Abstract
Purpose:
This review sought to (a) describe definitions of long-term opioid therapy (LTOT) outcome measures, and (b) identify the predictors associated with the transition from short-term opioid use to LTOT for opioid-naïve individuals.
Methods:
We conducted a systematic review of the peer-reviewed literature (January 2007 to July 2018). We included studies examining opioid use for more than 30 days. We classified operationalization of LTOT based on criteria used in the definitions. We extracted LTOT predictors from multivariate models in studies of opioid-naïve individuals.
Results:
The search retrieved 5,221 studies, and 34 studies were included. We extracted 41 unique variations of LTOT definitions. About 36% of definitions required a cumulative duration of opioid use of 3 months. Only 17% of definitions considered consecutive observation periods, 27% used days’ supply, and no definitions considered dose.
We extracted 76 unique predictors of LTOT from seven studies of opioid-naïve patients. Common predictors included pre-existing comorbidities (21.1%), non-opioid prescription medication use (13.2%), substance use disorders (10.5%), and mental health disorders (10.5%).
Conclusions:
Most LTOT definitions aligned with the chronic pain definition (pain more than 3 months), and used cumulative duration of opioid use as a criterion, although most did not account for consistent use. Definitions were varied and rarely accounted for prescription characteristics, such as days’ supply. Predictors of LTOT were similar to known risk factors of opioid abuse, misuse, and overdose. As LTOT becomes a central component of quality improvement efforts, researchers should incorporate criteria to identify consistent opioid use to build the evidence for safe and appropriate use of prescription opioids.
1 |. INTRODUCTION
For the nearly 20% of Americans and Canadians suffering from chronic non-cancer pain (CNCP), pain that lasts more than 3 months, prescription opioids are commonly used to relieve pain.1–3 Although prescription opioids may be appropriate for short-term pain relief, long-term opioid therapy (LTOT) is not associated with improvements in pain or function.4,5 LTOT can lead to tolerance and escalating doses, and is associated with greater risk of opioid-related overdose and mortality.6 Across populations and health care settings, the rising prevalence of LTOT has paralleled increases with opioid-related overdose and mortality rates.7–13 Previous studies, which defined LTOT as opioid use of more than 90 days, found that the prevalence of LTOT was 5.4% in the United States and 17% in Canada.11,14
Defining LTOT is critical to understanding the risk factors that lead to LTOT and the evaluation of clinical practices and policies aimed at reducing risky opioid use that may occur with LTOT (eg, high doses, concurrent benzodiazepine prescriptions, or other forms of opioid use that are associated with behaviors related to abuse, misuse, and addiction).15 The concept of LTOT has become a central component to assess quality of care. In 2018, the National Committee for Quality Assurance added “Risk of Continued Opioid Use” as a quality of care measure.16 The Centers for Disease Control and Prevention’s (CDC) guide to monitor quality of the implementation of the 2016 Guideline for Prescribing Opioids for Chronic Pain defines nine measures corresponding to different types of risky LTOT.17,18
Adapted from the 2008 seminal observational study by Von Korff and colleagues on LTOT, clinical guidelines define LTOT as “use of opioids on most days for more than 3 months.”18–21 Yet, in research and clinical practice, the criteria used to define LTOT vary. Similar to other measures of medication use (eg, adherence, persistence, and compliance), researchers seeking to define LTOT need to select cutoffs for the duration of medication use, length of time for follow-up, frequency of fills, quantity, and days’ supply to define LTOT.22–24 Previous systematic reviews of pain medication use (covering studies published before 2014) for CNCP, examined constructs relating to adherence such as overuse, underuse, and misuse of opioid and non-opioid medications, but did not specifically examine LTOT.25,26 Reviews also examined predictors of opioid misuse and abuse, and overdose, but predictors may differ for LTOT.27–32
Little is known about the criteria used to define LTOT for research purposes, and there has been no synthesis of the risk factors associated with transition from short-term opioid therapy to LTOT. Without an understanding of the criteria or the predictors of LTOT, it is difficult to understand the strengths and limitations regarding the evidence on the risk of LTOT, distinguish between episodic and long-term opioid use, and monitor the prevalence and incidence of LTOT. The purpose of this systematic review is to (a) document the varied definitions of LTOT used as an outcome in observational research and (b) identify the risk factors associated with transition from short-term opioid therapy to LTOT.
2 |. METHODS
2.1 |. Database and search strategy
We systematically reviewed the peer-reviewed literature to identify empirical studies investigating LTOT as an outcome following an indication for pain or initiation of prescription opioid therapy. We searched Pubmed, Medline, and Scopus databases for peer-reviewed articles published from January 2007 to July 2018. We chose this time period because the Food and Drug Administration received additional authority for post-approval monitoring in 2007, and there was specific attention to LTOT as demonstrated by the publication of clinical guidelines and recognition that LTOT was an important clinical outcome of prescribed opioid use for patients with CNCP during this time period.20,21,33 We worked with a research librarian to develop a search strategy based on synonyms for three concepts: opioids, long-term opioid therapy, and risk factors (Appendix 1).
2.2 |. Study selection
We considered LTOT definitions that examined prescription opioid therapy over 30 days or more of calendar time follow-up. We did not require specific study designs but required studies to measure LTOT as an outcome to understand the methodology used to define LTOT. To extract the predictors of LTOT, the study had to compare LTOT with short-term opioid therapy either by including a comparison group or examining opioid therapy over time to capture the shift from short-term to LTOT and have LTOT as an outcome. Articles were excluded if they were not written in English or were not original research. We also excluded studies not conducted in the United States or Canada to focus on countries with similar population characteristics and trends in opioid use.13,34 We excluded studies where the outcome was chronic pain but not LTOT. Studies with pediatric or cancer patients were excluded because guidelines for LTOT may differ for these populations compared with patients with CNCP.18,19 Within included studies, we identified a subset of studies with a new user study design to examine transitions to LTOT from an opioid-naïve state, defined using a washout period, or the period of time before the index date without any opioid use.35 Two authors screened each study based on the title, abstract, and full text. We had group discussions to resolve conflicts and reach consensus for inclusion.
2.3 |. Data extraction
For each included study, we extracted information about the sample (size and characteristics), data source, comparison group, and LTOT prevalence. We indicated whether studies applied a new user study design. If studies did not use a new user study design, we examined how opioid use was assessed during the lookback period or time period before the index date.
We identified distinct definitions of LTOT used in each study and deconstructed the components of each definition, which may capture the following:
type of event used to identify the index date for assessing LTOT;
follow-up time: calendar time observed after the index date;
start of the measurement of LTOT: time points after the index date to collect information for LTOT definition;
cumulative duration of opioid use: total amount of calendar time used to define LTOT;
use of multiple, distinct observation periods;
use of consecutive observation periods;
number of prescriptions;
days’ supply: total number of days covered by a prescription;
gaps or overlaps between prescriptions.
The CDC guideline indicates that the risk of opioid use increases with dose.18 Therefore, we examine whether LTOT definitions use dose, reported in morphine milligrams equivalent (MME). We also examined whether definitions identified specific opioid types (eg, oxycodone, hydrocodone, long-acting, etc.). Both dose and opioid type may be used to distinguish between high- and low-risk LTOT.
Two authors independently identified study and definition characteristics and conflicts were resolved by group discussion. Since many studies had more than one definition, we present the percentage of definitions with each criterion.
Among the subset of studies with a new user study design, we identify the predictors associated with the transition from short-term opioid therapy to LTOT from multivariate models. We classified predictors according to the conceptual framework developed by Hooten and colleagues to understand the gaps in evidence on the predictors of LTOT.36 Predictors were grouped into three domains: patient characteristics, practice characteristics, and prescriber characteristics.36 Within each domain, we grouped similar predictors into categories. We describe the number of times a predictor was used across the models, whether the odds ratio for each predictor was less than 1, more than 1, not significant, or not reported.
3 |. RESULTS
The search retrieved 5221 studies (Figure 1). After removing duplicates, we reviewed 3555 studies and selected 183 studies for full text review. Thirty-four studies were included for data extraction (Table 1).
FIGURE 1.
