Highlights
-
•
The varietal composition of olive oil blends was quantified.
-
•
Quantification of olive oil adulteration with oils of different plant origin.
-
•
The quantitative authentication of olive oil blends has limitations.
-
•
The SNP-HRM showed higher authentication ability for oil blends compared to SSR-HRM.
-
•
SNP-HRM indicated a 5% limit of detection for varietal identification of olive oil.
Keywords: Olive oil, Quantitative authentication, High resolution melting, Molecular markers, SSRs, SNPs, Adulteration, Sunflower oil, Maize oil
Abstract
A plethora of biotechnological methodologies is used to authenticate quality olive oils. Among the DNA-based approaches, SNPs and SSRs combined with high resolution melting (HRM) provide certain advantages such as speed, simplicity and reliability. SNP-HRM and SSR-HRM were used for the authentication of monovarietal olive oils as well as the quantification of varietal composition in olive oil DNA admixtures and olive oil blends of two different cultivars. The SSR-HRM was more efficient in distinguishing monovarietal olive oils while the SNP-HRM assay was more reliable in discriminating olive oil blends. HRM was also used for the detection of adulteration of olive oil with oils of different plant origin by using plastid trnL indels and SNPs. The trnL-indels-HRM showed higher discrimination power than the trnL-SNP-HRM in determining adulteration in olive oil. These results indicate that traceability of adulteration might be more reliable than authentication of the varietal origin in olive oil blends.
1. Introduction
Olive oil is a valuable agricultural commodity of the Mediterranean basin and is considered the major component of the Mediterranean diet not only due to its health benefits but also for its great nutritional value. The olive oil production has a high social-economic impact, especially the extra virgin olive oil (EVOO) which is considered of premium quality and possess higher prices in the market (Kalaitzis & El-Zein, 2016). The identity of such processed products is practically difficult to be determined because is influenced by several factors; olive cultivars, pedoclimatic conditions, environment, agricultural practices, fruit maturation and methods of extraction of olive oil (Avramidou, Doulis, & Petrakis, 2018). Protected designation of origin (PDO) and protected geographical indication (PGI) are important labels referring to the quality and identity of olive oils. Therefore, authenticity and traceability of high quality PDO and PGI monovarietal extra virgin olive oils is a major concern for markets and consumers. A major part of authentication efforts concentrate on the identification of the varietal origin as well as the adulteration with oils of different plant origin.
DNA-based approaches are considered complementary to analytical chemistry methodologies for olive oil varietal authentication due to their sensitivity, specificity and reliability (Agrimonti and Marmiroli, 2019, Avramidou et al., 2018, Lo and Shaw, 2018). In this context, various molecular markers were used for food authenticity and traceability but the single nucleotide polymorphisms (SNPs) and the microsatellites or single sequence repeats (SSRs) became the markers of choice for olive oil traceability purposes (Bazakos et al., 2016, Montemurro et al., 2015, Pasqualone et al., 2016). These molecular markers are considered as ideal analytical targets in partially degraded DNA preparations such as those of olive oil since they require the amplification of short length PCR amplicons. Therefore, many studies have used the SSRs for the identification of varietal origin of olive oil with various technological platforms (Alba et al., 2009, Ben-Ayed et al., 2013, Pasqualone et al., 2007).
Recent reports explored the potential of SNPs in olive oil varietal discrimination by using various methodologies (Consolandi et al., 2007, Reale et al., 2006) such as polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) combined with capillary electrophoresis (CE) (Bazakos et al., 2012, Bazakos et al., 2016). Moreover, a multiplex SNPs assay was also used for the varietal identification of monovarietal olive oils in combination with an innovative approach of fluorescence-encoded microspheres (Kalogianni et al., 2015).
Plastid markers have also been used for the detection of adulteration of olive oil (Besnard et al., 2011, Pérez-Jiménez et al., 2013). One of the analytical targets of extra interest is the plastid trnL (UAA) intron for various reasons. The plastid trnL (UAA) intron has been used as an analyte molecule to identify the identity of specific food crops (Spaniolas, Bazakos, Spano, Zoghby, & Kalaitzis, 2010). It has been used in the past for the identification of plant species (Spaniolas et al., 2010). This intron shows acceptable discrimination efficiency since it is sufficiently variable among species and conserved enough within species (Taberlet et al., 2006). Alternatively, a set of 40 polymorphic loci were identified after sequencing 8 plastid genomes of olive showing very low variability in olive tree cultivars (Besnard et al., 2011). Such loci might be efficiently used for detection of adulteration with oils of different plant origin.
The development of methodologies that require minimum manipulation is mandatory when dealing with samples destined for authenticity testing. Therefore, the High resolution melting (HRM) technology might be an approach which allows the genotyping of varieties in a closed-tube reaction, without further analysis, as long as the variants have been previously identified (Druml & Cichna-Markl, 2014). The HRM analysis curves can be distinguished on the basis of their shape, due to polymorphisms on single nucleotides and/or amplicon length, even though the PCR products might have similar Tm values (Ganopoulos et al., 2012, Pereira et al., 2017). A barcode-HRM approach was established by Madesis, Ganopoulos, Anagnostis, and Tsaftaris (2012) on chloroplastic DNA to detect Lupinus adulterants present in Glycine max flour. Moreover, the HRM assay combined with molecular markers allowed a quick and high-throughput detection of adulteration of monovarietal olive oils (Ganopoulos, Bazakos, Madesis, Kalaitzis, & Tsaftaris, 2013). In addition, the SSR-HRM approach was reported to efficiently determine the traceability of olive oils (Montemurro et al., 2015) while recently, the SSR-HRM methodology was proposed as an efficient, fast, simple and reliable approach to authenticate a high number of monovarietal olive oils (Gomes, Breia, Carvalho, Carnide, & Martins-Lopes, 2018).
