Visual Abstract
Keywords: chronic kidney disease, kidney function decline, tubule injury biomarkers, male, humans, human LCN2 protein, lipocalin-2, glomerular filtration rate, interleukin-18, albuminuria, human CCL2 protein, chemokine CCL2, human CHI3L1 protein, chitinase-3-like protein 1, kidney transplantation, proportional hazards models, blood pressure, follow-up studies, confidence intervals, human HAVCR1 protein, hepatitis A virus cellular receptor 1, acute kidney injury, chronic renal insufficiency, kidney tubules, inflammation
Abstract
Background and objectives
eGFR and albuminuria primarily reflect glomerular function and injury, whereas tubule cell atrophy and interstitial fibrosis on kidney biopsy are important risk markers for CKD progression. Kidney tubule injury markers have primarily been studied in hospitalized AKI. Here, we examined the association between urinary kidney tubule injury markers at baseline with subsequent loss of kidney function in persons with nondiabetic CKD who participated in the Systolic Blood Pressure Intervention Trial (SPRINT).
Design, setting, participants, & measurements
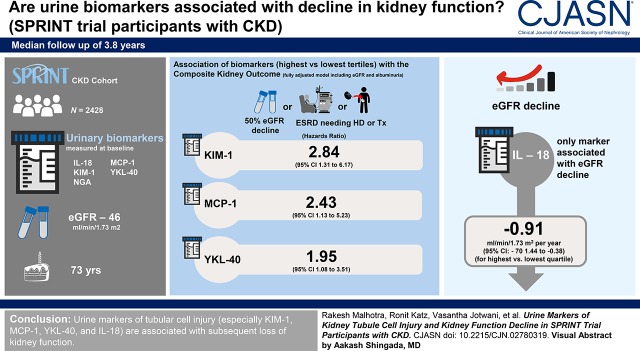
Among 2428 SPRINT participants with CKD (eGFR<60 ml/min per 1.73 m2) at baseline, we measured urine markers of tubule injury (IL-18, kidney injury molecule-1 [KIM-1], neutrophil gelatinase-associated lipocalin [NGAL]), inflammation (monocyte chemoattractant protein-1 [MCP-1]), and repair (human cartilage glycoprotein-40 [YKL-40]). Cox proportional hazards models evaluated associations of these markers with the kidney composite outcome of 50% eGFR decline or ESKD requiring dialysis or kidney transplantation, and linear mixed models evaluated annualized change in eGFR.
Results
Mean participant age was 73±9 (SD) years, 60% were men, 66% were white, and mean baseline eGFR was 46±11 ml/min per 1.73 m2. There were 87 kidney composite outcome events during a median follow-up of 3.8 years. Relative to the respective lowest quartiles, the highest quartiles of urinary KIM-1 (hazard ratio, 2.84; 95% confidence interval [95% CI], 1.31 to 6.17), MCP-1 (hazard ratio, 2.43; 95% CI, 1.13 to 5.23), and YKL-40 (hazard ratio, 1.95; 95% CI, 1.08 to 3.51) were associated with higher risk of the kidney composite outcome in fully adjusted models including baseline eGFR and urine albumin. In linear analysis, urinary IL-18 was the only marker associated with eGFR decline (−0.91 ml/min per 1.73 m2 per year for highest versus lowest quartile; 95% CI, −1.44 to −0.38), a finding that was stronger in the standard arm of SPRINT.
Conclusions
Urine markers of tubule cell injury provide information about risk of subsequent loss of kidney function, beyond the eGFR and urine albumin.
Introduction
Despite improvements in staging of CKD, the ability to provide prognostic information about kidney disease progression remains challenging. eGFR and albuminuria are used to diagnose and stage CKD (1,2); however, they primarily reflect glomerular function and injury and correlate poorly with tubular atrophy and tubulointerstitial fibrosis on biopsy (3). These later pathologic factors are highly prognostic for CKD progression across multiple different causes of CKD (4,5). Thus, we hypothesized that there may be opportunities to improve CKD risk assessment through noninvasive evaluation of kidney tubule health.
Recent studies have identified urinary proteins that reflect tubular injury, fibrosis, and repair (6,7). Although initially used to risk stratify patients with AKI, higher urine concentrations of these markers have been associated with longitudinal loss of kidney function in ambulatory settings (8,9). These studies have typically evaluated tubule injury markers individually (10–12), and therefore broader evaluations of multiple candidate tubular markers are needed.
The Systolic Blood Pressure Intervention Trial (SPRINT) was a randomized clinical trial where hypertensive individuals were randomized to intensive (<120 mm Hg) or standard (<140 mm Hg) systolic BP targets for prevention of cardiovascular disease (13). Randomization to the intensive systolic BP arm of SPRINT reduced risk for cardiovascular disease and mortality; however, more rapid loss of kidney function was seen during follow-up (14). The later finding is of concern for persons with established CKD. We examined a panel of kidney tubule injury markers measured at baseline among SPRINT participants with CKD to determine whether they provided information for risk of CKD progression, beyond that available from the eGFR, urine albumin, and other CKD risk factors. Our secondary aim was to examine whether the urinary kidney tubule injury markers at baseline have different associations with CKD progression in the intensive versus standard arms of SPRINT.
Materials and Methods
The design of the SPRINT trial has been reported elsewhere (13). Briefly, SPRINT is an open-label, clinical trial that randomized hypertensive persons at high risk for cardiovascular disease events to a systolic BP target of <120 mm Hg (“intensive” arm) versus <140 mm Hg (“standard” arm). Participants were recruited from 102 clinical centers in the United States and Puerto Rico. Inclusion criteria required age ≥50 years, systolic BP 130–180 mm Hg depending on the number of antihypertensive medications used at the time of recruitment, and increased risk for cardiovascular disease events (prior clinical or subclinical cardiovascular disease other than stroke, 10-year risk of cardiovascular disease of ≥15% on the basis of the Framingham risk score, CKD defined as eGFR 20–59 ml/min per 1.73 m2, or age ≥75 years). Major exclusion criteria included diabetes mellitus, proteinuria >1 g/d, polycystic kidney disease, prior stroke or transient ischemic attack, symptomatic heart failure, or a left ventricular ejection fraction <35%. A total of 9361 participants were enrolled between November 2010 and March 2013 (15). All participants provided written informed consent and were randomly assigned (1:1 ratio) to the intensive or standard systolic BP arm. The antihypertensive regimens were adjusted to achieve and maintain systolic BP according to randomized targets. Participants attended visits monthly for the first 3 months and every 3 months thereafter, and clinical data and venous blood and random spot urine samples were subsequently obtained every 6 months. Venous blood and random urine specimens were immediately processed and shipped overnight on ice packs. Plasma and urine samples were stored at −80°C at the central laboratory at the University of Minnesota for future analysis. Institutional Review Boards at all participating institutions approved the study.
