Short abstract
Introduction
Severe obesity is a growing epidemic that causes significant morbidity and mortality, and is particularly difficult to reverse. Efficacious and cost-effective interventions are needed to combat this epidemic. This study hypothesized that obese people (body mass index (BMI) ≥35 kg/m2) using a remote weight-loss program combining a mobile application, wireless scales, and low-calorie meal replacement would experience clinically significant weight loss.
Methods
This study was a retrospective observational analysis of 8275 individuals with a baseline BMI ≥35 kg/m2 who used a remote weight-loss program combining mobile applications, frequent self-weighing, and calorie restriction via meal replacement for a minimum of 35 days. Weight changes were evaluated at multiple intervals (42, 60, 90, and 120 days), and weight loss was evaluated for all and for pre-specified subgroups based on demographic features and frequency of self-weighing.
Results
Mean weight loss at 42 days (N = 6781) was 8.1 kg (margin of error (MOE) = 0.126 kg) with 73.6% of users experiencing >5% total body weight loss. Both men (9.1 kg; MOE = 0.172 kg; 7.9% from baseline) and women (7.1 kg; MOE = 0.179 kg; 7.2% from baseline) experienced significant weight loss. At the 120-day interval (N = 2914), mean weight loss was 14 kg (MOE = 0.340 kg), 13% total body weight loss from baseline, and 82.3% of participants had lost >5% of their initial body weight. The decrease in body-fat percent correlated well with weight loss (R = 0.92; p < 0.001).
Conclusions
In a large cohort of individuals with class II or III obesity, a remote weight-loss program combining mobile applications, daily self-weighing, and calorie restriction via meal replacement resulted in dramatic weight loss among subjects who were active users when evaluated through a retrospective observational analysis.
Keywords: Digital health, weight loss, severe obesity, wireless scales, mobile application
Introduction
Over the past five decades, obesity has continued to swell to epidemic proportions, nearly tripling in that time frame worldwide.1 More than 640 million people worldwide are considered obese, constituting approximately 13% of the adult population.2 The prevalence of class III obesity (body mass index (BMI) ≥40 kg/m2) has increased even more dramatically, with more than a fivefold increase.3 The obesity epidemic is increasingly present in the developing world as well, with an estimated 62% of all obese adults residing in low- and middle-income countries.3 Obesity has been linked to increased risk of cardiovascular disease, diabetes, and some cancers, as well as an increased overall mortality4,5—risks that are further amplified in severely obese individuals.6 Weight loss has been shown to be effective in reducing the burden of comorbidities caused or worsened by obesity,7 even with as little as 5% body weight loss.8
The current guidelines for obesity management recommend comprehensive life-style intervention for patients with class II (BMI 35–40 kg/m2) and class III (BMI ≥40 kg/m2) obesity followed by consideration for bariatric surgery in those with class II obesity and comorbidities or those with class III obesity.9 Unfortunately, life-style interventions in these groups of patients have been largely ineffective, leaving few therapeutic alternatives and making many of them candidates for bariatric surgery. While effective and relatively safe,10 bariatric surgery bears a significant cost and requires access to a capable medical institution, limiting its ability to scale effectively.11 It is also not without risks, including anemia, thromboembolism, re-intervention, and, in rare cases, death.12
The increasing availability of Internet access via smartphones affords an emerging avenue for the treatment of severe obesity. Several smartphone applications have shown at least some effectiveness in attaining weight loss in overweight and obese individuals.13–15 Routine weighing with the use of wireless scales has also been shown to help people achieve clinically significant weight loss through behavior change.16,17 Unfortunately, there is a paucity of information on digital health weight-loss interventions specifically in the severely obese individuals, who are at highest risk of obesity-related complications.
We sought to investigate weight loss in obese people (BMI ≥35 kg/m2) participating in a digital health weight-loss program utilizing a mobile application, wireless scales, and a nutritional program through a retrospective observational study.
