Abstract
The population of the United States is shaped by centuries of migration, isolation, growth, and admixture between ancestors of global origins. Here, we assemble a comprehensive view of recent population history by studying the ancestry and population structure of more than 32,000 individuals in the US using genetic, ancestral birth origin, and geographic data from the National Geographic Genographic Project. We identify migration routes and barriers that reflect historical demographic events. We also uncover the spatial patterns of relatedness in subpopulations through the combination of haplotype clustering, ancestral birth origin analysis, and local ancestry inference. Examples of these patterns include substantial substructure and heterogeneity in Hispanics/Latinos, isolation-by-distance in African Americans, elevated levels of relatedness and homozygosity in Asian immigrants, and fine-scale structure in European descents. Taken together, our results provide detailed insights into the genetic structure and demographic history of the diverse US population.
Keywords: population genetics, human history, human genomics, USA, birth records, demography, identity-by-descent
Introduction
The United States population is a diverse collection of global ancestries shaped by migration from distant continents and admixture of recent migrants and Native Americans. Throughout the past few centuries, continuous migration and gene flow have played major roles in shaping the diversity of the US. Mixing between groups that have historically been genetically and spatially distinct have resulted in individuals with complex ancestries, while within-country migration has led to genetic differentiation.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13
Deeply characterizing population history is important for understanding human evolution and demographic history, as well as for adequate study design when associating genotypes to phenotypes.14, 15, 16, 17 Earlier population genetic studies in the US broadly characterized this structure, typically using a limited set of ancestry-informative markers or uniparental mtDNA and Y chromosome DNA data.18 As the cost of genetic technologies have dropped, more recent studies have inferred population history with more complete genome-wide data, typically using more than 100,000 SNPs ascertained via sequencing or genotyping.
Previous genetic studies of the US population have sought to infer genetic ancestry and population history primarily in European Americans, African Americans, and Hispanics/Latinos.7, 8, 9,19,20 European American ancestry is characterized by substantial mixing between different ancestral European populations and, to a lesser extent, admixture with non-European populations.6,8,9 Isolation among certain European population, such as Ashkenazi Jewish, French Canadian, and Finnish populations, have also resulted in founder effects.21, 22, 23, 24 The mixing of European settlers with Native Americans has contributed to large variations in the admixture proportions of different Hispanic/Latino populations.1,4,9 Among Hispanics/Latinos, Mexicans and Central Americans have more Native American ancestry; Puerto Ricans and Dominicans have more African ancestry; and Cubans have more European ancestry.1,4 In African Americans, proportions of African, European, and Native American ancestry vary across the country and reflect migration routes, slavery, and patterns of segregation between states.2,3,7,9,25 Although much effort has been made to understand the genetic diversity in the US, fine-scale patterns of demography, migration, isolation, and founder effects are still being uncovered with the growing scale of genetic data, particularly for Latin American and African descendants with complex admixture histories.26,27 At the same time, there has been little research on the population structure of individuals with East Asian, South Asian, and Middle Eastern ancestry in the US.
Many previous studies have investigated specific population histories in the US at relatively small scales—on the order of hundreds to thousands of individuals. These studies have provided deep insights into many specific populations, with some well-powered to infer population history across a breadth of ancestries. Some of these insights have been made by applying methods that are computationally tractable only at smaller scales.28,29 More recently, however, important insights highlight the need for broader and more comprehensive investigations of population history. For example, recent studies have shown that population structure is inaccurately captured in small sample sizes.14,17 Additionally, millions of Americans have been interested enough in their genetic ancestry to pay direct-to-consumer companies for individual-level genetic ancestry reports.8,9 The reliability of these reports is high for many individuals, but they are dependent on (1) the representativeness of their reference panel or customer database, (2) completeness and accuracy of multigenerational birth origin data, and (3) the application of multiple approaches to gain holistic insights into population history.
In this study, we comprehensively evaluate the population history of more than 32,000 genotyped individuals in the US who partook in the National Geographic Genographic Project, a not-for-profit public participation research initiative to study human migration history. This project has several distinct advantages compared to other large-scale population genetics datasets. Participants were genotyped with the GenoChip, a validated array of ∼150,000 markers designed for genetic anthropology that excludes medically related SNPs to protect the health privacy of participants.30 Individual-level genetic data are accessible to researchers around the world to answer anthropological questions. Additionally, most participants report birthplace and ethnicity data for themselves, their parents, and their grandparents, enabling fine-scale insights into recent history. Furthermore, participants report their postal code when they participated in the study, enabling analysis of intragenerational migration. These data therefore enable high spatiotemporal resolution into historical migration patterns. While these trends are consistent with US history at the population scale, we note that genetic ancestry patterns are not commensurate with individual-level ethnicity (i.e., cultural identity).
Here, we leverage these advantages over existing data to identify patterns of genetic ancestry by studying pairwise sharing among the project participants. We combine these comparative patterns with ancestral birth origin records and geographic information to uncover recent demographic and migration trends. By comprehensively analyzing these data to learn about recent migration events, we gain deeper insights into ancestral origins than in many existing studies, especially into Latin America. We also provide early insights into Asian Americans often ignored in genetic studies of the US, including South Asians, East Asians, and Middle Easterners. We also identify detailed patterns among European and African American populations, recapitulating some similar trends reported previously. Taken together, we use accessible individual-level genetic and birth record data to provide insights into the ancestral origins and complex population histories in the US.
Material and Methods
Human Subjects
The Genographic Project and Geno 2.0 Project received full approval from the Social and Behavioral Sciences Institutional Review Board (IRB) at the University of Pennsylvania Office of Regulatory Affairs on April 12, 2005. The IRB operates in compliance with applicable laws, regulations, and ethical standards necessary for research involving human participants. All DNA samples included in this study came from customers of the National Geographic Genographic Project, who have consented to have their results used in scientific research. To participate in the Genographic Project, participants would first order a DNA Ancestry Kit through the Genographic Project website. To ensure anonymity, each DNA Ancestry Kit is encoded with a randomly generated, nonsequential, Genographic Participant ID number. Prior to providing a sample, participants must read an IRB-approved consent form and provide written consent. Participants would then give a saliva sample, mix the saliva sample with a stabilization buffer solution, and return it along with their completed consent form via postal mail. DNA is then extracted from the saliva sample and genome-wide genotyping was performed (Genotyping and Quality Control). Once participants obtain their results, they can voluntarily provide an additional separate consent on the Genographic Project website to make their genotype data anonymously available for qualified anthropological and genetic research.
In addition to providing a DNA sample, participants also provided geographic location (postal code) data and, optionally, family history information in the form of ancestral birth origin and ethnicity (up to grandparental level). All data of individuals who consented to research were deidentified prior to its inclusion in the Genographic research database. We limited our study to those individuals who provided valid geographic locations in the United States. Approximately 75% of individuals selected provided complete pedigrees and family history data (see Supplemental Material and Methods for further detail).
Genotyping and Quality Control
Participants of the Genographic project were genotyped with the GenoChip array,30 an Illumina iSelect HD custom genotyping bead array with approximately 150,000 markers that are Ancestry Informative Markers. It excludes markers that are medically related to protect the health privacy of participants and minimize the improper translation of direct-to-customer genetic ancestry results to clinical care.31 The ability of the Genochip array to discern subpopulations was validated by producing concordant ancestry patterns with samples from the 1000 Genomes Project and demonstrating similar FST distributions and higher mean FST values when compared to the Affymetrix Axiom Human Origins array (used in HGDP-CEPH) and the Illumina Human660W-Quad BeadChip.30 Raw genotype data were quality controlled (QC) using PLINK v1.90b3.39.32 We filtered to keep samples with ≤ 0.1 missingness, sites with = 0.0 missingness, and MAF ≥ 0.05. A total of 32,589 individuals and 108,003 SNPs passed quality control.
Ancestry Reference Panels
We leveraged a variety of reference populations to help better infer and interpret the genetic ancestry, admixture proportions, and population structure in the Genographic cohort. Data from the 1000 Genomes Project was used to help identify genetic ancestry and estimate admixture proportions.1 108,003 SNPs were shared between the Genographic samples and the 1000 Genomes Project samples. We also used data from the Population Reference Sample (POPRES) to help understand the population structure of individuals with European ancestry in the Genographic cohort.33 All analysis with the POPRES data was limited to the 46,710 SNPs that are shared between the two datasets. We also leveraged recently released sequence data for the Human Genome Diversity Project (HGDP) to expand the available set of ancestral populations from Asia.34 All analyses using the HGDP data was performed using the 105,944 SNPs shared between the samples in Genographic Project and HGDP.
Principal Component Analysis
We performed principal component analysis (PCA) on the quality-controlled samples using FlashPCA v.2.0.35 For PCA of all Genographic Project individuals, we used the genotypes of all 2,504 individuals from the 1000 Genomes Project as reference samples. We first computed PCs across the 108,003 shared sites for 1000 Genomes Project individuals. We then projected the Genographic Project individuals on the same principal component space using the flag: --project.
For PCA analyses of East Asian and South Asian populations, we used samples from 1000 Genomes Project that correspond to the East Asian and South Asian super populations. Similar to above, we first compute PCs for the 1000 Genomes Project samples separately for East Asians and South Asians. We then projected East Asian and South Asian Genographic Project individuals onto the respective principal component space using: --project.
Continental Ancestry Assignment
We assigned continental ancestry to each Genographic sample by using a random forest classifier. Using the PCs and known super population assignments (AFR, African; EUR, European; EAS, East Asian; AMR, admixed American; and SAS, South Asian) from the 1000 Genomes Project samples as training data, we applied the classifier to assign ancestry to each Genographic sample at 90% probability. We considered unassigned ancestries as “other” (OTH).
Comparison of Continental Ancestry Assignment with Self-Reported Data
To evaluate the concordance between continental ancestry assignments based on genetics and self-reported ethnicity, we standardized self-reported ancestral ethnicities and estimated the proportion of assigned individuals within each continental ancestry groups that have at least one grandparent with a matching continental ancestry. Since ancestral ethnicity data were provided in the form of free text and was therefore not standardized across participants, we manually cleaned and mapped the reported ethnicities to continent level ancestries. For example, African ancestry can include a country (e.g., Jamaican, Nigerian, Cape Verdean), an ethnic group (e.g., Amhara or Tigray from Ethiopia), a historical term used to describe African descendants in America (e.g., Melungeon, Maroon, Mulatto), or the commonly used terms of African American or Black.
Genetic Ancestry Proportion Estimation
We estimated admixture proportions using ADMIXTURE by first analyzing samples from the 1000 Genomes Project in unsupervised mode to learn allele frequencies.36 Then, we projected the learned allele frequencies onto the Genographic samples to obtain the admixture proportions using the flag: -P. We ran ADMIXTURE with K = 2–9 and chose K = 5 as the most stable representation based on cross-validation.
