Abstract
Background
The exhaustive collection of new sarcoma cases and their second histologic review offer a unique opportunity to study their incidence and time trends in France according to the major subtypes.
Methods
Data were collected from population-based cancer registries covering 22% of the French population. Crude and world age-standardized incidence rates (ASR) were estimated according to anatomic, histological and genetic groups, age and sex over the 2010–2013 period.
Results
Time trends in incidence were calculated by the annual percent change over the 2000–2013 period. During the most recent period (2010–2013), 3942 patients with sarcoma were included. The ASR of soft-tissue and bone sarcomas, and gastro-intestinal stromal tumors (GIST) were 2.1, 1.0 and 0.6, respectively. For the four most frequent histological subtypes (unclassified, leiomyosarcoma, GIST and liposarcoma), the ASR ranged from 0.4 to 0.7. ASRs were 1.9 for complex genomic and 1.3 for recurrent translocation sarcomas. The time-trend analysis showed a significant increase of sarcoma incidence rate between 2000 and 2005, which stabilized thereafter. Incidence rates increased for four histological subtypes (GIST, chondrosarcoma, myxofibrosarcoma, solitary fibrous tumors) and decreased for three (leiomyosarcomas, Kaposi sarcoma and fibrosarcoma).
Conclusion
To our knowledge, this study is the first to investigate sarcoma incidence based on a systematic pathological review of these cancers and on the updated sarcoma classifications. Due to the paucity of literature on sarcomas, future studies using data from population-based cancer registries should consider a standardized inclusion criterion presented in our study to better describe and compare data between countries.
Keywords: Sarcoma, Incidence, Trends in incidence, France, Cancer registry
Background
Sarcomas are a heterogeneous group of rare malignant tumors derived from primitive mesenchymal cells. These tumors arise from muscle, connective tissue, supportive tissue and vascular tissue, and more than 80 histologic subtypes are included in the 2013 World Health Organization (WHO) Classification of Tumors of Soft Tissue and Bone [1]. In addition to having a multiple and complex histology, these tumors can occur in almost any anatomic site. In spite of these facts, sarcomas account for less than 1% of all adult cancers and for about 20% of all malignant solid tumors in children, adolescents and young adults [2].
From an epidemiological point of view, the lack of a unified method of reporting sarcomas has led to considerable variations in the reported incidence and time trends Sarcomas are sometimes mistaken for carcinomas of the same organ, and can involve a variety of localizations. As a consequence, 30 % of sarcomas are misclassified at initial diagnosis [3]. In addition, sarcomas encompass a wide variety of histological and molecular subtypes and are categorized in rapidly evolving phenotypic and molecular subgroup classification schemas now used for sarcoma diagnosis, which has a growing impact on the management of patients [4]. Furthermore, innovation in immune-histochemistry and molecular biology techniques in the last three decades has led to major changes in the diagnosis and classification of sarcoma subtypes.
Currently, data for sarcomas in the French population are provided by the reference networks for sarcomas that collect and manage cases of soft tissue, bone and visceral sarcomas. Reference networks propose a systematic second histologic review by expert pathologists [5–7]. A few French studies carried out by these reference networks provided world age-standardized incidence rates of 4.8 and 3.3 per 100,000 inhabitants per year for all sarcomas and soft-tissue sarcomas (STS) respectively [8, 9]. However, data from these reference networks based on the voluntary participation are not totally exhaustive.
Besides reference networks, cancer surveillance information is coming from the French Network of population-based cancer registries that exhaustively collects all newly diagnosed and confirmed cancer cases within geographical areas in France [10]. The exhaustive collection of sarcoma cases from population-based cancer registries and the systematic second review of diagnosis from reference centers offer an optimal framework to study the incidence and time trends of sarcomas in France. The incidence trends have never been studied in France and the results from other countries are divergent [11]. We undertook this study to describe sarcoma entity according to anatomic sites, histologic subtypes and genetic groups based on guidelines developed by sarcoma specialists.
Methods
Data sources
Cases included in this study were children and adults with sarcoma diagnosed between January 1, 2000 and December 31, 2013, and living in one of the administrative areas covered by a population-based cancer registry of the French Network (details in online supplementary material). The French sarcoma pathological reference network (RRePS) and the French reference Network for bone sarcoma and rare bone tumors (RESOS) propose a systematic second histologic review and confirmation for all diagnoses of sarcomas across France [6].
Data collection and classification
The following data were collected for each case: general demographic characteristics of the patients (age, sex, and residence area), the date of diagnosis, the anatomical site, and the histology of the tumor according to the International Classification of Diseases for Oncology, third edition (ICD-O-3) (12).
This study included intermediate (only with a “/3” behavior) and malignant sarcomas presenting morphologic criteria described in the 2013 WHO Classification of Tumors of Soft Tissue and Bone (fourth edition), regardless of the anatomic site [1]. This recent classification includes histologic updates not defined in ICD-O-3 and new terms, synonyms, morphology and behavior codes. For this reason, and whenever possible, cases were reclassified according to the updated version. The alignments from ICD-O-3 to the 2013 WHO standard classification of tumors have been validated by a panel of sarcoma specialists (clinical and pathological experts) from sarcoma Networks (NP, JMC and IRC).
Certain alignments could not be performed: ten morphological terms not described in this updated classification (e.g. sarcoma NOS, periosteal fibrosarcoma, fascial fibrosarcoma …) have been maintained for analyses. Conversely, well differentiated liposarcoma and chondroblastoma have been changed from malignant to borderline diseases. In the same way, behaviors for dermatofibrosarcoma protuberans and pigmented dermatofibrosarcoma protuberans have been also changed from malignant to borderline with henceforth, only fibrosarcomatous dermatofibrosarcoma protuberans which is coded as malignant behavior. In our analyses, we have made the choice to keep all dermatofibrosarcomas. Indeed, we do not have the possibility to differentiate if this is a dermatofibrosarcoma borderline or malignant. Besides, endometrial stromal sarcoma NOS (89303), low grade endometrial stromal sarcoma (89313) and stromal sarcoma (89353) not described in the WHO 2013 have been also included. Additional details on the list and choice of classification systems are provided in the online supporting material (see Additional File 1).