Methods
MetaWell
The MetaWell program (Weijian Technologies, Inc., Hangzhou, PR China) is an entirely remote weight-loss program, without any face-to-face interaction, which consists of a free mobile application combined with wireless home scales and a nutrition program. The MetaWell application is available on the Google Play and Apple app stores in multiple languages, including Chinese and English. Upon download, users are prompted to register and provide basic demographic information, including age and sex. They are also prompted to purchase the program, which includes wireless scales and six weeks’ worth of meal-replacement biscuits and available support for US$770. The wireless scales captures body weight and bioimpedance measurements, including water content and fat percent. The program focuses predominantly on weight loss via diet by providing a low-calorie meal plan centered on meal-replacement biscuits (MetaWell biscuits) supplemented with healthy recipes available in the application. A standard meal plan is initially prescribed via the application, recommending up to three MetaWell biscuits daily along with a selection of other recommended foods, with the aim of achieving a negative caloric balance. Based on weight-loss progression, the program is individualized to aim for 4 kg of weight loss per month. If weight loss is achieved too rapidly, a less rigorous plan is recommended.
MetaWell biscuits are low-calorie, high-nutrition meal replacements with a low glycemic index. Nutritional information as well as sample diet plans provided in the app are detailed in Appendix 1 in the Supplemental Material. Activity is encouraged during the program, but no specific exercise program is provided. Users are prompted by the application to weigh themselves and simultaneously take bioimpedence measurements on a daily basis via the wireless scales during their weight-loss program, which lasts for six weeks or longer. This is subsequently liberalized to every three days after the weight-loss program and finally weekly after three months. In the application, participants can view a record of their weight-loss progress, as well as a “Health Status Overview” that provides a snapshot of their current health data and optimal measures using a goal BMI of 22.0 kg/m2. Screen captures of the application are presented in Figure 1. Though not a requirement of the program, daily urinary ketone measurement is recommended to users as a means to monitor ketone production. Participants are instructed to measure each morning, and the urinary concentration of ketones is measured on a scale from 0 (0 mg/dL), meaning no ketosis, to 4 (160 mg/dL), meaning potentially unsafe ketosis, with a goal of 2 (40 mg/dL) to 3 (80 mg/dL). If the ketone measurement is 4, the recommended diet is adjusted to increase carbohydrate intake. If level 4 is measured on three consecutive measurements, the user is recommended to stop the plan and to consume a diet higher in carbohydrates.
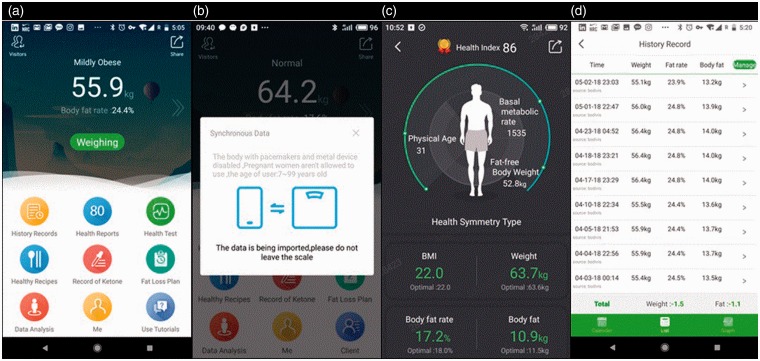
Figure 1.
Screenshots of the MetaWell mobile application. (a) Home screen of application. (b) Weighing screen, shown as users are positioned on the scale. (c )Health summary screen, providing measurement data of current session. (d) Record of weight change over time for an individual user.
Study protocol
Using a retrospective design, we probed the data to determine if MetaWell users with an initial reported BMI ≥35 kg/m2 on average achieved weight loss and if any recorded factors were associated with more or less weight loss among program users. A complete, de-identified record of application users collected by Weijian Technologies, Inc., was provided for research purposes. Participants provided electronic consent to have their data used for research when downloading the application, which was obtained at their own discretion from an online app store. Participants had chosen to be a part of the commercial weight-loss program at their own discretion. They received no subsidized products and no financial compensation for the program. This analysis included adult MetaWell users in China with an initial BMI of ≥35 kg/m2 from October 27, 2016, to December 31, 2017. Subjects also needed to have met a minimum of recorded participation, including a baseline weight and a weight at 35 days or beyond. The study design, strategy to analyze the data, actual data analysis, and the writing of the manuscript occurred without company input. Institutional Review Board (IRB) exemption was obtained via the Mayo Clinic IRB because of the de-identified data and retrospective observational nature of the analysis.