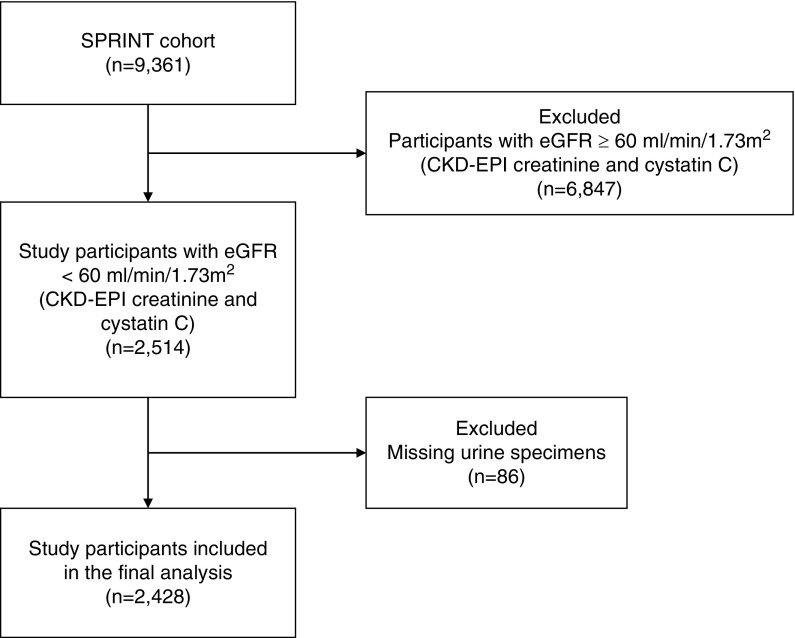
For this ancillary study, serum cystatin C concentrations were measured in all SPRINT participants in baseline specimens. We then defined a subset of participants with eGFR<60 ml/min per 1.73 m2 calculated using the CKD Epidemiology Collaboration creatinine and cystatin C equation (16); on these, we measured tubule injury markers at baseline using frozen urine samples. Among the 2514 participants with baseline eGFR <60 ml/min per 1.73 m2, 86 lacked urine specimens and were excluded. The final sample for this study included 2428 participants (Figure 1).
Figure 1.
Flowchart of the study. CKD-EPI, CKD Epidemiology Collaboration; SPRINT, Systolic Blood Pressure Intervention Trial.
Kidney Tubule Injury Marker Measurements
The urine markers of tubule injury (IL-18, kidney injury molecule-1 [KIM-1], and neutrophil gelatinase-associated lipocalin [NGAL]), inflammation (monocyte chemoattractant protein-1 [MCP-1]), and repair (human cartilage glycoprotein-40 [YKL-40]) were measured centrally at the Laboratory for Clinical Biochemistry Research at the University of Vermont by personnel blinded to clinical information. Measurements were performed using multiplex assays on a MESO Scale Diagnostics platform (MSD, Rockville, MD). Each marker was measured twice, and results were averaged to improve precision. Duplicate measurements were used to determine the interassay coefficients of variation, which were as follows: urinary IL-18, 4.9%–13.7%; KIM-1, 6.1%–13.0%; NGAL, 12.9%–16.2%; MCP-1, 7.1%–12.0%; and YKL-40, 6.5%–11.1%. Urine creatinine was measured by an enzymatic procedure (Roche, Indianapolis, IN), and urine albumin was measured by a nephelometric method (Siemens, Tarrytown, NY) (16). Interassay coefficients of variation for urine creatinine and urine albumin measurements were 1.5%–4.3% and 2.2%–6.9%, respectively.
Longitudinal Change in Kidney Function
Changes in eGFR were on the basis of the serum creatinine concentration, which was measured centrally at the University of Minnesota from stored venous plasma samples collected every 3 months. The study outcomes included (1) a prespecified kidney composite outcome of the SPRINT study protocol, defined as ≥50% eGFR decline (confirmed by repeat testing ≥90 days later) or ESKD requiring dialysis or kidney transplantation (14); and (2) annualized eGFR change, expressed as percentage change from baseline.
Statistical Analyses
Continuous variables were described as the mean (SD) or median and interquartile ranges (IQRs) and categorical variables as absolute (n) and relative (%) frequency. Cox proportional hazards models and linear mixed models were used to examine associations between baseline urine kidney tubule injury markers and time to the binary kidney composite outcome and annualized percent eGFR change, respectively. We initially evaluated each urinary tubule injury marker as a continuous variable after a log base 2 transformation and reported results as “per two-fold higher.” We also evaluated each marker across quartiles, with the lowest quartile set as the reference group to evaluate the functional form of the observed relationships. A series of models were developed. Model 1 was adjusted for age, sex, race, randomization arm, and urine creatinine concentration (to account for urine tonicity). Model 2 additionally included baseline eGFR, urine albumin, systolic BP and diastolic BP, number of antihypertensive medications, history of cardiovascular disease, smoking status, body mass index, and serum concentrations of HDL and total cholesterol. In exploratory analyses, we evaluated an additional model where we adjusted for model 2 variables plus all the urine tubule injury biomarkers concurrently; we viewed this model as potentially overadjusted, as the markers all capture tubule injury, and therefore provide the data in the Supplemental Material. Model 2, therefore, was considered our fully adjusted model. We performed secondary analyses where biomarkers were indexed to urine creatinine as biomarker/creatinine ratios. Finally, to determine whether the markers of interest had similar relationships with kidney disease progression in each randomized treatment arm, we explored stratified analyses and tested multiplicative interaction terms. All analyses were conducted using Stata (Stata Statistical Software, release 13; StataCorp LP, College Station, TX) and SPSS (released 2016, IBM SPSS Statistics for Windows, version 24.0; IBM Corp, Armonk, NY). P values <0.05 were considered statistically significant for all analyses including interaction terms.
