728]. [DOI] [PubMed] [Google Scholar]
- 55.Guerrero-Bosagna C., Savenkova M., Haque M.M., Nilsson E., Skinner M.K. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLoS One. 2013;8(3):e59922. doi: 10.1371/journal.pone.0059922. [http://dx.doi.org/10.1371/journal.pone.0059922]. [PMID: 23555832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mendonca K., Hauser R., Calafat A.M., Arbuckle T.E., Duty S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health. 2014;87(1):13–20. doi: 10.1007/s00420-012-0834-9. [http://dx.doi.org/10.1007/s00420-012-0834-9]. [PMID: 23212895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobrzyńska M.M., Gajowik A., Radzikowska J., Tyrkiel E.J., Jankowska-Steifer E.A. Male-mediated F1 effects in mice exposed to bisphenol A, either alone or in combination with X-irradiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;789-790:36–45. doi: 10.1016/j.mrgentox.2015.06.015. [http://dx.doi.org/10.1016/j.mrgentox.2015.06.015]. [PMID: 26232256]. [DOI] [PubMed] [Google Scholar]
- 58.Marczylo E.L., Amoako A.A., Konje J.C., Gant T.W., Marczylo T.H. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7(5):432–439. doi: 10.4161/epi.19794. [http://dx.doi.org/10.4161/epi.19794]. [PMID: 22441141]. [DOI] [PubMed] [Google Scholar]
- 59.Mielke H., Partosch F., Gundert-Remy U. The contribution of dermal exposure to the internal exposure of bisphenol A in man. Toxicol. Lett. 2011;204(2-3):190–198. doi: 10.1016/j.toxlet.2011.04.032. [http://dx.doi.org/10.1016/j.toxlet.2011.04.032]. [PMID: 21571050]. [DOI] [PubMed] [Google Scholar]
- 60.Marquet F., Payan J.P., Beydon D., Wathier L., Grandclaude M.C., Ferrari E. In vivo and ex vivo percutaneous absorption of [14C]-bisphenol A in rats: a possible extrapolation to human absorption? Arch. Toxicol. 2011;85(9):1035–1043. doi: 10.1007/s00204-011-0651-z. [http://dx.doi.org/10.1007/s00204-011-0651-z]. [PMID: 21287149]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 2010;118(9):1196–1203. doi: 10.1289/ehp.0901575. [http://dx.doi.org/10.1289/ehp.0901575]. [PMID: 20382578]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schönfelder G., Wittfoht W., Hopp H., Talsness C.E., Paul M., Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110(11):A703–A707. doi: 10.1289/ehp.110-1241091. [http://dx.doi.org/10.1289/ehp.021100703]. [PMID: 12417499]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi O., Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ. Health Perspect. 2000;108(10):931–935. doi: 10.1289/ehp.00108931. [http://dx.doi.org/10.1289/ehp.00108931]. [PMID: 11049811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Teeguarden J.G., Twaddle N.C., Churchwell M.I., Doerge D.R. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem. Toxicol. 2016;92:129–142. doi: 10.1016/j.fct.2016.03.023. [http://dx.doi.org/10.1016/j.fct.2016.03.023]. [PMID: 27038865]. [DOI] [PubMed] [Google Scholar]
- 65.Grandin F.C., Lacroix M.Z., Gayrard V., Viguié C., Mila H., de Place A., Vayssière C., Morin M., Corbett J., Gayrard C., Gely C.A., Toutain P.L., Picard-Hagen N. Is bisphenol S a safer alternative to bisphenol A in terms of potential fetal exposure? Placental transfer across the perfused human placenta. Chemosphere. 2019;221:471–478. doi: 10.1016/j.chemosphere.2019.01.065. [http://dx.doi.org/10.1016/j.chemosphere.2019.01.065]. [PMID: 30654261]. [DOI] [PubMed] [Google Scholar]
- 66.Roen E.L., Wang Y., Calafat A.M., Wang S., Margolis A., Herbstman J., Hoepner L.A., Rauh V., Perera F.P. Bisphenol A exposure and behavioral problems among inner city children at 7-9 years of age. Environ. Res. 2015;142:739–745. doi: 10.1016/j.envres.2015.01.014. [http://dx.doi.org/10.1016/j.envres.2015.01.014]. [PMID: 25724466]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kundakovic M., Gudsnuk K., Franks B., Madrid J., Miller R.L., Perera F.P., Champagne F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA. 2013;110(24):9956–9961. doi: 10.1073/pnas.1214056110. [http://dx.doi.org/10.1073/pnas.1214056110]. [PMID: 23716699]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCaffrey K.A., Jones B., Mabrey N., Weiss B., Swan S.H., Patisaul H.B. Sex specific impact of perinatal bisphenol A (BPA) exposure over a range of orally administered doses on rat hypothalamic sexual differentiation. Neurotoxicology. 2013;36(36):55–62. doi: 10.1016/j.neuro.2013.03.001. [http://dx.doi.org/10.1016/j.neuro.2013.03.001]. [PMID: 23500335]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohwi M., Doe C.Q. Temporal fate specification and neural progenitor competence during development. Nat. Rev. Neurosci. 2013;14(12):823–838. doi: 10.1038/nrn3618. [http://dx.doi.org/10.1038/nrn3618]. [PMID: 24400340]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohtsuka T., Kageyama R. Regulation of temporal properties of neural stem cells and transition timing of neurogenesis and gliogenesis during mammalian neocortical development. Semin. Cell Dev. Biol. 2019;S1084-9521(18):30062–4. doi: 10.1016/j.semcdb.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Kempermann G., Jessberger S., Steiner B., Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27(8):447–452. doi: 10.1016/j.tins.2004.05.013. [http://dx.doi.org/10.1016/j.tins.2004.05.013]. [PMID: 15271491]. [DOI] [PubMed] [Google Scholar]
- 72.Kee N., Teixeira C.M., Wang A.H., Frankland P.W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [http://dx.doi.org/10.1038/nn1847]. [PMID: 17277773]. [DOI] [PubMed] [Google Scholar]
- 73.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [http://dx.doi.org/10.1016/j.cell.2008.01.033]. [PMID: 18295581]. [DOI] [PubMed] [Google Scholar]
- 74.Stuchlik A. Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front. Behav. Neurosci. 2014;8:106. doi: 10.3389/fnbeh.2014.00106. [http://dx.doi.org/10.3389/fnbeh.2014.00106]. [PMID: 24744707]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cassé F., Richetin K., Toni N. Astrocytes' contribution to adult neurogenesis in physiology and alzheimer's disease. Front. Cell. Neurosci. 2018;12(432) doi: 10.3389/fncel.2018.00432. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Negri-Cesi P., Bisphenol A. Bisphenol a interaction with brain development and functions. Dose Response. 2015;13(2):1559325815590394. doi: 10.1177/1559325815590394. [http://dx.doi.org/10.1177/1559325815590394]. [PMID: 26672480]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tiwari S.K., Agarwal S., Seth B., Yadav A., Ray R.S., Mishra V.N., Chaturvedi R.K. Inhibitory effects of bisphenol-a on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/β-Catenin pathway. Mol. Neurobiol. 2015;52(3):1735–1757. doi: 10.1007/s12035-014-8940-1. [http://dx.doi.org/10.1007/s12035-014-8940-1]. [PMID: 25381574]. [DOI] [PubMed] [Google Scholar]
- 78.Kim K., Son T.G., Kim S.J., Kim H.S., Kim T.S., Han S.Y., Lee J. Suppressive effects of bisphenol A on the proliferation of neural progenitor cells. J. Toxicol. Environ. Health A. 2007;70(15-16):1288–1295. doi: 10.1080/15287390701434216. [http://dx.doi.org/10.1080/15287390701434216]. [PMID: 17654246]. [DOI] [PubMed] [Google Scholar]
- 79.Kim K., Son T.G., Park H.R., Kim S.J., Kim H.S., Kim H.S., Kim T.S., Jung K.K., Han S.Y., Lee J. Potencies of bisphenol A on the neuronal differentiation and hippocampal neurogenesis. J. Toxicol. Environ. Health A. 2009;72(21-22):1343–1351. doi: 10.1080/15287390903212501. [http://dx.doi.org/10.1080/15287390903212501]. [PMID: 20077206]. [DOI] [PubMed] [Google Scholar]
- 80.Agarwal S., Tiwari S.K., Seth B., Yadav A., Singh A., Mudawal A., Chauhan L.K., Gupta S.K., Choubey V., Tripathi A., Kumar A., Ray R.S., Shukla S., Parmar D., Chaturvedi R.K. Activation of autophagic flux against xenoestrogen Bisphenol-A-induced hippocampal neurodegeneration via AMP kinase (AMPK)/mammalian target of Rapamycin (mTOR) pathways. J. Biol. Chem. 2015;290(34):21163–21184. doi: 10.1074/jbc.M115.648998. [http://dx.doi.org/10.1074/jbc.M115.648998]. [PMID: 26139607]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Zhao Y.G., Zhang H. The incredible ULKs: Autophagy and beyond. Mol. Cell. 2016;62(4):475–476. doi: 10.1016/j.molcel.2016.05.005. [http://dx.doi.org/10.1016/j.molcel.2016.05.005]. [PMID: 27203174]. [DOI] [PubMed] [Google Scholar]