The program directs users to start an initial six-week weight-loss program. However, it was commonly continued for longer. All users who had weight recorded both at baseline and at ≥35 days were included in the analysis. From this group, users with a weight recorded in 14-day intervals centered on 42-, 60-, 90-, and 28-day intervals around 120 days were identified. The sets of users in each time window were not identical. Exceptionally high BMI (≥80 kg/m2) observations were excluded, as they were presumed to represent erroneous measurements. Users with a stated age <18 or >100 years were also excluded.
Subgroups were evaluated based on age, sex, baseline weight status, and frequency of use. The frequency of use was defined by the number of weights recorded for each person during each time interval studied divided by the number of days a person was in that time period. Tertiles of these frequencies were then made within the time period to create high, medium, and low frequency of use categories. We calculated percent weight loss from baseline, percent excess body weight, and percent excess body weight lost according to published recommendations.18
Statistical analysis
Means and standard deviations were used to describe continuous variables; counts and percentages were used to summarize categorical variables, both across strata and within stratum. Weight and body-fat percentage at baseline were determined by taking the median measurements within a three-day period of first user observation. Measurements at the end of the time intervals were constructed in a similar fashion but extending the end window to seven days (or 14 days in the case of the 120 stratum) before or after the end-date mark. Fourteen days was chosen for the 120 stratum due to weighing frequency recommended by the program. Excess weight was defined as any weight that exceeded that maximum healthy weight as determined by a BMI of 23 kg/m2 for Asian populations. Spearman correlations were used to assess the association between total body weight loss and body-fat percentage decrease. Chi-square tests were utilized to investigate if there was an average shift in weights from time 1 to time 2 within the stratum, and then further to probe for differences between men and women, age categories, overweight categories, and low-, medium-, or high-frequency weight recorders.
Results
From the 16,354 individuals with a baseline BMI ≥35 kg/m2, 8275 (50.6%) had a weight recorded at 35 days or beyond. Of these, 50.5% (n = 4179) of the cohort were females and had mean age of 33.8 years (standard deviation (SD = 9.7 years). The mean baseline weight was 108 kg (SD = 14.3 kg) with a BMI of 38.1 kg/m2 (SD = 2.84 kg/m2). A total of 79.5% (n = 6575) had a baseline BMI of 35–40 kg/m2, with 20.5% (1700) having a BMI ≥40 kg/m2. Baseline demographics are displayed in Table 1.