Results
Among 2428 SPRINT participants with baseline eGFR <60 ml/min per 1.73 m2, the mean age was 73±9 years, 60% were men, 66% were non-Hispanic whites, and 25% had prevalent cardiovascular disease. The mean baseline eGFR was 46±11 ml/min per 1.73 m2, and median urine albumin-to-creatinine ratio was 15 (IQR, 7–47) mg/g. The mean baseline systolic BP and diastolic BP were 139±16 and 74±12 mm Hg, respectively. The distributions of all five urinary kidney tubule injury markers were right-skewed, with medians (IQRs) of 30 (16–57) ng/g for IL-18, 849 (387–1596) pg/g for KIM-1, 28 (15–59) ng/g for NGAL, 181 (89–327) pg/g for MCP-1, and 543 (214–1243) ng/g for YKL-40. Among the study sample, 1242 participants were randomized to the intensive systolic BP arm and 1186 to the standard systolic BP arm. Baseline demographics and clinical characteristics and laboratory characteristics were similar across treatment arms within the analytic sample (Table 1).
Table 1.
Baseline characteristics by randomized treatment arm in Systolic Blood Pressure Intervention Trial participants with CKD
| Variables | Total, n=2428 | Intensive Arm, n=1242 | Standard Arm, n=1186 |
|---|---|---|---|
| Age, yr | 73±9 | 73±9 | 73±9 |
| Male | 1449 (60) | 742 (60) | 707 (60) |
| White | 1605 (66) | 813 (66) | 792 (67) |
| Current smoker | 212 (9) | 111 (9) | 101 (9) |
| History of cardiovascular disease | 611 (25) | 309 (25) | 302 (26) |
| Systolic BP, mm Hg | 139±16 | 139±16 | 140±16 |
| Diastolic BP, mm Hg | 74±12 | 75±12 | 74±12 |
| No. of BP medications, n (%) | |||
| 0 | 100 (4) | 50 (4) | 50 (4) |
| 1 | 544 (22) | 277 (22) | 267 (23) |
| 2 | 880 (36) | 461 (37) | 419 (35) |
| 3 | 693 (29) | 349 (28) | 344 (29) |
| ≥4 | 211 (9) | 105 (9) | 106 (9) |
| Total cholesterol, mg/dl | 184±41 | 184±41 | 183±40 |
| HDL, mg/dl | 52±14 | 53±15 | 52±14 |
| Triglycerides, mg/dl | 112 [82–154] | 111 [79–150] | 113 [83–156] |
| Body mass index, kg/m2 | 30±6 | 30±6 | 29±6 |
| eGFR, ml/min per 1.73 m2 | 46±11 | 46±11 | 46±11 |
| UACR, mg/g | 15 [7–47] | 14 [7–46] | 15 [7–50] |
| IL-18, pg/ml | 30 [16–57] | 29 [15–54] | 32 [17–59] |
| KIM-1, pg/ml | 849 [387–1596] | 857 [377–1568] | 836 [399–1623] |
| NGAL, ng/ml | 28 [15–59] | 28 [14–61] | 27 [15–57] |
| MCP-1, pg/ml | 181 [89–327] | 176 [85–337] | 186 [95–320] |
| YKL-40, pg/ml | 543 [214–1243] | 540 [204–1251] | 549 [221–1243] |
Data presented as median [interquartile range], mean±SD, or numbers (percent). UACR, urine albumin-to-creatinine ratio; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein 1; YKL-40, chitinase-3-like protein-1.
Associations of the urinary tubule injury markers with the binary kidney composite outcome of 50% eGFR decline or ESKD requiring dialysis or kidney transplantation were assessed. During the 3.8-year median follow-up, 87 kidney composite events were seen (30 in standard and 57 in intensive arm; 67 had 50% decline, 19 had ESKD events, and one had a kidney transplant). In quartile analyses, relatively flat relationships were observed across quartiles 1–3 for all tubule injury markers except urinary IL-18 and NGAL, but there was consistently a marked increase in risk among individuals in the highest quartile. Therefore, we focus our interpretation on quartile analyses. In models adjusted for demographics, randomization arm, and urine creatinine, individuals in the highest quartiles of all five markers were at higher risk for the kidney composite end point compared with the lowest quartile. In the fully adjusted models, the highest quartiles of urinary KIM-1, MCP-1, and YKL-40 remained significantly associated with an approximate two-fold risk of the kidney composite end point (Table 2). The attenuation observed, from the least-adjusted to the fully adjusted models, was mainly attributed to adjusting for urine albumin. However, even after adjusting for urine albumin in addition to eGFR and other CKD risk factors, the strengths of the associations of the tubule injury biomarkers approached that of the highest quartile of urine albumin for the composite kidney end point (Table 2). The quartile 3 of urinary IL-18 was also associated with the kidney composite end point, but IL-18 did not achieve statistical significance for comparison of the highest to lowest quartiles. When we adjusted biomarkers for each other, the highest quartiles of urinary IL-18, KIM-1, and YKL-40 were independently associated with risk of the kidney composite end point, as was urine albumin (Supplemental Table 1). When the biomarkers were indexed to urine creatinine (Supplemental Table 2), participants in the highest versus lowest quartile of YKL-40/creatinine had higher risk of composite events (hazard ratio [HR], 2.43; 95% confidence interval [95% CI], 1.35 to 4.37). Urinary IL-18/creatinine, KIM-1/creatinine, NGAL/creatinine, and MCP-1/creatinine were not associated with composite events in fully adjusted models. When we evaluated death as a competing risk, it did not meaningfully change the point estimates or the confidence intervals (data not shown).
Table 2.