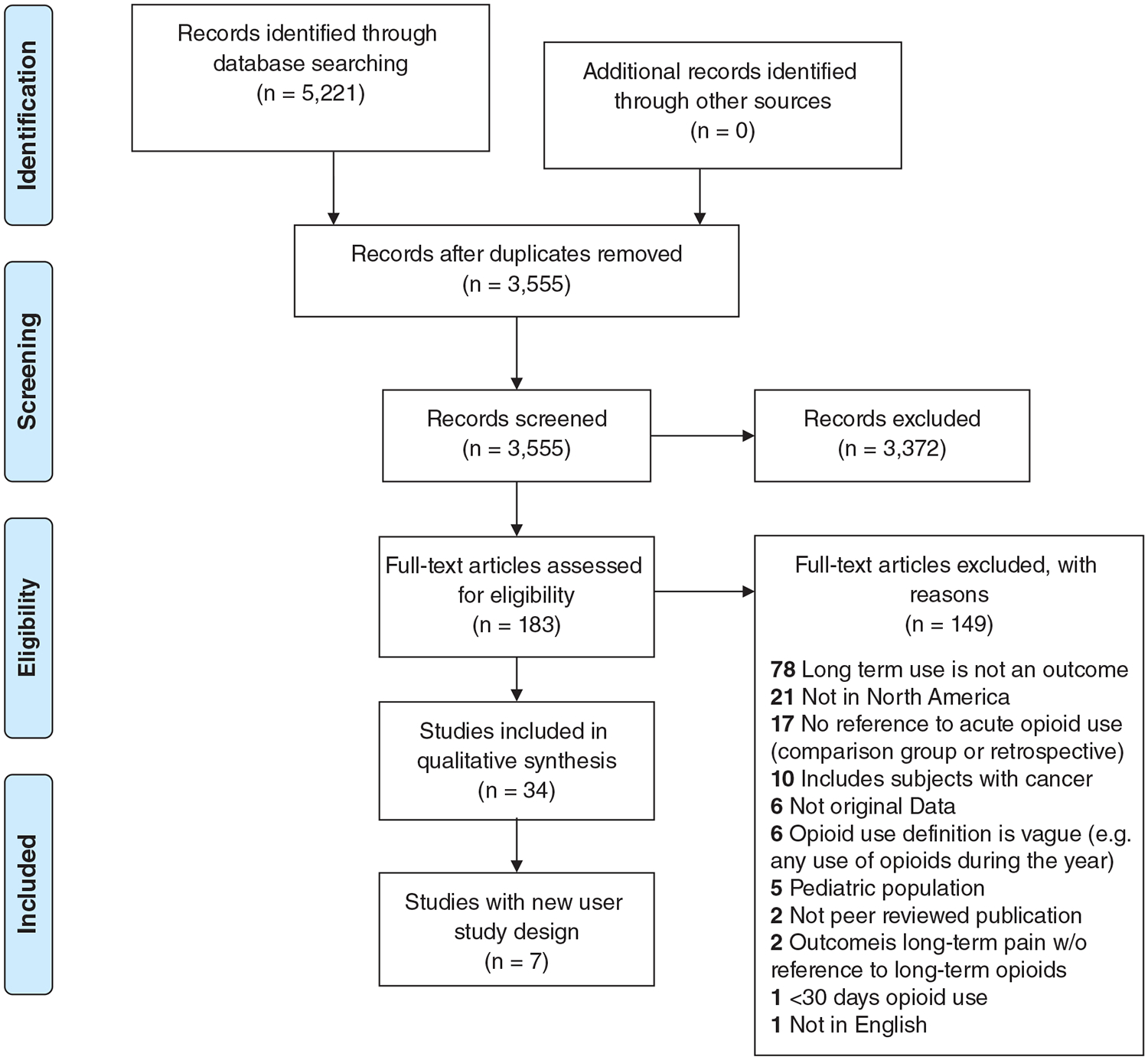
Prisma flow diagram
TABLE 1.
Characteristics of included studies
| Article | Study Sample Description (N) | Source of Data for Long-Term Use Measure | Definition Summary | Comparison Group | Percent Long-Term Users |
|---|---|---|---|---|---|
| Alghnam64 | Adults (18+) (N = 36 824) | Survey data (Medical Expenditure Panel Survey) | Self-reported opioid use in two of the final three rounds of the Medical Expenditure Panel Survey (totaling 8 mo of time) | Patients without long-term use | 15.60% |
| Anderson65 | Workers’ compensation recipients in Ohio who had lumbar fusion from 1993 to 2013 (N = 1002) | Administrative claims database (Ohio Bureau of Workers’ Compensation) | Post-operative long-term use: starting 6 wk after the surgery, more than 365 days’ opioid supply in the 3-y follow-up period Pre-operative long-term use: 120 days’ opioid supply in the year before surgery. |
Less than 365 days’ supply of opioids in the year following a 6-wk post-operative period. | 575/1002 = 57% |
| Bartels53 | Patients with surgery during October 2011 and September 2013 (N = 6003) | Electronic health record (University of Colorado Hospital) | At least one opioid prescription fill 31 to 180 d after surgery | Patients without long-term prescription | 43.6% |
| Brumett66 | Opioid-naïve, US adults age 19 to 64 with a surgical procedure between 2013 and 2014 (N = 36 177) | Administrative claims data (Clinformatics Data Mart) | At least one opioid prescription fill in each of two time periods: 30 d before through 14 d after surgery and 90–180 d after surgery | Patients without surgery, anesthesia, or an opioid prescription in a 12-mo period | Minor surgery group: 5.9% Major surgery group: 6.5% Nonoperative control group: 0.4% |
| Cancienne49 | Patients who underwent primary total knee arthroplasty from 2007 to the first quarter of 2016 (N = 113 337) | Administrative claims data (Pearldiver patient records) | At least one opioid prescription fill 3 to 6 mo after surgery. | Patients without long-term use | 35 770/113 337 = 32% |
| Connolly67 | US adults with lumbar spinal fusion surgery during 2009 to 2012 (N = 8377) | Administrative claims data (Clinformatics Data Mart) | At least 365 days’ supply in the 2-y follow-up period after surgery. | Patients without long-term opioid use | 29.34% |
| Crocker68 | Patients with Crohn disease and functional gastrointestinal disorder diagnosis from 2006 to 2011 (N = 931) | Electronic medical record (University of Virginia Digestive Health Center) and the prescription monitoring program database (Virginia and West Virginia) | One opioid prescription fill per month for 3 consecutive months in a 12-mo follow-up period or more than two prescription opioid fills in any 6-mo period of the 12-mo follow-up period. | Two groups- Crohn disease with and without functional gastrointestinal disorders | 192/931 = 21% |
| Deyo69 | Patients with lumbar fusion from October 2012 to September 2013 (N = 2491) | Administrative data (Oregon prescription drug monitoring program) | Pre-operative long-term use was at least four opioid prescription fills in the 7 mo before the index date where three fills occur within 180 d before the index date. Post-operative long-term use was at least four opioid prescription fills in 7 mo after the index date with at least three prescriptions 30 d after the hospitalization |
Patients without postoperative long-term opioid use | 12.8% |
| Daoust44 | Canadian adults, age 65+, with a trauma admission from April 2004 to March 2014 (N = 84 241) | Administrative database (Medical Consultations and Medication database in Quebec) | At least one opioid prescription fill 305 to 425 d after hospital discharge | Patients without opioid prescription 1 y after injury. | 4337/39 833 = 10.9% |
| Dunn70 | Patients who received elective spinal fusion from March 2011 to February 2016 (N = 1477) | Electronic health record (University of Virginia) | Documented opioid use at each of the following postoperative time periods: 1–3 d, 1 mo, 6 mo, and 12 mo | Patients without opioid use at 12 mo | (67 + 498)/1477 = 38% |
| Franklin38 | Workers with acute back injury at risk for long-term disability from 2002 to 2004 (N = 1296) | Administrative pharmacy claims data (Washington workers’ compensation medical billing database) | At least one opioid prescription fill in each of four consecutive calendar quarters | Patients with opioid use during fewer than three quarters | 111/1843 = 6.02% |
| Goesling43 | Patients with total knee arthroplasty and total hip arthroplasty from March 2010 to May 2013 (N = 574) | Medical records and confirmation with patients during follow-up (telephone surveys and validated questionnaires) | No explicit definition. Self-reported opioid use was assessed at 30 d, 90 d, and 180 d after surgery. | Patients without opioid use at 6 mo | Not information to calculate rate |
| Fritz71 | Opioid-naïve patients with low back pain from 2012 to 2014 (N = 707) | Administrative data (University of Utah Health Plan) | More than 120 d supply or more than 10 opioid prescription fills with more than 90 d supply during 1-y follow-up. | Patients without long-term use | 24.3% |
| Granadillo48 | Patients with hip arthroscopy from 2007 to 2015 (N = 1708) | Administrative claims data (Pearldiver patient records) | At least one opioid prescription fill 3 to 6 mo after surgery. | Patients without long-term use | 468/1708 = 27.4% |
| Holman45 | Patients with orthopedic trauma from June 2005 to June 2007 (N = 748) | Administrative data (Utah Controlled Substance Database) | No explicit definition. Presence of at least one opioid prescription fill was assessed during three time periods in the 6-mo follow-up period after surgery: 0 to 6 wk, 6 to 12 wk, and more than 12 wk. | Patients without continued use of opioids 6 wk after surgery | LTOT prevalence for more than 12 wk: 19.7% |
| Jain72 | Patients with primary cervical fusion for degenerative pathology from 2007 to the third quarter of 2015 (N = 29,101) | Administrative claims data (Pearldiver Humana database) | Preoperative long-term use is at least one prescription during the following preoperative time periods: 0 to two weeks, 2 wk to 1 mo, 1–3 mo, 3–6 mo, and 6 mo. Postoperative long-term use is at least one prescription during the following postoperative time periods: 0–6 wk, 6 wk to 3 mo, 3–6 mo, 6–9 mo, and 9–12 mo. |
Patients without preoperative long-term use Patients without postoperative long-term use |
49.2% |
| Jiang46 | Adults who received surgical care from 2010 to 2011 (N = 79,123) | Electronic medical record (University of Pennsylvania Health system) | At least 90 d of opioid use between the first and last recorded outpatient visit occurring within a 2-y observation period | Patients without chronic opioid use | 9.2% |
