Abstract
Kawasaki disease (KD) is an inflammatory disorder of young children, associated with vasculitis of the coronary arteries with subsequent aneurysm formation in up to one-third of untreated patients. Those who develop aneurysms are at life-long risk of coronary thrombosis or the development of stenotic lesions, which may lead to myocardial ischaemia, infarction or death. The incidence of KD is increasing worldwide, and in more economically developed countries, KD is now the most common cause of acquired heart disease in children. However, many clinicians in the UK are unaware of the disorder and its long-term cardiac complications, potentially leading to late diagnosis, delayed treatment and poorer outcomes. Increasing numbers of patients who suffered KD in childhood are transitioning to the care of adult services where there is significantly less awareness and experience of the condition than in paediatric services. The aim of this document is to provide guidance on the long-term management of patients who have vascular complications of KD and guidance on the emergency management of acute coronary complications. Guidance on the management of acute KD is published elsewhere.
Keywords: Kawasaki disease, lifetime cardiovascular management, coronary artery aneurysm, late sequelae, acute coronary syndrome, cardiovascular risk, person specific protocol, transitional care
Background
Kawasaki disease (KD) was first described in Japan in 1967, predominantly affects young children and has potential life-long consequences.1–4 Its incidence in children under 5 years ranges from 322/100 000 in Japan and South East Asian countries, to 4.5–25/100 000 in Europe and USA3–5 and the disease has become increasingly common in the UK.6 7 Its cause is unknown, but epidemiological observations suggest an environmental agent causing an inflammatory process in genetically predisposed individuals.8 Although the acute febrile and exanthematous illness resolves spontaneously, 30% of untreated patients develop coronary artery aneurysms (CAA).9
Treatment of the acute illness with intravenous immunoglobulin (IVIG) reduces the risk of CAA,10 and is now the standard recommended treatment.2 11 The 10%–15% of patients who are unresponsive to IVIG may be treated with corticosteroids, infliximab or other immunosuppressive agents2 and are at increased risk of CAA, as are those in whom treatment is delayed.12 13 Following an acute episode of KD, British Paediatric Surveillance Unit data suggest that 19% of children overall and 39% of those aged under 1 year, still develop coronary involvement6 despite IVIG, partly related to delayed diagnosis and treatment. Such children are at long-term risk of coronary thrombosis, acute coronary syndrome and progressive coronary stenoses.13–15 Comparably high rates of CAA have also recently been reported from Sweden, Russia, Germany and North America.16–18
Although paediatricians are familiar with acute KD, there is less awareness of its long-term consequences and management of any subsequent acute coronary syndrome, in both paediatric and adult services. To help raise awareness a guidance document was produced by NHS England London Cardiac Strategic Clinical Network in 201519 and a national NHS Patient Safety Alert in 2016.20
Methodology
A writing group was convened to obtain consensus from experts in the UK and USA, concerning the long-term management of patients who had coronary artery complications from KD. A literature search was performed and data reviewed by convened experts resulting in wide ranging consensus across the UK and USA. Clinical and other specialists were in the areas of Paediatric Cardiology (RMT/OM/JCB), Adult Cardiology (TWJ/VD/HG/JG/PM/IM), Paediatric Rheumatology (PG/DE), Paediatric infectious disease (ML), NHS England (JC/HG), Societi patient charity (RM). Face to face meetings were held to derive consensus and external expert advice sought from individuals including emergency medicine, ambulance services, patient charities and pharmacy. In addition, endorsement and/or support was obtained from the organisations of the Royal College of Paediatrics and Child Health, Royal College of Physicians, British Cardiovascular society and the Royal College of Emergency Medicine.
Cardiovascular consequences of Kawasaki disease
All cardiac tissues are involved in the acute inflammatory phase of the disease.9 Vasculitis causes destruction of the normal arterial architecture and is followed by aneurysmal dilatation, particularly affecting the proximal coronary arteries.21 22 Pathological studies in patients with previous KD reveal widespread changes23 including inflammatory cell infiltration of the arterial wall, disruption of the intima and media, intimal myofibroblastic proliferation and replacement of myocytes with fibroblasts and connective tissue. Fibrotic changes occur in the myocardium even in regions not closely related to aneurysms, probably reflecting widespread cardiac inflammation. Arterial remodelling occurs and may progress over months to several years with the development of coronary stenoses, particularly at the junction between the aneurysm and normal artery.23 Calcification is common in the aneurysmal arterial wall. Aneurysms of non-coronary arteries (axillary, ilio-femoral, renal and popliteal arteries for example and rarely in visceral and cerebral arteries) may also occur and should be considered and investigated, particularly when coronary involvement is extensive.
Serial echocardiographic studies in acute KD show that CA dilation may be visible early in the illness, but maximal development is usually in the second and third week of the acute illness.2 Those with persistent CA aneurysm, defined as a Z score≥2.5 after 6 weeks (Z score=the internal dimension of the coronary artery expressed as the number of SD units normalised for body surface area) are considered to have suffered long-term arterial damage.11
The risk of thrombotic and stenotic complications is related to aneurysm size.24 Large or giant aneurysms (≥8 mm in diameter or Z score ≥10) are the least likely to undergo resolution, and within 30 years after the initial illness are associated with up to a 50% risk of thrombotic coronary occlusion, progressive stenoses requiring revascularisation or acute coronary syndrome.25 Even though the risk of coronary events is lower in those with smaller aneurysms longer-term follow-up is still needed. Heart failure and serious arrhythmias may also occur later in life.11
Assessment of cardiovascular risk
As most episodes of acute KD occur in young children, assessment for coronary artery involvement is by serial transthoracic echocardiography, at diagnosis, 2 weeks and 6 weeks following onset of the disease, as a minimum. If abnormal, more frequent echocardiography will be required (up to twice weekly) to identify rapidly progressive coronary involvement and/or coronary thromboses. Echocardiography should be undertaken by someone appropriately trained and designated as such by the congenital cardiac network. Echocardiographic imaging is less definitive in older children or adults, for whom CT angiography or MRI may be needed.
On the basis of echocardiography, patients are classified into defined risk groups according to the 2017 American Heart Association classification,11 each requiring different follow-up (table 1). It is recognised that echocardiography of coronary arteries can be demanding particularly in very young children. However, the most common locations of aneurysms are at the bifurcation of the left main coronary artery and in the proximal right coronary artery. These areas should be clearly imaged (as an absolute minimum). If technical issues limit the examination and it is not possible to obtain adequate views of the coronary arteries or calculate Z scores, the child should be referred for a repeat examination under sedation, performed by an expert echocardiographer. It should also be noted that the measurement of infant length must be carefully executed as the Z score calculation is extremely sensitive to variation in body surface area that incorporates both height and weight. Aneurysms of peripheral arteries may occur in patients with severe coronary involvement, so imaging of other vascular territories will also be required in these cases.