For the analysis of East Asian and South Asian, we combined samples from HGDP and 1000 Genomes Project together to build more comprehensive reference panels. Specifically, we combined 1000 Genomes Project populations under the East Asian (EAS) super population label with HGDP samples that have the East Asian and Oceania region label, and we combined 1000 Genomes Project samples under the South Asian super population label with Central South Asia labeled populations in HGDP. Similar to above, we first ran ADMIXTURE on the ancestral reference panels for East Asians and South Asians, separately. We then projected the learned allele frequencies onto the Genographic samples to obtain admixture proportions using the flag: -P. We tested a variety of clusters, K = 2–9, and chose K = 4 for East Asians and K = 3 for South Asians as the most stable representations.
UMAP
We applied the Uniform Manifold Approximation and Projection (UMAP) method to visualize subcontinental structure.37,38 We first combined the PCs of the Genographic samples and the 1000 Genomes Project samples into one dataset. We then applied UMAP on the first 20 PCs from the joint dataset to produce a two-dimensional plot. We tested various parameter choices for UMAP and found that the default nearest neighbor value of 15 and the minimum distance values of 0.5 delivered the clearest result. Coloring of UMAP plots are described in the Supplemental Material and Methods.
We further examined the subcontinental structure of Genographic Project individuals who were classified as European ancestry individuals with data from the Population Reference Sample (POPRES).33 Similar to the analyses with the 1000 Genomes Project data, we performed dimensionality reduction with PCA and UMAP, keeping the same parameter values. Coloring of POPRES data was grouped by continental regions: Southeast Europeans = Croatia, Yugoslavia, Bosnia-Herzegovina, Serbia, Romania, Hungary, Albania, Macedonia; Central Europe = Switzerland, France, Germany, Germany, Swiss-Italian, Belgium, Swiss-French, Netherlands, Swiss-German; British Isle = Scotland, Ireland, United Kingdom; South Europe = Italy, Cyprus, Turkey, Greece; Iberian = Portugal, Spain; Eastern Europe = Austria, Czech Republic, Poland, Russia; Scandinavia = Sweden, Norway.
Phasing and Haplotype Estimation
Genographic genotypes were phased with the Sanger Imputation Service using EAGLE239 and the Haplotype Reference Consortium reference panel.40 No genotype imputation was performed.
fineSTRUCTURE Analysis
For classified East Asian individuals and South Asian individuals, we inferred clusters of unrelated individuals with shared ancestries by applying the fineSTRUCTURE framework v.4.0.1, a model-based approach to estimate patterns of haplotype similarity and identify clusters of discrete populations.29 We performed fineSTRUCTURE analysis separately for the two populations. The first part of the fineSTRUCTURE framework uses ChromoPainter to measure shared ancestry between individuals and estimate a coancestry matrix. This matrix is then used in fineSTRUCTURE’s clustering and tree-building algorithm to hierarchically cluster individuals from fine levels of structuring to broader levels. We first applied ChromoPainter to phased genotypes to estimate the number of contiguous segments (chunks) shared and total amount of genome (in cM) shared between each pair of individuals within each population, as well as the normalization parameter (c). Using the coancestry matrix and normalized parameter, we then ran the fineSTRUCTURE with 2 million Markov Chain Monte Carlo (MCMC) iterations, of which 1 million are “burn-in” iterations, and every 2,000 iterations was sampled. Finally, we used fineSTRUCTURE to infer a hierarchical tree using 100,000 hill-climbing moves. We used the scripts accompanying the fineSTRUCTURE software as well as the ape package in R to visualize the coancestry matrix and dendrogram results.
To examine the properties of the inferred clusters, we sought to examine structure at both the broad-scale and fine-scale. There is no definitively correct level of the dendrogram to pick for examination. We examined clades at various levels of the tree and assessed broad structure at the levels in which clades had sufficient number of individuals (on average 50 or more samples). We further used a combination of PCA and analysis of ancestral origins to assess and define these clades. Some of the clusters are small but genetically distinct as evident by the branch length and height of the split (i.e., Girmitiyas, Bangladesh), and therefore, they were kept as separate clades.
Unlike traditional PCA, PCA using the coancestry matrix (i.e., chunk counts matrix) can better discern fine-scale population structure and provide greater interpretability.29 We performed PCA on the chunk counts matrix using in the Python library scikit-learn. Individual markers are colored and labeled based on their respective grouping.
Estimating Effective Migration Surfaces
We estimated migration and diversity relative to geographic distance using the estimating effective migration surfaces (EEMS) method for Genographic Project individuals that were classified under African, European, and admixed American ancestries.41 We excluded East Asian and South Asian ancestries due to low sample size and density. We used unrelated individuals with available postal code data. We first computed pairwise genetic dissimilarities with the EEMS bed2diffs tool and then ran EEMS with runeems_snps, setting the number of demes to 250 and to 500. Per the recommendation in the manual, we adjusted the variance for all proposed distributions of diversity, migration, and degree-of-freedom parameters such that all were accepted 10%–40% of the time. We increased the number of Markov chain Monte Carlo (MCMC) iterations until it converged.
To evaluate the robustness of EEMS to sampling bias, we simulated three different sampling schemes. We used individuals classified with African ancestry as it is the smallest of the three ancestries and therefore more likely to be impacted by sampling bias. In the first sampling scheme, we randomly subsampled individuals to 80% of the original sample size. In the second scheme, we used the US Census Regions assignments for states and explored the impact of even sampling across the four major Census Regions. We subsampled African Americans so that each Census Region was represented in equal proportions. In the last sampling scheme, we explored the scenario of overrepresentation in the South by subsampling at 80% but this time with half of the subsamples being from the South and the remaining samples are evenly distributed across the three regions.
Haplotype Calling and Network Construction
We used IBDSeq v.r1206 to generate shared identity-by-descent (IBD) segments from genotype data for all unrelated individuals.42 Unlike other IBD detection algorithms, IBDseq does not rely on phased genotype data and is less susceptible to switch errors in phasing that can cause erroneous haplotype breaks. We filter for IBD segments greater than 3 cM. We removed segments that overlapped with long chromosomal regions (1 Mb) that had no SNPs across all unrelated individuals. These sites can result in false positives IBD sharing and likely correspond to centromeres and telomeres. We calculate the cumulative IBD sharing between individuals by summing the length of all shared IBD segments. We then constructed a haplotype network of unrelated individuals by defining vertices an individuals and edge weights between vertices as the cumulative IBD sharing between individuals. We filtered for edges with cumulative IBD sharing is ≥12 cM and ≤72 cM, as previously described.8
Detection of IBD Clusters
While fineSTRUCTURE can identify population structure in admixed cohorts using haplotype similarity,28 fineSTRUCTURE does not scale to large sample sizes and is not recommended for samples >10,000.29 We therefore sought to identify clusters of related individuals in the haplotype network using the Louvain Method implemented in the igraph package for R. The Louvain Method is a greedy iterative algorithm that assigns vertices of a graph into clusters to optimize modularity (a measure of the density of edges within a community to edges between communities).43 The Louvain Method begins by first assigning each node as its own community and then adds node i to a neighbor community j. It then calculates the change in modularity and places i in the community that maximizes modularity. The algorithm repeats this continuously and terminates when no vertices can be reassigned.
We partitioned the haplotype network into clusters by recursively applying the Louvain Method within subcommunities. At the highest level, we take the full, unpartitioned haplotype graph and identify a set of subcommunities. We isolate the vertices within each subcommunity, keeping only the edges between those vertices to create separate new networks. We then apply the Louvain Method to the new subgraphs. We repeat this process up to four levels. We combined subcommunities with low genetic divergence based on FST values of < 0.0001.
Annotation of IBD Clusters
We used a combination of ancestral birth origins and self-reported ethnicities to discern demographic characteristics of each cluster. For each cluster, we quantified the proportion of each birth origin (i.e., country of origin) among all four grandparents, treating each grandparent’s origin equality. We use these proportions to inform population labels. Clusters in which a single non-US birth origin was in high proportions was labeled with that country. In cases where multiple non-US birth locations exists in approximately equally high proportions, we assigned a label representing the broader region (e.g., Eastern Europeans for Poland, Lithuania, Ukraine, and Slovakia; East Asia for Japan, China). For certain clusters, annotations could not be easily discerned by birth origin data. In these cases, we relied on self-reported ethnicities to label the clusters as these populations were found to be less associated with a non-US country (e.g., Ashkenazi Jews) or the population has resided in the US for generations (e.g., African Americans, Acadians).
Runs of Homozygosity
We used PLINK v.1.90b3.39 to infer runs of homozygosity with a window of 25 SNPs.32 By default, PLINK reports only the runs of homozygosity with lengths ≥1 Mb. For each individual, we calculated the sum of runs of homozygosity (sROH) by summing the lengths of homozygous segments. We compared ROH segments inferred by PLINK with homozygosity-by-descent (HBD) segments inferred using IBDSeq. The two approaches largely agreed in ROH lengths (Spearman correlation = 0.94; p = 7.24 × 10−12), with the exception that the median sROH lengths for the Greece-Italy and Italy clusters were lower in IBDSeq while the median sROH length for East Asians were higher in IBDSeq when compared to PLINK (Table S1).
Local Ancestry Inference
We inferred local ancestry with RFMix v.1.5.4 for Genographic samples in clusters that were annotated as Hispanics/Latinos and African Americans.44 We used samples of African (LWK, MSL, GWD, YRI, ESN, ACB, and ASW; n = 661), European (CEU, GBR, FIN, IBS, and TSI; n = 503), and Native American (MXL, PUR, CLM, and PEL; n = 347) ancestry from the 1000 Genomes Project to build the reference panel for classifying genomic segments. We ran RFMix with the default minimum window size (0.2 centimorgans, cM) and a node size of 5 with the flags: -w 0.2, -n 5. We then collapsed the output of RFMix, which denotes the classified ancestry of each SNP for each individual, into local ancestry segments/tracts (in cM) for each individual. We then derived global ancestry proportions for each individual using that individual’s local ancestry tracts; we summed the length of local ancestry tracts for each ancestry (EUR, AFR, AMR) dividing by the total length of the genome to get the global proportion of each ancestry. Global ancestry proportions were visualized using the python-ternary package in Python (see Web Resources).
Genetic Divergence
We computed weighted Weir-Cockerham FST estimates for each pair of haplotype clusters using PLINK v.1.90b3.39.32 Using the distance matrix of FST values between clusters, we constructed an unrooted phylogenetic tree using the neighbor joining method implemented in scikit-bio (see Web Resources). We visualized the tree using Interactive Tree of Life (see Web Resources).
Effective Population Size
We estimated effective population size with IBDNe.45,46 Using the inferred IBD segments between individuals for each cluster, we ran IBDNe in default mode separately for each cluster to infer the effective population size over time along with confidence intervals.