| Johnson37 | Adults with elective and trauma related hand surgery procedures from 2010 to 2012 (N = 77,573) | Administrative data (Truven Market-Scan Commercial Claims Research Database) | At least one opioid prescription fill in each of two time periods: 30 d before through 14 d after surgery and 90–180 d after surgery | Patients without long-term opioid use | Of the opioid naïve patients with a perioperative opioid prescription (N = 59 735), 13% |
| Kim55 | Patients with hip or knee arthroplasty from 2004 to 2013 (N = 57 545) | Administrative data (United Healthcare/Optum Clinformatics Data Mart Database) | Monthly opioid use for 12 consecutive months | Patients without long-term opioid use | 4394/57 545 = 7.6% |
| Koeppe 201173 | Opioid naïve, US Adults with HIV from 11 clinics from 1998 to 2008 (N = 931) | Medical Records (data from the HIV Outpatient Study) | Measured prolonged analgesic use, defined as 90 or more consecutive days of opioid or nonopioid analgesic medication use. | Patients without long-term opioid use | 931/4180 = 22% |
| Kulshrestha74 | Adults who have kidney transplant from 2004 to 2008 (N = 1045) | Electronic medical record (single center study, name not reported) | 1. Opioid use reported during three time periods after surgery: 1–2 mo, 3–6 mo, and 10–12 mo. 2. Opioid use reported during the 1–2-mo and 3–6-mo follow-up period only if patient had a graft loss or died during the 3–12 mo follow-up period |
Patients without long-term opioid use | 119/(119 + 926) = 11% |
| Mosher39 | Patients with an outpatient opioid prescription during FY 2011 that was preceded by a 1-y opioid-free period (N = 43 027) | Administrative data (Veterans Health Administration) | More than 90 days of continuous opioid use (determined by the days’ supply), with the initiation of the episode beginning within the first 30 d of the first prescription. | Patients without long-term use | Medicine: 15.2% Surgery: 5.3% Total sample: 9.10% |
| Mueller50 | Patients with total shoulder arthroplasty and shoulder hemiarthroplasty during 2002 to 2012 (N = 6988) | Administrative claims data (Marketscan) | Preoperative long-term use was more than 10 opioid prescription fills or 120 d opioid supply during the year before surgery. Postoperative long-term use was at least one prescription during the follow-up |
Patients with and without nerve block | 50.1% of patients without nerve block; 49.5% among patients with nerve block |
| Mulligan75 | Patients with ankle or hindfoot reconstruction (N = 132) | Electronic medical record (study site not reported) | Opioid prescription from 90 d to 2 y after surgery | Patients without continued use 90 d after surgery | 35% |
| Namba76 | Patients with unilateral primary total knee arthroplasty from 2008 to 2011 (N = 23 726) | Electronic medical record (Kaiser Permanente Northern California, Southern California, and Hawaii) | No explicit definition. Assessed presence of opioid prescription during each quarter of the follow-up year after surgery. | Patients without an opioid prescription in that quarter (separate models for each quarter) | Days 1–90: 92.7% Days 91–180: 42.1% Days 181–270: 32.2% Days 271–360: 30.4% |
| Pugley51 | Patients with cervical spinal arthrodesis from 2007 to 2014 (N = 17 391) | Administrative data (Humana Inc via PearlDiver) | One or more opioid prescription fill at 1 y after surgery. | Patients without opioid use in the 3 mo before surgery | LTOT at 12 mo: 27.6% |
| Qureshi52 | Patients with discectomy from 2007 to 2015 (N = 1321) | Administrative data (PearlDiver) | Opioid prescription fill 3–6 mo after surgery | Patients without long-term prescription | (621/621 + 700) = 47% |
| Raebel77 | Adults (21+) with bariatric surgery without chronic opioid use before surgery from nine health systems from 2005 to 2009 (N = 10 643) | Electronic medical record (outpatient pharmacy dispensing data) (nine health systems participating in the Scalable Partnering Network (SPAN)) | Ten or more opioid prescription fills over more than 90 d, or more than 120 d opioid supply during 1 y of follow-up More than 120 d supply during the 1-y follow-up. | Patients without chronic opioid use the year after surgery | 4% |
| Ray 201741 | Opioid naïve patients (+19) with at least 1 opioid prescription 2011 (N = 2 480 030) | Electronic medical record (Kaiser Permanente Northern California) | At least 3 mo of opioid use with either more than 120 d opioid supply or 10 or more opioid prescription fills during the 3-y follow-up period. | Cohort of patients without opioid fill in 2011 | 112 089/455 693 = 25% |
| Rosenbloom47 | Adults with musculoskeletal surgery (n = 122) | Patient reported survey (2 large level I trauma centers (Sunnybrook Health Sciences Centre [SHSC] and St. Michael’s Hospital [SMH]), in Toronto, Canada) | Self-reported opioid use in the past week at 4 mo after injury | Patients without opioid prescription at 4 mo after surgery | 35% |
| Rozet54 | US veterans 18 to 50 with elective ambulatory knee arthroscopic surgery from 2007 to October 2010 (N = 145) | Electronic medical record (Veterans Affairs’ Tertiary Medical Center (Seattle Division, VA PSHCS)) | At least 3 mo of “uninterrupted” opioid use after surgery. | Patients with and without post-traumatic stress disorder | 30% |
| Rao78 | Patients with shoulder arthroplasty registry from 2008 to 2014 (N = 4243) | Shoulder Arthroplasty Registry which includes data collected from electronic intraoperative forms, electronic health records, administrative claims, membership data, and mortality records (Kaiser Permanente Hawaii, Northern California, and Southern California | No explicit definition. Assessed presence of opioid prescription during each quarter of the follow-up year after surgery. | Patients without an opioid prescription in that quarter (separate models for each quarter) | Opioid use in the rehabilitation period ranged from 38% to 42% though specific percentages are not reported. |
| Sun42 | Patients with total knee arthroplasty from 2002 to 2012 (N = 120 080) | Administrative claims data (Marketscan) | More than 10 opioid prescription fills or 120 d opioid supply 91–365 d post-surgery | Patients without long-term use | Unable to calculate LTOT |
| Thornton40 | Patients with an opioid prescription from 2007 to May 2015 (N = 491 442) | Administrative Claims Data (IMS Health/Quintiles, IQVIA) | More than 90 d supply during the 4 mo follow up period | Patients without long-term use | 1.30% |
Over 75% (n = 27) of the studies examined LTOT among patients who underwent surgery, and 25% (n = 7) examined LTOT among patients with CNCP. Seven studies had a new user study design. Data sources included administrative claims data (N = 20), medical records (N = 13), and survey data (N = 3).
