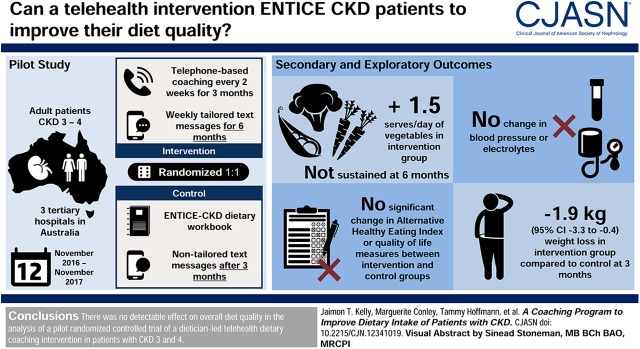
Visual Abstract
Keywords: chronic kidney disease, diet, dietary pattern, mobile health, self-efficacy, smartphone, telehealth, telemedicine, text messaging, trial, weight loss, male, humans, vegetables, healthy diet, self-management, blood pressure, mentoring, nutritionists, control groups, confidence intervals, pilot projects, body weight, telephone, chronic renal insufficiency
Abstract
Background and objectives
The dietary self-management of CKD is challenging. Telehealth interventions may provide an effective delivery method to facilitate sustained dietary change.
Design, setting, participants, & measurements
This pilot, randomized, controlled trial evaluated secondary and exploratory outcomes after a dietitian-led telehealth coaching intervention to improve diet quality in people with stage 3–4 CKD. The intervention group received phone calls every 2 weeks for 3 months (with concurrent, tailored text messages for 3 months), followed by 3 months of tailored text messages without telephone coaching, to encourage a diet consistent with CKD guidelines. The control group received usual care for 3 months, followed by nontailored, educational text messages for 3 months.
Results
Eighty participants (64% male), aged 62±12 years, were randomized to the intervention or control group. Telehealth coaching was safe, with no adverse events or changes to serum biochemistry at any time point. At 3 months, the telehealth intervention, compared with the control, had no detectable effect on overall diet quality on the Alternative Health Eating Index (3.2 points, 95% confidence interval, −1.3 to 7.7), nor at 6 months (0.5 points, 95% confidence interval, −4.6 to 5.5). There was no change in clinic BP at any time point in any group. There were significant improvements in several exploratory diet and clinical outcomes, including core food group consumption, vegetable servings, fiber intake, and body weight.
Conclusions
Telehealth coaching was safe, but appeared to have no effect on the Alternative Healthy Eating Index or clinic BP. There were clinically significant changes in several exploratory diet and clinical outcomes, which require further investigation.
Clinical Trial registry name and registration number:
Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD (ENTICE-CKD), ACTRN12616001212448.
Introduction
Dietary modification is a key management strategy in CKD to treat electrolyte abnormalities and potentially prevent CKD progression and cardiovascular events (1,2). It is also considered to be a top research priority by clinicians, patients, and caregivers (3). However, patients frequently experience dietary management as overwhelming, difficult to follow, and report contradictory messages that thwart implementation (4).
The majority of dietary interventions for predialysis patients with CKD are delivered in one-off dietary education sessions, without ongoing follow-up (5). Telephone coaching is a basic form of telehealth shown to be effective at promoting adherence to complex dietary recommendations in chronic disease (6). Telephone coaching in combination with one-on-one support can reduce dietary sodium intake (7) and increase knowledge of self-management behavior in people with CKD (8). m-Health also falls under the definition of telehealth, but extends more broadly to the use of mobile phones for health care delivery, is perceived as flexible and cost-effective (9), and has improved diet quality in people with coronary heart disease (10). Although there has been no telehealth intervention demonstrated to effectively support diet quality improvements in CKD, recent patient engagement studies suggest that people with CKD are open to using telephone and m-health methods for their dietary self-management (11,12). However, it is uncertain whether telehealth coaching interventions can improve diet quality in people with stage 3–4 CKD.
A pilot, randomized, controlled trial (Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD [ENTICE-CKD] study) recently demonstrated the feasibility and acceptability of a telehealth-delivered intervention to improve dietary self-management (13). Eighty participants were randomized to the intervention and control group, with 4% attrition and 96% of all coaching calls completed to protocol. All participants in the intervention arm (100%) believed the tailored text messages were useful in supporting their dietary self-management. A total of 69% of participants in the control group felt that the nontailored text messages were useful in supporting diet change. Participants viewed the intervention as an acceptable, personalized alternative to face-face clinic consultations, and were satisfied with the frequency of contact (13). This study aims to evaluate the secondary and exploratory outcomes of the ENTICE-CKD telehealth coaching intervention to improve diet quality in people with stage 3–4 CKD.
Materials and Methods
Study Design
ENTICE-CKD was a 6-month, parallel-group, randomized, controlled trial conducted across three tertiary hospitals in Australia (November 2016 to November 2017). This study was designed as a pilot trial to primarily test the feasibility and acceptability of the telehealth program to improve diet quality (13). This work reports on the diet-related outcomes and exploratory clinical outcomes listed in the clinical trial registration (trial identifier: ACTRN12616001212448). Ethics was approved by the Metro South Human Research Ethics Committee.
Participants
A detailed description of participant selection, screening, and intervention materials are described elsewhere (13). Any participant aged >18 years with stage 3–4 CKD (eGFR 15–59 ml/min per 1.73 m2 calculated using the CKD Epidemiology Collaboration formula) (14), with access to a mobile phone and ability to provide written informed consent, were eligible to participate. People on dialysis, non-English speaking, or deemed unsafe to participate were excluded.
Randomization and Allocation Concealment
The randomization schedule was created in RedCap (15) and participants were allocated on a 1:1 ratio, stratified by recruitment site and diabetes status using a computer-generated random number list. Allocation was concealed from the site investigators. An offsite trial coordinator randomized participant and notified the intervention coach (who had never met the participant and did not work in the same hospital), who in turn notified participants of their allocation.