- 82.Li Z., Zhao K., Lv X., Lan Y., Hu S., Shi J., Guan J., Yang Y., Lu H., He H., Gao F., He W. Ulk1 governs nerve growth factor/trka signaling by mediating Rab5 GTPase activation in porcine hemagglutinating encephalomyelitis virus-induced neurodegenerative disorders. J. Virol. 2018;92(16):e00325–e18. doi: 10.1128/JVI.00325-18. [http://dx.doi.org/10.1128/JVI.00325-18]. [PMID: 29875237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agarwal S., Yadav A., Tiwari S.K., Seth B., Chauhan L.K., Khare P., Ray R.S., Chaturvedi R.K. Dynamin-related Protein 1 inhibition mitigates Bisphenol A-mediated alterations in mitochondrial dynamics and neural stem cell proliferation and differentiation. J. Biol. Chem. 2016;291(31):15923–15939. doi: 10.1074/jbc.M115.709493. [http://dx.doi.org/10.1074/jbc.M115.709493]. [PMID: 27252377]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang Y.J., Park H.R., Kim T.H., Yang W.J., Lee J.J., Choi S.Y., Oh S.B., Lee E., Park J.H., Kim H.P., Kim H.S., Lee J. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296(1-3):73–82. doi: 10.1016/j.tox.2012.03.007. [http://dx.doi.org/10.1016/j.tox.2012.03.007]. [PMID: 22484357]. [DOI] [PubMed] [Google Scholar]
- 85.Kumar D., Thakur M.K. Effect of perinatal exposure to Bisphenol-A on DNA methylation and histone acetylation in cerebral cortex and hippocampus of postnatal male mice. J. Toxicol. Sci. 2017;42(3):281–289. doi: 10.2131/jts.42.281. [http://dx.doi.org/10.2131/jts.42.281]. [PMID: 28496034]. [DOI] [PubMed] [Google Scholar]
- 86.Feng J., Zhou Y., Campbell S.L., Le T., Li E., Sweatt J.D., Silva A.J., Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 2010;13(4):423–430. doi: 10.1038/nn.2514. [http://dx.doi.org/10.1038/nn.2514]. [PMID: 20228804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campbell R.R., Wood M.A. How the epigenome integrates information and reshapes the synapse. Nat. Rev. Neurosci. 2019;20(3):133–147. doi: 10.1038/s41583-019-0121-9. [http://dx.doi.org/10.1038/s41583-019-0121-9]. [PMID: 30696992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keverne E.B. Significance of epigenetics for understanding brain development, brain evolution and behaviour. Neuroscience. 2014;264:207–217. doi: 10.1016/j.neuroscience.2012.11.030. [http://dx.doi.org/10.1016/j.neuroscience.2012.11.030]. [PMID: 23201253]. [DOI] [PubMed] [Google Scholar]
- 89.Bale T.L. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015;16(6):332–344. doi: 10.1038/nrn3818. [http://dx.doi.org/10.1038/nrn3818]. [PMID: 25921815]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheong A., Johnson S.A., Howald E.C., Ellersieck M.R., Camacho L., Lewis S.M., Vanlandingham M.M., Ying J., Ho S.M., Rosenfeld C.S. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics. 2018;13(7):704–720. doi: 10.1080/15592294.2018.1497388. [http://dx.doi.org/10.1080/15592294.2018.1497388]. [PMID: 30001178]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kitraki E., Nalvarte I., Alavian-Ghavanini A., Rüegg J. Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response. Mol. Cell. Endocrinol. 2015;417:191–199. doi: 10.1016/j.mce.2015.09.028. [http://dx.doi.org/10.1016/j.mce.2015.09.028]. [PMID: 26427651]. [DOI] [PubMed] [Google Scholar]
- 92.Alavian-Ghavanini A., Lin P.I., Lind P.M., Risén Rimfors S., Halin Lejonklou M., Dunder L., Tang M., Lindh C., Bornehag C.G., Rüegg J. Prenatal bisphenol a exposure is linked to epigenetic changes in glutamate receptor subunit gene Grin2b in female rats and humans. Sci. Rep. 2018;8(1):11315. doi: 10.1038/s41598-018-29732-9. [http://dx.doi.org/10.1038/s41598-018-29732-9]. [PMID: 30054528]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [http://dx.doi.org/10.1038/nrn3504]. [PMID: 23686171]. [DOI] [PubMed] [Google Scholar]
- 94.Aiba T., Saito T., Hayashi A., Sato S., Yunokawa H., Maruyama T., Fujibuchi W., Ohsako S. Does the prenatal bisphenol A exposure alter DNA methylation levels in the mouse hippocampus? An analysis using a high-sensitivity methylome technique. Genes Environ. 2018;40:12. doi: 10.1186/s41021-018-0099-y. [http://dx.doi.org/10.1186/s41021-018-0099-y]. [PMID: 29881475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hajszan T., Leranth C. Bisphenol A interferes with synaptic remodeling. Front. Neuroendocrinol. 2010;31(4):519–530. doi: 10.1016/j.yfrne.2010.06.004. [http://dx.doi.org/10.1016/j.yfrne.2010.06.004]. [PMID: 20609373]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacLusky N.J., Hajszan T., Leranth C. The environmental estrogen bisphenol a inhibits estradiol-induced hippocampal synaptogenesis. Environ. Health Perspect. 2005;113(6):675–679. doi: 10.1289/ehp.7633. [http://dx.doi.org/10.1289/ehp.7633]. [PMID: 15929888]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leranth C., Petnehazy O., MacLusky N.J. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23(5):1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [http://dx.doi.org/10.1523/JNEUROSCI.23-05-01588.2003]. [PMID: 12629162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leranth C., Hajszan T., Szigeti-Buck K., Bober J., MacLusky N.J. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc. Natl. Acad. Sci. USA. 2008;105(37):14187–14191. doi: 10.1073/pnas.0806139105. [http://dx.doi.org/10.1073/pnas.0806139105]. [PMID: 18768812]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Santoro A., Spinelli C.C., Martucciello S., Nori S.L., Capunzo M., Puca A.A., Ciaglia E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018;103(3):509–524. doi: 10.1002/JLB.3MR0118-003R. [http://dx.doi.org/10.1002/JLB.3MR0118-003R]. [PMID: 29389023]. [DOI] [PubMed] [Google Scholar]
- 100.Allen N.J., Lyons D.A. Glia as architects of central nervous system formation and function. Science. 2018;362(6411):181–185. doi: 10.1126/science.aat0473. [http://dx.doi.org/10.1126/science.aat0473]. [PMID: 30309945]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramon-Cañellas P., Peterson H.P., Morante J. From early to late neurogenesis: neural progenitors and the glial niche from a fly’s point of view. Neuroscience. 2019;399:39–52. doi: 10.1016/j.neuroscience.2018.12.014. [http://dx.doi.org/10.1016/j.neuroscience.2018.12.014]. [PMID: 30578972]. [DOI] [PubMed] [Google Scholar]
- 102.Schwarz J.M., Sholar P.W., Bilbo S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120(6):948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [http://dx.doi.org/10.1111/j.1471-4159.2011.07630.x]. [PMID: 22182318]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williamson L.L., Sholar P.W., Mistry R.S., Smith S.H., Bilbo S.D. Microglia and memory: modulation by early-life infection. J. Neurosci. 2011;31(43):15511–15521. doi: 10.1523/JNEUROSCI.3688-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI.3688-11.2011]. [PMID: 22031897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosin J.M., Kurrasch D.M. Bisphenol A and microglia: could microglia be responsive to this environmental contaminant during neural development? Am. J. Physiol. Endocrinol. Metab. 2018;315(2):E279–E285. doi: 10.1152/ajpendo.00443.2017. [http://dx.doi.org/10.1152/ajpendo.00443.2017]. [PMID: 29812986]. [DOI] [PubMed] [Google Scholar]
- 105.Sadowski R.N., Wise L.M., Park P.Y., Schantz S.L., Juraska J.M. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience. 2014;279:122–131. doi: 10.1016/j.neuroscience.2014.08.038. [http://dx.doi.org/10.1016/j.neuroscience.2014.08.038]. [PMID: 25193849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wise L.M., Sadowski R.N., Kim T., Willing J., Juraska J.M. Long-term effects of adolescent exposure to bisphenol A on neuron and glia number in the rat prefrontal cortex: Differences between the sexes and cell type. Neurotoxicology. 2016;53:186–192. doi: 10.1016/j.neuro.2016.01.011. [http://dx.doi.org/10.1016/j.neuro.2016.01.011]. [PMID: 26828634]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Takahashi M., Komada M., Miyazawa K., Goto S., Ikeda Y. Bisphenol A exposure induces increased microglia and microglial related factors in the murine embryonic dorsal telencephalon and hypothalamus. Toxicol. Lett. 2018;284:113–119. doi: 10.1016/j.toxlet.2017.12.010. [http://dx.doi.org/10.1016/j.toxlet.2017.12.010]. [PMID: 29248573]. [DOI] [PubMed] [Google Scholar]
- 108.Luo G., Wang S., Li Z., Wei R., Zhang L., Liu H., Wang C., Niu R., Wang J. Maternal bisphenol a diet induces anxiety-like behavior in female juvenile with neuroimmune activation. Toxicol. Sci. 2014;140(2):364–373. doi: 10.1093/toxsci/kfu085. [http://dx.doi.org/10.1093/toxsci/kfu085]. [PMID: 24824810]. [DOI] [PubMed] [Google Scholar]
- 109.Zhu J., Jiang L., Liu Y., Qian W., Liu J., Zhou J., Gao R., Xiao H., Wang J. MAPK and NF-κB pathways are involved in bisphenol A-induced TNF-α and IL-6 production in BV2 microglial cells. Inflammation. 2015;38(2):637–648. doi: 10.1007/s10753-014-9971-5. [http://dx.doi.org/10.1007/s10753-014-9971-5]. [PMID: 25047101]. [DOI] [PubMed] [Google Scholar]