Table 1.
Classification of coronary artery dilation or aneurysms (after AHA guidance with modification)11
| Classification of risk level | Description of coronary arteries | Follow-up interval | Imaging required to assess for inducible ischaemia (stress echo or stress MRI) | PSP | Regional specialist Kawasaki disease clinic |
| 1 | No involvement at any time point (Z score<2) |
2 weeks 6 weeks 6 months 12 months Discharge if normal at 12 months. |
None | No | No—annual cardiac and general health review with GP recommended* |
| 2 | Dilation only (2<Z score≤2.5): resolves within 1 year |
2 weeks 6 weeks 6 months 12 months Discharge if normal at 12 months |
None | No | No—annual cardiac and general health review with GP recommended* |
| 3 | Small aneurysm (2.5≤Z score<5): (a) current or persistent, (b) decreased to normal or Z score <2.5 |
2 weeks 6 weeks 6 months 12 months Annual review |
Coronary angiography (preferably CT) at 12 months as baseline. Consider stress imaging for inducible myocardial ischaemia every 2 years. Imaging (echo) for coronary surveillance annually |
Yes | Yes |
| 4 | Medium aneurysm (5≤Z score<10): (a) persistent aneurysm, (b) decreased to normal or Z score<2.5 |
2 weeks 6 weeks 6 months 12 months Annual review |
Coronary angiography (preferably CT) at 12 months as baseline. Consider stress imaging for inducible myocardial ischaemia annually. Imaging (echo, CT† or MRI) for coronary thrombus surveillance annually. |
Yes | Yes |
| 5 | Giant aneurysm (Z score≥10 or ≥8 mm): (a) persistent giant aneurysm, (b) persistent aneurysm (but regressed to medium or small aneurysms), (c) regressed to normal dimensions |
2 weeks 6 weeks 3 months 6 months 9 months 12 months Then every 6 months |
Coronary angiography (preferably CT) at 6–12 months as baseline. Consider stress imaging for inducible myocardial ischaemia annually. Imaging (echo, CT† or MRI) for coronary thrombus surveillance 6 monthly. |
Yes | Yes |
*GP review should include clinical examination, blood pressure measurement, general health discussion and advice on avoidance of cardiovascular risk factors and lifestyle choices—including maintaining a healthy weight, reducing risk of diabetes, avoiding smoking and taking regular exercise. This provides the opportunity to discuss any parent or patient questions and concerns.
†CT should not be used repeatedly if possible. Use MRI or ultrasound where possible, to reduce radiation exposure.
ADP, Adenine di-Phosphate; AHA, American Heart Association; FBC, Full blood count; GP, General Practioner; PSP, person-specific protocol.
Defining patient groups
Acute KD in children results in varying levels of future risk following recovery from the acute phase. This guidance has been prepared focussing on patients of risk level 3 and above (see table 1) who require lifetime specialist management within cardiology services. Patients who have had KD but are at risk levels 1 or 2 should have follow-up through their General Practitioner. See also notes in table 1.
Patients who have had KD with subsequent coronary or other arterial involvement require continued input of a clinician with expertise in KD, complementing routine postintervention care and taking account of guidance given in table 1. KD presents lifelong risks for patients irrespective of intervention on localised aneurysms. Given the low prevalence of children with CAA following KD, specific studies in this population are difficult, and posology is generally based on adult cardiology principles and some mainly retrospective studies of these drugs used in children. The drugs and doses proposed are clinically reasonable suggestions for consideration by clinicians, based on available literature evidence, and in the absence of trial data (noting that some trial data may become available in future for direct oral anticoagulants (DOACs) in KD). Many of the indications are not licensed in the paediatric population, but it is acknowledged that are no licensed alternatives and children with CAA are at risk of cardiovascular events. Thrombolysis of a coronary event in a child is rare but medically critical and it is preferable to have an indication of agents that might be considered in this situation, acknowledging that it is clear there is a lack of evidence surrounding paediatric use.
Long-term management
The major long-term risks for patients with CAA are thrombosis within the aneurysm or coronary stenosis, either of which can result in myocardial ischaemia.11 The risk of aneurysm thrombosis is greatest in the first 2 years after the acute episode of KD but persists life-long.26 Long-term management is based on prevention of thrombosis, early detection of thrombosis or stenosis when they occur, general measures to lower cardiovascular risk (such as lipid lowering, control of hypertension, smoking cessation) and support for patients and their families to pursue a healthy lifestyle. Evidence levels vary considerably, and it is explicit that there is the need for clinicians to consider evidence levels and individual benefit risk, including discussions with patients and/or their guardian.
Antithrombotic therapy
Antiplatelet agents
All patients with KD receive aspirin during the acute illness, along with gastric protection if needed. Those who have CAA (persisting after 6 weeks) should remain on long-term aspirin (see online supplementary drug appendix for all doses), including those in whom there is later remodelling of the CAA. It needs to be remembered that the switch from high-dose to low-dose aspirin is to minimise the risk of thrombosis in situ, following evidence from those who have had myocardial infarction.27 Those with giant CAA should remain on low dose aspirin (or clopidogrel) and have an anticoagulant added. Other antiplatelet medications (such as ticagrelor) have been used but on the basis of evidence derived from non-KD populations. The alternative of an antagonist of ADP mediated activation of platelet aggregation such as clopidogrel may be considered in individuals where the use of aspirin is problematic, for example, in patients requiring non-steroidal anti-inflammatory drugs (NSAIDs) for other comorbidities such as arthritis, since NSAIDs interfere with the antiplatelet effect of aspirin.11 In addition, clopidogrel (or other thienopyridine) may be added to aspirin therapy for those with large but not giant aneurysms, based on the inference from adult trials that this would be a more effective antiplatelet strategy.11
heartjnl-2019-315925supp001.pdf (241KB, pdf)
Anticoagulants
There are no randomised controlled trials of anticoagulation therapy in KD but there is a lower rate of CAA thrombosis and better outcome for patients with giant aneurysms maintained on long-term warfarin.11 Although warfarin is the most widely used anticoagulant, it is difficult to use in very young children, and a subcutaneous low molecular weight heparin (such as Enoxaparin) is preferable converting to warfarin in older children.28 29 Although clinical trials are underway, direct oral antithrombins (or DOACs) are increasingly used in adults, under expert supervision, when warfarin is felt to be inappropriate or insufficiently effective (for instance, due to failure to achieve an INR consistently in the therapeutic range or thrombus formation while on warfarin). These may become a future alternative to heparin or warfarin in children and young adults, but trials regarding safety and efficacy are currently lacking and therefore this cannot be routinely recommended at the time of writing.