Table 1.
Baseline characteristics.
| N | 8275 |
|---|---|
| Age (years), M (SD) | 33.8 (9.74) |
| Female, n (%) | 4179 (50.5%) |
| Baseline weight (kg), M (SD) | 108 (14.3) |
| Baseline BMI (kg/m2), M (SD) | 38.1 (2.84) |
| Obesity class II (35–40 kg/m2), n (%) | 6575 (79.5%) |
| Obesity class III (≥40 kg/m2), n (%) | 1700 (20.5%) |
SD: standard deviation; BMI: body mass index.
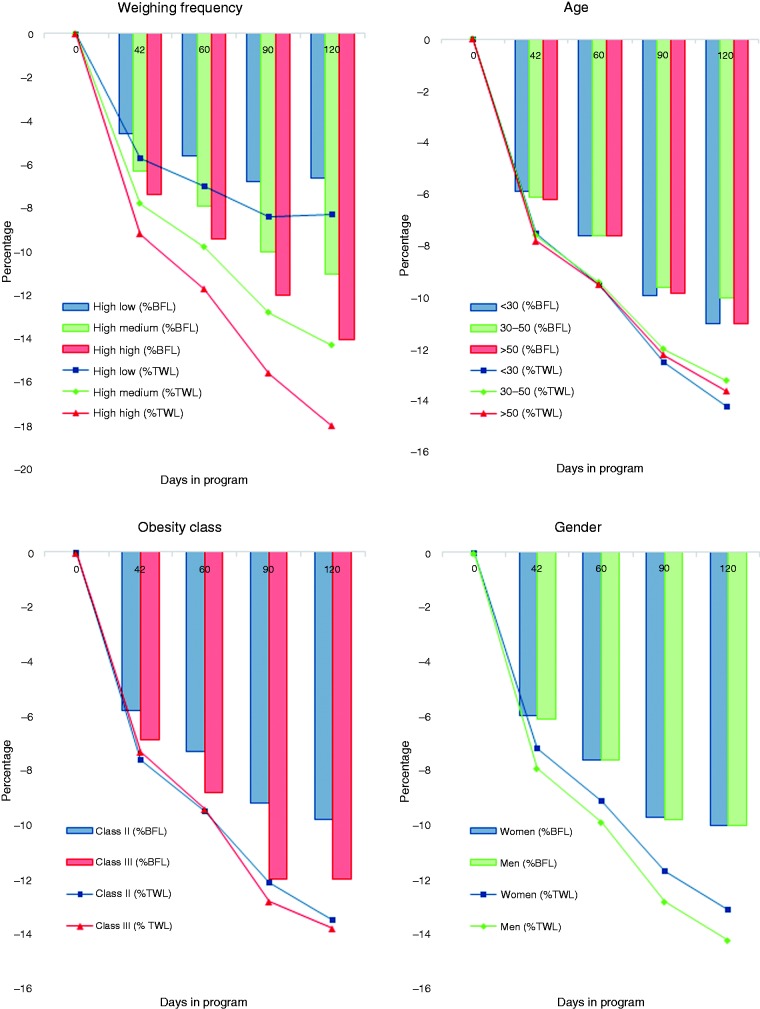
At the 42-day interval, the mean weight loss was 8.1 kg (margin of error (MOE) = 0.126 kg), with 73.6% of users experiencing >5% weight loss from baseline. Men (9.1 kg; MOE = 0.172kg; 7.9% total body weight loss (TWL)) and women (7.1 kg; MOE = 0.179 kg; 7.2% TWL) both displayed a similar and significant weight loss. The percentage of body fat loss (% BFL) was also similar between sexes (men 6.1%, SD = 3.7; women 6.0%, SD = 4.6). Weight loss was similar across age groups, with a mean loss among those aged <30 years, 30–50 years, and >50 years all between 7.9 and 8.3 kg (7.5–7.8% TWL). At the 120-day interval (n = 2914), the mean weight loss was 14 kg (MOE = 0.340 kg; 13% TWL; 10% BFL) for men and 12 kg (MOE = 0.44 kg; 12.6% TWL; 10% BFL) for women, respectively. A total of 82.3% of participants with a measurement at 120 days had lost >5% of their initial body weight. Cumulative weight-loss data and % BFL at 42-, 60-, 90-, and 120-day intervals are shown in Figure 2. At each time interval studied, the correlation between weight loss and BFL was significant (R > 0.92; p < 0.001).
Figure 2.
Percentage of total body weight loss (% TWL) displayed as lines, and percentage of body fat loss (% BFL) displayed as bars, among application users classified by sex, age, frequency of application use, and baseline body mass index.
Increased frequency of use, as categorized by tertile, was associated with significantly greater weight loss at each interval studied (Figure 2). At 42 days, the mean frequency of use for the highest tertile groups was 1.26 weights per day compared to 0.43 weights per day in the lowest tertile (Figure 2).
Compared to people with a BMI of 35–40 kg/m2, those with a BMI of ≥40 kg/m2 had greater weight loss at 42, 60, 90, and 120 days (p < 0.001) Total body weight loss, percentage excess weight loss (% EWL), and % BFL were similar between the groups at 42, 60, 90, and 120 days. Results are detailed in Table 2.
Table 2.
Weight loss and percentage excess weight loss among class II and class III obesity.