Associations of kidney tubule injury markers, categorized by quartiles, with CKD progression in SPRINT, defined by 50% eGFR decline or ESKD requiring dialysis or kidney transplant
| Biomarkers | Quartiles | Events, n | Incidence Rate, %/yr | Model 1 | Model 2 |
|---|---|---|---|---|---|
| Urine IL-18 | 1 | 18 | 0.92 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 25 | 0.28 | 2.24 (1.18 to 4.27)a | 1.76 (0.92 to 3.35) | |
| 3 | 25 | 1.30 | 3.05 (1.52 to 6.11)a | 2.24 (1.13 to 4.42) | |
| 4 | 19 | 0.99 | 3.21 (1.52 to 6.11)a | 1.91 (0.95 to 3.82) | |
| Urine KIM-1 | 1 | 22 | 1.14 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 22 | 1.12 | 2.17 (1.13 to 4.16)a | 1.12 (0.59 to 2.14) | |
| 3 | 18 | 0.92 | 2.82 (1.32 to 6.06)a | 1.30 (0.61 to 2.76) | |
| 4 | 25 | 1.31 | 8.25 (3.62 to 18.82)a | 2.84 (1.31 to 6.17)a | |
| Urine NGAL | 1 | 19 | 0.97 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 21 | 1.07 | 1.49 (0.79 to 2.82) | 0.90 (0.47 to 1.72) | |
| 3 | 20 | 1.02 | 1.65 (0.85 to 23.19) | 1.04 (0.52 to 2.06) | |
| 4 | 27 | 1.43 | 2.37 (1.27 to 4.43)a | 1.11 (0.58 to 2.14) | |
| Urine MCP-1 | 1 | 25 | 1.29 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 21 | 1.06 | 1.50 (0.80 to 2.83) | 0.81 (0.43 to 1.51) | |
| 3 | 18 | 0.93 | 2.12 (1.05 to 4.30)a | 0.94 (0.46 to 1.90) | |
| 4 | 23 | 1.20 | 4.51 (2.14 to 9.52)a | 2.43 (1.13 to 5.23)a | |
| Urine YKL-40 | 1 | 25 | 1.26 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 19 | 0.98 | 1.11 (0.59 to 2.11) | 1.31 (0.68 to 2.53) | |
| 3 | 15 | 0.77 | 1.11 (0.55 to 2.22) | 1.14 (0.56 to 2.32) | |
| 4 | 28 | 1.48 | 2.40 (1.31 to 4.40)a | 1.95 (1.08 to 3.51)a | |
| Urine albumin | 1 | 13 | 0.63 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 14 | 0.71 | 1.64 (0.77 to 3.52) | 1.31 (0.60 to 2.86) | |
| 3 | 15 | 0.82 | 2.08 (0.99 to 4.40) | 1.48 (0.68 to 3.24) | |
| 4 | 45 | 2.45 | 5.35 (2.91 to 9.85)a | 2.88 (1.49 to 5.55)a |
Model 1: adjusted for age, sex, race, intervention arm, and urine creatinine. Model 2: additionally adjusted for baseline eGFR, urine albumin, smoking status, history of cardiovascular disease, baseline number of antihypertensive medications, systolic BP, diastolic BP, body mass index, HDL, and total cholesterol. Urine IL-18, pg/ml (quartile 1: ≤16.30; quartile 2: 16.31–30.48; quartile 3: 30.49–56.60; quartile 4: >56.60), urine KIM-1, pg/ml (quartile 1: ≤386.73; quartile 2: 386.74–849.01; quartile 3: 849.02–1595.84; quartile 4: >1595.84), urine NGAL, ng/ml (quartile 1: ≤14.71; quartile 2: 14.72–27.69; quartile 3: 27.70–59.27; quartile 4: >59.27), urine MCP-1, pg/ml (quartile 1: ≤89.42; quartile 2: 89.43–180.78; quartile 3: 180.79–326.99; quartile 4: >326.99), urine YKL-40, pg/ml (quartile 1: ≤213.95; quartile 2: 213.96–542.59; quartile 3: 542.60–1243.42; quartile 4: >1243.42), and urine albumin, mg/dl (quartile 1: ≤7; quartile 2: 8–16; quartile 3: 17–50, quartile 4: >50). SPRINT, Systolic Blood Pressure Intervention Trial; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; YKL-40, chitinase-3-like protein-1.
Represents significant results.
During a median 3.8-year follow-up, participants had a mean of 8.0±2.3 eGFR assessments, and the mean eGFR decline was 0.57±1.18 ml/min per 1.73 m2 per year. In general, we observed that associations of urine tubule injury markers with percent change in eGFR were weaker compared with the binary outcome. When comparing the highest to the lowest quartile, only urinary IL-18 was significantly associated with eGFR decline in fully adjusted models. The findings remained similar even after adjustment of the tubule injury biomarkers for one another (Supplemental Table 3). None of the five markers were associated with annual eGFR decline when the tubule markers were evaluated as continuous variables (Table 3). Supplemental Table 4 shows result for annualized change in eGFR when the biomarkers were indexed to urine creatinine. IL-18/creatinine was significantly associated with eGFR decline in fully adjusted models both when analyzed as continuous variable and by quartiles. None of the remaining biomarkers were associated with eGFR decline when indexed to urine creatinine.
Table 3.
Association of quartiles of kidney tubule injury markers with annualized relative change in eGFR in SPRINT
| Biomarkers | Mean eGFR Decline, %/yr | Model 1 | Model 2 | ||
|---|---|---|---|---|---|
| Urine IL-18 | Linear model | Per two-fold higher biomarker concentration |
−0.38 (−0.51 to −0.25) | −0.11 (−0.25 to 0.02) | |
| Quartiles | 1 | −1.06 (−1.36 to −0.76) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −1.17 (−1.47 to −0.87) | −0.51 (−0.94 to −0.07)a | −0.06 (−0.49 to 0.37) | ||
| 3 | −1.75 (−2.05 to −1.45) | −1.38 (−1.83 to −0.92) | −0.81 (−1.26 to − 0.35)a | ||
| 4 | −1.77 (−2.08 to −1.46) | −1.88 (−2.38 to −1.36) | −0.91 (−1.44 to −0.38)a | ||