In this report, a comparative authentication and adulteration of olive oil approach was performed at the quantitative level by using SSR- and SNP-based HRM. Initially, HRM combined with DNA markers was used for the discrimination and quantitative determination of blends of monovarietal olive oils. In this analysis, the discriminatory capacity of SNPs compared to SSRs was found to be higher. Moreover, the efficiency of HRM was also investigated for the detection of adulteration of olive oil with oils of different plant origin. For this approach, plastid trnL (insertions/deletions) indels and SNPs were used and it was found that the indels region is more efficient in the discrimination of adulterants. Overall, our findings suggest that depending on the authentication and adulteration objective, one DNA marker might provide advantages over another.
2. Materials and methods
2.1. Plant tissues and oil samples
The leaf tissue of three Greek olive varieties, Koroneiki, Tsounati and Kalamon, was collected from an olive collection maintained at the Mediterranean Agronomic Institute of Chania, Crete, Greece and used for DNA extraction. The monovarietal olive oil samples were provided by Pamako S.A.and Kolympari SA, Chania, Crete and by the Union of Agricultural Cooperatives of Lakonia, Sparta, Peloponnese. Commercial maize and sunflower oils were used for the adulteration experiments.
For the authentication of the olive oil DNA admixtures, genomic DNA was extracted from three monovarietal olive oils and diluted to equal concentrations separately. Subsequently, the DNA of Koroneiki with Kalamon and Koroneiki with Tsounati were blended in ratios of 50–50%, 75–25%, 85–15% and 95–5%. Monovarietal olive oil DNA of each variety was also used as positive control. For the authentication of olive oil blends, monovarietal olive oils of Koroneiki and Kalamon as well as Koroneiki and Tsounati were mixed in ratios of 50–50%, 75–25%, 85–15% and 95–5% and then DNA was extracted from the mixtures. In addition, monovarietal olive oils of each variety were also used as positive controls. Finally, for the detection of olive oil adulteration, two different vegetable oils were used, maize and sunflower. Monovarietal Koroneiki olive oil was blended with maize and sunflower oils in ratios of 99–1%, 95–5%, 90–10%, 85–15% and 75–25% and subsequently DNA extraction was performed in the mixtures. The monovarietal olive oil and the 100% maize and sunflower oils were used as positive controls.
2.2. Isolation of DNA from olive oil
Genomic DNA was extracted from leaves of the three olive varieties with a standard CTAB (cetyltrimethylammonium bromide) protocol as described in Woolley, James, and Manning (2001). DNA extraction from olive oil samples was performed using two different methods: the CTAB/hexane/chloroform protocol developed by Giménez, Pistón, Martín, and Atienza (2010) and Norgen’s Olive Oil DNA Isolation Kit (Norgen Biotek co., Canada). DNA quantity and purity were estimated on a Nano photometer (Pearl, Implen GmbH Munich Germany)
2.3. PCR amplification from olive oil DNA
The amplification of DNA templates comprising the SSR and SNP molecular markers (Supplementary Table 1) was performed by PCR reaction in a DYAD thermocycler (BIORAD) using 5x Phusion HF Buffer, 4 mM dNTP, 10 μM for each primer, 0.4 units of Phusion High-Fidelity DNA Polymerase (Applied Biosystems, Foster City, CA) and 20 ng of olive DNA in a final volume of 25 μl. The PCR conditions were 98 °C for 30 s, followed by 45 cycles of 98 °C for 10 s, Tm of the primers for 30 s and 72 °C for 30 s/Kb, with a final extension step at 72 °C for 5 min. The amplicons were run on a 1.5% agarose gel and purified with the QIAquick Gel Extraction Kit (QİAGEN, Germany) according to the manufacturer’s instructions based on the expected lentgh despite the fact that were not visualized.
2.4. High resolution melting analysis
The purified PCR amplicons and the sets of primers used for their amplification were also used for the subsequent HRM assays. HRM analysis was conducted in a 96-well plate using the CFX Connect™ Real-Time PCR Detection System (Bio-Rad). Duplicates of each sample were prepared in a final volume of 10 μl containing 2x Precision Melt Supermix (Bio-Rad), 0.2 μM of each primer and 20 ng/μL of amplified and purified template DNA. A negative control was included in each assay. An initial 2 min step at 95 °C was followed by 40 cycles of 95 °C for 10 s, Tm of the primers for 30 s and 72 °C for 30 s. The melting curve was obtained in continuous, performed as follow: 95 °C for 30 s, 65 °C for 1 min rising 0.2 °C/s, 95 °C for 10 s. During the incremental melting step, fluorescence data were continuously acquired. Precision Melt Analysis™ Software (Bio-Rad) was used to analyze the data. After normalization and temperature shift determination, the different melting curves of the several plots were generated. Three biological replicates were performed for each HRM assay.
3. Results
3.1. Detection of monovarietal olive oils using SSR- and SNP-HRM analysis
The major bottleneck of the DNA-based approaches for the identification of the varietal origin of olive oil as well as the detection of adulteration with oils of different plant origin is the isolation of adequate quality DNA for PCR amplification from the oil samples. Therefore, two different methodologies were initially tested, the CTAB/hexane/chloroform protocol (Giménez et al., 2010) and the Norgen kit for olive oil (Norgen Biotek, Thorold, Canada) using 600 µl (Bazakos et al., 2016) and 500 µl of olive oil sample, respectively. The Norgen DNA extraction procedure required less time compared to the CTAB protocol and was considered more reliable in providing olive oil DNA isolates which were more consistently leading to successful PCR amplifications. Therefore, the Norgen kit was used for the isolation of DNA from the monovarietal olive oils as well as their blends in order to be PCR amplified and purified after fractionation in agarose gel electrophoresis. Although the visualization of the PCR amplicons was not possible in the agarose gel, the PCR products were purified as gel fragments according to their expected length (Supplementary Fig. 1). These purified PCR products were successfully re-amplified and used for HRM analysis (Supplementary Fig. 1). The same set of primers was used for the initial and the additional PCR amplification for the HRM analysis.