Data from Japan show that the risk of thrombosis in children with giant CAA is greatest in the first 2 years after disease onset, but persists throughout life26 and a US study showed that aneurysm size was the strongest predictor of major cardiac events.18 Combined anticoagulation and antiplatelet therapy are therefore recommended for all patients with KD with a coronary artery Z score ≥10.11
Other drug therapies
Beta-blockers
Not routinely prescribed but may be appropriate for some cases of CAA when associated with myocardial ischaemia or if antiarrhythmic therapy is indicated. The current advice is for beta blockers to be used lifelong in patients with prior infarction and depressed (<40%) ejection fraction.
Statins
Although the pathology of KD differs from that of atherosclerotic heart disease, and despite a lack of definitive evidence, statins should be considered for patients with persisting CAA due to the potential benefit of their anti-inflammatory effect.30
For all the drug therapies, physicians should consult the SmPC, consider BNFc posology where available and also check for any relevant Direct Healthcare Professional Communications.
Avoidance of cardiovascular risk factors
Patients who have ever had CAA should be counselled to lower their cardiovascular risk from an early age, by measures such as eating a diet low in animal fat, taking regular and appropriate exercise, maintenance of ideal weight (reducing the risk of diabetes), avoidance of smoking and monitoring for hypertension and hyperlipidaemia.
Exercise
After the acute illness, patients with previous KD should be encouraged to undertake regular aerobic exercise which is tailored to their disease severity. Competitive sports may need to be restricted in those with giant aneurysms, and body contact sports should be avoided in patients on anticoagulants.
Psychological support
Children may suffer psychological stress adjusting to teenage and young adult life with the constant threat of an acute coronary ischaemic event. Care teams have to balance providing honest advice on the risks, and the need for urgent action in the event of changing symptoms, with the value of reassurance and helping families to live as normal a life as possible. Multidisciplinary team involvement, including access to counselling and psychological support should be part of the specialist service provided to patients with KD.
Regular clinical assessment and investigations
Table 1 suggests the frequency of cardiovascular follow-up and recommends imaging/stress testing, by level of patient risk. Patients with small or remodelled aneurysms may be seen less frequently, but those with giant aneurysms need regular imaging and assessment to detect developing thrombi within aneurysms, particularly in the early years after the acute KD illness, when the risk of thrombosis is greatest. Table 2 suggests additional tests to be undertaken during a visit, and in the transition period from paediatric to adult care, before the decision is made on the appropriate long-term follow-up regime. Each assessment should focus on evaluating the size of persisting aneurysms, the detection of thrombi within aneurysms and whether there is evidence of impaired myocardial perfusion suggesting the development of coronary stenoses.
Table 2.
Follow-up assessments
| Assessment | Each visit | Additionally at transition |
| Clinical | History Examination Medication review |
|
| ECG | 12 lead | |
| Imaging (see also table 1) |
Echocardiography | CT calcium scoring and angiography with ischaemia testing (stress MRI, stress echo, CTFFR) if indicated prior to transition to adult services |
| Blood tests | Lipid profile every 5 years HbA1c | |
| Psychological | Family and patient dialogue | During transition process from 13 to 18 years—patient focused dialogue |
| Advice | Smoking Exercise Diet Family planning PSP review |
ADP, Adenine di-Phosphate; AHA, American Heart Association; CTFFR, CT fractional flow reserve; PSP, person-specific protocol.
Imaging considerations
Patients with CAA require repeated assessment throughout life and imaging should minimise cumulative radiation exposure, using modalities such as echocardiography and MRI. While modern multidetector CT can achieve high-resolution coronary imaging at much lower radiation doses than in the past, CT (or even invasive) coronary angiography should be undertaken only when other modalities cannot be used to define stenotic lesions or plan interventions. Cardiac MRI has no known risk unless gadolinium enhancement is used31 and in addition to providing detailed information on aneurysm size and presence of thrombi, it can detect small myocardial scars and fibrosis that cannot be detected by other modalities.32 Adenosine or exercise stress MRI should be used in older children and adults for the investigation of myocardial ischaemia. In addition, there may be a role for positron emission tomography or nuclear stress imaging in order to determine the haemodynamic and perfusion defects in KD, but large trials have not yet been performed (only isolated studies), so the role of these techniques is not fully established.32–34
If CT angiography at transition demonstrates evidence of coronary artery stenosis or if there is admission with chest pain, then ischaemia testing (Stress MRI, Stress echo, CT fractional flow reserve) could be considered to determine if there is evidence of functional tissue hypoperfusion.35 If this is abnormal, then progression to stress imaging with MRI or echocardiography should be undertaken. In addition, MRI/MRA should be used to screen for non-cardiac aneurysms in cases of severe disease.11
Person-specific protocol
All patients with a history of KD at risk level 3 and above (see table 1) require a person-specific protocol (PSP). The PSP (see online supplementary file 2) is a guidance document held by the patient, parents and school (if a child) the Congenital Cardiac Surgical Centre (children)—or Heart Attack Centre (adults) and their emergency medical services (including Ambulance Services) so they have prior knowledge of the patients’ KD history and can act quickly in the event of a suspected cardiac emergency. The PSP includes the patient’s KD history and highlights the specific instructions to the Ambulance Service regarding the specialist centre to which the patient should be transported, where the necessary age-appropriate expertise, facilities and imaging are available without delay. A suggested template for the KD PSP is given as an online supplementary file 2. It is recommended that the patient should hold a copy of their most recent ECG and their coronary imaging (either digitally or as printed copy), to facilitate decision making in the emergency situation.
heartjnl-2019-315925supp002.pdf (123.4KB, pdf)
The PSP should be agreed with the patient (if adult) or carers (if child) and should include direct phone contact numbers (24 hours) for the specialist KD service, and clear instructions regarding who to contact for advice out of hours. It should be agreed by the local hospital and ambulance services and should be provided to the patient and, as relevant, the patient’s school/university and/carers parents.
Engagement with primary care
Good communication between the specialist cardiac centre and the patient’s Primary Care team is essential. All health, care and school services should be aware of plans for the emergency management of complications, as documented in the PSP.