| Obesity class II (BMI 35–40 kg/m2) | Obesity class III BMI (≥40 kg/m2) | |
|---|---|---|
| Baseline | ||
| Weight (SD) | 104 (11.8) | 122 (14.4) |
| % EW (SD) | 37.8 (2.26) | 46.2 (2.76) |
| % BF (SD) | 52.2 (7.46) | 64 (8.87) |
| n | 5361 | 1420 |
| 42 days | ||
| WL (MOE) | 7.9 (0.138) | 8.9 (0.304) |
| % EWL (MOE) | 20.3 (0.343) | 16.3 (0.624) |
| % TWL (MOE) | 7.6 (0.128) | 7.3 (0.260) |
| % BFL (MOE) | 5.8 (0.103) | 6.9 (0.267) |
| n | 4211 | 1093 |
| 60 days | ||
| WL (MOE) | 9.9 (0.178) | 11 (0.404) |
| % EWL (MOE) | 25.4 (0.447) | 20.7 (0.794) |
| % TWL (MOE) | 9.5 (0.147) | 9.4 (0.307) |
| % BFL (MOE) | 7.3 (0.130) | 8.8 (0.337) |
| n | 2435 | 638 |
| 90 days | ||
| WL (MOE) | 13 (0.288) | 16 (0.665) |
| % EWL (MOE) | 32.2 (0.723) | 28.4 (1.273) |
| % TWL (MOE) | 12.1 (0.182) | 12.8 (0.374) |
| % BF (MOE) | 9.2 (0.208) | 12 (0.536) |
| n | 2280 | 634 |
| 120 days | ||
| WL (MOE) | 13 (0.367) | 16 (0.817) |
| % EWL (MOE) | 34.5 (0.915) | 29.3 (1.518) |
| % TWL (MOE) | 13 (0.225) | 13.3 (0.453) |
| % BF (MOE) | 9.8 (0.261) | 12 (0.638) |
% EW: % excess weight; % BF: % body fat; WL: weight loss; % EWL: % excess weight loss; % TWL: % total weight loss; % BFL: % body fat loss; MOE: margin of error.
The mean duration of use was 118 days (SD = 72.5 days) based on the last available weight record. Male users continued the program for a shorter average duration (113 days; SD = 69.5) than women did (122 days; SD = 75.1 days; p < 0.001). The highest frequency tertile of users continued the program for an average of 161 days (SD = 72 days), which was longer than medium-frequency tertile users (101 days; SD = 62.7) and low-frequency tertile users (92 days; SD = 62.5 days; p < 0.001).
A total of 2531 (30.6%) participants recorded at least one urinary ketone measurement during the study. The group with at least one record made a mean of 10.8 measurements, of which 48.7% (n = 13,287) were in the goal range (2–3), while 45.2% (n = 12,335) were below goal ketone level (0–1), and 6.1% (n = 1664) were unfavorably high (4). Of those individuals recording a potentially unsafe level of 4, 5.2% (n = 86) had it on their last day of record. Those with at least one ketone recording of 4 lost more weight on average than those with a ketone reading that never reached 4 (14.88 kg vs. 12.69 kg, respectively; p < 0.001).
Discussion
Severe obesity is a growing problem that puts individuals at high risk for obesity-related complications. In this study, we investigated the use of a novel smartphone-based program combined with wireless digital scales and nutritional program in a large cohort, and we found dramatic weight loss driven by body fat loss up to 120 days. The program requires no face-to-face interaction and is widely accessible, making it a potentially useful strategy to combat severe obesity in large populations. This study is unique in its scale, with more than 8000 participants, for a life-style intervention in severe obesity. The only other similarly sized studies in severe obesity have evaluated bariatric surgical procedures. Also notable is the Chinese cohort, a nation that is home to more than one fifth of the obese individuals in the world.19.
Management of class II (BMI 35–40 kg/m2) and class III (BMI ≥40 kg/m2) obesity is exceptionally challenging to intervene on effectively.20 As these conditions grow in prevalence worldwide, especially in lower-resource countries, effective strategies that can be employed at scale are needed.1,9 The approach studied herein takes a multi-pronged intervention to treat severe obesity by employing several components with documented success: mobile applications,13 frequent self-weighing,21 and calorie restriction via meal replacement.22 Among active users, this program yielded impressive weight loss, with a mean of 14 kg (13% TWL) at 120 days, and 82.3% of participants losing >5% of their initial body weight. As a comparator in the control arm of the Swedish Obesity Subjects Study, evaluating the effectiveness of bariatric surgery in severe obesity, the untreated control group lost on average a mere 1 kg at six months.23 An intensive life-style program for severe obesity, with intense face-to-face interaction, incorporating reduced caloric intake via meal replacements and increased physical activity evaluated via a randomized control trial, showed 10.9 kg of weight loss at six months, with 80% achieving >5% weight loss.24 The similar degree of weight loss raises the potential of a less costly intervention at achieving clinically significant weight loss for some users. However, applicability and efficacy across a broad population requires further study. The addition of bioelectrical impedance measurements to weight loss provides important confirmation that a large proportion of the weight loss during the study in all groups was fat loss as opposed to lean body mass. Bioelectrical impedance can be inaccurate in single measurements, but is a useful tool in serial assessments, such as in our study.25
Frequency of self-weighing has previously been studied in groups with a larger diversity in baseline BMI and has been associated with increased weight loss.26 Our study found similar results, with the highest frequency tertile losing approximately twice the weight over 120 days of their low-frequency counterparts. This relationship could be motivational, as those more motivated to lose weight likely weighed themselves more frequently. Alternatively, high-frequency self-weighing may have driven a feedback loop of being reminded about current weight more frequently and increasing motivation. Further work is needed to parse this relationship and to determine any causality so that it can be exploited to increase likelihood of success in achieving weight loss.