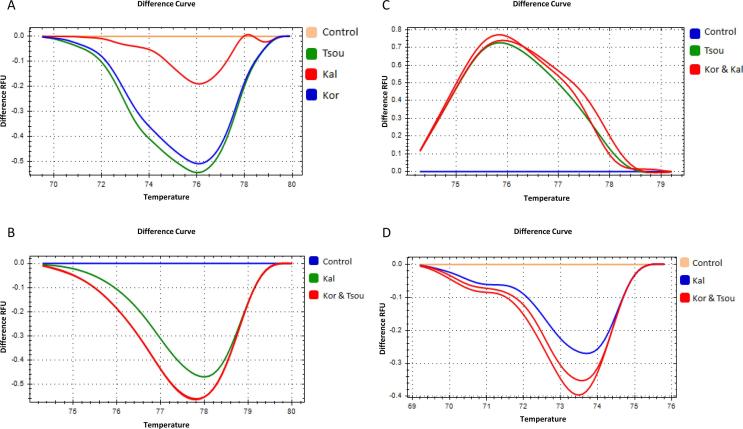
The olive SSR loci ssrOe-DCA3, ssrOe-DCA9, ssrOe-DCA16 and ssrOe-DCA18 (Sefc et al., 2000) were selected among several others such as ssrDCA5, ssrEMO90 and ssrGAPU71 (Carriero, Fontanazza, Cellini, & Giorio, 2002) because they were more consistent in the PCR amplification of the SSR marker fragments. The nucleotide sequence of the SSR amplicons was determined in order to validate amplification of the selected SSR loci. Representative sequences of ssrOe-DCA3 and ssrOe-DCA18 which were amplified from the Kalamon, Koroneiki and Tsounati are shown in Supplementary Fig. 2. The HRM difference plot with the ssrOe-DCA18 showed three different melting profiles of various shapes, one for every variety, allowing their discrimination (Fig. 1A). Moreover, the HRM assay with the ssrOe-DCA3 generated two different melting profiles of similar shape distinguishing the Kalamon (Tm = 70.40 ˚C) from Koroneiki and Tsounati both with a Tm of 70.20˚C (Fig. 1B). Leaf DNA of Koroneiki was used as a positive control template (Fig. 1A, B).
Fig. 1.
High resolution melting difference plots after normalization of Koroneiki (Kor), Kalamon (Kal) and Tsounati (Tsou) monovarietal olive oils using four different DNA markers, two SSRs and two SNPs. (A) ssrOe-DCA18, (B) ssrOe-DCA3, (C) SNP1 and (D) SNP3. The Koroneiki (Kor) leaf DNA was used as control.
The SNP1, SNP2 and SNP3 were successfully used for the determination of the varietal origin of Kalamon, Koroneiki and Tsounati as was previously reported after validation by sequencing of the PCR amplicons comprising these SNPs (Bazakos et al., 2012, Bazakos et al., 2016).
The use of SNPs for HRM analysis generated two melting plots per SNP, as expected. Specifically, the SNP1 and SNP3 (Bazakos et al., 2016) discriminated Tsounati from Koroneiki and Kalamon and Kalamon from Koroneiki and Tsounati, respectively (Fig. 1C, D).
3.2. Authentication of olive oil DNA admixtures using SSR- and SNP-HRM analysis
Genomic DNA was extracted from three monovarietal olive oils and diluted to equal concentrations for admixtures preparations as described in materials and methods. The admixtures and the positive control samples were used for PCR amplification in order to generate the templates for HRM analysis.
Three SSRs and three SNPs markers were used for the discrimination of the two sets of admixtures in a preliminary, comparative study to determine which DNA marker might be more suitable for authentication of olive oil admixtures.
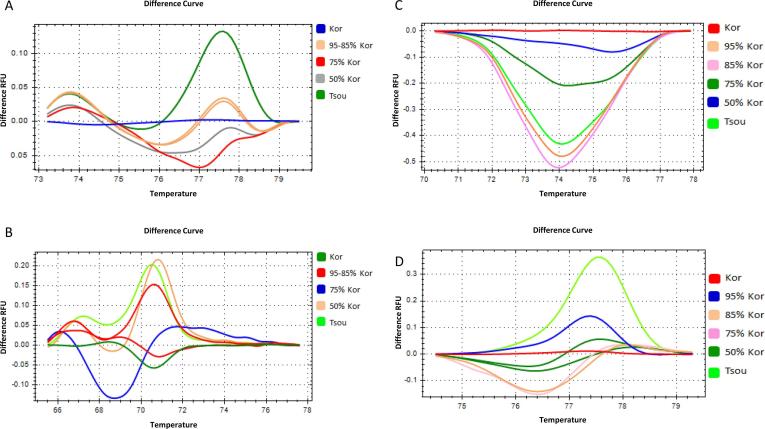
The ssrOe-DCA18 and ssrOe-DCA16 exhibited 5 different HRM melting profiles out of 6 samples for the DNA mixtures of Koroneiki-Tsounati (Fig. 2A, B). The melting curves of 95–5% and 85–15% ratios showed overlapping profiles and could not be discriminated (Fig. 2A, B). Although, curves of variable shapes were generated for some ratios compared to others, the discrimination of all ratios was not possible (Fig. 2A, B). The SNP1 and SNP2 (Bazakos et al., 2016) generated six different melting profiles distinguishing the four ratios and the two monovarietal samples generating curves of similar shape (Fig. 2C, D).
Fig. 2.
High resolution melting analysis of DNA mixtures. DNA extracted from Kor and Tsou monovarietal olive oil was mixed in four different ratios; 95% Kor + 5% Tsou, 85% Kor + 15% Tsou, 75% Kor + 25% Tsou and 50% Kor + 50% Tsou. Curves of different ratio mixtures in normalized difference plots using four different DNA markers, two SSRs and two SNPs. (A) ssrOe-DCA18, (B) ssrOe-DCA16, (C) SNP1 and (D) SNP2.