Transition from paediatric to adult services
All patients with a history of KD and who are in risk level 3 and above (table 1) require planned transition to adult cardiac follow-up at age 16–18 years. The timing of such transition should reflect the developmental needs of the individual concerned. During transition, joint paediatric and adult clinical supervision is recommended, until such time as safe transfer of care can occur. Transition should be to a specialist KD clinic, led by staff with a specific interest in KD, with access to interventional cardiology, assessment of anticoagulation and 24 hours availability of cardiac CT or MRI.
Many children with CAA during the acute illness or persisting during early follow-up may have undergone resolution of their CAA at the time of transition or their cardiac status may be unknown. A rational approach to transition includes making a cardiovascular assessment (table 1) together with specific testing to detect subclinical ischaemia, valve dysfunction and myocardial fibrosis. Because arterial calcification is a feature of KD vascular lesions, the CT calcium score is particularly useful for risk stratification of young adults (table 2).36
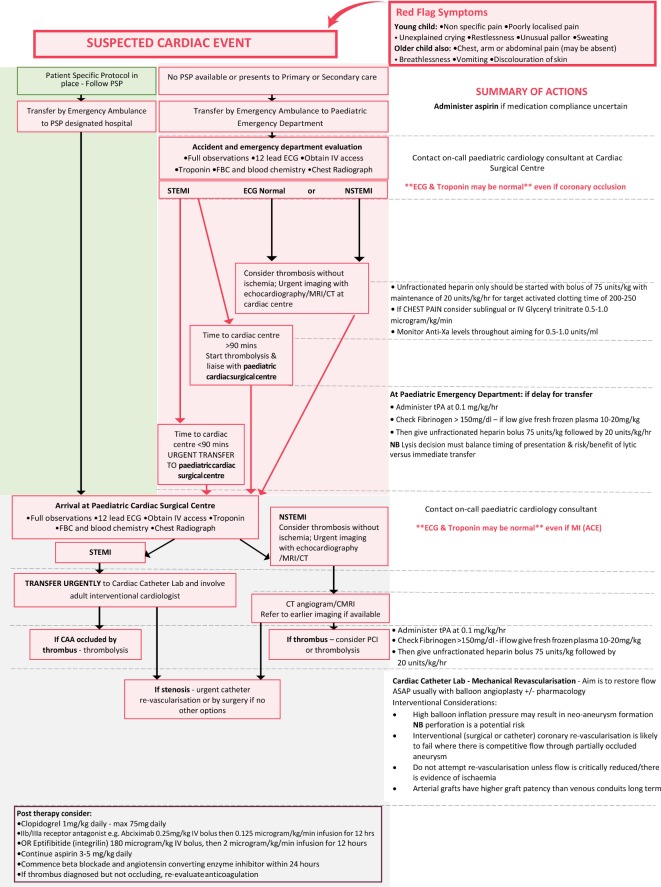
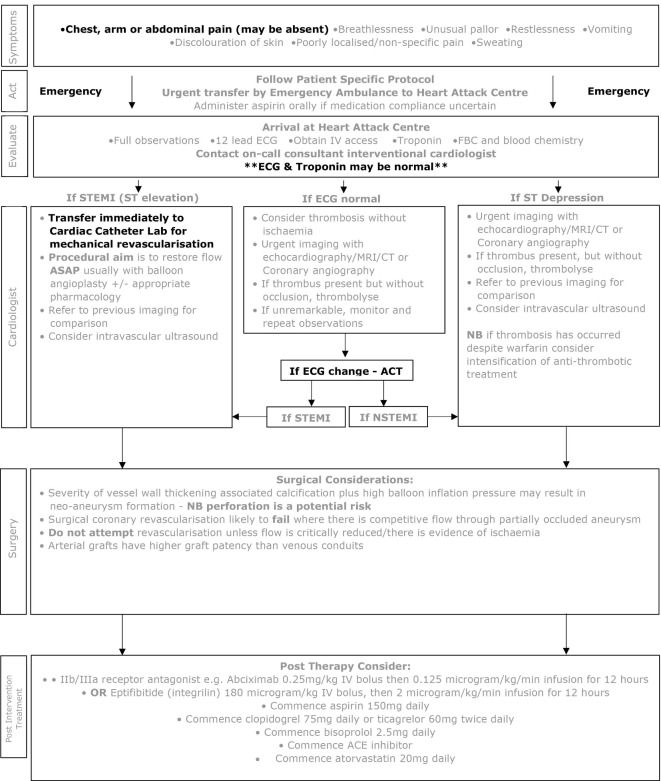
Myocardial ischaemia may develop in patients with CAA at any age, either due to thrombosis within the aneurysm or slow development of a coronary stenosis during vascular remodelling. As clinical features cannot reliably determine the underlying aetiology, urgent imaging should be undertaken to rule out coronary thrombosis in all patients with new onset suspected myocardial ischaemia (10) (figures 1 and 2). It is important to stress that:
Figure 1.
Kawasaki disease emergency management of suspected myocardial ischaemia in children with previous Kawasaki disease and possible coronary artery aneurysms. CAA, coronary artery aneurysms; nSTEMI, non-STEMI; PSP, person-specific protocol; STEMI, ST-elevation myocardial infarction.
Figure 2.
Kawasaki disease emergency management of suspected myocardial ischaemia in adults with previous Kawasaki disease and possible coronary artery aneurysms. nSTEMI, non-STEMI; STEMI, ST-elevation myocardial infarction.
The classical presentation of myocardial ischaemia in adults (chest pain, arm or jaw pain, decreased exercise tolerance, breathlessness) may be absent in young children, whose symptoms may be non-specific, including poorly localised pain, unexplained crying, restlessness, unusual pallor or sweating.
Children and young adults with stenotic CAA may have well-developed collaterals, a greater tolerance for ischaemia and may present with atypical symptoms even when extensive thrombus is present in the CAA.11
In all patients with a previous history of KD CAA, an initial ECG and troponin may be unremarkable, even with significant myocardial ischaemia.
Thrombosis within aneurysms can occur in children and adults even when taking anticoagulant and antiplatelet agents.
For these reasons, a high index of suspicion is needed in all patients with persisting or resolved aneurysms whenever new symptoms occur that could represent myocardial ischaemia, as potentially life-threatening coronary thrombosis may be the cause. Rapid access to urgent expert assessment and imaging is needed.
An unusual feature of children with persistent significant coronary involvement as a consequence of KD is that they can develop significant coronary collaterals over time, such that even complete thrombotic occlusion of a coronary artery may not result in myocardial ischaemia. Coronary thrombosis per se is therefore not necessarily a call to action if there is no myocardial territory at risk but always requires rapid review of antithrombotic strategy and of the overall management plan. The presence of myocardial ischaemia is the most important factor that should prompt consideration of coronary intervention.