This classification also provides new genetic and molecular data for each histologic entity allowing a better characterization of sarcomas. The same group of experts were consulted with the aim of proposing the optimal classification system for sarcomas based on the genetic profile. Two main distinct genetic groups were defined: (i) sarcomas defined with simple genetics based on recurrent translocations (e.g. Ewing sarcoma, myxoïd liposarcoma), activating or inactivating mutations (e.g epithelioid sarcoma, gastrointestinal stromal tumor), MDM2 amplification (e.g. dedifferentiated liposarcoma, low-grade central osteosarcoma); and (ii) sarcomas with complex genomic profiles (e.g. angiosarcoma, leiomyosarcoma). Another group was defined for miscellaneous and undefined alterations. The list of histology codes according to their genetic groups is presented in the supplementary material.
This study is based on data from cancer registries gathered in the French network of cancer registries and a representative of each registry was involved in the study and approved the use of its data All French registries received an authorization to collect patient data from the data protection authority (Commission Nationale de l’Informatique et des Libertés). Ethics approval and consent to participate were not required for this study which is an observational research without direct contact with patient.
Statistical analyses
Two datasets were used: i) the first one was used to estimate the incidence of patients diagnosed during the 2010–13 period and that included data from 19 registries; and ii) the second one was used to examine trends in the incidence from 2000 to 2013 in only 11 registries for which data were available over the entire studied period. Incidence rates were presented per 100,000 person-years.
The incidence of sarcomas was described according to 1) the anatomic group (i.e. soft-tissue, bone, gastro-intestinal, skin, female genital organs, other viscera and other sites), and to 2) histologic and 3) genetic groups based on guidelines developed by sarcoma specialists (see Additional File 1).
Age-standardized incidence rates (ASR) were estimated using direct standardization and were calculated using the population data for each age group and year supplied by the National Institute of Statistics and Economic Studies (www.insee.fr) and the European (ASR-E), Segi World (ASR-W), and the US (ASR-US) standard populations. The analyses presented here describe the overall ASR and the ASR by sex. Age-specific incidence rates are provided by age groups (0–14; 15–24; 25–39; 40–64; 65–74 and 75 and more) and by sex and presented in figures.
Time trends were calculated using Joinpoint Trend Analysis Software setting a maximum of a single Joinpoint (details in online supplementary material). The annual percent change (APC) with the 95% confidence interval (CI) was estimated according to topographic and histologic groups.
Results
Over the 2010–13 period, sarcomas accounted for 1.3% (3942/307,862) of all malignant tumors diagnosed over the French registry area. The male/female ratio for overall sarcomas was 1.0 but ranged from 0.5 for angiosarcomas to 6.2 for Kaposi sarcomas (KS) (Table 1). The median age was 63 years (range: 0–106) with large intergroup variations. About 9% of subjects were under 24 years and 27% were older than 75 years. Almost half of the cases were soft tissue sarcomas (45%). The most frequent histological subtypes were undifferentiated or unclassified sarcomas (16%), leiomyosarcoma (14%) and GIST (13%). Sarcomas with complex genomics accounted for the most frequent molecular profile (40%).
Table 1.
Gender distribution of sarcoma patients according to age and topographic, genomic and histologic groups. FRANCIM network data 2010–2013 (19 registries)
| Male | Female | Overall |
Sex ratio M/F |
||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age group (in years) | |||||||
| 0–14 | 81 | 4.1 | 81 | 4.1 | 162 | 4.1 | 1.0 |
| 15–24 | 99 | 5.0 | 79 | 4.0 | 178 | 4.5 | 1.3 |