Intervention
All specific details relating to the intervention are reported according to the template for intervention description and replication items 1–10 (16), detailed in Table 1. Details about intervention fidelity (items 11 and 12) are published elsewhere (13). Briefly, after a face-to-face baseline assessment with the local site investigator, all participants received the ENTICE-CKD workbook (main content summarized in Table 1), which was designed by dietitians specialized in kidney disease, with extensive academic, clinician, and consumer input. Participants randomized to the intervention group completed the telehealth intervention in two phases. The first phase involved individualized, telephone-based coaching from a dietitian (referred to as coaches) every 2 weeks for 3 months (total of six calls), with weekly tailored text messages (detailed in Table 1) delivered within a set protocol at a frequency determined by the participant. Phase 2 of the intervention involved no further telephone coaching calls; however, participants continued to receive the same tailored text messages as phase 1 at a different frequency (Table 1).
Table 1.
Description of the ENTICE-CKD intervention according to the TIDieR checklist (16)
| Item Name/Number | Item Description | |||||
|---|---|---|---|---|---|---|
| Item 1: Brief name | ||||||
| ENTICE-CKD | ||||||
| Item 2: Why | ||||||
| Telehealth intervention may support patients with stage 3–4 CKD to improve their diet quality through access to education, coaching, and regular contact with a health professional. Improving access to dietary education may assist people with stage 3–4 CKD reduce their dietary sodium intake <100 mmol/d and improve their overall diet quality in line with the Australian Dietary Guidelines (17). These dietary changes are complex and different levels of telehealth tailoring and intensity may be needed to support and sustain dietary behavior change. | ||||||
| Item 3: What | ||||||
| ENTICE-CKD program workbook (available on request) | ||||||
| About ENTICE | Introduction page “The focus of the ENTICE program is to help you make gradual changes to your eating and physical activity habits that work for YOU – changes that become lifelong.” | |||||
| Section 1: Setting your goals and keeping track | “Use the following steps every time you set a SMART goal…” | |||||
| Section 2: Eating well for healthy kidneys | “The ENTICE program will help you to gradually make changes to your diet to meet the daily recommended serves of fruit, vegetables and wholegrain breads/cereals.” | |||||
| Section 3: Active living | “Participating in regular physical activity and reducing sitting time is very important for your health and wellbeing.” | |||||
| Section 4: Why is healthy eating important for my kidneys? | Did you know? “<4% of the Australians meet the recommended daily intake for vegetables. Research has shown that increasing your intake of vegetables by as little as ONE serve per day can help you live longer and stronger.” | |||||
| Section 5: Plan for success | “There are a number of things that affect what we eat and our overall energy intake. It is important to be aware of, pay attention to and plan for: How you eat; Where/why you eat?” | |||||
| Section 6: Self-monitoring and setting goals | Smart snacking | |||||
| Reflections | ||||||
| Tracking my food intake | ||||||
| Section 7: Additional healthy eating resources | Useful websites; healthy recipes | |||||
| Useful apps for mobiles or tablets | ||||||
| High/low potassium/phosphate foods (if required) | ||||||
| Healthier verse unhealthy takeaway options | ||||||
| Item 4: What, procedures | ||||||
| Primary care physicians and treating nephrologists retained full responsibility over their patients’ medical care at all times. The ENTICE-CKD intervention was adjunct to the usual care received, which in Australia, typically involves medical care with 3-mo follow-up of patients with stage 4 CKD, and 3–6 mo follow-up for patients with stage 3 CKD. Patients with stage 3–4 CKD do not typically receive dietary consultation with a dietitian, unless there is clinical indication (18). | ||||||
| Before receiving allocated intervention: after a face-to-face baseline assessment with the local site investigator, all participants received the ENTICE-CKD workbook after receiving the randomized intervention, which was designed by dietitians specialized in kidney disease, with extensive academic, clinician, and consumer input. | ||||||
| Phase 1: Intensive coaching using telephone calls and tailored text messages. | ||||||
| Each call was designed to align with each section of the workbook, and structured on the basis of the 5As framework (Assess, Advise, Agree, Assist, Arrange) (49). The overall sequence of calls had the purpose of aligning participants’ diets with a reduced dietary sodium intake to <100 mmol/d and improving their overall diet quality in line with the Australian Dietary Guidelines (17). | ||||||
| Intervention calls | ||||||
| Call 1 | ||||||
| Welcome to ENTICE-CKD | ||||||
| Information about the program | ||||||
| Feedback on baseline outcome measures | ||||||
| Complete section 1: goal setting | ||||||
| Discuss section 6: self-monitoring | ||||||
| Begin section 2: introduction the five food groups | ||||||
| Call 2 | ||||||
| Revisit goals | ||||||
| Recap Australia Guide to Healthy Eating, answer any questions | ||||||
| Continue section 2 (plate model, snacks, salt, label reading, potassium, and phosphate) | ||||||
| Call 3 | ||||||
| Revisit goals | ||||||
| Answer any questions on healthy eating | ||||||
| Complete section 3: active living | ||||||
| Call 4 | ||||||
| Revisit goals | ||||||
| Revisit any questions about active living/healthy eating | ||||||
| Complete section 4: why is healthy eating important for my kidneys | ||||||
| Complete section 5: planning for success; how, why, and where you eat and managing slips | ||||||
| Call 5 | ||||||
| Revisit goals | ||||||
| Answer any dietary or active living questions | ||||||
| Discuss section 7: additional information/ resources | ||||||
| Call 6 | ||||||
| Revisit goals | ||||||
| Revisit any questions participant may have | ||||||
| Discuss where to from here | ||||||
| Adjust text message frequency if desired | ||||||
| Text message component | ||||||
| Text message type | SCT construct | Example text | Intervention | Control | ||
| Phase 1 | Phase 2 | Phase 1 | Phase 2 | |||
| Education | Outcome expectations | Dietary fiber intake reduces ur cholesterol levels and controls ur blood sugar. Include wholegrain breads and cereals, fruits and veg regularly | 2–6 | 1–4 | NA | 6–8 |
| Self-monitor | Self-regulation | Hi [name], are u keeping track of ur fruit/vegetable intake every day? Remember ur goal to have 5 serves this week | 0–2 | 1–4 | NA | NA |
| Goal check | Self-regulation | Hi [name], did u reach ur goal to eat 5 fruits/vegetables 4 times this week? Text me back yes or no to let me know | 2 | 2–4 | NA | NA |