Existing care pathways—gaps in paediatric provision
In the UK, adults with suspected acute coronary syndrome (ST-elevation myocardial infarction (STEMI) or high-risk non-ST elevation myocardial infarction (nSTEMI)) are usually taken by the Ambulance Service to a designated Heart Attack Centre. Patients presenting to general hospitals undergo emergency triage and are usually then transported to a Heart Attack Centre if appropriate. There is no similar arrangement for coordinated management of suspected acute coronary syndromes in children. Paediatric services are largely unfamiliar with the detection and management of myocardial ischaemia due to its rarity in children, and many hospital emergency departments for children have no on-site paediatric cardiology. Children’s specialist cardiac centres are often located away from accident and emergency services or adult interventional cardiology centres.
Addressing gaps
It is therefore essential that, together with general raised awareness of the risk of acute coronary syndrome in people who have had a past episode of acute KD, a PSP and local pathway are in place for every patient with persistent or remodelled CAA so that they can reach the required cardiac expertise rapidly in the event of a suspected acute coronary syndrome. These should form and inform part of the PSP (see above).
Myocardial ischaemia—presentation to primary or secondary care
It is inevitable that some patients, both adults and children, will present to a primary or secondary care service where the required cardiac expertise is not available. We summarise below the potential complications and actions required to ensure patients reach appropriate care rapidly.
If the patient does not have a PSP or the deterioration occurs at work or school, the patient is likely to be transported by ambulance services to the nearest Heart Attack Centre (adult patient) if paramedics diagnose STEMI or high risk nSTEMI or the nearest children’s accident and emergency centre, where the required cardiac expertise may not be available.
Patients with new onset chest pain, exercise induced chest pain or (particularly in young children) unusual pallor, restlessness, breathlessness, poorly localised pain or unexplained crying or collapse should be transported urgently to the designated Heart Attack Centre (adults) or nearest paediatric cardiac surgical centre or as specified on their PSP.
Unless presentation is clearly due to a non-cardiac condition (such as acute gastroenteritis, bacterial infection, acute abdomen, epilepsy or trauma) patients with known CAA, whether persistent or remodelled, should always be evaluated at a Heart Attack Centre (adults) or paediatric cardiac surgical centre, as specified on their PSP.
Acute investigations—paediatric
A pre-existing paediatric KD acute coronary syndrome pathway should be in place at the designated centre and the child comanaged by the paediatric cardiology team and coronary intervention service. Urgent assessment should include clinical examination, an ECG, serial high sensitivity troponins and an echocardiogram, with awareness and acknowledgement of their PSP, and an understanding that absence of ECG changes or rise in troponin does not exclude a KD-related cardiac event. If ST elevation is present on the ECG, urgent contact with the appropriate interventional cardiologist should be made, with likely triage direct to the cardiac catheter laboratory (see below). If obvious STEMI is not present, but acute myocardial ischaemia is suspected, imaging by CT angiography or cardiac MRI should be undertaken to establish whether thrombus formation within a CAA has occurred or if coronary stenosis is the cause of symptoms, as the therapeutic options differ. Those with thrombus present in the coronary aneurysm, but without complete vessel occlusion, should be considered for intravenous thrombolysis and intensification of antithrombotic measures. Successful thrombolysis can be achieved in over 50% of patients with aneurysms using repeated daily infusion of tissue plasminogen activator (tPA) (alteplase).37 38 If thrombosis has occurred despite anticoagulation with warfarin, intensification of antithrombotic treatment by transfer to unfractionated heparin, switching to a DOAC and addition of other antiplatelet agents should be considered in adults and might be a possibility in children and young adults if their safety and efficacy is confirmed in the future.
Emergency management of paediatric acute coronary syndrome
Experience from the management of acute coronary syndrome in adults has demonstrated the importance of time to coronary reperfusion. Broadly, the longer the time to restoration of normal coronary blood flow, the greater the extent of myocardial damage and the worse are outcomes. Most paediatric cardiologists have little experience in managing acute myocardial ischaemia, so close collaboration with adult interventional cardiology services is essential. With the advent of smaller guide catheters and devices, patients with coronary artery internal dimensions of at least 1.5 mm are suitable candidates for percutaneous coronary intervention (PCI) in the setting of STEMI. Technical considerations and the size of the child will determine which patients are candidates for PCI or when thrombolysis is the preferred initial treatment. A carefully mapped pathway, set out and agreed in advance of any emergency event and taking account of local context, should be made and documented within the PSP in order to ensure a timely and smoothly orchestrated response by the paediatric cardiology and supporting adult interventional cardiology teams.
Paediatric protocol
Although clear guidelines for adult STEMI have been issued, guidance for the paediatric population is limited; box 1 suggests a protocol for STEMI or other coronary ischaemic events in children
Box 1. Suggested protocol for ST-elevation myocardial infarction or other coronary ischaemic events in children.
As most children with known coronary artery aneurysms will already be on aspirin, additional oral aspirin should only be administered if there is uncertainty about compliance or the child is not already on aspirin.
If there is ST elevation on the initial ECG, cardiovascular collapse or clinical suspicion of myocardial ischaemia and expected transport time to a congenital cardiac surgical centre with colocated interventional cardiology or Heart Attack Centre is more than 90 min, then tissue plasminogen activator (tPA) should be administered prior to transport. For tPA to be effective, fibrinogen must be >1500 mg/L. If low, then intravenous fresh frozen plasma (10–20 mL/kg) should be given and a further check performed. While giving tPA, unfractionated heparin (UFH) should be commenced through a separate line.
If there is ST depression on the ECG, UFH should be started. There should be an initial intravenous bolus of 75 units/kg, with maintenance of 20 units/kg/hour for target activated clotting time 200–250 or an activated partial thromboplastin time ratio in the range 1.5–2.5. If there is chest pain, sublingual or intravenous glyceryl trinitrate should be started.