| Urine KIM-1 | Linear model | Per two-fold higher biomarker concentration |
−0.29 (−0.40 to −0.18) | 0.06 (−0.05 to 0.17) | |
| Quartiles | 1 | −1.09 (−1.39 to −0.79) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −1.95 (−2.25 to −1.65) | −1.33 (−1.30 to −0.44) | −0.54 (−0.98 to −0.09)a | ||
| 3 | −1.21 (−1.51 to −0.91) | −0.83 (−1.32 to −0.35)a | 0.17 (−0.32 to 0.66) | ||
| 4 | −1.48 (−1.79 to −1.17) | −1.65 (−2.21 to −1.08) | −0.21 (−0.80 to 0.38) | ||
| Urine NGAL | Linear model | Per two-fold higher biomarker concentration |
−0.14 (−0.24 to −0.04) | 0.10 (0.01 to 0.21)a | |
| Quartiles | 1 | −1.33 (−1.64 to −1.03) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −1.49 (−1.78 to −1.19) | −0.35 (−0.79 to 0.08) | −0.30 (−0.13 to 0.74) | ||
| 3 | −0.91 (−1.21 to −0.61) | 0.24 (−0.22 to 0.69) | 0.80 (−0.35 to 1.25) | ||
| 4 | −2.04 (−2.35 to −1.73) | −0.91 (−1.39 to −0.44) | 0.27 (−0.21 to 0.76) | ||
| Urine MCP-1 | Linear model | Per two-fold higher biomarker concentration |
−0.23 (−0.36 to −0.11) | 0.07 (−0.05 to 0.20) | |
| Quartiles | 1 | −1.26 (−1.56 to −0.96) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −1.34 (−1.64 to −1.04) | −0.39 (−0.83 to 0.06) | 0.28 (−0.16 to 0.73) | ||
| 3 | −1.64 (−1.95 to −1.34) | −1.03 (−1.53 to −0.54) | −0.19 (−0.68 to 0.31) | ||
| 4 | −1.48 (−1.79 to −1.17) | −1.25 (−1.83 to −0.67) | −0.17 (−0.76 to 0.42) | ||
| Urine YKL-40 | Linear model | Per two-fold higher biomarker concentration |
−0.14 (−0.22 to −0.07) | 0.03 (−0.11 to 0.04) | |
| Quartiles | 1 | −1.61 (−1.90 to −1.32) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −0.83 (−1.14 to −0.52) | 0.60 (0.16 to 1.04) | 0.41 (−0.02 to 0.84) | ||
| 3 | −1.30 (−1.61 to −1.00) | 0.01 (−0.45 to 0.46) | 0.02 (−0.43 to 0.46) | ||
| 4 | −1.97 (−2.28 to −1.66) | −0.83 (−1.30 to −0.33) | −0.11 (−0.61 to 0.38) | ||
| Urine albumin | Linear model | Per two-fold higher biomarker concentration |
−0.79 (−0.86 to −0.71) | −0.67 (−0.75 to −0.59)a | |
| Quartiles | 1 | −0.30 (−0.59 to −0.01) | 0.0 (Reference) | 0.0 (Reference) | |
| 2 | −0.60 (−0.89 to −0.31) | −0.67 (−1.09 to −0.24)a | −0.40 (−0.83 to 0.03) | ||
| 3 | −1.38 (−1.68 to −1.07) | −1.50 (−1.94 to −1.05) | −1.08 (−1.53 to −0.63)a | ||
| 4 | −3.68 (−3.98 to −3.37) | −3.75 (−4.19 to −3.32) | −3.05 (−3.51 to −2.59)a |
Model 1: adjusted for age, sex, race, intervention arm, and urine creatinine. Model 2: additionally adjusted for baseline eGFR, urine albumin, smoking status, history of cardiovascular disease, baseline number of antihypertensive medications, systolic BP, diastolic BP, body mass index, HDL, and total cholesterol. Urine IL-18, pg/ml (quartile 1: ≤16.30; quartile 2: 16.31–30.48; quartile 3: 30.49–56.60; quartile 4: >56.60), urine KIM-1, pg/ml (quartile 1: ≤386.73; quartile 2: 386.74–849.01; quartile 3: 849.02–1595.84; quartile 4: >1595.84), urine NGAL, ng/ml (quartile 1: ≤14.71; quartile 2: 14.72–27.69; quartile 3: 27.70–59.27; quartile 4: >59.27), urine MCP-1, pg/ml (quartile 1: ≤89.42; quartile 2: 89.43–180.78; quartile 3: 180.79–326.99; quartile 4: >326.99), urine YKL-40, pg/ml (quartile 1: ≤213.95; quartile 2: 213.96–542.59; quartile 3: 542.60–1243.42; quartile 4: >1243.42), and urine albumin mg/dl (quartile 1: ≤7; quartile 2: 8–16; quartile 3: 17–50, quartile 4: >50). SPRINT, Systolic Blood Pressure Intervention Trial; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemo attractant protein-1; YKL-40, chitinase-3-like protein-1.
Represents significant results.
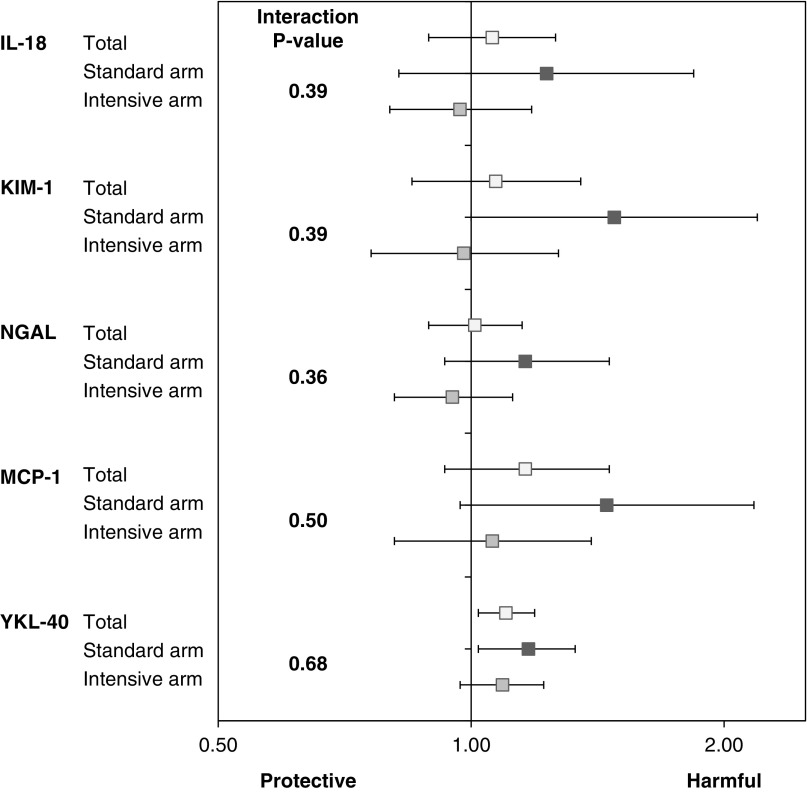
No statistically significant interactions were observed for the systolic BP intervention and the urine tubule injury markers with the binary kidney composite end point (interaction P values 0.30–0.70); however, the point estimates were consistently greater in magnitude within the standard arm, particularly for urinary KIM-1, MCP-1, and YKL-40 (Figure 2). There were statistically significant interactions of urinary IL-18 and KIM-1 by treatment arm for the continuous eGFR change end point. Among the five biomarkers, urinary IL-18 had the strongest association with eGFR decline within the standard arm stratum (Table 4).
Figure 2.
Forest plot of the fully adjusted model to assess the association of tubule injury markers with 50% kidney function decline, ESKD requiring dialysis, or kidney transplantation stratified by randomization arm in the Systolic Blood Pressure Intervention Trial. Horizontal bars represent the 95% confidence intervals. Model adjusted for age, sex, race, intervention arm, urine creatinine, baseline eGFR, urine albumin, smoking status, history of cardiovascular disease, baseline number of antihypertensive medications, systolic BP, diastolic BP, body mass index, HDL, and total cholesterol. KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; YKL-40, chitinase-3-like protein-1.
Table 4.