3.1 |. Characteristics of LTOT definitions
We extracted 41 unique variations of definitions of LTOT outcome measures (Table 2). Common definitions of the index date included the surgery date (71% of definitions), the first opioid prescription (10%), and the first visit (10%). Follow-up times for studies varied from 3.5 months to 3 years, and the most common follow-up time was 1 year (44%). Most definitions had at least one measurement on the index date (51%), and 37% of definitions had at least one measurement at 3 months after the index date. Cumulative duration of opioid use to define LTOT varied from 6 weeks to 1 year, and most definitions used 3 months (46%).
TABLE 2.
Characteristics of definitions of long-term opioid use (n = 41)
| Index event for Follow-up | ||
|---|---|---|
| Surgery | 29 | 71% |
| First visit | 4 | 10% |
| First prescription | 4 | 10% |
| Hospital discharge | 2 | 5% |
| Acute injury | 1 | 2% |
| 14 days after hospitalization | 1 | 2% |
| Unclear | 2 | 5% |
| Study follow-up time | ||
| 3.5 mo | 1 | 2% |
| 4 mo | 2 | 5% |
| 6 mo | 10 | 24% |
| 7 mo | 1 | 2% |
| 1 y | 18 | 44% |
| 14 mo | 1 | 2% |
| 2 y | 3 | 7% |
| 3 y | 3 | 7% |
| Unclear | 2 | 5% |
| Time points of measurement: time points used to collect information for LTOT definition. | ||
| 1 month before index date | 1 | 2% |
| Index date | 21 | 51% |
| First visit with first prescription | 1 | 2% |
| 14 days after index date | 1 | 2% |
| 1 mo after index date | 6 | 15% |
| 6 wk after index date | 3 | 7% |
| 3 mo after index date | 15 | 37% |
| 4 mo after index date | 2 | 5% |
| 6 mo after index date | 5 | 12% |
| 8 mo after index date | 1 | 2% |
| 9 mo after index date | 3 | 7% |
| 10 mo after index date | 1 | 2% |
| 305 d after index date | 1 | 2% |
| 12 mo after index date | 4 | 10% |
| 16 mo after index date | 1 | 2% |
| Cumulative duration of opioid use: total amount of calendar time used to define LTOT | ||
| 6 wk | 1 | 2% |
| 3 mo | 19 | 46% |
| 4 mo | 5 | 12% |
| 5 mo | 1 | 2% |
| 6 mo | 3 | 7% |
| 7 mo | 2 | 5% |
| 8 mo | 1 | 2% |
| 9 mo | 2 | 5% |
| 1 y | 7 | 17% |
| Unclear | 2 | 5% |
| Use of multiple, distinct observation periods (required more than 1, not including pre/post op) | ||
| Yes | 12 | 29% |
| No | 29 | 71% |
| Consecutive time periods (explicitly states consecutive) | ||
| Yes | 7 | 17% |
| Daily | 3 | 7% |
| Monthly | 2 | 5% |
| Quarterly | 1 | 2% |
| Other | 1 | 2% |
| No/not explicitly stated in the definition | 34 | 83% |
| Number of prescriptions | ||
| At least 1 | 25 | 61% |
| At least 2 | 6 | 15% |
| At least 3 | 2 | 5% |
| At least 4 | 3 | 7% |
| At least 5 | 1 | 2% |
| At least 10 | 4 | 10% |
| At least 12 | 1 | 2% |
| Used days’ supply | ||
| Yes | 11 | 27% |
| ≥90 d supply | 5 | 12% |
| ≥120 d supply | 4 | 10% |
| ≥365 d supply | 2 | 5% |
| No | 30 | 73% |
| Accounts for overlapping prescription | ||
| Yes | 3 | 7% |
| No | 38 | 93% |
| Used dose | ||
| Yes | 0 | 0% |
| No | 41 | 100% |
| Identified opioid product type | ||
| Yes | 28 | 68% |
| No | 13 | 32% |
| Long-term use is self-reported (from patient surveys) | ||
| Yes | 4 | 10% |
| No | 37 | 90% |
| Type of data used | ||
| Administrative data | 39 | 95% |
| Survey data | 4 | 10% |
| Washout period (and length of time required without opioid use) | ||
| Yes | 10 | 24% |
| 3 mo before the index | 3 | 7% |
| 11 mo preceding the month before surgery | 2 | 5% |
| 12 mo before index | 2 | 5% |
| 3 y before index | 2 | 5% |
| Unclear | 1 | 2% |
| No | 31 | 76% |
| Opioid use in look back period (and length of time required for look back period to detect opioid use) | ||
| Yes | 26 | 63% |
| 3 mo | 7 | 17% |
| 4 mo | 3 | 7% |
| 6 mo | 1 | 2% |
| >250 d | 1 | 2% |
| 1 y | 9 | 22% |
| unclear time period | 5 | 12% |
| No | 15 | 37% |
Almost 30% of the definitions required more than one distinct observation period during study follow-up. For example, one definition used two observation periods: between 30 days before surgery and 2 weeks after the surgery and 3 to 6 months after surgery.37 Only 17% of definitions explicitly specified consecutive observation periods. For example, one definition required a prescription fill for four consecutive 90-day time periods in the follow-up year.38
The number of prescriptions required for LTOT varied from one or more (61%) to 12 or more (2%). Days’ supply was used for 27% of the definitions, and 90 days’ supply was the most common cutoff (12%). No definitions considered dose and 68% of definitions considered product type. Only 7% of definitions accounted for the possibility of overlapping prescriptions. For 10% of the definitions, LTOT was self-reported from patient surveys.
About 64% of the definitions specified opioid use in the lookback period. The length of time used for the lookback period varied from 3 months (17%) to 1 year (22%), and most definitions had a 1-year lookback period.
Only 24% of definitions specified a washout period for opioid use, or time without opioid use before the index date. The amount of time for the washout period varied from 3 months (7%) to 3 years (5%), and most definitions had a 3-month washout period (7%).
3.2 |. Prevalence of LTOT
Estimates of LTOT prevalence varied across studies by the population studied and LTOT definition used (Table 1). For studies involving opioid-naïve patients with surgery (n = 3), the prevalence of LTOT during study follow-up ranged from 5.3% to 13%.37,39 The prevalence of LTOT among studies of opioid-naïve patients (n = 4) with CNCP ranged from 1.3% to 25%.40,41 Two studies did not provide enough information to calculate the LTOT prevalence.42,43
3.3 |. Predictors of LTOT
For the seven studies with opioid-naïve individuals, we identified 76 unique possible predictors of LTOT across eight analytic models that examined LTOT as an outcome (Table 3). According to the domains of the conceptual framework by Hooten and colleagues for LTOT, all predictors were classified as patient characteristics and none of the predictors captured the practice environment or prescriber characteristics.36 Within the patient characteristics domains, 44.7% of predictors were classified as medical and mental health conditions, 19.7% were sociodemographic factors, and 11.8% were pain etiology. There were no predictors that assessed individual responses to pain and opioids. Although not included in the conceptual framework, 23% of the predictors referred to prior or baseline health service and medication use.36
TABLE 3.