Results
Genetic Ancestry and Diversity across the United States
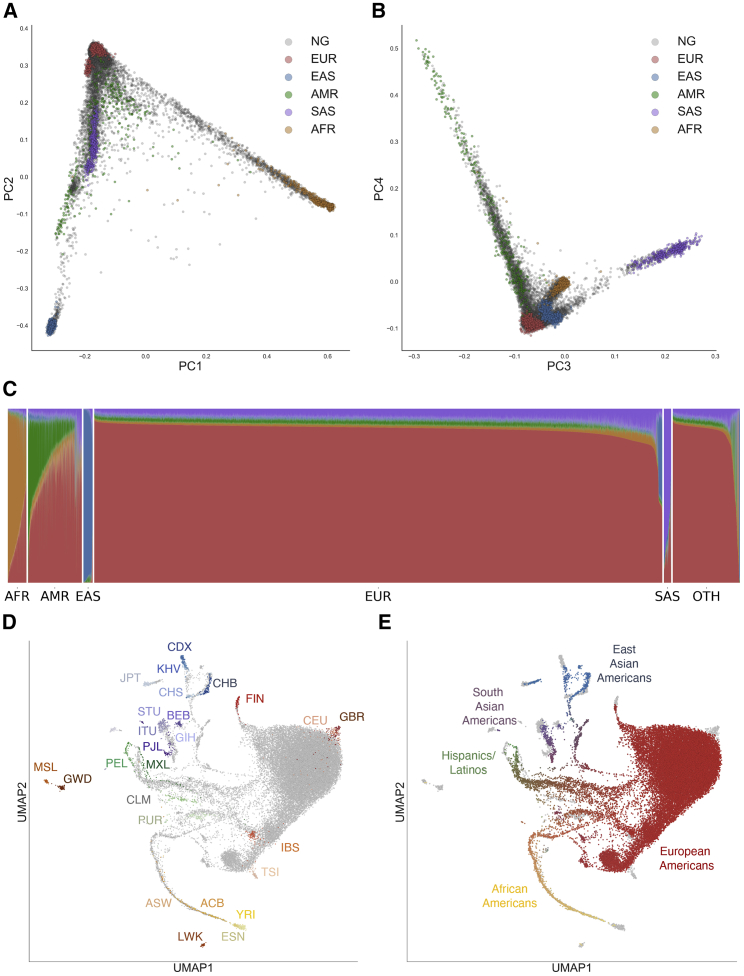
To assess the diversity of ancestries among individuals in the Genographic Project, we first performed principal component analysis, projecting Genographic samples into the same principal component (PC) space as that of the 1000 Genomes Project samples (Figures 1A–1C, S1, and S2).35,36 Since self-reported ancestry was not consistently provided across all Genographic Project individuals, we leveraged the 1000 Genomes Project data to assign continental ancestry to each Genographic sample (Material and Methods). We first trained a Random Forest classifier on the first 10 PCs of the 1000 Genomes Project samples with super population groupings as ancestry labels (EUR, European; AMR, Admixed American; AFR, African; EAS, East Asian; SAS, South Asian). We then used the trained model to assigned continental ancestry to each individual in the Genographic cohort at > 90% confidence. A total of 3,028 individuals (9.3% of total) did not meet the classification threshold (Table S2). The inability to classify these individuals may be due to variable levels of admixture not reflected in the 1000 Genomes reference populations. No particular bias was found in the ancestral birth origin records for these individuals, as the top non-US origins are Germany (3.0%), Italy (2.6%), Poland (2.5%), UK (2.5%), and Mexico (2.0%). Overall, the assigned continental ancestry was largely consistent with the self-reported ancestral ethnicity, as 95% of classified African-ancestry individuals and 85% of classified Hispanic-ancestry individuals who reported ancestral data had at least one grandparent of that ancestry (Material and Methods).
Figure 1.
Genetic Diversity of the US Population
(A) Principal Components Analysis (PCA) of individuals in the United States and in the 1000 Genomes Project. Each individual is represented by a single dot. Individuals in this study (NG) are colored in gray while 1000 Genomes Project individuals are colored by super population (EUR, red; AFR, yellow; AMR, green; EAS, blue; SAS, purple). Principal components (PC) 1 and PC 2 are shown.
(B) Similar to (A), except principal components (PC) 3 and PC 4 are shown.
(C) ADMIXTURE analysis at K = 5 of individuals in this study. Each individual was assigned a continent-level ancestry label using a random forest model trained on the super population labels and the first 10 PCs of the 1000 Genomes Project dataset. OTH, individuals who did not meet the 90% confidence threshold for classification.
(D and E) UMAP projection of the first 20 PCs. Each dot represents one individual. In (D), individuals in the 1000 Genomes Project are colored by population while US individuals from the National Geographic Genographic Project are in gray. In (E), 1000 Genomes Project individuals are colored in gray while US individuals from the National Geographic Genographic Project are colored based on their admixture proportions from ADMIXTURE. The color for each dot was calculated as a linear combination of each individual’s admixture proportion and the RGB values for the colors assigned to each continental ancestry (EUR, red; AFR, yellow; NAM, green; EAS, blue; SAS, purple). See Material and Methods for specific population labels.
Regional differences in genetic ancestry correspond to historical demographic trends. We evaluated the distributions of classified individuals across the four designated US Census regions: South, Northeast, Midwest, and West (Table S2). Classified individuals of European descent make up the majority (78.5%) of the Genographic cohort and are the most prevalent in the Midwest (82.8% of individuals in the Midwest; p < 0.01, Fisher’s exact test; Table S2). Admixed American ancestry individuals are most prominent in the West and South (9.7% and 7.8% of total individuals in the West and South, respectively; p < 0.05, Fisher’s exact test). Individuals classified as having African ancestry are most common in the South (3.2%), followed by the Northeast (3.0%). East Asians mostly reside in the West (2.1%), while South Asians are most abundant in the Northeast (1.0%). While the proportion of individuals classified as of European descent in the Genographic cohort (78.5%) are similar to the proportions of individuals reported as “White” in the US Census Data (76.1%; Table S3), we note that genetic ancestry is not a direct measure of ethnicity and race, and the two are not fully comparable (Supplemental Material and Methods). The large proportion of unclassified individuals also hinders our ability to properly compare the Genographic cohort to the US Census and understand how representative the Genographic cohort is of the US population. Overall, the distribution of Genographic Project participants by state reflects the US population distribution reported in the Census (Spearman’s ρ = 0.91, p = 1.5 × 10−20; Figure S3). However, the states of Washington, California, Virginia, Maryland, and Colorado have higher proportions (>1% difference) of participants when compared to the US population distribution while Texas and Ohio have lower proportions of participants (Table S4). For certain ancestries, some ascertainment bias exists. For example, individuals with African ancestry are overrepresented in California but are absent in Idaho, Maine, Nebraska, North Dakota, South Dakota, and Wyoming.
To uncover population substructure, we performed dimensionality reduction with Uniform Manifold Approximation and Projection (UMAP) on the first 20 PCs of a combined Genographic and 1000 Genomes Project dataset.37,38 By leveraging multiple PCs at once, UMAP can disentangle subcontinental structure (Figures 1D, 1E, S4, and S5). Similar to a previous analysis,38 populations in the 1000 Genomes Project form distinct clusters corresponding to ancestry and geography. The Genographic Project individuals project into several clusters, overlapping with the 1000 Genomes Project clusters. Consistent with the PCA and ADMIXTURE analysis, the largest clusters correspond to European ancestry and cluster closely with the 1000 Genomes CEU and GBR populations (CEU = Utah Residents with Northern and Western European Ancestry, GBR = British in England and Scotland).
While UMAP is a visualization tool with no direct interpretation of genetic distance, the continuum of points connecting UMAP clusters reflects the varying degrees of estimated admixture between different continental ancestries. In particular, the complex population structure of Hispanics/Latinos is shown by the points spanning between the clusters of European, Native American, and African ancestry. Coloring of these points based on ancestry proportions affirms the relationship between the degree of admixture and their relative position between reference clusters. Interestingly, African American individuals from both datasets form a single continuum from the European cluster to the Yoruba (YRI) and Esan (ESN) populations of Nigeria in the 1000 Genomes Project, indicative of the West African origins of most African Americans. This observation is consistent with and further expands the previous finding that the African tracts in the admixed 1000 Genomes Project populations of ACB and ASW are similar to the Nigerian YRI and ESN populations.2,47
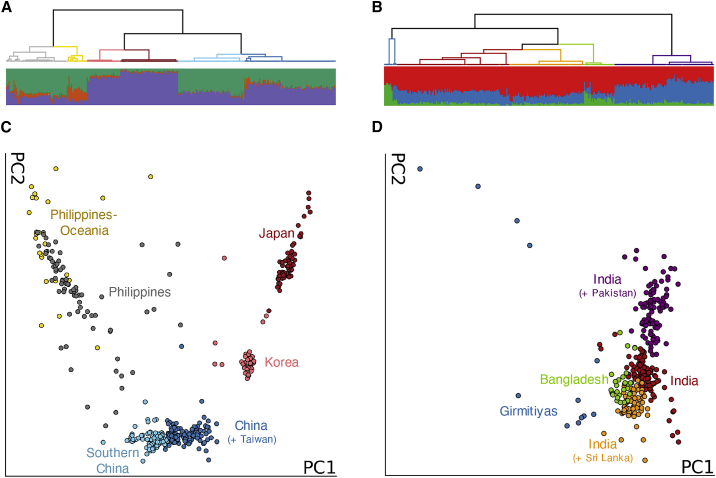
Fine-Scale Structure among US Individuals of Asian Ancestry
Existing genetic studies of the US population have largely overlooked East Asian and South Asian populations, likely due to their underrepresentation in datasets. We therefore explored the population structure of Genographic Project individuals classified as East Asians and South Asians. We used fineSTRUCTURE to first estimate patterns of haplotype similarities between individuals, taking into consideration linkage disequilibrium, and then hierarchically clustered individuals based on these patterns of shared ancestry to identify clusters of populations and their relationships.29 We applied fineSTRUCTURE to unrelated individuals in each population and inferred a total of 40 East Asian clusters (Figure 2A) and 26 South Asian clusters (Figure 2B). These clusters further organized into clades on the tree to reveal broader genetic structure. To visualize these structures, we performed PCA on the fineSTRUCTURE coancestry matrix. Compared to traditional PCA, distinctions between groups of individuals were clearer with fineSTRUCTURE PCA, particularly at the broader levels of genetic differentiation (Figures 2C and S6A; Figures 2D and S7A; Material and Methods). We also estimated subcontinental admixture proportions with ADMIXTURE using the East Asian and South Asian populations in the 1000 Genomes Project and the Human Genome Diversity Project (HGDP) as reference populations (Figures S6B, S6C, S7B, and S7C). Finally, we leveraged data from individuals who provided grandparental birth origin to help annotate and interpret the clusters and clades.
Figure 2.
Population Structure of East Asian and South Asian Individuals in the US
(A and B) fineSTRUCTURE dendrogram showing the hierarchical relationship between clusters inferred using the genotypes of classified East Asian individuals (A) and South Asian individuals (B). Branch colors represent clades with shared ancestral origins. The admixture proportion of each individual is displayed as a bar plot in the corresponding position below the dendrogram. The number of ancestral populations, K, is four for East Asians (A) and three for South Asians (B).