Patients too ill to transfer, or in whom delay in transfer to a paediatric cardiac surgical centre is likely, should be discussed with an interventional cardiologist and thrombolysis considered prior to transfer.47
Interventional cardiology—all ages
Management of suspected myocardial ischaemia in patients with KD aneurysms differs from standard management of adult chest pain because of the high risk of large thrombi within coronary aneurysms, and the different anatomy of the artery damaged by inflammation, calcification and fibrosis as a consequence of KD. The procedural aim in the emergency setting should be to restore flow as quickly as possible. In the presence of acute occlusion with a large thrombus burden, angioplasty without stenting may be the preferred option. Routine thrombus aspiration is not supported but may be required to achieve recanalisation, acknowledging the associated risk of thromboembolisation.39 Intravascular ultrasound (IVUS) is essential to assess true vessel size and guide management. If the thrombus is not occlusive, medical therapy with aspirin, clopidogrel, tPA and IIb/IIIa platelet inhibitor should be considered. Other oral antiplatelets (such as ticagrelor) have been used but on the basis of evidence derived from non-KD populations.40
Patients with angina due to stenotic lesions (ie, thrombus within the aneurysm is not the cause of ischaemic symptoms) may require PCIs. However, a number of issues specific to KD pathology need to be considered before any procedure is undertaken. The intense luminal myofibroblastic proliferation and calcification in KD can pose particular challenges to interventionists; debulking or modification of calcification with rotational atherectomy or cutting balloon technology may be required. Due to the severity of vessel wall thickening and associated calcification, high balloon inflation pressures may lead to neo-aneurysm formation. Moreover, the frequency of a heavy thrombus load and the large calibre of the aneurysms themselves present additional challenges for coronary stent deployment.
There is limited experience with the use of covered or drug-eluting stents in this patient population.41 Intravascular imaging, either by IVUS or optical coherence tomography, plays an important role in guiding treatment, as inadequate appreciation of the diameter of vessels due to thrombus may lead to undersizing and/or inappropriate stent placement.14 15 23 42 43
Clopidogrel should be given orally, in addition to aspirin, prior to intervention. For additional or postprocedure therapy, consider:
Abciximab (currently in short supply): or
Eptifibatide (Integrilin).
Aspirin continued at low dose along with Clopidogrel.
For patients at increased risk of thrombosis (such as those with large or giant aneurysms and recent coronary thrombosis) ‘triple therapy’ with aspirin, a second antiplatelet agent and anticoagulation with warfarin, low molecular weight heparin or a direct oral anticoagulant (DOAC) should be considered. Trials of DOACs for this clinical indication are underway; in adults, many already prefer a DOAC to warfarin.
Atorvastatin for possible additional anti-inflammatory effect.
In order to reduce the risk of postprocedural heart failure and arrhythmia, then beta blockade and ACE inhibitor within 24 hours of procedure.
Cardiac surgery
Revascularisation is likely to fail when flow through a partially occluded aneurysm competes with graft flow and so bypass grafting should not be considered unless flow through the aneurysm is critically reduced. When surgery has been undertaken in this patient population both saphenous vein and arterial grafts have been used44 but arterial grafts have a much higher rate of graft patency over time compared with venous conduits.45 46 A saphenous graft might supply adequate flow acutely, but the conduit may degenerate while the patient is young.
‘Excluding’ an aneurysm (by surgically occluding the native coronary artery) at the time of bypass grafting has been undertaken in some past cases in an attempt to reduce the potential for bypass graft occlusion due to competitive flow. This approach is not recommended as it has significant associated risks; if the aneurysm is excluded, an immature internal mammary LIMA graft may not supply adequate blood flow to the left ventricular anterior wall at the time of surgery, resulting in continuing ischaemia.
Elective coronary interventions should be planned after careful discussion with a broad multidisciplinary team. Cardiac transplantation has been successfully performed for the patient with rare KD with end-stage cardiomyopathy and inoperable multivessel coronary artery disease.
Non-cardiac complications
Thrombosis within extracardiac aneurysms
Patients with aneurysms of extracardiac arteries (most commonly axillary and iliac/femoral) are at risk of luminal thrombosis. This may present with features of peripheral ischaemia such as claudication, pallor, pain, loss of pulses or discolouration of peripheral limbs or digits. Any acute symptoms compatible with thrombosis should lead to discussion with a vascular specialist and imaging studies to exclude thrombosis or occlusion should be considered.
Bleeding
Patients with giant CAA on warfarin and antiplatelet agents are at risk of external or internal bleeding, spontaneously or following trauma. Internal bleeding may present with swelling over limbs or joints, GI bleeding or haemorrhagic stroke. New symptoms or lesions should undergo imaging by ultrasound or CT, and INR should be checked. Any CNS symptoms with or without a history of trauma such as persistent headache, impaired consciousness or neurological signs requires imaging to exclude haemorrhagic or thrombotic stroke.
Summary
Patients with CAA as a result of KD in childhood are at lifelong risk of cardiac complications and require lifetime follow-up at specialist regional KD clinics. Those with established CAA have a continuing increased risk at all ages; in Japan, it has been reported that major adverse cardiovascular events occur in 64% of patients within 30 years of diagnosis.26The management of suspected myocardial ischaemic events in these patients differs from that of adults, who have acute coronary syndromes due to atherosclerotic heart disease. Each patient at risk requires a PSP to ensure that they reach an appropriately equipped centre with specialist expertise and that they do so without delay (Box 2).
Box 2. Key elements for management of previous Kawasaki disease (KD) in children and adults.
Those who have coronary aneurysms following acute KD, whether persisting or remodelled, are at lifelong risk of coronary thrombosis, coronary stenoses and acute coronary syndromes.
An individual’s lifetime risk is related to the severity of residual cardiac pathology (particularly coronary aneurysms) after the initial illness.
Every child or adult who has had coronary artery aneurysms (CAA) following KD, whether persisting or remodelled, requires lifelong uninterrupted follow-up by a cardiology team within a specialist KD clinic with services agreed by the relevant Congenital Cardiac Network and adult interventional cardiology service.
A child with a past history of KD aneurysms, who presents with any symptoms or signs which could be due to aneurysm thrombosis or acute coronary syndrome, should be managed using a pathway of care predefined by the local specialist children’s congenital cardiac network in accordance with this guidance.
An adult with a past history of KD and CAA, who presents with any symptoms or signs which could be due to aneurysm thrombosis or acute coronary syndrome, should be taken directly to a Heart Attack Centre (HAC). Those who present to a local hospital should be transferred urgently to a HAC to rule out CAA thrombosis, progressive coronary stenoses or acute coronary syndrome. Delay is likely to have an adverse effect on outcome.
Every child or adult followed-up for CAA should have a person-specific protocol (PSP) written, detailing the pathway of care to be followed if a suspected acute coronary syndrome should occur.
Aneurysm thrombosis and acute coronary syndromes in patients with previous KD may present with atypical symptoms and initial absence of typical changes on the ECG or changes in cardiac enzymes. All patients with chest pain or suspected acute coronary syndrome should be imaged urgently to rule out thrombus.