| Education (Safety protocol) | Low potassium diet | Choose high fiber, low potassium breakfast cereals. Good choices are Multigrain Weetbix, Rolled Oats, Guardian, Oatbritz, Special K | 0–2a | 0–2a | NA | 0–2a |
| Phase 2: Extended contact using tailored text messages only. At the end of phase 1 (3-mo study midpoint), participants completed their final coaching call and discussed their preferences for the timing and frequency of the phase 2 text messages. At 18 wk, participants received another tailoring call (no dietary coaching) to make individualized adjustments to their text message timing and frequency for the remaining 6 wk of the intervention. | ||||||
| Item 5: Provider | ||||||
| Two accredited practicing dietitians (RD equivalent) with additional training in behavior change, motivational interviewing, and kidney nutrition. Each participant in the intervention was assigned to one dietitian for the duration of the intervention. | ||||||
| Item 6: How | ||||||
| Phase 1 (mo 0–3) | Intervention: One-to-one coaching provided through six phone calls every 2 wk, and tailored text messages at a frequency requested by the participant (TIDieR item 4: text message component). | |||||
| Phase 2 (mo 3–6) | Intervention: Tailored text messages at a frequency requested by the participant (TIDieR item 4: text message component). | |||||
| Item 7: Where | ||||||
| Participants were in locations of their choosing as the intervention was delivered by telephone/mobile. | ||||||
| Item 8: When and how much | ||||||
| Phone calls: Intervention group participants received phone calls every 2 wk for 3 mo. | ||||||
| Text messages: Intervention participants received text messages every 2 wk for 6 mo. Control group participants received text messages for 3 mo (in phase 2 only) (TIDieR item 4: text message component). | ||||||
| Item 9: Tailoring | ||||||
| Telephone calls: Coaches could tailor the dietary guidelines to participants’ individual comorbidities and goals. Coaches documented any tailoring to the intervention in call logs. | ||||||
| Text messages: Tailored text messages were tailored to participants’ names, set goals and barriers to achieving each goal (examples can be seen under TIDieR item 4: text message component). | ||||||
| Safety tailoring: where required, coaches tailored the food group suggestions to be electrolyte controlled, only when clinically indicated or directly requested by participants. | ||||||
| Item 10: Modifications | ||||||
| Some participants who replied to the goal check text messages in a way the system could not recognize (i.e., not a yes/no response) were giving a tailored goal check reply message instead of the automatic system generated reply. No other modifications were made to the intervention during the course of the study. | ||||||
Educational permutations were only available for coaches to use if a participant experienced hyperkalaemia or hyperphosphataemia.
ENTICE-CKD, Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD study; TIDieR, template for intervention description and replication; SCT, social cognitive theory; NA, not applicable; RD, registered dietitian.
Control Group
Participants allocated to the control group received two phases of care. In phase 1 (months 0–3), they received the same ENTICE-CKD workbook as the intervention group and continued to receive usual care, but no other intervention. After 3 months, participants commenced phase 2 (months 3–6), a delayed contact intervention, including nontailored text messages in which participants could choose the text frequency. The content of the text messages was on the basis of improving dietary quality as per the Australian Dietary Guidelines (17) and the Kidney Health Australia Caring for Australasians with a Renal Impairment guidelines (19). Although these text messages are not considered “usual care,” they were utilized as a retention mechanism, and to generate hypotheses around the potential benefit of tailored text messages compared with nontailored text messages to improve dietary intake in CKD.
Outcome Assessment
The primary outcome of the trial was feasibility and acceptability, which have been previously reported (13). In this study, we report all secondary and exploratory outcomes of ENTICE-CKD (Supplemental Figure 1). Data were collected at baseline, and 3 and 6 months. Participants’ study visits were scheduled hours apart to mitigate contamination bias. To maintain blinding of allocation for site investigators, participants were asked not to disclose which group they had been assigned to during their study visits. Site investigators performed all objective clinical measures, whereas serum and urine pathology were performed as part of usual care (using random samples) by local National Association of Testing Authorities, Australia (NATA)-accredited laboratories. Self-reported surveys and health care utilization were completed by participants at all times, either on site, returned via registered post, or completed via an online email link.
Secondary Outcomes
Overall change in diet quality was measured by the Alternative Healthy Eating Index 2010, which assesses adherence to dietary guidelines (20). The Australian Eating Survey (21) was used to capture and measure all diet outcome measures, including eight of the ten Alternative Healthy Eating Index food scoring components, except for trans-fat and n-3 polyunsaturated fatty acids, which were calculated using FoodWorks (version 18). All ten food scoring components of the Alternative Healthy Eating Index were summed to generate an Alternative Healthy Eating Index score ranging from 0 to 110 (higher score reflecting higher adherence) (20).
Change in clinic BP was measured with a calibrated digital BP monitor in accordance with the recommended protocol from the American Heart Association (22).
All serum investigations were performed at local National Association of Testing Authorities, Australia (NATA)-accredited laboratories and used to assess changes in potassium, bicarbonate, and phosphate for safety monitoring.
Exploratory Outcomes
Data collection methods for the exploratory measures of diet quality, nutrient intake, antihypertensive medication requirements, body weight, waist circumference, eGFR, albuminuria, Assessment of Quality of Life questionnaire (23), and economic evaluation (program costs, participant food costs, and health care expenditure), are reported in Supplemental Table 1.
Safety Monitoring
Participants were trained to identify symptoms of hypotensive episodes between study visits, defined as systolic BP <100 mm Hg or diastolic BP <60 mm Hg. Assigned coaches monitored symptoms and/or large changes in potassium (increase in potassium intake >50 mmol/d) or sodium intake during each coaching call (24,25). If concerns were identified, participants were advised to see their general practitioner.
