Abstract
IMPORTANCE
Considerable efforts have been undertaken to relate single nutrients to bone health. To this point, results are inconsistent. Suboptimal single nutrient intake does not occur in isolation but rather reflects a poor diet quality.
OBJECTIVE
To assess the association between adherence to a diet quality index constructed on the basis of dietary recommendations or existing healthy dietary patterns and fractures in postmenopausal women.
DESIGN, SETTING, AND PARTICIPANTS
Post hoc analysis was conducted of longitudinal data from 40 clinical centers throughout the United States included in the Women’s Health Initiative (WHI) observational study. Participants in the prospective cohort included 93 676 women who were eligible for the WHI if they were aged 50 to 79 years. Recruitment was conducted from October 1, 1993, to December 31, 1998, with the study ending August 29, 2014. The WHI food frequency questionnaire was used to derive nutrient and food intake at baseline. Diet quality and adherence were assessed by scores on the alternate Mediterranean Diet (aMED), a 9-category measure of adherence to a Mediterranean dietary pattern; the Healthy Eating Index 2010 (HEI-2010), a 100-point measure of 12 food components; the 11-item Alternate Healthy Eating Index 2010 (AHEI-2010); or the 8-component Dietary Approaches to Stop Hypertension (DASH) diet score.
MAIN OUTCOMES AND MEASURES
Outcome measures included incident total and hip fractures. Hazard ratios (HRs) by quintiles of dietary index scores were estimated using Cox proportional hazards regression analyses.
RESULTS
Of the 93 676 participants, 90 014 were included in the analysis (mean [SD] age, 63.6 [7.4]) years. During a median follow-up time of 15.9 years, there were 2121 cases of hip fractures and 28 718 cases of total fractures. Women scoring in the highest quintile (Q5) of the aMED index had a lower risk for hip fractures (HR, 0.80; 95% CI, 0.66–0.97), with an absolute risk reduction of 0.29% and a number needed to treat of 342 (95% CI, 249–502). No association between the aMED score and total fractures was observed (Q5 HR, 1.01; 95% CI, 0.95–1.07). Higher HEI-2010 or DASH scores tended to be inversely related to hip fracture risk, but the results were nonsignificant (Q5 HR, 0.87; 95% CI, 0.75–1.02; and Q5 HR, 0.89; 95% CI, 0.75–1.06, respectively). The AHEI-2010 score was associated with neither hip nor total fractures.
CONCLUSIONS AND RELEVANCE
Higher adherence to a Mediterranean diet is associated with a lower risk for hip fractures. These results support that a healthy dietary pattern may play a role in maintaining bone health in postmenopausal women.
Osteoporotic fractures constitute a major burden for health care systems in aging societies. Although considerable research1–6 has examined whether intake of nutrients involved in bone metabolism, such as protein, calcium, or unsaturated fat, can prevent fracture events, the findings are not consistent. However, suboptimal single nutrient intake does not occur in isolation but rather reflects a poor-quality diet.7
Several descriptive epidemiologic studies8–10 have shown that the incidence of osteoporosis and osteoporosis-related fractures varies across nations, with a tendency of lower rates in Mediterranean compared with northern European countries. These differences have been attributed to life-style factors, including specific dietary patterns. The traditional Mediterranean-style diet emphasizes the consumption of dietary components, such as plant foods, fish, nuts, and monounsaturated fat, which have been shown11,12 to impart beneficial effects on bone health. Adherence to a Mediterranean diet was previously operationalized by a dietary scoring system and modified to be applied to non-Mediterranean populations.13 This Mediterranean diet score has been associated with a decreased hip fracture risk, particularly among men,14 but overall evidence is inconclusive.15 Moreover, data are sparse as to whether other dietary scoring systems that characterize a high-quality diet preserve bone health.16 Comprehensive analyses investigating the association between various commonly recommended dietary quality indexes and fracture risk in the United States are warranted.
The primary aim of this study was to examine the association between adherence to a diet quality index constructed on the basis of dietary recommendations or existing healthy dietary patterns and bone outcomes (hip or total fractures) in a large population of postmenopausal women. Specifically, diet quality was assessed using the alternate Mediterranean Diet (aMED) score,13,17 the Healthy Eating Index 2010 (HEI-2010),18 the Alternate Healthy Eating Index 2010 (AHEI-2010),19,20 or the Dietary Approaches to Stop Hypertension (DASH) score.21 As a secondary aim, the associations between diet quality, bone mineral density (BMD), and lean body mass measurements were examined. Given prior epidemiologic data14,16 and the composition of the aMED index, we hypothesized that high aMED scoring would be associated with a lower fracture risk.
Methods
Study Population
The study population consisted of 93 676 women enrolled in the Women’s Health Initiative observational study (WHI-OS) (clinicaltrials.gov, ).22–25 The WHI-OS24 examined the indicators and natural history of important causes of morbidity and mortality in postmenopausal women. Women were recruited from October 1, 1993, to December 31, 1998, at 40 clinical centers in the United States and were eligible for the WHI-OS if they were aged 50 to 79 years, were generally healthy, and were postmenopausal at the time of enrollment. Institutional review boards at participating institutions approved all protocols, and all participants provided written informed consent.