The ssrOe-DCA9 and ssrOe-DCA18 showed 5 different HRM melting profiles out of 6 samples for the Koroneiki-Kalamon blends (Fig. 3). The melting curves of 95–5% and 85–15% ratios also showed overlapping curves and could not be discriminated (Fig. 3A, B). However, the SNP2 and SNP3 (Bazakos et al., 2016) resulted in six different melting profiles distinguishing the four ratios and the two monovarietal samples generating curves of similar, though, distinct shapes (Fig. 3).
Fig. 3.
High resolution melting analysis of DNA mixtures. DNA extracted from Kor and Kal monovarietal olive oil was mixed in four different ratios; 95% Kor + 5% Kal, 85% Kor + 15% Kal, 75% Kor + 25% Kal and 50% Kor + 50% Kal. Curves of different ratios mixtures in normalized difference plots using four different DNA markers, two SSRs and two SNPs. (A) ssrOe-DCA18, (B) ssrOe-DCA9, (C) SNP3 and (D) SNP2.
These results indicate higher discriminatory capacity of SNPs compared to SSRs in DNA admixtures. Although the monovarietal DNA samples exhibited melting curves of similar shape, they were distinguished based on the Tm differences (Fig. 2, Fig. 3). However, the discrimination of the ratios was mainly based on the shape of the visualized melting profiles (Fig. 2, Fig. 3).
Moreover, the limit of detection for both DNA markers was determined at the levels of 5% since the 95–5% ratios of both blends were discriminated efficiently and reliably (Fig. 2, Fig. 3).
3.3. Authentication of olive oil blends
Olive oil blends were prepared and DNA extraction was performed as described in detail in Materials & Methods. The two-cultivar blends at four different ratios and their two monovarietal oils were used for SSR- and SNP-HRM analysis.
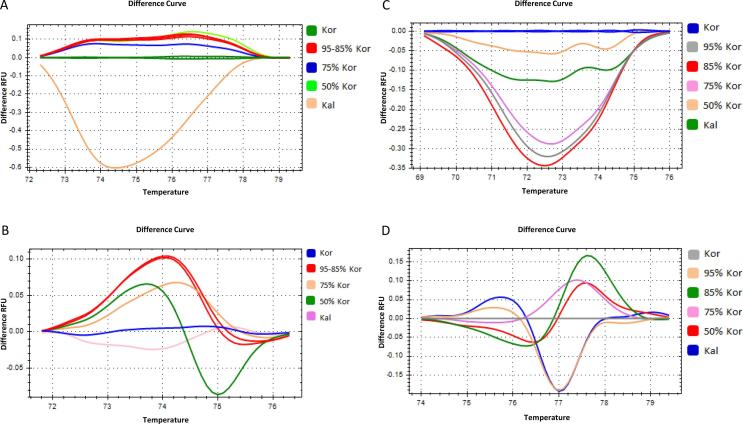
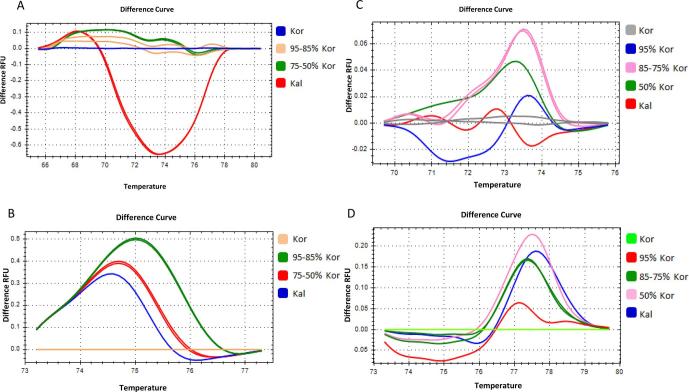
Initially the ssrOe-DCA16, ssrOe-DCA18 and ssrOe-DCA9, ssrOe-DCA18 pair of markers were used with blends of Koroneiki, Tsounati and Koroneiki, Kalamon, respectively (Fig. 4, Fig. 5). The blends of 95–5% and 85–15% and those of 75–25% and 50–50% showed overlapping melting profiles and therefore could not be discriminated (Fig. 4, Fig. 5). These results indicate that blends with ratios in the range between 95 and 85% and between 75 and 50% could not be distinguished by using these specific pairs of SSR markers (Fig. 4, Fig. 5).
Fig. 4.
High resolution melting analysis of monovarietal Olive oil mixtures. DNA extracted from mixtures of Kor and Tsou monovarietal olive oil at four different ratios; 95% Kor + 5% Tsou, 85% Kor + 15% Tsou, 75% Kor + 25% Tsou and 50% Kor + 50% Tsou. Curves of different ratio mixtures in normalized difference plots using four different DNA markers, two SSRs and two SNPs. (A) ssrOe-DCA18, (B) ssrOe-DCA16, (C) SNP1 and (D) SNP2.
Fig. 5.
High resolution melting analysis of monovarietal Olive oil mixtures. DNA was extracted from mixtures of Kor and Kal monovarietal olive oils at four different ratios; 95% Kor + 5% Kal, 85% Kor + 15% Kal, 75% Kor + 25% Kal and 50% Kor + 50% Kal. Curves of different ratio mixtures in normalized difference plots using four different DNA markers, two SSRs and two SNPs. (A) ssrOe-DCA18, (B) ssrOe-DCA9, (C) SNP3 and (D) SNP2.
Moreover, the SNP1, SNP2 and the SNP2, SNP3 pair of markers were used with blends of Koroneiki, Tsounati and Koroneiki, Kalamon, respectively (Fig. 4, Fig. 5). The blends of 95–5% and 50–50% showed distinct melting profiles and therefore could be discriminated by these combinations of SNP markers (Fig. 4, Fig. 5). However, the blends of 85–15% and 75–25% showed overlapping melting profiles and therefore could not be discriminated (Fig. 4, Fig. 5). These results indicate that blends with ratios in the range between 85 and 75% could not be distinguished with these specific pair of SNP markers (Fig. 4). Overall, the SNPs showed higher capacity to distinguish olive oil blends compared to the SSR markers.