(C and D) Principal component analysis (PCA) of the fineSTRUCTURE co-ancestry matrix. Each individual (point) corresponds to a Genographic Project individual classified as either East Asian (C) or South Asian (D). The color of each point corresponds to a clade in the fineSTRUCTURE dendrogram shown in (A) and (B).
The patterns of shared ancestry among these US individuals capture the genetic diversity of East Asia and South Asia. The East Asian clusters broadly organize into six major clades, reflecting the different countries of ancestral origin (Figure 2A). At the highest level of genetic differentiation (top level of the hierarchical tree), individuals from Southeast Asia separate from East Asians. This Southeast Asian clade is predominantly represented by Filipinos with a branch of individuals with more Oceanic origins (shown in gray and yellow, respectively). Admixture proportions vary among the Southeast Asian individuals, likely due to the large number of ethnolinguistic groups that are found in the Philippines and neighboring islands. The East Asian clade further separates into individuals of Chinese descent (light blue and dark blue) and those from Japan (dark red) and Korea (light red). While the two Chinese-related groups share a branch on the tree, Taiwanese ancestral origins are more prevalent in one of the groups (dark blue), the “China (+ Taiwan)” group, while the other group (light blue), labeled “Southern China,” also contains some individuals from Laos and Vietnam. Lower levels of hierarchy did not differentiate these ancestral origins into separate groups. PCA and ADMIXTURE analysis for these two groups show that the China (+ Taiwan) cluster resembles the Han Chinese (CHB) population in the 1000 Genomes Project while the Southern China group resembles the Southern Han Chinese (CHS) population (Figure S6). Among the South Asian individuals, we observed genetic differentiation between individuals with ancestral origins from India, reflecting the diverse population structure previously observed in India.1,48 Of the three clades with majority Indian ancestral origin, ancestral origins from Pakistan was observed in the “India (+ Pakistan)” clade, while Sri Lankan ancestral origins were present in the “India (+ Sri Lanka)” clade. Individuals in these two clades resemble the Punjabi from Lahore, Pakistan (PJL) and Sri Lankan Tamil (STU) populations in the 1000 Genomes Project, respectively (Figure S7). Similarly, we also find a clade of individuals with Bangladesh ancestral origins that is similar to the 1000 Genomes Project Bengali from Bangladesh (BEB). Interestingly, we also inferred a small, but genetically distinct “Girmitiyas” clade (N = 12; blue branch in Figure 2B). While the small sample size makes it difficult to accurately assess this clade, we note that many former British colonies (e.g., Trinidad and Tobago, Fiji, Barbados, Guyana) are represented in the ancestral origins of these individuals. We therefore hypothesize that these individuals may potentially be descendants of Girmitiyas, indentured Indian laborers brought to those former colonies.49
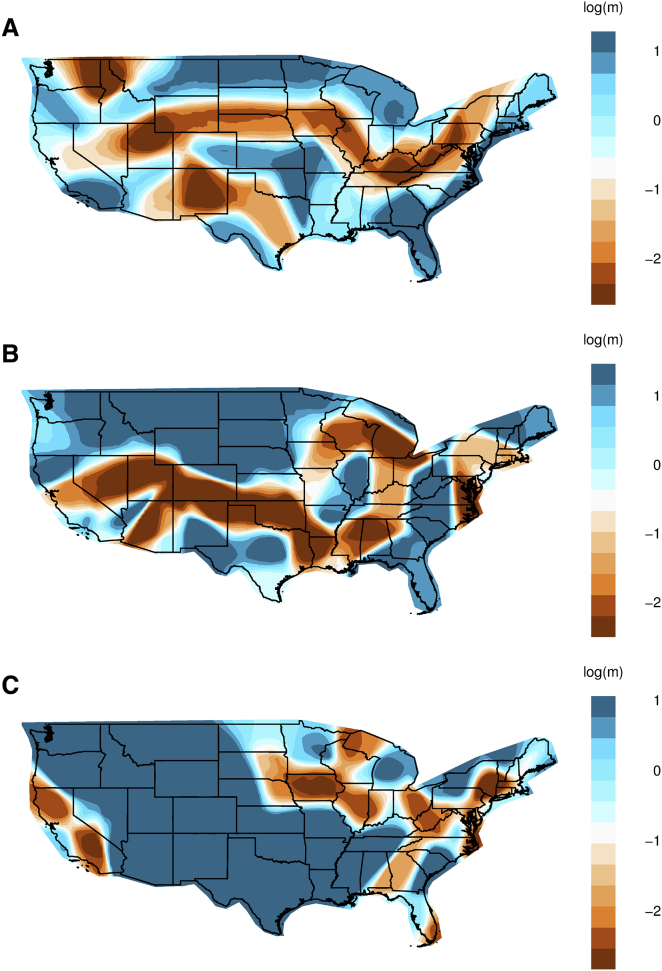
Population Differentiation and Migration Rate Inference across the United States
Understanding the relationship between genetics and geography can provide insights into demographic history. Previous analyses of this relationship in the US population have primarily compared data aggregated at the state or regional level.7,9 Such approaches, however, do not capture the fine-scale patterns of genetic similarity that are not influenced by discrete political boundaries. We therefore sought to infer population structure across continuous space with the estimating effective migration surfaces (EEMS) method.41 EEMS statistically measures effective migration rates by overlaying a dense grid of evenly spaced demes and calculating genetic differentiation (i.e., resistance distance) between neighboring demes. Higher rates of migration are inferred in locations where genetic similarity is high (colored in blue in Figure 3) while lower rates of migration are inferred in locations where genetic similarity is low (colored in dark orange). Areas with low effective migration are also referred to in EEMS as “barriers,” which can be intuitively interpreted as regions in which neighboring populations are more genetically dissimilar than expected. In more homogeneous populations, these barriers tend to indicate isolation by distance, while in more heterogenous populations, they may reflect differences in population structure. We applied EEMS to genetically classified Europeans, African Americans, and Hispanic/Latinos across the contiguous 48 states. We excluded East Asians and South Asians due to low sample density.
Figure 3.
Effective Migration Rates of African Americans, Hispanics/Latinos, and Europeans within the United States.
Migration rates inferred with EEMS for African Americans (A), Hispanics/Latinos (B), and Europeans (C). EEMS models the relationship between genetics and geography by assessing the decay of genetic similarity with respect to geographic distance. Colors and values correspond to inferred rates, m, relative to the overall migration rate across the country. Shades of blue indicate higher migration (i.e., log(m) = 1 represents effective migration that is 10-fold faster than the average) and higher levels of genetic similarity while shades of orange indicate migration barriers and lower levels of genetic similarity.
The inferred migration rates for African Americans reveal genetic signatures of historical demographic events (Figures 3A, S8, and S9). Along the Atlantic coast from the Florida Panhandle to southern Maine, genetic similarity and effective migration rates are relatively high, indicating the constant migration and similar effective population sizes of African Americans in these states. However, we also observe a strong north-south barrier to migration starting along the Appalachian Mountain Range, continuing north up the Mississippi River, and extending west across the rest of the country. This migration barrier, along with the migration barrier spanning Texas and New Mexico, reveals a pattern of genetic relatedness across geography that is consistent with the Great Migration from the 1910s to the 1960s in which an estimated 6 million African Americans migrated out of the South to cities across the Northeast, Midwest, and West.7,50 To understand whether this migration barrier is influenced by sampling bias, we subsampled individuals and simulated three different sampling schemes (Material and Methods). We found that the north-south migration barrier was consistently present in all three sampling schemes, confirming that the inferred migration results of EEMS are robust to irregular sampling (Figure S10).41
A highly complex pattern of genetic similarity exists among present-day Hispanics/Latinos across the country, capturing regional genetic structure. Across the southwestern states, two regions bordering Mexico—one in California and another extending from New Mexico to Texas—exhibit high levels of genetic similarity and effective migration rates (Figures 3B, S8, and S9). Separated by a migration barrier in Arizona, these two distinct regions likely reflect known differences in the northward migration from east versus west Mexico.8,51 High genetic similarity and relative rates of effective migration are also observed in Florida and continue northward. However, barriers to migration are observed in states immediately east of the Mississippi River, likely resulting from varying degrees of admixture.
The patterns of genetic similarity for Europeans capture subcontinental structure. With the exception of the states in the Midwest and along the Atlantic coast, elevated levels of genetic similarity and relative migration rates are observed across most of the country. We find low effective migration rates surrounding Minnesota and Michigan, likely due to the genetic dissimilarity of Finnish and Scandinavian ancestry that is abundant in the region (Figures 3C, S8, and S9).8 We also find reduced migration rates across Ohio, West Virginia, and Virginia, suggesting the existence of genetic differentiation along the Appalachian Mountains. Many of the major cities, such as Washington, DC, Philadelphia, and Miami, also exhibit low genetic similarity, perhaps due to greater genetic diversity and admixture within cities.
Coupling Fine-Scale Haplotype Clusters and Multigenerational Birth Records Uncovers Distinct Subcontinental Structure
To disentangle more recent and subtle population structure, we performed identity-by-descent (IBD) clustering on the Genographic cohort and annotated clusters using multigenerational self-reported birth origin data. We first built an IBD network from pairwise IBD sharing among 31,783 unrelated individuals, where vertices represent individuals and edges represent the cumulative IBD (in centimorgans, cM) between pairs of individuals. We employed the Louvain method, a greedy heuristic algorithm, to recursively partition vertices in the graph into clusters that maximize modularity for each iteration.8,43 The clusters of individuals resulting from each iteration can be interpreted as having greater amounts of cumulative IBD shared between individuals within the cluster than with those outside of the cluster. To aid in the interpretation of the clusters, we merged clusters with low genetic differentiation (FST < 0.0001), resulting in a final set of 25 clusters (Table 1). We annotated each cluster based on ancestral birth origin and ethnicity data and constructed a neighbor-joining tree based on the FST values (Figure S11). 98% of the 3,028 individuals that were not classified by our Random Forest model were assigned to a haplotype cluster. No single cluster was overrepresented by unclassified individuals, as unclassified individuals comprised of 8%–11% of each cluster.
Table 1.