Emergency access to interventional cardiology services will be required to manage suspected acute coronary syndromes in children and should be part of the care pathway defined. The congenital cardiac centre and the HAC should ideally be colocated, but where not, arrangements for emergency access should be clear, agreed in advance of any need and documented in a PSP.
Congenital Cardiac Networks should take the lead on disseminating learning and best practice in line with this guidance, to all those who may be involved in the care of patients who have had KD. Where these do not exist, they should be established with adult and paediatric cardiology input and ensure access to specialist cardiac imaging, interventional cardiology and cardiac surgery.
Centralising the follow-up of affected patients will help concentrate and build expertise, enable the development of care pathways for the emergency management of acute complications and facilitate research.
Transition of care from a paediatric to adult service should be planned in advance and be well-coordinated.
Person-specific protocol
Footnotes
Contributors: All authors contributed to the design of the manuscript and attended multiple meetings over a 2-year period in order to achieve a consensus document. All authors have approved the final version of the manuscript. Members of the writing group are; Brogan PA, Burns JC, Cornish J, Diwakar V, Eleftheriou D, Gordon JB, Gray HH, Johnson T, Levin M, Malik I, MacCarthy P, McCormack R, Miller OI, Tulloh RMR.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PB has received institutional grants from SOBI, Roche, Novartis and Novimmune and consultancy fees from SOBI, Novartis, Roche and UCB. RMRT has received grants and speaker fees from Actelion, Abbvie, GSK, Bayer, Pfizer, Jansen. Societi Foundation (RMcC) has received grants from SOBI and Roche.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. de Graeff N, Groot N, Ozen S, et al. . European consensus based recommendations for the diagnosis and treatment of Kawasaki disease - the Share initiative. Rheumatology 2018. [DOI] [PubMed] [Google Scholar]
- 2. Eleftheriou D, Levin M, Shingadia D, et al. . Management of Kawasaki disease. Arch Dis Child 2014;99:74–83. 10.1136/archdischild-2012-302841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harnden A, Tulloh R, Burgner D. Kawasaki disease. BMJ 2014;349:g5336 10.1136/bmj.g5336 [DOI] [PubMed] [Google Scholar]
- 4. Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol 2012;22:79–85. 10.2188/jea.JE20110131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura Y, Yashiro M, Uehara R, et al. . Epidemiologic features of Kawasaki disease in Japan: results of the 2009–2010 nationwide survey. Journal of Epidemiology 2012;22:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tulloh RMR, Mayon-White R, Harnden A, et al. . Kawasaki disease: a prospective population survey in the UK and ireland from 2013 to 2015. Arch Dis Child 2019;104:640–6. 10.1136/archdischild-2018-315087 [DOI] [PubMed] [Google Scholar]
- 7. Hall GC, Tulloh LE, Tulloh RMR. Kawasaki disease incidence in children and adolescents: an observational study in primary care. British Journal of General Practice 2016;66:e271–6. 10.3399/bjgp16X684325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodo X, Curcoll R, Robinson M, et al. . Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc Natl Acad Sci U S A 2014;111:7952–7. 10.1073/pnas.1400380111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics 1978;61:100–7. [PubMed] [Google Scholar]
- 10. Newburger JW, Takahashi M, Beiser AS, et al. . A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 1991;324:1633–9. 10.1056/NEJM199106063242305 [DOI] [PubMed] [Google Scholar]
- 11. McCrindle BW, Rowley AH, Newburger JW, et al. . Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation 2017;135:e927–99. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 12. Uehara R, Belay ED, Maddox RA, et al. . Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J 2008;27:155–60. 10.1097/INF.0b013e31815922b5 [DOI] [PubMed] [Google Scholar]
- 13. Kato H, Sugimura T, Akagi T, et al. . Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 1996;94:1379–85. 10.1161/01.cir.94.6.1379 [DOI] [PubMed] [Google Scholar]
- 14. Daniels LB, Gordon JB, Burns JC. Kawasaki disease: late cardiovascular sequelae. Curr Opin Cardiol 2012;27:572–7. 10.1097/HCO.0b013e3283588f06 [DOI] [PubMed] [Google Scholar]
- 15. Gordon JB, Kahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol 2009;54:1911–20. 10.1016/j.jacc.2009.04.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyskina G, Bockeria O, Shirinsky O, et al. . Cardiovascular outcomes following Kawasaki disease in Moscow, Russia: a single center experience. Glob Cardiol Sci Pract 2017;2017:e201723 10.21542/gcsp.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mossberg M, Segelmark M, Kahn R, et al. . Epidemiology of primary systemic vasculitis in children: a population-based study from southern Sweden. Scand J Rheumatol 2018;47:295–302. 10.1080/03009742.2017.1412497 [DOI] [PubMed] [Google Scholar]
- 18. Friedman KG, Gauvreau K, Hamaoka‐Okamoto A, et al. . Coronary artery aneurysms in Kawasaki disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc 2016;5 10.1161/JAHA.116.003289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NHSEngland New congenital heart disease review, 2015. Available: https://www.england.nhs.uk/wp-content/uploads/2015/07/Item-4-CHD-Report.pdf
- 20. NHSEngland Failure to recognise coronary syndromes in Kawasaki disease, 2016. Available: https://improvement.nhs.uk/news-alerts/failure-recognise-acute-coronary-syndromes-kawasaki-disease-patients/ [Accessed 20 Sep 2016].