For the present analysis, data from women with extreme energy intake (ie, <600 kcal/d or >5000 kcal/d) were excluded because these reported intakes were judged to be implausible (n = 3662).26 Our final study population included 90 014 women who were monitored through August 29, 2014, with a median follow-up time of 15.9 years (Figure).
Figure. Study Inclusion Criteria.
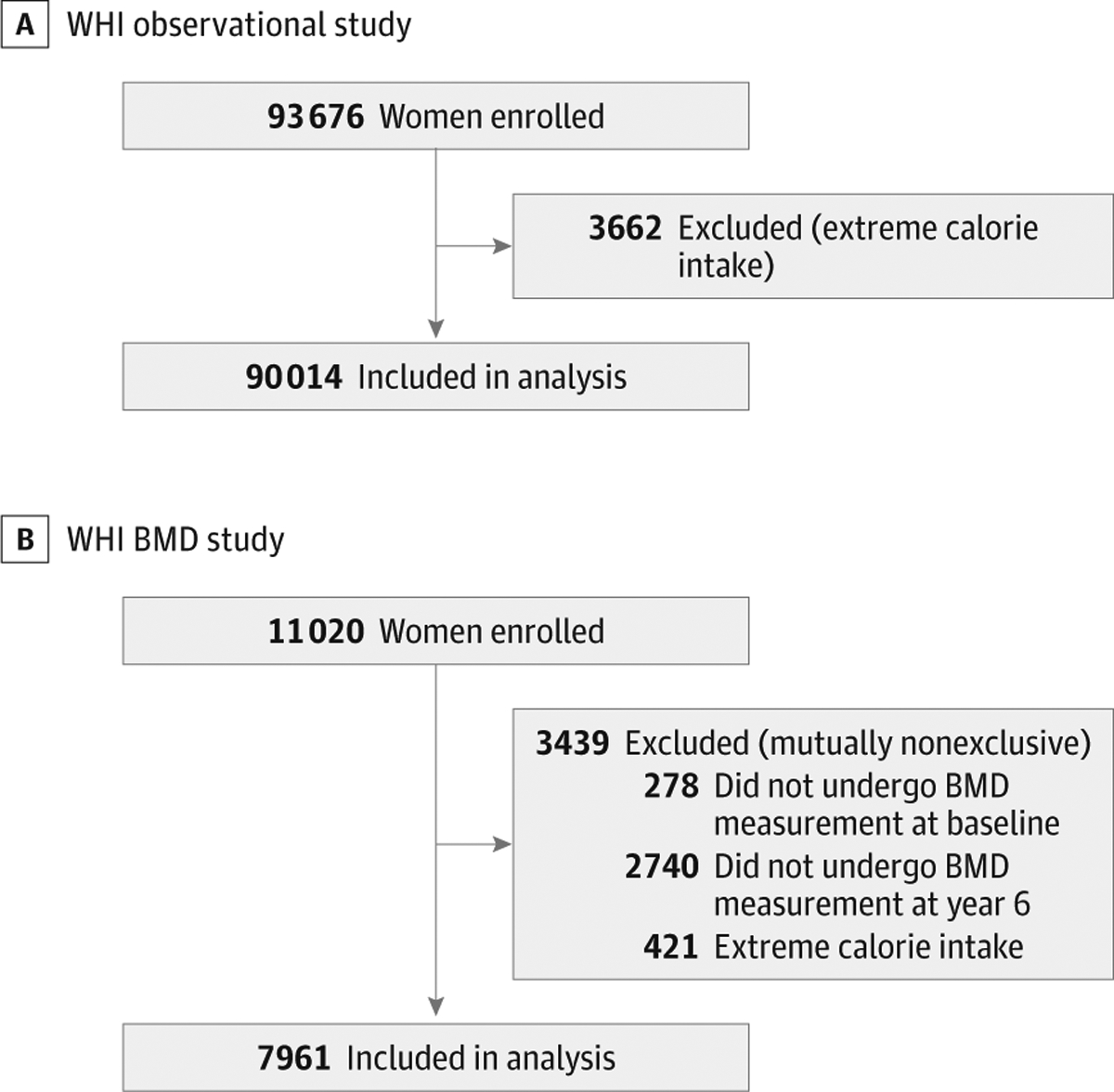
A, Women’s Health Initiative (WHI) observational study; B, WHI bone mineral density (BMD) study.
Food Frequency Questionnaires
Nutrient and food intake was derived from self-report through WHI food frequency questionnaires (WHI-FFQs) at baseline.26 The WHI-FFQ is based on the Block FFQ.26,27 The main differences between the measures are the addition of questions to make the WHI-FFQ more sensitive to fat intake (including low-fat food preparation methods and reduced-fat foods) and fruit and vegetable consumption.26 The WHI-FFQ nutrient database was derived from the University of Minnesota Nutrition Coordinating Center food and nutrient database.28 The WHI-FFQ has demonstrated good validity as a measurement of dietary intake compared with 24-hour dietary recalls and food records.26 The WHI-FFQ has also been validated against biomarkers of nutrients important to bone health, including protein and polyunsaturated fatty acids.29,30
Assessment of Dietary Patterns
Based on nutrient and food items intake, dietary indexes (aMED, HEI-2010, AHEI-2010, and DASH) were used to assess the extent of adherence to various dietary patterns.13,18,19,31–33 Food items were transformed into standardized quantities with the help of the MyPyramid Equivalents Database.32,34
The aMED score was designed to assess adherence to a Mediterranean dietary pattern. Total aMED scoring ranges from 0 (nonadherence) to 9 (perfect adherence). It includes the following food items13,17,32,33: (1) fruits, (2) vegetables, (3) nuts, (4) legumes, (5) whole grains, (6) fish, (7) ratio of monounsaturated to saturated fat, (8) red and processed meats, and (9) alcohol. Participants whose intake was above the median for fruits, vegetables, nuts, legumes, whole grains, fish, or ratio of monounsaturated to saturated fat received 1 point for each category. Consumption of red and processed meat below the median was awarded 1 point, and alcohol intake between 5 and 15 g/d was awarded 1 point; otherwise, women received 0 points.