Association of kidney tubule injury markers with annualized relative change in eGFR stratified by randomization arm in SPRINT
| Total, n=2428 | Standard Arm, n=1179 | Intensive Arm, n=1236 | ||
|---|---|---|---|---|
| Biomarkers (per doubling) | Model, β (95% CI)a | Model, β (95% CI)a | Model, β (95% CI)a | P Value for Interaction |
| Urine IL-18 | −0.09 (−0.23 to 0.04) | −0.26 (−0.45 to −0.07)b | −0.01 (−0.19 to 0.18) | 0.005 |
| Urine KIM-1 | 0.07 (−0.04 to 0.18) | −0.003 (−0.16 to 0.15) | 0.14 (−0.02 to 0.30) | 0.004 |
| Urine NGAL | 0.09 (−0.01 to 0.19) | 0.08 (−0.07 to 0.23) | 0.13 (−0.02 to 0.27) | 0.22 |
| Urine MCP-1 | 0.07 (−0.05 to 0.20) | 0.26 (0.09 to 0.43)b | −0.03 (−0.21 to 0.15) | 0.90 |
| Urine YKL-40 | −0.04 (−0.11 to 0.04) | −0.08 (−0.18 to 0.03) | −0.01 (−0.12 to 0.11) | 0.37 |
SPRINT, Systolic Blood Pressure Intervention Trial; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; MCP-1, monocyte chemoattractant protein-1; YKL-40, chitinase-3-like protein-1.
Adjusted for age, sex, race, intervention arm, urine creatinine, baseline eGFR, urine albumin, smoking status, history of cardiovascular disease, baseline number of antihypertensive medications, systolic BP, diastolic BP, body mass index, HDL, and total cholesterol.
Represents significant results.
Discussion
Among hypertensive individuals with nondiabetic CKD in the SPRINT trial, urine markers of tubule cell injury, fibrosis, and repair provided information about risk of the composite kidney end point (50% eGFR decline or ESKD requiring dialysis or kidney transplant) independent of baseline eGFR, urine albumin, and other CKD risk factors. The associations of urinary KIM-1, MCP-1, and YKL-40 with this end point appeared nonlinear and were particularly strong among those with levels in the highest quartile of the study sample. The associations of the highest quartiles of the tubule injury biomarkers with CKD progression were similar in strength to that of urine albumin with the CKD progression end point, despite the fact that the urine tubule biomarker models were adjusted for urine albumin. The highest quartile of urinary IL-18 was also associated with the continuous eGFR decline outcome, an association that appeared stronger in participants in the standard arm of the trial.
We found that urinary biomarker concentrations were only associated with CKD progression when they exceeded the cut-off for the high quartile. In addition, with the exception of urinary IL-18, kidney tubule makers were not associated with linear eGFR decline. Collectively, these findings suggest that the tubule injury biomarkers evaluated herein must exceed a threshold of severity of ongoing injury, inflammation, or repair to identify the subset at risk for more rapid CKD progression. These findings require confirmation in other settings, but if confirmed, the markers may assist in risk stratification for CKD progression and could potentially be useful for enriching clinical trial enrollment for studies designed to affect kidney end points.
Urinary IL-18 was the only marker associated with linear eGFR decline, a finding that was stronger in the standard arm of SPRINT. Participants with CKD who were randomized to the intensive arm had acute declines in eGFR in the 6 months after randomization (14), which likely reflect acute hemodynamic changes in eGFR (17), a trajectory less often observed in the standard arm. Thus, the determinants for eGFR changes in the two arms of SPRINT differ somewhat. We hypothesize that urinary IL-18 may mark kidney tubule cell injury, which may be more closely reflected by longitudinal changes in eGFR in the standard arm than in the intensive arm. If so, it may not be surprising that urinary IL-18 measured at baseline before BP changes, was not associated with acute eGFR changes owing to hemodynamic effects in the intensive arm. This hypothesis is supported by recent findings in the The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial among individuals with diabetes but predominantly without CKD, who were randomized to the same BP targets as SPRINT. Among ACCORD participants experiencing incident CKD during follow-up, there were no significant changes in urinary IL-18 in the intensive arm over 2 years, whereas urinary IL-18 concentrations increased in the standard arm (18). Thus, urinary IL-18 may hold promise for distinguishing changes in eGFR related to intrinsic tubule damage from changes driven by hemodynamic fluctuations. We evaluated baseline levels of IL-18 in this study only, so future studies are required to evaluate longitudinal trajectories of IL-18 in response to different causes of eGFR change to confirm this hypothesis.
Several studies reported associations of single urinary tubule cell injury markers with kidney disease progression in ambulatory persons (10–12,19). However, few evaluated multiple markers in parallel to compare their relative strengths of association with CKD progression (8,9,18,20–25). In a study from the Chronic Renal Insufficiency Cohort (CRIC) that evaluated 2466 participants with CKD, the investigators jointly evaluated urinary KIM-1, NGAL, N-acetyl β-D-glucosaminidase, and liver fatty acid binding protein with rates of CKD progression defined similarly to our binary end point (incident ESKD or halving of eGFR) (21). In unadjusted models, the highest quintile of urine tubule injury markers were strongly associated with CKD progression with HRs ranging from 7 to 15, compared with the lowest quintile. However, adjustment for eGFR and albuminuria in CRIC resulted in marked attenuation of these relationships; none of the markers remained independently associated with CKD progression (21). Of the markers that were common between CRIC and our SPRINT data (urinary KIM-1 and NGAL), we found no association of urinary NGAL with CKD progression. However, the highest quartile of urinary KIM-1 was associated with a nearly three-fold risk of CKD progression in SPRINT, whereas the highest quintile was associated with a nonsignificant HR of 1.36 (95% CI, 0.93 to 2.00) in the fully adjusted model in CRIC. As the point estimate in CRIC was in the same direction as ours, one explanation is that a true association in CRIC was missed. Similarly, among 5367 individuals with type 2 diabetes mellitus and recent acute coronary syndromes enrolled in the Examination of Cardiovascular Outcomes with Alogliptin versus Standard of Care trial, Garlo et al. showed no association of urinary NGAL (adjusted HR, 1.03; 95% CI, 0.94 to 1.14) and KIM-1 (adjusted HR, 0.97; 95% CI, 0.85 to 1.10) with CKD progression (25). These differences may be explained by the fact that we measured markers using different assay platforms and made two measurements in each sample to improve precision. These technical aspects may have improved our ability to detect associations with CKD end points. The study populations were also different as SPRINT excluded those with proteinuria >1 g/d or diabetes mellitus. In contrast, approximately 50% of CRIC participants and all of those in the study by Garlo et al. had diabetes.