- 110.Bilbo S.D., Frank A., Frank A. Beach award: programming of neuroendocrine function by early-life experience: a critical role for the immune system. Horm. Behav. 2013;63(5):684–691. doi: 10.1016/j.yhbeh.2013.02.017. [http://dx.doi.org/10.1016/j.yhbeh.2013.02.017]. [PMID: 23474365]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patisaul H.B., Sullivan A.W., Radford M.E., Walker D.M., Adewale H.B., Winnik B., Coughlin J.L., Buckley B., Gore A.C. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7(9):e43890. doi: 10.1371/journal.pone.0043890. [http://dx.doi.org/10.1371/journal.pone.0043890]. [PMID: 22957036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rebuli M.E., Gibson P., Rhodes C.L., Cushing B.S., Patisaul H.B. Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen. Comp. Endocrinol. 2016;238:39–46. doi: 10.1016/j.ygcen.2016.04.018. [http://dx.doi.org/10.1016/j.ygcen.2016.04.018]. [PMID: 27102938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xu X.B., Fan S.J., He Y., Ke X., Song C., Xiao Y., Zhang W.H., Zhang J.Y., Yin X.P., Kato N., Pan B.X. Loss of hippocampal oligodendrocytes contributes to the deficit of contextual fear learning in adult rats experiencing early bisphenol A exposure. Mol. Neurobiol. 2017;54(6):4524–4536. doi: 10.1007/s12035-016-0003-3. [http://dx.doi.org/10.1007/s12035-016-0003-3]. [PMID: 27364615]. [DOI] [PubMed] [Google Scholar]
- 114.Hu F., Li T., Gong H., Chen Z., Jin Y., Xu G., Wang M. Bisphenol A impairs synaptic plasticity by both pre- and postsynaptic mechanisms. Adv. Sci. (Weinh.) 2017;4(8):1600493. doi: 10.1002/advs.201600493. [http://dx.doi.org/10.1002/advs.201600493]. [PMID: 28852612]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishido M., Yonemoto J., Morita M. Mesencephalic neurodegeneration in the orally administered bisphenol A-caused hyperactive rats. Toxicol. Lett. 2007;173(1):66–72. doi: 10.1016/j.toxlet.2007.06.014. [http://dx.doi.org/10.1016/j.toxlet.2007.06.014]. [PMID: 17689037]. [DOI] [PubMed] [Google Scholar]
- 116.Chen Z., Li T., Zhang L., Wang H., Hu F. Bisphenol A exposure remodels cognition of male rats attributable to excitatory alterations in the hippocampus and visual cortex. Toxicology. 2018;410:132–141. doi: 10.1016/j.tox.2018.10.002. [http://dx.doi.org/10.1016/j.tox.2018.10.002]. [PMID: 30312744]. [DOI] [PubMed] [Google Scholar]
- 117.Zhou Y., Wang Z., Xia M., Zhuang S., Gong X., Pan J., Li C., Fan R., Pang Q., Lu S. Neurotoxicity of low bisphenol A (BPA) exposure for young male mice: Implications for children exposed to environmental levels of BPA. Environ. Pollut. 2017;229:40–48. doi: 10.1016/j.envpol.2017.05.043. [http://dx.doi.org/10.1016/j.envpol.2017.05.043]. [PMID: 28577381]. [DOI] [PubMed] [Google Scholar]
- 118.Eilam-Stock T., Serrano P., Frankfurt M., Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav. Neurosci. 2012;126(1):175–185. doi: 10.1037/a0025959. [http://dx.doi.org/10.1037/a0025959]. [PMID: 22004261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuwahara R., Kawaguchi S., Kohara Y., Cui H., Yamashita K. Perinatal exposure to low-dose bisphenol A impairs spatial learning and memory in male rats. J. Pharmacol. Sci. 2013;123(2):132–139. doi: 10.1254/jphs.13093fp. [http://dx.doi.org/10.1254/jphs.13093FP]. [PMID: 24077108]. [DOI] [PubMed] [Google Scholar]
- 120.Diaz Weinstein S., Villafane J.J., Juliano N., Bowman R.E. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain Res. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [http://dx.doi.org/10.1016/j.brainres.2013.07.018]. [PMID: 23872220]. [DOI] [PubMed] [Google Scholar]
- 121.Xu X.H., Wang Y.M., Zhang J., Luo Q.Q., Ye Y.P., Ruan Q. Perinatal exposure to bisphenol-A changes N-methyl-D-aspartate receptor expression in the hippocampus of male rat offspring. Environ. Toxicol. Chem. 2010;29(1):176–181. doi: 10.1002/etc.18. [http://dx.doi.org/10.1002/etc.18]. [PMID: 20821433]. [DOI] [PubMed] [Google Scholar]
- 122.Kubo K., Arai O., Ogata R., Omura M., Hori T., Aou S. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neurosci. Lett. 2001;304(1-2):73–76. doi: 10.1016/s0304-3940(01)01760-8. [http://dx.doi.org/10.1016/S0304-3940(01)01760-8]. [PMID: 11335058]. [DOI] [PubMed] [Google Scholar]
- 123.Kubo K., Arai O., Omura M., Watanabe R., Ogata R., Aou S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 2003;45(3):345–356. doi: 10.1016/s0168-0102(02)00251-1. [http://dx.doi.org/10.1016/S0168-0102(02)00251-1]. [PMID: 12631470]. [DOI] [PubMed] [Google Scholar]
- 124.Elsworth J.D., Jentsch J.D., Groman S.M., Roth R.H., Redmond E.D., Jr, Leranth C. Low circulating levels of bisphenol-A induce cognitive deficits and loss of asymmetric spine synapses in dorsolateral prefrontal cortex and hippocampus of adult male monkeys. J. Comp. Neurol. 2015;523(8):1248–1257. doi: 10.1002/cne.23735. [http://dx.doi.org/10.1002/cne.23735]. [PMID: 25557059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braun J.M., Yolton K., Dietrich K.N., Hornung R., Ye X., Calafat A.M., Lanphear B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009;117(12):1945–1952. doi: 10.1289/ehp.0900979. [http://dx.doi.org/10.1289/ehp.0900979]. [PMID: 20049216]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braun J.M., Kalkbrenner A.E., Calafat A.M., Yolton K., Ye X., Dietrich K.N., Lanphear B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [http://dx.doi.org/10.1542/peds.2011-1335]. [PMID: 22025598]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perera F., Vishnevetsky J., Herbstman J.B., Calafat A.M., Xiong W., Rauh V., Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ. Health Perspect. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [http://dx.doi.org/10.1289/ehp.1104492]. [PMID: 22543054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lim Y.H., Bae S., Kim B.N., Shin C.H., Lee Y.A., Kim J.I., Hong Y.C. Prenatal and postnatal bisphenol A exposure and social impairment in 4-year-old children. Environ. Health. 2017;16(1):79. doi: 10.1186/s12940-017-0289-2. [http://dx.doi.org/10.1186/s12940-017-0289-2]. [PMID: 28747197]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miodovnik A., Engel S.M., Zhu C., Ye X., Soorya L.V., Silva M.J., Calafat A.M., Wolff M.S. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [http://dx.doi.org/10.1016/j.neuro.2010.12.009]. [PMID: 21182865]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carter C.J., Blizard R.A. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem. Int. 2016;101:83–109. doi: 10.1016/j.neuint.2016.10.011. [http://dx.doi.org/10.1016/j.neuint.2016.10.011]. [PMID: 27984170]. [DOI] [PubMed] [Google Scholar]
- 131.Thongkorn S., Kanlayaprasit S., Jindatip D., Tencomnao T., Hu V.W., Sarachana T. Sex differences in the effects of prenatal bisphenol A exposure on genes associated with autism spectrum disorder in the hippocampus. Sci. Rep. 2019;9(1):3038. doi: 10.1038/s41598-019-39386-w. [http://dx.doi.org/10.1038/s41598-019-39386-w]. [PMID: 30816183]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harley K.G., Gunier R.B., Kogut K., Johnson C., Bradman A., Calafat A.M., Eskenazi B. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [http://dx.doi.org/10.1016/j.envres.2013.06.004]. [PMID: 23870093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ghassabian A., Bell E.M., Ma W.L., Sundaram R., Kannan K., Buck Louis G.M., Yeung E. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ. Pollut. 2018;243(Pt B):1629–1636. doi: 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Matsushima A., Liu X., Okada H., Shimohigashi M., Shimohigashi Y. Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ. Health Perspect. 2010;118(9):1267–1272. doi: 10.1289/ehp.0901819. [http://dx.doi.org/10.1289/ehp.0901819]. [PMID: 20427257]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li Y., Burns K.A., Arao Y., Luh C.J., Korach K.S. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ. Health Perspect. 2012;120(7):1029–1035. doi: 10.1289/ehp.1104689. [http://dx.doi.org/10.1289/ehp.1104689]. [PMID: 22494775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molina-Molina J.M., Amaya E., Grimaldi M., Sáenz J.M., Real M., Fernández M.F., Balaguer P., Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013;272(1):127–136. doi: 10.1016/j.taap.2013.05.015. [http://dx.doi.org/10.1016/j.taap.2013.05.015]. [PMID: 23714657]. [DOI] [PubMed] [Google Scholar]
- 137.Danzl E., Sei K., Soda S., Ike M., Fujita M. Biodegradation of bisphenol A, bisphenol F and bisphenol S in seawater. Int. J. Environ. Res. Public Health. 2009;6(4):1472–1484. doi: 10.3390/ijerph6041472. [http://dx.doi.org/10.3390/ijerph6041472]. [PMID: 19440529]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liao C., Liu F., Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ. Sci. Technol. 2012;46(12):6515–6522. doi: 10.1021/es300876n. [http://dx.doi.org/10.1021/es300876n]. [PMID: 22591511]. [DOI] [PubMed] [Google Scholar]