The HEI-2010 aligns with the US Dietary Guidelines for Americans of 2010, and scores range from 0 (nonadherence) to 100 (perfect adherence) points.18,32 The HEI-2010 consists of 12 components as previously outlined: 6 components—total vegetables, total fruit, whole fruit, seafood proteins, plant proteins, and total protein foods—are worth 0 to 5 points each; 5 components—whole grains, low-fat dairy, fatty acids ratio ([polyunsaturated fatty acids plus monounsaturated fatty acids] to saturated fatty acids), refined grains, and sodium—are worth 0 to 10 points each; and 1 component—empty calories (energy from solid fats, added sugars, and any alcohol in excess of 13 g per 1000 kcal)—are worth 0 to 20 points. All food components except for the fatty acids ratio are scored on a density basis (per 1000 kcal or as a percentage of energy). Three components (sodium, refined grains, and empty calories) are reverse scored (ie, higher intakes receive lower scores).18
The AHEI-2010 was designed as an alternative to the HEI-2010, and scoring can range from 0 (nonadherence) to 110 (perfect adherence).19,20,31,32 The AHEI-2010 includes 11 items, and each component intake is evaluated from 0 (worst) to 10 (best). The AHEI-2010 emphasizes vegetables, fruits, whole grains, nuts, legumes, vegetable proteins, long-chain ω−3 polyunsaturated fatty acids, polyunsaturated fatty acids (excluding long-chain ω−3 polyunsaturated fatty acids), moderate alcohol intake, and lower intakes of sugar-sweetened beverages and fruit juice, red and processed meats, and sodium as well as avoidance of trans-fat.
The DASH diet score considers intake of (1) fruits, (2) vegetables, (3) nuts and legumes, (4) low-fat dairy, (5) whole grains, (6) sodium, (7) sweetened beverages, and (8) red and processed meats.21,32,33 The score is based on quintile rankings within the population. For fruits, vegetables, nuts and legumes, low-fat dairy, and whole grains, participants in the highest quintile receive a score of 5, those in the second-highest quintile receive a score of 4, and so on. For sodium, sweetened beverages, and red and processed meats, scoring is reversed (ie, women in the highest quintile receive a minimum score of 1, whereas participants in the lowest quintile receive a maximum score of 5). The score for each component is summed, and the overall score ranges from 8 (no adherence) to 40 (perfect adherence).
Outcome Ascertainment
Primary outcome measures included incident hip and total fractures. In the WHI-OS, all fracture outcomes were self-reported except hip fractures, which were assigned a diagnosis by local trained physician adjudicators and centrally confirmed by a second medical record review.35,36 Toe, finger, sternum, and clavicle fractures as total fracture events were excluded since these fractures are less likely related to osteoporosis.37,38
Bone mineral density at the femoral neck (hip) and total body as well as lean body mass were measured at baseline and after 6 years in a subset of WHI participants (WHI-BMD cohort [n = 11 020]) at 3 of the 40 clinical centers of the WHI (Pittsburgh, Pennsylvania; Birmingham, Alabama; and Phoenix and Tucson, Arizona) with dual-energy x-ray absorptiometry (Hologic QDR densitometer; Hologic Inc).39,40 Of the 11 020 participants in the WHI-BMD cohort, 278 women did not undergo BMD measurements at baseline, whereas 2740 were not measured at the 6-year follow-up visit. A total of 421 women were excluded owing to extreme energy intake. The final sample size for the BMD analysis was 7961 participants (Figure). Exclusion criteria were not mutually exclusive.
Covariate Assessment
Information on age, race/ethnicity, educational level, family income, personal history of fracture, history of falls, self-rated health, and smoking status was obtained by self-report questionnaires at baseline.22,24 Physical function was assessed by a 36-item Short-Form Health Survey.41,42 Current medication use was assessed by clinic interviewers.22,24 History of cardiovascular disease was coded as positive if the participant reported a history of myocardial infarction, angina pectoris, atrial fibrillation, heart failure, peripheral vascular disease, coronary bypass surgery, angioplasty, or carotid endarterectomy. Women were classified as having diabetes mellitus on the basis of self-report of diabetes or self-report of diabetes treatment. For each participant, the number of self-reported chronic medical conditions (ie, stroke, any cancer, history of cardiovascular disease, arthritis, hypertension, diabetes, and emphysema) and the number of psychoactive medications (ie, anxiolytics, hypnotics, antidepressants, antipsychotics, and antiepileptic agents) was calculated.