| 25–39 | 197 | 10.0 | 160 | 8.1 | 357 | 9.1 | 1.2 |
| 40–64 | 671 | 34.1 | 738 | 37.4 | 1409 | 35.7 | 0.9 |
| 65–74 | 376 | 19.1 | 379 | 19.2 | 755 | 19.2 | 1.0 |
| 75 and more | 546 | 27.7 | 535 | 27.1 | 1081 | 27.4 | 1.0 |
| Sarcoma topographic groups | |||||||
| Soft tissue | 972 | 49.3 | 812 | 41.2 | 1784 | 45.3 | 1.2 |
| Bone | 310 | 15.7 | 259 | 13.1 | 569 | 14.4 | 1.2 |
| Skin | 262 | 13.3 | 167 | 8.5 | 429 | 10.9 | 1.6 |
| Viscera | |||||||
| Gastro-intestinal organs | 291 | 14.8 | 287 | 14.6 | 578 | 14.7 | 1.0 |
| Female genital organs | – | – | 282 | 14.3 | 282 | 7.2 | – |
| Others visceral organs | 102 | 5.2 | 129 | 6.5 | 231 | 5.9 | 0.8 |
| Other anatomic sites | 33 | 1.7 | 36 | 1.8 | 69 | 1.8 | 0.9 |
| Sarcoma genomic groups | |||||||
| Complex genomic alterations | 723 | 36.6 | 847 | 43.0 | 1570 | 39.8 | 0.9 |
| MDM2 amplification | 135 | 6.9 | 81 | 4.1 | 216 | 5.5 | 1.7 |
| Mutations | 274 | 13.9 | 276 | 14.0 | 550 | 14.0 | 1.0 |
| Recurrent translocations | 340 | 17.3 | 438 | 22.2 | 778 | 19.7 | 0.8 |
| Undefined/Miscellaneous alterations | 498 | 25.3 | 330 | 16.7 | 828 | 21.0 | 1.5 |
| Sarcoma histologic groups | |||||||
| Unclassified sarcomaa | 327 | 16.6 | 308 | 15.6 | 635 | 16.1 | 1.1 |
| Leiomyosarcoma | 205 | 10.4 | 346 | 17.5 | 551 | 14.0 | 0.6 |
| GIST | 246 | 12.5 | 250 | 12.7 | 496 | 12.6 | 1.0 |
| Liposarcoma | 228 | 11.6 | 130 | 6.6 | 358 | 9.1 | 1.8 |
| Dedifferentiated liposarcoma | 125 | 6.3 | 69 | 3.5 | 194 | 4.9 | 1.8 |
| Round cell \ Myxoid liposarcoma | 42 | 2.1 | 29 | 1.5 | 71 | 1.8 | 1.4 |
| Pleomorphic liposarcoma | 18 | 0.9 | 7 | 0.4 | 25 | 0.6 | 2.6 |
| Liposarcoma NOS | 43 | 2.2 | 25 | 1.3 | 68 | 1.7 | 1.7 |
| Chondrosarcoma | 123 | 6.2 | 118 | 6.0 | 241 | 6.1 | 1.0 |
| Dermatofibrosarcoma | 101 | 5.1 | 124 | 6.3 | 225 | 5.7 | 0.8 |
| Kaposi sarcoma | 156 | 7.9 | 25 | 1.3 | 181 | 4.6 | 6.2 |
| Angiosarcoma | 54 | 2.7 | 115 | 5.8 | 169 | 4.3 | 0.5 |
| Osteosarcoma | 84 | 4.3 | 71 | 3.6 | 155 | 3.9 | 1.2 |
| Ewing sarcoma | 72 | 3.7 | 65 | 3.3 | 137 | 3.5 | 1.1 |
| Myxofibrosarcoma | 75 | 3.8 | 49 | 2.5 | 124 | 3.1 | 1.5 |
| Rhabdomyosarcoma | 66 | 3.4 | 51 | 2.6 | 117 | 3.0 | 1.3 |
| Embryonal rhabdomyosarcoma | 27 | 1.4 | 16 | 0.8 | 43 | 1.1 | 1.7 |
| Alveolar rhabdomyosarcoma | 10 | 0.5 | 12 | 0.6 | 22 | 0.6 | 0.8 |
| Pleomorphic rhabdomyosarcoma | 14 | 0.7 | 7 | 0.4 | 21 | 0.5 | 2.0 |
| Spindle cell rhabdomyosarcoma | 7 | 0.4 | 7 | 0.4 | 14 | 0.4 | 1.0 |
| Rhabdomyosarcoma NOS | 8 | 0.4 | 9 | 0.5 | 17 | 0.4 | 0.9 |
| Nerve Sheath Tumors | 38 | 1.9 | 44 | 2.2 | 82 | 2.1 | 0.9 |
| Endometrial stromal sarcoma | – | – | 81 | 4.1 | 81 | 2.1 | – |
| Synovial sarcoma | 37 | 1.9 | 40 | 2.0 | 77 | 2.0 | 0.9 |
| Spindle cell synovial sarcoma | 19 | 1.0 | 20 | 1.0 | 39 | 1.0 | 1.0 |
| Biphasic synovial sarcoma | 3 | 0.1 | 7 | 0.3 | 10 | 0.2 | 0.4 |
| Synovial sarcoma NOS | 15 | 0.8 | 13 | 0.7 | 28 | 0.7 | 1.2 |
| Chordoma | 40 | 2.0 | 27 | 1.4 | 67 | 1.7 | 1.5 |
| Solitary fibrous tumor, malignant | 33 | 1.7 | 33 | 1.7 | 66 | 1.7 | 1.0 |
| Fibrosarcoma | 15 | 0.8 | 16 | 0.8 | 31 | 0.8 | 0.9 |
| Malignant myoepithelioma | 12 | 0.6 | 11 | 0.6 | 23 | 0.6 | 1.1 |
| Epithelioid haemangioendothelioma | 9 | 0.5 | 11 | 0.6 | 20 | 0.5 | 0.8 |
| Other (with fewer than 20 cases) | 49 | 2.5 | 57 | 2.9 | 106 | 2.7 | 0.7 |
| Overall | 1970 | 100.0 | 1972 | 100.0 | 3942 | 100.0 | 1.0 |
aUnclassified sarcomas include: Sarcoma NOS (ICDO-88003), undifferentiated spindle cell sarcoma (ICDO-88013), undifferentiated pleomorphic sarcoma (ICDO-88023), undifferentiated round cell sarcoma (ICDO-88033), epithelioid sarcoma (ICDO-88043), undifferentiated sarcoma NOS (ICDO-88053)
The crude incidence rate and ASR-W of sarcomas were 7.4 and 5.0, respectively (Table 2). The ASR-W of soft tissue, bone and gastro-intestinal sarcomas were 2.1, 1.0 and 0.6, respectively. For the five most frequent histological subtypes, the ASR-W ranged from 0.3 to 0.7 with gender variations. For the two most frequent genomic profiles (over 60% of all sarcoma cases) the ASR-W was 1.9 for complex genomic and 1.3 for recurrent translocation events.