Statistical Analyses
The study was designed as a pilot study (26). A sample size of 30–40 participants per study arm was determined so as to reliably interpret data to inform power calculations for future intervention studies (27). A one-way analysis of covariance was conducted to assess the main effects of the categorical independent variable (tailored telephone coaching and text messages versus control [phase 1]; or tailored text messages versus nontailored, education-only text messages [phase 2]) on a single, continuous, dependent variable, after controlling for the effects of the baseline covariate. Categorical outcomes were assessed by the standard chi-squared test. Given the small amount of missing data across the sample (<10%), all analyses were performed using an available case analysis. As a form of sensitivity analysis, we also performed intention to treat using the last value carried forward technique. Both this and the available case analyses reported the same overall statistical (and point-estimate) significance (data available on request). There were no further subgroup or additional analyses completed. Statistical analyses were performed using SPSS Statistics for Windows (version 22.0), with a two-sided P value (≤0.05) considered statistically significant. The economic evaluation was conducted using the mean costs and the health outcomes of (1) quality-adjusted life-years (QALYs) gained over 3 months, and (2) the proportion of participants achieving a five-point improvement in diet quality over 3 months. Incremental cost-effectiveness ratios (ICERs) were calculated as the incremental cost per QALY gained, and the incremental cost per additional patient achieving a meaningful improvement in diet quality, using the following formula:
 |
All cost-effectiveness analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington, DC).
Results
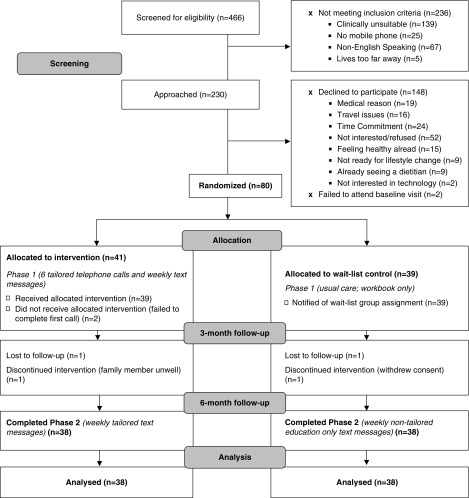
Eighty participants were randomized and 76 completed the 6-month study (Figure 1). Except for servings of vegetables per day, where intervention participants had a 1.2-serving lower intake at baseline, all other baseline characteristics were not statistically different between groups (Table 2). However, there was evident clinical differences between the groups which may be meaningful in practice. Mean age of the intervention group was 63±12 years compared with 61 years in the control arm, the intervention arm also had a higher proportion of Asian, white, and Indigenous ethnicities, whereas the control arm had a higher proportion of European and other ethnicities. Intervention participants had a 5.8-kg higher weight, and a 5.9-cm higher waist circumference compared with control participants, which are important clinical characteristics to note. Participants had a high diet quality at baseline, with overall mean Alternative Healthy Eating Index values of 71.4 and 69.6 points for the intervention and control groups, respectively.
Figure 1.
Consolidated Standards of Reporting Trials diagram showing the flow of the 80 enrolled (n=41 intervention and n=39 control) participants through the Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD study.
Table 2.
Baseline characteristics of participants in the ENTICE-CKD study
| Characteristic | Intervention, n=41 | Control, n=39 |
|---|---|---|
| Age, yr | 63±12 | 61±13 |
| Sex, % male | 63 | 64 |
| Ethnicity, n (%) | ||
| Asian | 2 (5) | |
| White | 35 (85) | 1 (3) |
| European | 2 (5) | 29 (74)3 (8) |
| Indigenous | 1 (2.5) | 0 (0) |
| Other | 1 (2.5) | 6 (15) |
| Diabetes, n (%) | 16 (39) | 15 (39) |
| Cardiovascular disease, n (%) | 14 (34) | 12 (31) |
| Hypertension, n (%) | 34 (83) | 31 (80) |
| Antihypertensive medication, n (%) | 38 (93) | 34 (87) |
| Angiotensin-converting enzyme inhibitor | 12 | 13 |
| Angiotensin receptor blocker | 20 | 16 |
| Alpha-blocker | 9 | 1 |
| Beta-blocker | 20 | 18 |
| Calcium channel blocker | 15 | 15 |
| Diuretic | 19 | 17 |
| Other | 7 | 8 |
| Total antihypertensive medication pill count at baseline, n | 102 | 88 |
| Serum potassium, mEq/L | 4.6±0.6 | 4.7±0.5 |
| Serum bicarbonate, mEq/L | 26±3 | 26±3 |
| Serum phosphate, mg/dl | 3.7±0.6 | 3.7±0.6 |
| Serum creatinine, mg/dl | 2.0±0.6 | 1.9±0.7 |
| eGFR, ml/min per 1.73 (2) | 36±12 | 39±12 |
| Systolic BP, mm Hg | 136±18 | 131±19 |
| Diastolic BP, mm Hg | 80±12 | 78±11 |
| Weight, kg | 96±22 | 90±19 |
| BMI, kg/m2 | 33±7 | 31±6 |
| Waist circumference | 113±18 | 107±18 |
| Urine albuminuria, mg/L | 120.0 (8.0–442.0) | 38.5 (13.0–143.5) |
| Alternative Healthy Eating Index, score between 1 and 110 | 71.4 (11.8) | 69.6 (12.1) |
| Energy intake from the core food groups, % | 62.8±17.4 | 65.7±13.9 |
| Fruit, servings/d | 1.5±0.9 | 1.9±1.5 |
| Vegetables, servings/d | 4.2±2.0 | 5.4±2.5a |
| Sodium, mg | 2379.0±1392.0 | 2260.0±907.0 |
| Fiber, g | 24.1±9.1 | 26.7±9.6 |
| Daily food costs, AU$b | 12.8±4.4 | 13.3±3.7 |
| Energy, kJ | 9472.0±4837.0 | 9571.9±2909.0 |
| Energy, kJ/kg | 100.5±55.3 | 111.4±41.8 |
| Protein, g | 104.8±51.3 | 111.3±31.5 |
| Protein, g/kg | 1.1±0.5 | 1.3±0.5 |
Data are reported as mean±SD or median (interquartile range). ENTICE-CKD, Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD study; BMI, body mass index.