Summary of Haplotype Clusters
| Cluster | Samples (Count) | Median Sum of ROH (Mb) | Median Cumulative IBD (cM) |
|---|---|---|---|
| Northwest Europe 1 | 11,725 | 2.88 | 15.23 |
| Northwest Europe 2 | 1,571 | 2.80 | 15.15 |
| Ireland | 2,137 | 2.85 | 15.42 |
| Central Europe | 3,116 | 2.83 | 15.06 |
| Eastern Europe | 2,471 | 3.16 | 15.37 |
| Southern Europe | 1,626 | 2.73 | 14.98 |
| Italy | 697 | 6.91 | 14.64 |
| Greece-Italy | 238 | 7.28 | 15.02 |
| Scandinavia | 717 | 3.02 | 15.54 |
| Finland | 314 | 3.67 | 17.50 |
| Acadia | 249 | 3.89 | 19.48 |
| French Canadian | 314 | 2.89 | 16.60 |
| Ashkenazi Jewish | 1,475 | 11.26 | 31.75 |
| Admixed Jewish | 445 | 2.75 | 15.50 |
| Hispanics/Latinos | 810 | 3.53 | 16.38 |
| Hispanics/Latinos in California | 573 | 4.10 | 17.11 |
| Hispanics/Latinos in New Mexico | 163 | 5.52 | 21.92 |
| Hispanics/Latinos in Texas | 177 | 6.27 | 23.65 |
| Puerto Rico | 350 | 8.01 | 26.23 |
| African Americans South | 761 | 3.34 | 19.56 |
| African Americans North | 420 | 2.94 | 15.90 |
| East Asia | 561 | 3.65 | 19.63 |
| Southeast Asia | 325 | 8.44 | 17.90 |
| South Asia | 389 | 10.42 | 14.82 |
| Greater Middle East | 93 | 9.01 | 17.16 |
Sum of runs of homozygosity (sROH) was calculated by summing the lengths of continuous homozygous segments ≥1 Mb. Cumulative IBD was determined by summing IBD segments of ≥3 cM and filtering for only pairs ≥12 cM and ≤72 cM. Statistics were determined within haplotype clusters, rather than across the ancestrally heterogeneous and imbalanced full network.
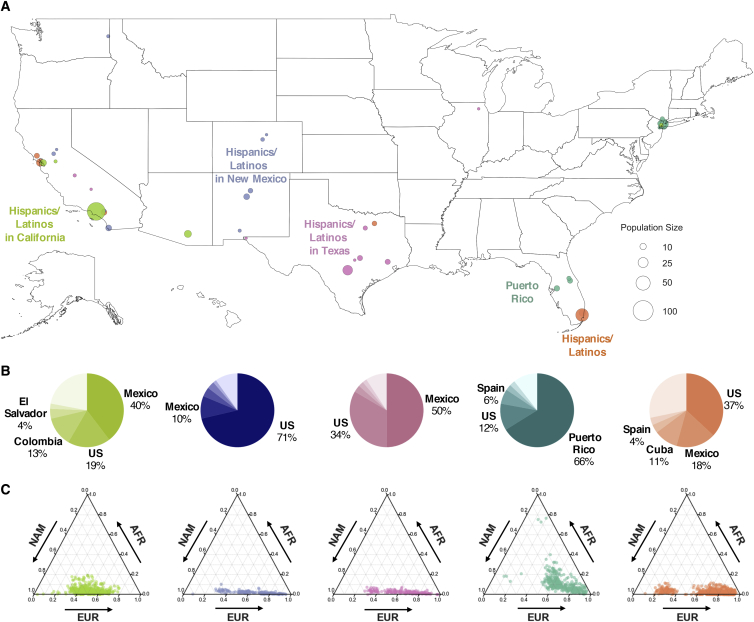
Genetic and geographic differences are greatest among Hispanic/Latino haplotype clusters. We identified a total of five Hispanic-related clusters (Figure 4). The largest of these cluster (n = 810; orange in Figure 4A) is strongly associated with south Florida (OR = 10.4; p = 2.5 × 10−25; Table S5) but is also found in California and Texas (OR ≥ 2; p < 0.05; Table S5). No single ancestral birthplace characterizes this cluster, as the US, Mexico, and Cuba each make up more than 10% of the birth origin labels (Figure 4B). Proportions of European ancestry tracts inferred with RFMix44 are higher in this cluster (mean = 72.7%, SD = 20.4%; Figure 4C) than in the other Hispanic/Latino clusters (mean = 48.0%–67.4%; Figure 4C). Puerto Ricans characterize a substantial proportion of another Hispanic/Latino cluster associated with Florida (OR > 4) as well as New York City (OR > 5). Unlike the other Hispanic clusters, the Puerto Rican cluster shares the same branch on the FST tree as the African American clusters (Figure S11), likely due to relatively high proportions of African ancestry (mean = 11.2%, SD = 9.0%) among Puerto Ricans. Median lengths of sROH and cumulative IBD in Puerto Ricans are also the highest among the Hispanic clusters (8.01 Mb and 26.23 cM, respectively; Table 1). Consistent with other studies,45,52 we found evidence of a strong bottleneck in Puerto Ricans approximately 9–14 generations ago (Figure S12), coinciding with the colonization of America and likely explaining the elevated levels of IBD and sROH.
Figure 4.
Geographical Distribution of Hispanic/Latino Haplotype Clusters
(A) Each dot corresponds to a county containing present-day individuals and the size of the dot signifies the number of samples of the particular cluster in that county. Only the Hispanic/Latino cluster with the highest odds ratio is shown for each county, and for clarity, only the top ten locations with the highest odds ratio are shown for each cluster. Maps showing the full distribution for each haplotype cluster can be found in the supplement (Figure S15).
(B) Ancestral birth origin proportions of each cluster for individuals with complete pedigree annotations, up to grandparent level. Proportions were calculated from aggregating the birth locations of all grandparents corresponding to members of each haplotype cluster. For each chart, only the top five birth origins are shown as individual proportions; the remaining birth origins are aggregated into one slice (lightest color).
(C) Ternary plots of ancestry proportions based on local ancestry inference for each haplotype cluster. Each dot represents one individual.
Three distinct clusters of Hispanics/Latinos were found in the Southwest (Figure 4A): one strongly associated with New Mexico (OR > 4; p < 0.05), another primarily in Texas (OR > 3; p < 0.05), and the third associated with Southern California (OR > 2; p < 0.05). Combined with the EEMS analysis, these clusters confirm our observation of parallel migration routes from east and west Mexico into Southwestern United States. While genetic differentiation between these three clusters are subtle (FST = 0.001–0.003), comparison of the ancestral birth origin patterns and local ancestry proportions of these clusters reveal meaningful differences in their population history. Whereas the majority of Hispanics/Latinos in New Mexico report US ancestral origins, the recent ancestors of Hispanics/Latinos in Texas are predominantly from Mexico. Nonetheless, these two clusters share similar local ancestry proportions with only slight genetic dissimilarity that result in a moderate decrease in migration rate (from darker blue to light blue in Figure 3B). Unlike the Hispanic/Latino clusters associated with New Mexico and Texas, the Hispanics/Latinos in California cluster contain greater proportions of ancestors from Central and South America (e.g., Colombia and El Salvador). Proportions of Native American ancestry (Figure 4C) and effective population size (Figure S12) are also higher in this cluster, but median cumulative IBD and sROH length are shorter (Table 1), similar to Central/South Americans found in New York City.52 Taken together, these two differences further explain the presence of the migration barrier in Arizona between Hispanic/Latino individuals in California and those in New Mexico.
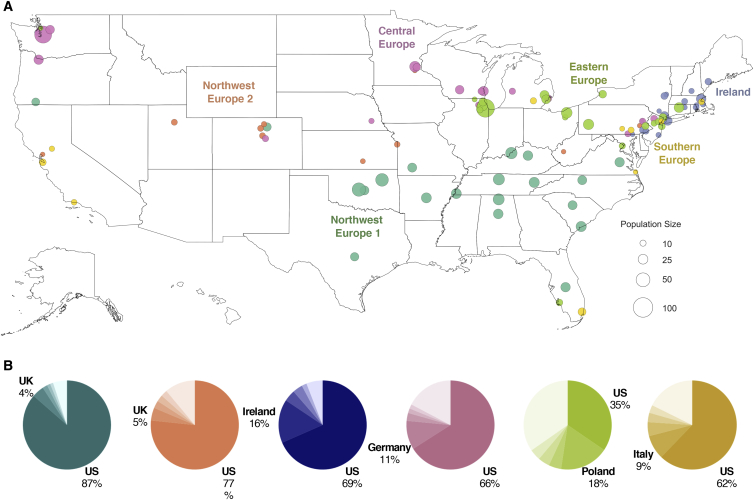
Historical immigration of Europeans into the US occurred in successive waves, with northern and western Europeans making up one wave from the 1840s to 1880s and another wave comprised of southern and eastern Europeans occurring from the 1880s to 1910s.53 Consistent with this immigration pattern, haplotype clusters with ancestries from northwest and central Europe have higher proportions of US ancestral birth origins than haplotype clusters from southern and eastern Europe, suggesting earlier immigration (Figures 5A and 5B). The two clusters with the highest proportion (>75%) of US ancestral birth origin (“Northwest Europe 1” and “Northwest Europe 2”) have ∼4.5% of UK ancestral origins. The central European cluster and the Irish cluster both have 66.1% and 68.5% of US ancestral origins, respectively (Figure 5B). In contrast, the US makes up only 62.2% and 34.5% of ancestral birth origin for the clusters of southern Europeans and eastern Europeans, respectively.
Figure 5.
Geographical Distribution of European American Haplotype Clusters
(A) Each dot represents a county containing present-day individuals. The size of the dot represents the number of individuals of the particular cluster in that county. For each cluster, the top 20 locations with the highest odds ratio are shown. Maps showing the full distribution for each cluster can be found in the supplement (Figure S16).
(B) Ancestral birth origin proportions for each cluster in (A). Only individuals with complete pedigree annotations, up to grandparent level, are included. For each chart, only the top five birth origins are shown as individual proportions; the remaining birth origins are aggregated into one slice (lightest color).
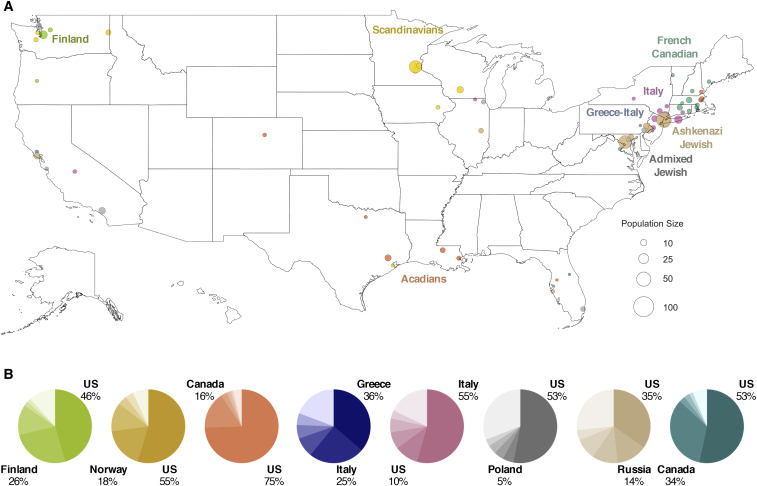
Unlike the larger European clusters, the smaller European clusters reflect the structure of recent immigrants and genetically isolated populations, recapitulating earlier findings.8 The geographic distributions of these subpopulations are more concentrated, and their ancestral birth origin proportions are overrepresented by specific countries and ethnicities (Figures 6A and 6B). Specifically, Finns and Scandinavians are abundant in the Upper Midwest and Washington; French Canadians are found in the Northeast; Acadians are present in the Northeast and Louisiana; and Italians, Greeks, and Jews are mostly located in the metropolitan area of New York City (Figure 6A). Of the European clusters, median cumulative IBD sharing and sROH lengths are highest among Ashkenazi Jews (31.8 cM and 11.3 Mb, respectively; Table 1), reflective of past founding events and endogamy.21,54 The two Jewish-related clusters were identified using self-reported ancestral ethnicity data rather than birth origin data, since Jewish ancestry is not specific to any single location. Jewish ancestry, particularly Ashkenazi Jewish ancestry, is more consistently reported on both sides of the family in the larger cluster, while individuals in the smaller cluster more commonly reported Jewish ancestry on only one side of the family, suggesting the presence of admixture with non-Jewish ancestries. Therefore, the larger cluster is labeled “Ashkenazi Jewish” and the smaller cluster is labeled “Admixed Jewish.”