Statistical Analysis
To assess the associations of aMED, HEI-2010, AHEI-2010, and DASH with incident fractures, quintiles of each exposure variable of interest were formed based on the distribution of non-cases in the WHI-OS cohort. Hazard ratios (HRs) for the risk of hip and total fractures by quintiles of dietary pattern scores were obtained using covariate-adjusted Cox proportional hazards regression models. Incident time to event was defined as the time from enrollment to the first occurrence of an incident hip fracture and a fracture at any anatomic site. Follow-up was censored at the date of the outcome event, end of follow-up, or date of death, whichever came first. Potential confounding was addressed by adjusting for linear age, race/ethnicity, body mass index, smoking, physical activity, self-reported health, treated diabetes mellitus, history of fracture when younger than 55, physical function score, number of chronic medical conditions, number of psychoactive medications, use of menopausal hormone therapy, and use of bisphosphonates, calcitonin, or selective-estrogen receptor modulators at baseline. The proportional hazards regression assumption was found to be valid for all analyses. With use of data from the WHI-BMD cohort, general linear regression models were applied to examine the associations of dietary scoring indexes with BMD and lean body mass at baseline and year 6 with multivariable adjustment as described above.
Statistical analyses were performed using SAS, version 9.3 (SAS Institute Inc). A 2-sided t test value of P < .05 was considered statistically significant.
Results
Baseline characteristics of the study participants and BMD measurements by lowest and highest quintiles of the various dietary scores are reported in Table 1 and Table 2. Women scoring in the highest quintile were more likely to be older and white, to have a high physical function score, and to have less than 1 chronic medical condition; they were also more likely to be physically active and to have a lower body mass index. Women in the highest quintile were additionally less likely to have never used menopausal hormone therapy and slightly more likely to have used bisphosphonates. Total body and hip BMD values were slightly higher in women in the highest quintiles.
Table 1.
Baseline Characteristics by Lowest and Highest Quintiles (Qs) of Dietary Pattern Scoring in the WHI Health Observational Study
| Dietary Pattern, No. (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|
| aMED | HEI-2010 | AHEI-2010 | DASH | |||||
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| Participantsb | 6545 (7.3) | 20819 (23.1) | 17 584 (19.5) | 18 409 (20.5) | 16 517 (18.3) | 21047 (23.4) | 13 365 (14.8) | 20 499 (22.8) |
| Age, y | ||||||||
| 50–59 | 2230 (34.1) | 6381 (30.7) | 6758 (38.4) | 4534 (24.6) | 5820 (35.2) | 6391 (30.4) | 5243 (39.2) | 5693 (27.8) |
| 60–69 | 2819 (43.1) | 9355 (44.9) | 7483 (42.6) | 8319 (45.2) | 7074 (42.8) | 9411 (44.7) | 5638 (42.2) | 9105 (44.4) |
| 70–79 | 1496 (22.9) | 5083 (24.4) | 3343 (19.0) | 5556 (30.2) | 3623 (21.9) | 5245 (24.9) | 2484(18.6) | 5701 (27.8) |
| Race/ethnicity | ||||||||
| White | 5431 (83.0) | 18 140 (87.1) | 13 996 (79.6) | 15 877 (86.3) | 12 947 (78.4) | 18 693 (88.8) | 9793 (73.3) | 18 255 (89.1) |
| African American | 584 (8.9) | 1145 (5.5) | 1866 (10.6) | 1242 (6.8) | 2041 (12.4) | 901 (4.3) | 2019 (15.1) | 886 (4.3) |
| Hispanic | 307 (4.7) | 410 (2.0) | 1046 (6.0) | 352 (1.9) | 937 (5.7) | 370 (1.8) | 950 (7.1) | 387 (1.9) |
| Other | 223 (3.4) | 1124 (5.4) | 676 (3.8) | 938 (5.1) | 592 (3.6) | 1083 (5.2) | 603 (4.5) | 971 (4.7) |
| BMI≥30 | 2161 (33.4) | 3752 (18.3) | 5995 (34.5) | 3178 (17.5) | 6107 (37.4) | 2971 (14.3) | 5037 (38.2) | 3232 (16.0) |
| Smoking, never | 3199 (49.6) | 10492 (51.1) | 7683 (4.4) | 10 214 (56.2) | 8670 (53.2) | 9590 (46.2) | 6203 (47.1) | 10 835 (53.5) |
| Physical activity, ≥19 METs | 922 (14.2) | 7689 (37.3) | 2889 (16.6) | 6500 (35.7) | 2120 (13.0) | 8525 (40.9) | 1825 (13.8) | 7963 (39.3) |