- 139.Inadera H. Neurological Effects of Bisphenol A and its Analogues. Int. J. Med. Sci. 2015;12(12):926–936. doi: 10.7150/ijms.13267. [http://dx.doi.org/10.7150/ijms.13267]. [PMID: 26664253]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim B., Colon E., Chawla S., Vandenberg L.N., Suvorov A. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ. Health. 2015;14:64. doi: 10.1186/s12940-015-0051-6. [http://dx.doi.org/10.1186/s12940-015-0051-6]. [PMID: 26242739]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ohtani N., Iwano H., Suda K., Tsuji E., Tanemura K., Inoue H., Yokota H. Adverse effects of maternal exposure to bisphenol F on the anxiety- and depression-like behavior of offspring. J. Vet. Med. Sci. 2017;79(2):432–439. doi: 10.1292/jvms.16-0502. [http://dx.doi.org/10.1292/jvms.16-0502]. [PMID: 28025458]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Catanese M.C., Vandenberg L.N., Bisphenol S. BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology. 2017;158(3):516–530. doi: 10.1210/en.2016-1723. [PMID: 28005399]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Castro B., Sánchez P., Torres J.M., Ortega E. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ. Res. 2015;142:281–287. doi: 10.1016/j.envres.2015.07.001. [http://dx.doi.org/10.1016/j.envres.2015.07.001]. [PMID: 26186136]. [DOI] [PubMed] [Google Scholar]
- 144.Lee S., Kim Y.K., Shin T.Y., Kim S.H. Neurotoxic effects of bisphenol AF on calcium-induced ROS and MAPKs. Neurotox. Res. 2013;23(3):249–259. doi: 10.1007/s12640-012-9353-4. [http://dx.doi.org/10.1007/s12640-012-9353-4]. [PMID: 22996013]. [DOI] [PubMed] [Google Scholar]
- 145.Pierantoni R., Cobellis G., Meccariello R., Fasano S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002;218:69–141. doi: 10.1016/s0074-7696(02)18012-0. [http://dx.doi.org/10.1016/S0074-7696(02)18012-0]. [PMID: 12199520]. [DOI] [PubMed] [Google Scholar]
- 146.Pierantoni R., Cobellis G., Meccariello R., Cacciola G., Chianese R., Chioccarelli T., Fasano S. CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitam. Horm. 2009;81:367–387. doi: 10.1016/S0083-6729(09)81014-5. [http://dx.doi.org/10.1016/S0083-6729(09)81014-5]. [PMID: 19647119]. [DOI] [PubMed] [Google Scholar]
- 147.Pierantoni R., Cobellis G., Meccariello R., Cacciola G., Chianese R., Chioccarelli T., Fasano S. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Ann. N. Y. Acad. Sci. 2009;1163:279–291. doi: 10.1111/j.1749-6632.2008.03617.x. [http://dx.doi.org/10.1111/j.1749-6632.2008.03617.x]. [PMID: 19456349]. [DOI] [PubMed] [Google Scholar]
- 148.Cacciola G., Chianese R., Chioccarelli T., Ciaramella V., Fasano S., Pierantoni R., Meccariello R., Cobellis G. Cannabinoids and reproduction: A lasting and intriguing history. Pharmac. 2010;3:3275–3323. [http://dx.doi.org/10.3390/ph3103275]. [Google Scholar]
- 149.Chianese R., Chioccarelli T., Cacciola G., Ciaramella V., Fasano S., Pierantoni R., Meccariello R., Cobellis G. The contribution of lower vertebrate animal models in human reproduction research. Gen. Comp. Endocrinol. 2011;171(1):17–27. doi: 10.1016/j.ygcen.2010.12.011. [http://dx.doi.org/10.1016/j.ygcen.2010.12.011]. [PMID: 21192939]. [DOI] [PubMed] [Google Scholar]
- 150.Meccariello R., Chianese R., Chioccarelli T., Ciaramella V., Fasano S., Pierantoni R., Cobellis G. Intra-testicular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. (Lausanne) 2014;5:69. doi: 10.3389/fendo.2014.00069. [http://dx.doi.org/10.3389/fendo.2014.00069]. [PMID: 24847312]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chianese R., Cobellis G., Chioccarelli T., Ciaramella V., Migliaccio M., Fasano S., Pierantoni R., Meccariello R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016;23(36):4070–4091. doi: 10.2174/0929867323666160902155434. [http://dx.doi.org/10.2174/0929867323666160902155434]. [PMID: 27593959]. [DOI] [PubMed] [Google Scholar]
- 152.Cobellis G., Meccariello R., Chianese R., Chioccarelli T., Fasano S., Pierantoni R. Effects of neuroendocrine CB1 activity on adult Leydig cells. Front. Endocrinol. (Lausanne) 2016;7:47. doi: 10.3389/fendo.2016.00047. [http://dx.doi.org/10.3389/fendo.2016.00047]. [PMID: 27375550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chianese R., Colledge W.H., Fasano S., Meccariello R. Editorial: The Multiple Facets of Kisspeptin Activity in Biological Systems. Front. Endocrinol. (Lausanne) 2018;9:727. doi: 10.3389/fendo.2018.00727. [http://dx.doi.org/10.3389/fendo.2018.00727]. [PMID: 30559719]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meccariello R., Fasano S., Pierantoni R., Cobellis G. Modulators of hypothalamic-pituitary-gonadal axis for the control of spermatogenesis and sperm quality in vertebrates. Front. Endocrinol. (Lausanne) 2014;5:135. doi: 10.3389/fendo.2014.00135. [http://dx.doi.org/10.3389/fendo.2014.00135]. [PMID: 25183961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ciaramella V., Chianese R., Pariante P., Fasano S., Pierantoni R., Meccariello R. Expression analysis of gnrh1 and gnrhr1 in spermatogenic cells of rat. Int. J. Endocrinol. 2015;2015:982726. doi: 10.1155/2015/982726. [http://dx.doi.org/10.1155/2015/982726]. [PMID: 25861269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cobellis G., Meccariello R., Pierantoni R., Fasano S. Intratesticular signals for progression of germ cell stages in vertebrates. Gen. Comp. Endocrinol. 2003;134(3):220–228. doi: 10.1016/s0016-6480(03)00281-8. [http://dx.doi.org/10.1016/S0016-6480(03)00281-8]. [PMID: 14636628]. [DOI] [PubMed] [Google Scholar]
- 157.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Kisspeptin regulates steroidogenesis and spermiation in anuran amphibian. Reproduction. 2017;154(4):403–414. doi: 10.1530/REP-17-0030. [http://dx.doi.org/10.1530/REP-17-0030]. [PMID: 28878091]. [DOI] [PubMed] [Google Scholar]
- 158.Chianese R., Ciaramella V., Fasano S., Pierantoni R., Meccariello R. Kisspeptin drives germ cell progression in the anuran amphibian Pelophylax esculentus: a study carried out in ex vivo testes. Gen. Comp. Endocrinol. 2015;211:81–91. doi: 10.1016/j.ygcen.2014.11.008. [http://dx.doi.org/10.1016/j.ygcen.2014.11.008]. [PMID: 25452028]. [DOI] [PubMed] [Google Scholar]
- 159.Huo X., Chen D., He Y., Zhu W., Zhou W., Zhang J. Bisphenol-A and Female Infertility: A Possible Role of Gene-Environment Interactions. Int. J. Environ. Res. Public Health. 2015;12(9):11101–11116. doi: 10.3390/ijerph120911101. [http://dx.doi.org/10.3390/ijerph120911101]. [PMID: 26371021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cariati F., D’Uonno N., Borrillo F., Iervolino S., Galdiero G., Tomaiuolo R. Bisphenol a: an emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019;17(1):6. doi: 10.1186/s12958-018-0447-6. [http://dx.doi.org/10.1186/s12958-018-0447-6]. [PMID: 30660193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Franssen D., Gérard A., Hennuy B., Donneau A.F., Bourguignon J.P., Parent A.S. Delayed neuroendocrine sexual maturation in female rats after a very low dose of bisphenol a through altered GABAergic neurotransmission and opposing effects of a high dose. Endocrinology. 2016;157(5):1740–1750. doi: 10.1210/en.2015-1937. [http://dx.doi.org/10.1210/en.2015-1937]. [PMID: 26950200]. [DOI] [PubMed] [Google Scholar]
- 162.Oliveira I.M., Romano R.M., de Campos P., Cavallin M.D., Oliveira C.A., Romano M.A. Delayed onset of puberty in male offspring from bisphenol A-treated dams is followed by the modulation of gene expression in the hypothalamic-pituitary-testis axis in adulthood. Reprod. Fertil. Dev. 2017;29(12):2496–2505. doi: 10.1071/RD17107. [http://dx.doi.org/10.1071/RD17107]. [PMID: 28641706]. [DOI] [PubMed] [Google Scholar]
- 163.de Roux N., Genin E., Carel J.C., Matsuda F., Chaussain J.L., Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA. 2003;100(19):10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seminara S.B., Messager S., Chatzidaki E.E., Thresher R.R., Acierno J.S., Jr, Shagoury J.K., Bo-Abbas Y., Kuohung W., Schwinof K.M., Hendrick A.G., Zahn D., Dixon J., Kaiser U.B., Slaugenhaupt S.A., Gusella J.F., O’Rahilly S., Carlton M.B., Crowley W.F., Jr, Aparicio S.A., Colledge W.H. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003;349(17):1614–1627. doi: 10.1056/NEJMoa035322. [http://dx.doi.org/10.1056/NEJMoa035322]. [PMID: 14573733]. [DOI] [PubMed] [Google Scholar]
- 165.Pinilla L., Aguilar E., Dieguez C., Millar R.P., Tena-Sempere M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012;92(3):1235–1316. doi: 10.1152/physrev.00037.2010. [http://dx.doi.org/10.1152/physrev.00037.2010]. [PMID: 22811428]. [DOI] [PubMed] [Google Scholar]