Table 2.
Sarcoma crude and age-standardized incidence rate per 100,000 person-years according to topographic, genomic and histological groups by sex. FRANCIM network data 2010–2013 (19 registries)
| Median Age | Male | Female | Overall | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIR | ASR-W (segi) | ASR-E | ASR-US | CIR | ASR-W (segi) | ASR-E | ASR-US | CIR | ASR-W (segi) | ASR-E | ASR-US | ||
| Sarcomas by topographic groups | |||||||||||||
| Soft tissue | 65 | 3.70 | 2.43 | 3.12 | 3.31 | 2.90 | 1.91 | 2.34 | 2.38 | 3.30 | 2.15 | 2.68 | 2.78 |
| Bone | 47 | 1.20 | 1.09 | 1.16 | 1.17 | 0.90 | 0.85 | 0.87 | 0.88 | 1.10 | 0.96 | 1.01 | 1.01 |
| Skin | 54 | 1.00 | 0.70 | 0.88 | 0.91 | 0.60 | 0.46 | 0.54 | 0.54 | 0.80 | 0.59 | 0.72 | 0.73 |
| Viscera | |||||||||||||
| Gastro-intestinal organs | 69 | 1.10 | 0.65 | 0.91 | 0.95 | 1.00 | 0.57 | 0.77 | 0.79 | 1.10 | 0.58 | 0.81 | 0.84 |
| Female genital organs | 62 | – | – | – | – | 1.00 | 0.62 | 0.82 | 0.81 | 1.00 | 0.62 | 0.82 | 0.81 |
| Other visceral organs | 65 | 0.40 | 0.26 | 0.33 | 0.33 | 0.50 | 0.28 | 0.36 | 0.37 | 0.40 | 0.26 | 0.34 | 0.35 |
| Others anatomic sites | 55 | 0.10 | 0.15 | 0.13 | 0.13 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Sarcomas by genomic groups | |||||||||||||
| Complex genomic alterations | 65 | 2.80 | 1.78 | 2.30 | 2.43 | 3.10 | 1.93 | 2.44 | 2.47 | 2.90 | 1.87 | 2.38 | 2.45 |
| MDM2 amplification | 68 | 0.50 | 0.30 | 0.42 | 0.45 | 0.30 | 0.18 | 0.23 | 0.23 | 0.40 | 0.23 | 0.31 | 0.33 |
| Mutations | 68 | 1.10 | 0.66 | 0.87 | 0.90 | 1.00 | 0.58 | 0.76 | 0.77 | 1.00 | 0.62 | 0.80 | 0.83 |
| Recurrent translocations | 46 | 1.30 | 1.23 | 1.30 | 1.30 | 1.60 | 1.44 | 1.56 | 1.54 | 1.50 | 1.34 | 1.44 | 1.43 |
| Undefined/Miscellaneous alterations | 65 | 1.90 | 1.27 | 1.62 | 1.70 | 1.20 | 0.73 | 0.90 | 0.94 | 1.60 | 0.97 | 1.22 | 1.26 |
| Sarcomas by histologic groups | |||||||||||||
| Unclassified sarcomaa | 69 | 1.30 | 0.71 | 1.00 | 1.08 | 1.10 | 0.62 | 0.82 | 0.85 | 1.20 | 0.65 | 0.90 | 0.94 |
| Leiomyosarcoma | 66 | 0.80 | 0.43 | 0.62 | 0.68 | 1.30 | 0.74 | 0.99 | 0.99 | 1.00 | 0.58 | 0.79 | 0.81 |
| GIST | 69 | 0.90 | 0.52 | 0.75 | 0.78 | 0.90 | 0.47 | 0.65 | 0.67 | 0.90 | 0.50 | 0.70 | 0.72 |
| Liposarcoma | 67 | 0.90 | 0.48 | 0.68 | 0.72 | 0.50 | 0.28 | 0.37 | 0.37 | 0.70 | 0.38 | 0.51 | 0.53 |
| Dedifferentiated liposarcoma | 69 | 0.50 | 0.24 | 0.35 | 0.38 | 0.30 | 0.11 | 0.16 | 0.16 | 0.40 | 0.17 | 0.25 | 0.26 |
| Round cell \ Myxoid liposarcoma | 51 | 0.20 | 0.13 | 0.16 | 0.16 | 0.10 | 0.09 | 0.11 | 0.11 | 0.10 | 0.10 | 0.12 | 0.12 |
| Pleomorphic liposarcoma | 73 | 0.10 | 0.03 | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.02 |
| Liposarcoma, NOS | 67 | 0.20 | 0.09 | 0.13 | 0.14 | 0.10 | 0.04 | 0.05 | 0.06 | 0.10 | 0.05 | 0.08 | 0.08 |
| Chondrosarcoma | 55 | 0.50 | 0.32 | 0.41 | 0.40 | 0.40 | 0.30 | 0.36 | 0.36 | 0.50 | 0.34 | 0.41 | 0.41 |
| Dermatofibrosarcoma | 44 | 0.40 | 0.31 | 0.35 | 0.35 | 0.50 | 0.40 | 0.45 | 0.44 | 0.40 | 0.37 | 0.42 | 0.41 |
| Kaposi sarcoma | 63 | 0.60 | 0.40 | 0.52 | 0.54 | 0.10 | 0.04 | 0.06 | 0.06 | 0.30 | 0.20 | 0.27 | 0.28 |
| Angiosarcoma | 73 | 0.20 | 0.10 | 0.15 | 0.16 | 0.40 | 0.17 | 0.25 | 0.27 | 0.30 | 0.16 | 0.23 | 0.25 |
| Osteosarcoma | 34 | 0.30 | 0.34 | 0.33 | 0.33 | 0.30 | 0.25 | 0.24 | 0.25 | 0.30 | 0.28 | 0.27 | 0.27 |
| Ewing sarcoma | 19 | 0.30 | 0.35 | 0.30 | 0.30 | 0.20 | 0.32 | 0.27 | 0.27 | 0.30 | 0.33 | 0.28 | 0.28 |
| Myxofibrosarcoma | 66 | 0.30 | 0.16 | 0.22 | 0.22 | 0.20 | 0.10 | 0.14 | 0.14 | 0.20 | 0.14 | 0.19 | 0.19 |
| Rhabdomyosarcoma | 25 | 0.30 | 0.26 | 0.24 | 0.25 | 0.20 | 0.21 | 0.18 | 0.18 | 0.20 | 0.25 | 0.22 | 0.23 |
| Embryonal rhabdomyosarcoma | 12 | 0.10 | 0.15 | 0.11 | 0.11 | 0.10 | 0.09 | 0.06 | 0.06 | 0.10 | 0.11 | 0.08 | 0.08 |
| Alveolar rhabdomyosarcoma | 22 | 0.00 | 0.05 | 0.04 | 0.04 | 0.00 | 0.08 | 0.06 | 0.06 | 0.00 | 0.05 | 0.04 | 0.04 |