P value for between group difference at baseline <0.05.
Daily food costs were calculated from each participants daily food intake using the Australian Eating Survey.
Change in Dietary Intake
Compared with controls, telehealth coaching did not significantly change Alternative Healthy Eating Index at 3 or 6 months. However, individual measures of diet quality were significantly changed after the telehealth intervention, including an increase in energy intake from the core food groups at 3 months (5.8%; 95% confidence interval [95% CI], 0.9 to 10.7) and 6 months (5.4%; 95% CI, 1.0 to 9.8), increased vegetable intake at 3 months by 1.5 servings/d (95% CI, 0.6 to 2.3), and increased grams of dietary fiber by 6.1 g/d (95% CI, 3.2 to 8.9). These improvements were not sustained at 6 months (Table 3).
Table 3.
Change from baseline in indices of diet quality and clinical assessment at 3 and 6 mo, adjusted for baseline values
| Variable | 3 mo | 6 mo | ||||
|---|---|---|---|---|---|---|
| Intervention, n=38 | Control, n=37 | Mean Difference (95% Confidence Interval) | Intervention, n=38 | Control, n=37 | Mean Difference (95% Confidence Interval) | |
| Diet quality index (secondary outcomes) | ||||||
| Alternative Healthy Eating Index (score between 1 and 110) | 4.3 (1.2 to 7.5)a | 2.1 (−1.7 to 5.8) | 3.2 (−1.3 to 7.7) | 3.4 (−0.3 to 7.2) | 3.8 (−0.1 to 7.8) | 0.5 (−4.6 to 5.5) |
| Proportion of participants achieving a 5-point change in Alternative Healthy Eating Index, n (%) | 19 (50.0) | 14 (36.8) | φ=−0.13b | 15 (39.5) | 17 (44.7) | φ=−0.05b |
| Food groups (exploratory outcomes) | ||||||
| Energy intake from the core food groups, % | 10.3 (6.2 to 14.4)c | 3.3 (−1.2 to 7.7) | 5.8 (0.9 to 10.7)a | 11.3 (7.7 to 14.8)c | 4.2 (−0.1 to 8.5) | 5.4 (1.0 to 9.8)a |
| Fruit, servings/dd | 0.7 (0.3 to 1.0)e | 0.1 (−0.3 to 0.6) | 0.4 (−0.1 to 0.9) | 0.3 (0.0 to 0.7) | 0.2 (−0.3 to 0.7) | 0.0 (−0.6 to 0.5) |
| Vegetables, servings/df | 1.3 (0.7 to 1.9)c | −0.3 (−0.9 to 0.2) | 1.5 (0.6 to 2.3)e | 0.8 (0.2 to 1.4)a | −0.1 (−0.8 to 0.7) | 0.3 (−0.6 to 1.3) |
| Clinical measure | ||||||
| Clinic BP | ||||||
| Systolic BP, mm Hg | −2 (−8 to 5) | 2 (−3 to 8) | −1 (−8 to 6) | −1 (−8 to 7) | 2 (−5 to 8) | 2 (−7 to 10) |
| Diastolic BP, mm Hg | −3 (−6 to 1) | 0 (−3 to 4) | −2 (−6 to 1) | −1 (−5 to 3) | 0 (−3 to 3) | 1 (−3 to 4) |
| Serum blood chemistry | ||||||
| Serum potassium, mEq/Lg | −0.01 (−0.12 to 0.10) | −0.01 (−0.15 to 0.13) | −0.01 (−0.20 to 0.20) | 0.05 (−0.09 to 0.20) | −0.04 (−0.18 to 0.10) | 0.08 (−0.10 to 0.30) |
| Serum bicarbonate, mEq/Lg | 0.18 (−0.74 to 1.11) | −0.03 (−0.94 to 0.88) | 0.07 (−1.10 to 1.25) | 0.32 (−0.51 to 1.16) | 1.79 (−2.34 to 5.92) | −1.56 (−5.76 to 2.66) |
| Serum phosphate, mEq/Lh | −0.03 (−0.25 to 0.19) | −0.09 (−0.04 to 0.19) | 0.00 (−0.34 to 0.31) | 0.12 (−0.12 to 0.37) | −0.19 (−0.43 to 0.06) | 0.22 (−0.06 to 0.37) |
| Exploratory outcomes | ||||||
| Quality of life (measure name) | ||||||
| Overall change in quality of lifei | 0.10 (0.03 to 0.16)a | 0.03 (−0.03 to 0.09) | 0.07 (−0.01 to 0.15) | 0.07 (0.01 to 0.14)a | 0.05 (−0.01 to 0.12) | 0.02 (−0.06 to 0.10) |
P≤0.05.
φ: φ coefficient used to determine a relationship between two binary variables. A φ<0.19 distinguishes a weak-to-negligible relationship.
P≤0.0001.
Sample size at 3 mo (n=37 for intervention; n=37 for control). Sample size at 6 mo (n=34 for intervention; n=35 for control).
P≤0.001; P≤0.001.
Sample size at 3 mo (n=38 for intervention; n=36 for control). Sample size at 6 mo (n=35 for intervention; n=36 for control).
Sample size at 3 mo (n=38 for intervention; n=36 for control). Sample size at 6 mo (n=37 for intervention; n=38 for control).
Sample size at 3 mo (n=32 for intervention; n=35 for control). Sample size at 6 mo (n=32 for intervention; n=37 for control).
Sample size at 3 mo (n=36 for intervention; n=36 for control). Sample size at 6 mo (n=37 for intervention; n=36 for control).
BP and Serum Electrolytes
There was no change in BP or serum electrolytes in either group at any time point. There were no incidences of hypotensive episodes or any other adverse events (including hyperkalemia).