- 166.Patisaul H.B., Todd K.L., Mickens J.A., Adewale H.B. Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30(3):350–357. doi: 10.1016/j.neuro.2009.02.010. [http://dx.doi.org/10.1016/j.neuro.2009.02.010]. [PMID: 19442818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cao J., Mickens J.A., McCaffrey K.A., Leyrer S.M., Patisaul H.B. Neonatal bisphenol a exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33(1):23–36. doi: 10.1016/j.neuro.2011.11.002. [http://dx.doi.org/10.1016/j.neuro.2011.11.002]. [PMID: 22101008]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Arambula S.E., Fuchs J., Cao J., Patisaul H.B. Effects of perinatal bisphenol A exposure on the volume of sexually-dimorphic nuclei of juvenile rats: A CLARITY-BPA consortium study. Neurotoxicology. 2017;63:33–42. doi: 10.1016/j.neuro.2017.09.002. [http://dx.doi.org/10.1016/j.neuro.2017.09.002]. [PMID: 28890130]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arambula S.E., Belcher S.M., Planchart A., Turner S.D., Patisaul H.B. Impact of low dose oral exposure to bisphenol a (BPA) on the neonatal rat hypothalamic and hippocampal transcriptome: A CLARITY-BPA consortium study. Endocrinology. 2016;157(10):3856–3872. doi: 10.1210/en.2016-1339. [http://dx.doi.org/10.1210/en.2016-1339]. [PMID: 27571134]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kurian J.R., Keen K.L., Kenealy B.P., Garcia J.P., Hedman C.J., Terasawa E. Acute influences of bisphenol a exposure on hypothalamic release of gonadotropin-releasing hormone and kisspeptin in female rhesus monkeys. Endocrinology. 2015;156(7):2563–2570. doi: 10.1210/en.2014-1634. [http://dx.doi.org/10.1210/en.2014-1634]. [PMID: 25853665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klenke U., Constantin S., Wray S. BPA directly decreases GnRH neuronal activity via noncanonical pathway. Endocrinology. 2016;157(5):1980–1990. doi: 10.1210/en.2015-1924. [http://dx.doi.org/10.1210/en.2015-1924]. [PMID: 26934298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.McIlwraith E.K., Loganathan N., Belsham D.D. Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol a in immortalized hypothalamic neurons. Front. Neurosci. 2018;12:838. doi: 10.3389/fnins.2018.00838. [http://dx.doi.org/10.3389/fnins.2018.00838]. [PMID: 30524225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McIlwraith E.K., Loganathan N., Belsham DD. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol. Cell. Endocrinol. 2019;S0303-7207(19):30038–3. doi: 10.1016/j.mce.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 174.Legeay S., Faure S. Is bisphenol A an environmental obesogen? Food Chem. Toxicol. 2018;114:98–107. [Google Scholar]
- 175.Errico S., Portaccio M., Nicolucci C., Meccariello R., Chianese R., Scafuro M., Lepore M., Diano N. A novel experimental approach for liver analysis in rats exposed to Bisphenol A by means of LC-mass spectrometry and infrared spectroscopy. J. Pharm. Biomed. Anal. 2019;165:207–212. doi: 10.1016/j.jpba.2018.12.011. [http://dx.doi.org/10.1016/j.jpba.2018.12.011]. [PMID: 30553981]. [DOI] [PubMed] [Google Scholar]
- 176.Shu L., Meng Q., Diamante G., Tsai B., Chen Y.W., Mikhail A., Luk H., Ritz B., Allard P., Yang X. Prenatal bisphenol a exposure in mice induces multitissue multiomics disruptions linking to cardiometabolic disorders. Endocrinology. 2019;160(2):409–429. doi: 10.1210/en.2018-00817. [http://dx.doi.org/10.1210/en.2018-00817]. [PMID: 30566610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Friedman J.M., Halaas J.L. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [http://dx.doi.org/10.1038/27376]. [PMID: 9796811]. [DOI] [PubMed] [Google Scholar]
- 178.Smith J.T., Acohido B.V., Clifton D.K., Steiner R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006;18(4):298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [http://dx.doi.org/10.1111/j.1365-2826.2006.01417.x]. [PMID: 16503925]. [DOI] [PubMed] [Google Scholar]
- 179.Castellano J.M., Navarro V.M., Fernández-Fernández R., Nogueiras R., Tovar S., Roa J., Vazquez M.J., Vigo E., Casanueva F.F., Aguilar E., Pinilla L., Dieguez C., Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146(9):3917–3925. doi: 10.1210/en.2005-0337. [http://dx.doi.org/10.1210/en.2005-0337]. [PMID: 15932928]. [DOI] [PubMed] [Google Scholar]
- 180.Castellano J.M., Navarro V.M., Roa J., Pineda R., Sánchez-Garrido M.A., García-Galiano D., Vigo E., Dieguez C., Aguilar E., Pinilla L., Tena-Sempere M. Alterations in hypothalamic KiSS-1 system in experimental diabetes: early changes and functional consequences. Endocrinology. 2009;150(2):784–794. doi: 10.1210/en.2008-0849. [http://dx.doi.org/10.1210/en.2008-0849]. [PMID: 18845637]. [DOI] [PubMed] [Google Scholar]
- 181.Dudek M., Kołodziejski P.A., Pruszyńska-Oszmałek E., Sassek M., Ziarniak K., Nowak K.W., Sliwowska J.H. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides. 2016;56:41–49. doi: 10.1016/j.npep.2016.01.005. [http://dx.doi.org/10.1016/j.npep.2016.01.005]. [PMID: 26853724]. [DOI] [PubMed] [Google Scholar]
- 182.Roepke T.A., Yang J.A., Yasrebi A., Mamounis K.J., Oruc E., Zama A.M., Uzumcu M. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats. Reprod. Toxicol. 2016;62:18–26. doi: 10.1016/j.reprotox.2016.04.014. [http://dx.doi.org/10.1016/j.reprotox.2016.04.014]. [PMID: 27103539]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Desai M., Ferrini M.G., Han G., Jellyman J.K., Ross M.G. In vivo maternal and in vitro BPA exposure effects on hypothalamic neurogenesis and appetite regulators. Environ. Res. 2018;164:45–52. doi: 10.1016/j.envres.2018.02.011. [http://dx.doi.org/10.1016/j.envres.2018.02.011]. [PMID: 29476947]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cornejo M.P., Hentges S.T., Maliqueo M., Coirini H., Becu-Villalobos D., Elias C.F. Neuroendocrine Regulation of Metabolism. J. Neuroendocrinol. 2016;28(7):12395. doi: 10.1111/jne.12395. [http://dx.doi.org/10.1111/jne.12395]. [PMID: 27114114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wilson J.L., Enriori P.J. A talk between fat tissue, gut, pancreas and brain to control body weight. Mol. Cell. Endocrinol. 2015;418(Pt 2):108–119. doi: 10.1016/j.mce.2015.08.022. [http://dx.doi.org/10.1016/j.mce.2015.08.022]. [PMID: 26316427]. [DOI] [PubMed] [Google Scholar]
- 186.Salehi A., Loganathan N., Belsham D.D. Bisphenol A induces Pomc gene expression through neuroinflammatory and PPARγ nuclear receptor-mediated mechanisms in POMC-expressing hypothalamic neuronal models. Mol. Cell. Endocrinol. 2019;479:12–19. doi: 10.1016/j.mce.2018.08.009. [http://dx.doi.org/10.1016/j.mce.2018.08.009]. [PMID: 30149043]. [DOI] [PubMed] [Google Scholar]
- 187.MacKay H., Patterson Z.R., Abizaid A. Perinatal Exposure to low-dose bisphenol-a disrupts the structural and functional development of the hypothalamic feeding circuitry. Endocrinology. 2017;158(4):768–777. doi: 10.1210/en.2016-1718. [http://dx.doi.org/10.1210/en.2016-1718]. [PMID: 28323920]. [DOI] [PubMed] [Google Scholar]
- 188.Rezg R., Abot A., Mornagui B., Aydi S., Knauf C. Effects of Bisphenol S on hypothalamic neuropeptides regulating feeding behavior and apelin/APJ system in mice. Ecotoxicol. Environ. Saf. 2018;161:459–466. doi: 10.1016/j.ecoenv.2018.06.001. [http://dx.doi.org/10.1016/j.ecoenv.2018.06.001]. [PMID: 29909315]. [DOI] [PubMed] [Google Scholar]
- 189.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr. Rev. 2006;27(1):73–100. doi: 10.1210/er.2005-0009. [http://dx.doi.org/10.1210/er.2005-0009]. [PMID: 16306385]. [DOI] [PubMed] [Google Scholar]
- 190.Battista N., Meccariello R., Cobellis G., Fasano S., Di Tommaso M., Pirazzi V., Konje J.C., Pierantoni R., Maccarrone M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012;355(1):1–14. doi: 10.1016/j.mce.2012.01.014. [http://dx.doi.org/10.1016/j.mce.2012.01.014]. [PMID: 22305972]. [DOI] [PubMed] [Google Scholar]
- 191.Meccariello R., Battista N., Bradshaw H.B., Wang H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014;2014:412354. doi: 10.1155/2014/412354. [http://dx.doi.org/10.1155/2014/412354]. [PMID: 24550985]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bovolin P., Cottone E., Pomatto V., Fasano S., Pierantoni R., Cobellis G., Meccariello R. Endocannabinoids are involved in male vertebrate reproduction: regulatory mechanisms at central and gonadal level. Front. Endocrinol. (Lausanne) 2014;5:54. doi: 10.3389/fendo.2014.00054. [http://dx.doi.org/10.3389/fendo.2014.00054]. [PMID: 24782832]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meccariello R., Franzoni M.F., Chianese R., Cottone E., Scarpa D., Donna D., Cobellis G., Guastalla A., Pierantoni R., Fasano S. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology. 2008;149(5):2149–2158. doi: 10.1210/en.2007-1357. [http://dx.doi.org/10.1210/en.2007-1357]. [PMID: 18218699]. [DOI] [PubMed] [Google Scholar]
- 194.Chianese R., Cobellis G., Pierantoni R., Fasano S., Meccariello R. Non-mammalian vertebrate models and the endocannabinoid system: relationships with gonadotropin-releasing hormone. Mol. Cell. Endocrinol. 2008;286(1-2) Suppl. 1:S46–S51. doi: 10.1016/j.mce.2008.01.009. [http://dx.doi.org/10.1016/j.mce.2008.01.009]. [PMID: 18325658]. [DOI] [PubMed] [Google Scholar]