- 21. Amano S, Hazama F, Hamashima Y. Pathology of Kawasaki disease: II. distribution and incidence of the vascular lesions. Jpn Circ J 1979;43:741–8. 10.1253/jcj.43.741 [DOI] [PubMed] [Google Scholar]
- 22. Chung KJ, Brandt L, Fulton DR, et al. . Cardiac and coronary arterial involvement in infants and children from new England with mucocutaneous lymph node syndrome (Kawasaki disease). Angiocardiographic-echocardiographic correlations. Am J Cardiol 1982;50:136–42. 10.1016/0002-9149(82)90019-4 [DOI] [PubMed] [Google Scholar]
- 23. Mitani Y, Ohashi H, Sawada H, et al. . In vivo plaque composition and morphology in coronary artery lesions in adolescents and young adults long after Kawasaki disease: a virtual histology-intravascular ultrasound study. Circulation 2009;119:2829–36. 10.1161/CIRCULATIONAHA.108.818609 [DOI] [PubMed] [Google Scholar]
- 24. Miura M, Kobayashi T, Kaneko T, et al. . Association of severity of coronary artery aneurysms in patients with Kawasaki disease and risk of later coronary events. JAMA Pediatr 2018;172:e180030 10.1001/jamapediatrics.2018.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suda K, Iemura M, Nishiono H, et al. . Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation 2011;123:1836–42. 10.1161/CIRCULATIONAHA.110.978213 [DOI] [PubMed] [Google Scholar]
- 26. Tsuda E, Hamaoka K, Suzuki H, et al. . A survey of the 3-decade outcome for patients with giant aneurysms caused by Kawasaki disease. Am Heart J 2014;167:249–58. 10.1016/j.ahj.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 27. Krumholz HM, et al. Aspirin for secondary prevention after acute myocardial infarction in the elderly: prescribed use and outcomes. Ann Intern Med 1996;124:292–8. 10.7326/0003-4819-124-3-199602010-00002 [DOI] [PubMed] [Google Scholar]
- 28. Su D, Wang K, Qin S, et al. . Safety and efficacy of warfarin plus aspirin combination therapy for giant coronary artery aneurysm secondary to Kawasaki disease: a meta-analysis. Cardiology 2014;129:55–64. 10.1159/000363732 [DOI] [PubMed] [Google Scholar]
- 29. Levin M, Burns JC, Gordon JB. Warfarin plus aspirin or aspirin alone for patients with giant coronary artery aneurysms secondary to Kawasaki disease? Cardiology 2014;129:174–7. 10.1159/000366052 [DOI] [PubMed] [Google Scholar]
- 30. Suda K, Tahara N, Honda A, et al. . Statin reduces persistent coronary arterial inflammation evaluated by serial 18fluorodeoxyglucose positron emission tomography imaging long after Kawasaki disease. Int J Cardiol 2015;179:61–2. 10.1016/j.ijcard.2014.10.057 [DOI] [PubMed] [Google Scholar]
- 31. Kanda T, Ishii K, Kawaguchi H, et al. . High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted Mr images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270:834–41. 10.1148/radiol.13131669 [DOI] [PubMed] [Google Scholar]
- 32. Tacke CE, Kuipers IM, Groenink M, et al. . Cardiac magnetic resonance imaging for noninvasive assessment of cardiovascular disease during the follow-up of patients with Kawasaki disease. Circulation 2011;4:712–20. [DOI] [PubMed] [Google Scholar]
- 33. Mostafa MS, Sayed AO, Al Said YM. Assessment of coronary ischaemia by myocardial perfusion dipyridamole stress technetium-99 M tetrofosmin, single-photon emission computed tomography, and coronary angiography in children with Kawasaki disease: pre- and post-coronary bypass grafting. Cardiol Young 2015;25:927–34. 10.1017/S1047951114001292 [DOI] [PubMed] [Google Scholar]
- 34. Kashyap R, Mittal BR, Bhattacharya A, et al. . Exercise myocardial perfusion imaging to evaluate inducible ischaemia in children with Kawasaki disease. Nucl Med Commun 2011;32:137–41. 10.1097/MNM.0b013e3283411c67 [DOI] [PubMed] [Google Scholar]
- 35. Ko BS, Cameron JD, Munnur RK, et al. . Noninvasive CT-Derived FFR based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging 2017;10:663–73. 10.1016/j.jcmg.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 36. Kahn AM, Budoff MJ, Daniels LB, et al. . Usefulness of calcium scoring as a screening examination in patients with a history of Kawasaki disease. Am J Cardiol 2017;119:967–71. 10.1016/j.amjcard.2016.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harada M, Akimoto K, Ogawa S, et al. . National Japanese survey of thrombolytic therapy selection for coronary aneurysm: intracoronary thrombolysis or intravenous coronary thrombolysis in patients with Kawasaki disease. Pediatr Int 2013;55:690–5. 10.1111/ped.12187 [DOI] [PubMed] [Google Scholar]
- 38. Kandan SR, Johnson TW. Management of percutaneous coronary intervention complications. Heart 2019;105:75–86. 10.1136/heartjnl-2017-311155 [DOI] [PubMed] [Google Scholar]
- 39. Ge J, Schafer A, Ertl G, et al. . Thrombus aspiration for ST-segment-elevation myocardial infarction in modern era: still an issue of debate? Circ Cardiovasc Interv 2017;10. [DOI] [PubMed] [Google Scholar]
- 40. Li D-D, Wang X-Y, Xi S-Z, et al. . Relationship between ADP-induced platelet-fibrin clot strength and anti-platelet responsiveness in ticagrelor treated ACS patients. J Geriatr Cardiol 2016;13:282–9. 10.11909/j.issn.1671-5411.2016.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Okuno S, Ishihara T, Iida O, et al. . Satisfactory arterial healing after second-generation drug-eluting stent implantation for segmental stenosis in a patient with Kawasaki disease. Cardiovasc Interv Ther 2019;34:83–4. 10.1007/s12928-018-0520-2 [DOI] [PubMed] [Google Scholar]
- 42. Gordon JB, Daniels LB, Kahn AM, et al. . The Spectrum of Cardiovascular Lesions Requiring Intervention in Adults After Kawasaki Disease. JACC: Cardiovascular Interventions 2016;9:687–96. 10.1016/j.jcin.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 43. Abdolmanafi A, Duong L, Dahdah N, et al. . Characterization of coronary artery pathological formations from OCT imaging using deep learning. Biomed Opt Express 2018;9:4936–60. 10.1364/BOE.9.004936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muta H, Ishii M. Percutaneous coronary intervention versus coronary artery bypass grafting for stenotic lesions after Kawasaki disease. J Pediatr 2010;157:120–6. 10.1016/j.jpeds.2010.01.032 [DOI] [PubMed] [Google Scholar]
- 45. Nishida H, Endo M, Hayashi H, et al. . Early occlusion of saphenous vein grafts due to marked intimal proliferation in Kawasaki disease. Prog Clin Biol Res 1987;250:527–8. [PubMed] [Google Scholar]
- 46. Tsuda E, Kitamura S, Kimura K, et al. . Long-Term patency of internal thoracic artery grafts for coronary artery stenosis due to Kawasaki disease: comparison of early with recent results in small children. Am Heart J 2007;153:995–1000. 10.1016/j.ahj.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 47. Dionne A, Bakloul M, Manlhiot C, et al. . Coronary artery bypass grafting and percutaneous coronary intervention after Kawasaki disease: the pediatric Canadian series. Pediatr Cardiol 2017;38:36–43. 10.1007/s00246-016-1480-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2019-315925supp001.pdf (241KB, pdf)
heartjnl-2019-315925supp002.pdf (123.4KB, pdf)