Body Mass
Telehealth coaching participants had a greater decrease in body weight at 3 months (−1.9 kg; 95% CI, −3.3 to −0.4) compared with control group participants, which slightly attenuated at 6 months (−1.5 kg; 95% CI, −3.5 to 0.4) (Supplemental Table 2). The intervention appeared to make little difference to waist circumference at both time points (Supplemental Table 2).
Clinical Parameters
The total number of antihypertensive medications use at 3 months in the intervention and control groups increased in one (3%) and nine (24%) participants and decreased in five (13%) and two (5%) participants (φ=0.3; P=0.02; Supplemental Table 2). No difference in antihypertensive medication use was observed at 6 months (P=0.1; Supplemental Table 2).
Quality of Life
Telehealth coaching appeared to result in no significant difference in Assessment of Quality of Life questionnaire score (Table 3) or QALYS gained (Supplemental Table 2) between groups at both 3 and 6 months.
Cost-Effectiveness
Over the 6-month trial period, the intervention cost AU$ 297.76 (US$ 204.01) compared with AU$ 42.93 (US$ 29.41) in the control group; total costs per participant were AU$ 1780.88 (US$ 1220.20) for the intervention group and AU$ 1789.74 (US$ 1226.15) for the control group (Supplemental Table 3). At 3 months, the intervention was both less costly and more effective (dominant) than the control group in terms of both the proportion of patients achieving a meaningful change in diet quality, and in QALYs gained.
Adverse Events
There were no adverse events or unintended consequences to any participant in any group throughout the study.
Discussion
This paper aimed to evaluate the secondary and exploratory outcomes of the ENTICE-CKD telehealth coaching intervention to improve diet quality in people with stage 3–4 CKD. The intervention was established to be safe, but did not lead to result in a significant improvement in the secondary outcome of diet quality, as measured by the Alternative Healthy Eating Index, or clinic BP at any time point. There were a number of exploratory dietary outcomes that did significantly improve after intensive telehealth coaching, including energy intake from the core food groups and vegetables and dietary fiber intake, compared with controls at 3 months. It is important to not overstate the intervention effects observed as a large number of secondary and exploratory outcomes were examined in this pilot study, with a small number showing statistically significant effects (some of which could be by chance), and notably, the majority of these effects were not sustained at the 6-month conclusion of the study. As the examination of dietary outcomes was a secondary aim of the ENTICE-CKD study, it was underpowered to detect meaningful change in these outcomes. However, these observed effects in diet and clinical outcomes highlight the potential to examine this further in larger, adequately powered clinical trials.
Although the intervention group improved their overall diet quality compared with their baseline intake, there was no statistically significant difference compared with the control group at any time point. Although noting that the study was underpowered, it is interesting that the mean intervention effect at 3 months equated to an improvement in Alternative Healthy Eating Index score of approximately one quarter of an SD in favor of the intervention group, with the 95% CIs encompassing effects in the order of up to half an SD improvement. Although not statistically significant effect, the observed within-group changes in intervention participants’ Alternative Healthy Eating Index scores are consistent with the minimal clinically important changes suggested to translate to reduced risk of morbidity and mortality (28). It is also plausible that participants’ high diet quality at baseline (in both study groups) resulted in ceiling effects, limiting the magnitude of improvement possible through further dietary intervention. In fact, both study groups at a baseline had a mean Alternative Healthy Eating Index score that would be in the highest quintile in observational studies, which have demonstrated kidney and cardiovascular benefit with diet quality (20,29).
Improving vegetable and fiber intake are important characteristics of diet quality (30). In our study, medium to large (d>0.5) statistically significant effects were observed for these exploratory outcomes. In an Australian cohort study, consuming more vegetables and fruit was shown to be associated with a 65% lower risk of death over a 4-year follow-up period in stage 3–4 CKD (1). In the only available randomized trial, people with stage 4 CKD randomized to an intervention targeting higher vegetable and fruit intakes, had lower rates of metabolic acidosis, and greater reductions in BP and body weight compared with a standard sodium bicarbonate prescription over a 5-year follow-up period (31,32). Intervention group participants significantly increased their consumption of dietary fiber by >6 g/d at 3 months to exceed the 30 g/d as recommended in current guidelines (33). Meta-analysis in CKD populations has shown that dietary fiber consumption of approximately 27 g/d can significantly reduce serum urea and creatinine levels (34). Long-term exposure to a diet higher in fiber may also reduce inflammation and mortality risk (35).
BP is a controllable risk factor for CKD progression, but was unchanged throughout the study. Intervention participants did reduce their overall antihypertensive medication pill burden at 3 months, which may reflect a possible benefit of improving BP control in CKD. For example, the efficacy of some antihypertensive medications (notably angiotensin-converting enzyme inhibitors and angiotensin receptor blockers) are enhanced with effective sodium restriction (36). As sodium reduction was observed in both study groups, the reduction in antihypertensive medication prescriptions in the intervention group may have attenuated a potential change in BP, which we could attribute to the intervention itself. However, antihypertensive medication burden was only an exploratory outcome and therefore this is not conclusive.
Excessive weight gain carries a higher burden of cardiovascular risk and CKD progression (37). Although weight change was an exploratory outcome and not a focus of the intervention, the magnitude of change (−1.9 kg) was similar to that of two recent diet and physical activity randomized trials, in which both studies demonstrated a weight loss of 1.8 kg in 4- and 12-month interventions (38,39). Although the intervention effect on weight was statistically significant, this magnitude of weight loss is unlikely to be associated with clinical benefit, with weight losses of at least 5% of body weight associated with improvements in health outcomes (40).
The tailored telehealth program (telephone coaching plus text messages) was both less costly and more effective at 3 months for improving diet quality compared with the control group. We were unable to analyze the 6-month cost-effectiveness of the ENTICE-CKD program, as the tailored telehealth intervention was delivered in two phases, both of which had different interventions (in both the intervention and control). Although this is a promising finding, a larger study with greater participant numbers is needed to confirm the cost-effectiveness of the intervention.