- 195.Ciaramella V., Meccariello R., Chioccarelli T., Sirleto M., Fasano S., Pierantoni R., Chianese R. Anandamide acts via kisspeptin in the regulation of testicular activity of the frog, Pelophylax esculentus. Mol. Cell. Endocrinol. 2016;420:75–84. doi: 10.1016/j.mce.2015.11.011. [http://dx.doi.org/10.1016/j.mce.2015.11.011]. [PMID: 26586207]. [DOI] [PubMed] [Google Scholar]
- 196.Osei-Hyiaman D., Depetrillo M., Harvey-White J., Bannon A.W., Cravatt B.F., Kuhar M.J., Mackie K., Palkovits M., Kunos G. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81(4):273–282. doi: 10.1159/000087925. [http://dx.doi.org/10.1159/000087925]. [PMID: 16131814]. [DOI] [PubMed] [Google Scholar]
- 197.Suglia A., Chianese R., Migliaccio M., Ambrosino C., Fasano S., Pierantoni R., Cobellis G., Chioccarelli T. Bisphenol A induces hypothalamic down-regulation of the the cannabinoid receptor 1 and anorexigenic effects in male mice. Pharmacol. Res. 2016;113(Pt A):376–383. doi: 10.1016/j.phrs.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 198.Loganathan N., Salehi A., Chalmers J.A., Belsham D.D., Bisphenol A. Bisphenol a alters bmal1, Per2, and Rev-Erba mRNA and requires bmal1 to increase neuropeptide Y expression in hypothalamic neurons. Endocrinology. 2019;160(1):181–192. doi: 10.1210/en.2018-00881. [http://dx.doi.org/10.1210/en.2018-00881]. [PMID: 30500912]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen W., Lau S.W., Fan Y., Wu R.S.S., Ge W. Juvenile exposure to bisphenol A promotes ovarian differentiation but suppresses its growth - Potential involvement of pituitary follicle-stimulating hormone. Aquat. Toxicol. 2017;193:111–121. doi: 10.1016/j.aquatox.2017.10.008. [http://dx.doi.org/10.1016/j.aquatox.2017.10.008]. [PMID: 29055862]. [DOI] [PubMed] [Google Scholar]
- 200.Maffini M.V., Rubin B.S., Sonnenschein C., Soto A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006;254-255:179–186. doi: 10.1016/j.mce.2006.04.033. [http://dx.doi.org/10.1016/j.mce.2006.04.033]. [PMID: 16781053]. [DOI] [PubMed] [Google Scholar]
- 201.Zhang H-Q., Zhang X-F., Zhang L-J., Chao H-H., Pan B., Feng Y-M., Li L., Sun X.F., Shen W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol. Biol. Rep. 2012;39(5):5651–5657. doi: 10.1007/s11033-011-1372-3. [http://dx.doi.org/10.1007/s11033-011-1372-3]. [PMID: 22187349]. [DOI] [PubMed] [Google Scholar]
- 202.Susiarjo M., Hassold T.J., Freeman E., Hunt P.A. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3(1):e5. doi: 10.1371/journal.pgen.0030005. [http://dx.doi.org/10.1371/journal.pgen.0030005]. [PMID: 17222059]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hunt P.A., Lawson C., Gieske M., Murdoch B., Smith H., Marre A., Hassold T., VandeVoort C.A. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA. 2012;109(43):17525–17530. doi: 10.1073/pnas.1207854109. [http://dx.doi.org/10.1073/pnas.1207854109]. [PMID: 23012422]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Karavan J.R., Pepling M.E. Effects of estrogenic compounds on neonatal oocyte development. Reprod. Toxicol. 2012;34(1):51–56. doi: 10.1016/j.reprotox.2012.02.005. [http://dx.doi.org/10.1016/j.reprotox.2012.02.005]. [PMID: 22406039]. [DOI] [PubMed] [Google Scholar]
- 205.Rodríguez H.A., Santambrosio N., Santamaría C.G., Muñoz-de-Toro M., Luque E.H. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 2010;30(4):550–557. doi: 10.1016/j.reprotox.2010.07.008. [http://dx.doi.org/10.1016/j.reprotox.2010.07.008]. [PMID: 20692330]. [DOI] [PubMed] [Google Scholar]
- 206.Chao H-H., Zhang X-F., Chen B., Pan B., Zhang L-J., Li L., Sun X-F., Shi Q-H., Shen W. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 2012;137(2):249–259. doi: 10.1007/s00418-011-0894-z. [http://dx.doi.org/10.1007/s00418-011-0894-z]. [PMID: 22131059]. [DOI] [PubMed] [Google Scholar]
- 207.Laing L.V., Viana J., Dempster E.L., Trznadel M., Trunkfield L.A., Uren Webster T.M., van Aerle R., Paull G.C., Wilson R.J., Mill J., Santos E.M. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics. 2016;11(7):526–538. doi: 10.1080/15592294.2016.1182272. [http://dx.doi.org/10.1080/15592294.2016.1182272]. [PMID: 27120497]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu Y., Yuan C., Chen S., Zheng Y., Zhang Y., Gao J., Wang Z. Global and cyp19a1a gene specific DNA methylation in gonads of adult rare minnow Gobiocypris rarus under bisphenol A exposure. Aquat. Toxicol. 2014;156:10–16. doi: 10.1016/j.aquatox.2014.07.017. [http://dx.doi.org/10.1016/j.aquatox.2014.07.017]. [PMID: 25125231]. [DOI] [PubMed] [Google Scholar]
- 209.Santangeli S., Maradonna F., Gioacchini G., Cobellis G., Piccinetti C.C., Dalla Valle L., Carnevali O. BPA-induced deregulation of epigenetic patterns: Effects on female zebrafish reproduction. Sci. Rep. 2016;6:21982. doi: 10.1038/srep21982. [http://dx.doi.org/10.1038/srep21982]. [PMID: 26911650]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kandaraki E., Chatzigeorgiou A., Livadas S., Palioura E., Economou F., Koutsilieris M., Palimeri S., Panidis D., Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011;96(3):E480–E484. doi: 10.1210/jc.2010-1658. [http://dx.doi.org/10.1210/jc.2010-1658]. [PMID: 21193545]. [DOI] [PubMed] [Google Scholar]
- 211.Rutkowska A., Rachoń D., Bisphenol A., Bisphenol A. BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2014;30(4):260–265. doi: 10.3109/09513590.2013.871517. [http://dx.doi.org/10.3109/09513590.2013.871517]. [PMID: 24397396]. [DOI] [PubMed] [Google Scholar]
- 212.Takeuchi T., Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem. Biophys. Res. Commun. 2002;291(1):76–78. doi: 10.1006/bbrc.2002.6407. [http://dx.doi.org/10.1006/bbrc.2002.6407]. [PMID: 11829464]. [DOI] [PubMed] [Google Scholar]
- 213.Takeuchi T., Tsutsumi O., Ikezuki Y., Takai Y., Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr. J. 2004;51(2):165–169. doi: 10.1507/endocrj.51.165. [http://dx.doi.org/10.1507/endocrj.51.165]. [PMID: 15118266]. [DOI] [PubMed] [Google Scholar]
- 214.Zhou W., Liu J., Liao L., Han S., Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell. Endocrinol. 2008;283(1-2):12–18. doi: 10.1016/j.mce.2007.10.010. [http://dx.doi.org/10.1016/j.mce.2007.10.010]. [PMID: 18191889]. [DOI] [PubMed] [Google Scholar]
- 215.Fernández M., Bourguignon N., Lux-Lantos V., Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ. Health Perspect. 2010;118(9):1217–1222. doi: 10.1289/ehp.0901257. [http://dx.doi.org/10.1289/ehp.0901257]. [PMID: 20413367]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Seachrist D.D., Bonk K.W., Ho S-M., Prins G.S., Soto A.M., Keri R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016;59:167–182. doi: 10.1016/j.reprotox.2015.09.006. [http://dx.doi.org/10.1016/j.reprotox.2015.09.006]. [PMID: 26493093]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mallozzi M., Leone C., Manurita F., Bellati F., Caserta D. Endocrine disrupting chemicals and endometrial cancer: An overview of recent laboratory evidence and epidemiological studies. Int. J. Environ. Res. Public Health. 2017;14(3):14. doi: 10.3390/ijerph14030334. [http://dx.doi.org/10.3390/ijerph14030334]. [PMID: 28327540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aghajanova L., Giudice L.C. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod. Biomed. Online. 2011;22(3):249–256. doi: 10.1016/j.rbmo.2010.12.007. [http://dx.doi.org/10.1016/j.rbmo.2010.12.007]. [PMID: 21273127]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pollock T., deCatanzaro D. Presence and bioavailability of bisphenol A in the uterus of rats and mice following single and repeated dietary administration at low doses. Reprod. Toxicol. 2014;49:145–154. doi: 10.1016/j.reprotox.2014.08.005. [http://dx.doi.org/10.1016/j.reprotox.2014.08.005]. [PMID: 25181699]. [DOI] [PubMed] [Google Scholar]
- 220.Diamanti-Kandarakis E., Bourguignon J-P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002. [http://dx.doi.org/10.1210/er.2009-0002]. [PMID: 19502515]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Aldad T.S., Rahmani N., Leranth C., Taylor H.S. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil. Steril. 2011;96(1):175–179. doi: 10.1016/j.fertnstert.2011.04.010. [http://dx.doi.org/10.1016/j.fertnstert.2011.04.010]. [PMID: 21536273]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hiroi H., Tsutsumi O., Takeuchi T., Momoeda M., Ikezuki Y., Okamura A., Yokota H., Taketani Y. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr. J. 2004;51(6):595–600. doi: 10.1507/endocrj.51.595. [http://dx.doi.org/10.1507/endocrj.51.595]. [PMID: 15644579]. [DOI] [PubMed] [Google Scholar]
- 223.Han T.S., Lean M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016;5:2048004016633371. doi: 10.1177/2048004016633371. [http://dx.doi.org/10.1177/2048004016633371]. [PMID: 26998259]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shafiee M.N., Seedhouse C., Mongan N., Chapman C., Deen S., Abu J., Atiomo W. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Mol. Cell. Endocrinol. 2016;424:94–101. doi: 10.1016/j.mce.2016.01.019. [http://dx.doi.org/10.1016/j.mce.2016.01.019]. [PMID: 26802879]. [DOI] [PubMed] [Google Scholar]