| Pleomorphic rhabdomyosarcoma | 69 | 0.10 | 0.02 | 0.03 | 0.03 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 | 0.02 |
| Spindle cell rhabdomyosarcoma | 40 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.03 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rhabdomyosarcoma NOS | 64 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | 0.01 | 0.02 | 0.02 |
| Nerve Sheath Tumors | 55 | 0.10 | 0.10 | 0.13 | 0.13 | 0.20 | 0.12 | 0.15 | 0.15 | 0.20 | 0.12 | 0.15 | 0.15 |
| Endometrial stromal sarcoma | 62 | – | – | – | – | 0.30 | 0.17 | 0.23 | 0.23 | 0.30 | 0.17 | 0.23 | 0.23 |
| Synovial sarcoma | 47 | 0.10 | 0.16 | 0.17 | 0.17 | 0.10 | 0.13 | 0.15 | 0.14 | 0.10 | 0.15 | 0.16 | 0.16 |
| Spindle cell synovial sarcoma | 49 | 0.1 | 0.07 | 0.05 | 0.05 | 0.10 | 0.05 | 0.06 | 0.06 | 0.10 | 0.05 | 0.07 | 0.07 |
| Biphasic synovial sarcoma | 44 | 0.0 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Synovial sarcoma NOS | 44 | 0.1 | 0.06 | 0.04 | 0.06 | 0.00 | 0.05 | 0.05 | 0.05 | 0.10 | 0.05 | 0.05 | 0.05 |
| Chordoma | 61 | 0.20 | 0.11 | 0.14 | 0.15 | 0.10 | 0.06 | 0.08 | 0.08 | 0.10 | 0.09 | 0.12 | 0.12 |
| Solitary fibrous tumor. Malignant | 63 | 0.10 | 0.11 | 0.14 | 0.13 | 0.10 | 0.08 | 0.10 | 0.10 | 0.10 | 0.10 | 0.12 | 0.12 |
| Fibrosarcoma | 60 | 0.10 | 0.03 | 0.05 | 0.05 | 0.10 | 0.03 | 0.04 | 0.04 | 0.10 | 0.03 | 0.04 | 0.04 |
| Malignant myoepithelioma | 56 | 0.00 | 0.03 | 0.04 | 0.04 | 0.00 | 0.03 | 0.04 | 0.04 | 0.00 | 0.03 | 0.04 | 0.04 |
| Epithelioid haemangioendothelioma | 47 | 0.00 | 0.03 | 0.03 | 0.03 | 0.00 | 0.05 | 0.05 | 0.05 | 0.00 | 0.05 | 0.06 | 0.06 |
| Other (with fewer than 20 cases) | 46 | 0.20 | 0.19 | 0.17 | 0.18 | 0.20 | 0.19 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Total | 63 | 7.60 | 5.27 | 6.54 | 6.80 | 7.20 | 4.81 | 5.83 | 5.90 | 7.40 | 5.00 | 6.12 | 6.26 |
Abreviations: GIST Gastro-Intestinal Stromal Tumors, CIR Crude Incidence Rate per 100,000 persons-years, ASR-W, ASR-E and ASR-US Age-Standardized incidence Rate from three reference populations (W, World Segi; E, European; US, United-States)
aUnclassified sarcomas include: Sarcoma not otherwise specified (ICDO-88003), undifferentiated spindle cell sarcoma (ICDO-88013), undifferentiated pleomorphic sarcoma (ICDO-88023), undifferentiated round cell sarcoma (ICDO-88033), epithelioid sarcoma (ICDO-88043), undifferentiated sarcoma NOS (ICDO-88053)
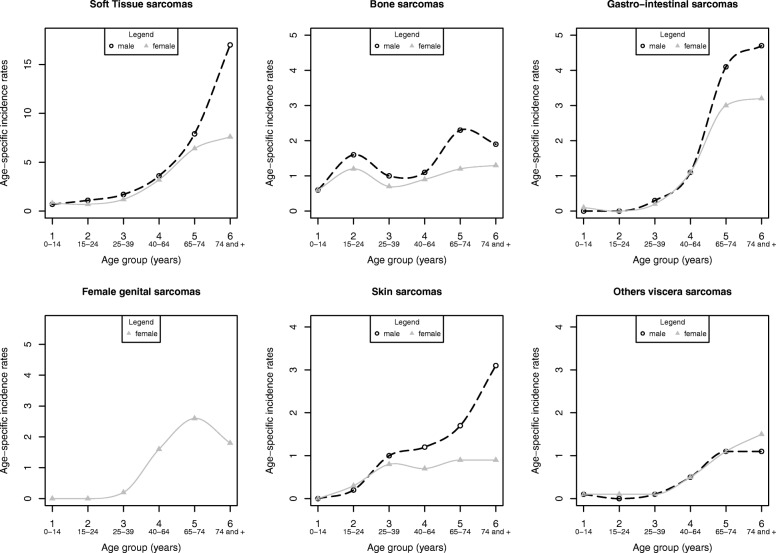
The overall sarcoma incidence peaked at 22 in patients aged 75 or over (data not shown). Age-specific rates for soft tissue, viscera and skin sarcomas were relatively stable among patients aged between 0 and 40 years, and then increased with age (Fig. 1). This increase was less pronounced in women. In men, bone sarcomas presented a biphasic profile with a first peak in young people between 15 and 25 years of age and a second peak in adults aged between 65 and 74 years of age. With respect to histological subtypes, age-specific incidence rates had various profiles (see Additional File 2). According to the genomic profile, the incidence increased steadily with age, except for tumors harboring recurrent translocations and MDM2 amplification among women (see Additional File 3).
Fig. 1.
Age-specific incidence rates of sarcomas per 100,000 person-years according to topographic groups. FRANCIM network data 2010–2013 (19 registries)
The ASR-W for all sarcomas increased between 2000 and 2005 (APC = 3.6%), and remained stable since 2005 (non-significant APC, Table 3). According to the anatomic site, the ASR-W decreased for skin sarcomas (APC = -2.0%) and female genital tumors between 2005 and 2013 (APC = -2.2%). Stratifying by major histological subtypes, the ASR-W increased for GIST (APC = 3.7%), chondrosarcoma (APC = 4.1%), myxofibrosarcoma (8.2%) and solitary fibrous tumors (12.2%) and decreased for leiomyosarcoma (APC = -2.6%), Kaposi sarcoma (− 4.1%) and fibrosarcoma (APC = -9.2%). All trend figures are provided in the online supplementary material (see Additional Files 4 and 5).
Table 3.