Figure 6.
Geographical Distribution of Genetically Differentiated European American Haplotype Clusters
(A) Similar to Figure 5A but corresponding to European populations that are more genetically isolated. For clarity, the top ten locations with the highest odds ratio are shown for each cluster. Full distributions for each cluster can be found in the supplement (Figure S17).
(B) Ancestral birth origin proportions for each cluster in (A). Only individuals with complete pedigree annotations, up to grandparent level, are shown. For each chart, only the top five birth origins are shown as individual proportions; the remaining birth origins are aggregated into one slice (lightest color).
We inferred two haplotype clusters of African Americans separated along a north-south cline, recapitulating the EEMS migration barrier inference. One cluster is primarily distributed among the northern and western states (“African Americans North”), while the other is distributed among the states southeast of the Appalachian Mountains (“African Americans South”) (Figure S13). The proportion of US birth origin is higher in the northern cluster than the southern cluster, providing further evidence of isolation-by-distance among African Americans in the north.7 These two clusters share similar sROH lengths but differ in admixture proportions and median IBD sharing (Table 1), pointing to a cluster with consistent African American ancestors and a cluster with more admixed ancestors. Median cumulative IBD sharing is higher among African Americans in the south (median cumulative IBD = 19.6 cM, median sROH = 3.3 Mb) than in the north (median = 15.9 cM; Table 1), resulting in different patterns of effective population size over antecedent generations (Figure S12),45,46 while the average proportion of African ancestry is higher in the northern cluster than the southern cluster.
Four of the clusters reflect recent immigrants from Asia (Figure S14), which grew rapidly in the mid-20th century after the elimination of national origin quotas.55 The recency of immigration among these clusters is supported by the observation that fewer than 30% of grandparents were born in the US. Geographically, individuals in these clusters primarily reside in major cities. East Asians predominantly inhabit the metropolitan areas of the west and northeast (OR > 2), Southeast Asians are enriched in the west (OR > 2.5), and South Asians are strongly associated with the northeast (OR > 2.5). Despite its small size, the cluster of Greater Middle East individuals reflects many of the known demographic patterns of Arab Americans, as individuals in this cluster are primarily of Lebanese origin and are distributed in the northeast as well as metropolitan Detroit. sROH lengths are particularly long for South Asians (median sROH = 10.3 cM; Table 1), Southeast Asians (median sROH = 7.8 cM), and Middle Easterners (median sROH = 8.2 cM), potentially reflecting patterns of consanguinity and inbreeding in their ancestral regions.56 In particular, the median sROH length in the South Asia cluster is the second highest among all clusters, but the median cumulative IBD length is similar to most clusters (Table 1). The population of South Asia is large and diverse, with many endogamous groups making up the 1.5 billion people living in the region.57,58 The pattern of IBD and sROH among individuals in South Asian cluster thus may reflect the result of recent consanguinity in a large population.48,59
Discussion
As the US population is becoming increasingly diverse, genomic studies are simultaneously growing in scale and relevance; to increase scientific and ethical parity, these studies must move beyond the current practice of evaluating genetically homogeneous groups in isolation.47,60 Here, we provide an integrative framework for analyzing population structure in ancestrally heterogeneous individuals. Our comprehensive approach has allowed us to capture spatial patterns of gene flow within and between subpopulations that are difficult to infer from a single method alone. For example, while EEMS enabled us to examine genetic similarity at a finer scale than previous studies and identify genetic differentiation within a state, EEMS can only compare neighboring demes and does not directly evaluate the genetic similarity of geographically distant individuals. Haplotype clustering, on the other hand, can identify population structures over long distances, but it does not measure genetic similarity with respect to geography. Since individuals are exclusively assigned to a single cluster, information regarding admixture, especially between neighboring clusters, are lost during haplotype clustering. An integrative approach can thus enable greater insights into populations with complex histories, as well as populations typically overlooked in previous studies such as Asian Americans.
The genetic structure and history of Hispanic/Latino populations is particularly complex due to many historical migration and admixture events.4,9 This complexity is reflected in the variable migration rates across the country and the large variations in admixture proportions within and between subpopulations. While prior analysis of Hispanics/Latinos in the US found differences in ancestry proportions aggregated at the state level,9 we demonstrate that considerable differences in genetic ancestry also exist within a state. For example, two distinct clusters—Puerto Rico and Hispanics/Latinos—are found in Florida, with the Puerto Rico cluster having higher average African ancestry proportions than the Hispanics/Latinos cluster (9.0% versus 2.5%, respectively). EEMS also enabled direct measures of genetic similarity within states and between subpopulations. While the mean ancestry proportions are similar between the New Mexican cluster and the Texan cluster, individuals in northern New Mexico are more genetically differentiated than individuals in southern New Mexico, as indicated by the migration barrier. The individuals in northern New Mexico are likely Nuevomexicanos, descendants of Spanish colonial settlers, while those in the south are more genetically similar to Hispanic/Latino individuals in central Texas, likely because they share a common ancestral origin (i.e., Mexico). We also built upon the use of pedigree annotation8 by quantifying ancestral origins to better understand the differences in genetic ancestry between subpopulations. For example, in the Hispanics/Latinos in California cluster, the mean proportion of European ancestry is smaller when compared to the New Mexican and Texan clusters, reflecting the lower proportion of US ancestral origins. Comparison of sROH and IBD lengths of these clusters further reveal evidence of founder effects. Puerto Ricans, Hispanics/Latinos in Texas, and Hispanics/Latinos in New Mexico had the highest median IBD lengths and showed evidence of recent bottleneck (Figure S12), consistent with prior studies.45,52 In general, median sROH and IBD lengths were higher in Hispanic-related clusters than European clusters, reflecting the patterns found in reference populations56,61 and in line with recent findings in New York City.52
The demographic history of African Americans is characterized by large-scale migration and admixture, primarily due to the transatlantic slave trade and racial segregation.50,62 The patterns of genetic ancestry and relatedness between states and regions of the US reflect these events.3,7,9 Our results show, at a finer scale, the barriers to migration and gene flow, particularly along the Appalachian Mountains. This migration barrier overlaps with the boundary between slave states and free states, as well as the boundary between states that enacted laws enforcing racial segregation and states that forbade segregation. The north-south separation of two African American clusters further emphasize this divide. The African Americans South cluster contains more recent ancestors from outside the US, particularly from the Caribbean, than the African Americans North cluster. These insights further emphasize the impact of historical migration and socioeconomic divide on the present-day patterns of genetic relatedness among African Americans.
Despite accounting for more than 5% of the US population, individuals with Asian ancestries are underrepresented in US population genetics studies, hindering the ability of prior studies to investigate of their ancestry.8 Our analyses of these individuals therefore provide new insights into their genetic structure. Many of these individuals are descendants of recent immigrants, as indicated by the high proportions of non-US grandparental ancestral origin; therefore, they likely reflect the population of their ancestral region. The genetic structure of these individuals is particularly diverse. Using fineSTRUCTURE, genetic differentiation was found between East Asian and South Asian individuals of different ancestral origin as well as between individuals with the same ancestral origin. At the same time, longer sROH was observed in the Southeast Asia, South Asia, and Greater Middle East haplotype clusters, likely reflecting consanguinity or endogamy patterns in their ancestral countries. For example, the long sROH in South Asians may reflect endogamy related to the caste system in India, while similar patterns among the Middle Eastern and Southeast Asian clusters may be capturing consanguineous marriage practices in those regions.48,63,64 Understanding population genetic structure and patterns of homozygosity are important in determining the genetic profile of diseases within subpopulations, especially since these recent immigrants are becoming less similar to those in their ancestral countries due to outbreeding, admixture, and population growth.65,66 As populations mix, heterozygosity increases and allele frequencies change. This, in turn, can alter the prevalence of certain diseases, particularly rare recessive disorders that are often more prevalent in populations with increased homozygosity.67 At the same time, changes in allele frequencies can also reduce the accuracy of genetic predictors of complex traits (i.e., polygenic risk scores), especially if the prediction model was built using a homogeneous cohort of individuals from a divergent ancestry.60
Population history in the US is best characterized among individuals of European descent. Genetic diversity tends to be highest in more densely populated regions, likely due to multiple populations living in the same place. Many of the European subpopulations we identified are similar to those previously found—e.g., French Canadians, Acadians, Scandinavians, and Ashkenazi Jews.8 The geographic distribution of these subpopulations, particularly those that are more genetically diverged, overlap in the metropolitan areas of the Northeast, Midwest, and California. These overlaps may explain the presence of certain EEMS-inferred migration barriers. For example, the migration barrier and lower genetic similarity encompassing metropolitan New York City may be explained in part by the large presence of Greeks, Italians, and Ashkenazi Jews in that area.
The precision of population labels assigned to clusters of individuals is a function of demographic complexity and sample size. For example, Finnish ancestry is clearly European but genetically distinct from several other European populations due to historical bottlenecks, making this ancestry cluster relatively easily separable. By contrast, most Americans of European descent have heterogeneous ancestors from several northwestern European countries who have admixed over time, resulting in relatively evenly distributed ancestry overlapping that of present-day Europeans from multiple primarily northwestern countries. Additionally, while we identify and describe some substantial structure among Hispanic/Latino populations, considerably more is likely to exist and remains to be learned from larger and more diverse future studies. Similarly, sub-regional resolution into the ancestry of recent Asian immigrants has been relatively limited in population genetics studies, and the structure of this immigration will be learned from larger future studies. Interestingly, we found that fineSTRUCTURE was able to disentangle Asian subpopulations at a finer resolution than haplotype clustering, demonstrating the tradeoff between resolution and scale of these two methods and further highlighting the value of an integrated approach. The accuracy of self-reported birth records and variable granularity of geopolitical boundaries also provide additional considerations regarding the precision of population labels.