We found different effect sizes across the biomarkers when evaluated as urine creatinine-adjusted biomarker concentrations versus creatinine-indexed biomarkers. Previous studies have more often indexed urine biomarker concentrations to urine creatinine (26). However, urine creatinine not only reflects urine tonicity but is also influenced by muscle mass, and urine creatinine is itself associated with higher risk of adverse outcomes in some prior studies (27). These nontonicity factors may influence the ratio of biomarkers to creatinine and interpretation of the ratio’s relationship with end points if they are assumed to be driven by the numerator only. For these reasons, we have consistently evaluated biomarker concentrations that adjusted for urine creatinine concentration in the multivariable models rather than indexing as our primary analysis.
A unique feature of this study is measurement of urinary IL-18, MCP-1, and YKL-40; markers reflecting the interwoven axes of kidney tubule injury, inflammation, and repair within the kidney. Urinary MCP-1 and YKL-40 have not been extensively studied in the setting of ambulatory CKD; however, existing studies support their utility as markers of tubule disease (28,29). We reported that urinary MCP-1 was independently associated with risk of allograft failure in stable kidney transplant recipients (28). Similarly Puthumana et al. (29) demonstrated that higher urinary YKL-40 was associated with delayed graft function in kidney transplant recipients. As these markers were more strongly associated with CKD progression than urinary NGAL or IL-18, we believe our finds suggest that they hold promise as markers of tubule damage in ambulatory CKD populations.
Strengths of this study include its assessment of multiple urinary markers reflecting distinct aspects of kidney tubule biology, including tubule injury, inflammation, and repair. The finding of stronger signals at the highest quartile, the consistent direction of signals with CKD progression, and the independence of these associations from eGFR and urine albumin across the aggregate panel of markers support that noninvasive assessments of kidney tubule health can identify risk of CKD progression beyond eGFR and albuminuria. Close monitoring and frequent assessment of kidney function during follow-up in SPRINT provided useful measurement of eGFR changes, and minimized informative loss due to death and other comorbidities. All kidney tubule injury markers were measured in duplicate and averaged to improve precision. This study also has limitations. SPRINT excluded persons with diabetes mellitus, stroke, or proteinuria >1 g/d. Finally, because urine tubule injury markers were measured at baseline, longitudinal trajectories of these markers and potential changes in concentrations in response to interventions requires future study.
In summary, concentrations of three of five markers of kidney tubule cell injury among the highest quartile were associated with 50% eGFR decline or ESKD requiring dialysis or transplant in SPRINT participants with CKD. These associations were independent of CKD risk factors, eGFR, and urine albumin. Higher urinary IL-18 was independently associated with annualized eGFR decline, a finding confined to the standard arm of SPRINT. Thus, extremes of urinary KIM-1, MCP-1, and YKL-40 may mark risk for subsequent large eGFR declines. In contrast, urinary IL-18 may help to distinguish subtler changes in eGFR. Overall, these data demonstrate that markers of tubule cell injury provide information on risk of CKD progression independent of the glomerular markers of kidney health (eGFR and urine albumin).
Disclosures
Dr. Freedman reports receiving consultant fees from AstraZeneca and RenalytixAI and an issued patent for APOL1 gene testing, outside of the submitted work. Dr. Ix reports receiving a grant from Baxter outside of the submitted work. Dr. Rastogi reports receiving research grants and speaker’s bureau fees from and an advisory board position with both AstraZeneca and Sanofi; receiving a research grant from and an advisory board position with GlaxoSmithKline; and a research grant from Pfizer, all outside of the submitted work. Dr. Rocco reports receiving clinical trial grants from Bayer, Boehringer Ingelheim, and GlaxoSmithKline; receiving consultant fees from Baxter; and positions on an adjudication committee at Abbvie, Beacon Bioscience, and George Clinical, all outside of the submitted work. Dr. Shlipak reports a position of Scientific Advisor at and holds stock options with Cricket Health, Inc. and TAI Diagnostics. Dr. Ambrosius, Dr. Cheung, Dr. Haley, Dr. Jotwani, Dr. Katz, Dr. Malhotra, Dr. Punzi, and Dr. Raphael have nothing to disclose.
Funding
This study was funded by National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK098234 (to J. Ix and M. Shlipak). The Systolic Blood Pressure Intervention Trial is funded by federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute, the NIDDK, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke, under contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement number A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. We also acknowledge the support from the following Clinical and Translational Science Awards funded by the National Center for Advancing Translational Sciences: Case Western Researve University: UL1TR000439; Ohio State University: UL1RR025755; University of Pennsylvania: UL1RR024134 and UL1TR000003; Boston University Medical Center: UL1RR025771; Stanford: UL1TR000093; Tufts: UL1RR025752, UL1TR000073, and UL1TR001064; University of Illinois: UL1TR000050; University of Pittsburgh: UL1TR000005; UT Southwestern: 9U54TR000017-06; University of Utah: UL1TR000105-05; Vanderbilt University: UL1TR000445; George Washington University: UL1TR000075; University of California, Davis: UL1TR000002; University of Florida: UL1TR000064; University of Michigan: UL1TR000433; Tulane University: P30GM103337 Centers of Biomedical Research Excellence Award National Institute of General Medical Sciences; Wake Forest University: UL1TR001420. Dr. Ambrosius is supported by a grant from the NIH. Dr. Freedman is supported by a grant from the NIH. Dr. Ix is supported by NIDDK grants R01DK098234 and K24DK110427, and American Heart Association grant 14EIA18560026. Dr. Katz is supported by a grant from Veterans Medical Research Foundation San Diego. Dr. Malhotra is supported by the Satellite Coplon award and a grant from the Academic Community of the University of California, San Diego. Dr. Punzi is supported by a grant from the NIH. Dr. Rastogi is supported by a grant from the NIH. Dr. Rocco is supported by a grant from the NIH.
Supplementary Material
Acknowledgments
The Systolic Blood Pressure Intervention Trial (SPRINT) investigators acknowledge the contribution of study medications (azilsartan and azilsartan combined with chlorthalidone) from Takeda Pharmaceuticals International, Inc. All components of the SPRINT study protocol were designed and implemented by the investigators. The investigative team collected, analyzed, and interpreted the data. All aspects of manuscript writing and revision were carried out by the coauthors.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Veterans Affairs, or the US Government.
For a full list of contributors to SPRINT, please see the supplementary acknowledgment list: https://www.sprinttrial.org/public/dspScience.cfm.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “When Increase in Serum Creatinine Doesn’t Imply Kidney Damage,” on pages 304–305.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02780319/-/DCSupplemental.