Annual percentage change of world age-standardized incidence rate by topographic groups, histologic types. FRANCIM network data 2000–2013 (11 registries)
| n | Joinpoint | APC | 95% CI | |
|---|---|---|---|---|
| Sarcomas by topographic groups | ||||
| Soft Tissue | 3766 | 0.8 | (−0.4; 2.0) | |
| Bone | 1193 | 1.2 | (−0.4; 2.9) | |
| Skin | 1062 | -2.0a | (−3.5; −0.4) | |
| Viscera tumors organs | ||||
| Gastro-intestinal organs | 1053 | 1.5 | (−0.3; 3.3) | |
| Female genital organs | 297 | 2000–2005 | 4.2 | (−4.2; 13.2) |
| 376 | 2005–2013 | −6.7a | (−10.4; −2.7) | |
| Other visceral organs | 540 | −1.7 | (−5.1; 1.8) | |
| Other anatomic sites | 171 | 1.0 | (−3.4; 5.6) | |
| Sarcomas by histologic groups | ||||
| Unclassified sarcoma | 1513 | −1.6 | (−3.6; 0.3) | |
| Leiomyosarcoma | 1281 | −2.6a | (−4.6; −0.6) | |
| GIST | 822 | 3.7a | (0.8; 6.8) | |
| Liposarcoma | 713 | 1.3 | (−1.1; 3.7) | |
| Dermatofibrosarcoma | 496 | 0.6 | (−1.4; 2.7) | |
| Chondrosarcoma | 454 | 4.1a | (1.6; 6.6) | |
| Kaposi sarcoma | 419 | −4.1a | (−6.8; −1.4) | |
| Osteosarcoma | 359 | −0.6 | (−3.7; 2.6) | |
| Angiosarcoma | 335 | 2.2 | (−1.2; 5.7) | |
| Ewing sarcoma | 330 | −0.2 | (−4.1; 3.8) | |
| Rhabdomyosarcoma | 286 | −1.1 | (−6.2; 4.4) | |
| Others (with fewer than 20 cases) | 226 | 4.1 | (−1.3; 9.8) | |
| Synovial sarcoma | 219 | 1.2 | (−4.2; 6.9) | |
| Nerve Sheath Tumors | 191 | −0.1 | (−4.3; 4.4) | |
| Myxofibrosarcoma | 183 | 8.2a | (0.4; 16.6) | |
| Endometrial stromal sarcoma | 173 | −3.7 | (−7.4; 0.1) | |
| Fibrosarcoma | 151 | −9.2a | (−15.7; −2.3) | |
| Chordoma | 126 | 0.8 | (−4.8; 6.6) | |
| Solitary fibrous tumor. Malignant | 102 | 12.2a | (6.2; 18.5) | |
| Epithelioid haemangioendothelioma | 55 | – | – | |
| Myoepithelial carcinoma | 24 | – | – | |
| Total | 3359 | 2000–2005 | 3.6a | (0.2; 7.1) |
| 5099 | 2005–2013 | −1.4 | (−2.9; 0.1) | |
Note. Joinpoint = years when statistically significant changes in incidence trend occurred
APC Annual Percent Change, CI Confidence Interval
aIndicates that the APC is significantly different from 0 at the alpha = 0.05 level
Discussion
In this study, we precisely described the incidence of sarcomas according to different classifications (anatomic, histologic and genetic) using data from population-based cancer registries. To our knowledge, this is one of the first reports on sarcomas based on a systematic pathological review of these cancers while taking into account the updated sarcoma classifications.
In this study, sarcomas accounted for 1.3% of all malignant tumors (1.1% for soft tissue -including skin and viscera- and 0.2% for bone) and had an ASR-E of 6.1 per 100,000 person-years over the 2010–2013 period (European population standard). The ASR-E was slightly higher than that reported in Europe [12]. Data comparison between countries is difficult due to the heterogeneity of sarcoma definition used as inclusion criteria. This heterogeneity is mainly related to some analysis characteristics: i) certain specific histological subtypes are not consistently included in analyses (e.g. Kaposi sarcoma or dermatofibroma sarcoma); ii) some studies consider adults and children separately, while others mix them; and iii) anatomic sites may be limited to specific sites such as STS. The current approach to describe sarcomas using registry data based on expert recommendations are expected to better follow epidemiological indicators and to carry out reliable comparisons between countries.
With respect to the anatomic site, ASR-E for STS (2.7) in our study was below most published international incidence rates. This may be explained by the exclusion of visceral sarcomas of soft tissue and the different description of well-differentiated liposarcoma compared to the WHO 2013 classification. In the current study, ASR-Ws for bone sarcomas among males and females (1.1 and 0.9 respectively) were close to those recently reported in five continents (2010–13 period, ASR-W 0.8–1.2 in males and 0.5–1.0 in females) [13]. For visceral sarcomas, the comparison between studies with inclusion periods close to that in the present study showed ASR-E similar to ours [8, 14]. In contrast, the ASR was greater than that reported in the RARECARE project, which may be due to differences in the definition of visceral sarcomas (GIST not included) [14].
The comparison of ASRs for main histologic groups between studies with a shorter inclusion period showed that the ASR-E for leiomyosarcoma (0.8; 0.6 for males and 1.0 for females) was greater than that reported in France (0.6) and was similar to that reported in three European regions (0.5 for males and 1.0 for females) [8, 14]. ASR-E for liposarcoma in our study (0.5; 0.7 for males and 0.4 for females), was lower than that reported in France (0.8) and in three European regions (1.06 for males and 0.59 for females), which may be attributed to differences in the definition of liposarcoma as inclusion criteria [8, 14]. In our study, we found an ASR-W for osteosarcoma slightly lower than that of chondrosarcoma (0.28 versus 0.34). For male, ASR-W was equivalent (0.34 versus 0.32). A recent population-based study from Swiss cancer registries showed similar results [15]. In contrast, others studies based on older inclusion period of sarcoma diagnosis found an ASR-W slightly higher for osteosarcoma [8, 16]. However, looking at the trend in our study (Additional File 5), we can notice that the ASR-W of osteosarcoma was actually higher over the period 2000–2005 than the ASR-W of chondrosarcoma in accordance with these studies. The increasing trend in the ASR of chondrosarcoma and the stabilization of the ASR of osteosarcomas may logically explain why the incidence of chondrosarcomas has been higher than that of osteosarcomas in recent years.
Molecular biology of sarcomas, available for diagnosis in France since 2010 is a complementary approach and has led to a molecular classification for sarcomas [17]. For the first time, we provided ASR at national level and showed molecular profiles by age groups.
This study provides the first time trend analysis of sarcomas in France and shows that ASR-W for sarcomas increased between 2000 and 2005 (APC = 3.6%) and stabilized from 2005. The current study has not shown an increase in ASR-W for soft-tissue sarcomas. This is in contrast to reports in others countries covering different periods: in the United States APC was 1.2% for males and 0.8% for females between 1978 and 2001, in Japan APC was 0.6% between 1978 and 2007 and in Serbia APC was 0.77% between 1985 and 2009 [18–20]. We report a significant decrease in incidence for skin sarcomas over the study period and for female genital sarcomas since 2005. Some histological subtypes have shown a significant decrease over the study period: leiomyosarcoma, KS and fibrosarcoma. The decline for KS has also been described in the population from the United States over the same period [21]. These changes are consistent with the improvement in access for antiretroviral therapy among HIV-infected patients and the declining AIDS incidence in developed countries. The decrease in incidence of leiomyosarcoma and fibrosarcoma could be explained by a histological classification published by the WHO in 2002 that includes new data of immunohistochemistry and new histological subtypes. Similarly, we report an increase in incidence of GIST, likely related to the introduction in the early 2000s of an immunohistochemical diagnostic test specific to GIST tumors (KIT-activating mutations). Further, the increase in GIST was more noticeable before 2005 and stabilised after 2005. The time trend analysis also revealed a significant increase for chondrogenic sarcomas (APC = 4.4%). Such increase has been reported in a study from the United States including only women (1976–2005) [20], whereas a study from the United Kingdom showed the same trend in incidence for both sexes (1988–2007) [13]. The strongest hypothesis to explain the increased risk of chondrogenic sarcoma in women is the introduction of exogenous estrogen exposures (oral contraceptives, hormone therapy), whereas other factors has to be identified in men [13, 16].