In addition to being of anthropological interest, understanding fine-scale human history and its role in shaping genetic variation is also important for interpreting the genetic basis of biomedical traits. The emergence of biobank-scale genomic data is enabling the imputation of pedigree structure regardless of whether some relatives have contributed DNA,68 greater insights into the impact of fine-scale population structure on genetic associations with disease,14,17,27,60,69 and population-based screening for individuals with serious genetic and health-related associations.70 Standard practice in genetic studies to date has involved identifying the largest genetically homogeneous population in a study (typically European ancestry) and conducting genetic analysis excluding other populations.71,72 However, as genetic studies become increasingly promising for clinical translation, this practice has led to concerns about genetic tools exacerbating health disparities, particularly for populations underrepresented in genetic studies.47,60 Participation in genetic programs is increasing in the US, for example with the All of Us Research Program (Web Resources) or with direct-to-consumer genetic tests that an estimated 26 million people have taken, and many of these participants are of diverse non-European ancestry.73,74 As a result, the need for including more diverse populations in genetic studies and for inferring more granular demographic histories in diverse study cohorts is becoming greater. Understanding such structure is important to account for stratification in association studies, prevent the overgeneralization of potentially confounded results, and avoid exacerbating existing Eurocentric study biases.60,71,75,76 This study demonstrates how genetic data can be coupled with geographic and birth origin data to reconstruct such demographic histories, particularly in a large and heterogeneous population.
Data and Code Availability
Genotype data and associated metadata are available to researchers through an application process and data usage agreement. We encourage qualified researchers to email the Genographic team at National Geographic Society (genographic@ngs.org) for information on and access to the Genographic database. For more information, please visit the Genographic Project website (https://genographic.nationalgeographic.com/for-scientists/).
Custom scripts generated to analyze the data in this paper are available through GitHub (https://github.com/chengdai/genographic_ancestry).
Declaration of Interests
M.G.V. is the Senior Program Officer for the National Geographic Society and lead scientist for the Genographic Project. R.S.W. was the former Director of the Genographic Project and is a cofounder for Insitome. M.J.D. is a member of the Scientific Advisory Board at Ancestry.com LLC.
Acknowledgments
We thank the National Geographic Genographic Project participants who consented to research for making this study possible. We also thank Gregory Vilshansky for helping organize and manage the data for the Genographic Project. This work was supported by funding from the National Institutes of Health (K99MH117229 to A.R.M.). C.L.D., M.M.V., R.T., and C.R. would also like to thank all the members of the MIT Senseable City Lab Consortium for supporting this research. M.G.V. acknowledges support from the National Geographic Society.
Published: March 5, 2020
Footnotes
Supplemental Data can be found online at https://doi.org/10.1016/j.ajhg.2020.02.002.
Web Resources
1000 Genomes Project, https://www.internationalgenome.org
All of Us Research Hub, https://www.researchallofus.org/
Ancestry pipeline, https://github.com/armartin/ancestry_pipeline
Human Genome Diversity Project, https://www.hagsc.org/hgdp/
Interactive Tree of Life, https://itol.embl.de/
POPRES: Population Reference Sample, https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000145.v4.p2
python-ternary, https://github.com/marcharper/python-ternary
scikit-bio, http://scikit-bio.org/
Supplemental Data
Figures S1–S19 and Supplemental Material and Methods
Tables S1–S7
Article plus Supplemental Information
References
- 1.Auton A., Brooks L.D., Durbin R.M., Garrison E.P., Kang H.M., Korbel J.O., Marchini J.L., McCarthy S., McVean G.A., Abecasis G.R., 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tishkoff S.A., Reed F.A., Friedlaender F.R., Ehret C., Ranciaro A., Froment A., Hirbo J.B., Awomoyi A.A., Bodo J.-M., Doumbo O. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryc K., Auton A., Nelson M.R., Oksenberg J.R., Hauser S.L., Williams S., Froment A., Bodo J.-M., Wambebe C., Tishkoff S.A., Bustamante C.D. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc. Natl. Acad. Sci. USA. 2010;107:786–791. doi: 10.1073/pnas.0909559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryc K., Velez C., Karafet T., Moreno-Estrada A., Reynolds A., Auton A., Hammer M., Bustamante C.D., Ostrer H. Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc. Natl. Acad. Sci. USA. 2010;107(Suppl 2):8954–8961. doi: 10.1073/pnas.0914618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reich D., Patterson N., Campbell D., Tandon A., Mazieres S., Ray N., Parra M.V., Rojas W., Duque C., Mesa N. Reconstructing Native American population history. Nature. 2012;488:370–374. doi: 10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novembre J., Johnson T., Bryc K., Kutalik Z., Boyko A.R., Auton A., Indap A., King K.S., Bergmann S., Nelson M.R. Genes mirror geography within Europe. Nature. 2008;456:98–101. doi: 10.1038/nature07331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baharian S., Barakatt M., Gignoux C.R., Shringarpure S., Errington J., Blot W.J., Bustamante C.D., Kenny E.E., Williams S.M., Aldrich M.C., Gravel S. The Great Migration and African-American Genomic Diversity. PLoS Genet. 2016;12:e1006059. doi: 10.1371/journal.pgen.1006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han E., Carbonetto P., Curtis R.E., Wang Y., Granka J.M., Byrnes J., Noto K., Kermany A.R., Myres N.M., Barber M.J. Clustering of 770,000 genomes reveals post-colonial population structure of North America. Nat. Commun. 2017;8:14238. doi: 10.1038/ncomms14238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryc K., Durand E.Y., Macpherson J.M., Reich D., Mountain J.L. The genetic ancestry of African Americans, Latinos, and European Americans across the United States. Am. J. Hum. Genet. 2015;96:37–53. doi: 10.1016/j.ajhg.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakharia F., Basu A., Absher D., Assimes T.L., Go A.S., Hlatky M.A., Iribarren C., Knowles J.W., Li J., Narasimhan B. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10:R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaff C.L., Parra E.J., Bonilla C., Hiester K., McKeigue P.M., Kamboh M.I., Hutchinson R.G., Ferrell R.E., Boerwinkle E., Shriver M.D. Population structure in admixed populations: effect of admixture dynamics on the pattern of linkage disequilibrium. Am. J. Hum. Genet. 2001;68:198–207. doi: 10.1086/316935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parra E.J., Marcini A., Akey J., Martinson J., Batzer M.A., Cooper R., Forrester T., Allison D.B., Deka R., Ferrell R.E., Shriver M.D. Estimating African American admixture proportions by use of population-specific alleles. Am. J. Hum. Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price A.L., Patterson N., Yu F., Cox D.R., Waliszewska A., McDonald G.J., Tandon A., Schirmer C., Neubauer J., Bedoya G. A genomewide admixture map for Latino populations. Am. J. Hum. Genet. 2007;80:1024–1036. doi: 10.1086/518313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg J.J., Harpak A., Sinnott-Armstrong N., Joergensen A.M., Mostafavi H., Field Y., Boyle E.A., Zhang X., Racimo F., Pritchard J.K., Coop G. Reduced signal for polygenic adaptation of height in UK Biobank. eLife. 2019;8:e39725. doi: 10.7554/eLife.39725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henn B.M., Cavalli-Sforza L.L., Feldman M.W. The great human expansion. Proc. Natl. Acad. Sci. USA. 2012;109:17758–17764. doi: 10.1073/pnas.1212380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohail M., Maier R.M., Ganna A., Bloemendal A., Martin A.R., Turchin M.C., Chiang C.W., Hirschhorn J., Daly M.J., Patterson N. Polygenic adaptation on height is overestimated due to uncorrected stratification in genome-wide association studies. eLife. 2019;8:e39702. doi: 10.7554/eLife.39702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayser M., Brauer S., Weiss G., Schiefenhövel W., Underhill P., Shen P., Oefner P., Tommaseo-Ponzetta M., Stoneking M. Reduced Y-chromosome, but not mitochondrial DNA, diversity in human populations from West New Guinea. Am. J. Hum. Genet. 2003;72:281–302. doi: 10.1086/346065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montinaro F., Busby G.B.J., Pascali V.L., Myers S., Hellenthal G., Capelli C. Unravelling the hidden ancestry of American admixed populations. Nat. Commun. 2015;6:6596. doi: 10.1038/ncomms7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Estrada A., Gravel S., Zakharia F., McCauley J.L., Byrnes J.K., Gignoux C.R., Ortiz-Tello P.A., Martínez R.J., Hedges D.J., Morris R.W. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray S.M., Mulle J.G., Dodd A.F., Pulver A.E., Wooding S., Warren S.T. Signatures of founder effects, admixture, and selection in the Ashkenazi Jewish population. Proc. Natl. Acad. Sci. USA. 2010;107:16222–16227. doi: 10.1073/pnas.1004381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peltonen L., Palotie A., Lange K. Use of population isolates for mapping complex traits. Nat. Rev. Genet. 2000;1:182–190. doi: 10.1038/35042049. [DOI] [PubMed] [Google Scholar]
- 23.Scriver C.R. Human genetics: lessons from Quebec populations. Annu. Rev. Genomics Hum. Genet. 2001;2:69–101. doi: 10.1146/annurev.genom.2.1.69. [DOI] [PubMed] [Google Scholar]
- 24.Martin A.R., Karczewski K.J., Kerminen S., Kurki M.I., Sarin A.-P., Artomov M., Eriksson J.G., Esko T., Genovese G., Havulinna A.S. Haplotype Sharing Provides Insights into Fine-Scale Population History and Disease in Finland. Am. J. Hum. Genet. 2018;102:760–775. doi: 10.1016/j.ajhg.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patin E., Lopez M., Grollemund R., Verdu P., Harmant C., Quach H., Laval G., Perry G.H., Barreiro L.B., Froment A. Dispersals and genetic adaptation of Bantu-speaking populations in Africa and North America. Science. 2017;356:543–546. doi: 10.1126/science.aal1988. [DOI] [PubMed] [Google Scholar]