Predictors of long-term opioid therapy
| Category | Variable | Interpretation | Number of Time Odds Reported Greater Than 1 | Number of Time Odds Reported Less Than 1 | Number of Time Odds Reported Equal to 1 | Number of Models | References |
|---|---|---|---|---|---|---|---|
| Demographics | Age | Older age; lower risk (measured as categorical variable) | 5 | 4 | 4 | 8 | [37, 39–41, 64, 66, 71] |
| Demographics | Sex | Females have greater risk | 2 | 3 | 3 | 8 | [37, 39–41, 64, 66, 71] |
| Opioid use | Opioid dose | greater dose, greater risk | 1 | 3 | 2 | 4 | [39, 40, 66] |
| Pain diagnosis | Arthritis | Presence of arthritis, greater risk | 4 | 0 | 0 | 4 | [40, 41, 66] |
| Demographics | Race | Compared to White, Black have a greater risk | 0 | 1 | 4 | 4 | [39, 41, 64, 66] |
| Pain chronicity | Chronic pain | Presence of chronic pain have greater risk | 4 | 0 | 0 | 4 | [39–41] |
| Medication use | Benzodiazepine use | Presence of benzodiazepine prescription vs none | 2 | 1 | 0 | 3 | [39, 40] |
| Mental illness | Anxiety disorder | Presence of anxiety disorder, greater risk | 2 | 0 | 1 | 3 | [39, 66, 71] |
| Substance use issue | Tobacco | Tobacco or Smoking increases risk | 3 | 0 | 0 | 3 | [40, 66, 71] |
| Substance use issue | Alcohol or substance use disorder | Presence of alcohol or substance use disorder, greater risk | 2 | 0 | 1 | 3 | [39, 66, 71] |
| Mental illness | Depression | Presence of depression increases risk | 1 | 0 | 2 | 3 | [39, 64, 71] |
| Demographics | Race | Compared to white, Other/multiracial have a greater risk | 0 | 2 | 3 | 3 | [39, 41, 64] |
| Opioid use1 | Opioid type | N/A | 3 | [39, 40] | |||
| Comorbidities | Charleson comorbidities | Higher score, greater risk | 1 | 0 | 1 | 2 | [66, 71] |
| Demographics | Race | Compared to white, Asian have a greater risk | 0 | 1 | 2 | 2 | [41, 66] |
| Demographics | Race | Compared to white, Hispanic have a greater risk | 0 | 1 | 1 | 2 | [41, 66] |
| Mental illness | Mood disorder | Presence of mood disorder, greater risk | 1 | 0 | 1 | 2 | [66, 71] |
| Insurance | Type of insurance plan | compared to PPO, HMO coverage has greater risk | 0 | 0 | 2 | 2 | [37, 40] |
| Substance use issue | Drug disorder | Presence of drug disorder has greater risk | 2 | 0 | 0 | 2 | [40] |
| Substance use issue | Alcohol dependence/abuse | Presence of alcohol dependence/abuse, greater risk | 0 | 0 | 2 | 2 | [37, 40] |
| Medication use | Non opioid analgesic | Presence of non-opioid analgesic, greater risk | 1 | 0 | 1 | 2 | [39, 40] |
| Surgery2 | Surgery | N/A | [37, 66] | ||||
| Insurance | Type of insurance plan | compared to PPO, other coverage has greater risk | 0 | 1 | 1 | 2 | [37, 40] |
| Mental illness | Mental health disorder (schizophrenia, mood disorders, neurotic disorders, and depression) | Presence of mental health disorder, greater risk | 2 | 0 | 0 | 2 | [37, 40] |
| Utilization | Primary care visit (within 30 days) | Presence of primary care visit (within 30 days) has greater risk | 1 | 1 | 0 | 2 | [41, 71] |
| Comorbidities | Asthma | Presence of asthma, greater risk | 0 | 1 | 1 | 2 | [40, 41] |
| Pain diagnosis | Osteoporosis | Presence of osteoporosis, greater risk | 1 | 0 | 1 | 2 | [40, 41] |
Note. This table only includes predictor variables that were in more than one model. Studies with opioid-naïve sample37,39–41,64,66,71 Results from Koeppe 2011 are not included because the definition of opioid-naïve sample differed from the other studies.73
Excludes variables that referenced opioid type, but models examined long-acting, tramadol, codeine, oxycodone, hydrocodone, hydrocodeine.
Excludes surgery related variables, but models examined varicose vein removal, laparoscopic cholecystectomy, laparoscopic appendectomy, hemorrhoidectomy, thyroidectomy, transurethral prostate surgery, parathyroidectomy carpal tunnel, ventral incisional hernia repair, colectomy reflux surgery, bariatric surgery, hysterectomy, nonoperative comparisons, carpal tunnel release, carpometacarpal arthroplasty/arthrodesis, cubital tunnel release, trigger finger release, closed distal radial fracture fixation, flexor tendon repair, metacarpal fracture fixation, phalangeal fracture fixation.
The most common predictors included age (eight models), sex (eight models), opioid dose at baseline (four models), arthritis (four models), black race (four models), and presence of chronic pain (four models). Arthritis, chronic pain, tobacco use, drug disorders, and mental health disorders were consistently associated with greater risk of LTOT.
4 |. DISCUSSION
This systematic review identified 41 unique variations of definitions of LTOT across 34 studies. The definition of LTOT differed by the follow-up time, cumulative duration of opioid use for LTOT, the time points used to define LTOT, and consistency of opioid use. All studies included follow-up time of at least 3 months, but half of the definitions had follow-up times that were less than 1 year. Shorter follow-up times make it difficult to observe LTOT, which is defined as longer than 3 months. For example, LTOT that begins near the end of a follow-up period would be misclassified as short-term opioid therapy. Longer follow-up times would improve LTOT identification and distinguish LTOT from episodic opioid therapy.
Most definitions used more than 3 months as the cumulative duration of opioid use for LTOT, which aligns with guideline definitions of chronic pain.3,18,19 However, the length of time required for LTOT does not always account for consistent opioid therapy during follow-up. The definitions of opioid therapy during the time windows within the follow-up time were not strict and many definitions only required one opioid prescription during a predefined time period for LTOT.40,43–54 For example, one study defined LTOT as at least one prescription 305 to 425 days after a hospital discharge.44
By only requiring one prescription during a specified window of time after the index date, these definitions fail to identify consistent use. Studies often used EHR data or claims data which contain rich information about prescriptions, but LTOT definitions rarely incorporated prescription characteristics such as the frequency of fills, fill dates, and days’ supply. These characteristics capture consistent opioid use and overlapping prescriptions which exemplify differing levels of risky LTOT.18 Eleven definitions used days’ supply, and only three definitions used daily use to identify LTOT, a stark contrast to traditional measures of adherence which often require days’ supply in the definition.23 Only three definitions described methods used to account for gaps between prescriptions and overlapping prescriptions.39,41 Identifying consistent use is important because it more closely reflects actual opioid therapy characteristics, compared with fills at various time points during follow-up.
While length of time of opioid therapy is the only necessary criterion to define LTOT, context about the nature of LTOT is important to identify patients who are at the highest risk for adverse outcomes. According to clinical guidelines, dose and opioid type are associated with greater risk of adverse events.18 None of the included studies used dose, and 68% of definitions distinguished opioid type in the definition of LTOT. Patients with LTOT may become tolerant or increase doses over time.18 Similarly, patients may also switch between long and short acting opioids during opioid use. Although technically reflective of LTOT, measures that do not account for these characteristics may not identify high-risk populations.27 For example, identifying patients with doses above recommended thresholds (more than 90 MME) during LTOT allows providers to identify patients who could benefit from tapering opioid doses.18
Assessing clinical appropriateness of LTOT is challenging because definitions lacked clinical information (eg, pain scores or functional status). Clinical information may not be available or easily accessible in claims or EHR data. Both claims and EHR data provide “as-prescribed” information, but they do not include patient behaviors (whether and how medications are consumed, disposed of, or diverted). For example, claims data only has data on prescriptions reimbursed by insurers, and does not have data on medications that were prescribed and never filled, or prescriptions that were paid for out-of-pocket or by other insurers. EHRs may not contain information about all medications dispensed. Without data on clinical indicators and all opioid prescriptions, clinically appropriate LTOT is difficult to distinguish from risky LTOT.
Very few LTOT definitions were described and implemented in exactly the same way. The definition of LTOT by Von Korff and colleagues was cited by several included studies.20 LTOT was defined as “more than 90 days and more than 10 opioid prescriptions or more than 120 days’ supply” during a 6-month follow-up time.20 The definition also incorporated average daily doses by delineating between high-doses (more than 20 MME) and low doses (less than 20 MME).20 The application of these definitions in the included studies was not always clear. Interestingly, one study took a data-driven approach for LTOT definitions by using group-based trajectory modeling, which identifies types of opioid use depending on changes in opioid use over time.55–57 Data-driven approaches may improve the classification of LTOT, but studies have not compared the performance of data-driven approaches to a simple, binary measures defined at prespecified time points. These definitions have not been validated with patient self-reports or monitoring with urine drug screens.