This study has important limitations to consider. First, the ENTICE-CKD study was primarily designed to test the intervention’s feasibility (26). As such, the dietary outcomes were nominated as secondary outcomes and all clinical outcomes were exploratory to generate hypotheses for future studies. Second, reliable comparisons to usual care can only be drawn at 3 months, as our delayed-contact control participants received an active intervention from 3 to 6 months. It is therefore unclear whether a longer-term lower intensity intervention, such as the nontailored, education-only text messages, may lead to similar results as observed in the tailored intervention over 6 months which could be more cost-effective. Third, instead of using standard 24-hour ambulatory BP, clinic BP was measured as a pragmatic approach to align with routine clinical practice. Finally, the majority of participants reported following a healthy diet at baseline, as reflected in their high baseline diet quality score rating (above the median of “high adherence” in population studies) and dietary sodium intake (in line with the suggested 100 mmol/d), which raises the possibility of healthy volunteer bias and diminished potential for benefit from dietary intervention in a more representative cohort.
In conclusion, telehealth coaching appeared to have no effect on overall diet quality (as measured by the Alternative Healthy Eating Index) or clinic BP at any time point, compared with usual care. Other exploratory markers of diet quality were improved, including the energy intake from the core food groups, number of servings of vegetables, dietary fiber, and reduction of body weight at 3 months. All of these outcomes were exploratory, so there remains uncertainty in the findings, which require further investigation. The findings from this study can be used to inform hypotheses for future telehealth coaching interventions to initiate and sustain improvements in dietary intake across the complexity of dietary recommendations for patients with CKD.
Disclosures
Dr. Johnson reports consultancy fees from AstraZeneca, AWAK, Baxter Healthcare, and Fresenius Medical Care; grants and speakers’ honoraria from Baxter Healthcare and Fresenius Medical Care; and travel sponsorship from Amgen, all outside of the submitted work. Dr. Krishnasamy reports receiving travel honoraria from Amgen Australia and consulting and speaking fees and travel honoraria from Baxter International outside of the submitted work. Dr. Campbell, Dr. Conley, Dr. Craig, Dr. Hoffmann, Dr. Howard, Dr. Kelly, Dr. Kurtkoti, Dr. Palmer, Dr. Reeves, Dr. Reidlinger, and Dr. Tong have nothing to disclose.
Funding
This study was supported by a research support grant awarded by Kidney Health Australia via Australasian Kidney Trial Network, and a Vice-Chancellors Research Award through Bond University. Dr. Johnson is supported by a National Health and Medical Research Council Practitioner Fellowship. Dr. Kelly is supported by an Australian Government Research Training Program Scholarship. Dr. Palmer is supported by a Rutherford Discovery Fellowship from the Royal Society of New Zealand.
Supplementary Material
Acknowledgments
The authors would like to thank the people who participated in the Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in CKD study. They would also like to thank the health professionals who assisted in participant recruitment, the Australasian Kidney Trials Network CKD Working Group, including Professor Nigel Toussaint, Professor Carmel Hawley, and Dr. Sunil Badve for their contribution to the study design, and Elaine Pascoe from Australasian Kidney Trial Network, who carried out the independent randomization.
Dr. Kelly wrote the first draft of the manuscript and takes responsibility for the integrity of the data. Dr. Kelly, Dr. Campbell, Dr. Johnson, Dr. Hoffmann, Dr. Reidlinger, Dr. Reeves, Dr. Craig, Dr. Tong, and Dr. Palmer assisted in the conceptualization of the trial design. Dr. Kelly and Dr. Conley designed the intervention materials and were responsible for the management of the trial at their respective sites. Dr. Kurtkoti and Dr. Krishnasamy provided recruitment logistic expertise and revised drafts of the manuscript. All authors contributed to revisions of the manuscript and approved the final version for submission.
Data sharing statement
The deidentified, individual participant data that underlie the results reported in this publication will be made available for sharing 24 months after article publication. The study protocol and statistical analysis plan can be requested by contacting the corresponding author. To request deidentified individual data, researchers will need to submit a proposal, which will be assessed for appropriateness according to the following criteria: (1) methodologically sound proposal and data analysis plan, and (2) gained ethicalclearance. Proposals may be submitted up to 24 months after article publication, on request, by contacting Bond University Research Data Management (research@bond.edu.au). Requesting researchers will need to sign a data access agreement before being approved to access the data for 12 months.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12341019/-/DCSupplemental.
Supplemental Table 1. Description of data collection methods for the exploratory outcomes in the ENTICE-CKD study.
Supplemental Table 2. Exploratory dietary and clinical outcomes in the ENTICE-CKD study.
Supplemental Table 3. Cost-effectiveness of the tailored intervention (Phase 1 (0–3 months): workbook, telephone coaching with tailored text messages; Phase 2 (3–6 months) tailored text messages only) versus non-tailored intervention (Phase 1 (0–3 months) usual care plus workbook; Phase 2 (3–6 months) non-tailored text messages).
Supplemental Figure 1. Summary of ENTICE-CKD program delivery.