Supplemental Table 1. Associations of kidney tubule injury markers, categorized by quartiles, with CKD progression in SPRINT, defined by 50% kidney function decline, ESKD requiring dialysis or kidney transplantation (additional adjustment with biomarkers).
Supplemental Table 2. Associations of kidney tubule injury markers, categorized by quartiles indexed to urine creatinine, with CKD progression in SPRINT, defined by 50% kidney function decline, ESKD requiring dialysis or kidney transplantation.
Supplemental Table 3. Association of quartiles of kidney tubule injury markers with annualized relative change in eGFR in SPRINT (additional adjustment with biomarkers).
Supplemental Table 4. Association of quartiles of kidney tubule injury markers indexed to urine creatinine with annualized relative change in eGFR in SPRINT.
References
- 1.Norris KC, Smoyer KE, Rolland C, Van der Vaart J, Grubb EB: Albuminuria, serum creatinine, and estimated glomerular filtration rate as predictors of cardio-renal outcomes in patients with type 2 diabetes mellitus and kidney disease: A systematic literature review. BMC Nephrol 19: 36, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babazono T, Nyumura I, Toya K, Hayashi T, Ohta M, Suzuki K, Kiuchi Y, Iwamoto Y: Higher levels of urinary albumin excretion within the normal range predict faster decline in glomerular filtration rate in diabetic patients. Diabetes Care 32: 1518–1520, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassirer JP: Clinical evaluation of kidney function--glomerular function. N Engl J Med 285: 385–389, 1971 [DOI] [PubMed] [Google Scholar]
- 4.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Howie AJ, Ferreira MA, Adu D: Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 16: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Malhotra R, Siew ED: Biomarkers for the Early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 12: 149–173, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa S: [Identification of biomarkers for tubular injury and interstitial fibrosis in chronic kidney disease]. Yakugaku Zasshi 137: 1355–1360, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherzer R, Lin H, Abraham A, Thiessen-Philbrook H, Parikh CR, Bennett M, Cohen MH, Nowicki M, Gustafson DR, Sharma A, Young M, Tien P, Jotwani V, Shlipak MG: Use of urine biomarker-derived clusters to predict the risk of chronic kidney disease and all-cause mortality in HIV-infected women. Nephrol Dial Transplant 31: 1478–1485, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khatir DS, Bendtsen MD, Birn H, Nørregaard R, Ivarsen P, Jespersen B, Buus NH: Urine liver fatty acid binding protein and chronic kidney disease progression. Scand J Clin Lab Invest 77: 549–554, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY; Chronic Renal Insufficiency Cohort (CRIC) study investigators: Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT Jr., Whelton PK; SPRINT Study Research Group: The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: The Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 11: 532–546, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, Oparil S, Rocco MV, Sink KM, Whelton PK, Wright JT Jr., Basile J, Beddhu S, Bhatt U, Chang TI, Chertow GM, Chonchol M, Freedman BI, Haley W, Ix JH, Katz LA, Killeen AA, Papademetriou V, Ricardo AC, Servilla K, Wall B, Wolfgram D, Yee J; SPRINT Research Group: Effects of intensive BP control in CKD. J Am Soc Nephrol 28: 2812–2823, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 373: 2103–2116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, Chonchol M, Sarnak M, Parikh CR, Shlipak MG, Ix JH; SPRINT Research Group: Effects of intensive blood pressure Lowering on kidney tubule injury in CKD: A longitudinal subgroup analysis in SPRINT. Am J Kidney Dis 73: 21–30, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, Cantley L, Devarajan P, Parikh CR, Coca SG: Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: The ACCORD trial. Clin J Am Soc Nephrol 11: 1343–1352, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, Katz R, Delaney JA, Chaves P, Rifkin DE, Hughes-Austin JM, Garimella PS, Sarnak MJ, Shlipak MG, Kizer JR: Urine Collagen Fragments and CKD progression-the cardiovascular health study. J Am Soc Nephrol 26: 2494–2503, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhavsar NA, Köttgen A, Coresh J, Astor BC: Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu CY, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, Mifflin TE, Coresh J, Diamantidis CJ, He J, Lora CM, Miller ER, Nelson RG, Ojo AO, Rahman M, Schelling JR, Wilson FP, Kimmel PL, Feldman HI, Vasan RS, Liu KD; CRIC Study Investigators; CKD Biomarkers Consortium: Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 91: 196–203, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Bonventre JV, Sabbisetti V, Waikar SS, Mifflin TE, Zhang X, Xie D, Hsu CY, Feldman HI, Coresh J, Vasan RS, Kimmel PL, Liu KD; Chronic Kidney Disease Biomarkers Consortium Investigators: Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia 58: 188–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster MC, Coresh J, Bonventre JV, Sabbisetti VS, Waikar SS, Mifflin TE, Nelson RG, Grams M, Feldman HI, Vasan RS, Kimmel PL, Hsu CY, Liu KD; CKD Biomarkers Consortium: Urinary biomarkers and risk of ESRD in the Atherosclerosis risk in Communities study. Clin J Am Soc Nephrol 10: 1956–1963, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobato GR, Lobato MR, Thomé FS, Veronese FV: Performance of urinary kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and N-acetyl-β-D-glucosaminidase to predict chronic kidney disease progression and adverse outcomes. Braz J Med Biol Res 50: e6106, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garlo KG, White WB, Bakris GL, Zannad F, Wilson CA, Kupfer S, Vaduganathan M, Morrow DA, Cannon CP, Charytan DM: Kidney biomarkers and decline in eGFR in patients with type 2 diabetes. Clin J Am Soc Nephrol 13: 398–405, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang KW, Toh QC, Teo BW: Normalisation of urinary biomarkers to creatinine for clinical practice and research--when and why. Singapore Med J 56: 7–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ix JH, de Boer IH, Wassel CL, Criqui MH, Shlipak MG, Whooley MA: Urinary creatinine excretion rate and mortality in persons with coronary artery disease: The Heart and Soul Study. Circulation 121: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ix JH, Katz R, Bansal N, Foster M, Weiner DE, Tracy R, Jotwani V, Hughes-Austin J, McKay D, Gabbai F, Hsu CY, Bostom A, Levey AS, Shlipak MG: Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: A Case-Cohort ancillary study of the FAVORIT trial. Am J Kidney Dis 69: 410–419, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puthumana J, Hall IE, Reese PP, Schröppel B, Weng FL, Thiessen-Philbrook H, Doshi MD, Rao V, Lee CG, Elias JA, Cantley LG, Parikh CR: YKL-40 Associates with Renal Recovery in Deceased Donor Kidney Transplantation. J Am Soc Nephrol 28: 661–670, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.