Only a handful of included studies used prescription characteristics to define LTOT, potentially reflecting a lack of guidance on using prescription characteristics, limited analytic resources, or belief that prescriptions characteristics do not improve the sensitivity of the measure. Existing guidance for researchers and health systems contains conflicting definitions for LTOT.16–18,58,59 As illustrated in one study using prescription registry data to create different LTOT definitions based on the dose, number of prescriptions, consistency of use, population characteristics vary depending on the criteria used.60 Inconsistent definitions of LTOT inhibit our understanding of the utility of LTOT as an indicator of high-risk opioid use. We found that application of the various definitions has led to highly variable estimates of the prevalence of LTOT and inconclusive evidence on the risk factors of LTOT.
Across the studies of opioid-naïve individuals, the evidence regarding the predictors associated with LTOT is limited to patient characteristics, reflecting the data elements available in administrative data. The lack of evidence about individual responses to pain and opioid use as well as the patient’s preferences, knowledge, beliefs, and psychosocial stressors indicates limited self-reported data available from opioid-naïve patients who develop LTOT. There was no evidence about prescriber characteristics (eg, pain management training, beliefs about opioid prescriptions, and professionalism) and the practice environment (eg, health care team composition, capacity to deliver care, incentives, guideline adoption, prescribing laws, and prescription drug monitoring use) in the included studies. Further research is needed to understand how patient characteristics, prescriber characteristics, and practice environment characteristics interact with one another to influence LTOT.
The most common predictors used in studies include age, sex, opioid dose at baseline, and race, but the direction of the association varied depending on the model. However, in these studies, arthritis, chronic pain, tobacco use, drug disorders, and mental health disorders were consistently identified as risk factors for LTOT, many of which are similar to the risk factors associated with misuse, abuse, and overdose.27–32 Finally, only three predictors examined prior opioid prescriptions, suggesting limited evidence on how initial prescribing may lead to LTOT. In a study not included in this review because it included pediatric patients, the number of fills and total dose during the first month of opioid use were associated with greater risk of LTOT.61
This review has several limitations. Our focus on the transition from short-term to LTOT means we did not examine studies of the time to opioid discontinuation. We also excluded studies where LTOT definitions were used solely to identify populations to target for interventions or where LTOT was a predictor. A meta-analysis of the predictors of LTOT was not feasible because of heterogeneity in study design and populations across the studies. Comparing findings for predictors across studies may be difficult because of the variation in the LTOT definition. Finally, we excluded studies that were published prior to 2007, which may have resulted in the exclusion of additional definitions of LTOT. The evidence from this review is limited to only the definitions of LTOT and does not assess how these definitions may impact the estimates for the risk of adverse events associated with LTOT. The above limitations are unlikely to alter our overall findings that the heterogeneity in study designs, populations, and LTOT definitions prevent the meta-analysis and comparison of predictors of LTOT across studies.
The implications of measurement error and misclassification, particularly for opioid research using large administrative datasets, are not unique to defining LTOT, and have been discussed in reviews of other measures such as opioid abuse and nonmedical prescription opioid use.30,62,63 This study does not identify a common length of time for LTOT, nor did it aim to address the appropriate cutoff for the length of time used to define LTOT. At a minimum, researchers should include criteria that reflect consistent opioid use over time. Validation of LTOT definitions can better support efforts to predict and prevent LTOT. Researchers should consider incorporating clinical information available from data sources, such as patient-reported outcome measures (eg, pain and functioning) or EHR data, to better distinguish between clinically appropriate and inappropriate LTOT as well as low-risk and high-risk LTOT. Incorporating opioid dose exposure into LTOT definitions can distinguish the risks associated with LTOT. Patient reported measures and opioid dose can create risk-stratified definitions of LTOT to inform the selection of interventions for LTOT based on risk. Diverse data sources, including primary collection, could identify multilevel risk factors that influence LTOT. Researchers should perform sensitivity analyses around their selected definition of LTOT to evaluate the robustness of their measure. Applying these recommendations will reduce bias and improve the internal validity of studies that examine LTOT as an outcome.63
5 |. CONCLUSION
LTOT is quickly becoming a component used to assess quality of care for CNCP patients. We found that across studies of CNCP patients from the last decade, definitions of LTOT have been inconsistent, often lacking key prescription characteristics, such as opioid product type (eg, long acting vs short acting), frequency of fills, dose, and days’ supply, that could distinguish between low- and high-risk LTOT. Use of unstandardized LTOT definitions has led to inconsistent estimates of the prevalence of LTOT and weak evidence on the risk factors of LTOT. Without addressing the potential bias resulting from misclassification and measurement error of LTOT measures, translating findings into policies and clinical practices to prevent adverse events that stem from LTOT will be difficult.
Key Points.
Despite no gold standard definitions for long-term opioid therapy (LTOT), it is increasingly used as a component of quality improvement efforts to identify populations at risk for opioid-related adverse events and inform interventions to promote appropriate opioid therapy.
LTOT definitions often lacked information about dose and consistency of use, despite availability of these elements in secondary data sources.
Inconsistent definitions of LTOT have led to differences in estimates of the prevalence of LTOT and inconclusive evidence of the risk factors for LTOT.
Researchers should consider incorporating criteria that indicate consistency of opioid use when defining LTOT and perform sensitivity analyses to evaluate the robustness of LTOT measures.
A consensus about LTOT definitions is needed to identify patients with LTOT, understand the risk factors that lead to LTOT, and evaluate clinical practices and policies aimed at reducing risky LTOT.
ACKNOWLEDGEMENTS
R.N.K was supported by The Permanente Medical Group through the Delivery Science Post-Doctoral Fellowship and the Duke Clinical Research Institute through the Pre-Doctoral Fellowship. A.W.R. was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (#KL2TR002367). None of the other authors received funding for this work.
FUNDING DISCLOSURE
R.N.K. was supported by The Permanente Medical Group through the Delivery Science Post-Doctoral Fellowship and the Duke Clinical Research Institute. S.R.R. received research funds from GlaxoSmithKline for work outside of the current work. A.W.R. was supported by a CTSA grant from NCATS awarded to the University of Kansas for Frontiers: University of Kansas Clinical and Translational Science Institute (#KL2TR002367). C.I.C. has received support through her institution for work outside the current work from the Industry PMR Consortium, a consortium of companies working together to conduct postmarketing studies required by the Food and Drug Administration that assesses known risks related to opioid analgesic use.