- 26.Mooney J.A., Huber C.D., Service S., Sul J.H., Marsden C.D., Zhang Z., Sabatti C., Ruiz-Linares A., Bedoya G., Freimer N., Lohmueller K.E., Costa Rica/Colombia Consortium for Genetic Investigation of Bipolar Endophenotypes Understanding the Hidden Complexity of Latin American Population Isolates. Am. J. Hum. Genet. 2018;103:707–726. doi: 10.1016/j.ajhg.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belbin G.M., Odgis J., Sorokin E.P., Yee M.-C., Kohli S., Glicksberg B.S., Gignoux C.R., Wojcik G.L., Van Vleck T., Jeff J.M. Genetic identification of a common collagen disease in puerto ricans via identity-by-descent mapping in a health system. eLife. 2017;6:e25060. doi: 10.7554/eLife.25060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hellenthal G., Busby G.B.J., Band G., Wilson J.F., Capelli C., Falush D., Myers S. A genetic atlas of human admixture history. Science. 2014;343:747–751. doi: 10.1126/science.1243518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson D.J., Hellenthal G., Myers S., Falush D. Inference of population structure using dense haplotype data. PLoS Genet. 2012;8:e1002453. doi: 10.1371/journal.pgen.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elhaik E., Greenspan E., Staats S., Krahn T., Tyler-Smith C., Xue Y., Tofanelli S., Francalacci P., Cucca F., Pagani L., Genographic Consortium The GenoChip: a new tool for genetic anthropology. Genome Biol. Evol. 2013;5:1021–1031. doi: 10.1093/gbe/evt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royal C.D., Novembre J., Fullerton S.M., Goldstein D.B., Long J.C., Bamshad M.J., Clark A.G. Inferring genetic ancestry: opportunities, challenges, and implications. Am. J. Hum. Genet. 2010;86:661–673. doi: 10.1016/j.ajhg.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., Sham P.C. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson M.R., Bryc K., King K.S., Indap A., Boyko A.R., Novembre J., Briley L.P., Maruyama Y., Waterworth D.M., Waeber G. The Population Reference Sample, POPRES: a resource for population, disease, and pharmacological genetics research. Am. J. Hum. Genet. 2008;83:347–358. doi: 10.1016/j.ajhg.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergström A., McCarthy S.A., Hui R., Almarri M.A., Ayub Q., Danecek P., Chen Y., Felkel S., Hallast P., Kamm J. Insights into human genetic variation and population history from 929 diverse genomes. bioRxiv. 2019 doi: 10.1126/science.aay5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraham G., Qiu Y., Inouye M. FlashPCA2: principal component analysis of Biobank-scale genotype datasets. Bioinformatics. 2017;33:2776–2778. doi: 10.1093/bioinformatics/btx299. [DOI] [PubMed] [Google Scholar]
- 36.Alexander D.H., Novembre J., Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19:1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McInnes L., Healy J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv. 2018 1802.03426. [Google Scholar]
- 38.Diaz-Papkovich A., Anderson-Trocmé L., Ben-Eghan C., Gravel S. UMAP reveals cryptic population structure and phenotype heterogeneity in large genomic cohorts. PLoS Genet. 2019;15:e1008432. doi: 10.1371/journal.pgen.1008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loh P.-R., Danecek P., Palamara P.F., Fuchsberger C., A Reshef Y., K Finucane H., Schoenherr S., Forer L., McCarthy S., Abecasis G.R. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016;48:1443–1448. doi: 10.1038/ng.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy S., Das S., Kretzschmar W., Delaneau O., Wood A.R., Teumer A., Kang H.M., Fuchsberger C., Danecek P., Sharp K., Haplotype Reference Consortium A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016;48:1279–1283. doi: 10.1038/ng.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petkova D., Novembre J., Stephens M. Visualizing spatial population structure with estimated effective migration surfaces. Nat. Genet. 2016;48:94–100. doi: 10.1038/ng.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browning B.L., Browning S.R. Detecting identity by descent and estimating genotype error rates in sequence data. Am. J. Hum. Genet. 2013;93:840–851. doi: 10.1016/j.ajhg.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blondel V.D., Guillaume J.-L., Lambiotte R., Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008;2008:P10008. [Google Scholar]
- 44.Maples B.K., Gravel S., Kenny E.E., Bustamante C.D. RFMix: a discriminative modeling approach for rapid and robust local-ancestry inference. Am. J. Hum. Genet. 2013;93:278–288. doi: 10.1016/j.ajhg.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browning S.R., Browning B.L., Daviglus M.L., Durazo-Arvizu R.A., Schneiderman N., Kaplan R.C., Laurie C.C. Ancestry-specific recent effective population size in the Americas. PLoS Genet. 2018;14:e1007385. doi: 10.1371/journal.pgen.1007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Browning S.R., Browning B.L. Accurate Non-parametric Estimation of Recent Effective Population Size from Segments of Identity by Descent. Am. J. Hum. Genet. 2015;97:404–418. doi: 10.1016/j.ajhg.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin A.R., Gignoux C.R., Walters R.K., Wojcik G.L., Neale B.M., Gravel S., Daly M.J., Bustamante C.D., Kenny E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017;100:635–649. doi: 10.1016/j.ajhg.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moorjani P., Thangaraj K., Patterson N., Lipson M., Loh P.-R., Govindaraj P., Berger B., Reich D., Singh L. Genetic evidence for recent population mixture in India. Am. J. Hum. Genet. 2013;93:422–438. doi: 10.1016/j.ajhg.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lal B.V. J. Pac. Hist.; 1983. Girmitiyas: The Origins of the Fiji Indians. [Google Scholar]
- 50.US Census Bureau The Great Migration, 1910 to 1970. https://www.census.gov/dataviz/visualizations/020/ Retrieved February 21, 2019.
- 51.Massey D.S., Rugh J.S., Pren K.A. The Geography of Undocumented Mexican Migration. Mex. Stud. 2010;26:129–152. doi: 10.1525/msem.2010.26.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belbin G.M., Wenric S., Cullina S., Glicksberg B.S., Moscati A., Wojcik G.L., Shemirani R., Beckmann N.D., Cohain A., Sorokin E.P. Towards a fine-scale population health monitoring system. bioRxiv. 2019 doi: 10.1016/j.cell.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 53.Passel J.S., Fix M. U.S. Immigration in a Global Context: Past, Present, and Future. Indiana J. Glob. Leg. Stud. 1994;2:5–19. [Google Scholar]
- 54.Ostrer H., Skorecki K. The population genetics of the Jewish people. Hum. Genet. 2013;132:119–127. doi: 10.1007/s00439-012-1235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grieco E.M., Trevelyan E., Larsen L., Acosta Y.D., Gambino C. US Census Bureau; 2012. The Size, Place of Birth, and Geographic Distribution of the Foreign-Born Population in the United States: 1960 to 2010.https://www.census.gov/content/dam/Census/library/working-papers/2012/demo/POP-twps0096.pdf [Google Scholar]
- 56.Pemberton T.J., Absher D., Feldman M.W., Myers R.M., Rosenberg N.A., Li J.Z. Genomic patterns of homozygosity in worldwide human populations. Am. J. Hum. Genet. 2012;91:275–292. doi: 10.1016/j.ajhg.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakatsuka N., Moorjani P., Rai N., Sarkar B., Tandon A., Patterson N., Bhavani G.S., Girisha K.M., Mustak M.S., Srinivasan S. The promise of discovering population-specific disease-associated genes in South Asia. Nat. Genet. 2017;49:1403–1407. doi: 10.1038/ng.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wall J.D., Stawiski E.W., Ratan A., Kim H.L., Kim C., Gupta R., Suryamohan K., Gusareva E.S., Purbojati R.W., Bhangale T., GenomeAsia100K Consortium The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature. 2019;576:106–111. doi: 10.1038/s41586-019-1793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auton A., Bryc K., Boyko A.R., Lohmueller K.E., Novembre J., Reynolds A., Indap A., Wright M.H., Degenhardt J.D., Gutenkunst R.N. Global distribution of genomic diversity underscores rich complex history of continental human populations. Genome Res. 2009;19:795–803. doi: 10.1101/gr.088898.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin A.R., Kanai M., Kamatani Y., Okada Y., Neale B.M., Daly M.J. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 2019;51:584–591. doi: 10.1038/s41588-019-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ceballos F.C., Joshi P.K., Clark D.W., Ramsay M., Wilson J.F. Runs of homozygosity: windows into population history and trait architecture. Nat. Rev. Genet. 2018;19:220–234. doi: 10.1038/nrg.2017.109. [DOI] [PubMed] [Google Scholar]
- 62.National Archives . 2016. The Slave Trade.https://www.archives.gov/education/lessons/slave-trade.html [Google Scholar]
- 63.Tadmouri G.O., Nair P., Obeid T., Al Ali M.T., Al Khaja N., Hamamy H.A. Consanguinity and reproductive health among Arabs. Reprod. Health. 2009;6:17. doi: 10.1186/1742-4755-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hussain R., Bittles A.H. Assessment of association between consanguinity and fertility in Asian populations. J. Health Popul. Nutr. 2004;22:1–12. [PubMed] [Google Scholar]
- 65.López G., Ruiz N., Patten E. 2017. Key facts about Asian Americans, a diverse and growing population. Pew Research Center Fact Tank, September 8, 2017.https://www.pewresearch.org/fact-tank/2017/09/08/key-facts-about-asian-americans/ [Google Scholar]
- 66.Bialik K. 2017. Key facts about race and marriage, 50 years after Loving v. Virginia. Pew Research Center Fact Tank, June 12, 2017.https://www.pewresearch.org/fact-tank/2017/06/12/key-facts-about-race-and-marriage-50-years-after-loving-v-virginia/ [Google Scholar]
- 67.Nalls M.A., Simon-Sanchez J., Gibbs J.R., Paisan-Ruiz C., Bras J.T., Tanaka T., Matarin M., Scholz S., Weitz C., Harris T.B. Measures of autozygosity in decline: globalization, urbanization, and its implications for medical genetics. PLoS Genet. 2009;5:e1000415. doi: 10.1371/journal.pgen.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erlich Y., Shor T., Pe’er I., Carmi S. Identity inference of genomic data using long-range familial searches. Science. 2018;362:690–694. doi: 10.1126/science.aau4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathieson I., McVean G. Differential confounding of rare and common variants in spatially structured populations. Nat. Genet. 2012;44:243–246. doi: 10.1038/ng.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bastarache L., Hughey J.J., Hebbring S., Marlo J., Zhao W., Ho W.T., Van Driest S.L., McGregor T.L., Mosley J.D., Wells Q.S. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. Science. 2018;359:1233–1239. doi: 10.1126/science.aal4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Popejoy A.B., Fullerton S.M. Genomics is failing on diversity. Nature. 2016;538:161–164. doi: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Need A.C., Goldstein D.B. Next generation disparities in human genomics: concerns and remedies. Trends Genet. 2009;25:489–494. doi: 10.1016/j.tig.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 73.Regalado A. MIT Technology Review, February 11, 2019. 2019. More than 26 million people have taken an at-home ancestry test.https://www.technologyreview.com/s/612880/more-than-26-million-people-have-taken-an-at-home-ancestry-test/ [Google Scholar]
- 74.Parker K., Horowitz J.M., Mortin R., Lopez M.H. 2015. Multiracial in America: Proud, Diverse and Growing in Numbers. Pew Research Center, June 11, 2015.https://www.pewsocialtrends.org/2015/06/11/multiracial-in-america/ [Google Scholar]
- 75.Manrai A.K., Funke B.H., Rehm H.L., Olesen M.S., Maron B.A., Szolovits P., Margulies D.M., Loscalzo J., Kohane I.S. Genetic Misdiagnoses and the Potential for Health Disparities. N. Engl. J. Med. 2016;375:655–665. doi: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caswell-Jin J.L., Gupta T., Hall E., Petrovchich I.M., Mills M.A., Kingham K.E., Koff R., Chun N.M., Levonian P., Lebensohn A.P. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet. Med. 2018;20:234–239. doi: 10.1038/gim.2017.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S19 and Supplemental Material and Methods
Tables S1–S7
Article plus Supplemental Information
Data Availability Statement
Genotype data and associated metadata are available to researchers through an application process and data usage agreement. We encourage qualified researchers to email the Genographic team at National Geographic Society (genographic@ngs.org) for information on and access to the Genographic database. For more information, please visit the Genographic Project website (https://genographic.nationalgeographic.com/for-scientists/).
Custom scripts generated to analyze the data in this paper are available through GitHub (https://github.com/chengdai/genographic_ancestry).