The different incidence trends for sarcomas reported over the world may partly be explained by variations in diagnosis practices and the classification used. The impact of environmental factors in the etiology of these cancers may also be a point at issue. However, the large heterogeneity of histological subtypes and the rarity of sarcomas prevent examining this association and drawing conclusions from existing environmental epidemiological studies. A national French study on the etiology of sarcomas (Etiosarc) has been launched to study the possible effect of environmental factors [22].
A major strength in this study is that the incidence of sarcomas was estimated using the 2013 WHO classification [1]. Whenever possible, registry data was converted to the latest classification to take into account changes and evolutions between different classifications (e.g. new morphological terms, obsolete morphological codes and terms).
Moreover, this study is the first to describe sarcomas in a geographic area where an expert sarcoma pathologist reviews the pathologic diagnosis. Contrary to imperfectly estimated sarcoma incidence rates, this review allows to provide a consistent incidence of sarcomas. A French study, confirmed these results and indicated that 45% of sarcomas are misclassified at initial diagnosis and that 19% have complete discordance [3]. For this reason, the review for sarcoma diagnosis is necessary to estimate a consistent incidence and more so for the different subgroups. In France, the second review was based on voluntary participation before the year 2010. Thereby, we cannot be certain that the review was obtained for all sarcomas in the period 2000–2010, even if significant efforts were made by French sarcoma network in order that pathologists systematically send slides of any newly diagnosed of sarcomas. For this reason, the estimated incidence over the 2010–2013 seems to be most relevant and reliable.
Conclusion
This study provided the opportunity to precisely describe the incidence of sarcomas according to three different groups (anatomic, histologic and genetic) defined by sarcoma specialists using data from population-based cancer registries. To our knowledge, this study is the first to report sarcoma incidence based on a systematic pathological review of these cancers and taking into account the updated sarcoma classifications. Due to literature paucity on sarcomas, future studies using data from population-based cancer registries will have to consider a strict inclusion criterion presented in our study to better describe and compare data between countries. The molecular classification will be useful for etiological studies as incidence studies.
Supplementary information
Additional file 1. Complementary information on data collection and statistical analyses.
Additional file 2: Figure S1. Age-specific incidence rates of sarcomas per 100,000 person-years according to histologic groups. FRANCIM network data 2010–2013 (19 registries).
Additional file 3: Figure S2. Age-specific incidence rates of sarcomas per 100,000 person-years according to genomic groups. FRANCIM network data 2010–2013 (19 registries).
Additional file 4: Figure S3. Sarcoma trends and annual percentage change (APC) of world age-standardized incidence rate according to topographic group. FRANCIM network data 2000–2013 (11 registries).
Additional file 5: Figure S4. Sarcoma trends and annual percentage change (APC) of world age-standardized incidence rate according to histologic group. FRANCIM network data 2000–2013 (11 registries).
Acknowledgments
We thank Vianney Jouhet for advice about classification alignements and Marie Poiseuil for datamanagement. Thanks to Jone Iriondo-Alberdi for proofreading and comments.
We thank the Francim Network for their collaboration in the study: J Jégu, M Velten (Bas-Rhin General Cancer Registry); E Cornet, X Troussard (Registre Régional des Hémopathies Malignes de Basse Normandie); A M Bouvier (Registre Bourguignon des Cancers Digestifs); A V Guizard (Registre Général des Tumeurs du Calvados); V Bouvier, G Launoy (Registre des Tumeurs Digestives du Calvados); P Arveux (Breast cancers registry of Côte-d’Or France); M Maynadié, M Mounier (Hémopathies Malignes de Côte d’Or); A S Woronoff (Doubs and Belfort Territory General Cancer Registry); M Daoulas, M Robaszkiewicz (Finistère Cancer Registry); J Clavel, S Goujon (French National Registry of Childhood Hematopoietic Malignancies); B Lacour (National Registry of Childhood Solid Tumors); I Baldi, C Pouchieu (Gironde Registry of Primary Central Nervous System Tumors); B Amadeo, G Coureau (General Cancer Registry of Gironde Department); S Leguyader, A Monnereau, S Orazio (Registre des Hémopathies Malignes de la Gironde); P M Preux, F Rharbaoui (Registre Général des Cancers de Haute-Vienne); E Marrer (Haut-Rhin Cancer Registry); B Trétarre (Registre des Tumeurs de l’Hérault); M Colonna, P Delafosse (Registre du Cancer du Département de l’Isère); K Ligier, S Plouvier (Registre Général des Cancers de Lille et de sa Region); A Cowppli-Bony, F Molinié (Loire-Atlantique-Vendée Cancer Registry); S Bara (Manche Cancer Registry); O Ganry, B Lapôtre-Ledoux (Registre du Cancer de la Somme); P Grosclaude (Tarn Cancer Registry); N Bossard, Z Uhry (Hospices Civils de Lyon). We thank all pathologists, clinicians, and clinical research assistants of French sarcoma networks (RRePS, NetSarc and ReSos).
Abbreviations
- APC
Annual percentage change
- ASR
Age-standardized incidence rates
- CI
Confident interval
- GIST
Gastro-intestinal stromal tumors
- ICD-O-3
International Classification of Diseases for Oncology, third edition
- KS
Kaposi sarcoma
Authors’ contributions
BA performed the statistical analyses and wrote the original draft. ED, SMP and NP conceived of the study and contributed to revising the manuscript for intellectual content. JMC, IRC, NP (sarcoma specialists) validated ICD–O3 codes to include in the study. Francim network participated in the data acquisition. JG contributed to manuscript preparation and writing review. KL, PD, AMB, SP, AL, GC and AM contributed to manuscript validation and writing-review. All authors read and approval the final manuscript.