- 225.Dickerson S.M., Gore A.C. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev. Endocr. Metab. Disord. 2007;8(2):143–159. doi: 10.1007/s11154-007-9048-y. [http://dx.doi.org/10.1007/s11154-007-9048-y]. [PMID: 17674209]. [DOI] [PubMed] [Google Scholar]
- 226.Rich A.L., Phipps L.M., Tiwari S., Rudraraju H., Dokpesi P.O. The increasing prevalence in intersex variation from toxicological dysregulation in fetal reproductive tissue differentiation and development by endocrine-disrupting chemicals. Environ. Health Insights. 2016;10:163–171. doi: 10.4137/EHI.S39825. [http://dx.doi.org/10.4137/EHI.S39825]. [PMID: 27660460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Strakovsky R.S., Schantz S.L. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet. 2018;4(3):dvy022. doi: 10.1093/eep/dvy022. [http://dx.doi.org/10.1093/eep/dvy022]. [PMID: 30210810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Newbold R.R., Jefferson W.N., Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009;117(6):879–885. doi: 10.1289/ehp.0800045. [http://dx.doi.org/10.1289/ehp.0800045]. [PMID: 19590677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pinney S.E., Mesaros C.A., Snyder N.W., Busch C.M., Xiao R., Aijaz S., Ijaz N., Blair I.A., Manson J.M. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod. Toxicol. 2017;67:1–9. doi: 10.1016/j.reprotox.2016.11.007. [http://dx.doi.org/10.1016/j.reprotox.2016.11.007]. [PMID: 27829162]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Snijder C.A., Heederik D., Pierik F.H., Hofman A., Jaddoe V.W., Koch H.M., Longnecker M.P., Burdorf A. Fetal growth and prenatal exposure to bisphenol A: the generation R study. Environ. Health Perspect. 2013;121(3):393–398. doi: 10.1289/ehp.1205296. [http://dx.doi.org/10.1289/ehp.1205296]. [PMID: 23459363]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Burstyn I., Martin J.W., Beesoon S., Bamforth F., Li Q., Yasui Y., Cherry N.M. Maternal exposure to bisphenol-A and fetal growth restriction: a case-referent study. Int. J. Environ. Res. Public Health. 2013;10(12):7001–7014. doi: 10.3390/ijerph10127001. [http://dx.doi.org/10.3390/ijerph10127001]. [PMID: 24336026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Casas M., Valvi D., Ballesteros-Gomez A., Gascon M., Fernández M.F., Garcia-Esteban R., Iñiguez C., Martínez D., Murcia M., Monfort N., Luque N., Rubio S., Ventura R., Sunyer J., Vrijheid M. Exposure to bisphenol a and phthalates during pregnancy and ultrasound measures of fetal growth in the INMA-sabadell cohort. Environ. Health Perspect. 2016;124(4):521–528. doi: 10.1289/ehp.1409190. [http://dx.doi.org/10.1289/ehp.1409190]. [PMID: 26196298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Xu X., Chiung Y.M., Lu F., Qiu S., Ji M., Huo X. Associations of cadmium, bisphenol A and polychlorinated biphenyl co-exposure in utero with placental gene expression and neonatal outcomes. Reprod. Toxicol. 2015;52:62–70. doi: 10.1016/j.reprotox.2015.02.004. [http://dx.doi.org/10.1016/j.reprotox.2015.02.004]. [PMID: 25687722]. [DOI] [PubMed] [Google Scholar]
- 234.Morrissey R.E., George J.D., Price C.J., Tyl R.W., Marr M.C., Kimmel C.A. The developmental toxicity of bisphenol A in rats and mice. Fundam. Appl. Toxicol. 1987;8(4):571–582. doi: 10.1016/0272-0590(87)90142-4. [http://dx.doi.org/10.1016/0272-0590(87)90142-4]. [PMID: 3609543]. [DOI] [PubMed] [Google Scholar]
- 235.Miao M., Yuan W., He Y., Zhou Z., Wang J., Gao E., Li G., Li D-K. In utero exposure to bisphenol-A and anogenital distance of male offspring. Birth Defects Res. A Clin. Mol. Teratol. 2011;91(10):867–872. doi: 10.1002/bdra.22845. [http://dx.doi.org/10.1002/bdra.22845]. [PMID: 21987463]. [DOI] [PubMed] [Google Scholar]
- 236.Huo W., Xia W., Wan Y., Zhang B., Zhou A., Zhang Y., Huang K., Zhu Y., Wu C., Peng Y., Jiang M., Hu J., Chang H., Xu B., Li Y., Xu S. Maternal urinary bisphenol A levels and infant low birth weight: A nested case-control study of the Health Baby Cohort in China. Environ. Int. 2015;85:96–103. doi: 10.1016/j.envint.2015.09.005. [http://dx.doi.org/10.1016/j.envint.2015.09.005]. [PMID: 26382648]. [DOI] [PubMed] [Google Scholar]
- 237.Troisi J., Mikelson C., Richards S., Symes S., Adair D., Zullo F., Guida M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta. 2014;35(11):947–952. doi: 10.1016/j.placenta.2014.08.091. [http://dx.doi.org/10.1016/j.placenta.2014.08.091]. [PMID: 25227326]. [DOI] [PubMed] [Google Scholar]
- 238.Behnia F., Peltier M., Getahun D., Watson C., Saade G., Menon R. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J. Matern. Fetal Neonatal Med. 2016;29(22):3583–3589. doi: 10.3109/14767058.2016.1139570. [http://dx.doi.org/10.3109/14767058.2016.1139570]. [PMID: 26911979]. [DOI] [PubMed] [Google Scholar]
- 239.Cantonwine D.E., Ferguson K.K., Mukherjee B., McElrath T.F., Meeker J.D. Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. Environ. Health Perspect. 2015;123(9):895–901. doi: 10.1289/ehp.1408126. [http://dx.doi.org/10.1289/ehp.1408126]. [PMID: 25815860]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Weinberger B., Vetrano A.M., Archer F.E., Marcella S.W., Buckley B., Wartenberg D., Robson M.G., Klim J., Azhar S., Cavin S., Wang L., Rich D.Q. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J. Matern. Fetal Neonatal Med. 2014;27(4):323–327. doi: 10.3109/14767058.2013.815718. [http://dx.doi.org/10.3109/14767058.2013.815718]. [PMID: 23795657]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Padmanabhan V., Siefert K., Ransom S., Johnson T., Pinkerton J., Anderson L., Tao L., Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J. Perinatol. 2008;28(4):258–263. doi: 10.1038/sj.jp.7211913. [http://dx.doi.org/10.1038/sj.jp.7211913]. [PMID: 18273031]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Smarr M.M., Grantz K.L., Sundaram R., Maisog J.M., Kannan K., Louis G.M.B. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ. Health. 2015;14:73. doi: 10.1186/s12940-015-0060-5. [http://dx.doi.org/10.1186/s12940-015-0060-5]. [PMID: 26362861]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Guida M., Troisi J., Ciccone C., Granozio G., Cosimato C., Di Spiezio Sardo A., Ferrara C., Guida M., Nappi C., Zullo F., Di Carlo C. Bisphenol A and congenital developmental defects in humans. Mutat. Res. 2015;774:33–39. doi: 10.1016/j.mrfmmm.2015.02.007. [http://dx.doi.org/10.1016/j.mrfmmm.2015.02.007]. [PMID: 25796969]. [DOI] [PubMed] [Google Scholar]
- 244.Balakrishnan B., Henare K., Thorstensen E.B., Ponnampalam A.P., Mitchell M.D. Transfer of bisphenol A across the human placenta. Am. J. Obstet. Gynecol. 2010;202(4):393.e1–393.e7. doi: 10.1016/j.ajog.2010.01.025. [http://dx.doi.org/10.1016/j.ajog.2010.01.025]. [PMID: 20350650]. [DOI] [PubMed] [Google Scholar]
- 245.Machtinger R., Combelles C.M.H., Missmer S.A., Correia K.F., Williams P., Hauser R., Racowsky C. Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 2013;28(10):2735–2745. doi: 10.1093/humrep/det312. [http://dx.doi.org/10.1093/humrep/det312]. [PMID: 23904465]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Christiansen S., Axelstad M., Boberg J., Vinggaard A.M., Pedersen G.A., Hass U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction. 2014;147(4):477–487. doi: 10.1530/REP-13-0377. [http://dx.doi.org/10.1530/REP-13-0377]. [PMID: 24298045]. [DOI] [PubMed] [Google Scholar]
- 247.Timms B.G., Howdeshell K.L., Barton L., Bradley S., Richter C.A., vom Saal F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA. 2005;102(19):7014–7019. doi: 10.1073/pnas.0502544102. [http://dx.doi.org/10.1073/pnas.0502544102]. [PMID: 15867144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ho S-M., Tang W-Y., Belmonte de Frausto J., Prins G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [http://dx.doi.org/10.1158/0008-5472.CAN-06-0516]. [PMID: 16740699]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen J., Wu S., Wen S., Shen L., Peng J., Yan C., Cao X., Zhou Y., Long C., Lin T., He D., Hua Y., Wei G. The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS One. 2015;10(6):e0126403. doi: 10.1371/journal.pone.0126403. [http://dx.doi.org/10.1371/journal.pone.0126403]. [PMID: 26035430]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fernández M.F., Arrebola J.P., Jiménez-Díaz I., Sáenz J.M., Molina-Molina J.M., Ballesteros O., Kortenkamp A., Olea N. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod. Toxicol. 2016;59:89–95. doi: 10.1016/j.reprotox.2015.11.002. [http://dx.doi.org/10.1016/j.reprotox.2015.11.002]. [PMID: 26602963]. [DOI] [PubMed] [Google Scholar]