| Self-reported health, excellent | 807 (12.4) | 4671 (22.6) | 2443 (14.0) | 3840 (21.0) | 1771 (10.8) | 5399 (25.8) | 1504 (11.3) | 4866 (23.9) |
| Diabetes mellitus, treated | 280 (4.3) | 647 (3.1) | 652 (3.7) | 759 (4.1) | 889 (5.4) | 526 (2.5) | 659 (4.9) | 707 (3.4) |
| Menopausal hormone therapy use, never | 2969 (45.4) | 7782 (37.4) | 7855 (44.7) | 7214 (39.2) | 7548 (45.7) | 7670 (36.5) | 6262 (46.9) | 7706 (37.6) |
| No. of psychoactive medications, <1c | 5571 (85.1) | 18 582 (89.3) | 15 016 (85.4) | 16 405 (89.1) | 13 937 (84.4) | 18 885 (89.7) | 11485 (85.9) | 18 201 (88.8) |
| No. of chronic medical conditions, <1d | 2731 (41.7) | 10 162 (48.8) | 7578 (43.1) | 8397 (45.6) | 6305 (38.2) | 10841 (51.5) | 5248 (39.3) | 10184 (49.7) |
| History of fracture at age ≥55 y | 824 (16.2) | 2951 (17.7) | 2096 (16.0) | 2779 (18.2) | 2038 (16.0) | 3043 (18.2) | 1510 (15.1) | 2999 (18.0) |
| Physicalfunction, SF-36 score >90 | 1868 (29.2) | 9219 (45.0) | 5459 (31.7) | 7690 (42.6) | 4406 (27.3) | 10 046 (48.5) | 3775 (28.9) | 9108 (45.2) |
| Bisphosphonate use | 127 (1.9) | 592 (2.8) | 303 (1.7) | 619 (3.4) | 284 (1.7) | 665 (3.2) | 216 (1.6) | 656 (3.2) |
| Calcitonin use | 22 (0.3) | 91 (0.4) | 56 (0.3) | 87 (0.5) | 50 (0.3) | 91 (0.4) | 34(0.3) | 97 (0.5) |
| Selective estrogen receptor modulator use | 0 | 8 (0.04) | 4(0.02) | 7 (0.04) | 5 (0.03) | 12 (0.06) | 5 (0.04) | 10 (0.05) |
| Alcohol intake, mean (SD), g/d | 5(12) | 6(10) | 9(17) | 3(6) | 4(12) | 7(9) | 6(13) | 5 (9) |
| Total energy intake, mean (SD), kcal | 1332 (507) | 1804 (598) | 1782 (742) | 1428 (469) | 1574 (628) | 1567 (538) | 1657 (653) | 1590 (530) |
Abbreviations: AHEI-2010, Alternate Healthy Eating Index 201019,20; aMED, alternate Mediterranean Diet13,17; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DASH, Dietary Approaches to Stop Hypertension21; HEI-2010, Healthy Eating Index 201018; METs, metabolic equivalent of tasks; SF-36, 36-item Short-Form Health Survey41,42; WHI, Women’s Health Initiative.
Q1 represents the least healthy quintile; Q5, the healthiest quintile.
Not all data were available on some characteristics.
Medications included anxiolytics, hypnotics, antidepressants, antipsychotics, and antiepileptic agents.
Conditions included treated diabetes mellitus, stroke, any cancer, history of cardiovascular disease, arthritis, hypertension, and emphysema.
Table 2.
Baseline BMD and Body Composition Measurements by Lowest and Highest Quintiles (Qs) of Dietary Pattern Scoring in the WHI BMD Study
| Dietary Patterna | ||||||||
|---|---|---|---|---|---|---|---|---|
| aMED | HEI-2010 | AHEI-2010 | DASH | |||||
| Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | Q1 | Q5 | |
| Participants, No. (%) | 1534 (19.3) | 1840 (23.1) | 1565 (19.7) | 1653 (20.8) | 1473 (18.5) | 1747 (21.9) | 1199 (15.1) | 1801 (22.6) |
| BMD of the total hip, corrected, mean (SE), g/cm2b | 0.85 (0) | 0.86 (0) | 0.85 (0) | 0.87 (0) | 0.85 (0) | 0.86 (0) | 0.85 (0) | 0.86 (0) |
| Hip T score, No. (%) | ||||||||
| Normal BMD, T score ≥−1.0 | 909 (59.3) | 1015 (55.2) | 957 (61.2) | 887 (53.7) | 916 (62.2) | 901 (51.6) | 786 (65.6) | 923 (51.2) |
| Low BMD, −2.5 ≤ T score < −1.0 | 567 (37.0) | 739 (40.2) | 552 (35.3) | 665 (40.2) | 510 (34.6) | 750 (42.9) | 381 (31.8) | 770 (42.8) |
| Osteoporosis, T score <−2.5 | 58 (3.8.) | 86 (4.7) | 56 (3.6) | 101 (6.1) | 47 (3.2) | 96 (5.5) | 32 (2.7) | 108 (6.0) |
| Whole-body BMD, corrected, mean (SE), g/cm2b | 1.01 (0) | 1.03 (0) | 1.01 (0) | 1.03 (0) | 1.00 (0) | 1.03 (0) | 1.01 (0) | 1.03 (0) |
| Lean mass, mean (SE), kg | 37.36 (0.10) | 38.24 (0.09) | 37.48 (0.10) | 38.06 (0.10) | 37.32 (0.11) | 38.33 (0.10) | 37.61 (0.12) | 38.22 (0.10) |
| Lean mass, mean (SE), % | 54.68 (0.12) | 55.67 (0.11) | 54.73 (0.12) | 55.53 (0.12) | 54.57 (0.13) | 55.94 (0.12) | 54.74 (0.14) | 55.80 (0.12) |
Abbreviations: AHEI-2010, Alternate Healthy Eating Index 201019; aMED, alternate Mediterranean Diet13,17; BMD, bone mineral density; DASH, Dietary Approaches to Stop Hypertension21; HEI-2010, Healthy Eating Index 201018; WHI, Women’s Health Initiative.