Funding information
The Permanente Medical Group, the Duke Clinical Research Institute; National Center for Advancing Translational Sciences, Grant/Award Number: KL2TR002367
APPENDIX 1:
SEARCH TERMS
| Key Term | Search Words |
|---|---|
| Opioids | Analgesics, Opioid[MeSH] OR opiate, opiates, opioid, opioids, addnok, belbuca, buprenex, buprenophine, buprenorfina, buprenorphine, buprenorphinum, buprigesic, butrans, morgesic, norphin, norspan, probuphine, subutex, temgesic, tidigesic, butorphanol, codeine, methylmorphine, “morphine monomethyl ether”, codhydrine, cohydrin, dehacodin, didrate, dihidrocodeina, dihydrin, dihydrocodeine, dihydrocodeinum, dihydrocodeinum, dihydrokodein, dihydrokodein, dihydroneopine, diidrocodeina, drocode, hydrocodeine, hydrocodin, nadeine, novicodin, novicondin, paracodin, paracodine, parzone, rapacodin, “alpha-hydrocodol”, dihydromorfin, dihydromorphine, hydromorphine, paramorfan, paramorphan, difenossilato, difenoxilato, diphenoxalate, diphenoxylate, diphenoxylatum, abstral, duragesic, durogesic, durotep, fentanest, fentanil, fentanila, fentanyl, fentanylum, fentora, lazanda, matrifen, pecfent, phentanyl, recuvyra, sentonil, sublimase, subsys, codinovo, dicodid, dihydrocodeinone, hycodan, hycon, hydrocodeinonebitartrate, hydrocodon, hydrocodone, hydrocon, robidone, dihydromorfinon, dihydromorphinone, dilaudid, dimorphone, exalgo, hidromorfona, hydromorfona, hydromorph, hydromorphone, hydromorphonum, idromorfone, jurnista, palladone, aromarone, levorfanol, levorfanolo, levorphan, levorphanol, levorphanolum, demerol, dolantin, dolcontral, dolosal, dolsin, isonipecaine, lydol, meperidine, mialgin, pethidin, pethidine, pethidinum, petidin, petidina, petydyna, sauteralgyl, spasmedal, spasmodolin, adanon, algovetin, althose, amidon, amidone, dextromethadone, diaminon, dolophin, dolophine, fenadone, heptadone, heptanon, ketalgin, levometadona, levomethadonum, levothyl, metadona, metadone, metasedin, methadon, methadona, methadonum, methaquaione, phenadone, polamidone, polamivet, sedo-rapide, tussol, westadone, methadone, ambenyl, avinza, bromanyl, chembl70, codrix, depodur, dimetane, infumorph, kadian, morphine, mybanil, triacin, nalbuphine, nubain, opium, codeinone, combunox, dihydrohydroxycodeinone, dihydrohydroxycondeinone, dihydrone, dihydroxycodeinone, dinarkon, diphydrone, endine, endone, eubine, eucodal, eucodalum, hydroxycodeinon, ossicodone, oxanest, oxicodona, oxicon, oxicone, oxiconum, oxyir, oxycodeinon, oxycodeinone, oxycodon, oxycodonum, oxycone, oxycontin, oxyfast, oxynorm, pancodine, pancodone, percobarb, percodan, percolone, remoxy, roxicet, roxicodone, supendol, tekodin, theocodin, oxycodone, oxymorphone, pentazocine, talacen, talwin, biomadol, contramid, labesfal, ralivia, tradonal, tramadis, tramadol, tramadolum, tramadon, tramaliv, trapidol, ultram, zytram, darvon, dextropropoxyphene, propoxyphene, Nucynta, tapentadol |
| Long-term opioid therapy | long term persistent, episodic, prolonged, chronic |
| Risk factors | risk factor, risk factors, predictive, predict, predicts, prediction, population at risk, populations at risk, at risk population, at risk populations |
REFERENCES
- 1.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. Morbidity and Mortality Weekly Report. 2018;67(36):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain research and Management. 2011;16(6):445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Association for the Study of Pain. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986(3): S1–226. [PubMed] [Google Scholar]
- 4.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, Tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958–968. [DOI] [PubMed] [Google Scholar]
- 5.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Prim Care Companion CNS Disord. 2012;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in opioid prescriptions among part D medicare recipients from 2007 to 2012. The American Journal of Medicine. 2016;129(2):221.e221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Axeen S. Trends in opioid use and prescribing in Medicare, 2006–2012. Health Serv Res. 2018;October;53(5):3309–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smolina K, Gladstone EJ, Rutherford K, Morgan SG. Patterns and trends in long-term opioid use for non-cancer pain in British Columbia, 2005–2012. Canadian Journal of Public Health. 2016;107(4–5): e404–e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiology and Drug Safety. 2018;27(5):526–534. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States Surveillance Special Report. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; Published August 31, 2018. Accessed 3/2/2019 from https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf.2018. [Google Scholar]
- 14.Canadian Institute for Health Information. Pan-Canadian trends in the prescribing of opioids, 2012 to 2016. Ottawa, ON: CIHI; 2017. [Google Scholar]
- 15.Von Korff MR. Long-term use of opioids for complex chronic pain. Best Pract Res Clin Rheumatol. 2013;27(5):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HEDIS. NCQA Updates Quality Measures for HEDIS® 2019. 2018; https://www.ncqa.org/news/ncqa-updates-quality-measures-for-hedis-2019/. Accessed April 1, 2019.
- 17.Centers for Disease Control and Prevention. Quality Improvement and Care Coordination: Implementing the CDC Guideline for Prescribing Opioids for Chronic Pain 2018 National Center for Injury Prevention and Control. Atlanta, GA: Division of Unintentional Injury Prevention. [Google Scholar]
- 18.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1). [DOI] [PubMed] [Google Scholar]
- 19.Busse JW, Craigie S, Juurlink DN, et al. Guideline for opioid therapy and chronic noncancer pain. Canadian Medical Association Journal. 2017;189(18):E659–E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. discussion 575–567 [DOI] [PubMed] [Google Scholar]
- 23.Lam WY, Fresco P. Medication adherence measures: an overview. BioMed research international. 2015;2015:217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008;11(1):44–47. [DOI] [PubMed] [Google Scholar]
- 25.Timmerman L, Stronks D, Groeneweg J, Huygen F. Prevalence and determinants of medication non-adherence in chronic pain patients: a systematic review. Acta Anaesthesiologica Scandinavica. 2016;60(4): 416–431. [DOI] [PubMed] [Google Scholar]
- 26.Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Medication adherence in patients with chronic non-malignant pain: is there a problem? European Journal of Pain. 2009;13(2):115–123. [DOI] [PubMed] [Google Scholar]
- 27.Park TW, Lin LA, Hosanagar A, Kogowski A, Paige K, Bohnert AS. Understanding risk factors for opioid overdose in clinical populations to inform treatment and policy. J Addict Med. 2016;Nov-Dec(10):6. [DOI] [PubMed] [Google Scholar]
- 28.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24(6):497–508. [DOI] [PubMed] [Google Scholar]
- 29.Sehgal N, Manchikanti L, Smith HS. Prescription Opioid Abuse in Chronic Pain: A Review of Opioid Abuse Predictors and Strategies to Curb Opioid Abuse. Pain Physician. 2012;15. [PubMed] [Google Scholar]
- 30.Alzeer AH, Jones J, Bair MJ. Review of factors, methods, and outcome definition in designing opioid abuse predictive models. Pain Med. 2017;19(5):997–1009. [DOI] [PubMed] [Google Scholar]
- 31.Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse: part 1. Pain Physician. 2017;20(2S):S93–S109. [PubMed] [Google Scholar]
- 32.Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2S): S111–S133. [PubMed] [Google Scholar]
- 33.Food and Drug Administration Amendments Act of 2007, 110, §901–909. [Google Scholar]
- 34.Canadian Institute for Health Information. Pan-Canadian trends in the prescribing of opioids and benzodiazepines, 2012 to 2017. Ottawa, ON: CIHI; 2018. [Google Scholar]
- 35.Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiology and drug safety. 2013;22(1):1–6. [DOI] [PubMed] [Google Scholar]
- 36.Hooten WM, Brummett CM, Sullivan MD, et al. A conceptual framework for understanding unintended prolonged opioid use. Mayo Clinic proceedings. 2017;92(12):1822–1830. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naive patients following common hand surgery procedures. The Journal of Hand Surgery. 2016;41(10):947–957.e943. [DOI] [PubMed] [Google Scholar]
- 38.Franklin GM, Rahman EA, Turner JA, Daniell WE, Fulton-Kehoe D. Opioid use for chronic low back pain: a prospective, population-based study among injured workers in Washington state, 2002–2005. The Clinical Journal of Pain. 2009;25(9):743–751. [DOI] [PubMed] [Google Scholar]