Representative amplification plots for the ssrOe-DCA18 and the SNP2 indicate differences in the amplification efficiency but not in the shape of the plot which might be attributed to the quality of the DNA template of the olive oil samples and their blends considering that the same primers and similar HRM reaction conditions were used for each SSR and SNP marker (Supplementary Fig. 3).
3.4. Detection of maize and sunflower oil adulteration in extra virgin olive oil
Two different polymorphic molecular markers of the same chloroplastic trnL intron region were used for the detection of adulteration (Spaniolas et al., 2010). The trnL-indels and the trnL-SNPs primers were designed to amplify two different regions of this intron which can discriminate between different species based on length polymorphisms and single nucleotide polymorphisms, respectively (Supplementary Table 1). The trnL-SNPs amplicon was comprising two SNPs (Spaniolas et al., 2010).
DNA was extracted from the five blends for each adulterant and from the three different plant species oil, and then used as a template for the amplification and the HRM analysis of the two DNA markers, trnL-indels and trnL-SNPs.
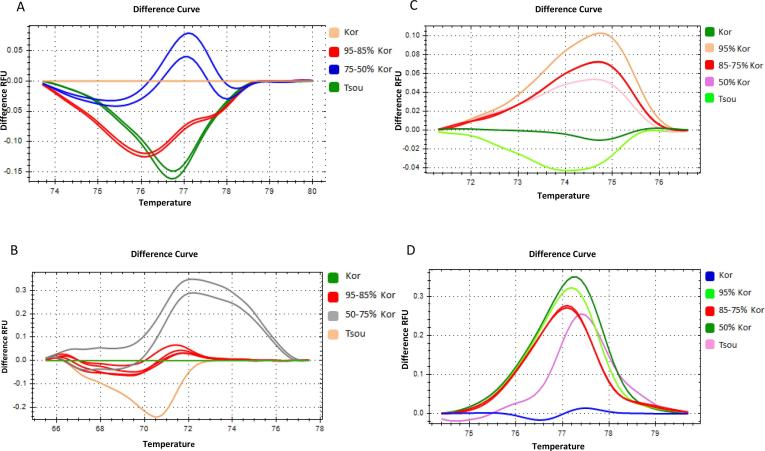
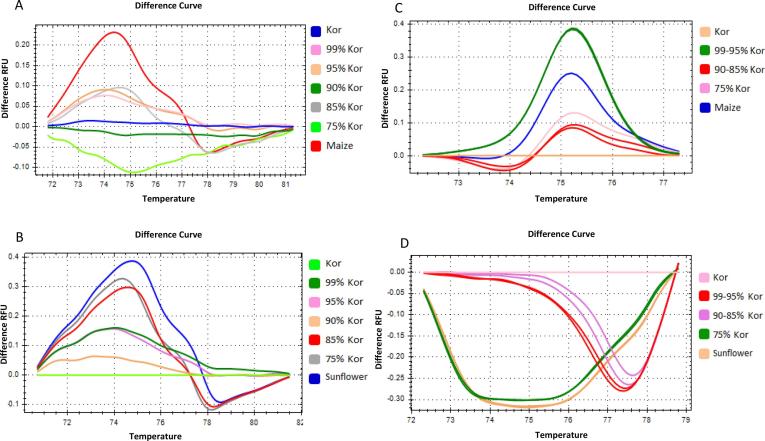
The HRM-trnL-indels difference plots of Koroneiki with maize and sunflower adulteration mixtures exhibited discrimination of all seven samples, four ratios and three 100% oil samples (Fig. 6). The shape of the melting profiles was similar in all blends regardless of the botanical origin of the adulterant and the percentage of adulteration (Fig.. 6 A, B).
Fig. 6.
High resolution melting analysis of olive oil adulteration with maize and sunflower oil. DNA was extracted from oil mixtures of Kor and either maize or sunflower adulterant (Adu) at five different ratios; 99% Kor + 1% Adu, 95% Kor + 5% Adu, 90% Kor + 10% Adu, 85% Kor + 15% Adu and 75% Kor + 25% Adu. Curves of different ratio mixtures in normalized difference plots using two different plastid DNA markers. (A) trnL-intels locus for maize adulteration (B) trnL-intels locus for sunflower adulteration, (C) trnL-SNPs locus for maize adulteration and (D) trnL-SNPs locus for sunflower adulteration.
However, the HRM- trnL-SNPs difference plots of the same samples detected five out of seven samples (Fig. 6C, D). The ratios of 99–1% with 95–5% and of 90–10% with 85–15% showed overlapping melting profiles indicating the higher discriminatory capacity of the trnL-indels marker compared to the trnL-SNPs (Fig. 6C, D). The shape of the melting curves was different in the maize adulterant plot compared to the sunflower plot (Fig. 6C, D). Moreover, in the sunflower difference plot the profiles of 100% and 25% sunflower oil exhibited different shape compared to the profiles of the other samples (Fig. 6C, D).
According to these results, the limit of detection for the HRM-trnL-indels analysis was determined at the levels of 1%. For the HRM-trnL-SNPs limit of detection was set at the range of 1–5% due to the overlapping curves of 99–1% and 95–5% blends (Fig. 6).
Representative amplification plots indicate differences in the efficiency of amplification but not in the shape of the plots which might be explained by differences in the quality of the DNA templates (Supplementary Fig. 3).
4. Discussion
Three monovarietal olive oils were selected for varietal authentication by using SSR-HRM and SNP-HRM analysis not only at the monovarietal level but also at the admixtures level of various ratios between two varieties each time. Moreover, this comparative study between SSRs and SNPs suggested possible advantages of one DNA marker over the other depending on the authentication objective.
Many studies revealed the efficiency of SSR-HRM analysis for the identification and differentiation of cultivars and closely related species (Bosmali et al., 2012, Distefano et al., 2012, Gomes et al., 2018, Li et al., 2018, Mackay et al., 2008). In this study, one SSR locus was adequate to discriminate the three olive varieties while two SNP loci were required to distinguish among them. Usually more SNP loci are required in order to acquire the same level of discriminatory power compared to SSRs (Avramidou et al., 2018). Therefore, the SSR-HRM might be considered the molecular marker of choice combined with HRM analysis if the objective is to distinguish a higher number of monovarietal olive oils.