Q1 represents the least healthy quintile; Q5, the healthiest quintile.
Adjusted for age, race/ethnicity, body mass index, and WHI clinical trial assignment.
During a median follow-up period of 15.9 years, WHI-OS documented 2121 cases of hip fractures and 28 718 cases of self-reported total fractures. The multivariate-adjusted HRs for incident hip fractures or total fractures by quintiles of dietary pattern scores are presented in Table 3. After controlling for confounding variables, women scoring in the highest quintile (Q5), reported as HR (95% CI), of aMED were at a lower risk for hip fractures (0.80 [0.66–0.97]), with an absolute risk reduction of 0.29% and a number needed to treat of 342 (95% CI, 249–502). No association between aMED and total fractures was observed (Q5 HR, 1.01 [0.95–1.07]). Higher HEI-2010 or DASH scoring tended to be inversely related to hip fracture risk (Q5 HR, 0.87 [0.75–1.02] and 0.89 [0.75–1.06], respectively), but the results were nonsignificant. No association between HEI-2010, DASH, and total fracture risk (Q5 HR, 0.98 [0.93–1.02] and 0.98 [0.94–1.03]), respectively, was found. Scores within the highest quintile of AHEI-2010 were not significantly associated with hip or total fractures (Q5 HR, 0.94 [0.80–1.09] and 1.01 [0.96–1.05], respectively). To account for the propensity to fall, the fall history was further included in our statistical modeling (eTable 1 in the Supplement); the main results did not change.
Table 3.
HRs for Hip or Total Fractures by Quintiles (Qs) of Dietary Pattern Scores in the WHI Observational Study
| Hip Fracture | Total Fracture | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Individuals | No. of Cases | Mean Follow-up, y | HR (95% CI)b | No. of Cases | Mean Follow-up, y | HR (95% CI)b | ||
| aMED | ||||||||
| Q1 | <2 | 6545 | 177 | 12.35 | 1 [Reference] | 1960 | 10.61 | 1 [Reference] |
| Q2 | 2–4 | 27 984 | 680 | 12.78 | 0.84 (0.70–1.01) | 8694 | 10.91 | 1.01 (0.96–1.07) |
| Q3 | 4–5 | 18050 | 396 | 13.15 | 0.77 (0.63–0.94) | 5714 | 11.25 | 0.99 (0.93–1.05) |
| Q4 | 5–6 | 16616 | 366 | 13.39 | 0.76 (0.62–0.92) | 5336 | 11.43 | 0.98 (0.92–1.04) |
| Q5 | >6 | 20819 | 502 | 13.78 | 0.80 (0.66–0.97) | 7014 | 11.65 | 1.01 (0.95–1.07) |
| HEI-2010 | ||||||||
| Q1 | <53 | 17 584 | 405 | 12.64 | 1 [Reference] | 5328 | 10.82 | 1 [Reference] |
| Q2 | 53–60 | 17871 | 406 | 13.06 | 0.93 (0.80–1.08) | 5617 | 11.14 | 0.98 (0.94–1.02) |
| Q3 | 60–66 | 18 083 | 419 | 13.28 | 0.84 (0.72–0.99) | 5826 | 11.32 | 0.97 (0.93–1.01) |
| Q4 | 66–72 | 18 067 | 392 | 13.40 | 0.79 (0.67–0.92) | 5796 | 11.44 | 0.95 (0.91–0.99) |
| Q5 | >72 | 18 409 | 499 | 13.43 | 0.87 (0.75–1.02) | 6151 | 11.39 | 0.98 (0.93–1.02) |
| AHEI-2010 | ||||||||
| Q1 | <47 | 16517 | 381 | 12.25 | 1 [Reference] | 4862 | 10.51 | 1 [Reference] |
| Q2 | 47–53 | 15 997 | 392 | 12.79 | 0.99 (0.85–1.16) | 4975 | 10.95 | 0.98 (0.94–1.03) |
| Q3 | 53–59 | 18 892 | 440 | 13.11 | 0.94 (0.80–1.09) | 5936 | 11.23 | 0.97 (0.93–1.01) |
| Q4 | 59–65 | 17561 | 399 | 13.60 | 0.92 (0.78–1.08) | 5733 | 11.56 | 0.99 (0.95–1.04) |
| Q5 | >65 | 21 047 | 509 | 13.86 | 0.94 (0.80–1.09) | 7212 | 11.70 | 1.01 (0.96–1.05) |
| DASH | ||||||||
| Q1 | <20 | 13 365 | 277 | 12.26 | 1 [Reference] | 3834 | 10.53 | 1 [Reference] |
| Q2 | 20–23 | 18 704 | 456 | 12.84 | 1.02 (0.86–1.20) | 5814 | 10.98 | 0.97 (0.92–1.01) |
| Q3 | 23–25 | 15 441 | 350 | 13.24 | 0.85 (0.71–1.02) | 4933 | 11.29 | 0.95 (0.91–1.00) |
| Q4 | 25–28 | 22 005 | 504 | 13.46 | 0.85 (0.72–1.00) | 7098 | 11.48 | 0.94 (0.89–0.98) |
| Q5 | >28 | 20 499 | 534 | 13.69 | 0.89 (0.75–1.06) | 7039 | 11.57 | 0.98 (0.94–1.03) |
Abbreviations: AHEI-2010, Alternate Healthy Eating Index 201019; aMED, alternate Mediterranean Diet13,17; DASH, Dietary Approaches to Stop Hypertension21; HEI-2010, Healthy Eating Index 201018; HR, hazard ratio; WHI, Women’s Health Initiative.