Funding
This work was supported by the French National Cancer Institute (in the framework of INCa-BCB 2012 grant for constitution of multicentre clinical and biological databases nationwide in cancer. Funding bodies had no role in the design of the study, collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to national regulations. Permission to use French cancer registry data was provided by the National Cancer Institute after consultation with the data protection authority.
Ethics approval and consent to participate
This study is based on data from cancer registries gathered in the French network of cancer registries and a representative of each registry was involved in the study and approved the use of its data. All French registries received an authorization to collect patient data from the data protection authority (Commission Nationale de l’Informatique et des Libertés). Ethics approval and consent to participate were not required for this study which is an observational research without direct contact with patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-6683-0.
References
- 1.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of Tumours of soft tissue and bone. 4. Lyon: IARC Press; 2013. [Google Scholar]
- 2.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lurkin A, Ducimetiere F, Vince DR, et al. Epidemiological evaluation of concordance between initial diagnosis and central pathology review in a comprehensive and prospective series of sarcoma patients in the Rhone-Alpes region. BMC Cancer. 2010;10:150. doi: 10.1186/1471-2407-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonescu CR. The role of genetic testing in soft tissue sarcoma. Histopathology. 2006;48:13–21. doi: 10.1111/j.1365-2559.2005.02285.x. [DOI] [PubMed] [Google Scholar]
- 5.Honoré C, Méeus P, Stoeckle E, Bonvalot S. Soft tissue sarcoma in France in 2015: epidemiology, classification and organization of clinical care. J Visc Surg. 2015;152:223–230. doi: 10.1016/j.jviscsurg.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 6.French reference network of sarcoma [Internet]. [cited 2019 Dec 06]; Available from: https://netsarc.sarcomabcb.org/.
- 7.Blay J-Y, Coindre J-M, Ducimetière F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17:e62–e69. doi: 10.1016/S1470-2045(15)00388-5. [DOI] [PubMed] [Google Scholar]
- 8.Ducimetiere F, Lurkin A, Ranchere-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathoulin-Pelissier S, Chevreau C, Bellera C, et al. Adherence to consensus-based diagnosis and treatment guidelines in adult soft-tissue sarcoma patients: a French prospective population-based study. Ann Oncol. 2014;25:225–231. doi: 10.1093/annonc/mdt407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institut National du Cancer. Francim Network. [cited 2019 Dec 06]; Available from: http://lesdonnees.e-cancer.fr/Informations/Sources/SOURCE-Reseau-FRANCIM.
- 11.Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol. 2010;21:1106–1111. doi: 10.1093/annonc/mdp415. [DOI] [PubMed] [Google Scholar]
- 12.Stiller CA, Trama A, Serraino D, et al. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Valery PC, Laversanne M, Bray F. Bone cancer incidence by morphological subtype: a global assessment. Cancer Causes Control. 2015;26:1127–1139. doi: 10.1007/s10552-015-0607-3. [DOI] [PubMed] [Google Scholar]
- 14.Mastrangelo G, Coindre JM, Ducimetiere F, et al. Incidence of soft tissue sarcoma and beyond: a population-based prospective study in 3 European regions. Cancer. 2012;118:5339–5348. doi: 10.1002/cncr.27555. [DOI] [PubMed] [Google Scholar]
- 15.Kollár A, Rothermundt C, Klenke F, et al. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019;63:101596. doi: 10.1016/j.canep.2019.101596. [DOI] [PubMed] [Google Scholar]
- 16.Anfinsen KP, Devesa SS, Bray F, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976-2005) Cancer Epidemiol Biomark Prev. 2011;20:1770–1777. doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 17.Coindre J-M, Ducimetière F, Mathoulin-Pélissier S, et al. Illustration de partenariat public/privé. Prises en charge des sarcomes des tissus mous en France–une analyse rétrospective de la « base clinico-biologique sarcomes ». Rev Epidemiol Sante Publique. 2016;64:S278. doi: 10.1016/j.respe.2016.04.022. [DOI] [Google Scholar]
- 18.Toro JR, Travis LB, Wu HJ, Zhu K, Fletcher CD, Devesa SS. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978-2001. An analysis of 26,758 cases. Int J Cancer. 2006;119:2922–2930. doi: 10.1002/ijc.22239. [DOI] [PubMed] [Google Scholar]
- 19.Nomura E, Ioka A, Tsukuma H. Incidence of soft tissue sarcoma focusing on gastrointestinal stromal sarcoma in Osaka, Japan, during 1978-2007. Jpn J Clin Oncol. 2013;43:841–845. doi: 10.1093/jjco/hyt073. [DOI] [PubMed] [Google Scholar]
- 20.Dugandzija T, Mikov MM, Solajic N, Nikolin B, Trifunovic J, Ilic M. Increasing frequency of soft tissue sarcomas in Vojvodina - comparison with the literature. Asian Pac J Cancer Prev. 2014;15:1011–1014. doi: 10.7314/APJCP.2014.15.2.1011. [DOI] [PubMed] [Google Scholar]
- 21.Royse KE, El Chaer F, Amirian ES, et al. Disparities in Kaposi sarcoma incidence and survival in the United States: 2000-2013. PLoS One. 2017;12:e0182750. doi: 10.1371/journal.pone.0182750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacourt A, Amadéo B, Gramond C, et al. ETIOSARC study : environmental aetiology of sarcomas from a French prospective multicentric population-based case-control study-study protocol. BMJ Open. 2019;9:e030013. doi: 10.1136/bmjopen-2019-030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Complementary information on data collection and statistical analyses.
Additional file 2: Figure S1. Age-specific incidence rates of sarcomas per 100,000 person-years according to histologic groups. FRANCIM network data 2010–2013 (19 registries).
Additional file 3: Figure S2. Age-specific incidence rates of sarcomas per 100,000 person-years according to genomic groups. FRANCIM network data 2010–2013 (19 registries).
Additional file 4: Figure S3. Sarcoma trends and annual percentage change (APC) of world age-standardized incidence rate according to topographic group. FRANCIM network data 2000–2013 (11 registries).
Additional file 5: Figure S4. Sarcoma trends and annual percentage change (APC) of world age-standardized incidence rate according to histologic group. FRANCIM network data 2000–2013 (11 registries).
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to national regulations. Permission to use French cancer registry data was provided by the National Cancer Institute after consultation with the data protection authority.