Blends of specific varieties grown in certain regions are considered premium olive oils of higher value. Therefore there is a pressing need for reliable identification of the genetic identity of premium quality olive oils (Kalaitzis & El-Zein, 2016). In the current study, the limit of detection of artificial DNA admixtures of two olive oil cultivars was determined down to 5% for the SNP-HRM assays while for the SSR-HRM assays the limit of detection ranged between 5 and 15%. This is an improvement considering that Bazakos et al. (2016) determined the limit of detection of olive oil blends at the levels of 10% by using a SNP-based PCR-RFLP approach.
Significant differences in the discrimination accuracy of HRM analysis was detected between DNA mixtures and monovarietal olive oil mixtures. The SSR-HRM assay of olive oil admixtures of Koroneiki-Kalamon and Koroneiki-Tsounati revealed similar results; the HRM profiles of blends of 5% and 15% ratios were overlapping as well as the blends of 25% and 50% ratios indicating that there are limits in the discriminatory power of the SSR markers. However, the SNP-HRM assay distinguished all ratios except the 5% and 15% indicating that this analytical approach might be more efficient in olive oil traceability and authenticity efforts with strong commercial application potential as was previously described by Reed and Wittwer (2004).
The greatest challenges one faces while using DNA technology is the low quality and highly degraded DNA recovered from the fatty matrices and the impact of oil extraction processing on the size of the recovered DNA (Enferadi & Rabiei, 2013). The critical steps of the extraction process that affects the most the DNA, are the malaxation and separation steps where oil might be exposed to high temperatures causing higher degradation of DNA. This can result in variable quality DNA isolates. In these cases, a blend comprised of differentially processed monovarietal olive oils might result in DNA isolates which might not be representative of the blend ratios. Consequently, false estimation of DNA isolate for each variety from the blend might be obtained. Thus, this might be a limiting factor for olive oil authenticity at the quantitative level potentially leading to inconclusive results.
Various qualities of isolated DNA from monovarietal olive oils or blends might lead to significant variation in the amplification plots in the HRM reactions. In addition, there is the possibility that the presence of contaminants might interfere with the PCR amplification leading to a reduction in the amplification efficiency (Scollo et al., 2016). However, the difference plots provided by the HRM software represent the melting kinetics of each PCR amplicon which is affected by the SNP- or SSR-based polymorphic nature of the sequence of the amplicon and is not related to the amplification plots which are affected by the DNA templates per se.
The low quality and partially degraded DNA in olive oil samples make the traceability more challenging and less accurate due to the mostly short DNA templates present in the extracts. In this context, the amplicon length is important in determining the outcome of PCR-based amplification of DNA templates. Therefore, the shorter the amplicon is, the higher the probability of successful amplification (Spaniolas et al., 2008). Therefore, it is suggested that the SNP-HRM might be considered the molecular marker of choice compared to the SSR-HRM for authentication of olive and vegetable oil blends due to the requirement for relatively shorter DNA templates.
DNA mixtures extracted from adequately high quality olive oil DNA samples might result in the detection of the ratios of two varieties and distinguish among 5%, 15%, 25% and 50% by using SNP-HRM assays. However, it was suggested that CE electrophoretograms are more suitable for large data sets due to many samples compared to SNP-HRM because it is easier to digitalize and quantify peaks than melting curves (Lian & Zeng, 2017).
It was demonstrated that the discrimination of various percentages of olive oil mixtures is possible at limits of detection set at 5% by using this efficient, closed tube HRM approach. However, mixtures which differ in the range of 10 to 25% might not be possible to be discriminated regardless of high or low ratio percentages neither by SSR-HRM nor by SNPs-HRM.
The use of plastidial trnL (UAA) intron as an analytical target to discriminate among oils of different botanical origin by using HRM analysis proved to be an accurate and effective approach. The various polymorphisms in this plastid DNA region among different plant oils were previously reported by Spaniolas et al., 2008, Spaniolas et al., 2010 using a CE assay indicating thus the potential use of this intron in the detection of adulteration of olive oil.
Moreover, the HRM assay with trnL-indels locus and trnL-SNPs locus revealed a limit of detection of 1% (v/v) of plant oil adulterant in olive oil. Similar results were obtained by Ganopoulos et al. (2013) which reported a barcode-HRM analysis with an rbcL marker determining the limit of detection at 1% of canola oil in olive oil. In addition, they were able to distinguish different ratio admixtures of olive oil and canola.
In this preliminary, comparative report it was demonstrated that the trnL-indels locus revealed better discrimination power than the trnL-SNPs locus, distinguishing all mixture ratios, even though it requires a longer DNA template.
The fact that HRM analysis of DNA mixtures is more accurate compared to olive oil mixtures indicates that the quantitative determination of the varietal origin of olive oils has limitations which are directly related to the olive oil production process and/or storage conditions. However, the SNP-based HRM approach might be considered of higher discriminatory potential compared to SSR-based approaches for quantitative authentication purposes.
The use of trnL intel-HRM analysis showed higher discrimination power than the trnL SNP-HRM determining all mixture ratios of adulteration with maize oil as well as sunflower oil indicating that traceability of adulteration might be more reliable compared to authentication of the varietal origin of olive oil blends.
5. Conclusion
The authentication, traceability and adulteration of olive oil are of major importance in order to monitor for fraudulent practices. The SSR-HRM and SNP-HRM assays were both efficiently determined the varietal origin of monovarietal olive oils. In two cultivar admixtures, SNP-HRM showed higher efficiency to discriminate olive oil blends of various ratios except those of 95–5% and 85–15%. Moreover, the limit of detection was determined at the level of 5% only for the SNP-HRM assay. The HRM-based comparative quantitative determination of adulteration of olive oil with oils of different plant origin indicated that the indels within the trnL region were more efficient compared to trnL SNPs. The limit of detection for adulteration was determined at the level of 1% in accordance with previous reports. These results indicate that the quantitative validation of the varietal composition of olive oil blends might be possible, though not always reliable due to variations in the processing and/or storage of olive oil samples. However, the quantitative detection of adulteration might be considered more efficient despite the heavily processed nature of oils of plant origin.