Q1 represents the least healthy quintile; Q5, the healthiest quintile.
Adjusted for age, race/ethnicity, body mass index, smoking status, physical activity, self-reported health, diabetes mellitus status, history of fracture at 55 years or older, physical function score, number of chronic medical conditions, number of psychoactive medications, and use of hormone therapy, bisphosphonates, calcitonin, and selective estrogen receptor modulators.
The BMD and lean body mass measurements at baseline and year 6 by quintiles of dietary pattern scoring are presented in eTable 2 and eTable 3, respectively, in the Supplement. No clinically significant differences in BMD loss and no clinically significant changes of lean body mass over time were found.
Discussion
The primary aim of this study was to investigate the association between adherence to a diet quality index and fracture risk in a large sample of postmenopausal women. Women who were highly adherent to a Mediterranean dietary pattern (aMED) that emphasizes the consumption of fruits, vegetables, fish, nuts, legumes, and whole grains; intake of monounsaturated fat; and avoidance of red and processed meats were found to have a lower risk for hip fracture, but the absolute risk reduction was small.
At this time, epidemiologic evidence8–10 suggests that fracture rates vary geographically. Lifestyle differences, including diet quality, may be part of an explanation for regional and local discrepancies. Previous research16 on diet quality scores based on case-control data in a Chinese population suggests that avoiding a low-quality diet is associated with a lower risk of hip fractures in elderly individuals and that the aMED score appears to be the best scoring system for consumers because of its simplicity. The aMED, HEI-2010, AHEI-2010, and DASH dietary measures have many similarities since all dietary patterns include vegetables, fruits, vegetable protein, and whole grains, but there are also distinctive differences.13,18,19,21 Whereas the AHEI-2010 emphasizes low intake of red and processed meats and high intake of polyunsaturated fatty acids, the aMED promotes intake of monounsaturated fat, largely from olive oil, and fish intake. Similarly, the HEI-2010 includes an increased emphasis on seafood and plant proteins.18 Both plant proteins and unsaturated fatty acids have been shown4,6 to be beneficial for bone health. However, aMED does not limit sodium intake as do the HEI-2010, AHEI-2010, or, specifically, the DASH diet. Data from randomized clinical trials43 suggest that adherence to the DASH diet lowers bone turnover and imparts beneficial effects on bone health.
Our data support an association between the extent of adherence to a healthy diet characterized by adherence to a Mediterranean diet and lower fracture risk. However, given the apparent risk reductions across various dietary patterns, a specific dietary index may not be associated with lower risk; rather, high diet quality reflected by various dietary indexes and their common components may achieve a lower risk. The lack of an association with total fractures may be explained by the wide heterogeneity of fracture types in our analyses. However, because the propensity to fall is a major risk factor for fractures in the senior population, additional analyses accounting for fall history were undertaken—the main results did not change. Finally, since diet may also relate to muscle mass and BMD and thereby prevent fractures, we further investigated whether higher diet quality was associated with greater lean body mass or less loss of BMD. No clinically significant changes over time were observed.
Strengths of our analysis include a large, well-characterized study cohort with long-term follow-up and adjudicated hip fracture outcome events. Conversely, there are several limitations. First, because our study included only postmenopausal women in overall good health, external validity may be limited and residual confounding may explain parts of the findings since risk reduction was small. Our data showed marked differences in fracture risk across quintiles of multiple indicators of fractures; these differences may suggest the possibility of other confounding factors. Second, assessment of dietary patterns was based on indexes that operationalize various food items derived from FFQs at baseline. Exposure variability is therefore limited. Moreover, assessment of certain nutrients, such as sodium or potassium, with FFQs is problematic.44,45 Finally, outcome events on fractures other than the hip were self-reported, and misclassification bias may be present. However, previous data from WHI36 show that agreements between self-reports for single-site fractures and medical records were generally high; thus, this bias can be considered as low.
Conclusions
High diet quality characterized by adherence to a Mediterranean diet is associated with a lower risk for hip fractures. These results support the notion that following a healthy dietary pattern may play a role in the maintenance of bone health in postmenopausal women.
Supplementary Material
Key Points.
Question
Does diet quality affect bone health in postmenopausal women?
Findings
In the Women’s Health Initiative observational study, of 90 014 postmenopausal women, higher diet quality based on a Mediterranean diet that emphasizes the consumption of fruits, vegetables, fish, nuts, legumes, whole grains, and intake of monounsaturated fat, as well as avoidance of red and processed meats, was found to be associated with a lower risk for hip fractures.
Meaning
A healthy dietary pattern may play a role in maintaining bone health in postmenopausal women.
Funding/Support:
The Women’s Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health, US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Role of the Funder/Sponsor: The WHI project office at the NHLBI, which was the sponsor of the study, had a role in the design and conduct of the study and in the collection and management of the data. The sponsor did not have a role in analysis and interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication. Review and approval of the manuscript was carried out by committees composed of WHI investigators and NHLBI representatives.