- 39.Mosher HJ, Hofmeyer BA, Hadlandsmyth K, Richardson KK, Lund BC. Predictors of long-term opioid use after opioid initiation at discharge from medical and surgical hospitalizations. Journal of Hospital Medicine. 2018;13(4):243–248. [DOI] [PubMed] [Google Scholar]
- 40.Thornton JD, Dwibedi N, Scott V, et al. Predictors of transitioning to incident chronic opioid therapy among workingage adults in the United States. Am Health Drug Benefits. 2018;11(1):12–21. [PMC free article] [PubMed] [Google Scholar]
- 41.Ray GT, Bahorik AL, VanVeldhuisen PC, Weisner CM, Rubinstein AL, Campbell CI. Prescription opioid registry protocol in an integrated health system. Am J Managed Care. 2017;23(5):e146–e155. [PMC free article] [PubMed] [Google Scholar]
- 42.Sun EC, Bateman BT, Memtsoudis SG, Neuman MD, Mariano ER, Baker LC. Lack of association between the use of nerve blockade and the risk of postoperative chronic opioid use among patients undergoing total knee arthroplasty: evidence from the marketscan database. Anesth Analg. 2017;125(3):999–1007. [DOI] [PubMed] [Google Scholar]
- 43.Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6): 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daoust R, Paquet J, Moore L, et al. Incidence and risk factors of long-term opioid use in elderly trauma patients. Ann Surg. 2018;268(6): 985–991. [DOI] [PubMed] [Google Scholar]
- 45.Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. The Journal of Bone and Joint Surgery American Volume. 2013;95(12):1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Orton M, Feng R, et al. Chronic opioid usage in surgical patients in a large academic center. Ann Surg. 2017;265(4):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbloom BN, McCartney CJL, Canzian S, Kreder HJ, Katz J. Predictors of prescription opioid use 4 months after traumatic musculoskeletal injury and corrective surgery: a prospective study. J Pain. 2017;18(8):956–963. [DOI] [PubMed] [Google Scholar]
- 48.Anciano Granadillo V, Cancienne JM, Gwathmey FW, Werner BC. Perioperative opioid analgesics and hip arthroscopy: trends, risk factors for prolonged use, and complications. Arthroscopy. 2018;34(8): 2359–2367. [DOI] [PubMed] [Google Scholar]
- 49.Cancienne JM, Patel KJ, Browne JA, Werner BC. Narcotic use and total knee arthroplasty. J Arthroplasty. 2018;33(1):113–118. [DOI] [PubMed] [Google Scholar]
- 50.Mueller KG, Memtsoudis SG, Mariano ER, Baker LC, Mackey S, Sun EC. Lack of association between the use of nerve blockade and the risk of persistent opioid use among patients undergoing shoulder arthroplasty: evidence from the marketscan database. Anesth Analg. 2017;125(3):1014–1020. [DOI] [PubMed] [Google Scholar]
- 51.Pugely AJ, Bedard NA, Kalakoti P, et al. Opioid use following cervical spine surgery: trends and factors associated with long-term use. Spine J. 2018. [DOI] [PubMed] [Google Scholar]
- 52.Qureshi R, Werner B, Puvanesarajah V, et al. Factors affecting long-term postoperative narcotic use in discectomy patients. World Neurosurg. 2018;112:e640–e644. [DOI] [PubMed] [Google Scholar]
- 53.Bartels K, Fernandez-Bustamante A, McWilliams SK, Hopfer CJ, Mikulich-Gilbertson SK. Long-term opioid use after inpatient surgery—A retrospective cohort study. Drug Alcohol Depend. 2018; 187:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rozet I, Nishio I, Robbertze R, Rotter D, Chansky H, Hernandez AV. Prolonged opioid use after knee arthroscopy in military veterans. Anesthesia and Analgesia. 2014;119(2):454–459. [DOI] [PubMed] [Google Scholar]
- 55.Kim SC, Choudhry N, Franklin JM, et al. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthritis Cartilage. 2017;25(9):1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franklin JM, Shrank WH, Pakes J, et al. Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Medical Care. 2013;51(9):789–796. [DOI] [PubMed] [Google Scholar]
- 57.Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods & Research. 2007;35(4):542–571. [Google Scholar]
- 58.U.S. Department of Health & Human Services, Office of Inspector General. Using data analysis to calculate opioid levels and identify patients at risk of misuse or overdose. (Report No. OEI-02-17-005600). retrieved from https://oig.hhs.gov/oei/reports/oei-02-17-00560.pdf. In. Washington D.C. 2018.
- 59.Kaiser Foundation Health Plan of Washington. Patients on chronic opioid therapy for chronic non-cancer pain safety guideline. retrieved from https://wa.kaiserpermanente.org/static/pdf/public/guidelines/opioid.pdf. In:2016.
- 60.Svendsen K, Skurtveit S, Romundstad P, Borchgrevink PC, Fredheim OM. Differential patterns of opioid use: defining persistent opioid use in a prescription database. Eur J Pain. 2012;16(3):359–369. [DOI] [PubMed] [Google Scholar]
- 61.Deyo RA, Hallvik SE, Hildebran C, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naive patients: a statewide retrospective cohort study. J Gen Intern Med. 2017;32(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cochran G, Woo B, Lo-Ciganic WH, Gordon AJ, Donohue JM, Gellad WF. Defining nonmedical use of prescription opioids within health care claims: a systematic review. Subst Abus. 2015;36(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranapurwala SI, Naumann RB, Austin AE, Dasgupta N, Marshall SW. Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiology and drug safety. 2019;28(1):4–12. [DOI] [PubMed] [Google Scholar]
- 64.Alghnam S, Castillo R. Traumatic injuries and persistent opioid use in the USA: findings from a nationally representative survey. Inj Prev. 2017;23(2):87–92. [DOI] [PubMed] [Google Scholar]
- 65.Anderson JT, Haas AR, Percy R, Woods ST, Ahn UM, Ahn NU. Chronic opioid therapy after lumbar fusion surgery for degenerative disc disease in a workers’ compensation setting. Spine. 2015;40(22): 1775–1784. [DOI] [PubMed] [Google Scholar]
- 66.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Connolly J 3rd, Javed Z, Raji MA, Chan W, Kuo YF, Baillargeon J. Predictors of long-term opioid use following lumbar fusion surgery. Spine (Phila Pa 1976). 2017;42(18):1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crocker JA, Yu H, Conaway M, Tuskey AG, Behm BW. Narcotic use and misuse in Crohn’s disease. Inflammatory Bowel Diseases. 2014; 20(12):2234–2238. [DOI] [PubMed] [Google Scholar]
- 69.Deyo RA, Hallvik SE, Hildebran C, et al. Use of prescription opioids before and after an operation for chronic pain (lumbar fusion surgery). Pain. 2018;159(6):1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn LK, Yerra S, Fang S, et al. Incidence and risk factors for chronic postoperative opioid use after major spine surgery: a cross-sectional study with longitudinal outcome. Anesth Analg. 2018;127(1):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fritz JM, King JB, McAdams-Marx C. Associations between early care decisions and the risk for long-term opioid use for patients with low back pain with a new physician consultation and initiation of opioid therapy. Clin J Pain. 2018;34(6):552–558. [DOI] [PubMed] [Google Scholar]
- 72.Jain N, Brock JL, Phillips FM, Weaver T, Khan SN. Chronic preoperative opioid use is a risk factor for increased complications, resource use, and costs after cervical fusion. Spine J. 2018. [DOI] [PubMed] [Google Scholar]
- 73.Koeppe J, Lichtenstein K, Armon C, Chmiel JS, Buchacz K, Wood K, Brooks JT, HOPS Investigators. Factors associated with initiation of prolonged analgesic use among patients in the HIV Outpatient Study (HOPS). The Clinical journal of pain. 2011;27(8):699–706. [DOI] [PubMed] [Google Scholar]
- 74.Kulshrestha S, Barrantes F, Samaniego M, Luan FL. Chronic opioid analgesic usage post-kidney transplantation and clinical outcomes. Clinical Transplantation. 2014;28(9):1041–1046. [DOI] [PubMed] [Google Scholar]
- 75.Mulligan RP, McCarthy KJ, Grear BJ, Richardson DR, Ishikawa SN, Murphy GA. Psychosocial risk factors for postoperative pain in ankle and hindfoot reconstruction. Foot & Ankle International. 2016;37(10): 1065–1070. [DOI] [PubMed] [Google Scholar]
- 76.Namba RS, Singh A, Paxton EW, Inacio MCS. Patient factors associated with prolonged postoperative opioid use after total knee arthroplasty. J Arthroplasty. 2018;33(8):2449–2454. [DOI] [PubMed] [Google Scholar]
- 77.Raebel MA, Newcomer SR, Bayliss EA, et al. Chronic opioid use emerging after bariatric surgery. Pharmacoepidemiology and Drug Safety. 2014;23(12):1247–1257. [DOI] [PubMed] [Google Scholar]
- 78.Rao AG, Chan PH, Prentice HA, et al. Risk factors for postoperative opioid use after elective shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27(11):1960–1968. [DOI] [PubMed] [Google Scholar]