CRediT authorship contribution statement
Elsa Chedid: Investigation, Validation, Writing - original draft. Myrto Rizou: Investigation, Validation, Writing - original draft, Writing - review & editing. Panagiotis Kalaitzis: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research has been financed by Greek national funds through the Public Investments Program (PIP) of General Secretariat for Research & Technology (GSRT), Greece under the Emblematic Αction “The Οlive Road” (project code: 2018ΣΕ01300000)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100082.
Contributor Information
Myrto Rizou, Email: myrto@maich.gr.
Panagiotis Kalaitzis, Email: panagiot@maich.gr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agrimonti C., Marmiroli N. Food genomics for the characterization of PDO and PGI virgin olive oils. European Journal of Lipid Science and Technology. 2019;121(3) [Google Scholar]
- Alba V., Sabetta W., Blanco A., Pasqualone A., Montemurro C. Microsatellite markers to identify specific alleles in DNA extracted from monovarietal virgin olive oils. European food research and technology = Zeitschrift fur Lebensmittel-Untersuchung und -Forschung. A. 2009;229(3):375–382. [Google Scholar]
- Avramidou E.V., Doulis A.G., Petrakis P.V. Chemometrical and molecular methods in olive oil analysis: A review. Journal of food processing and preservation. 2018;42(11) [Google Scholar]
- Bazakos C., Dulger A.O., Uncu A.T., Spaniolas S., Spano T., Kalaitzis P. A SNP-based PCR–RFLP capillary electrophoresis analysis for the identification of the varietal origin of olive oils. Food Chemistry. 2012;134(4):2411–2418. doi: 10.1016/j.foodchem.2012.04.031. [DOI] [PubMed] [Google Scholar]
- Bazakos C., Khanfir E., Aoun M., Spano T., Zein Z.E., Chalak L.…Grati Kammoun N. The potential of SNP-based PCR-RFLP capillary electrophoresis analysis to authenticate and detect admixtures of Mediterranean olive oils. Electrophoresis. 2016;37(13):1881–1890. doi: 10.1002/elps.201500537. [DOI] [PubMed] [Google Scholar]
- Ben-Ayed R., Kamoun-Grati N., Rebai A. An overview of the authentication of olive tree and oil. Comprehensive Reviews in Food Science and Food Safety. 2013;12(2):218–227. [Google Scholar]
- Besnard G., Hernández P., Khadari B., Dorado G., Savolainen V. Genomic profiling of plastid DNA variation in the Mediterranean olive tree. BMC Plant Biology. 2011;11(1):80. doi: 10.1186/1471-2229-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmali I., Ganopoulos I., Madesis P., Tsaftaris A. Microsatellite and DNA-barcode regions typing combined with High Resolution Melting (HRM) analysis for food forensic uses: A case study on lentils (Lens culinaris) Food Research International. 2012;46(1):141–147. [Google Scholar]
- Carriero F., Fontanazza G., Cellini F., Giorio G. Identification of simple sequence repeats (SSRs) in olive (Olea europaea L.) Theoretical and Applied Genetics. 2002;104(2–3):301–307. doi: 10.1007/s001220100691. [DOI] [PubMed] [Google Scholar]
- Consolandi C., Palmieri L., Doveri S., Maestri E., Marmiroli N., Reale S.…Severgnini M. Olive variety identification by ligation detection reaction in a universal array format. Journal of Biotechnology. 2007;129(3):565–574. doi: 10.1016/j.jbiotec.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Distefano G., Caruso M., La Malfa S., Gentile A., Wu S.-B. High resolution melting analysis is a more sensitive and effective alternative to gel-based platforms in analysis of SSR–an example in citrus. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druml B., Cichna-Markl M. High resolution melting (HRM) analysis of DNA–Its role and potential in food analysis. Food Chemistry. 2014;158:245–254. doi: 10.1016/j.foodchem.2014.02.111. [DOI] [PubMed] [Google Scholar]
- Enferadi S.T., Rabiei Z. The Mediterranean Genetic Code: Grapevine and Olive. 2013. Challenges for genetic identification of olive oil; p. 201. [Google Scholar]
- Ganopoulos I., Bazakos C., Madesis P., Kalaitzis P., Tsaftaris A. Barcode DNA high-resolution melting (Bar-HRM) analysis as a novel close-tubed and accurate tool for olive oil forensic use. Journal of the Science of Food and Agriculture. 2013;93(9):2281–2286. doi: 10.1002/jsfa.6040. [DOI] [PubMed] [Google Scholar]
- Ganopoulos I., Bosmali I., Madesis P., Tsaftaris A. Microsatellite genotyping with HRM (High Resolution Melting) analysis for identification of the PGI common bean variety Plake Megalosperma Prespon. European Food Research and Technology. 2012;234(3):501–508. [Google Scholar]
- Giménez M.J., Pistón F., Martín A., Atienza S.G. Application of real-time PCR on the development of molecular markers and to evaluate critical aspects for olive oil authentication. Food Chemistry. 2010;118(2):482–487. [Google Scholar]
- Gomes S., Breia R., Carvalho T., Carnide V., Martins-Lopes P. Microsatellite high-resolution melting (SSR-HRM) to track olive genotypes: From field to olive oil. Journal of Food Science. 2018;83(10):2415–2423. doi: 10.1111/1750-3841.14333. [DOI] [PubMed] [Google Scholar]
- Kalaitzis P., El-Zein Z. Olive oil authentication, traceability and adulteration detection using DNA-based approaches. Lipid Technology. 2016;28(10–11):173–176. [Google Scholar]