Footnotes
Conflict of Interest Disclosures: None reported.
Data Sharing Statement: Women’s Health Initiative Study data are available via the BioLINCC website of the NHLBI at https://biolincc.nhlbi.nih.gov/home/.
REFERENCES
- 1.Beasley JM, LaCroix AZ, Larson JC, et al. Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am J Clin Nutr. 2014;99 (4):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86(6):1780–1790. [DOI] [PubMed] [Google Scholar]
- 3.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(12):2504–2512. [DOI] [PubMed] [Google Scholar]
- 4.Orchard TS, Cauley JA, Frank GC, et al. Fatty acid consumption and risk of fracture in the Women’s Health Initiative. Am J Clin Nutr. 2010;92(6):1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155(7):636–644. [DOI] [PubMed] [Google Scholar]
- 6.Weikert C, Walter D, Hoffmann K, Kroke A, Bergmann MM, Boeing H. The relation between dietary protein, calcium and bone health in women: results from the EPIC-Potsdam cohort. Ann Nutr Metab. 2005;49(5):312–318. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002; 13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 8.Dhanwal DK, Cooper C, Dennison EM. Geographic variation in osteoporotic hip fracture incidence: the growing importance of Asian influences in coming decades. J Osteoporos. 2010; 2010:757102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel-H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10 (6):338–351. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C; IOF Working Group on Epidemiology and Quality of Life. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int. 2012;23(9): 2239–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6)(suppl): 1402S–1406S. [DOI] [PubMed] [Google Scholar]
- 12.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–2608. [DOI] [PubMed] [Google Scholar]
- 13.Trichopoulou A, Orfanos P, Norat T, et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ. 2005; 330(7498):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benetou V, Orfanos P, Pettersson-Kymmer U, et al. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos Int. 2013;24(5):1587–1598. [DOI] [PubMed] [Google Scholar]
- 15.Feart C, Lorrain S, Ginder Coupez V, et al. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos Int. 2013;24(12):3031–3041. [DOI] [PubMed] [Google Scholar]
- 16.Zeng FF, Xue WQ, Cao WT, et al. Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: a case-control study. Osteoporos Int. 2014;25(8):2131–2141. [DOI] [PubMed] [Google Scholar]
- 17.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119 (8):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76(6):1261–1271. [DOI] [PubMed] [Google Scholar]
- 21.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7): 713–720. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9)(suppl): S5–S17. [DOI] [PubMed] [Google Scholar]
- 23.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9) (suppl):S107–S121. [DOI] [PubMed] [Google Scholar]
- 24.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998; 19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov. Women’s Health Initiative. . https://clinicaltrials.gov/ct2/show/NCT00000611. Accessed February 13, 2016.
- 26.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9(3):178–187. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327–1335. [DOI] [PubMed] [Google Scholar]
- 28.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–1271. [PubMed] [Google Scholar]
- 29.Orchard TS, Ing SW, Lu B, et al. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the Women’s Health Initiative. J Bone Miner Res. 2013;28(3):505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prentice RL, Mossavar-Rahmani Y, Huang Y, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174(5):591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belin RJ, Greenland P, Allison M, et al. Diet quality and the risk of cardiovascular disease: the Women’s Health Initiative (WHI). Am J Clin Nutr. 2011;94(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.George SM, Ballard-Barbash R, Manson JE, et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative observational study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitan EB, Lewis CE, Tinker LF, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: the Women’s Health Initiative. Circ Heart Fail. 2013;6(6):1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman SAFJ, Mosfegh A, eds. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004. Beltsville, MD: US Dept of Agriculture; 2008. [Google Scholar]
- 35.Curb JD, McTiernan A, Heckbert SR, et al. ; WHI Morbidity and Mortality Committee. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13 (9)(suppl):S122–S128. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Kooperberg C, Pettinger MB, et al. Validity of self-report for fractures among a multiethnic cohort of postmenopausal women: results from the Women’s Health Initiative observational study and clinical trials. Menopause. 2004;11(3):264–274. [DOI] [PubMed] [Google Scholar]
- 37.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 38.Warriner AH, Patkar NM, Curtis JR, et al. Which fractures are most attributable to osteoporosis? J Clin Epidemiol. 2011;64(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck TJ, Petit MA, Wu G, LeBoff MS, Cauley JA, Chen Z. Does obesity really make the femur stronger? BMD, geometry, and fracture incidence in the Women’s Health Initiative–observational study. J Bone Miner Res. 2009;24(8):1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21(6):919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): I: conceptual framework and item selection. Med Care. 1992;30 (6):473–483. [PubMed] [Google Scholar]
- 42.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3): 217–227. [DOI] [PubMed] [Google Scholar]
- 43.Lin PH, Ginty F, Appel LJ, et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr. 2003;133(10):3130–3136. [DOI] [PubMed] [Google Scholar]
- 44.Cobb LK, Anderson CA, Elliott P, et al. ; American Heart Association Council on Lifestyle and Metabolic Health. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129(10):1173–1186. [DOI] [PubMed] [Google Scholar]
- 45.Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24-hour urinary excretion. Hypertension. 2014;63(2):238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