The use of a meal-replacement biscuit as a part of the prescribed weight-loss program is notable. Its direct contribution to the observed weight loss is unable to be determined at this time. However, previous work has shown meal replacements to be a viable strategy for both weight loss and weight-loss maintenance.27,28 Future studies directed at comparing the weight-loss program with the nutritional biscuit versus a diet based on real food would be useful.
This initial observational study shows excellent short-term weight loss among individuals actively engaged in this remote weight-loss program. However, the number of participants actively engaged in this program at 120 days represented 35% of initial participants. Further investigation is warranted to identify factors that would enhance participant engagement in order to increase the number of individuals that achieve clinically significant weight loss. A randomized clinical trial of longer duration is warranted to confirm these findings and to explore the impact on long-term weight maintenance further. Investigation into a more diverse cohort of severely obese individuals would also be warranted to examine the effects in other patient populations. The remote, easily accessible nature of the intervention makes further large-scale studies in these contexts feasible, with the possible addition of relevant demographic information and medical comorbidities, as well as remotely collected data such as activity, specific meal replacement use, or blood pressure.
Limitations
This study is limited by its observational design, which does not allow for a casual association between application use and weight loss. The use of a smartphone application for data collection through the study provides an important and evolving avenue for clinical research. It is notable that the safety and confidentiality of application use appears reliable. However, continued monitoring is warranted. The limited demographic information available in this study limits generalizability of the results. The inclusion criterion to have recorded weights for at least 35 days introduces motivation and participation bias, as this cohort represents a group of probably more motivated individuals. More than half of the individuals with access to this tool did not fully engage in its use, limiting applicability. However, it is not unusual for weight-loss clinical trials to have run-in periods to preselect individuals who may be more compliant with the therapy under study. The lack of comparison to a control group is a limitation because we cannot rule out confounding variables that may account for the results reported. Trials evaluating weight loss with calorie-restrictive diets have shown a tendency toward initial weight loss followed by a plateau.29,30 This study is limited in duration and unable to account for extended weight-loss outcomes. We are also unable to capture potential adverse effects of the dietary intervention implemented, specifically for those individuals who introduced significant carbohydrate restriction and developed subsequent ketosis. Direct measures of supplement use during the period studied were unavailable for this study. However, in the future, they represent an important measure for the program.
Multiple factors contribute to weight loss,31 but during this study, only application use was evaluated. Extended duration of follow-up would allow for further investigation into the possible prolonged benefits of application use. Information gathered for the study is inherently user generated via the app and thus is likely prone to some error. Failure to self-report weight information is likely confounded with weight loss and is a potential source of bias.
Conclusion
The use of a remote weight-loss program combining mobile applications, frequent self-weighing, and calorie restriction via meal replacements was associated with significant weight loss at three months among active users. Smartphone based weight-loss programs may provide an individualized and easily accessible avenue for weight loss in severely obese patients.
Supplemental Material
Supplemental material, DHJ910279 Supplemental Material for A digital health weight-loss intervention in severe obesity by Conor Senecal, Maria Collazo-Clavell, Beth R Larrabee, Mariza de Andrade, Weihua Lin, Bing Chen, Lilach O. Lerman, Amir Lerman and Francisco Lopez-Jimenez in Digital Health
Contributorship
C.S., L.L., A.L. and F.L.J. researched literature and conceived the study. C.S., M.dA., B.L., L.L., A.L., and F.L.J. were involved in protocol development and data analysis. C.S. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.L. and L.L. serve as consultants to Weijian Technologies, Inc., Hangzhou, PR China. W.L. and B.C. are employed by Hangzhou MetaWell Technology Co., Hangzhou, PR China. The MetaWell mobile application and Yufit meal-replacement biscuits evaluated in this study are both licensed and owned by a single commercial enterprise, Weijian Technologies, Inc., Hangzhou, PR China.