- Kalogianni D.P., Bazakos C., Boutsika L.M., Targem M.B., Christopoulos T.K., Kalaitzis P., Ioannou P.C. Olive oil DNA fingerprinting by multiplex SNP genotyping on fluorescent microspheres. Journal of Agriculture and Food Chemistry. 2015;63(12):3121–3128. doi: 10.1021/jf5054657. [DOI] [PubMed] [Google Scholar]
- Li J., Xiong C., He X., Lu Z., Zhang X., Chen X., Sun W. Using SSR-HRM to identify closely related species in herbal medicine PRODUCTS: A case study on licorice. Frontiers in Pharmacology. 2018;9:407. doi: 10.3389/fphar.2018.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian D.S., Zeng H.S. Capillary electrophoresis based on nucleic acid detection as used in food analysis. Comprehensive Reviews in Food Science and Food Safety. 2017;16(6):1281–1295. doi: 10.1111/1541-4337.12297. [DOI] [PubMed] [Google Scholar]
- Lo Y.-T., Shaw P.-C. DNA-based techniques for authentication of processed food and food supplements. Food Chemistry. 2018;240:767–774. doi: 10.1016/j.foodchem.2017.08.022. [DOI] [PubMed] [Google Scholar]
- Mackay J.F., Wright C.D., Bonfiglioli R.G. A new approach to varietal identification in plants by microsatellite high resolution melting analysis: Application to the verification of grapevine and olive cultivars. Plant Methods. 2008;4(1):8. doi: 10.1186/1746-4811-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madesis P., Ganopoulos I., Anagnostis A., Tsaftaris A. The application of Bar-HRM (Barcode DNA-High Resolution Melting) analysis for authenticity testing and quantitative detection of bean crops (Leguminosae) without prior DNA purification. Food Control. 2012;25(2):576–582. [Google Scholar]
- Montemurro C., Miazzi M.M., Pasqualone Antonella, Fanelli V., Sabetta W., di Rienzo V. Traceability of PDO Olive Oil “Terra di Bari” Using High Resolution Melting. Journal of Chemistry. 2015;2015:1–7. [Google Scholar]
- Pasqualone A., Montemurro C., di Rienzo V., Summo C., Paradiso V.M., Caponio F. Evolution and perspectives of cultivar identification and traceability from tree to oil and table olives by means of DNA markers. Journal of the Science of Food and Agriculture. 2016;96(11):3642–3657. doi: 10.1002/jsfa.7711. [DOI] [PubMed] [Google Scholar]
- Pasqualone A., Montemurro C., Summo C., Sabetta W., Caponio F., Blanco A. Effectiveness of microsatellite DNA markers in checking the identity of protected designation of origin extra virgin olive oil. Journal of Agriculture and Food Chemistry. 2007;55(10):3857–3862. doi: 10.1021/jf063708r. [DOI] [PubMed] [Google Scholar]
- Pereira L., Gomes S., Castro C., Eiras-Dias J.E., Brazão J., Graça A.…Martins-Lopes P. High Resolution Melting (HRM) applied to wine authenticity. Food Chemistry. 2017;216:80–86. doi: 10.1016/j.foodchem.2016.07.185. [DOI] [PubMed] [Google Scholar]
- Pérez-Jiménez M., Besnard G., Dorado G., Hernandez P. Varietal tracing of virgin olive oils based on plastid DNA variation profiling. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0070507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale S., Doveri S., Díaz A., Angiolillo A., Lucentini L., Pilla F.…Lee D. SNP-based markers for discriminating olive (Olea europaea L.) cultivars. Genome. 2006;49(9):1193–1205. doi: 10.1139/g06-068. [DOI] [PubMed] [Google Scholar]
- Reed G.H., Wittwer C.T. Sensitivity and specificity of single-nucleotide polymorphism scanning by high-resolution melting analysis. Clinical Chemistry. 2004;50(10):1748–1754. doi: 10.1373/clinchem.2003.029751. [DOI] [PubMed] [Google Scholar]
- Scollo F., Egea A.L., Gentile A., La Malfa S., Dorado G., Hernandez P. Absolute quantification of olive oil DNA by droplet digital-PCR (ddPCR): Comparison of isolation and amplification methodologies. Food Chemistry. 2016;213:388–394. doi: 10.1016/j.foodchem.2016.06.086. [DOI] [PubMed] [Google Scholar]
- Sefc K., Lopes M., Mendonça D., Santos M.R.D., Machado M.L.D.C., Machado A.D.C. Identification of microsatellite loci in olive (Olea europaea) and their characterization in Italian and Iberian olive trees. Molecular Ecology. 2000;9(8):1171–1173. doi: 10.1046/j.1365-294x.2000.00954.x. [DOI] [PubMed] [Google Scholar]
- Spaniolas S., Bazakos C., Awad M., Kalaitzis P. Exploitation of the chloroplast trn L (UAA) intron polymorphisms for the authentication of plant oils by means of a Lab-on-a-Chip capillary electrophoresis system. Journal of Agriculture and Food Chemistry. 2008;56(16):6886–6891. doi: 10.1021/jf8008926. [DOI] [PubMed] [Google Scholar]
- Spaniolas S., Bazakos C., Ntourou T., Bihmidine S., Georgousakis A., Kalaitzis P. Use of lambda DNA as a marker to assess DNA stability in olive oil during storage. European Food Research and Technology. 2008;227(1):175–179. [Google Scholar]
- Spaniolas S., Bazakos C., Spano T., Zoghby C., Kalaitzis P. The potential of plastid trnL (UAA) intron polymorphisms for the identification of the botanical origin of plant oils. Food Chemistry. 2010;122(3):850–856. [Google Scholar]
- Taberlet P., Coissac E., Pompanon F., Gielly L., Miquel C., Valentini A.…Willerslev E. Power and limitations of the chloroplast trn L (UAA) intron for plant DNA barcoding. Nucleic Acids Research. 2006;35(3) doi: 10.1093/nar/gkl938. e14 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley L.C., James D.J., Manning K. Purification and properties of an endo-β-1, 4-glucanase from strawberry and down-regulation of the corresponding gene, cel1. Planta. 2001;214(1):11–21. doi: 10.1007/s004250100577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.