- 251.Fiorini C., Tilloy-Ellul A., Chevalier S., Charuel C., Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 2004;18(3):413–421. doi: 10.1016/j.reprotox.2004.01.002. [http://dx.doi.org/10.1016/j.reprotox.2004.01.002]. [PMID: 15082077]. [DOI] [PubMed] [Google Scholar]
- 252.Salian S., Doshi T., Vanage G. Neonatal exposure of male rats to Bisphenol A impairs fertility and expression of sertoli cell junctional proteins in the testis. Toxicology. 2009;265(1-2):56–67. doi: 10.1016/j.tox.2009.09.012. [http://dx.doi.org/10.1016/j.tox.2009.09.012]. [PMID: 19782717]. [DOI] [PubMed] [Google Scholar]
- 253.Chianese R., Viggiano A., Urbanek K., Cappetta D., Troisi J., Scafuro M., Guida M., Esposito G., Ciuffreda L.P., Rossi F., Berrino L., Fasano S., Pierantoni R., De Angelis A., Meccariello R. Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via SIRT1 modulation. Sci. Rep. 2018;8(1):2961. doi: 10.1038/s41598-018-21076-8. [http://dx.doi.org/10.1038/s41598-018-21076-8]. [PMID: 29440646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhang G.L., Zhang X.F., Feng Y.M., Li L., Huynh E., Sun X.F., Sun Z.Y., Shen W. Exposure to bisphenol A results in a decline in mouse spermatogenesis. Reprod. Fertil. Dev. 2013;25(6):847–859. doi: 10.1071/RD12159. [http://dx.doi.org/10.1071/RD12159]. [PMID: 22951085]. [DOI] [PubMed] [Google Scholar]
- 255.Xie M., Bu P., Li F., Lan S., Wu H., Yuan L., Wang Y. Neonatal bisphenol A exposure induces meiotic arrest and apoptosis of spermatogenic cells. Oncotarget. 2016;7(9):10606–10615. doi: 10.18632/oncotarget.7218. [http://dx.doi.org/10.18632/oncotarget.7218]. [PMID: 26863571]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Liu C., Duan W., Li R., Xu S., Zhang L., Chen C., He M., Lu Y., Wu H., Pi H., Luo X., Zhang Y., Zhong M., Yu Z., Zhou Z. Exposure to bisphenol A disrupts meiotic progression during spermatogenesis in adult rats through estrogen-like activity. Cell Death Dis. 2013 doi: 10.1038/cddis.2013.203. 4e676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Allard P., Colaiácovo M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA. 2010;107(47):20405–20410. doi: 10.1073/pnas.1010386107. [http://dx.doi.org/10.1073/pnas.1010386107]. [PMID: 21059909]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Horan T.S., Pulcastro H., Lawson C., Gerona R., Martin S., Gieske M.C., Sartain C.V., Hunt P.A. Replacement bisphenols adversely affect mouse gametogenesis with consequences for subsequent generations. Curr. Biol. 2018;28(18):2948–2954.e3. doi: 10.1016/j.cub.2018.06.070. [http://dx.doi.org/10.1016/j.cub.2018.06.070]. [PMID: 30220498]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sasaki M., Lange J., Keeney S. Genome destabilization by homologous recombination in the germ line. Nat. Rev. Mol. Cell Biol. 2010;11(3):182–195. doi: 10.1038/nrm2849. [http://dx.doi.org/10.1038/nrm2849]. [PMID: 20164840]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mínguez-Alarcón L., Hauser R., Gaskins A.J. Effects of bisphenol A on male and couple reproductive health: A review. Fertil. Steril. 2016;106(4):864–870. doi: 10.1016/j.fertnstert.2016.07.1118. [http://dx.doi.org/10.1016/j.fertnstert.2016.07.1118]. [PMID: 27498136]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Meeker J.D., Ehrlich S., Toth T.L., Wright D.L., Calafat A.M., Trisini A.T., Ye X., Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010;30(4):532–539. doi: 10.1016/j.reprotox.2010.07.005. [http://dx.doi.org/10.1016/j.reprotox.2010.07.005]. [PMID: 20656017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rahman M.S., Kwon W.S., Lee J.S., Yoon S.J., Ryu B.Y., Pang M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015;5:9169. doi: 10.1038/srep09169. [http://dx.doi.org/10.1038/srep09169]. [PMID: 25772901]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li J., Mao R., Zhou Q., Ding L., Tao J., Ran M.M., Gao E.S., Yuan W., Wang J.T., Hou L.F. Exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of ERK signal pathway. Toxicol. Mech. Methods. 2016;26(3):180–188. doi: 10.3109/15376516.2016.1139024. [http://dx.doi.org/10.3109/15376516.2016.1139024]. [PMID: 26862991]. [DOI] [PubMed] [Google Scholar]
- 264.Kotwicka M., Skibinska I., Piworun N., Jendraszak M., Chmielewska M., Jedrzejczak P. Bisphenol A modifies human spermatozoa motility in vitro. J. Medical. Sci. 2016;85:39–45. [http://dx.doi.org/10.20883/jms.2016.5]. [Google Scholar]
- 265.Lan H.C., Wu K.Y., Lin I.W., Yang Z.J., Chang A.A., Hu M.C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere. 2017;185:237–246. doi: 10.1016/j.chemosphere.2017.07.004. [http://dx.doi.org/10.1016/j.chemosphere.2017.07.004]. [PMID: 28697429]. [DOI] [PubMed] [Google Scholar]
- 266.Feng Y., Jiao Z., Shi J., Li M., Guo Q., Shao B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere. 2016;147:9–19. doi: 10.1016/j.chemosphere.2015.12.081. [http://dx.doi.org/10.1016/j.chemosphere.2015.12.081]. [PMID: 26751127]. [DOI] [PubMed] [Google Scholar]
- 267.Ullah A., Pirzada M., Jahan S., Ullah H., Turi N., Ullah W., Siddiqui M.F., Zakria M., Lodhi K.Z., Khan M.M. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 2018;121:24–36. doi: 10.1016/j.fct.2018.08.024. [http://dx.doi.org/10.1016/j.fct.2018.08.024]. [PMID: 30120946]. [DOI] [PubMed] [Google Scholar]
- 268.Desdoits-Lethimonier C., Lesné L., Gaudriault P., Zalko D., Antignac J.P., Deceuninck Y., Platel C., Dejucq-Rainsford N., Mazaud-Guittot S., Jégou B. Parallel assessment of the effects of bisphenol A and several of its analogs on the adult human testis. Hum. Reprod. 2017;32(7):1465–1473. doi: 10.1093/humrep/dex093. [http://dx.doi.org/10.1093/humrep/dex093]. [PMID: 28482050]. [DOI] [PubMed] [Google Scholar]
- 269.Roelofs M.J., van den Berg M., Bovee T.F., Piersma A.H., van Duursen M.B. Structural bisphenol analogues differentially target steroidogenesis in murine MA-10 Leydig cells as well as the glucocorticoid receptor. Toxicology. 2015;329:10–20. doi: 10.1016/j.tox.2015.01.003. [http://dx.doi.org/10.1016/j.tox.2015.01.003]. [PMID: 25576683]. [DOI] [PubMed] [Google Scholar]
- 270.Eladak S., Grisin T., Moison D., Guerquin M.J., N’Tumba-Byn T., Pozzi-Gaudin S., Benachi A., Livera G., Rouiller-Fabre V., Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015;103(1):11–21. doi: 10.1016/j.fertnstert.2014.11.005. [http://dx.doi.org/10.1016/j.fertnstert.2014.11.005]. [PMID: 25475787]. [DOI] [PubMed] [Google Scholar]
- 271.Kokkinaki M., Lee T.L., He Z., Jiang J., Golestaneh N., Hofmann M.C., Chan W.Y., Dym M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol. Reprod. 2009;80(4):707–717. doi: 10.1095/biolreprod.108.073809. [http://dx.doi.org/10.1095/biolreprod.108.073809]. [PMID: 19109221]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Liang S., Yin L., Yu S. K.; Hofmann, M.C.; Yu, X. High-Content Analysis Provides Mechanistic Insights into the Testicular Toxicity of Bisphenol A and Selected Analogues in Mouse Spermatogonial Cells. Toxicol. Sci. 2017;155(1):43–60. doi: 10.1093/toxsci/kfw178. [http://dx.doi.org/10.1093/toxsci/kfw178]. [PMID: 27633978]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sidorkiewicz I., Czerniecki J., Jarząbek K., Zbucka-Krętowska M., Wołczyński S. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line. Toxicol. Appl. Pharmacol. 2018;359:1–11. doi: 10.1016/j.taap.2018.09.006. [http://dx.doi.org/10.1016/j.taap.2018.09.006]. [PMID: 30196065]. [DOI] [PubMed] [Google Scholar]
- 274.Shi M., Sekulovski N., MacLean J.A., II, Hayashi K. Effects of bisphenol A analogues on reproductive functions in mice. Reprod. Toxicol. 2017;73:280–291. doi: 10.1016/j.reprotox.2017.06.134. [http://dx.doi.org/10.1016/j.reprotox.2017.06.134]. [PMID: 28676390]. [DOI] [PubMed] [Google Scholar]
- 275.Shi M., Sekulovski N., MacLean J.A., II, Hayashi K. Prenatal exposure to bisphenol a analogues on male reproductive functions in mice. Toxicol. Sci. 2018;163(2):620–631. doi: 10.1093/toxsci/kfy061. [http://dx.doi.org/10.1093/toxsci/kfy061]. [PMID: 29741722]. [DOI] [PubMed] [Google Scholar]
- 276.Jégou B. The Sertoli-germ cell communication network in mammals. Int. Rev. Cytol. 1993;147:25–96. [http://dx.doi.org/10.1016/S0074-7696(08)60766-4]. [PMID: 8225836]. [PubMed] [Google Scholar]
- 277.Liu C., Wang H., Shang Y., Liu W., Song Z., Zhao H., Wang L., Jia P., Gao F., Xu Z., Yang L., Gao F., Li W. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy. 2016;12(5):814–832. doi: 10.1080/15548627.2016.1159377. [http://dx.doi.org/10.1080/15548627.2016.1159377]. [PMID: 26986811]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ullah H., Ambreen A., Ahsan N., Jahan S. Bisphenol S induces oxidative stress and DNA damage in rat spermatozoa in vitro and disrupts daily sperm production in vivo. J. Toxicol. Environmental. Chem. 2017;99:953–965. [Google Scholar]