Ethical approval
IRB exception was obtained via Mayo Clinic Institutional Review Board because of the de-identified data and retrospective observational nature of the analysis.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by an unrestricted research grant from Weijian Technologies Inc., Hangzhou, PR China. The company had no influence in the study design, analysis, or interpretation of the data.
Guarantor
F.L.J.
Peer review
Marta Supervía, Hospital General Universitario Gregorio Marañeón reviewed this paper.
ORCID iD
Conor Senecal https://orcid.org/0000-0002-5000-6254
Supplemental material
Supplemental material for this article is available online.
References
- 1.NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016; 387: 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017; 390: 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrington De Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. New Engl J Med 2010; 363: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitahara CM, Flint AJ, Berrington De Gonzalez A, et al. Association between class III obesity (BMI of 40–59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med 2014; 11: e1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poobalan AS, Aucott LS, Smith WC, et al. Long-term weight loss effects on all cause mortality in overweight/obese populations. Obes Rev 2007; 8: 503–513. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129: S102–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontiroli AE, Morabito A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann Surg 2011; 253: 484–487. [DOI] [PubMed] [Google Scholar]
- 11.Doble B, Wordsworth S, Rogers CA, et al. What are the real procedural costs of bariatric surgery? A systematic literature review of published cost analyses. Obes Surg 2017; 27: 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014; 349: g3961–g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores Mateo G, Granado-Font E, Ferré-Grau C, et al. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta-analysis. J Med Internet Res 2015; 17: e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Xue H, Huang Y, et al. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self-management. Adv Nutr 2017; 8: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu F, Kong X, Cao J, et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta-analysis of randomized controlled trials. Am J Epidemiol 2015; 181: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas JG, Raynor HA, Bond DS, et al. Weight loss and frequency of body-weight self-monitoring in an online commercial weight management program with and without a cellular-connected “smart” scale: a randomized pilot study. Obes Sci Pract 2017; 3: 365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg DM, Tate DF, Bennett GG, et al. The efficacy of a daily self-weighing weight loss intervention using smart scales and email. Obesity (Silver Spring) 2013; 21: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brethauer SA, Kim J, El Chaar M, et al. Standardized outcomes reporting in metabolic and bariatric surgery. Obes Surg 2015; 25: 587–606. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y. Overweight and obesity in China. BMJ 2006; 333: 362–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Roundtable on Obesity Solutions. The challenge of treating obesity and overweight: proceedings of a workshop. Washington, DC: National Academies Press, 2017. [PubMed] [Google Scholar]
- 21.Steinberg DM, Bennett GG, Askew S, et al. Weighing every day matters: daily weighing improves weight loss and adoption of weight control behaviors. J Acad Nutr Diet 2015; 115: 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006; 14: 1283–1293. [DOI] [PubMed] [Google Scholar]
- 23.Sjöström L, Lindroos A-K, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. New Engl J Med 2004; 351: 2683–2693. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, DeLany JP, Otto AD, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA 2010; 304: 1795–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdich C, Barbe P, Petersen M, et al. Changes in body composition during weight loss in obese subjects in the NUGENOB study: comparison of bioelectrical impedance vs. dual-energy X-ray absorptiometry. Diabetes Metab 2011; 37: 222–229. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Klem ML, Sereika SM, et al. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring) 2015; 23: 256–265. [DOI] [PubMed] [Google Scholar]
- 27.LeCheminant JD, Jacobsen DJ, Hall MA, et al. A comparison of meal replacements and medication in weight maintenance after weight loss. J Am Coll Nutr 2005; 24: 347–353. [DOI] [PubMed] [Google Scholar]
- 28.Davis LM, Coleman C, Kiel J, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J 2010; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrobelli A, Schoeller DA, Beetsch JW, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am J Clin Nutr 2007; 85: 346–354. [DOI] [PubMed] [Google Scholar]
- 30.Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003; 289: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 31.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005; 6: 67–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DHJ910279 Supplemental Material for A digital health weight-loss intervention in severe obesity by Conor Senecal, Maria Collazo-Clavell, Beth R Larrabee, Mariza de Andrade, Weihua Lin, Bing Chen, Lilach O. Lerman, Amir Lerman and Francisco Lopez-Jimenez in Digital Health