References
- 1.Wai SN, Kelly JT, Johnson DW, Campbell KL: Dietary patterns and clinical outcomes in chronic kidney disease: The CKD. QLD nutrition study. J Ren Nutr 27: 175–182, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JT, Palmer SC, Wai SN, Ruospo M, Carrero JJ, Campbell KL, Strippoli GF: Healthy dietary patterns and risk of mortality and ESRD in CKD: A meta-analysis of cohort studies. Clin J Am Soc Nephrol 12: 272–279, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong A, Crowe S, Chando S, Cass A, Chadban SJ, Chapman JR, Gallagher M, Hawley CM, Hill S, Howard K, Johnson DW, Kerr PG, McKenzie A, Parker D, Perkovic V, Polkinghorne KR, Pollock C, Strippoli GF, Tugwell P, Walker RG, Webster AC, Wong G, Craig JC: Research priorities in CKD: Report of a national workshop conducted in Australia. Am J Kidney Dis 66: 212–222, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SC, Hanson CS, Craig JC, Strippoli GF, Ruospo M, Campbell K, Johnson DW, Tong A: Dietary and fluid restrictions in CKD: A thematic synthesis of patient views from qualitative studies. Am J Kidney Dis 65: 559–573, 2015. [DOI] [PubMed] [Google Scholar]
- 5.St Peter WL, Schoolwerth AC, McGowan T, McClellan WM: Chronic kidney disease: Issues and establishing programs and clinics for improved patient outcomes. Am J Kidney Dis 41: 903–924, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Desroches S, Lapointe A, Ratté S, Gravel K, Légaré F, Turcotte S: Interventions to enhance adherence to dietary advice for preventing and managing chronic diseases in adults. Cochrane Database Syst Rev 2: CD008722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meuleman Y, Hoekstra T, Dekker FW, Navis G, Vogt L, van der Boog PJM, Bos WJW, van Montfrans GA, van Dijk S; ESMO Study Group: Sodium restriction in patients with CKD: A randomized controlled trial of self-management support. Am J Kidney Dis 69: 576–586, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Chen SH, Tsai YF, Sun CY, Wu IW, Lee CC, Wu MS: The impact of self-management support on the progression of chronic kidney disease--a prospective randomized controlled trial. Nephrol Dial Transplant 26: 3560–3566, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Spark LC, Fjeldsoe BS, Eakin EG, Reeves MM: Efficacy of a text message-delivered extended contact intervention on maintenance of weight loss, physical activity, and dietary behavior change. JMIR Mhealth Uhealth 3: e88, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, Jan S, Graves N, de Keizer L, Barry T, Bompoint S, Stepien S, Whittaker R, Rodgers A, Thiagalingam A: Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: A randomized clinical trial. JAMA 314: 1255–1263, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Kelly JT, Campbell KL, Hoffmann T, Reidlinger DP: Patient experiences of dietary management in chronic kidney disease: A focus group study. J Ren Nutr 28: 393–402, 2018. [DOI] [PubMed] [Google Scholar]
- 12.Stevenson J, Tong A, Campbell KL, Craig JC, Lee VW: Perspectives of healthcare providers on the nutritional management of patients on haemodialysis in Australia: An interview study. BMJ Open 8: e020023, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JT, Warner MM, Conley M, Reidlinger DP, Hoffmann T, Craig J, Tong A, Reeves M, Johnson DW, Palmer S, Campbell KL: Feasibility and acceptability of telehealth coaching to promote healthy eating in chronic kidney disease: A mixed-methods process evaluation. BMJ Open 9: e024551, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S: Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 348: g1687, 2014 [DOI] [PubMed] [Google Scholar]
- 17.National Health and Medical Research Council: Australian Dietary Guidelines, Canberra, Australia, Department of Health and Ageing, Commonwealth of Australia, 2013 [Google Scholar]
- 18.Whitlock EP, Orleans CT, Pender N, Allan J: Evaluating primary care behavioral counseling interventions: An evidence-based approach. Am J Prev Med 22: 267–284, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Chan M, Johnson D: Modification of Lifestyle and Nutrition Interventions for Management of Early Chronic Kidney Disease, 2013. Available at: http://www.cari.org.au/CKD/CKD%20early/Modification_of_Llifestyle_Nutrition_ECKD.pdf. Accessed February 2019
- 20.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC: Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 142: 1009–1018, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins CE, Boggess MM, Watson JF, Guest M, Duncanson K, Pezdirc K, Rollo M, Hutchesson MJ, Burrows TL: Reproducibility and comparative validity of a food frequency questionnaire for Australian adults. Clin Nutr 33: 906–914, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research: Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45: 142–161, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Hawthorne G, Richardson J, Osborne R: The Assessment of Quality of Life (AQoL) instrument: A psychometric measure of health-related quality of life. Qual Life Res 8: 209–224, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Musso CG: Potassium metabolism in patients with chronic kidney disease (CKD), Part I: Patients not on dialysis (stages 3-4). Int Urol Nephrol 36: 465–468, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Keith NM, Osterberg AE: The tolerance for potassium in severe renal in-sufficiency: A study of ten cases. J Clin Invest 26: 773–783, 1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Australian New Zealand Clinical Trials Registry (ANZCTR) : The Evaluation of Individualized Telehealth Intensive Coaching to Promote Healthy Eating and Lifestyle in Chronic Kidney Disease (ACTRN12616001212448), 2016. Available at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371359&showOriginal=true&isReview=true. Accessed February 2019
- 27.Lancaster GA, Dodd S, Williamson PR: Design and analysis of pilot studies: Recommendations for good practice. J Eval Clin Pract 10: 307–312, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB: Association of changes in diet quality with total and cause-specific mortality. N Engl J Med 377: 143–153, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth A, Griffin M, Yusuf S, Mann JF, Reddan D, Canavan M, Newell J, O’Donnell M: Diet and major renal outcomes: A prospective cohort study. The NIH-AARP diet and health study. J Ren Nutr 26: 288–298, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Wirt A, Collins CE: Diet quality--what is it and does it matter? Public Health Nutr 12: 2473–2492, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Goraya N, Simoni J, Jo C-H, Wesson DE: A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8: 371–381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE: Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol 49: 438–448, 2019 [DOI] [PubMed] [Google Scholar]
- 33.National Health and Medical Research Council: Nutrient Reference Values for Australia and New Zealand, Canberra, Australia, Australian Government Department of Health and Ageing, New Zealand Ministry of Health, National Health and Medical Research Council, 2006 [Google Scholar]
- 34.Chiavaroli L, Mirrahimi A, Sievenpiper JL, Jenkins DJA, Darling PB: Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 69: 761–768, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S: High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81: 300–306, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal R: Resistant hypertension and the neglected antihypertensive: Sodium restriction. Nephrol Dial Transplant 27: 4041–4045, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Ikizler TA, Robinson-Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, Evans EE, Milch CM, Moody KA, Germain M, Limkunakul C, Bian A, Stewart TG, Himmelfarb J: Metabolic effects of diet and Exercise in patients with moderate to severe CKD: A randomized clinical trial. J Am Soc Nephrol 29: 250–259, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH: Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Neprol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson RE, Haines PS, Popkin BM: Health lifestyle patterns of U.S. adults. Prev Med 23: 453–460, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.